Gut Microbiota: A Contributing Factor to Obesity
- 1Special Infectious Agents Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
- 2Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
- 3Experimental Biochemistry Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
- 4Department of Animal and Veterinary Sciences, Faculty of Agricultural and Food Sciences, American University of Beirut, Beirut, Lebanon
- 5Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
- 6Biology Department, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
- 7Clinical Biochemistry Department, College of Medicine, Nutrition Unit-King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
- 8King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia
- 9Department of Medical Laboratory Technology, Faculty of Applied Medical Science, King Abdulaziz University, Jeddah, Saudi Arabia
Obesity, a global epidemic of the modern era, is a risk factor for cardiovascular diseases (CVD) and diabetes. The pervasiveness of obesity and overweight in both developed as well as developing populations is on the rise and placing a huge burden on health and economic resources. Consequently, research to control this emerging epidemic is of utmost importance. Recently, host interactions with their resident gut microbiota (GM) have been reported to be involved in the pathogenesis of many metabolic diseases, including obesity, diabetes, and CVD. Around 1014 microorganisms reside within the lower human intestine and many of these 1014 microorganisms have developed mutualistic or commensal associations with the host and actively involved in many physiological processes of the host. However, dysbiosis (altered gut microbial composition) with other predisposing genetic and environmental factors, may contribute to host metabolic disorders resulting in many ailments. Therefore, delineating the role of GM as a contributing factor to obesity is the main objective of this review. Obesity research, as a field is expanding rapidly due to major advances in nutrigenomics, metabolomics, RNA silencing, epigenetics, and other disciplines that may result in the emergence of new technologies and methods to better interpret causal relationships between microbiota and obesity.
Introduction
Pervasiveness of obesity and the other disorders associated to this, for example, metabolic syndrome, have become a great challenge for health care throughout the world (Kovatcheva-Datchary and Arora, 2013). Obesity is a multifaceted and multi-factorial condition that results from the interaction of one's genotype with environmental exposures.
It was estimated by the World Health Organization that 1.9 billion adult people were overweight, out of which 600 millions were obese in 2014. Futhermore, recent estimates of 2014 indicate that, since 1980, incidence of obesity has more than doubled (WHO, 2015). Globally, obesity is rising as an epidemic and is identified to be an emerging public health threat in the Middle Eastern region (Lobstein et al., 2004). In the United States, obesity and overweight individuals are prone to premature mortality, possibly arising from multiple etiologies (Fat et al., 1998) while in Canada, 57% of adult men and 35% of adult women are overweight or obese (Canning et al., 2004). Recent data reported that around 18.3% of the adult Canadian population could be classified as obese (Twells et al., 2014). Moreover, it was reported in 2007 that about 40% of adults in Tehran were overweight and 23.1% of these individuals were obese (Rezaeian and Salem, 2007). The problem is more severe in most of the European countries. Data collected from European countries claimed that around more than half of the European population were obese or overweight (Eurostat, 2015). Latest national data from the USA as of November 2015, on obesity among adults and youth revealed that around 36% of adults and 17% percent of children and adolescents are obese (Ogden et al., 2014).
Obesity has appeared as an endemic disorder that is prominent in developed countries and is quickly emerging as a major problem in some of the developing countries such as Saudi Arabia.
For the past three decades, Saudi Arabia has experienced considerable economic growth drastically impacting lifestyle of citizens, specifically eating and exercise habits (Darwish et al., 2014) giving rise to major nutritional changes among the Saudis (Musaiger, 2011) leading to a higher prevalence of overweight and obesity to alarming levels (Al-Nuaim et al., 1997). According to the local news agency, around 70% of adult Saudis are obese or overweight (Arab News, 2014).
A study conducted in 2012 indicated that percent body fat among Saudi children and teenagers is rising and thus resulting in an increase in obesity (Al-Hazzaa et al., 2012). The estimates show that Saudi children and teenagers are 26.6 and 10.6% overweight or obese, respectively (El Mouzan et al., 2010). Saudi adolescents between the ages from 15 to 19 years were reported to suffer from sleep deprivation and thus are exposed to high risk of overweight and obesity (Al-Hazzaa et al., 2012).
Recently, the resident microbial communities, known as Gut Microbiota (GM), has been suggested to be a major driving force in the pathogenesis of metabolic disease and obesity, in particular (Kovatcheva-Datchary and Arora, 2013; Khan et al., 2014; Nguyen et al., 2015). Besides genetic, environmental, and the immune system related factors, GM is one of the contributing factor in developing obesity (Giovanni et al., 2010). Thus, scientific interest has been directed toward understanding the contributions of GM in obesity and metabolic disease. The GM exerts their role through several integrated pathways including the host immune system, responses to the environment including diet and their genome in addition to several factors (Ferreira et al., 2011; Khan et al., 2014).
Around 1014 microorganisms colonize various parts of the human body and human body is estimated to be around 90% bacterial cells of all cells (Khan et al., 2014). Generally, they colonize exterior and interior mucosal surfaces of the human body. Microbes colonizes skin, gastrointestinal, genitourinary, and upper respiratory tracts as well as other sites on the human body. They are found in high concentrations in the lower gastrointestinal tract (Frank et al., 2007; Hattori and Taylor, 2009). The GM are primarily bacterial though fungal, protozoan, and archaeal species have been isolated too (Khan et al., 2014). Furthermore, the collective metagenome content is 150-fold larger on a gene basis than that of the human genome. Bacteria constitute 99% of the total GM, in which 90% fall under two main phyla the Firmicutes and Bacteroidetes. However, a small population of bacteria belongs to the phyla Proteobacteria, Actinobacteria, Verrucomicrobia, and Fusobacteria (Khan et al., 2014).
Humans have coevolved and thus developed several symbiotic associations with their GM that accounts for the high concentrations of GM. The GM association with their hosts could be commensal (negligible net effect on host physiology) leading to enhancing the overall ability of the body to be a better fit for survival. It has been reported that GM plays an important role in enhancing the digestion and absorption of otherwise indigestible nutrients as well as energy turnover (Dethlefsen et al., 2006; Round and Mazmanian, 2009; Foster and Neufeld, 2013). GM diversity and composition are profoundly influenced by host diet, lifestyle, and environmental factors (Maslowski and Mackay, 2011; Graf et al., 2015). Advanced analytical platforms such as metagenomics and metabolomics, revealed that GM help host in harvesting energy and lead to increasing adiposity (Dumas et al., 2006; Martin et al., 2008; Turnbaugh et al., 2008; Turnbaugh and Gordon, 2009). Accordingly, a 20% increase in Firmicutes and a corresponding decrease in Bacteroidetes were found to be associated with the increase in energy intake, thus inducing obesity (Rautava et al., 2012). GM population surveys primarily are basedon clinical data, as well as data obtained from gnotobiotic mice and epidemiological studies. This includes studies conducted on various human populations and risk groups such as formula vs. breastfed infants and Cesarean- vs. vaginally delivered infants (Koenig et al., 2011; Maynard et al., 2012). However, emerging research indicates that modern dietary, hygienic, and medicinal practices strongly affect the variations in GM composition. GM composition is involved with a number of chronic diseases and ailments including obesity among populations in the developed countries (Backhed et al., 2004; Turnbaugh et al., 2006), diabetes (Ehehalt et al., 2010), inflammatory and immune disorders (Penders et al., 2007), and cancer (Zhang et al., 2012; Khan et al., 2014). This review examines the contributing role of GM in the development of obesity. Various factors are discussed that contribute to the development of obesity.
Diet Effects on GM Composition
Despite multiple paths and physiological factors, obesity is tightly linked to diet, and lifestyle that could promote several other metabolic disorders if not properly managed (Kovatcheva-Datchary and Arora, 2013; Boursier and Diehl, 2015). It is now a fact that GM is playing important role in harvesting energy efficiently from the diet (Khan et al., 2014; Graf et al., 2015). The GM participates within various biochemical pathways to assist in digestion and metabolism of food. Interestingly, the GM of obese individuals exhibits aberrant carbohydrate and lipids metabolism (Rautava et al., 2012; Caesar et al., 2016). Recent studies showed that the consumption of diet emulsifiers have been associated with changes in the GM composition (Chassaing et al., 2015). Carbohydrates are a vital source of dietary energy. However, humans are not capable of digesting all linkages inherent to oligo- or polysaccharides. Non-digestible carbohydrates include plant-derived fibers such as xylans, cellulose, inulin, and resistant starch. GM degrades these carbohydrates for harvesting energy and providing the host with a variety of metabolites such as short-chain fatty acids (SCFAs) propionate, acetate, and butyrate (Tremaroli and Bäckhed, 2012). These SCFAs affect glucose, cholesterol, and lipid metabolism in different body tissues (den Besten et al., 2013). Polysaccharide fermentation by GM impacts host adiposity through modulating energy derived from dietary substrates (Tremaroli and Bäckhed, 2012).
The type of food intake by the human host influences the GM composition and diversity (Carmody et al., 2015; Gérard, 2016). For instance, the western diet (high fat) results in a reduction of Bacteroidetes and an increase in Firmicutes, especially Mollicutes (DiBaise et al., 2012). The latter is associated with an enrichment genes involved in carbohydrate catabolism (Turnbaugh et al., 2008; Bested et al., 2013). Accordingly, obese individuals are known to have a microbiota rich in Firmicutes and lower in Bacteroidetes as compared to the GM of lean individuals (Scott et al., 2015). Similarly, Prevotellaceae, a hydrogen-producing bacteria, and archaeal species were abundant in obese individuals. It was demonstrated that the transfer of hydrogen between archaeal and bacterial species might enhance energy harvest efficiency within the gut (DiBaise et al., 2012; Doré and Blottière, 2015). Interestingly, high-fat diets modulate the microbiome composition to increase circulatory lipopolysaccharides coinciding with general inflammation (Cani and Van Hul, 2015). General dysbiosis within the gut is associated with a high level of plasma endotoxin and inflammation that eventually promotes metabolic disorder (Zhang et al., 2010, 2013). Zhang et al. (2013) showed that one of the endotoxins producing bacteria (i.e., Enterobacter), when inoculated into germ-free mice resulted in the induction of obesity and insulin resistance (Zhang et al., 2010). Moreover, the host genetics, diet, and different environmental factors influence the etiology of liver disorders. As such, the GM has been recognized as being important in greatly influencing the host metabolism. GM may have a double-edged sword and may exert both beneficial and deleterious extra-intestinal influence to the host.
Furthermore, GM actively participate in bile acid (BA) activation, metabolism, and regulation (Sayin et al., 2013). BA is secreated from liver to digestive system and help in lipid absorption (Inagaki et al., 2005; Kuribayashi et al., 2012). Abnormal BA level has been linked with various metabolic diseases including obesity (Kuribayashi et al., 2012). Gut bacteria, especially Clostridium scindens, Clostridium sordellii, and Bacillus fragilis have a proven role in the biotransformation of BA (Miyata et al., 2009). Any aberration in GM composition can modulate BA level and can accordingly manipulate obesity (Ridlon et al., 2014). However, theantimicrobial activity of conjugated-BAs is suppressed during deconjugation through the bile salt hydrolase enzyme, which is excreated by the GM, especially bacteria from the phyla Firmicutes, Bacteroidetes, and Actinobacteria.
GM Modulate Energy Intake
Obesity is a consequence of a prolonged imbalance between energy intake and expenditure caused by a complexinterplay between genetic susceptibility and nutritional, physiological, social, and environmental factors (Brahe et al., 2016). In addition, it has been estimated that 40% of the gut microbial gene pool is shared among individuals (Qin et al., 2010), and there seems to exist a core microbiome, which is a set of microbial genes shared among the vast majority of healthy individuals that enables conservation of several important functional pathways including those involved in energy metabolism (Brahe et al., 2016). The connection between GM and energy homeostasis and inflammation and its role in the pathogenesis of obesity-related disorders are becoming increasingly recognized.
Mammalian evolution includes innovation of mechanisms to store energy in the form of adipose tissue for when needed and providing physical protection during exercise (Chaput et al., 2010). Maintaining energy homeostasis requires strict regulation of energy intake and the amount consumed. Obesity has increased in developed countries, in part, due to lifestyle changes and the availability of energy high caloric foods. A significant change in GM composition has been reported to be associated with obesity. In addition, germ-free mice do not develop diet-induced obesity. Such a finding indicates that the effect of GM on the host metabolism and obesity induction is achieved by more efficient harvest of energy yield from consumed food and modulating dietary or the host-derived compounds that alter the host's metabolic pathways (Tremaroli and Bäckhed, 2012; Graf et al., 2015).
No significant increase in weight was observed when germ-free mice were fed a high-fat diet, intestinal SCFAs were reduced, and calories were lost through urine and fecal elimination (Brun et al., 2007; DiBaise et al., 2012).
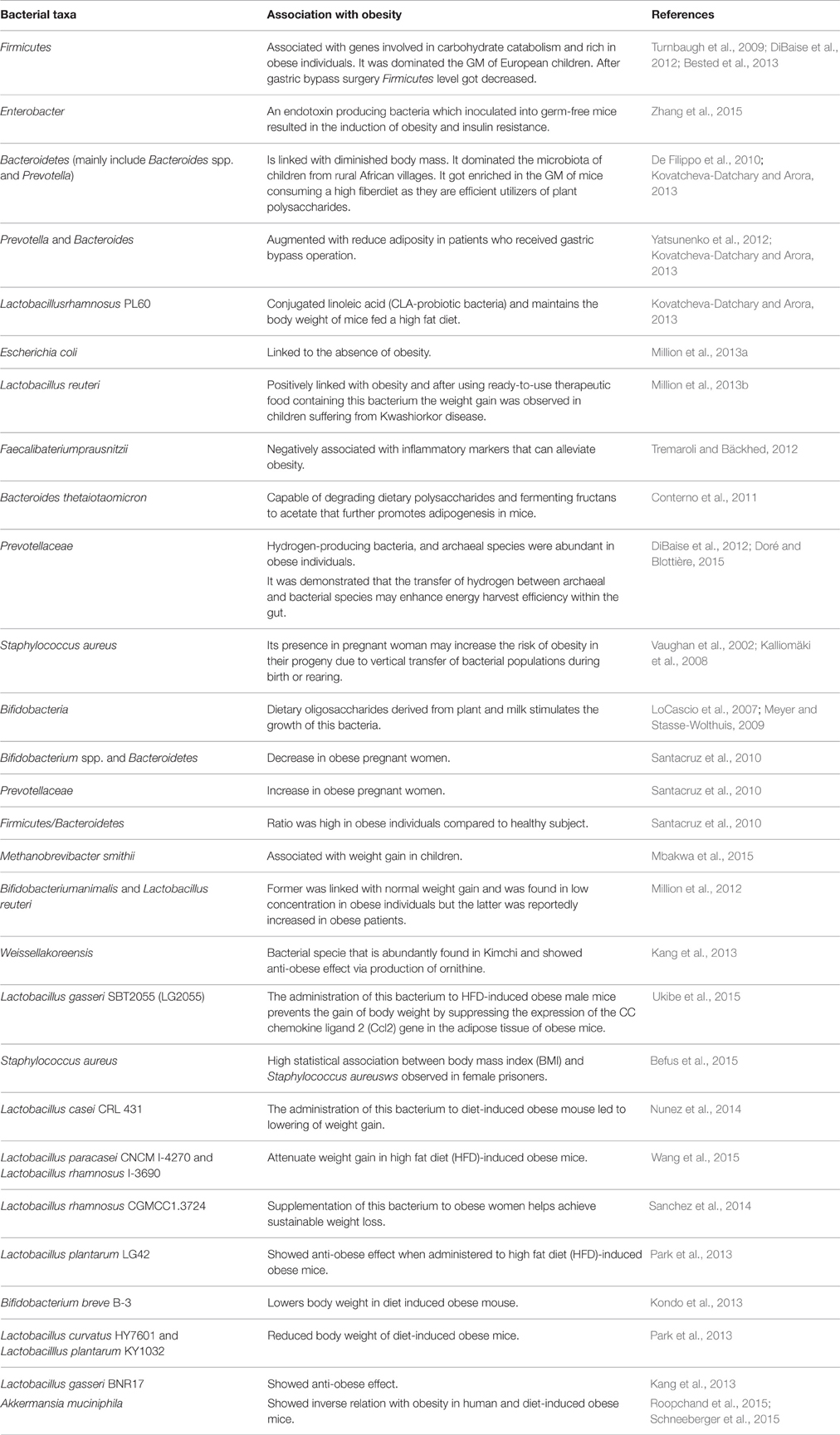
It has been clearly demonstrated in animal models that GM play a role in inducing obesity through a more efficient way for better energy yield from consumed foods and by modulating dietary or the host-derived compounds that alter host metabolic pathway (Tremaroli and Bäckhed, 2012; Table 1). The same results were observed after a patient had a Roux-en-Y gastric bypass operation, where there was an increase in the presence of Prevotella and Bacteroides which correlated in a negative manner with energy intake and adiposity (Kovatcheva-Datchary and Arora, 2013). Accordingly, The increased ratio of Bacteroidetes to Firmicutes was linked with diminished body mass (De Filippo et al., 2010; Kovatcheva-Datchary and Arora, 2013). There are several Firmicutes genera/species typically isolated or observed in the gut. This includes various genera associated with Clostridia class (e.g., Ruminococcus) that may secrete butyrate. In general, the primary Bacteroidetes genera associated with the gut are Bacteroides spp. and Prevotella. The same results were observed when a patient received a gastric bypass operation with Prevotella and Bacteroides were augmented with reducing adiposity (Yatsunenko et al., 2012; Kovatcheva-Datchary and Arora, 2013). There is, however, disagreement in the scientific literature (Schwiertz et al., 2010). The role of GM composition in relation to obesity is not clear. This is because there are different etiological factors that are behind obesity and can be linked to different microbes (Tremaroli and Bäckhed, 2012).
Conjugated linoleic acid (CLA) is a derivative of linoleic acid that is naturally found in dairy products and has been involved in increasing the metabolic rate in mice (West et al., 1998; Kovatcheva-Datchary and Arora, 2013). The CLA-producing probiotic bacteria (Lactobacillus rhamnosus PL60) maintain the body weight of mice fed a high-fat diet. The same diet had resulted in induction of obesity in mice that were not colonized with either L. rhamnosus or Lactobacillus plantarum (Kovatcheva-Datchary and Arora, 2013). Similarly, Escherichia coli were linked to the absence of obesity (Million et al., 2013b). In contrast, there are bacteria that have a positive linkage to obesity, such as Lactobacillus reuteri and were linked to weight gain in children affected with Kwashiorkor using ready-to-use therapeutic food (Million et al., 2013a). Moreover, certain strains like Faecalibaterium prausnitzii which are less abundant in obese and diabetic patients may modulate obesity through different mechanisms which are negatively correlated with inflammatory markers proving that that F. prausnitzii may lead to the modulation of obesity (Tremaroli and Bäckhed, 2012).
GM has the potential to synthesize a vast amount of enzymes like glycoside which hydrolyses the breakdown of complex plant polysaccharides to monosaccharides. This is in addition to the production of ligands made of short-chain fatty acids like acetate, propionate, and butyrate. When these ligands for two G-proteins-coupled receptors Gpr41 and Gpr43, of gut enteroendocrine cells bind, they result in the stimulation of the gut peptide PYY which inhibits gut motility and slows intestinal transit and it allow the better absorption of nutrients and may be used as a treatment for obesity (Batterham et al., 2003).
Obesity can be Regulated by Modulating GM
The hypothesis that obesity could be controlled by guiding the composition and function of GM holds much potential for therapeutic interventions (Burcelin et al., 2015; Wang et al., 2015). For example, Bacteroides thetaiotaomicron is capable of degrading dietary polysaccharides and fermenting fructans to acetate that promotes adipogenesis in mice (Conterno et al., 2011). In addition, the co-colonization of B. thetaiotaomicron with other fermentable strains such as Methanobrevibacter smithii can potentiate the process of adipose tissue build-up and thus potentiates obesity (DiBaise et al., 2012).
In order to control obesity in relation to the GM composition, certain ecological and functional parameters remain to be fully elucidated (Cani and Van Hul, 2015; Brahe et al., 2016). Investigations into the ecological role of specific and individual microbiota populations will likely yield targets for probiotic interventions in communities that are incorrectly assembled (Carding et al., 2015). There are several probiotic strategies that have been developed containing Lactobacillus acidophilus tailored toward reducing the serum cholesterol levels in humans (Parvez et al., 2006; Cani and Van Hul, 2015). Interestingly, in a study to characterize possible determinants for childhood obesity, it was found that the common skin bacterium Staphylococcus aureus in feces was a marker for obesity risk during development (DiBaise et al., 2012). In a similar study of pregnant women, overweight individuals harbored higher concentrations of S. aureus. This may increase the risk of obesity in their progeny due to a vertical transfer of bacterial populations during birth or rearing (Vaughan et al., 2002; Kalliomäki et al., 2008).
Based on animal studies, it was reported that the G-protein-coupled receptor (GPR) deficient mice were abundantly colonized by two commensals bacteria i.e., B. thetaiotaomicron and M. smithii. Those mice were significantly leaner than their wild-type counterpart littermates. This is possibly because Gpr deficient mice had a lower expression of peptide tyrosine tyrosine (PYY), fast intestinal rate, and reduced energy intake from the consumed food. There was an increase in the glucagon-like peptide (GLP)-1 as a response to GM fermentation of prebiotics, which promoted L-cell differentiation in the rat's colon. The treatment of ob/ob mice with prebiotic crbohydrates has resulted in the alteration of the GM composition and an increase in the circulating GLP-1 and GLP-2. The GLP-1 was important in mediating the prebiotic action and prevented its effects on body weight gain, glucose metabolism, and inflammatory pathway activation. Studies showed that GLP-2, a 33-amino acid peptide, may cause the GM to modulate the gut barrier and endotoxinemia and is known for its intestinotrophic properties (Samuel et al., 2008; De Silva and Bloom, 2012).
The presence of Akkermansia mucin I Phila is associated with a more efficient glucose metabolism and leanness in mice. However, this has not yet been proven in humans. The new findings indicate that the higher abundance of A. mucin I Phila results in healthier metabolic status in overweight/obese humans and is associated with improved glucose homeostasis, blood lipids, and body composition after calorie restriction. The current data need to be investigated further to allow for a more conclusive link to ascertain the therapeutic applicability of A. muciniphila in the treatment of insulin resistance. In addition to the possible use of A. muciniphila as a diagnostic or prognostic tool to predict the potential success of dietary interventions (Dao et al., 2016). Obesity is likely to be related to: (a) an increase in Firmicutes phylum, (b) a reduced abundance of Bacteroidetes, (c) a higher level of Actinobacteria phylum, (d) a Lower proportion of Verrucomicrobia, and (e) a reduction in the abundance of Faecalibacterium prausnitzii spec. (Chakraborti, 2015).
Relationship between GM and Obesity
There are several well-established risk factors for obesity including genetics, lifestyle, and dietary habits. However, recently, and due to new advances in nucleic acid sequencing technology, there has been an emerging field to examine GM's role in obesity. Fecal transplantation studies conducted on mice, humans, and human-to-mouse have led to several molecular links between GM composition and obesity (Conterno et al., 2011). It has been reported that a healthy woman (BMI 26) who had contracted a reoccurring Clostridium difficile infection undergone fecal transplant from her overweight yet healthy daughter became obese (BMI 34.5) 36 months after receiving the fecal transplant. The mother failed to lose weight after following a “medically supervised liquid protein diet and exercise program” (Brandt, 2012; Alang and Kelly, 2015).
Inter-individual variation in microbiome composition and methodological tools has led to some difficulties in reconciling large taxonomic surveys of healthy or obese associated microbiota. This includes impactful studies conducted by the Human Microbiome Project and the Metagenomics of the Human Intestinal Tract (MetaHIT). Thus, a scientific consensus on what constitutes a conserved obese enterotype is not fully realized.
In obese patients, a bimodal distribution of microbial gene richness has been noted. Obese individuals are stratified as either having high Gene Count (HGC) or Low Gene Count (LGC). A high prevalence of presumed anti-inflammatory species of F. prausnitzii was noted in 32 HGC individuals with higher production potential of like butyrate as well as other organic acids. A different pattern was seen in LGC individuals who demonstrated a higher relative abundance of potentially proinflamatory Bacteroides spp. and genes associated with oxidative stress response. A significant association of biochemical obesity-associated variables, such as insulin resistance, was observed with gene counts. However, weight and BMI did not show such an association. Such a phenomenon underscores the inadequacy of using BMI as an indicator of “Obesity and its Associated Metabolic Disorders” (OAMD). Later it was shown that in a diet used for the induction of weight-loss significantly resulted in higher gene richness in individuals with LGC associated with improved metabolic status. Although gene richness was not fully restored, the data further support the reported link between GM structure and the long-term dietary habits. Based on that, it was concluded that diet can play a crucial role in the permanent adjustment of the microbiota (Marchesi et al., 2016).
It was published that GM-promoted storage of circulating triglycerides into adipocytes. Such an activity was caused by suppressing the intestinal secretions of an inhibitor of adipose tissue lipoprotein lipase FIAF, which is angiopoietin-like protein. Consistently, in FIAF-deficient knockout (KO) mice, only a 10% increase in total body fat was observed(Giovanni et al., 2010). This is in comparison to a 57% fat gain noted in wild-type littermates. Experiments on germ-free FIAF KO mice fed with a high fat, containing high-carbohydrate levels were not protected from diet-induced obesity. Consequently, the blunted FIAF expression might have led to the accumulation of triglyceride in adipocytes and adipose tissue hypertrophy of conventionalized germ-free mice (Giovanni et al., 2010).
It is well-established that environmental factors including host diet influence the relative representation of certain bacterial phyla in the gut. Beyond population structure, it is clear that functional attributes of a community may drastically influence host metabolism (Martin and Sela, 2013). Nutrition influences the composition of the gut microbiome such as high fiber food that passes to the colon (Khan et al., 2014; Song et al., 2016). For this reason, studies showed more diversified levels of GM in rats reared on high fiber diets (Blaut and Klaus, 2012). Fermentable fibers are utilized by specific microbiota capable of metabolizing the molecular structure of a given substrate. As an example, dietary oligosaccharides including plant and milk-derived stimulate the growth of Bifidobacteria (LoCascio et al., 2007; Meyer and Stasse-Wolthuis, 2009). Mouse studies confirm this, as the GM shifts when standard chow is changed to a semi-synthetic high-fat diet due to a lower percentage of fermentable dietary fibers (Fleissner et al., 2010).
There are several disparities regarding the relative ratio of Firmicutes/Bacteroidetes in obese individuals compared to healthy subjects (Santacruz et al., 2010). Interestingly studies conducted in developed countries, higher ratios of Firmicutes to Bacteroidetes have been linked to obesity. In a comparative study, Bacteroidetes dominated the microbiota of children from rural African villages, while Firmicutes dominated the microbiota of European children (De Filippo et al., 2010). Differential GM composition was attributed to diet as well as ethnicity, sanitation, hygiene, geography, and/or climate (De Filippo et al., 2010). Bacteroidetes dominated the microbiota of children from rural African villages while Firmicutes dominated the microbiota of European children. Differential GM composition was attributed to diet as well as ethnicity, sanitation, hygiene, geography, and/or climate (De Filippo et al., 2010). Other studies did not identify such a correlation and reported that the relative abundance of Firmicutes decreased following gastric bypass surgery (De Filippo et al., 2010). Moreover, obese pregnant women exhibited a decrease in Bifidobacterium sp. and Bacteroidetes with an increase in Prevotellaceae (Santacruz et al., 2010).
There have been attempts to define a “core microbiome” as some have speculated that deviation from a core structure may lead to pathologies such as obesity (Turnbaugh et al., 2009; Kovatcheva-Datchary and Arora, 2013). Although a conserved core microbiome structure is difficult to identify due to high inter-individual variation (Qin et al., 2010), community function may be conserved within this variation. It is noteworthy that GM of obese individuals exhibit less taxonomic richness and potentially diminished metabolic capacity than the microbiota of lean individuals (Turnbaugh et al., 2009; Greenblum et al., 2012; Kovatcheva-Datchary and Arora, 2013).
In studies of obese and lean twins, microbial groups were found correspondingly enriched or depleted in the lean and obese subjects (Turnbaugh et al., 2009). Most of the microbial genes that were linked with obesogenic pathways did not belong to Bacteroidetes but came from either Actinobacteria (75%) or Firmicutes (25%). In a follow-up study conducted on mice, the microbiome composition shifts in just 1 day after switching mice from low fat (rich in fiber) to a high-fat diet with increased adiposity occurring just 2 weeks (Turnbaugh et al., 2009). Bacteroidetes were enriched in the GM in mice consuming a high fiber diet, as they are efficient utilizers of plant polysaccharides (Maslowski and Mackay, 2011). This is in contrast to the Firmicute enrichment and associated obesogenic microbiota in high caloric diets typically encountered in developed countries. Future research should focus on assessing the possibility of altering the Firmicutes to Bacteroidetes ratios as a therapeutic solution to obesity. Such an alteration may be achieved by the use of either antimicrobial agents (Morgun et al., 2015) and/or the use of probiotics in conjunction with changing the selective environment induced by switching to a low-fat/high-fiber diet.
Aspects related to understanding the relationship between GM in relation to Obesity:
There are still lots of pitfalls regarding the understanding of the relationship between diet, lifestyle activities, GM, and obesity. Most of the investigations concentrated on GM in the gastrointestinal tract. However, other important aspects have been overlooked and include the following (Conlon and Bird, 2014):
1. Better understanding of the functions of GM and their physiological relevance to obesity.
2. Identification of the different types of genes of the current GM and the many more newly discovered ones and their function in breaking down of the different types of foods, their role in energy harvest and the resulting by-product(s)in relation to obesity.
3. Focus should also be made on understanding the role of bacteria in the small intestine (ileum and cecum) and the role of diet in the overall health of the small intestine, which may become inflamed because of imbalances in gut microbial populations in addition to delineating the involvement of bacteria in the small intestine enteropathies, leaky gut, and obesity.
4. Understanding the influence of the different types of foods that have the ability to compromise the integrity of the gut mucosa, which is considered of critical importance to the overall health and to obesity. Diets should be tailored toward foods that keep the gut mucosa intact and prevent invading microbes from causing inflammation in the gut mucosa.
5. Delineating the association between GM and immune system; such an understanding will reveal how GM can contribute to obesity or not and such an information can be used to design diets to promote optimal body weight.
6. Understanding the bidirectional communication of data between GM on the satiety center in the brain and their effect on obesity.
7. Effect of dietary manipulation affects GM composition among individuals and their effects on mode swings and food intake and their consequences on obesity.
8. Food by-products effect on GM and in turn on obesity.
9. Understanding the inter-individual variations of GM and their role in obesity.
10. The modulation of the microbial profile based on the understanding the origins and variations to fight obesity.
11. Understanding the genetic predisposition to obesity and how environmental factors and diet can be modified to overcome this problem. This may result in the development of diets with an optimum composition to enhance the growth of certain bacteria that will counteract the effect of the genetic factors of the host that can better resist obesity.
12. The effect of long-term dietary habits on the microbial populations in the GM.
13. Effect of long-term administration of vitamins, amino acids, and micronutrients including phytobiologics on the GM composition and their consequences on obesity.
14. Understand how the main constituents of the diet affect GM composition and their effect on obesity.
15. The effect of food matrix, structure, the particle size, and the interaction of the food matrix with the gastric enzymes found in the gut.
16. The influence of cooking food or consuming it raw and its effects on GM and its relation to obesity.
17. The effects of prebiotics and probiotics on the GM and their relationship to obesity.
The role of GM may contribute to the increased incidence of autoimmune and metabolic diseases in developed countries. An example is Type-1 and type-2 diabetes. Understanding the role that GM plays in these two diseases may result in prevention and intervention strategies to control those two diseases. However, well-controlled prospective human studies are required for the understanding of the role of the GM and its response to environmental factors in order to identify effective preventive strategies targeting specific component of the gut ecosystem (Nobel et al., 2015).
Conclusion
During the last decade or two, there has been an exponential increase due to “omics” technologies in our understanding of the composition and functions of the human GM. The “omics” technologies have made it easier to analyzed on a large large-scale of the genetic and metabolic profile of microbial communities, thus offering the possibility of a new route for therapeutic intervention. Their association is similar to what has been seen in the immune system, which comprises a collection of cells that work in harmony either with the host in order to promote health or in other instances cause disease. Obesity is a major problem worldwide and any possible was should be used to control it. Scientists worldwide are trying to use different and latest technologies to treat obesity. It well-documented that there are different factors that contribute to obesity other than the increased energy harvest from the diet. It is extremely necessary to decipher the underlying association between the adipose tissue and pathophysiology in relation to different metabolic disorders including but not only limited to obesity (DiBaise et al., 2012; Pekkala et al., 2015).
As such, different mechanisms have been proposed to link to the composition. This field is still is in its early stages and require elaborately controlled studies to substantiate the available data that link obesity to GM. Based on the above, it is evident that better understanding of the role of GM in obesity would lead to possible solutions to manage this disorder. The GM compositions are genetically predisposed and are affected by the type of diet consumed and its role in the treatment of obesity should not be overlooked. Future work should be conducted in a very controlled manner to have a reproducible data that will delineate the role of microbiota in obesity across the globe. For this reason, there is an urgent need for an open and enhanced dialogue between experimental scientists and computational scientists to uncover possible reasons behind the conflicting results that are published and to: get novel findings in the field, synthesize diverse information related to GM and obesity and accelerate the future of discovering new therapeutic applications.
Finally, it would be of interest to identify different bacterial phyla that are involved with obesity and treatment of obesity can be achieved via the modification of the microbiota of the obese persons. Such a process may be achieved by transplantation of known microbiota that are not involved in obesity.
Author Contributions
SH and IK contributed to main content of design and drafting of this article. EB and TK helped in critically reviewing the article. SBA, SB, SMA, GA, and EA contributed to literature survey and writing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors express their sincere gratitude and gratefulness for the efforts of Dr. David Sela (Department of Food Science, University of Massachusetts, Amherst, USA) for his contribution in the preparation and revision of the manuscript. This work was funded by grant from King Abdulaziz city for Science and Technology, Saudi Arabia (Grant #AR-34-191).
References
Alang, N., and Kelly, C. R. (2015). Weight gain after fecal Microbiota transplantation. Open Forum Infect. Dis. 2, 1–2. doi: 10.1093/ofid/ofv004
Al-Hazzaa, H. M., Abahussain, N. A., Al-Sobayel, H. I., Qahwaji, D. M., and Musaiger, A. O. (2012). Lifestyle factors associated with overweight and obesity among Saudi adolescents. BMC Public Health 12:354. doi: 10.1186/1471-2458-12-354
Al-Nuaim, A. A., Bamgboye, E. A., al-Rubeaan, K. A., and al-Mazrou, Y. (1997). Overweight and obesity in Saudi Arabian adult population, role of socio-demographic variables. J. Community Health 22, 211–223. doi: 10.1023/A:1025177108996
Arab News (2014). 70% of Saudis are Obese, Says Study. Available Online at: http://www.arabnews.com/news/527031 (Accessed April 1, 2014).
Backhed, F., Ding, H., Wang, T., Hooper, L. V., Koh, G. Y., Nagy, A., et al. (2004). The GM as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U.S.A. 101, 15718–15723. doi: 10.1073/pnas.0407076101
Batterham, R. L., Cohen, M. A., Ellis, S. M., Le Roux, C. W., Withers, D. J., Frost, G. S., et al. (2003). Inhibition of food intake in obese subjects by peptide YY3-36. N. Engl. J. Med. 349, 941–948. doi: 10.1056/NEJMoa030204
Befus, M., Lowy, F. D., Miko, B. A., Mukherjee, D. V., Herzig, C. T. A., and Larson, E. L. (2015). Obesity as a determinant of Staphylococcus aureus colonization among inmates in maximum-security prisons in New York State. Am. J. Epidemiol. 182, 494–502. doi: 10.1093/aje/kwv062
Bested, A. C., Logan, A. C., and Selhub, E. M. (2013). Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part III—convergence toward clinical trials. Gut Pathog. 5:4. doi: 10.1186/1757-4749-5-4
Blaut, M., and Klaus, S. (2012). Intestinal microbiota and obesity. Handb. Exp. Pharmacol. 2012, 251–273. doi: 10.1007/978-3-642-24716-3_11
Boursier, J., and Diehl, A. M. (2015). Implication of GM in nonalcoholic fatty liver disease. PLoS Pathog. 11:e1004559–e1004559. doi: 10.1371/journal.ppat.1004559
Brahe, L. K., Astrup, A., and Larsen, L. H. (2016). Can we prevent obesity-related metabolic diseases by dietary modulation of the GM? Adv. Nutr. 7, 90–101. doi: 10.3945/an.115.010587
Brandt, L. (2012). Faecal microbiota transplant. Gastrointest. Nurs. 10, 6–7. doi: 10.12968/gasn.2012.10.2.6
Brun, P., Castagliuolo, I., Di Leo, V., Buda, A., Pinzani, M., Palù, G., et al. (2007). Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Liver Physiol. 292, G518–G525. doi: 10.1152/ajpgi.00024.2006
Burcelin, R., Courtney, M., and Amar, J. (2015). “GM and metabolic diseases: from pathogenesis to therapeutic perspective,” in Metabonomics and Gut Microbiota in Nutrition and Disease, eds S. Kochhar and F.-P. Martin (London: Springer), 199–234.
Caesar, R., Nygren, H., Orešič, M., and Bäckhed, F. (2016). Interaction between dietary lipids and GM regulates hepatic cholesterol metabolism. J. Lipid Res. 57, 474–481. doi: 10.1194/jlr.M065847
Cani, P. D., and Van Hul, M. (2015). Novel opportunities for next-generation probiotics targeting metabolic syndrome. Curr. Opin. Biotechnol. 32, 21–27. doi: 10.1016/j.copbio.2014.10.006
Canning, P. M., Courage, M. L., and Frizzell, L. M. (2004). Prevalence of overweight and obesity in a provincial population of Canadian preschool children. CMAJ 171, 240–242. doi: 10.1503/cmaj.1040075
Carding, S., Verbeke, K., Vipond, D. T., Corfe, B. M., and Owen, L. (2015). Dysbiosis of the GM in disease. Microb. Ecol. Health Dis. 26. doi: 10.3402/mehd.v26.26191
Carmody, R. N., Gerber, G. K., Luevano, J. M., Gatti, D. M., Somes, L., Svenson, K. L., et al. (2015). Diet dominates host genotype in shaping the murine GM. Cell Host Microbe 17, 72–84. doi: 10.1016/j.chom.2014.11.010
Chakraborti, C. K. (2015). New-found link between microbiota and obesity. World J. Gastrointest Pathophysiol. 6, 110–119. doi: 10.4291/wjgp.v6.i4.110
Chaput, J.-P., Klingenberg, L., Rosenkilde, M., Gilbert, J.-A., Tremblay, A., and Sjödin, A. (2010). Physical activity plays an important role in body weight regulation. J. Obes. 2011:360257. doi: 10.1155/2011/360257
Chassaing, B., Koren, O., Goodrich, J. K., Poole, A. C., Srinivasan, S., Ley, R. E., et al. (2015). Dietary emulsifiers impact the mouse GM promoting colitis and metabolic syndrome. Nature 519, 92–96. doi: 10.1038/nature14232
Conlon, M. A., and Bird, A. R. (2014). The impact of diet and lifestyle on gm and human health. Nutrients 7, 17–44. doi: 10.3390/nu7010017
Conterno, L., Fava, F., Viola, R., and Tuohy, K. M. (2011). Obesity and the GM: does up-regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 6, 241–260. doi: 10.1007/s12263-011-0230-1
Dao, M. C., Everard, A., Aron-Wisnewsky, J., Sokolovska, N., Prifti, E., Verger, E. O., et al. (2016). Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65, 426–436. doi: 10.1136/gutjnl-2014-308778
Darwish, M. A., Al-Saif, G., Albahrani, S., and Sabra, A. A. (2014). Lifestyle and dietary behaviors among saudi preschool children attending primary health care centers, Eastern Saudi Arabia. Int. J. Family Med. 2014:e432732. doi: 10.1155/2014/432732
De Filippo, C., Cavalieri, D., Di Paola, M., Ramazzotti, M., Poullet, J. B., Massart, S., et al. (2010). Impact of diet in shaping GM revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U.S.A. 107, 14691–14696. doi: 10.1073/pnas.1005963107
De Silva, A., and Bloom, S. R. (2012). Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver 6, 10–20. doi: 10.5009/gnl.2012.6.1.10
den Besten, G., van Eunen, K., Groen, A. K., Venema, K., Reijngoud, D. J., and Bakker, B. M. (2013). The role of short-chain fatty acids in the interplay between diet, GM, and host energy metabolism. J. Lipid Res. 54, 2325–2340. doi: 10.1194/jlr.R036012
Dethlefsen, L., Eckburg, P. B., Bik, E. M., and Relman, D. A. (2006). Assembly of the human intestinal microbiota. Trends Ecol. Evol. 21, 517–523. doi: 10.1016/j.tree.2006.06.013
DiBaise, J. K., Frank, D. N., and Mathur, R. (2012). Impact of the gut microbiota on the development of obesity: current concepts. Am. J. Gastroenterol. (Suppl. 1), 1, 22–27. doi: 10.1038/ajgsup.2012.5. Available Online at: http://www.nature.com/ajgsup/journal/v1/n1/full/ajgsup20125a.html
Doré, J., and Blottière, H. (2015). The influence of diet on the GM and its consequences for health. Curr. Opin. Biotechnol. 32, 195–199. doi: 10.1016/j.copbio.2015.01.002
Dumas, M.-E., Barton, R. H., Toye, A., Cloarec, O., Blancher, C., Rothwell, A., et al. (2006). Metabolic profiling reveals a contribution of GM to fatty liver phenotype in insulin-resistant mice. Proc. Natl. Acad. Sci. U.S.A. 103, 12511–12516. doi: 10.1073/pnas.0601056103
Ehehalt, S., Dietz, K., Willasch, A. M., and Neu, A. (2010). Epidemiological perspectives on type 1 diabetes in childhood and adolescence in germany: 20 years of the Baden-wurttemberg Diabetes Incidence Registry (DIARY). Diabetes Care 33, 338–340. doi: 10.2337/dc09-1503
El Mouzan, M. I., Foster, P. J., Al Herbish, A. S., Al Salloum, A. A., Al Omer, A. A., Qurachi, M. M., et al. (2010). Prevalence of overweight and obesity in saudi children and adolescents. Ann. Saudi Med. 30, 203–208. doi: 10.4103/0256-4947.62833
Eurostat (2015). Overweight and Obesity–BMI Statistics. Last modified on 25 November 2015. Available online at: http://ec.europa.eu/eurostat/statistics-explained/index.php/Overweight_and_obesity_-_BMI_statistics
Fat, B., Class, O., and Obesity, E. (1998). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. WMJ 97, 20–21, 24–25, 27–37. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/9813653
Ferreira, R. B. R., Gill, N., Willing, B. P., Antunes, L. C. M., Russell, S. L., Croxen, M. A., et al. (2011). The intestinal microbiota plays a role in salmonella-induced colitis independent of pathogen colonization. PLoS ONE 6:e20338. doi: 10.1371/journal.pone.0020338
Fleissner, C. K., Huebel, N., Abd El-Bary, M. M., Loh, G., Klaus, S., and Blaut, M. (2010). Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br. J. Nutr. 104, 919–929. doi: 10.1017/S0007114510001303
Foster, J. A., and Neufeld, K.-A. M. (2013). Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 36, 305–312. doi: 10.1016/j.tins.2013.01.005
Frank, D. N., St Amand, A. L., Feldman, R. A., Boedeker, E. C., Harpaz, N., and Pace, N. R. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. U.S.A. 104, 13780–13785. doi: 10.1073/pnas.0706625104
Giovanni, M., Gambino, R., and Cassader, M. (2010). Obesity, diabetes and GM. Diabetes Care 33, 2277–2284. doi: 10.2337/dc10-0556
Graf, D., Di Cagno, R., Fåk, F., Flint, H. J., Nyman, M., Saarela, M., et al. (2015). Contribution of diet to the composition of the human GM. Microb. Ecol. Health Dis. 26, 26164–26175. doi: 10.3402/mehd.v26.26164
Greenblum, S., Turnbaugh, P. J., and Borenstein, E. (2012). Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc. Natl. Acad. Sci. U.S.A. 109, 594–599. doi: 10.1073/pnas.1116053109
Hattori, M., and Taylor, T. D. (2009). The human intestinal microbiome: a new frontier of human biology. DNA Res. 16, 1–12. doi: 10.1093/dnares/dsn033
Inagaki, T., Choi, M., Moschetta, A., Peng, L., Cummins, C. L., Mcdonald, J. G., et al. (2005). Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2, 217–225. doi: 10.1016/j.cmet.2005.09.001
Kalliomäki, M., Collado, M. C., Salminen, S., and Isolauri, E. (2008). Early differences in fecal microbiota composition in children may predict overweight. Am. J. Clin. Nutr. 87, 534–538. Available Online at: http://ajcn.nutrition.org/content/87/3/534.long
Kang, J. H., Yun, S. I., Park, M. H., Park, J. H., Jeong, S. Y., and Park, H. O. (2013). Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS ONE 8:e54617. doi: 10.1371/journal.pone.0054617
Khan, I., Yasir, M., Azhar, E. I., Kumosani, T., Barbour, E. K., Bibi, F., et al. (2014). Implication of GM in human health. CNS Neurol. Disord. Drug Targets 13, 1325–1333. doi: 10.2174/1871527313666141023153506
Koenig, J. E., Spor, A., Scalfone, N., Fricker, A. D., Stombaugh, J., Knight, R., et al. (2011). Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. U.S.A. 108, 4578–4585. doi: 10.1073/pnas.1000081107
Kondo, S., Kamei, A., Xiao, J. Z., Iwatsuki, K., and Abe, K. (2013). Bifidobacterium breve B-3 exerts metabolic syndrome-suppressing effects in the liver of diet-induced obese mice: a DNA microarray analysis. Benef. Microbes 4, 247–252. doi: 10.3920/BM2012.0019
Kovatcheva-Datchary, P., and Arora, T. (2013). Nutrition, the gut microbiome and the metabolic syndrome: EBSCOhost. Best Pract. Res. Clin. Gastroenterol. 27, 59–72. doi: 10.1016/j.bpg.2013.03.017
Kuribayashi, H., Miyata, M., Yamakawa, H., Yoshinari, K., and Yamazoe, Y. (2012). Enterobacteria-mediated deconjugation of taurocholic acid enhances ileal farnesoid X receptor signaling. Eur. J. Pharmacol. 697, 132–138. doi: 10.1016/j.ejphar.2012.09.048
Lobstein, T., Baur, L., and Uauy, R. (2004). Obesity in children and young people : a crisis in public health. Obes. Rev. 5, 4–85. doi: 10.1111/j.1467-789X.2004.00133.x
LoCascio, R. G., Ninonuevo, M. R., Freeman, S. L., Sela, D. A., Grimm, R., Lebrilla, C. B., et al. (2007). Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J. Agric. Food Chem. 55, 8914–8919. doi: 10.1021/jf0710480
Marchesi, J. R., Adams, D. H., Fava, F., Hermes, G. D. A., Hirschfield, G. M., Hold, G., et al. (2016). The GM and host health: a new clinical frontier. Gut 65, 330–339. doi: 10.1136/gutjnl-2015-309990
Martin, F. P., Wang, Y., Sprenger, N., Yap, I. K., Lundstedt, T., Lek, P., et al. (2008). Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol. Syst. Biol. 4, 157. doi: 10.1038/msb4100190
Martin, M. A., and Sela, D. A. (2013). “Infant GM: developmental influences and health outcomes,” in Building Babies, eds K. B. H. Clancy, K. Hinde, and J. N. Rutherford (New York, NY: Springer), 233–256. Available online at: http://link.springer.com/book/10.1007%2F978-1-4614-4060-4
Maslowski, K. M., and Mackay, C. R. (2011). Diet, GM and immune responses. Nat. Immunol. 12, 5–9. doi: 10.1038/ni0111-5
Maynard, C. L., Elson, C. O., Hatton, R. D., and Weaver, C. T. (2012). Reciprocal interactions of the intestinal microbiota and immune system. Nature 489, 231–241. doi: 10.1038/nature11551
Mbakwa, C. A., Penders, J., Savelkoul, P. H., Thijs, C., Dagnelie, P. C., Mommers, M., et al. (2015). Gut colonization with Methanobrevibacter smithii is associated with childhood weight development. Obesity. 23, 2508–2516. doi: 10.1002/oby.21266
Meyer, D., and Stasse-Wolthuis, M. (2009). The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur. J. Clin. Nutr. 63, 1277–1289. doi: 10.1038/ejcn.2009.64
Million, M., Lagier, J. C., Yahav, D., and Paul, M. (2013a). Gut bacterial microbiota and obesity. Clin. Microbiol. Infect. 19, 305–313. doi: 10.1111/1469-0691.12172
Million, M., Maraninchi, M., Henry, M., Armougom, F., Richet, H., Carrieri, P., et al. (2012). Obesity-associated GM is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. (Lond). 36, 817–825. doi: 10.1038/ijo.2011.153
Million, M., Thuny, F., Angelakis, E., Casalta, J.-P., Giorgi, R., Habib, G., et al. (2013b). Lactobacillus reuteri and Escherichia coli in the human GM may predict weight gain associated with vancomycin treatment. Nutr. Diabetes 3:e87. doi: 10.1038/nutd.2013.28
Miyata, M., Matsuda, Y., Nomoto, M., Takamatsu, Y., Sato, N., Hamatsu, M., et al. (2009). Cholesterol feeding prevents hepatic accumulation of bile acids in cholic acid-fed farnesoid X receptor (FXR)-null mice: FXR-independent suppression of intestinal bile acid absorption. Drug Metab. Dispos. 37, 338–344. doi: 10.1124/dmd.108.022590
Morgun, A., Dzutsev, A., Dong, X., Greer, R. L., Sexton, D. J., Ravel, J., et al. (2015). Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut. 64, 1732–1743. doi: 10.1136/gutjnl-2014-308820
Musaiger, A. O. (2011). Overweight and obesity in Eastern Mediterranean Region: prevalence and possible causes. J. Obes. 2011:407237. doi: 10.1155/2011/407237
Nguyen, T. L. A., Vieira-Silva, S., Liston, A., and Raes, J. (2015). How informative is the mouse for human GM research? Dis. Model. Mech. 8, 1–16. doi: 10.1242/dmm.017400
Nobel, Y. R., Cox, L. M., Kirigin, F. F., Bokulich, N. A., Yamanishi, S., Teitler, I., et al. (2015). Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat. Commun. 6:7486. doi: 10.1038/ncomms8486
Nunez, I. N., Galdeano, C. M., de LeBlanc Ade, M., and Perdigon, G. (2014). Evaluation of immune response, microbiota, and blood markers after probiotic bacteria administration in obese mice induced by a high-fat diet. Nutrition 30, 1423–1432. doi: 10.1016/j.nut.2014.03.025
Ogden, C. L., Carroll, M. D., Kit, B. K., and Flegal, K. M. (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. J. Am. Med. Assoc. 311, 806–814. doi: 10.1001/jama.2014.732
Park, D. Y., Ahn, Y. T., Park, S. H., Huh, C. S., Yoo, S. R., Yu, R., et al. (2013). Supplementation of Lactobacillus curvatus KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS ONE 8:e59470. doi: 10.1371/journal.pone.0059470
Parvez, S., Malik, K. A., Ah Kang, S., and Kim, H. (2006). Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 100, 1171–1185. doi: 10.1111/j.1365-2672.2006.02963.x
Pekkala, S., Munukka, E., Kong, L., Pöllänen, E., Autio, R., Roos, C., et al. (2015). Toll-like receptor 5 in obesity: the role of GM and adipose tissue inflammation. Obesity 23, 581–590. doi: 10.1002/oby.20993
Penders, J., Thijs, C., van den Brandt, P. A., Kummeling, I., Snijders, B., Stelma, F., et al. (2007). GM composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut 56, 661–667. doi: 10.1136/gut.2006.100164
Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K. S., Manichanh, C., et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. doi: 10.1038/nature08821
Rautava, S., Luoto, R., Salminen, S., and Isolauri, E. (2012). Microbial contact during pregnancy, intestinal colonization and human disease. Nat. Rev. Gastroenterol. Hepatol. 9, 565–576. doi: 10.1038/nrgastro.2012.144
Rezaeian, M., and Salem, Z. (2007). Prevalence of obesity and abdominal obesity in a sample of urban adult population within South East of Iran. Pak. J. Med. Sci. 23, 193–197. Available Online at: http://pjms.com.pk/issues/aprjun107/abstract/article9.html
Ridlon, J. M., Kang, D. J., Hylemon, P. B., and Bajaj, J. S. (2014). Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 30, 332. doi: 10.1097/MOG.0000000000000057
Roopchand, D. E., Carmody, R. N., Kuhn, P., Moskal, K., Rojas-Silva, P., Turnbaugh, P. J., et al. (2015). Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 64, 2847–2858. doi: 10.2337/db14-1916
Round, J. L. J., and Mazmanian, S. S. K. (2009). The GM shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323. doi: 10.1038/nri2515
Samuel, B. S., Shaito, A., Motoike, T., Rey, F. E., Backhed, F., Manchester, J. K., et al. (2008). Effects of the GM on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. U.S.A. 105, 16767–16772. doi: 10.1073/pnas.0808567105
Sanchez, M., Darimont, C., Drapeau, V., Emady-Azar, S., Lepage, M., Rezzonico, E., et al. (2014). Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 111, 1507–1519. doi: 10.1017/S0007114513003875
Santacruz, A., Collado, M. C., García-Valdés, L., Segura, M. T., Martín-Lagos, J. A., Anjos, T., et al. (2010). GM composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Brit. J. Nutr. 104, 83–92. doi: 10.1017/S0007114510000176
Sayin, S. I., Wahlström, A., Felin, J., Jäntti, S., Marschall, H. U., Bamberg, K., et al. (2013). GM regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235. doi: 10.1016/j.cmet.2013.01.003
Schneeberger, M., Everard, A., Gomez-Valades, A. G., Matamoros, S., Ramirez, S., Delzenne, N. M., et al. (2015). Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 5:16643. doi: 10.1038/srep16643
Schwiertz, A., Taras, D., Schafer, K., Beijer, S., Bos, N. A., Donus, C., et al. (2010). Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 18, 190–195. doi: 10.1038/oby.2009.167
Scott, K. P., Antoine, J.-M., Midtvedt, T., and van Hemert, S. (2015). Manipulating the GM to maintain health and treat disease. Microb. Ecol. Health Dis. 26:25877. doi: 10.3402/mehd.v26.25877
Song, H., Han, W., Yan, F., Xu, D., Chu, Q., and Zheng, X. (2016). Dietary Phaseolus vulgaris extract alleviated diet-induced obesity, insulin resistance and hepatic steatosis and alters GM composition in mice. J. Funct. Foods 20, 236–244. doi: 10.1016/j.jff.2015.10.022
Tremaroli, V., and Bäckhed, F. (2012). Functional interactions between the GM and host metabolism. Nature 489, 242–249. doi: 10.1038/nature11552
Turnbaugh, P. J., Bäckhed, F., Fulton, L., and Gordon, J. I. (2008). Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223. doi: 10.1016/j.chom.2008.02.015
Turnbaugh, P. J., and Gordon, J. I. (2009). The core gut microbiome, energy balance and obesity. J. Physiol. 587, 4153–4158. doi: 10.1113/jphysiol.2009.174136
Turnbaugh, P. J., Hamady, M., Yatsunenko, T., Cantarel, B. L., Duncan, A., Ley, R. E., et al. (2009). A core gut microbiome in obese and lean twins. Nature 457, 480–484. doi: 10.1038/nature07540
Turnbaugh, P. J., Ley, R. E., Mahowald, M. A., Magrini, V., Mardis, E. R., and Gordon, J. I. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. doi: 10.1038/nature05414
Twells, L. K., Gregory, D. M., Reddigan, J., and Midodzi, W. K. (2014). Current and predicted prevalence of obesity in Canada: a trend analysis. Can. Med. Assoc. Open Access J. 2, E18–E26. doi: 10.9778/cmajo.20130016
Ukibe, K., Miyoshi, M., and Kadooka, Y. (2015). Administration of Lactobacillus gasseri SBT2055 suppresses macrophage infiltration into adipose tissue in diet-induced obese mice. Br. J. Nutr. 114, 1180–1187. doi: 10.1017/s0007114515002627
Vaughan, E., de Vries, M., Zoetendal, E., Ben-Amor, K., Akkermans, A. L., and de Vos, W. (2002). The intestinal LABs. Antonie Van Leeuwenhoek 82, 341–352. doi: 10.1023/A:1020672724450
Wang, J., Tang, H., Zhang, C., Zhao, Y., Derrien, M., Rocher, E., et al. (2015). Modulation of GM during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 9, 1–15. doi: 10.1038/ismej.2014.99
West, D. B., Delany, J. P., Camet, P. M., Blohm, F., Truett, A. A., and Scimeca, J. (1998). Effects of conjugated linoleic acid on body fat and energy metabolism in the mouse. Am. J. Physiol. Integr. Comp. Physiol. 275, R667–R672.
WHO (2015). Fact sheet: Obesity and Overweight. Available online at: http://www.who.int/mediacentre/factsheets/fs311/en/ (Accessed June 2016).
Yatsunenko, T., Rey, F. E., Manary, M. J., Trehan, I., Dominguez-Bello, M. G., Contreras, M., et al. (2012). Human gut microbiome viewed across age and geography. Nature 486, 222–227. doi: 10.1038/nature11053
Zhang, C., Zhang, M., Wang, S., Han, R., Cao, Y., Hua, W., et al. (2010). Interactions between GM, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 4, 232–241. doi: 10.1038/ismej.2009.112
Zhang, D., Huang, Y., and Ye, D. (2015). Intestinal dysbiosis: an emerging cause of pregnancy complications? Med. Hypotheses 84, 223–226. doi: 10.1016/j.mehy.2014.12.029
Zhang, H.-L., Yu, L.-X., Yang, W., Tang, L., Lin, Y., Wu, H., et al. (2012). Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J. Hepatol. 57, 803–812. doi: 10.1016/j.jhep.2012.06.011
Keywords: Saudi Arabia, obesity, gut microbiota, food, GM-obesity dilema
Citation: Harakeh SM, Khan I, Kumosani T, Barbour E, Almasaudi SB, Bahijri SM, Alfadul SM, Ajabnoor GMA and Azhar EI (2016) Gut Microbiota: A Contributing Factor to Obesity. Front. Cell. Infect. Microbiol. 6:95. doi: 10.3389/fcimb.2016.00095
Received: 28 February 2015; Accepted: 17 August 2016;
Published: 30 August 2016.
Edited by:
Yongqun “Oliver” He, University of Michigan Health System, USAReviewed by:
Roberto Mauricio Vidal, University of Chile, ChileValerio Iebba, Sapienza University of Rome, Italy
Askin Erdogan, Georgia Regents University, USA
Copyright © 2016 Harakeh, Khan, Kumosani, Barbour, Almasaudi, Bahijri, Alfadul, Ajabnoor and Azhar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steve M. Harakeh, sharakeh@gmail.com
 Steve M. Harakeh
Steve M. Harakeh Imran Khan
Imran Khan Taha Kumosani2,3
Taha Kumosani2,3  Suhad M. Bahijri
Suhad M. Bahijri Sulaiman M. Alfadul
Sulaiman M. Alfadul Esam I. Azhar
Esam I. Azhar