H-NS Nucleoid Protein Controls Virulence Features of Klebsiella pneumoniae by Regulating the Expression of Type 3 Pili and the Capsule Polysaccharide
- 1Unidad de Investigación Médica en Enfermedades Infecciosas y Parasitarias, Hospital de Pediatría, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, Hospital de Pediatría, Mexico City, Mexico
- 2Departamento de Microbiología, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Mexico City, Mexico
- 3Unidad de Investigación en Medicina Experimental, Facultad de Medicina, Universidad Nacional Autónoma de México, Mexico City, Mexico
- 4Cell Biology Institute, University of Bonn, Bonn, Germany
Klebsiella pneumoniae is an opportunistic pathogen causing nosocomial infections. Main virulence determinants of K. pneumoniae are pili, capsular polysaccharide, lipopolysaccharide, and siderophores. The histone-like nucleoid-structuring protein (H-NS) is a pleiotropic regulator found in several gram-negative pathogens. It has functions both as an architectural component of the nucleoid and as a global regulator of gene expression. We generated a Δhns mutant and evaluated the role of the H-NS nucleoid protein on the virulence features of K. pneumoniae. A Δhns mutant down-regulated the mrkA pilin gene and biofilm formation was affected. In contrast, capsule expression was derepressed in the absence of H-NS conferring a hypermucoviscous phenotype. Moreover, H-NS deficiency affected the K. pneumoniae adherence to epithelial cells such as A549 and HeLa cells. In infection experiments using RAW264.7 and THP-1 differentiated macrophages, the Δhns mutant was less phagocytized than the wild-type strain. This phenotype was likely due to the low adherence to these phagocytic cells. Taken together, our data indicate that H-NS nucleoid protein is a crucial regulator of both T3P and CPS of K. pneumoniae.
Introduction
Klebsiella pneumoniae is an opportunistic Gram-negative bacterium belonging to the Enterobacteriaceae family causing nosocomial infections such as septicemia, pneumonia, urinary tract infections, surgical site infections and catheter-related infections (Han, 1995; Podschun and Ullmann, 1998; Schelenz et al., 2007; Ares et al., 2013). In addition, K. pneumoniae has been implicated in pyogenic liver abscesses in patients with meningitis, endophthalmitis and malignancies (Fung et al., 2002; Chang et al., 2008; Tsai et al., 2008; Alcántar-Curiel and Girón, 2015). Numerous nosocomial outbreaks caused by multiple-drug resistant K. pneumoniae have been reported (Nordmann et al., 2009; Hirsch and Tam, 2010; Schwaber et al., 2011). The main virulence determinants of K. pneumoniae are: capsular polysaccharide (CPS), lipopolysaccharide, siderophores, and pili (Gerlach et al., 1989; Podschun and Ullmann, 1998; Brisse et al., 2009). The K. pneumoniae genome codes for different pili such as Type 1 pili (T1P), Type 3 pili (T3P), and E. coli common pilus (ECP; Allen et al., 1991; Schurtz et al., 1994; Struve et al., 2009; Alcántar-Curiel et al., 2013). In contrast to T1P, the T3P can cause mannose-resistant agglutination of tannic acid-treated human erythrocytes (Podschun and Ullmann, 1998). The biogenesis of T3P is dependent on the mrkABCDF operon (Hornick et al., 1995; Huang et al., 2009). The filament is composed of the major pilus subunit MrkA and the tip adhesion protein MrkD (Gerlach et al., 1989). K. pneumoniae T3P mediate adherence to tracheal epithelial cells, renal tubular cells, basolateral surfaces of lung tissue and are crucial in biofilm formation (Tarkkanen et al., 1997; Sebghati et al., 1998; Langstraat et al., 2001; Jagnow and Clegg, 2003; Schroll et al., 2010). While the pili are required during the initial colonization of the host, the CPS impairs macrophage-mediated phagocytosis (Highsmith and Jarvis, 1985; Podschun and Ullmann, 1998; Alvarez et al., 2000). CPS is a complex layer of surface-associated polysaccharides which is important for the pathogenesis of K. pneumoniae in both, animal models as well as in infections of cultured cells (Cortés et al., 2002; Lawlor et al., 2005; Regueiro et al., 2006; March et al., 2013).
The histone-like nucleoid-structuring protein (H-NS) is a DNA-binding protein found in enteropathogens such as Escherichia, Salmonella, Shigella, Vibrio, and Yersinia (Tendeng and Bertin, 2003). It has functions as an architectural component of the nucleoid and as a global regulator of gene expression (Tendeng and Bertin, 2003; Dorman, 2004). It has been proposed that H-NS affects bacterial evolution by direct repression of AT-rich foreign DNA (i.e., pathogenicity islands) acquired by horizontal transfer events, to facilitate tolerance of these foreign sequences and to integrate them into a pre-existing regulatory network (Navarre et al., 2006, 2007; Dorman, 2007). Mutations in hns-like genes have pleiotropic effects in several bacteria and may cause defects in their growth or even bacterial cell death (Zhang et al., 1996; Tendeng et al., 2000; Heroven et al., 2004; Ellison and Miller, 2006; Lucchini et al., 2006; Navarre et al., 2006; Baños et al., 2008; Castang and Dove, 2012). Similar to other enterobacteria, K. pneumoniae is known to possess regions of horizontally acquired genetic sequences. However, there are no reports about the role of H-NS in this pathogen.
In this work we describe the effect of H-NS protein on the expression of both T3P and CPS, two of the main virulence determinants of K. pneumoniae. The absence of H-NS down-regulated the T3P and affected biofilm formation. In contrast, expression of CPS was derepressed in a Δhns mutant, conferring a hypermucoviscous phenotype. Finally, the absence of H-NS resulted in low adherence to epithelial cells and macrophages and in high resistance to macrophage phagocytosis.
Material and Methods
Bacterial Strains and Culture Conditions
Bacterial strains and plasmids used in this study are listed in Table 1. Bacterial cultures were routinely grown in Luria-Bertani (LB) broth with or without antibiotics [200 μg/ml (ampicillin), 50 μg/ml (kanamycin), 30 μg/ml (chloramphenicol), or 10 μg/ml (tetracycline)] after overnight growth with shaking at 37°C.
Construction of K. pneumoniae Mutants
K. pneumoniae 123/01 was isolated from a patient with pneumonia by bronchoalveolar washing. Capsular serotype K39 was determined by sequencing of wzc gene as previously described (Pan et al., 2013). K. pneumoniae was targeted for mutagenesis of hns, mrkA and cps following the procedure reported by Datsenko and Wanner (2000) with some modifications. Each purified PCR product was electroporated into competent K. pneumoniae carrying the lambda-Red recombinase helper plasmid pKD119, whose expression was induced by adding L-(+)-arabinose (Sigma) at a final concentration of 1.0%. For the Δcps mutant, we deleted the chromosomal region from galF to wzi [Δ(galF-orf2-wzi)]. PCR fragments containing hns, mrkA and cps sequences flanking a kanamycin cassette were generated using gene-specific primer pairs (Table 2), and DNA of the pKD4 plasmid was used as template. For the Δhns Δcps double mutant, we generated a PCR fragment containing cps sequence flanking a chloramphenicol cassette using the pKD3 plasmid as template. The respective mutations were confirmed by PCR and sequencing.
Construction of Plasmids
The pT3-H-NS plasmid was generated by cloning a PCR product containing the corresponding hns region of K. pneumoniae into the pMPM-T3 plasmid (see primers in Table 2). The PCR product was digested with XhoI and EcoRI and ligated into pMPM-T3 digested with the same enzymes. pT6-MrkH was constructed by cloning a PCR product containing the mrkH region, which was digested with NcoI and HindIII and ligated into pMPM-T6 (see Table 2). The identities of the inserts were confirmed by DNA sequencing.
Quantitative RT-PCR
Total RNA extraction was performed using the hot phenol method as previously described (Jahn et al., 2008). DNA was removed with TURBO DNA-free (Ambion, Inc.) and the quality of RNA was assessed using a NanoDrop (ND-1000; Thermo Scientific) and an Agilent 2100 bioanalyzer with a Picochip (Agilent Technologies). The absence of contaminating DNA was controlled by lack of amplification products after 35 qPCR cycles. cDNA was prepared using 1 μg of RNA, random hexamer primers (0.2 μg/μl), and M-MulV-RT (20 U/μl, reverse transcriptase of Moloney Murine leukemia Virus; Thermo Scientific). Specific primers were designed with the Primer3Plus software (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi/) and are listed in Table 2. For LightCycler reactions, a master mix of the following components was prepared: 3.0 μl PCR-grade water, 1.0 μl (10 μM) forward primer, 1.0 μl (10 μM) reverse primer, 10 μl 2x SYBR Green I Master Mix, and 5.0 μl cDNA (50–100 ng). A multiwell plate was sealed with sealing foil, centrifuged at 1500 g for 2 min and loaded into the LightCycler 480 instrument (Roche). Amplification was performed in triplicate wells for each sample analyzed. Control reactions with no template (water) and minus-reverse transcriptase (RNA) were run with all reactions. Real-time PCR analysis was performed using the following conditions: denaturation (95°C for 10 min); amplification and quantification repeated for 45 cycles (95°C for 10 s, 57°C for 20 s, 72°C for 30 s with a single fluorescence measurement); melting curve (95°C for 10 s, 65°C for 1 min with continuous fluorescence measurement at 97°C); and finally a cooling step at 40°C for 10 s. Melting curve analysis was performed after each run to confirm specificity of the primers. 16S rRNA was used as a reference gene for normalization and the relative gene expression was calculated using the 2−ΔCt method (Livak and Schmittgen, 2001).
Mucoviscosity
The mucoviscosities of K. pneumoniae strains were determined by a string test and measured by centrifugation as described previously (Pan et al., 2011; Lin et al., 2012). The string test was performed stretching a colony that had been grown overnight on a blood agar plate, using a loop. To further measure the levels of mucoviscosity, a low speed centrifugation was performed. Briefly, equal numbers of exponential phase-cultured bacteria were centrifuged at 1000 g for 5 min. The supernatant was subjected to measurement of the absorbance at 600 nm.
Glucuronic Acid Analysis
Capsular polysaccharides were extracted and quantified using a colorimetric assay for glucuronic acid as previously described (Lin et al., 2009). Basically, 500 μl of bacterial cultures were mixed with 100 μl of 1% zwittergent 3–14 in 100 mM citric acid and then the mixtures were incubated at 50°C for 20 min. After centrifugation, 250 μl of supernatants were transferred into new tubes, and 1 ml of absolute ethanol was added to precipitate the CPS. The pellets were dissolved in 200 μl of distilled water, and then 1200 μl of 12.5 mM borax in concentrated H2SO4 were added. The mixtures were vigorously vortexed, boiled for 5 min, and then cooled. 20 μl of 0.15% 3-hydroxydiphenol in 0.5% NaOH were added to the mixture and the absorbance was measured at 520 nm. The glucuronic acid concentration in each sample was determined from a standard curve of glucuronic acid and expressed in micrograms/109 CFU.
Adherence Assays to Cultured Eukaryotic Cells
Monolayers of HeLa (ATCC CCL-2) human cervix epithelial and A549 (ATCC CCL-185) human lung epithelial cell lines (7 × 105) were infected with the indicated strains of an LB broth overnight culture at a multiplicity of infection (MOI) of 100. Epithelial cells were grown in DMEM (Invitrogen) with 10% fetal bovine serum (FBS). After infection, eukaryotic cells were incubated in DMEM with no FBS for 2 h at 37°C under an atmosphere of 5% CO2. After the 2 h incubation period, cells were rinsed three times with PBS to remove unbound bacteria. For quantification of adherence, the cells were lysed with a solution of 0.1% Triton X-100. After homogenization, 10-fold serial dilutions were plated onto LB agar plates to determine total CFUs. The results shown are the mean of at least three experiments performed in triplicate on different days.
Phagocytosis of Bacteria by Macrophages
THP-1 (ATCC TIB-202) human monocytes (differentiated to macrophages with 200 nM of phorbol 12-myristate 13-acetate for 24 h) and RAW264.7 (ATCC TIB-71) murine macrophages (6 × 105) were seeded into 24-well tissue culture plates. Bacteria were grown in 5 ml of LB broth to the exponential phase. Macrophages were infected with a MOI of 100 in a final volume of 1 ml RPMI 1640 tissue culture medium supplemented with 10% heat-inactivated FBS. To synchronize infection, plates were centrifuged at 200 g for 5 min. Plates were incubated at 37°C under an humidified 5% CO2 atmosphere. After 2 h, cells were rinsed three times with PBS and incubated for an additional 60 min with 1 ml of RPMI 1640 containing 10% FBS and gentamicin (100 μg/ml) to eliminate extracellular bacteria. Cells were then rinsed again three times with PBS and lysed with 0.1% Triton X-100. After homogenization, 10-fold serial dilutions were plated onto LB agar plates to determine total CFUs. Adherence of K. pneumoniae strains (grown to the exponential phase) to macrophages was performed as previously described (Rosales-Reyes et al., 2012), incubating 1 h at 4°C to inhibit phagocytosis.
Biofilm Formation Assay on Abiotic Surface
Adhesion to abiotic surface (polystyrene) was analyzed using 96-well plates as described previously (Saldaña et al., 2014). Overnight cultures of bacteria grown in LB broth (10 μl) were added to 1 ml of LB. This volume was distributed in quintuples (100 μl per well) into a 96-well plate and incubated at room temperature for 24 h. Unbound bacteria were removed by washing the wells three times with PBS, and bound bacteria were stained with 1% crystal violet (CV) for 20 min. Wells were thoroughly rinsed three times with PBS, and the dye was solubilized in 100 μl of ethanol 70%. Finally, the amount of extracted crystal violet was determined by measuring the OD600 using an enzyme-linked immunosorbent assay (ELISA) Multiskan plate reader.
Statistical Analysis
For statistical differences, one-way ANOVA followed by the Tukey's comparison test was performed using Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). P ≤ 0.05 was considered statistically significant.
Results
Generation of an hns Mutant of K. pneumoniae
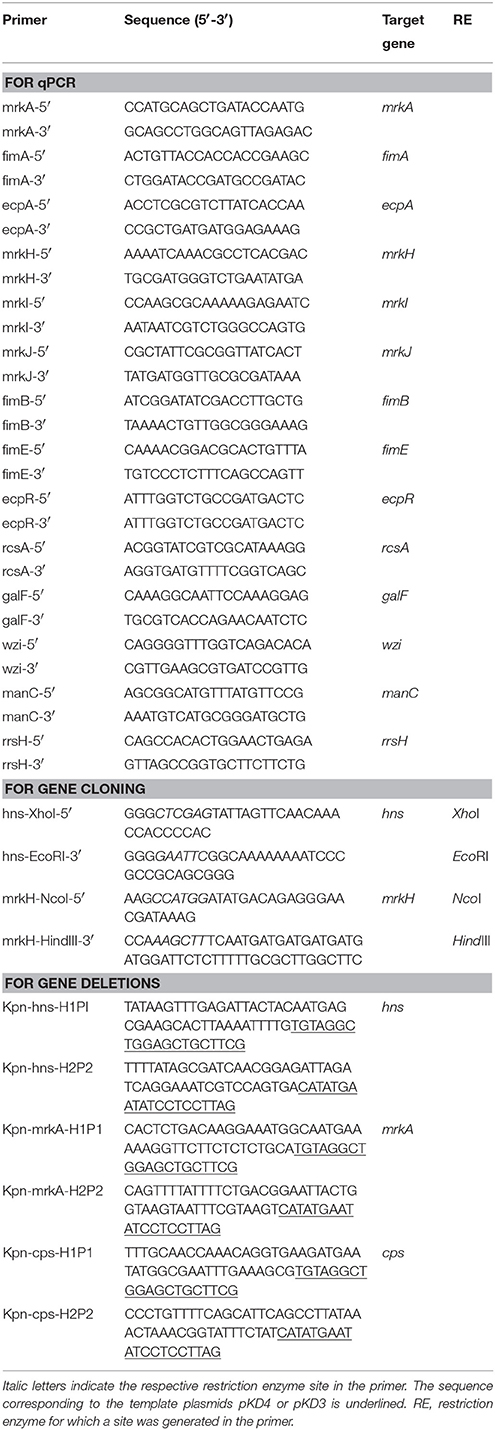
H-NS amino acid sequences of K. pneumoniae strains were homologous to H-NS proteins of enteric bacteria such as Salmonella, Yersinia, Shigella, and E. coli (Figure 1A). The hns gene of the strain 123/01 used in this study was completely identical to all K. pneumoniae sequenced strains (data not shown). By using the λ-red homolog recombinase (Datsenko and Wanner, 2000), we were able to replace the hns gene of the K. pneumoniae genome with a kanamycin resistance cassette. The hns gene of K. pneumoniae was cloned into a plasmid yielding pT3-H-NS to complement the Δhns mutant. In terms of resistance to antibiotics, the Δhns mutant did not differ with respect to the wild type strain (data not shown). The growth in LB broth of K. pneumoniae strains was followed over a period of 8 h at 37 and 25°C. Growth of the Δhns mutant was slightly attenuated at 37°C, mainly in the exponential phase but reaching the stationary phase like the wild-type strain (Figure 1B). In contrast, at 25°C the Δhns mutant did not reach the growth of the wild-type strain in the stationary phase (Figure 1C). Growth of the complemented strain harboring pT3-H-NS was restored to wild-type levels at both temperatures. This observation indicates that H-NS is required for optimal growth of K. pneumoniae as has been shown for other enterobacteria (Zhang et al., 1996; Tendeng et al., 2000; Heroven et al., 2004; Ellison and Miller, 2006; Lucchini et al., 2006; Navarre et al., 2006; Baños et al., 2008; Castang and Dove, 2012).
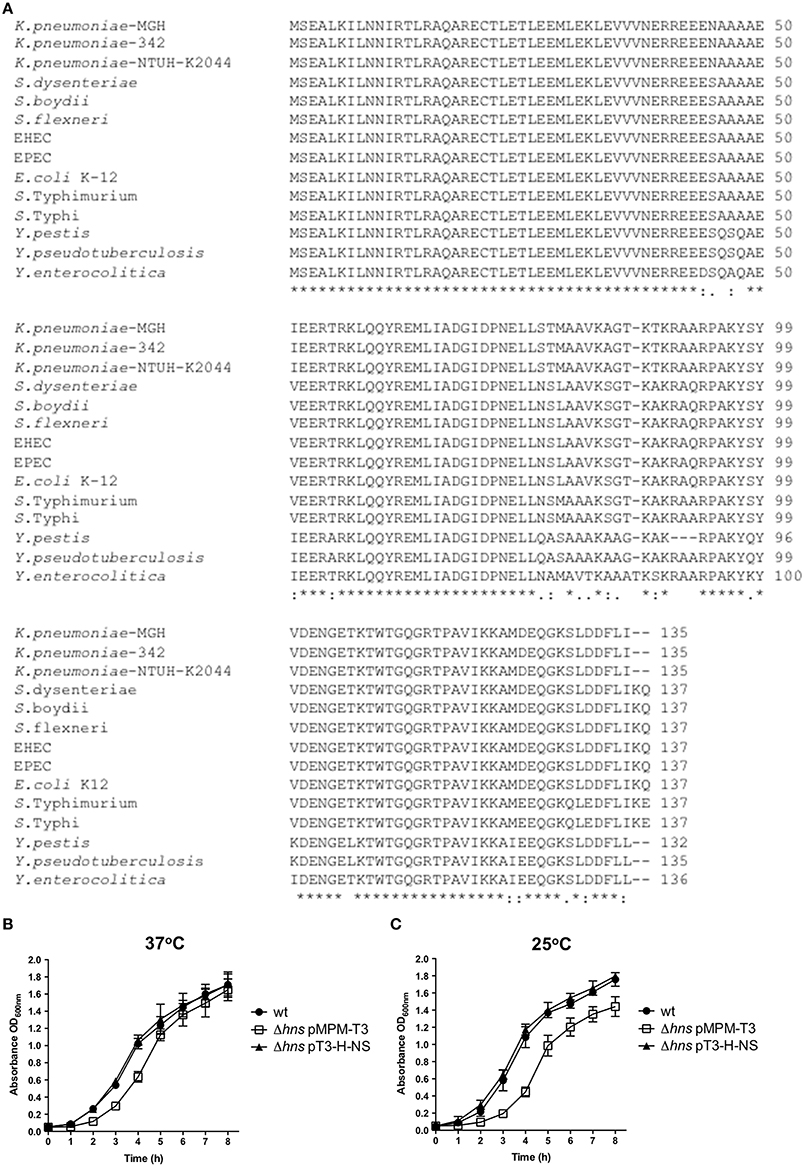
Figure 1. H-NS protein in K. pneumoniae. (A) Alignment of amino acid sequences of H-NS proteins from several enterobacteria: K. pneumoniae (MGH, 342 and NTUH-K2044), Shigella dysenteriae (Sd197), Shigella boydii (Sb227), Shigella flexneri (2a str. 2457T), Enterohemorragic E. coli (EDL933), Enteropathogenic E. coli (E2348/69), E. coli K-12 (MG1655), Salmonella enterica serovar Typhimurium (LT2), Salmonella enterica serovar Typhi (CT18), Yersinia pestis (KIM 10), Yersinia pseudotuberculosis (IP 32953) and Yersinia enterocolitica (8081). Analysis was performed using the ClustalW2 software (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Growth kinetics of wild-type K. pneumoniae, and the isogenic mutants at 37°C (B) and 25°C (C). Bacterial cultures were grown for 8 h in LB medium.
H-NS Differentially Regulates the Fimbrial Repertoire of K. pneumoniae
We reported previously that ecpA, mrkA and fimA fimbrial genes are highly prevalent in K. pneumoniae strains (Alcántar-Curiel et al., 2013). To demonstrate the role of H-NS in regulation of fimbrial genes, we determined transcriptional expression levels of ecpA, mrkA and fimA by qRT-PCR. Both, ecpA and fimA were derepressed in the absence of H-NS. In contrast to ecpA and fimA, mrkA was repressed in the absence of H-NS (Figure 2A). In addition to pilin genes themselves, we evaluated the expression of genes regulating expression of three pili types. Regulatory genes for the pilins analyzed were up-regulated in the absence of H-NS. The mrkJ gene codes for a phosphodiesterase (Johnson and Clegg, 2010) and negatively regulates mrkA expression by degrading c-di-GMP, thereby inhibiting MrkH activity, which is the central activator of T3P (Wilksch et al., 2011). To corroborate decreased expression of the mrkA pilin gene in absence of H-NS, we performed biofilm formation assays. Biofilm formation in K. pneumoniae is MrkA-dependent (Langstraat et al., 2001; Wilksch et al., 2011). Indeed, the absence of MrkA reduced K. pneumoniae biofilm formation by 10-fold (Figure 2B). The Δhns mutant was impaired in biofilm formation (5-fold) similar to the ΔmrkA mutant, while this phenotype was counteracted by complementing the Δhns mutant with the pT3-H-NS plasmid (Figure 2B). In addition to T3P, the capsule polysaccharide (CPS) is a crucial virulence determinant in K. pneumoniae. To evaluate the role of CPS in biofilm formation, we assayed the Δcps and Δhns Δcps mutants. The Δcps mutant was not affected in biofilm formation. However, the Δhns Δcps double mutant was impaired in biofilm formation similar to the Δhns single mutant (Figure 2B). These observations suggest that CPS has no role in biofilm formation and that downregulation of T3P expression is the main reason for the biofilm phenotypes observed in the Δhns mutant. To corroborate that the effect of H-NS on biofilm formation is due to the transcriptional repression of mrkA and not due to overexpression of the capsule possibly resulting in steric hindrance of T3P, we generated wild type and Δhns mutant strains overexpressing the MrkH activator protein. Indeed, MrkH overexpression positively affected biofilm formation (~2-fold) in the wild-type strain (Figure 2C). Interestingly, overexpression of the MrkH protein counteracted the decrease in biofilm formation observed in the Δhns mutant (~9-fold) regardless of the excess production of CPS (Figure 2C). These data corroborated H-NS as a positive regulator of TP3 gene expression.
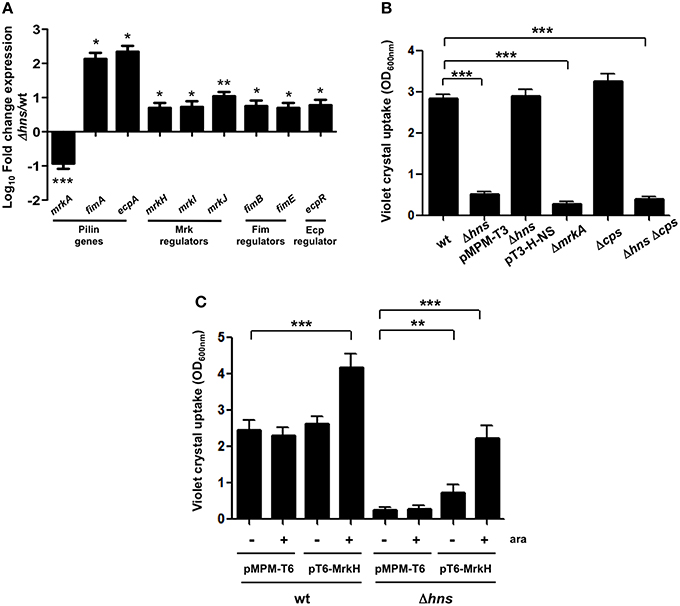
Figure 2. H-NS positively regulates mrkA expression. (A) Fold-change expression (qRT-PCR) of the pilin genes and their regulators in the Δhns mutant as compared to the K. pneumoniae wild-type strain. (B) Quantification of biofilm formation by measuring Crystal Violet uptake. (C) Quantification of biofilm formation by measuring Crystal Violet uptake overexpressing the MrkH activator protein (0.1% arabinose) in both wild-type and hns background. Results shown represent mean and standard deviations of 3 independent experiments performed. ns, not significant; statistically significant with respect to the wild-type strain ***p < 0.001; **p < 0.01; *p < 0.05.
The Absence of H-NS Results in a Hypermucoviscous Phenotype of K. pneumoniae
The Δhns mutant colony morphology was hypermucoviscous compared to wild-type bacteria on agar plates while the complemented strain was similar to the wild-type strain (data not shown). To determine the levels of mucoviscosity, we measured the supernatant of suspensions of wild-type, Δhns mutant and complemented Δhns mutant bacteria centrifuged at low speed. Indeed, the absence of H-NS resulted in higher mucoviscosity levels compared to the wild-type strain (~3-fold), while complementation restored the wild-type phenotype (Figures 3A,B). To determine the amount of CPS quantitatively, we performed a biochemical assay, which measured the capsular glucuronic acid. As shown in Figure 3C, the CPS level increased in the absence of H-NS (~3-fold), while the complemented Δhns strain had similar amounts of CPS compared to the wild-type strain. The absence of MrkA did not alter the CPS production; however in both the Δcps and Δhns Δcps mutants the CPS amount was considerably diminished (~4-fold), indicating that increasing of the capsule in the hns background is dependent on capsular genes. Genetically, capsule-generating genes of K. pneumoniae are encoded in the cps cluster, including three transcriptional units, being galF, wzi, and manC the first genes for each promoter (Chou et al., 2004; Chuang et al., 2006; Pan et al., 2011). K. pneumoniae strains belonging to serotype K39 contain the three capsule-generating genes described above (Pan et al., 2013, 2015). In addition, cps genes are activated by the RcsA regulatory protein (Wehland and Bernhard, 2000; Lin et al., 2011, 2013). Using qRT-PCR we monitored the expression of rcsA, galF, wzi, and manC in wild-type K. pneumoniae, the Δhns mutant and the complemented Δhns mutant strain. Expression levels of rcsA, galF, wzi, and manC were derepressed ~6 to 8-fold in the absence of H-NS (Figure 3D). The complemented Δhns mutant presented expression levels similar to the wild-type strain. These observations supported an inhibitory effect of H-NS on the expression/production of a polysaccharide capsule in K. pneumoniae.
Figure 3. H-NS represses capsular polysaccharide in K. pneumoniae. (A,B) Mucoviscosity of K. pneumoniae wild-type, Δhns mutant, complemented Δhns mutant, ΔmrkA mutant, Δcps mutant, and Δhns Δcps mutant. The mucoviscosity was determined by low speed centrifugation and is expressed as OD600 of the supernatant. (C) Capsule quantification of K. pneumoniae strains. The glucuronic acid concentration in each strain was determined from capsular polysaccharides extracted of 0.5 ml bacterial cultures. (D) Transcriptional expression (qRT-PCR) of the rcsA, galF, wzi, and manC genes in the WT K. pneumoniae strain, Δhns mutant and complemented Δhns mutant. Data represent the mean of at least three independent experiments (mean ± SD). Statistically significant with respect to the wild-type strain ***p < 0.001; **p < 0.01; *p < 0.05.
Fimbrial and Capsular Genes of K. pneumoniae are Thermoregulated by H-NS
H-NS nucleoid protein has been reported to be an essential component in thermoregulation of virulence factors in several pathogenic bacteria (Falconi et al., 1998; Umanski et al., 2002; Ono et al., 2005; Duong et al., 2007). To analyze if the repressor effect of H-NS was temperature-dependent, we performed qRT-PCR experiments determining the transcriptional expression of both fimbrial (mrkA, fimA, ecpA) and capsule-generating (galF, wzi, manC) genes in the wild-type and Δhns mutant at 37 and 25°C. At 25°C, the repressor effect of H-NS was higher than at 37°C, specifically 3.33-, 8.72-, and 9.66-fold for mrkA, fimA, and ecpA, respectively (Figure 4A). For capsular genes, H-NS-mediated repression was about 4-fold higher at 25°C compared to 37°C (Figure 4B). These data clearly indicate that at low temperatures (25°C), H-NS efficiently represses both fimbrial and capsular genes as compared to 37°C by maintaining down-regulation of these virulence genes.
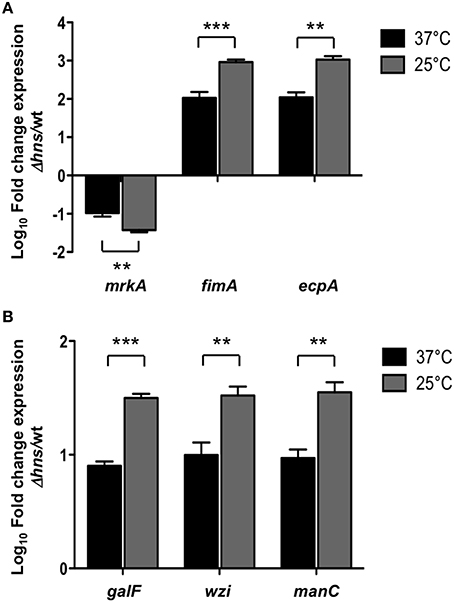
Figure 4. H-NS thermoregulates both fimbrial and capsular genes. Fold-change expression (qRT-PCR) of the fimbrial (A) and capsular genes (B) in the Δhns mutant as compared to the K. pneumoniae wild-type strain. Data represent the mean of at least three independent experiments (mean ± SD). Statistically significant with respect to the wild-type strain ***p < 0.001; **p < 0.01.
H-NS is Involved in K. pneumoniae Adherence to and Phagocytosis by Eukaryotic Cells
K. pneumoniae is able to adhere to different epithelial cell lines such as A549 and HeLa (Moranta et al., 2010; Alcántar-Curiel et al., 2013). We evaluated the adherence of the K. pneumoniae wild-type bacteria, the Δhns mutant, the complemented Δhns mutant, the ΔmrkA mutant, the Δcps mutant and the Δhns Δcps double mutant to A549 or HeLa cells. Surprisingly, the absence of H-NS dramatically impaired the adherence of K. pneumoniae to both, A549 and HeLa cells (~4500-fold), while the complemented Δhns mutant showed an adherence that was similar to that of the wild-type strain (Figure 5A). Interestingly, the ΔmrkA mutant was less adherent than the wild-type strain (~9-fold) but not comparable to the Δhns mutant, suggesting that the phenotype observed with the Δhns mutant cannot be explained solely by the decrease of MrkA expression (Figure 5A). Similar to the biofilm assay, we overexpressed the MrkH activator protein to exclude possible effects of capsule-mediated steric hindrance on T3P activity. Overexpression of MrkH enhanced the adherence of K. pneumoniae to HeLa cells for both, wild-type bacteria (~14-fold) and Δhns mutant (~263-fold) (Figure 5B), supporting the notion that H-NS affects the adherence by transcriptional control of mrkA pilin. The absence of CPS resulted in a slight increase in adherence of K. pneumoniae to both epithelial cell types (~5-fold). Bacteria deficient in both hns and cps genes adhered in higher numbers to epithelial cells compared to those deficient in hns alone (~15-fold). However, the levels of adherence of the Δhns Δcps double mutant did not reach the numbers of the wild-type strain, indicating that CPS is partially involved in the low adherence observed with the Δhns single mutant (Figure 5A). To discard that biofilm formation on plastic surfaces did not affect the adherence assays during 2 h of incubation, we quantified the CFU/ml of adhered bacteria on plastic wells at this time with no eukaryotic cells. We were unable to find adhered bacteria on the plastic surface during 2h. Furthermore, biofilm formation assays were performed and all strains examined did not form biofilm at 2 h of incubation in the conditions examined for eukaryotic cells (DMEM or RPMI, 37°C, 5% CO2). A crucial event in the pathogenesis of K. pneumoniae is the evasion of macrophage phagocytosis. We used THP-1 differentiated macrophages and RAW264.7 cells to evaluate the effect of H-NS deletion on phagocytosis. A Δhns mutant was considerably less phagocytized by macrophages (~55-fold) and the complemented strain was recovered in numbers similar to the wild-type strain (Figure 5C). Bacterial growth rates during adherence and phagocytosis were similar in the different strains analyzed (data not shown), indicating that the low levels of phagocytosis observed with the Δhns mutant were not due to growth defects. The absence of MrkA did not alter the phagocytosis of K. pneumoniae by macrophages, while the absence of CPS increased the phagocytosis by both cell lines (~67-fold). In contrast, a Δhns Δcps double mutant was phagocytosed in similar bacterial numbers as the Δhns single mutant. To determine if the low level of phagocytosis showed by the Δhns Δcps double mutant was due to the initial stage of adherence, we performed adherence assays using both macrophages cell lines. The Δhns and the Δhns Δcps mutants presented the same low levels of adherence to macrophages, indicating that the evasion of phagocytosis is mainly due to impaired adherence (Figure 5D). These observations suggest that the H-NS nucleoid protein in K. pneumoniae is relevant for both, adherence to and phagocytosis by eukaryotic cells.
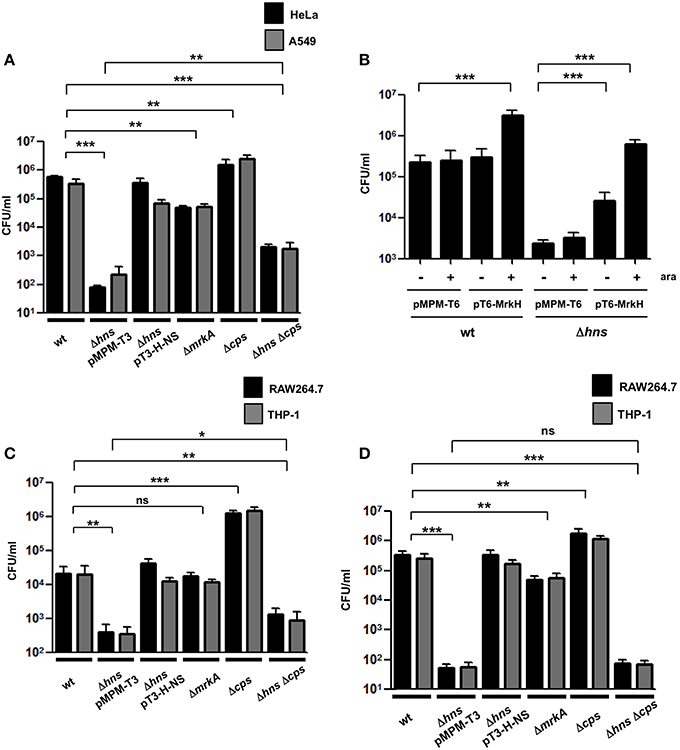
Figure 5. Adherence and phagocytosis of the K. pneumoniae Δhns mutant. (A) Comparison of adherence levels of K. pneumoniae wild-type, Δhns mutant, complemented Δhns mutant, ΔmrkA mutant, Δcps mutant, and Δhns Δcps mutant to HeLa and A549 cells. (B) Adherence levels of wild-type strain and isogenic Δhns mutant to HeLa cells, overexpressing the MrkH activator protein (0.1% arabinose). (C) Comparison of phagocytic uptake of indicated K. pneumoniae strains by RAW264.7 and THP-1 macrophages. (D) Adherence levels of K. pneumoniae wild-type strain and isogenic mutants to RAW264.7 and THP-1 macrophages. Results represent means and standard deviations of the results obtained from the 3 experiments performed in triplicates. ns, not significant; statistically significant with respect to the wild-type strain ***p < 0.001; **1p < 0.01; *p < 0.05.
Discussion
H-NS is a pleiotropic regulator, which modulates expression of virulence determinants of several enteropathogens (Dorman, 2004). This study describes for the first time the role of H-NS in expression of K. pneumoniae virulence features. The K. pneumoniae H-NS protein is homologous to other H-NS-like proteins of different enteropathogens such as E. coli, Salmonella, Yersinia, and Shigella. In terms of bacterial fitness, the absence of H-NS resulted in a slight defect in K. pneumoniae growth in LB broth at 37°C, but more evident at low temperature such as 25°C. As previously reported in other hns mutants, this could be due to dysregulated expression of non-related genes (Navarre et al., 2007). Pili are relevant for the adherence to host cells as well as in biofilm formation (Podschun and Ullmann, 1998; Langstraat et al., 2001; Wilksch et al., 2011). Interestingly, a Δhns mutant differentially regulated fimbrial genes, as we observed an increase of both fimA and ecpA and a repression of mrkA expression. FimA and EcpA pilin subunits were previously reported to be repressed by H-NS in E. coli, showing similar regulation for pilin genes as for regulatory genes in both bacteria (Dorman and Ní Bhriain, 1992; Schembri et al., 1998; Martínez-Santos et al., 2012; Lehti et al., 2013). The mrkA expression was down-regulated in the absence of H-NS, likely by up-regulation of mrkJ, which is a negative regulator of T3P (Johnson and Clegg, 2010), albeit MrkH and MrkI transcriptional regulators were also derepressed. In the absence of H-NS, high levels of MrkJ may degrade c-di-GMP affecting the MrkH activity as transcriptional activator of the mrkA gene (Wilksch et al., 2011). The positive effect of H-NS on mrkA suggests a post-transcriptional process indirectly affecting MrkA expression, as has been previously described for other genes regulated by H-NS (Bertin et al., 1994; Suzuki et al., 1996; Johansson et al., 1998; Park et al., 2010). The positive role of H-NS on mrkA expression was corroborated by the fact that the Δhns mutant was impaired in biofilm formation similarly to the mutant deficient in mrkA. Since pili are important in the early stage of infection, we analyzed the contribution of H-NS and Mrk to the adherence to human epithelial cells. A Δhns mutant was dramatically affected in the adherence to both, A549 and HeLa cells, while the absence of MrkA led to a significant but comparatively mild decrease in adherence. T3P have been expressed in an E. coli background (Tarkkanen et al., 1997), yet this is the first report about the contribution of MrkA pilin to the adherence to A549 and HeLa epithelial cells using a ΔmrkA mutant. The low adherence levels of the Δhns mutant could not be observed with the ΔmrkA mutant, suggesting that this decrease in adherence to human epithelial cells may be caused by different factors. In addition to transcriptional repression, hypermucoviscosity observed in the Δhns mutant could block the exposition of Mrk pili and therefore interfere with their attachment to the abiotic surface. Previous studies showed that T1P function can be inhibited by the presence of the capsule by steric overcrowding and it has been suggested that this would also affect exposition of Mrk pili (Schembri et al., 2005; Wilksch et al., 2011). Overexpression of MrkH activator protein showed, however, that T3P are not affected by capsule-mediated steric hindrance in the hns background neither with respect to biofilm formation, nor during adherence to epithelial cells. Hypervirulent K. pneumoniae strains produce large amounts of CPS, which confer both, a mucoviscous phenotype and resistance to phagocytosis (Lin et al., 2004; Regueiro et al., 2006). We found that the Δhns mutant was hypermucoviscous with respect to the wild-type strain. This increase in CPS correlated with the derepression of capsular genes (Chuang et al., 2006; Ho et al., 2011; Lin et al., 2013). Controversial results regarding the involvement of capsule on biofilm formation in K. pneumoniae have been reported (Schembri et al., 2005; Boddicker et al., 2006; Balestrino et al., 2008; Wu et al., 2011; Wang et al., 2015). However, our data support observations stating that CPS is not related with biofilm formation.
As to CPS regulation by H-NS, a mucoid morphology in an E. coli hns background has been shown in previous reports (Ebel and Trempy, 1999). This phenotype was due to up-regulation of rcsA, which activates the cps locus (Sledjeski and Gottesman, 1995). Interestingly, we observed the same phenomenon in K. pneumoniae, since capsular structural genes (galF, wzi, and manC) and a capsular regulator (rcsA) were derepressed in the absence of H-NS. Moreover, the K. pneumoniae Δhns mutant was phagocytized in lower numbers as the wild-type strain, likely related to its high mucoviscosity which confers resistance to phagocytic uptake by macrophages (Williams et al., 1983; Cortés et al., 2002). Surprisingly, uptake of bacteria deficient in both, hns and cps was similar to that of mutants deficient in hns alone. This decrease was likely due to impaired adherence to macrophages, indicating that H-NS regulates the initial stages of K. pneumoniae recognition by phagocytic cells. Our data show that there are probably others factors involved in macrophage adherence in addition to CPS. Pili of gram-negative bacteria such as E. coli T1P, Porphyromonas gingivalis FimA (also called T2P), Gram-positive Lactobacillus rhamnosus ScaCBA pili and Streptococcus pneumoniae RrgA pili have been described to be required for the phagocytic uptake by macrophages (Baorto et al., 1997; Wang et al., 2007; Orrskog et al., 2012; Vargas García et al., 2015). The absence of MrkA, however, did not affect macrophage phagocytosis, indicating that T3P are not required for this phenomenon. LPS and outer membrane proteins (OMPs) of K. pneumoniae have been reported to be involved in resistance to phagocytosis (March et al., 2013). Our group currently studies the effect of H-NS on other K. pneumoniae virulence factors such as LPS, OMPs, and siderophores.
Change of temperature is an environmental condition that affects the oligomerization state of H-NS and subsequently its DNA-binding properties, being crucial for control of transcriptional regulation (Ono et al., 2005; Stella et al., 2005, 2006). In bacteria such as E. coli, Shigella and Salmonella, virulence genes are thermoregulated by H-NS: down-regulated at low temperature and expressed at mammalian body temperature (37°C) (Maurelli and Sansonetti, 1988; Falconi et al., 1998; Umanski et al., 2002; Ono et al., 2005; Duong et al., 2007). In agreement with the thermoregulatory functions of H-NS on various virulence factors, fimbrial and capsular genes of K. pneumoniae were differentially repressed at 37 and 25°C, showing that H-NS-mediated repression is higher at the lower temperatures likely encountered outside of the host.
In summary, we have described for the first time the important role of H-NS in K. pneumoniae. Our data show that similar to other pathogens, K. pneumoniae H-NS is a master regulator assuring optimal expression of both T3P and CPS whose uncontrolled expression may severely impact bacterial fitness.
Author Contributions
Conceived and designed the experiments: MA, JF RR, MJ, MA, MD. Performed the experiments: MA, JF, RR, MJ MD. Analyzed the data: MA, JT, JG, MA, MD. Wrote the paper: MA, KV, MA, MD.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by grant No. 2003-C1-63 and 104804-M from Consejo Nacional de Ciencia y Tecnología (CONACyT), México. We thank Thais L. Lacerda for critical reading of the manuscript.
References
Alcántar-Curiel, M. D., Blackburn, D., Saldaña, Z., Gayosso-Vazquez, C., Iovine, N. M., De La Cruz, M. A., et al. (2013). Multi-functional analysis of Klebsiella pneumoniae fimbrial types in adherence and biofilm formation. Virulence 4, 129–138. doi: 10.4161/viru.22974
Alcántar-Curiel, M. D., and Girón, J. A. (2015). Klebsiella pneumoniae and the pyogenic liver abscess: implications and association of the presence of rpmA genes and expression of hypermucoviscosity. Virulence 6, 407–409. doi: 10.1080/21505594.2015.1030101
Alvarez, D., Merino, S., Tomás, J. M., Benedí, V. J., and Albertí, S. (2000). Capsular polysaccharide is a major complement resistance factor in lipopolysaccharide O side chain-deficient Klebsiella pneumoniae clinical isolates. Infect. Immun. 68, 953–955. doi: 10.1128/IAI.68.2.953-955.2000
Allen, B. L., Gerlach, G. F., and Clegg, S. (1991). Nucleotide sequence and functions of mrk determinants necessary for expression of type 3 fimbriae in Klebsiella pneumoniae. J. Bacteriol. 173, 916–920.
Ares, M. Á., Alcántar-Curiel, M. D., Jiménez-Galicia, C., Rios-Sarabia, N., Pacheco, S., and De la Cruz, M. Á. (2013). Antibiotic resistance of gram-negative bacilli isolated from pediatric patients with nosocomial bloodstream infections in a Mexican tertiary care hospital. Chemotherapy 59, 361–368. doi: 10.1159/000362085
Balestrino, D., Ghigo, J. M., Charbonnel, N., Haagensen, J. A., and Forestier, C. (2008). The characterization of functions involved in the establishment and maturation of Klebsiella pneumoniae in vitro biofilm reveals dual roles for surface exopolysaccharides. Environ. Microbiol. 10, 685–701. doi: 10.1111/j.1462-2920.2007.01491.x
Baños, R. C., Pons, J. I., Madrid, C., and Juárez, A. (2008). A global modulatory role for the Yersinia enterocolitica H-NS protein. Microbiology 154, 1281–1289. doi: 10.1099/mic.0.2007/015610-0
Baorto, D. M., Gao, Z., Malaviya, R., Dustin, M. L., van der Merwe, A., Lublin, D. M., et al. (1997). Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 389, 636–639. doi: 10.1038/39376
Bertin, P., Terao, E., Lee, E. H., Lejeune, P., Colson, C., Danchin, A., et al. (1994). The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J. Bacteriol. 176, 5537–5540.
Boddicker, J. D., Anderson, R. A., Jagnow, J., and Clegg, S. (2006). Signature-tagged mutagenesis of Klebsiella pneumoniae to identify genes that influence biofilm formation on extracellular matrix material. Infect. Immun. 74, 4590–4597. doi: 10.1128/IAI.00129-06
Brisse, S., Fevre, C., Passet, V., Issenhuth-Jeanjean, S., Tournebize, R., Diancourt, L., et al. (2009). Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS ONE 4:e4982. doi: 10.1371/journal.pone.0004982
Castang, S., and Dove, S. L. (2012). Basis for the essentiality of H-NS family members in Pseudomonas aeruginosa. J. Bacteriol. 194, 5101–5109. doi: 10.1128/JB.00932-12
Cortés, G., Borrell, N., de Astorza, B., Gómez, C., Sauleda, J., and Albertí, S. (2002). Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect. Immun. 70, 2583–2590. doi: 10.1128/IAI.70.5.2583-2590.2002
Chang, C. M., Lee, H. C., Lee, N. Y., Lee, I. W., Wu, C. J., Chen, P. L., et al. (2008). Community-acquired Klebsiella pneumoniae complicated skin and soft-tissue infections of extremities: emphasis on cirrhotic patients and gas formation. Infection 36, 328–334. doi: 10.1007/s15010-008-7272-3
Chou, H. C., Lee, C. Z., Ma, L. C., Fang, C. T., Chang, S. C., and Wang, J. T. (2004). Isolation of a chromosomal region of Klebsiella pneumoniae associated with allantoin metabolism and liver infection. Infect. Immun. 72, 3783–3792. doi: 10.1128/IAI.72.7.3783-3792.2004
Chuang, Y. P., Fang, C. T., Lai, S. Y., Chang, S. C., and Wang, J. T. (2006). Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J. Infect. Dis. 193, 645–654. doi: 10.1086/499968
Datsenko, K. A., and Wanner, B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645. doi: 10.1073/pnas.120163297
Dorman, C. J. (2004). H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2, 391–400. doi: 10.1038/nrmicro883
Dorman, C. J. (2007). H-NS, the genome sentinel. Nat. Rev. Microbiol. 5, 157–161. doi: 10.1038/nrmicro1598
Dorman, C. J., and Ní Bhriain, N. (1992). Thermal regulation of fimA, the Escherichia coli gene coding for the type 1 fimbrial subunit protein. FEMS Microbiol. Lett. 78, 125–130. doi: 10.1111/j.1574-6968.1992.tb05554.x
Duong, N., Osborne, S., Bustamante, V. H., Tomljenovic, A. M., Puente, J. L., and Coombes, B. K. (2007). Thermosensing coordinates a cis-regulatory module for transcriptional activation of the intracellular virulence system in Salmonella enterica serovar Typhimurium. J. Biol. Chem. 282, 34077–34084. doi: 10.1074/jbc.M707352200
Ebel, W., and Trempy, J. E. (1999). Escherichia coli RcsA, a positive activator of colanic acid capsular polysaccharide synthesis, functions To activate its own expression. J. Bacteriol. 181, 577–584.
Ellison, D. W., and Miller, V. L. (2006). H-NS represses inv transcription in Yersinia enterocolitica through competition with RovA and interaction with YmoA. J. Bacteriol. 188, 5101–5112. doi: 10.1128/JB.00862-05
Falconi, M., Colonna, B., Prosseda, G., Micheli, G., and Gualerzi, C. O. (1998). Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H-NS. EMBO J. 17, 7033–7043. doi: 10.1093/emboj/17.23.7033
Fung, C. P., Chang, F. Y., Lee, S. C., Hu, B. S., Kuo, B. I., Liu, C. Y., et al. (2002). A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 50, 420–424. doi: 10.1136/gut.50.3.420
Gerlach, G. F., Clegg, S., and Allen, B. L. (1989). Identification and characterization of the genes encoding the type 3 and type 1 fimbrial adhesins of Klebsiella pneumoniae. J. Bacteriol. 171, 1262–1270.
Han, S. H. (1995). Review of hepatic abscess from Klebsiella pneumoniae. An association with diabetes mellitus and septic endophthalmitis. West. J. Med. 162, 220–224.
Heroven, A. K., Nagel, G., Tran, H. J., Parr, S., and Dersch, P. (2004). RovA is autoregulated and antagonizes H-NS-mediated silencing of invasin and rovA expression in Yersinia pseudotuberculosis. Mol. Microbiol. 53, 871–888. doi: 10.1111/j.1365-2958.2004.04162.x
Highsmith, A. K., and Jarvis, W. R. (1985). Klebsiella pneumoniae: selected virulence factors that contribute to pathogenicity. Infect. Control 6, 75–77.
Hirsch, E. B., and Tam, V. H. (2010). Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J. Antimicrob. Chemother. 65, 1119–1125. doi: 10.1093/jac/dkq108
Ho, J. Y., Lin, T. L., Li, C. Y., Lee, A., Cheng, A. N., Chen, M. C., et al. (2011). Functions of some capsular polysaccharide biosynthetic genes in Klebsiella pneumoniae NTUH K-2044. PLoS ONE 6:e21664. doi: 10.1371/journal.pone.0021664
Hornick, D. B., Thommandru, J., Smits, W., and Clegg, S. (1995). Adherence properties of an mrkD-negative mutant of Klebsiella pneumoniae. Infect. Immun. 63, 2026–2032.
Huang, Y. J., Liao, H. W., Wu, C. C., and Peng, H. L. (2009). MrkF is a component of type 3 fimbriae in Klebsiella pneumoniae. Res. Microbiol. 160, 71–79. doi: 10.1016/j.resmic.2008.10.009
Jagnow, J., and Clegg, S. (2003). Klebsiella pneumoniae MrkD-mediated biofilm formation on extracellular matrix- and collagen-coated surfaces. Microbiology 149, 2397–2405. doi: 10.1099/mic.0.26434-0
Jahn, C. E., Charkowski, A. O., and Willis, D. K. (2008). Evaluation of isolation methods and RNA integrity for bacterial RNA quantitation. J. Microbiol. Methods 75, 318–324. doi: 10.1016/j.mimet.2008.07.004
Johansson, J., Dagberg, B., Richet, E., and Uhlin, B. E. (1998). H-NS and StpA proteins stimulate expression of the maltose regulon in Escherichia coli. J. Bacteriol. 180, 6117–6125.
Johnson, J. G., and Clegg, S. (2010). Role of MrkJ, a phosphodiesterase, in type 3 fimbrial expression and biofilm formation in Klebsiella pneumoniae. J. Bacteriol. 192, 3944–3950. doi: 10.1128/JB.00304-10
Langstraat, J., Bohse, M., and Clegg, S. (2001). Type 3 fimbrial shaft (MrkA) of Klebsiella pneumoniae, but not the fimbrial adhesin (MrkD), facilitates biofilm formation. Infect. Immun. 69, 5805–5812. doi: 10.1128/IAI.69.9.5805-5812.2001
Lawlor, M. S., Hsu, J., Rick, P. D., and Miller, V. L. (2005). Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol. Microbiol. 58, 1054–1073. doi: 10.1111/j.1365-2958.2005.04918.x
Lehti, T. A., Bauchart, P., Kukkonen, M., Dobrindt, U., Korhonen, T. K., and Westerlund-Wikström, B. (2013). Phylogenetic group-associated differences in regulation of the common colonization factor Mat fimbria in Escherichia coli. Mol. Microbiol. 87, 1200–1222. doi: 10.1111/mmi.12161
Lin, C. T., Chen, Y. C., Jinn, T. R., Wu, C. C., Hong, Y. M., and Wu, W. H. (2013). Role of the cAMP-dependent carbon catabolite repression in capsular polysaccharide biosynthesis in Klebsiella pneumoniae. PLoS ONE 8:e54430. doi: 10.1371/journal.pone.0054430
Lin, C. T., Wu, C. C., Chen, Y. S., Lai, Y. C., Chi, C., Lin, J. C., et al. (2011). Fur regulation of the capsular polysaccharide biosynthesis and iron-acquisition systems in Klebsiella pneumoniae CG43. Microbiology 157, 419–429. doi: 10.1099/mic.0.044065-0
Lin, J. C., Chang, F. Y., Fung, C. P., Xu, J. Z., Cheng, H. P., Wang, J. J., et al. (2004). High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess. Microbes Infect. 6, 1191–1198. doi: 10.1016/j.micinf.2004.06.003
Lin, M. H., Hsu, T. L., Lin, S. Y., Pan, Y. J., Jan, J. T., Wang, J. T., et al. (2009). Phosphoproteomics of Klebsiella pneumoniae NTUH-K2044 reveals a tight link between tyrosine phosphorylation and virulence. Mol. Cell. Proteomics 8, 2613–2623. doi: 10.1074/mcp.M900276-MCP200
Lin, T. L., Yang, F. L., Yang, A. S., Peng, H. P., Li, T. L., Tsai, M. D., et al. (2012). Amino acid substitutions of MagA in Klebsiella pneumoniae affect the biosynthesis of the capsular polysaccharide. PLoS ONE 7:e46783. doi: 10.1371/journal.pone.0046783
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lucchini, S., Rowley, G., Goldberg, M. D., Hurd, D., Harrison, M., and Hinton, J. C. (2006). H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2:e81. doi: 10.1371/journal.ppat.0020081
March, C., Cano, V., Moranta, D., Llobet, E., Pérez-Gutiérrez, C., Tomás, J. M., et al. (2013). Role of bacterial surface structures on the interaction of Klebsiella pneumoniae with phagocytes. PLoS ONE 8:e56847. doi: 10.1371/journal.pone.0056847
Martínez-Santos, V. I., Medrano-López, A., Saldaña, Z., Girón, J. A., and Puente, J. L. (2012). Transcriptional regulation of the ecp operon by EcpR, IHF, and H-NS in attaching and effacing Escherichia coli. J. Bacteriol. 194, 5020–5033. doi: 10.1128/JB.00915-12
Maurelli, A. T., and Sansonetti, P. J. (1988). Identification of a chromosomal gene controlling temperature-regulated expression of Shigella virulence. Proc. Natl. Acad. Sci. U.S.A. 85, 2820–2824. doi: 10.1073/pnas.85.8.2820
Mayer, M. P. (1995). A new set of useful cloning and expression vectors derived from pBlueScript. Gene 163, 41–46. doi: 10.1016/0378-1119(95)00389-N
Moranta, D., Regueiro, V., March, C., Llobet, E., Margareto, J., Larrarte, E., et al. (2010). Klebsiella pneumoniae capsule polysaccharide impedes the expression of beta-defensins by airway epithelial cells. Infect. Immun. 78, 1135–1146. doi: 10.1128/IAI.00940-09
Navarre, W. W., McClelland, M., Libby, S. J., and Fang, F. C. (2007). Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev. 21, 1456–1471. doi: 10.1101/gad.1543107
Navarre, W. W., Porwollik, S., Wang, Y., McClelland, M., Rosen, H., Libby, S. J., et al. (2006). Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313, 236–238. doi: 10.1126/science.1128794
Nordmann, P., Cuzon, G., and Naas, T. (2009). The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9, 228–236. doi: 10.1016/S1473-3099(09)70054-4
Ono, S., Goldberg, M. D., Olsson, T., Esposito, D., Hinton, J. C., and Ladbury, J. E. (2005). H-NS is a part of a thermally controlled mechanism for bacterial gene regulation. Biochem. J. 391, 203–213. doi: 10.1042/BJ20050453
Orrskog, S., Rounioja, S., Spadafina, T., Gallotta, M., Norman, M., Hentrich, K., et al. (2012). Pilus adhesin RrgA interacts with complement receptor 3, thereby affecting macrophage function and systemic pneumococcal disease. MBio 4, e00535–12. doi: 10.1128/mBio.00535-12
Pan, Y. J., Lin, T. L., Chen, C. T., Chen, Y. Y., Hsieh, P. F., Hsu, C. R., et al. (2015). Genetic analysis of capsular polysaccharide synthesis gene clusters in 79 capsular types of Klebsiella spp. Sci. Rep. 5, 15573. doi: 10.1038/srep15573
Pan, Y. J., Lin, T. L., Chen, Y. H., Hsu, C. R., Hsieh, P. F., Wu, M. C., et al. (2013). Capsular types of Klebsiella pneumoniae revisited by wzc sequencing. PLoS ONE 8:e80670. doi: 10.1371/journal.pone.0080670
Pan, Y. J., Lin, T. L., Hsu, C. R., and Wang, J. T. (2011). Use of a Dictyostelium model for isolation of genetic loci associated with phagocytosis and virulence in Klebsiella pneumoniae. Infect. Immun. 79, 997–1006. doi: 10.1128/IAI.00906-10
Park, H. S., Ostberg, Y., Johansson, J., Wagner, E. G., and Uhlin, B. E. (2010). Novel role for a bacterial nucleoid protein in translation of mRNAs with suboptimal ribosome-binding sites. Genes Dev. 24, 1345–1350. doi: 10.1101/gad.576310
Podschun, R., and Ullmann, U. (1998). Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11, 589–603.
Regueiro, V., Campos, M. A., Pons, J., Albertí, S., and Bengoechea, J. A. (2006). The uptake of a Klebsiella pneumoniae capsule polysaccharide mutant triggers an inflammatory response by human airway epithelial cells. Microbiology 152, 555–566. doi: 10.1099/mic.0.28285-0
Rosales-Reyes, R., Skeldon, A. M., Aubert, D. F., and Valvano, M. A. (2012). The Type VI secretion system of Burkholderia cenocepacia affects multiple Rho family GTPases disrupting the actin cytoskeleton and the assembly of NADPH oxidase complex in macrophages. Cell. Microbiol. 14, 255–273. doi: 10.1111/j.1462-5822.2011.01716.x
Saldaña, Z., De La Cruz, M. A., Carrillo-Casas, E. M., Durán, L., Zhang, Y., Hernandez-Castro, R., et al. (2014). Production of the Escherichia coli common pilus by uropathogenic E. coli is associated with adherence to HeLa and HTB-4 cells and invasion of mouse bladder urothelium. PLoS ONE 9:e101200. doi: 10.1371/journal.pone.0101200
Schelenz, S., Bramham, K., and Goldsmith, D. (2007). Septic arthritis due to extended spectrum beta lactamase producing Klebsiella pneumoniae. Joint Bone Spine 74, 275–278. doi: 10.1016/j.jbspin.2006.08.007
Schembri, M. A., Blom, J., Krogfelt, K. A., and Klemm, P. (2005). Capsule and fimbria interaction in Klebsiella pneumoniae. Infect. Immun. 73, 4626–4633. doi: 10.1128/IAI.73.8.4626-4633.2005
Schembri, M. A., Olsen, P. B., and Klemm, P. (1998). Orientation-dependent enhancement by H-NS of the activity of the type 1 fimbrial phase switch promoter in Escherichia coli. Mol. Gen. Genet. 259, 336–344. doi: 10.1007/s004380050820
Schroll, C., Barken, K. B., Krogfelt, K. A., and Struve, C. (2010). Role of type 1 and type 3 fimbriae in Klebsiella pneumoniae biofilm formation. BMC Microbiol. 10:179. doi: 10.1186/1471-2180-10-179
Schurtz, T. A., Hornick, D. B., Korhonen, T. K., and Clegg, S. (1994). The type 3 fimbrial adhesin gene (mrkD) of Klebsiella species is not conserved among all fimbriate strains. Infect. Immun. 62, 4186–4191.
Schwaber, M. J., Lev, B., Israeli, A., Solter, E., Smollan, G., Rubinovitch, B., et al. (2011). Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin. Infect. Dis. 52, 848–855. doi: 10.1093/cid/cir025
Sebghati, T. A., Korhonen, T. K., Hornick, D. B., and Clegg, S. (1998). Characterization of the type 3 fimbrial adhesins of Klebsiella strains. Infect. Immun. 66, 2887–2894.
Sledjeski, D., and Gottesman, S. (1995). A small RNA acts as an antisilencer of the H-NS-silenced rcsA gene of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 92, 2003–2007. doi: 10.1073/pnas.92.6.2003
Stella, S., Falconi, M., Lammi, M., Gualerzi, C. O., and Pon, C. L. (2006). Environmental control of the in vivo oligomerization of nucleoid protein H-NS. J. Mol. Biol. 355, 169–174. doi: 10.1016/j.jmb.2005.10.034
Stella, S., Spurio, R., Falconi, M., Pon, C. L., and Gualerzi, C. O. (2005). Nature and mechanism of the in vivo oligomerization of nucleoid protein H-NS. EMBO J. 24, 2896–2905. doi: 10.1038/sj.emboj.7600754
Struve, C., Bojer, M., and Krogfelt, K. A. (2009). Identification of a conserved chromosomal region encoding Klebsiella pneumoniae type 1 and type 3 fimbriae and assessment of the role of fimbriae in pathogenicity. Infect. Immun. 77, 5016–5024. doi: 10.1128/IAI.00585-09
Suzuki, T., Ueguchi, C., and Mizuno, T. (1996). H-NS regulates OmpF expression through micF antisense RNA in Escherichia coli. J. Bacteriol. 178, 3650–3653.
Tarkkanen, A. M., Virkola, R., Clegg, S., and Korhonen, T. K. (1997). Binding of the type 3 fimbriae of Klebsiella pneumoniae to human endothelial and urinary bladder cells. Infect. Immun. 65, 1546–1549.
Tendeng, C., Badaut, C., Krin, E., Gounon, P., Ngo, S., Danchin, A., et al. (2000). Isolation and characterization of vicH, encoding a new pleiotropic regulator in Vibrio cholerae. J. Bacteriol. 182, 2026–2032. doi: 10.1128/JB.182.7.2026-2032.2000
Tendeng, C., and Bertin, P. N. (2003). H-NS in Gram-negative bacteria: a family of multifaceted proteins. Trends Microbiol. 11, 511–518. doi: 10.1016/j.tim.2003.09.005
Tsai, F. C., Huang, Y. T., Chang, L. Y., and Wang, J. T. (2008). Pyogenic liver abscess as endemic disease, Taiwan. Emerging Infect. Dis. 14, 1592–1600. doi: 10.3201/eid1410.071254
Umanski, T., Rosenshine, I., and Friedberg, D. (2002). Thermoregulated expression of virulence genes in enteropathogenic Escherichia coli. Microbiology 148, 2735–2744. doi: 10.1099/00221287-148-9-2735
Vargas García, C. E., Petrova, M., Claes, I. J., De Boeck, I., Verhoeven, T. L., Dilissen, E., et al. (2015). Piliation of Lactobacillus rhamnosus GG promotes adhesion, phagocytosis, and cytokine modulation in macrophages. Appl. Environ. Microbiol. 81, 2050–2062. doi: 10.1128/AEM.03949-14
Wang, H., Wilksch, J. J., Strugnell, R. A., and Gee, M. L. (2015). Role of capsular polysaccharides in biofilm formation: an AFM nanomechanics study. ACS Appl. Mater. Interfaces 7, 13007–13013. doi: 10.1021/acsami.5b03041
Wang, M., Shakhatreh, M. A., James, D., Liang, S., Nishiyama, S., Yoshimura, F., et al. (2007). Fimbrial proteins of porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J. Immunol. 179, 2349–2358. doi: 10.4049/jimmunol.179.4.2349
Wehland, M., and Bernhard, F. (2000). The RcsAB box. Characterization of a new operator essential for the regulation of exopolysaccharide biosynthesis in enteric bacteria. J. Biol. Chem. 275, 7013–7020. doi: 10.1074/jbc.275.10.7013
Wilksch, J. J., Yang, J., Clements, A., Gabbe, J. L., Short, K. R., Cao, H., et al. (2011). MrkH, a novel c-di-GMP-dependent transcriptional activator, controls Klebsiella pneumoniae biofilm formation by regulating type 3 fimbriae expression. PLoS Pathog. 7:e1002204. doi: 10.1371/journal.ppat.1002204
Williams, P., Lambert, P. A., Brown, M. R., and Jones, R. J. (1983). The role of the O and K antigens in determining the resistance of Klebsiella aerogenes to serum killing and phagocytosis. J. Gen. Microbiol. 129, 2181–2191. doi: 10.1099/00221287-129-7-2181
Wu, M. C., Lin, T. L., Hsieh, P. F., Yang, H. C., and Wang, J. T. (2011). Isolation of genes involved in biofilm formation of a Klebsiella pneumoniae strain causing pyogenic liver abscess. PLoS ONE 6:e23500. doi: 10.1371/journal.pone.0023500
Keywords: H-NS, K. pneumoniae, T3P, capsule, adherence, phagocytosis, virulence
Citation: Ares MA, Fernández-Vázquez JL, Rosales-Reyes R, Jarillo-Quijada MD, von Bargen K, Torres J, González-y-Merchand JA, Alcántar-Curiel MD and De la Cruz MA (2016) H-NS Nucleoid Protein Controls Virulence Features of Klebsiella pneumoniae by Regulating the Expression of Type 3 Pili and the Capsule Polysaccharide. Front. Cell. Infect. Microbiol. 6:13. doi: 10.3389/fcimb.2016.00013
Received: 24 November 2015; Accepted: 22 January 2016;
Published: 09 February 2016.
Edited by:
Matthew S. Francis, Umeå University, SwedenReviewed by:
Antonio Juárez, Universidad de Barcelona, SpainYichyi Lai, Chung Shan Medical University, Taiwan
Timo A. Lehti, University of Helsinki, Finland
Copyright © 2016 Ares, Fernández-Vázquez, Rosales-Reyes, Jarillo-Quijada, von Bargen, Torres, González-y-Merchand, Alcántar-Curiel and De la Cruz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: María D. Alcántar-Curiel, alcantar@unam.mx;
Miguel A. De la Cruz, miguel_angel_81@live.com
 Miguel A. Ares
Miguel A. Ares José L. Fernández-Vázquez
José L. Fernández-Vázquez Roberto Rosales-Reyes
Roberto Rosales-Reyes Ma. Dolores Jarillo-Quijada3
Ma. Dolores Jarillo-Quijada3  Kristine von Bargen
Kristine von Bargen Jorge A. González-y-Merchand
Jorge A. González-y-Merchand María D. Alcántar-Curiel
María D. Alcántar-Curiel Miguel A. De la Cruz
Miguel A. De la Cruz