Environmental Drivers of Dissolved Organic Matter Molecular Composition in the Delaware Estuary
- 1ICBM-MPI Bridging Group for Marine Geochemistry, Institute for Chemistry and Biology of the Marine Environment, Carl von Ossietzky University, Oldenburg, Germany
- 2School of Marine Science and Policy, University of Delaware, Lewes, DE, USA
Estuaries as connectors of freshwater and marine aquatic systems are hotspots of biogeochemical element cycling. In one of the best studied temperate estuaries, the Delaware Estuary (USA), we investigated the variability of dissolved organic matter (DOM) over five sampling cruises along the salinity gradient in August and November of 3 consecutive years. Dissolved organic carbon (DOC) concentrations were more variable in the upper reaches of the estuary (245 ± 49 μmol DOC L−1) than at the mouth of the estuary (129 ± 14 μmol L−1). Bulk DOC decreased conservatively along the transect in November but was non-conservative with increased DOC concentrations mid-estuary in August. Detailed analysis of the solid-phase extractable DOM pool via ultrahigh resolution mass spectrometry (Fourier-transform ion cyclotron resonance mass spectrometry, FT-ICR-MS) revealed compositional differences at the molecular level that were not reflected in changes in concentration. Besides the mixing of terrestrial and marine endmember signatures, river discharge levels and biological activity impacted DOM molecular composition. DOM composition changed less between August and November than along the salinity gradient. Relative contributions of presumed photolabile DOM compounds did not reveal non-conservative behavior indicative of photochemical processing, suggesting that on the timescales of estuarine mixing photochemical removal of molecules plays a minor role in the turbid Delaware Bay. Overall, a large portion of molecular formulae overlapped between sampling campaigns and persisted during estuarine passage. Extending the analysis to the structural level via the fragmentation of molecular masses in the FT-ICR-MS, we found that the relative abundance of isomers along the salinity gradient did not change, indicating a high structural similarity of aquatic DOM independent of the origin. These results point toward a recalcitrant character of the DOM supplied by the Delaware River. We demonstrate that in addition to bulk DOC quantification, detailed information on molecular composition is essential for constraining sources of DOM and to identify the processes that impact estuarine DOM, thereby controlling amount and composition of DOM eventually discharged to the ocean through estuaries.
Introduction
Large amounts of terrigenous organic matter are channeled through rivers and estuaries into the oceans—approximately 0.25 × 1015 g are transported in the form of dissolved organic carbon (DOC) annually (Hedges et al., 1997). The true amount of the DOC exported from land is likely higher as some regions (for example northern high-latitude rivers, Holmes et al., 2011), diffuse flows and flood events (Cole et al., 2007; Raymond and Saiers, 2010) are not well constrained to date. During transit, the size and composition of the riverine dissolved organic matter (DOM) pool is transformed through a multitude of biotic and abiotic processes. Despite decades of research on estuarine and coastal DOM cycling, we still lack understanding of the mechanisms and magnitudes of these modifications, thereby possibly resulting in misinterpretation of characteristics of the riverine DOM discharged to the coastal seas and ultimately reaching the deep ocean (Bauer and Bianchi, 2011).
Generally, bulk DOC concentrations decrease along estuaries from the river to the ocean while at the same time, the composition of the DOM pool changes. On molecular level, the aromaticity of the DOM decreases (Abdulla et al., 2013), while molecular weight, carbohydrate content (Abdulla et al., 2010), heteroelement content (Sleighter and Hatcher, 2008) and lability increase (D'Andrilli et al., 2015) with increasing salinity. Positive and negative deviations from a conservative mixing line defined by high-DOC river water and the low-DOC marine endmember are frequently observed (Cadée, 1982; Middelburg and Herman, 2007; Sharp et al., 2009). Estuaries are complex systems where, in addition to mixing of fresh- and marine waters, diffuse sources such as subterranean groundwater discharge (Taniguchi et al., 2002), aeolian and anthropogenic input (Liu et al., 2005; Tzortziou et al., 2015) add to the intricacy of biogeochemical cycling. Abiotic processes shaping the DOM pool along the estuarine gradient of pH, ionic strength and turbidity include the adsorption on and desorption from mineral surfaces (Keil et al., 1994; Mayer, 1994), flocculation (Eisma, 1986), aggregation or precipitation. Loss of organic carbon from the dissolved phase in the estuarine turbidity maximum (e.g., Miller, 1999), where the concentration of particulate organic carbon is usually the highest, is accompanied by a loss in aromaticity and nitrogen-containing compounds, as these compounds are preferentially adsorbed (Aufdenkampe et al., 2001; Riedel et al., 2012). Photodegradation processes, although generally thought to be minor in riverine systems due to the low transmission (Spencer et al., 2009), exhibit the highest impact on aromatic moieties of terrestrial origin (Stubbins et al., 2010). By decreasing the average molecular weight and increasing the contribution of aliphatic compounds, the bioavailability of the DOM can increase through photochemically mediated breakdown of molecules especially in tropical regions (Medeiros et al., 2015b). Biological processes, in temperate regions influenced by seasonality, such as selective uptake and transformation by heterotrophic microbes (Azam et al., 1994; Bourgoin and Tremblay, 2010), as well as addition of compounds via autochthonous production (Pennock and Sharp, 1986) likewise shape the estuarine DOM pool. Medeiros et al. (2015b) for example showed that phytoplankton-derived DOM inputs introduce saturated compounds in the tropical Amazon plume. DOM export by the Yukon River to the Bering Sea was highly stable over seasons, with enhanced DOM photo- and biolability in spring due to higher inputs of carbohydrates and aromatic moieties (Cao et al., 2016). Previous studies have furthermore shown that the DOC concentration in rivers increases with discharge (Schiff et al., 1998; Raymond and Saiers, 2010), superimposing the influence of most other environmental drivers. At high discharge, riverine DOM composition tends to carry a stronger terrigenous signal (Spencer et al., 2008). A similar observation was made by Medeiros et al. (2015a) who found strong seasonal variability in molecular DOM composition in the marsh-dominated Altamaha-Doboy-Sapelo estuary. This variability was driven mainly by river flow, leading to a stronger terrigenous signature at high discharge.
In our study, we investigate estuarine carbon cycling in the tidal Delaware River and Bay, located on the east coast of the USA. The freshwater flow into the estuary is dominated by the Delaware River, which supplies nearly 60% of the freshwater to the bay; the Schuylkill and other smaller rivers supply another 14% (Sutton et al., 1996). In total, these rivers account for >80% of freshwater input into the estuary. The water column of the Delaware Estuary is usually well-mixed, although stratification can build up near the mouth of the bay, particularly during summer (Garvine et al., 1992). The bay has an estimated flushing time of about 80 to 180 days (Ketchum, 1952; Sharp et al., 1982; Cifuentes et al., 1990). In parts heavily urbanized, its waters carry a high nutrient loading from the Philadelphia area and saline waters reach as far as ~125 km from the mouth (Biggs et al., 1983). The estuary has been extensively monitored over the last 30 years with a focus on carbon and nutrient fluxes (Sharp et al., 2009). Different sources of the DOM and particulate organic matter (POM) have been identified along the estuary: isotope and biomarker studies of ultrafiltered DOM have identified a terrestrial signal in the river and turbid middle estuary transitioning to a more algal and zooplankton-dominated signal in the lower estuary and coastal ocean (Mannino and Harvey, 1999, 2000b). Further compositional changes of the DOM pool have been examined at the level of chemical classes, e.g., total amino acids and polysaccharides (Hoch and Kirchman, 1995; Mannino and Harvey, 2000a; Kirchman and Borch, 2003), which in addition to mixing of marine and terrestrial signatures reveal a seasonal imprint as well as processing in the estuarine turbidity maximum. Of the rivers draining into the Middle Atlantic Bight, the Delaware River however carries a high proportion of allochthonous DOC and aged carbon (Hossler and Bauer, 2013).
We characterized the main drivers of DOM concentration and composition between August and November over 3 years (five sampling cruises) along the salinity gradient of the Delaware estuary, thereby allowing inferences about the modified terrestrial signal ultimately reaching the Atlantic Ocean. In addition to bulk DOC concentrations, we assessed the compositional variation of solid-phase extractable (SPE) DOM using ultrahigh resolution mass spectrometry (Fourier-Transform Ion Cyclotron Resonance Mass Spectrometry, FT-ICR-MS), which allowed the assignment of thousands of different molecular formulae to a water sample (Stenson et al., 2003; Koch et al., 2005, 2007). Further, we applied collision-induced fragmentation to reveal possible structural changes within the SPE-DOM pool along the salinity gradient that might be hidden on molecular formula level. The different levels of analytical resolution provided unprecedented insight into the DOM cycling in the Delaware estuary. Overall, we used the new opportunities presented by FT-ICR-MS to build on the previous body of work in the Delaware and other study systems and to gain a more detailed understanding of DOM at the molecular level.
Materials and Methods
Site Description and Sample Collection
Sampling was carried out from about 160 km upstream from the mouth of the estuary to a station just outside of the Delaware Bay during five cruises over three years (November 7–10 2011, August 9–13 2012, November 12–16 2012, August 3–7 2013, and November 18–21 2013). Water was obtained by a CTD rosette-mounted Niskin bottle from ~0.5 m depth. Ammonium, nitrate, phosphate, and silicate concentrations were measured with a SEAL Analytical AA3 Continuous Segmented Flow Analyzer using standard procedures (Sharp et al., 2009). The concentration of total chlorophyll a was estimated in acetone extracts by fluorometry. Light attenuation was estimated from the intensity of photosynthetically active radiance over a depth profile measured with a Biospherical PNF-210 radiometer. A microcentrifuge approach was used to estimate leucine incorporation (added concentration of 20 nM) (Kirchman, 2001). Sampling locations and environmental parameters are summarized in Table S1 and Figure S1.
River discharge at Trenton and Schuylkill gage stations was obtained from the USGS Water Information System (waterdata.usgs.gov). We calculated the sum of discharge for 7, 14, 28, 56, and 112 days before the start of each sampling campaign and found the same general trend (Figure S2). The summed discharge of 112 days was chosen for further calculations because it lies well within the residence time of the estuary which is between 80 to 180 days (Ketchum, 1952; Sharp et al., 1982; Cifuentes et al., 1990).
Dissolved Organic Matter Analysis
For quantification and molecular analysis of the DOM, water was filtered through precombusted glass fiber filters (GF/F, Whatman), acidified to pH 2 with 25% HCl (p.a. grade), and stored at 4°C in the dark before being shipped to Germany for analysis. Concentrations of DOC and total dissolved nitrogen (TDN) were measured via high temperature catalytic oxidation on a Shimadzu TOC-VCPH analyzer equipped with a TDN module. Accuracy and precision were monitored with consensus reference material (DSR, D. Hansell, University of Miami) and were better than 5%. DON was estimated by subtracting nitrate and ammonium concentrations from TDN. The DOM was solid-phase extracted according to Dittmar et al. (2008) using 1 g PPL columns (Agilent Bond Elut), and the resulting methanol extracts were stored at −20°C in the dark. The concentration of extractable DOC was determined from the extracts after complete removal of the methanol and dissolution in ultrapure water. Methanol extracts were diluted to a DOC concentration of 20 mg C L−1 in a methanol:water mixture of 1:1 (v/v) and submitted to Fourier Transform Ion Cyclotron Resonance Mass Spectrometry on a solariX FT-ICR-MS (Bruker Daltonik GmbH) equipped with an electrospray ionization source (Bruker Apollo II) applied in negative mode. 500 scans in a mass window from 150 to 2000 Da were accumulated and molecular formulae were calculated with the following restrictions: 12C1-1301H1-200O1-5014N0-4S0-2P0-1 to masses above the method detection limit after Riedel and Dittmar (2014). Additionally, masses detected in less than three samples were removed prior to further analysis.
Each sample was normalized to the sum of FT-ICR-MS signal intensities and corrected for dilution per sample. The modified aromaticity index (AImod, Koch and Dittmar, 2006 as corrected and reported in the erratum published in 2016), double bond equivalents (DBE) and weighted molar ratios were calculated for each sample. The molecular formulae were assigned to compound groups according to their O/C and H/C ratios as described in Seidel et al. (2014). Here, the seven main groups with their subcategories include (1) polycyclic aromatics (PCA, AImod > 0.66) subdivided into PCA containing more than 15 C atoms indicative of dissolved black carbon, PCA with less than 15 C atoms, or PCA containing a heteroelement; (2) polyphenols (0.5 < AImod ≤ 0.66) with high (O/C ≥ 0.5) or low oxygen (O/C < 0.5) content; (3) highly unsaturated aliphatics (AImod ≤ 0.5, H/C < 1.5, O/C < 0.9) with high (O/C ≥ 0.5) or low (O/C < 0.5) oxygen content; (4) unsaturated aliphatics (1.5 < H/C ≤ 2, O/C < 0.9, N = 0) with high (O/C ≥ 0.5) or low (O/C < 0.5) oxygen content; (5) saturated fatty acids (H/C > 2, O/C < 0.9) with or without heteroelements; (6) carbohydrate-like formulae (O/C > 0.9) with or without (N,S,P = 0) heteroelements; and (7) peptide-like molecular formulae (1.5 < H/C < 2, O/C < 0.9, N > 0). The assignment of a formula to a compound class is not unambiguous since the grouping does not take into account all possible isomers. Calculated and intensity-weighted molecular composition and molecular categories per sample are provided in Table S1.
In order to investigate the structural DOM composition along the salinity gradient, we fragmented six nominal masses of two homologous series (m/z 269, 283, 297, 341, 365, and 379 Da) of three samples from the sampling campaign in November 2012 (salinities 0.8, 14.7, and 30.7) via collision-induced fragmentation in the FT-ICR-MS. The nominal masses were chosen to compare to a previous publication using the same approach (Osterholz et al., 2015), to span a broad mass range and to have a high relative signal intensity suitable for fragmentation. The November 2012 cruise was chosen for the fragmentation experiment as the DOC concentrations behaved conservatively, being representative of the conditions in the estuary most of the time except for bloom or very high discharge conditions. For the fragmentation, methanol extracts were diluted to a DOC concentration of 60 mg C L−1 in a methanol:water mixture of 1:1 (v/v). The collision voltage was chosen to yield similar signal intensities of the most intense fragments and varied between 13 V and 18 V. 150 scans were accumulated for each analysis performed in duplicates. Molecular formula assignment to fragmentation mass spectra was done as described above. We identified 423 fragments resulting from the neutral losses of H2O, CH4O, 2H2O, CO2, CO2+H2O, CO2+CH4O, 2CO2, 2CO2+H2O, 2CO2+CH4O, 3CO2, 3CO2+H2O or 3CO2+CH4O. Only molecular formulae that were detected in the two replicate analyses were analyzed in further detail. The relative signal intensity of each fragment ion was divided by the sum of the intensities of all major fragment signal intensities plus the precursor ion signal intensity for better comparison between samples. The normalized fragment intensities were then grouped by neutral loss and compared between samples.
Statistical Analysis
Conservative mixing lines (Figures 1, 2) were constructed using the respective mean concentrations/values of the two samples of highest and lowest salinity. The deviation of each sample from the theoretical mixing line was then calculated and averaged over the whole transect. A high value therefore represents a high variability of the parameter in relation to the values expected for a conservative mixing scenario, similar as described in Seidel et al. (2015a).
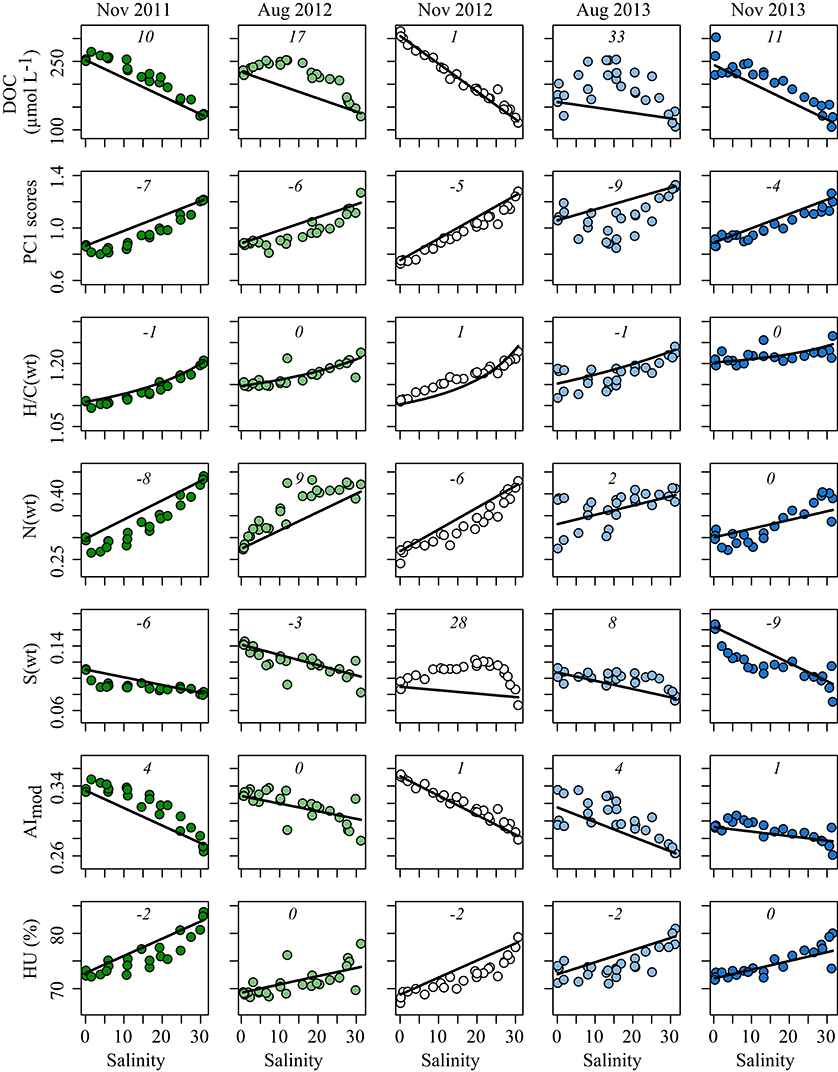
Figure 1. DOC concentration and characteristics of DOM molecular composition (rows) vs. salinity for all five cruises (columns). PC1 scores are from Figure 3 (1 was added to each score for data representation); H/C ratio, N- and S-content of molecular formulae were weighted by peak intensity; AImod, modified aromaticity index; HU, percentage of highly unsaturated compounds. Solid lines represent conservative mixing lines calculated using averages of the two samples of lowest and highest salinities, respectively. Numbers in italics denote percentage of deviation between the conservative mixing line and the measured values (see section Materials and Methods).
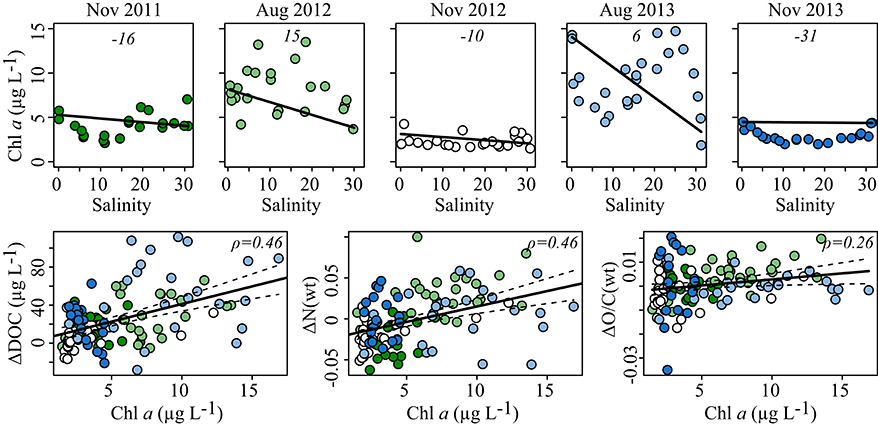
Figure 2. (Top row) Chlorophyll a concentration vs. salinity for all five cruises. Solid lines represent conservative mixing lines calculated using averages of the two samples of lowest and highest salinities, respectively. Numbers in italics denote percentage of deviation between the conservative mixing line and the measured values (see Methods). (Bottom row) Chlorophyll a concentration vs. the deviation from conservative mixing of DOC concentration, weighted N content and weighted O/C ratio noted as Δ. The color of the symbols in the bottom row indicates the cruise as defined in the top row. Solid lines represent linear regression, and dashed lines denote standard error. Spearman rank correlations for each parameter vs. chlorophyll a concentration are given. Significance of correlations is p < 0.01.
Multivariate statistical analyses were performed using R (version 3.2.2) and the package vegan (Oksanen et al., 2015). All analyses were run on normalized intensities of signals with molecular formula assignment which were corrected for dilution. Four extraction efficiencies (on carbon basis) above 100 and below 40% were likely erroneous and were replaced by the average value of extraction efficiencies of other samples (67%). Principal coordinate analyses (PCoA) were performed on Bray Curtis distance matrices (Bray and Curtis, 1957). Environmental vectors were fitted to the respective PCoA scores using the envfit function in the vegan package. Spearman rank correlations were calculated between single molecular formulae intensities and environmental parameters. Only correlations between a formula and an environmental parameter with a p < 0.01 were considered in further analysis and discussion. The non-parametric Wilcoxon rank-sum test was used for comparisons between groups of samples.
Results
Environmental Characterization of the Delaware Bay
DOC, TDN and nitrate concentrations as well as turbidity generally decreased along the salinity gradient (Figure 1, Figure S1). Several biological parameters influenced by water temperature and light availability varied greatly between the two months (August and November) we sampled. These parameters included microbial abundance, chlorophyll a concentration and leucine incorporation as well as silicate concentrations (Figure 2, Figure S1). Freshwater discharge did not vary consistently between the two seasons.
Most environmental parameters deviated from conservative mixing along the salinity gradient. Nitrate concentrations, for example, were always high in the oligohaline zone, decreasing toward the ocean with a negative deviation from conservative mixing at intermediate salinities. Most of the total dissolved nitrogen in the Delaware estuary is inorganic. DON concentration did not vary consistently along the estuarine salinity gradient or with season.
Silicate concentrations were depleted at the freshwater end of the estuary in August, while in November concentrations were high because the river contributes large amounts of dissolved silicate into the estuary. Chlorophyll a concentration (Figure 2) and leucine incorporation (Figure S1) differed between the two campaigns that took place in August, yet covaried significantly (ρ = 0.82, p < 0.01) with highest values observed in the oligo- and polyhaline zones in August 2013 and overall high values during August 2012.
DOC concentrations decreased linearly (conservatively) during estuarine mixing in November (Figure 1). In August, however, changes in DOC concentrations were non-conservative; deviations from conservative mixing were significantly higher in August than in November (Wilcoxon rank-sum test, p < 0.01). Overall, DOC concentrations were more variable at the freshwater end; the coefficient of variation (CV) at low salinity was 20% (salinity < 1, mean DOC 245 ± 49 μmol L−1, n = 14) while it was 11% in marine waters (salinity > 30, mean DOC 129 ± 14 μmol L−1, n = 14). At salinities of less than 1, DOC concentrations were significantly lower in August than in November by 1.4-fold (Wilcoxon rank-sum test, p < 0.01). DOC concentrations in marine waters did not vary substantially between the 2 months. DOC concentrations at the high or low salinity extremes did not correlate significantly with discharge.
A turbidity maximum was apparent at salinities between 0 and 5 during all cruises; turbidity then decreased toward the ocean. Turbidity patterns were neither related to discharge nor revealed an impact on phytoplankton growth or DOC concentration.
Dissolved Organic Matter Molecular Composition
In the whole dataset consisting of 121 solid-phase extracted DOM samples, 12666 unique molecular formulae (MF) within the mass range of 154–885 Da were identified. Of those, 3093 MF (24%) were present in all 121 samples. Further, 78% of all MF were detected at least once during each of the five cruises. Considering each cruise separately, 34–45% of the MF were observed in every sample (November 2011: n = 22, August 2012: n = 26, November 2012: n = 25, August 2013: n = 26, November 2013: n = 22).
Principal coordinate analysis was performed on a Bray-Curtis dissimilarity matrix (Bray and Curtis, 1957) calculated using relative signal intensities of all samples (Figure 3). The two major axes of variation together encompassed almost 80% of the variability of the dataset. Salinity was highly positively correlated to principal coordinate PC1 (ρ = 0.78, p < 0.001, Figure 1), whereas silicate and DOC concentration were negatively correlated with PC1 (ρ = −0.95 and −0.57, respectively, p < 0.001). Discharge was strongly associated with PC2 (ρ = −0.75, p < 0.001, Figure 4), the second axis of variation comprising 25% of the variability of the DOM molecular data. Microbial abundance, bacterial production (leucine incorporation) and chlorophyll a concentration were correlated with the third and/or fourth PCs, encompassing 6 and 3% of the variation in the data, respectively (Figures S3, S5E,F).
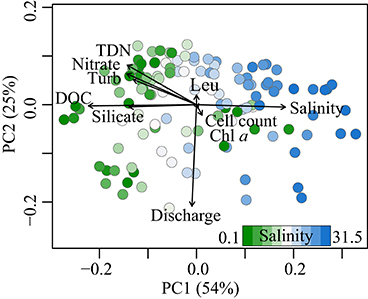
Figure 3. Principal coordinate analysis based on Bray-Curtis dissimilarities of relative peak intensities, color-coded by salinity of each sample. Shown are the first two principal coordinates, accounting for 54 and 25% of the total variability in the dataset. Environmental parameters (black) are fitted to the ordination. Leu, Leucine incorporation; Turb, Turbidity.
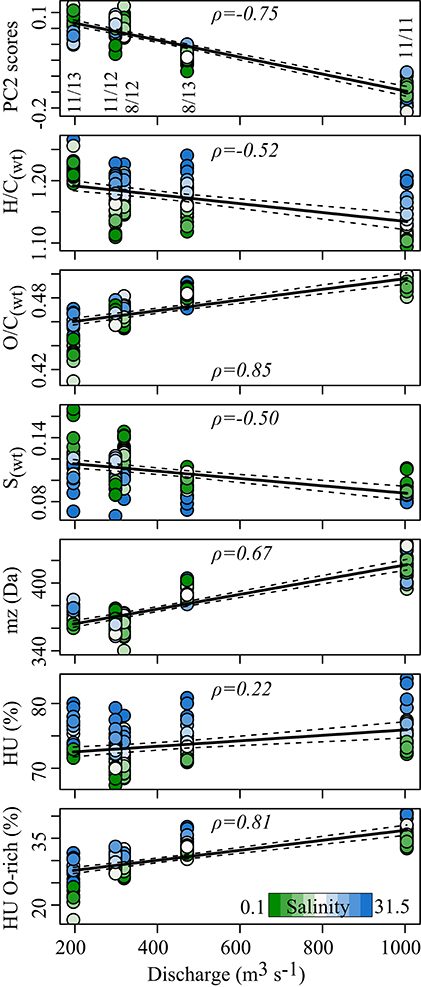
Figure 4. Characteristics of DOM molecular formulae vs. discharge. All samples per cruise are plotted and colored according to salinity. Cruise is marked in the top panel as MM/YY. Solid lines represent linear regression, dashed lines denote standard error. Spearman rank correlations for each parameter vs. discharge are given (ρ). Significance of correlations is p < 0.001, except for HU where p < 0.05. HU, highly unsaturated compounds; PC2 scores are taken from Figure 3.
Three environmental parameters (salinity, discharge, chlorophyll a concentration) were chosen for a more detailed investigation of changes in molecular DOM composition. These three parameters each represent one group of co-correlating environmental variables identified via hierarchical clustering (Figure S4).
Almost half of all identified molecular formulae, capturing 85 ± 5% of the total signal intensity of each sample, decreased in intensity with increasing salinity. The overall aromaticity of the SPE-DOM, assessed via the modified aromaticity index AImod, strongly decreased (Figure 1, Table 1). A molecular formulae based measure for terrigenous input, Iterr proposed by Medeiros et al. (2016), decreased from ~0.5 at the freshwater end to ~0.2 in the most marine waters and was highly negatively correlated with salinity (ρ = −0.79, p < 0.001). The relative contribution of sulfur to DOM molecular composition exhibited a weak relationship with salinity, overall decreasing toward the ocean. SPE-DOM N-containing molecular formulae, average molecular mass and H/C ratio all increased with increasing salinity (Figure 1, Figures S5A,B, Table S1). The relative abundance of highly unsaturated compounds, representing the largest compound class, likewise increased. The index for labile compounds above the molecular lability boundary (MLB) of H/C > 1.5, the MLBwL proposed by D'Andrilli et al. (2015), slightly increased toward the ocean for all cruises except in November 2012 (ρ = 0.23, p < 0.05).
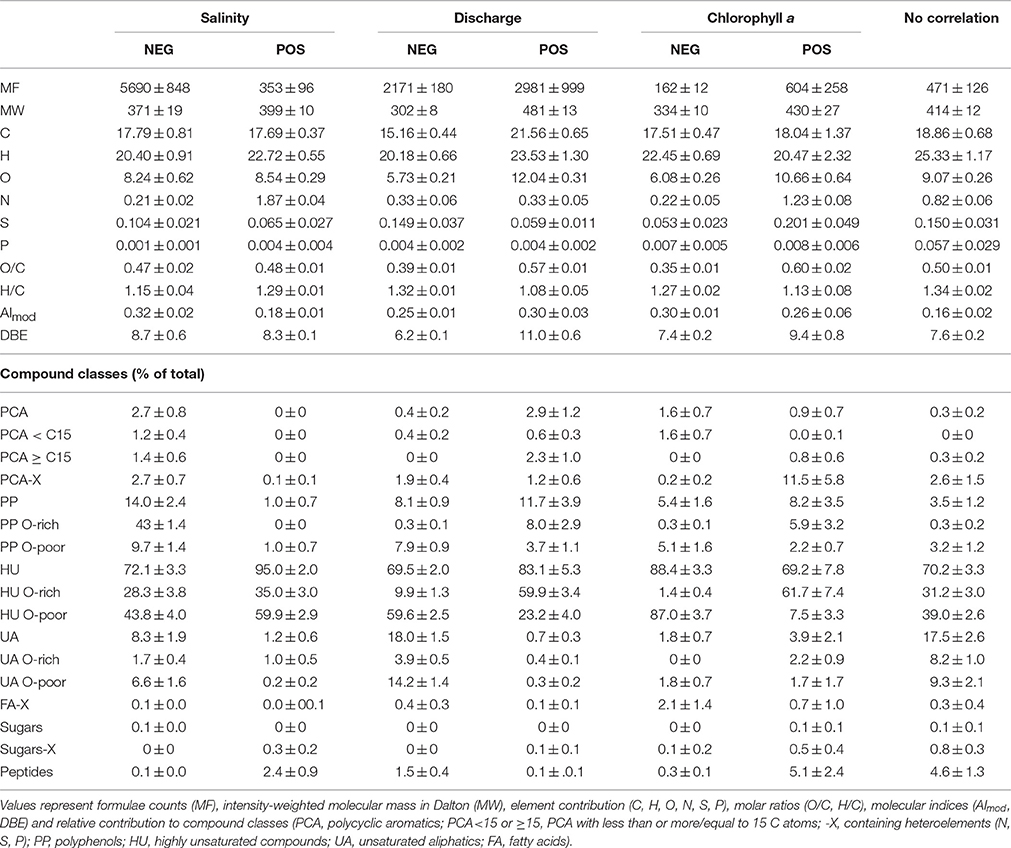
Table 1. Characteristics of molecular formulae correlating positively (POS) or negatively (NEG) with salinity, discharge and chlorophyll a concentration as well as of those molecules exhibiting no correlation to either one of the three environmental parameters.
At high discharge in the Delaware Bay, we found high values for O/C ratio, molecular mass and highly unsaturated compounds (Figure 4). The subgroup of oxygen-rich highly unsaturated compounds emphasizes this trend (Figure 4, Table 1, Figures S5C,D). We observed only a slight increase in aromaticity, while the Iterr did not correlate significantly with discharge (p > 0.05). The MLBwL was highly negatively linked with discharge (ρ = −0.80, p < 0.001). About 2000 molecular formulae were positively affected by discharge and another 3000 were negatively affected, in total accounting for ~60% of total signal intensity (Table 1).
Chlorophyll a concentration and microbial activity were highly correlated; therefore only chlorophyll a was chosen for further investigation. Overall, chlorophyll a concentration was variable along the salinity gradient, but consistently displayed positive deviations from conservative mixing in August and negative deviations in November (Figure 2). Only a few molecules varied consistently with chlorophyll a concentration and accounted for ~6% of the total signal intensity (Figure S5E, Table 1). The molecular variation associated with the chlorophyll a gradient was, however, markedly different from random correlations as tested with a dataset of normally distributed, random variables (Figure S5H). During periods of high phytoplankton biomass and microbial production, DOM was enriched in N, S, O, unsaturated aliphatics, polyphenolic compounds as well as sugar- and peptide-like molecular formulae (Table 1). To explore the correlations in more detail, the deviations from conservative mixing of DOC concentrations and molecular parameters were calculated (ΔDOC, ΔN, ΔO/C); significant increases are shown in Figure 2. The MLBwL and Iterr did not correlate significantly with chlorophyll a concentrations (p > 0.05).
The 471 ± 126 molecular formulae that correlated with one of the three factors (p > 0.01) accounted for ~3% of the total signal intensity and were widely distributed over the van Krevelen space occupied by a typical DOM sample, but were less prevalent in the low-H/C and high-O/C region (Figure S5G).
Dissolved Organic Matter Structural Analysis
Fragmentation of six nominal masses from three samples along the salinity gradient in Nov 2012 was used to detect potential preferential removal or addition of DOM structural features along the salinity gradient of the Delaware Estuary. A total of 423 detected fragments were normalized and grouped by neutral loss (H2O, CO2, CH4O as well as combinations and multiples thereof). We did not detect any of the OCH2 functionalities described by Liu et al. (2011). These neutral losses represent a component of lignin biopolymers generated from wood extract and C18-extracted black waters and were thus presumed to occur in the terrigenous DOM. In pairwise comparisons of the relative signal intensities per neutral loss, a ratio of 1 indicates that no differences in neutral losses were observed between samples. This in turn suggests that a formula with the same molecular mass has a similar structure, regardless of its sampling location within the salinity gradient of the estuary. Although there were some deviations from the ratio of 1 among samples taken at different salinities, the fragmentation patterns did not differ significantly (Wilcoxon rank-sum test, p > 0.05, Figure 5).
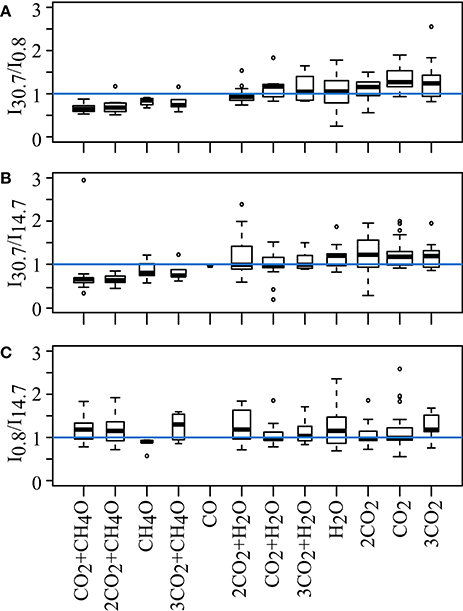
Figure 5. Fragmentation of 18 nominal masses at different salinities (0.8, 14.7, 30.7, Nov 2012), resulting in 423 identified fragments. Boxplots show normalized intensities of fragments grouped by neutral loss; pairwise comparisons are (A) salinity 30.7 to 0.8, (B) salinity 30.7 to 14.7, and (C) salinity 0.8 to 14.7. Blue line denotes ratio of 1 in pairwise comparisons of samples. Relative intensities of averaged neutral losses did not differ significantly between samples (Wilcoxon rank-sum test, p < 0.01). Box widths are proportional to the number of observations in the groups.
Discussion
In order to investigate the environmental drivers of DOM quantity and quality in a temperate estuarine system, we combined quantitative analysis of DOC concentrations with ultrahigh resolution mass spectrometric analysis of solid-phase extractable (SPE) DOM from samples from the entire salinity gradient of the Delaware estuary over 3 years. The analytical window provided by the combination of PPL-SPE and FT-ICR-MS allowed us to examine a large, otherwise inaccessible portion of the DOM pool; in this study on average 67 ± 6% of the DOC was captured with our solid-phase extraction method. The PPL columns used here poorly recover very small and polar compounds (Raeke et al., 2016), but absorb a wide range of compounds of varying polarity and are thought to provide a major fraction of the natural DOM (Green et al., 2014; Li et al., 2016). The extraction efficiency did not significantly change along the salinity gradient of the estuary, indicating that our method captured a reproducible fraction of the estuarine DOM pool. FT-ICR-MS analysis of our SPE-DOM samples allowed us to gain valuable information on molecular composition and explore variation with biogeochemical properties. Additional insights into DOM structural features were revealed with in-cell fragmentation.
Mixing of Terrigenous and Marine DOM along the Salinity Gradient
On its way from soils to the ocean margins, DOM can undergo transformations in molecular composition that are not reflected in the DOC concentrations along the salinity gradient. Besides a slight decrease in molecular formulae richness (ρ = −0.29, p < 0.01) and the Shannon diversity index (ρ = −0.44, p < 0.001) along the salinity gradient, condensed aromatic compounds, AImod, and polyphenols indicating DOM of terrigenous origin were higher in the low salinity region. Proportions of highly unsaturated compounds and molecular mass increased with increasing salinity, as did the contributions of nitrogen-containing molecular formulae and sugar- and peptide-like compounds that are thought to be products of more recent primary production (Amon et al., 2001). Our results are overall in line with previous studies applying ultrahigh resolution mass spectrometry to molecularly characterize DOM. These have shown that DOM tends to become more aliphatic, has a higher heteroelement content (N, S, P), and contains lower abundances of oxygen-rich molecules along gradients of increasing salinity in temperate (Sleighter and Hatcher, 2008) and tropical estuaries (Medeiros et al., 2015b; Seidel et al., 2015b). Highly unsaturated compounds, however, are thought to mainly comprise soil-derived “humics” and other highly unsaturated compounds according to Šantl-Temkiv et al. (2013). It has to be kept in mind that FT-ICR-MS detects relative signal intensities and grouping into molecular classes is reported in proportions, i.e., if one compound class such as the polyphenols strongly decreases in a sample, another compound class or classes must increase. The less oxygenated polyphenols decrease more strongly with increasing salinity than the oxygen-rich subgroup (Table 1) which includes many phenolic acids. This finding illustrates that the classification into broad compound groups does not unambiguously identify the origin and fate of the molecules. Further, although unsaturated aliphatic compounds have previously been shown to be abundant in the ocean supposedly due to the DOM from marine organisms (Sleighter and Hatcher, 2008; Seidel et al., 2015a), they exhibited no relationship with salinity in our study.
Data on DON concentration and the molecular composition data give a different picture about sources of DOM in the estuary. DON concentrations were similar along the whole transect whereas increased DON concentrations have been observed previously in freshwater parts of estuaries (Badr et al., 2008). However, there was a strong positive correlation between relative intensities of N-containing molecular masses and salinity (ρ = 0.77, p < 0.001). This is consistent with the hypothesis that marine DOM is mainly derived from autochthonous production and contains more heteroelements than DOM from terrestrial sources (Sleighter and Hatcher, 2008). In addition to a higher N content of the SPE-DOM molecular formulae, we also observed a slightly positive correlation between the relative abundance of P in the assigned molecular formulae and salinity (ρ = 0.18, p < 0.05).
We propose that variation in the endmembers (river and ocean) is preserved more clearly in the DOM molecular composition than in DOC concentration. The coefficient of variation (CV) of DOC concentrations was 20% (245 ± 49 μmol L−1) in waters with salinities <1 and 11% (129 ± 14 μmol L−1) in waters with salinity > 30. In contrast, the CV of the Bray-Curtis dissimilarity of the SPE-DOM samples was similar for the low and high salinity samples (35 and 31%, respectively). Put differently, DOC concentrations decreased along the salinity gradient and reached similarly low levels in the marine waters while DOM molecular composition remained diverse among the five cruises. This discordance in trends of concentration and composition could be caused by the removal of DOM fractions not captured with our SPE-method. A more likely explanation is the consistent removal or dilution or both of SPE-DOM over the whole captured mass range, supported by the strong correlation of DOM molecular composition with salinity (e.g., Figures 1, 3).
The fact that few molecules increase in relative intensity toward the ocean indicates that dilution and/or degradation processes are by far more important than new production of DOM compounds in shaping DOM composition in this system. Many compounds present at the freshwater source were detected along the whole salinity gradient (34–45%, calculated separately for each cruise). Together with the high percentage of compounds that were detected repeatedly during each cruise (78%), these data indicate a common source of DOM from land that is preserved due to the recalcitrant nature of these compounds. Hossler and Bauer (2013) indeed report a high degree of allochthonous (40–96%) and aged DOC (1–23%) in the Delaware River and Bay. A second possibility is that some compounds are actually removed while others with different structures but the same molecular formulae are produced during the transit through the estuary, thus masking a lower-level DOM variability in the system (Sleighter and Hatcher, 2008). Since structure can be deduced from molecular formula only in extreme cases, i.e., for molecules with very low H/C ratios (Koch and Dittmar, 2006), we conducted fragmentation experiments applying FT-ICR-MS/MS to three samples along the salinity gradient at low, mid and high salinity of the November 2012 cruise. Collision-induced fragmentation has been shown to distinguish samples with similar molecular formulae fingerprints, but different structural composition (Osterholz et al., 2015). The neutral losses of H2O, CO2, and CH4O as well as combinations and multiples thereof are markedly different in relative intensity and exhibit ratios up to 4 when comparing relative fragment intensities of fresh, biologically produced and aged oceanic DOM. The same neutral losses were detected in the fragmentation experiments with Delaware Estuary DOM (Figure 5). However, none of the fragment intensities differed significantly between samples. This finding indicates that structures in aquatic DOM were highly similar, irrespective of their origin from marine or terrestrial sources. For our study, we infer that during estuarine mixing, there were no changes in DOM composition at the structural level that were overlooked at molecular formula level.
The Influence of Discharge Levels
During our five sampling campaigns we did not observe correlations between discharge regimes and DOC concentrations. This remained true even if only the low-salinity samples (salinity <1, n = 14) were taken into account, which would be most directly affected by discharge. For forested streams in the eastern United States as well as the Amazon, high DOC concentrations at the onset of high discharge events and low DOC concentrations at low discharge have been reported (Raymond and Saiers, 2010; Ward et al., 2013). Other studies using a similar analysis approach to the one used here have detected positive correlations between DOC concentration and discharge (Raymond and Bauer, 2001; Medeiros et al., 2015b). The Delaware Estuary in general shows a weak axial salinity response to freshwater input, probably due to vertical shear flow dispersion and the action of lateral shear coupled to a strong lateral salinity gradient (Garvine et al., 1992). DOC, mostly exhibiting conservative behavior, accordingly showed no strong influence of discharge.
Although discharge did not affect DOC concentrations it affected DOM molecular composition. According to correlation analysis of the principal coordinates, discharge was identified as the second most important driver of DOM molecular composition responsible for about a quarter of the total variability. We observed a pronounced shift in DOM composition between low/normal discharge situations where the discharge was around or below the yearly mean flow of ~ 400 m3 s−1 provided by the Delaware and Schuylkill River (Polis and Kupferman, 1973) and high discharge regimes (> 400 m3 s−1, Figure 4). DOM associated with high river discharge was enriched in condensed aromatics, oxygen-rich polyphenols, oxygen-rich highly unsaturated compounds and a higher AImod, all indicative of higher terrigenous DOM input (Meyers-Schulte and Hedges, 1986; Koch et al., 2005). The specific positive correlation with oxygen-rich aromatic compounds presumably relates to the input of lignin- and tannin-like compounds with high O/C ratio (Table 1, Figure 4). Lignins are complex aromatic biopolymers that make up a major component of vascular plant biomass (Sarkanen and Ludwig, 1971). They are an important component of riverine DOM (Ertel et al., 1986). Tannins are produced by some algae but primarily are constituents of higher plant tissue (Hedges and Weliky, 1989). They are thought to be quickly removed from aquatic environments via decomposition, precipitation, and sorption to sediments (Maie et al., 2008). Litterfall and plant debris are degraded in forest soils, from which rainfall events can mobilize tannins and lignin, leading to high concentrations in the Delaware River when discharge is high. The average mass of the compounds associated with discharge rates was higher than of those compounds negatively correlating with discharge. This is opposite of the expected trend as terrigenous SPE-DOM tends to have a lower molecular weight when compared to marine DOM analyzed via FT-ICR-MS (Koch et al., 2005; Medeiros et al., 2015b). Tannins, in molecular weight from 500 to 3000 Da (Bate-Smith, 1968), and lignin polymers of similar or higher molecular weight increased in abundance at high discharge and therefore could contribute to this increase in average molecular mass of the entire DOM pool. Forest and wetland coverage of the watershed decreases from the upper basin toward the bay but nevertheless accounts for the major land use of the Delaware basin (49 and 8%, respectively, Partnership for the Delaware Estuary, 2012). Forests and wetlands are possible sources for this terrigenous DOM. Another much debated source of DOC to the Delaware Estuary are the Spartina alterniflora-dominated wetlands surrounding especially the lower estuary. Hossler and Bauer (2012) reported a contribution of carbon originating from plants with a C4-photosynthetic mechanism to DOC of up to 51% in the Delaware River. Since the production of maize, a C4 plant, is low in the Delaware watershed, the salt marshes might be a large source of this C4-organic matter. According to Benner et al. (1987) and Cifuentes (1991) and references therein, the marshes do not seem to export large quantities of organic matter to the estuarine water column. These sources, however, cannot be deciphered through the methods applied in our study.
Further, the river tended to carry more S-containing DOM to the freshwater end of the estuary at low to intermediate discharge scenarios (ρ = −0.54, p < 0.001, Figure 4, Table 1). The anthropogenically influenced portion of the Delaware River (land cover: 26% agriculture, 15% developed land, Partnership for the Delaware Estuary, 2012) especially in the central and lower watershed, may introduce dissolved organic sulfur (DOS) compounds originating from e.g., wastewater input and agriculture (Gonsior et al., 2011; Wagner et al., 2015), which would be less diluted at low river discharge. In the same manner, DOS compounds formed during early diagenesis in sediments could be released into the water column (Seidel et al., 2014) or be exported from the salt marshes surrounding the Delaware Bay (Luther et al., 1986). The Delaware Bay nevertheless carried SPE-DOS in ranges comparable to less anthropogenically influenced rivers such as Lena, Amazon and Congo (Wagner et al., 2015).
Seasonal DOM Imprint Due to Biological Processes
The impact of biological processes on DOM concentration and composition was assessed through chlorophyll a concentration as a proxy for phytoplankton biomass and bacterial activity measured via leucine incorporation. Due to their high co-correlation, these two factors cannot be distinguished in our correlative approach (ρ = 0.82, p < 0.001). There were no significant correlations between DOC and chlorophyll a concentrations or with leucine incorporation rates.
Only a small portion of molecular formulae comprising about 6% of the total signal intensity correlated positively or negatively with chlorophyll a (Table 1). This number is similar to the percentage of molecular formulae attributed to phytoplankton and/or biodegradation-related processes in the Amazon (Medeiros et al., 2015b). The authors report selective removal of compounds with high O/C ratios via biodegradation (Sun et al., 1997; Kim et al., 2006) and input of phytoplankton-derived material of low O/C ratio (Landa et al., 2014). This was not observed for the Delaware system, where we found that oxygen-rich polyphenols and highly unsaturated compounds increased with chlorophyll a concentration. The higher heteroelement content and occurrence of saturated compounds are consistent with phytoplankton-derived DOM (Sleighter and Hatcher, 2008). Phytoplankton classes differed significantly not only between August and November sampling, but also between the different years (Kirchman et al., in press), possibly producing different DOM compounds and thereby hampering the detection of a uniform “biological signature.”
Abiotic Factors Influencing DOM Composition
At increasing ionic strength, abiotic processes such as flocculation and absorption to particles may influence DOM composition (Hernes and Benner, 2003). Selective removal of terrestrially derived compounds such as humic acids (Fox, 1983; Ertel et al., 1986) has been reported during estuarine mixing, as well as sorption of amino acids and dissolved polysaccharides to particles in the turbidity maximum zone of the Delaware Estuary (Mannino and Harvey, 2000a). Flocculation with metal salts, for example, has been shown to selectively remove more aromatic compounds (Riedel et al., 2012). Cifuentes (1991) proposed the release of lignin phenols from resuspended sediments in the turbid regions of the Delaware Estuary. We were not able to attribute changes in DOC concentration or molecular DOM composition to adsorption processes in this zone through our correlative approach, i.e., correlating relative FT-ICR-MS signal intensities or bulk DOC concentrations with an environmental factor (here: turbidity). Likely, extensive DOM processing before it reaches the river and estuary (Goñi and Gardner, 2003), the small relative contribution of those compound classes to the total DOC pool, the low spatial resolution of the sampling points in and around the turbidity maximum and the strong superimposing signal of the mixing of fresh- and saltwater in combination all hinder the recognition of a turbidity maximum signal. In a recent publication, Hermes and Sikes (2016) showed that POC concentrations are highest in the bottom waters of the estuarine turbidity maximum and sampling at that depth might have revealed a stronger imprint of DOM-POM interaction that are not evident at the 0.5 m sampling depth chosen for our study.
Additionally, photochemical processes are known to shape the DOM pool by removing (condensed) aromatic structures, especially in rivers with watersheds dominated by forests and grassland (Hernes and Benner, 2003; Stubbins et al., 2010; Riedel et al., 2016). This process would yield a negative concave relationship of, for example, condensed aromatics and polyphenols, vs. salinity as both sunlight exposure potential and time increase while the more colored freshwater is diluted along the estuary. As these compound classes decreased linearly along the salinity gradient, there was no evidence of a significant contribution of photochemical alteration to estuarine DOM processing in the Delaware Estuary. Further, we analyzed the relative contribution of molecular formulae identified as photo-labile, photo-resistant and photo-produced by Stubbins et al. (2010) for the Congo River, but no pattern indicative of photochemical alteration emerged (data not shown).
In line with our findings, no large in situ sources or sinks of chromophoric DOM in the Delaware Estuary were reported by Del Vecchio and Blough (2004). These and other authors found photochemical processing to be more important in clearer waters offshore with higher light penetration depths for the Delaware, Amazon, and Mississippi river plumes (Hernes and Benner, 2003; Medeiros et al., 2015b; Seidel et al., 2015b). Indeed, an extensive photochemical DOM sink during August months was described also for the Delaware Estuary plume by Vodacek et al. (1997). The authors further assume oceanic and terrestrial DOM pools mixing in the estuary and on the shelf to originate from constant or very similar sources, which is consistent with a high overlap of detected molecular formulae described in this study.
Conclusion
In our study, we revealed DOM molecular-level variations that are masked by looking at DOC concentrations alone. Mixing of fresh- and marine waters, hydrological conditions, and also biological processes alter the composition of the DOM discharged through the Delaware Estuary into the Atlantic Ocean. For the first time, we applied collision-induced fragmentation to nominal masses along a salinity gradient, demonstrating that the chemical structures behind DOM molecular formulae exhibit a high similarity independent of their origin from marine or terrestrial environments.
Understanding the numerous processes occurring during land-to-ocean carbon transport is of utmost significance to the global carbon cycle as riverine discharge of DOC alone is sufficient to support the turnover of DOC throughout the entire marine environment (Williams and Druffel, 1987). Future increase in discharge due to increased precipitation and storm events projected by climate change scenarios and land use change reducing forest cover will most probably alter the signature of the DOM reaching the ocean, corroborating the need for further monitoring especially of such well-understood land-to-ocean connectors as the Delaware Estuary.
Resource Identification Initiative
CRAN: RRID:SCR_003005, vegan: RRID:SCR_011950.
Author Contributions
DK, TD, and JN conceived the study. HO analyzed the samples and wrote the manuscript with considerable input of all coauthors.
Funding
This work was supported by NSF grants OCE-1030306 and OCE-1261359 to DK. HO, JN, and TD were funded by the University of Oldenburg, Germany.
Conflict of Interest Statement
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past co-authorship with the author TD and states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
The field work was only possible with the help of Matt Cottrell, Monica Stegman, Thomas Lankiewicz, Katie Kalis, Raphaël Lami, and Dave Kieber while Liying Yu was essential in the laboratory analyses. We are grateful to Matthias Friebe and Ina Ulber for DOC/TDN analysis and to Katrin Klaproth for FT-ICR-MS analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/feart.2016.00095/full#supplementary-material
References
Abdulla, H. A. N., Minor, E. C., Dias, R. F., and Hatcher, P. G. (2013). Transformations of the chemical compositions of high molecular weight DOM along a salinity transect: using two dimensional correlation spectroscopy and principal component analysis approaches. Geochim. Cosmochim. Acta 118, 231–246. doi: 10.1016/j.gca.2013.03.036
Abdulla, H. A. N., Minor, E. C., Dias, R. F., and Hatcher, P.G. (2010). Changes in the compound classes of dissolved organic matter along an estuarine transect: a study using FTIR and C-13 NMR. Geochim. Cosmochim. Acta 74, 3815–3838. doi: 10.1016/j.gca.2010.04.006
Amon, R. M. W., Fitznar, H. P., and Benner, R. (2001). Linkages among the bioreactivity, chemical composition, and diagenetic state of marine dissolved organic matter. Limnol. Oceanogr. 46, 287–297. doi: 10.4319/lo.2001.46.2.0287
Aufdenkampe, A. K., Hedges, J. I., Richey, J. E., Krusche, A. V., and Llerena, C. A. (2001). Sorptive fractionation of dissolved organic nitrogen and amino acids onto fine sediments within the Amazon Basin. Limnol. Oceanogr. 46, 1921–1935. doi: 10.4319/lo.2001.46.8.1921
Azam, F., Smith, D. C., Steward, G. F., and Hagström, Å. (1994). Bacteria-organic matter coupling and its significance for oceanic carbon cycling. Microb. Ecol. 28, 167–179. doi: 10.1007/BF00166806
Badr, E.-S. A., Tappin, A. D., and Achterberg, E. P. (2008). Distributions and seasonal variability of dissolved organic nitrogen in two estuaries in SW England. Mar. Chem. 110, 153–164. doi: 10.1016/j.marchem.2008.04.007
Bate-Smith, E. C. (1968). The phenolic constituents of plants and their taxonomic significance. J. Linn. Soc. Lond. Bot. 60, 325–356. doi: 10.1111/j.1095-8339.1968.tb00094.x
Bauer, J. E., and Bianchi, T. S. (2011). “Dissolved organic carbon cycling and transformation,” in Treatise on Estuarine and Coastal Science, Vol. 5, eds E. Wolanski and D. S. McLusky (Waltham, MA: Academic Press), 7–67.
Benner, R., Fogel, M. L., Sprague, E. K., and Hodson, R. E. (1987). Depletion of 13C in lignin and its implications for stable carbon isotope studies. Nature 329, 708–710. doi: 10.1038/329708a0
Biggs, R. B., Sharp, J. H., Church, T. M., and Tramontano, J. M. (1983). Optical properties, suspended sediments, and chemistry associated with the turbidity maxima of the Delaware Estuary. Can. J. Fish. Aquat. Sci. 40, 172–179. doi: 10.1139/f83-279
Bourgoin, L.-H., and Tremblay, L. (2010). Bacterial reworking of terrigenous and marine organic matter in estuarine water columns and sediments. Geochim. Cosmochim. Acta 74, 5593–5609. doi: 10.1016/j.gca.2010.06.037
Bray, J. R., and Curtis, J. T. (1957). An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27, 326–349. doi: 10.2307/1942268
Cadée, G. C. (1982). Tidal and seasonal variation in particulate and dissolved organic carbon in the western dutch Wadden Sea and Marsdiep tidal inlet. Neth. J. Sea Res. 15, 228–249. doi: 10.1016/0077-7579(82)90006-0
Cao, X., Aiken, G. R., Spencer, R. G. M., Butler, K., Mao, J., and Schmidt-Rohr, K. (2016). Novel insights from NMR spectroscopy into seasonal changes in the composition of dissolved organic matter exported to the Bering Sea by the Yukon River. Geochim. Cosmochim. Acta 181, 72–88. doi: 10.1016/j.gca.2016.02.029
Cifuentes, L. A. (1991). Spatial and temporal variations in terrestrially-derived organic-matter from sediments of the Delaware Estuary. Estuaries 14, 414–429. doi: 10.2307/1352266
Cifuentes, L. A., Schemel, L. E., and Sharp, J. H. (1990). Qualitative and numerical analyses of the effects of river inflow variations on mixing diagrams in estuaries. Estuar. Coast. Shelf Sci. 30, 411–427. doi: 10.1016/0272-7714(90)90006-D
Cole, J. J., Prairie, Y. T., Caraco, N. F., Mcdowell, W. H., Tranvik, L. J., Striegl, R. G., et al. (2007). Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10, 172–185. doi: 10.1007/s10021-006-9013-8
D'Andrilli, J., Cooper, W. T., Foreman, C. M., and Marshall, A. G. (2015). An ultrahigh-resolution mass spectrometry index to estimate natural organic matter lability. Rapid Commun. Mass Spectrom. 29, 2385–2401. doi: 10.1002/rcm.7400
Del Vecchio, R., and Blough, N. V. (2004). Spatial and seasonal distribution of chromophoric dissolved organic matter and dissolved organic carbon in the Middle Atlantic Bight. Mar. Chem. 89, 169–187. doi: 10.1016/j.marchem.2004.02.027
Dittmar, T., Koch, B., Hertkorn, N., and Kattner, G. (2008). A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol. Oceanogr. Methods 6, 230–235. doi: 10.4319/lom.2008.6.230
Eisma, D. (1986). Flocculation and de-flocculation of suspended matter in estuaries. Neth. J. Sea Res. 20, 183–199. doi: 10.1016/0077-7579(86)90041-4
Ertel, J. R., Hedges, J. I., Devol, A. H., Richey, J. E., and Ribeiro, M. D. N. G. (1986). Dissolved humic substances of the Amazon River system. Limnol. Oceanogr. 31, 739–754. doi: 10.4319/lo.1986.31.4.0739
Fox, L. E. (1983). The removal of dissolved humic acid during estuarine mixing. Estuar. Coast. Shelf Sci. 16, 431–440. doi: 10.1016/0272-7714(83)90104-X
Garvine, R. W., Mccarthy, R. K., and Wong, K.-C. (1992). The axial salinity distribution in the Delaware Estuary and its weak response to river discharge. Estuar. Coast. Shelf Sci. 35, 157–165. doi: 10.1016/S0272-7714(05)80110-6
Goñi, M. A., and Gardner, I. R. (2003). Seasonal dynamics in dissolved organic carbon concentrations in a Coastal Water-table aquifer at the forest-marsh interface. Aquat. Geochem. 9, 209–232. doi: 10.1023/B:AQUA.0000022955.82700.ed
Gonsior, M., Zwartjes, M., Cooper, W. J., Song, W., Ishida, K. P., Tseng, L. Y., et al. (2011). Molecular characterization of effluent organic matter identified by ultrahigh resolution mass spectrometry. Water Res. 45, 2943–2953. doi: 10.1016/j.watres.2011.03.016
Green, N. W., Perdue, E. M., Aiken, G. R., Butler, K. D., Chen, H., Dittmar, T., et al. (2014). An intercomparison of three methods for the large-scale isolation of oceanic dissolved organic matter. Mar. Chem. 161, 14–19. doi: 10.1016/j.marchem.2014.01.012
Hedges, J. I., Keil, R. G., and Benner, R. (1997). What happens to terrestrial organic matter in the ocean? Org. Geochem. 27, 195–212.
Hedges, J. I., and Weliky, K. (1989). Diagenesis of conifer needles in a coastal marine environment. Geochim. Cosmochim. Acta 53, 2659–2673. doi: 10.1016/0016-7037(89)90137-3
Hermes, A. L., and Sikes, E. L. (2016). Particulate organic matter higher concentrations, terrestrial sources and losses in bottom waters of the turbidity maximum, Delaware Estuary, U.S.A. Estuar. Coast. Shelf Sci. 180, 179–189. doi: 10.1016/j.ecss.2016.07.005
Hernes, P. J., and Benner, R. (2003). Photochemical and microbial degradation of dissolved lignin phenols: implications for the fate of terrigenous dissolved organic matter in marine environments. J. Geophys. Res. Oceans 108:3291. doi: 10.1029/2002JC001421
Hoch, M. P., and Kirchman, D. L. (1995). Ammonium uptake by heterotrophic bacteria in the Delaware Estuary and adjacent coastal waters. Limnol. Oceanogr. 40, 886–897. doi: 10.4319/lo.1995.40.5.0886
Holmes, R. M., Mcclelland, J. W., Peterson, B. J., Tank, S. E., Bulygina, E., Eglinton, T. I., et al. (2011). Seasonal and annual fluxes of nutrients and organic matter from large Rivers to the Arctic Ocean and surrounding Seas. Estuar. Coasts 35, 369–382. doi: 10.1007/s12237-011-9386-6
Hossler, K., and Bauer, J. E. (2012). Estimation of riverine carbon and organic matter source contributions using time-based isotope mixing models. J. Geophys. Res. Biogeosci. 117, G03035. doi: 10.1029/2012jg001988
Hossler, K., and Bauer, J. E. (2013). Amounts, isotopic character, and ages of organic and inorganic carbon exported from rivers to ocean margins: 1. Estimates of terrestrial losses and inputs to the Middle Atlantic Bight. Global Biogeochem. Cycles 27, 331–346. doi: 10.1002/gbc.20033
Keil, R. G., Montlucon, D. B., Prahl, F. G., and Hedges, J. I. (1994). Sorptive preservation of labile organic matter in Marine Sediments. Nature 370, 549–552. doi: 10.1038/370549a0
Ketchum, B. H. (1952). The distribution of salinity in the estuary of the Delaware River. Woods Hole Oceanogra. Instit. Rep. 52–103. doi: 10.1575/1912/5339
Kim, S., Kaplan, L. A., and Hatcher, P. G. (2006). Biodegradable dissolved organic matter in a temperate and a tropical stream determined from ultra-high resolution mass spectrometry. Limnol. Oceanogr. 51, 1054–1063. doi: 10.4319/lo.2006.51.2.1054
Kirchman, D. L. (2001). “Measuring bacterial biomass production and growth rates from leucine incorporation in natural aquatic environments,” in Methods in Microbiology, ed J. H. Paul (St. Petersburg, FL: Academic Press), 227–237.
Kirchman, D. L., and Borch, N. H. (2003). Fluxes of dissolved combined neutral sugars (polysaccharides) in the Delaware Estuary. Estuaries 26, 894–904. doi: 10.1007/BF02803348
Koch, B. P., and Dittmar, T. (2006). From mass to structure: an aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun. Mass Spectrom. 20, 926–932. doi: 10.1002/rcm.2386
Koch, B. P., Dittmar, T., Witt, M., and Kattner, G. (2007). Fundamentals of molecular formula assignment to ultrahigh resolution mass data of natural organic matter. Anal. Chem. 79, 1758–1763. doi: 10.1021/ac061949s
Koch, B. P., Witt, M. R., Engbrodt, R., Dittmar, T., and Kattner, G. (2005). Molecular formulae of marine and terrigenous dissolved organic matter detected by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Geochim. Cosmochim. Acta 69, 3299–3308. doi: 10.1016/j.gca.2005.02.027
Landa, M., Cottrell, M. T., Kirchman, D. L., Kaiser, K., Medeiros, P. M., Tremblay, L., et al. (2014). Phylogenetic and structural response of heterotrophic bacteria to dissolved organic matter of different chemical composition in a continuous culture study. Environ. Microbiol. 16, 1668–1681. doi: 10.1111/1462-2920.12242
Li, Y., Harir, M., Lucio, M., Kanawati, B., Smirnov, K., Flerus, R., et al. (2016). Proposed guidelines for solid phase extraction of suwannee River dissolved organic matter. Anal. Chem. 88, 6680–6688. doi: 10.1021/acs.analchem.5b04501
Liu, W., Wang, Y., Russell, A., and Edgerton, E. S. (2005). Atmospheric aerosol over two urban–rural pairs in the southeastern United States: chemical composition and possible sources. Atmos. Environ. 39, 4453–4470. doi: 10.1016/j.atmosenv.2005.03.048
Liu, Z., Sleighter, R. L., Zhong, J., and Hatcher, P. G. (2011). The chemical changes of DOM from black waters to coastal marine waters by HPLC combined with ultrahigh resolution mass spectrometry. Estuar. Coast. Shelf Sci. 92, 205–216. doi: 10.1016/j.ecss.2010.12.030
Luther, G. W. III., Church, T. M., Scudlark, J. R., and Cosman, M. (1986). Inorganic and organic sulfur cycling in salt-marsh pore waters. Science 232, 746–749. doi: 10.1126/science.232.4751.746
Maie, N., Pisani, O., and Jaffé, R. (2008). Mangrove tannins in aquatic ecosystems: their fate and possible influence on dissolved organic carbon and nitrogen cycling. Limnol. Oceanogr. 53, 160–171. doi: 10.4319/lo.2008.53.1.0160
Mannino, A., and Harvey, H. R. (1999). Lipid composition in particulate and dissolved organic matter in the Delaware Estuary: sources and diagenetic patterns. Geochim. Cosmochim. Acta 63, 2219–2235. doi: 10.1016/S0016-7037(99)00128-3
Mannino, A., and Harvey, H. R. (2000a). Biochemical composition of particles and dissolved organic matter along an estuarine gradient: Sources and implications for DOM reactivity. Limnol. Oceanogr. 45, 775–788. doi: 10.4319/lo.2000.45.4.0775
Mannino, A., and Harvey, H. R. (2000b). Terrigenous dissolved organic matter along an estuarine gradient and its flux to the coastal ocean. Org. Geochem. 31, 1611–1625. doi: 10.1016/S0146-6380(00)00099-1
Mayer, L. M. (1994). Relationships between mineral surfaces and organic carbon concentrations in soils and sediments. Chem. Geol. 114, 347–363. doi: 10.1016/0009-2541(94)90063-9
Medeiros, P. M., Seidel, M., Dittmar, T., Whitman, W. B., and Moran, M. A. (2015a). Drought-induced variability in dissolved organic matter composition in a marsh-dominated estuary. Geophys. Res. Lett. 42, 6446–6453. doi: 10.1002/2015GL064653
Medeiros, P. M., Seidel, M., Niggemann, J., Spencer, R. G. M., Hernes, P. J., Yager, P. L., et al. (2016). A novel molecular approach for tracing terrigenous dissolved organic matter into the deep ocean. Global Biogeochem. Cycles 30, 689–699. doi: 10.1002/2015GB005320
Medeiros, P. M., Seidel, M., Ward, N. D., Carpenter, E. J., Gomes, H. R., Niggemann, J., et al. (2015b). Fate of the Amazon River dissolved organic matter in the tropical Atlantic Ocean. Global Biogeochem. Cycles 29, 677–690. doi: 10.1002/2015GB005115
Meyers-Schulte, K. J., and Hedges, J. I. (1986). Molecular evidence for a terrestrial component of organic matter dissolved in ocean water. Nature 321, 61–63. doi: 10.1038/321061a0
Middelburg, J. J., and Herman, P. M. J. (2007). Organic matter processing in tidal estuaries. Mar. Chem. 106, 127–147. doi: 10.1016/j.marchem.2006.02.007
Miller, A. E. J. (1999). Seasonal investigations of dissolved organic carbon dynamics in the Tamar Estuary, U.K. Estuar. Coast. Shelf Sci. 49, 891–908. doi: 10.1006/ecss.1999.0552
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., and O'Hara, R. B. (2015). Vegan: Community Ecology Package, R Package Version 2.3-1. Available online at: http://CRAN.R-project.org/package=vegan
Osterholz, H., Niggemann, J., Giebel, H. A., Simon, M., and Dittmar, T. (2015). Inefficient microbial production of refractory dissolved organic matter in the ocean. Nat. Commun. 6, 7422. doi: 10.1038/ncomms8422
Partnership for the Delaware Estuary (2012). Technical Report for the Delaware Estuary and Basin. A complete section author list is available at the end of the report.
Pennock, J. R., and Sharp, J. H. (1986). Phytoplankton production in the Delaware Estuary - Temporal and spatial variability. Mar. Ecol. Prog. Ser. 34, 143–155. doi: 10.3354/meps034143
Polis, D., and Kupferman, S. L. (1973). Physical Oceanography. Newark, NJ: College of Marine Studies, University of Delaware.
Raeke, J., Lechtenfeld, O. J., Wagner, M., Herzsprung, P., and Reemtsma, T. (2016). Selectivity of solid phase extraction of freshwater dissolved organic matter and its effect on ultrahigh resolution mass spectra. Environ. Sci. Process. Impacts 18, 918–927. doi: 10.1039/c6em00200e
Raymond, P. A., and Bauer, J. E. (2001). DOC cycling in a temperate estuary: a mass balance approach using natural 14C and 13C isotopes. Limnol. Oceanogr. 46, 655–667. doi: 10.4319/lo.2001.46.3.0655
Raymond, P. A., and Saiers, J. E. (2010). Event controlled DOC export from forested watersheds. Biogeochemistry 100, 197–209. doi: 10.1007/s10533-010-9416-7
Riedel, T., Biester, H., and Dittmar, T. (2012). Molecular fractionation of dissolved organic matter with metal salts. Environ. Sci. Technol. 46, 4419–4426. doi: 10.1021/es203901u
Riedel, T., and Dittmar, T. (2014). A Method detection limit for the analysis of natural organic matter via fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 86, 8376–8382. doi: 10.1021/ac501946m
Riedel, T., Zark, M., Vähätalo, A. V., Niggemann, J., Spencer, R. G. M., Hernes, P. J., et al. (2016). Molecular signatures of biogeochemical transformations in dissolved organic matter from ten World Rivers. Front. Earth Sci. 4:85. doi: 10.3389/feart.2016.00085
Sarkanen, K., and Ludwig, C. (1971). Lignins: Occurrence, Formation, Structure, and Reactions. New York, NY: John Wiley and Sons, Inc.
Schiff, S., Aravena, R., Mewhinney, E., Elgood, R., Warner, B., Dillon, P., et al. (1998). Precambrian shield Wetlands: hydrologic control of the sources and export of dissolved organic matter. Clim. Change 40, 167–188. doi: 10.1023/A:1005496331593
Seidel, M., Beck, M., Greskowiak, J., Riedel, T., Waska, H., Suryaputra, I. G. N. A., et al. (2015a). Benthic-pelagic coupling of nutrients and dissolved organic matter composition in an intertidal sandy beach. Mar. Chem. 176, 150–163. doi: 10.1016/j.marchem.2015.08.011
Seidel, M., Beck, M., Riedel, T., Waska, H., Suryaputra, I. G. N. A., Schnetger, B., et al. (2014). Biogeochemistry of dissolved organic matter in an anoxic intertidal creek bank. Geochim. Cosmochim. Acta 140, 418–434. doi: 10.1016/j.gca.2014.05.038
Seidel, M., Yager, P. L., Ward, N. D., Carpenter, E. J., Gomes, H. R., Krusche, A. V., et al. (2015b). Molecular-level changes of dissolved organic matter along the Amazon River-to-ocean continuum. Mar. Chem. 177, 218–231. doi: 10.1016/j.marchem.2015.06.019
Sharp, J. H., Culberson, C. H., and Church, T. M. (1982). The chemistry of the Delaware Estuary - General considerations. Limnol. Oceanogr. 27, 1015–1028. doi: 10.4319/lo.1982.27.6.1015
Sharp, J. H., Yoshiyama, K., Parker, A. E., Schwartz, M. C., Curless, S. E., Beauregard, A. Y., et al. (2009). A biogeochemical view of Estuarine Eutrophication: seasonal and spatial trends and correlations in the Delaware Estuary. Estuar. Coasts 32, 1023–1043. doi: 10.1007/s12237-009-9210-8
Sleighter, R. L., and Hatcher, P. G. (2008). Molecular characterization of dissolved organic matter (DOM) along a river to ocean transect of the lower Chesapeake Bay by ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Mar. Chem. 110, 140–152. doi: 10.1016/j.marchem.2008.04.008
Spencer, R. G. M., Aiken, G. R., Wickland, K. P., Striegl, R. G., and Hernes, P. J. (2008). Seasonal and spatial variability in dissolved organic matter quantity and composition from the Yukon River basin, Alaska. Global Biogeochem. Cycles 22, GB4002. doi: 10.1029/2008GB003231
Spencer, R. G. M., Stubbins, A., Hernes, P. J., Baker, A., Mopper, K., Aufdenkampe, A. K., et al. (2009). Photochemical degradation of dissolved organic matter and dissolved lignin phenols from the Congo River. J. Geophys. Res. Biogeosci. 114, G03010. doi: 10.1029/2009JG000968
Stenson, A. C., Marshall, A. G., and Cooper, W. T. (2003). Exact masses and chemical formulas of individual Suwannee River fulvic acids from ultrahigh resolution electrospray ionization fourier transform ion cyclotron resonance mass spectra. Anal. Chem. 75, 1275–1284. doi: 10.1021/ac026106p
Stubbins, A., Spencer, R. G. M., Chen, H., Hatcher, P. G., Mopper, K., Hernes, P. J., et al. (2010). Illuminated darkness: molecular signatures of Congo River dissolved organic matter and its photochemical alteration as revealed by ultrahigh precision mass spectrometry. Limnol. Oceanogr. 55, 1467–1477. doi: 10.4319/lo.2010.55.4.1467
Sun, L., Perdue, E. M., Meyer, J. L., and Weis, J. (1997). Use of elemental composition to predict bioavailability of dissolved organic matter in a Georgia River. Limnol. Oceanogr. 42, 714–721. doi: 10.4319/lo.1997.42.4.0714
Sutton, C., O'Herron, J., and Zappalorti, R. (1996). The Scientific Characterization of the Delaware Estuary. Technical Report (DRBC Project No. 321; HA File No. 93.21). Delaware Estuary Program, U.S. Environmental Protection Agency, New York.
Taniguchi, M., Burnett, W. C., Cable, J. E., and Turner, J. V. (2002). Investigation of submarine groundwater discharge. Hydrol. Process. 16, 2115–2129. doi: 10.1002/hyp.1145
Šantl-Temkiv, T., Finster, K., Dittmar, T., Hansen, B. M., Thyrhaug, R., Nielsen, N. W., et al. (2013). Hailstones: a window into the microbial and chemical inventory of a storm cloud. PLoS ONE 8:e53550. doi: 10.1371/journal.pone.0053550
Tzortziou, M., Zeri, C., Dimitriou, E., Ding, Y., Jaffé, R., Anagnostou, E., et al. (2015). Colored dissolved organic matter dynamics and anthropogenic influences in a major transboundary river and its coastal wetland. Limnol. Oceanogr. 60, 1222–1240. doi: 10.1002/lno.10092
Vodacek, A., Blough, N. V., Degrandpre, M. D., Degrandpre, M. D., and Nelson, R. K. (1997). Seasonal variation of CDOM and DOC in the Middle Atlantic Bight: terrestrial inputs and photooxidation. Limnol. Oceanogr. 42, 674–686. doi: 10.4319/lo.1997.42.4.0674
Wagner, S., Riedel, T., Niggemann, J., Vähätalo, A. V., Dittmar, T., and Jaffé, R. (2015). Linking the molecular signature of heteroatomic dissolved organic matter to watershed characteristics in world rivers. Environ. Sci. Technol. 49, 13798–13806. doi: 10.1021/acs.est.5b00525
Ward, N. D., Keil, R. G., Medeiros, P. M., Brito, D. C., Cunha, A. C., Dittmar, T., et al. (2013). Degradation of terrestrially derived macromolecules in the Amazon River. Nat. Geosci. 6, 530–533. doi: 10.1038/ngeo1817
Keywords: dissolved organic matter, Delaware Estuary, FT-ICR-MS, discharge, conservative mixing, seasonal variation, collision-induced fragmentation
Citation: Osterholz H, Kirchman DL, Niggemann J and Dittmar T (2016) Environmental Drivers of Dissolved Organic Matter Molecular Composition in the Delaware Estuary. Front. Earth Sci. 4:95. doi: 10.3389/feart.2016.00095
Received: 26 August 2016; Accepted: 20 October 2016;
Published: 17 November 2016.
Edited by:
Nicholas David Ward, University of Florida, USAReviewed by:
Yina Liu, Pacific Northwest National Laboratory, USAElizabeth Ann Canuel, Virginia Institute of Marine Science, USA
Copyright © 2016 Osterholz, Kirchman, Niggemann and Dittmar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helena Osterholz, helena.osterholz@uni-oldenburg.de
 Helena Osterholz
Helena Osterholz David L. Kirchman
David L. Kirchman Jutta Niggemann
Jutta Niggemann Thorsten Dittmar
Thorsten Dittmar