- Department of Cell and Developmental Biology, University College London, London, UK
Controlled ATP release has been demonstrated from many neuronal and non-neuronal cell types. Once released, extracellular ATP acts on cells in a paracrine manner via purinergic receptors. Considerable evidence now suggests that extracellular nucleotides, signaling via P2 receptors, play important roles in bone homeostasis modulating both osteoblast and osteoclast function. In this study, we demonstrate that mouse osteoclasts and their precursors constitutively release ATP into their extracellular environment. Levels were highest at day 2 (precursor cells), possibly reflecting the high number of red blood cells and accessory cells present. Mature osteoclasts constitutively released ATP in the range 0.05–0.5 pmol/ml/cell. Both osteoclasts and osteoblasts express mRNA and protein for the P2X7 receptor. We found that in osteoclasts, expression levels are fourfold higher in mature cells relative to precursors, whilst in osteoblasts expression remains relatively constant during differentiation. Selective antagonists (0.1–100 μM AZ10606120, A438079, and KN-62) were used to determine whether this release was mediated via P2X7 receptors. AZ10606120, A438079, and KN-62, at 0.1–10 μM, decreased ATP release by mature osteoclasts by up to 70, 60, and 80%, respectively. No differences in cell viability were observed. ATP release also occurs via vesicular exocytosis; inhibitors of this process (1–100 μM NEM or brefeldin A) had no effect on ATP release from osteoclasts. P2X7 receptor antagonists (0.1–10 μM) also decreased ATP release from primary rat osteoblasts by up to 80%. These data show that ATP release via the P2X7 receptor contributes to extracellular ATP levels in osteoclast and osteoblast cultures, suggesting an important additional role for this receptor in autocrine/paracrine purinergic signaling in bone.
Introduction
The idea that purines act as extracellular signaling molecules was first suggested in 1929, yet it was not until 1972 that the concept of purinergic neurotransmission was proposed (Burnstock, 1972). It is now widely accepted that extracellular nucleotides, signaling via the P2 receptors, participate in a wide number of biological processes in both neuronal and non-neuronal tissues. P2 receptors are subdivided into the P2X ligand-gated ion channels and P2Y G-protein-coupled receptors (Kennedy and Burnstock, 1985; Abbracchio and Burnstock, 1994). Currently, seven P2X receptors (P2X1-7) and eight P2Y (P2Y1,2,4,6,11,12,13,14) receptors have been identified; each of these receptors has been cloned, characterized, and displays distinct tissue expression and pharmacology (Ralevic and Burnstock, 1998; Burnstock, 2007). P2 receptors respond to a range of adenine and uridine-containing nucleotides including adenosine triphosphate (ATP), adenosine diphosphate (ADP), uridine triphosphate (UTP), and uridine diphosphate (UDP).
In recent years, it has become evident that extracellular nucleotides play a significant role in bone biology modulating both osteoblast and osteoclast function (see reviews by Grol et al., 2009; Orriss et al., 2010). Expression of multiple P2 receptor subtypes by osteoblasts (Maier et al., 1997; Hoebertz et al., 2000; Nakamura et al., 2000; Gartland et al., 2001; Ke et al., 2003; Ihara et al., 2005; Orriss et al., 2006, 2010; Alqallaf et al., 2009) and osteoclasts has now been reported (Bowler et al., 1995; Naemsch et al., 1999; Buckley et al., 2002; Gartland et al., 2003a; Korcok et al., 2005; Orriss et al., 2010, 2011b). Functional effects of purinergic signaling on bone cells include increased osteoblast proliferation (Nakamura et al., 2000), decreased bone mineralization by osteoblasts (Orriss et al., 2007), the modulation of osteoblast responses to systemic factors such as parathyroid hormone (Bowler et al., 2001; Buckley et al., 2001), induction of osteoblastic membrane blebbing (Panupinthu et al., 2007), and the production of lipid mediators (Panupinthu et al., 2008). In osteoclasts, activation of the P2Y1 and P2Y6 receptor subtypes has been shown to enhance formation, activity, and survival (Hoebertz et al., 2001; Korcok et al., 2005; Orriss et al., 2011b). The P2X7 receptor, which has a more complex role in osteoclast function, has been implicated in cell fusion (Gartland et al., 2003b), apoptosis (Penolazzi et al., 2005), the translocation, and activation of NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells; Korcok et al., 2004) and PKC (Armstrong et al., 2009) and intercellular communication (Jorgensen et al., 2002). The important role of P2 receptors in bone homeostasis is further highlighted by the recent studies of several knockout mouse models (P2Y1, P2Y2, P2Y6, P2X7), all of which show significant changes in bone architecture and cell function (reviewed by Orriss et al., 2011a).
ATP is present in cell cytoplasm at concentrations between 2 and 5 mM. Following membrane damage or necrosis, all cells can potentially release ATP into the extracellular environment, which can then act in an autocrine/paracrine manner to influence local purinergic signaling. In addition, numerous excitatory and non-excitatory cells including epithelial and endothelial cells (Bodin and Burnstock, 2001; Knight et al., 2002), platelets (Beigi et al., 1999), fibroblasts (Gerasimovskaya et al., 2002), chondrocytes (Graff et al., 2000), erythrocytes (Sprague et al., 1998), and astrocytes (Coco et al., 2003) release ATP in a controlled manner. Constitutive ATP release has also been reported from primary osteoblasts (Orriss et al., 2009), a number of osteoblast-like cells lines (Romanello et al., 2001; Buckley et al., 2003; Genetos et al., 2005) and MLO-Y4 osteocyte-like cells (Genetos et al., 2007; Thompson et al., 2011). Controlled release of ATP from mature osteoclasts has not been described.
Cellular release of ATP is thought to occur via several mechanisms including (1) ATP binding cassette (ABC) transporters (Lazarowski et al., 2003), (2) vesicular exocytosis, perhaps involving lysosomes (Zhang et al., 2007), (3) gap junctions, connections, and/or pannexin hemichannels (Lazarowski et al., 2003; Spray et al., 2006), and (4) the P2X7 receptor (Suadicani et al., 2006). Several ABC proteins are potential candidates for mediating ATP release including the cystic fibrosis transmembrane conductance regulator (CFTR). The CFTR was initially thought to mediate ATP release from several cell types including erythrocytes (Sprague et al., 1998); however, later work indicated that the CFTR regulates rather than mediates the release of ATP (Sugita et al., 1998; Watt et al., 1998; Braunstein et al., 2001). Controlled vesicular exocytosis is implicated in ATP release from many cell types including epithelial and endothelial cells (Bodin and Burnstock, 2001; Knight et al., 2002). Additionally, it has been suggested that ATP release from osteoblasts occurs, at least in part, via vesicular mechanisms (Genetos et al., 2005; Romanello et al., 2005; Orriss et al., 2009). Connexin hemichannels and gap junctions, which allow the movement of molecules less than 1 kDa, reportedly act as a conductive pathway to mediate ATP release from astrocytes (Cotrina et al., 1998; Bowler et al., 2001; Coco et al., 2003).
The P2X7 receptor is distinct from other P2X receptors in that it has a relatively low sensitivity for ATP (>100 μM) and preferentially binds to 2′,3′-O-(benzoyl-4-benzoyl)-ATP (Bz-ATP). Prolonged or repeated exposure to high concentrations of P2X7 agonist mediates the formation of cytolytic pores (Murgia et al., 1992), whereas, transient receptor stimulation causes the formation of non-selective membrane pores permeable to molecules up to 900 kDa in size (Di Virgilio, 1995). Recently, involvement of the P2X7 receptor in ATP release has been reported in astrocytes (Suadicani et al., 2006) and osteoclast monocyte precursors (Pellegatti et al., 2011). In osteoclast precursors, the release of ATP via the P2X7 receptor is thought to be an important source of extracellular adenosine which acts to promote cell fusion (Pellegatti et al., 2011).
The aims of this study were to investigate (1) whether osteoclasts release ATP under normal conditions, (2) the effect of osteoclast differentiation on constitutive ATP release, and (3) the role of the P2X7 receptor in the release of ATP from bone cells.
Materials and Methods
Reagents
All tissue culture reagents were purchased from Gibco (Paisley, UK). Unless otherwise mentioned, all chemicals were purchased from Sigma Aldrich (Poole, Dorset, UK). The CytoTox 96® non-radioactive cytotoxicity assay and CellTiter-Glo® luminescent assay were obtained from Promega UK (Southampton, UK). The P2X7 receptor antagonists were purchased from Tocris Bioscience (Bristol, UK). All molecular biology reagents were purchased from Invitrogen (Paisley, UK) and all primers from MWG Biotech (Ebersberg, Germany).
Cell Culture
Primary rat osteoblast cells were obtained from the calvaria of 2-day-old neonatal Sprague-Dawley rats and cultured as described previously (Orriss et al., 2012). Osteoclasts were isolated from the long bones of two 6-week-old mice and cultured as we have previously reported (Orriss and Arnett, 2012).
Measurement of ATP Release
Prior to measurement of ATP release, culture medium was removed, cell layers washed, and cells incubated with serum-free DMEM (osteoblasts: 1 ml/well) or MEM (osteoclasts: 250 μl/well). Samples were collected after 1 h and immediately snap-frozen on dry ice for later ATP quantification. In the experiments examining release mechanisms, P2X7 receptor antagonists (1 nM–100 μM AZ10606120, KN-62, A438079, A740003), and vesicular inhibitors [0.1–50 μM brefeldin A, n-ethylmaleimide (NEM)] were added to the serum-free DMEM. ATP release was measured luminetrically using the luciferin–luciferase assay as described previously (Orriss et al., 2009).
Cell Proliferation and Viability Assay
Cell number and viability was determined in all samples using the CytoTox 96® colorimetric cytotoxicity assay (Promega UK, Southampton, UK). This assay quantifies cellular lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released on cell lysis. LDH oxidizes lactate into pyruvate, generating NADH, which is then used to convert a tetrazolium salt into a red formazan product in proportion to the number of lysed cells.
Following measurement of ATP release, cell supernatants were collected to determine medium LDH levels (cell viability). To establish total cellular LDH levels, cells were lysed with 1% Triton X-100 in water (lysis buffer, 15 μl/ml of medium) for 1 h. The LDH content of the supernatants and cell lysates were measured colorimetrically (490 nm; ELX800 plate reader, Bio-tek International) as per manufacturer’s instructions. A standard curve for determination of cell numbers was constructed using cells seeded at 102–106/well. Manual cell counts were performed in parallel for assay validation. By expressing medium LDH as a percentage of the total cellular LDH cell viability could be also calculated.
Quinacrine Staining
The acridine derivative, quinacrine, is a weak base that binds ATP with a high affinity. When excited by light at 476 nm it fluoresces in the 500- to 540-nm range and is widely used to visualize ATP-containing subcellular compartments in live cells (Irvin and Irvin, 1954; Olson et al., 1976). Osteoblasts and osteoclasts were seeded onto sterile 1 cm diameter disks, cut from Melinex (Du Pont Teijin Films, Dumfries, UK) clear polyester film, in 24-well trays at 2.5 × 104 cells/disk and 106 cells/disk, respectively, and cultured until the formation of mature cells. To visualize ATP-filled vesicles, Melinex disks were twice washed with PBS before incubation with 30 μM quinacrine for 1 h; disks were washed twice more and mounted onto microscope slides. The cells were immediately observed using fluorescence microscopy with a digital camera attachment (AxioCam MRC5, Imaging Associates Ltd., Bicester, UK).
Total RNA Extraction and DNase Treatment
Osteoclasts were cultured on large dentine disks in 24-well trays and total RNA extracted at 2, 5, 7, and 9 days of culture using TRIZOL® reagent (Invitrogen, Paisley, UK) according to the manufacturer’s instructions. Extracted RNA was treated with RNase-free DNase I (35 U/ml) for 30 min at 37°C. The reaction was terminated by heat inactivation at 65°C for 10 min. Total RNA was quantified spectrophotometrically by measuring absorbance at 260 nM. RNA was stored at –80°C until amplification by qPCR.
Quantitative Real Time Polymerase Chain Reaction (qPCR)
Osteoclast RNA (50 ng) was transcribed and amplified using the iScript one-step qRT-PCR kit with SYBR green (Bio-rad Laboratories Ltd., Hemel Hempstead, UK), which allows cDNA synthesis and PCR amplification to be carried out sequentially. qRT-PCR was performed according to the manufacturer’s instructions, with initial cDNA synthesis (50°C for 10 min) and reverse transcriptase inactivation (95°C for 5 min), followed by 40 cycles of denaturation (95°C for 10 s) and detection (60°C for 30 s). Gene expression was investigated in cells cultured for 2, 5, 7, and 9 days. Data were analyzed using the Pfaffl method and are shown as changes in the level of gene expression relative to that in precursor cells. All reactions were carried out in triplicate using RNAs derived from four different osteoclast cultures. Primer sequences: B actin S: gat ctg gca cca cac ctt ct/AS: ggg gtg aag gtc tca aa; P2X7 S: ggc act gga gga aaa ttt ga/AS: tga gca agt caa tgc aca ca.
Western Blot
Osteoclasts were cultured for 9 days and protein was extracted at 2, 5, 7, and 9 days. Cell layers were lysed in ice-cold radio-immunoprecipitation (RIPA) lysis buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.1% SDS 1 mM phenyl methyl sulfonyl fluoride (PMSF), 1 mg/ml aprotinin, 1 mM Na3VO4, and 2.5 mg/ml deoxycholic acid). Cell homogenates were sonicated for 5 min and stored at −80°C for at least half an hour before use. Protein concentrations from lysates were determined using the Bradford assay (Sigma Aldrich, Gillingham, Dorset, UK). Prior to loading total protein samples were denatured by incubating at 95°C for 5 min in the presence of 5× reducing sample buffer (60 mM Tris–HCl pH 6.8, 25% glycerol, 2% SDS, 10% β-mercaptoethanol, and 0.1% bromophenol blue). Protein samples (30 μg/lane) were loaded into SDS-PAGE (10%) gels and transferred onto a polyvinyldifluoride (PVDF) membrane (Amersham, Buckinghamshire, UK) by the use of a wet tank blotter (Bio-Rad, Hercules, CA, USA) at 150 V for 1 h. Membranes were then blocked with 5% non-fat milk and incubated with the P2X7 (1:200) antibody overnight at room temperature (P2X7 extracellular, Alomone, Jerusalem, Israel). After washing, blots were incubated in horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 1 h at room temperature. A peroxidase detection system (Immobilon™ Western, Millipore UK, Watford, UK) was used for the visualization of the immunoreactivity. Western blotting performed in the presence of an antibody-specific blocking peptide or without primary antibody served as negative controls. Membranes were subsequently stained with ponceau red to ensure equal loading of the protein.
Statistical Analysis
Statistical comparisons were made by one-way analysis of variance and adjusted using the Bonferroni method. Representative data are presented as means ± SEM for 6–12 replicates. Results presented are for representative experiments that were each repeated at least three times.
Results
Constitutive ATP Release from Bone Cells
Controlled ATP release from osteoblasts has been reported by many groups (Romanello et al., 2001; Buckley et al., 2003; Genetos et al., 2005). We have shown previously that ATP release from osteoblasts occurs, at least in part, by vesicular exocytosis (Orriss et al., 2009). Baseline results confirmed that osteoblasts constitutively release ATP in the range 0.5–8 pmol/ml/cell (Figure 1A). Furthermore, the amount of ATP released was increased up to 12-fold in differentiating osteoblasts (day 7 and 10) and 16-fold in mature, bone-forming osteoblasts (day 14) relative to precursor cells (day 4). Cell viability remained constant throughout the culture period (Figure 1A). In mature osteoblasts, abundant cytoplasmic quinacrine staining, with a clear granular–vesicular appearance was observed, indicating the presence of intracellular ATP stores (Figures 1C,D). Widespread quinacrine staining was also evident in precursor cells and differentiating osteoblasts (not shown).
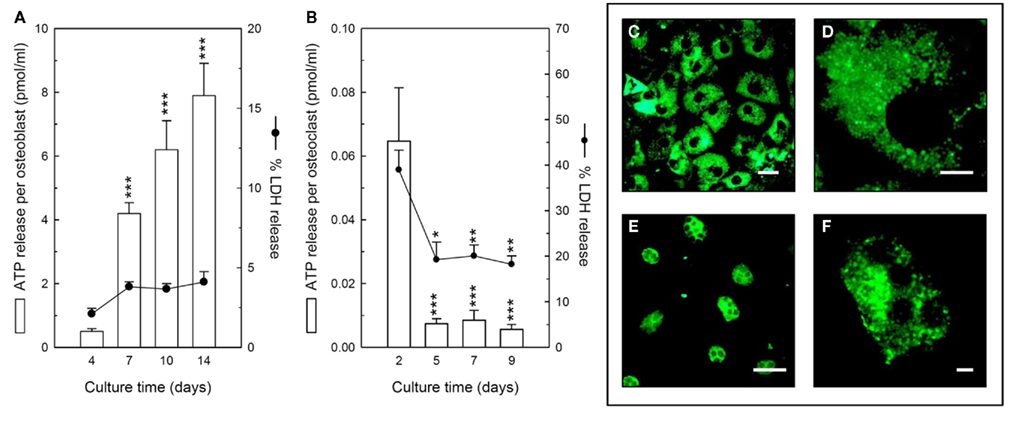
Figure 1. Constitutive ATP release from bone cells. (A) Osteoblasts released ATP constitutively throughout the culture period, with peak release from mature, bone-forming cells (day 14); cell viability remained similar at every stage of development. (B) Osteoclasts also constitutively released ATP throughout the culture period. The highest level of ATP release was detected from precursor cells (day 2), although this was accompanied by higher LDH levels indicating a reduced cell viability. The amount of ATP release from early osteoclasts (day 5) was the same as mature, resorbing cells (day 9). Values are means ± SEM (n = 10–12 replicate wells or disks), significantly different from controls: *p < 0.05, **p < 0.01, ***p < 0.001. Quinacrine staining showing intracellular ATP in the cytosol of (C) osteoblasts, and (E) osteoclasts, scale bars = 10 μm. Clear, granular staining shows ATP is probably localized to vesicles in (D) a single osteoblast, and (F) a single osteoclast, scale bars = 2.5 μm.
We found that osteoclasts also constitutively released ATP throughout the culture period (Figure 1B). The levels detected were up to 10-fold lower than osteoblasts, typically in the range 0.05–0.5 pmol/ml/cell. The highest levels of extracellular ATP were detected in early-stage cultures of precursor cells (day 2), possibly reflecting some initial cell damage during the isolation procedure and the high number of red blood cells present at this stage. The amount of ATP release per cell did not appear to change during osteoclastogenesis, with release from early osteoclasts (day 5) being the same as from mature, resorbing osteoclasts (day 9; Figure 1B). Quinacrine staining indicated the presence of abundant ATP in the cytosol of mature osteoclasts (Figures 1E,F).
Expression of the P2X7 Receptor by Osteoclasts
We have previously demonstrated expression of the P2X7 receptor by primary rat osteoblasts (Orriss et al., 2006) and mouse osteoclasts (Orriss et al., 2011b). We observed that P2X7 receptor mRNA expression increased fourfold in mature, non-resorbing osteoclasts relative to precursor cells (Figure 2A). When mature osteoclasts were activated to resorb by acid, P2X7 receptor expression decreased slightly, although still remaining 2.5-fold higher than in precursor cells. Expression of P2X7 protein was not detected in precursor cells or early osteoclasts, but only in mature osteoclasts (Figure 2B).
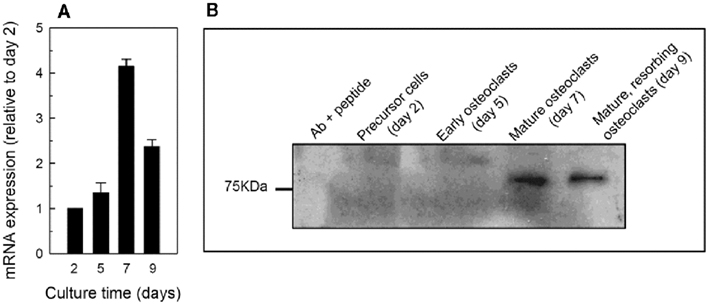
Figure 2. Expression of the P2X7 receptor by osteoclasts. (A) Levels of P2X7 mRNA were increased fourfold in mature osteoclasts relative to precursor cells. Osteoclast activation by acid caused levels of the P2X7 receptor to be decreased in resorbing cells (day 9) compared to mature non-activated cells (day 7). (B) P2X7 receptor protein was not detected in precursor cells or early osteoclasts but was present in mature cells. Unlike the mRNA, no differences in protein level were detected between mature and mature, resorbing cells.
P2X7 Receptor Antagonists Block ATP Release from Osteoclasts
To determine whether the P2X7 receptor mediates ATP release from osteoclasts, mature cells (day 7) were incubated with four different P2X7 receptor antagonists prior to measurement of extracellular ATP levels. All four inhibitors, which block the P2X7 receptor via different mechanisms, reduced ATP release from osteoclasts (Figure 3). AZ10606120, which acts as a negative allosteric modulator of the P2X7 receptor, inhibited ATP release by 80% at 1–10 μM (Figure 3A). KN-62, a non-competitive P2X7 receptor antagonist, reduced ATP efflux by up to 90% in the concentration range 0.1–10 μM (Figure 3B). A438079, a competitive P2X7 receptor antagonist, decreased ATP release 70–80% at concentrations ≥0.1 μM (Figure 3C). Finally, the potent, competitive P2X7 receptor antagonist, A740003, inhibited ATP release up to 85% at ≥1 nM (Figure 3D). For all four antagonists, treatment with concentrations up to 10 μM did not affect osteoclast viability (Figures 3A–D); however, AZ10606120, KN-62, and A740003 at concentrations >10 μM were toxic, causing significant cell death.
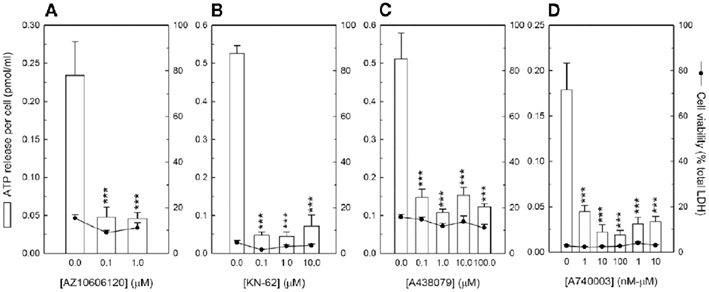
Figure 3. P2X7 receptor antagonists inhibit ATP release from osteoclasts. Cells were cultured on dentine disks for 7 days before the effect of P2X7 receptor antagonists on ATP release from mature osteoclasts was investigated. (A) AZ10606120, (B) KN-62, (C) A438079, and (D) A740003 decreased ATP release by up to 80, 90, 80, and 85%, respectively. At the concentrations shown no effects on cell viability were observed. A740003 was the most potent of the P2X7 receptor antagonists tested blocking ATP release from 1 nM. Values are means ± SEM (n = 10 replicate disks), significantly different from controls: ***p < 0.001.
Inhibitors of Vesicular Exocytosis Do Not Influence ATP Release from Osteoclasts
To determine whether ATP release from mature osteoclasts could involve vesicular exocytosis, the cells were incubated with two selective inhibitors, NEM and Brefeldin A, and the effects on extracellular ATP levels measured. NEM, which inhibits vesicular fusion with the plasma membrane, and Brefeldin A, which disrupts vesicular trafficking by blocking protein transport from the endoplasmic reticulum to the Golgi apparatus, inhibit ATP release from osteoblasts by up to 80% at 100 μM (Figure 4A). No effect was seen at concentrations below 100 μM. In contrast, NEM (Figure 4B) and Brefeldin A (Figure 4C), had no effect on ATP release from osteoclasts at concentrations up to 50 μM. At the concentrations tested, no effects of these inhibitors on cell viability were observed. Concentrations of ≥100 μM caused significant cell death in osteoclasts but not in osteoblasts.
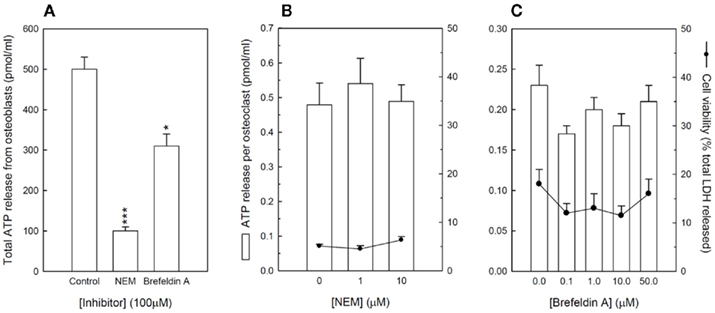
Figure 4. Inhibitors of vesicular exocytosis do not block ATP release from osteoclasts. (A) NEM and brefeldin A (100 μM) inhibit ATP release from mature osteoblasts by 80 and 40%, respectively. Cells were cultured on dentine disks for 7 days before the effect vesicular exocytosis inhibitors on ATP release from mature osteoclasts was investigated. (B) NEM and, (C) brefeldin A had no effect on ATP release from osteoclasts. At the concentrations shown no effects on cell viability were observed. Values are means ± SEM (n = 10 replicate disks/wells).
P2X7 Receptor Antagonists Reduce ATP Release from Osteoblasts
To determine whether the P2X7 receptor could contribute toward ATP release from mature osteoblasts, the antagonists AZ10606120, A438079, and A740003 were tested at between 10 nM and 100 μM; at these concentrations, no effects on cell viability were observed. Treatment with ≥100 nM AZ10606120 decreased ATP release by 65–80% (Figure 5A), whilst ≥10 nM A438079 reduced ATP levels by ∼25% (Figure 5B). A740003, at concentrations ≥1 μM, was also inhibitory, decreasing ATP levels by 60–80% (Figure 5C).
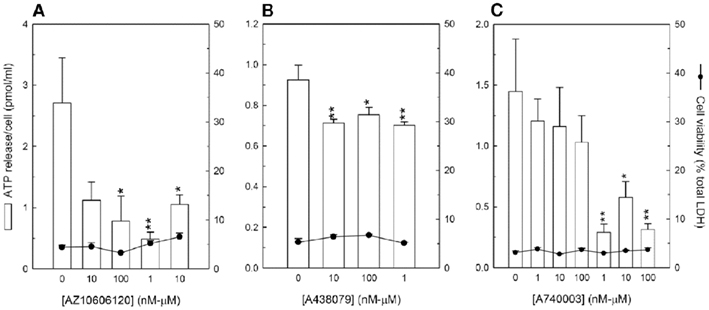
Figure 5. P2X7 receptor antagonists decrease ATP release from osteoblasts. Osteoblasts were cultured until the onset of bone formation (∼10 days) before the effect of P2X7 receptor antagonists on ATP release was investigated. (A) AZ10606120, (B) A438079, and (C) A740003 decreased ATP release by up to 80, 25, and 80%, respectively. At the concentrations shown no effects on cell viability were observed. Values are means ± SEM (n = 12 replicate wells), significantly different from controls: *p < 0.05, **p < 0.01.
Discussion
The aim of this investigation was to study controlled ATP release from bone cells, particularly osteoclasts, and to determine the mechanisms mediating this process. We demonstrated that osteoclasts contain extensive intracellular ATP stores and constitutively release ATP in the range 0.05–0.5 pmol/ml/cell. Pharmacological studies indicate that this efflux is primarily meditated via the P2X7 receptor, rather than vesicular exocytosis. This constitutes the first report of controlled ATP release from mature, resorbing osteoclasts in vitro. In contrast, our earlier work has suggested that osteoblasts release ATP principally by exocytosis (Orriss et al., 2009). Here, however, we demonstrate that the P2X7 receptor also contributes toward ATP efflux from osteoblasts.
ATP release was measured at all stages throughout the osteoclast culture, using cells grown on a natural, resorbable substrate (dentine) over a 9-day period. At day 2 of culture, the majority of cells present were precursors along with some residual red blood cells from the bone marrow; by day 4 some of the cells exhibited multinucleated osteoclast-like morphology; at day 7 mature, inactive osteoclasts were observed, and by day 9, mature, resorbing osteoclasts were present in acidified cultures as described recently (Orriss et al., 2011b; Orriss and Arnett, 2012). We found that osteoclasts constitutively released ATP at all times, although levels were highest at the beginning of the culture period (day 2). This increase in extracellular ATP levels was accompanied by a large decrease in cell viability and is probably a consequence of the initial cell damage during the isolation procedure. Thus, uncontrolled rather than regulated, ATP release from damaged cells most likely artificially enhanced the extracellular levels of ATP. Furthermore, since red blood cells also release ATP (Sprague et al., 1998), the residual erythrocytes seen in these cultures at day 2 could also have contributed to the increased extracellular ATP levels seen at this stage. By day 4 of culture there are no longer any red blood cells present in these cultures.
Cellular differentiation did not affect basal ATP release in osteoclast-forming cultures with early osteoclasts releasing similar amounts to mature, resorbing cells. In contrast, ATP release from osteoblasts is strongly dependent on differentiation, with extracellular levels up to sevenfold higher in cultures of mature, bone-forming cells relative to precursor cells (Orriss et al., 2009). Differentiated osteoblasts have also been shown to have intracellular ATP levels that are fivefold higher than precursor cells (Komarova et al., 2000). Thus, the increased ATP release from mature osteoblasts could be a consequence of the higher intracellular ATP concentration. In both cell types, the observed pattern of quinacrine staining (used to visualize intracellular ATP) was granular and cytoplasmic, suggesting a vesicular localization of ATP.
Expression of the P2X7 receptor by bone cells has been widely reported (see review by Grol et al., 2009). In this study, we confirmed the expression of the P2X7 receptor by primary mouse osteoclasts. Furthermore, we demonstrated that expression levels were dependent on differentiation, with the highest mRNA and protein levels present in mature osteoclasts. This contrasts to osteoblasts, where P2X7 receptor expression remains constant throughout differentiation (Orriss et al., 2006).
In several cell types, the P2X7 receptor has been implicated as a potential mechanism for the release of ATP (Suadicani et al., 2006; Pellegatti et al., 2011). The role of the P2X7 receptor, polymorphisms of which have been associated with increased fracture risk (Ohlendorff et al., 2007), in the regulation of bone cell function is complex (Grol et al., 2009). This study investigated whether the P2X7 receptor could mediate the release of ATP from bone cells. A number of selective P2X7 receptor antagonists are commercially available, each of which inhibits the receptor via a slightly different pharmacological mechanism. All four of the antagonists tested here (1 nM–1 μM AZ10606120, KN-62, A438079, A740003) reduced ATP release from mature osteoclasts by up to 90%, without affecting cell viability. In contrast, the inhibitors of vesicular exocytosis NEM and Brefeldin A had no effect on ATP release from osteoclasts. A previous study demonstrated that the P2X7 receptor mediates the release of ATP from osteoclast monocyte precursors (Pellegatti et al., 2011). Our investigation was performed on mature osteoclasts rather than precursor cells but agrees with the findings of Pellegatti et al. (2011). Combined these data suggest that efflux via the P2X7 receptor is the primary mechanism for ATP release from osteoclasts under normal conditions. However, since none of the P2X7 receptor antagonists completely abolished ATP efflux, it is possible that another pathway, such as gap junctions, could also be involved.
Controlled ATP release from osteoblasts under normal conditions has been widely reported (Romanello et al., 2001; Buckley et al., 2003; Genetos et al., 2005; Orriss et al., 2009). Evidence from these and our own studies suggests that vesicular exocytosis is the major mechanism mediating ATP release from osteoblasts. We previously demonstrated that inhibitors of vesicular exocytosis reduced ATP release by up to 90% (Orriss et al., 2009). However, the failure of these agents to completely block the process indicated that another mechanism could also be involved. In this study, we have demonstrated that P2X7 receptor antagonists also block ATP release from osteoblast by between 25 and 80%. The degree to which these agents inhibited ATP efflux was quite variable between experiments. This is most likely due to the heterogeneity of receptor expression seen between different primary osteoblast isolations and the observation that only a subset of osteoblasts express the P2X7 receptor (Gartland et al., 2001). Thus not all osteoblasts will have the ability to release ATP via the P2X7 receptor. Consequently, release from some cells will be unaffected by receptor antagonists. Overall, our data suggest that efflux via the P2X7 receptor contributes to the controlled release of ATP from osteoblasts but this release is likely to be in addition to that which occurs via vesicular exocytosis.
Exactly how activation of the P2X7 receptor mediates ATP release from bone cells is unclear. Transient activation of the P2X7 receptor results in the formation of membrane pores permeable to molecules up to 900 kDa in size (Di Virgilio, 1995), thus a likely mechanism for ATP release is directly through these pores (Suadicani et al., 2006). P2X7 receptors have also been shown to activate connexin hemichannels (Baroja-Mazo et al., 2012); since, these hemichannels can also mediate the release of ATP (Lazarowski et al., 2003; Spray et al., 2006) the participation of the P2X7 receptor in ATP release from bone cells may be indirect. However, because several of the widely used connexin hemichannel inhibitors also partly block the P2X7 receptor it is difficult to determine which of these P2X7-mediated pathways is responsible for the observed ATP release (Suadicani et al., 2006).
In many cell types, including osteoblasts, endothelial cells, and fibroblasts, levels of ATP release are increased by external stimuli such as fluid shear stress and hypoxia (Bodin et al., 1992; Bodin and Burnstock, 1998; Gerasimovskaya et al., 2002; Genetos et al., 2005; Orriss et al., 2009). Although not studied here, it is possible that ATP release from osteoclasts could also be increased following exposure to similar external stimuli. This would then have the potential to influence the local concentration of ATP (and its breakdown products) and subsequent purinergic signaling.
In summary, our work indicates that both osteoclasts and osteoblasts release ATP constitutively by multiple mechanisms which include efflux via the P2X7 receptor. This finding highlights further the important role of the P2X7 receptor in the regulation of bone cell function and extracellular ATP levels.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Arthritis Research UK and the European Commission under the 7th Framework Program for their financial support. Grant support: Arthritis Research UK EU Framework 7 Programme.
References
Abbracchio, M. P., and Burnstock, G. (1994). Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol. Ther. 64, 445–475.
Alqallaf, S. M., Evans, B. A., and Kidd, E. J. (2009). Atypical P2X receptor pharmacology in two human osteoblast-like cell lines. Br. J. Pharmacol. 156, 1124–1135.
Armstrong, S., Pereverzev, A., Dixon, S. J., and Sims, S. M. (2009). Activation of P2×P2 7 receptors causes isoform-specific translocation of protein kinase C in osteoclasts. J. Cell Sci. 122, 136–144.
Baroja-Mazo, A., Barbera-Cremades, M., and Pelegrin, P. (2012). The participation of plasma membrane hemichannels to purinergic signaling. Biochim. Biophys. Acta. Available at: http://dx.doi.org/10.1016/j.bbamem.2012.01.002
Beigi, R., Kobatake, E., Aizawa, M., and Dubyak, G. R. (1999). Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am. J. Physiol. 276, C267–C278.
Bodin, P., and Burnstock, G. (1998). Increased release of ATP from endothelial cells during acute inflammation. Inflamm. Res. 47, 351–354.
Bodin, P., and Burnstock, G. (2001). Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J. Cardiovasc. Pharmacol. 38, 900–908.
Bodin, P., Milner, P., Winter, R., and Burnstock, G. (1992). Chronic hypoxia changes the ratio of endothelin to ATP release from rat aortic endothelial cells exposed to high flow. Proc. R. Soc. Lond. B Biol. Sci. 247, 131–135.
Bowler, W. B., Birch, M. A., Gallagher, J. A., and Bilbe, G. (1995). Identification and cloning of human P2U purinoceptor present in osteoclastoma, bone, and osteoblasts. J. Bone Miner. Res. 10, 1137–1145.
Bowler, W. B., Buckley, K. A., Gartland, A., Hipskind, R. A., Bilbe, G., and Gallagher, J. A. (2001). Extracellular nucleotide signaling: a mechanism for integrating local and systemic responses in the activation of bone remodeling. Bone 28, 507–512.
Braunstein, G. M., Roman, R. M., Clancy, J. P., Kudlow, B. A., Taylor, A. L., Shylonsky, V. G., Jovov, B., Peter, K., Jilling, T., Ismailov, I. I., Benos, D. J., Schwiebert, L. M., Fitz, J. G., and Schwiebert, E. M. (2001). Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J. Biol. Chem. 276, 6621–6630.
Buckley, K. A., Golding, S. L., Rice, J. M., Dillon, J. P., and Gallagher, J. A. (2003). Release and interconversion of P2 receptor agonists by human osteoblast-like cells. FASEB J. 17, 1401–1410.
Buckley, K. A., Hipskind, R. A., Gartland, A., Bowler, W. B., and Gallagher, J. A. (2002). Adenosine triphosphate stimulates human osteoclast activity via upregulation of osteoblast-expressed receptor activator of nuclear factor-kappa B ligand. Bone 31, 582–590.
Buckley, K. A., Wagstaff, S. C., McKay, G., Gaw, A., Hipskind, R. A., Bilbe, G., Gallagher, J. A., and Bowler, W. B. (2001). Parathyroid hormone potentiates nucleotide-induced [Ca2]i release in rat osteoblasts independently of Gq activation or cyclic monophosphate accumulation. A mechanism for localizing systemic responses in bone. J. Biol. Chem. 276, 9565–9571.
Coco, S., Calegari, F., Pravettoni, E., Pozzi, D., Taverna, E., Rosa, P., Matteoli, M., and Verderio, C. (2003). Storage and release of ATP from astrocytes in culture. J. Biol. Chem. 278, 1354–1362.
Cotrina, M. L., Lin, J. H., Alves-Rodrigues, A., Liu, S., Li, J., Azmi-Ghadimi, H., Kang, J., Naus, C. C., and Nedergaard, M. (1998). Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. U.S.A. 95, 15735–15740.
Di Virgilio, F. (1995). The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol. Today 16, 524–528.
Gartland, A., Buckley, K. A., Hipskind, R. A., Bowler, W. B., and Gallagher, J. A. (2003a). P2 receptors in bone – modulation of osteoclast formation and activity via P2×P2 7 activation. Crit. Rev. Eukaryot. Gene Expr. 13, 237–242.
Gartland, A., Buckley, K. A., Bowler, W. B., and Gallagher, J. A. (2003b). Blockade of the pore-forming P2×P2 7 receptor inhibits formation of multinucleated human osteoclasts in vitro. Calcif. Tissue Int. 73, 361–369.
Gartland, A., Hipskind, R. A., Gallagher, J. A., and Bowler, W. B. (2001). Expression of a P2×P27 receptor by a subpopulation of human osteoblasts. J. Bone Miner. Res. 16, 846–856.
Genetos, D. C., Geist, D. J., Liu, D., Donahue, H. J., and Duncan, R. L. (2005). Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J. Bone Miner. Res. 20, 41–49.
Genetos, D. C., Kephart, C. J., Zhang, Y., Yellowley, C. E., and Donahue, H. J. (2007). Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J. Cell. Physiol. 212, 207–214.
Gerasimovskaya, E. V., Ahmad, S., White, C. W., Jones, P. L., Carpenter, T. C., and Stenmark, K. R. (2002). Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J. Biol. Chem. 277, 44638–44650.
Graff, R. D., Lazarowski, E. R., Banes, A. J., and Lee, G. M. (2000). ATP release by mechanically loaded porcine chondrons in pellet culture. Arthritis Rheum. 43, 1571–1579.
Grol, M. W., Panupinthu, N., Korcok, J., Sims, S. M., and Dixon, S. J. (2009). Expression, signaling, and function of P2×P27 receptors in bone. Purinergic Signal. 5, 205–221.
Hoebertz, A., Meghji, S., Burnstock, G., and Arnett, T. R. (2001). Extracellular ADP is a powerful osteolytic agent: evidence for signaling through the P2Y1 receptor on bone cells. FASEB J. 15, 1139–1148.
Hoebertz, A., Townsend-Nicholson, A., Glass, R., Burnstock, G., and Arnett, T. R. (2000). Expression of P2 receptors in bone and cultured bone cells. Bone 27, 503–510.
Ihara, H., Hirukawa, K., Goto, S., and Togari, A. (2005). ATP-stimulated interleukin-6 synthesis through P2Y receptors on human osteoblasts. Biochem. Biophys. Res. Commun. 326, 329–334.
Irvin, J. L., and Irvin, E. M. (1954). The interaction of quinacrine with adenine nucleotides. J. Biol. Chem. 210, 45–56.
Jorgensen, N. R., Henriksen, Z., Sorensen, O. H., Eriksen, E. F., Civitelli, R., and Steinberg, T. H. (2002). Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2×P2 7 receptors. J. Biol. Chem. 277, 7574–7580.
Ke, H. Z., Qi, H., Weidema, A. F., Zhang, Q., Panupinthu, N., Crawford, D. T., Grasser, W. A., Paralkar, V. M., Li, M., Audoly, L. P., Gabel, C. A., Jee, W. S., Dixon, S. J., Sims, S. M., and Thompson, D. D. (2003). Deletion of the P2×P2 7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol. Endocrinol. 17, 1356–1367.
Kennedy, C., and Burnstock, G. (1985). Evidence for two types of P2-purinoceptor in longitudinal muscle of the rabbit portal vein. Eur. J. Pharmacol. 111, 49–56.
Knight, G. E., Bodin, P., De Groat, W. C., and Burnstock, G. (2002). ATP is released from guinea pig ureter epithelium on distension. Am. J. Physiol. Renal Physiol. 282, F281–F288.
Komarova, S. V., Ataullakhanov, F. I., and Globus, R. K. (2000). Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am. J. Physiol. Cell Physiol. 279, C1220–C1229.
Korcok, J., Raimundo, L. N., Du, X., Sims, S. M., and Dixon, S. J. (2005). P2Y6 nucleotide receptors activate NF-{kappa}B and increase survival of osteoclasts. J. Biol. Chem. 280, 16909–16915.
Korcok, J., Raimundo, L. N., Ke, H. Z., Sims, S. M., and Dixon, S. J. (2004). Extracellular nucleotides act through P2×P2 7 receptors to activate NF-kappaB in osteoclasts. J. Bone Miner. Res. 19, 642–651.
Lazarowski, E. R., Boucher, R. C., and Harden, T. K. (2003). Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 64, 785–795.
Maier, R., Glatz, A., Mosbacher, J., and Bilbe, G. (1997). Cloning of P2Y6 cDNAs and identification of a pseudogene: comparison of P2Y receptor subtype expression in bone and brain tissues. Biochem. Biophys. Res. Commun. 240, 298–302.
Murgia, M., Pizzo, P., Steinberg, T. H., and Di Virgilio, F. (1992). Characterization of the cytotoxic effect of extracellular ATP in J774 mouse macrophages. Biochem. J. 288(Pt 3), 897–901.
Naemsch, L. N., Weidema, A. F., Sims, S. M., Underhill, T. M., and Dixon, S. J. (1999). P2×P2 4 purinoceptors mediate an ATP-activated, non-selective cation current in rabbit osteoclasts. J. Cell Sci. 112(Pt 23), 4425–4435.
Nakamura, E., Uezono, Y., Narusawa, K., Shibuya, I., Oishi, Y., Tanaka, M., Yanagihara, N., Nakamura, T., and Izumi, F. (2000). ATP activates DNA synthesis by acting on P2X receptors in human osteoblast-like MG-63 cells. Am. J. Physiol. Cell Physiol. 279, C510–C519.
Ohlendorff, S. D., Tofteng, C. L., Jensen, J. E., Petersen, S., Civitelli, R., Fenger, M., Abrahamsen, B., Hermann, A. P., Eiken, P., and Jorgensen, N. R. (2007). Single nucleotide polymorphisms in the P2×P2 7 gene are associated to fracture risk and to effect of estrogen treatment. Pharmacogenet. Genomics 17, 555–567.
Olson, L., Alund, M., and Norberg, K. A. (1976). Fluorescence-microscopical demonstration of a population of gastro-intestinal nerve fibres with a selective affinity for quinacrine. Cell Tissue Res. 171, 407–423.
Orriss, I. R., and Arnett, T. R. (2012). Rodent osteoclast cultures. Methods Mol. Biol. 816, 103–117.
Orriss, I. R., Burnstock, G., and Arnett, T. R. (2010). Purinergic signalling and bone remodelling. Curr. Opin. Pharmacol. 10, 322–330.
Orriss, I. R., Knight, G. E., Ranasinghe, S., Burnstock, G., and Arnett, T. R. (2006). Osteoblast responses to nucleotides increase during differentiation. Bone 39, 300–309.
Orriss, I. R., Knight, G. E., Utting, J. C., Taylor, S. E. B., Burnstock, G., and Arnett, T. R. (2009). Hypoxia stimulates vesicular ATP release from rat osteoblasts. J. Cell. Physiol. 220, 155–162.
Orriss, I. R., Syberg, S., Wang, N., Robaye, B., Gartland, A., Jorgensen, N., Arnett, T. R., and Boeynaems, J. M. (2011a). Bone phenotypes displayed by P2 receptor knockout mice. Front. Biosci. S3, 1038–1046.
Orriss, I. R., Wang, N., Burnstock, G., Arnett, T. R., Gartland, A., Robaye, B., and Boeynaems, J. M. (2011b). The P2Y6 receptor stimulates bone resorption by osteoclasts. Endocrinology 152, 3706–3716.
Orriss, I. R., Taylor, S. E., and Arnett, T. R. (2012). Rat osteoblast cultures. Methods Mol. Biol. 816, 31–41.
Orriss, I. R., Utting, J. C., Brandao-Burch, A., Colston, K., Grubb, B. R., Burnstock, G., and Arnett, T. R. (2007). Extracellular nucleotides block bone mineralization in vitro: evidence for dual inhibitory mechanisms involving both P2Y2 receptors and pyrophosphate. Endocrinology 148, 4208–4216.
Panupinthu, N., Rogers, J. T., Zhao, L., Solano-Flores, L. P., Possmayer, F., Sims, S. M., and Dixon, S. J. (2008). P2×P2 7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J. Cell Biol. 181, 859–871.
Panupinthu, N., Zhao, L., Possmayer, F., Ke, H. Z., Sims, S. M., and Dixon, S. J. (2007). P2×P2 7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. J. Biol. Chem. 282, 3403–3412.
Pellegatti, P., Falzoni, S., Donvito, G., Lemaire, I., and Di Virgilio, F. (2011). P2×P2 7 receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB J. 25, 1264–1274.
Penolazzi, L., Bianchini, E., Lambertini, E., Baraldi, P. G., Romagnoli, R., Piva, R., and Gambari, R. (2005). N-Arylpiperazine modified analogues of the P2×7 receptor KN-62 antagonist are potent inducers of apoptosis of human primary osteoclasts. J. Biomed. Sci. 12, 1013–1020.
Ralevic, V., and Burnstock, G. (1998). Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492.
Romanello, M., Codognotto, A., Bicego, M., Pines, A., Tell, G., and D’Andrea, P. (2005). Autocrine/paracrine stimulation of purinergic receptors in osteoblasts: contribution of vesicular ATP release. Biochem. Biophys. Res. Commun. 331, 1429–1438.
Romanello, M., Pani, B., Bicego, M., and D’Andrea, P. (2001). Mechanically induced ATP release from human osteoblastic cells. Biochem. Biophys. Res. Commun. 289, 1275–1281.
Sprague, R. S., Ellsworth, M. L., Stephenson, A. H., Kleinhenz, M. E., and Lonigro, A. J. (1998). Deformation-induced ATP release from red blood cells requires CFTR activity. Am. J. Physiol. 275, H1726–H1732.
Spray, D. C., Ye, Z. C., and Ransom, B. R. (2006). Functional connexin “hemichannels”: a critical appraisal. Glia 54, 758–773.
Suadicani, S. O., Brosnan, C. F., and Scemes, E. (2006). P2×P2 7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 26, 1378–1385.
Sugita, M., Yue, Y., and Foskett, J. K. (1998). CFTR Cl- channel and CFTR-associated ATP channel: distinct pores regulated by common gates. EMBO J. 17, 898–908.
Thompson, W. R., Majid, A. S., Czymmek, K. J., Ruff, A. L., Garcia, J., Duncan, R. L., and Farach-Carson, M. C. (2011). Association of the alpha(2)delta(1) subunit with Ca(v)3.2 enhances membrane expression and regulates mechanically induced ATP release in MLO-Y4 osteocytes. J. Bone Miner. Res. 26, 2125–2139.
Watt, W. C., Lazarowski, E. R., and Boucher, R. C. (1998). Cystic fibrosis transmembrane regulator-independent release of ATP. Its implications for the regulation of P2Y2 receptors in airway epithelia. J. Biol. Chem. 273, 14053–14058.
Keywords: osteoclast, osteoblast, ATP release, P2X7 receptor
Citation: Brandao-Burch A, Key ML, Patel JJ, Arnett TR and Orriss IR (2012) The P2X7 receptor is an important regulator of extracellular ATP levels. Front. Endocrin. 3:41. doi: 10.3389/fendo.2012.00041
Received: 19 October 2011; Accepted: 29 February 2012;
Published online: 19 March 2012.
Edited by:
Alison Gartland, The University of Sheffield, UKReviewed by:
Pablo Pelegrin, Hospital Universitario Virgen Arrixaca, SpainElena Adinolfi, University of Ferrara, Italy
Helen Knowles, University of Oxford, UK
Copyright: © 2012 Brandao-Burch, Key, Patel, Arnett and Orriss. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Isabel R. Orriss, Department of Cell and Developmental Biology, University College London, London WC1E 6BT, UK. e-mail: i.orriss@ucl.ac.uk
 Andrea Brandao-Burch
Andrea Brandao-Burch