YopN and TyeA Hydrophobic Contacts Required for Regulating Ysc-Yop Type III Secretion Activity by Yersinia pseudotuberculosis
- 1Department of Molecular Biology, Umeå University, Umeå, Sweden
- 2Umeå Centre for Microbial Research, Umeå University, Umeå, Sweden
- 3Department of Molecular Biology, Uppsala BioCenter, Swedish University of Agricultural Sciences, Uppsala, Sweden
- 4Joint Biotechnology Laboratory, Department of Chemistry, University of Turku, Turku, Finland
- 5Laboratory for Molecular Infection Medicine Sweden, Umeå University, Umeå, Sweden
Yersinia bacteria target Yop effector toxins to the interior of host immune cells by the Ysc-Yop type III secretion system. A YopN-TyeA heterodimer is central to controlling Ysc-Yop targeting activity. A + 1 frameshift event in the 3-prime end of yopN can also produce a singular secreted YopN-TyeA polypeptide that retains some regulatory function even though the C-terminal coding sequence of this YopN differs greatly from wild type. Thus, this YopN C-terminal segment was analyzed for its role in type III secretion control. Bacteria producing YopN truncated after residue 278, or with altered sequence between residues 279 and 287, had lost type III secretion control and function. In contrast, YopN variants with manipulated sequence beyond residue 287 maintained full control and function. Scrutiny of the YopN-TyeA complex structure revealed that residue W279 functioned as a likely hydrophobic contact site with TyeA. Indeed, a YopNW279G mutant lost all ability to bind TyeA. The TyeA residue F8 was also critical for reciprocal YopN binding. Thus, we conclude that specific hydrophobic contacts between opposing YopN and TyeA termini establishes a complex needed for regulating Ysc-Yop activity.
Introduction
Human pathogenic Yersinia are represented by the species Yersinia pestis, the causative agent of bubonic, septicemic, and pneumonic plague (Zhou et al., 2006), as well as Yersinia enterocolitica and Yersinia pseudotuberculosis that are responsible for mild self-limiting gastrointestinal infections that are rarely systemic (Naktin and Beavis, 1999). A common virulence strategy among these bacteria is the plasmid-borne Ysc-Yop type III secretion system (T3SS; Portnoy et al., 1984; Cornelis et al., 1998). This confers to Yersinia a tropism for immune cell rich lymphatic tissue where they resist phagocytosis to preferentially remain in an extracellular replicative niche (Fällman and Gustavsson, 2005). Numerous other Gram-negative bacteria also employ T3SSs to establish parasitic or mutualistic interactions with eukaryotic hosts (Pallen et al., 2005b; Troisfontaines and Cornelis, 2005). Most T3SSs consist of 20–25 proteins that assemble into a hollow protein transport channel traversing the bacterial envelope and protruding out from the bacterial surface to connect with target eukaryotic cells. Through this portal cytoplasmic anti-host effectors can be injected direct to the host cell interior in a one-step process, or surface-located proteins can be delivered into the eukaryotic cell via a two-step process (Edgren et al., 2012).
Assembly is a coordinated process involving the build-up of different sub-parts that eventually connect to form one coherent structure (Kosarewicz et al., 2012). Assembly may either start in the inner membrane and build from the inside-out (Schraidt et al., 2010; Wagner et al., 2010), or it may begin with the simultaneous formation of structures in both the inner and outer membranes (Diepold et al., 2011). When completed, a universal specificity switch mechanism involving auto-processing of the inner membrane embedded YscU family of homologous proteins dictates the secretion of needle components followed by the distal needle tip proteins and the hydrophobic translocator proteins that dock with the host cell to form a translocon pore in the plasma membrane (Frost et al., 2012; Hughes, 2012).
Less defined are mechanisms that delay effector protein secretion until the translocon pore has assembled. Almost all secreted substrates require a dedicated T3S chaperone to prevent premature protein interactions in the cytoplasm and also to ensure their efficient secretion (Francis, 2010). In some cases, the secretion of hydrophobic translocators allows their free cognate T3S chaperone to act as a cofactor to induce subsequent transcription of effector genes (Darwin and Miller, 2001; Mavris et al., 2002; Pilonieta and Munson, 2008). A number of studies also propose mechanisms for enhancing the secretion efficiency of translocator proteins over effector proteins. This can involve recognition of their customized chaperones by a cytoplasmic sorting platform that comprises a complex of the SpaO (FliN/HrcQ/Spa33/YscQ), OrgA (HrpD/MxiK/YscK), and OrgB (FliH/HrpE/MxiN/YscL) protein families (Lara-Tejero et al., 2011). It could also involve free translocator T3S chaperone interacting with the FliJ family of proteins at the cytoplasmic face of the inner membrane to improve their ability to reload with their translocator cargo and expedite secretion (Evans and Hughes, 2009). In addition, specific sequences within the translocator proteins may have evolved into distinctive secretion signals that are preferentially recognized by the T3SS to prioritize their secretion (Munera et al., 2010; Amer et al., 2011; Tomalka et al., 2012). In other cases, this recognition might occur via direct interaction with members of the InvE family of proteins (Kubori and Galán, 2002; Kim et al., 2013). Some members of this protein family also bind effector substrates to delay their secretion (O'Connell et al., 2004; Deng et al., 2005; Wang et al., 2008) or even to the system ATPase at the base of the T3SS channel to physically block effector secretion (Botteaux et al., 2009; Martinez-Argudo and Blocker, 2011; Cherradi et al., 2013).
In the Ysc-Yop T3SS of Yersinia, YopN, and TyeA possess homology to the N- and C-terminus of InvE-like proteins, respectively (Pallen et al., 2005a). Consistent with this homology, a complex of YopN and TyeA, in cooperation with the cognate YopN secretion pilot chaperone composed of a SycN and YscB heterodimer, control substrate secretion by plugging the secretion channel (Forsberg et al., 1991; Day and Plano, 1998; Jackson et al., 1998; Iriarte and Cornelis, 1999; Cheng and Schneewind, 2000; Cheng et al., 2001; Ferracci et al., 2005; Schubot et al., 2005; Joseph and Plano, 2013). The significance of this secretion control function is reflected in the deregulated secretion profiles exhibited by bacterial strains harboring full length deletions of the yopN and/or tyeA alleles (Forsberg et al., 1991; Day and Plano, 1998; Iriarte et al., 1998; Jackson et al., 1998; Cheng et al., 2001; Lee et al., 2001; Sundberg and Forsberg, 2003; Ferracci et al., 2004, 2005; Amer et al., 2013). Until recently it was not known how the YopN-TyeA complex tethers to the T3S apparatus to plug the export channel. Now it has been revealed that Pcr1, the TyeA homolog in Pseudomonas aeruginosa, complexes with PcrG (LcrG in Yersinia) and then co-assembles with the integral inner membrane protein PcrD (YscV) to block access of substrates to the secretion channel (Lee et al., 2014).
Curiously, YopN and TyeA can be synthesized as a singular YopN-TyeA polypeptide (Ferracci et al., 2004; Amer et al., 2013). Probably this occurs via transcriptional strand slippage to introduce a +1 frameshift after codon 278 of yopN that contributes to YopN-TyeA hybrid production, although this is not yet experimentally verified (Figure 1; Ferracci et al., 2004; Amer et al., 2013). In all three Yersinia species known to be pathogenic to humans, the yopN DNA sequence where the frameshift is believed to occur contains stretches of T's that may contribute to strand slippage. Despite this, some strains of Y. enterocolitica do not produce a natural hybrid of YopN and TyeA, most likely because of a defined single nucleotide difference that would place a TAA termination codon upstream of tyeA following a + 1 frameshift event (Ferracci et al., 2004). Hence, on the basis of these anomalies it is unclear whether the YopN-TyeA hybrid has evolved a role in Yersinia T3SS function. Mutants of Y. pseudotuberculosis designed to produce only the YopN-TyeA hybrid alone maintained in vitro low Ca2+-dependent control of substrate T3S, but were unable to control fully the polarized translocation of effectors into the cytosol of eukaryotic cells, and this impinged on their ability to survive in vivo infections of mice (Amer et al., 2013). The non-functional hybrids contained a C-terminal YopN sequence beyond residue 278 that barely resembled native YopN. In this study, scrutiny of this C-terminal region revealed a small segment necessary for full YopN function, within which was the W279 residue that specifically established hydrophobic contacts with the N-terminus of TyeA to maintain Ysc-Yop regulatory control.
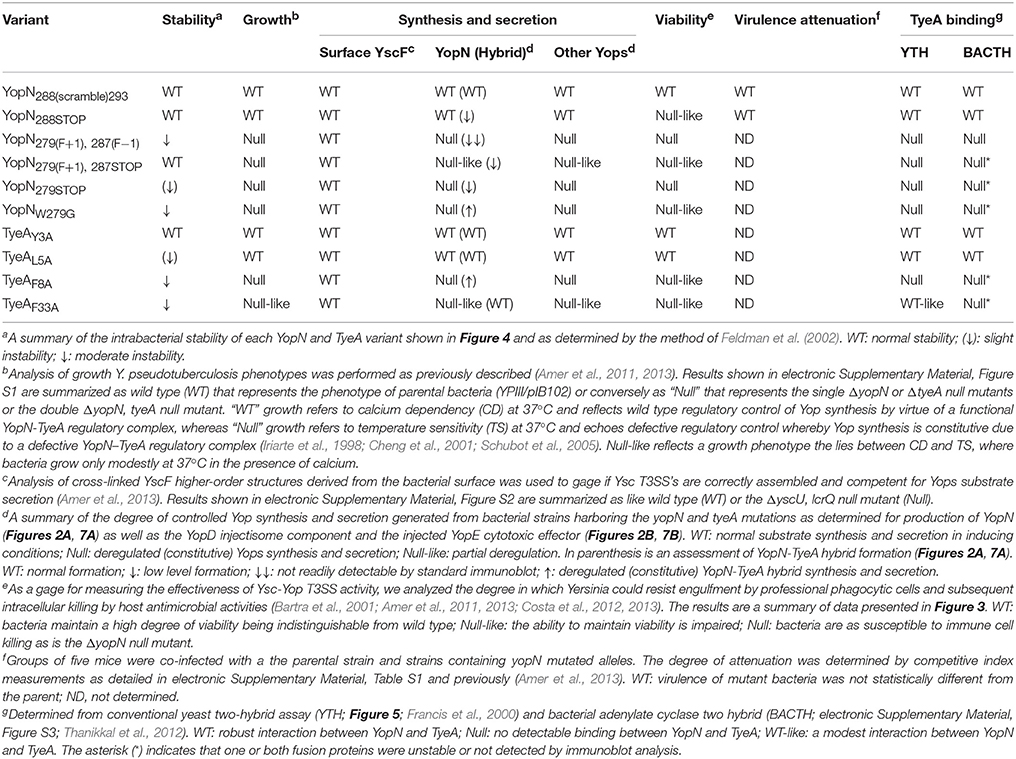
Figure 1. A comparison of the nucleotide and amino acid sequence changes in the key in cis yopN mutations used in this study. Shown is nucleotide (lower case font) and amino acid (upper case font; single letter code) sequence encompassing codon positions 277–293 of YopN and the overlapping 1–7 codons of TyeA. Derived from the native sequence (Parent), three different polypeptides can be generated—YopNnative, TyeA native, and a YopN-TyeA hybrid fusion product resulting from an unconfirmed +1 frameshift mutation after codon 279 (Ferracci et al., 2004; Amer et al., 2013). Shading in light gray indicates the YopNnative amino acid sequence. Amino acids shaded in light blue are YopN sequences that differ from the native protein due to a natural or engineered alteration to the codon sequence. Introduced site-directed nucleotide substitutions are highlighted by an overlying filled-in circle. Open arrowheads above the nucleotide sequence specifically locate positions of nucleotide deletions that result in a +1 frameshift, and filled-in arrowheads identify nucleotide insertions that serve as compensatory −1 frameshifts. No mutation altered the coding sequence of overlapping tyeA as shown by routine retention of the first 6–7 TyeA residues in green (TyeAnative); the start codon of which is highlighted in bold italic font. However, bacteria producing Mutant 2 (YopN288STOP) and Mutant 3 [YopN279(F+1), 287(F−1)] have a displaced tyeA initiation codon relative to a putative Shine-Dalgarno sequence (“agaggg” in bold purple font) by n + 2 [TyeAnative(n+2)] and n + 1 [TyeAnative(n+1)], respectively. Also note that in these bacteria and in bacteria producing Mutant 4 [YopN279(F+1), 287STOP], tyeA coding sequence assumes a different reading from the native sequence. Native or introduced yopN termination codons are indicated by an asterisk (red shade). Two additional mutations were genetically engineered and are designated Mutant 1 (YopN288(scramble)293) and Mutant 5 (YopN279STOP).
Materials and Methods
Bacterial Strains and Growth Conditions
Bacterial strains used in this study are listed in electronic Supplementary Material, Table S2. Bacteria were routinely cultivated in Luria Bertani (LB) agar or broth at either 26°C (Y. pseudotuberculosis) or 37°C (E. coli) with aeration. Where required, appropriate antibiotics were added at the final concentrations of carbenicillin (Cb; 100 μg per ml), kanamycin (Km; 50 μg per ml) and chloramphenicol (Cm; 25 μg per ml).
PCR Amplification and Sequence Analysis
Amplified DNA fragments were obtained by PCR using the various oligonucleotide combinations listed in electronic Supplementary Material (Table S3), which were earlier synthesized by Sigma-Aldrich Co (Dorset, England). All amplified DNA fragments where quality controlled by sequence analysis (Eurofins MWG Operon AG, Ebersberg, Germany) of clones generated using the InsTAclone PCR cloning kit (Thermo Fisher Scientific, Inc.).
Construction of yopN and tyeA Mutations
Various site-directed and deletion mutations in the yopN and tyeA alleles were first generated by the classical two-step overlap PCR procedure. For analysis of mutated alleles in trans, PCR amplified and sequenced DNA fragments were cloned directly into appropriate expression vectors. To generate in cis mutations of yopN or tyeA, sequence-confirmed DNA fragments were subsequently cloned into the SalI-XbaI digested suicide mutagenesis vector, pDM4 (a gift from Debra Milton; electronic Supplementary Material, Table S2), and using E. coli S17-1λpir as the donor in conjugal matings, were then transferred into parental Y. pseudotuberculosis (YPIII/pIB102). Allelic exchange of the virulence plasmid-encoded wild type yopN or tyeA copy with individual yopN or tyeA mutations was selected for using conventional sacB-mediated sensitivity to 5% sucrose. Mutants were confirmed by a combination of diagnostic PCR and sequence analysis.
Protein Stability
To measure stability of accumulated cytoplasmic YopN or TyeA exposed to endogenous proteases, de novo protein synthesis was inhibited by the addition of 50 μg/ml chloramphenicol prior to sample collection as described previously (Feldman et al., 2002).
Type III Secretion Substrate Synthesis and Secretion
Analysis of T3SS by Y. pseudotuberculosis was performed according to standard protocol (Amer et al., 2011) after growth at 37°C in Brain heart infusion (BHI) broth. Media containing Ca2+ ions was the non-inducing condition (BHI supplemented with 2.5 mM CaCl2), while media devoid of Ca2+ ions was the inducing condition (BHI supplemented with 20 mM MgCl2 and 5 mM Ethylene glycol-bis-(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid). Total protein associated with whole bacterial culture was assessed by sampling direct from the bacterial suspension. Sampling of the cleared supernatant provided an assessment of the secreted protein levels. All protein fractions were separated by SDS-PAGE and subjected to immunoblotting using the semi-dry transfer technique onto PDVF membranes. Detection of Yersinia substrates used rabbit polyclonal antisera raised against the secreted YopN, YopD, and YopE (a gift from Hans Wolf-Watz) or non-secreted TyeA (a gift from Gregory Plano), an anti-rabbit antibody conjugated to horseradish peroxidase, and chemiluminescent detection with the Pierce ECL 2 Western Blotting Substrate.
Assessment of T3S Activity in the Presence of Eukaryotic Cells
To indirectly assess the efficiency of the Ysc-Yop T3SS to translocate effectors into eukaryotic cells we measured the viability of Yersinia in the presence of murine macrophage-like J774 cells (Bartra et al., 2001; Amer et al., 2011, 2013; Costa et al., 2012, 2013). This assay capitalizes on the anti-phagocytic properties of the Ysc-Yop T3SS. Bacteria lacking a fully functional T3SS are therefore more efficiently phagocytosed and these intracellular bacteria are susceptible to the antimicrobial killing effects of J774 cells. This assay tests the total recovery of bacteria associated with host cells, which includes both surface attached and intracellular bacteria. Hence any reduction in bacterial viability as determined by CFU counts reflects the amount of bacteria that were susceptible to immune cell killing following phagocytosis.
Plasmid Construction, Transformation, and Yeast Two-Hybrid Analysis
To facilitate YopN and TyeA interaction studies in yeast, wild type and mutated yopN alleles were cloned into the EcoRI/BamHI restricted GAL4 DNA-binding domain plasmid pGBKT7 (Clontech Laboratories, Palo Alto, CA, USA), while wild type and mutated tyeA alleles were cloned into the EcoRI/BamHI restricted the GAL4 activation domain plasmid pGADT7 (Clontech Laboratories). Transformation of the Saccharomyces cerevisiae reporter strain AH109 and analysis of protein-protein interactions was performed as described in detail earlier (Francis et al., 2000). Verification of protein stability by isolation and analysis of yeast protein extracts has also been described (Francis et al., 2000).
Cysteine Cross-Linking
In vivo disulphide cross-linking was performed as essentially described previously (Lee et al., 2006; Gueguen et al., 2011), but with some slight modifications. Briefly, Yersinia bacteria containing engineered YopN and TyeA with strategically placed cysteine substitutions were grown in inducing condition (BHI supplemented with 20 mM MgCl2 and 5 mM EDTA). Cells were harvested by centrifugation and washed with 10 ml of 20 mM sodium phosphate (NaP) buffer, pH 6.8 [20.29 mM NaH2PO4.H2O (monobasic), 19.57 mM Na2HPO4 (dibasic)]. After washing, the cells were resuspended in 1.6 ml of NaP and aliquoted into three samples of 300 μl each. For a control, cells were incubated only with buffer. For the oxidized sample, cells were treated with 0.3 mM dichloro(1,10-phenanthroline) copper(II; Cu-oP; Sigma-Aldrich) for 20 min at room temperature. The reaction was subsequently quenched by addition of 2.5 mM N-ethyl-maleimide (NEM; Sigma-Aldrich) for 15 min at room temperature to quench the reaction. To the reduced sample was added 0.3 mM Cu-oP and 2.5 mM NEM simultaneously, centrifuged and resuspended in SDS-PAGE sample buffer containing 10 mM DTT as reducing agent. After centrifugation of the control and the oxidized samples, they were resuspended in SDS-PAGE sample buffer without the DTT reducing agent.
Structure Modeling and Analysis
The model of the YopN-TyeA fusion protein was constructed based on the crystal structure of the YopN-TyeA complex (RCSB PDB accession code 1XL3; Schubot et al., 2005) using program O (Jones et al., 1991). The connecting loop was designed based on search of the loop library, keeping high restrains for stereochemistry. The side chains of residues at the C-terminus that are altered due to the +1 frame-shift were modeled using the most frequently found rotamer conformations. The interactive surfaces were analyzed using the AREAIMOL program from the CCP4 crystallography suite (CCP4, 1994).
Statistics
An unpaired t-test with Welch's correction performed by means of GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com was used to analyse the differences in data sets. Differences with a probability value of P < 0.05 were considered significant.
Ethics Statement
Infection studies were performed in strict accordance with the Swedish Bioethical Guidelines for care and use of laboratory animals. The protocol was approved by the Umeå Committee on the Ethics of Animal Experiments (Permit Number: A-60-10).
Results
Site-Directed Mutagenesis of the YopN C-Terminus
Genetically engineered YopN-TyeA hybrids were compromised for Ysc-Yop T3SS activity in the presence of host cells and in the mouse infection model (Amer et al., 2013). As these were constructed via an introduced +1 frameshift mutation that caused altered coding potential in yopN after codon 278, it suggested that the extreme YopN C-terminus might be needed for proper T3S activity in Y. pseudotuberculosis (Amer et al., 2013). To investigate this, we generated five site-directed mutations localized within the 3-prime end of yopN (Table 1). To avoid any copy number effects, mutated versions of the yopN gene were used to replace the wild type allele on the virulence plasmid in Yersinia.
One set of mutants targeted the six codon overlapping region between the YopN C-terminus and the TyeA N-terminus (Figure 1). The first mutation scrambled all possible nucleotides in the codon wobble position to specifically alter the C-terminal codon potential of YopN only, thereby generating a YopN288(scramble)293 variant (Mutant 1). The second mutation introduced the “TAG” stop codon after yopN codon 287, which gave rise to bacteria producing YopN288STOP that lacked the extreme C-terminal residues 288–293 (Mutant 2).
A second set of mutants was focused on the region of YopN incorporating residues 279–287 (Figure 1). The first of these, YopN279(F+1), 287(F−1), contained the same +1 frameshift deletion after codon 278 that was followed by a compensatory insertion of an “A” nucleotide to restore the reading frame after codon 287 (Mutant 3). The second of these, YopN279(F+1), 287STOP, was constructed by a +1 frameshift in which a “T” nucleotide was deleted immediately after codon 278 followed by the insertion of a stop codon “TGA” in place of codon 287 (Mutant 4). The third mutant of these, YopN279STOP, was generated via the introduction of the “TAG” stop codon after residue 278 resulting in YopN lacking the C-terminal residues 279–293 (Mutant 5).
Critically, all these allelic variants left the integrity of the partially overlapping tyeA coding sequence intact. However, mutant 2 and mutant 3 altered the position of the putative Shine-Dalgarno sequence (“agaggg”) relative to the tyeA start codon from the customary 8 nucleotides to 10 nucleotides (e.g., n + 2) and 9 nucleotides (e.g., n + 1), respectively (Figure 1). We then performed a functional analysis of the YopN C-terminus using both in vitro and in vivo phenotypic assays. A summary of the YopN mutant phenotypes is provided in Table 1.
Null Phenotypes Caused by Mutations that Disrupt the Region of YopN Encompassing Residues 279–287
Mutants 3–5 that respectively produced the YopN279(F+1), 287(F−1), YopN279(F+1), 287STOP, and YopN279STOP variants, exhibited essentially null phenotypes with respect to in vitro and in vivo T3SS activity. We first assayed the growth phenotype of these strains, in terms of temperature-sensitivity and calcium-dependence. Typically wild type strains are unable to grow without the addition of Ca2+, while yopN and tyeA null mutants are temperature-sensitive, able to grow at 26°C but not at 37°C even in the presence of Ca2+ (electronic Supplementary Material, Figure S1; Forsberg et al., 1991; Lee et al., 1998; Cheng and Schneewind, 2000; Ferracci et al., 2005; Amer et al., 2013). Similar to these previous reports of defective YopN mutants, our three yopN mutant strains were severely growth restricted at elevated temperature—a growth phenotype known as temperature sensitive (electronic Supplementary Material, Figure S1, Mutants 3–5).
Temperature sensitive Yersinia are usually deregulated for Yop synthesis, causing constitutive protein production regardless of Ca2+ levels. For this yopN mutant set, we investigated the impact of temperature sensitivity on Yop synthesis and secretion in two ways. First, using a procedure involving chemical cross-linking and YscF immunoblots we determined the amount of the outermost YscF needle appendage assembled at the distal extremity of T3SS structures spanning the bacterial envelope of the various yopN mutant strains (electronic Supplementary Material, Figure S2A; Amer et al., 2013). This revealed that all three strains assembled YscF at the bacterial surface, at levels comparable to full length yopN null mutants, and these levels far exceeded the amounts observed for parental bacteria (electronic Supplementary Material, Figure S2A, Mutants 3–5). Second, we used a combination of fractionation and immunoblotting to measure the amount of total Yops production (in raw culture media that contains both bacteria associated Yops and freely secreted Yops) and the amount of free Yops secreted into the cleared culture supernatants of the various mutant strains grown in in vitro laboratory media (Figure 2). This demonstrated that the YopN279(F+1), 287(F–1), YopN279(F+1), 287STOP and YopN279STOP variants could no longer maintain Ca2+-dependent control of Yops synthesis and secretion in vitro (Figure 2, Mutants 3–5). The extent of Yops deregulation was most severe for bacteria producing the YopN279(F+1), 287(F–1) and YopN279STOP variants, which mirrored the degree of deregulation caused by the complete removal of the yopN allele or the tyeA allele (Figure 2; Forsberg et al., 1991; Lee et al., 1998; Cheng and Schneewind, 2000; Ferracci et al., 2005; Amer et al., 2013). The deregulation of Yops synthesis and secretion in these strains is corroborated by the corresponding elevated levels of surface localized YscF (see Figure S2A).
Figure 2. Yop synthesis and secretion by in vitro grown Yersinia. Bacteria were grown in BHI medium either with (+) or without (−) Ca2+. Collected samples consisted of a mix of proteins contained within intact bacteria and associated with the outer bacterial surface that were retained in the bacterial pellet (Synthesis) or Yop proteins secreted free into the extracellular medium obtained from the cleared culture supernatants (Secretion). These were fractionated on a long 12% SDS-PAGE, wet-blotted onto PDVF membrane and then analyzed by immunoblot using polyclonal rabbit anti-YopN antiserum (A) or polyclonal rabbit anti-YopD and anti-YopE antiserum (B). The single asterisk (*) highlights the singular YopN (~32 kDa) polypeptide, while the double asterisk (**) reveals the naturally produced and secreted ~42 kDa YopN-TyeA hybrid. The arrows (←) indicate a non-specific protein band recognized by the anti-YopN antiserum and the anti-YopD antiserum. The band appearing just above the nonspecific band in the ΔtyeA strain likely represents a frameshifting event that causes full-length YopN to be fused with the TyeAΔ19−59 deletion remnant resulting in a hybrid product that has a predicted molecular weight of ~38 kDa. Strains: Parent (YopNnative), YPIII/pIB102; ΔyscU, lcrQ double mutant, YPIII/pIB75-26; ΔyopN null mutant, YPIII/pIB82; ΔtyeA null mutant, YPIII/pIB801a; ΔyopN, tyeA double mutant, YPIII/pIB8201a; Mutant 1–YopN288(scramble)293, YPIII/pIB8213; Mutant 2–YopN288STOP, YPIII/pIB8212; Mutant 3–YopN279(F+1), 287(F−1), YPIII/pIB8208; Mutant 4–YopN279(F+1), 287STOP, YPIII/pIB8207; Mutant 5–YopN279STOP, YPIII/pIB8209. The theoretical molecular masses predicted from amino acid sequence are given in parentheses.
Quite probably, Yops secretion into laboratory media is an in vitro artifact. To compensate for this, we also assessed the ability of the T3SS to permit the extracellular survival of bacteria in the presence of professional phagocyte monolayers (Figure 3; Bartra et al., 2001; Amer et al., 2011, 2013; Costa et al., 2012, 2013). Hence, deregulation of Yops synthesis and secretion was manifested in an ineffective bacterial defense against killing by immune cells in vivo. In particular, the bacterial mutant producing the YopN279STOP form was as susceptible to immune cell killing as the full length yopN null mutant and the tyeA null mutant at both 2 and 6 h time points (Figures 3A,B, Mutant 5). Additionally at the 6 h time point, bacteria producing YopN279(F+1), 287(F−1) and YopN279(F+1), 287STOP were also more susceptible than parental bacteria to immune cell killing, but to a lesser degree than was observed for the full length null mutants (Figure 3B, Mutants 3 and 4). We also considered to examine the effect that Yops deregulation in this set of three mutants has on virulence attenuation in a mouse model of infection. However, studying a ΔyopN null mutant had earlier revealed that a temperature sensitive growth defect caused severe attenuation during competitive infections of mice; we have previously measured a competitive index (CI) of 0.00007 for this strain, which is >11000 fold less virulent than parental bacteria that displayed a CI of 0.83 (electronic Supplementary Material, Table S1; Amer et al., 2013). Consequently, we opted not to perform infection studies with these additional temperature sensitive strains harboring yopN mutated alleles.
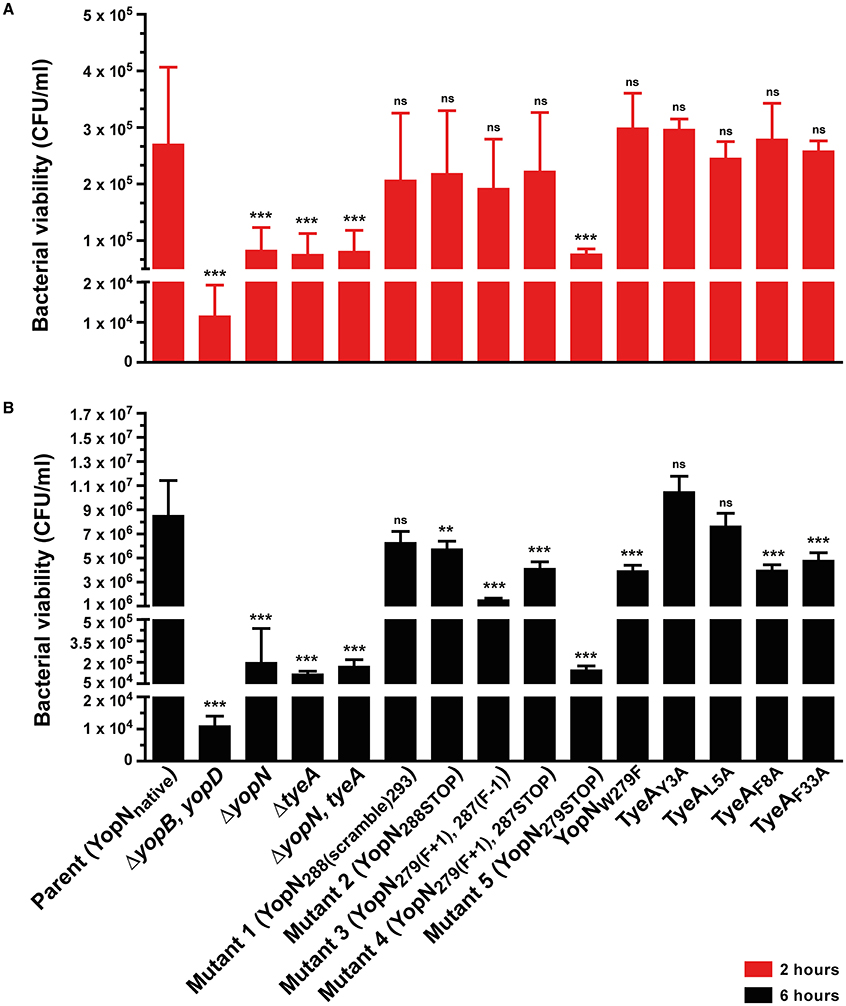
Figure 3. Yersinia susceptibility to killing by macrophages. Y. pseudotuberculosis strains were used to infect murine macrophage-like J774-1 cells. Bacterial cells with a compromised T3SS were more rapidly phagocytosed and killed by these immune cells. Bacterial survival as measured by CFU/ml was determined at 2 h (A) and 6 h (B) post-infection. The results are expressed as a mean of at least six independent assays ± the standard deviation (Unpaired t-test with Welch's correction; n = 12–22 cells; α = 0.05; ns, not significant, P > 0.05; **P ≤ 0.01; ***P ≤ 0.001). Of all the site-directed point mutants examined, bacteria producing YopN288(scramble)293, TyeAY3A and TyeAL5A still show comparable viability to parental bacteria after 6 h, whereas Mutant 5 (YopN279STOP) is particularly compromised to the extent of null mutants in yopN or tyeA.. Strains: Parent (YopNnative), YPIII/pIB102; ΔyopB, yopD double mutant, YPIII/pIB619; ΔyopN null mutant, YPIII/pIB82; ΔtyeA null mutant, YPIII/pIB801a; ΔyopN, tyeA double mutant, YPIII/pIB8201a; Mutant 1–YopN288(scramble)293, YPIII/pIB8213; Mutant 2–YopN288STOP, YPIII/pIB8212; Mutant 3–YopN279(F+1), 287(F−1), YPIII/pIB8208; Mutant 4–YopN279(F+1), 287STOP, YPIII/pIB8207; Mutant 5–YopN279STOP, YPIII/pIB8209; YopNW279G, YPIII/pIB8223; TyeAY3A, YPIII/pIB8221; TyeAL5A, YPIII/pIB8222; TyeAF8A, YPIII/pIB8220; TyeAF33A, YPIII/pIB8219.
Critically, targeting the region encoding resides 279–287 by site-directed mutagenesis did not cause a general increase in their in vivo susceptibility to proteolysis, at least as measured by the fact that both YopN279(F+1), 287STOP and YopN279STOP displayed a stability that was reminiscent of wild type protein (Figure 4, Mutants 4 and 5). However, the variant YopN279(F+1), 287(F−1) did displayed some reduction in stable protein levels when compared to native YopN (Figure 4, Mutant 3). This mutant has therefore a heightened sensitivity to proteolysis.
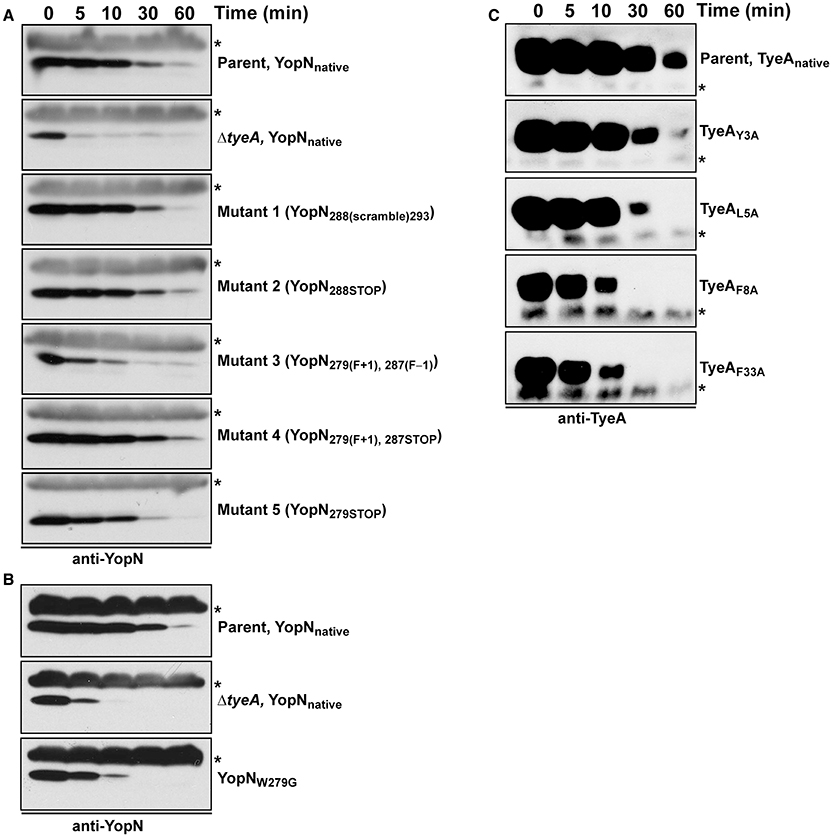
Figure 4. Intrabacterial stability of pre-formed pools of YopN and TyeA variants. Bacteria were first cultured for 1 h in non-inducing (plus 2.5 mM CaCl2) BHI broth at 37°C. The protein synthesis inhibitor chloramphenicol (50 μg/ml) was added at time point 0 min (min). Samples were then collected at this and subsequent time points. Protein levels associated with pelleted bacteria were detected by Western blot using rabbit polyclonal anti-YopN antiserum (A,B) or anti-TyeA antiserum (C). Non-specific cross-reacting bands were used as convenient loading controls and are indicated by an asterisk (*). Parent (YopNnative and TyeAnative), YPIII/pIB102; ΔtyeA null mutant (YopNnative), YPIII/pIB801a; Mutant 1–YopN288(scramble)293, YPIII/pIB8213; Mutant 2–YopN288STOP, YPIII/pIB8212; Mutant 3–YopN279(F+1), 287(F−1), YPIII/pIB8208; Mutant 4–YopN279(F+1), 287STOP, YPIII/pIB8207; Mutant 5–YopN279STOP, YPIII/pIB8209; YopNW279G, YPIII/pIB8223; TyeAY3A, YPIII/pIB8221; TyeAL5A, YPIII/pIB8222; TyeAF8A, YPIII/pIB8220; TyeAF33A, YPIII/pIB8219.
Disruption of the YopN-TyeA Regulatory Complex
Current thinking suggests that a TyeA anchor aids stable YopN to form a plug in the T3S channel that serves to prevent Yop substrate entry into the secretion channel until appropriate environmental cues such as target cell contact have been sensed and interpreted by Yersinia (Cheng and Schneewind, 2000; Cheng et al., 2001; Ferracci et al., 2005; Joseph and Plano, 2013; Lee et al., 2014). Upon encountering inducing cues the YscF needle may alter conformation, opening the channel to release YopN (Day et al., 2003) that then permits the secretion of other Yop substrates. The TyeA binding site on YopN is thought to encompass the C-terminal residues 248–293 (Iriarte et al., 1998; Cheng et al., 2001), as well as a secondary region involving residues 212–222 (Schubot et al., 2005). Hence, the deregulation of Yop synthesis observed in our strains with mutated yopN alleles could be explained by loss of YopN-TyeA binding.
Consequently, we used the yeast two-hybrid system to investigate YopN-TyeA complex formation. Native yopN and manipulated alleles were translationally fused to the C-terminus of the Gal4 transcriptional activator DNA binding domain (BD) in pGBKT7, whereas the native tyeA allele was fused to the Gal4 activation domain in pGADT7. As indicated by yeast growth on selective media lacking either histidine or adenine, a strong interaction between native YopN and native TyeA corroborated previous studies (Figure 5A; Iriarte et al., 1998; Cheng et al., 2001; Schubot et al., 2005). In contrast, all three variants YopN279(F+1), 287(F−1), YopN279(F+1), 287STOP, and YopN279STOP completely lost an ability to engage with TyeA (Figure 5A, Mutants 3–5). This was similar to the lost TyeA binding by a YopN variant having a deletion of residues 248–272 encoding a coiled-coil domain that serves as an established TyeA anchor point (Figure 5A; Iriarte et al., 1998; Cheng et al., 2001; Schubot et al., 2005). Importantly, disruption of binding was not due to protein instability because these Gal4 BD fusions accumulated to levels in yeast that were comparable to the fusion made with native YopN (Figure 5B, Mutants 3–5). We also noted that even though the N-terminus of TyeA is the region that engages with YopN (Schubot et al., 2005), the AD-TyeA fusion that appends an additional domain at this position did not perturb the interaction. We also verified this interaction using the independent bacterial adenylate cyclase two-hybrid (BACTH) system. In this case, the T18 domain was appended to the YopN N-terminus and the T25 domain appended to the TyeA C-terminus (i.e., leaving a free YopN C-terminus to interact with a free TyeA N-terminus). Critically, the truncated YopNΔ248−272 deletion and all three YopN279(F+1), 287(F−1), YopN279(F+1), 287STOP, and YopN279STOP variants were once again unable to engage with TyeA, while a robust interaction between the two wild type proteins was readily apparent (electronic Supplementary Material, Figures S3A,C). Based on this information, we conclude that in Mutants 3–5 producing the YopN279(F+1), 287(F−1), YopN279(F+1), 287STOP, and YopN279STOP variants respectively, the YopN-TyeA regulatory complex is disrupted and this causes the deregulation of Yops synthesis and secretion, which in turn compromises T3S activity against immune cells.
Figure 5. Interaction analysis of YopN and TyeA fusions used in the Y2H assay. All YopN variants were expressed fused to the GAL4 binding domain plasmid pGBKT7, while all TyeA variants were expressed fused to the GAL4 activation domain plasmid pGADT7 (A). Containing HIS3 and ADE2 as reporter genes, the yeast strain S. cerevisiae AH109 was used as host for the two hybrid assay. Plasmid pairs were maintained in this strain via growth on dropout media lacking tryptophan (Trp) and leucine (Leu). Activation of the HIS3 reporter was measured by yeast growth on this media also lacking histidine (His), whereas independent activation of the ADE2 reporter was measured by yeast growth on equivalent media also lacking adenine (Ade). The extent of growth was recorded after 4 days following the plating of 2-fold serial dilutions of logarithmic phase yeast cultures. Results reflect trends in growth from three independent experiments in which different transformants were tested on each separate occasion. Yeast containing no vector or only YopN or TyeA, routinely failed to grow on the assay medium. To examine for stable expression of each variant in AH109, protein extracts were generated from yeast and separated by SDS-PAGE (B). The YopN and TyeA fusions were identified by immunoblot analysis using anti-YopN and anti-TyeA antibody, respectively. In each case, a protein extract from AH109 harboring the relevant vector alone was included as a negative control. The approximate molecular weight of the BD::YopN variants were predicted to be around 48.0 kDa and the AD::TyeA variants around 22.0 kDa.
A New Hydrophobic Contact that Supports YopN Binding to TyeA
We wanted to explore why these three mutated yopN alleles produced a YopN product incapable of engaging with TyeA. A three dimensional structure of the YopN-TyeA complex has been reported (Schubot et al., 2005). The majority of the intermolecular interface sites concern hydrophobic contacts between the YopN residues W216, Y213, I212, V271, and F278 with the respective partner residues S6, G10, V13, F55, and M51 from TyeA (Schubot et al., 2005; Joseph and Plano, 2007). Thus, the region of YopN encompassing residues 279–287 may represent an extension of the TyeA contact site. To investigate this, we analyzed the available crystal structure of the YopN C-terminus in complex with TyeA and observed two additional C-terminal YopN residues W279 and F292 that seemingly form extensive hydrophobic interactions with TyeA and may thus contribute significantly to the binding energy (Figure 6A). The importance of these two residues were overlooked in the initial report (Schubot et al., 2005). Residue F292 remains unchanged following an engineered frame-shift (Amer et al., 2013) and in the mutants YopN279(F+1), 287STOP and YopN279(F+1), 287(F−1) generated herein (see Table 1), and therefore cannot be responsible for failed TyeA binding. However, in these variants the residue W279 is consistently exchanged for a G. Hence, we predicted that this W279 residue would be critical for YopN binding to TyeA. To test this, two yopN alleles were established that had only the single W279G mutation or a Δ279W mutation in which the entire codon was deleted. In parallel, an additional mutated yopN allele was created were W was exchanged for F (W279F) that possessed similar side chain properties. All three YopN variants were then assessed in parallel Y2H assays (Figure 5A) and BACTH assays (electronic Supplementary Material, Figure S3B) for their ability to bind TyeA. Data from both Y2H and BACTH analysis consistently revealed that YopNW279G and YopNΔ279W had completely lost the ability to engage TyeA, whereas the conservative mutant variant of YopNW279F maintained robust TyeA binding akin to native YopN. Once again, this phenotype was not due to poor or unstable fusion expression in either assay host (Figure 5B and electronic Supplementary Material, Figure S3C). Thus, we have identified the residue W279 as a dominant hydrophobic contact point that contributes to stabilizing YopN-TyeA interactions.
Figure 6. Predicted molecular interactions between the C-terminus of YopN and the N-terminus of TyeA in the YopN-TyeA complex and YopN-TyeA fusion protein. Cartoon diagram of a fragment of the crystal structure of the YopN-TyeA complex (RCSB PDB accession code 1XL3; A). The C-terminal helix of YopN is painted in magenta and TyeA is shown in green. Two C-terminal residues of YopN, significantly contributing to the binding interface, Trp279 and Phe282, are shown as balls-on-sticks with carbon and nitrogen atoms painted in pink and blue, respectively. Each of these two residues contributes about 10% to the total interactive area (1099 Å2), establishing hydrophobic interactions with TyeA. In addition, the nitrogen atom of the side chain of Trp279 forms a hydrogen bond with the main chain carbonyl group of Tyr3 in TyeA. Residues that interact with Trp279 and Phe282 are shown in sticks or balls-on-sticks (Phe8) with carbon, nitrogen, and oxygen atoms painted in yellow, blue, and red, respectively. The hydrogen bond between Trp279 and Tyr3 is shown with a dashed line (length 3.0 Å). Our study demonstrates the pivotal role of Trp279 of YopN and Phe8 of TyeA in the YopN-TyeA binding. The ten-residue C-terminus of YopN is unstructured (indicated by a blue dashed line) and, as we show here, plays no role in the binding. Cartoon diagram of a model of the YopN-TyeA fusion protein as a consequence of a mutated yopN allele containing an engineered in cis +1 frameshift mutation immediately downstream of codon 278 (Amer et al., 2013; B). The model was produced based on the crystal structure of the YopN-TyeA complex using program O. The connecting loop (cyan) was designed based on the search of loop library, keeping high restrains for stereochemistry. The side chains of residues at the C-terminus that are altered due to the +1 frame-shift were modeled using the most frequently found rotamer conformations. Only Cα and Cβ atoms are shown for the connecting loop residues. The interactive residues are shown as in (A). The figure was generated by PYMOL (http://www.pymol.org/).
Identifying TyeA Residues That Reciprocate Contacts with the YopN C-Terminus
The TyeA residues S6, G10, V13, F55, and M51 had previously been identified as contact points for YopN (Joseph and Plano, 2007). Our own analysis of the YopN-TyeA structure showed that the residues Y3, L5, F8 and F33 were also potential hydrophobic contact points on TyeA (Figure 6A). To study the importance of these interactions, all four TyeA residues were mutated to alanine, and then assessed for YopN binding in both Y2H assay (Figure 5A) and BACTH assay (electronic Supplementary Material, Figure S3D). Both assays consistently revealed that TyeA residue F8 was needed for interfacing with YopN. Importantly, at least for the Y2H assay we could confirm that the failure to detect an interaction was not due to poor protein production or unstable protein (Figure 5B), although this was not true for the BACTH assay where detection of these proteins was not possible (electronic Supplementary Material, Figure S3B). On the other hand, Y3, L5, and F33 seemed not to be required, although again we could not confirm production of the F33 fusion in the BACTH assay (electronic Supplementary Material, Figure S3B), but all three were detected in the Y2H assay (Figure 5B).
This interaction data suggests that TyeAF8 makes direct contact with YopNW279 and the resultant hydrophobic contact contributes to stable YopN-TyeA complex formation. However, attempts to verify this using a cysteine crosslinking experiment on protein lysate from Y. pseudotuberculosis design to co-produce the engineered variants YopNW279C and TyeAF8C were inconclusive (data not shown). As a consequence, we examined closely the molecular surface of TyeA using available structural data. This revealed a definitive hydrophobic pocket that housed the F8 residue, and into which clearly projected the W279 side chain of YopN (Figure 7). Hence, TyeAF8 and YopNW279 are in close proximity where they most likely make direct and specific contact. Interestingly, both residues are a part of a large cluster of aromatic side chains that includes Y3, F8, F33, and F44 in TyeA and W279 and F282 in YopN. These residues form nearly optimal T-shaped conformations, suggesting an important contribution of pi stacking interactions in this structure (Figure 6A). Hence, our data suggests that F8 and W279 are particularly important for stabilizing the YopN-TyeA complex by engaging in both hydrophobic and pi stacking interactions.
Figure 7. Hydrophobic interaction between the C-terminus of YopN and TyeA. TyeA is shown by electrostatic surface potential representation with negatively (red) and positively (blue) charged residues (A). The C-terminal chain of YopN (magenta) is shown by ribbon view with Trp279 locating in the TyeA hydrophobic pocket. The insert (B) is a close up view of the TyeA hydrophobic pocket highlighting the close proximity between TyeA(Phe8) and YopN(Trp279).
The YopNW279-TyeAF8 Hydrophobic Contact is Necessary for Controlled T3SS Activity
On the basis of their role in establishing a hydrophobic contact between YopN and TyeA, we would predict that their respective residues W279 and F8 are critical for T3SS activity. To test this we generated in cis mutations in Y. pseudotuberculosis to enable production of YopNW279G and TyeAF8A, respectively. As controls, we also generated a further three isogeneic in cis mutations in Y. pseudotuberculosis to produce the TyeAY3A, TyeAL5A, and TyeAF33A variants, respectively. All five mutants were then compared to parental bacteria in a range of tests for T3SS activity, and the results are summarized in Table 1.
When examined for their ability to assemble YscF-needles at the bacterial surface and to control the production and secretion of components associated with the Ysc-Yop T3SS. It was evident that bacteria producing the TyeAY3A and TyeAL5A variants maintain tight control of both YscF assembly (electronic Supplementary Material, Figure S2B), as well as Yops synthesis and secretion (Figure 8), to an extent that mirrored parental bacteria. Even bacteria producing TyeAF33A displayed a near typical calcium dependent Yops synthesis and secretion profile, although a slight depression was observed during bacterial growth in the presence of calcium (Figure 8), and this corroborates elevated levels of surface-located YscF (electronic Supplementary Material, Figure S2B). Clearly however, surface assembled YscF (electronic Supplementary Material, Figure S2B) as well as Yops synthesis and secretion was deregulated in bacteria producing YopNW279G or TyeAF8A (Figure 8), and this was almost to the level observed for Yersinia bacteria harboring full length deletions of yopN or tyeA. These data were corroborated by the growth phenotype of these strains. The regulatory proficient TyeAY3A- and TyeAL5A-producing bacteria were unable to grow without the addition of Ca2+, and this calcium dependent growth phenotype is shared by parental Yersinia (electronic Supplementary Material, Figure S1). In contrast, the regulatory deficient YopNW279G- and TyeAF8A-producing bacteria were unable to grow at 37°C irrespective of calcium, and this temperature sensitive growth phenotype is shared by Yersinia null mutants lacking yopN and/or tyeA (electronic Supplementary Material, Figure S1). Moreover, TyeAF33A-producing bacteria were modestly impaired in calcium dependent growth (electronic Supplementary Material, Figure S1), and this intermediate calcium dependent-like growth phenotype is consistent with elevated levels of surface located YscF and the slight defect observed in regulatory control of Yops synthesis and secretion.
Figure 8. The YopNW279-TyeAF8 contact is required for controlled Yop synthesis and secretion by in vitro grown Yersinia. Bacteria were grown in BHI medium either with (+) or without (−) Ca2+. Collected samples consisted of a mix of proteins contained within intact bacteria and associated with the outer bacterial surface that were retained in the bacterial pellet (Synthesis) or Yop proteins secreted free into the extracellular medium obtained from the cleared culture supernatants (Secretion). These were fractionated on a long 12% SDS-PAGE, wet-blotted onto PDVF membrane and then analyzed by immunoblot using polyclonal rabbit anti-YopN antiserum (A) or polyclonal rabbit anti-YopD and anti-YopE antiserum (B). The single asterisk (*) highlights the singular YopN (~32 kDa) polypeptide, while the double asterisk (**) reveals the naturally produced and secreted ~42 kDa YopN-TyeA hybrid. Arrows (←) indicate non-specific protein bands recognized by the anti-YopN antiserum and the anti-YopD antiserum. The band appearing just above the nonspecific band in the ΔtyeA strain likely represents a frameshifting event that causes full-length YopN to be fused with the TyeAΔ19−59 deletion remnant resulting in a hybrid product that has a predicted molecular weight of ~38 kDa. Strains: Parent (YopNnative, TyeAnative), YPIII/pIB102; ΔyscU, lcrQ double mutant, YPIII/pIB75-26; ΔyopN null mutant, YPIII/pIB82; ΔtyeA null mutant, YPIII/pIB801a; ΔyopN, tyeA double mutant, YPIII/pIB8201a; YopNW279G, YPIII/pIB8223; TyeAY3A, YPIII/pIB8221; TyeAL5A, YPIII/pIB8222; TyeAF8A, YPIII/pIB8220; TyeAF33A, YPIII/pIB8219. The theoretical molecular masses predicted from amino acid sequence are given in parentheses.
To determine if compromised control of T3SS assembly and activity in vitro was an indicator of impaired in vivo function, we assessed bacterial viability in the presence of immune cells. After 6 h post-infection, it was evident that the mutants with most pronounced deregulation of T3SS activity—i.e., bacteria producing producing YopNW279G or TyeAF8A and to a lesser extent TyeAF33A, were all defective in their ability to resist immune cell killing (Figure 3B). Taken altogether, we suggest that at least residues F8 of TyeA and W279 of YopN promote fully controlled T3SS activity because they make a hydrophobic contact essential for stabilizing a YopN-TyeA interaction. Consistent with this finding is that alteration of these residues impact on the structural integrity of the two proteins as measured by YopNW279G (Figure 4B) and TyeAF8A (Figure 4C) being more prone to proteolytic digestion by endogenous proteases.
C-Terminal YopN Contains Functionally Redundant Sequence
We also examined the six residue coding sequence in the extreme C-terminus of YopN that overlaps by six codons with the N-terminal coding region of downstream tyeA. The generated Mutant 1 and Mutant 2 that produced YopN288(scramble)293 and YopN288STOP respectively, both maintained appropriate control of T3S synthesis and secretion (Figure 2, Mutants 1 and 2) and protected bacteria from immune cell engulfment and intracellular killing by various antimicrobial processes as efficiently as bacteria harboring the native yopN allele, although the latter mutant was slightly impaired (Figure 3, Mutants 1 and 2). As these two mutant strains were also capable of a normal growth profile (electronic Supplementary Material, Figure S1, Mutants 1 and 2), we deliberately tested their ability to maintain T3SS activity during competitive infections of mice. Bacteria producing YopN288STOP had a CI value of 0.33 and bacteria producing YopN288(scramble)293 had a CI value of 0.61 that was statistically not different from the CI value of 0.83 recorded for parental bacteria (electronic Supplementary Material, Table S1, P = 0.2222 and P = 0.5476, respectively). Thus, from these data it is apparent that both Mutant 1 and Mutant 2 producing YopN288(scramble)293 and YopN288STOP respectively, still permit full T3S activity, even when forced to compete with parental bacteria for limiting nutrients and colonization niches, while simultaneously thwarting a directed and varied attack from the host immune response. These conclusions are reinforced by the fact that both the YopN288(scramble)293 and YopN288STOP variants maintained robust binding to TyeA in both the Y2H assay (Figure 5A, Mutants 1 and 2) and the BACTH assay (electronic Supplementary Material, Figure S3B, Mutants 1 and 2).
Disruption of YopN-TyeA Hybrid Formation
Despite the fact that the YopN C-terminus contains functionally redundant sequence, we considered the possibility that these six terminal residues that overlap with N-terminal TyeA sequence might be relevant in the context of YopN and TyeA being synthesized as a singular YopN-TyeA polypeptide in both Y. pestis and Y. pseudotuberculosis (Ferracci et al., 2004; Amer et al., 2013). As homologs of YopN and TyeA are commonly made as a singular polypeptide in other bacteria (Pallen et al., 2005a), it is possible that YopN-TyeA hybrid formation is functionally relevant under certain conditions. The structural consequence of this +1 frameshift has been modeled in Figure 6B. The altered C-terminal YopN sequence can act as a linker that maintains both YopN and TyeA structural integrity in the hybrid fusion that compensates for loosing pivotal hydrophobic contacts necessary for complex formation of the singularly produced polypeptides (e.g., between YopNW279 and TyeAF8). Hence, we inspected YopN-TyeA hybrid formation in our six C-terminal mutated YopN mutants after growth in BHI broth restrictive (plus Ca2+) and permissive (minus Ca2+) for T3S. Bacteria producing YopN288(scramble)293 (Figure 2A) or YopNW279G (Figure 8A) formed a natural chimera with TyeA to similar levels as produced by parental bacteria. However, relative to the single YopN polypeptide the level of hybrid production was severely reduced in bacteria producing the four other YopN mutants (Figure 2A). In fact, hybrid formation with YopN279(F+1), 287(F−1) was undetected (Figure 2A). Thus, it is possible to manipulate YopN amounts produced alone relative to when produced as a YopN-TyeA hybrid fusion, and the latter appears to be influenced by the six codon overlap between the end of YopN and the beginning of TyeA.
Discussion
We have performed a functional characterization of the YopN C-terminus. This revealed a segment encompassing residues 279–287 that performs important functions in the control of T3S activity. Likely this occurs through the positioning of the residue W279 to facilitate hydrophobic intermolecular contact with the F8 residue of TyeA and stabilization of an aromatic cluster at the TyeA-YopN interface. The consequence of these interactions is to contribute to the formation of a functional YopN conformation. On the other hand, YopN has evolved with six terminal residues (288–293) that serve no obvious function. However, we speculate that this strategically situates the end of yopN in overlap with the start of tyeA, which may aid in controlling a programmed +1 frameshifting event that serves to join YopN with TyeA to form a larger chimeric protein and also control the production of singular TyeA.
Mutants 3–5 that altered YopN sequence between residues 279–287 (i.e., generating the YopN279(F+1), 287(F−1), YopN279(F+1), 287STOP, and YopN279STOP variants respectively) resulted in bacteria with dysfunctional T3SSs, as measured by both in vitro and in vivo tests. The variants YopN279(F+1), 287STOP and YopN279STOP did not display any increase in in vivo susceptibility to proteolysis, indicating that their defective phenotypes are caused more likely by a defect in YopN function per se, rather than by disrupting the structural integrity of YopN folding. However, the variant YopN279(F+1), 287(F−1) did displayed some reduction in stable protein levels when compared to native YopN. Hence, the introduced mutations have probably brought about some modest structural change, or even altered the ability to bind target proteins, which in turn has heighten its sensitivity to proteolysis. On this note, it is interesting that in bacteria lacking the YopN anchor, TyeA, native YopN was considerably more unstable then any of our engineered mutants. This cannot be due to low levels of YopN production—perhaps by residual YopN plugging the secretion channel to cause feedback inhibition of Yop synthesis—because this tyeA mutant is quite obviously de-regulated for Yops production and secretion (this study; Amer et al., 2013). Rather, it suggests that TyeA targets YopN, and this interaction stabilizes YopN cytoplasmic pools. This stabilizing effect of TyeA must function conjointly with the T3S SycN-YscB cochaperone, which is a known stabilizer and secretion pilot of YopN (Day and Plano, 1998; Cheng et al., 2001; Day et al., 2003). Thus, TyeA would serve at least two functions in complex with YopN—the first to stabilise YopN and the second to anchor YopN as it plugs the secretion channel. Thus, an inability to bind TyeA renders the YopN279(F+1), 287STOP, YopN279(F+1), 287(F−1), and YopN279STOP variants incapable of plugging the T3S channel, thus surrendering any possibility to impart meticulous environmental control of T3S in Yersinia.
A previous study had identified the YopN residues W216, Y213, I212, V271, and F278 as being critical for engaging with TyeA (Schubot et al., 2005). In one other study, the TyeA residues S6, G10, V13, F55, and M51 were revealed to be important for YopN binding (Joseph and Plano, 2007). Herein, we have combined analyses of available structural data with various protein-protein interaction assays to identify a specific hydrophobic contact between YopNW279 and TyeAF8. So important is this interaction to YopN function that alteration of either residue severely disrupts T3SS activity by Y. pseudotuberculosis. Interestingly, a BLASTP analysis of all known YopN amino acid sequences revealed a prominent foci of sequence diversity in the C-terminus that also incorporates the TyeA binding domain between residues 248 and 272 (data not shown; Iriarte et al., 1998; Cheng et al., 2001; Schubot et al., 2005). Yet a similar analysis of TyeA revealed it to be generally well conserved across all pathogenic Yersinia isolates (data not shown). Hence, we speculate that this YopN C-terminal region may have evolved specific sequence variations as a means to strategically modulate TyeA binding avidity to customize the extent of Ysc-Yop T3S control imparted by the YopN-TyeA complex in the different pathogenic variants of human pathogenic Yersinia. We are currently testing this hypothesis experimentally, with the idea that this type of fine-tuning of T3S control may afford certain Yersinia isolates the potential to facilitate unique niche adaptations.
On the other hand, the extreme terminal six residues of YopN appeared to serve no obvious purpose in the control and/or activity of the Ysc-Yop T3SS of Y. pseudotuberculosis, at least under the in vitro and in vivo experimental conditions tested herein. These data corroborate studies that have appended fusions to the C-terminus of YopN without loss of function (Day et al., 2003; Garcia et al., 2006). Yet this region strategically overlaps with the N-terminus of TyeA, such that upon a +1 frameshifting event can produce a YopN-TyeA hybrid (Ferracci et al., 2004). Engineered mutants of Y. pseudotuberculosis designed to mimic this endogenous +1 frameshift to produce only the YopN-TyeA hybrid have been examined (Amer et al., 2013). These mutants maintained in vitro low Ca2+-dependent control of substrate T3S, although they were unable to control polarized translocation of effectors into the cytosol of eukaryotic cells, which reduced their ability to survive in vivo infections of mice (Amer et al., 2013). Hence, the formation of a YopN-TyeA hybrid in Yersinia can have functional consequences for T3SS activity. This corroborates other studies showing that programmed translational +1 frameshifting is a way to regulate the production or diversity of various protein entities (Farabaugh, 1996; Baranov et al., 2002; Namy et al., 2004; Buchan and Stansfield, 2007; Dinman, 2012). As nucleic acid architecture and environmental factors influence frameshifting events (Schwartz and Curran, 1997; Björk et al., 1999; Kontos et al., 2001; McNulty et al., 2003; Higashi et al., 2006; Hansen et al., 2007), the identification of such factors that modulate YopN-TyeA hybrid formation in Yersinia would have biological relevance.
Our data herein suggests two architectural features that potentially influence hybrid formation. The first is the six codon overlap between the end of YopN and the beginning of TyeA. Even though this region appears functionally redundant, the disparity in hybrid formation in Mutant 1 bacteria producing YopN288(scramble)293 compared to Mutant 2 and Mutant 4 bacteria producing YopN288STOP and YopN279(F+1), 287STOP respectively, suggests that this region obviously has cause to affect YopN and TyeA production as singular entities and as a fused unit. The second feature concerns the position of the tyeA Shine-Dalgarno (SD) sequence relative to the upstream potential +1 frameshifting site (codons 278 and 279 of yopN), the downstream tyeA initiation codon, and the downstream yopN termination codon. Particularly for the YopN288STOP variant, the tyeA initiation codon is displaced relative to a putative Shine-Dalgarno sequence such that a +1 frameshift may no longer give productive translation if the ribosome encounters a premature stop codon. This is relevant given how the SD location relative to other architectural features of the coding sequence does affect +1 frameshifting frequency (Weiss et al., 1988; Chen et al., 1994; Li et al., 2012). Thus, a future goal of ours is to investigate whether the length and position of the tyeA SD sequence relative to the tyeA start and the yopN stop may have evolved to promote YopN-TyeA hybrid formation.
In summary, this study has identified a critical point of contact between YopN and TyeA that is necessary for ensuring the correct functional orientation of YopN. A YopN-TyeA hybrid is also produced possibly via a translational +1 frameshift after codon 278 of yopN (Ferracci et al., 2004; Amer et al., 2013). A YopN-TyeA hybrid produced by Y. pseudotuberculosis is stable, but does not retain full function in vivo (Amer et al., 2013). Structural modeling of this singular hybrid polypeptide indicated an altered conformation compared to the YopN-TyeA heterocomplex. Therefore, we believe that the YopN-TyeA heterocomplex has a defined conformation conferred by specific hydrophobic contacts, and this is critical for full YopN function, the importance of which we have demonstrated here.
Author Contributions
AA, JG, TC, and ÅF carried out the laboratory work. TC and AZ performed the structural modeling. AA, JG, and MF designed the experiments and wrote the manuscript; all authors helped draft the manuscript, and gave their final approval for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was performed within the virtual framework of the Umeå Center for Microbial Research Linnaeus Program and Molecular Infection Medicine Sweden. This work was supported in part by grants from the Swedish Research Council (MF), Foundation for Medical Research at Umeå University (MF) and J C Kempe Memorial Fund (AA, JG, and TC). We express gratitude to Hans Wolf-Watz for the gifts of antisera specific to various YscF, YopD, YopE, and YopN antigens, as well as to Gregory Plano for the gift of anti-TyeA antiserum and to Debra Milton for plasmid pDM4. Monika Francis is also thanked for her constructive comments on some aspects of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fcimb.2016.00066
References
Amer, A. A., Åhlund, M. K., Broms, J. E., Forsberg, Å., and Francis, M. S. (2011). Impact of the N-terminal secretor domain on YopD translocator function in Yersinia pseudotuberculosis type III secretion. J. Bacteriol. 193, 6683–6700. doi: 10.1128/JB.00210-11
Amer, A. A., Costa, T. R., Farag, S. I., Avican, U., Forsberg, Å., and Francis, M. S. (2013). Genetically engineered frameshifted YopN-TyeA chimeras influence type III secretion system function in Yersinia pseudotuberculosis. PLoS ONE 8:e77767. doi: 10.1371/journal.pone.0077767
Baranov, P. V., Gesteland, R. F., and Atkins, J. F. (2002). Recoding: translational bifurcations in gene expression. Gene 286, 187–201. doi: 10.1016/S0378-1119(02)00423-7
Bartra, S., Cherepanov, P., Forsberg, A., and Schesser, K. (2001). The Yersinia YopE and YopH type III effector proteins enhance bacterial proliferation following contact with eukaryotic cells. BMC Microbiol. 1:22. doi: 10.1186/1471-2180-1-22
Björk, G. R., Durand, J. M., Hagervall, T. G., Leipuviene, R., Lundgren, H. K., Nilsson, K., et al. (1999). Transfer RNA modification: influence on translational frameshifting and metabolism. FEBS Lett. 452, 47–51. doi: 10.1016/S0014-5793(99)00528-1
Botteaux, A., Sory, M. P., Biskri, L., Parsot, C., and Allaoui, A. (2009). MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 71, 449–460. doi: 10.1111/j.1365-2958.2008.06537.x
Buchan, J. R., and Stansfield, I. (2007). Halting a cellular production line: responses to ribosomal pausing during translation. Biol. Cell 99, 475–487. doi: 10.1042/BC20070037
CCP4 (1994). The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763.
Chen, H., Bjerknes, M., Kumar, R., and Jay, E. (1994). Determination of the optimal aligned spacing between the Shine-Dalgarno sequence and the translation initiation codon of Escherichia coli mRNAs. Nucleic Acids Res. 22, 4953–4957. doi: 10.1093/nar/22.23.4953
Cheng, L. W., Kay, O., and Schneewind, O. (2001). Regulated secretion of YopN by the type III machinery of Yersinia enterocolitica. J. Bacteriol. 183, 5293–5301. doi: 10.1128/JB.183.18.5293-5301.2001
Cheng, L. W., and Schneewind, O. (2000). Yersinia enterocolitica TyeA, an intracellular regulator of the type III machinery, is required for specific targeting of YopE, YopH, YopM, and YopN into the cytosol of eukaryotic cells. J. Bacteriol. 182, 3183–3190. doi: 10.1128/JB.182.11.3183-3190.2000
Cherradi, Y., Schiavolin, L., Moussa, S., Meghraoui, A., Meksem, A., Biskri, L., et al. (2013). Interplay between predicted inner-rod and gatekeeper in controlling substrate specificity of the type III secretion system. Mol. Microbiol. 87, 1183–1199. doi: 10.1111/mmi.12158
Cornelis, G. R., Boland, A., Boyd, A. P., Geuijen, C., Iriarte, M., Neyt, C., et al. (1998). The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62, 1315–1352.
Costa, T. R., Amer, A. A., Fallman, M., Fahlgren, A., and Francis, M. S. (2012). Coiled-coils in the YopD translocator family: a predicted structure unique to the YopD N-terminus contributes to full virulence of Yersinia pseudotuberculosis. Infect. Genet. Evol. 12, 1729–1742. doi: 10.1016/j.meegid.2012.07.016
Costa, T. R., Amer, A. A., Farag, S. I., Wolf-Watz, H., Fallman, M., Fahlgren, A., et al. (2013). Type III secretion translocon assemblies that attenuate Yersinia virulence. Cell. Microbiol. 15, 1088–1110. doi: 10.1111/cmi.12100
Darwin, K. H., and Miller, V. L. (2001). Type III secretion chaperone-dependent regulation: activation of virulence genes by SicA and InvF in Salmonella typhimurium. EMBO J. 20, 1850–1862. doi: 10.1093/emboj/20.8.1850
Day, J. B., Ferracci, F., and Plano, G. V. (2003). Translocation of YopE and YopN into eukaryotic cells by Yersinia pestis yopN, tyeA, sycN, yscB and lcrG deletion mutants measured using a phosphorylatable peptide tag and phosphospecific antibodies. Mol. Microbiol. 47, 807–823. doi: 10.1046/j.1365-2958.2003.03343.x
Day, J. B., and Plano, G. V. (1998). A complex composed of SycN and YscB functions as a specific chaperone for YopN in Yersinia pestis. Mol. Microbiol. 30, 777–788. doi: 10.1046/j.1365-2958.1998.01110.x
Deng, W., Li, Y., Hardwidge, P. R., Frey, E. A., Pfuetzner, R. A., Lee, S., et al. (2005). Regulation of type III secretion hierarchy of translocators and effectors in attaching and effacing bacterial pathogens. Infect. Immun. 73, 2135–2146. doi: 10.1128/IAI.73.4.2135-2146.2005
Diepold, A., Wiesand, U., and Cornelis, G. R. (2011). The assembly of the export apparatus (YscR,S,T,U,V) of the Yersinia type III secretion apparatus occurs independently of other structural components and involves the formation of an YscV oligomer. Mol. Microbiol. 82, 502–514. doi: 10.1111/j.1365-2958.2011.07830.x
Dinman, J. D. (2012). Mechanisms and implications of programmed translational frameshifting. Wiley Interdiscip. Rev. RNA 3, 661–673. doi: 10.1002/wrna.1126
Edgren, T., Forsberg, A., Rosqvist, R., and Wolf-Watz, H. (2012). Type III secretion in Yersinia: injectisome or not? PLoS Pathog. 8:e1002669. doi: 10.1371/journal.ppat.1002669
Evans, L. D., and Hughes, C. (2009). Selective binding of virulence type III export chaperones by FliJ escort orthologues InvI and YscO. FEMS Microbiol. Lett. 293, 292–297. doi: 10.1111/j.1574-6968.2009.01535.x
Fällman, M., and Gustavsson, A. (2005). Cellular mechanisms of bacterial internalization counteracted by Yersinia. Int. Rev. Cytol. 246, 135–188. doi: 10.1016/S0074-7696(05)46004-0
Farabaugh, P. J. (1996). Programmed translational frameshifting. Annu. Rev. Genet. 30, 507–528. doi: 10.1146/annurev.genet.30.1.507
Feldman, M. F., Muller, S., Wuest, E., and Cornelis, G. R. (2002). SycE allows secretion of YopE-DHFR hybrids by the Yersinia enterocolitica type III Ysc system. Mol. Microbiol. 46, 1183–1197. doi: 10.1046/j.1365-2958.2002.03241.x
Ferracci, F., Day, J. B., Ezelle, H. J., and Plano, G. V. (2004). Expression of a functional secreted YopN-TyeA hybrid protein in Yersinia pestis is the result of a +1 translational frameshift event. J. Bacteriol. 186, 5160–5166. doi: 10.1128/JB.186.15.5160-5166.2004
Ferracci, F., Schubot, F. D., Waugh, D. S., and Plano, G. V. (2005). Selection and characterization of Yersinia pestis YopN mutants that constitutively block Yop secretion. Mol. Microbiol. 57, 970–987. doi: 10.1111/j.1365-2958.2005.04738.x
Forsberg, Å., Viitanen, A. M., Skurnik, M., and Wolf-Watz, H. (1991). The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol. Microbiol. 5, 977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x
Francis, M. S. (2010). “Type III secretion chaperones: a molecular toolkit for all occasions,” in Handbook of Molecular Chaperones: Roles, Structures and Mechanisms, eds P. Durante and L. Colucci (Hauppauge, NY: Nova Science Publishers, Inc.), 79–147.
Francis, M. S., Aili, M., Wiklund, M. L., and Wolf-Watz, H. (2000). A study of the YopD-LcrH interaction from Yersinia pseudotuberculosis reveals a role for hydrophobic residues within the amphipathic domain of YopD. Mol. Microbiol. 38, 85–102. doi: 10.1046/j.1365-2958.2000.02112.x
Frost, S., Ho, O., Login, F. H., Weise, C. F., Wolf-Watz, H., and Wolf-Watz, M. (2012). Autoproteolysis and intramolecular dissociation of Yersinia YscU precedes secretion of its C-terminal polypeptide YscU(CC). PLoS ONE 7:e49349. doi: 10.1371/journal.pone.0049349
Garcia, J. T., Ferracci, F., Jackson, M. W., Joseph, S. S., Pattis, I., Plano, L. R., et al. (2006). Measurement of effector protein injection by type III and type IV secretion systems by using a 13-residue phosphorylatable glycogen synthase kinase tag. Infect. Immun. 74, 5645–5657. doi: 10.1128/IAI.00690-06
Gueguen, E., Flores-Kim, J., and Darwin, A. J. (2011). The Yersinia enterocolitica phage shock proteins B and C can form homodimers and heterodimers in vivo with the possibility of close association between multiple domains. J. Bacteriol. 193, 5747–5758. doi: 10.1128/JB.05080-11
Hansen, T. M., Reihani, S. N., Oddershede, L. B., and Sorensen, M. A. (2007). Correlation between mechanical strength of messenger RNA pseudoknots and ribosomal frameshifting. Proc. Natl. Acad. Sci. U.S.A. 104, 5830–5835. doi: 10.1073/pnas.0608668104
Higashi, K., Kashiwagi, K., Taniguchi, S., Terui, Y., Yamamoto, K., Ishihama, A., et al. (2006). Enhancement of +1 frameshift by polyamines during translation of polypeptide release factor 2 in Escherichia coli. J. Biol. Chem. 281, 9527–9537. doi: 10.1074/jbc.M513752200
Hughes, K. T. (2012). The locus of enterocyte effacement type III secretion specificity switch: the devil's in the data for a common mechanism. J. Bacteriol. 194, 6019–6022. doi: 10.1128/JB.01520-12
Iriarte, M., and Cornelis, G. R. (1999). Identification of SycN, YscX, and YscY, three new elements of the Yersinia yop virulon. J. Bacteriol. 181, 675–680.
Iriarte, M., Sory, M. P., Boland, A., Boyd, A. P., Mills, S. D., Lambermont, I., et al. (1998). TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 17, 1907–1918. doi: 10.1093/emboj/17.7.1907
Jackson, M. W., Day, J. B., and Plano, G. V. (1998). YscB of Yersinia pestis functions as a specific chaperone for YopN. J. Bacteriol. 180, 4912–4921.
Jones, T. A., Zou, J. Y., Cowan, S. W., and Kjeldgaard, M. (1991). Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47(Pt 2), 110–119. doi: 10.1107/s0108767390010224
Joseph, S. S., and Plano, G. V. (2007). Identification of TyeA residues required to interact with YopN and to regulate Yop secretion. Adv. Exp. Med. Biol. 603, 235–245. doi: 10.1007/978-0-387-72124-8_21
Joseph, S. S., and Plano, G. V. (2013). The SycN/YscB chaperone-binding domain of YopN is required for the calcium-dependent regulation of Yop secretion by Yersinia pestis. Front. Cell. Infect. Microbiol. 3:1. doi: 10.3389/fcimb.2013.00001
Kim, J. S., Jang, J. I., Eom, J. S., Oh, C. H., Kim, H. G., Kim, B. H., et al. (2013). Molecular characterization of InvE regulator in the secretion of type III secretion translocases in Salmonella enterica serovar Typhimurium. Microbiology 159, 446–461. doi: 10.1099/mic.0.061689-0
Kontos, H., Napthine, S., and Brierley, I. (2001). Ribosomal pausing at a frameshifter RNA pseudoknot is sensitive to reading phase but shows little correlation with frameshift efficiency. Mol. Cell. Biol. 21, 8657–8670. doi: 10.1128/MCB.21.24.8657-8670.2001
Kosarewicz, A., Königsmaier, L., and Marlovits, T. C. (2012). The blueprint of the type-3 injectisome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 1140–1154. doi: 10.1098/rstb.2011.0205
Kubori, T., and Galán, J. E. (2002). Salmonella type III secretion-associated protein InvE controls translocation of effector proteins into host cells. J. Bacteriol. 184, 4699–4708. doi: 10.1128/JB.184.17.4699-4708.2002
Lara-Tejero, M., Kato, J., Wagner, S., Liu, X., and Galan, J. E. (2011). A sorting platform determines the order of protein secretion in bacterial type III systems. Science 331, 1188–1191. doi: 10.1126/science.1201476
Lee, P. A., Orriss, G. L., Buchanan, G., Greene, N. P., Bond, P. J., Punginelli, C., et al. (2006). Cysteine-scanning mutagenesis and disulfide mapping studies of the conserved domain of the twin-arginine translocase TatB component. J. Biol. Chem. 281, 34072–34085. doi: 10.1074/jbc.M607295200
Lee, P. C., Zmina, S. E., Stopford, C. M., Toska, J., and Rietsch, A. (2014). Control of type III secretion activity and substrate specificity by the cytoplasmic regulator PcrG. Proc. Natl. Acad. Sci. U.S.A. 111, E2027–E2036. doi: 10.1073/pnas.1402658111
Lee, V. T., Anderson, D. M., and Schneewind, O. (1998). Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol. Microbiol. 28, 593–601. doi: 10.1046/j.1365-2958.1998.00822.x
Lee, V. T., Mazmanian, S. K., and Schneewind, O. (2001). A program of Yersinia enterocolitica type III secretion reactions is activated by specific signals. J. Bacteriol. 183, 4970–4978. doi: 10.1128/JB.183.17.4970-4978.2001
Li, G. W., Oh, E., and Weissman, J. S. (2012). The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature 484, 538–541. doi: 10.1038/nature10965
Martinez-Argudo, I., and Blocker, A. J. (2011). The Shigella T3SS needle transmits a signal for MxiC release, which controls secretion of effectors. Mol. Microbiol. 78, 1365–1378. doi: 10.1111/j.1365-2958.2010.07413.x
Mavris, M., Page, A. L., Tournebize, R., Demers, B., Sansonetti, P., and Parsot, C. (2002). Regulation of transcription by the activity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 43, 1543–1553. doi: 10.1046/j.1365-2958.2002.02836.x
McNulty, D. E., Claffee, B. A., Huddleston, M. J., Porter, M. L., Cavnar, K. M., and Kane, J. F. (2003). Mistranslational errors associated with the rare arginine codon CGG in Escherichia coli. Protein Expr. Purif. 27, 365–374. doi: 10.1016/S1046-5928(02)00610-1
Munera, D., Crepin, V. F., Marches, O., and Frankel, G. (2010). N-terminal type III secretion signal of enteropathogenic Escherichia coli translocator proteins. J. Bacteriol. 192, 3534–3539. doi: 10.1128/JB.00046-10
Naktin, J., and Beavis, K. G. (1999). Yersinia enterocolitica and Yersinia pseudotuberculosis. Clin. Lab. Med. 19, 523–536.
Namy, O., Rousset, J. P., Napthine, S., and Brierley, I. (2004). Reprogrammed genetic decoding in cellular gene expression. Mol. Cell 13, 157–168. doi: 10.1016/S1097-2765(04)00031-0
O'Connell, C. B., Creasey, E. A., Knutton, S., Elliott, S., Crowther, L. J., Luo, W., et al. (2004). SepL, a protein required for enteropathogenic Escherichia coli type III translocation, interacts with secretion component SepD. Mol. Microbiol. 52, 1613–1625. doi: 10.1111/j.1365-2958.2004.04101.x
Pallen, M. J., Beatson, S. A., and Bailey, C. M. (2005a). Bioinformatics analysis of the locus for enterocyte effacement provides novel insights into type-III secretion. BMC Microbiol. 5:9. doi: 10.1186/1471-2180-5-9
Pallen, M. J., Beatson, S. A., and Bailey, C. M. (2005b). Bioinformatics, genomics and evolution of non-flagellar type-III secretion systems: a Darwinian perpective. FEMS Microbiol. Rev. 29, 201–229. doi: 10.1016/j.femsre.2005.01.001
Pilonieta, M. C., and Munson, G. P. (2008). The chaperone IpgC copurifies with the virulence regulator MxiE. J. Bacteriol. 190, 2249–2251. doi: 10.1128/JB.01824-07
Portnoy, D. A., Wolf-Watz, H., Bölin, I., Beeder, A. B., and Falkow, S. (1984). Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect. Immun. 43, 108–114.
Schraidt, O., Lefebre, M. D., Brunner, M. J., Schmied, W. H., Schmidt, A., Radics, J., et al. (2010). Topology and organization of the Salmonella typhimurium type III secretion needle complex components. PLoS Pathog. 6:e1000824. doi: 10.1371/journal.ppat.1000824
Schubot, F. D., Jackson, M. W., Penrose, K. J., Cherry, S., Tropea, J. E., Plano, G. V., et al. (2005). Three-dimensional structure of a macromolecular assembly that regulates type III secretion in Yersinia pestis. J. Mol. Biol. 346, 1147–1161. doi: 10.1016/j.jmb.2004.12.036
Schwartz, R., and Curran, J. F. (1997). Analyses of frameshifting at UUU-pyrimidine sites. Nucleic Acids Res. 25, 2005–2011. doi: 10.1093/nar/25.10.2005
Sundberg, L., and Forsberg, A. (2003). TyeA of Yersinia pseudotuberculosis is involved in regulation of Yop expression and is required for polarized translocation of Yop effectors. Cell. Microbiol. 5, 187–202. doi: 10.1046/j.1462-5822.2003.00267.x
Thanikkal, E. J., Mangu, J. C., and Francis, M. S. (2012). Interactions of the CpxA sensor kinase and cognate CpxR response regulator from Yersinia pseudotuberculosis. BMC Res. Notes 5:536. doi: 10.1186/1756-0500-5-536
Tomalka, A. G., Stopford, C. M., Lee, P. C., and Rietsch, A. (2012). A translocator-specific export signal establishes the translocator-effector secretion hierarchy that is important for type III secretion system function. Mol. Microbiol. 86, 1464–1481. doi: 10.1111/mmi.12069
Troisfontaines, P., and Cornelis, G. R. (2005). Type III secretion: more systems than you think. Physiology (Bethesda) 20, 326–339. doi: 10.1152/physiol.00011.2005
Wagner, S., Königsmaier, L., Lara-Tejero, M., Lefebre, M., Marlovits, T. C., and Galan, J. E. (2010). Organization and coordinated assembly of the type III secretion export apparatus. Proc. Natl. Acad. Sci. U.S.A. 107, 17745–17750. doi: 10.1073/pnas.1008053107
Wang, D., Roe, A. J., McAteer, S., Shipston, M. J., and Gally, D. L. (2008). Hierarchal type III secretion of translocators and effectors from Escherichia coli O157:H7 requires the carboxy terminus of SepL that binds to Tir. Mol. Microbiol. 69, 1499–1512. doi: 10.1111/j.1365-2958.2008.06377.x
Weiss, R. B., Dunn, D. M., Dahlberg, A. E., Atkins, J. F., and Gesteland, R. F. (1988). Reading frame switch caused by base-pair formation between the 3' end of 16S rRNA and the mRNA during elongation of protein synthesis in Escherichia coli. EMBO J. 7, 1503–1507.
Keywords: protein-protein interaction, molecular modeling, protein secretion, mutagenesis, bacterial pathogenesis, regulation
Citation: Amer AAA, Gurung JM, Costa TRD, Ruuth K, Zavialov AV, Forsberg Å and Francis MS (2016) YopN and TyeA Hydrophobic Contacts Required for Regulating Ysc-Yop Type III Secretion Activity by Yersinia pseudotuberculosis. Front. Cell. Infect. Microbiol. 6:66. doi: 10.3389/fcimb.2016.00066
Received: 02 March 2016; Accepted: 03 June 2016;
Published: 21 June 2016.
Edited by:
Thomas A. Ficht, Texas A&M University, USAReviewed by:
William D. Picking, University of Kansas, USAGregory Plano, University of Miami Miller School of Medicine, USA
Copyright © 2016 Amer, Gurung, Costa, Ruuth, Zavialov, Forsberg and Francis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew S. Francis, matthew.francis@umu.se
†Present Address: Ayad A. A. Amer, Helmholtz Centre for Infection Research, Braunschweig, Germany; Tiago R. D. Costa, Institute of Structural and Molecular Biology, University College London and Birkbeck, London, UK
‡These authors have contributed equally to this work.
 Ayad A. A. Amer
Ayad A. A. Amer Jyoti M. Gurung
Jyoti M. Gurung Tiago R. D. Costa
Tiago R. D. Costa Kristina Ruuth1,2
Kristina Ruuth1,2  Anton V. Zavialov
Anton V. Zavialov Matthew S. Francis
Matthew S. Francis