The PorX Response Regulator of the Porphyromonas gingivalis PorXY Two-Component System Does Not Directly Regulate the Type IX Secretion Genes but Binds the PorL Subunit
- Laboratoire d'Ingénierie des Systèmes Macromoléculaires, Institut de Microbiologie de la Méditerranée, Aix-Marseille Université – Centre National de la Recherche Scientifique (UMR7255), Marseille, France
The Type IX secretion system (T9SS) is a versatile multi-protein complex restricted to bacteria of the Bacteriodetes phylum and responsible for the secretion or cell surface exposition of diverse proteins that participate to S-layer formation, gliding motility or pathogenesis. The T9SS is poorly characterized but a number of proteins involved in the assembly of the secretion apparatus in the oral pathogen Porphyromonas gingivalis have been identified based on genome substractive analyses. Among these proteins, PorY, and PorX encode typical two-component system (TCS) sensor and CheY-like response regulator respectively. Although the porX and porY genes do not localize at the same genetic locus, it has been proposed that PorXY form a bona fide TCS. Deletion of porX in P. gingivalis causes a slight decrease of the expression of a number of other T9SS genes, including sov, porT, porP, porK, porL, porM, porN, and porY. Here, we show that PorX and the soluble cytoplasmic domain of PorY interact. Using electrophoretic mobility shift, DNA-protein co-purification and heterologous host expression assays, we demonstrate that PorX does not bind T9SS gene promoters and does not directly regulate expression of the T9SS genes. Finally, we show that PorX interacts with the cytoplasmic domain of PorL, a component of the T9SS membrane core complex and propose that the CheY-like PorX protein might be involved in the dynamics of the T9SS.
Introduction
Periodontitis and gum diseases are considered as major public health concerns as a recent prevalence study of the Centers for Disease Control and Prevention reported that roughly 47% of the USA adults aged 30 years or older suffer of serious tooth tissue damages and tooth decay (Eke et al., 2012). These diseases are due to a lack of dental hygiene and by the action of deleterious bacteria. The major bacterium responsible for periodontitis, gingivitis, and other gum diseases is Porphyromonas gingivalis (Kamma et al., 1994; Lo Bue et al., 1999; Hussain et al., 2015). P. gingivalis is an anaerobic bacterial oral pathogen that causes severe lesions in periodontal tissues such as the gingiva or the alveolar bone by disrupting the tooth-supporting structure (Bostanci and Belibasakis, 2012). In addition, recent studies reported links between periodontitis and systemic health issues, such as higher risks of cardiovascular diseases or rheumatoid arthritis (Janssen et al., 2013; Koziel et al., 2014). Rheumatoid arthritis is caused by the citrullination activity of a specific enzyme, the peptidylarginine deiminase (PPAD; Koziel et al., 2014; Gabarrini et al., 2015). By contrast, tissue damages are mainly induced by a cocktail of specialized toxin proteins secreted by the bacterium, collectively known as gingipains (Fitzpatrick et al., 2009). Gingipains act as adhesins or proteases that help the bacterium to adhere to periodontal tissues and to promote gingival tissue invasion by the degradation of matrix proteins such as fibrinogen and collagen (Fitzpatrick et al., 2009; Nakayama, 2015). The active release of gingipains at the bacterial cell surface and of the PPAD is catalyzed by a multi-protein complex, the Type IX secretion system (T9SS, Sato et al., 2010; Nakayama, 2015). Eleven genes, called por, have been identified in the P. gingivalis strain ATCC 33277 using substractive genome analyses, mutagenesis, and proteomic studies and have been shown to be involved in gingipain and PPAD transport to the cell surface (Sato et al., 2010, 2013; Taguchi et al., 2015; Gorasia et al., 2016). It is therefore thought that these 11 subunits assemble a trans-envelope channel that specifically recruits the toxins and transports them to the cell surface. A complex composed of the PorK, PorL, PorM, and PorN proteins has been isolated and visualized by blue-native gel electrophoresis (Sato et al., 2010). However, the T9SS is not restricted to P. gingivalis strains. A number of studies have reported the presence of T9SS genes in bacteria of the Bacteriodetes phylum, including species of the Flavobacterium, Cytophaga, Cellulophaga, Capnocytophaga, or Tannerella genus (McBride and Zhu, 2013). In these strains, the T9SS is responsible for the cell surface exposition, attachment, or external release of very diverse proteins (Sato et al., 2010; Shrivastava et al., 2013; Narita et al., 2014; Tomek et al., 2014; Zhu and McBride, 2014; Kita et al., 2016). This machine has been therefore adapted to the specific needs of each bacterium. In T. forsythia, the T9SS is necessary for the transport of the components of the outmost S-layer (Narita et al., 2014; Tomek et al., 2014). In F. johnsioniae, the T9SS is responsible for the transport and propulsion of adhesins at the cell surface allowing the bacterium to glide on solid surfaces (Nakane et al., 2013; Shrivastava et al., 2013; McBride and Nakane, 2015). The F. johnsoniae adhesins are rotative filaments (Nakane et al., 2013; Shrivastava et al., 2015). Similarly to the flagellum, the T9SS is thought to act as a proton-motive force-dependent trans-envelope motor and to power the rotation of the adhesins (Nakane et al., 2013; McBride and Nakane, 2015; Shrivastava and Berg, 2015; Shrivastava et al., 2015).
In addition to structural subunits of the transport apparatus, the substractive analyses performed with P. gingivalis revealed two additional genes, porY and porX (Sato et al., 2010). These two genes encode a two-component sensor and response regulator respectively. Although the two genes are not encoded within a single genetic unit, it has been proposed that these two proteins form a two-component system responsible for regulation of the por genes. Indeed, microarray analyses showed that PorX and PorY contribute to the regulation of the T9SS por genes as a 1.8-fold decrease of their expression is observed in porX and porY mutant cells compared to the WT strain (Sato et al., 2010). Here, we build up on this result and show that PorX and PorY interact and likely constitute a bona fide two-component system. We did not detect binding of PorX to the promoter regions of the sov, porT, and porP genes, and we did not observe increased activity of this promoters in presence of PorX suggesting that PorX does not directly regulate these genes. Indeed, domain analyses of PorX did not reveal any DNA binding motif, but rather a CheY-like receiver domain. We further show that PorX binds to the cytoplasmic domain of the T9SS PorL proteins, and that a specific patch of hydrophobic residues of PorL mediate this interaction.
Materials and Methods
Bacterial Strains, Plasmids, Medium, and Growth Conditions
Strains and plasmids used in this study are listed in the Supplemental Table. The P. gingivalis ATCC33277 (DSM-20709) wild-type strain used in this study was obtained from the German collection of microorganisms and cell cultures (DSMZ). The DH5α, BTH101, W3110, and BL21(DE3) E. coli K-12 strains were used for cloning, bacterial two-hybrid, co-immune-precipitation, and protein production and purification respectively. E. coli cells were routinely grown in Luria Broth (LB) supplemented with antibiotics when necessary (kanamycin 50 μg/mL, ampicillin 100 μg/mL, chloramphenicol 30 μg/mL). P. gingivalis cells were grown anaerobically in Brain Heart Infusion medium supplemented with hemin (5 μg/mL) and menadione (0.5 μg/mL) in Hungate tubes.
Plasmid Construction
Polymerase Chain Reactions (PCR) were performed using a Biometra thermocycler using the Q5 (New England Biolabs) or Pfu Turbo (Agilent Technologies) DNA polymerases. Restriction enzymes were purchased from New England Biolabs and used according to the manufacturer's instructions. Custom oligonucleotides were synthesized by Sigma Aldrich and are listed in the Supplemental Table. P. gingivalis chromosomal DNA was used as a template for PCRs. Bacterial two-hybrid vectors have been constructed by ligation of EcoRI-BamHI fragments into the vectors pUT18C and pKT25 (Karimova et al., 1998). Cloning of porX into the pETG20A vector was performed according to standard Gateway protocols. Other DNA fragment insertions into the pBAD24 (Guzman et al., 1995) and pUA66 (Zaslaver et al., 2006) vectors have been performed by restriction-free cloning (van den Ent and Löwe, 2006). Briefly, the gene of interest was amplified using oligonucleotides introducing extensions annealing to the target vector. The double-stranded product of the first PCR has then been used as oligonucleotides for a second PCR using the target vector as template. PCR products were then treated with DpnI to eliminate template plasmids and transformed into DH5α-competent cells. All constructs have been verified by restriction analyses and DNA sequencing (Eurofins or MWG). The expression of the cloned genes or fragments was induced with IPTG (0.5 mM), L-arabinose (0.02%), or anhydrotetracycline (AHT, 0.02 μg/mL).
Bacterial Two-Hybrid (Bacth)
The adenylate cyclase-based bacterial two-hybrid technique (Karimova et al., 1998) was used as previously published (Battesti and Bouveret, 2008, 2012; Zoued et al., 2013). Briefly, the proteins to be tested were fused to the isolated T18 and T25 catalytic domains of the Bordetella adenylate cyclase. After introduction of the two plasmids producing the fusion proteins into the reporter BTH101 strain, plates were incubated at 30°C for 48 h. Three independent colonies for each transformation were inoculated into 600 μL of LB medium supplemented with ampicillin, kanamycin and IPTG (0.5 mM). After overnight growth at 30°C, 10 μL of each culture were dropped onto LB plates supplemented with ampicillin, kanamycin, IPTG, and X-Gal and incubated for 16 h at 30°C. The experiments were done at least in triplicate and a representative result is shown.
Co-Immune-Precipitation
100 mL of E. coli W3110 cells bearing the plasmid of interest were grown to an optical density at λ = 600 nm (A600) ~ 0.4 and the expression of the cloned genes were induced with AHT or L-arabinose for 45 min. The cells were harvested, and the pellets were resuspended in Tris-HCl 20 mM pH 8.0, NaCl 100 mM, sucrose 30%, EDTA 1 mM, lysozyme 100 μg/mL, DNase 100 μg/mL, RNase 100 μg/mL supplemented with protease inhibitors (Complete, Roche) to an A600 of 80, incubated on ice for 20 min and cells were lysed by three passages at the French Press (800 psi). The lysates were clarified by centrifugation at 20,000 × g for 20 min. and the supernatants containing the soluble proteins were used for co-immunoprecipitation using anti-FLAG M2 affinity gel (Sigma-Aldrich). After 3 h of incubation on a wheel, the beads were washed three times with 1 mL of Tris-HCl 20 mM pH 8.0, NaCl 100 mM, sucrose 15%, air-dried, resuspended in 25 μL of Laemmli loading buffer, boiled for 10 min, and subjected to SDS-PAGE and immunodetection analyses.
Protein Purification
E. coli BL21(DE3) cells carrying pETG20A-PorX or pRSF-PorYC were grown at 37°C in LB to an A600 ~ 0.7 and gene expression was inducted with 0.5 mM IPTG for 16 h at 22°C. Cells were harvested, resuspended in Tris-HCl 50 mM pH 8.0, NaCl 300 mM supplemented with lysozyme (100 μg/mL), DNase (100 μg/mL), MgCl2 5 mM, and imidazole 10 mM, and broken by three passages at the French press (800 psi). Soluble proteins were separated from inclusion bodies and cell debris by centrifugation 30 min at 16,000 × g. The His-tagged fusions were purified using ion metal Ni2+ affinity chromatography (IMAC) using a 5-mL HisTrap column (GE Healthcare) and eluted with a step gradient of imidazole. For PorYC, the collected fractions were analyzed by SDS-PAGE and Coomassie blue staining and the fractions of interest were pooled and dialized for 16 h at 4°C in Tris-HCl 50 mM pH 8.0, NaCl 150 mM. The final concentration of PorYC was 1.23 mg/mL. The PorX protein, purified from the pETG20A vector, is fused to a Tobacco Etch Virus (TEV) protease-cleavable thioredoxin-6 × His tag. After the first IMAC, the fusion PorX proteins were digested overnight at 4°C by a 6 × His-tagged TEV protease using a 1:10 (w/w) protease:protein ratio. The TEV protease and contaminants were retained by a second IMAC and the purified proteins were collected in the flow through. Proteins were further separated on preparative Superdex 200 or Superose 6 gel filtration column (GE Healthcare) equilibrated in Tris-HCl 20 mM pH 8.0, NaCl 150 mM. The fractions containing the purified protein were pooled and concentrated by ultrafiltration using the Amicon technology (Millipore, California, USA). The final concentration of PorX was 11.6 mg/mL. The E. coli Ferric uptake regulator (Fur) and sequence signal-less Peptidoglycan-associated lipoprotein (Pal) were purified as previously described (Abergel et al., 2001; Brunet et al., 2011).
Protein-Protein Co-Purification
The purified PorX and 6 × His-tagged PorYC or Pal proteins were mixed and incubated on ice for 10 min. The mixture was then applied to Co2+-NTA beads (Talon, Clontech) in Tris-HCl 50 mM pH 8.0, NaCl 150 mM and incubated at 25°C for 45 min. The beads were washed three times with Tris-HCl 50 mM pH 8.0, NaCl 150 mM supplemented with imidazole 10 mM, air-dried, resuspended in 25 μL of Laemmli loading buffer, boiled for 10 min, and subjected to SDS-PAGE and immunodetection analyses.
DNA-Protein Co-Purification
PCR fragments were obtained using a biotinylated 5′ oligonucleotide, and immobilized on streptavidin beads (DyNAbeads, Lifescience) in Tris-HCl 10 mM pH 7.5, EDTA 0.5 mM, NaCl 1 mM. Unbound biotinylated probes were eliminated by three washes in phosphate-buffered saline (PBS). The purified PorX and Fur proteins were mixed, and the mixture was then applied to the DNA-coated streptavidin beads in PBS at 25°C for 45 min on a wheel. The beads were washed three times in PBS, air-dried, resuspended in 25 μL of Laemmli loading buffer, boiled for 10 min, and subjected to SDS-PAGE and immunodetection analyses.
Electrophoretic Mobility Shift Assay
PCR products were purified using the Wizard Gel and PCR clean-up kit (Promega). 250 ng of PCR products were incubated in a final volume of 10 μl in Tris-HCl 40 mM pH 7.2, NaCl 50 mM, KCl 5 mM, Glycerol 5%, MgCl2 6 mM, CaCl2 0.5 mM, acetylphosphate 10 mM, dithiothreitol 1 mM, bovine serum albumine 100 μg/mL in presence of purified PorX (0, 50, 100, 200, or 400 nM). After incubation for 25 min at 25°C, the samples were loaded on a pre-run 8% non denaturing polyacrylamide (Tris-borate) gel, and DNA and DNA-complexes were separated at 100 V in Tris-Borate buffer (45 mM Tris base, 45 mM boric acid, 100 μM MnCl2).
Heterologous Host Regulation Assay
W3110 cells carrying the empty vector pBAD24 or pBAD-PorX and the promoter-pUA66 derivatives were grown in 96-well microplates to an A600nm ~ 0.4, treated with L-arabinose for 45 min, and the A600nm and the levels of fluorescence were acquired at 505 nm after excitation at 488 nm using a TECAN microplate reader. Reported values represent the average of technical triplicates from three independent biological cultures and standard deviation are shown on the graphs.
Computer Analyses
Domain homology analyses were performed with pfam (Finn et al., 2016; http://pfam.xfam.org/), HHpred (Söding et al., 2005; http://toolkit.tuebingen.mpg.de/hhpred), and Phyre2 (Kelley et al., 2015; http://www.sbg.bio.ic.ac.uk/phyre2). Predictions of trans-membrane domains were performed with TMPred (Hofmann and Stoffel, 1993; http://www.ch.embnet.org/software/TMPRED_form.html), TMHMM (Sonnhammer et al., 1998; http://www.cbs.dtu.dk/services/TMHMM/), and HHMTop (Tusnády and Simon, 1998; http://sbcb.bioch.ox.ac.uk/TM_noj/TM_noj.html).
Miscellaneous
SDS-polyacrylamide gel electrophoresis was performed using standard protocols. For immunostaining, proteins were transferred onto nitrocellulose membranes, and immunoblots were probed with primary antibodies and goat secondary antibodies coupled to alkaline phosphatase, and developed in alkaline buffer in presence of 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium. The anti-FLAG (M2 clone, Sigma-Aldrich), anti-VSV-G (P5D4 clone, Sigma-Aldrich) monoclonal antibodies, and alkaline phosphatase-conjugated goat anti-mouse secondary antibodies (Beckman Coulter) have been purchased as indicated and used as recommended by the manufacturers.
Results and Discussion
Sequence Analyses of PorX and PorY
To gain insights onto the PorX and PorY proteins, we performed a sequence and structural analysis using the HHPred and Phyre2 algorithms. The 518-aminoacid PorX protein [PGN_1019, gene accession (GI): 188594563] is a putative cytoplasmic protein that is constituted of two domains: a N-terminal receiver domain of the CheY family (pfam identifier: PF00072) and a C-terminal effector domain of the PglZ family (pfam: PF08665) (Figure 1A). CheY is a phospho-protein involved in chemotaxis, coupling signal sensing via chemoreceptors to the control of the directionality of the flagellar rotation (Sourjik and Wingreen, 2012). This regulation occurs via the phosphorylation of a conserved aspartate residue that is also present in PorX, at position 58 (Figure 1A). The function of PglZ domains is still unknown but these domains do not bear typical motif responsible for DNA binding. The 395-aminoacid PorY protein (PGN_2001, GI: 188595544) is predicted to be anchored to the inner membrane via two trans-membrane segments located between residues 14 and 36, and 149 and 173, delimitating a ~115-aminoacid periplasmic loop probably involved in signal detection (Figures 1B,C). The PorY cytoplasmic domain is anticipated to adopt a classical signal transduction histidine kinase fold of the BaeS family with an ATP binding site and a phospho-acceptor domain that bears a conserved histidine residue at position 193 (Figure 1B).
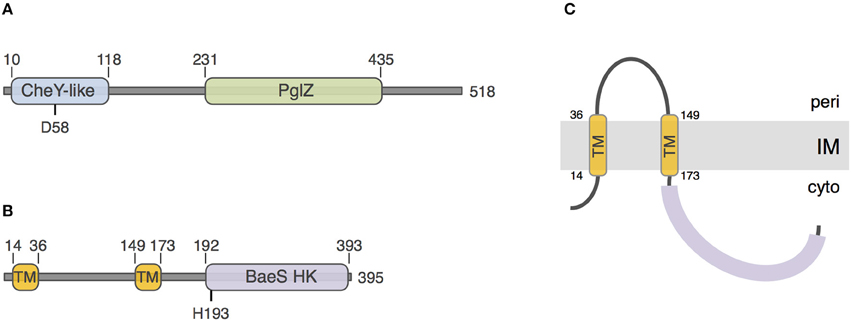
Figure 1. Sequence analyses of PorX and PorY. Schematic representation of the PorX (A) and PorY (B) proteins. The number of amino-acids of the protein is indicated on the right. The predicted receiver CheY-like, PglZ, and BaeS histidine kinase (BaeS HK) domains and transmembrane helices (TM) are indicated, as well as their boundaries. The putative residues involved in phospho-transfer [Aspartate-58 (D58) of PorX and Histidine-193 (H193) of PorY] are indicated. (C) Predicted topology of the PorY protein at the inner membrane (IM).
PorY and PorX Constitute a Bona Fide Two-Component System
The porY and porX genes are separated by ~1000 genes on the P. gingivalis chromosome, a genetic organization that is uncommon for cognate TCS, which are usually arranged as dicistronic units. This organization raised the question whether these two proteins are partners. To test the interaction between PorX and PorY, we performed bacterial two-hybrid experiments. The PorX protein was fused to the T18 domain, and its interaction with the cytoplasmic soluble domain of PorY (PorYC) fused to the T25 domain was assayed on reporter plates. Figure 2A shows that the PorX-PorYC interaction reconstitutes the activity of the adenylate cyclase. This interaction was confirmed by co-purification. PorX and PorYC were first purified to homogeneity. Figure 2B shows that the immobilization of the 6 × His-tagged PorYC protein—but not that of the 6 × His-tagged Pal unrelated control protein—on the Ni-NTA affinity resin retains the untagged PorX protein. Taken together, these results demonstrate that PorY and PorX interact and likely constitute a bona fide TCS. This result is consistent with the observation that the deletions of the porX and porY genes have similar effects on T9SS gene expression (Sato et al., 2010).
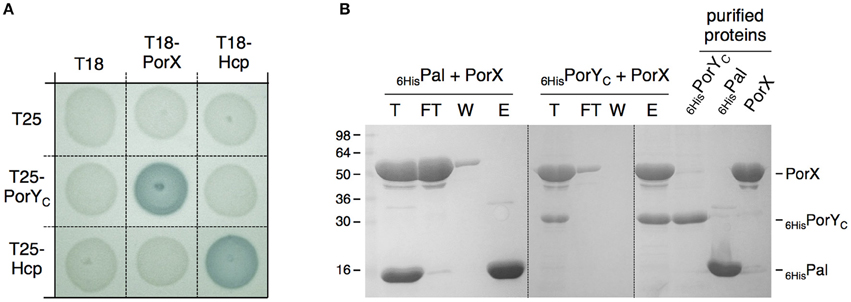
Figure 2. PorX and PorY interact. (A) Bacterial two-hybrid assay. BTH101 reporter cells carrying pairs of plasmids producing the indicated proteins fused to the T18 or T25 domain of the Bordetella adenylate cyclase were spotted on X-Gal-IPTG reporter LB agar plates. Controls include T18 and T25 fusions to Hcp (Zoued et al., 2013), a T6SS protein from enteroaggregative E. coli and unrelated to the T9SS. (B) Co-purification assay. The indicated proteins (individually shown on the right) were mixed, and the total sample (T) was incubated with Ni-NTA beads. Unbound [flow through (FT) and wash (W)] and bound (E) proteins were collected and analyzed by SDS-PAGE and Coomassie blue staining. The different proteins are indicated on the right. Molecular weight markers (in kDa) are indicated on the left.
PorX is Not a Direct Regulator of T9SS Genes
We then asked whether PorX regulates directly the T9SS genes by binding to their promoter regions. Sequence analyses of the promoter sequences of a subset of por genes highlighted a conserved palindromic motif that may constitute a binding box for a transcriptional regulator. 400-bp DNA fragments encompassing the promoter regions of sov (−373 to +33 relative to the putative +1 transcriptional start site), porT (−413 to +60) and of the porPKLMN operon (−309 to +34) were amplified and used for electrophoretic mobility shift assays (EMSA) with the purified PorX protein. We did not observe retardation of these DNA probes with up to 400 nM of PorX (Figure 3A), suggesting that no binding occurs. To test por promoters-PorX interaction by an alternative approach, the promoters were biotinylated, incubated with a mixture of PorX and of the E. coli Fur protein and then immobilized on a streptavidin-loaded resin. Figure 3B shows that the sov, porT, and porP promoters did not retain PorX whereas Fur was specifically retained by the Fur-dependent enteroaggregative E. coli T6SS sci1 promoter (Brunet et al., 2011). These results therefore suggest that PorX does not bind to the por gene promoters. This conclusion was strengthened by a promoter/PorX reconstitution approach in the E. coli K-12 heterologous host. Translational reporter fusions between the promoters and the GFP-encoding gene were engineered and the expression of the gfp (under the control of the cloned fragments) was followed at 505 nm (Figure 3C). In absence of PorX, the levels of fluorescence of the reporter fusions were similar to that of the parental promoterless pUA66 vector or the Fur-repressed pUA66-sci1 construct, suggesting that the por genes are not expressed (Figure 3C, white bars). In presence of PorX, the levels of fluorescence of the reporter fusions were comparable to that in absence of PorX (Figure 3C, blue bars), demonstrating that PorX does not increase the activity of the promoters, and that these promoters are not under the direct control of PorX. This result is in agreement with the observation that PorX lacks DNA binding motifs.
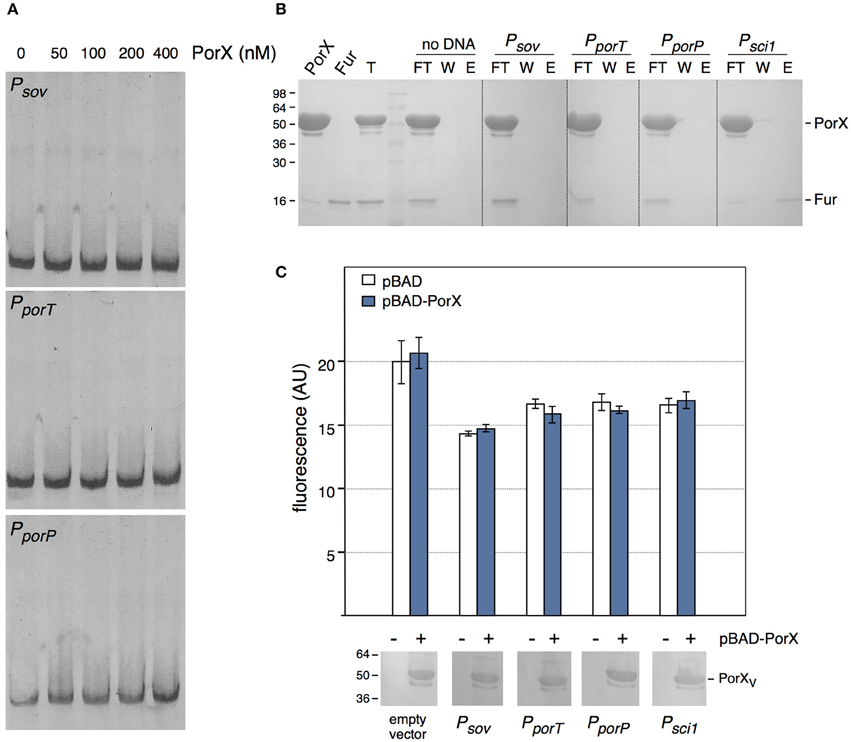
Figure 3. PorX is not a direct regulator of T9SS genes. (A) Electrophoretic mobility shift assay (EMSA) of the sov (Psov), porT (PporT), or porP (PporP) promoter with the indicated concentrations of the purified PorX protein. (B) DNA-protein co-purification assay. The purified PorX and Fur proteins (individually shown on the left) were mixed, and the total sample (T) was incubated with streptavidin beads coated with the indicated biotinylated promoter fragment (no DNA, streptavidin beads alone; Psci1, promoter fragment of the Fur-regulated enteroaggregative E. coli sci1 T6SS gene cluster). Unbound [flow through (FT) and wash (W)] and bound (E) proteins were collected and analyzed by SDS-PAGE and Coomassie blue staining. The different proteins are indicated on the right. Molecular weight markers (in kDa) are indicated on the left. (C) Promoter/regulator reconstitution assay in the E. coli heterologous host. Relative fluorescence levels (in arbitrary units) of E. coli cells carrying the indicated pUA66-promoter derivatives (GFP under the control of the indicated promoter) were measured in absence (−, white bars) or presence (+, blue bars) or PorX. Is represented the mean of fluorescence levels obtained from three independent experiments (each measured in triplicate). The production of VSV-G-tagged PorX is shown on bottom (immunodetection with anti-VSV-G monoclonal antibody; molecular weight markers on left).
The CheY-Like PorX Protein Interacts with the Cytoplasmic Domain of the PorL T9SS Membrane Core Subunit
The fact that PorX is not a direct regulator of T9SS genes prompted us to investigate further how PorX could influence the T9SS. The initial sequence analyses defined that the N-terminal domain of PorX is similar to response regulators of the CheY family, which are involved in chemotaxis. Once phosphorylated, CheY binds to the flagellar C-ring (Roman et al., 1992; Sagi et al., 2003), and more specifically to the FliM and FliN switch complex proteins (Shukla et al., 1998; Paul et al., 2006; Sarkar et al., 2010) hence reversing the direction of rotation of the filament. Binding of CheY to FliN is mediated by an hydrophobic patch within FliN (Paul et al., 2006; Sarkar et al., 2010). Interestingly, it has been recently reported that the T9SS is also a rotary machine, and specifically that it promotes rotary movement of the SprB filamentous adhesin at the cell surface of F. johnsioniae (Shrivastava and Berg, 2015). We therefore asked whether, similarly to CheY, PorX is recruited to one of the basal components of the T9SS. The T9SS subunits are poorly characterized but the system is principally constituted of outer membrane-associated proteins (McBride and Zhu, 2013; McBride and Nakane, 2015; Nakayama, 2015; Gorasia et al., 2016). However, two proteins, PorL and PorM, are anchored to the inner membrane and have therefore cytoplasmic regions or domains (Sato et al., 2010). We therefore tested whether PorX contacts PorL and/or PorM. Bacterial two-hybrid experiments revealed that PorX and PorL interact whereas no interaction was detected between PorX and PorM (Figure 4A). In addition, we showed that PorX forms a precipitable complex with the cytoplasmic domain of PorL (PorLC (amino-acids 73–309), Figures 4A,B). Interestingly, the cytoplasmic domain of PorL carries a hydrophobic patch near its C-terminus (residues 279–298; LLQALTTNVGLPGMPGNFGA). Deletion of this hydrophobic region [PorLC△Ct (amino-acids 73–274)] abolished the interaction with PorX (Figure 4A). We therefore conclude that PorX is recruited to the basal part of the T9SS core apparatus via direct interaction with an hydrophobic patch of PorL, a situation reminiscent to the CheY recruitment to the flagellar switch complex.

Figure 4. PorX interacts with the cytoplasmic domain of the PorL T9SS subunit. (A) Bacterial two-hybrid assay. BTH101 reporter cells carrying pairs of plasmids producing the indicated proteins fused to the T18 or T25 domain of the Bordetella adenylate cyclase were spotted on X-Gal-IPTG reporter LB agar plates. Controls include T18 and T25 fusions to Pal and TolB, two proteins unrelated to the T9SS. (B) Co-immune precipitation assay. Lysates producing the indicated proteins were mixed and subjected to immune precipitation with anti-FLAG antibodies. The total input (T) and immunoprecipitated (IP) material were analyzed by SDS-PAGE and immuno-detected with anti VSV-G (upper panel) and anti-FLAG (lower panel) monoclonal antibodies. The proteins are indicated on the right. The molecular weight markers are indicated on the left.
Concluding Remarks
Sequence analyses of the P. gingivalis PorX and PorY proteins showed that PorX is composed of a CheY-like receiver domain and a PglZ domain of unknown function, whereas the cytoplasmic histidine kinase domain of PorY is anchored to the inner membrane through a trans-membrane hairpin. This prediction is in agreement with recent results published by Kadowaki et al. who showed that PorY co-fractionates with the inner membrane fraction (Kadowaki et al., 2016). Using bacterial two-hybrid and co-purification experiments, we then demonstrate that PorY and PorX interact and likely assemble a cognate signal sensor/response regulator pair. Our results are in agreement with surface plasmon resonance studies that revealed that the two proteins interact with a KD of 1.41 μM and that PorY is capable of phospho-transfer onto PorX (Kadowaki et al., 2016). Using electrophoretic mobility shift and DNA-co-purification assays, we showed that PorX does not bind to the promoter regions of three T9SS genes or operons, sov, porT, and porP, suggesting that PorX does not directly regulate these genes. This hypothesis was validated by promoter/PorX reconstitution experiments in the E. coli heterologous host that showed that the presence of PorX does not increase the activity of these promoters. Kadowaki et al. recently identified a direct regulator of T9SS genes, SigP (Kadowaki et al., 2016). SigP is a transcriptional regulator of the ECF sigma factor family that binds and activates the expression of the T9SS genes. The authors nicely showed that PorX interacts and stabilizes SigP (Kadowaki et al., 2016).
Finally, we showed that PorX also interacts with the cytoplasmic domain of an inner membrane component of the T9SS, PorL. This interaction is mediated by a hydrophobic patch located at the C-terminal extremity of PorL. This behavior is reminiscent of the interaction of CheY with the switch complex that constitutes the basal C-ring structure of the flagellum (Roman et al., 1992; Shukla et al., 1998; Sagi et al., 2003; Paul et al., 2006; Sarkar et al., 2010; Figure 5A). CheY binding on the C-ring induces the reversal of flagellum rotation (Roman et al., 1992; Sourjik and Wingreen, 2012). Based on (i) the homology of the receiver domain of PorX with CheY, (ii) its interaction with PorL, and (iii) the recent observation that the F. johnsioniae T9SS is a rotary machine (Nakane et al., 2013; Shrivastava and Berg, 2015; Shrivastava et al., 2015), we propose a working model in which phosphorylated PorX binds to PorL, and similarly to CheY, induces T9SS rotation reversal (Figure 5B). It would be interesting to test this hypothetical model in F. johnsioniae by following T9SS rotation and by tracking the gliding activity of porX mutant cells. Understanding how the T9SS activity is controlled would provide insights for the design of drugs that interfere with T9SS function and for alternative therapeutic treatments for individuals suffering of gum diseases.
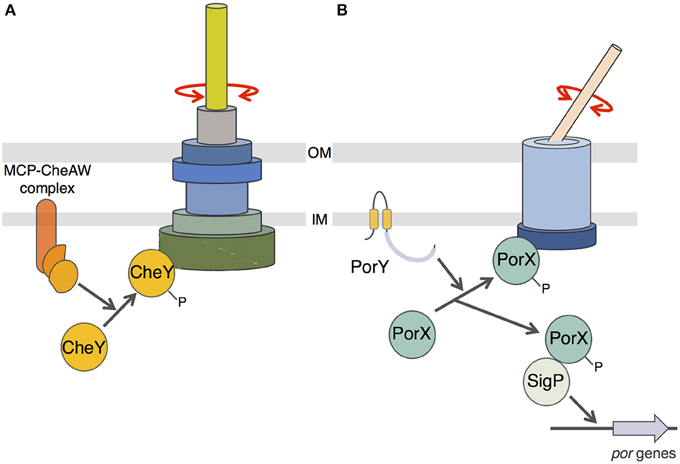
Figure 5. Schematic comparison between flagellum and T9SS rotary mechanisms. (A) CheY-dependent chemotaxis-flagellum rotation coupling. Phosphorylation of the CheY protein by the MCP/CheA/CheW complex induces recruitment of phospho-CheY to the C-ring switch complex and reversal of the flagellum filament rotation. (B) Putative model of PorX function. Phosphorylation of PorX protein by PorY induces (i) PorX-SigP complex formation and stabilization of the SigP sigma factor involved in por genes regulation, and (ii) recruitment of phospho-PorX to the T9SS complex and reversal of the cell surface filament adhesin rotation.
Author Contributions
MV and EC designed the study. MV and ED performed the experiments. EC wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Laure Journet for critical reading of the manuscript, Laure Journet, Bérengère Ize and Laetitia Houot and the members of the Cascales, Lloubès, Cambillau/Roussel, Bouveret and Sturgis research groups for discussions, Isabelle Bringer, Annick Brun, and Olivier Uderso for technical assistance and Martine Scort-Seize for encouragements. This work was supported by the CNRS, the Aix-Marseille Université and a grant from the Agence Nationale de la Recherche to EC (ANR-15-CE11-0019-01). MV is a recipient of a doctoral fellowship from the French Ministry of Research. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fcimb.2016.00096
References
Abergel, C., Walburger, A., Chenivesse, S., and Lazdunski, C. (2001). Crystallization and preliminary crystallographic study of the peptidoglycan-associated lipoprotein from Escherichia coli. Acta Crystallogr. D Biol. Crystallogr. 57, 317–319. doi: 10.1107/S0907444900019739
Battesti, A., and Bouveret, E. (2008). Improvement of bacterial two-hybrid vectors for detection of fusion proteins and transfer to pBAD-tandem affinity purification, calmodulin binding peptide, or 6-histidine tag vectors. Proteomics 8, 4768–4771. doi: 10.1002/pmic.200800270
Battesti, A., and Bouveret, E. (2012). The bacterial two-hybrid system based on adenylate cyclase reconstitution in Escherichia coli. Methods 58, 325–334. doi: 10.1016/j.ymeth.2012.07.018
Bostanci, N., and Belibasakis, G. N. (2012). Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 333, 1–9. doi: 10.1111/j.1574-6968.2012.02579.x
Brunet, Y. R., Bernard, C. S., Gavioli, M., Lloubès, R., and Cascales, E. (2011). An epigenetic switch involving overlapping fur and DNA methylation optimizes expression of a type VI secretion gene cluster. PLoS Genet. 7:e1002205. doi: 10.1371/journal.pgen.1002205
Eke, P. I., Dye, B. A., Wei, L., Thornton-Evans, G. O., Genco, R. J., and the C. D. C Periodontal Disease Surveillance workgroup (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 91, 914–920. doi: 10.1177/0022034512457373
Finn, R. D., Coggill, P., Eberhardt, R. Y., Eddy, S. R., Mistry, J., Mitchell, A. L., et al. (2016). The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44, D279–D285. doi: 10.1093/nar/gkv1344
Fitzpatrick, R. E., Wijeyewickrema, L. C., and Pike, R. N. (2009). The gingipains: scissors and glue of the periodontal pathogen, Porphyromonas gingivalis. Future Microbiol. 4, 471–487. doi: 10.2217/fmb.09.18
Gabarrini, G., de Smit, M., Westra, J., Brouwer, E., Vissink, A., Zhou, K., et al. (2015). The peptidylarginine deiminase gene is a conserved feature of Porphyromonas gingivalis. Sci. Rep. 5:13936. doi: 10.1038/srep13936
Gorasia, D. G., Veith, P. D., Hanssen, E. G., Glew, M. D., Sato, K., Yukitake, H., et al. (2016). Structural insights into the PorK and PorN components of the Porphyromonas gingivalis Type IX secretion system. PLoS Pathog. 12:e1005820. doi: 10.1371/journal.ppat.1005820
Guzman, L. M., Belin, D., Carson, M. J., and Beckwith, J. (1995). Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177, 4121–4130.
Hofmann, K., and Stoffel, W. (1993). TMbase – A database of membrane spanning proteins segments. Biol. Chem. 374, 166.
Hussain, M., Stover, C. M., and Dupont, A. (2015). P. gingivalis in periodontal disease and atherosclerosis – scenes of action for antimicrobial peptides and complement. Front. Immunol. 6:45. doi: 10.3389/fimmu.2015.00045
Janssen, K. M., Vissink, A., de Smit, M. J., Westra, J., and Brouwer, E. (2013). Lessons to be learned from periodontitis. Curr. Opin. Rheumatol. 25, 241–247. doi: 10.1097/BOR.0b013e32835d833d
Kadowaki, T., Yukitake, H., Naito, M., Sato, K., Kikuchi, Y., Kondo, Y., et al. (2016). A two-component system regulates gene expression of the type IX secretion component proteins via an ECF sigma factor. Sci. Rep. 6:23288. doi: 10.1038/srep23288
Kamma, J. J., Nakou, M., and Manti, F. A. (1994). Microbiota of rapidly progressive periodontitis lesions in association with clinical parameters. J Periodontol. 65, 1073–1078. doi: 10.1902/jop.1994.65.11.1073
Karimova, G., Pidoux, J., Ullmann, A., and Ladant, D. (1998). A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U.S.A. 95, 5752–5756. doi: 10.1073/pnas.95.10.5752
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N., and Sternberg, M. J. (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858. doi: 10.1038/nprot.2015.053
Kita, D., Shibata, S., Kikuchi, Y., Kokubu, E., Nakayama, K., Saito, A., et al. (2016). Involvement of the Type IX secretion system in Capnocytophaga ochracea gliding motility and biofilm formation. Appl. Environ. Microbiol. 82, 1756–1766. doi: 10.1128/AEM.03452-15
Koziel, J., Mydel, P., and Potempa, J. (2014). The link between periodontal disease and rheumatoid arthritis: an updated review. Curr. Rheumatol. Rep. 16, 408. doi: 10.1007/s11926-014-0408-9
Lo Bue, A. M., Nicoletti, G., Toscano, M. A., Rossetti, B., Calì, G., and Condorelli, F. (1999). Porphyromonas gingivalis prevalence related to other micro-organisms in adult refractory periodontitis. New Microbiol. 22, 209–218.
McBride, M. J., and Nakane, D. (2015). Flavobacterium gliding motility and the type IX secretion system. Curr. Opin. Microbiol. 28, 72–77. doi: 10.1016/j.mib.2015.07.016
McBride, M. J., and Zhu, Y. (2013). Gliding motility and Por secretion system genes are widespread among members of the phylum bacteroidetes. J. Bacteriol. 195, 270–278. doi: 10.1128/JB.01962-12
Nakane, D., Sato, K., Wada, H., McBride, M. J., and Nakayama, K. (2013). Helical flow of surface protein required for bacterial gliding motility. Proc. Natl. Acad. Sci. U.S.A. 110, 11145–11150. doi: 10.1073/pnas.1219753110
Nakayama, K. (2015). Porphyromonas gingivalis and related bacteria: from colonial pigmentation to the type IX secretion system and gliding motility. J. Periodont. Res. 50, 1–8. doi: 10.1111/jre.12255
Narita, Y., Sato, K., Yukitake, H., Shoji, M., Nakane, D., Nagano, K., et al. (2014). Lack of a surface layer in Tannerella forsythia mutants deficient in the type IX secretion system. Microbiology 160, 2295–2303. doi: 10.1099/mic.0.080192-0
Paul, K., Harmon, J. G., and Blair, D. F. (2006). Mutational analysis of the flagellar rotor protein FliN: identification of surfaces important for flagellar assembly and switching. J. Bacteriol. 188, 5240–5248. doi: 10.1128/JB.00110-06
Roman, S. J., Meyers, M., Volz, K., and Matsumura, P. (1992). A chemotactic signaling surface on CheY defined by suppressors of flagellar switch mutations. J. Bacteriol. 174, 6247–6255.
Sagi, Y., Khan, S., and Eisenbach, M. (2003). Binding of the chemotaxis response regulator CheY to the isolated, intact switch complex of the bacterial flagellar motor: lack of cooperativity. J. Biol. Chem. 278, 25867–25871. doi: 10.1074/jbc.M303201200
Sarkar, M. K., Paul, K., and Blair, D. (2010). Chemotaxis signaling protein CheY binds to the rotor protein FliN to control the direction of flagellar rotation in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 107, 9370–9375. doi: 10.1073/pnas.1000935107
Sato, K., Naito, M., Yukitake, H., Hirakawa, H., Shoji, M., McBride, M. J., et al. (2010). A protein secretion system linked to bacteroidete gliding motility and pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 276–281. doi: 10.1073/pnas.0912010107
Sato, K., Yukitake, H., Narita, Y., Shoji, M., Naito, M., and Nakayama, K. (2013). Identification of Porphyromonas gingivalis proteins secreted by the Por secretion system. FEMS Microbiol. Lett. 338, 68–76. doi: 10.1111/1574-6968.12028
Shrivastava, A., and Berg, H. C. (2015). Towards a model for Flavobacterium gliding. Curr. Opin. Microbiol. 28, 93–97. doi: 10.1016/j.mib.2015.07.018
Shrivastava, A., Johnston, J. J., van Baaren, J. M., and McBride, M. J. (2013). Flavobacterium johnsoniae GldK, GldL, GldM, and SprA are required for secretion of the cell surface gliding motility adhesins SprB and RemA. J. Bacteriol. 195, 3201–3212. doi: 10.1128/JB.00333-13
Shrivastava, A., Lele, P. P., and Berg, H. C. (2015). A rotary motor drives Flavobacterium gliding. Curr. Biol. 25, 338–341. doi: 10.1016/j.cub.2014.11.045
Shukla, D., Zhu, X. Y., and Matsumura, P. (1998). Flagellar motor-switch binding face of CheY and the biochemical basis of suppression by CheY mutants that compensate for motor-switch defects in Escherichia coli. J. Biol. Chem. 273, 23993–23999. doi: 10.1074/jbc.273.37.23993
Söding, J., Biegert, A., and Lupas, A. N. (2005). The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33, W244–W248. doi: 10.1093/nar/gki408
Sonnhammer, E. L., von Heijne, G., and Krogh, A. (1998). A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 6, 175–182.
Sourjik, V., and Wingreen, N. S. (2012). Responding to chemical gradients: bacterial chemotaxis. Curr. Opin. Cell Biol. 24, 262–268. doi: 10.1016/j.ceb.2011.11.008
Taguchi, Y., Sato, K., Yukitake, H., Inoue, T., Nakayama, M., Naito, M., et al. (2015). Involvement of an Skp-like protein, PGN_0300, in the Type IX secretion system of Porphyromonas gingivalis. Infect. Immun. 84, 230–240. doi: 10.1128/IAI.01308-15
Tomek, M. B., Neumann, L., Nimeth, I., Koerdt, A., Andesner, P., Messner, P., et al. (2014). The S-layer proteins of Tannerella forsythia are secreted via a type IX secretion system that is decoupled from protein O-glycosylation. Mol. Oral Microbiol. 29, 307–320. doi: 10.1111/omi.12062
Tusnády, G. E., and Simon, I. (1998). Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J. Mol. Biol. 283, 489–506. doi: 10.1006/jmbi.1998.2107
van den Ent, F., and Löwe, J. (2006). RF cloning: a restriction-free method for inserting target genes into plasmids. J. Biochem. Biophys. Methods 67, 67–74. doi: 10.1016/j.jbbm.2005.12.008
Zaslaver, A., Bren, A., Ronen, M., Itzkovitz, S., Kikoin, I., Shavit, S., et al. (2006). A comprehensive library of fluorescent transcriptional reporters for Escherichia coli. Nat. Methods 3, 623–628. doi: 10.1038/nmeth895
Zhu, Y., and McBride, M. J. (2014). Deletion of the Cytophaga hutchinsonii type IX secretion system gene sprP results in defects in gliding motility and cellulose utilization. Appl. Microbiol. Biotechnol. 98, 763–775. doi: 10.1007/s00253-013-5355-2
Zoued, A., Durand, E., Bebeacua, C., Brunet, Y. R., Douzi, B., Cambillau, C., et al. (2013). TssK is a trimeric cytoplasmic protein interacting with components of both phage-like and membrane anchoring complexes of the type VI secretion system. J. Biol. Chem. 288, 27031–27041. doi: 10.1074/jbc.M113.499772
Keywords: transcriptional regulation, type IX secretion, gingivitis, periodontitis, Porphyromonas gingivalis, two-component system, bacterial pathogenesis
Citation: Vincent MS, Durand E and Cascales E (2016) The PorX Response Regulator of the Porphyromonas gingivalis PorXY Two-Component System Does Not Directly Regulate the Type IX Secretion Genes but Binds the PorL Subunit. Front. Cell. Infect. Microbiol. 6:96. doi: 10.3389/fcimb.2016.00096
Received: 21 July 2016; Accepted: 19 August 2016;
Published: 31 August 2016.
Edited by:
Georgios N. Belibasakis, Karolinska Institutet, SwedenReviewed by:
Hua Xie, Meharry Medical College, USAMaribasappa Karched, Kuwait University, Kuwait
Joe Aduse-Opoku, Queen Mary University of London, UK
Copyright © 2016 Vincent, Durand and Cascales. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maxence S. Vincent, mvincent@imm.cnrs.fr
Eric Cascales, cascales@imm.cnrs.fr
 Maxence S. Vincent
Maxence S. Vincent Eric Durand
Eric Durand Eric Cascales
Eric Cascales