Transformations and Decomposition of MnCO3 at Earth's Lower Mantle Conditions
- 1Geological Sciences, Stanford University, Stanford, CA, USA
- 2Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, Menlo Park, CA, USA
- 3Stanford Nano Shared Facilities, Stanford University, Stanford, CA, USA
- 4EDF Lab Les Renardieres, Dpt MMC, Moret sur Loing, France
- 5Centre National de la Recherche Scientifique, UMR Centre National de la Recherche Scientifique 7590, Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie, IRD, Sorbonne Universités—Université Pierre et Marie Curie, Muséum National d'Histoire Naturelle, Paris, France
- 6European Synchrotron Radiation Facility (ESRF), Grenoble, France
Carbonates have been proposed as the principal oxidized carbon-bearing phases in the Earth's interior. Their phase diagram for the high pressure and temperature conditions of the mantle can provide crucial constraints on the deep carbon cycle. We investigated the behavior of MnCO3 at pressures up to 75 GPa and temperatures up to 2200 K. The phase assemblage in the resulting run products was determined in situ by X-ray diffraction (XRD), and the recovered samples were studied by analytical transmission electron microscopy (TEM) and X-ray absorption near edge structure (XANES) imaging. At moderate temperatures below 1400 K and pressures above 50 GPa, MnCO3 transformed into the MnCO3-II phase, with XANES data indicating no change in the manganese oxidation state in MnCO3-II. However, upon heating above 1400 K at the same pressure conditions, both MnCO3 and MnCO3-II undergo decomposition and redox reactions which lead to the formation of manganese oxides and reduced carbon.
Introduction
Carbonates represent the main oxidized carbon-bearing phases which are transported into the mantle during subduction. The high-pressure behavior of carbonates can provide insight on crystal chemistry of carbon-bearing phases relevant to Earth's deep interior. In particular, the stability of carbonates vs. their decomposition and melting provides critical constraints for understanding the global carbon cycle. For all these reasons, the thermodynamic properties and phase diagrams for relevant carbonate compositions are needed down to core-mantle boundary conditions, i.e., megabar pressures and temperatures up to 3000 K.
The high-pressure behavior of various divalent cation-bearing carbonates has been the subject of a large number of studies. Systematic differences in compressibility and high pressure and high temperature polymorphs that depend on cation type have been observed in previous studies demonstrating that the polymorphism of carbonates is likely more complex than previously thought (see Shatskiy et al., 2015 and references therein). No single structural parameter or electronic property of the cation can account for the behavior of carbonates, suggesting that a combination of factors must be considered in explaining compressibility trends among members of the calcite-structure type (Zhang et al., 1998; Zhang and Reeder, 1999). With a Mn2+ cation size that lies between those of Mg2+ and Ca2+, rhodochrosite (MnCO3) represents a potential model compound for understanding the differences in the high-pressure behavior of the two main carbonate compositions (Mg and Ca carbonates). The interplay between these two species has indeed been the subject of many studies (e.g., de Capitani and Peters, 1981; Wang et al., 2011). At ambient conditions, MnCO3 crystallizes with the calcite-type structure, R-3c. Santillán and Williams (2004) reported that MnCO3 is stable in its rhombohedral calcite-type structure up to 50 GPa. However, more recently, Farfan et al. (2013) reported a possible electronic transition in rhodochrosite in the pressure interval 25–40 GPa which may be related to fine structural changes in the MnO6 octahedra in the rhombohedral structure (Merlini et al., 2015). Evidence of a first order phase transition above ~40 GPa at room temperature in which MnCO3 transforms into a CaCO3-VI structure was reported by Boulard et al. (2015a) and confirmed by Merlini et al. (2015). Finally, Ono (2007) reported a phase transition at 50 GPa, after heating at 1500–2000 K and proposed an orthorhombic symmetry, the structure of which, however, could not be refined. To clarify the high pressure and high temperature behavior of MnCO3, we combined in situ XRD using laser-heated diamond anvil cells and ex situ analyses using analytical TEM and XANES tomography for conditions up to ~75 GPa and 2200 K, the results of which are reported in this work.
Materials and Methods
Sample Preparation
Powdered samples of rhodochrosite were loaded between two thermal insulating layers into symmetric diamond anvil cells (DAC) with 300 μm diamonds culets. Both NaCl and KCl were used as the thermal insulators and also served as pressure calibrants using the thermal equations of state from Dorogokupets and Dewaele (2007) and Dewaele et al. (2012), respectively. Hot spots with a diameter larger than 20 μm (FWHM) were obtained by two YAG lasers with excellent power stability aligned on both sides of the sample. The X-ray spot, spectrometer entrance and the heating laser spot were carefully aligned before the experiments. Temperatures were obtained by fitting the sample thermal emission spectrum from the central 2 × 2 μm2 of the hotspot to the Planck's function using the wavelength range 600–900 nm. Reflective lenses were used for measurement in order to prevent any chromatic aberration (Benedetti and Loubeyre, 2004). Temperature stability was checked by measuring continuously during heating and X-ray acquisition. Uncertainties are of about 13% (Dewaele et al., 2012) on the pressure and 150 K on the temperature (Morard et al., 2008). For each run, the sample was first compressed at ambient temperature to the target pressure and then heated between 1000 and 2200 K using double-sided infrared laser heating. Because of their high stiffness, the high-pressure phase of NaCl and KCl do not guarantee hydrostatic conditions. Therefore, high temperature annealing of the sample have been performed in order to partly reduce the contribution of deviatoric stresses which may have built up at high pressure. Samples were heated between 5 and 10 min at each temperature step. Some runs were performed with the addition of platinum black to the MnCO3 sample in order to heat at a lower temperature.
In situ X-Ray Diffraction
Angle-dispersive XRD spectra were collected in situ at high pressure and high-temperature at beamline 12.2.2 of the Advanced Light Source (ALS), Lawrence Berkeley National Laboratory (LBNL), and beamline ID27 of the European Synchrotron Radiation Facility (ESRF) using a monochromatic incident X-ray beam with a wavelength of 0.4959 or 0.6199 Å at ALS and 0.3738 Å at ESRF. The monochromatic X-ray beam was focused to a smaller size than the laser heating spot in order to reduce both the radial and axial temperature gradients, typically: 3 × 3 μm at ID27 beamline and 10 × 10 μm at 12.2.2 beamline. The diffraction images were integrated with the Fit2d software (Hammersley et al., 1996), and the one-dimensional diffraction patterns were treated with the General Structure Analysis System (GSAS) software package (Larson and Von Dreele, 2004) using the LeBail method to identify the different phases and refine their lattice parameters.
X-Ray Absorption Near Edge Structure (XANES)
In order to constrain possible changes in the redox state of Mn, XANES spectra were collected on the recovered sample at the Mn-K edge using the nanoscale X-ray transmission microscope (nanoTXM) at the beamline 6-2c of the Stanford Synchrotron Radiation Lightsource (SSRL), SLAC National Acceleratory Laboratory. This microscope is equipped with optics optimized for photon energies ranging from ~5 to 14 keV, it provides a spatial resolution as high as 30 nm and a single flat field of view of 30 × 30 μm. Depth of focus is ~50 μm. More details about the instrument can be found in Andrews et al. (2009) and Liu et al. (2011). NanoTXM is capable of nondestructive spectroscopic imaging, and the use of hard x-rays allows it to image the entire sample thickness avoiding possible contamination which could occur if special sample preparation were required. Transmission images were collected at small energy steps from the pre-edge region through the electronic edge (from 6470 to 6693 eV), enabling us to map out the oxidation state for Mn from its XANES signal.
Focused Ion Beam and Transmission Electron Microscopy
In order to be analyzed by TEM, the recovered samples were thinned to electron transparency (~150 nm) with a Ga+ focused ion beam (FIB) operating at 30 kV and currents from 20 nA to 1 pA for final thinning. FIB milling was performed with a FEI Helios NanoLab 600i DualBeam FIB/SEM at the Stanford Nano Shared Facilities. FIB thin sections were extracted from the center of laser heated spots. Analytical TEM was carried out on the FIB thin sections in order to help with phase identification and to obtain chemical analyses on individual phases. TEM was performed with FEI Titan 80–300 operated at 300 keV, equipped with an extra high brightness field emission gun. Semi-quantitative information on the sample chemical composition was obtained by energy dispersive X-ray spectrometry (EDXS).
Results
Lower Pressure Experiments (P < 33 GPa)
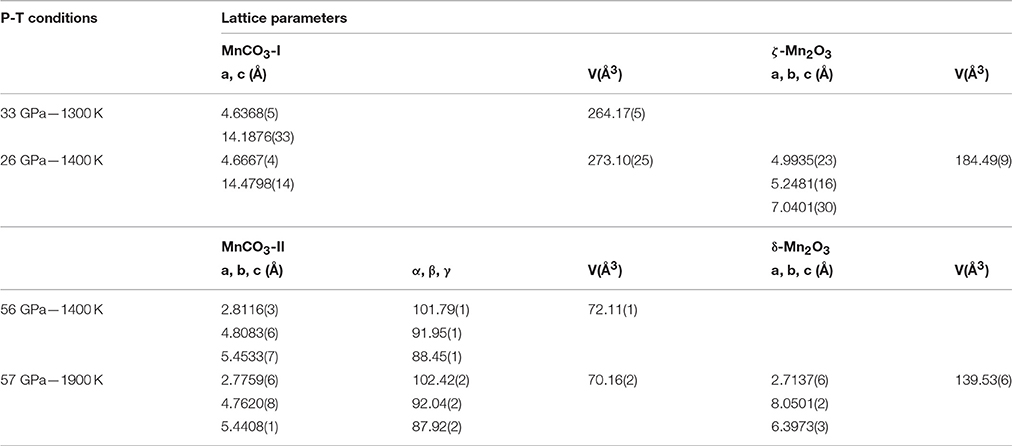
Figure 1 shows XRD patterns collected in situ at the different pressure ranges and at low or high temperature. These XRD patterns show that the rhombohedral MnCO3 structure—rhodochrosite—is stable up to 33 GPa. For simplicity we will refer to this phase as MnCO3-I. When heating at relatively low temperature (~1300 K) only MnCO3-I is observed; however upon heating above 1400 K, the XRD patterns reveal the presence of another phase which does not correspond to the high pressure phase of MnCO3 (marked by stars in Figure 1), MnO, cubic α-Mn2O3 (bixbyite), or Mn3O4 (hausmanite) manganese oxides. In a recent study, Ovsyannikov et al. (2013) reported the transformation of α-Mn2O3 into a perovskite-like structure (ζ-Mn2O3) at these pressure and temperature (P-T) conditions. Although they used a supercell in order to fit their diffraction patterns, here we found that a single cell of an orthorhombic perovskite structure can be used to index the new diffraction peaks (Figure 2 and Table 1).
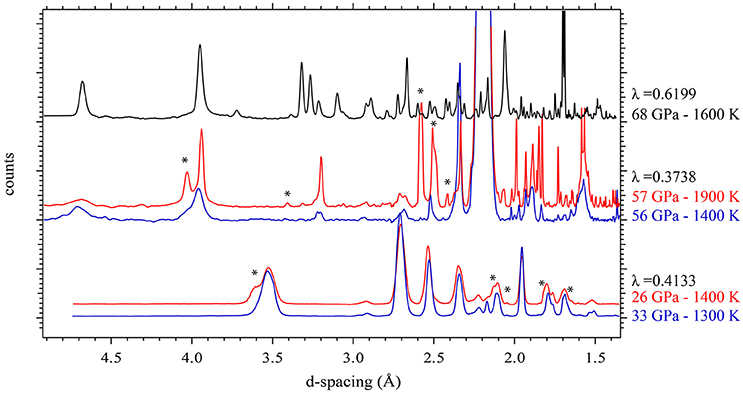
Figure 1. In situ high P-T XRD patterns collected in the three pressure ranges: P < 33 GPa, 49 < P < 65 GPa, P > 65 GPa at lower and higher temperature. Stars indicate the main new diffraction peaks observed upon increase in temperature.
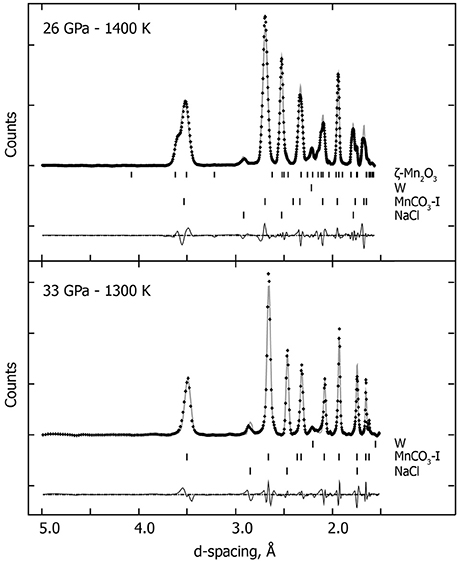
Figure 2. In situ high P-T XRD patterns. Crosses represent observed data (background subtracted), and the gray solid lines show the profile refinements. Residuals between experiment and fit are shown below the diffraction pattern. At 33 GPa and 1300 K, we used an assemblage of NaCl and W (gasket) and rhodochrosite (MnCO3-I). At higher temperature (26 GPa and 1400 K), we used the same assemblage with an additional high pressure manganese oxide polymorph (ζ-Mn2O3).
Medium Pressure Experiments (49 < P < 65 GPa)
Upon compressing MnCO3 at ambient temperature and pressures above ~45 GPa, the XRD patterns change drastically, as the rhombohedral MnCO3-I transforms into the high-pressure phase MnCO3-II which is triclinic. This is in good agreement with previous high pressure studies performed at ambient temperature (Boulard et al., 2015a; Merlini et al., 2015). Similar to the lower pressure results, when heated at moderately low temperature, i.e., 1300 K at 57 GPa, a single carbonate phase, MnCO3-II is observed (Figure 1). However upon heating above 1400 K, new XRD peaks appear which can be indexed to the high pressure manganese oxide phase, δ-Mn2O3, with a post-perovskite like structure described by Santillán et al. (2006). We identified this assemblage (MnCO3-II + δ-Mn2O3) up to 57 GPa and 2100 K (Figure 3 and Table 1). When recovered to ambient pressure and temperature, XRD patterns show the back transformation of MnCO3-II into MnCO3-I as well as δ-Mn2O3 into the cubic α-Mn2O3 phase.
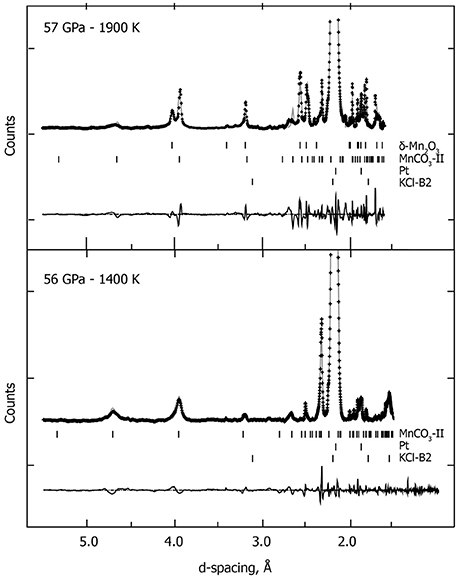
Figure 3. In situ high P-T XRD patterns collected at 56 GPa—1400 K and at 57 GPa—1900 K. We used the following assemblage: the high-pressure polymorph MnCO3-II, KCl-B2 high pressure polymorph and W (gasket), and the post-perovskite like high pressure polymorph of the manganese oxide (δ-Mn2O3). Crosses represent observed data (background subtracted), and the solid lines show the profile refinements. Residuals between experiment and fit are shown below the diffraction pattern.
Highest Pressure Experiments (P > 65 GPa)
Above 65 GPa, new changes in the diffraction patterns are observed as a new phase appears together with the previous assemblage (Figure 1). Above 70 GPa, the diffraction peaks from δ-Mn2O3 disappear and we observed an assemblage of MnCO3-II plus this new phase (Figure 4). This new phase may correspond to a new high-pressure polymorph of Mn2O3. To our knowledge, no experimental studies exist on Mn2O3 at such high pressure and temperature conditions and theoretical studies support the stability of δ-Mn2O3 up to at least 120 GPa (Xu et al., 2015). Therefore, the composition and structure of this new phase remain unresolved. After transformation 68 GPa and 1600 K, diffraction patterns were collected on the sample once recovered to ambient pressure and temperature, and MnCO3-I, α-Mn2O3 as well as another unknown phase were observed.
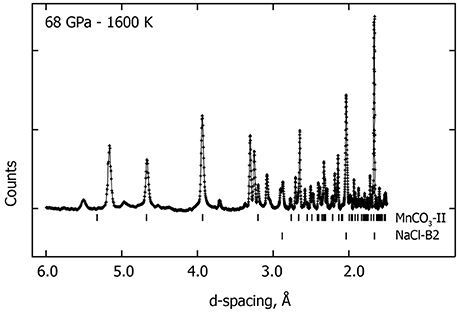
Figure 4. Observed in situ XRD pattern collected at 68 GPa—1600 K. Upper-ticks below the pattern indicate the high-pressure phase MnCO3-II peak positions and the lower-ticks correspond to the peak positions of the NaCl-B2 high pressure phase. Several new XRD peaks (unmarked) are observed in addition to MnCO3-II and NaCl-B2 which may correspond to a new high pressure polymorph of Mn3O4.
Further, ex situ analyses were performed on these samples to understand the highest pressure phase assemblage. XANES imaging at the manganese K-edge (6545 eV) was conducted in order to get insight on the manganese oxidation state. These measurements were collected in the heating spot, the area that has been laser heated during the in situ experiments, directly on the sample that had been recovered from 68 GPa and 1600 K without special sample preparation. The results indicate three areas with different absorption contrast characterized by distinct Mn-K edge spectra. The background corrected and normalized spectra are presented in Figure 5. A spectrum from the first area (shown in black in Figure 5) reveals a peak at 6549 eV, another at 6560 eV as well as a weak pre-edge peak at 6539 eV. This spectrum is consistent with Mn2+ in octahedral sites as in a rhodochrosite MnCO3-I structure. In the spectrum for the second area (show in blue in Figure 5), one can see a broad peak at ~6559 eV and a shift to higher energy of the Mn K-edge (from 6547 to 6548 eV) which indicate an increase in Mn oxidation state. This spectrum is consistent with the Mn-K edge for Mn3O4 (Ressler et al., 1999; Jiao and Frei, 2010). Finally the spectrum collected on the third area (in red in Figure 5) show an Mn-K edge at 6550 eV and broad peak at 6555 eV which is consistent with Mn2O3 (Nam et al., 2007; Jiao and Frei, 2010).
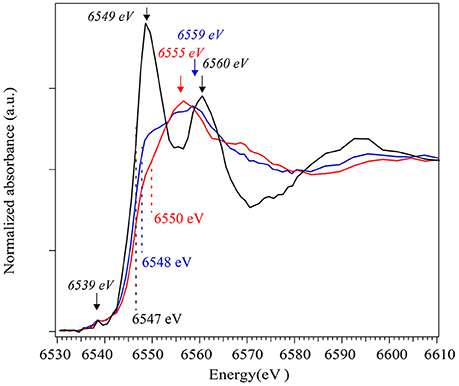
Figure 5. On the left, XANES spectra collected at the Mn K-edge on different grains in the recovered samples using nanoTXM. The positions of the Mn-K edges are noted for each spectrum. Stars indicate XANES peaks for which the positions are reported on the right side of the Figure: the second derivative of the experimental spectra. Spectrum in black corresponds to Mn2+ in a carbonate structure, blue to Mn3O4 oxide and red to Mn2O3.
A thin section of the same sample was then prepared by FIB in order to perform analytical TEM. A global high-angle annular dark-field imaging scanning transmission electron microscopy (HAADF-STEM) image of the thin section is presented in Figure 6A. In this image, the grayscale is related to the mean Z of each phase. It highlights the presence of four different phases: a manganese and carbon rich phase that corresponds to the carbonate (Figure 6B); a carbon rich phase (Figure 6C) that appears in dark in the STEM image indicating the presence of a reduced form of carbon that could not be detected by in situ XRD; finally TEM-XEDS analyses confirm the presence of two manganese oxides (Figures 6D,E). Semi-quantitative analyses show that the experimental spectrum in Figure 6D has an O/Mn ratio of 1.68 while that from the spectrum in Figure 6E is 1.22. These numbers are consistent with the elemental ratio expected in Mn2O3 (theoretical O/Mn = 1.5) and Mn3O4 (theoretical O/Mn = 1.33), respectively.
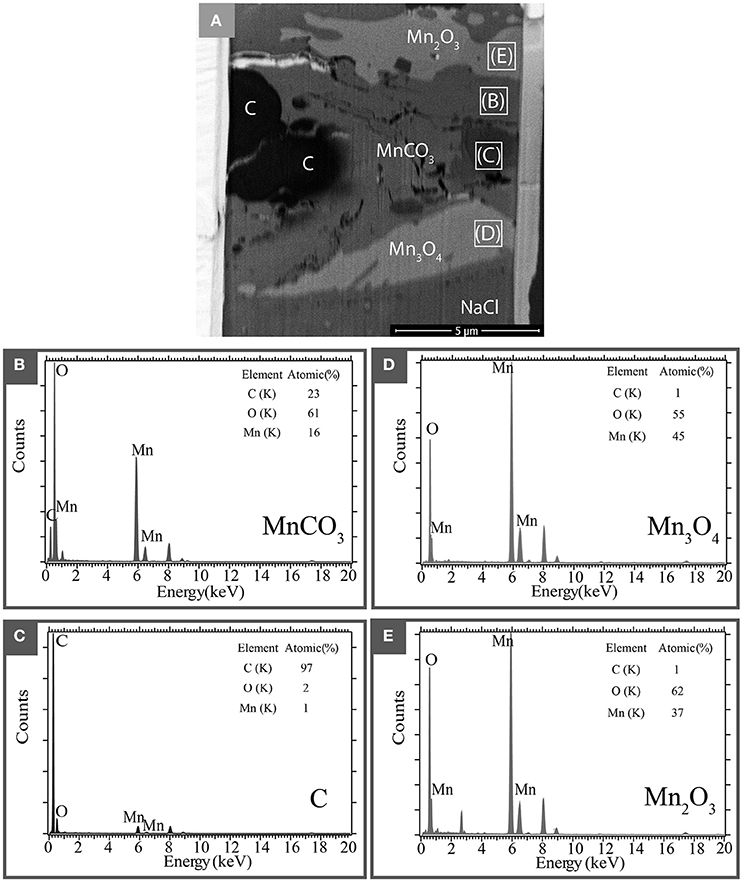
Figure 6. Analytical TEM electron microscopy of a recovered sample after transformation at 68 GPa and 1600 K. (A) STEM picture of the FIB thin section. Four different phases are identified: a carbonate area marked as MnCO3, reduced carbon area marked by C and manganese oxides Mn2O3 and Mn3O4. (B–E) show the XEDS measurements (spectra and quantification) collected in these different areas.
Discussion
New High Pressure and Temperature Phase Diagram
The run products for all high-temperature experiments are summarized in Figure 7. The Mn2O3 phase diagram from Ovsyannikov et al. (2013) is also represented. To our knowledge, no study exists on manganese oxides above 25 GPa at high temperature. The P-T conditions of the different polymorphs α-Mn2O3, ζ-Mn2O3, and δ-Mn2O3 (respectively red, yellow, and purple P-T field in Figure 6) reported in the present study are in very good agreement with Ovsyannikov et al. (2013). In addition, our study shows that δ-Mn2O3 is stable up to at least 75 GPa—1600 K. This is in good agreement with theoretical prediction of the stability of the post-perovskite structure δ-Mn2O3 up to 120 GPa from Xu et al. (2015). Above 65 GPa—1700 K, we report evidences of an additional manganese oxide: a high pressure polymorph of Mn3O4. The presence of a fourth phase Mn3O4 as observed by TEM and XANES imaging is in agreement with the in situ diffraction which show an unknown structure in addition to MnCO3-II and δ-Mn2O3: a new high-pressure polymorph of Mn3O4 which does not back transform into the tetragonal hausmanite (Mn3O4). The structure of this polymorph is beyond the scope of this study and was not resolved. Further, studies on Mn3O4 at high pressure would be necessary in order to elucidate this new phase.
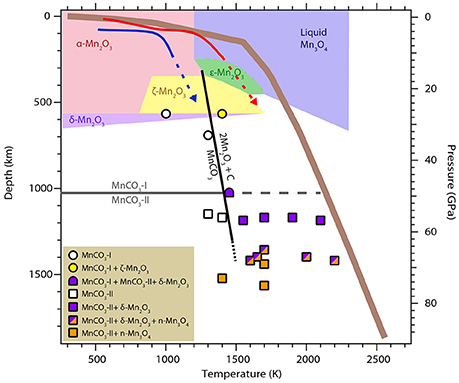
Figure 7. Summary of the P–T conditions for the different experimental points collected in this present work. The phase diagram for Mn2O3 as described in Ovsyannikov et al. (2013) is also shown for comparison. A typical mantle geotherm (solid brown line) as well as the P-T paths for hot and cold subducted slabs are plotted in red and blue, respectively.
The phase transition boundary MnCO3 → MnCO3-II is indicated in gray on Figure 7. MnCO3-I is observed up to 33 GPa—1300 K (circles in Figure 7). The high pressure polymorph MnCO3-II appears for P-T condition above 49 GPa—1500 K (squares in Figure 7). This is in good agreement with previous studies performed at room temperature that reported the phase transition of rhodochrosite into the triclinic structure MnCO3-II at pressure above ~40 GPa (Boulard et al., 2015a and Merlini et al., 2015). Here, we show that MnCO3-II is also observed at high temperature and up to 75 GPa—1700 K. This phase is isostructural to CaCO3-VI which is metastable for the calcium composition (CaCO3) and only observed at room temperature. We did not observe any evidence of the aragonite or post-aragonite structures upon heating. In fact, Oganov et al. (2006) showed that the several metastable structures of CaCO3 are similar in energy with aragonite and are almost as stable as aragonite and post-aragonite at these conditions. Due to the large cation site in aragonite it is not surprising that MnCO3 adopts the CaCO3-VI structure instead of aragonite.
Our study show that the carbonate as well as its high pressure polymorph MnCO3-II are stable for temperature bellow 1400 K. Above 1400 K, we observed the decomposition of MnCO3 and the reduction of carbon corresponding to the two reactions:
The persistence of a carbonate phase in our experiments may indicate that the decomposition reaction was not complete by the time we stopped laser-heating.
The corresponding decomposition line is shown in black in Figure 7. Liu et al. (2001) also reported the decomposition of MnCO3 and formation of diamond at 6–8 GPa and temperatures above 2300 K. According to the Mn2O3 phase diagram represented in Figure 6, their P-T conditions falls into the liquid area (in blue in Figure 7) for which Ovsyannikov et al. (2013) reported that Mn2O3 is irreversibly reduced into Mn3O4. This is in good agreement with their observations of a Mn3O4, C and O2 assemblage. Finally, Ono (2007) reported experiments at 54 GP and 1500/2000 K in which he observed new diffraction peaks and proposed a new structure for MnCO3. We found very good agreement between our diffraction patterns at these P-T conditions and his, however our ex situ analyses indicate the presence of oxides as well as carbonate at these temperatures and part of these new diffraction peaks actually belong to the manganese oxide.
Implications for the Deep Carbon Cycle
Carbon in the Earth may exist in various forms including carbides, diamond, graphite, lonsdaleite, hydrocarbons, CO2, and carbonates depending on the oxygen fugacity and P-T conditions (Dasgupta and Hirschmann, 2010; Hazen et al., 2013; Jones et al., 2016). Although it is generally thought that the conditions of the Earth's deep interior and of the subducting slab materials may be not compatible with the stability of carbonates or carbonate-rich liquid (Anzolini et al., 2016; Thomson et al., 2016), the observation of carbonate inclusions in diamonds potentially brought up to the Earth's surface from the deep mantle indicates that carbonates can exist at least locally in the mantle (e.g., Stachel et al., 1998, 2000; Leost et al., 2003; Bulanova et al., 2010). Carbonate compositions at the surface of the planet are mainly calcite and dolomite. When transported into the deep Earth via subducting slabs, dolomite breaks down into aragonite and magnesite at a depth of around 250 km (e.g., Hammouda et al., 2011). These two end members display very different behavior at high pressure: magnesite is now known to be stable down to about 2000 km depth, below which it transforms into a tetrahedrally coordinated CO4 structure (Boulard et al., 2015b) while aragonite undergoes a phase transition into a CO3-bearing post-aragonite structure at about 1000 km depth that remains stable down to core mantle boundary conditions (Ono et al., 2007).
Together with previous studies on MnCO3-II, we show that metastable structures of CaCO3 such as CaCO3-VI may play an important role in the transport of carbon into our planet's deep interior as it can host intermediate sized cations such as Mn2+ in contrast to aragonite and post-aragonite structures. Possible miscibility between CaCO3 and other smaller cation-bearing components, such as MnCO3, or MgCO3 and FeCO3 may make the CaCO3-VI structure more abundant in the planet's deep interior than previously thought. One should note that MnCO3 and CaCO3 display miscibility gap at ambient conditions (de Capitani and Peters, 1981) and the possibility of a miscibility in CaCO3-VI structure merits further investigation. However, as the slabs undergo subduction and reach temperatures closer to the mantle geotherm (brown line in Figure 6), this phase will decompose into manganese oxides and diamonds. Reduction of carbon into diamond at high P-T was also reported for other 3d metal-bearing carbonates such as FeCO3 and (Mg,Fe)CO3 (Boulard et al., 2012). However, formation of diamond in FeCO3 and (Mg,Fe)CO3 system is due to the fact that Fe is mainly incorporated as Fe3+ in their high-pressure polymorphs leading to partial reduction of carbon and a coexistence of oxidized and reduced carbon. Here, we show that there is no evidence of a change in the oxidation state of manganese in MnCO3-II. The redox reaction (2) that would lead to diamond formation in the subducted slabs is in fact due to decomposition of MnCO3 into MnO and CO2 oxides. It therefore seems probable that even if miscibility between MnCO3, FeCO3, MgCO3 in the CaCO3-VI structure takes place, this structure will only be present as an intermediate stage of subduction. The high-pressure polymorph of FeCO3 and (Mg,Fe)CO3 would likely represent the main oxidized carbon host at lower mantle conditions.
Conclusions
Our study brings new insight into the phase diagram of MnCO3 at high pressure and temperature. Rhombohedral MnCO3-I as well as its high pressure polymorph MnCO3-II are stable at temperatures up to 1400 K. At higher temperatures however, both MnCO3-I and MnCO3-II break down into oxides and redox reactions take place which lead to the formation of manganese oxides such as Mn2O3 and Mn3O4 and diamond. These reactions occur at P-T conditions close to the mantle geotherm. The CaCO3-VI structure that can host small cations at relatively high pressures can only be encountered at an intermediate stage of subduction, thus, the high pressure tetrahedral carbonate phase of FeCO3 and (Mg,Fe)CO3 would represent the main mineral host for oxidized carbon in the deep Earth.
Author Contributions
EB and WM designed the research project; EB, YL, AK, MR, JS, GM, and MM performed experiments and analysis; EB and YL analyzed data; and EB and WM wrote the paper with input from all co-authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
EB and WM acknowledge support from the Deep Carbon Observatory. WM is supported by NSF-EAR-1055454. Portion of the XRD work was performed at the high-pressure beamline 12.2.2, ALS which is supported by the DOE-BES under contract DE-AC02-05CH11231. We thank J. Yan, J. Knight, and A. MacDowell for their assistance with XRD experiments at ALS. We also thank M. Scott for providing the rhodochrosite sample.
References
Andrews, J. C., Brennan, S., Pianetta, P., Ishii, H., Gelb, J., Feser, M., et al. (2009). Full-field transmission x-ray microscopy at SSRL. J. Phys. 186:12002. doi: 10.1088/1742-6596/186/1/012002
Anzolini, C., Angel, R. J., Merlini, M., Derzsi, M., Tokár, K., Milani, S., et al. (2016). Depth of formation of CaSiO3-walstromite included in super-deep diamonds. Lithos. 265, 138–147. doi: 10.1016/j.lithos.2016.09.025
Benedetti, L. R., and Loubeyre, P. (2004). Temperature gradients, wavelength-dependent emissivity, and accuracy of high and very-high temperatures measured in the laser-heated diamond cell. High Press. Res. 24, 423–445. doi: 10.1080/08957950412331331718
Boulard, E., Goncharov, A. F., Blanchard, M., and Mao, W. L. (2015a). Pressure-induced phase transition in MnCO3 and its implications on the deep carbon cycle. J. Geophys. Res. Solid Earth 120, 4069–4079. doi: 10.1002/2015JB011901
Boulard, E., Menguy, N., Auzende, A. L., Benzerara, K., Bureau, H., Antonangeli, D., et al. (2012). Experimental investigation of the stability of Fe-rich carbonates in the lower mantle. J. Geophys. Res. 117, B02208. doi: 10.1029/2011jb008733
Boulard, E., Pan, D., Galli, G., Liu, Z., and Mao, W. L. (2015b). Tetrahedrally coordinated carbonates in Earth's lower mantle. Nat. Commun. 6, 6311. doi: 10.1038/ncomms7311
Bulanova, G. P., Walter, M. J., Smith, C. B., Kohn, S. C., Armstrong, L. S., Blundy, J., et al. (2010). Mineral inclusions in sublithospheric diamonds from Collier 4 kimberlite pipe, Juina, Brazil: subducted protoliths, carbonated melts and primary kimberlite magmatism. Contrib. Minerl. Petrol. 160, 489–510, doi: 10.1007/s00410-010-0490-6
Dasgupta, R., and Hirschmann, M. M. (2010). The deep carbon cycle and melting in Earth's interior. Earth Planet. Sci. Lett. 298, 1–13. doi: 10.1016/j.epsl.2010.06.039
de Capitani, C., and Peters, T. (1981). The solvus in the System MnCO3-CaCO. Contrib. Mineral. Petrol. 76, 394–400, doi: 10.1007/bf00371481
Dorogokupets, P. I., and Dewaele, A. (2007). Equations of state of MgO, Au, Pt, NaCl-B1, and NaCl-B2: internally consistent high-temperature pressure scales. High Pressure Res. 27, 431–446. doi: 10.1080/08957950701659700
Farfan, G. A., Boulard, E., Wang, S., and Mao, W. L. (2013). Bonding and electronic changes in rhodochrosite at high pressure. Am. Mineral. 98, 1817–1823. doi: 10.2138/am.2013.4497
Dewaele, A., Belonoshko, A., Garbarino, G., Occelli, F., Bouvier, P., Hanfland, M., et al. (2012). High-pressure–high-temperature equation of state of KCl and KBr. Phys. Rev. 85, 1–7. doi: 10.1103/physrevb.85.214105
Hammersley, A. P., Svensson, S. O., Hanfland, M., Fitch, A. N., and Hausermann, D. (1996). Two-dimensional detector software: from real detector to idealised image or two-theta scan. High Press. Res. 14, 235–248.
Hammouda, T., Andrault, D., Koga, K., Katsura, T., and Martin, A. M. (2011). Ordering in double carbonates and implications for processes at subduction zones. Contrib. Mineral. Petrol. 161, 439–450, doi: 10.1007/s00410-010-0541-z
Hazen, R. M., Downs, R. T., Jones, A. P., and Kah, L. (2013). Carbon Mineralogy and Crystal Chemistry. Rev. Mineral. Geochem. 75, 7–46. doi: 10.2138/rmg.2013.75.2
Jiao, F., and Frei, H. (2010). Nanostructured manganese oxide clusters supported on mesoporous silica as efficient oxygen-evolving catalysts. Chem. Commun. 46, 2920–2922. doi: 10.1039/b921820c
Jones, A. P., McMillan, P. F., Salzmann, C. G., Alvaro, M., Nestola, F., Prencipe, M., et al. (2016). Structural characterization of natural diamond shocked To 60GPa; implications for earth and planetary systems. Lithos. 265, 214–221. doi: 10.1016/j.lithos.2016.09.023
Larson, A. C., and Von Dreele, R. B. (2004). General Structure Analysis System (GSAS). Los Alamos National Laboratory Report LAUR, 86–748.
Leost, I., Stachel, T., Brey, G. P., Harris, J. W., and Ryabchikov, I. D. (2003). Diamond formation and source carbonation: mineral associations in diamonds from Namibia. Contrib. Mineral. Petrol. 145, 15–24. doi: 10.1007/s00410-003-0442-5
Liu, L., Lin, C., and Yang, Y. (2001). Formation of diamond by decarbonation of MnCO3. Solid State Commun. 118, 195–198. doi: 10.1016/s0038-1098(01)00068-0
Liu, Y., Andrews, J. C., Wang, J., Meirer, F., Zhu, P., Wu, Z., et al. (2011). Phase retrieval using polychromatic illumination for transmission X-ray microscopy. Opt. Express 19, 540–545. doi: 10.1364/OE.19.000540
Merlini, M., Hanfland, M., and Gemmi, M. (2015). The MnCO3 -II high-pressure polymorph of rhodocrosite. Am. Mineral. 100, 2625–2629. doi: 10.2138/am-2015-5320
Morard, G., Andrault, D., Guignot, N., Sanloup, C., Mezouar, M., Petitgirard, S., et al. (2008). In situ determination of Fe–Fe3S phase diagram and liquid structural properties up to 65 GPa. Earth Planet. Sci. Lett. 272, 620–626. doi: 10.1016/j.epsl.2008.05.028
Nam, K., Kim, M. G., and Kim, K. (2007). In Situ Mn K-edge X-ray absorption spectroscopy studies of electrodeposited manganese oxide films for electrochemical capacitors. J. Phys. Chem. C 111, 749–758. doi: 10.1021/jp063130o
Oganov, A. R., Glass, C. W., and Ono, S. (2006). High-pressure phases of CaCO3. Crystal structure prediction and experiment. Earth Planet. Sci. Lett. 241, 95–103. doi: 10.1016/j.epsl.2005.10.014
Ono, S. (2007). High-pressure phase transformation in MnCO3. a synchrotron XRD study. Mineral. Mag. 71, 105–111. doi: 10.1180/minmag.2007.071.1.105
Ono, S., Kikegawa, T., and Ohishi, Y. (2007). High-pressure transition of CaCO3. Am. Mineral. 92, 1246–1249. doi: 10.2138/am.2007.2649
Ovsyannikov, S. V., Abakumov, A. M., Tsirlin, A. A., Schnelle, W., Egoavil, R., Verbeeck, J., et al. (2013). Perovskite-like Mn2O3. A path to new manganites. Angew. Chem. Int. Ed. Eng. 52, 1494–1498. doi: 10.1002/anie.201208553
Ressler, T., Brock, S. L., Wong, J., and Suib, S. L. (1999). Multiple-Scattering EXAFS analysis of tetraalkylammonium manganese oxide colloids. J. Phys. Chem. B 103, 6407–6420. doi: 10.1021/jp9835972
Santillán, J., Shim, S. H., Shen, G., and Prakapenka, B. (2006). High-pressure phase transition in Mn2O3: application for the crystal structure and preferred orientation of the CaIrO3 type. Geophys. Res. Lett. 33, 1–5. doi: 10.1029/2006GL026423
Santillán, J., and Williams, Q. (2004). A high-pressure infrared and X-ray study of FeCO3 and MnCO3: comparison with CaMg(CO3)2-dolomite. Phys. Earth Planet. Interiors 143–144, 291–304. doi: 10.1016/j.pepi.2003.06.007
Shatskiy, A. F., Litasov, K. D., and Palyanov, Y. N. (2015). Phase relations in carbonate systems at pressures and temperatures of lithospheric mantle : review of experimental data. Russ. Geol. Geophys. 56, 113–142. doi: 10.1016/j.rgg.2015.01.007
Stachel, T., Brey, G. P., and Harris, J. W. (2000). Kankan diamonds (Guinea) I : from the lithosphere down to the transition zone. Contrib. Mineral. Petrol. 140, 1–15. doi: 10.1007/s004100000173
Stachel, T., Harris, J. W., and Brey, G. P. (1998). Rare and unusual mineral inclusions in diamonds from Mwadui, Tanzani. Contrib. Mineral. Petrol. 132, 34–47, doi: 10.1007/s004100050403
Thomson, A. R., Walter, M. J., Kohn, S. C., and Brooker, R. A. (2016). Slab melting as a barrier to deep carbon subduction. Nature 529, 76–79. doi: 10.1038/nature16174
Wang, Q., Grau-Crespo, R., and de Leeuw, N. H. (2011). Mixing thermodynamics of the calcite-structured (Mn,Ca)CO3 solid solution: a computer simulation study. J. Phys. Chem. B 115, 13854–13861. doi: 10.1021/jp200378q
Xu, C., Xu, B., Yang, Y., Dong, H., Oganov, A. R., Wang, S., et al. (2015). Prediction of a stable post-post-perovskite structure from first principles. Phys. Rev. B 91, 1–5. doi: 10.1103/physrevb.91.020101
Zhang, J., Martinez, I., Guyot, F., and Reeder, R. J. (1998). Effects of Mg-Fe 2+ substitution in calcite-structure carbonates : thermoelastic properties. Am. Mineral. 83, 280–287.
Keywords: carbonate, phase transition, redox reaction, deep carbon cycle, high pressure
Citation: Boulard E, Liu Y, Koh AL, Reagan MM, Stodolna J, Morard G, Mezouar M and Mao WL (2016) Transformations and Decomposition of MnCO3 at Earth's Lower Mantle Conditions. Front. Earth Sci. 4:107. doi: 10.3389/feart.2016.00107
Received: 22 August 2016; Accepted: 28 November 2016;
Published: 15 December 2016.
Edited by:
Benjamin Alexander Black, City College of New York, USAReviewed by:
Matteo Alvaro, University of Pavia, ItalyZachary M. Geballe, Geophysical Laboratory (CIS), USA
Copyright © 2016 Boulard, Liu, Koh, Reagan, Stodolna, Morard, Mezouar and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eglantine Boulard, eglantine.boulard@synchrotron-soleil.fr
†Present Address: Eglantine Boulard, Synchrotron SOLEIL, L'Orme les Merisiers, St. Aubin, France
 Eglantine Boulard
Eglantine Boulard Yijin Liu2
Yijin Liu2  Mary M. Reagan
Mary M. Reagan Wendy L. Mao
Wendy L. Mao