Effects of Foliar Selenite on the Nutrient Components of Turnip (Brassica rapa var. rapa Linn.)
- 1Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany (CAS), Kunming, China
- 2China Germplasm Bank of Wild Species, Kunming Institute of Botany (CAS), Kunming, China
We administered foliar applications of 50, 100, and 200 mg L−1 selenium (Se, selenite) on turnip (Brassica rapa var. rapa Linn.) and detected the changes in the main nutrient components in fleshy roots. Results showed that the foliar application of Se (IV) significantly increased the Se content in turnip, and Se (IV) positively affected the uptake of several mineral elements, including magnesium, phosphorus, iron, zinc, manganese, and copper. Se (IV) treatments also improved the synthesis of protein and multiple amino acids instead of crude fat and total carbohydrate in turnip, indicating that the foliar application of Se (IV) could enhance Se biofortification in turnip and promote its nutritional value. We recommended 50–100 mg L−1 Se treatment for foliar application on turnip based on the daily intake of Se for adults (96–139 μg person−1 day−1) and its favorable effects on the nutrient components of turnip.
Introduction
Selenium (Se) is an essential micronutrient for humans (White and Brown, 2010). Se deficiency can weaken the immune system and increase the risks of several diseases, including hypothyroidism, cardiovascular disease, or various cancers (Fairweather-Tait et al., 2011; Rayman, 2012; White, 2016). Excessive dietary Se intake can also cause selenosis in humans (Fairweather-Tait et al., 2011; Rayman, 2012; Sperotto et al., 2014), and its symptoms are similar to those caused by heavy metals (White, 2016). As such, the World Health Organization (WHO) has recommended the dietary allowance of ~55–200 μg Se day−1 for adults (Wu et al., 2015), and the Institute of Medicine (USA) has suggested a tolerable upper intake of 400 μg Se day−1 for adults (White, 2016). Se supplementation greatly depends on the production of crops, vegetables, or edible mushrooms on soils with substantial Se content or phytoavailability (Broadley et al., 2006; Chilimba et al., 2011; Joy et al., 2015; Dogan et al., 2016). Se accumulation in tissues can be used as a basis for the classification of angiosperm species into three ecological types: non-accumulator, Se-indicator, and Se-accumulator species (White et al., 2007; White, 2016). Some studies have focused on the potential of plants, such as Se-accumulator species, for the phytoremediation of Se-contaminated soils (Banuelos and Dhillon, 2011; Wu et al., 2015). However, much interest has been directed toward developing Se-biofortified agricultural products because of severe Se deficiency worldwide (Wu et al., 2015). For example, inorganic Se fertilizers have been applied to effectively increase Se contents in diets and thus improve the Se status and health of humans (White and Broadley, 2009; Alfthan et al., 2015).
For plants, Se is a beneficial element because it can stimulate plant growth and enhance plant tolerance or resistance to abiotic or biotic stress (Quinn et al., 2007; Pilon-Smits et al., 2009; Feng et al., 2013). However, Se toxicity in plants has also been reported (Fu et al., 2011; Mao et al., 2011; Longchamp et al., 2015). A low Se concentration is considered advantageous for plant growth and development, whereas a high Se concentration can be toxic to plants (Fu et al., 2011; Mao et al., 2011; Gupta and Gupta, 2017). These effects may be attributed to changes in biochemical and metabolic processes caused by Se. For example, Se can mediate reactive oxygen species metabolism and trace element uptake in plants (Gupta and Gupta, 2017). Appropriate Se concentrations also improve the nutrient components, including mineral elements, polysaccharides, proteins, amino acids, and vitamin C, of some vegetables or edible mushrooms (Shang et al., 1998; Du et al., 2004; Zhao et al., 2004; Wang et al., 2005, 2006). Se enhances the synthesis of glucosinolates, which are important secondary metabolites found mainly in cruciferous plants (Sams et al., 2011; Malagoli et al., 2015; Schiavon et al., 2016). These reports fully support the conclusion that Se assimilation affects sulfur (S) and nitrogen (N) metabolic pathways in plants (Malagoli et al., 2015).
Turnip (Brassica rapa var. rapa Linn.), a cruciferous biennial plant, has been widely cultivated as a long-term vegetable or fodder in Europe, America, and Asia. It is rich in vitamin C, riboflavin, dietary fiber, and various mineral elements but is low in calories (Parveen et al., 2015; Ma et al., 2016). This biennial plant also contains antioxidants and can reduce the risk of high blood pressure, diabetes, and different cancers (Parveen et al., 2015). In China, turnip is mainly cultivated in the Qinghai–Tibet Plateau and its surrounding areas. However, these areas are exposed to severe Se deficiency. In a previous study, we analyzed the absorption and translocation characteristics of selenite and selenate in turnip by soil addition or foliar spraying (Li et al., 2018). We reported that turnip may be a potential Se-indicator species, and Se (IV) should be mainly selected as artificial Se fertilizer for turnips (Li et al., 2018). These results provided preliminary information on Se intake from turnip for local people, but the risk of Se biofortification, such as the assimilation of Se in plants and the effects of Se on turnip quality, is still unknown in turnips. In the present study, we examined the changes in nutrient components in turnips treated with Se (IV) concentration gradient via foliar application. We assumed that suitable Se concentrations can promote the nutrition level of turnip. Our results would further improve the understanding of the potential and value of Se biofortification in turnips.
Materials and Methods
Plant Cultivation and Treatment
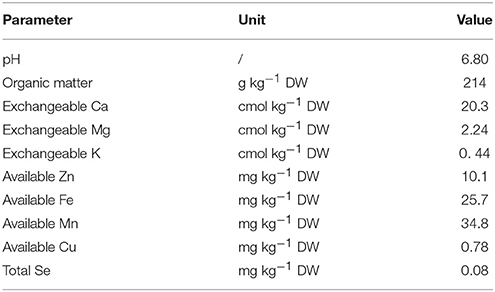
Turnip seeds from Ninglang County of China (Landrace No. KTRG-B54) were germinated and grown in a natural environment. After 25 days, seedlings with consistent growth were neatly transplanted into uniform flowerpots (d = 18.5 cm, h = 17.5 cm) with equal amounts of Se-free mucky soil (one seedling in each pot). The soil condition is shown in Table 1. The pots were divided into four groups, and each group contained three pots. The different groups of plants were supplied with 0, 50, 100, and 200 mg L−1 Se (IV) solutions by foliar application 25 and 40 days after the plants were transplanted. For each treatment, the Se (IV) solutions were sprayed until water droplets formed on the surface of the leaves. The pots were then placed in a transparent plastic shed with appropriate watering. The plants in each treatment were harvested 60 days after transplantation for subsequent measurement.
Sample Preparation and Biomass Measurement
The fleshy roots of plants were harvested and washed with distilled water. The fresh weights of the root samples were measured, and the samples were subsequently dried in an oven at 80°C for 48 h to determine their dry biomasses. The conversion factors necessary to convert the fresh weight to the dry weight (DW) of the experimental plants were calculated for each treatment. The dry samples were then used for the following measurements. Three biological replicates were prepared for each treatment.
Mineral Analysis
Calcium (Ca), potassium (K), magnesium (Mg), and phosphorus (P) contents were determined in accordance with the method described by Li et al. (2016) by using an inductively-coupled plasma spectrometer (ICP-OES Optima 8000, Perkin Elmer, USA). The contents of Se, copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn) were determined in accordance with the method of Li et al. (2017) by using an inductively-coupled plasma mass spectrometer (ICP-MS, Thermo Fisher Scientific, USA). The daily intake of Se for adults was calculated on the basis of the Se concentrations as follows: daily intake of Se = Se concentrations in plants (μg kg−1 DW) × conversion factor × daily intake of vegetables. The conversion factors 0.120, 0.116, 0.104, and 0.093 were used to convert the fresh weight to DW of the samples Se0, Se50, Se100, and Se200 in this study, respectively. The average daily vegetable intake for adults is 0.345 kg person−1 day−1 (Parveen et al., 2015).
Macronutrient Analysis
The crude protein content (N × 4.38) was determined using Kjeldahl method (Du et al., 2004; Liu et al., 2016). The crude fat content was determined through Soxhlet extraction with petroleum ether as a solvent (Du et al., 2004; Liu et al., 2016). The total carbohydrate content was measured using a phenol–sulphuric acid method (Liu et al., 2016). The total energy was calculated on the basis of the following equation: total energy (kJ) = 17 × (g crude protein + g total carbohydrate) + 37 × (g crude fat) (Liu et al., 2016).
Amino Acid Analysis
The hydrolyzed amino acid compositions of the different samples were determined using a Thermo Fisher U3000 high-performance liquid chromatography system. Approximately 2 g of the samples was mixed with 16 mL of 6 M HCl in 20 mL hydrolysis tubes and subsequently vacuum degassed for 30 min. The tubes were then sealed with N2 and hydrolyzed at 110°C for 22–24 h. When cooled, the mixtures were transferred to 50 mL volumetric flasks, and their volumes were fixed to the scale. The hydrolysate (1 mL) of each sample was vacuum dried, and the precipitate was dissolved with 1 mL of 0.02 M HCl. Afterwards, 500 μL of the solution was mixed with 250 μL of 1 M triethylamine acetonitrile solution, and 250 μL of 0.1 M phenyl isothiocyanate acetonitrile solution. The obtained mixture was subsequently left at room temperature for 1 h. The mixture was added with 2 mL of n-hexane, intensely shaken and allowed to stand for 10 min. The resulting solution was filtered with a 0.22 μm aqueous filter membrane for analysis.
Statistical Analysis
Statistical analyses were performed using SPSS version 18.0. One-way ANOVA was conducted to analyse significant differences among multiple samples at a 0.05 level.
Results
Contents of Mineral Elements
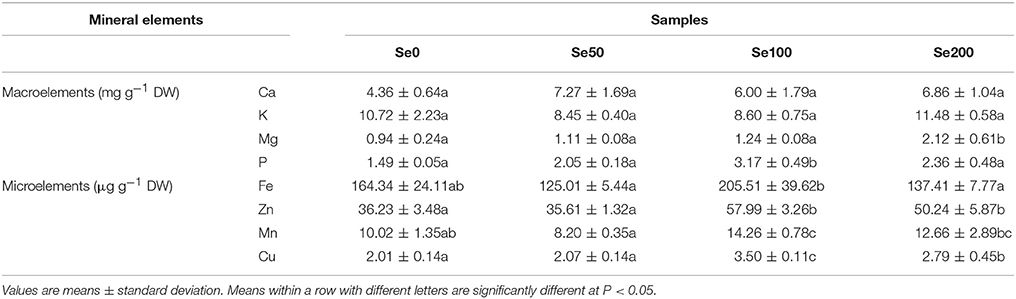
The contents of the mineral elements expressed on a DW basis in different samples are shown in Figure 1A and Table 2. The results of Se concentrations indicated that the foliar application of selenite could significantly increase Se accumulation in turnip fleshy roots (0.09 μg g−1 DW in the control samples and 2.39–7.54 μg g−1 DW in the Se-treated samples; P < 0.05; Figure 1A). On the basis of the Se concentrations in the fleshy roots, we estimated the following daily intake of Se for adults by feeding the turnip samples treated with the respective foliar applications of 0, 50, 100, and 200 mg L−1 Se (IV): 3.89, 96.08, 139.23, and 242.77 μg (Figure 1B).
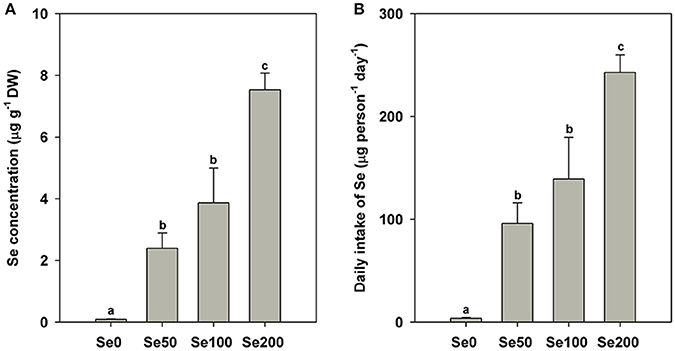
Figure 1. Se contents in different samples (A) and estimated daily intake of Se for adults from different samples (B). Bars are means ± standard deviation. Bars labeled with different letters are significantly different at P < 0.05.
To assess the effects of Se biofortification on the nutrients of turnip, we detected other essential mineral elements in the samples. In this study, the foliar application of Se (IV) did not elicit significant effects on the uptake of the macroelement Ca (4.36–7.27 mg g−1 DW) and K (8.45–11.48 mg g−1 DW) (Table 2). The Mg content in turnip significantly increased at 200 mg L−1 Se (IV) treatment (2.12 mg g−1 DW) (Table 2). The Se (IV) treatment at 100 mg L−1 increased the P content (3.17 mg g−1 DW) in the turnip fleshy roots, but a higher Se (IV) concentration (200 mg L−1) did not produce the same stimulus effect (Table 2). By comparison, the Se (IV) treatment further affected the uptake of microelements (Table 2). Furthermore, 100 mg L−1 Se (IV) treatment significantly improved the contents of Fe, Zn, Mn, and Cu in the turnip fleshy roots (P < 0.05) compared with those of the control samples, whereas 50 mg L−1 Se (IV) treatment had no such effects (Table 2). The contents of all of the four microelements decreased at 200 mg L−1 Se (IV) compared with those at 100 mg L−1 Se (IV) (P < 0.05). However, the contents of Zn and Cu remained higher than those of the control samples (P < 0.05; Table 2).
Contents of Macronutrients
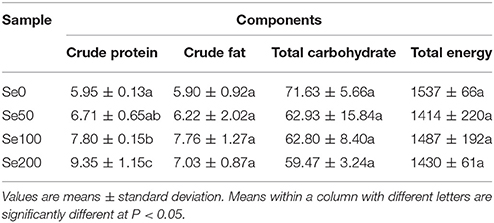
The macronutrients and the total energy in different samples are presented in Table 3. The determined contents in a descending order were total carbohydrate (59.47–71.63 g/100 g DW), crude protein (5.95–9.35 g/100 g DW), and crude fat (5.90–7.76 g/100 g DW). The total energy (1414–1537 kJ/100 g DW) was also estimated. The crude protein content was significantly higher at 100 and 200 mg L−1 Se (IV) treatment (increased by 31.09 and 57.14%, respectively) than at the control concentration (Table 3). By contrast, Se (IV) treatment did not significantly affect the changes in the contents of total carbohydrate and crude fat and the amount of total energy (Table 3).
Contents of Hydrolyzed Amino Acids
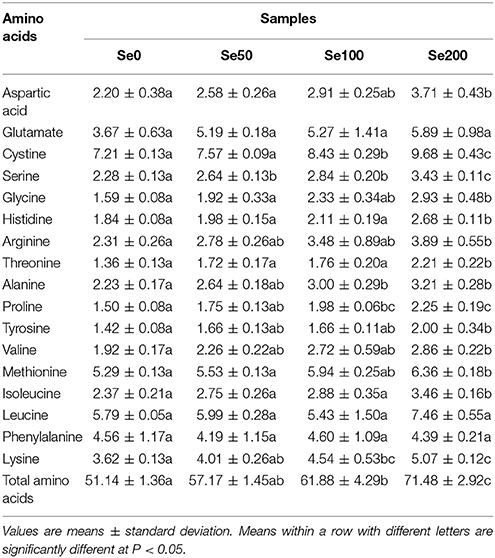
The changes in the hydrolyzed amino acids (17 amino acids) in the turnip fleshy roots that were treated with different Se concentrations are presented in Table 4. The results showed that the foliar application of 50–200 mg L−1 Se (IV) did not change the contents of glutamate (3.67–5.89 g kg−1 DW), leucine (5.79–7.46 g kg−1 DW), and phenylalanine (4.19–4.60 g kg−1 DW) in turnip (Table 4). The contents of the 13 amino acids were improved by Se (IV) application at various levels. The contents of cystine, alanine, proline and lysine significantly increased when Se (IV) reached 100 and 200 mg L−1 (P < 0.05). By comparison, the contents of aspartic acid, glycine, histidine, arginine, threonine, tyrosine, valine, methionine, and isoleucine were not markedly induced until 200 mg L−1 Se (IV) was applied (P < 0.05; Table 4). The content of serine began to significantly increase at 50 mg L−1 Se (IV) (P < 0.05; Table 4). Overall, the contents of the total amino acids increased as the Se treatment concentrations increased (Table 4).
Discussion
Effects of Se (IV) on the Contents of Mineral Elements
Consistent with the results from our previous study (Li et al., 2018), the foliar application of Se (IV) could significantly increase Se accumulation in the turnip fleshy roots. On the basis of the Se concentrations in the fleshy roots, we estimated the daily intake of Se for adults according to the formula used by Parveen et al. (2015). The daily Se intake from the turnip treated with the foliar application of 50 and 100 mg L−1 Se (IV) was in accordance with the dietary allowance of 55–200 μg Se day−1 for adults recommended by WHO (Wu et al., 2015). However, in our previous study, the daily Se intake from turnip treated with similar methods reaches 239 and 340 μg (Li et al., 2018). These values did not exceed the tolerable upper intake of 400 μg Se day−1 for adults as suggested by the Institute of Medicine (USA) (White, 2016), indicating that the foliar application of selenite should be an advisable method to consider turnip as Se-enriched food by biofortification. However, Se concentrations should be further validated according to actual environmental conditions, especially soil properties.
Interactions between Se uptake and other mineral elements have been studied in many plants, but some studies have reported ambiguous conclusions on these interactions. Some reports have supported our results that suitable Se concentrations could improve the uptake of various mineral elements, such as Mg, P, Fe, Zn, Mn, and Cu. Arvy (1992) considered that Se content is positively correlated with Mn, Zn, cobalt (Co), P, and molybdenum (Mo) concentrations in plants. Similarly, Chen and Liu (1996) reported that Se within the physiological concentration range can improve the uptake of P, K, Ca, Mg, S, Fe, Mn, Cu, Zn, and Mo in plants. Hu et al. (2015) also showed that Mn, Zn, Cu, Ni, and Co in the roots of Danshen are high when Se (Na2SeO4) is added. However, some of these studies have demonstrated that Se uptake can subsequently decrease the concentrations of other elements. For example, Arvy (1992) reported that Se content is negatively correlated with Fe, aluminum, and arsenic concentrations in plants. Singh and Singh (1978) also found that Se application reduces Fe, Mn, and Zn concentrations. Thus far, the interaction mechanism between Se uptake and other mineral elements is undefined. Se-induced decrease in the concentrations of some other elements may be attributed to ion antagonism. Fu et al. (2011) found that low Se (selenate or selenite) concentrations can improve the uptake of N, P, K, S, Mg, and Zn, whereas high Se concentrations inhibit the uptake of these elements in pak choi. In addition, some environmental factors may regulate the antagonism among different microelements. Singh and Singh (1978) found that increased Se levels reduce Zn and Cu contents in plants with a low P level, whereas Se application significantly increases Zn, Cu, and Mn contents when P fertilizer is applied. Thus, the effects of Se on the uptake of other mineral elements possibly depend on Se concentrations and soil conditions. However, our results provided evidence that the foliar application of Se (IV) could help improve mineral nutrient contents in turnip.
Effects of Se (IV) on the Contents of Macronutrients
Our results showed that Se could improve protein synthesis in turnip, and similar results have been reported in several mushroom or plant species. Zhao et al. (2004) reported that Se can enhance protein content in Ganoderma lucidum. Yin et al. (2015) found that the crude protein content in potato increases by 4.87–5.44% after Se (0.379 kg hm−2) is applied. Nawaz et al. (2016) found that Se foliar spray (40 mg L−1) increases crude protein content by 47% in maize plants under drought stress conditions. These results indicated that Se affects N metabolism in mushrooms and plants. In contrast to our results, previous findings showed that Se can improve the contents of total carbohydrate, polysaccharide, or soluble sugar in mushrooms or plants (Shang et al., 1998; Zhao et al., 2004; Wang et al., 2006; Yin et al., 2015). For example, Shang et al. (1998) found that Se application can increase the total carbohydrate content in lettuce, and similar results are observed in the fleshy root of carrot (Wang et al., 2006). Related studies have shown that Se application positively affects the production of some organic nutrients, including carotene, crude fiber, vitamin C, and amino acids (Shang et al., 1998; Zhao et al., 2004; Wang et al., 2006; Yin et al., 2015). However, few studies have reported that Se can change the contents of total carbohydrate or crude fat. Du et al. (2004) revealed that 0.60 and 3.00 mg kg−1 of soil Se fertilizer significantly increase the content of crude fat in eggplant. Overall, our results were consistent with existing knowledge that Se can improve organic nutrients in plants. The Se-induced enhancement of organic nutrients may be closely related to Se assimilation and metabolism in plants, but the exact mechanism by which Se improves organic nutrition remains unclear.
Effects of Se (IV) on the Contents of Hydrolysis Amino Acids
In this study, Se uptake elicited various positive effects on the synthesis of different amino acids, and the results were consistent with the change in crude protein. Previous studies indicated that Se can improve the contents of amino acids in some mushroom and plant species. Zhao et al. (2004) showed that moderate amounts of Se added in a culture medium can increase the contents of total amino acids and most components, except cystine in G. lucidum. Similar results have also been reported in two other studies (Niu et al., 1998; Wang et al., 2001). Du et al. (2004) found that soil Se fertilizer (0.60 and 3.00 mg kg−1) significantly improves the contents of total essential amino acids but does not obviously influence total amino acids in eggplant. However, they also observed that 0.15 mg kg−1 of soil Se fertilizer negatively affects the contents of amino acids in eggplant. In addition, Nawaz et al. (2016) demonstrated that 40 mg L−1 Se application enhanced the accumulation of total free amino acids (40%) in water-stressed maize plants. Thus, our results were partly consistent with previous findings, indicating that the effects of Se on amino acid synthesis were related to Se treatment concentrations and environmental conditions for different species. Se-induced changes in S and N metabolism in plants may be an important factor causing an increase in amino acid contents (Malagoli et al., 2015). For example, the contents of methionine and cystine can be improved to synthesize selenomethionine and selenocystine by inorganic Se because these amino acids contain S (Du et al., 2004). This conclusion is supported by the detected Se-methyl-SeCys in foliar selenate-treated radish (Schiavon et al., 2016) because the metabolism of inorganic Se in plants is usually carried out via the S metabolism pathway (Singh and Singh, 1979).
Among the Se-induced amino acids in our study, threonine, valine, lysine, methionine, histidine, and isoleucine are essential amino acids for humans (Galili et al., 2016). In addition, alanine, glycine, serine, and proline are considered as sweeteners, whereas aspartic acid exhibits umami characteristics (Ma et al., 2016). Thus, Se (IV) application for turnip might help improve amino acid nutrition and textures. The promotional effects of Se on amino acids were also observed at 50 and 100 mg L−1 Se (IV) (Table 4), which are the recommended concentrations for Se biofortification in turnip.
Conclusion
This study demonstrated that the foliar application of selenite on turnip could significantly increase the Se content in turnip fleshy roots. Se (IV) application positively affected the uptake of several other mineral elements, such as Mg, P, Fe, Zn, Mn, and Cu. The foliar application of Se (IV) could improve the synthesis of proteins and multiple amino acids, including several essential amino acids, in turnip. Although Se (IV) treatment did not affect the contents of Ca, K, crude fat, and total carbohydrate and the amount of total energy, the results indicated that the foliar application of Se could enhance Se biofortification in turnip and promote its nutritional value. We recommended 50–100 mg L−1 Se (IV) treatment for foliar applications on turnip based on the daily intake of Se for adults. These Se treatment concentrations elicited favorable effects on the nutrient components of turnip. We also considered that the effects of Se on the nutrient components of plants are closely related to the soil environment. This study presented the feasibility and benefits of Se biofortification in Chinese turnip.
Author Contributions
YY and XL conceived and designed the experiments. XL and BL performed the experiments. XL analyzed the data and wrote the manuscript.
Funding
This work was financially supported by the Major Projects of the National Natural Science Foundation of China (31590823).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alfthan, G., Eurola, M., Ekholm, P., Venäläinen, E. R., Root, T., Korkalainen, K., et al. (2015). Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: from deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 31, 142–147. doi: 10.1016/j.jtemb.2014.04.009
Arvy, M. P. (1992). Some aspects of selenium relationships in soils and plants. Commun. Soil Sci. Plant Anal. 23, 1397–1407. doi: 10.1080/00103629209368675
Bañuelos, G. S., and Dhillon, K. S. (2011). Developing a sustainable phytomanagement strategy for excessive selenium in western United States and India. Int. J. Phytoremediation 13 (Suppl. 1), 208–228. doi: 10.1080/15226514.2011.568544
Broadley, M. R., White, P. J., Bryson, R. J., Meacham, M. C., Bowen, H. C., Johnson, S. E., et al. (2006). Biofortification of UK food crops with selenium. Proc. Nutr. Soc. 65, 169–181. doi: 10.1079/PNS2006490
Chen, M., and Liu, G. L. (1996). Selenium nutrition in higher plants and its role in the food chain (II). Chin. J. Soil Sci. 27, 185–188.
Chilimba, A. D., Young, S. D., Black, C. R., Rogerson, K. B., Ander, E. L., Watts, M. J., et al. (2011). Maize grain and soil surveys reveal suboptimal dietary selenium intake is widespread in Malawi. Sci. Rep. 1:72. doi: 10.1038/srep00072
Dogan, H., Coteli, E., and Karatas, F. (2016). Determination of glutathione, selenium, and malondialdehyde in different edible mushroom species. Biol. Trace Elem. Res. 174, 459–463. doi: 10.1007/s12011-016-0715-2
Du, Z. Y., Shi, Y. X., and Wang, Q. H. (2004). Effects of selenium application on the selenium absorption and transformation of eggplant and its qualities. Plant Nutr. Fertiliz. Sci. 10, 298–301. doi: 10.11674/zwyf.2004.0315
Fairweather-Tait, S. J., Bao, Y., Broadley, M. R., Collings, R., Ford, D., Hesketh, J. E., et al. (2011). Selenium in human health and disease. Antioxid. Redox Signal. 14, 1337–1383. doi: 10.1089/ars.2010.3275
Feng, R. W., Wei, C. Y., and Tu, S. X. (2013). The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 87, 58–68. doi: 10.1016/j.envexpbot.2012.09.002
Fu, D. D., Duan, M. L., Liang, D. L., Wang, S. S., and Wu, X. P. (2011). Effects of selenite and selenate on growth and nutrient absorption of pakchoi. Plant Nutr. Fertiliz. Sci. 17, 358–365. doi: 10.11674/zwyf.2011.0251
Galili, G., Amir, R., and Fernie, A. R. (2016). The regulation of essential amino acid synthesis and accumulation in plants. Annu. Rev. Plant Biol. 67, 153–178. doi: 10.1146/annurev-arplant-043015-112213
Gupta, M., and Gupta, S. (2017). An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 7:2074. doi: 10.3389/fpls.2016.02074
Hu, X. R., Dong, W. B., and Liu, R. (2015). Effects of the addition of selenium on trace element concentrations in Danshen (Salvia miltiorrhiza). Anal. Lett. 48, 513–525. doi: 10.1080/00032719.2014.947536
Joy, E. J., Broadley, M. R., Young, S. D., Black, C. R., Chilimba, A. D., Ander, E. L., et al. (2015). Soil type influences crop mineral composition in Malawi. Sci. Tot. Environ. 505, 587–595. doi: 10.1016/j.scitotenv.2014.10.038
Li, X., Wu, Y. S., Li, B. Q., Yang, Y. H., and Yang, Y. P. (2018). Selenium accumulation characteristics and biofortification potentiality in Turnip (Brassica rapa var. rapa) Supplied with selenite or selenate. Front. Plant Sci. 8:2207. doi: 10.3389/fpls.2017.02207
Li, X., Zhang, X. M., Li, B. Q., Wu, Y. S., Sun, H., and Yang, Y. P. (2017). Cadmium phytoremediation potential of turnip compared with three common high Cd-accumulating plants. Environ. Sci. Pollut. R. 24, 21660–21670. doi: 10.1007/s11356-017-9781-z
Li, X., Zhang, X. M., Yang, Y., Li, B. Q., Wu, Y. S., Sun, H., et al. (2016). Cadmium accumulation characteristics in Turnip landraces from China and assessment of their Phytoremediation potential for contaminated soils. Front. Plant Sci. 7:1862. doi: 10.3389/fpls.2016.01862
Liu, Y. T., Chen, D., You, Y. X., Zeng, S. Q., Li, Y. W., Tang, Q. Q., et al. (2016). Nutritional composition of boletus mushrooms from Southwest China and their antihyperglycemic and antioxidant activities. Food Chem. 211, 83–91. doi: 10.1016/j.foodchem.2016.05.032
Longchamp, M., Castrec-Rouelle, M., Biron, P., and Bariac, T. (2015). Variations in the accumulation, localization and rate of metabolization of selenium in mature Zea mays plants supplied with selenite or selenate. Food Chem. 182, 128–135. doi: 10.1016/j.foodchem.2015.02.137
Ma, G. C., Wang, Y. R., and Xuan, Z. Y. (2016). Analysis and comparison of nutritional compositions in Xinjiang turnip (Brassica rapa L.). Sci. Technol. Food Ind. 37, 360–364. doi: 10.13386/j.issn1002-0306.2016.04.064
Malagoli, M., Schiavon, M., dall'Acqua, S., and Pilon-Smits, E. A. H. (2015). Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 6:280. doi: 10.3389/fpls.2015.00280
Mao, H., Wang, Z. H., Graham, L., and Glenn, M. (2011). Effects of selenium valence states and concentration on germination and root growth of six crop species. J. Agro Environ. Sci. 30, 1958–1965.
Nawaz, F., Naeem, M., Ashraf, M. Y., Tahir, M. N., Zulfiqar, B., Salahuddin, M., et al. (2016). Selenium supplementation affects physiological and biochemical processes to improve fodder yield and quality of Maize (Zea mays L.) under water deficit conditions. Front. Plant Sci. 7:1438. doi: 10.3389/fpls.2016.01438
Niu, L. Y., Shi, Y. E., Liu, Z. G., Wang, X. G., Wang, R. B., Song, X. Y., et al. (1998). Analysis of the content of trace elements and amino acids in Ganoderma lucidum. Edible Fungi China 17, 31–32.
Parveen, T., Hussain, A., and Rao, M. S. (2015). Growth and accumulation of heavy metals in turnip (Brassica rapa) irrigated with different concentrations of treated municipal wastewater. Hydrol. Res. 46, 60–71. doi: 10.2166/nh.2014.140
Pilon-Smits, E. A., Quinn, C. F., Tapken, W., Malagoli, M., and Schiavon, M. (2009). Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 12, 267–274. doi: 10.1016/j.pbi.2009.04.009
Quinn, C. F., Galeas, M. L., Freeman, J. L., and Pilon-Smits, E. A. (2007). Selenium: deterrence, toxicity, and adaptation. Integr. Environ. Assess. Manag. 3, 460–462. doi: 10.1002/ieam.5630030317
Rayman, M. P. (2012). Selenium and human health. Lancet 379, 1256–1268. doi: 10.1016/S0140-6736(11)61452-9
Sams, C. E., Panthee, D. R., Charron, C. S., Kopsell, D. A., and Yuan, J. S. (2011). Selenium regulates gene expression for glucosinolate and carotenoid biosynthesis in Arabidopsis. J. Am. Soc. Hortic. Sci. 136, 23–34.
Schiavon, M., Berto, C., Malagoli, M., Trentin, A., Sambo, P., Dall'Acqua, S., et al. (2016). Selenium biofortification in radish enhances nutritional quality via accumulation of methyl-selenocysteine and promotion of transcripts and metabolites related to glucosinolates, phenolics, and amino acids. Front. Plant Sci. 7:1371. doi: 10.3389/fpls.2016.01371
Shang, Q. M., Gao, L. H., and Li, S. J. (1998). Effect of selenium on quality of hydroponics lettuce. J. China Agric. Univ. 3, 67–71.
Singh, M., and Singh, N. (1978). Selenium toxicity in plants and its detoxication by phosphorus. Soil Sci. 126, 255–262. doi: 10.1097/00010694-197811000-00001
Singh, M., and Singh, N. (1979). Effect of forms of selenium on the accumulation of selenium, sulfur, and forms of nitrogen and phosphorus in Forage Cowpea (Vigna-Sinensis). Soil Sci. 127, 264–269. doi: 10.1097/00010694-197905000-00002
Sperotto, R. A., Ricachenevsky, F. K., Williams, L. E., Vasconcelos, M. W., and Menguer, P. K. (2014). From soil to seed: micronutrient movement into and within the plant. Front. Plant Sci. 5:438. doi: 10.3389/fpls.2014.00438
Wang, G. L., Shang, D. J., and Yang, W. X. (2001). Study on the nutritional components and antioxidant of Ganoderma lucidum rich in selenium. Acta Nutr. Sin. 32, 73–75.
Wang, J. M., Zhao, Z. Z., and Li, G. R. (2006). Effects of selenium application on the selenium content, yield and qualities of carrot. Plant Nutr. Fertiliz. Sci. 12, 240–244. doi: 10.11674/zwyf.2006.0216
Wang, X. F., Dai, C. C., Jiang, H. L., Chen, Z. L., Shi, H. J., and Li, J. (2005). The effect of the Se to the fruiting-body of Pleurotus ostreatus and nutrition composition. Food Sci. 26, 91–95.
White, P. J. (2016). Selenium accumulation by plants. Ann. Bot. 117, 217–235. doi: 10.1093/aob/mcv180
White, P. J., Bowen, H. C., Marshall, B., and Broadley, M. R. (2007). Extraordinarily high leaf selenium to sulfur ratios define ‘Se-accumulator’ plants. Ann. Bot. 100, 111–118. doi: 10.1093/aob/mcm084
White, P. J., and Broadley, M. R. (2009). Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 182, 49–84. doi: 10.1111/j.1469-8137.2008.02738.x
White, P. J., and Brown, P. H. (2010). Plant nutrition for sustainable development and global health. Ann. Bot. 105, 1073–1080. doi: 10.1093/aob/mcq085
Wu, Z., Bañuelos, G. S., Lin, Z. Q., Liu, Y., Yuan, L., Yin, X., et al. (2015). Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 6:136. doi: 10.3389/fpls.2015.00136
Keywords: selenium, biofortification, turnip, nutritional value, human health
Citation: Li X, Li B and Yang Y (2018) Effects of Foliar Selenite on the Nutrient Components of Turnip (Brassica rapa var. rapa Linn.). Front. Chem. 6:42. doi: 10.3389/fchem.2018.00042
Received: 18 December 2017; Accepted: 19 February 2018;
Published: 02 March 2018.
Edited by:
Holger Hintelmann, Trent University, CanadaReviewed by:
Susana Casal, Universidade do Porto, PortugalMarcin Szymański, Poznan University of Medical Sciences, Poland
Copyright © 2018 Li, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongping Yang, yangyp@mail.kib.ac.cn
 Xiong Li
Xiong Li Boqun Li
Boqun Li Yongping Yang
Yongping Yang