Computational Screening of MOFs for Acetylene Separation
- Department of Chemical and Biological Engineering, Koc University, Istanbul, Turkey
Efficient separation of acetylene (C2H2) from CO2 and CH4 is important to meet the requirement of high-purity acetylene in various industrial applications. Metal organic frameworks (MOFs) are great candidates for adsorption-based C2H2/CO2 and C2H2/CH4 separations due to their unique properties such as wide range of pore sizes and tunable chemistries. Experimental studies on the limited number of MOFs revealed that MOFs offer remarkable C2H2/CO2 and C2H2/CH4 selectivities based on single-component adsorption data. We performed the first large-scale molecular simulation study to investigate separation performances of 174 different MOF structures for C2H2/CO2 and C2H2/CH4 mixtures. Using the results of molecular simulations, several adsorbent performance evaluation metrics, such as selectivity, working capacity, adsorbent performance score, sorbent selection parameter, and regenerability were computed for each MOF. Based on these metrics, the best adsorbent candidates were identified for both separations. Results showed that the top three most promising MOF adsorbents exhibit C2H2/CO2 selectivities of 49, 47, 24 and C2H2/CH4 selectivities of 824, 684, 638 at 1 bar, 298 K and these are the highest C2H2 selectivities reported to date in the literature. Structure-performance analysis revealed that the best MOF adsorbents have pore sizes between 4 and 11 Å, surface areas in the range of 600–1,200 m2/g and porosities between 0.4 and 0.6 for selective separation of C2H2 from CO2 and CH4. These results will guide the future studies for the design of new MOFs with high C2H2 separation potentials.
Introduction
Metal organic frameworks (MOFs), nanoporous materials that are composed of metal clusters connected with organic linkers, have attracted significant interest in the last decade. MOFs offer a wide range of pore sizes, permanent porosities, very large surface areas, and good chemical stabilities (Li et al., 1999; Eddaoudi et al., 2002). The most important characteristic of MOFs is that their physical, chemical and structural properties can be tuned during synthesis. This controllable synthesis leads to a large diversity of materials having different geometry, pore size, and chemical functionality (Mondloch et al., 2013). Due to these advantageous physical and chemical properties, MOFs have emerged as strong alternatives to traditional nanoporous materials in various gas separation applications. MOFs have been widely examined for CO2 separation because of the growing environmental concerns on the removal of CO2 from natural gas (CO2/CH4), flue gas (CO2/N2), and from other gases (CO2/H2). Experimentally measured selectivities and gas uptake capacities of several MOFs for separation of CO2 from CH4 and N2 have been reported and results showed that MOFs can be strong alternatives to traditional porous materials in CO2 separations (Li et al., 2011). Comparison of CO2 separation performances of MOFs, zeolites and activated carbons showed that CO2/N2 selectivity changes from low in zeolites to moderate in carbon-based absorbents and becomes high in MOFs (Ben-Mansour et al., 2016). Although a significant number of studies exist on the CO2 separation with MOFs, acetylene separation with these new porous materials has not been thoroughly investigated and research on MOFs for acetylene separation left behind that for CO2 separation.
Acetylene (C2H2) is the simplest member of unsaturated hydrocarbons and it is produced by different processes such as reaction of water with calcium carbide from coal, partial oxidation of natural gas, or as a byproduct of ethylene steam cracking (Zhang et al., 2011). C2H2 is a very important raw material for the synthesis of various industrial chemicals such as polyurethane and polyester plastics, consumer products, and oxy-acetylene welding and cutting in metal fabrication. Since high purity C2H2 is strongly needed for these processes, C2H2 separation is important in the industry. C2H2 is traditionally separated from CO2 and CH4 using cryogenic distillation, however this process is very costly. C2H2/CO2 separation is specifically challenging because both gas molecules have similar molecular sizes (3.4 × 3.4 × 5.5 and 3.4 × 3.4 × 5.3 Å) and boiling points (189.3 and 194.7 K) (Foo et al., 2016). The energy and equipment costs associated with these gas separations could be significantly reduced by the development of alternative separation methods such as adsorption-based gas separations which provide very large reductions in energy consumption and costs of these processes. The greatest limitation in the applications of adsorption-based gas separation technologies is the low selectivity of the materials used as adsorbents. Therefore, identification of new adsorbent materials that can achieve C2H2 separation from other gases with high selectivity has gained significant attention.
An ideal adsorbent material should offer a good combination of high adsorption selectivity and high uptake capacity in addition to good stability. There is a wide range for C2H2 uptake capacities of MOFs from 25 to 200 cm3/g reported at 1 bar and 298 K (Zhang et al., 2011). Recent studies on MOFs showed that it is difficult to simultaneously achieve both high C2H2/CO2 selectivity and high C2H2 uptake capacity (Wen et al., 2016). For example, a widely studied MOF, HKUST-1 (also known as CuBTC in the MOF literature) was reported to exhibit high C2H2 uptake, 201 cm3/g at 1 bar and 298 K (Xiang et al., 2009). However, its C2H2/CO2 selectivity was found to be low, 6, based on the ideal adsorbed solution theory (IAST) calculations (Myers, 2002) for equimolar C2H2/CO2 mixture (Li et al., 2014). A MOF named as UTSA-50 was shown to exhibit higher C2H2/CO2 selectivity, 13.3 at 1 bar and 296 K based on the Henry's law ratios of C2H2 and CO2 but its C2H2 uptake was low, 91 cm3/g, which was attributed to its low surface area (Xu et al., 2013). Wen et al. (2016) synthesized a new MOF and measured its C2H2 uptake as 216 cm3/g at 1 bar and 298 K, which was one of the highest C2H2 uptakes of MOFs reported to date. They also calculated the C2H2/CO2 selectivity of the MOF using IAST based on the single-component adsorption isotherms data of C2H2 and CO2. Results showed that C2H2 selectivity is 11.5 at 1 bar for separation of an equimolar C2H2/CO2 mixture. Li et al. (2014) studied C2H2/CO2 separation performance of hydrogen bonded organic frameworks (HOFs) and calculated selectivity of HOF-3 using IAST as 21 at 1 bar and 296 K. Isostructural MOF-74 materials having different metal sites were reported to have high C2H2 uptakes, 120–197 cm3/g at 1 bar and 295 K, however their C2H2/CO2 selectivities were not reported. Xiang et al. (2010) studied the effect of metal sites on C2H2 storage performance of four isostructural MOFs and reported the highest C2H2 uptake capacity as 198 cm3/g. Similar to C2H2/CO2 separation, C2H2/CH4 separation is an important process because C2H2 is mainly derived from the cracking of crude oil and residual oils. Purification is necessary to meet the requirement of high-purity C2H2 for the organic synthesis (Zhang et al., 2011). Separation of C2H2 from CH4 using MOFs has been very rarely studied in the literature. UTSA-50 was reported to have a high C2H2/CH4 selectivity, 68, based on the ratio of Henry's constants of gases (Xu et al., 2013).
As can be seen from this literature review, most experiments only reported the C2H2 uptake of MOFs and estimated MOFs' selectivities using the single-component data without performing the adsorption measurements for gas mixtures. In reality, gases exist as mixtures and selectivities should be calculated for gas mixtures. Considering the large number and variety of available MOFs, it is very challenging to identify the most promising MOF materials for adsorption-based separation of C2H2/CO2 and C2H2/CH4 mixtures using purely experimental manners. Molecular simulations play an important role in studying adsorption of various gas molecules in a large number of MOFs in a time effective manner to identify the best materials for a target gas separation (Colón and Snurr, 2014). There are some computational studies on C2H2 storage performance of MOFs in the literature (Pang et al., 2015; Chen et al., 2016; Zhang et al., 2017) however, the number of molecular simulation studies on C2H2 separation is very limited. Fischer et al. (2010) performed the first molecular simulation study to obtain adsorption isotherm of equimolar C2H2/CO2 mixture in HKUST-1. The C2H2/CO2 selectivity was calculated from the mixture data as 2.4, which was much lower than the one calculated from the single-component adsorption data (6). Yeganegi et al. (2017) carried out Grand Canonical Monte Carlo (GCMC) simulations for adsorption of equimolar C2H2/CH4 mixture in MOF-5, MOF-505, and HKUST-1. The C2H2/CH4 selectivity of HKUST-1 (66) was computed to be significantly higher than that of MOF-505 (6) and MOF-5 (2) at 1 bar and 295 K. Ji et al. (2017) recently performed GCMC simulations to calculate adsorption isotherms for single-component C2H2, CO2, CH4, and equimolar C2H2/CO2 and C2H2/CH4 mixtures at 298 K. They considered 11 MOFs having the same metal and showed that all MOFs except MOF-505 have C2H2/CH4 selectivities lower than 7 and C2H2/CO2 selectivities lower than 2. Selectivity of MOF-505 for C2H2/CH4 was computed to be around 9 whereas selectivity for C2H2/CO2 was found to be <2 at 1 bar. As can be seen from this review, current molecular simulation studies calculated C2H2 selectivities of at most 11 different MOF structures having the same metal. There is no large-scale computational screening study to assess C2H2/CO2 and C2H2/CH4 selectivities of MOFs that span a large variety in structural properties. It is also important to note that although selectivity is a widely used metric to assess the gas separation performances of adsorbents, several other metrics such as working capacity and regenerability determine the practical usability of MOF adsorbents in separation processes. These metrics have not been examined for adsorption-based C2H2 separation performances of MOFs to date.
In this work, we performed molecular simulations for a large number and variety of MOFs to examine their separation potentials for C2H2/CO2 and C2H2/CH4 mixtures. Adsorption data of C2H2/CO2 and C2H2/CH4 mixtures obtained from the GCMC simulations were used to calculate several adsorbent performance metrics of MOFs including adsorption selectivity, working capacity, adsorbent performance score (APS), sorbent selection parameter and regenerability. Separation performances of MOFs were evaluated based on these metrics and the top performing MOF adsorbents were identified for C2H2/CO2 and C2H2/CH4 separations. We then examined the relations between structural properties of MOFs such as pore sizes, porosities, surface areas and their C2H2 selectivities to provide the structure-performance relationships that can serve as a map for experimental synthesis of new MOFs with better C2H2 separation performances.
Materials and Methods
MOFs
We used the MOFs that represent a large variety in structure and chemistry from our previous work (Sumer and Keskin, 2016) and the crystallographic information of these MOFs were obtained from the literature (Chung et al., 2014). We also included some newly synthesized MOFs for which experimental C2H2 uptakes were reported and crystallographic information of these MOFs were taken from the Cambridge Structural Database (CSD) (Groom and Allen, 2014). As a result, we considered 174 different MOF structures in this work. Structural properties of MOFs such as pore limiting diameter (PLD), the largest cavity diameter (LCD), accessible surface area, pore volume and density were calculated using Zeo++ software (Willems et al., 2012). We only considered MOFs with LCDs larger than 4 Å so that all three gas molecules can be adsorbed in the pores of materials. The PLDs, LCDs, surface areas, pore volumes, and densities of MOFs range from 2.5 to 15.6 Å, 4.1 to 28.7 Å, 103 to 5,800 m2/g, 0.08 to 3.3 cm3/g, and 0.18 to 5.05 g/cm3, respectively. The complete list of the MOFs studied in this work and their calculated structural properties are given in Table S1.
Simulation Details
We performed GCMC simulations to compute adsorption isotherms of gas mixtures in MOFs (Frenkel and Smit, 2002). These simulations were carried out as implemented in the RASPA simulation code (Dubbeldam, 2014). Five different types of moves, translation, reinsertion, rotation, swap, and identity exchange of molecules were considered. The Lorentz-Berthelot mixing rules were employed. The cut-off distance for truncation of the intermolecular interactions was set to 12.5 Å. The simulation cell lengths were increased to at least 25 Å along each dimension and periodic boundary conditions were applied in all simulations. For each MOF, simulations were carried out for 60,000 cycles with the first 10,000 cycles for initialization. Figure S1 shows that molecular simulation reached equilibrium at 10,000 cycles and increasing the cycle number does not affect the number of adsorbed gas molecules. Peng-Robinson equation of state was used to convert the pressure to the corresponding fugacity. More details of these simulations can be found in the literature (Frenkel and Smit, 2002; Dubbeldam, 2014).
C2H2 molecule was represented as a two-site rigid and linear model with the Lennard-Jones (LJ) positions located on the carbon atoms and partial charges located on each atom (Fischer et al., 2010). Unsaturated C=C and C–H bond lengths were considered as 1.211 and 1.071 Å, respectively. CO2 molecule was modeled as a linear molecule with three LJ sites and partial charges were centered on each atom (Potoff and Siepmann, 2001). The rigid C–O bond length used in this model was 1.16 Å. Single-site spherical LJ 12–6 potential was used to model CH4 molecules (Chen and Siepmann, 1999). All LJ parameters and atomic partial charges of gas molecules are given in Table S2.
The potential parameters of MOF atoms were taken from the Universal Force Field (UFF) (Rappé et al., 1992) since UFF has been successful in predicting gas adsorption and separation performances of a large number of MOFs in previous studies (Keskin et al., 2009). Furthermore, UFF contains potential parameters for all elements of the periodic table and applicable to all types of MOFs having a variety of atoms. Potential parameters of Cu atoms of MOFs were taken from a molecular simulation study (Fischer et al., 2010) in which modified parameters for Cu was shown to better represent the interaction of carbon site of C2H2 and oxygen site of CO2 with the unsaturated Cu sites of MOFs. Electrostatic interactions were taken into account using the Coulomb potential. In order to compute the electrostatic interactions between gas molecules and MOFs, partial point charges were assigned to MOF atoms using the extended charge equilibration method (EQeq; Wilmer et al., 2012). MOFs were assumed to be rigid in their reported crystallographic structures in the simulations. This assumption has been used in all large-scale molecular simulation studies of MOFs to save significant computational time. Since we only considered the MOFs that have pore sizes larger than the kinetic diameters of the gas molecules, flexibility is expected to have a negligible effect on the gas adsorption results. All GCMC simulations were performed at an adsorption pressure of 1 bar and desorption pressure of 0.1 bar at 298 K since the compression limit for the safe storage of C2H2 is 2 bar. These conditions were chosen to mimic vacuum swing adsorption process following the literature (Bae and Snurr, 2011). Equimolar C2H2/CO2 and C2H2/CH4 mixtures were considered in all molecular simulations. It was recently discussed that the two most important factors in molecular simulations are the force field and the degree of sampling in the relevant configuration space (van Gunsteren et al., 2017). In order to show the good sampling of our simulations, we reported the deviations of GCMC results for the top three promising MOFs for C2H2/CO2 and C2H2/CH4 separations in Table S3 and results showed that the uncertainty for the simulated C2H2 adsorption is <3%.
Adsorbent Evaluation Metrics
Results obtained from GCMC simulations were used to compute several adsorbent evaluation metrics that are defined in Table 1. Adsorption selectivity (Sads) is the most widely used metric to evaluate adsorbents and it is simply defined as the ratio of compositions of the adsorbed gases (x) in the adsorbent normalized by the ratio of bulk phase compositions (y). The subscript 1 represents the strongly adsorbed gas and subscript 2 represents the weakly adsorbed gas. Since the aim of our work is to identify the MOFs that are able to selectively separate C2H2 from CO2 and CH4, we reported C2H2/CO2 and C2H2/CH4 selectivities of MOFs. Working capacity (ΔN) is defined as the difference between the gas uptakes (N) at the adsorption and desorption pressures in the unit of mol gas per kg adsorbent (Bae and Snurr, 2011). We computed C2H2 working capacity of all MOFs. APS was defined as the product of selectivity and working capacity to easily identify the top performing adsorbent materials that combine high selectivities with high working capacities (Chung et al., 2016). Sorbent selection parameter (Ssp) includes the ratio of working capacities and selectivities computed at adsorption and desorption pressures and it is useful for studying the performance of adsorbents in pressure swing adsorption processes (PSA; Rege and Yang, 2001). Per cent regenerability (R%) describes the regeneration of the adsorption sites and shows the reusability of the adsorbent in the cyclic processes (Bae and Snurr, 2011).
Results and Discussions
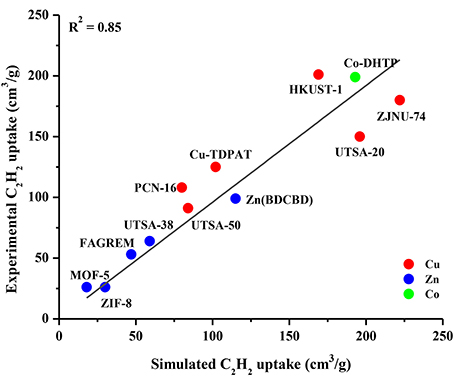
The accuracy of our GCMC simulations to assess the adsorption of CO2 and CH4 molecules in various MOFs was validated in our previous works (Sezginel et al., 2015; Altintas and Keskin, 2016; Sumer and Keskin, 2016) by comparing the results of our molecular simulations with the available experimental data from different research groups. In this work, we aim to validate the GCMC simulations for C2H2 adsorption in MOFs. We collected experimental C2H2 uptake data of several different MOFs from the literature and performed GCMC simulations for these MOFs under the same conditions with the experiments at 1 bar and 298 K (in some cases 296 K). We specifically included the widely studied MOFs such as MOF-5 (IRMOF-1), HKUST-1, ZIF-8, UTSA-50 and the MOFs identified as promising due to their high C2H2 uptakes such as Co-DHTP in this comparison. Figure 1 shows that there is a good agreement between single-component adsorption experiments and simulations for C2H2 uptakes of different MOFs, indicating the appropriate choice of the force fields used in the simulations. The good agreement between experimentally reported and simulated C2H2 adsorption isotherms of IRMOF-1 and HKUST-1 up to 1 bar is also shown in Figure S2. There was no experimental data on adsorption of equimolar C2H2/CO2 and C2H2/CH4 mixtures in the literature to the best of our knowledge, therefore it was not possible to make a comparison for the mixture adsorption. The good agreement we showed in Figure 1 suggests that molecular simulations can be used to make accurate estimates about the adsorption of C2H2/CO2 and C2H2/CH4 mixtures in MOFs.
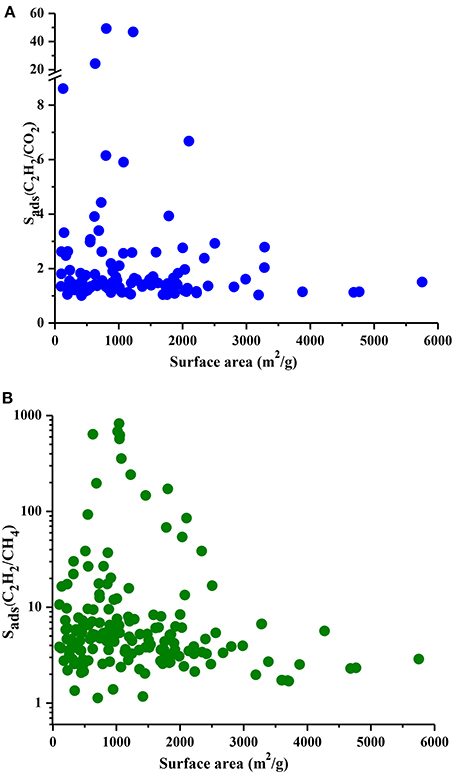
We performed the GCMC simulations of MOFs at 0.1 and 1 bar considering equimolar C2H2/CO2 and C2H2/CH4 mixtures and computed their C2H2 selectivities and working capacities. All 174 MOFs were found to be C2H2 selective for C2H2/CH4 separation whereas 121 MOFs were found to be C2H2 selective for C2H2/CO2 separation. Since our aim is to identify C2H2 selective MOFs in this work, we only show these 121 MOFs in Figure 2A. Figure 2A shows that most of the MOFs have C2H2/CO2 selectivities lower than 2. These low selectivities can be attributed to the similarity of the C2H2 and CO2 molecules which makes the adsorption-based C2H2/CO2 separation challenging. There are 25 MOFs that show C2H2/CO2 selectivities in the range of 2–10 and widely studied HKUST-1 is among these MOFs. We calculated its selectivity as 2.9 which is in good agreement with the literature value of 2.4 (Fischer et al., 2010). This slight difference can be attributed to (a) the different potential parameters used for CO2, (b) the different charge assignment methods used for CuBTC and/or (c) different crystal structures of CuBTC used in two simulation studies. Three MOFs, UWUTIQ, CUVTUJ [also known as Co2(DHTP)] and GUXQAS, were found to show the highest C2H2/CO2 selectivities of 49, 47, and 24, respectively. The highest C2H2/CO2 selectivities were reported for UTSA-50 and HOF-3 using IAST calculations as 13 and 21, respectively (Xu et al., 2013; Li et al., 2014). The three MOFs mentioned above outperform UTSA-50 and HOF-3 in terms of selectivity. On the other hand, Figure 2A shows that MOFs with high C2H2/CO2 selectivities generally suffer from low C2H2 working capacities. A large number of MOFs was found to exhibit C2H2 working capacities lower than 2 mol/kg. The best MOF adsorbents are expected to offer both high C2H2 selectivities and high C2H2 working capacities. In order to identify the MOFs that offer a good combination of C2H2 selectivity and C2H2 working capacity, we color-coded Figure 2A with APS values to separate low and high-performance regions within the MOF search space. Three different regions were defined to provide a reference for quantitatively identifying a number of promising MOFs. MOFs with high C2H2 selectivity but low C2H2 working capacity and MOFs with low C2H2 selectivity but high C2H2 working capacity were located in the green region. These MOFs have APS values lower than 2 for separation of equimolar C2H2/CO2 mixtures. MOFs showing moderate selectivities (1.2–49) and working capacities (0.1–3.25 mol/kg) result in APS values of 2.14–4.76 and they are shown in the blue region. Finally, the most promising MOFs with the best selectivity and working capacity combinations are shown in the red region with APS > 5.25. There are 7 MOFs in this region and except one all have C2H2/CO2 selectivities in the range of 2–7 and their working capacities are between 1.5 and 3 mol/kg. Three MOFs with the highest APSs, CUVTUJ, OMORUE, and DOTSOV can be considered as the best candidates for C2H2/CO2 separations.
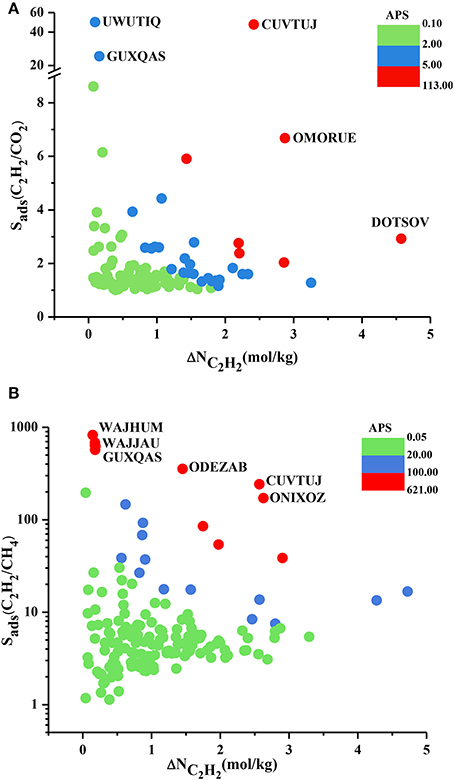
Figure 2. C2H2 selectivity and C2H2 working capacity of MOFs for separation of equimolar (A) C2H2/CO2 (B) C2H2/CH4 mixtures.
Figure 2B shows that C2H2/CH4 selectivities of MOFs have a wide range from 1.2 to 824, and most of the MOF adsorbents have C2H2 selectivities lower than 10. The C2H2 working capacities of MOFs were also calculated to span a wide range, from 0.04 to 4.7 mol/kg. For equimolar C2H2/CH4 mixture, Alduhaish et al. (2017) reported the highest selectivity, 98, for a MOF (VAQXUJ) using IAST at 1 bar and 296 K. Ten MOFs we studied exhibit higher selectivities than this record. The top three selective MOFs were identified to be WAJHUM, WAJJAU, GUXQAS with C2H2/CH4 selectivities of 824, 684, 638, respectively. These MOFs outperform the widely studied MOFs such as HKUST-1 and UTSA-50 which were reported to have C2H2/CH4 selectivities of ~66–68 at 1 bar, 295/296 K as we discussed above. The four MOFs with the highest selectivities suffer from low C2H2 working capacities, ~0.17 mol/kg. The combination of high selectivity and low working capacity of these MOFs can be attributed to their relatively low pore volumes (<0.45 cm3/g). CUVTUJ and ONIXOZ exhibit both high C2H2/CH4 selectivities (242 and 171, respectively) and high C2H2 working capacities (~2.6 mol/kg). Similar to Figure 2A, we color-coded the APSs of MOFs where green represents the MOFs with low performance (APSs < 20), blue represents the promising MOFs with C2H2/CH4 selectivities of 7.5–147, C2H2 working capacities of 0.6–5 mol/kg, resulting in 20 < APSs < 92. Finally, the most promising MOFs for separation of equimolar C2H2/CH4 mixtures are located in the red region with APSs > 102. The best candidates for C2H2/CH4 separations were identified as CUVTUJ, ONIXOZ (high selectivity and high working capacity), and ODEZAB (high selectivity and moderate working capacity). These MOFs were computed to have APSs of 621, 450, and 515, respectively.
At that point it is also useful to compare the C2H2 working capacities of MOFs with each other. Zhang et al. (2017) calculated single-component C2H2 working capacities of 7 MOF structures as 110–180 cm3 (STP)/g at an adsorption pressure of 1 bar and desorption pressure of 0.1 bar. The highest C2H2 working capacities of the 174 MOFs we considered in this work were calculated to be as 103 cm3 (STP)/g and 106 cm3 (STP)/g for C2H2/CO2 and C2H2/CH4, respectively, under the same conditions. These values belong to a well-known MOF, HKUST-1. It is important to note that our C2H2 working capacities were calculated for equimolar mixtures, not for the single-component gas adsorption and therefore they are less than the ones reported for single-component cases.
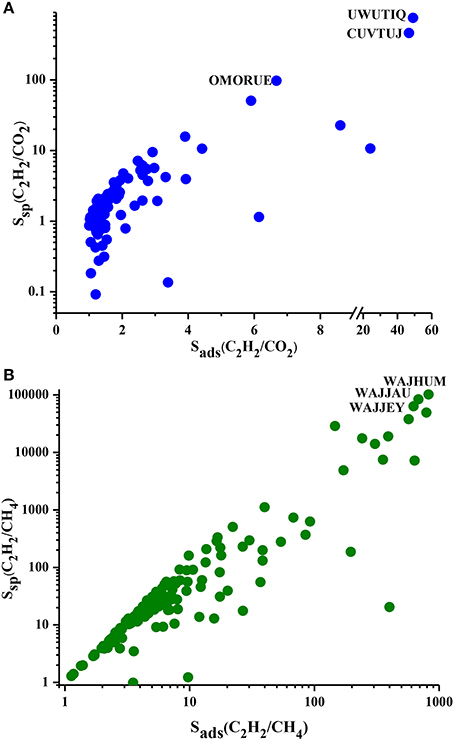
We showed the Ssp values of MOFs for separation of C2H2/CO2 and C2H2/CH4 mixtures in Figure 3 as a function of selectivity. Ssp values of MOF adsorbents are in the range of 0.1–755 and 1.2–101,362 for C2H2/CO2 and C2H2/CH4 separations, respectively. Ssp increases with selectivity as described in Table 1. Most of the MOFs have Ssp values lower than 10 for C2H2/CO2 separation due to the low selectivities as shown in Figure 3A. Ssp values of MOFs are <100 for C2H2/CH4 separation as shown in Figure 3B. It is important to note that a MOF which has been widely studied as adsorbent in the literature, IRMOF-1 (SAHYIK), has a low Ssp (3.1) for C2H2/CH4 separation. This means there are many other MOFs with better separation potentials than this widely studied MOF. The most promising MOFs are located at the top right corner of Figure 3 which have both high Ssp and high selectivity such as UWUTIQ (Ssp: 754.88, Sads:49) for C2H2/CO2 and WAJHUM for C2H2/CH4 (Ssp: 101,36 and Sads: 824). To summarize, APS identifies CUVTUJ, OMORUE, and DOTSOV (CUVTUJ, ODEZAB, and ONIXOZ) as the best adsorbents for C2H2/CO2 (C2H2/CH4) separation whereas Ssp identifies UWUTIQ, CUVTUJ, and OMORUE (WAJHUM, WAJJAU, and WAJJEY) as the best adsorbents for C2H2/CO2 (C2H2/CH4) separation. These results show that for C2H2/CO2, CUVTUJ, and OMORUE are the promising adsorbents based on both APS and Ssp. The low working capacity of UWUTIQ, which has the highest selectivity and Ssp, leads to a low APS value. MOFs that are promising for separation of C2H2/CH4 were identified to be different based on these two metrics because MOFs with high selectivities generally have low working capacities (as shown in Figure 2B) resulting in high Ssp but low APS values.
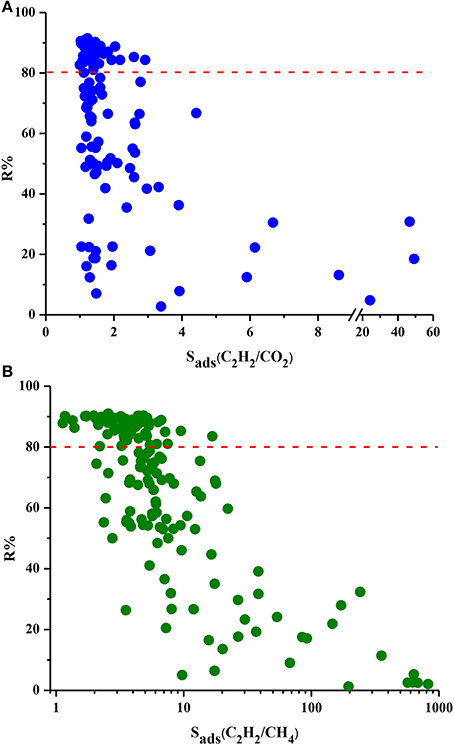
Figure 4 shows the relation between R% and selectivity of MOFs. MOFs show a very wide range of R%, from 2 to 91% for these two mixtures and the red dotted line represents R% = 80%. We chose this value as the minimum desired R% since lower R% values result in high cost in adsorption-based gas separation applications (Li et al., 2009). There is generally an inverse relation between R% and Sads. As the MOF strongly adsorbs one gas component over other, desorption becomes difficult resulting in low working capacities and low regenerabilities. Almost half of the MOFs exhibit R% higher than 80%, however these MOFs have low selectivities. For example, the three most selective MOFs for C2H2/CO2 separation have very low R% values of 18, 31, and 5% as can be seen from Figure 4A. Similarly, three MOFs with the highest C2H2/CH4 selectivities suffer from very low R% (2, 3, and 5%, respectively) as shown in Figure 4B. These results show that choosing the best adsorbent material based on solely selectivity is not completely accurate and other metrics such as R% should be considered. Among the MOFs which have R% higher than 80%, DOTSOV shows the highest selectivity of 3 and 17 for C2H2/CO2 and C2H2/CH4 mixtures, respectively. This MOF also exhibits high C2H2 working capacity for C2H2/CO2 and C2H2/CH4 (4.6 and 4.73 mol/kg) suggesting that it is a promising adsorbent for both separations considering the cost of regeneration.
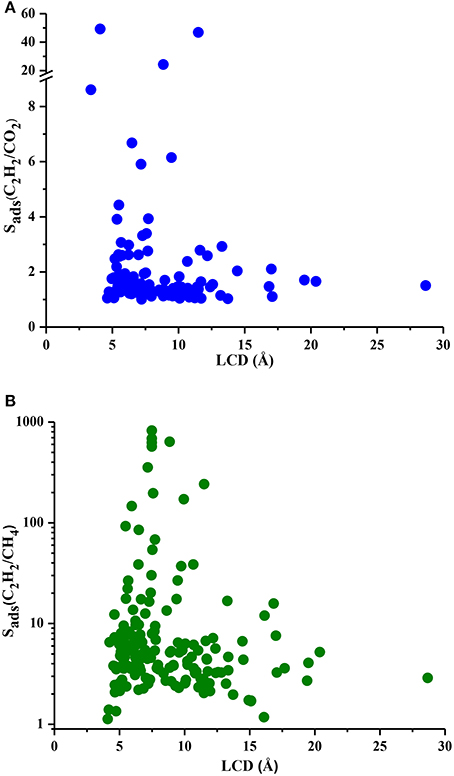
Understanding the correlations between separation performances of MOF adsorbents and their structural properties is crucial for identification of the best candidates with pre-determined structural features. Since selectivity is the most widely considered metric in choosing adsorbents, we examined the relation between LCDs of MOFs and their selectivities in Figure 5. Results show that MOFs having pores in the range of 5–10 Å generally have high selectivities both for C2H2/CO2 and C2H2/CH4 separations whereas MOFs with LCDs > 10 Å exhibit low adsorption selectivities. This result is in agreement with the findings of a recent study in which MOFs with LCDs of 6.7–10 Å were found to have the highest single-component C2H2 uptakes (Zhang et al., 2017). Figure 6 shows the relation between accessible surface areas of MOFs and their selectivities. MOFs having surface areas between 180 and 1,200 m2/g have higher C2H2/CO2 selectivities whereas MOFs with surface areas of 550–1,800 m2/g are more promising for selective separation of C2H2 from CH4. Although there is not a very strong relation between surface area and selectivity, Figure 6B suggests that MOFs with large surface areas (>3,500 m2/g) are not very selective. We also investigated the relation between APSs and accessible surface areas of MOFs in Figure S3. Similar to selectivity, there is not an obvious relation but MOFs having high APSs generally have surface areas of 1,100–3,200 and 1,000–2,300 m2/g for C2H2/CO2 and C2H2/CH4 separations, respectively. No obvious relation was found between selectivity, APS and porosity of MOFs as shown in Figures S4, S5 but lower porosities generally lead to higher selectivities. As a result, we concluded that pore sizes smaller than 10 Å, surface areas <2,000 m2/g and low porosities (0.41–0.64) provide a stronger confinement for the C2H2 molecules compared to CO2 and CH4 and lead to higher C2H2 selectivities. At that point, it is important to note that selectivity of a material is determined by the interplay of various factors and cannot be easily correlated to only a few structural properties as we attempted to do. For example, chemical composition and topology of MOFs strongly affect the affinity of materials for specific gas molecules but these correlations are very complex and they can be only captured if a very large number and variety of structures are investigated. The simple correlations that we demonstrated in Figures 5, 6 will provide useful information to accelerate the design of new high-performance MOFs for C2H2 separation applications.
We finally investigated the effect of composition of the gas mixture on the separation performance of the MOFs. CUVTUJ and GUXQAS are the promising MOFs for C2H2/CO2 and C2H2/CH4 separations due to their high selectivities and high APSs. Outstanding performance of these two MOFs motivated us to investigate their separation performance for mixtures with different compositions. We performed GCMC simulation for these two MOFs at 1 bar, 298 K by changing the C2H2 mole fraction (yC2H2) in the bulk mixture. Figure 7A shows that as yC2H2 increases, selectivities of CUVTUJ and GUXQAS decrease. There are sharp decreases in the selectivities of both MOFs with increasing yC2H2 from 0.1 to 0.5. In this region, increasing yC2H2 results in an increase in the adsorbed C2H2 (xC2H2) and decrease in the adsorbed CO2 (xCO2). Since the selectivity was normalized by the bulk composition, C2H2/CO2 selectivities decrease with yC2H2. Figure 7B shows that GUXQAS has a very high C2H2/CH4 selectivity for mixtures with yC2H2 < 0.5. xC2H2 of GUXQAS does not change remarkably by increasing yC2H2, however xCH4 decreases resulting in sharp reductions in the C2H2/CH4 selectivities. The selectivity of CUVTUJ does not have a very significant dependence on the yC2H2 as can be seen from Figure 7B.
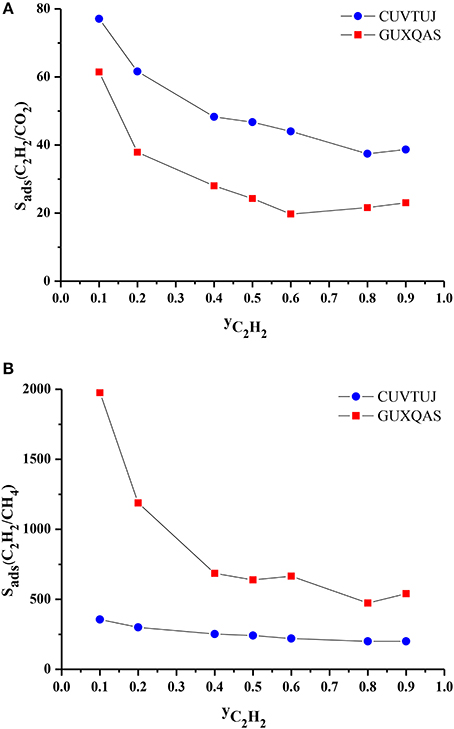
Figure 7. (A) C2H2/CO2 and (B) C2H2/CH4 selectivity of promising MOFs as a function of C2H2 composition in the bulk mixtures.
Finally, it is important to discuss the effect of force field selection on the results of molecular simulations. Generic force fields were found to be less successful in predicting gas adsorption in materials having strong binding sites, such as open metal sites (Getman et al., 2012). Specific force fields derived from quantum chemical calculations are required to describe the interactions between gas molecules and MOFs having open metal sites. The key challenge in developing such force fields using quantum chemistry calculations is selecting the appropriate level of theory and balancing it with the computational expense. There may be several MOFs having open metal sites and showing strong binding for C2H2 molecules but we did not define a specific force field for them and screened the MOFs using generic force fields. The value of our calculations is to efficiently identify the most promising MOF materials using generic, off-the-shelf-force fields, in a time-efficient manner. More detailed quantum chemistry calculations can be further performed for the best candidates to understand the underlying mechanism in future studies.
Our molecular simulations give no information about stability of MOFs, however an adsorbent should be stable in order to find place in practical applications. Therefore, we collected the stability information of the top three promising MOFs from their experimental synthesis articles. There is no specific stability information in the literature for CUVTUJ, OMORUE, and ONIXOZ. DOTSOV was reported to be thermally stable up to 240°C (Wu et al., 2008), UMUTIQ (Das et al., 2011) and GUXQAS (Ling et al., 2009) were reported to save their stabilities up to high temperatures. WAJHUM and WAJJAU were reported in the same experimental work and their thermal stabilities were investigated in detail (Xie et al., 2010). ODEZAB was reported to be thermally stable up to around 300°C after evacuating solvent molecules from its structure (Duan et al., 2016). Stabilities of the most promising materials identified in this work are most likely to be examined under practical gas separation experiments in future studies.
Conclusions
Developing effective adsorbents for challenging separations of C2H2/CO2 and C2H2/CH4 is crucial in order to meet the requirement of high purity C2H2 in various industries. MOFs are strong candidates for storage of C2H2, but limited information was available about their C2H2 separation potentials. We performed the first large-scale molecular simulation study to examine the potential of 174 different MOF structures for separation of C2H2/CO2 and C2H2/CH4 mixtures. Several MOFs were identified to show high C2H2 selectivities. The top three most promising MOF adsorbents were computed to have C2H2/CO2 selectivities of 49, 47, 24, and C2H2/CH4 selectivities of 824, 684, 638 at 1 bar, 298 K. These are the highest C2H2 selectivities reported to date in the literature. Two MOFs, CUVTUJ and GUXQAS, were found to be very promising both for C2H2/CO2 and C2H2/CH4 separations leading to selectivities of 47 (242) and 24 (638), respectively. In addition to selectivity, other adsorbent evaluation metrics such as APS, Ssp, R% were computed for all MOFs. Results showed that highly selective MOFs suffer from low R% and therefore choosing the best adsorbent material based on solely selectivity is not completely accurate. We also examined the structure-performance relations of MOFs and showed that MOFs with pore sizes <10 Å, surface areas <2,000 m2/g and low porosities (0.41–0.64) lead to higher C2H2 selectivities. We believe that these results will motivate extensive research on MOF adsorbents for C2H2 separation processes.
Author Contributions
AN: performed the molecular simulations and contributed to writing of the manuscript; SK: discussed the results and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
SK acknowledges ERC-2017-Starting Grant. This study has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (ERC-2017-Starting Grant, grant agreement No 756489-COSMOS).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2018.00036/full#supplementary-material
References
Alduhaish, O., Li, B., Arman, H., Lin, R.-B., Zhao, J. C.-G., and Chen, B. (2017). A two-dimensional microporous metal–organic framework for highly selective adsorption of carbon dioxide and acetylene. Chin. Chem. Lett. 28, 1653–1658. doi: 10.1016/j.cclet.2017.04.025
Altintas, C., and Keskin, S. (2016). Computational screening of MOFs for C2H6/C2H4 and C2H6/CH4 separations. Chem. Eng. Sci. 139, 49–60. doi: 10.1016/j.ces.2015.09.019
Bae, Y. S., and Snurr, R. Q. (2011). Development and evaluation of porous materials for carbon dioxide separation and capture. Angew. Chem. 50, 11586–11596. doi: 10.1002/anie.201101891
Ben-Mansour, R., Habib, M. A., Bamidele, O. E., Basha, M., Qasem, N. A. A., Peedikakkal, A., et al. (2016). Carbon capture by physical adsorption: materials, experimental investigations and numerical modeling and simulations - a review. Appl. Energy 161, 225–255. doi: 10.1016/j.apenergy.2015.10.011
Chen, B., and Siepmann, J. I. (1999). Transferable potentials for phase equilibria. 3. explicit-hydrogen description of normal alkanes. J. Phys. Chem. B 103, 5370–5379. doi: 10.1021/jp990822m
Chen, D. M., Tian, J. Y., Liu, C., Sen, C., M., and Du, M. (2016). Charge control in two isostructural anionic/cationic CoIICoordination frameworks for enhanced acetylene capture. Chem. Eur. J. 22, 15035–15041. doi: 10.1002/chem.201603054
Chung, Y. G., Camp, J., Haranczyk, M., Sikora, B. J., Bury, W., Krungleviciute, V., et al. (2014). Computation-ready, experimental metal-organic frameworks: a tool to enable high-throughput screening of nanoporous crystals. Chem. Mater. 26, 6185–6192. doi: 10.1021/cm502594j
Chung, Y. G., Gómez-Gualdrón, D. A., Li, P., Leperi, K. T., Deria, P., Zhang, H., et al. (2016). In silico discovery of metal-organic frameworks for precombustion CO2 capture using a genetic algorithm. Sci. Adv. 2:e1600909. doi: 10.1126/sciadv.1600909
Colón, Y. J., and Snurr, R. Q. (2014). High-throughput computational screening of metal–organic frameworks. Chem. Soc. Rev. 43, 5735–5749. doi: 10.1039/C4CS00070F
Das, M. C., Xu, H., Xiang, S., Zhang, Z., Arman, H. D., Qian, G., et al. (2011). A new approach to construct a doubly interpenetrated microporous metal-organic framework of primitive cubic net for highly selective sorption of small hydrocarbon molecules. Chem. Eur. J. 17, 7817–7822. doi: 10.1002/chem.201100350
Duan, X., Cui, Y., Yang, Y., and Qian, G. (2016). A novel methoxy-decorated Metal-Organic Framework exhibiting high acetylene and carbon dioxide storage capacities. CrystEngComm 2, 1464–1469. doi: 10.1039/C6CE02291J
Dubbeldam, D. (2014). RASPA 2.0: Molecular software package for adsorption and diffusion in (Flexible) nanoporous materials (Basel). Mol. Simulat. 1–145.
Eddaoudi, M., Kim, J., Rosi, N., Vodak, D., Wachter, J., Keeffe, M. O., et al. (2002). Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage published by : American association for the advancement of science linked references are available on JSTOR for this article : systematic design. Science 295, 469–472. doi: 10.1126/science.1067208
Fischer, M., Hoffmann, F., and Fröba, M. (2010). New microporous materials for acetylene storage and C2H2/CO2 separation: insights from molecular simulations. Chemphyschem 11, 2220–2229. doi: 10.1002/cphc.201000126
Foo, M. L., Matsuda, R., Hijikata, Y., Krishna, R., Sato, H., Horike, S., et al. (2016). An adsorbate discriminatory gate effect in a flexible porous coordination polymer for selective adsorption of CO2 over C2H2. J. Am. Chem. Soc. 138, 3022–3030. doi: 10.1021/jacs.5b10491
Frenkel, D., and Smit, B. (2002). Understanding Molecular Simulation: From Algorithms to Applications. San Diego, CA: Academic Press.
Getman, R. B., Bae, Y.-S., Wilmer, C. E., and Snurrr, R. Q. (2012). Review and analysis of molecular simulations of methane, hydrogen, and acetylene storage in metal–organic frameworks. Chem. Rev. 112, 703–723. doi: 10.1021/cr200217c
Groom, C. R., and Allen, F. H. (2014). The cambridge structural database in retrospect and prospect. Angew. Chem. 53, 662–671. doi: 10.1002/anie.201306438
Ji, Y., Ding, L., Cheng, Y., Zhou, H., Yang, S., Li, F., et al. (2017). Understanding the effect of ligands on C2H2 storage and C2H2/CH4, C2H2/CO2 separation in metal-organic frameworks with open Cu(II) sites. J. Phys. Chem. C. 121, 24104–24113. doi: 10.1021/acs.jpcc.7b08370
Keskin, S., Liu, J., Rankin, R. B., Johnson, J. K., and Sholl, D. S. (2009). Progress, opportunities, and challenges for applying atomically detailed modeling to molecular adsorption and transport in metal–organic framework materials. Ind. Eng. Chem. Res. 48, 2355–2371. doi: 10.1021/ie800666s
Li, H., Eddaoudi, M., O'Keeffe, M., and Yaghi, O. M. (1999). Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402, 276–279.
Li, J. R., Kupler, R. J., and Zhou, H. C. (2009). Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 38, 1477–1504. doi: 10.1039/B802426J
Li, J. R., Ma, Y., McCarthy, M. C., Sculley, J., Yu, J., Jeong, H. K., et al. (2011). Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 255, 1791–1823. doi: 10.1016/j.ccr.2011.02.012
Li, P., He, Y., Zhao, Y., Weng, L., Wang, H., Krishna, R., et al. (2014). A Rod-packing microporous hydrogen-bonded organic framework for highly selective separation of C2H2/CO2 at room temperature. Angew. Chem. 54, 574–577. doi: 10.1002/anie.201410077
Ling, Y., Zhang, L., Li, J., and Hu, A. X. (2009). Three-fold-interpenetrated diamondoid coordination frameworks with torus links constructed by tetranuclear building blocks. Cryst. Growth Des. 9, 2043–2046. doi: 10.1021/cg801188r
Mondloch, J. E., Karagiaridi, O., Farha, O. K., and Hupp, J. T. (2013). Activation of metal- organic framework materials. CrystEngComm. 15, 9258–9264. doi: 10.1039/c3ce41232f
Myers, A. L. (2002). Thermodynamics of adsorption in porous materials. AIChE J. 48, 145–160. doi: 10.1002/aic.690480115
Pang, J., Jiang, F., Wu, M., Liu, C., Su, K., Lu, W., et al. (2015). A porous metal-organic framework with ultrahigh acetylene uptake capacity under ambient conditions. Nat. Commun. 6:7575. doi: 10.1038/ncomms8575
Potoff, J. J., and Siepmann, J. I. (2001). Vapor–liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen. AIChE J. 47, 1676–1682. doi: 10.1002/aic.690470719
Rappé, A. K., Casewit, C. J., Colwell, K. S., Goddard, W. A., and Skiff, W. M. (1992). UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 114, 10024–10035. doi: 10.1021/ja00051a040
Rege, S., and Yang, R. (2001). a Simple parameter for selecting an adsorbent for gas separation by pressure swing adsorption. Sep. Sci. Technol. 36, 3355–3365. doi: 10.1081/SS-100107907
Sezginel, K. B., Uzun, A., and Keskin, S. (2015). Multivariable linear models of structural parameters to predict methane uptake in metal-organic frameworks. Chem. Eng. Sci. 124, 125–134. doi: 10.1016/j.ces.2014.10.034
Sumer, Z., and Keskin, S. (2016). Ranking of MOF Adsorbents for CO2 separations: a molecular simulation study. Ind. Eng. Chem. Res. 55, 10404–10419. doi: 10.1021/acs.iecr.6b02585
van Gunsteren, W., Daura, X., Hansen, N., Mark, A., Oostenbrink, C., Riniker, S., et al. (2017). Validation of molecular simulation: an overview of issues. Angew. Chem. 57, 884–902. doi: 10.1002/anie.201702945
Wen, H.-M., Wang, H., Li, B., Cui, Y., Wang, H., Qian, G., et al. (2016). A microporous metal–organic framework with lewis basic nitrogen sites for high C2H2 storage and significantly enhanced C2H2/CO2 separation at ambient conditions. Inorg. Chem. 55, 7214–7218. doi: 10.1021/acs.inorgchem.6b00748
Willems, T. F., Rycroft, C. H., Kazi, M., Meza, J. C., and Haranczyk, M. (2012). Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials. Microporous Mesoporous Mater. 149, 134–141. doi: 10.1016/j.micromeso.2011.08.020
Wilmer, C. E., Kim, K. C., and Snurr, R. Q. (2012). An extended charge equilibration method. J. Phys. Chem. Lett. 3, 2506–2511. doi: 10.1021/jz3008485
Wu, Y., Kobayashi, A., Halder, G. J., Peterson, V. K., Chapman, K. W., Lock, N., et al. (2008). Negative thermal expansion in the metal-organic framework material Cu 3(1,3,5-benzenetricarboxylate)2. Angew. Chem. 47, 8929–8932. doi: 10.1002/anie.200803925
Xiang, S. C., Zhou, W., Gallegos, J. M., Liu, Y., and Chen, B. L. (2009). Exceptionally high acetylene uptake in a microporous metal-organic framework with open metal sites. J. Am. Chem. Soc. 131, 12415–12419. doi: 10.1021/ja904782h
Xiang, S., Zhou, W., Zhang, Z., Green, M. A., Liu, Y., and Chen, B. (2010). Open metal sites within isostructural metal-organic frameworks for differential recognition of acetylene and extraordinarily high acetylene storage capacity at room temperature. Angew. Chem. Int. Edn. 49, 4615–4618. doi: 10.1002/anie.201000094
Xie, L. H., Lin, J., Bin, L. X. M., Wang, Y., Zhang, W. X., Zhang, J. P., et al. (2010). Porous coordination polymer with flexibility imparted by coordinatively changeable lithium ions on the pore surface. Inorg. Chem. 49, 1158–1165. doi: 10.1021/ic902077j
Xu, H., He, Y., Zhang, Z., Xiang, S., Cai, J., Cui, Y., et al. (2013). A microporous metal–organic framework with both open metal and Lewis basic pyridyl sites for highly selective C2H2/CH4 and C2H2/CO 2 gas separation at room temperature. J. Mater. Chem. A 1, 77–81. doi: 10.1039/C2TA00155A
Yeganegi, S., Gholami, M., and Sokhanvaran, V. (2017). Molecular simulations of adsorption and separation of acetylene and methane and their binary mixture on MOF-5, HKUST-1 and MOF-505 metal–organic frameworks. Mol. Simul. 43, 260–266. doi: 10.1080/08927022.2016.1262036
Zhang, C., Lan, Y., Guo, X., Yang, Q., and Zhong, C. (2017). Materials genomics-guided ab initio screening of MOFs with open copper sites for acetylene storage. AIChE J. doi: 10.1002/aic.16025. [Epub ahead of print].
Keywords: metal organic frameworks, C2H2 separation, adsorption, selectivity, molecular simulation
Citation: Nemati Vesali Azar A and Keskin S (2018) Computational Screening of MOFs for Acetylene Separation. Front. Chem. 6:36. doi: 10.3389/fchem.2018.00036
Received: 16 January 2018; Accepted: 12 February 2018;
Published: 27 February 2018.
Edited by:
Alexei Lapkin, University of Cambridge, United KingdomReviewed by:
Nils E. R. Zimmermann, Lawrence Berkeley National Laboratory (LBNL), United StatesRavichandar Babarao, RMIT University, Australia
Copyright © 2018 Nemati Vesali Azar and Keskin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seda Keskin, skeskin@ku.edu.tr
 Ayda Nemati Vesali Azar
Ayda Nemati Vesali Azar Seda Keskin
Seda Keskin