Electrophysiological correlates of learning-induced modulation of visual motion processing in humans
1
Neurobionics Research Group, Hungarian Academy of Sciences – Péter Pázmány Catholic University – Semmelweis University, Budapest, Hungary
2
Faculty of Information Technology, Péter Pázmány Catholic University, Budapest, Hungary
3
MR Research Center, Szentágothai J. Knowledge Center – Semmelweis University, Budapest, Hungary
4
Department of Psychology, University of California, San Diego, CA, USA
Training on a visual task leads to increased perceptual and neural responses to visual features that were attended during training as well as decreased responses to neglected distractor features. However, the time course of these attention-based modulations of neural sensitivity for visual features has not been investigated before. Here we measured event related potentials (ERP) in response to motion stimuli with different coherence levels before and after training on a speed discrimination task requiring object-based attentional selection of one of the two competing motion stimuli. We found that two peaks on the ERP waveform were modulated by the strength of the coherent motion signal; the response amplitude associated with motion directions that were neglected during training was smaller than the response amplitude associated with motion directions that were attended during training. The first peak of motion coherence-dependent modulation of the ERP responses was at 300 ms after stimulus onset and it was most pronounced over the occipitotemporal cortex. The second peak was around 500 ms and was focused over the parietal cortex. A control experiment suggests that the earlier motion coherence-related response modulation reflects the extraction of the coherent motion signal whereas the later peak might index accumulation and readout of motion signals by parietal decision mechanisms. These findings suggest that attention-based learning affects neural responses both at the sensory and decision processing stages.
Training on a visual perceptual task can induce long-lasting improvements in our ability to detect, discriminate or identify visual stimuli (for review see Fahle and Poggio, 2002
; Fine and Jacobs, 2002
). However, learning effects are not restricted to the trained task conditions, but will also affect overall perceptual sensitivity to the visual features present during training based on their strength and task relevance, i.e. whether they were attended or neglected during training (Watanabe et al., 2001
, 2002
; Seitz and Watanabe, 2003
; Vidnyánszky and Sohn, 2005
; Paffen et al., 2008
; Tsushima et al., 2008
; Gál et al., 2009
). It was shown that training to discriminate the color or speed of a specific motion direction in the presence of a spatially overlapping task-irrelevant motion direction will lead to decreased and increased motion coherence detection threshold for the attended and neglected directions, respectively (Vidnyánszky and Sohn, 2005
; Paffen et al., 2008
; Gál et al., 2009
). Importantly, motion directions that were attended during training evoke stronger fMRI responses in early visual cortical areas, including the motion-sensitive human area MT+, than directions that were neglected during training (Gál et al., 2009
). These results show that object-based attentional selection during training guides learning processes that will affect overall perceptual sensitivity and neural responses both to the task-relevant and the task-irrelevant visual features present during training.
An important unresolved question concerns the temporal dynamics of these attention-based learning effects on the neural responses to attended and neglected visual features. Computational models (Smith and Ratcliff, 2004
; Beck et al., 2008
) and experimental studies (for reviews, Glimcher, 2003
; Gold and Shadlen, 2007
; Heekeren et al., 2008
) suggest that the neural events underlying detection or discrimination of visual stimuli consist of two stages: a first stage where the low-level sensory properties of stimuli are computed in the early visual cortical areas, followed by a second stage in which this sensory evidence is accumulated and integrated so that a perceptual decision can be formed (this evidence accumulation is thought to occur primarily in downstream feature-specific visual cortical areas and the parietal and frontal cortex). The main goal of the current study was to test whether attention-based learning influences perceptual sensitivity for the visual features present during training via modulating the sensory gain for the different features at the early stages of visual cortical processing and/or by biasing the decision processes at the higher processing stages.
Previous electrophysiological studies (Skrandies and Fahle, 1994
; Skrandies et al., 1996
, 2001
; Shoji and Skrandies, 2006
; Pourtois et al., 2008
) investigating the timecourse of learning effects in the trained task condition revealed perceptual learning effects on the processing of task-relevant information starting early, from ∼100 ms after stimulus onset. Based on these results it was suggested that perceptual leaning might modulate the earliest cortical stages of visual information processing. On the other hand, recent monkey neurophysiological (Law and Gold, 2008
) and modelling results (Law and Gold, 2009
), suggest that perceptual learning in a motion direction discrimination task primary affects the later, decision-related processes and in particular the readout of the directional information by the lateral intraparietal (LIP) neurons. Based on these results we hypothesized that attention-based learning might affect both the visual cortical extraction and the parietal integration of the visual feature information that was present during training. More specifically, we predicted that as a result of attention-based learning neural responses to the visual information that was task-irrelevant during training will be reduced as compared to the responses to the task-relevant information both at the stage of early visual cortical processing as well as at the later stage of decision-related processing.
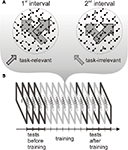
To test this prediction, we measured event related potential (ERP) responses to motion directions that were present as task-relevant or task-irrelevant features during training. Subjects were trained on a speed discrimination task, which required attending to one of the components of a bidirectional transparent motion display (i.e. task-relevant direction) and ignoring the other component (task-irrelevant direction) throughout several practice sessions (see Figure 1
A). The two components of the transparent motion display were moving in orthogonal directions and thus perceptually were segmented into two transparent surfaces gliding over each other. This allowed object-based selection of the task-relevant motion direction during the training trials (Valdes-Sosa et al., 1998
; Sohn et al., 2004
). To examine the effect of training on the processing of task-relevant and task-irrelevant motion directions, ERP responses to the two motion directions were measured before and after training while subjects performed a motion direction discrimination task. We varied the strength of the task-relevant and task-irrelevant motion signal during the test sessions by modulating the number of dots moving coherently in a given trial. This allowed us to measure motion coherence-dependent modulation of the ERP responses, i.e. the sensitivity of the ERP responses to the strength of coherent motion signal. This is important because previous monkey electrophysiological studies have shown that motion coherence modulates neural responses both in the motion sensitive visual cortical area MT (Newsome et al., 1989
; Britten et al., 1992
, 1996
) as well as in the LIP (Shadlen et al., 1996
; Gold and Shadlen, 2000
; Shadlen and Newsome, 2001
), which is involved in the accumulation and integration of the sensory evidence for decision making. Furthermore, in agreement with the monkey electrophysiological results, recent MEG studies revealed strong motion coherence-dependent modulation of neural responses starting from about 200 ms after the onset of the coherent motion stimuli and the results of the source localization analysis suggested that the primary source of this modulation might be localized in the human area MT+ (Aspell et al., 2005
; Händel et al., 2007
). Importantly, in the Händel et al. study motion coherence-dependent modulation was also present in a later time window (between 400–700 ms), however, the source of this late modulation was not reported. Taken together, these results suggest that motion coherence-dependent modulation of the neural responses might be a good marker of the neural sensitivity for the motion directional signal both at the early stage of visual cortical processing as well as at the later decision-related parietal processing stages. Accordingly, in the current study we quantified the magnitude of the motion strength dependent ERP modulations and used this measure to investigate the effects of training on responses to task-relevant and task-irrelevant motion directions both before and after training. We observed learning effects within two time-windows, and we posit that modulations of the ERPs in the earlier time window reflect the extraction of coherent motion signals in early visual cortical areas (most likely in human area MT+; Aspell et al., 2005
; Händel et al., 2007
), and that activity in the later time window reflects the integration of sensory evidence by decision-related mechanisms in parietal cortex (e.g. Shadlen and Newsome, 2001
; reviewed by Gold and Shadlen, 2007
). Critically, in both time-windows modulation of the ERP responses by motion coherence was significantly weaker for the task-irrelevant compared to the task-relevant direction.
Figure 1. Schematic representation of the stimuli during training and the experimental procedure. (A) Transparent random dot motion display used during the training sessions. One of the motion directions was task-relevant and the other direction was task-irrelevant throughout training. The different length of the arrows indicate that dot speed was different in the two intervals both, in the case of task-relevant and task-irrelavant direction. (B) The experimental protocol consisted of a training phase and two testing phases, one before and another after training. During training (six 1-h sessions), subjects performed a speed discrimination task. Before and after training, the test phase included an ERP recording session.
Subjects
Fourteen subjects (six females; age range 22–25 years) participated in the main experiment and nine subjects (three females, age range 22–30) took part in the control experiment. All had normal or corrected to normal visual acuity and reported no history of neurological problems. Subjects gave informed consent to participate in the study, which was approved by the local ethics committee of the Semmelweis University.
Stimuli and Apparatus
Stimuli were programmed in MATLAB 7.1. (MathWorks, Inc., Sherborn, MA, USA) using the Cogent 2000 Software Toolbox (Cogent, www.vislab.ucl.ac.uk/Cogent/
) and were presented on generic PCs. All visual stimuli were rendered in white on a black background. The luminance of the background and the moving dots was <2 cd/m2 and 32.2 cd/m2, respectively. In all experiments subjects were instructed to maintain gaze on a central fixation square subtending 0.25° visual angle present for the entire duration of each experiment. In all experiments, moving dots (N = 200) were presented within a 20° (diameter) circular field centered on the fixation square, with a 1.6° (diameter) circular blank region around the fixation point. Dots subtended 0.15° in diameter, and had a limited lifetime of seven frames. Behavioral responses were collected by means of mouse button presses.
During the psychophysical and ERP experiments visual stimuli were presented at 75 Hz on a 21” Syncmaster 1100 mb CRT monitor (Samsung Electronics, Seoul, Korea); the monitor was the only light source in the room. Eye movements were recorded in these sessions using an iView X™ HI-Speed eye tracker (Sensomotoric Instruments, Berlin, Germany) at a sampling rate of 240 Hz. The eye tracker also served as a head rest that fixed the viewing distance at 50 cm.
General Procedure
The experiment protocol consisted of a training phase and two testing phases, one before and another after training (see Figure 1
). The testing phases consisted of a psychophysical testing session to estimate motion coherence detection thresholds, an ERP session, and an fMRI scanning session. Training phase comprised six 1-h sessions of psychophysical testing during which subjects performed the speed discrimination task.
The post-training testing sessions were separated by two ‘top-up’ learning sessions to ensure that learning effects were maintained. Each testing session was performed on a different day and their order was randomized across subjects. Psychophysical testing and training sessions lasted for 1 h, while ERP and fMRI experiments lasted for 1.5 h. Part of the behavioral and the fMRI results obtained in the current experiment were reported earlier (Gál et al., 2009
).
Training
In the training sessions subjects performed a 2-interval forced choice speed discrimination tasks. In each trial the two 500 ms stimulus presentation intervals were separated by a 200 ms inter-stimulus interval. There was an inter-trial interval (jittered between 300–500 ms) between the subject’s response button press and the beginning of the next trial. Each stimulus interval contained two populations of spatially superimposed dots moving in a direction either +45° or −45° tilted from the upward direction (Figure 1
). Subjects were instructed to attend to dots moving in one of the directions (task-relevant direction) while simultaneously ignoring dots that moved in the orthogonal direction (task-irrelevant direction). They were asked to indicate which of the two intervals contained faster motion in the task-relevant direction. The speed of the task-relevant direction was fixed for one of the two intervals (at 6°/s), while that of the other interval was varied using a QUEST adaptive staircase procedure (Watson and Pelli, 1983
) arriving at a value providing 75% correct performance. The speed of the task-irrelevant motion direction was also changing across the two stimulus intervals: it was jitter between 6° and 7°/s. Every training session consisted of eight experimental blocks of 80 trials each. Task-relevant and irrelevant directions were randomized across subjects, but kept constant across training sessions.
Testing motion coherence detection threshold
We measured motion coherence thresholds within the same block for three different motion directions: for the two directions present during training (±45° from the upward direction) and for a third, control direction (180°, downward direction). A single trial consisted of two 250 ms stimulus presentation intervals, separated by a 250 ms ISI. There was an inter-trial interval (jittered between 300–500 ms) between the subject’s response button press and the beginning of the next trial. Motion coherence for each direction was varied independently by using the QUEST adaptive staircase procedures to converge at 75% correct performance in 60 steps. Two staircases (one starting at 0% and the other starting at 100% coherence) were randomly interleaved within an experimental block for each motion direction. Data were analyzed with repeated measures ANOVA with factors of test session (before training, after training), and task relevance (task-relevant, task-irrelevant).
Main EEG experiment
During EEG recordings motion discrimination thresholds were measured using the method of constant stimuli in a 2-alternative forced choice procedure. Motion directions (+45° or −45°) were displayed at six different coherence levels (5, 10, 15, 20, 30, and 45%). The six different coherence levels for both motion directions were presented randomly within a single block, resulting in 12 different trial types. Each EEG experimental session contained five blocks and each block contained 40 repetitions for each trial type (for a total of 2400 trials per session). The subject’s task was to report whether they perceived coherent motion in the +45° or −45° directions. All subjects gave responses with their right hand. They were required to press the left mouse button to indicate that coherent motion was perceived in the −45° (northwest) direction and press the right mouse button for +45° (northeast) direction. Stimuli were displayed for 250 ms. Between the manual response and the subsequent stimulus there was a short delay, jittered between 200–300 ms. Reaction times were measured starting from the stimulus onset.
Control experiment
The stimuli and the procedure were the same as those used in the main EEG experiment except that only two motion coherence levels (10% and 45%) were used and in each trial all the dots appearing on the screen were colored either red or green in an unpredictable way. In separate blocks subjects either performed a motion direction discrimination task, just as in the main experiment or a color discrimination task, i.e. the subject’s task was to report whether the color of the dots was red or green. The control EEG experimental session contained three blocks of 40 trials for both motion and color discrimination tasks conditions.
EEG Data Acquisition
EEG data were acquired using a BrainAmp MR EEG system (Brain Products GmbH) from 60 (Ag/AgCl) scalp electrodes mounted in an EasyCap (Easycap GmbH, Herrsching-Breitbrunn, Germany, extended 10–20 System). Horizontal and vertical EOGs were monitored using four electrodes placed on the outer canthi of the eyes and in the inferior and superior areas of the right orbit. All channels were referenced to linked earlobes with input impedance of ≤5 kΩ and a forehead electrode was used as ground. Data were sampled at 1000 Hz with an analog band-pass filter of 0.016–250 Hz and were digitally band-pass filtered and rereferenced to average reference for the subsequent analysis (butterworth zero phase; high cutoff: 30 Hz, 12 dB/oct; low cutoff: 0.1 Hz, 12-dB/oct attenuation and 50-Hz notch filter). Trials containing blinks, movements, A/D saturation or EEG baseline drift were rejected on the basis of [+100 �V−100 �V] rejection criterion and visual inspection of each recording by semi-automatic artifact detection.
EEG Data Analysis
For each subject, averaged epochs ranging from −100 to 600 ms relative to the onset of the stimuli and containing no EEG artefacts were computed for each combination of motion direction, motion coherence and training session separately and baseline corrected using the 100 ms prestimulus time window.
To quantify the strength of the motion coherence-dependent modulation of ERP responses the area under the average ERP curve was calculated in successive 10 ms time-bins for each of the six different motion coherence levels. Linear regression was used separately for each time-bin to estimate the beta value (slope) of the best fitting line that relates the area under the curve to motion coherence level. The beta value indicates the degree to which motion coherence modulated the ERP responses, with a slope of zero indicating no effect. We constructed scalp maps of beta values to visualize their spatial distribution. All scalp maps were plotted by commercially available EEG software BESA 5.2 (MEGIS Software GmbH) that uses spline interpolation designed for irregularly spaced data points.
Eye Movement Data Analysis
During the ERP recordings, we tracked the eye position of four randomly selected subjects while they performed the motion discrimination task before training, and of eleven randomly selected subjects after training. We calculated the mean eye position using an interactive computer program. Artifacts like drifts or blinks were identified by visual analysis and removed. Trials were binned based on motion direction and we calculated the mean eye position (x and y values) for the period when the motion stimulus was present on each trial. We compared these values between the different conditions using Student’s t-test.
In addition EOG channels were also used for eye movement analysis: bipolar EOG signals were derived by computing the difference between the voltages at the four electrodes placed around the right orbit: horizontal (HEOG) and vertical (VEOG) channels were calculated. The averaged EEG epochs – we obtained after training for the different conditions and subjects in the main EEG data analysis – were quantified for the bipolar EOG channels (as in Khoe et al., 2005
) within the same time-windows that were selected for the analysis of the ERP responses. Average amplitudes were analyzed using repeated measures ANOVA with factors of task relevance separately for both bipolar channels.
The behavioral results obtained during the ERP recording sessions before training revealed no difference in the subjects’ motion direction discrimination performance between the task-relevant and the task-irrelevant directions Figure 2
A. On the other hand, after training observers more often reported seeing the task-relevant than the task-irrelevant direction Figure 2
A.
Figure 2. (A) Motion direction discrimination performance during the ERP recording sessions. Before training. (solid line), there was no difference between the performance in the case of task-relevant (red) and task-irrelevant (blue) directions. After training (dashed line), subjects more often reported seeing the task-relevant than the task-irrelevant direction. Data were modeled by Weibull psychometric functions. (B) Reaction times in the motion direction discrimination task. Learning led to overall reduction of reaction times after training (bars with solid outlines). There was no difference in subjects’ reaction times between task-relevant (light shaded bars) and task-irrelevant direction (dark shaded bars) neither before nor after training. Error bars indicate the SEM.
ANOVA revealed that the main effect of test session did not quite reach significance (before and after training, F(1,13) = 4.26, p = 0.059); however, there was a significant main effect of task relevance (task-relevant and task-irrelevant, F(1,13) = 4.91, p = 0.045); and a significant interaction between these variables (F(1,13) = 16.6, p < 0.002). Importantly, even though learning led to an overall reduction of reaction times after training, there was no difference in subjects’ reaction times between task-relevant and task-irrelevant direction either before or after training (Figure 2
B). ANOVA showed no significant main effect of test session (before and after training, F(1,13) = 2.345, p = 0.149); no significant main effect of task relevance (task-relevant and task-irrelevant, F(1,13) = 0.035, p = 0.855); and no significant interaction between these variables (F(1,13) = 2.352, p < 0.149). Taken together, the behavioral results obtained during the ERP sessions are in agreement with the results of the motion coherence detection threshold measurements obtained in the current experiment and presented earlier (Gál et al., 2009
). In this previous report we showed that learning resulted in decreased coherence detection thresholds for the task-relevant motion direction as well as increased detection thresholds for motion in a direction that was continuously present as a task-irrelevant distractor during training.
Effect of Training on the ERP Responses
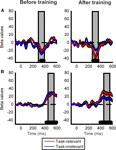
We next examined how training influences the sensitivity of ERP responses to coherent motion signals for task-relevant and task-irrelevant motion directions. Average ERPs were computed at each of six different motion coherence levels from the data obtained before and after training. Over occipito-temporal electrodes, ERP responses were modulated by motion strength both before and after training (as illustrated in Figures 3
A–D for electrode PO8) in a time interval peaking approximately 330 ms after stimulus onset: ERPs were more negative as the motion coherence increased. On the other hand, over the parietal electrodes, ERP responses were modulated by motion strength both before and after training (as illustrated in Figures 3
E–H for electrode Pz) in a time interval peaking approximately 500 ms after stimulus onset: ERPs were more positive as the motion coherence increased.
Figure 3. Grand average ERP responses shown for the PO8 (A–D) and Pz (E–H) electrodes. There was no difference between the ERP responses to the task-relevant (A,E) and task-irrelevant (B,F) directions before training. After training, the magnitude of motion signal strength dependent modulation of the ERP responses in the 300–550 ms time interval is reduced in the case of task-irrelevant direction (D,H) compared to that in the case of task-relevant direction (C,G). Different colors represent different motion coherence levels. Grey shaded bars indicate the time-windows where motion signal strength dependent modulations are most pronounced.
Next, we quantified the magnitude of the motion strength dependent ERP modulations and used this measure to investigate the effect of training on responses to task-relevant and task-irrelevant motion directions. The area under the curve was calculated in successive 10 ms time-bins for each of the six different motion coherence levels. We then used linear regression separately for each time-bin to estimate the slope of the best fitting line that relates the area under the curve to the motion coherence level. The regression coefficient (beta value) indicates the degree to which motion coherence modulated the ERP responses, with a slope of zero indicating no effect. We constructed scalp maps of beta values to visualize their spatial distribution; Figure 4
illustrates the distribution of beta values related to task-relevant motion before training (the scalp map was similar to the map obtained in response to task-irrelevant motion). The two peaks of motion coherence-dependent modulation of ERP responses that were observed in the average ERP waveform can clearly be identified by examining the beta value maps. The first peak is at 330 ms, it is bilateral, and is most pronounced over the lateral occipito-temporal cortex. The second peak is around 500 ms and is strongest over the parietal cortex.
Figure 4. Spatial distribution of motion strength dependent modulation of the ERP responses: scalp maps of beta values related to task-relevant motion before training (the scalp map was similar to the map obtained in response to task-irrelevant motion. ). The temporal evolution of the distribution shows an early (320–360 ms) bilateral occipital and a late (480–520 ms) parietal peak.
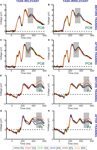
Next, we examined the influence of training by computing motion strength dependent modulations within a cluster of occipito-temporal (O1, O2, PO3, PO4, PO7, PO8, P7, P8) and a cluster of parietal (Pz, P1, P2, P3, P4) electrodes. These two clusters of electrodes were selected because in the data obtained before training they showed the largest beta values during the early and late peaks of the motion strength dependent modulation, respectively (collapsed across task-relevant and task-irrelevant directions). There were one significant peak of motion strength dependent modulation observed at 330 ms after stimulus onset in the occipito-temporal electrodes (Figure 5
A) and one significant peak in the parietal electrodes at 500 ms after stimulus onset (Figure 5
B).
Figure 5. Learning effects on the motion strength dependent modulation of the ERP responses. Time courses of the beta values for the task-relevant (red) and the task-irrelevant (blue) direction are shown; computed within a cluster of occipito-temporal (A) and parietal (B) electrodes. Black filled dots at the bottom of the figure indicate the intervals where beta values averaged across the two conditions are significantly different from zero (Student t-tests, corrected for multiple comparison, FDR = 0.05). Data from the time interval indicated by the vertical grey shaded bars placed at the peaks of the beta values were used for ANOVA. Red and blue shaded bands around the time courses indicate the SEM.
To further investigate the effect of training on ERP responses, we performed a repeated measures ANOVA on the beta values averaged across 100 ms time-windows centered on the significant peaks (as shown in Figures 5
A,B). Although there was a clear trend of higher beta values in the occipito-temporal electrodes (Figure 5
A) after but not before training, ANOVA revealed a nearly significant interaction between test session and task relevance (F(1,13) = 4.651, p = 0.052). However, a closer examination of the data revealed that the modest size of this interaction might be due to the fact that learning effects on the occipito-temporal electrodes were lateralized to the right hemisphere (interaction between test session and task relevance for the right hemisphere: F(1,13) = 6.894, p = 0.021; and for left hemisphere F(1,13) = 1.037, p = 0.326). Importantly, training also had a strong effect on the late parietal motion coherence-related peak of the ERP responses (Figure 5
B): beta values associated with the task-irrelevant direction were significantly reduced compared to the task-relevant direction after training but not before training (significant interaction between test session and task relevance: F(1,13) = 6.465, p = 0.0245 for parietal electrodes).
The behavioural findings showing no difference in the subjects’ reaction times between the task-relevant and task-irrelevant directions after (as well as before) training speak against a possible explanation of the learning effects found on the ERP responses based on training induced differential modulation of motor responses to the two motion direction. Nevertheless, to further investigate this possibility we tested the relationship between the motion coherence-dependent modulation of the ERP responses and subjects’ RTs. Similarly to the calculation of the motion coherence-dependent modulation of the ERP responses, for each subject, direction and test session we calculated beta values based on the average RTs obtained in the case of the six different motion coherence levels. Our analysis revealed no correlation between the motion coherence-dependent modulation of the ERP responses and RTs: r(12) < 0.3 and p > 0.3 in all cases (both test sessions, directions and hemispheres, tested separately).
To verify that subjects were able to maintain fixation during the ERP recordings, we tracked the eye position of four randomly selected subjects while they performed the motion discrimination task before training, and of eleven randomly selected subjects after training. We found no significant difference in the mean eye position for the different motion directions (paired t test, before training: t(3) = −0.299, p = 0.784 for x coordinates and t(3) = −0.438, p = 0.691 for y coordinates; after training: t(10) = −0.347, p = 0.735 for x coordinates and t(10) = 0.294, p = 0.774 for y coordinates) indicating that there was no systematic bias in eye position induced by the direction of the motion stimulus.
Furthermore, since we did not apply an EOG correction to our EEG data and we measured eye position only in a subset of subjects we performed an additional analysis of the EOG signal obtained before as well as after training. We tested whether there are any differences in the EOG signals between the case of task-relevant and task-irrelevant motion directions. Repeated measures of ANOVA were calculated over the average EOG amplitudes within the same two time-windows that were selected for the analysis of the ERP responses. ANOVA revealed no significant difference between the two motion directions: p > 0.29 and F(1,13) < 1.19 for either of the EOG channels and time-windows. These results provide further evidence against the explanation of the learning-induced modulation of the processing of task-relevant and task-irrelevant motion directions based on eye movements.
Control Experiment
A control experiment was performed to determine whether attending to the motion directional signal and performing the motion discrimination task is required to evoke the observed motion coherence-related ERP peaks. The stimuli were the same as those used in the main experiment except that only two motion coherence levels (10% and 45%) were used and in each trial all dots were colored either red or green in an unpredictable way. In separate blocks subjects either performed a motion direction discrimination task, just as in the main experiment or a color discrimination task (red vs. green). Behavioral results showed that in the motion direction discrimination task, but not in the color discrimination task subjects’ performance was significantly better at the higher than at the lower motion coherence level (at 10% motion coherence: 60.44%; at 45% motion coherence: 94.29%; main effect of motion coherence levels: F(1,8) = 301.993, p = 0.0001), whereas performance in the color discrimination task was similar at the two different motion coherence levels (at 10% motion coherence: 98.27% and at 45% motion coherence: 97.66%; F(1,8) = 2.47, p = 0.154).
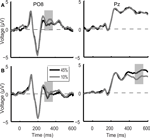
In the case of direction discrimination task ERP responses to the low and high motion coherence stimuli differed in two time intervals, which closely corresponded to the two peaks of motion coherence-related modulation of the ERP responses observed in the main experiment (Figure 6
). On the other hand, in the case of color discrimination task, ERP responses differed between the low and high motion coherence stimuli only in a temporal interval corresponding to the first coherence-related peak found in the motion direction discrimination task both in the main and in the control experiment (Figure 6
). Accordingly, ANOVA revealed no significant difference in modulation of the first motion coherence-related ERP peak between the direction and color discrimination conditions (occipital-temporal electrodes interaction between direction and color discrimination: F(1,8) = 0.732, p = 0.417). However, there was a significant difference in modulation of the late motion coherence-related ERP peak between the direction and color discrimination condition (parietal electrodes: F(1,8) = 6.3, p = 0.036). Post hoc analysis showed that ERP responses to the high and low motion coherence stimuli in the time interval corresponding to the late coherence-related ERP peak differed during the motion direction discrimination task (F(1,8) = 14.569, p = 0.005) but not during the color discrimination condition task (F(1,8) = 0.054, p = 0.823).
Figure 6. Control experiment grand average ERP waveforms during the color discrimination task (A) and the motion direction discrimination task (B) shown for the PO8 and Pz electrodes. In the case of color discrimination task (A), ERP responses differed between the 10% (grey line) and 45% (black line) motion coherence stimuli only in an early temporal interval (330 ms after stimulus onset, grey shaded bar). During the direction discrimination task (B) ERP responses to the low and high motion coherence stimuli differed in two time intervals (indicated by grey shaded bars) which closely corresponded to the two peaks of motion coherence-related modulation of the ERP responses observed in the main experiment.
Our ERP results revealed that training on a task which requires object-based attentional selection of one of the two competing, spatially superimposed motion stimuli will lead to strong modulation of the neural responses to these motion directions when measured in a training-unrelated motion direction discrimination task. Motion direction that was task-relevant during training evoked significantly stronger modulation of the earliest motion coherence-related peak of the ERP responses over the right hemisphere peaking around 330 ms as compared to the motion direction that was present as a distractor during practice. The latency of the first motion coherence-related peak found in the present study is in agreement with the results of previous studies showing that motion coherence-related modulation of the neural responses starts more than 200 ms after stimulus onset (Aspell et al., 2005
; Händel et al., 2007
). Lateralization of the learning-induced modulation of the first motion coherence-related ERP peak to the right hemisphere appears to be in line with the results of previous studies showing right hemisphere dominance in visual motion processing (Kubová et al., 1990
; Aspell et al., 2005
).
Our control experiment showed that this first peak of motion coherence-related modulation in the conditions where subjects perform a task in which motion information is task-irrelevant (color discrimination task) is very similar to that found in the condition where the motion signal is attended (direction discrimination task). This suggests that the first motion coherence-related peak reflects the initial, feed-forward stage of representing the coherent motion signal in visual cortex. The fact that the learning effects related to this early motion–related ERP peak was most pronounced over the occipital cortex is in agreement with previous electrophysiological and neuroimaging studies suggesting that perceptual learning effects act on early visual cortical stages of information processing (Skrandies and Fahle, 1994
; Skrandies et al., 1996
; Dolan et al., 1997
; Vaina et al., 1998
; Gauthier et al., 1999
; Schiltz et al., 1999
; Schwartz et al., 2002
; Furmanski et al., 2004
; Kourtzi et al., 2005
; Sigman et al., 2005
; Shoji and Skrandies, 2006
; Pourtois et al., 2008
). Our ERP results are also in agreement with previously reported effects of learning on fMRI responses associated with task-relevant and task-irrelevant motion directions (Gál et al., 2009
). It was found that after training the task-irrelevant motion direction evoked weaker fMRI responses than the task-relevant direction in early visual cortical areas, including the human area MT + , where neural responses are sensitive to motion coherence and are associated with the perceived strength of the global coherent motion signal (for review see Serences and Boynton, 2007
).
Learning also had a strong effect on the late motion strength dependent peak of the ERP responses. Our control experiment revealed that the late motion coherence-related modulation of the ERP responses was present only in the motion discrimination but not in the color discrimination task. This suggests that this late peak of motion coherence-dependent modulation might reflect decision processes related to the motion direction discrimination task. This interpretation is also supported by our results showing that the late ERP response peaked over the parietal cortex. For example, Shadlen and coworkers have shown that oculomotor circuits in parietal cortex are involved in accumulating and integrating sensory evidence about different motion directions during decision making (e.g. Shadlen and Newsome, 2001
; reviewed by Gold and Shadlen, 2007
). In agreement with this, recently it was also reported that in humans different regions of the posterior parietal cortex are involved in accumulation of sensory evidence for perceptual decisions depending on whether subjects were required to respond by eye movements or by hand-pointing (Tosoni et al., 2008
). Furthermore, the results of recent studies that examine the neural mechanisms of object discrimination in humans provide additional support for the notion that the late peak of motion coherence-dependent modulation reported here might be related to perceptual decision making. For example, a late stage of recurrent processing has been observed during the accumulation of sensory evidence about object-related processing under degraded viewing conditions consists (Murray et al., 2006
; Philiastides and Sajda, 2006
; Philiastides et al., 2006
; Fahrenfort et al., 2008
). Importantly, the marker for this late processing stage is an ERP component that starts between 300–400 ms after stimulus onset (Murray et al., 2006
; Philiastides and Sajda, 2006
; Philiastides et al., 2006
). Although the onset of the late motion strength dependent ERP modulation that we observed in the present study starts approximately 100 ms after the late component observed during visual object processing (Murray et al., 2006
; Philiastides and Sajda, 2006
; Philiastides et al., 2006
), we suggest that both modulations might reflect similar neural mechanisms. The differential onset times might be due to the fact that the motion stimuli we used were made up of limited lifetime dots and embedded in distracting noise; this noise likely delayed the formation of a decision about the direction of the global motion signal. If we posit that the motion coherence-dependent modulation in our study started around 250 ms – which is in agreement with earlier findings (Aspell et al., 2005
) – the delay between our early and late time window of motion coherence-dependent modulation (which started between 400–500 ms) corresponds closely to that found in the case of object processing: 150–200 ms (Murray et al., 2006
; Philiastides and Sajda, 2006
; Philiastides et al., 2006
; Carmel and Carrasco, 2008
; Fahrenfort et al., 2008
).
In conjunction with these previous reports, the present demonstration of a significant training-related modulation of the late peak of motion coherence-dependent modulation of ERP responses suggests that learning affects the integration and evaluation of motion information at decisional stages in the parietal cortex. This conclusion appears to be in agreement with recent monkey neurophysiological (Law and Gold, 2008
) and modelling results (Law and Gold, 2009
), suggesting that perceptual learning in a motion discrimination task requiring an eye movement response primary affects the decision processes and in particular the readout of the directional information by the LIP neurons. Based on previous results demonstrating human posterior parietal cortex is involved in accumulating sensory evidence in a task requiring manual responses, it is reasonable to suppose that the modulation of the late peak of motion coherence-dependent modulation of ERP responses we observe in the current study reflects the influence of learning on the parietal decision processes involved in performing the motion discrimination task.
From a broader perspective, our results are also in agreement with the growing body of psychophysical, neuroimaging and modelling results suggesting a close relationship between perceptual learning and attention (Ahissar and Hochstein, 1993
, 1997
; Li et al., 2004
, 2009
; Vidnyánszky and Sohn, 2005
; Lu et al., 2006
; Petrov et al., 2006
; Mukai et al., 2007
; Law and Gold, 2008
, 2009
; Paffen et al., 2008
; Xiao et al., 2008
; Gál et al., 2009
; Gutnisky et al., 2009
; for review see: Tsushima and Watanabe, 2009
). It was proposed that visual perceptual learning affects visual attentional selection mechanisms leading to more efficient processing of the task-relevant as well as more efficient suppression and exclusion of the task-irrelevant visual information as a result of training. The possibility that plasticity of attentional selection might be involved in the learning effects found in the current study are supported by previous results showing that attention can modulate processing of motion information in the visual cortical areas, including the human area MT+ (Valdes-Sosa et al., 1998
; O’Craven et al., 1999
; Corbetta and Shulman, 2002
; Pessoa et al., 2003
; Händel et al., 2008
). Furthermore, it is also known that the parietal cortex plays a critical role in attentional functions (Serences and Yantis, 2006
) and thus learning-induced changes in the parietal responses to motion information might reflect modulation of the attentional selection processes involved in decision making as a result of training. In fact, in the previous study investigating the effect of perceptual learning on visual motion direction discrimination (Law and Gold, 2008
) one possible explanation for the observed modulation of motion-driven responses of neurons in area LIP by perceptual learning was based on improved attention to appropriate features of the motion representation used to form the decision.
In conclusion, our results provide evidence that attention-based perceptual learning leads to reduced neural sensitivity for visual motion directions that were neglected compared to those that were attended during training by modulating the efficacy of visual cortical extraction of the coherent motion signal as well as the accumulation and readout of motion directional information by parietal decision processes.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by a grant from the Hungarian Scientific Research Fund (T048949) and by the Bolyai Fellowship to Zoltán Vidnyánszky.