The Proteome of Biologically Active Membrane Vesicles from Piscirickettsia salmonis LF-89 Type Strain Identifies Plasmid-Encoded Putative Toxins
- 1Laboratorio de Patología de Organismos Acuáticos y Biotecnología Acuícola, Universidad Andrés Bello, Viña del Mar, Chile
- 2Facultad de Ciencias, Instituto de Bioquímica y Microbiología, Universidad Austral de Chile, Valdivia, Chile
- 3Interdisciplinary Center for Aquaculture Research, Concepción, Chile
- 4Austral-OMICS, Facultad de Ciencias, Universidad Austral de Chile, Valdivia, Chile
- 5Center of Integrative Microbiology and Evolution, University of Oslo, Oslo, Norway
- 6Department of Pharmaceutical Biosciences, School of Pharmacy, University of Oslo, Oslo, Norway
- 7Microbiology and Immunology Department, Dalhousie University, Halifax, NS, Canada
- 8Department of Biochemistry and Molecular Biology, School of Medicine, University of Kansas Medical Center, Kansas City, KS, United States
Piscirickettsia salmonis is the predominant bacterial pathogen affecting the Chilean salmonid industry. This bacterium is the etiological agent of piscirickettsiosis, a significant fish disease. Membrane vesicles (MVs) released by P. salmonis deliver several virulence factors to host cells. To improve on existing knowledge for the pathogenicity-associated functions of P. salmonis MVs, we studied the proteome of purified MVs from the P. salmonis LF-89 type strain using multidimensional protein identification technology. Initially, the cytotoxicity of different MV concentration purified from P. salmonis LF-89 was confirmed in an in vivo adult zebrafish infection model. The cumulative mortality of zebrafish injected with MVs showed a dose-dependent pattern. Analyses identified 452 proteins of different subcellular origins; most of them were associated with the cytoplasmic compartment and were mainly related to key functions for pathogen survival. Interestingly, previously unidentified putative virulence-related proteins were identified in P. salmonis MVs, such as outer membrane porin F and hemolysin. Additionally, five amino acid sequences corresponding to the Bordetella pertussis toxin subunit 1 and two amino acid sequences corresponding to the heat-labile enterotoxin alpha chain of Escherichia coli were located in the P. salmonis MV proteome. Curiously, these putative toxins were located in a plasmid region of P. salmonis LF-89. Based on the identified proteins, we propose that the protein composition of P. salmonis LF-89 MVs could reflect total protein characteristics of this P. salmonis type strain.
Introduction
Salmonid rickettsial septicemia, also known as piscirickettsiosis, is a multi-systemic infectious disease that produces septicemia in infected salmonids, ultimately affecting the kidney, liver, spleen, intestine, brain, skeletal muscle, ovaries, and gills. This disease causes high mortality rates in Atlantic salmon (Salmo salar), coho salmon (Oncorhynchus kisutch), and rainbow trout (Oncorhynchus mykiss), ultimately translating into significant economic losses for the salmon industry in Chile (Rozas and Enríquez, 2014). Piscirickettsiosis is caused by the Gram-negative, facultative intracellular bacterium Piscirickettsia salmonis. This fastidious pathogen was first reported in coho salmon in Chile and has since been found in Canada, Ireland, Norway, and Scotland (Fryer and Hedrick, 2003). Currently, piscirickettsiosis is the most important fish disease affecting marine aquaculture in Chile (Sernapesca, 2015).
Gram-negative bacteria produce membrane vesicles (MVs) during both in vitro growth and in vivo infection (Lee et al., 2008), including Escherichia coli, Pseudomonas aeruginosa, Shigella flexneri, Helicobacter pylori (Hoekstra et al., 1976; Fiocca et al., 1999; Kadurugamuwa and Beveridge, 1999), and the fish pathogens Francisella noatunensis (Bakkemo et al., 2011) and Vibrio anguillarum (Hong et al., 2009). MVs, small spherical structures that range in size from 10 to 300 nm in diameter, are released from the surface of Gram-negative bacteria. These structures are mainly composed of outer membrane proteins, lipopolysaccharides, phospholipids, and periplasmic proteins and are a reduced composition of inner membrane and cytoplasmic proteins (Deatherage et al., 2009). Interestingly, bacterial MVs can also contain toxins or effector proteins involved in survival and pathogenesis (Bomberger et al., 2009). Indeed, MVs are implicated in the pathogenicity of several bacteria, such as Acinetobacter baumannii (Kwon et al., 2009) and Edwardsiella tarda (Park et al., 2011). Importantly, MVs have been licensed for use in humans and for example to control outbreaks of disease caused by Neisseria meningitidis (Holst et al., 2009, 2013). It was recently reported that P. salmonis can produce MVs during normal growth in liquid media and during the infection of CHSE-214 cells. Interestingly, purified MVs are cytotoxic for CHSE-214 cells (Oliver et al., 2016) and zebrafish (Danio rerio) (Tandberg et al., 2016). However, despite the increasing research concerning MVs and bacterial pathogenicity, the mechanisms underlying the pathophysiological roles of MVs have not been clearly defined.
Multiple mass spectrometry (MS) methods for the proteomic characterization of bacterial MVs have been reported, including liquid chromatography-MS/MS (Kwon et al., 2009; Pierson et al., 2011; Choi et al., 2014), matrix-assisted laser desorption/ionization, time-of-flight MS (Galka et al., 2008), and multidimensional protein identification technology (MudPIT) (McCaig et al., 2013). Additionally, several proteins involved in virulence were recently pinpointed through the partial proteomic characterization of MVs from the P. salmonis LF-89 type strain using liquid chromatography-MS/MS (Oliver et al., 2016; Tandberg et al., 2016). Nevertheless, identification remains pending for the full P. salmonis-purified MVs proteome, as well as for toxins or virulence-related proteins that could contribute to pathogenicity. Therefore, the aim of this study was to extensively characterize the proteome of MVs purified from P. salmonis LF-89 using highly sensitive MudPIT technology.
Materials and Methods
Bacterial Culture
The P. salmonis LF-89 (equivalent to ATCC VR-1361) type strain was grown on AUSTRAL-TSFe agar plates at 18°C for 10 days (Yañez et al., 2013). After this period, bacteria were growth in AUSTRAL- salmonid rickettsial septicemia broth until reaching the logarithmic phase (Yañez et al., 2012). Finally, the culture (4 mL) was inoculated in a minimal liquid medium (400 mL) and incubated at 18°C with agitation (50 rpm) until the early stationary phase (Oliver et al., 2016).
Isolation and Purification of MVs from Culture Supernatant
MVs were isolated from the culture supernatant following the method described by Oliver et al. (2016). Briefly, P. salmonis cells were removed through low-speed centrifugation at 5,000 × g for 10 min at 4°C. The supernatant was sequentially filtered through a 0.45 and 0.22 μm/pore-filter to remove residual cells. Finally, MVs were isolated and concentrated through ultracentrifugation at 125,000 × g for 2 h at 4°C. The pelleted MVs were resuspended in phosphate-buffered saline (PBS) with 0.05% sodium azide. The protein concentration obtained from MVs purification was equivalent to ~166.9 ± 44.5 mg per liter of bacterial culture. The purified MVs were stored at −80°C until use. The purity of MVs after purification was confirmed by transmission electron microscopy.
Intraperitoneal Injection of P. salmonis-Derived MVs in Adult Zebrafish
A total of 120 healthy male and female wild-type, strain AB zebrafish (Danio rerio; 10–11 months-old) were obtained from the Model Fish Unit at the Norwegian University of Life Science. Fish were acclimatized for 2 weeks at room temperature (20 ± 2°C) prior to the experiment. The fish were fed every morning with brine shrimp (Scanbur AS, Nittedal, Norway) and afternoon with SDS 400 Scientific Fish Food (Scanbur AS). After acclimation, zebrafish were randomly allocated among 6 experimental groups containing 20 fish each. All fish groups were anesthetized by immersion in water containing tricaine methanesulfonate (100 mg/mL; MS-222, Sigma Aldrich St. Louis, MO, USA) buffered with bicarbonate to pH 7–7.5. Then, three experimental groups were intraperitoneally injected (27 G needle) with 20 μL of 10, 20, or 40 μg of P. salmonis LF-89 MVs in PBS (Cosma et al., 2006; Brudal et al., 2015). As a positive control, an additional group was intraperitoneally injected with 20 μL of P. salmonis LF-89 (equivalent to 109 colony forming units [CFU]/mL). Additionally, a group of 20 fish were injected with PBS as negative control.
After injection, the 6 fish groups (n = 20 fish) were separately placed into polycarbonate recovery tanks (6 L; Pentair, Minneapolis, MN, USA), in which 50% of the water was manually changed daily. Tank water was provided by the Model Fish Unit at the Norwegian University of Life Science and was supplemented with Instant Ocean sea salt (0.55 g/L; Spectrum Brands, Blacksburg, VA, USA), sodium bicarbonate (0.053 g/L), and calcium chloride (0.015 g/L). Water parameters (i.e., pH, , , NH3/, and hardness) were monitored every third day using commercial TetraTest Kits (Spectrum Brands). The tanks were maintained at 20°C with a 14:10 light:dark cycle. Tank wastewater was decontaminated through chlorination and tested for sterility before disposal.
The fish were closely monitored, and animal health was recorded twice daily. Fish that did not resume normal behavior after injections were removed from the experiment and euthanized with an overdose of tricaine methanesulfonate (250 mg/mL; Sigma Aldrich). These fish included those that were moribund or that clearly showed deviant behaviors/clinical symptoms inconsistent with good animal welfare (e.g., greatly reduced activity levels, environmental responses, and/or appetite). All experimental procedures were approved by The Norwegian Animal Research Authority.
Sample Preparation for Proteomics Analysis
Purified MVs were incubated in lysis buffer (50 mM Tris–HCl, pH 7.5; 150 mM NaCl; 1% NP-40; 0.5% sodium deoxicolate; and 1% SDS) for 1 h at 4°C. Finally, the solution was sonicated for 10 min at 4°C at a frequency of 20 kHz, lyophilized and stored at −20°C until use. All samples were analyzed by SDS-PAGE.
Lyophilized MVs proteins were resolubilized in 6 M guanidine hydrochloride and 25 mM NH4HCO3, pH 7.5. Subsequently, proteins were reduced at room temperature for 30 min with 2 mM dithiothreitol and alkylated in the dark at room temperature for 30 min with 10 mM iodoacetamide. The reaction was diluted seven times with 25 mM NH4HCO3, pH 7.5; 2 μL of 0.1 ng/mL modified trypsin (Promega, Madison, WI, USA) was added, and the reaction was incubated at 37°C for 16 h. The reaction was stopped by adding acetic acid, pH 2.0.
Identification of MV Proteins by MudPIT
All samples were concentrated on a CentriVap Concentrator (Labconco, Kansas City, MO, USA) to a final volume of 20 μL and loaded on a 350 μm ID fused silica 2D high-performance liquid chromatography triphasic peptide trap column packed in-house with 3 cm of a reverse-phase desalting C18 (100 Å, 5 μm Magic C18 particles; Michrom Bioresources, Auburn, CA, USA), 3 cm of a strong cation exchange column (300 Å, 5 μm, PolySULFOETHYL A; PolyLC Inc., Columbia, MD, USA), and, finally, 3 cm of reversed phase resolving C18. The peptide trap was mounted on the loop of a nanoLC (Thermo Finnigan LLC, Waltham, WA, USA). Following a wash with 0.1% formic acid for 30 min at 0.5 μL/min, the efflux of the peptide trap column was directed to a 10 cm resolving reversed-phase column (100 Å, 5 μm Magic C18 particles, Michrom Bioresources), which was mounted on the electrospray stage of a FT ICR mass spectrometer (LTQ FT, Thermo Finnigan LLC). The peptides were separated on-line using 15 salt steps (0, 10, 30, 50, 100, 150, 200, 250, 300, 350, 400, 500, 1,000, 1,500, and 2,000 mM NH4CH3OO) followed by a 0–90% acetonitrile gradient for 120 min at a flow rate of 350 nL/min. An electrospray voltage of 1.9 kV was used, with the ion transfer temperature set to 250°C. The mass spectrometer was controlled by the Xcalibur software, which continuously performed mass-scan analysis of the FT and, subsequently, of the six most intense ions during MS/MS scans of the ion traps. For this, one repeat scan of the same ion was dynamically excluded, using a 30 s repeat duration and 90 s exclusion duration. Normalized collision energy for the MS/MS was set to 35%. Details of the proteome analysis by MudPIT are available (Supplementary Data 2).
Data Analysis Using Database Search Algorithm
All tandem mass spectra MS/MS samples were analyzed using SEQUEST (v1.4.0.288; Thermo Fisher Scientific, San Jose, CA, USA) and X! Tandem (vCYCLONE 2010.12.01.1; The GPM, thegpm.org). SEQUEST searched the National Center for Biotechnology Information (NCBI) Piscirickettsia salmonis 12-21-2015.fasta database (10,012 entries) assuming digestion of the enzyme trypsin. X! Tandem searched a subset of the Piscirickettsia salmonis NCBI 11-03-2016 database, also assuming trypsin digestion. SEQUEST and X! Tandem were searched with a fragment ion mass tolerance of 0.80 Da and a parent ion tolerance of 50 PPM. Carbamidomethyl-cysteine was a fixed modification in SEQUEST and X! Tandem. In SEQUEST, asparagine and glutamine deamidation and methionine oxidation were variable modifications. In X! Tandem, Glu->pyro-Glu of the N-terminus, ammonia-loss of the n-terminus, gln->pyro-Glu of the N-terminus, asparagine and glutamine deamidation, and methionine oxidation were variable modifications.
Criteria for Protein Identification
Scaffold (v.4.5.0; Proteome Software Inc., Portland, OR, USA) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if the Peptide Prophet algorithm, with Scaffold delta-mass correction, established a >95.0% probability (Keller et al., 2002). Protein identifications were accepted if presenting a >99.9% probability, as assigned by the Protein Prophet algorithm, and containing at least two identified peptides (Nesvizhskii et al., 2003). Proteins containing similar peptides that could not be differentiated based on MS/MS analysis alone were grouped. Proteins sharing significant peptide evidence were grouped into clusters. Proteins were annotated with NCBI Gene Ontology terms (downloaded 17-03-2016) (Ashburner et al., 2000). Secondary and tertiary structure prediction were make using I-TASSER (https://zhanglab.ccmb.med.umich.edu/I-TASSER/). The crystal structures predictions and alignments were visualized using multiseq extension through VMD 1.9.3 (Visual Molecular Dinamics, Illinois University).
In Silico Analysis of Toxins Contained in P. salmonis MVs
Amino acid sequences from seven putative proteins annotated as toxins, and separated into two groups, were retrieved from the NCBI database and mapped on the P. salmonis LF-89 plasmid 1 (CP011850) using the TBLASTn tool with default parameters. Additionally, five putative pertussis toxin sequences (≈685 amino acids) were aligned using Clustal Omega and phylogenetically analyzed. Phylogenetic calculations and tree building were performed in the CLC Sequence Viewer v7.7 using the UPGMA method and applying a Jukes-Cantor Model. A total of 10,000 bootstrap replicates were performed to evaluate node-support values.
Statistical Analysis
For zebrafish infection, dataset analyses and graphing were completed using Graphpad Prism v7 (GraphPad Software Inc., La Jolla, CA, USA). Mortality curves were used for analyzing the percent mortality, and differences between groups were deemed statistically significant at p-value < 0.05, as established using the Gehan-Breslow-Wilcoxon and Log-rank tests.
Results
Proteome Analysis of MVs Derived from P. salmonis
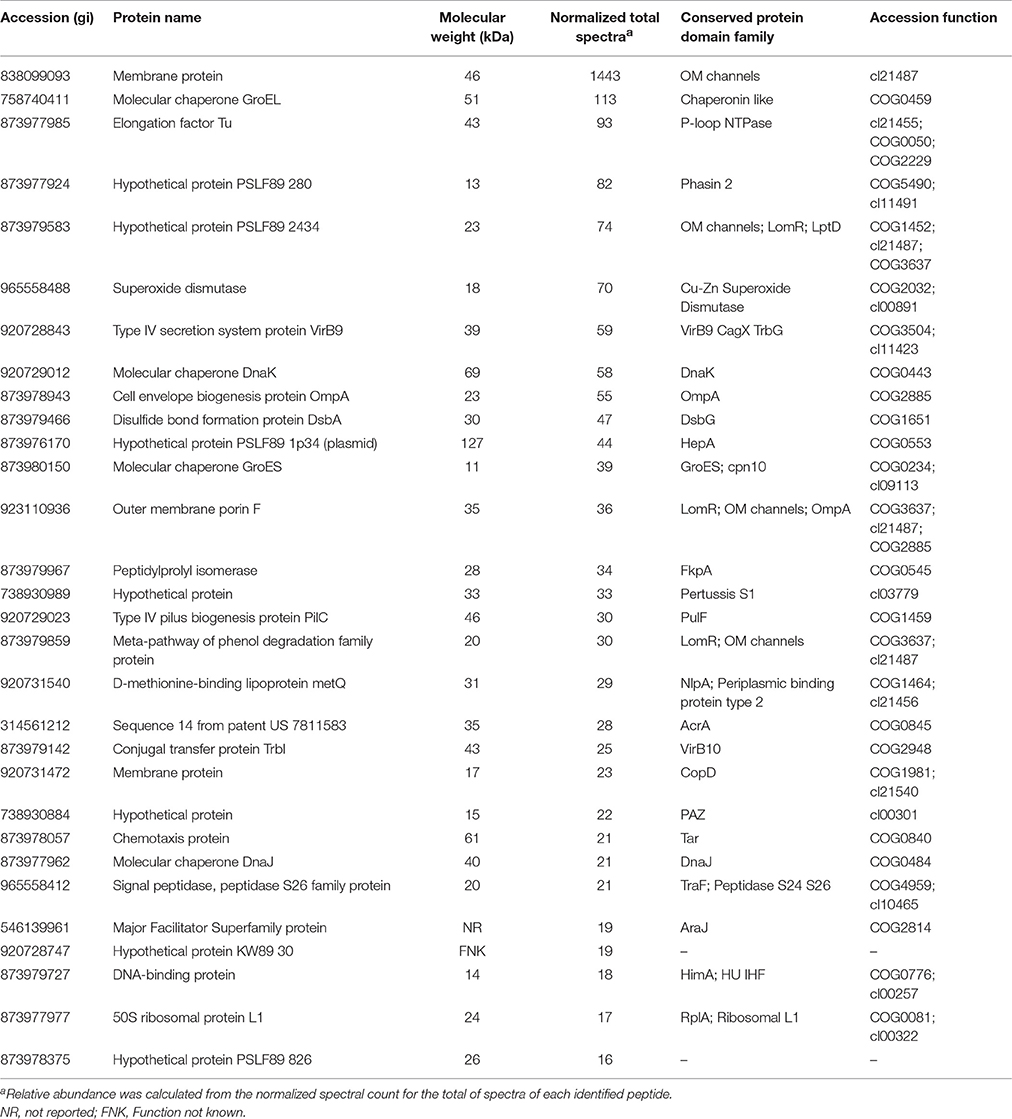
MudPIT analysis was performed to identify proteome components in the MVs purified from P. salmonis LF-89 (Supplementary Figure 1). A total of 452 unique MV-associated proteins were identified (Supplementary Table 1 and Supplementary Data 1). The 30 most-abundant proteins from the purified MVs are listed in Table 1.
To determine the subcellular localization of the identified proteins, the proteins were classified by the subcellular localization prediction tool (PSORTb v.3.0.2). This resulted in the following six groups, which were classified according to protein localization in the bacterium: (1) cytoplasmic proteins, (2) cytoplasmic membrane proteins, (3) periplasmic proteins, (4) outer membrane proteins, (5) extracellular proteins, and (6) proteins of unknown localization or multiple localization sites. Of the 452 proteins identified in MVs, 7 (1.3%) were extracellular, 27 (4.9%) were from the outer membrane, 15 (2.7%) were periplasmic, 143 (26%) were from the inner membrane, 209 (38%) were cytoplasmic, and 149 (27.1%) were from an unknown localization group (Figure 1A). The 5 most-abundant proteins from each subcellular compartment are listed in Table 2. Although a large amount of outer membrane proteins was expected, these results indicate that cytoplasmic proteins are the predominant component in MVs. Furthermore, the high representation of inner membrane and cytoplasmic proteins suggests that P. salmonis LF-89 MVs composition is derived from multiple bacterial compartments.
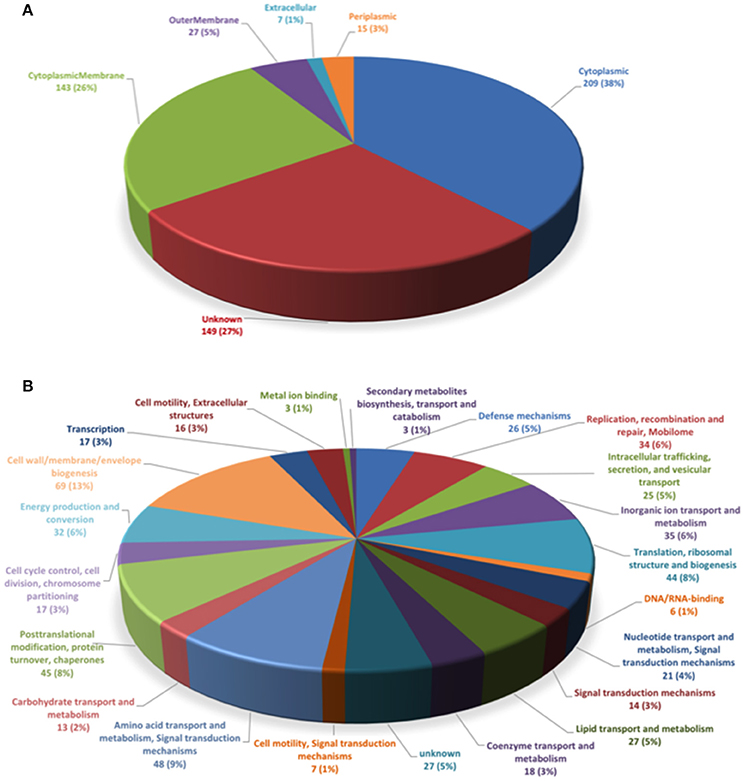
Figure 1. Classification of P. salmonis LF-89 membrane vesicles proteins. (A) Subcellular locations of membrane vesicle proteins identified by MudPIT. Predicted subcellular locations of the 452 membrane vesicle proteins identified using PSORT3b. (B) Functional classification of P. salmonis LF-89 membrane vesicles proteins. The 452 proteins identified by MudPIT were sorted according to the indicated clusters of orthologous groups.
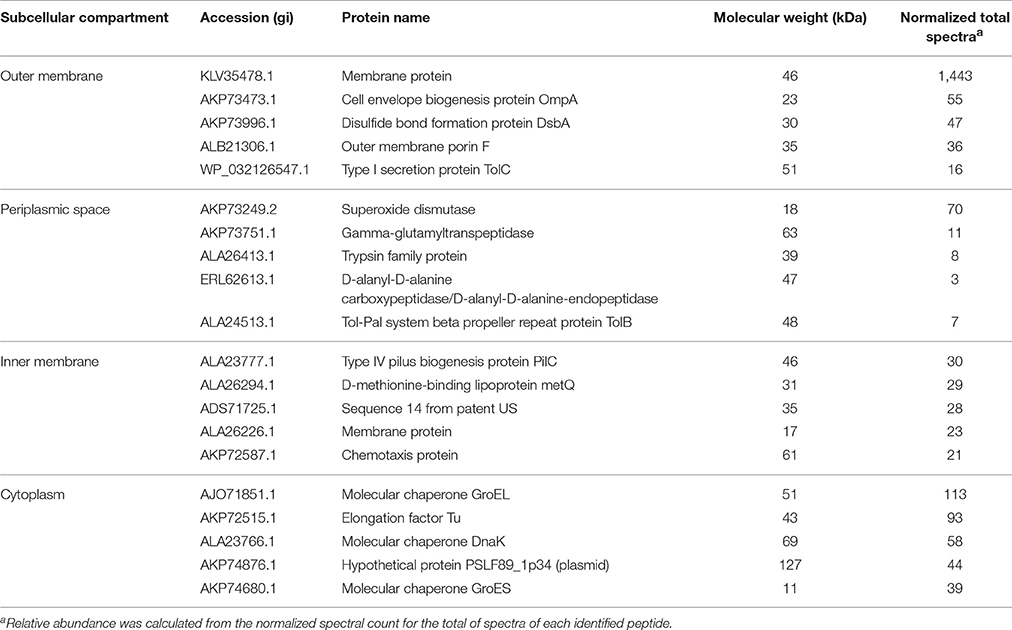
Table 2. Five most abundant proteins from each subcellular compartment identified in P. salmonis LF-89 type strain membrane vesicles.
Functional Classification of Identified Proteins in P. salmonis MVs
To determine the putative functions of the 452 proteins in the P. salmonis LF-89 MVs proteome, the proteins were analyzed according to Clusters of Orthologous Groups (COGs) definitions (http://www.ncbi.nlm.nih.gov/COG/). The results showed that the six largest COGs recognized in P. salmonis MVs (Figure 1B) were mainly involved in cell wall, membrane, and envelope biogenesis (69 proteins). Furthermore, 48 proteins were involved in the transport and metabolism of amino acids and signal transduction mechanisms; 45 proteins were involved in post-translational modifications, protein turnover, and chaperone activities; 44 were involved in translation, ribosomal structuring, and biogenesis; 35 were involved in inorganic ion transport and metabolism; and 34 proteins were related to the replication, recombination, and repair of the mobilome. Details for the functional classifications of these proteins are shown in Supplementary Table 1. Additionally, some proteins were involved in functions such as defense mechanisms, metal ion binding, and DNA/RNA binding. Interestingly, 25 proteins were identified in relation to intracellular trafficking, secretion, and vesicular transport. Taken together, these results suggest that MVs may exhibit multiple, specific functions inside the host.
Virulence-Associated Proteins Contained in P. salmonis MVs
To gain insight into the virulence potential of P. salmonis LF-89 MVs, the 452 proteins identified by MudPIT were subjected to in silico analysis using the virulent factor database (Chen et al., 2016), which is designed to predict virulent proteins of pathogenic bacteria. Analysis showed that 64 of the MV proteins (≈14%) (Table 3) had a predicted association with bacterial virulence, including members of the heat-shock families GroEL and GroES, which are strong immunogenic proteins. Additionally, other molecular chaperones, such as GrpE, Hsp33, DnaJ, DnaK, and HtpG, were also identified together with the outer membrane protein OmpA and outer membrane porin OmpF. The latter two are integral outer membrane proteins that are highly immunogenic. Some components of the flagellar structure, such as FlhA, FliF, FliM, FliL, and FliH, and proteins involved in type IV pilus biogenesis, such as PilC, PilT, PilB, PilW, and FimV were found present in P. salmonis LF-89 MVs. Furthermore, MVs purified from P. salmonis LF-89 also contained siderophores such as SufD, a TonB-dependent siderophore receptor family protein, and other proteins related to iron transport and metabolism. The presence of multiple proteins involved in the secretion of virulence factors supports a role of P. salmonis MVs in the pathogenesis of piscirickettsiosis.
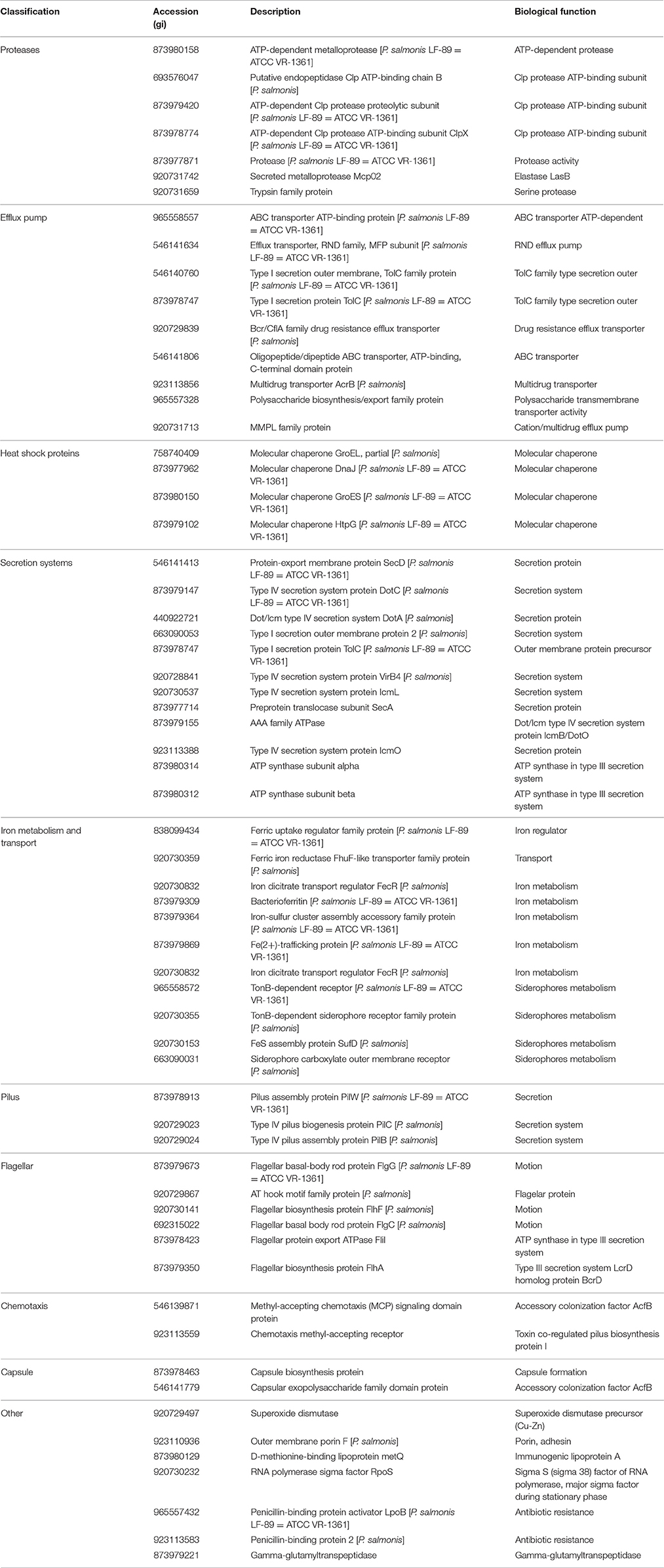
Table 3. Classification of virulence-related proteins identified from Piscirickettsia salmonis LF-89 type strain membrane vesicles.
Putative Bacterial Toxins Secreted in P. salmonis LF-89 MVs
A total of seven putative toxin sequences (Table 4) were detected in the proteome of MVs purified from P. salmonis LF-89 through an NCBI conservative domain database search (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). The identified toxin-related peptides are listed in Supplementary Table 2. Five amino acids sequences corresponding to Bordetella pertussis toxin subunit 1 were identified in the P. salmonis LF-89 plasmid pPSLF89-1 (accession number CP011850.1) (Figure 2A). Interestingly, three of these sequences (i.e., WP_032126894.1, ERL60989.1, and WP_036817364.1) corresponded to Ps-Tox1 and were located in the same plasmid region (between ≈9,500 and 11,600 base pairs). TBLASTn analyses of these sequences showed high identity percentages (>95%) with the P. salmonis LF-89 plasmid sequence. Similarly, two identical sequences (accession number AKP74948.2 and WP_036817009.1) corresponding to Ps-Tox2 were located in the plasmid region between 123,047 and 124,891 base pairs, with 100% identity. Additionally, multiple alignments of amino acid sequences showed that Ps-Tox1.1, Ps-Tox1.2, and Ps-Tox1.3 were identical to the B. pertussis toxin subunit 1 (accession number AMT50644.1), evidencing 7.5, 8.9, and 11.6% identities, respectively (Supplementary Figure 2 and Supplementary Data 3). In the case of Ps-Tox2.1 and Ps-Tox2.2, the identity percentage was 8.6%. Despite the low identities of these putative toxins, most changes in the amino acids sequences were conservative modifications. Subsequently, a phylogenetic tree was constructed according to amino acid sequence alignment of the five putative toxins corresponding to Ps-Tox (Figure 2B). As could be expected from the previously obtained data, these putative toxins were classified into two clusters, namely Ps-Tox1, containing Ps-Tox1.1, Ps-Tox1.2, and Ps-Tox1.3; and Ps-Tox2, conformed by Ps-Tox2.1 and Ps-Tox2.2.
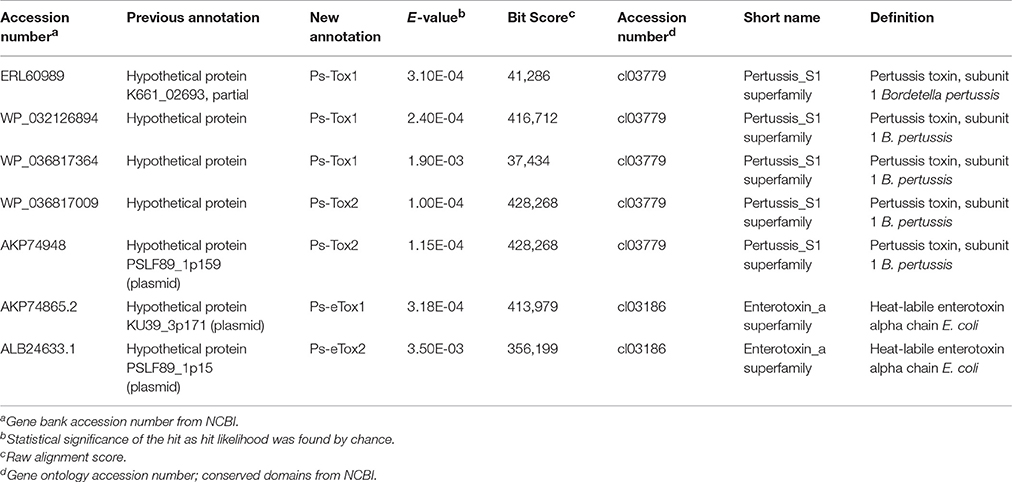
Table 4. Toxins identified by MudPIT analysis from Piscirickettsia salmonis LF-89 type strain membrane vesicles.
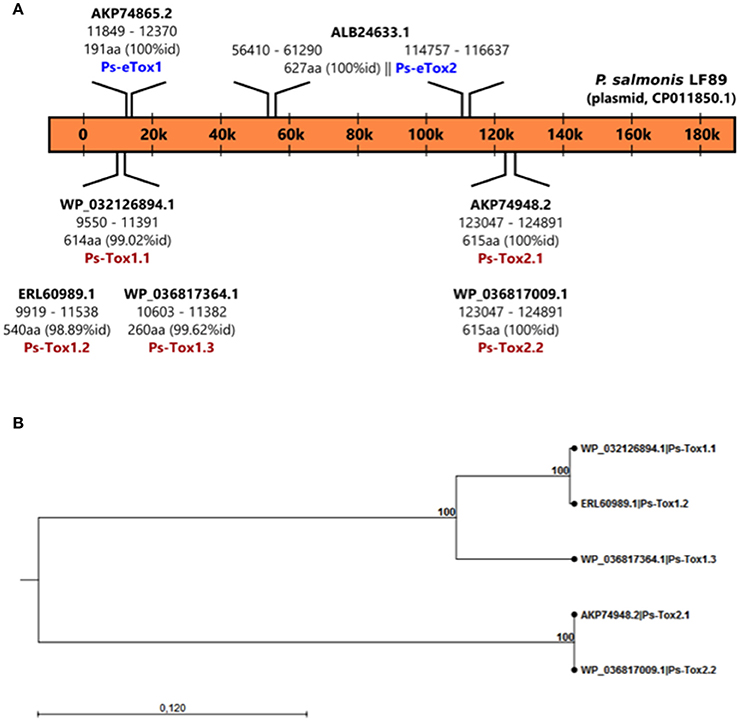
Figure 2. Identification of Ps-Tox genes in the P. salmonis LF-89 plasmid. (A) Schematic representation of Ps-Tox genes in the P. salmonis LF-89 plasmid. Two genomic regions containing three amino acid copies of Ps-Tox 1 (Ps-Tox1.1; Ps-Tox1.2; and Ps-Tox1.3) and two amino acid copies of Ps-Tox 2 (Ps-Tox2.1 and Ps-Tox2.2) were identified after TBLASTn analysis. The identity of each Ps-Tox1 and Ps-Tox2 is indicated in parenthesis. (B) Phylogenetic relationship between five Ps-Tox copies. A phylogenetic tree was constructed using the neighbor joining method with 1,000 bootstrap replicates according to the alignment of the Ps-tox amino acid sequence. Bootstrap support values are indicated at the nodes.
On the other hand, another two amino acid sequences corresponding to the heat-labile enterotoxin alpha chain of E. coli were also identified in the P. salmonis LF-89 plasmid. One sequence (100% identity) for Ps-eTox1 (accession number AKP74865.2) was located in the plasmid region between ≈11,850 and 12,370 base pairs, while the second amino acid sequence (100% identity) was for Ps-eTox2 (accession number ALB24633.1) and matched two different plasmid regions (≈56,400 and 61,300 base pairs, and ≈114,750 and 116,640 base pairs). Multiple alignments of these amino acid sequences showed that Ps-eTox1 and Ps-eTox2 were identical to the enterotoxin alpha from E. coli (13.7 and 16.6%, respectively) (Supplementary Figure 3).
Interestingly, the analysis of the secondary and tertiary structure of Ps-Tox and Ps-eTox generated by i-tasser revealed a high structural similarity with CARDS toxin, which is present in Mycoplasma pneumonia, and also common with the adhesion domain of B. pertussis toxin and heat-labile enterotoxin alpha chain of E. coli (Supplementary Figure 4).
Effect of MVs Isolated from P. salmonis in Adult Zebrafish
To evaluate the toxicity of MVs isolated from the pathogenic P. salmonis LF89 strain, different MV concentrations were injected into an adult zebrafish infection model. The cumulative mortality of zebrafish injected with MVs showed a dose-dependent pattern (Figure 3). Fish injected with 10 μg of MVs registered < 5% mortality 14 days post-injection. In contrast, fish injected with 40 μg of MVs showed a rapid onset of mortalities (≈20%) just 3 days post-injection. After 14 days, the 40 μg MV group registered ≈45% mortality, which was higher than the final 5 and 15% in the 10 and 20 μg MV groups, respectively. Interestingly, zebrafish challenged with live P. salmonis (109 CFU/mL, positive control) had a cumulative mortality of ≈30% 7 days post-injection, a rate similar to that for the 40 μg MV group. Nevertheless, the mortality of the positive control group reached ≈60% by the end of the challenge period. These findings support that MVs isolated from P. salmonis are cytotoxic for zebrafish.
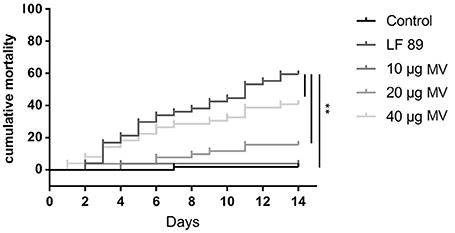
Figure 3. Cumulative mortality (%) of adult zebrafish challenged with membrane vesicles (MVs) isolated from Piscirickettsia salmonis LF-89 type strain. Adult zebrafish were injected with 10, 20, and 40 μg of MVs isolated from P. salmonis. PBS and live P. salmonis LF-89 were used as controls (n = 20). Asterisks indicate statistical significance (P < 0.05).
Discussion
Membrane vesicles are produced by several Gram-negative bacteria, and the pathogenic role of MVs during bacterial infection has been extensively reported (Lim and Yoon, 2015). The main focus of this study was to expand on the knowledge available for proteins contained in P. salmonis MVs. In the present work, a total of 452 proteins were identified using MudPIT analysis, 28 of which were within the outer membrane, as indicated by the PSORTb algorithm. Interestingly, most proteins (209) in P. salmonis LF-89 MVs corresponded to the cytoplasmic compartment, and 143 proteins were associated with the inner membrane. Finally, the locations of 149 proteins could not be identified. Despite some reports suggesting that Gram-negative bacterial MV proteomes consist mainly of outer membrane and periplasmic proteins (Horstman and Kuehn, 2000; Deatherage et al., 2009), other proteomic approaches indicate that MVs contain proteins with different cellular origins, including the cytoplasmic, inner membrane, outer membrane, and periplasmic proteins (Lee et al., 2007; Galka et al., 2008). Other studies still report that MVs contain mostly cytoplasmic and periplasmic proteins (Choi et al., 2011; Bai et al., 2014), such as specifically observed in the Aggregatibacter actinomycetemcomitans MV proteome (Kieselbach et al., 2015). A possible explanation for this phenomenon may be related to a physiological role of MVs, which have been found to help bacteria expel useless and harmful waste that has accumulated inside bacterial cells via MVs (McBroom and Kuehn, 2007). However, further studies are needed to elucidate this hypothesis and the production of MVs by P. salmonis. On the other hand, the presence of cytoplasmic proteins in the MV proteome may be due to moonlighting abilities, which is the capacity to perform additional biological activities distinct from those they normally occupy (Mani et al., 2014). These multitasking bacterial proteins include metabolic proteins/enzymes and molecular chaperones, which could to play a role in bacterial interaction with host cells by serving as adhesins and invasins (Henderson and Martin, 2011; Wang et al., 2014). Thus, this possibility needs to be assessed through further studies on P. salmonis MV cytoplasmic proteins and respective potential moonlighting functions. Nevertheless, the present findings support that the P. salmonis LF-89 MV proteome includes proteins from different subcellular origins.
In relation to differential origins, the identified P. salmonis LF-89 MV proteins presented varied functions. Thus, a total of 69 proteins were classified through COG definitions with functions in cell wall, membrane, and envelope biogenesis, including VirB9, required for type IV secretion (Jakubowski et al., 2003), which has been shown to induce humoral and cellular immunity in A. marginale (Zhao et al., 2016). Likewise, the inner membrane-associated ATPase VirB4, essential for pilus biogenesis and protein transport in type IV secretion systems was also identified (Peña et al., 2012). In turn, 48 proteins were related to amino acids transport and metabolism, as well as to signal transduction mechanisms. Other proteins were associated with functions of post-translational modification, protein turnover, and chaperone activity (45 proteins), including GroEL, GroES, DnaJ, and HtpG, homolog of the ubiquitous HSP90 family of proteins; translation, ribosomal structure, and biogenesis (44 proteins); inorganic ion transport and metabolism (35 proteins); and the replication, recombination, and repair of the mobilome (34 proteins). Additionally, other proteins were involved in defense mechanisms, metal ion binding, and DNA/RNA binding. Overall, the high amount of P. salmonis MV proteins involved in key functions for pathogen survival is in accordance with findings from previously reported MV proteomes for several Gram-negative bacteria, such as Pseudomonas syringae (Kulkarni et al., 2014) and A. actinomycetemcomitans (Kieselbach et al., 2015).
From a functional point of view, many vesicle-associated proteins are virulence factors, playing diverse bacterial roles in pathogenicity such as invasion, adherence, antibiotic resistance, damage to host cells, modulation of the host immune response, biofilm formation, and promotion of virulence. Thus, several antibiotic resistance-related proteins have been identified in the P. salmonis MVs proteome including the transporter AcrB, TolC, and the MFP subunit, members of the RND-type multidrugs efflux pumps, which have been previously described in P. salmonis (Sandoval et al., 2016). Furthermore, the Bcr/CflA family drug resistance efflux transporter, described as resistance to bicyclomycin in E. coli (Bentley et al., 1993), and chloramphenicol and florfenicol in Salmonella typhimurium (Braibant et al., 2005) were also identified. Additionally, several proteins involved in iron metabolism and uptake (FhuF-like transporter, the regulator FecR, and Bacterioferritin), and siderophores metabolism (TonB-dependent siderophore receptor and FeS assembly protein SufD) were identified. These proteins are highly important for intracellular bacterial pathogens, which use multiple strategies to obtain nutritional iron from the intracellular environment in order to use this element for its replication, in the same way as it does P. salmonis (Pulgar et al., 2015; Almarza et al., 2016). Although, the flagellar basal-body rod protein Flagellin G (FlgG) and the chaperone GroEL are present in P. salmonis MVs and they were chosen in early vaccine studies (Wilhelm et al., 2006), the field results suggest that these two proteins are not suitable as a vaccine candidates for P. salmonis. It is possible that some combination of these and other immunogenic and/or virulence-associated antigens may be needed as has been reported for the fish pathogen Flavobacterium psychrophilum (Plant et al., 2011).
Interestingly, our study identified the outer membrane proteins OmpA, that has been involved in adhesion, invasion and replication of several bacterial pathogens; and OmpF, with porin activity forming small water-filled channels (Buehler et al., 1991; Cowan et al., 1992). These highly immunogenic proteins are found across genera in Gram-negative bacteria such as Yersinia enterocolitica (Gu et al., 2012), Salmonella enterica (Toobak et al., 2013), and Coxiella burnetii (Martinez et al., 2014), and several successful bacterial OMP-based vaccines have used OmpA and OmpF in its formulation (Camacho et al., 2013; Liu et al., 2016). Thus, P. salmonis MVs containing OmpA and OmpF proteins could serve as protective antigens and should be further assessed as potential vaccine candidates against piscirickettsiosis.
Furthermore and importantly, this is the first study demonstrating that the B. pertussis toxin subunit 1 and heat-labile enterotoxin alpha chain of E. coli are proteins carried by P. salmonis LF-89 MVs. It has been widely demonstrated that important bacterial toxins are secreted via bacterial MVs, including heat-labile enterotoxin from E. coli (Horstman and Kuehn, 2000), the anthrax toxin from Bacillus anthracis (Rivera et al., 2010), the cholera toxin from Vibrio cholerae (Chatterjee and Chaudhuri, 2011), listeriolysin O (Listeria monocytogenes), and alpha-hemolysin from Staphylococcus aureus (Lee et al., 2013). More specifically, B. pertussis toxin subunit 1 (28 kDa) is an important virulence factor that exercises NAD-dependent ADP-ribosyltransferase activity, which plays a crucial role in B. pertussis pathogenesis by causing the suppression/modulation of the host immune and inflammatory responses (Higgs et al., 2012; Melvin et al., 2014). Indeed, ADP-ribosylation of target substrates in eukaryotic cells is a common action mechanism of many bacterial protein toxins, including the cholera toxin from V. cholerae (Chatterjee and Chaudhuri, 2011) and exotoxin A from P. aeruginosa (Allured et al., 1986). In turn, expression of the heat-labile enterotoxin by enterotoxigenic E. coli promotes bacterial adherence to intestinal epithelial cells (Johnson et al., 2009), causing diarrhea in infected subjects (Nataro, 2005). This action is mediated by an ADP-ribosylation activity of the A subunit of heat-labile enterotoxin. Additionally, further reports support that enterotoxigenic E. coli secretes physiologically active heat-labile enterotoxin via MVs (Horstman and Kuehn, 2000). Similar to P. salmonis, it was recently found that B. pertussis can survive and replicate inside human macrophages. Furthermore, the bactericidal and inflammatory response of infected macrophages is progressively downregulated, and the pertussis toxin is involved in manipulating the host-cell response (Valdez et al., 2016). Likewise, once P. salmonis is inside the host cell, can modulate the expression of several pro-inflammatory cytokines (Tacchi et al., 2011; Salazar et al., 2016). Furthermore, IL-10 is upregulated in the RTS-11 monocyte/macrophage cell line during P. salmonis infection, thus promoting the bacterial survival inside the cell through macrophage inactivation (Álvarez et al., 2016). Likewise, Tandberg et al. (2016) demonstrated an upregulation of several pro-inflammatory genes in the spleen and kidney of adult zebrafish after immunization with MVs from P. salmonis. Additionally, it has been revealed that tnf-a, il-1b, il-6 and il-10 display significant differences in MV-immunized fish (Tandberg et al., 2017). The modulation of these genes might therefore indicate the functionality of B. pertussis toxin subunit 1 and E. coli heat-labile enterotoxin alpha in the modulation of the host immune response and in the pathogenicity of P. salmonis. However, the presence of these putative toxins in P. salmonis MVs, the toxicity induced by these toxins, toxin regulations, and the modulation of the host immune and inflammatory responses by these putative toxins should be explored in future studies.
Our group previously reported the production of MVs by the fish pathogen P. salmonis through microscopic characterization and liquid chromatography-MS/MS, the first proteomic approach in this bacterium (Oliver et al., 2016). In the present study, the cytotoxicity of MVs purified from the P. salmonis LF-89 type strain was confirmed in an in vivo model. Specifically, the cumulative mortality of adult zebrafish was ≈40% 14 days after MV injection (40 μg). This finding was similar to Tandberg et al. (2016), who reported a mortality of ≈50% in zebrafish injected with the same quantity of MVs purified from P. salmonis isolates. Additionally, the presently purified MVs induced dose-dependent mortality rates in fish. However, the inflammatory response, other immune issues, and the putative protection induced by the MVs, a point imperative to the possible vaccine application of MVs against piscirickettsiosis, were not evaluated in this study and should be considered in future investigations.
On the other hand, MV-based vaccines have successfully been used for epidemic control against serogroup B meningococcal disease (Holst et al., 2013). MVs used in vaccination of fish have also been reported to give good protection against several fish pathogens (Lagos et al., 2017; Tandberg et al., 2017), inducing up-regulation of immune-related genes, showing MVs as potential activator of the host's immune system. However, whether this activation is mediated by i.e., toll-like receptors (TLRs) or not, is still not known. Thus, considering the composition of MVs, which contain several molecules and proteins identified as pathogen associated molecular pattern (PAMPS) including LPS, carbohydrates, HSPs, and nuclei sequence motifs suggest the participation of TLRs as a bridge between innate and adaptive immunity, making P. salmonis MVs interesting as a vaccine candidate. Interestingly, it has been described different effects on mortality induced by MVs purified from three different P. salmonis strains, been LF-89 MVs the most toxic (Tandberg et al., 2016). However, whether the difference in mortality are caused by differences in the LPS, it is unknown. Thus, there is still a lack of knowledge regarding the immunogenic effect of LPS from fish pathogens, and studies of P. salmonis derived LPS would be interesting to follow up in future studies Recent studies have shown that LPS is one of the most abundant components of OMVs, being able to exceed the total protein content of vesicles by ratios as high as 10:1 (Ellis and Kuehn, 2010). Given the high LPS content, all investigations into immune responses to OMVs must define the contribution of LPS to the host response. MVs, as LPS delivery vehicles, have the capacity to enhance either bacterial clearance or cause host tissue damage by activating an inflammatory response. Recent studies have identified MVs as the vehicle that mediates the cytosolic localization of LPS during extra cellular Gram-negative bacterial infections, demonstrating a necessary role for MVs for intracellular LPS release during bacterial infections (Vanaja et al., 2016). To date, no studies have demonstrated LPS purified from either P. salmonis or MVs directly impacting the host responses. However, the present study identified several proteins in MVs, including toxins, which would be able to stimulate the fish immune system. However, the importance of each of these components in virulence and pathogenesis of P. salmonis it is until now, unexplored.
Collectively, these results suggest that MV secretion might have an association with P. salmonis virulence. Although not specifically tested herein, we speculate that MVs secretion might contribute to the transport and dissemination of key virulence factors and putative bacterial toxins to host cells during bacterial infection.
In conclusion, the present study identified 452 proteins in P. salmonis MVs, which, to our knowledge, is the most comprehensive report on a bacterial MV proteome. Notably, a relatively large number of cytoplasmic proteins were found in the vesicles. Taken together, the present results support that the P. salmonis MVs purified from the LF-89 type strain contain numerous virulence factors that can stimulate the host immune system, as well as some proteins involved in antibiotic resistance, invasion into host cells, and, interestingly, intracellular trafficking. Two putative toxins were also identified in the MVs, which might be involved in P. salmonis cytotoxicity, as previously reported by our research group. Overall, the currently presented results suggest that the protein composition of the MVs in P. salmonis LF-89 may reflect the characteristics of the total P. salmonis proteome. This valuable information provides a basis for future studies toward elucidating key pathogenic roles of P. salmonis MVs. Moreover, this study should contribute to the development of vaccines or vaccine adjuvants against this fastidious fish pathogen.
Author Contributions
CO planned and performed most of the experiments and participated in the writing of the manuscript. MH, JT, KV, LL, RH, and PS planned and performed some of the experiments. CS, MC, and PR performed some of the experiments and participated in the writing of the manuscript. MV, AA, HW, RA, and AY planned some of the experiments and participated in writing of the manuscript. All authors have approved the final article.
Funding
This work was financially supported by the Fondo de Financiamiento de Centros de Investigación (FONDAP) Interdisciplinary Center for Aquaculture Research (INCAR) (Grant No. 15110027), Dirección de Investigación of the Universidad Austral de Chile (DID-UACh), Fondo de Inversión Estratégica (Grant No. FIE V014) and the University of Oslo. The Research Council of Norway; Biotek2021 Program (Grant No. 233849), and Havbruksprogrammet Program (Grant No. 268201). CO acknowledges reception of a Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) Postdoctoral Scholarship (No. 3160849).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fcimb.2017.00420/full#supplementary-material
References
Allured, V. S., Collier, R. J., Carroll, S. F., and McKay, D. B. (1986). Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc. Natl. Acad. Sci. U.S.A. 83, 1320–1324. doi: 10.1073/pnas.83.5.1320
Almarza, O., Valderrama, K., Ayala, M., Segovia, C., and Santander, J. (2016). A functional ferric uptake regulator (Fur) protein in the fish pathogen Piscirickettsia salmonis. Int. Microbiol. 19, 49–55. doi: 10.2436/20.1501.01.263
Álvarez, C. A., Gomez, F. A., Mercado, L., Ramírez, R., and Marshall, S. H. (2016). Piscirickettsia salmonis imbalances the innate Immune response to succeed in a productive infection in a salmonid cell line model. PLoS ONE 11:e0163943. doi: 10.1371/journal.pone.0163943
Ashburner, M., Ball, C. A., Blake, J. A., Botstein, D., Butler, H., Cherry, J. M., et al. (2000). Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29. doi: 10.1038/75556
Bai, J., Kim, S. I., Ryu, S., and Yoon, H. (2014). Identification and characterization of outer membrane vesicle-associated proteins in Salmonella enterica serovar Typhimurium. Infect. Immun. 82, 4001–4010. doi: 10.1128/IAI.01416-13
Bakkemo, K. R., Mikkelsen, H., Bordevik, M., Torgersen, J., Winther-Larsen, H. C., Vanberg, C., et al. (2011). Intracellular localisation and innate immune responses following Francisella noatunensis infection of Atlantic cod (Gadus morhua) macrophages. Fish Shellfish Immunol. 31, 993–1004. doi: 10.1016/j.fsi.2011.08.020
Bentley, J., Hyatt, L., Ainley, K., Parish, J., Herbert, R., and White, G. (1993). Cloning and sequence analysis of an Escherichia coli gene conferring bicyclomycin resistance. Gene 127, 117–120. doi: 10.1016/0378-1119(93)90625-D
Bomberger, J. M., MacEachran, D. P., Coutermarsh, B. A., Ye, S., O'Toole, G. A., and Stanton, B. A. (2009). Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5:e1000382. doi: 10.1371/journal.ppat.1000382
Braibant, M., Chevalier, J., Chaslus-Dancla, E., Pagès, J.-M., and Cloeckaert, A. (2005). Structural and functional study of the phenicol-specific efflux pump FloR belonging to the major facilitator superfamily. Antimicrob. Agents Chemother. 49, 2965–2971. doi: 10.1128/AAC.49.7.2965-2971.2005
Brudal, E., Lampe, E. O., Reubsaet, L., Roos, N., Hegna, I. K., Thrane, I. M., et al. (2015). Vaccination with outer membrane vesicles from Francisella noatunensis reduces development of francisellosis in a zebrafish model. Fish Shellfish Immunol. 42, 50–57. doi: 10.1016/j.fsi.2014.10.025
Buehler, L., Kusumoto, S., Zhang, H., and Rosenbusch, J. (1991). Plasticity of Escherichia coli porin channels. Dependence of their conductance on strain and lipid environment. J. Biol. Chem. 266, 24446–24450.
Camacho, A., Irache, J., de Souza, J., Sánchez-Gómez, S., and Gamazo, C. (2013). Nanoparticle-based vaccine for mucosal protection against Shigella flexneri in mice. Vaccine 31, 3288–3294. doi: 10.1016/j.vaccine.2013.05.020
Chatterjee, D., and Chaudhuri, K. (2011). Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett. 585, 1357–1362. doi: 10.1016/j.febslet.2011.04.017
Chen, L., Zheng, D., Liu, B., Yang, J., and Jin, Q. (2016). VFDB 2016: hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 44, D694–D697. doi: 10.1093/nar/gkv1239
Choi, C.-W., Park, E. C., Yun, S. H., Lee, S.-Y., Lee, Y. G., Hong, Y., et al. (2014). Proteomic characterization of the outer membrane vesicle of Pseudomonas putida KT2440. J. Proteome Res. 13, 4298–4309. doi: 10.1021/pr500411d
Choi, D. S., Kim, D. K., Choi, S. J., Lee, J., Choi, J. P., Rho, S., et al. (2011). Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics 11, 3424–3429. doi: 10.1002/pmic.201000212
Cosma, C. L., Swaim, L. E., Volkman, H., Ramakrishnan, L., and Davis, J. (2006). Zebrafish and frog models of Mycobacterium marinum infection. Curr. Protoc. Microbiol. Chapter 10:Unit 10B.2. doi: 10.1002/0471729256.mc10b02s3
Cowan, S., Schirmer, T., Rummel, G., Steiert, M., Ghosh, R., Pauptit, R., et al. (1992). Crystal structures explain functional properties of two E. coli porins. Nature 358:727. doi: 10.1038/358727a0
Deatherage, B. L., Lara, J. C., Bergsbaken, T., Barrett, S. L. R., Lara, S., and Cookson, B. T. (2009). Biogenesis of bacterial membrane vesicles. Mol. Microbiol. 72, 1395–1407. doi: 10.1111/j.1365-2958.2009.06731.x
Ellis, T. N., and Kuehn, M. J. (2010). Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74, 81–94. doi: 10.1128/MMBR.00031-09
Fiocca, R., Necchi, V., Sommi, P., Ricci, V., Telford, J., Cover, T. L., et al. (1999). Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 188, 220–226.
Fryer, J., and Hedrick, R. (2003). Piscirickettsia salmonis: a Gram-negative intracellular bacterial pathogen of fish. J. Fish Dis. 26, 251–262. doi: 10.1046/j.1365-2761.2003.00460.x
Galka, F., Wai, S. N., Kusch, H., Engelmann, S., Hecker, M., Schmeck, B., et al. (2008). Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles. Infect. Immun. 76, 1825–1836. doi: 10.1128/IAI.01396-07
Gu, W., Wang, X., Qiu, H., Luo, X., Xiao, D., Xiao, Y., et al. (2012). Comparative antigenic proteins and proteomics of pathogenic Yersinia enterocolitica bio-serotypes 1B/O: 8 and 2/O: 9 cultured at 25° C and 37° C. Microbiol. Immunol. 56, 583–594. doi: 10.1111/j.1348-0421.2012.00478.x
Henderson, B., and Martin, A. (2011). Bacterial virulence in the moonlight: multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect. Immun. 79, 3476–3491. doi: 10.1128/IAI.00179-11
Higgs, R., Higgins, S., Ross, P., and Mills, K. (2012). Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. 5, 485–500. doi: 10.1038/mi.2012.54
Hoekstra, D., van der Laan, J. W., de Leij, L., and Witholt, B. (1976). Release of outer membrane fragments from normally growing Escherichia coli. Biochim. et Biophys. Acta 455, 889–899. doi: 10.1016/0005-2736(76)90058-4
Holst, J., Martin, D., Arnold, R., Huergo, C. C., Oster, P., O'Hallahan, J., et al. (2009). Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27, B3–B12. doi: 10.1016/j.vaccine.2009.04.071
Holst, J., Oster, P., Arnold, R., Tatley, M., Næss, L., Aaberge, I., et al. (2013). Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum. Vaccin. Immunother. 9, 1241–1253. doi: 10.4161/hv.24129
Hong, G. E., Kim, D. G., Park, E. M., Nam, B. H., Kim, Y. O., and Kong, I. S. (2009). Identification of Vibrio anguillarum outer membrane vesicles related to immunostimulation in the Japanese flounder, Paralichthys olivaceus. Biosci. Biotechnol. Biochem. 73, 437–439. doi: 10.1271/bbb.80580
Horstman, A. L., and Kuehn, M. J. (2000). Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 275, 12489–12496. doi: 10.1074/jbc.275.17.12489
Jakubowski, S. J., Krishnamoorthy, V., and Christie, P. J. (2003). Agrobacterium tumefaciens VirB6 protein participates in formation of VirB7 and VirB9 complexes required for type IV secretion. J. Bacteriol. 185, 2867–2878. doi: 10.1128/JB.185.9.2867-2878.2003
Johnson, A. M., Kaushik, R. S., Francis, D. H., Fleckenstein, J. M., and Hardwidge, P. R. (2009). Heat-labile enterotoxin promotes Escherichia coli adherence to intestinal epithelial cells. J. Bacteriol. 191, 178–186. doi: 10.1128/JB.00822-08
Kadurugamuwa, J. L., and Beveridge, T. J. (1999). Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other gram-negative bacteria. Microbiology 145, 2051–2060. doi: 10.1099/13500872-145-8-2051
Keller, A., Purvine, S., Nesvizhskii, A. I., Stolyar, S., Goodlett, D. R., and Kolker, E. (2002). Experimental protein mixture for validating tandem mass spectral analysis. Omics J. Integrat. Biol. 6, 207–212. doi: 10.1089/153623102760092805
Kieselbach, T., Zijnge, V., Granström, E., and Oscarsson, J. (2015). Proteomics of Aggregatibacter actinomycetemcomitans outer membrane vesicles. PLoS ONE 10:e0138591. doi: 10.1371/journal.pone.0138591
Kulkarni, H. M., Swamy, C. V., and Jagannadham, M. V. (2014). Molecular characterization and functional analysis of outer membrane vesicles from the antarctic bacterium Pseudomonas syringae suggest a possible response to environmental conditions. J. Proteome Res. 13, 1345–1358. doi: 10.1021/pr4009223
Kwon, S.-O., Gho, Y. S., Lee, J. C., and Kim, S. I. (2009). Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol. Lett. 297, 150–156. doi: 10.1111/j.1574-6968.2009.01669.x
Lagos, L., Tandberg, J. I., Repnik, U., Boysen, P., Ropstad, E., Varkey, D., et al. (2017). Characterization and vaccine potential of membrane vesicles produced by Francisella noatunensis subsp. orientalis in an adult zebrafish model. Clin. Vaccine Immunol. 24, e00557–e00516. doi: 10.1128/CVI.00557-16
Lee, E. Y., Bang, J. Y., Park, G. W., Choi, D. S., Kang, J. S., Kim, H. J., et al. (2007). Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics. 7, 3143–3153. doi: 10.1002/pmic.200700196
Lee, E. Y., Choi, D. S., Kim, K. P., and Gho, Y. S. (2008). Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom. Rev. 27, 535–555. doi: 10.1002/mas.20175
Lee, J.-H., Cho, H. S., Kim, Y., Kim, J.-A., Banskota, S., Cho, M. H., et al. (2013). Indole and 7-benzyloxyindole attenuate the virulence of Staphylococcus aureus. Appl. Microbiol. Biotechnol. 97, 4543–4552. doi: 10.1007/s00253-012-4674-z
Lim, S., and Yoon, H. (2015). Roles of outer membrane vesicles (OMVs) in bacterial virulence. J. Bacteriol. Virol. 45, 1–10. doi: 10.4167/jbv.2015.45.1.1
Liu, F., Tang, X., Sheng, X., Xing, J., and Zhan, W. (2016). Edwardsiella tarda outer membrane protein C: an immunogenic protein induces highly protective effects in flounder (Paralichthys olivaceus) against Edwardsiellosis. Int. J. Mol. Sci. 17:1117. doi: 10.3390/ijms17071117
Mani, M., Chen, C., Amblee, V., Liu, H., Mathur, T., Zwicke, G., et al. (2014). MoonProt: a database for proteins that are known to moonlight. Nucleic Acids Res. 43, D277–D282. doi: 10.1093/nar/gku954
Martinez, E., Cantet, F., Fava, L., Norville, I., and Bonazzi, M. (2014). Identification of OmpA, a Coxiella burnetii protein involved in host cell invasion, by multi-phenotypic high-content screening. PLoS Pathog. 10:e1004013. doi: 10.1371/journal.ppat.1004013
McBroom, A. J., and Kuehn, M. J. (2007). Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63, 545–558. doi: 10.1111/j.1365-2958.2006.05522.x
McCaig, W. D., Koller, A., and Thanassi, D. G. (2013). Production of outer membrane vesicles and outer membrane tubes by Francisella novicida. J. Bacteriol. 195, 1120–1132. doi: 10.1128/JB.02007-12
Melvin, J. A., Scheller, E. V., Miller, J. F., and Cotter, P. A. (2014). Bordetella pertussis pathogenesis: current and future challenges. Nat. Rev. Microbiol. 12, 274–288. doi: 10.1038/nrmicro3235
Nataro, J. P. (2005). Enteroaggregative Escherichia coli pathogenesis. Curr. Opin. Gastroenterol. 21, 4–8. doi: 10.3201/eid0402.980212
Nesvizhskii, A. I., Keller, A., Kolker, E., and Aebersold, R. (2003). A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658. doi: 10.1021/ac0341261
Oliver, C., Valenzuela, K., Hernández, M., Sandoval, R., Haro, R. E., Avendaño-Herrera, R., et al. (2016). Characterization and pathogenic role of outer membrane vesicles produced by the fish pathogen Piscirickettsia salmonis under in vitro conditions. Vet. Microbiol. 184, 94–101. doi: 10.1016/j.vetmic.2015.09.012
Park, S. B., Jang, H. B., Nho, S. W., Cha, I. S., Hikima, J.-I., Ohtani, M., et al. (2011). Outer membrane vesicles as a candidate vaccine against edwardsiellosis. PLoS ONE 6:e17629. doi: 10.1371/journal.pone.0017629
Peña, A., Matilla, I., Martín-Benito, J., Valpuesta, J. M., Carrascosa, J. L., De la Cruz, F., et al. (2012). The hexameric structure of a conjugative VirB4 protein ATPase provides new insights for a functional and phylogenetic relationship with DNA translocases. J. Biol. Chem. 287, 39925–39932. doi: 10.1074/jbc.M112.413849
Pierson, T., Matrakas, D., Taylor, Y. U., Manyam, G., Morozov, V. N., Zhou, W., et al. (2011). Proteomic characterization and functional analysis of outer membrane vesicles of Francisella novicida suggests possible role in virulence and use as a vaccine. J. Proteome Res. 10, 954–967. doi: 10.1021/pr1009756
Plant, K., LaPatra, S., Call, D., and Cain, K. (2011). Immunization of rainbow trout, Oncorhynchus mykiss (Walbaum), with Flavobacterium psychrophilum proteins elongation factor-Tu, SufB Fe-S assembly protein and ATP synthaseβ. J. Fish Dis. 34, 247–250. doi: 10.1111/j.1365-2761.2010.01235.x
Pulgar, R., Hödar, C., Travisany, D., Zuñiga, A., Domínguez, C., Maass, A., et al. (2015). Transcriptional response of Atlantic salmon families to Piscirickettsia salmonis infection highlights the relevance of the iron-deprivation defence system. BMC Genomics 16:495. doi: 10.1186/s12864-015-1716-9
Rivera, J., Cordero, R. J., Nakouzi, A. S., Frases, S., Nicola, A., and Casadevall, A. (2010). Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. U.S.A. 107, 19002–19007. doi: 10.1073/pnas.1008843107
Rozas, M., and Enríquez, R. (2014). Piscirickettsiosis and Piscirickettsia salmonis in fish: a review. J. Fish Dis. 37, 163–188. doi: 10.1111/jfd.12211
Salazar, C., Haussmann, D., Kausel, G., and Figueroa, J. (2016). Molecular cloning of Salmo salar Toll-like receptors (TLR1, TLR22, TLR5M and TLR5S) and expression analysis in SHK-1 cells during Piscirickettsia salmonis infection. J. Fish Dis. 39, 239–248. doi: 10.1111/jfd.12354
Sandoval, R., Oliver, C., Valdivia, S., Valenzuela, K., Haro, R. E., Sánchez, P., et al. (2016). Resistance-nodulation-division efflux pump acrAB is modulated by florfenicol and contributes to drug resistance in the fish pathogen Piscirickettsia salmonis. FEMS Microbiol. Lett. 363:fnw102. doi: 10.1093/femsle/fnw102
Sernapesca (2015). Informe Sanitario Salmonicultura en Centros Marinos. Available online at: http://www.sernapesca.cl/index.php?option=com_remository&Itemid=246&func=startdown&id=14754
Tacchi, L., Bron, J. E., Taggart, J. B., Secombes, C. J., Bickerdike, R., Adler, M. A., et al. (2011). Multiple tissue transcriptomic responses to Piscirickettsia salmonis in Atlantic salmon (Salmo salar). Physiol. Genomics 43, 1241–1254. doi: 10.1152/physiolgenomics.00086.2011
Tandberg, J. I., Lagos, L. X., Langlete, P., Berger, E., Rishovd, A.-L., Roos, N., et al. (2016). Comparative analysis of membrane vesicles from three Piscirickettsia salmonis Isolates reveals differences in vesicle characteristics. PLoS ONE 11:e0165099. doi: 10.1371/journal.pone.0165099
Tandberg, J., Oliver, C., Lagos, L., Gaarder, M., Yáñez, A. J., Ropstad, E., et al. (2017). Membrane vesicles from Piscirickettsia salmonis induce protective immunity and reduce development of salmonid rickettsial septicemia in an adult zebrafish model. Fish Shellfish Immunol. 67, 189–198. doi: 10.1016/j.fsi.2017.06.015
Toobak, H., Rasooli, I., Talei, D., Jahangiri, A., Owlia, P., and Astaneh, S. D. A. (2013). Immune response variations to Salmonella enterica serovar Typhi recombinant porin proteins in mice. Biologicals 41, 224–230. doi: 10.1016/j.biologicals.2013.05.005
Valdez, H. A., Oviedo, J. M., Gorgojo, J. P., Lamberti, Y., and Rodriguez, M. E. (2016). Bordetella pertussis modulates human macrophage defense gene expression. Pathog. Dis. 74:ftw073. doi: 10.1093/femspd/ftw073
Vanaja, S. K., Russo, A. J., Behl, B., Banerjee, I., Yankova, M., Deshmukh, S. D., et al. (2016). Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165, 1106–1119. doi: 10.1016/j.cell.2016.04.015
Wang, G., Xia, Y., Cui, J., Gu, Z., Song, Y., Chen, Y. Q., et al. (2014). The roles of moonlighting proteins in bacteria. Curr. Issues Mol. Biol. 16, 15–22. doi: 10.21775/cimb.016.015
Wilhelm, V., Miquel, A., Burzio, L. O., Rosemblatt, M., Engel, E., Valenzuela, S., et al. (2006). A vaccine against the salmonid pathogen Piscirickettsia salmonis based on recombinant proteins. Vaccine 24, 5083–5091. doi: 10.1016/j.vaccine.2006.03.027
Yañez, A., Silva, H., Valenzuela, K., Pontigo, J., Godoy, M., Troncoso, J., et al. (2013). Two novel blood-free solid media for the culture of the salmonid pathogen Piscirickettsia salmonis. J. Fish Dis. 36, 587–591. doi: 10.1111/jfd.12034
Yañez, A., Valenzuela, K., Silva, H., Retamales, J., Romero, A., Enriquez, R., et al. (2012). Broth medium for the successful culture of the fish pathogen Piscirickettsia salmonis. Dis. Aquat. Organ. 97, 197–205. doi: 10.3354/dao02403
Keywords: Piscirickettsia salmonis, SRS, bacterial toxins, mass spectrometry, MudPIT, zebrafish
Citation: Oliver C, Hernández MA, Tandberg JI, Valenzuela KN, Lagos LX, Haro RE, Sánchez P, Ruiz PA, Sanhueza-Oyarzún C, Cortés MA, Villar MT, Artigues A, Winther-Larsen HC, Avendaño-Herrera R and Yáñez AJ (2017) The Proteome of Biologically Active Membrane Vesicles from Piscirickettsia salmonis LF-89 Type Strain Identifies Plasmid-Encoded Putative Toxins. Front. Cell. Infect. Microbiol. 7:420. doi: 10.3389/fcimb.2017.00420
Received: 20 June 2017; Accepted: 12 September 2017;
Published: 28 September 2017.
Edited by:
Matthew S. Francis, Umeå University, SwedenReviewed by:
Hai Xia Xie, Institute of Hydrobiology (CAS), ChinaJavier Santander, Memorial University of Newfoundland, Canada
Myron Christodoulides, University of Southampton, United Kingdom
Copyright © 2017 Oliver, Hernández, Tandberg, Valenzuela, Lagos, Haro, Sánchez, Ruiz, Sanhueza-Oyarzún, Cortés, Villar, Artigues, Winther-Larsen, Avendaño-Herrera and Yáñez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruben Avendaño-Herrera, reavendano@yahoo.com; ravendano@unab.cl
Alejandro J. Yáñez, ayanez@uach.cl
†These authors have contributed equally to this work.
 Cristian Oliver
Cristian Oliver Mauricio A. Hernández
Mauricio A. Hernández Julia I. Tandberg
Julia I. Tandberg Karla N. Valenzuela
Karla N. Valenzuela Leidy X. Lagos
Leidy X. Lagos Ronie E. Haro
Ronie E. Haro Patricio Sánchez
Patricio Sánchez Pamela A. Ruiz
Pamela A. Ruiz Constanza Sanhueza-Oyarzún
Constanza Sanhueza-Oyarzún Marcos A. Cortés
Marcos A. Cortés María T. Villar
María T. Villar Antonio Artigues
Antonio Artigues Hanne C. Winther-Larsen5,6
Hanne C. Winther-Larsen5,6  Ruben Avendaño-Herrera
Ruben Avendaño-Herrera Alejandro J. Yáñez
Alejandro J. Yáñez