Oral Immunization with a Multivalent Epitope-Based Vaccine, Based on NAP, Urease, HSP60, and HpaA, Provides Therapeutic Effect on H. pylori Infection in Mongolian gerbils
- 1Ningxia Key Laboratory of Clinical and Pathogenic Microbiology, General Hospital of Ningxia Medical University, Yinchuan, China
- 2Department of Medical Laboratory, School of Clinical Medicine, Ningxia Medical University, Yinchuan, China
- 3Ningxia Key Laboratory of Cerebrocranial Diseases, Ningxia Medical University, Yinchuan, China
- 4Research Center for High Altitude Medicine, Qinghai University, Xining, China
- 5Medical School of Nantong University, Nantong University, Nantong, China
- 6Department of Molecular Microbiology and Immunology, Johns Hopkins University, Baltimore, MD, United States
Epitope-based vaccine is a promising strategy for therapeutic vaccination against Helicobacter pylori (H. pylori) infection. A multivalent subunit vaccine containing various antigens from H. pylori is superior to a univalent subunit vaccine. However, whether a multivalent epitope-based vaccine is superior to a univalent epitope-based vaccine in therapeutic vaccination against H. pylori, remains unclear. In this study, a multivalent epitope-based vaccine named CWAE against H. pylori urease, neutrophil-activating protein (NAP), heat shock protein 60 (HSP60) and H. pylori adhesin A (HpaA) was constructed based on mucosal adjuvant cholera toxin B subunit (CTB), Th1-type adjuvant NAP, multiple copies of selected B and Th cell epitopes (UreA27–53, UreA183–203, HpaA132–141, and HSP60189–203), and also the epitope-rich regions of urease B subunit (UreB158–251 and UreB321–385) predicted by bioinformatics. Immunological properties of CWAE vaccine were characterized in BALB/c mice model. Its therapeutic effect was evaluated in H. pylori-infected Mongolian gerbil model by comparing with a univalent epitope-based vaccine CTB-UE against H. pylori urease that was constructed in our previous studies. Both CWAE and CTB-UE could induce similar levels of specific antibodies against H. pylori urease, and had similar inhibition effect of H. pylori urease activity. However, only CWAE could induce high levels of specific antibodies to NAP, HSP60, HpaA, and also the synthetic peptides epitopes (UreB158–172, UreB181–195, UreB211–225, UreB349–363, HpaA132–141, and HSP60189–203). In addition, oral therapeutic immunization with CWAE significantly reduced the number of H. pylori colonies in the stomach of Mongolian gerbils, compared with oral immunization using CTB-UE or H. pylori urease. The protection of CWAE was associated with higher levels of mixed CD4+ T cell (Th cell) response, IgG, and secretory IgA (sIgA) antibodies to H. pylori. These results indic ate that a multivalent epitope-based vaccine including Th and B cell epitopes from various H. pylori antigens could be a promising candidate against H. pylori infection.
Introduction
Helicobacter pylori (H. pylori) is a helix-shaped bacterium that infects more than half of the world's population (Vakil et al., 2010). H. pylori infection is closely associated with gastritis, peptic ulcer disease, and stomach cancer (Parsonnet et al., 1991). Current antibiotic-based triple therapies have many disadvantages such as high cost, poor patient compliance, increasing antibiotic resistance, and reinfection (Graham and Fischbach, 2010). Therefore, antibiotic-based triple therapies are not practical for global control. Vaccination against H. pylori infection, especially therapeutic vaccination, could be an effective and economic strategy, either as an alternative or a complementary to antibiotic-based triple therapies.
Many antigens from H. pylori, such as urease, heat shock protein 60 (HSP60), H. pylori adhesin A (HpaA) and neutrophil-activating protein (NAP), have been proved to be the excellent candidates for their ability to induce protective immune responses against H. pylori infection (Satin et al., 2000; Yamaguchi et al., 2000; Lucas et al., 2001; Flach et al., 2011; Vermoote et al., 2013). NAP is not only a major virulence factor, but also a protective antigen. Besides, NAP has potential application as a general vaccine adjuvant for inducing Th1 cell-mediated immunity (D'Elios et al., 2007). HpaA is essential for the adhesion of H. pylori to human gastric tissue. It has been reported that a lysine rich peptide fragment from HpaA is involved in receptor recognition, which is crucial for the binding of H. pylori to gastric epithelium (Chaturvedi et al., 2001). H. pylori produces large amounts of urease (Ure) which is composed of two subunits, UreA and UreB. Urease can hydrolyze urea to ammonia and carbon dioxide, thereby neutralizing gastric acid and facilitating H. pylori colonization (Suerbaum and Josenhans, 1999). Many antigenic epitopes from H. pylori urease, such as Th cell epitope UreA27–53 (Rizos et al., 2003) and B cell epitopes UreA183–203 (Fujii et al., 2004) and UreB321–339 (Hirota et al., 2001), have been identified and could be useful for epitope-based vaccine development. The main heat shock proteins (HSP) possessed by H. pylori are the GroEL/S (58 KD also called HSPB/HSP60 and 13 KD also called HSPA, respectively) and the Dna K/J (also called HSP70) chaperones (Suerbaum et al., 1994). Heat shock protein 60 (HSP60) has been demonstrated to be expressed on the surface of H. pylori, and facilitate adhesion to host cells (Yamaguchi et al., 1997). The epitope peptide recognized by the H9 MAb against HSP60 was mapped to the sequence of amino acids 189–203 (HSP60189–203; Yamaguchi et al., 2000). A univalent vaccine composed of a single H. pylori antigen has limited protective efficiency against H. pylori infection. Therefore, a multivalent vaccine containing various antigens from H. pylori has been well accepted to be superior to a univalent vaccine (Corthesy et al., 2005; Wu et al., 2008). However, there are still some drawbacks in multivalent recombinant subunit vaccines containing several antigens. For example, each subunit antigen from the pathogen has a large molecular weight so that it is difficult to construct and express recombinant subunit vaccine containing more than two antigens. Therefore, it is an effective approach to construct multivalent epitope-based vaccines by using the selected epitope peptides or the predicted epitope-rich regions, instead of using the whole antigens.
Animal models have been widely emphused to study H. pylori infection, such as mice, rats, beagle dogs, cats, or nonhuman primates (Czinn and Blanchard, 2011). The most widely used animal model involves infection of mice with H. pylori. The mouse is small, inexpensive and convenient, and the elegant genetics permits molecular dissection of the host response to H. pylori infection. However, the function of the H. pylori Cag-type IV secretion system (T4SS) is commonly lost during colonization of mice (Philpott et al., 2002). This occurs less frequently in the Mongolian gerbil (M. gerbil), indicating the Mongolian gerbil model seems more suitable for H. pylori infection (Rieder et al., 2005). In addition, the M. gerbil is an efficient and cost-effective rodent model that recapitulates many features of H. pylori-induced gastritis and carcinogenesis in humans (Liu et al., 2016; Jang et al., 2017).
In this study, our aim is to develop a multivalent epitope-based vaccine, CWAE against H. pylori, involving urease A and B subunit (UreA and UreB), NAP, HSP60, and HpaA, which are involved in the adhesion and virulence of H. pylori to gastric mucosa. Immunological properties of CWAE vaccine were evaluated in BALB/c mouse model, and its therapeutic effect was analyzed in Mongolian gerbils, in which H. pylori infection is similar to that in humans.
Materials and Methods
Multivalent Epitope-Based Vaccine Design
Based on a consensus approach which combines NN-align, SMM-align, and combinatorial library methods, potential immunodominant CD4+ T cell epitopes binding to human leukocyte antigen (HLA) class II molecules were screened from UreB by online T Cell Epitope Prediction Tools (IEDB Analysis Resource, http://tools.iedb.org/main/tcell/). The predicted CD4+ T cell epitopes with percentile rank ≤ 1 are considered high affinity and used as the candidate epitopes for vaccine design (Supplementary Data Sheet 1). At the same time, based on sequence characteristics of the UreB antigen using amino acid scales and Hidden Markov Model (HMM), potential linear B cell epitopes was screened by online B Cell Epitope Prediction Tools (IEDB Analysis Resource, http://tools.iedb.org/main/bcell/). An output threshold of 0.9 was used (sensitivity = 0.25, specificity = 0.91) for identification of linear B cell epitopes. This threshold was chosen to provide a high level of certainty for predicted B-cell epitopes from UreB. The predicted B cell epitopes are as shown in Supplementary Data Sheet 2. In addition, the known epitopes of UreB were also obtained from Immune Epitope Database (IEDB, http://www.iedb.org/home_v3.php), as shown in Supplementary Data Sheet 3. The final epitope-rich regions of UreB were determined by overall consideration on the predicted and known epitopes. Finally, the predicted epitope-rich regions (UreB158–251 and UreB321–385) and the other four B or Th cell epitopes [UreA27–53 (Rizos et al., 2003), UreA183–203 (Hifumi et al., 2006), HpaA132–141 (Chaturvedi et al., 2001), and HSP60189–203 (Yamaguchi et al., 2000)] were used to construct multivalent epitope-based vaccine. The reasonable combination of mucosal adjuvant CTB, Th1-type immune adjuvant NAP, the linkers (KK, GS, GGG, DPRVPSS), the tandem copies of Th or B cell epitopes (UreA27–53, UreA183–203, HpaA132–141, and HSP60189–203) and two predicted UreB epitope-rich regions (UreB158–251 and UreB321–385) were determined by modeling and prediction using RANKPEP, molecular operating environment (MOE), and DNAstar software.
Construction of Multivalent Epitope-Based Vaccine
To construct the vector expressing the multivalent epitope-based vaccine, a DNA fragment named WAE encoding NAP, multiple copies of selected B and Th cell epitopes (UreA27–53, UreA183–203, HpaA132–141, and HSP60189–203) and the UreB epitope-rich regions (UreB158–251 and UreB321–385) were synthesized and cloned into vector pETC containing cholera toxin B subunit (CTB) gene, generating the plasmid pETCWAE. The plasmid pETCWAE was transformed into E. coli BL21 for expression of the fusion protein CWAE. The CWAE protein was purified by Ni2+-NTA affinity chromatography according to according to the manufacturer's instructions.
Western Blot
Purified CWAE and CTB-UE, various H. pylori antigens (UreA, UreB, HpaA, HSP60, and NAP; Linc-Bio, Shanghai, China) were applied to 15% SDS-PAGE and transferred onto polyvinylidene difluoride membrane (PVDF, Millipore). Rabbit anti-H. pylori polyclonal antibody (Rabbit anti-Hp PcAb, Abace biology, Beijing, China) was used as primary antibody for CWAE and CTB-UE. Mice anti-CWAE polyclonal antibody (prepared by our laboratory) was for various H. pylori antigens (UreA, UreB, HpaA, Hsp60, and NAP). After washing, the membrane was then incubated with HRP-Goat Anti-Rabbit IgG (Proteintech) or HRP-Goat Anti-Mouse IgG (Proteintech). The positive signals were monitored using HRP-DAB Chromogenic Substrate Kit (Tiangen Biotech) according to manufacturer's reagent instructions.
Immunization and Infection
All animal experiments were approved by the Animal Ethical and Experimental Committee of Ningxia Medical University. Immunological properties of CWAE vaccine were characterized in BALB/c mice model. SPF BALB/c mice (male, 5–6 weeks old; n = 6) were immunized with 100 μg of the purified CWAE, CTB-UE, or CTB (Absin Bioscience Inc., Shanghai) by abdominal multipoint subcutaneous injection with Freund's adjuvant 3 times at 1 week's interval. The antigens in PBS without adjuvant were for the last booster immunization. Anti-serum were separated on the fifth day after the last booster and were used for detection of antibody level and specificity.
Mongolian gerbils (male, 6 weeks old) were used for evaluating therapeutic effect of the CWAE vaccine. The experimental design of therapeutic vaccination was shown in Figure 1. Firstly, Mongolian gerbils were infected with H. pylori SS1 (109 CFUs) using intubation, four times within the span of two weeks. For therapeutic vaccination, H. pylori-infected Mongolian gerbils were randomized into four groups (n = 7). Then H. pylori-infected Mongolian gerbils were vaccinated intra-gastrically with 100 μg of CWAE, CTB-UE or Urease in 500 μl aluminum hydroxide adjuvant for four times at 1-week interval. The infected Mongolian gerbils were also immunized with PBS, as control, using the same method. Two weeks after the final vaccination, Mongolian gerbils were sacrificed for evaluation of H. pylori infection.
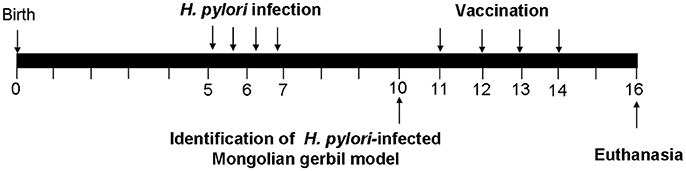
Figure 1. The experimental design of therapeutic vaccination. Briefly, Mongolian gerbils were infected with H. pylori SS1. We tested whether H. pylori-infected Mongolian gerbils are successful at the 10th week. H. pylori-infected Mongolian gerbils were administered orally with 100 μg of CWAE, CTB-UE, Urease in 500 μl aluminum hydroxide adjuvant on 4 occasions (weeks 11, 12, 13, and 14), and 500 μl PBS was also given orally on 4 occasions as control. Two weeks after the final immunization (At 16th weeks), Mongolian gerbils were sacrificed for various testing items.
Assay for Antigen-Specific Antibody and Peptide-Specific Antibody
Serum antibody levels were measured by ELISA. ELISA plates were coated with 0.5 μg/well of large antigen (Urease, UreA, UreB, HSP60, HpaA, NAP, or H. pylori lysates) or with 1 μg/well of synthetic epitope peptide (UreA183–203, UreB321–339, UreB158–172, UreB181–195, UreB211–225, UreB349–363, HpaA132–141, and HSP60189–203). To measure the serum IgG, blood was collected immediately before sacrifice. The serum was isolated and serially diluted before assay. The titer was determined as the reciprocal of the last dilution, with an OD >2 times the negative specimens (normal mouse serum). To detect secretory IgA (sIgA), the supernatants from the homogenized stomach tissue, intestinal tissue or feces were collected and diluted 1:5 in PBS for analysis of sIgA.
GM1-ELISA
The ability of the CTB component in CWAE to bind to its cellular receptor was assessed by GM1-ELISA as previously described (Areas et al., 2004). Briefly, ELISA plates were coated with 1 μg/well GM1 ganglioside or BSA for 24 h. After washing, ELISA plates were blocked by incubating with 5% (m/V) skim milk for 2 h. The CWAE, CTB-UE, CTB or UreB proteins (100 μg/ml) were then added to ELISA plates and incubated for 2 h. After that, a proper dilution of anti-CTB polyclonal antibody (Biomade Technology) was added to the plates and incubated for 1 h. After washing, HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, USA) was added to the plate and incubated for 1 h. Substrate tetramethylbenzidine (TMB, Tiangen Biotech) was then added and incubated for 10 min. The absorbance was measured at 450 nm.
H. pylori Urease Neutralization Assay
A protein A sepharose column (BioVision) was used to purify mouse IgG in the antiserum. H. pyloti urease were incubated with 50 μl serial dilutions of purified antiserum IgG (0–30 μg/ml) in ELISA plates for 12 h at 4°C. After that, The ELISA plates were incubated with 50 μl of 50 mM phosphate buffer (pH 6.8) containing 0.02% phenol red, 500 mM urea and 0.1 mM dithiothreitol (DTT) at 37°C. Color development was measured at 550 nm at 30 min intervals over a period of 3 h. Percentage inhibition was determined by the following equation: [(activity without antiserum − activity with antiserum)/(activity without antiserum) × 100].
H. pylori Quantification and Urease Activity Determination
Two weeks after the final therapeutic vaccination, mice were killed for determination of the H. pylori colonization in the stomachs. Briefly, the stomach was dissected into two tissue fragments along the lesser curvature. One fragment was homogenized in 2 ml of PBS by using a tissue homogenizer. Serial 10-fold dilutions of the homogenate were plated on H. pylori selective plate (QingDao Hopebio Technology) supplemented with 7% goat blood, trimethoprim (5 μg/ml), polymixin B (5 μg/ml), and vancomycin (10 μg/ml) under microaerobic conditions. After 4–6 days culture, colonies were counted and the number of Colony-Forming Units (CFU) per stomach was calculated. The degree of H. pylori colonization in the mouse stomach was also measured by rapid urease test. Briefly, the antral portion of the stomach was immediately immersed in 500 μl of sodium phosphate buffer containing 500 mM urea, 0.02 % phenol red, and 0.1 mM DTT. The stomach sample was incubated at 37°C for 3 h. The supernatant of specimens was used for quantification at 550 nm (A550).
Gastric Histology
One strip of stomach tissue was cut out and fixed with formalin. Then, the stomach tissue was embedded in paraffin and stained with hematoxylin and eosin (HE). For evaluation of gastritis, the slides were “blinded” and the extent of gastritis was graded as follows: 0, none; 1, a few leukocytes scattered in the deep mucosa; 2, moderate numbers of leukocytes in the deep to mid mucosa and occasional neutrophils in the gastric glands (microabscesses); 3, dense infiltrates in the deep to mid mucosa, a few microabscesses; and 4, dense, diffuse infiltrates throughout the lamina propria and into the submucosa, frequent microabscesses. The stomach tissue sections were also assessed for the presence of H. pylori infection by immunohistochemical (IHC) staining using polyclonal anti-H. pylori antibody (Linc-Bio, Shanghai, China) and polymer-HRP based detection system (BioGenex).
Specific T Lymphocyte Response and Cytokine Production
Splenic lymphocytes were isolated with lymphocyte separation medium (Dakewe Biotechnology Company) and cultured (2 × 105 cells/well) with synthetic peptides of the Th epitopes (UreB229–251, UreA27–53, or UreB373–385), NAP, Urease or H. pylori lysates (5 μg/ml) in plates at 37°C for 72 h. Then, 10 μl of the CCK-8 solution (Dojindo Molecular Technologies Inc) was added into plates for 4 h. The results were expressed as stimulation indices (SI), defined as the index of lymphocyte proliferation according to formula: SI = the absorbance 450 value of stimulated cultures/the absorbance 450 value of negative control cultures. To determine cytokine production, culture supernatants from splenic lymphocytes stimulated by H. pylori lysates for 72 h were collected to assay for IL-4, IFN-γ, and IL-17 by using ELISA kits (Shanghai Jiang Lai Biotechnology Co. Ltd., China) according to the manufacturer's instructions.
Statistical Analyses
All data were analyzed with the GraphPad Prism 5 software and expressed as mean ± SD. Statistical significance was tested using Student's t-test. p < 0.05 was considered as statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant).
Results
Design and Construction of Multivalent Vaccine CWAE
An univalent epitope-based vaccine CTB-UE against H. pylori urease composed of molecular adjuvants CTB and tandem copies of urease B and Th cell epitopes was constructed in previous study (Guo et al., 2014), as shown in Figure 2B. In order to obtain a vaccine with better protective effect, a multivalent epitope-based vaccine named CWAE (GenBank access number. MF402943) against Urease (Ure), NAP, HSP60, and HpaA was constructed. The structure diagram of multivalent epitope vaccine CWAE is shown in Figure 2A. The epitope-rich regions UreB158–251 and UreB321–385 in CWAE were obtained by using online T Cell Epitope Prediction Tools and B Cell Epitope Prediction Tools. The epitope-rich region UreB158–251 was found to contain four known Th or B cell epitopes [UreB158–172 and UreB181–195 (Qiu et al., 2010), UreB211–225 (Li et al., 2008), UreB229–251 (Shi et al., 2007)]. The epitope-rich region UreB321–385 was also found to contains three known Th or B cell epitopes [UreB327–334 (Hirota et al., 2001), UreB349–363 (Qiu et al., 2010), and UreB373–385 (Yang et al., 2013)]. In addition, the other four B or Th cell epitopes UreA27–53 (Rizos et al., 2003), UreA183–203 (Hifumi et al., 2006), HpaA132–141 (Chaturvedi et al., 2001), and HSP60189–203 (Yamaguchi et al., 2000) were also used as the components of multivalent vaccine. The theoretically optimal combination of cholera toxin B subunit (CTB), NAP, linkers, the tandem copies of the selected epitopes, and epitope-rich regions of UreB was determined by modeling and prediction using RANKPEP, MOE, and DNAstar software. CTB and NAP were selected as intra-molecular adjuvants, and linker (KK, GS, GGG, DPRVPSS) was used as a spacer between epitope tandem. In order to construct the recombinant expression vector pETCWAE containing the fusion gene CWAE, a synthetic WAE gene was synthesized and subcloned into pETC, as shown in Supplementary Figure 1a. The recombinant vectors pETC and pETCWAE was identified by enzyme restriction (Supplementary Figures 1b,c) and confirmed by DNA sequencing (data not shown).
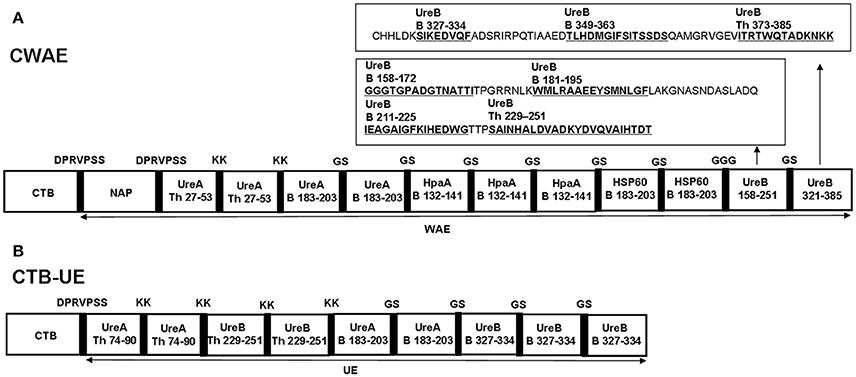
Figure 2. The structural diagrams of multivalent epitope-based vaccine CWAE and urease epitope-based vaccine CTB-UE. CWAE and CTB-UE share some same B or Th cell epitopes (UreB229–25, UreA183–203, and UreB327–334) which are marked in red fonts. (A) The CWAE vaccine is composed of two molecular adjuvants (CTB and NAP), tandem copies of the selected B and Th cell epitopes (UreA27–53, UreA183–203, HpaA132–141, and HSP60189–203), and epitope-rich regions UreB158–251 containing four known Th or B cell epitopes (UreB158–172, UreB181–195, UreB211–225, UreB229–251) and UreB321–385 containing three Th or B cell epitopes (UreB327–334, UreB349–363, UreB373–385). In order to avoid generating new epitopes at linkage sites, these linkers (DPRVPSS, KK, GGG, and GS) were designed to separate different epitopes or adjuvants. (B) The CTB-UE vaccine contains molecular adjuvants CTB and tandem copies of four different epitopes which are Th cell epitopes (UreA74–90 and UreB229–251) and B cell epitopes (UreA183–203 and UreB327–334) from H. pylori urease. In addition, the linkers (DPRVPSS, KK, and GS) were designed to retain the immunologic competence of each Th or B epitope and avoid the generation of new epitopes at linkage sites among epitopes.
Expression, Purification, and Antigenicity of CWAE
The CWAE protein (about 70 KD) was largely expressed in inclusion body of E. coli BL21(DE3)/pETCWAE. Based on the SDS-PAGE, the content of CWAE in the inclusion bodies was about 60% of the total protein (Figure 3A; Lanes 3 and 5). After purification by Ni2+-NTA affinity chromatography, the purity of the CWAE protein was 95.8% as analyzed by SDS-PAGE (Figure 3A; Lanes 6 and 7) and computer scan. Besides, the CTB-UE protein was also mainly expressed in inclusion body (Figure 4B; Lane 2, 3, and 5) and had a relative high level of expression. The purity of the CWAE protein was 96.2% as analyzed by SDS-PAGE (Figure 3B; Lane 6, 7, and 8) and computer scan. The antigenic characteristics of CWAE and CTB-UE was identified by Western blot and GM1-ELSIA. Both CWAE and CTB-UE protein could be recognized by rabbit anti-H. pylori polyclonal antibody (Figure 3Ci). Similarly, the polyclonal antibody induced by the CWAE protein could react with H. pylori UreA, UreB, HpaA, Hsp60, and NAP (Figure 3Cii). The adjuvanticity of CTB component was analyzed by GM1-ELSIA. When GM1 was used as the coating protein, both CWAE and CTB-UE were able to bind GM1 (Figure 3D), but the ability of CWAE and CTB-UE to combine GM1 decreased compared with the positive control CTB. In addition, UreB could not bind GM1.
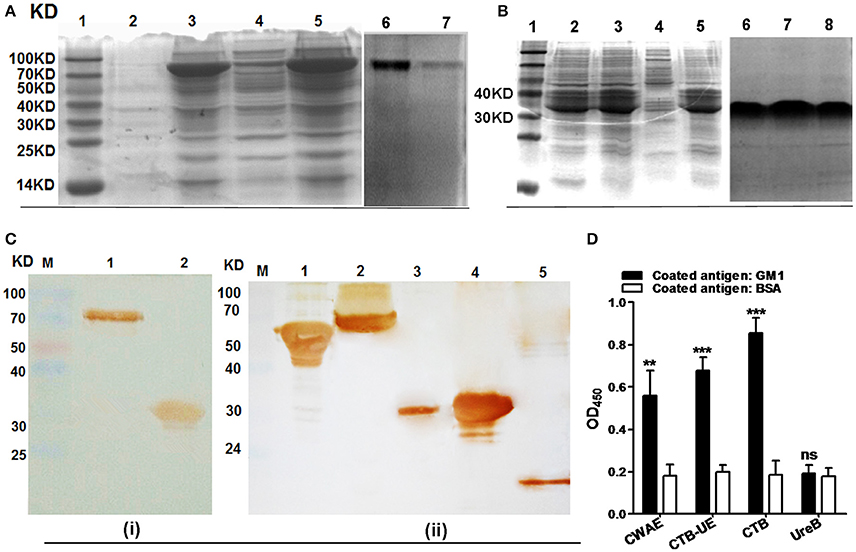
Figure 3. CWAE expression, purification, and antigen characteristics. (A) Protein expression and purification of CWAE. (Lane 1) protein marker, (lanes 3 and 5) the inclusion bodies of E. coli BL21(DE3)/pETCWAE, (lanes 2 and 4) the soluble proteins of E. coli BL21(DE3)/pETCWAE, (lane 6 and 7) the purified CWAE proteins. (B) Protein expression and purification of CTB-UE. (Lane 1) protein marker, (lanes 2, 3, and 5) the inclusion bodies of E. coli BL21(DE3)/pETCUE, (lane 4) the soluble proteins of E. coli BL21(DE3)/pETCUE, (lane 6, 7, and 8) the purified CTB-UE proteins. (C) Immunogenicity and immunoreactivity of CWAE analyzed by Western blot. (i) CWAE and CTB-UE reaction with Rabbit anti-H. pylori polyclonal antibody (Rabbit anti-Hp PcAb). (Lane M) protein marker, (lane 1) CWAE proteins, (lane 2) CTB-UE proteins. (ii) The H. pylori antigens (UreA, UreB, HpaA, Hsp60 and NAP) reaction with antiserum induced by CWAE vaccine. (Lane M) protein marker; (lane 1, 60 KD) Hsp60; (lane 2, 64 KD) UreB; (lane 3, 30 KD) UreA; (lane 4, 31 KD) HpaA; (lane 5, 14 KD) NAP. (D) The adjuvanticity of CTB component in CWAE and CTB-UE vaccine analyzed by GM1-ELSIA. In order to confirm the CTB component in CWAE or CTB-UE with the ability to bind GM1 gangliosides, GM1-ELISA was performed. ELISA plates were coated with 1 μg/well of GM1 ganglioside or BSA. The recombinant proteins CWAE, CTB-UE, CTB, and UreB with a concentration of 100 μg/ml were used to evaluate their capability of binding GM1. Data are mean ± SD. p < 0.05 was considered as statistically significant. **p < 0.01; ***p < 0.001; ns, not significant.
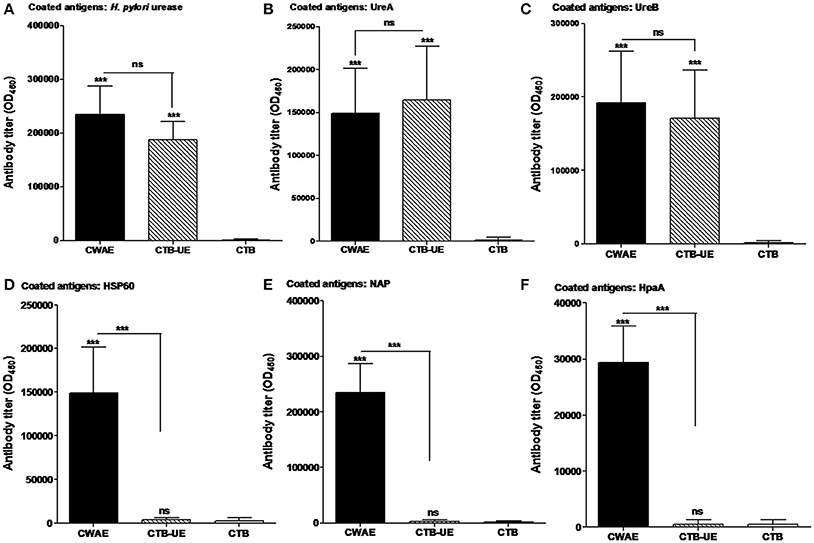
Figure 4. Assessment of antibodies specific for large antigens (H. pylori urease, UreA, UreB, Hsp60, NAP, and HpaA). The SPF BALB/c mice were immunized with CWAE, CTB-UE, or CTB by subcutaneous multi-point injection. (A) Detection of antibodies specific for H. pylori urease. ELISA plates were coated with 0.5 μg/well of native H. pylori urease. p <0.05 was considered as statistically significant. ***p < 0.001, ns, not significant. (B) Detection of antibodies specific for UreA. ELISA plates were coated with 0.5 μg/well of UreA. p < 0.05 was considered as statistically significant. ***p < 0.001, ns, not significant. (C) Detection of antibodies specific for UreB. ELISA plates were coated with 0.5 μg/well of UreB. p < 0.05 was considered as statistically significant. ***p < 0.001, ns, not significant. (D) Detection of antibodies specific for Hsp60. ELISA plates were coated with 0.5 μg/well of Hsp60. p < 0.05 was considered as statistically significant. ***p < 0.001, ns, not significant. (E) Detection of antibodies specific for NAP. ELISA plates were coated with 0.5 μg/well of NAP. p < 0.05 was considered as statistically significant. ***p < 0.001, ns not significant. (F) Detection of antibodies specific for HpaA. ELISA plates were coated with 0.5 μg/well of HpaA. p < 0.05 was considered as statistically significant. ***p < 0.001, ns, not significant.
Antigen-Specific Antibodies Induced by CWAE Vaccine
Mice were killed for determination of antibody level and specificity. Antibodies specific for large antigens (H. pylori urease, UreA, UreB, HSP60, HpaA, and NAP) were measured by ELISA. CWAE and CTB-UE could induce similar levels of antibodies specific to H. pylori urease, UreA, or UreB (Figures 4A–C). In addition, the CWAE vaccine could induce antibodies specific for HSP60 (Figure 5D), NAP (Figure 4E), and HpaA (Figure 4F), but CTB-UE could not induce these specific antibodies, which confirmed that CWAE vaccine could mount antibody responses against various virulence factors and adhesion factors of H. pylori.
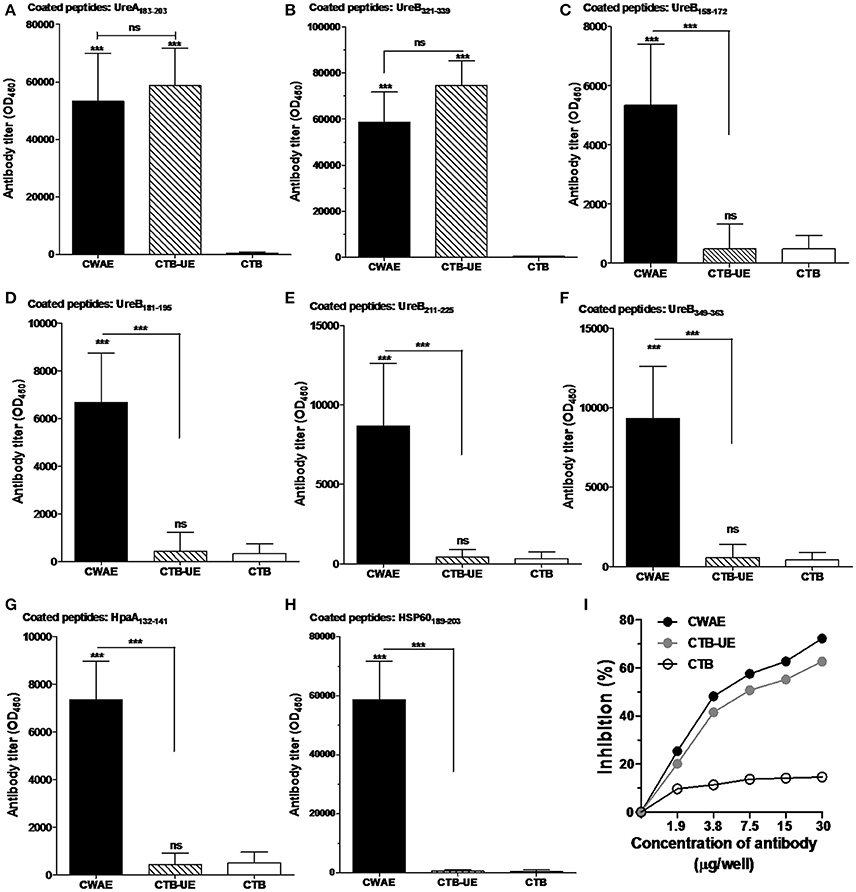
Figure 5. Detection of epitope-specific antibodies. The epitope peptides UreA183–203, UreB321–339, UreB158–172, UreB181–195, UreB211–225, UreB349–363, HpaA132–141, and HSP60189–203 were synthetized. Data are mean ± S.D. p < 0.05 was considered as statistically significant. ***p < 0.001, ns, not significant. (A) Measurement of antibodies specific for the UreA183–203 peptide. ELISA plates were coated with 1 μg/well of synthetic UreA183–203 peptides. (B) Measurement of antibodies specific for the UreB321–339 peptide. ELISA plates were coated with 1 μg/well of synthetic UreB321–339 peptides. (C) Measurement of antibodies specific for the UreB158–172 peptide. ELISA plates were coated with 1 μg/well of synthetic UreB158–172 peptides. (D) Measurement of antibodies specific for the UreB181–195 peptide. ELISA plates were coated with 1 μg/well of synthetic UreB181–195 peptides. (E) Measurement of antibodies specific for the UreB211–225 peptide. ELISA plates were coated with 1 μg/well of synthetic UreB211–225 peptides. (F) Measurement of antibodies specific for the UreB349–363 peptide. ELISA plates were coated with 1 μg/well of synthetic UreB349–363 peptides. (G) Measurement of antibodies specific for the HpaA132–141 peptide. ELISA plates were coated with 1 μg/well of synthetic HpaA132–141 peptides. (H) Measurement of antibodies specific for the HSP60189–203 peptide. ELISA plates were coated with 1 μg/well of synthetic HSP60189–203 peptides. (I) Inhibition of H. pylori urease activity by specific antibodies. Natural H. pylori urease was preincubated with a serial dilution of IgG from mice immunized with CWAE, CTB-UE, or rCTB. The optical density of the mixture was determined at 550 nm by the indicator of phenol red. The data are expressed as percentage inhibition.
Epitope-Specific Antibodies and Anti-Urease Neutralizing Antibodies
To examine the epitope-specific antibodies in mice induced by the CWAE vaccine, the B cell epitope peptides (UreA183–203, UreB321–339, UreB158–172, UreB181–195, UreB211–225, UreB349–363, HpaA132–141, and HSP60189–203) in the CWAE vaccine were synthesized and tested. The results indicated that both CWAE and CTB-UE vaccine were capable of generating similar levels of antibodies directed specifically against the epitope peptides UreA183–203 (Figure 5A) and UreB321–339 (Figure 5B). In addition, only CWAE vaccine could induce high levels of antibodies against UreB158–172 (Figure 5C), UreB181–195 (Figure 5D), UreB211–225 (Figure 5E), UreB349–363 (Figure 5F), HpaA132–141 (Figure 5G), and HSP60189–203 (Figure 5H). These results indicate that the B cell epitopes in CWAE vaccine retained their immunologic functions. To further confirm the effects of antibodies induced by CWAE and CTB-UE on H. pylori urease activity, a urease neutralization assay was performed. Natural H. pylori urease was incubated with a serial dilution of IgG induced by CWAE, CTB-UE, or rCTB. IgG antibodies induced by CWAE or CTB-UE could inhibit H. pylori urease activity dose-dependently, and the inhibitory effect of specific IgG from mice immunized with CWAE was stronger (Figure 5I). However, the IgG induced by CTB had no obvious inhibition. This result indicates that CWAE vaccine could induce higher levels of neutralizing antibodies against H. pylori urease than the whole urease antigen, which may be due to the B cell epitope UreA183–203, UreB321–339, UreB158–172, UreB181–195, UreB349–363, and UreB211–225 in CWAE vaccine.
Analysis of Bacterial Colonization in the Stomach of Mice
Since the CWAE vaccine showed good immunogenicity and immunological specificity, we next investigated whether oral immunization with CWAE vaccine could reduce the H. pylori load in the stomachs of Mongolian gerbils infected with H. pylori, and show better therapeutic effect than CTB-UE and Urease. Mongolian gerbils (already infected with H. pylori) were orally immunized with CWAE, urease or PBS. The H. pylori colonization in the stomach was analyzed by quantitative culture. The result showed that oral therapeutic immunization with CWAE, CTB-UE, or urease dramatically decreased the H. pylori loads in the stomachs of H. pylori-infected Mongolian gerbils compared with those using oral therapeutic immunization with PBS (Figure 6A). More importantly, the CWAE vaccine had a better reduction of bacterial burden than CTB-UE and Urease.
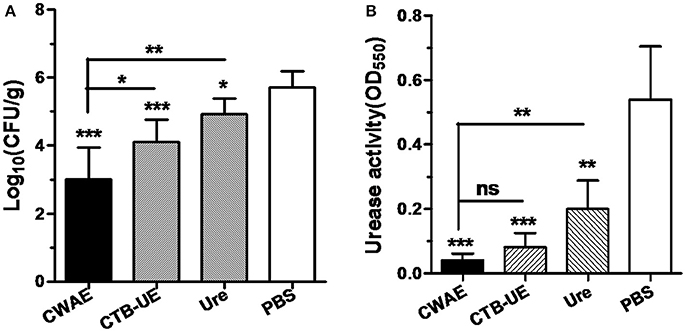
Figure 6. Evaluation of therapeutic effect by quantitative culture and rapid urease test. The H. pylori-infected Mongolian gerbils were orally immunized with CWAE, CTB-UE, Urease, or PBS. Data are mean ± S.D. p <0.05 was considered as statistically significant. *p <0.05, **p <0.01, ***p <0.001, ns, not significant. (A) Quantitative culture of H. pylori in the stomach after oral therapeutic immunization. The number of bacteria (CFU) per stomach was determined for individual mice in each group by quantitative culture. (B) The H. pylori urease activity in the stomach after oral therapeutic immunization. The H. pylori urease activity was measured by rapid urease test.
H. pylori can release large amounts of urease into the stomach. Urease decomposes urea into ammonia, which neutralizes gastric acid. Therefore, H. pylori urease is critical for H. pylori survival in the stomach. The results of rapid urease test showed that oral therapeutic immunization with CWAE, CTB-UE or urease could dramatically reduce the urease activity in the stomach, and the CWAE vaccine had a better treatment effect in decreasing urease activity in the stomach than Urease (Figure 6B). Unfortunately, there were not significant differences in urease activity between CWAE and CTB-UE vaccine, though the urease activity in the stomachs of Mongolian gerbils immunized with CWAE was lower than that of Mongolian gerbils immunized with CTB-UE.
Histological Analysis
Therapeutic effect of the CWAE vaccine was also analyzed by histopathological analysis of stomach tissue. High levels of leukocytes and neutrophils were found in the stomachs from H. pylori-infected Mongolian gerbils immunized with vehicle control PBS. In contrast, inflammation was weakened in the stomachs from H. pylori-infected Mongolian gerbils immunized with CWAE, CTB-UE, or Urease. Typical histological findings of gastric mucosa are shown in Figure 7A, and the results of histological scoring showed that the scoring grades of Mongolian gerbils immunized with CWAE were significantly lower than those of Mongolian gerbils immunized with Urease or CTB-UE (Figure 7B). Besides, Figure 7C show representative IHC results obtained with biopsies from mice immunized with CWAE, CTB-UE, Ure, and PBS, respectively. The IHC substantially confirmed the histopathologic observations.
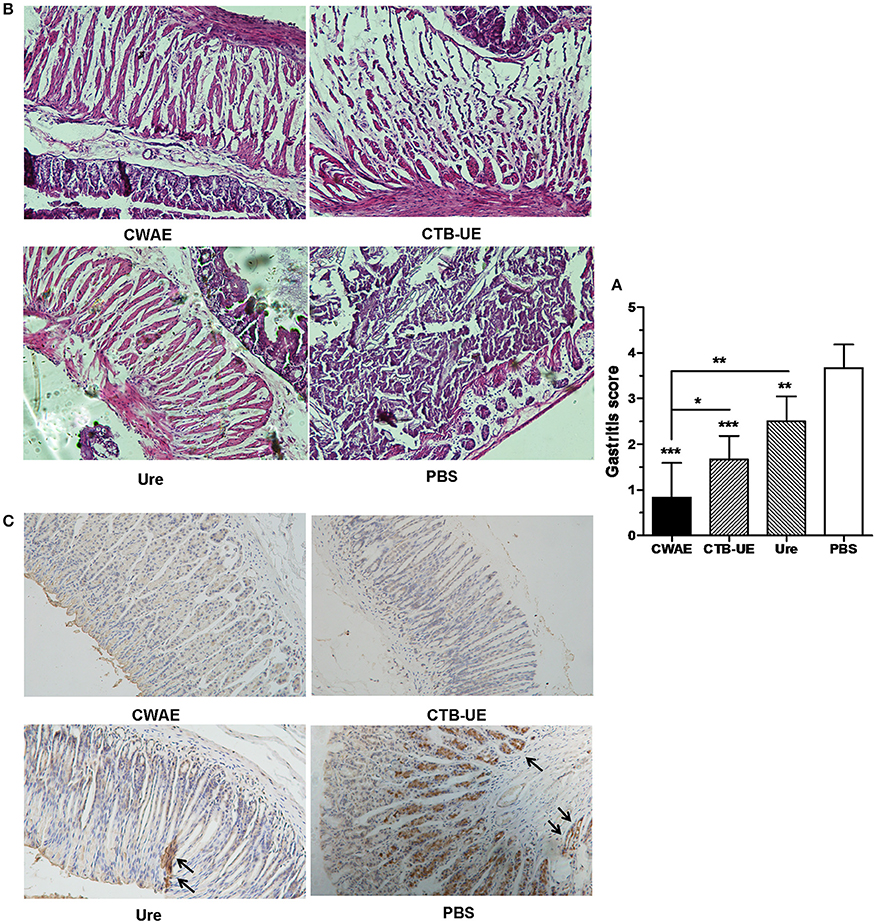
Figure 7. Histological analysis of stomach tissue. The H. pylori-infected Mongolian gerbils were orally immunized with CWAE, CTB-UE, Urease, or PBS. Data are mean ± S.D. p <0.05 was considered as statistically significant. *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant. (A) Assessment on therapeutic efficacy based on gastritis scores. The Inflammation score from Mongolian gerbils after immunization with CWAE is least. (B) Histopathological analysis after therapeutic vaccination. H. pylori-infected Mongolian gerbils after immunization with PBS showed severe inflammatory infiltrates (HE stain, 100×). However, H. pylori-infected Mongolian gerbils after immunization with CWAE, CTB-UE or Urease (Ure) showed mild inflammatory infiltrate. (C) Observation of H. pylori by IHC staining. The H. pylori-infected Mongolian gerbils after immunization with Urease (Ure) or PBS showed positively stained H. pylori within a glandular lumen. H. pylori colonizing in the stomach were denoted by black arrows.
Antibody Responses after Therapeutic Vaccination
Antibody responses in serum, gastric and intestinal mucus, and feces were analyzed in Mongolian gerbils by ELISA after therapeutic vaccination. Oral immunization with CWAE, CTB-UE, or Urease significantly raised the levels of serum IgG and IgA against H. pylori lysates compared with oral immunization with PBS. In addition, the CWAE vaccine could induce higher levels of IgG and IgA specific for H. pylori lysates than Urease and CTB-UE (Figures 8A,B), which may be due to NAP component and the B cell epitopes from HSP60 and HpaA. Secretory IgA (sIgA) antibodies in gastric mucus, intestinal mucus and feces was also measured. A modest level of sIgA was found in extracts from the gastric mucus, intestinal mucus, or feces. Oral immunization with the CWAE, CTB-UE, or Urease remarkably increased the levels of sIgA antibodies against H. pylori lysates. Moreover, the CWAE vaccine could induce higher levels of sIgA specific for H. pylori lysates than Urease and CTB-UE (Figure 8C).
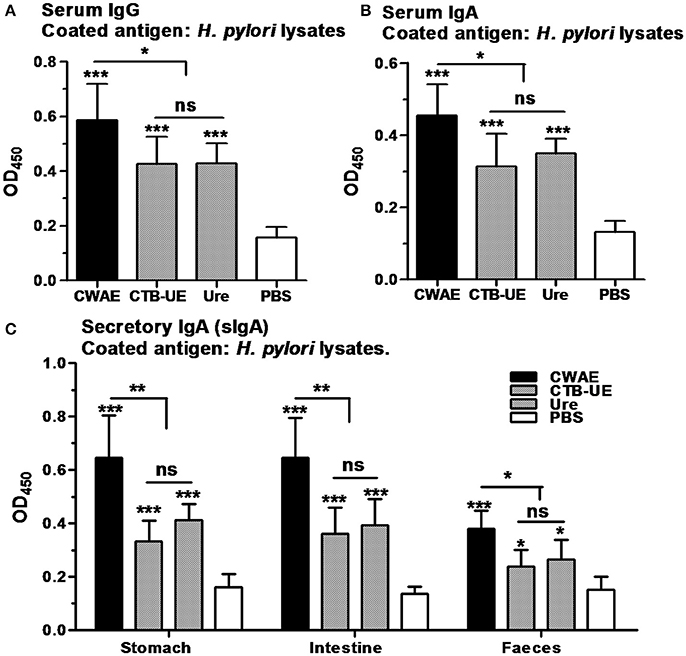
Figure 8. Antibody responses after oral therapeutic immunization. ELISA plates were coated with 0.5 μg/well of native H. pylori lysates. Data are mean ± S.D. p < 0.05 was considered as statistically significant. *p <0.05, **p < 0.01, ***p < 0.001, ns, not significant. (A) Detection of IgG against H. pylori lysates in serum. The different sera was diluted 1:800. (B) Detection of IgA against H. pylori lysates in serum. The different sera was diluted 1:800. (C) Detection sIgA against H. pylori lysates in the gastric mucus, intestinal mucus or feces. The supernatants from the homogenized stomach tissue, intestinal tissue or feces were diluted 1:5 in PBS for analysis of sIgA.
Lymphocyte Responses and Cytokine Production
To determine the ability of CWAE to elicit lymphocyte specific responses for H. pylori, splenic lymphocytes after therapeutic immunization with CWAE, CTB-UE, Urease, or PBS were stimulated with NAP, Urease, or H. pylori lysates (Figure 9A). Splenic lymphocytes from Mongolian gerbils immunized with CWAE, displayed significantly high proliferation after stimulation with NAP, Urease or H. pylori lysates, compared with lymphocytes from Mongolian gerbils immunized with PBS. However, lymphocytes from Mongolian gerbils, immunized with Urease or CTB-UE after stimulation with H. pylori NAP, had no significant proliferation compared with cells from Mongolian gerbils immunized with PBS. These results showed that the multivalent vaccine CWAE could induce lymphocyte responses against various H. pylori antigens, and NAP component in CWAE kept the function as molecular adjuvant.
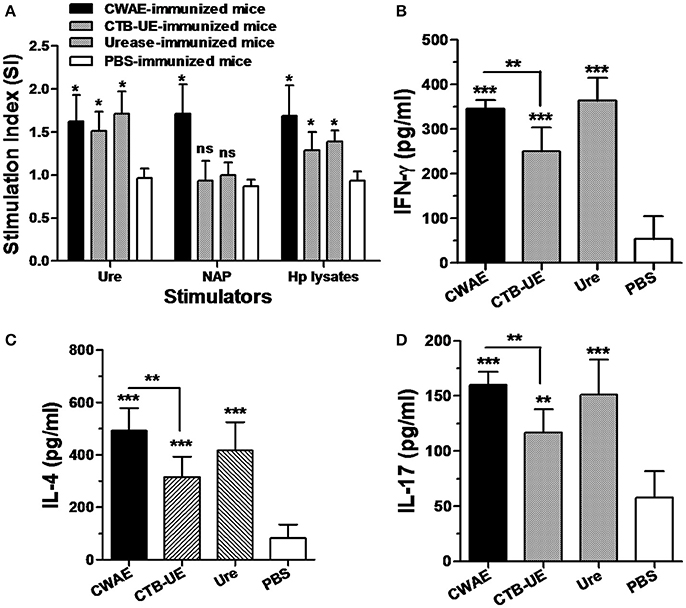
Figure 9. Lymphocyte responses and cytokine production after therapeutic vaccination. Data are mean ± S.D. p < 0.05 was considered as statistically significant. *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant. (A) Proliferation of splenic lymphocytes after stimulation with H. pylori antigens. Splenic lymphocytes were separated from H. pylori-infected Mongolian gerbils after therapeutic immunization with CWAE, CTB-UE, Urease, or PBS, and were incubated with NAP, Urease or H. pylori lysates (5 μg/ml). (B–D) Detection of IL-4, IFN-γ, and IL-17 cytokine production after therapeutic vaccination. The concentrations of cytokines in the supernatants of lymphocytes cultures were determined by ELISA. Splenic lymphocytes from H. pylori-infected Mongolian gerbils after therapeutic immunization with CWAE, CTB-UE, Urease, or PBS were stimulated with H. pylori lysates for 72 h, and cytokine production was detected by ELSA.
Cytokines IL-4, IFN-γ, and IL-17 in the supernatants of splenic lymphocyte cultures were measured by ELSIA after stimulation with H. pylori lysates. The splenic lymphocytes from Mongolian gerbils immunized with CWAE, CTB-UE, or H. pylori urease produced significantly high levels of IL-4, IFN-γ, and IL-17 cytokines. In addition, the CWAE vaccine could induce higher levels of IFN-γ (Figure 9B), IL-4 (Figure 9C), and IL-17 (Figure 9D) cytokines than CTB-UE, indicating that the CWAE vaccine induced comprehensive T cell responses of various types which were stronger than CTB-UE. However, there were no significant differences in the levels of IFN-γ, IL-4, and IL-17 cytokines between the CWAE- and Ure-immunized Mongolian gerbils.
Discussion
That antibiotic treatment of H. pylori infection has many apparent shortcomings, such as increasing antibiotic resistance, reinfection and high cost calls for an effective and economic vaccine that can eradicate H. pylori infection. Various proteins from H. pylori have been demonstrated to be excellent candidate antigens in animal model, such as UreA (Lucas et al., 2001; Rizos et al., 2003; Guo et al., 2012), UreB (Guo et al., 2013; Vermoote et al., 2013; Zhang et al., 2014), HSP60 (Yamaguchi et al., 2000; Kamiya et al., 2002), HpaA (Nystrom and Svennerholm, 2007; Flach et al., 2011), and NAP (Satin et al., 2000; Rossi et al., 2004). More importantly, it has been reported that an oral recombinant subunit vaccine containing UreB and mucosal immune adjuvant LTA2B was found to be effective in H. pylori-naive children aged between 6 and 15 years, in a phase-3 clinical trial (Zeng et al., 2015). Although, the univalent vaccines against H. pylori infection have made significant progress, the multivalent vaccines may have more advantages and potential to induce more comprehensive protection against H. pylori, especially as therapeutic vaccines. However, it is difficult to obtain a safe and effective multivalent vaccine for H. pylori proteins with large molecular weight (MW) and biological toxicity. For example, the active H. pylori urease comprising two subunits, UreA (MW, 30 KD) and UreB (MW, 66 KD), has a molecular weight of about 96 KD and induces damage to epithelial cells (Rutherford, 2014). Epitope-based vaccines represent an attractive strategy for controlling H. pylori infection, which has many potential advantages including safety, preferable immunological specificity, and the opportunity to design a multivalent epitope-based vaccine with increased potency and more abroad spectrum (Sette and Fikes, 2003). In this study, we constructed a multivalent epitope-based vaccine CWAE against H. pylori utilizing urease, HSP60, HpaA, and NAP, which are critical for H. pylori colonization and virulence. We found that oral immunization with the multivalent epitope-based vaccine CWAE could induce high levels of epitope-specific antibodies against various H. pylori antigens, and significantly reduced H. pylori colonization in Mongolian gerbils, compared with CTB-UE or Urease. The immune protection of CWAE vaccine was correlated with high levels of antigen-specific Th cell responses and epitope-specific IgG, IgA, or sIgA antibodies.
Earlier studies have demonstrated that antibody responses against H. pylori, especially sIgA antibodies, contributed to the protective immunity in the mouse models (Ferrero et al., 1997; Nystrom and Svennerholm, 2007). However, some studies showed that antibodies are not essential for protective immunity against H. pylori (Ermak et al., 1998). We speculated that antibodies with high specificity against various key H. pylori antigens might exhibit a certain protection against H. pylori. It has been reported that a trivalent vaccine including CagA, VacA, and NAP could reduce H. pylori colonization and gastritis in H. pylori-infected Beagle dogs by intramuscular injection with aluminum hydroxide adjuvant, which may be due to antibody responses against CagA, VacA, and NAP (Rossi et al., 2004). It is likely that effective immunity against H. pylori is correlated to specific antibody responses against various H. pylori antigens participating in different aspects of the pathogenesis of H. pylori infection. In addition, polyclonal IgG antibodies induced by purified urease did not induce inhibitory effect on urease activity (Nagata et al., 1992). However, several monoclonal antibodies against urease could inhibit the enzymatic activity of H. pylori urease (Hirota et al., 2001; Fujii et al., 2004), and epitope-based vaccines which can induce those monoclonal antibodies possessed protective effect against H. pylori. Thus, epitope-specific antibodies may play a role in the protection. In our study, the multivalent vaccine CWAE could induce antibodies specific for urease, HSP60, HpaA, and NAP, which are critical for H. pylori colonization and pathogenicity. Moreover, these antibodies belong to epitope-specific antibodies against specific amino acid fragment of H. pylori antigens. For example, antibodies induced by CWAE could recognize the UreA183–203, UreB321–339, UreB158–172, UreB181–195, UreB211–225, UreB349–363 fragments which are closely correlated to the enzymatic activity of H. pylori urease (Hirota et al., 2001; Fujii et al., 2004; Qiu et al., 2010), the HpaA132–141 fragments involved in receptor recognition with gastric epithelium, and the HSP60189–203 fragments which have all been proven to induce a protective immune response against H. pylori (Yamaguchi et al., 2000).
A critical role of CD4+ T cells (Th cells) in protection against H. pylori has been widely accepted (Ermak et al., 1998). However, whether Th1, Th2, or Th17 responses play dominant role in the protective immunity against H. pylori remains controversial. There is some evidence supporting that Th2 cell responses dominate the protective effect against H. pylori infection (Mohammadi et al., 1997; Saldinger et al., 1998). However, recent studies demonstrate that Th1 or Th17 cells mediate protection (DeLyria et al., 2009; Li et al., 2015). Meanwhile, other studies suggested that the protective immunity against H. pylori was mediated by mixed Th cell responses (Liu et al., 2011; Chen et al., 2014; Yang et al., 2015). Generally speaking, the immune protective mechanism against H. pylori infection still needs to be elucidated for the complementarity and complex network of immunologic system. In our study, oral immunization with CWAE had a significant therapeutic effect on H. pylori-infected Mongolian gerbils. Furthermore, analysis of the cytokine production showed that IL-4, IFN-γ, and IL-17 were all significantly induced by CWAE, indicating that the CWAE vaccine stimulated mixed Th cell responses. The mixed Th cell response induced by CWAE may be related to Th1-type cellular immune adjuvant (NAP) and mucosal adjuvant (CTB) within the vaccine, aluminum hydroxide adjuvant, and peptide components of CWAE. Furthermore, the lymphocyte proliferation results showed that splenic lymphocytes from Mongolian gerbils immunized with CWAE proliferated significantly after stimulation with NAP, Urease, and H. pylori lysates, indicating the CWAE can also induce lymphocyte responses against various H. pylori antigen. Interestingly, CWAE and Urease vaccinations elicited comparable production of IFN-γ, IL-4, and IL-17, while CWAE had significantly higher protective efficacy compared to URE vaccine, suggested that many factors, not only Urease, may be involved in H. pylori infection in vivo, and multi-target intervention in the process of H. pylori infection is critical for eradicating H. pylori colonization. The CWAE vaccine composed of HLA restricted CD4+ T (Th) cell epitopes could induce Th cell responses against H. pylori in Mongolian gerbils, implying the predicted epitope-rich regions of UreB may also contain some Mongolian gerbil Th cell epitopes. Besides, the CWAE vaccine also contains a lot of B cell epitopes, which can induce epitope-specific antibodies against H. pylori. Therefore, the immune protection of CWAE vaccine was correlated with high levels of Th cell responses and epitope-specific IgG, IgA, or sIgA antibodies.
In conclusion, a multivalent epitope-based vaccine CWAE against H. pylori was designed and constructed in this study. Oral immunization with CWAE significantly reduced H. pylori colonization in H. pylori-infected Mongolian gerbils, which may be related to mixed Th cell responses and epitope-specific antibodies against various H. pylori antigens. Given the complex nature of H. pylori infection, the CWAE vaccine will face many challenges on the road to a human vaccine. We will further evaluate the therapeutic effect of CWAE in other animal models and investigate other oral immune delivery systems, such as polysaccharide microsphere and food grade lactic acid bacteria, to help CWAE to effectively override or bypass enzymatic degradation in the human gastrointestinal tract. Furthermore, clinical trials of the therapeutic multivalent epitope-based vaccine are expected in the future. This multivalent epitope-based vaccine may be a promising vaccine candidate that may help to control H. pylori infection.
Author Contributions
Conceived and designed the experiments: LG, KL, and GX. Performed the experiments: FT, RY, HY, XG, and HL. Analyzed the data: LG and FT. Contributed reagents/materials/analysis tools: JW. Wrote the manuscript: LG, YZ, and RY.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by National Natural Science Foundation of China (grant no. 81360481; grant no. 31600744), Natural Science Foundation of Ningxia (grant no. NZ16054), Ministry of Education Chunhui Program (grant no. Z2016049), Science research project of Ningxia high school (grant no. NGY2015091), Science Foundation of Ningxia Medical University (grant no. XM2015006), Ningxia college students innovation and entrepreneurship training program (grant no. 201610752012), Preponderant Discipline Construction Project of Ningxia Medical University (grant no. XY201724), Ningxia 13th Plan of five-year Major Scientific Program (grant no. 2016BZ07) and Young Talents Cultivation Plan of Ningxia Medical University. We thank all of the researchers for their helpful suggestions during this project and Richard Ho for revising the manuscript text for grammar and style.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fcimb.2017.00349/full#supplementary-material
Supplementary Data Sheet 1. The predicted CD4+ T cell epitopes of UreB. The predicted CD4+ T cell epitopes with percentile rank ≤ 1 are considered high affinity and used as the candidate epitopes for vaccine design.
Supplementary Data Sheet 2. The predicted B cell epitopes of UreB. An output threshold of 0.9 was used (sensitivity = 0.25, specificity = 0.91) for identification of linear B cell epitopes of UreB.
Supplementary Data Sheet 3. The known CD4+ T and B epitopes of UreB. The known epitopes of UreB were obtained from Immune Epitope Database.
Supplementary Figure 1. Construction and identification of recombinant vectors. (a) The schematic flow of expression vector construction. The vector pETCWAE expressing the CWAE protein was obtained by inserting the synthetical gene WAE into pETC vector containing CTB gene. (b) Identification of the pETC vector. 1: DNA marker; 2: the recombinant plasmid pETC digested by Nco I and Xho I; After digestion, a 342 bp DNA fragment was obtained, which was consistent with the theoretical size of CTB-L fusion gene. (c) Identification of the pETCWAE vector. 1: DNA marker; 2: the recombinant plasmid pETCWAE digested by Nco I and Xho I; After digestion, a 1797 bp DNA fragment was obtained, which was consistent with the theoretical size of CWAE gene.
References
Areas, A. P., Oliveira, M. L., Miyaji, E. N., Leite, L. C., Aires, K. A., Dias, W. O., et al. (2004). Expression and characterization of cholera toxin B-pneumococcal surface adhesin A fusion protein in Escherichia coli: ability of CTB-PsaA to induce humoral immune response in mice. Biochem. Biophys. Res. Commun. 321, 192–196. doi: 10.1016/j.bbrc.2004.06.118
Chaturvedi, G., Tewari, R., Mrigank Agnihotri, N., Vishwakarma, R. A., and Ganguly, N. K. (2001). Inhibition of Helicobacter pylori adherence by a peptide derived from neuraminyl lactose binding adhesin. Mol. Cell. Biochem. 228, 83–89. doi: 10.1023/A:1013314604403
Chen, J., Li, N., and She, F. (2014). Helicobacter pylori outer inflammatory protein DNA vaccine-loaded bacterial ghost enhances immune protective efficacy in C57BL/6 mice. Vaccine 32, 6054–6060. doi: 10.1016/j.vaccine.2014.09.014
Corthesy, B., Boris, S., Isler, P., Grangette, C., and Mercenier, A. (2005). Oral immunization of mice with lactic acid bacteria producing Helicobacter pylori urease B subunit partially protects against challenge with Helicobacter felis. J. Infect. Dis. 192, 1441–1449. doi: 10.1086/444425
Czinn, S. J., and Blanchard, T. (2011). Vaccinating against Helicobacter pylori infection. Nat. Rev. Gastroenterol. Hepatol. 8, 133–140. doi: 10.1038/nrgastro.2011.1
D'Elios, M. M., Amedei, A., Cappon, A., Del Prete, G., and de Bernard, M. (2007). The neutrophil-activating protein of Helicobacter pylori (HP-NAP) as an immune modulating agent. FEMS Immunol. Med. Microbiol. 50, 157–164. doi: 10.1111/j.1574-695X.2007.00258.x
DeLyria, E. S., Redline, R. W., and Blanchard, T. G. (2009). Vaccination of mice against H pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology 136, 247–256. doi: 10.1053/j.gastro.2008.09.017
Ermak, T. H., Giannasca, P. J., Nichols, R., Myers, G. A., Nedrud, J., Weltzin, R., et al. (1998). Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188, 2277–2288. doi: 10.1084/jem.188.12.2277
Ferrero, R. L., Thiberge, J. M., and Labigne, A. (1997). Local immunoglobulin G antibodies in the stomach may contribute to immunity against Helicobacter infection in mice. Gastroenterology 113, 185–194. doi: 10.1016/S0016-5085(97)70094-5
Flach, C. F., Svensson, N., Blomquist, M., Ekman, A., Raghavan, S., and Holmgren, J. (2011). A truncated form of HpaA is a promising antigen for use in a vaccine against Helicobacter pylori. Vaccine 29, 1235–1241. doi: 10.1016/j.vaccine.2010.11.088
Fujii, R., Morihara, F., Oku, T., Hifumi, E., and Uda, T. (2004). Epitope mapping and features of the epitope for monoclonal antibodies inhibiting enzymatic activity of Helicobacter pylori urease. Biotechnol. Bioeng. 86, 434–444. doi: 10.1002/bit.20060
Graham, D. Y., and Fischbach, L. (2010). Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 59, 1143–1153. doi: 10.1136/gut.2009.192757
Guo, L., Liu, K., Xu, G., Li, X., Tu, J., Tang, F., et al. (2012). Prophylactic and therapeutic efficacy of the epitope vaccine CTB-UA against Helicobacter pylori infection in a BALB/c mice model. Appl. Microbiol. Biotechnol. 95, 1437–1444. doi: 10.1007/s00253-012-4122-0
Guo, L., Liu, K., Zhao, W., Li, X., Li, T., Tang, F., et al. (2013). Immunological features and efficacy of the reconstructed epitope vaccine CtUBE against Helicobacter pylori infection in BALB/c mice model. Appl. Microbiol. Biotechnol. 97, 2367–2378. doi: 10.1007/s00253-012-4486-1
Guo, L., Yin, R., Liu, K., Lv, X., Li, Y., Duan, X., et al. (2014). Immunological features and efficacy of a multi-epitope vaccine CTB-UE against H. pylori in BALB/c mice model. Appl. Microbiol. Biotechnol. 98, 3495–3507. doi: 10.1007/s00253-013-5408-6
Hifumi, E., Yamada, Y., and Uda, T. (2006). A catalytic antibody heavy chain HpU-2 degrading its epitope peptide and H. pylori urease. Immunol. Lett. 103, 68–74. doi: 10.1016/j.imlet.2005.10.016
Hirota, K., Nagata, K., Norose, Y., Futagami, S., Nakagawa, Y., Senpuku, H., et al. (2001). Identification of an antigenic epitope in Helicobacter pylori urease that induces neutralizing antibody production. Infect. Immun. 69, 6597–6603. doi: 10.1128/IAI.69.11.6597-6603.2001
Jang, S., Bak, E. J., and Cha, J. H. (2017). N-acetylcysteine prevents the development of gastritis induced by Helicobacter pylori infection. J. Microbiol. 55, 396–402. doi: 10.1007/s12275-017-7089-9
Kamiya, S., Osaki, T., Taguchi, H., and Yamaguchi, H. (2002). Immune response to heat shock protein of Helicobacter pylori–a candidate as a vaccine component. Keio J. Med 51(Suppl. 2), 24–25. doi: 10.2302/kjm.51.supplement2_24
Li, B., Chen, L., Sun, H., Yang, W., Hu, J., He, Y., et al. (2015). Immunodominant epitope-specific Th1 but not Th17 responses mediate protection against Helicobacter pylori infection following UreB vaccination of BALB/c mice. Sci. Rep. 5:14793. doi: 10.1038/srep14793
Li, H. X., Mao, X. H., Shi, Y., Ma, Y., Wu, Y. N., Zhang, W. J., et al. (2008). Screening and identification of a novel B-cell neutralizing epitope from Helicobacter pylori UreB. Vaccine 26, 6945–6949. doi: 10.1016/j.vaccine.2008.09.089
Liu, K. Y., Shi, Y., Luo, P., Yu, S., Chen, L., Zhao, Z., et al. (2011). Therapeutic efficacy of oral immunization with attenuated Salmonella typhimurium expressing Helicobacter pylori CagA, VacA and UreB fusion proteins in mice model. Vaccine 29, 6679–6685. doi: 10.1016/j.vaccine.2011.06.099
Liu, L. N., Ding, S. G., Shi, Y. Y., Zhang, H. J., Zhang, J., and Zhang, C. (2016). Helicobacter pylori with high thioredoxin-1 expression promotes stomach carcinogenesis in Mongolian gerbils. Clin. Res. Hepatol. Gastroenterol. 40, 480–486. doi: 10.1016/j.clinre.2015.11.001
Lucas, B., Bumann, D., Walduck, A., Koesling, J., Develioglu, L., Meyer, T. F., et al. (2001). Adoptive transfer of CD4+ T cells specific for subunit A of Helicobacter pylori urease reduces H. pylori stomach colonization in mice in the absence of interleukin-4 (IL-4)/IL-13 receptor signaling. Infect. Immun. 69, 1714–1721. doi: 10.1128/IAI.69.3.1714-1721.2001
Mohammadi, M., Nedrud, J., Redline, R., Lycke, N., and Czinn, S. J. (1997). Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology 113, 1848–1857. doi: 10.1016/S0016-5085(97)70004-0
Nagata, K., Mizuta, T., Tonokatu, Y., Fukuda, Y., Okamura, H., Hayashi, T., et al. (1992). Monoclonal antibodies against the native urease of Helicobacter pylori: synergistic inhibition of urease activity by monoclonal antibody combinations. Infect. Immun. 60, 4826–4831.
Nystrom, J., and Svennerholm, A. M. (2007). Oral immunization with HpaA affords therapeutic protective immunity against H. pylori that is reflected by specific mucosal immune responses. Vaccine 25, 2591–2598. doi: 10.1016/j.vaccine.2006.12.026
Parsonnet, J., Friedman, G. D., Vandersteen, D. P., Chang, Y., Vogelman, J. H., Orentreich, N., et al. (1991). Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325, 1127–1131. doi: 10.1056/NEJM199110173251603
Philpott, D. J., Belaid, D., Troubadour, P., Thiberge, J. M., Tankovic, J., Labigne, A., et al. (2002). Reduced activation of inflammatory responses in host cells by mouse-adapted Helicobacter pylory isolates. Cell. Microbiol. 4, 285–296. doi: 10.1046/j.1462-5822.2002.00189.x
Qiu, Y., Wang, Y. C., Tao, H. X., Zhan, D. W., Yuan, S. L., Wang, P., et al. (2010). Identification of B-cell epitopes in urease B subunit of Helicobacter pylori bound by neutralizing antibodies. Vaccine 28, 5220–5227. doi: 10.1016/j.vaccine.2010.05.067
Rieder, G., Merchant, J. L., and Haas, R. (2005). Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology 128, 1229–1242. doi: 10.1053/j.gastro.2005.02.064
Rizos, K., Lattemann, C. T., Bumann, D., Meyer, T. F., and Aebischer, T. (2003). Autodisplay: efficacious surface exposure of antigenic UreA fragments from Helicobacter pylori in Salmonella vaccine strains. Infect. Immun. 71, 6320–6328. doi: 10.1128/IAI.71.11.6320-6328.2003
Rossi, G., Ruggiero, P., Peppoloni, S., Pancotto, L., Fortuna, D., Lauretti, L., et al. (2004). Therapeutic vaccination against Helicobacter pylori in the beagle dog experimental model: safety, immunogenicity, and efficacy. Infect. Immun. 72, 3252–3259. doi: 10.1128/IAI.72.6.3252-3259.2004
Rutherford, J. C. (2014). The emerging role of urease as a general microbial virulence factor. PLoS Pathog. 10:e1004062. doi: 10.1371/journal.ppat.1004062
Saldinger, P. F., Porta, N., Launois, P., Louis, J. A., Waanders, G. A., Bouzourene, H., et al. (1998). Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacter infection. Gastroenterology 115, 891–897. doi: 10.1016/S0016-5085(98)70261-6
Satin, B., Del Giudice, G., Della Bianca, V., Dusi, S., Laudanna, C., Tonello, F., et al. (2000). The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J. Exp. Med. 191, 1467–1476. doi: 10.1084/jem.191.9.1467
Sette, A., and Fikes, J. (2003). Epitope-based vaccines: an update on epitope identification, vaccine design and delivery. Curr. Opin. Immunol. 15, 461–470. doi: 10.1016/S0952-7915(03)00083-9
Shi, Y., Wu, C., Zhou, W. Y., Mao, X. H., Guo, G., and Zou, Q. M. (2007). Identification of H-2d restricted Th epitopes in Urease B subunit of Helicobacter pylori. Vaccine 25, 2583–2590. doi: 10.1016/j.vaccine.2006.12.024
Suerbaum, S., and Josenhans, C. (1999). Virulence factors of Helicobacter pylori: implications for vaccine development. Mol. Med. Today 5, 32–39. doi: 10.1016/S1357-4310(98)01390-2
Suerbaum, S., Thiberge, J. M., Kansau, I., Ferrero, R. L., and Labigne, A. (1994). Helicobacter pylori hspA-hspB heat-shock gene cluster: nucleotide sequence, expression, putative function and immunogenicity. Mol. Microbiol. 14, 959–974. doi: 10.1111/j.1365-2958.1994.tb01331.x
Vakil, N., Malfertheiner, P., and Chey, W. D. (2010). Helicobacter pylori infection. N. Engl. J. Med. 363, 595–596. doi: 10.1056/NEJMc1006158
Vermoote, M., Flahou, B., Pasmans, F., Ducatelle, R., and Haesebrouck, F. (2013). Protective efficacy of vaccines based on the Helicobacter suis urease subunit B and gamma-glutamyl transpeptidase. Vaccine 31, 3250–3256. doi: 10.1016/j.vaccine.2013.05.047
Wu, C., Shi, Y., Guo, H., Zou, W. Y., Guo, G., Xie, Q. H., et al. (2008). Protection against Helicobacter pylori infection in mongolian gerbil by intragastric or intramuscular administration of H. pylori multicomponent vaccine. Helicobacter 13, 191–199. doi: 10.1111/j.1523-5378.2008.00609.x
Yamaguchi, H., Osaki, T., Kai, M., Taguchi, H., and Kamiya, S. (2000). Immune response against a cross-reactive epitope on the heat shock protein 60 homologue of Helicobacter pylori. Infect. Immun. 68, 3448–3454. doi: 10.1128/IAI.68.6.3448-3454.2000
Yamaguchi, H., Osaki, T., Kurihara, N., Taguchi, H., Hanawa, T., Yamamoto, T., et al. (1997). Heat-shock protein 60 homologue of Helicobacter pylori is associated with adhesion of H. pylori to human gastric epithelial cells. J. Med. Microbiol. 46, 825–831. doi: 10.1099/00222615-46-10-825
Yang, J., Dai, L. X., Pan, X., Wang, H., Li, B., Zhu, J., et al. (2015). Protection against Helicobacter pylori infection in BALB/c mice by oral administration of multi-epitope vaccine of CTB-UreI-UreB. Pathog. Dis. 73:ftv026. doi: 10.1093/femspd/ftv026
Yang, W. C., Chen, L., Li, H. B., Li, B., Hu, J., Zhang, J. Y., et al. (2013). Identification of two novel immunodominant UreB CD4+ T cell epitopes in Helicobacter pylori infected subjects. Vaccine 31, 1204–1209. doi: 10.1016/j.vaccine.2012.12.058
Zeng, M., Mao, X. H., Li, J. X., Tong, W. D., Wang, B., Zhang, Y. J., et al. (2015). Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386, 1457–1464. doi: 10.1016/S0140-6736(15)60310-5
Zhang, H. X., Qiu, Y. Y., Zhao, Y. H., Liu, X. T., Liu, M., and Yu, A. L. (2014). Immunogenicity of oral vaccination with Lactococcus lactis derived vaccine candidate antigen (UreB) of Helicobacter pylori fused with the human interleukin 2 as adjuvant. Mol. Cell. Probes 28, 25–30. doi: 10.1016/j.mcp.2013.08.003
Keywords: Helicobacter pylori, multivalent epitope-based vaccine, therapeutic vaccine, urease, NAP, HpaA, HSP60
Citation: Guo L, Yang H, Tang F, Yin R, Liu H, Gong X, Wei J, Zhang Y, Xu G and Liu K (2017) Oral Immunization with a Multivalent Epitope-Based Vaccine, Based on NAP, Urease, HSP60, and HpaA, Provides Therapeutic Effect on H. pylori Infection in Mongolian gerbils. Front. Cell. Infect. Microbiol. 7:349. doi: 10.3389/fcimb.2017.00349
Received: 10 May 2017; Accepted: 21 July 2017;
Published: 04 August 2017.
Edited by:
Alain Stintzi, University of Ottawa, CanadaReviewed by:
Chiung-Yu Hung, University of Texas at San Antonio, United StatesJere W. McBride, University of Texas Medical Branch, United States
Copyright © 2017 Guo, Yang, Tang, Yin, Liu, Gong, Wei, Zhang, Xu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Le Guo, guole@nxmu.edu.cn
Guangxian Xu, xuguangxian@nxmu.edu.cn
Kunmei Liu, lkm198507@126.com
†These authors have contributed equally to this work.
 Le Guo
Le Guo Hua Yang2†
Hua Yang2†  Guangxian Xu
Guangxian Xu