1
Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, Nijmegen, The Netherlands
2
Helen Wills Neuroscience Institute, University of California Berkeley, Berkeley, CA, USA
3
Max Planck Institute for Psycholinguistics, Nijmegen, The Netherlands
If motor imagery uses neural structures involved in action execution, then the neural correlates of imagining an action should differ between individuals who tend to execute the action differently. Here we report fMRI data showing that motor imagery is influenced by the way people habitually perform motor actions with their particular bodies; that is, motor imagery is ‘body-specific’ (Casasanto, 2009
). During mental imagery for complex hand actions, activation of cortical areas involved in motor planning and execution was left-lateralized in right-handers but right-lateralized in left-handers. We conclude that motor imagery involves the generation of an action plan that is grounded in the participant’s motor habits, not just an abstract representation at the level of the action’s goal. People with different patterns of motor experience form correspondingly different neurocognitive representations of imagined actions.
Studies employing behavioral, electrophysiological, as well as neuroimaging techniques indicate that motor imagery involves the generation of an action plan (Decety et al., 1989
, 1994
; Jeannerod, 1994
, 2001
; Parsons, 1994
; Beisteiner et al., 1995
; Lang et al., 1996
; Porro et al., 1996
; Bonnet et al., 1997
; Schnitzler et al., 1997
; Parsons et al., 1998
; Neuper et al., 1999
, 2005
; Pfurtscheller et al., 1999
; Gerardin et al., 2000
; de Lange et al., 2005
; Helmich et al., 2007
; Szameitat et al., 2007a
,b
; Munzert et al., 2009
). As such, motor imagery can be thought of as covert motor execution (Jeannerod, 1994
, 2001
). A remaining question is whether motor imagery is body-specific (Casasanto, 2008
, 2009
). That is, does the way one performs an action in the real world influence neural activation during motor imagery? Alternatively, it may be that the motor plan generated during motor imagery is abstracted away from individual motor experience or specific effectors and occurs at the level of goal of the imagined action (Rijntjes et al., 1999
). Here we aimed to distinguish between these possibilities by measuring cerebral activity in left- and right-handed participants while they imagined performing everyday motor activities.
Previous research on this issue is inconclusive. Consistent with a body-specific view of mental imagery, there is some work showing different lateralization when imagining actions with the right hand as compared to actions with the left hand (Szameitat et al., 2007a
), and decreased motor imagery performance specifically for the affected hand in Parkinson’s disease patients (Helmich et al., 2007
). Yet, other work suggests motor planning may occur at the level of an action’s goal instead of at a more specific level such as preferred hand (Rijntjes et al., 1999
), in which case the neural correlates of motor imagery should not necessarily vary with handedness.
In our own previous research, we have conducted fMRI experiments in left- and right-handers to investigate the influence of hand preference on the neural representation of action execution and action observation (Willems and Hagoort, 2009
), and on the neural basis of action semantics in language (Willems et al., in press
). In one study we showed that differences in motor cortex activation between left- and right-handers in terms of action execution were similarly observed when participants viewed movie clips of these hand actions (Willems and Hagoort, 2009
). Thus, differences in the way we act upon the world are reflected not only in the brain areas that subserve action production, but also in the neural substrates of action perception. In another study, we found that the motor component of action verb semantics is differently lateralized in right- and left-handers. When right-handers understand a verb that names a hand action (e.g. ‘grasp’, ‘throw’) they preferentially activate left premotor areas, whereas left-handers preferentially activate right premotor areas. At least by default, right- and left-handers represent action verb meanings from an egocentric perspective, which reflects the way they perform these actions with their dominant hands (Willems et al., in press
).
Here we investigate effects of handedness on motor imagery, which lies in between the concrete domains of action execution and perception and the more abstract domain of language. If the way one performs an action in the real world is reflected in neural activation during motor imagery (i.e., if motor imagery is body-specific), then left- and right-handed participants should show differently lateralized activity in brain areas involved in the planning and execution of hand actions. Alternatively, if motor imagery only involves generating a motor plan that is abstracted away from the participant’s own motor experience, we expect to see no lateralization differences between the two groups. Such a result would be expected for instance if motor imagery is mainly based on visual experience, provided that both left- and right-handers mainly observe right-handers perform actions in the world.
Participants
We tested 32 healthy participants with no known history of neurological problems, dyslexia or other language-related problems, and with normal or corrected-to-normal vision, all of whom gave informed consent. Half of the participants were left-handed (N = 16, 12 female, mean age 23.4 years, range 19–32 years, adapted Dutch version of Edinburgh Handedness Inventory (EHI) score (Oldfield, 1971
; Van Strien, 1992
): mean = −94.3, SD = 8.7, range −82 to −100, mode = −100), and half were right handed (N = 16, 10 female, mean age 23.2, range 20–29 years, EHI score: mean = 96.6, SD = 7.3, range 82–100, mode = 100). The groups did not differ in age [|t(30)| < 1], or in absolute EHI value [t(30) < 1]. The local ethics committee approved the study.
Materials
Stimuli were 96 Dutch verbs expressing concrete actions. Half of these were related to manual actions (MAN, e.g. to throw), half of them were not related to manual actions (NONMAN, e.g. to kneel, see the Appendix for the complete list of stimuli). The distinction between MAN and NONMAN was pretested in a group of raters who did not participate in the fMRI experiment (N = 16), who scored for each verb how much they associated that action with their hand(s) on a 1- to 7-point scale. MAN words were significantly more associated with hand actions than NONMAN words [t(94) = 23.60, p < 0.001; mean MAN = 5.55, SD = 0.53; mean NONMAN = 2.04, SD = 0.83]. Raters also indicated whether they preferentially acted out the hand actions with the left, right, or with both hands. Materials were selected to ensure that the number of raters indicating to use both hands in that particular action, was low (out of 16 raters: mean = 3.36, SD = 1.89, median = 3, mode = 2). MAN and NONMAN word lists did not differ in imageability (assessed by the same group of raters) [t(94) < 1], number of phonemes [t(94) < 1] or lexical frequency [t(94) < 1]; defined using the CELEX database; Baayen et al., 1993
).
Experimental Procedure
Stimuli were presented using Presentation software (version 10.2
1
). Each trial started with a fixation cross (250 ms) followed by presentation of a written verb (1500 ms) in the middle of the screen. Participants were instructed to read the word, close their eyes, imagine performing this action and open their eyes to indicate that they had finished motor imagery after which the next trial would start (after a variable intertrial interval between 2 and 6 s in steps of 250 ms (mean = 4 s) (Dale, 1999
). Participants were instructed to ‘vividly imagine performing this action several times and open your eyes when done’. This means that there was no constraint on the amount or duration of motor imagery, this was left to each individual participant. In terms of experimental design, we were hence vulnerable to the possible confound that participants would take longer in imagining one of the action types (MAN or NONMAN). This however turned out not to be the case (see Results).
Closing and opening of the eyes was monitored by an infrared IviewX eyetracker
2
and coded on-line by one of the experimenters. We used opening and closing of the eyes to signal start and finish of imagery instead of button presses since using the eyes as a response measure enables to measure imaging times and does not contaminate hand motor cortex activation due to button presses. Previous work shows that motor imagery with eyes closed entails similar processes as motor imagery with eyes open (Heremans et al., 2008
) and has been successfully used before in neuroimaging studies (Szameitat et al., 2007a
,b
; Bakker et al., 2008
). It is possible that opening and closing of the eyes leads to differential motor cortex lateralization in left- and right-handers. However, this is not problematic for the present study since opening and closing of the eyes was required for MAN and NONMAN trials alike and should hence cancel out when comparing these conditions with each other.
Stimuli were presented in pseudo-randomized order such that a condition was repeated maximally three times in a row. A mirrored presentation order was employed in half of the participants. Participants were familiarized with the procedure by 10 practice items containing different words than those used in the experiment.
Data Acquisition and Analysis
Whole-brain cho-Planar Images were acquired with a 8-channel head coil on a Siemens MR system with 3T magnetic field strength (TR = 2060 ms; TE = 30 ms; flip angle 85°, 31 transversal slices; voxel size 3.5 mm × 3.5 mm × 3 mm, 0.5 mm gap between slices). Data analysis was done using SPM5
3
. Preprocessing involved realignment through rigid body registration, slice timing correction to the onset of the first slice, normalization to Montreal Neurological Institute (MNI) space, interpolation of voxel sizes to 2 mm × 2 mm × 2 mm, and spatial smoothing (8 mm FWHM kernel). First-level analysis involved a multiple regression analysis with regressors describing the expected hemodynamic responses during imagery of MAN words and NONMAN words. Each trial was modeled as the actual duration of the trial, convolved with a canonical hemodynamic response function (Friston et al., 1998
). MR disturbances due to small head movements were accounted for by a series of nuisance regressors, namely the linear and exponential changes in the scan-by-scan estimated head motion, scan-by-scan average signals from outside the brain, white matter, and cerebro-spinal fluid (Verhagen et al., 2006
).
A second-level whole brain group analysis with subjects as a random factor (‘random effects analysis’) involved a model with factors VERB TYPE (MAN, NONMAN) and GROUP (left-handers, right-handers). Correction for multiple comparisons was applied by thresholding group maps at p < 0.005 uncorrected and subsequently taking the cluster extent into account by using the theory of Gaussian Random Fields to correct maps at p < 0.05 corrected for multiple comparisons (Poline et al., 1997
). Differential lateralization differences between the groups was tested by means of repeated measures analysis of variance to the mean contrast estimates from the regions sensitive to the MAN > NONMAN comparison with factors HEMISPHERE (left, right) and GROUP (left-handers, right-handers). We circumvented a bias in regions of interest selection, since the regions of interest were based on the overall contrast across the two groups (Kriegeskorte et al., 2009
). Follow-up one-sided planned comparisons of within group hemispheric differences (Right-handersleft hem > Right-handersright hem and Left-handersright hem > Left-handersleft hem) and between-group comparisons (Right-handersleft hem > Left-handersleft hem and Left-handersright hem > Right-handersright hem) were performed.
We also looked at common activations across the two groups. We implemented this as a conjunction analysis (Nichols et al., 2005
) testing for areas activated to MAN > NONMAN in left-handers as well as to MAN > NONMAN in right-handers (MAN > NONMANleft-handers ∩ MAN > NONMANright-handers). For general interest, we also conducted a conjunction analysis to investigate overlapping areas during imagery of MAN actions in both groups (MANleft-handers ∩ MANright-handers).
Given the heterogeneity of effectors to which the NONMAN verbs refer, we never compared NONMAN > MAN directly.
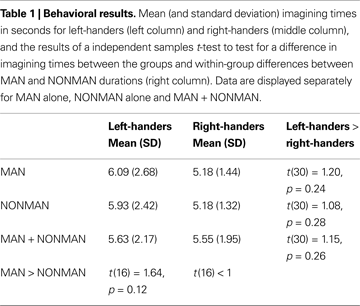
Behavioral
It took participants on average 5.63 s (SD = 2.17) to imagine the MAN verbs and 5.55 s (SD = 1.95) to imagine the NONMAN verbs (Table 1
). Right- and left-handers did not differ in imagining times, neither in overall times, nor in MAN or NONMAN times separately [MAN + NONMAN: t(30) = 1.15, p = 0.26; MAN: t(30) = 1.20, p = 0.24; NONMAN: t(30) = 1.08, p = 0.28]. There were no statistically significant differences within groups between MAN and NONMAN times [Left-handers: t(15) = 1.64, p = 0.12; Right-handers: t < 1].
Neural
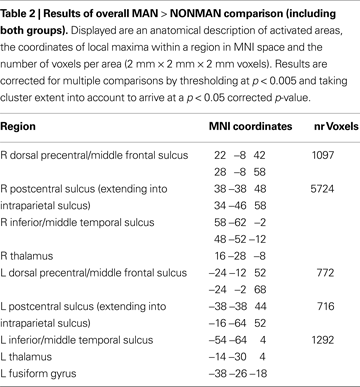
There were no areas sensitive to the main effect of GROUP. No areas were sensitive to the VERB TYPE × GROUP interaction at a whole-brain corrected statistical threshold. However, informal inspection at p < 0.005 uncorrected revealed such effects in bilateral postcentral sulcus. A wide-spread network of areas, including bilateral postcentral sulcus (mainly encompassing Brodmann Area 2; Eickhoff et al., 2005
), bilateral precentral sulcus (BA6) and bilateral inferior temporal cortex, was sensitive to the MAN > NONMAN comparison (Figure 1
; Table 2
).
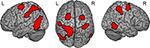
Figure 1. Result from whole brain analysis for both groups combined. Displayed are the results for comparing MAN > NONMAN across the two groups (MANleft-handers + MANright-handers > NONMANleft-handers + NONMANright-handers). Motor imagery of manual actions activated dorsal precentral sulcus, postcentral sulcus and inferior temporal sulcus bilaterally. Results are corrected for multiple comparisons at p < 0.05 corrected.
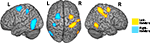
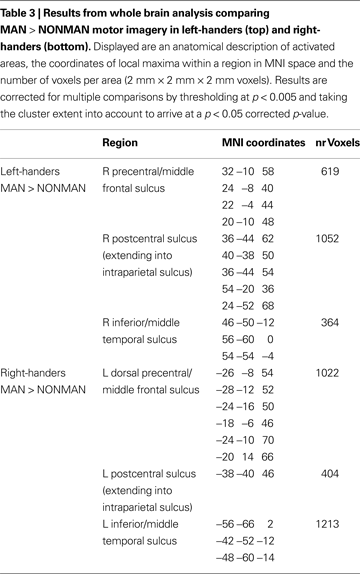
Second, we determined which regions showed sensitivity to the MAN > NONMAN contrast in each group in isolation. In the left-handers there was stronger activation to MAN as compared to NONMAN imagery in right postcentral sulcus (BA2) extending into intraparietal sulcus, right precentral sulcus (BA6) and right inferior temporal sulcus (Figure 2
; Table 3
). Conversely, for the right-handers there was stronger activation for MAN as compared to NONMAN imagery in left postcentral sulcus (BA2), left precentral sulcus (BA6) extending into intraparietal sulcus, and left inferior temporal sulcus (Figure 2
; Table 3
). Informal inspection at a liberal statistical threshold (p < 0.01 uncorrected) showed that in both groups, there was no activation in primary motor cortex (cytoarchitectonically defined BA4a and BA4p; Eickhoff et al., 2005
) to the MAN > NONMAN comparison. In previous work we observed that employing subject-specific regions of interest substantially improves sensitivity in detecting between-group differences (Willems et al., in press
; see also Aziz-Zadeh et al., 2006
). As an additional check on the involvement of primary motor cortex in body-specific motor imagery, we therefore determined subject-specific regions of interest as 4-mm spheres around local maxima to imagery of MAN trials in BA4 (again, using cytoarchitectonically defined BA4a and BA4p), and extracted the MAN > NONMAN contrast values for each subject separately. These were analyzed with repeated measures analysis of variance with factors HEMISPHERE (left, right) and GROUP (left-handers, right-handers). We did observe a HEMISPHERE × GROUP interaction to the MAN > NONMAN contrast values [F(1, 30) = 4.41, MSE = 0.06, p = 0.044], with each group showing greater activation in the hemisphere contralateral to the preferred hand.
Figure 2. Results from whole brain analysis for each group separately. Displayed are the results for the MAN > NONMAN comparison for left-handers (yellow) and right-handers (blue). Note the strong lateralization of responses in precentral, postcentral and inferior temporal sulcus. Results are corrected for multiple comparisons at p < 0.05 corrected.
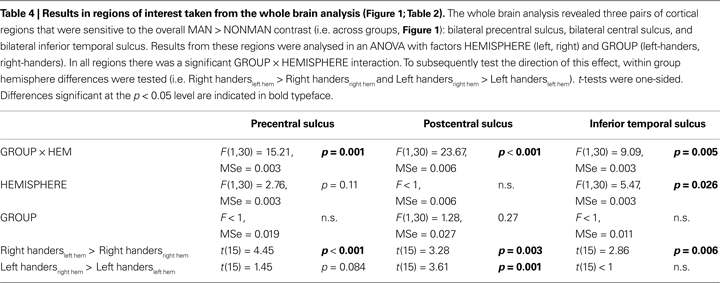
Third, to determine whether there were lateralization differences between the groups, we extracted MAN > NONMAN contrast values from precentral, postcentral and inferior/middle temporal regions sensitive to the overall MAN > NONMAN contrast (Figure 1
; Table 2
), and analyzed these in an ANOVA with factors HEMISPHERE (left, right) and GROUP (left-handers, right-handers). In all these regions, there was a significant HEMISPHERE × GROUP interaction (Table 4
; Figure 3
). Moreover, in postcentral and precentral structures, each group showed stronger activation in the hemisphere contralateral to the dominant hand than in the ipsilateral region (Table 4
; Figures 3
A,B), albeit that this difference was only a trend in precentral sulcus in left-handers (p = 0.08).
Figure 3. Results in bilateral pairs of regions activated in the whole brain analysis to the overall MAN > NONMAN comparison (Figure 1
). Displayed are the contrast values for the MAN > NONMAN contrast for: Left-handersleft hem, Left-handersright hem, Right-handersleft hem, Right-handersright hem. In all regions there is a HEMISPHERE × GROUP interaction (Table 4
). (A,B) In the precentral and postcentral sulcus, each group activated the hemisphere contralateral to their dominant hand more strongly than the other hemisphere. That is, right-handers activate these regions most strongly in the left-hemisphere, whereas left-handers activate them more strongly in the right-hemisphere. (C) For inferior/middle temporal cortex this within group difference was only present for right-handers (see text and Table 4
). Asterisks indicate statistical significance at the p < 0.05 level.
There were no areas commonly activated to MAN > NONMAN in both left and right-handers (MAN > NONMANleft-handers ∩ MAN > NONMANright-handers). However, at a lower p < 0.005 uncorrected statistical threshold, such overlap was observed in left dorsal premotor cortex. An extensive set of areas were commonly activated in left- as well as in right-handers during imagery of MAN actions (MANleft-handers ∩ MANright-handers). Activated areas include bilateral dorsal premotor cortex, bilateral inferior frontal gyrus, bilateral postcentral sulcus, bilateral inferior/middle temporal gyrus and bilateral middle occipital gyrus (Figure S1 and Table S1 in Supplementary Material).
To summarize, there were three bilateral pairs of regions (in precentral, postcentral and inferior/middle temporal sulci) which showed sensitivity to the MAN > NONMAN comparison either across the two groups (left-handers + right-handers), in left-handers (right-hemisphere regions), or in right-handers (left-hemisphere regions). Left-handers activated postcentral and precentral motor cortex more strongly in the right as compared to the left hemisphere, whereas the opposite pattern was observed in right-handers (left > right). A similar effect was observed in primary motor cortex (BA4) when employing subject-specific region of interest analysis, but not in the whole brain analysis.
In this study we investigated whether motor imagery involves the generation of a motor plan that is grounded in the way an individual typically performs the imagined action in the real world. Our results indicate that explicit motor imagery of everyday hand actions is body-specific (Casasanto, 2008
, 2009
). Left- and right-handers showed differential and opposite lateralization of activity in premotor and postcentral motor regions when they imagined performing one-handed manual actions, as compared to nonmanual actions. The hemisphere that primarily controls the dominant hand also subserves mental imagery for actions that people usually perform with this hand (see Szameitat et al., 2007a
for a compatible finding in right-handers).
Our results are in line with earlier work showing that present body posture influences motor imagery (de Lange et al., 2006
). Here we extend this by showing that long term motor history (i.e. a preference to execute an action with one hand) also influences motor imagery. In addition to finding body-specific laterality effects in dorsal premotor cortex (BA6), we also observed these effects in primary somatosensory cortex (S1, roughly corresponding with BA2), and, when using subject-specific regions of interest, in BA4. S1 activation during motor imagery has been argued to reflect the predicted somatosensory consequences of the imagined actions (i.e., a forward model, see Wolpert and Ghahramani, 2000
), but is observed only in some of the relevant neuroimaging studies (see Munzert et al., 2009
for review). Szameitat et al. (2007b)
also reported S1 activation during motor imagery when participants were required to perform imagery of everyday actions, just as in the present study (see also Sacco et al., 2006
). Some studies that did not observe S1 activation for instance employed the Parsons’ hand laterality judgment task, which arguably requires less elaborate motor imagery (e.g. Parsons et al., 1998
; de Lange et al., 2005
, 2006
). It is possible that the verbal instruction to imagine a relatively complex action for a more extended period of time (as in the present study and in Szameitat et al., 2007b
) leads to a more elaborate forward model and hence to stronger S1 activation
4
. A similar reasoning could be applied to the effects we observed in primary motor cortex (BA4). Involvement of primary motor cortex is observed in some (e.g. Tomasino et al., 2007
, 2008
), but not in other investigations of motor imagery (see Munzert et al., 2009
for overview). The findings in BA4 should be interpreted with caution since we did observe a Hemisphere × Group interaction in subject-specific regions of interest, but not in within-group comparisons in the whole brain analysis, even when assessed at liberal and uncorrected statistical thresholds.
It should be acknowledged that we did not measure EMG to ensure that there was no supra-threshold muscle activity during motor imagery. It is possible that the effects we observed in primary motor cortex/somatosensory cortex could be driven by supra-threshold muscle activation. It should be stressed however that participants were in no way encouraged to move and were explicitly instructed only to imagine performing the actions. Moreover, we checked visually that participants were not actually acting out the movements they were required to imagine. Hence primary motor cortex and somatosensory cortex activation cannot be due to actual acting out of these actions, which does not preclude subthreshold motor activation.
Interestingly, a similar lateralization difference was observed in inferior/middle temporal cortex. This suggests that the influence of hand preference extends beyond the cortical motor and language systems. Indeed in previous work we observed that lateralization of extrastriate regions involved in observation of faces (fusiform face area) and bodies (extrastriate body area) is influenced by handedness (Willems et al., in press
). However in that study extrastriate body area was right-lateralized in both groups, but to a lesser extent in left- as compared to right-handers. In the present study we observe a different lateralization pattern with a strong left-lateralization in inferior/middle temporal cortex for right-handers (Figure 3
). It is unclear what cognitive process drives these differences in temporal cortex.
The present findings suggest that when participants were asked to ‘vividly imagine performing an action’, they did so from their own (egocentric) perspective. While this may not be surprising, it was by no means a foregone conclusion. Although left-handers tend to perform actions like throwing with their left hands, they probably observe a far greater number of throws performed with the right hand, since the majority of throwers are right-handed. In principle, right- and left-handers could all generate motor images that reflect the statistics of observed actions rather than performed actions, which would result in similar patterns of motor activity across groups. The fact that participants generated body-specific images from their own perspective is consistent with our previous studies showing that people perceive actions and understand action language egocentrically, at least by default (Willems and Hagoort, 2009
; Willems et al., in press
). These findings should not be interpreted as indicating that people are only capable of imagining actions from their own perspective. It remains a question for future research how actions imagined from another’s perspective are instantiated in right- and left-handers’ motor systems (see Szameitat et al., 2007b
; Tomasino et al., 2007
).
On a methodological note, this study validates the use of differential lateralization in the motor system between left- and right-handers as an experimental tool (Longcamp et al., 2003
, 2005
; Lewis et al., 2006
). Earlier studies of motor execution differences in left- and right-handers did not observe such lateralization differences (Kim et al., 1993
; Kloppel et al., 2007
). However, a crucial difference with the present study is that in these previous studies very simple hand actions were used. For instance in Kim et al., the hand actions were simple finger-thumb oppositions. In Kloppel et al. (2007)
the hand actions involved pressing a button. Indeed we previously also did not observe the clear-cut difference in lateralization between left- and right-handers when they performed simple contractions and extension of the fingers (Willems and Hagoort, 2009
). Future research should more systematically investigate how the complexity of performed/imagined actions influences the amount of lateralization in left- and right-handers. For present purposes it is important to note that our finding of differently lateralized motor cortex activation in left- and right-handed participants lends support to using handedness-related lateralization to study the body-specificity of other cognitive processes as well (e.g. Casasanto, 2009
; Willems et al., in press
; Willems and Hagoort, 2007
). Moreover, this finding could have implications for clinical practice, in which motor imagery is sometimes used as a therapeutic tool (see Munzert et al., 2009
for comprehensive review).
Previous studies have suggested that the generation of an action plan occurs at the level of an action’s overall goal (Rijntjes et al., 1999
). Although some components of motor imagery may be abstracted away from motor experience, the present data show that motor imagery also involves generating an action plan consistent with the kinematics of actions as we tend to perform them with our particular bodies.
Stimuli used. Shown are the original stimuli in Dutch, with a translation in English. These verbs were matched and pretested on several measures, please see main text for details. Due to the literal translation in English it may be less obvious that same of the words are MAN or NONMAN verbs. It should be noted that the original Dutch stimulus set was carefully pretested to ensure that all MAN words were associated with hand actions and that the NONMAN words were not. This may not be fully captured by the translation in English and hence some of the verbs may not be obviously related to the hand(s) in the English translation. Moreover, the English translation sometimes has the same word appear twice, this was not the case in the Dutch originals.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supported by grants from European Union Joint-Action Science and Technology Project (IST-FP6-003747), the Dutch Organisation for Scientific research (NWO Rubicon 446-08-008) and the Niels Stensen foundation. We thank Martin Laverman, Jacqueline de Nooijer, Daan van Rooij and Paul Gaalman for assistance.
The Supplementary Material for this article can be found online at http://www.frontiersin.org/humanneuroscience/paper/10.3389/neuro.09/039.2009/
- ^ www.nbs.com
- ^ www.smi.de
- ^ http://www.fil.ion.ucl.ac.uk/spm/software/spm5/
- ^ This distinction is reminiscent of that between visual versus kinesthetic motor imagery (e.g. Guillot et al., 2009 ). In kinesthetic motor imagery participants are explicitly trained to focus on the kinesthetic consequences of the actions that they imagine as compared to visual motor imagery in which participants are instructed to focus on the visual aspects of motor imagery. Guillot et al. (2009) observed increased activations during kinesthetic motor imagery compared to a low-level control condition in somatosensory cortex. Such an effect was not observed when comparing visual motor imagery to the same control condition. A direct comparison between kinesthetic and visual motor imagery, however, did not reveal increased activation during kinesthetic motor imagery in S1. In our experiment we did not specifically instruct participants to focus/direct attention on one of these aspects of motor imagery and this interpretation is hence speculative.