Metabolism of the vacuolar pathogen Legionella and implications for virulence
- 1Max von Pettenkofer Institute, Faculty of Medicine, Ludwig-Maximilians University, Munich, Germany
- 2Institute of Medical Microbiology, Faculty of Medicine, University of Zürich, Zürich, Switzerland
Legionella pneumophila is a ubiquitous environmental bacterium that thrives in fresh water habitats, either as planktonic form or as part of biofilms. The bacteria also grow intracellularly in free-living protozoa as well as in mammalian alveolar macrophages, thus triggering a potentially fatal pneumonia called “Legionnaires' disease.” To establish its intracellular niche termed the “Legionella-containing vacuole” (LCV), L. pneumophila employs a type IV secretion system and translocates ~300 different “effector” proteins into host cells. The pathogen switches between two distinct forms to grow in its extra- or intracellular niches: transmissive bacteria are virulent for phagocytes, and replicative bacteria multiply within their hosts. The switch between these forms is regulated by different metabolic cues that signal conditions favorable for replication or transmission, respectively, causing a tight link between metabolism and virulence of the bacteria. Amino acids represent the prime carbon and energy source of extra- or intracellularly growing L. pneumophila. Yet, the genome sequences of several Legionella spp. as well as transcriptome and proteome data and metabolism studies indicate that the bacteria possess broad catabolic capacities and also utilize carbohydrates such as glucose. Accordingly, L. pneumophila mutant strains lacking catabolic genes show intracellular growth defects, and thus, intracellular metabolism and virulence of the pathogen are intimately connected. In this review we will summarize recent findings on the extra- and intracellular metabolism of L. pneumophila using genetic, biochemical and cellular microbial approaches. Recent progress in this field sheds light on the complex interplay between metabolism, differentiation and virulence of the pathogen.
Introduction
Legionella pneumophila is an environmental bacterium ubiquitously found in freshwater, where it is associated with biofilm communities (Lau and Ashbolt, 2009; Hilbi et al., 2011). Protozoan predators like amoebae are part of these communities and feed on bacteria residing within these biofilms. L. pneumophila has developed a way to survive and replicate within these free-living protozoa by forming a unique compartment called the Legionella-containing vacuole (LCV). The LCV is a pathogen vacuole, wherein L. pneumophila dodges lysosomal degradation by acquiring components of early and late endosomes, mitochondria, the endoplasmic reticulum and ribosomes (Isberg et al., 2009; Urwyler et al., 2009a; Hilbi and Haas, 2012). To establish this intracellular niche, the bacterial Icm/Dot type IV secretion system (T4SS) is essential, as it translocates around 300 different “effector” proteins into the host cell, many of which target central eukaryotic pathways like endocytic, secretory or retrograde vesicle trafficking by exploiting small GTPases, phosphoinositide lipids and other host factors (Hubber and Roy, 2010; Finsel et al., 2013; Haneburger and Hilbi, 2013; Rothmeier et al., 2013; Hoffmann et al., 2014a). Besides its natural protozoan hosts, L. pneumophila also replicates within human alveolar macrophages and epithelial cells, thus causing a severe pneumonia called Legionnaires' disease. Most processes involved in survival in protozoa or macrophages are very similar and appear to be evolutionarily conserved (Gao et al., 1997; Greub and Raoult, 2004; Hoffmann et al., 2014b). In addition to biofilm and protozoan niches, Legionella spp. are also naturally found in physically more challenging habitats, such as extremely acidic environments, antarctic freshwater lakes and water sources with temperatures over 60°C (Hilbi et al., 2011). Accordingly, these facultative intracellular bacteria are an example of a microorganism colonizing many different environmental niches.
To survive within its extra- and intracellular niches, L. pneumophila employs a biphasic life cycle, where it alternates between two different forms in response to environmental and metabolic stimuli (Molofsky and Swanson, 2004). In its transmissive form the pathogen is motile, resistant to environmental stress like nutrient starvation and infectious to host cells. In its replicative form the bacteria lack these traits but are able to replicate intracellularly (Rowbotham, 1986; Brüggemann et al., 2006). Further manifestations of L. pneumophila differentiation include a mature intracellular form (MIF) that develops late during infection (Garduno et al., 2002). MIFs are motile, metabolically inert, highly infectious and loaded with cytoplasmic inclusions of poly-3-hydroxybutyrate. Moreover, under harsh conditions, L. pneumophila appears to adopt a viable but non-culturable (VBNC) state (Steinert et al., 1997; Garcia et al., 2007; Al-Bana et al., 2014). To ensure bacterial survival in different environments, the biphasic life cycle of L. pneumophila is strictly regulated. Consequently, L. pneumophila employs a multitude of regulatory systems devoted to the control of gene expression, including transcriptional regulators and two-component systems (Molofsky and Swanson, 2004).
As L. pneumophila survives in various environmental niches, it is likely that the bacterium exploits numerous different carbon and energy sources. Furthermore, the intracellular milieu might represent a richly set table for pathogens, as eukaryotic host cells contain many different nutrients, which are potentially accessible to intracellular pathogens (Eisenreich et al., 2010; Rohmer et al., 2011; Abu Kwaik and Bumann, 2013). An intriguing aspect of intracellular metabolism is its compartmentalization into processes that occur within the host cytoplasm, the LCV lumen or the bacteria (Figure 1).
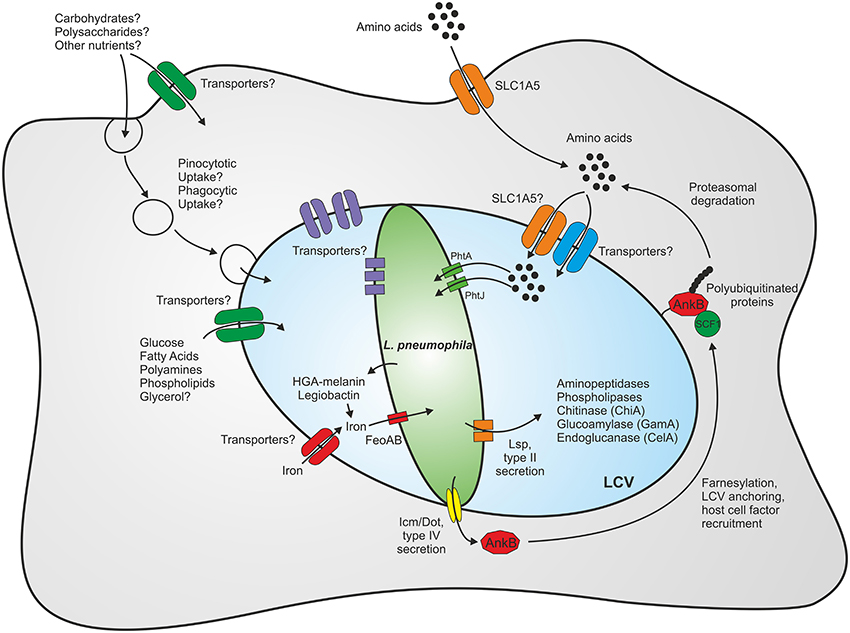
Figure 1. Compartmentalization of the metabolism of L. pneumophila. Within eukaryotic host cells L. pneumophila forms a membrane-bound replication-permissive compartment, the Legionella-containing vacuole. The bacteria gain access to nutrients through intrinsic membrane transporters localizing to the plasma membrane or the pathogen vacuole membrane, respectively, or through fusion with host vesicles and compartments such as endosomes/macropinosomes or the endoplasmic reticulum. Amino acids represent the main carbon and energy source of L. pneumophila, yet carbohydrates and complex polysaccharides are also catabolized. For details see text.
Early metabolic studies suggested that amino acids are the major if not only source of carbon and energy for L. pneumophila (Pine et al., 1979; Tesh and Miller, 1981; Tesh et al., 1983). However, the subsequent availability of genome sequences, transcriptome, proteome and metabolism data indicated that L. pneumophila possess much broader metabolic capacities (Cazalet et al., 2004; Chien et al., 2004; Urwyler et al., 2009b; Eylert et al., 2010; Faucher et al., 2011; Hoffmann et al., 2014a; Schunder et al., 2014). In this review we will summarize the metabolic capacities of L. pneumophila regarding amino acid and carbohydrate degradation. Moreover, we will highlight further nutrient requirements of the bacteria and assess the regulation of their life cycle by metabolites.
Amino Acid Metabolism
Initial studies of the nutrient requirements of L. pneumophila in chemically defined minimal media showed a preference for amino acids as main source of carbon and energy (Pine et al., 1979; Ristroph et al., 1981; Tesh and Miller, 1981; Tesh et al., 1983). A preference for amino acid utilization is also illustrated in the genome sequence of L. pneumophila, where around 12 classes of ATP binding cassette transporters, amino acid permeases and proteases can be found (Cazalet et al., 2004; Chien et al., 2004). Furthermore, genes involved in synthesis and transport of amino acids are highly induced during growth inside macrophages (Faucher et al., 2011). L. pneumophila employs transport systems to take up and utilize amino acids (Sauer et al., 2005), but also exploits host cell transporters (Wieland et al., 2005) and host proteolytic processes (Price et al., 2011).
L. pneumophila is an obligate aerobe organism and auxotroph for several amino acids including cysteine, arginine, isoleucine, leucine, threonine, valine, and methionine. The observed auxotrophy corresponds to the notion that cysteine biosynthetic genes and other anabolic genes are absent in the genomes of L. pneumophila (Cazalet et al., 2004; Chien et al., 2004; Glöckner et al., 2007; D'Auria et al., 2010; Schroeder et al., 2010) and L. longbeachae (Cazalet et al., 2010; Kozak et al., 2010). Compared to chemically defined media, the complex ACES-buffered yeast extract (AYE) broth routinely used to grow L. pneumophila contains several additional amino acids: alanine, asparagine, glutamine and glycine. The common solid growth medium for Legionella species is buffered charcoal-yeast extract (BCYE) agar, supplemented with L-cysteine and ferric pyrophosphate. L. pneumophila growth depends on excess cysteine in the medium (Feeley et al., 1979; George et al., 1980). Yet, the amount of cysteine added to the BCYE medium is much higher than what is required to support growth. The major part of cysteine in the Legionella growth medium is rapidly oxidized to cystine and becomes unavailable to the bacteria, as L. pneumophila is not able to utilize this compound (Ewann and Hoffman, 2006). The remaining concentration of cysteine is around 0.5 mM, which is enough to support Legionella growth. Furthermore, using radio-labeled cysteine and mutant strains, it was found that cysteine is not only imported by specific transporters but also consumed during L. pneumophila growth (Ewann and Hoffman, 2006).
L. pneumophila is also auxotroph for arginine, as the bacteria lack enzymes that allow synthesis of arginine from glutamate. However, the bacteria produce arginine in chemically defined medium supplemented with ornithine or citrulline, which are precursors of arginine emerging in the later steps of the synthesis from glutamate (Tesh and Miller, 1983; Hovel-Miner et al., 2010). Furthermore, L. pneumophila mutants lacking the arginine repressor ArgR fail to replicate within host cells. ArgR might sense the availability of arginine within the host. This leads to the expression of genes (many of them not involved in arginine metabolism), which are required for intracellular growth (Hovel-Miner et al., 2010).
The identification of the phagosomal transporter A (PhtA) revealed a major role of threonine not only for replication but also for differentiation of L. pneumophila (Sauer et al., 2005) (Figure 1). A mutant strain lacking phtA does not grow in a chemically defined medium, but is rescued by excess tryptone or dipeptides containing threonine, indicating that PhtA is not the only threonine uptake system. Intriguingly, phtA mutant bacteria are defective for intracellular replication in macrophages due to their inability to differentiate from the transmissive to the replicative state. Analogously, PhtJ was identified as a valine transporter also required for differentiation and replication within macrophages (Sauer et al., 2005; Chen et al., 2008). These findings highlight the role of the Pht transporters as means for L. pneumophila to scavenge amino acids from host cells.
Further evidence for the importance of the Pht transporter family for nutrient acquisition of L. pneumophila was obtained by investigating the phtC-phtD locus (Chen et al., 2008). The phtC and phtD genes are paralogs in an operon containing genes involved in nucleotide metabolism. The transporter genes are required for successful replication within macrophages and survival of thymidine deprivation. Expression of phtC and phtD in E. coli bestowed pyrimidine transport activity upon strains lacking all known nucleoside transporters, identifying PhtC and PhtD as thymidine transporters (Fonseca et al., 2014).
To take up and utilize amino acids, L. pneumophila does not only produce many own systems, but also exploits host metabolic functions (Figure 1). The eukaryotic neutral amino acid transporter SLC1A5 was found to be upregulated in L. pneumophila-infected cells, and blocking the transporter with the competitive inhibitor BCH (2-amino-2-bornonane-carboxylic acid) or depletion by RNA interference impaired intracellular growth of L. pneumophila (Wieland et al., 2005). This study demonstrated the requirement of a single host cell transporter for intracellular replication and also indicated that SLC1A5 may be recruited to the LCV, thus enabling L. pneumophila to import amino acids from the cytoplasm into the LCV lumen. Other host-cell transporters might be utilized in a similar manner. Notably, similar to L. pneumophila, Francisella tularensis modulates the expression of SLC1A5 upon infection of THP-1 human monocytes and is also impaired for intracellular replication when this transporter is downregulated (Barel et al., 2012).
L. pneumophila uses the Icm/Dot T4SS to translocate effector proteins across the LCV membrane to interfere with central host cell processes (Figure 1). The Icm/Dot substrate AnkB subverts amino acid metabolism and protein degradation by hijacking the host cell ubiquitination machinery and the proteasome to create nutrients for bacterial growth (Al-Khodor et al., 2010). AnkB harbors several eukaryotic domains: an F-box domain that allows interaction with the host SCF1 ubiquitin ligase complex, two ANK domains, which mediate protein-protein interactions in eukaryotes and a CaaX motif that is modified by farnesylation (Price et al., 2009, 2010a,b; Ensminger and Isberg, 2010; Ivanov et al., 2010; Lomma et al., 2010). Farnesylation of AnkB leads to localization of the effector to the LCV membrane, and intracellular replication of L. pneumophila fails when farnesylation is blocked. Anchoring of the effector to the LCV membrane recruits polyubiquitinated host cell proteins, which are degraded by the host proteasome generating a pool of amino acids utilized for intracellular bacterial replication (Price et al., 2011).
Isotopolog profiling is a powerful approach to study metabolic pathways. The method is based on the incorporation of carbon isotopes from stable isotope-labeled precursors such as [U-13C3]serine or [U-13C6]glucose. To elucidate the metabolic pathways and fluxes used, key metabolites such as protein-derived amino acids or storage compounds are then analyzed for the presence of labeled carbon atoms (Zamboni et al., 2009). Metabolomic flux analysis and isotopolog profiling have recently provided detailed insights into the metabolism of the pathogenic bacteria Listeria monocytogenes (Gillmaier et al., 2012) or Streptococcus pneumonia (Hartel et al., 2012), and the metabolic responses of infected host cells to the pathogens have also been investigated (Eisenreich et al., 2013).
Upon growth of L. pneumophila in AYE medium supplemented with [U-13C3]serine, incorporation of the 13C-label indicated that the amino acid was not only used for protein biosynthesis but also to synthesize other amino acids and poly-3-hydroxybutyrate (Eylert et al., 2010). Thus, in agreement with earlier studies (Pine et al., 1979; George et al., 1980; Ristroph et al., 1981) serine can serve as a major carbon source during growth of L. pneumophila in broth. Yet, no 13C-label was detected in isoleucine, leucine, phenylalanine, tyrosine, histidine, proline or valine, confirming the auxotrophy of L. pneumophila regarding these amino acids (Eylert et al., 2010). Finally, isotopolog profiling also revealed that L. pneumophila growing intracellularly in Acanthamoeba castellanii previously fed with [U-13C6]glucose utilizes amoebae-derived amino acids (e.g., phenylalanine, tyrosine) for protein biosynthesis (Schunder et al., 2014).
Carbohydrate and Polysaccharide Metabolism
While amino acids seem to represent the preferred carbon source of L. pneumophila, the bacteria can also metabolize carbohydrates, other small organic compounds and complex nutrients (Figure 1). Early studies using 14C-radio-labeled substrates indicated that glucose, α-ketoglutarate, pyruvate, glycerol and acetate are metabolized by L. pneumophila, yet only some of these compounds stimulated extracellular bacterial growth under the conditions used (Pine et al., 1979; Weiss et al., 1980; Tesh et al., 1983). Moreover, during infection of macrophages L. pneumophila genes required for glycerol catabolism—namely lpg1414 and glpD—were highly upregulated compared to growth in rich broth (Faucher et al., 2011). Therefore, glycerol likely plays a role during intracellular growth of L. pneumophila, similar to other intracellular bacteria such as L. monocytogenes (Eylert et al., 2008; Joseph et al., 2008) and Salmonella enterica (Steeb et al., 2013).
Glucose was not found to stimulate growth of Legionella spp.; however, the genomes of L. pneumophila (Cazalet et al., 2004; Chien et al., 2004; Glöckner et al., 2007; D'Auria et al., 2010; Schroeder et al., 2010) as well as L. longbeachae (Cazalet et al., 2010; Kozak et al., 2010) encode complete pathways required for metabolism of carbohydrates, including the Emden-Meyerhof-Parnas (EMP) pathway, the Entner-Doudoroff (ED) pathway, as well as an incomplete pentose phosphate (PP) pathway. In support of the notion that carbohydrate metabolism is crucial during infection, genes associated with the ED pathway, as well as a glucokinase and a glucoamylase, were upregulated upon intracellular growth of L. pneumophila in A. castellanii (Brüggemann et al., 2006). Another intracellular pathogen that depends on sugar assimilation via the ED pathway during intracellular growth is S. enterica. Yet, in this case the parallel exploitation of several different host nutrients enhances bacterial virulence (Steeb et al., 2013).
Using isotopolog profiling, it was recently shown that L. pneumophila indeed catabolizes glucose via the ED pathway (Eylert et al., 2010). Upon growth in a chemically defined medium containing [U-13C6]glucose, followed by analysis of the isotopolog pattern by mass spectrometry and NMR spectroscopy, the 13C-label was recovered with high efficiency in alanine and also in poly-3-hydroxybutyrate. In contrast, an L. pneumophila mutant lacking the glucose-6-phosphate dehydrogenase gene (zwf), the first gene of an operon comprising the genes of the ED pathway (zwf-pgl-edd-glk-eda-ywtG), did not incorporate label from glucose and was outcompeted by the wild-type strain in co-infection experiments using A. castellanii (Eylert et al., 2010). In line with these observations, L. pneumophila lacking other components of the ED pathway, either glucokinase (glk), phosphogluconate dehydratase (edd), 2-keto-3-deoxy-phosphogluconate aldolase (eda) or the putative sugar transporter (ywtG), was no longer able to metabolize glucose and was defective for growth in Acanthamoeba culbertsoni or mammalian cells (Harada et al., 2010). Together, these findings strongly support the notion that the ED pathway is essential for glucose metabolism and intracellular growth of L. pneumophila. The results also implicate that under the conditions prevailing within LCVs in host cells L. pneumophila does not solely grow on amino acids as carbon and energy sources, but rather, carbohydrates are also utilized (at least as co-metabolites). Yet, the relative contribution of amino acids and carbohydrates to intracellular growth is difficult to assess, and many carbohydrates do not support extracellular growth as sole source of carbon and energy.
The transporters promoting the uptake of sugars have not been studied in molecular detail at present. The gene ywtG (lpg0421) is conserved among L. pneumophila and L. longbeachae, and annotated as a putative D-xylose (galactose, arabinose)-proton symporter (Cazalet et al., 2004, 2010). However, arabinose appears to be barely taken up by L. pneumophila (excluding genetic approaches based on the arabinose promoter, Pbad). Moreover, glucose-1-phosphate is metabolized much faster than glucose-6-phosphate or glucose, suggesting that the former compound is transported efficiently into the cells (Weiss et al., 1980).
In addition to simple carbohydrates and small organic compounds, polymeric compounds also likely serve as carbon sources for L. pneumophila. The exogenous supply of polyamines during infection moderately favored intracellular replication of L. pneumophila (Nasrallah et al., 2011). Moreover, similar to other bacteria (Khosravi-Darani et al., 2013), L. pneumophila might use the intracellular “energy reserve” poly-3-hydroxybutyrate as an endogenous source of carbon and energy, which is synthesized via pyruvate and acetyl-coenzyme A (James et al., 1999; Eylert et al., 2010). Further support for the notion that Legionella spp. degrade complex polysaccharides stems from the genome sequences. L. longbeachae harbors a number of genes likely involved in cellulose degradation (Cazalet et al., 2010), and L. pneumophila contains genes putatively involved in the degradation of cellulose, chitin, starch and glycogen (Cazalet et al., 2004).
The Lsp type II secretion system (T2SS) is essential for intracellular growth of L. pneumophila in amoebae and macrophages (Hales and Shuman, 1999a; Liles et al., 1999) (Figure 1). Proteome studies on the type II “secretome” of L. pneumophila revealed that the bacteria secrete a chitinase (ChiA), as well as an endoglucanase, which metabolizes carboxymethyl cellulose (Debroy et al., 2006). An endoglucanase (CelA) was indeed found to degrade cellulose (Pearce and Cianciotto, 2009), and a eukaryotic-like glycoamylase (GamA) degraded carboxymethyl cellulose, glycogen and starch (Herrmann et al., 2010). Yet, neither CelA nor GamA was required for growth of L. pneumophila in amoebae. In summary, insights from genomics, transcriptomics, metabolomics, as well as biochemical experiments indicate that L. pneumophila utilizes simple and also complex carbohydrates as important sources of carbon and energy during extra- and intracellular growth.
Micronutrient Requirements
Iron is essential for growth of most if not all bacteria, as it is a co-factor for many enzymes of the central metabolism as part of prosthetic groups like heme or iron-sulfur clusters (Ratledge and Dover, 2000). Moreover, the availability of iron is especially important for pathogens, as iron limitation plays an important role in host defense against infections. For L. pneumophila iron represents an essential nutrient and has to be supplemented in high concentrations to growth media (Reeves et al., 1981; Ewann and Hoffman, 2006). The major iron-containing protein of L. pneumophila is aconitase of the tricarboxylic acid cycle (Mengaud and Horwitz, 1993). L. pneumophila grown under iron-limited conditions showed reduced virulence and was impaired for survival in host cells (James et al., 1995). Furthermore, host cells treated with iron chelators did not support growth of L. pneumophila, presumably due to iron limitation, as the addition of iron as iron-transferrin or ferric iron-nitrilotriacetate reversed growth inhibition (Gebran et al., 1994; Byrd and Horwitz, 2000; Viswanathan et al., 2000). Notably, patients with iron overload or smokers are at increased risk for Legionnaires' disease, probably because their lungs contain increased levels of iron (Fields et al., 2002; Vikram and Bia, 2002).
Iron exists in equilibrium between a ferrous (Fe2+) and a ferric (Fe3+) form, depending mostly on the pH and availability of oxygen (Williams, 2012). In L. pneumophila, many systems are devoted to iron metabolism and involved in iron reduction, complexation and transport (Figure 1). Iron reductase enzymes may promote iron assimilation in the periplasm and cytoplasm (Johnson et al., 1991; Poch and Johnson, 1993). Iron reduction is also catalyzed by the secreted compound homogentisic acid (HGA) and its polymerized derivative HGA-melanin (Chatfield and Cianciotto, 2007; Zheng et al., 2013). HGA is a product of the phenylalanine and tyrosine catabolism of L. pneumophila and was identified as the brown pigment secreted by L. pneumophila, which is produced from oxidative polymerization of HGA to HGA-melanin (Steinert et al., 2001). HGA and HGA-melanin stimulate growth of L. pneumophila under iron-limiting conditions, enhance the uptake of iron and can release ferrous iron from transferrin and ferritin, two major protein iron chelators of mammalian cells (Zheng et al., 2013).
L. pneumophila chelates and transports iron with the secreted high-affinity iron siderophore legiobactin (Liles et al., 2000; Starkenburg et al., 2004). The gene lbtA, which has homology with siderophore synthetases, and lbtB that encodes a homolog of a multidrug efflux pump, were identified as key players in the synthesis of legiobactin (Allard et al., 2006). Moreover, an L. pneumophila lbtA mutant strain showed reduced ability to infect lungs of A/J mice, demonstrating the importance of legiobactin in vivo (Allard et al., 2009). Iron is also transported in L. pneumophila via the FeoAB system (Robey and Cianciotto, 2002). The L. pneumophila feoAB operon bears homology to the E. coli system, a well-characterized ATP-driven ferrous iron transporter. An L. pneumophila feoB mutant was outcompeted by wild-type bacteria during infection of A/J mice, highlighting an important role of the transporter in vivo.
Finally, in addition to iron, the extracellular growth of L. pneumophila is also stimulated by calcium, magnesium and zinc. Calcium and magnesium might also play a role in biofilm formation, as the two metal ions enhance the adherence of Legionella to surfaces (Reeves et al., 1981; Koubar et al., 2013).
Metabolic Regulation of Differentiation and Virulence
The biphasic life cycle of L. pneumophila is regulated by a variety of environmental and metabolic stimuli (Molofsky and Swanson, 2004). As long as nutrients are not limiting, the post-transcriptional regulator CsrA suppresses transmission traits and promotes replication (Molofsky and Swanson, 2003). Given the importance of amino acids as carbon source for L. pneumophila, it is not surprising that these compounds are also main regulatory factors of the phenotypic switch (Byrne and Swanson, 1998; Sauer et al., 2005). Amino acid starvation or otherwise nutrient-limiting conditions trigger the shift from the replicative/non-motile to the virulent/motile form of L. pneumophila, which is mediated through the second messenger guanosine 3′,5′-bispyrophosphate (ppGpp) (Hammer and Swanson, 1999; Dalebroux et al., 2010) (Figure 2). The “alarmone” ppGpp is synthesized by the synthase RelA as part of the “stringent response” that senses the accumulation of uncharged tRNAs at the ribosome. A second stringent response enzyme called SpoT also synthesizes ppGpp (Dalebroux et al., 2009). However, rather than sensing amino acid shortage, SpoT monitors fatty acid biosynthesis by interacting with the acyl-carrier protein ACP (Edwards et al., 2009). In addition, SpoT hydrolyzes ppGpp during exponential growth to ensure that transmissive traits are not expressed during replication.
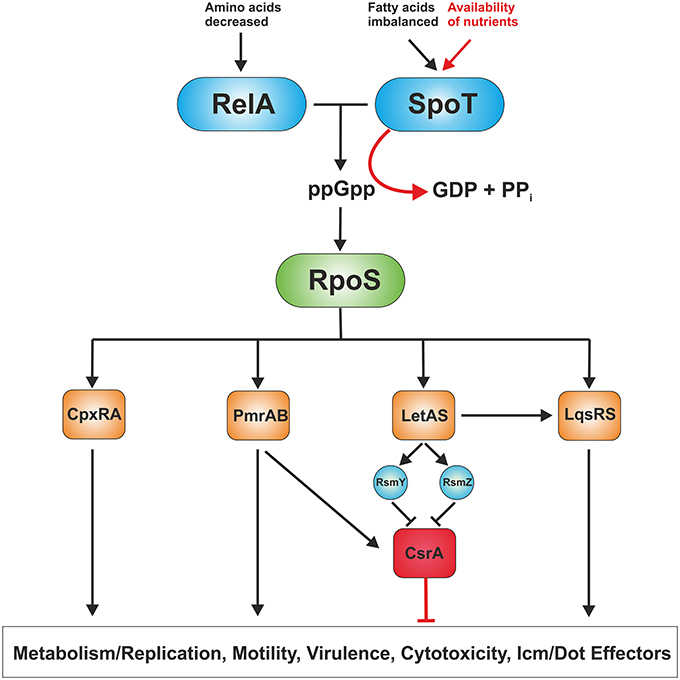
Figure 2. Regulation of replicative and transmissive traits of L. pneumophila. L. pneumophila senses its metabolic state by means of the two ppGpp synthases RelA and SpoT. RelA detects amino acid starvation, whereas SpoT monitors disturbances in fatty acid synthesis. When nutrients become limiting, the “alarmone” ppGpp acumulates in the bacteria leading to production of the alternative sigma factor RpoS. In turn, RpoS regulates the two component or quorum sensing systems CpxRA, PmrAB, LetAB, and LqsRS, which control metabolism/replication, motility as well as virulence traits, and hence, govern the transition from the replicative to the transmissive form. The RNA-binding global regulator CrsA controls the biphasic switch as an antagonist of the two component and quorum sensing systems.
The alternative sigma factor RpoS (σ38/σS) represents the pivotal transcriptional regulator of the L. pneumophila life cycle (Hales and Shuman, 1999b; Bachman and Swanson, 2001; Zusman et al., 2002). An L. pneumophila rpoS mutant is not affected regarding extracellular growth in broth and retains significant stress resistance, but is not able to replicate in amoebae. This severe defect in intracellular replication is not due to impaired Icm/Dot function or icm/dot gene expression, but because of major transcriptional changes affecting basic cellular processes and other central regulatory networks (Hovel-Miner et al., 2009). The transcription of more than 70 genes required for central metabolism, 40 of these associated with amino acid metabolism, was negatively regulated in the rpoS mutant. Furthermore, small regulatory RNAs (rsmY and rsmZ) (Rasis and Segal, 2009; Sahr et al., 2009), two component systems (CpxRA, PmrAB), the transcriptional regulator ArgR and the quorum sensing response regulator LqsR (Tiaden et al., 2007) are regulated by RpoS (Bachman and Swanson, 2004; Hovel-Miner et al., 2009) (Figure 2).
At least three two component systems and one quorum sensing system influence the virulence of L. pneumophila: CpxRA (Gal-Mor and Segal, 2003a; Altman and Segal, 2008), PmrAB (Zusman et al., 2007; Al-Khodor et al., 2009; Rasis and Segal, 2009), LetAS (GacAS) (Hammer et al., 2002; Gal-Mor and Segal, 2003b; Lynch et al., 2003) and the Legionella quorum sensing (lqs) gene cluster (Tiaden et al., 2010) (Figure 2). The lqs system of L. pneumophila comprises the autoinducer synthase LqsA, the sensor kinases LqsS and LqsT (Kessler et al., 2013), and the response regulator LqsR (Tiaden et al., 2007). LqsA produces the compound LAI-1 (Legionella autoinducer-1, 3-hydroxypentadecane-4-one) (Spirig et al., 2008), which presumably binds to the cognate sensor kinases. The kinase-mediated phosphorylation signal converges on LqsR (Schell et al., 2014), which among many other processes also controls the switch from the stationary phase to the replicative phase (Tiaden et al., 2007). Lqs-regulated processes include pathogen-phagocyte interactions, production of extracellular filaments, natural competence for DNA uptake and the expression of a 133 kb genomic “fitness island” (Tiaden and Hilbi, 2012). Furthermore, transcriptome analysis of L. pneumophila strains lacking lqsR, lqsS or lqsT or the entire lqs cluster indicates that the Lqs system also regulates a number of metabolic pathways (Tiaden et al., 2007, 2008; Kessler et al., 2013).
Conclusions and Perspectives
The amoebae-resistant bacterium L. pneumophila colonizes a variety of extra- and intracellular niches in the environment. Upon reaching the human lung, L. pneumophila grows in mammalian macrophages and possibly also in epithelial cells. Accordingly, the bacteria are equipped to utilize a broad range of compounds as carbon and energy sources. In addition to amino acids, which initially have been regarded as the main if not sole nutrients, carbohydrates have recently been shown to be catabolized by extra- and intracellularly growing L. pneumophila. Novel technological approaches such as isotopolog profiling allow analyzing metabolic fluxes with unprecedented resolution and sensitivity. Transcriptome and genome studies indicate that a number of other compounds, including complex polysaccharides, are also metabolized by L. pneumophila. Further studies will unravel the manifold and robust metabolic pathways that the bacteria employ to thrive in diverse environmental niches. Importantly, future investigations will also shed light on the intricate relationship between the physiology and pathogenesis of L. pneumophila, and thus might contribute to control Legionnaires' disease.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Ina Haneburger and Bernhard Steiner for critically reading the manuscript. Research in our laboratory was funded by the Max von Pettenkofer Institute, Ludwig-Maximilians University Munich, the Deutsche Forschungsgemeinschaft (DFG; SPP 1316, SPP 1617) and the Swiss National Science Foundation (31003A-125369).
References
Abu Kwaik, Y., and Bumann, D. (2013). Microbial quest for food in vivo: ‘nutritional virulence’ as an emerging paradigm. Cell. Microbiol. 15, 882–890. doi: 10.1111/cmi.12138
Al-Bana, B. H., Haddad, M. T., and Garduno, R. A. (2014). Stationary phase and mature infectious forms of Legionella pneumophila produce distinct viable but non-culturable cells. Environ. Microbiol. 16, 382–395. doi: 10.1111/1462-2920.12219
Al-Khodor, S., Kalachikov, S., Morozova, I., Price, C. T., and Abu Kwaik, Y. (2009). The PmrA/PmrB two-component system of Legionella pneumophila is a global regulator required for intracellular replication within macrophages and protozoa. Infect. Immun. 77, 374–386. doi: 10.1128/IAI.01081-08
Al-Khodor, S., Price, C. T., Kalia, A., and Abu Kwaik, Y. (2010). Functional diversity of ankyrin repeats in microbial proteins. Trends Microbiol. 18, 132–139. doi: 10.1016/j.tim.2009.11.004
Allard, K. A., Dao, J., Sanjeevaiah, P., McCoy-Simandle, K., Chatfield, C. H., Crumrine, D. S., et al. (2009). Purification of legiobactin and importance of this siderophore in lung infection by Legionella pneumophila. Infect. Immun. 77, 2887–2895. doi: 10.1128/IAI.00087-09
Allard, K. A., Viswanathan, V. K., and Cianciotto, N. P. (2006). lbtA and lbtB are required for production of the Legionella pneumophila siderophore legiobactin. J. Bacteriol. 188, 1351–1363. doi: 10.1128/JB.188.4.1351-1363.2006
Altman, E., and Segal, G. (2008). The response regulator CpxR directly regulates expression of several Legionella pneumophila icm/dot components as well as new translocated substrates. J. Bacteriol. 190, 1985–1996. doi: 10.1128/JB.01493-07
Bachman, M. A., and Swanson, M. S. (2001). RpoS co-operates with other factors to induce Legionella pneumophila virulence in the stationary phase. Mol. Microbiol. 40, 1201–1214. doi: 10.1046/j.1365-2958.2001.02465.x
Bachman, M. A., and Swanson, M. S. (2004). Genetic evidence that Legionella pneumophila RpoS modulates expression of the transmission phenotype in both the exponential phase and the stationary phase. Infect. Immun. 72, 2468–2476. doi: 10.1128/IAI.72.5.2468-2476.2004
Barel, M., Meibom, K., Dubail, I., Botella, J., and Charbit, A. (2012). Francisella tularensis regulates the expression of the amino acid transporter SLC1A5 in infected THP-1 human monocytes. Cell. Microbiol. 14, 1769–1783. doi: 10.1111/j.1462-5822.2012.01837.x
Brüggemann, H., Hagman, A., Jules, M., Sismeiro, O., Dillies, M. A., Gouyette, C., et al. (2006). Virulence strategies for infecting phagocytes deduced from the in vivo transcriptional program of Legionella pneumophila. Cell. Microbiol. 8, 1228–1240. doi: 10.1111/j.1462-5822.2006.00703.x
Byrd, T. F., and Horwitz, M. A. (2000). Aberrantly low transferrin receptor expression on human monocytes is associated with nonpermissiveness for Legionella pneumophila growth. J. Infect. Dis. 181, 1394–1400. doi: 10.1086/315390
Byrne, B., and Swanson, M. S. (1998). Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66, 3029–3034.
Cazalet, C., Gomez-Valero, L., Rusniok, C., Lomma, M., Dervins-Ravault, D., Newton, H. J., et al. (2010). Analysis of the Legionella longbeachae genome and transcriptome uncovers unique strategies to cause Legionnaires' disease. PLoS Genet. 6:e1000851. doi: 10.1371/journal.pgen.1000851
Cazalet, C., Rusniok, C., Brüggemann, H., Zidane, N., Magnier, A., Ma, L., et al. (2004). Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36, 1165–1173. doi: 10.1038/ng1447
Chatfield, C. H., and Cianciotto, N. P. (2007). The secreted pyomelanin pigment of Legionella pneumophila confers ferric reductase activity. Infect. Immun. 75, 4062–4070. doi: 10.1128/IAI.00489-07
Chen, D. E., Podell, S., Sauer, J. D., Swanson, M. S., and Saier, M. H. Jr. (2008). The phagosomal nutrient transporter (Pht) family. Microbiology 154, 42–53. doi: 10.1099/mic.0.2007/010611-0
Chien, M., Morozova, I., Shi, S., Sheng, H., Chen, J., Gomez, S. M., et al. (2004). The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305, 1966–1968. doi: 10.1126/science.1099776
Dalebroux, Z. D., Edwards, R. L., and Swanson, M. S. (2009). SpoT governs Legionella pneumophila differentiation in host macrophages. Mol. Microbiol. 71, 640–658. doi: 10.1111/j.1365-2958.2008.06555.x
Dalebroux, Z. D., Yagi, B. F., Sahr, T., Buchrieser, C., and Swanson, M. S. (2010). Distinct roles of ppGpp and DksA in Legionella pneumophila differentiation. Mol. Microbiol. 76, 200–219. doi: 10.1111/j.1365-2958.2010.07094.x
D'Auria, G., Jimenez-Hernandez, N., Peris-Bondia, F., Moya, A., and Latorre, A. (2010). Legionella pneumophila pangenome reveals strain-specific virulence factors. BMC Genomics 11:181. doi: 10.1186/1471-2164-11-181
Debroy, S., Dao, J., Soderberg, M., Rossier, O., and Cianciotto, N. P. (2006). Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc. Natl. Acad. Sci. U.S.A. 103, 19146–19151. doi: 10.1073/pnas.0608279103
Edwards, R. L., Dalebroux, Z. D., and Swanson, M. S. (2009). Legionella pneumophila couples fatty acid flux to microbial differentiation and virulence. Mol. Microbiol. 71, 1190–1204. doi: 10.1111/j.1365-2958.2008.06593.x
Eisenreich, W., Dandekar, T., Heesemann, J., and Goebel, W. (2010). Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat. Rev. Microbiol. 8, 401–412. doi: 10.1038/nrmicro2351
Eisenreich, W., Heesemann, J., Rudel, T., and Goebel, W. (2013). Metabolic host responses to infection by intracellular bacterial pathogens. Front. Cell. Infect. Microbiol. 3:24. doi: 10.3389/fcimb.2013.00024
Ensminger, A. W., and Isberg, R. R. (2010). E3 ubiquitin ligase activity and targeting of BAT3 by multiple Legionella pneumophila translocated substrates. Infect. Immun. 78, 3905–3919. doi: 10.1128/IAI.00344-10
Ewann, F., and Hoffman, P. S. (2006). Cysteine metabolism in Legionella pneumophila: characterization of an L-cystine-utilizing mutant. Appl. Environ. Microbiol. 72, 3993–4000. doi: 10.1128/AEM.00684-06
Eylert, E., Herrmann, V., Jules, M., Gillmaier, N., Lautner, M., Buchrieser, C., et al. (2010). Isotopologue profiling of Legionella pneumophila: role of serine and glucose as carbon substrates. J. Biol. Chem. 285, 22232–22243. doi: 10.1074/jbc.M110.128678
Eylert, E., Schar, J., Mertins, S., Stoll, R., Bacher, A., Goebel, W., et al. (2008). Carbon metabolism of Listeria monocytogenes growing inside macrophages. Mol. Microbiol. 69, 1008–1017. doi: 10.1111/j.1365-2958.2008.06337.x
Faucher, S. P., Mueller, C. A., and Shuman, H. A. (2011). Legionella pneumophila transcriptome during intracellular multiplication in human macrophages. Front. Microbiol. 2:60. doi: 10.3389/fmicb.2011.00060
Feeley, J. C., Gibson, R. J., Gorman, G. W., Langford, N. C., Rasheed, J. K., Mackel, D. C., et al. (1979). Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J. Clin. Microbiol. 10, 437–441.
Fields, B. S., Benson, R. F., and Besser, R. E. (2002). Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15, 506–526. doi: 10.1128/CMR.15.3.506-526.2002
Finsel, I., Ragaz, C., Hoffmann, C., Harrison, C. F., Weber, S., Van Rahden, V. A., et al. (2013). The Legionella effector RidL inhibits retrograde trafficking to promote intracellular replication. Cell Host Microbe 14, 38–50. doi: 10.1016/j.chom.2013.06.001
Fonseca, M. V., Sauer, J. D., Crepin, S., Byrne, B., and Swanson, M. S. (2014). The phtC-phtD locus equips Legionella pneumophila for thymidine salvage and replication in macrophages. Infect. Immun. 82, 720–730. doi: 10.1128/IAI.01043-13
Gal-Mor, O., and Segal, G. (2003a). Identification of CpxR as a positive regulator of icm and dot virulence genes of Legionella pneumophila. J. Bacteriol. 185, 4908–4919. doi: 10.1128/JB.185.16.4908-4919.2003
Gal-Mor, O., and Segal, G. (2003b). The Legionella pneumophila GacA homolog (LetA) is involved in the regulation of icm virulence genes and is required for intracellular multiplication in Acanthamoeba castellanii. Microb. Pathog. 34, 187–194. doi: 10.1016/S0882-4010(03)00027-5
Gao, L. Y., Harb, O. S., and Abu Kwaik, Y. (1997). Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect. Immun. 65, 4738–4746.
Garcia, M. T., Jones, S., Pelaz, C., Millar, R. D., and Abu Kwaik, Y. (2007). Acanthamoeba polyphaga resuscitates viable non-culturable Legionella pneumophila after disinfection. Environ. Microbiol. 9, 1267–1277. doi: 10.1111/j.1462-2920.2007.01245.x
Garduno, R. A., Garduno, E., Hiltz, M., and Hoffman, P. S. (2002). Intracellular growth of Legionella pneumophila gives rise to a differentiated form dissimilar to stationary-phase forms. Infect. Immun. 70, 6273–6283. doi: 10.1128/IAI.70.11.6273-6283.2002
Gebran, S. J., Yamamoto, Y., Newton, C., Klein, T. W., and Friedman, H. (1994). Inhibition of Legionella pneumophila growth by gamma interferon in permissive A/J mouse macrophages: role of reactive oxygen species, nitric oxide, tryptophan, and iron(III). Infect. Immun. 62, 3197–3205.
George, J. R., Pine, L., Reeves, M. W., and Harrell, W. K. (1980). Amino acid requirements of Legionella pneumophila. J. Clin. Microbiol. 11, 286–291.
Gillmaier, N., Gotz, A., Schulz, A., Eisenreich, W., and Goebel, W. (2012). Metabolic responses of primary and transformed cells to intracellular Listeria monocytogenes. PLoS ONE 7:e52378. doi: 10.1371/journal.pone.0052378
Glöckner, G., Albert-Weissenberger, C., Weinmann, E., Jacobi, S., Schunder, E., Steinert, M., et al. (2007). Identification and characterization of a new conjugation/type IVA secretion system (trb/tra) of Legionella pneumophila Corby localized on two mobile genomic islands. Int. J. Med. Microbiol. 298, 411–428. doi: 10.1016/j.ijmm.2007.07.012
Greub, G., and Raoult, D. (2004). Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17, 413–433. doi: 10.1128/CMR.17.2.413-433.2004
Hales, L. M., and Shuman, H. A. (1999a). Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect. Immun. 67, 3662–3666.
Hales, L. M., and Shuman, H. A. (1999b). The Legionella pneumophila rpoS gene is required for growth within Acanthamoeba castellanii. J. Bacteriol. 181, 4879–4889.
Hammer, B. K., and Swanson, M. S. (1999). Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33, 721–731. doi: 10.1046/j.1365-2958.1999.01519.x
Hammer, B. K., Tateda, E. S., and Swanson, M. S. (2002). A two-component regulator induces the transmission phenotype of stationary-phase Legionella pneumophila. Mol. Microbiol. 44, 107–118. doi: 10.1046/j.1365-2958.2002.02884.x
Haneburger, I., and Hilbi, H. (2013). Phosphoinositide lipids and the Legionella pathogen vacuole. Curr. Top. Microbiol. Immunol. 376, 155–173. doi: 10.1007/82_2013_341
Harada, E., Iida, K., Shiota, S., Nakayama, H., and Yoshida, S. (2010). Glucose metabolism in Legionella pneumophila: dependence on the Entner-Doudoroff pathway and connection with intracellular bacterial growth. J. Bacteriol. 192, 2892–2899. doi: 10.1128/JB.01535-09
Hartel, T., Eylert, E., Schulz, C., Petruschka, L., Gierok, P., Grubmüller, S., et al. (2012). Characterization of central carbon metabolism of Streptococcus pneumoniae by isotopologue profiling. J. Biol. Chem. 287, 4260–4274. doi: 10.1074/jbc.M111.304311
Herrmann, V., Eidner, A., Rydzewski, K., Blädel, I., Jules, M., Buchrieser, C., et al. (2010). GamA is a eukaryotic-like glucoamylase responsible for glycogen- and starch-degrading activity of Legionella pneumophila. Int. J. Med. Microbiol. 301, 133–139. doi: 10.1016/j.ijmm.2010.08.016
Hilbi, H., and Haas, A. (2012). Secretive bacterial pathogens and the secretory pathway. Traffic 13, 1187–1197. doi: 10.1111/j.1600-0854.2012.01344.x
Hilbi, H., Hoffmann, C., and Harrison, C. F. (2011). Legionella spp. outdoors: colonization, communication and persistence. Environ. Microbiol. Rep. 3, 286–296. doi: 10.1111/j.1758-2229.2011.00247.x
Hoffmann, C., Finsel, I., Otto, A., Pfaffinger, G., Rothmeier, E., Hecker, M., et al. (2014a). Functional analysis of novel Rab GTPases identified in the proteome of purified Legionella-containing vacuoles from macrophages. Cell. Microbiol. 16, 1034–1052. doi: 10.1111/cmi.12256
Hoffmann, C., Harrison, C. F., and Hilbi, H. (2014b). The natural alternative: protozoa as cellular models for Legionella infection. Cell. Microbiol. 16, 15–26. doi: 10.1111/cmi.12235
Hovel-Miner, G., Faucher, S. P., Charpentier, X., and Shuman, H. A. (2010). ArgR-regulated genes are derepressed in the Legionella-containing vacuole. J. Bacteriol. 192, 4504–4516. doi: 10.1128/JB.00465-10
Hovel-Miner, G., Pampou, S., Faucher, S. P., Clarke, M., Morozova, I., Morozov, P., et al. (2009). SigmaS controls multiple pathways associated with intracellular multiplication of Legionella pneumophila. J. Bacteriol. 191, 2461–2473. doi: 10.1128/JB.01578-08
Hubber, A., and Roy, C. R. (2010). Modulation of host cell function by Legionella pneumophila type IV effectors. Annu. Rev. Cell. Dev. Biol. 26, 261–283. doi: 10.1146/annurev-cellbio-100109-104034
Isberg, R. R., O'Connor, T. J., and Heidtman, M. (2009). The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7, 13–24. doi: 10.1038/nrmicro1967
Ivanov, S. S., Charron, G., Hang, H. C., and Roy, C. R. (2010). Lipidation by the host prenyltransferase machinery facilitates membrane localization of Legionella pneumophila effector proteins. J. Biol. Chem. 285, 34686–34698. doi: 10.1074/jbc.M110.170746
James, B. W., Mauchline, W. S., Dennis, P. J., Keevil, C. W., and Wait, R. (1999). Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low-nutrient environments. Appl. Environ. Microbiol. 65, 822–827.
James, B. W., Mauchline, W. S., Fitzgeorge, R. B., Dennis, P. J., and Keevil, C. W. (1995). Influence of iron-limited continuous culture on physiology and virulence of Legionella pneumophila. Infect. Immun. 63, 4224–4230.
Johnson, W., Varner, L., and Poch, M. (1991). Acquisition of iron by Legionella pneumophila: role of iron reductase. Infect. Immun. 59, 2376–2381.
Joseph, B., Mertins, S., Stoll, R., Schar, J., Umesha, K. R., Luo, Q., et al. (2008). Glycerol metabolism and PrfA activity in Listeria monocytogenes. J. Bacteriol. 190, 5412–5430. doi: 10.1128/JB.00259-08
Kessler, A., Schell, U., Sahr, T., Tiaden, A., Harrison, C., Buchrieser, C., et al. (2013). The Legionella pneumophila orphan sensor kinase LqsT regulates competence and pathogen-host interactions as a component of the LAI-1 circuit. Environ. Microbiol. 15, 646–662. doi: 10.1111/j.1462-2920.2012.02889.x
Khosravi-Darani, K., Mokhtari, Z. B., Amai, T., and Tanaka, K. (2013). Microbial production of poly(hydroxybutyrate) from C(1) carbon sources. Appl. Microbiol. Biotechnol. 97, 1407–1424. doi: 10.1007/s00253-012-4649-0
Koubar, M., Rodier, M. H., and Frère, J. (2013). Involvement of minerals in adherence of Legionella pneumophila to surfaces. Curr. Microbiol. 66, 437–442. doi: 10.1007/s00284-012-0295-0
Kozak, N. A., Buss, M., Lucas, C. E., Frace, M., Govil, D., Travis, T., et al. (2010). Virulence factors encoded by Legionella longbeachae identified on the basis of the genome sequence analysis of clinical isolate D-4968. J. Bacteriol. 192, 1030–1044. doi: 10.1128/JB.01272-09
Lau, H. Y., and Ashbolt, N. J. (2009). The role of biofilms and protozoa in Legionella pathogenesis: implications for drinking water. J. Appl. Microbiol. 107, 368–378. doi: 10.1111/j.1365-2672.2009.04208.x
Liles, M. R., Edelstein, P. H., and Cianciotto, N. P. (1999). The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol. Microbiol. 31, 959–970. doi: 10.1046/j.1365-2958.1999.01239.x
Liles, M. R., Scheel, T. A., and Cianciotto, N. P. (2000). Discovery of a nonclassical siderophore, legiobactin, produced by strains of Legionella pneumophila. J. Bacteriol. 182, 749–757. doi: 10.1128/JB.182.3.749-757.2000
Lomma, M., Dervins-Ravault, D., Rolando, M., Nora, T., Newton, H. J., Sansom, F. M., et al. (2010). The Legionella pneumophila F-box protein Lpp2082 (AnkB) modulates ubiquitination of the host protein parvin B and promotes intracellular replication. Cell. Microbiol. 12, 1272–1291. doi: 10.1111/j.1462-5822.2010.01467.x
Lynch, D., Fieser, N., Gloggler, K., Forsbach-Birk, V., and Marre, R. (2003). The response regulator LetA regulates the stationary-phase stress response in Legionella pneumophila and is required for efficient infection of Acanthamoeba castellanii. FEMS Microbiol. Lett. 219, 241–248. doi: 10.1016/S0378-1097(03)00050-8
Mengaud, J. M., and Horwitz, M. A. (1993). The major iron-containing protein of Legionella pneumophila is an aconitase homologous with the human iron-responsive element-binding protein. J. Bacteriol. 175, 5666–5676.
Molofsky, A. B., and Swanson, M. S. (2003). Legionella pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. Mol. Microbiol. 50, 445–461. doi: 10.1046/j.1365-2958.2003.03706.x
Molofsky, A. B., and Swanson, M. S. (2004). Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53, 29–40. doi: 10.1111/j.1365-2958.2004.04129.x
Nasrallah, G. K., Riveroll, A. L., Chong, A., Murray, L. E., Lewis, P. J., and Garduno, R. A. (2011). Legionella pneumophila requires polyamines for optimal intracellular growth. J. Bacteriol. 193, 4346–4360. doi: 10.1128/JB.01506-10
Pearce, M. M., and Cianciotto, N. P. (2009). Legionella pneumophila secretes an endoglucanase that belongs to the family-5 of glycosyl hydrolases and is dependent upon type II secretion. FEMS Microbiol. Lett. 300, 256–264. doi: 10.1111/j.1574-6968.2009.01801.x
Pine, L., George, J. R., Reeves, M. W., and Harrell, W. K. (1979). Development of a chemically defined liquid medium for growth of Legionella pneumophila. J. Clin. Microbiol. 9, 615–626.
Poch, M. T., and Johnson, W. (1993). Ferric reductases of Legionella pneumophila. Biometals 6, 107–114. doi: 10.1007/BF00140111
Price, C. T., Al-Khodor, S., Al-Quadan, T., and Abu Kwaik, Y. (2010a). Indispensable role for the eukaryotic-like ankyrin domains of the ankyrin B effector of Legionella pneumophila within macrophages and amoebae. Infect. Immun. 78, 2079–2088. doi: 10.1128/IAI.01450-09
Price, C. T., Al-Khodor, S., Al-Quadan, T., Santic, M., Habyarimana, F., Kalia, A., et al. (2009). Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa. PLoS Pathog. 5:e1000704. doi: 10.1371/journal.ppat.1000704
Price, C. T., Al-Quadan, T., Santic, M., Jones, S. C., and Abu Kwaik, Y. (2010b). Exploitation of conserved eukaryotic host cell farnesylation machinery by an F-box effector of Legionella pneumophila. J. Exp. Med. 207, 1713–1726. doi: 10.1084/jem.20100771
Price, C. T., Al-Quadan, T., Santic, M., Rosenshine, I., and Abu Kwaik, Y. (2011). Host proteasomal degradation generates amino acids essential for intracellular bacterial growth. Science 334, 1553–1557. doi: 10.1126/science.1212868
Rasis, M., and Segal, G. (2009). The LetA-RsmYZ-CsrA regulatory cascade, together with RpoS and PmrA, post-transcriptionally regulates stationary phase activation of Legionella pneumophila Icm/Dot effectors. Mol. Microbiol. 72, 995–1010. doi: 10.1111/j.1365-2958.2009.06705.x
Ratledge, C., and Dover, L. G. (2000). Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54, 881–941. doi: 10.1146/annurev.micro.54.1.881
Reeves, M. W., Pine, L., Hutner, S. H., George, J. R., and Harrell, W. K. (1981). Metal requirements of Legionella pneumophila. J. Clin. Microbiol. 13, 688–695.
Ristroph, J. D., Hedlund, K. W., and Gowda, S. (1981). Chemically defined medium for Legionella pneumophila growth. J. Clin. Microbiol. 13, 115–119.
Robey, M., and Cianciotto, N. P. (2002). Legionella pneumophila feoAB promotes ferrous iron uptake and intracellular infection. Infect. Immun. 70, 5659–5669. doi: 10.1128/IAI.70.10.5659-5669.2002
Rohmer, L., Hocquet, D., and Miller, S. I. (2011). Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends. Microbiol. 19, 341–348. doi: 10.1016/j.tim.2011.04.003
Rothmeier, E., Pfaffinger, G., Hoffmann, C., Harrison, C. F., Grabmayr, H., Repnik, U., et al. (2013). Activation of Ran GTPase by a Legionella effector promotes microtubule polymerization, pathogen vacuole motility and infection. PLoS Pathog. 9:e1003598. doi: 10.1371/journal.ppat.1003598
Rowbotham, T. J. (1986). Current views on the relationships between amoebae, legionellae and man. Isr. J. Med. Sci. 22, 678–689.
Sahr, T., Brüggemann, H., Jules, M., Lomma, M., Albert-Weissenberger, C., Cazalet, C., et al. (2009). Two small ncRNAs jointly govern virulence and transmission in Legionella pneumophila. Mol. Microbiol. 72, 741–762. doi: 10.1111/j.1365-2958.2009.06677.x
Sauer, J. D., Bachman, M. A., and Swanson, M. S. (2005). The phagosomal transporter A couples threonine acquisition to differentiation and replication of Legionella pneumophila in macrophages. Proc. Natl. Acad. Sci. U.S.A. 102, 9924–9929. doi: 10.1073/pnas.0502767102
Schell, U., Kessler, A., and Hilbi, H. (2014). Phosphorylation signalling through the Legionella quorum sensing histidine kinases LqsS and LqsT converges on the response regulator LqsR. Mol. Microbiol. 92, 1039–1055. doi: 10.1111/mmi.12612
Schroeder, G. N., Petty, N. K., Mousnier, A., Harding, C. R., Vogrin, A. J., Wee, B., et al. (2010). Legionella pneumophila strain 130b possesses a unique combination of type IV secretion systems and novel Dot/Icm secretion system effector proteins. J. Bacteriol. 192, 6001–6016. doi: 10.1128/JB.00778-10
Schunder, E., Gillmaier, N., Kutzner, E., Herrmann, V., Lautner, M., Heuner, K., et al. (2014). Amino acid uptake and metabolism of Legionella pneumophila hosted by Acanthamoeba castellanii. J. Biol. Chem. 289, 21040–21054. doi: 10.1074/jbc.M114.570085
Spirig, T., Tiaden, A., Kiefer, P., Buchrieser, C., Vorholt, J. A., and Hilbi, H. (2008). The Legionella autoinducer synthase LqsA produces an a-hydroxyketone signaling molecule. J. Biol. Chem. 283, 18113–18123. doi: 10.1074/jbc.M801929200
Starkenburg, S. R., Casey, J. M., and Cianciotto, N. P. (2004). Siderophore activity among members of the Legionella genus. Curr. Microbiol. 49, 203–207. doi: 10.1007/s00284-004-4342-3
Steeb, B., Claudi, B., Burton, N. A., Tienz, P., Schmidt, A., Farhan, H., et al. (2013). Parallel exploitation of diverse host nutrients enhances Salmonella virulence. PLoS Pathog. 9:e1003301. doi: 10.1371/journal.ppat.1003301
Steinert, M., Emody, L., Amann, R., and Hacker, J. (1997). Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl. Environ. Microbiol. 63, 2047–2053.
Steinert, M., Flügel, M., Schuppler, M., Helbig, J. H., Supriyono, A., Proksch, P., et al. (2001). The Lly protein is essential for p-hydroxyphenylpyruvate dioxygenase activity in Legionella pneumophila. FEMS Microbiol. Lett. 203, 41–47. doi: 10.1111/j.1574-6968.2001.tb10818.x
Tesh, M. J., and Miller, R. D. (1981). Amino acid requirements for Legionella pneumophila growth. J. Clin. Microbiol. 13, 865–869.
Tesh, M. J., and Miller, R. D. (1983). Arginine biosynthesis in Legionella pneumophila: absence of N-acetylglutamate synthetase. Can. J. Microbiol. 29, 1230–1233. doi: 10.1139/m83-190
Tesh, M. J., Morse, S. A., and Miller, R. D. (1983). Intermediary metabolism in Legionella pneumophila: utilization of amino acids and other compounds as energy sources. J. Bacteriol. 154, 1104–1109.
Tiaden, A., and Hilbi, H. (2012). a-Hydroxyketone synthesis and sensing by Legionella and Vibrio. Sensors 12, 2899–2919. doi: 10.3390/s120302899
Tiaden, A., Spirig, T., Carranza, P., Brüggemann, H., Riedel, K., Eberl, L., et al. (2008). Synergistic contribution of the Legionella pneumophila lqs genes to pathogen-host interactions. J. Bacteriol. 190, 7532–7547. doi: 10.1128/JB.01002-08
Tiaden, A., Spirig, T., and Hilbi, H. (2010). Bacterial gene regulation by a-hydroxyketone signaling. Trends. Microbiol. 18, 288–297. doi: 10.1016/j.tim.2010.03.004
Tiaden, A., Spirig, T., Weber, S. S., Brüggemann, H., Bosshard, R., Buchrieser, C., et al. (2007). The Legionella pneumophila response regulator LqsR promotes host cell interactions as an element of the virulence regulatory network controlled by RpoS and LetA. Cell. Microbiol. 9, 2903–2920. doi: 10.1111/j.1462-5822.2007.01005.x
Urwyler, S., Brombacher, E., and Hilbi, H. (2009a). Endosomal and secretory markers of the Legionella-containing vacuole. Commun. Integr. Biol. 2, 107–109. Available online at: http://www.landesbioscience.com/journals/cib/article/7713
Urwyler, S., Nyfeler, Y., Ragaz, C., Lee, H., Mueller, L. N., Aebersold, R., et al. (2009b). Proteome analysis of Legionella vacuoles purified by magnetic immunoseparation reveals secretory and endosomal GTPases. Traffic 10, 76–87. doi: 10.1111/j.1600-0854.2008.00851.x
Vikram, H. R., and Bia, F. J. (2002). Severe Legionella pneumophila pneumonia in a patient with iron overload. Scand. J. Infect. Dis. 34, 772–774. doi: 10.1080/00365540260348608
Viswanathan, V. K., Edelstein, P. H., Pope, C. D., and Cianciotto, N. P. (2000). The Legionella pneumophila iraAB locus is required for iron assimilation, intracellular infection, and virulence. Infect. Immun. 68, 1069–1079. doi: 10.1128/IAI.68.3.1069-1079.2000
Weiss, E., Peacock, M., and Williams, J. (1980). Glucose and glutamate metabolism of Legionella pneumophila. Curr. Microbiol. 4, 1–6. doi: 10.1007/BF02602882
Wieland, H., Ullrich, S., Lang, F., and Neumeister, B. (2005). Intracellular multiplication of Legionella pneumophila depends on host cell amino acid transporter SLC1A5. Mol. Microbiol. 55, 1528–1537. doi: 10.1111/j.1365-2958.2005.04490.x
Williams, R. J. (2012). Iron in evolution. FEBS Lett. 586, 479–484. doi: 10.1016/j.febslet.2011.05.068
Zamboni, N., Fendt, S. M., Ruhl, M., and Sauer, U. (2009). (13)C-based metabolic flux analysis. Nat. Protoc. 4, 878–892. doi: 10.1038/nprot.2009.58
Zheng, H., Chatfield, C. H., Liles, M. R., and Cianciotto, N. P. (2013). Secreted pyomelanin of Legionella pneumophila promotes bacterial iron uptake and growth under iron-limiting conditions. Infect. Immun. 81, 4182–4191. doi: 10.1128/IAI.00858-13
Zusman, T., Aloni, G., Halperin, E., Kotzer, H., Degtyar, E., Feldman, M., et al. (2007). The response regulator PmrA is a major regulator of the Icm/Dot type IV secretion system in Legionella pneumophila and Coxiella burnetii. Mol. Microbiol. 63, 1508–1523. doi: 10.1111/j.1365-2958.2007.05604.x
Keywords: amoeba, Dictyostelium, Legionella, macrophage, metabolism, nutrition, pathogen vacuole, type IV secretion
Citation: Manske C and Hilbi H (2014) Metabolism of the vacuolar pathogen Legionella and implications for virulence. Front. Cell. Infect. Microbiol. 4:125. doi: 10.3389/fcimb.2014.00125
Received: 26 June 2014; Paper pending published: 24 July 2014;
Accepted: 20 August 2014; Published online: 09 September 2014.
Edited by:
Wolfgang Eisenreich, Technische Universität München, GermanyReviewed by:
Marina Santic', University of Rijeka, CroatiaJoseph Vogel, Washington University School of Medicine, USA
Copyright © 2014 Manske and Hilbi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hubert Hilbi, Institute of Medical Microbiology, Faculty of Medicine, University of Zürich, Gloriastrasse 30/32, 8006 Zürich, Switzerland e-mail: hilbi@imm.uzh.ch
 Christian Manske
Christian Manske Hubert Hilbi
Hubert Hilbi