Construction of a New Phage Integration Vector pFIV-Val for Use in Different Francisella Species
- 1Division 2 (ZBS 2), Cellular Interactions of Bacterial Pathogens, Centre for Biological Threats and Special Pathogens, Robert Koch Institute, Berlin, Germany
- 2Division 2 (ZBS 2), Highly Pathogenic Microorganisms, Centre for Biological Threats and Special Pathogens, Robert Koch Institute, Berlin, Germany
We recently identified and described a putative prophage on the genomic island FhaGI-1 located within the genome of Francisella hispaniensis AS02-814 (F. tularensis subsp. novicida-like 3523). In this study, we constructed two variants of a Francisella phage integration vector, called pFIV1-Val and pFIV2-Val (Francisella Integration Vector-tRNAVal-specific), using the attL/R-sites and the site-specific integrase (FN3523_1033) of FhaGI-1, a chloramphenicol resistance cassette and a sacB gene for counter selection of transformants against the vector backbone. We inserted the respective sites and genes into vector pUC57-Kana to allow for propagation in Escherichia coli. The constructs generated a circular episomal form in E. coli which could be used to transform Francisella spp. where FIV-Val stably integrated site specifically into the tRNAVal gene of the genome, whereas pUC57-Kana is lost due to counter selection. Functionality of the new vector was demonstrated by the successfully complementation of a Francisella mutant strain. The vectors were stable in vitro and during host-cell infection without selective pressure. Thus, the vectors can be applied as a further genetic tool in Francisella research, expanding the present genetic tools by an integrative element. This new element is suitable to perform long-term experiments with different Francisella species.
Introduction
Francisella tularensis, the causative agent of tularemia, is found in a wide range of wild animals and can infect humans, causing various clinical expressions ranging from skin lesions to severe pneumonia, depending on the route of infection (Ellis et al., 2002). Infections in humans are mostly associated with the highly virulent F. tularensis subsp. (Ft.) tularensis and the less virulent subspecies Ft. holarctica (Fth) (Keim et al., 2007). Opportunistic infections by other Francisella species such as F. hispaniensis (Fhis), F. novicida (Fno), and F. philomiragia (Fph) have been reported in individuals with compromised immune systems (Hollis et al., 1989; Clarridge et al., 1996; Whipp et al., 2003). Recently, a new Francisella species (Francisella sp. strain W12-1067) has been identified in an aquatic habitat in Germany (Rydzewski et al., 2014). Yet it is not clear if the new species will be grouped into the genus Francisella or into the new genus “Allofrancisella” (Qu et al., 2013, 2016; Challacombe et al., 2017a). So far it is not known if this species is able to infect humans.
We recently identified and described the genomic island (GI) FhaGI-1, located in the genome of Fhis AS02-814 (Ft. subsp. novicida-like 3523) that contains a putative prophage (Schunder et al., 2013). We could show that the GI integrates site specifically into the tRNAVal gene of the genome and that it generates an episomal form in an integrase-dependent manner. Furthermore, we could demonstrate that small variants of FhaGI-1 are able to integrate site specifically into the genome of other Francisella species (Rydzewski et al., 2015). Therefore, we decided to create the first Francisella phage integration vector on the basis of this GI.
There are a number of tools to manipulate Francisella genetically. For the expression of genes and complementation in trans, there are several shuttle-vectors derived from the cryptic plasmid pFNL10. Although the second and third generation of these vectors are mostly stable without selective pressure, high copy numbers can still pose a problem (Norqvist et al., 1996; Pomerantsev et al., 2001; Maier et al., 2004; LoVullo et al., 2006). Vectors based on plasmids from Fph expand the repertoire of shuttle-vectors and make it possible to use more than one vector per organism (Le Pihive et al., 2009). Chromosomal integration is a way to circumvent the problems associated with high copy numbers. For many bacteria, integration systems based on the site-specific elements of bacteriophages have been described (Lee et al., 1991; Hoang et al., 2000; Lauer et al., 2002). In general, these vectors consist of the site-specific integrase of a bacteriophage together with its attP-site (Campbell, 2003), a resistance gene, and a multiple cloning site. For Francisella few chromosomal integration systems have been described so far. The existing systems are either based on allelic exchange or a mini-Tn7 vector. Both systems produce transformants that are stable without selective pressure, but they also require helper plasmids or multiple rounds of transformation and selection (Ludu et al., 2008; LoVullo et al., 2009a,b). Phage integration vectors have not been generated for Francisella since phages for this organism have not been described before (LoVullo et al., 2009a; Rydzewski et al., 2015). Further cryptic plasmids and a putative conjugative element have been described recently and may be used to generate further plasmids for Francisella in the future (Siddaramappa et al., 2014; Challacombe et al., 2017b).
Here we report the construction of two variants of a new phage integration vector pFIV-Val on the basis of the genomic island FhaGI-1 that replicate in Escherichia coli and integrate stably and site specifically into the genome of different Francisella species.
Materials and Methods
Strains and Growth Conditions
Strains used in this study were E. coli (DH10B) One Shot® TOP 10 (Invitrogen) and various Francisella strains (see Table 1). The iglC mutant strain of Fth strain LVS was kindly provided by Anders Sjöstedt (Golovliov et al., 2003). For genes and abbriviations used, see Table 1.
E. coli was cultivated in Luria-Bertani (LB) medium or on LB agar. The antibiotic concentrations used for E. coli were chloramphenicol (Cm) 40 μg ml−1 and kanamycin (Km) 40 μg ml−1. Francisella strains were cultivated in medium T (Pavlovich and Mishan'kin, 1987; Becker et al., 2016), on medium T-based agar plates (MT-KH agar: medium T containing 2.4 g l−1 of activated charcoal, 14.3 g l−1 of agar and 9.5 g l−1 of hemoglobin), or on HCA agar (Brain Heart Infusion Agar [Liofilchem, Roseto degli Abruzzi, Italy] with 10% sheep blood). The antibiotic concentrations used for Francisella were 10 μg ml−1 for chloramphenicol and 12 μg ml−1 for kanamycin.
The human macrophage-like cell line U937 (ATCC CRL-1593.2) (growth medium RPMI 1640 + 10% FCS [purchased from PAA, Pasching, Austria]) was used to investigate the intracellular multiplication of Francisella strains. U937 cells were cultivated at 37°C and 5% CO2.
Construction of Integration Vectors
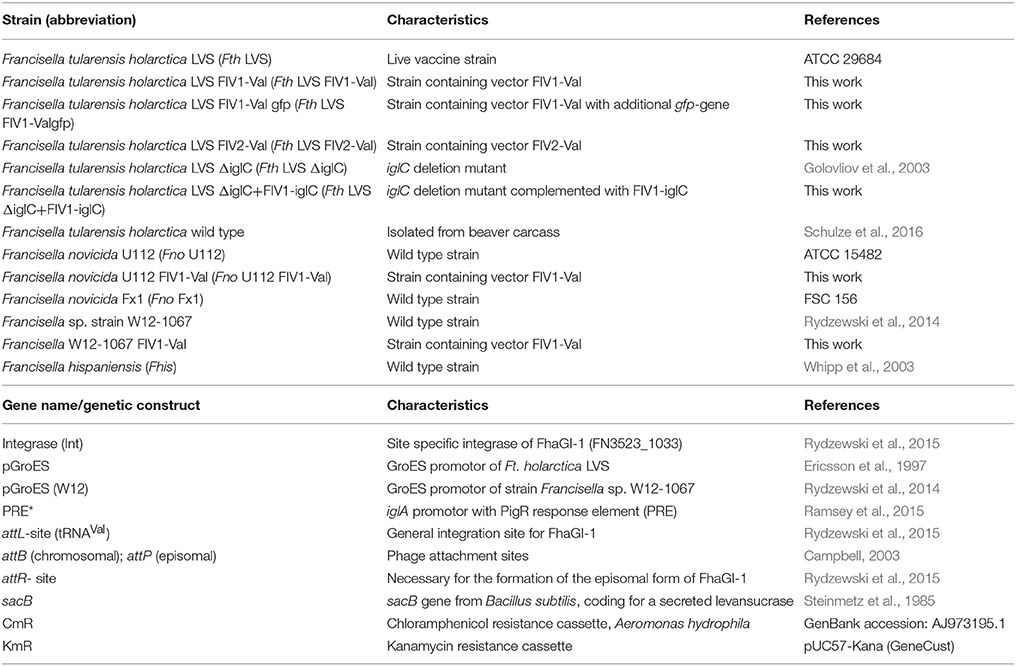
Three different DNA constructs used to generate pFIV-Val vectors were generated by in vitro DNA synthesis. DNA synthesis and DNA sequence verification by DNA sequencing were performed by GeneCust (Dudelang, Luxembourg). The different constructs were then used to generate pFIV-Val vectors 1, 2, and pFIV1-iglC, using pUC57-Kana as the back-bone (for details, see Table 1 and Figure 1). Construct 1: FhaGI-gfp-CmR is composed of 4,644 bp, exhibiting the tRNAVal gene of FhaGI-1 (Rydzewski et al., 2015), followed by restriction sites for NotI, BclI, and SnaBI, a gfp gene with promotor from vector pKK289KmGFP (Bönquist et al., 2008), restriction sites for NotI and SacII, the GroES promotor of Fth LVS (pGroES) (Ericsson et al., 1997), the iglA promotor with the PigR response element (PRE, underlined bps in PRE*), (PRE*: AGCTGTATAA ACATTGTGTT ATTGGCGTTA TTAAGGTAAC TT) (Ramsey et al., 2015), the GroES promotor from strain Francisella sp. strain W12-1067 (Rydzewski et al., 2014), followed by a Cm resistance cassette (952 bp) with promotor GroES from vector pKK289KmGFP (Bönquist et al., 2008), the integrase of FhaGI-1 (FN3523_1033), and the phage integration site attR (47 bp) (Rydzewski et al., 2015).
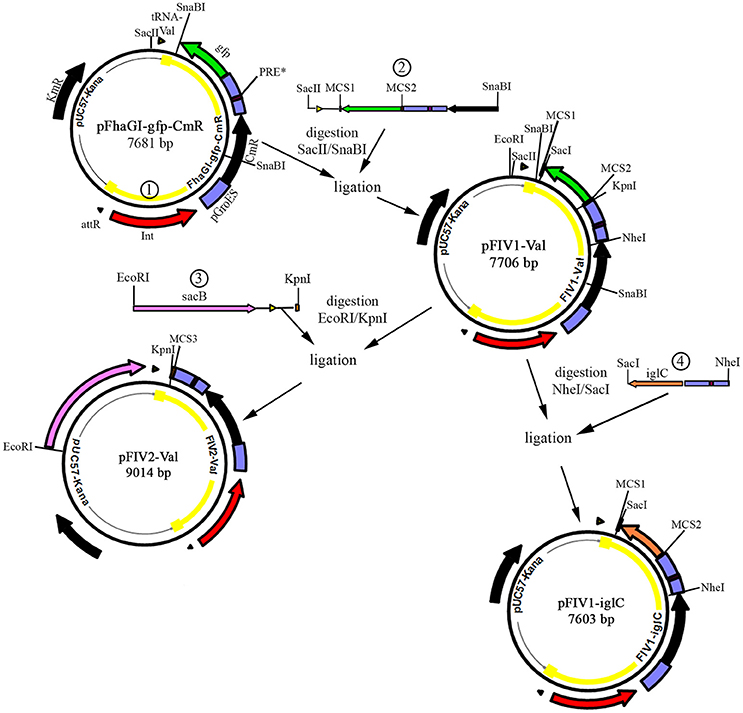
Figure 1. Construction of FhaGI-1-derived vectors and cloning. Vector maps and restriction fragments for the construction of the different vector variants are shown. Antibiotic resistance cassettes for kanamycin (KmR) and chloramphenicol (CmR) are given in black; triangles represent tRNA-Val and attR and promotors are shown in blue; the integrase gene is shown in red; genes inserted into the MCS are shown in green (gfp) and orange (iglC); the SacB gene is shown in pink; and the FIV-Val part of the vectors that integrates into the genome of Francisella transformants is highlighted by a yellow line. Restriction sites used in this study are indicated. Numbers indicate constructs (1–4) used for cloning, see also Materials and Methods section. Detailed vector maps with primer binding sites are given in Figure S1.
We introduced the PRE* site into the pFIV-Val vector to introduce a promotor that should be active during intracellular replication of Francisella. It has been published that the PRE element is an activator sequence for the expression of virulence genes, including genes (e.g., iglC) present on the Francisella pathogenicity island (FPI), and that genes of the FPI are induced during intracellular replication of Francisella (Ramsey et al., 2015).
Construct 2: The sequence (2389 bp) of construct 2 (see Figure 1) is identical to the sequence of FhaGI-gfp-CmR except for the restriction sequences surrounding the gfp gene, designated MCS1 with restriction sites for NotI, BclI, SacI, AatII, and MCS2 with restriction sites for KpnI, EcoRV, NotI, and NcoI. Construct 3 (in pFIV2-Val): SacB-tRNA-MCS3 (2502 bp) is composed of the complete sacB gene (2007 bp) of Bacillus subtilis (Steinmetz et al., 1985), the tRNAVal gene and a singular MCS3 including restriction sites for BclI, SacI, AatII, KpnI, EcoRV, NotI, and NcoI (Figure 1). In addition, the iglC gene of Ft. holarctica LVS (construct 4) was cloned into pFIV1-Val using SacI/NheI leading to pFIV1-iglC (Figure 1). The maps of pFIV-Val vectors are given in Figure S1.
For the handling of pFIV-Val vectors in in E. coli it is necessary to use chloramphenicol and kanamycin simultaneously to select for clones containing the whole vector. Otherwise, the episomal form, which is generated in E. coli will be lost, leading to strains containing only the ‘empty’ vector. This is because the episomal form (FIV-Val) is unable to integrate into the genome of E. coli or to replicate in E. coli. For further details, see Results and Discussion section.
DNA Techniques and PCR Analysis
Plasmid DNA for restriction digestion and PCR analysis was prepared using the Invisorb Plasmid Mini Two Kit (Stratec, Berlin, Germany), and preparation of genomic DNA was done with the Blood & Tissue kit (Qiagen, Hilden, Germany). Restriction enzymes were purchased from New England BioLabs and used according to the manufacturer's protocols (Frankfurt a. M., Germany). PCR was carried out using a Thermocycler TRIO-Thermoblock (Biometra, Göttingen, Germany) and the TopTaq DNA polymerase (Qiagen, Hilden, Germany). Analysis of E. coli transformants was done with primer pairs Fha-1P/Fha-2** (for pFIV1-Val), SacB_R_out/Fha-2** (pFIV2-Val) and Fha-3*/Fha-4P, for the presence of the complete construct and Fha-1P/Fha-4P (pFIV1-Val) and SacB_R_out/Fha-4P (pFIV2-Val), for presence of the “empty” vector. The presence of the episomal form was shown using primer pair Fha-2**/Fha-3*. Integration of the vectors into the genome of Francisella strains was shown using primer combinations Fha-1/Fha-2** and Fha-3* /Fha-4* (for integration into Fth LVS and Fno U112) and Fha-1W12/Fha-2** and Fha-3/Fha-4W12 (integration in Francisella sp. W12-1067). All mentioned primers are given in Table 2. In general, initial denaturation was performed at 94°C for 3 min and final extension was performed at 72°C for 10 min. The cycling conditions (35 cycles) were 94°C for 30 s, 57°C for 1 min and 72°C for 1 min, and ~ 100 ng of template DNA was used. Oligonucleotides were obtained from Eurofins MWG Operon (Ebersberg, Germany).
Transformation of Bacteria
Plasmid DNA was introduced into E. coli by thermal shock (30 min on ice, 30 s at 42°C, 2 min on ice) (Invitrogen). After transformation E. coli were incubated in LB medium for 1 h at 37°C and then plated onto agar containing 40 μg ml−1 of chloramphenicol and 40 μg ml−1 of kanamycin. Electroporation of Francisella strains was performed using a Gene Pulser system (Bio-Rad, Munich, Germany). Electroporation was done at 2.5 kV, 600 Ω and 25 μF. After transformation Francisella were incubated in medium T for 4 h at 37°C and then plated onto MT-KH or HCA agar plates containing 10 μg ml−1 of chloramphenicol and when appropriate 5% sucrose.
Testing the Stability of Vectors
To test the stability of the different vectors in Francisella transformants, they were cultured overnight in 3 ml of medium T with Cm (5 μg ml−1). The next day 200 μl of the overnight culture were used to inoculate 3 ml of fresh medium T without antibiotics. Bacteria were passaged in this manner every 12 h. After 10 passages the optical density at 600 nm (OD600) of the cultures was adjusted to 1, and cultures were diluted and plated on HCA agar with and without chloramphenicol to determine the number of bacteria still containing FIV-Val. Aliquots of the adjusted cultures were used for preparation of genomic DNA and for Western blot analysis. PCR analysis was performed to determine the presence of the integrated as well as the episomal form of FIV-Val.
SDS-PAGE and Immunoblotting
GFP detection was carried out by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. The SDS-PAGE assay was performed as described previously (Laemmli, 1970). Equal amounts of aliquots of Francisella strains from stability testing (20 μl) were boiled for 10 min in Laemmli buffer. A total of 25 μl of the solution was loaded onto a 12% SDS polyacrylamide gel. Western blotting was carried out using a polyclonal anti-GFP antibody (A-11122, Thermo Fisher Scientific, Darmstadt, Germany) diluted in 1% milk–Tris-buffered saline (TBS) (1:1,000). A horseradish peroxidase-conjugated goat anti-rabbit antibody was used as secondary antibody (1:1,000). Visualization was done using ECL Western blotting substrate (Thermo Fisher Scientific) and X-ray film.
Intracellular Replication in U937 Cells and Fluorescence Microscopy
For differentiation into macrophage-like cells, U937 cells were transferred into fresh RPMI medium containing 10% fetal calf serum (10% FCS), and PMA (phorbol-12-myristate-13-acetate, 1 mg/ml in dH2O [P-8139; Sigma-Aldrich Chemie, Munich, Germany]) was added at a concentration of 1:20,000. After incubation for 36 h at 37°C and 5% CO2, the supernatant was discarded and adherent cells were washed once with 10 ml of 0.2% EDTA in PBS. Cells were mechanically detached from the flask bottom and adjusted to 5 × 105 cells/ml with RPMI + 10% FCS. To each well of a 24-well plate 1 ml of the cell suspension was added and incubated for 2 h at 37°C and 5% CO2 for adhesion.
Overnight cultures of Francisella strains were diluted in plain RPMI medium, and the infection was done with a multiplicity of infection (MOI) of 10 (time point 0 h) for 2 h at 37°C and 5% CO2. Cells were washed three times with RPMI and incubated with 50 μg ml−1 of Gentamycin for 1 h to kill extracellular bacteria. Cells were washed again three times with RPMI and covered with 1 ml of RPMI. To determine the CFU at various time points of infection, coincubations of cells and bacteria were lysed by addition of 10 μl of 10% Saponin (S4521, Sigma-Aldrich Chemie) and serial dilutions were plated on HCA agar.
During the infection fluorescent images were obtained every 24 h using an inverse microscope (Carl Zeiss, Jena, Germany).
Copy Number
Copy numbers of FIV-Val were determined by qPCR-analysis. As target for the vector the CmR gene was used and as chromosomal reference the single copy gene fopA. Primers and hydrolysis probes are given in Table 2.
qPCRs were conducted in a total volume of 25 μl using the ABI 7500 Real Time PCR System and the TaqMan® Environmental Master Mix 2.0 (Applied Biosystems). Primers and hydrolysis probes were used at a final concentration of 0.3 mM and 0.1 mM, respectively. 5 μl of target DNA were added to each reaction. For each vector three decimal dilutions of DNA (0.1; 0.01; 0.001 ng) were pipetted in duplicate. Reactions were initiated with an incubation at 95°C for 10 min followed by 40 cycles of 95°C for 15 s (denaturation) and 60°C for 60 s (annealing and elongation, detection). Copy numbers were calculated using the ΔCt method.
Results and Discussion
Construction of pFIV-Val
Recently, we demonstrated that the att-sites of FhaGI-1 of Fhis AS02-814 in combination with the site-specific integrase are sufficient to generate the episomal form FIV-Val of the vector in E. coli and that after transformation into Fth LVS the element integrates site specifically into the tRNA-Val gene of transformants (Rydzewski et al., 2015). We now utilized this information to develop phage integration vectors to be used in Francisella research, with the idea that the constructs would be integrated site specifically and stable into the genome.
We constructed a first variation of the vector, called pFhaGI-gfp-CmR (7,681 bp) (Figure 1 and Figure S1A). The construct was composed of the following elements (see also Table 1): (1) the attL-site (tRNAVal) which is the general integration site for FhaGI-1; (2) a gfp gene flanked by restriction sites that serves as a place holder for the integration of future genes of interest (at this first stage) and as a control for gene expression during intracellular replication, (3) the Fth LVS GroES promotor; (4) the PRE* site that is used for the expression of genes during intracellular replication of Francisella in host cells; (5) the GroES promotor of strain Francisella sp. W12-1067 (pGroES-W12) for expression of genes in this species; (6) a chloramphenicol resistance marker for the selection process after transformation; (7) the site-specific integrase which is necessary for generating the episomal form and the integration into the attB-site (tRNAVal) of the acceptor strain (Francisella strain of interest) and the attR-site which is necessary for the formation of the episomal form of pFhaGI-gfp-CmR. For details, see also Materials and Methods. As a backbone, the plasmid pUC57-Kana was used to allow propagation of the construct in E. coli. Note, only the FhaGI-gfp-CmR construct (Figure 1 episomal form, marked in yellow) will be integrated site specifically into the genome of the acceptor strain (Rydzewski et al., 2015).
The construct was then introduced into E. coli by chemical transformation and plated on agar containing chloramphenicol and kanamycin. We isolated plasmid DNA from this strain and analyzed it for the presence of all three forms of pFhaGI-gfp-CmR using primer pairs 1P/2** and 3*/4P (pFIV-Val), 2**/3* (episomal form, FIV-Val), and 1P/4P (“empty” vector with attB-site) (Figures 2A,B). The PCRs confirmed that all three forms were present which means that the integrase is active in E. coli.
Figure 2. Forms of pFhaGI-1-gfp-CmR. (A) In E. coli transformants three forms of the vector are present: the complete construct (pFhaGI-gfp-CmR, representing pFIV-Val), the “empty” pUC57-Kana vector with attB-site, and the episomal form with attP-site (FhaGI-gfp-CmR, representing FIV-Val). After transformation into Francisella the FIV-Val part integrates site specifically into the genome. Arrows indicate primers used to detect the different forms of pFhaGI-1-gfp-CmR. (B) PCR analysis of an E. coli transformant containing pFhaGI-1-gfp-CmR. (C) PCR analysis of Ft. holarctica LVS transformant containing FhaGI-1-gfp-CmR. Primers Fha-2/3 (Fha-2**/Fha-3*) show the presence of the episomal form, primers 1/2 and 3/4 (Fha-1/Fha-2** and Fha-3*/Fha-4* or Fha-1P/Fha-2** and Fha-3*/Fha-4P) show the chromosomally integrated form of FhaGI-gfp-CmR, whereas primers 1/4 (Fha-1/Fha-4* or Fha-1P/Fha-4P) show the chromosomal attB-site.
The plasmid preparation of pFhaGI-gfp-CmR was introduced into Fth LVS by electroporation and plated onto agar plates containing chloramphenicol. One hundred clones were picked and then transferred to plates containing kanamycin for negative selection against transformants still harboring the “empty” vector. Of the picked clones ~30% were kanamycin sensitive and, therefore, maintained only the desired construct (FhaGI-gfp-CmR), the episomal or genomically integrated form of pFhaGI-gfp-CmR (Figure 2A). The loss of the “empty” pUC57-Kana vector was also confirmed by PCR analysis (Figure 2C, lane 5). To test these clones for site-specific integration into the tRNAVal gene they were further analyzed by PCR using species specific (acceptor strain) primers (Figure 2C and Table 2). PCR confirmed that the construct was successfully integrated into the genome of Fth LVS (Figure 2C, lanes 1 and 3) and that the episomal form was also present (Figure 2C, lane 2). In addition, the PCR product of about 500 bp using primers 1/4* (Figure 2C, lane 4) confirmed that after excision of FIV-Val, no copy of the GI is left in the genome (see also Rydzewski et al., 2015), indicating that the episomal form is not generated in a replicative way. The results demonstrated that the concept of a phage integration vector works at least in Fth.
Subsequently we optimized our vector by introducing an MCS on both sites of the gfp gene resulting in vector pFIV1-Val (7,706 bp) (Figure 1 and Figure S1B).
Functional Characterization of pFIV1-Val
An important characteristic of a vector is its stability without a selective pressure. To test the stability of FIV1-Val in Fth LVS without any selective pressure, we cultivated Fth LVS harboring FIV1-Val in medium T without antibiotics. After 10 passages, we plated cultures on HCA agar with and without chloramphenicol. As shown in Figure 3A, the CFU of tested strains on agar plates containing chloramphenicol was similar to that on agar without antibiotics, demonstrating that FIV1-Val remained stable without selective pressure. This was further verified by PCR analysis which confirmed the presence of the integrated as well as the episomal form of FIV1-Val (Figure 3B). To test if the gene of interest (e.g., gfp) cloned into pFIV1-Val was expressed in the acceptor strain, Western Blot analysis using an anti-GFP antibody was performed. The results demonstrated that the gfp gene was expressed (Figure 3C, lane 1), indicating that the used promotor element of FIV-Val is active in Fth. The wild type control proves that although the observed band is rather faint it is not due to background binding of the antibody in the strain (Figure 3C, lane 2).
Figure 3. Stability of FhaGI-1-based vectors. Strains Fth LVS FIV1-Val, Fth LVS FIV2-Val, Fno U112 FIV1-Val, and Francisella sp. W12-1067 FIV1-Val were passaged 10 times in mediumT without antibiotics. (A) CFU of strains on HCA agar and HCA agar containing Chloramphenicol (10 μg ml−1) after 10 passages in mediumT without antibiotics; results shown are means with SD of three independent experiments. (B) PCR analysis of genomic DNA of strains after 10 passages without antibiotics. Primers 2/3 (Fha-2**/Fha-3*) show the presence of the episomal form, primers 1/2 and 3/4 (Fha-1 or Fha-1W12/Fha-2** and Fha-3*/Fha-4* or Fha-4W12) show the chromosomally integrated form of FIV-Val, and primers 1/4 (Fha-1/Fha-4* or Fha-1W12/Fha-4W12) show the chromosomal attB-site. (C) Western Blot analysis of whole cell lysates with rabbit-α-gfp antibody (1:1,000).
To demonstrate further that the integration vector is functional in Francisella, we used it to complement a specific mutant strain of Fth. We chose an iglC mutant strain of Fth LVS which is known to be unable to replicate within host cells (Golovliov et al., 2003). We cloned the iglC gene into pFIV1-Val, resulting in pFIV1-iglC (Figure 1). FIV1-iglC was then successfully integrated into the iglC mutant strain, leading to strain Fth LVS ΔiglC+FIV1-iglC. The site-specific integration of the construct was confirmed by PCR analysis (data not shown). Then we performed infection assays with this strain as well as the Fth LVS wild-type strain and Fth LVS FIV1-Val, using the human macrophage-like cell line U937 (Figure 4A). As expected, the Fth LVS wild-type strain replicated in the macrophages while the iglC mutant strain did not. The complemented iglC mutant strain was able to replicate in U937 cells nearly as well as the wild-type strain (Figure 4A). The nearly complete complementation of the iglC mutant might be due to the fact that both copies of the iglC gene present in the wild-type strain had been inactivated (Golovliov et al., 2003; Lai et al., 2004) and that the expression of the gene from FIV1-Val was not high enough to complement both inactivated genes. However, the intracellular growth defect of the iglC mutant strain was complemented. In addition, we could demonstrate that the presence of FIV1-Val alone did not influence the intracellular replication of Fth LVS (Figure 4A). The results demonstrated that FIV-Val was stable without selective pressure and could be used to express genes of interest during intracellular replication of Francisella in host cells. In addition, since FIV-Val did not influence the ability of Francisella to replicate intracellularly in host cells, pFIV-Val can be used for successful complementation of specific mutant strains.
Figure 4. Infection assays using a human macrophage-like cell line (U937). (A) Replication of Fth LVS wild type (LVS), Fth LVS iglC mutant (LVS ΔiglC), complemented Fth LVS iglC mutant (LVS ΔiglC+FIV1-iglC) and Fth LVS wild type containing FIV1-Val (LVS FIV1-Val). Cells were infected at an MOI of 10, and CFU was determined by plating on HCA agar every 24 h. Results are mean standard deviations of duplicate samples and are representative of at least 3 independent experiments. *, done only twice. (B) Fluorescence microscopy of U937 cells infected with Fth LVS FIV1-Val or Fth LVS FIV1-Valgfp. f, fluorescence; b, bright field; m, merge.
In a further infection assay, we verified whether we could visualize the expression of the gfp gene during intracellular replication of Francisella. Activity of the gfp gene of FIV1-Val during the infection of U937 cells was visualized using a fluorescence microscope. As shown in Figure 4B, the activity of the GFP was low but macrophages containing fluorescent Fth LVS FIV1-Val could be demonstrated after 48 h and the number of fluorescent cells increased after 72 h. The results corroborated that the gene of interest cloned into pFIV-Val is expressed during intracellular replication. LoVullo and colleagues observed a similarly low number of fluorescent cells when using a Tn7-based chromosomal integration system to insert a gfp gene into the genome of Fth LVS (LoVullo et al., 2009a). They attributed the poor visualization to a multitude of factors including promotor strength and improper folding of the GFP. Another factor that might account for the rather weak fluorescent signal could be a low copy number of the gene. Experiments using a strain with an additional gfp gene cloned into pFIV1-Val (pFIV-Valgfp) seemed to support the theory of low copy numbers. With this strain a fluorescent signal was visible after 24 h and overall there seemed to be more fluorescent cells (Figure 4B, lane LVS FIV1-Valgfp). Altogether these results further confirmed that FIV1-Val remains stable in Fth without selective pressure and that it can be used to manipulate Fth strains genetically.
Functionality Test of pFIV-Val in Other Francisella Species
To validate whether pFIV-Val could also be used in other Francisella species, we transformed Fno U112, Fno Fx1, and Francisella sp. W12-1067 with pFIV-Val. For all three strains we verified the site-specific integration into the genome and the presence of the episomal form by PCR analysis (Figure 3B). We further analyzed Fno U112 and Francisella sp. W12-1067 for the stability of FIV1-Val (Figure 3A). In both species the construct remained stable integrated after 10 passages in medium T without antibiotics (Figure 3B, lanes U112 FIV1 and W12 FIV1, respectively). GFP activity in both strains was low but could be confirmed by Western-Blot analysis (Figure 3C, lanes 3 and 5). In Francisella sp. W12-1067 the amount of the GFP protein was higher than in both other species investigated, suggesting that the cloned promotor element is highly active in this Francisella species. However, the differences in GFP activity could be due to differences in vector copy number, expression or in protein stability (improper folding, LoVullo et al., 2009a). In our hands, similar results were also obtained with plasmids harboring a pGroES-gfp gene (unpublished results).
We also successfully introduced pFIV-Val into a wild-type strain of Fth (isolated from a beaver, Schulze et al., 2016) (data not shown). These results show that pFIV1-Val is suitable as an integration vector in different species and strains of Francisella.
Further Improvements and Determination of the Copy Number of pFIV-Val
To simplify selection of FIV-Val-positive clones after transformation, we decided to employ the idea of a negative selection step and introduced the sacB gene of Bacillus subtilis into that part of pFIV1-Val which does not integrate into the genome of transformants. The sacB gene codes for a secreted levansucrase which is toxic for Gram-negative bacteria when expressed in the presence of sucrose (Steinmetz et al., 1983, 1985). Using the levansucrase, only one selection step of transformants is needed since clones still harboring the “empty” or complete vector will die in the presence of sucrose. The construct “SacB-tRNA-MCS3” was cloned into pFIV1-Val, leading to the second vector called pFIV2-Val (9,014 bp). In addition, to generate a standard cloning vector pFIV2-Val, the gfp gene has been deleted and a singular MCS 3 has been introduced instead (Figure 1 and Figure S1D).
After transformation of pFIV2-Val into Fth LVS and selection on agar plates containing chloramphenicol and sucrose, only FIV2-Val-positive clones with the integrated form of FIV2-Val could be detected by PCR analysis (Figure 3B and data not shown). This demonstrates that the selection on sucrose was very efficient, thus eliminating the need for a second selection step. FIV2-Val was tested for its stability as described for FIV1-Val. The vector remained stable without selective pressure (Figure 3B, lanes LVS FIV2). However, since the integrase is still located on the FIV2-Val part of the vector, the episomal (excised) form of FIV-Val is still generated and detectable (Figure 3B, primers 2/3). Earlier we could show that the integrase is sufficient for the excision of a small variant of FhaGI-1, but excision still occurred in Fth LVS of an element missing the site-specific integrase. This may be due to the presence of further integrases or RecA in the genome sequence of the acceptor strain (Lesic and Carniel, 2005; Rydzewski et al., 2015). We obtained a comparable result for a genomic island (LpcGI-2) of Legionella pneumophila in which a similar mechanism was used for the excision of the GI from the genome (Lautner et al., 2013).
First qPCR analyses to quantify the copy number of the FIV-Val constructs (see section Materials and Methods) suggest that the copy number of FIV-Val in Fth LVS was 3.6 for both FIV1-Val and FIV2-Val (see Table S1). The results demonstrated that FIV-Val behaves in Francisella like a low-copy vector.
Conclusion
In this study we constructed two variants of a new phage integration vector (pFIV1-Val and pFIV2-Val), derived from FhaGI-1 of Fhis AS02-814 for the use in different Francisella species. Both constructs integrate site specifically into the genome of the acceptor species at the attB-site localized within the tRNAVal gene and remain stable without selective pressure. The introduction of a levansucrase into pFIV2-Val simplified the selection process of FIV-Val-positive strains after transformation of the acceptor with pFIV-Val. qPCR analysis suggests that there are about 3.6 copies of FIV-Val in Fth LVS. We used pFIV1-Val to complement successfully an iglC mutant strain of Fth LVS and could demonstrate that an introduced ‘gene of interest’ (gfp gene) was active in three different Francisella species.
GFP activity was not high in Fth, but the advantage of pFIV-Val is the site-specific integration, the low copy number and the stability without any selective pressure. In contrast to other plasmids or integration systems, pFIV-Val can be used without the help of “helper-plasmids” and in combination with other expression vectors, since no origin of replication is present on FIV-Val. Furthermore, pFIV-Val is usable in different Francisella strains of Fth and Fno and also different Francisella species. Thus, our results demonstrate that FhaGI-1-derived vectors can be used as a further genetic tool in Francisella research. With this new integration vector we now are able to perform research (in the laboratory) on persistence and reservoir research with Francisella spp. in long-term experiments.
Author Contributions
KH designed the study and RG provided facility and equipment. HT, KK, and KR performed the experiments. HT and KH wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Ursula Erikli for copy-editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2018.00075/full#supplementary-material
Figure S1. Detailed vector maps of FhaGI-derived vectors. Given are vector maps for the different versions of the vector. Antibiotic resistance cassettes for kanamycin (KmR) and chloramphenicol (CmR) are given in green; yellow triangles represent tRNA-Val and attR and promotors are shown in blue; the integrase gene is shown in red; the MCS is given in orange and the SacB gene is shown in pink; the FIV part of the vectors that integrates into the genome of Francisella transformants is highlighted by a yellow line; primer binding sites are indicated by blue arrows; and restriction sites of the MCS and others used in this study are indicated. Primer binding positions are given in the table below each map; restriction enzymes that do not cut the vectors are given in a list below each vector version. (A) Vector map of pFhaGI-gfp-CmR, (B) vector map of pFIV1-Val, (C) restriction digestion of the three pFIV-Val vectors with NheI/SacI. *, desired fragment; °, episomal form; [], empty vector; x, cut-out fragment, and (D) vector map of pFIV2-Val.
Table S1. Data of qPCR analysis determining the copy number of FIV-Val.
References
Becker, S., Lochau, P., Jacob, D., Heuner, K., and Grunow, R. (2016). Successful re-evaluation of broth medium T for the growth of Francisella tularensis ssp. and other higly pathogenic bacteria. J. Microbiol. Methods 121, 5–7. doi: 10.1016/j.mimet.2015.11.018
Bönquist, L., Lindgren, H., Golovliov, I., Guina, T., and Sjostedt, A. (2008). MglA and Igl proteins contribute to the modulation of Francisella tularensis live vaccine strain-containing phagosomes in murine macrophages. Infect. Immun. 76, 3502–3510. doi: 10.1128/IAI.00226-08
Campbell, A. (2003). Prophage insertion sites. Res. Microbiol. 154, 277–282. doi: 10.1016/S0923-2508(03)00071-8
Challacombe, J. F., Petersen, J. M., Gallegos-Graves, V., Hodge, D., Pillai, S., and Kuske, C. R. (2017a). Whole-Genome relationships among Francisella bacteria of diverse origins define new species and provide specific regions for detection. Appl. Environ. Microbiol. 83:e02589-16. doi: 10.1128/AEM.02589-16
Challacombe, J. F., Pillai, S., and Kuske, C. R. (2017b). Shared features of cryptic plasmids from environmental and pathogenic Francisella species. PLoS ONE 12:e0183554. doi: 10.1371/journal.pone.0183554
Clarridge, J. E. III., Raich, T. J., Sjosted, A., Sandstrom, G., Darouiche, R. O., Shawar, R. M., et al. (1996). Characterization of two unusual clinically significant Francisella strains. J. Clin. Microbiol. 34, 1995–2000.
Ellis, J., Oyston, P. C., Green, M., and Titball, R. W. (2002). Tularemia. Clin. Microbiol. Rev. 15, 631–646. doi: 10.1128/CMR.15.4.631-646.2002
Ericsson, M., Golovliov, I., Sandstrom, G., Tarnvik, A., and Sjostedt, A. (1997). Characterization of the nucleotide sequence of the groE operon encoding heat shock proteins chaperone-60 and−10 of Francisella tularensis and determination of the T-cell response to the proteins in individuals vaccinated with F. tularensis. Infect. Immun. 65, 1824–1829.
Golovliov, I., Sjostedt, A., Mokrievich, A., and Pavlov, V. (2003). A method for allelic replacement in Francisella tularensis. FEMS Microbiol. Lett. 222, 273–280. doi: 10.1016/S0378-1097(03)00313-6
Hoang, T. T., Kutchma, A. J., Becher, A., and Schweizer, H. P. (2000). Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43, 59–72. doi: 10.1006/plas.1999.1441
Hollis, D. G., Weaver, R. E., Steigerwalt, A. G., Wenger, J. D., Moss, C. W., and Brenner, D. J. (1989). Francisella philomiragia comb. nov. (formerly Yersinia philomiragia) and Francisella tularensis biogroup novicida (formerly Francisella novicida) associated with human disease. J. Clin. Microbiol. 27, 1601–1608.
Keim, P., Johansson, A., and Wagner, D. M. (2007). Molecular epidemiology, evolution, and ecology of Francisella. Ann. N. Y. Acad. Sci. 1105, 30–66. doi: 10.1196/annals.1409.011
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. doi: 10.1038/227680a0
Lai, X. H., Golovliov, I., and Sjostedt, A. (2004). Expression of IglC is necessary for intracellular growth and induction of apoptosis in murine macrophages by Francisella tularensis. Microb. Pathog. 37, 225–230. doi: 10.1016/j.micpath.2004.07.002
Lauer, P., Chow, M. Y., Loessner, M. J., Portnoy, D. A., and Calendar, R. (2002). Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184, 4177–4186. doi: 10.1128/JB.184.15.4177-4186.2002
Lautner, M., Schunder, E., Herrmann, V., and Heuner, K. (2013). Regulation, integrase-dependent excision, and horizontal transfer of genomic islands in Legionella pneumophila. J. Bacteriol. 195, 1583–1597. doi: 10.1128/JB.01739-12
Lee, C. Y., Buranen, S. L., and Ye, Z. H. (1991). Construction of single-copy integration vectors for Staphylococcus aureus. Gene 103, 101–105. doi: 10.1016/0378-1119(91)90399-V
Le Pihive, E., Blaha, D., Chenavas, S., Thibault, F., Vidal, D., and Valade, E. (2009). Description of two new plasmids isolated from Francisella philomiragia strains and construction of shuttle vectors for the study of Francisella tularensis. Plasmid 62, 147–157. doi: 10.1016/j.plasmid.2009.07.001
Lesic, B., and Carniel, E. (2005). Horizontal transfer of the high-pathogenicity island of Yersinia pseudotuberculosis. J. Bacteriol. 187, 3352–3358. doi: 10.1128/JB.187.10.3352-3358.2005
LoVullo, E. D., Molins-Schneekloth, C. R., Schweizer, H. P., and Pavelka, M. S. Jr. (2009a). Single-copy chromosomal integration systems for Francisella tularensis. Microbiology 155, 1152–1163. doi: 10.1099/mic.0.022491-0
LoVullo, E. D., Sherrill, L. A., and Pavelka, M. S. Jr. (2009b). Improved shuttle vectors for Francisella tularensis genetics. FEMS Microbiol. Lett. 291, 95–102. doi: 10.1111/j.1574-6968.2008.01440.x
LoVullo, E. D., Sherrill, L. A., Perez, L. L., and Pavelka, M. S. Jr. (2006). Genetic tools for highly pathogenic Francisella tularensis subsp. tularensis. Microbiology 152, 3425–3435. doi: 10.1099/mic.0.29121-0
Ludu, J. S., Nix, E. B., Duplantis, B. N., De Bruin, O. M., Gallagher, L. A., Hawley, L. M., et al. (2008). Genetic elements for selection, deletion mutagenesis and complementation in Francisella spp. FEMS Microbiol. Lett. 278, 86–93. doi: 10.1111/j.1574-6968.2007.00979.x
Maier, T. M., Havig, A., Casey, M., Nano, F. E., Frank, D. W., and Zahrt, T. C. (2004). Construction and characterization of a highly efficient Francisella shuttle plasmid. Appl. Environ. Microbiol. 70, 7511–7519. doi: 10.1128/AEM.70.12.7511-7519.2004
Norqvist, A., Kuoppa, K., and Sandstrom, G. (1996). Construction of a shuttle vector for use in Francisella tularensis. FEMS Immunol. Med. Microbiol. 13, 257–260. doi: 10.1111/j.1574-695X.1996.tb00248.x
O'shaughnessy, J. B., Chan, M., Clark, K., and Ivanetich, K. M. (2003). Primer design for automated DNA sequencing in a core facility. Biotechniques 35, 118–121.
Pavlovich, N. V., and Mishan'kin, B. N. (1987). [Transparent nutrient medium for culturing Francisella tularensis]. Antibiot. Med. Biotekhnol. 32, 133–137.
Pomerantsev, A. P., Golovliov, I. R., Ohara, Y., Mokrievich, A. N., Obuchi, M., Norqvist, A., et al. (2001). Genetic organization of the Francisella plasmid pFNL10. Plasmid 46, 210–222. doi: 10.1006/plas.2001.1548
Qu, P. H., Chen, S. Y., Scholz, H. C., Busse, H. J., Gu, Q., Kampfer, P., et al. (2013). Francisella guangzhouensis sp. nov., isolated from air-conditioning systems. Int. J. Syst. Evol. Microbiol. 63, 3628–3635. doi: 10.1099/ijs.0.049916-0
Qu, P. H., Li, Y., Salam, N., Chen, S. Y., Liu, L., Gu, Q., et al. (2016). Allofrancisella inopinata gen. nov., sp. nov. and Allofrancisella frigidaquae sp. nov., isolated from water-cooling systems, and transfer of Francisella guangzhouensis Qu et al. 2013 to the new genus as Allofrancisella guangzhouensis comb. nov. Int. J. Syst. Evol. Microbiol. 66, 4832–4838. doi: 10.1099/ijsem.0.001437
Ramsey, K. M., Osborne, M. L., Vvedenskaya, I. O., Su, C., Nickels, B. E., and Dove, S. L. (2015). Ubiquitous promoter-localization of essential virulence regulators in Francisella tularensis. PLoS Pathog. 11:e1004793. doi: 10.1371/journal.ppat.1004793
Rydzewski, K., Schulz, T., Brzuszkiewicz, E., Holland, G., Luck, C., Fleischer, J., et al. (2014). Genome sequence and phenotypic analysis of a first German Francisella sp. isolate (W12-1067) not belonging to the species Francisella tularensis. BMC Microbiol. 14:169. doi: 10.1186/1471-2180-14-169
Rydzewski, K., Tlapak, H., Niehaus, I. P., Dabrowski, P. W., Grunow, R., and Heuner, K. (2015). Identification and characterization of episomal forms of integrative genomic islands in the genus Francisella. Int. J. Med. Microbiol. 305, 874–880. doi: 10.1016/j.ijmm.2015.08.037
Schulze, C., Heuner, K., Myrtennas, K., Karlsson, E., Jacob, D., Kutzer, P., et al. (2016). High and novel genetic diversity of Francisella tularensis in Germany and indication of environmental persistence. Epidemiol. Infect. 144, 3025–3036. doi: 10.1017/S0950268816001175
Schunder, E., Rydzewski, K., Grunow, R., and Heuner, K. (2013). First indication for a functional CRISPR/Cas system in Francisella tularensis. Int. J. Med. Microbiol. 303, 51–60. doi: 10.1016/j.ijmm.2012.11.004
Siddaramappa, S., Challacombe, J. F., Petersen, J. M., Pillai, S., and Kuske, C. R. (2014). Comparative analyses of a putative Francisella conjugative element. Genome 57, 137–144. doi: 10.1139/gen-2013-0231
Steinmetz, M., Le Coq, D., Aymerich, S., Gonzy-Treboul, G., and Gay, P. (1985). The DNA sequence of the gene for the secreted Bacillus subtilis enzyme levansucrase and its genetic control sites. Mol. Gen. Genet. 200, 220–228. doi: 10.1007/BF00425427
Steinmetz, M., Le Coq, D., Djemia, H. B., and Gay, P. (1983). [Genetic analysis of sacB, the structural gene of a secreted enzyme, levansucrase of Bacillus subtilis Marburg]. Mol. Gen. Genet. 191, 138–144. doi: 10.1007/BF00330901
Keywords: Francisella tularensis, integrative vector, pFIV-Val, genomic island, episomal, phage
Citation: Tlapák H, Köppen K, Rydzewski K, Grunow R and Heuner K (2018) Construction of a New Phage Integration Vector pFIV-Val for Use in Different Francisella Species. Front. Cell. Infect. Microbiol. 8:75. doi: 10.3389/fcimb.2018.00075
Received: 27 October 2017; Accepted: 27 February 2018;
Published: 14 March 2018.
Edited by:
Marina Santic', University of Rijeka, CroatiaReviewed by:
Roger Derek Pechous, University of Arkansas for Medical Sciences, United StatesJanakiram Seshu, University of Texas at San Antonio, United States
Copyright © 2018 Tlapák, Köppen, Rydzewski, Grunow and Heuner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Klaus Heuner, heunerk@rki.de
 Hana Tlapák
Hana Tlapák Kristin Köppen1
Kristin Köppen1  Roland Grunow
Roland Grunow Klaus Heuner
Klaus Heuner