- 1Department of Neuroscience, Carleton University, Ottawa, ON, Canada
- 2Department of Chemistry, Syracuse University, Syracuse, NY, USA
- 3Department of Internal Medicine, Division of Hypothalamic Research, The University of Texas Southwestern Medical Center, Dallas, TX, USA
- 4Department of Internal Medicine, Division of Endocrinology and Metabolism, The University of Texas Southwestern Medical Center, Dallas, TX, USA
- 5Department of Psychiatry, The University of Texas Southwestern Medical Center, Dallas, TX, USA
Ghrelin is a 28 amino acid hormonal peptide that is intimately related to the regulation of food intake and body weight. Once secreted, ghrelin binds to the growth hormone secretagogue receptor-1a, the only known receptor for ghrelin and is capable of activating a number of signaling cascades, ultimately resulting in an increase in food intake and adiposity. Because ghrelin has been linked to overeating and the development of obesity, a number of pharmacological interventions have been generated in order to interfere with either the activation of ghrelin or interrupting ghrelin signaling as a means to reducing appetite and decrease weight gain. Here, we present a novel peptide, CF801, capable of reducing circulating acylated ghrelin levels and subsequent body weight gain and adiposity. To this end, we show that IP administration of CF801 is sufficient to reduce circulating plasma acylated ghrelin levels. Acutely, intraperitoneal injections of CF801 resulted in decreased rebound feeding after an overnight fast. When delivered chronically, they decreased weight gain and adiposity without affecting caloric intake. CF801, however, did cause a change in diet preference, decreasing preference for a high-fat diet and increasing preference for regular chow diet. Given the complexity of ghrelin receptor function, we propose that CF801, along with other compounds that regulate ghrelin secretion, may prove to be a beneficial tool in the study of the ghrelin system, and potential targets for ghrelin-based obesity treatments without altering the function of ghrelin receptors.
Introduction
One potential target for the control of body weight and appetite is the gut–brain peptide ghrelin. Ghrelin, a 28-amino-acid peptide produced in the X/A-like cells of the gastric oxyntic mucosa lining the stomach, is a key regulator of both short- and long-term energy homeostasis (1–3). Circulating ghrelin levels rise in anticipation of a meal and subside once the organism is satiated (4–7). Furthermore, ghrelin has been shown to regulate several physiological processes, including glucose metabolism (8–12), insulin secretion (8, 13, 14), gastric emptying (15), cell proliferation (16, 17), memory (18), stress (19–22), anxiety (18, 19, 22–25), and reward (26–29). Acutely, ghrelin promotes food intake through interactions with a subset of distinct first order hypothalamic nuclei (2, 3, 30–34) and injections of ghrelin result in vigorous feeding bouts (35–37). Ghrelin contributes to long-term energy homeostasis by increasing body weight and adiposity, presumably through a reduction of lipid oxidation (3, 21, 38, 39). Ghrelin increases motivation to obtain food (27–29, 40) and also increases the preference for highly palatable foods by acting in the VTA (28, 29, 41, 42).
The ability of ghrelin to carry out its physiological processes relies on its affinity for the growth hormone secretagogue receptor-1a (GHSR-1a), the only known ghrelin receptor. GHSR-1a is widely expressed in both rodents and humans, with highest expression found in the hypothalamus and pituitary gland, both areas heavily invested in energy homeostasis and growth hormone secretion (43). Within the hypothalamus, GHSR-1a expression is most concentrated to the arcuate nucleus (43–45), a region important in the regulation of food intake, metabolism and energy homeostasis (3, 46, 47). Interestingly, activation of GHSR-1a requires a post-translational modification of the proghrelin peptide (1), a characteristic unique to ghrelin amongst all other metabolically active hormones. Specifically, ghrelin must be acylated on the hydroxyl group of the third serine (Ser3) residue with an n-octanoic acid, or other medium-chain fatty acid (MCFA) containing 6–12 carbons, in order to bind to and activate GHSR-1a (1). Modification with an n-octanoyl group induces a conformational flexibility that accommodates the geometric specificity of GHSR-1a’s binding pocket (48).
The enzyme responsible for the acylation of the mature ghrelin peptide is called ghrelin-O-acyltransferase, or GOAT (49, 50), which belongs to a superfamily of enzymes known as the membrane-bound-O-acyltransferases (MBOATs) (51). GOAT expression has been demonstrated in many rat, mouse, and human tissues, including the ghrelin producing X/A-like cells of the gastric oxyntic mucosa lining the stomach (52–54). To date, GOAT is the only known enzyme capable of acylating, and therefore activating ghrelin, as evidenced by structure–activity analyses of ghrelin mimetic substrates and inhibitors as well as by studies demonstrating the complete absence of acylated ghrelin in GOAT null mice (49, 55). As such, GOAT has become a target for the development of drugs to curb appetite and reduce weight gain via a reduction in acylated ghrelin concentrations (55–59). Indeed, a number of compounds that reduce GOAT activity are successful in decreasing weight gain and adiposity. For instance, daily treatment with GO-CoA-TAT, a peptide GOAT inhibitor, was effective in reducing weight gain and adiposity in mice while decreasing acylated ghrelin concentrations (60).
In this paper, we present CF801, a synthetic peptide that was thought to be a novel GOAT inhibitor, and one that decreases plasma concentrations of acylated ghrelin in vitro and in vivo. While we demonstrate that CF801 is not an efficient inhibitor of GOAT octanoylation activity, CF801 produced a number of important metabolic effects, including a decrease in weight gain and adiposity associated with changes in appetite and energy expenditure associated with decreased ghrelin concentrations.
Materials and Methods
Design of CF801
An approach was undertaken to design a novel GOAT inhibitor, resulting in a compound we named CF801. The first five amino acids of CF801 derive from studies carried out in 2008 by Yang et al., who examined the inhibitory effect of certain pentapeptides (e.g., GSAFL-NH2) on GOAT using a microsomal assay (56). While these peptides showed inhibitory activity on GOAT, it was unlikely that the peptide could effectively cross membranes to reach the endoplasmic reticulum lumen, where GOAT performs its enzymatic reactions (50). In synthesizing CF801, we extended and modified the original GSAFL-NH2 sequence with the addition of five more amino acids that correspond to amino acids 6–10 of the full-length ghrelin peptide. The addition of these amino acids increases the similarity of CF801 to the non-acylated ghrelin peptide sequence as well as provides an appropriate spacer for attachment of additional modifications. The second modification from the GSAFL-NH2 peptide was the addition of an HIV trans-activator of transcription (Tat) sequence. CF801 contains the following Tat sequence: RKKRRQRRR, with an amide group retained on the C-terminus.
The full sequence of CF801 is as follows: GSAFLSPEHQRK KRRQRRR-NH2, where the N-terminus has the standard free amine group (–NH2). Variants of this molecule can be synthesized through the inclusion of (or perhaps exclusion of) additional amino acids from the mature ghrelin protein molecule and/or using variants of the Tat sequence or other cell-penetrating peptides. CF801 was custom synthesized by Peptides International (Louisville, KY, USA).
Cell-Based Ghrelin Secretion Assay
Ghrelin secretion studies were performed in stomach-derived ghrelinoma (SG-1) cells, cultured as described previously (61, 62). Cells were plated at a density of 5 × 104 cells/mL/well on to 24-well plates pre-coated with poly-d-lysine (day 0). On day 2, the cells were treated with the indicated concentrations of test compound CF801, 24 h ahead of the test period and with 50 μM sodium octanoate–bovine serum albumin (BSA), 16 h ahead of the test period. On day 3, the medium was aspirated and the cells were treated with the same concentrations of CF801 in 500 μL serum-free DMEM (Life Technologies, Grand Island, NY, USA) supplemented with 5 mM glucose and 50 μM sodium octanoate–BSA. After a 6-h incubation, the medium was collected, placed on ice, and immediately centrifuged at 800 × g for 5 min. Hydrochloric acid was added to the supernatant to achieve a final concentration of 0.1 N (for stabilization of acyl ghrelin) and stored at −80°C until analysis. Assay for acyl ghrelin and total were performed using commercial ELISA kits (EMD Millipore Corporation, Billerica, MA, USA) according to the manufacturer’s instructions and read in a PowerWave XS Microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
Fluorescence-Based GOAT Activity Assay
The effects of CF801 on GOAT activity were examined using a previously validated fluorescence-based assay, and in comparison with octanoyl-(Dap3)-ghrelin (1–5)–NH2, a well characterized GOAT inhibitor (56, 63). In this assay, membrane fractions from Sf9 insect cells were transfected to express either human (hGOAT) or mouse GOAT (mGOAT). Membranes fractions were thawed on ice and passed through an 18-gage needle 10 times enriched using a previously described method (63). Assays were performed with 20–30 μg membrane protein (for hGOAT) and 60–80 μg membrane protein (for mGOAT), 1.5 μM GSSFLCAcDan peptide substrate, 500 μM octanoyl-CoA, 0–10 μM CF801, octanoyl-(Dap3)-ghrelin (1–5)–NH2, or carrier control (50 mM HEPES pH = 7.0 or DMSO) and 50 mM HEPES pH 7.0 in a total volume of 50 μL. All components with the exception of the acrylodan-labeled peptide substrate were incubated in the reaction vessel for 30 min prior to assay initiation. Assays were initiated by addition of the acrylodanylated peptide substrate GSSFLCAcDan. Assays were then incubated at room temperature for 1 h and stopped by addition of 50 μL of 20% acetic acid in isopropanol. Assays were analyzed by reverse phase HPLC with fluorescence detection. Chromatogram analysis and integration of peptide substrate and product peaks were performed using Chemstation for LC (Agilent Technologies). The trials were run in triplicate, with the % activity calculated as the integrated fluorescence intensity of the octanoylated product in the CF801 or octanoyl-(Dap3)-ghrelin (1–5)–NH2 run normalized by the same quantity for the vehicle-only reaction.
In Vivo Animal Studies
Animals
In general, male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) weighing 20–25 g were used as experimental subjects. Throughout the duration of the studies, mice were housed under standard laboratory conditions and received ad libitum access to standard laboratory mouse chow and tap water. Lights in the facility were set to go on and off at 12 h intervals with the lights going on at 7:00 a.m. In some experiments, mice also had ad libitum access to a high-fat diet containing 60% caloric content from fat (TD 06414; Harlan Teklad, Indianapolis, IN, USA) in addition to regular chow to measure dietary preferences. All procedures were approved by the Carleton University Animal Care Committee and followed the guidelines of the Canadian Council on Animal Care.
Effects of CF801 on Plasma Ghrelin Concentrations and Rebound Food Intake in Fasted Mice
Mice (n = 16) were singly housed and given free access to water and regular chow for a 4-day baseline period. After this, mice were assigned to one of three groups: vehicle (n = 5), CF801 low dose (n = 5; 11 μmol⋅kg−1 – LD), or CF801 high dose (n = 6; 22 μmol⋅kg−1 – HD). These mice were injected intraperitoneally once a day for 4 days starting at 10:00 a.m. Following 4 days of treatment, animals were tested for rebound feeding after a fast. To do this, we removed the food from each cage 1 h before lights out (6:00 p.m.), and food was returned to the cage the following day 1 h after the last injection. Food intake was then monitored every hour for 4 h to determine the effects of CF801 on rebound feeding. Animals were then sacrificed by rapid decapitation to collect trunk blood and other tissues. Two samples were lost during sample collection, so the number of samples used to measure acylated ghrelin was reduced to n = 14.
Tissue and Blood Analysis
Following the treatment or recovery periods, mice were killed by rapid decapitation and plasma and tissue samples were collected. To measure glucose levels, glucose strips attached to a Contour glucose meter (Bayer Corp., Pittsburgh, PA, USA) were dipped in trunk blood collected before being centrifuged. The remaining trunk blood was collected in EDTA-coated tubes placed on ice and centrifuged at 800 × g for 15 min to separate plasma from red blood cells. Blood plasma was aliquoted separately to avoid multiple freeze/thaw cycles and stored at −80°C until processed. To protect the acylated ghrelin molecule, a 50 μL aliquot of blood plasma was treated with 2.7 μL of 1.0 N hydrochloric acid and 10 μL of 100 mM 4-(hydroxymercuric)benzoic acid prior to storage. Plasma acylated ghrelin for all animals was measured using an ELISA kit (Millipore). All samples analyzed had a coefficient of variation <10%.
Effects of CF801 on Food Intake, Weight Gain, and Adiposity
A second cohort of mice (n = 30) was acclimated to the laboratory conditions for 1 week prior to the onset of the experiment. Mice were housed individually and their body weight and food intake was recorded daily for a baseline period of 10 days. Given that ghrelin augments the intake of preferred diets (28), all mice received ad libitum access to a high-fat diet (60% of calories coming from fat), in addition to regular chow, to determine if CF801 altered dietary preference. Both chow and high-fat diet pellets were provided daily to mice ad libitum in pre-weighed amounts. Mice were then assigned to four different groups: non-injected control (NIC; n = 7), vehicle (0.9% saline) (VEH; n = 7), low-dose CF801 (11 μmol⋅kg−1 – LD; n = 8) and high-dose CF801 (22 μmol⋅kg−1 – HD; n = 8). Each day at 09:00 hours, mice were weighed and their food intake (both chow and high-fat diet) was recorded. After this, mice received an intraperitoneal injection of either CF801 or saline (except for the non-injected controls). Following the 13-day treatment period, half the animals in each group were sacrificed, while the remaining mice were allowed to recover from treatment. During the 8-day recovery period, mice were granted ad libitum access to standard laboratory chow, high-fat diet, and water, but did not receive any injections. At this point, the remaining mice were sacrificed. Carcasses from all animals were frozen and stored at −80°C until they were scanned for body composition analyses. Immediately prior to analysis, all carcasses were thawed to room temperature and weighed in order to obtain measures relative to total carcass mass. EchoMRI carcass analyses were performed at Health Canada, Nutrition Research Division facilities using the EchoMRI-1100 (System ID EF-020) scanner. Each carcass was analyzed separately and measurements of total fat mass, total lean mass, and total water weight were obtained for each mouse.
Effects of CF801 on Energy Expenditure
Mice (n = 10) were housed individually and their body weight and food intake was recorded daily for a baseline period of 10 days. As in Experiment 1, all mice had ad libitum access to regular lab chow and a high-fat diet (60% of calories coming from fat). At the end of the baseline, mice were housed in metabolic chambers (TSE Systems) for 48 h to examine metabolic rate using indirect calorimetry prior to the onset of the drug treatment. While indirect calorimetry measures were obtained throughout the 48-h period, we only analyzed measurements in the last 24 h to allow the animals to adjust to the chambers. Mice were then assigned to one of two groups: vehicle (0.9% saline) (VEH; n = 5) and CF801 (22 μmol⋅kg−1; n = 5). Each day at 09:00 hours, mice were weighed and their food intake (both chow and high-fat diet) was recorded, and then received an intraperitoneal injection of either CF801 or saline. Drug treatment continued for 16 days. On the eighth day, mice were again placed in the metabolic chambers for 48 h while still being monitored and injected with their respective treatment to determine differences in metabolism produced by the drug.
Statistical Analyses
In most experiments, group differences were analyzed using independent samples t-tests or one-way ANOVAs followed by Fisher LSD post hoc tests when significance was reported. Food intake and weight gain data over time were analyzed using repeated measures ANOVAs followed by post hoc one-way ANOVAs and Fisher LSD.
Results
CF801 Inhibits Acyl Ghrelin Secretion In Vitro and In Vivo
SG-1 cells are derived from stomach ghrelinomas induced by expression of the SV40 large T-antigen under the preproghrelin promoter. These cells retain many of the features of ghrelin cells within primary cultures of gastric mucosal cells from mice (61). They also show elevated expression of both preproghrelin and GOAT mRNA with high levels of acylated ghrelin synthesis and secretion (61). In order to test CF801’s ability to reduce acyl ghrelin secretion, we performed an in vitro cell-based assay using an SG-1 cell line as previously described (61, 62). No significant effects were found after a 6-h incubation period with CF801 (p > 0.05). However, following a 24-h incubation, CF801 inhibited acyl ghrelin secretion from SG-1 cells with an IC50 of 347 ± 40 μM, based on log-dose versus response non-linear fit. Significant decreases in secretion were found at concentrations of 100 μM (p < 0.05) and 300 μM (p < 0.05) CF801 (Figure 1).
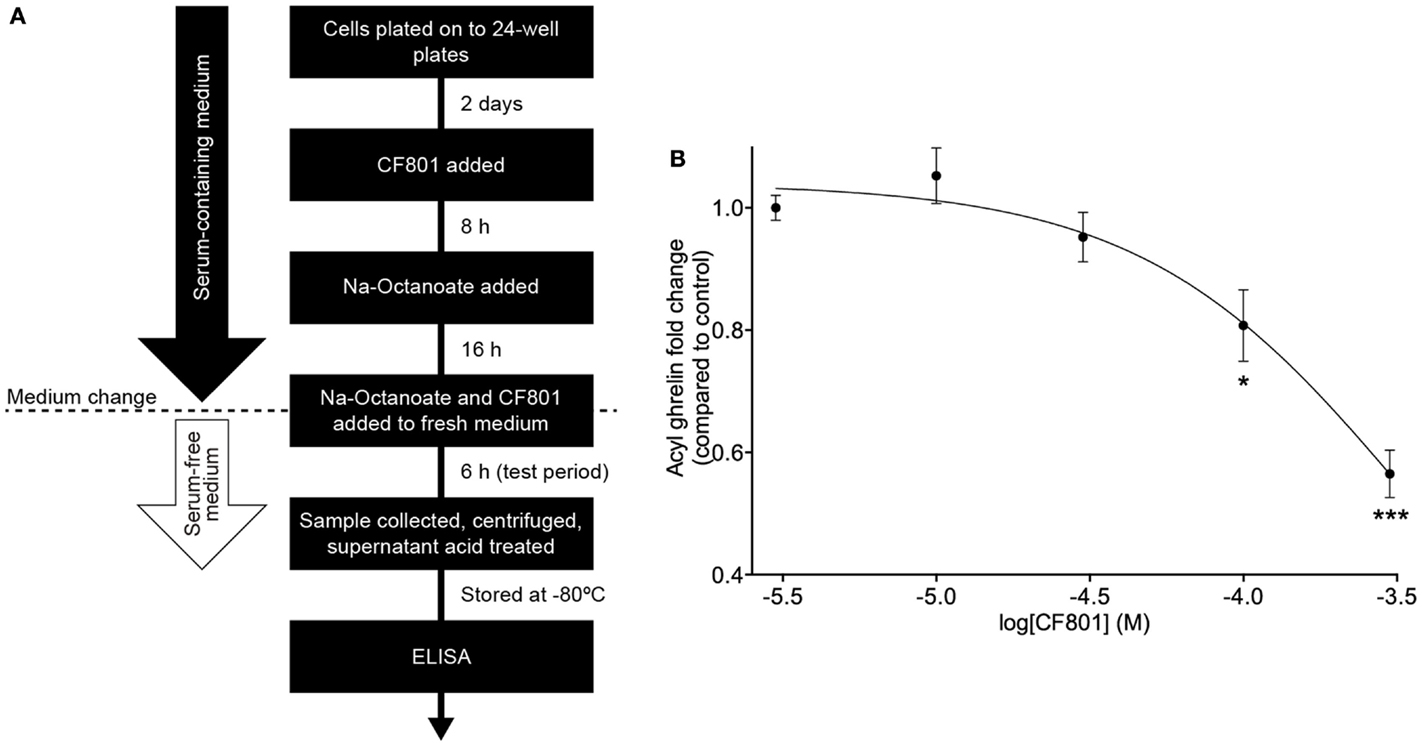
Figure 1. CF801 inhibits acyl ghrelin secretion from SG-1 cells following 24 h of incubation. (A) depicts the in vitro assay design. (B) shows Acyl ghrelin concentration in the culture medium collected during a 6 h test period following 24 h incubation at the indicated concentrations of CF801. All values are expressed as mean ± SEM. *p < 0.05, ***p < 0.001 significant difference in acyl ghrelin concentrations with CF801 treatment when compared to untreated control (n = 6 wells).
To determine if CF801 also altered acylated ghrelin concentrations, we conducted a study where mice were injected daily with saline or CF801 at one of two doses for 4 days. No effects of CF801 on food intake or weight gain were observed during the first 3 days of treatment (data not shown, p > 0.05). Before the last day of treatment, mice were fasted overnight, treated with their last injection, given access to food for 4 h and then sacrificed. Figure 2A shows plasma acylated ghrelin concentrations in mice treated with i.p. injections of saline, LD, or HD of CF801. As shown in the figure, CF801 caused a dose-dependent decrease in plasma ghrelin concentrations that was statistically significant [F(2,24) = 2.90 p < 0.05, partial eta2 = 0.22] in mice treated with the high dose (p < 0.05 Fisher LSD). This decrease in acylated ghrelin was also associated with a lower amount of food consumed by mice treated with the high dose of CF801 during the first hour of refeeding [see Figure 2B; F(2,24) = 2.394, p < 0.05, partial eta2 = 0.22, p < 0.05]. This was in correlation with acyl ghrelin concentrations (r = 0.492, p < 0.05). No significant effects were seen at any other time point examined (p > 0.05). Nevertheless, there was a significant overall dose-dependent treatment effect on the total amount of chow consumed during the 4-h period of food access after the fast [F(2,24) = 4.527, partial eta2 = 0.411; p > 0.05]. This effect was statistically significant when examining differences in total chow consumed between the HD and saline treated groups (p < 0.01, Fisher LSD). No significant differences were observed between the groups in blood glucose concentrations [F(1,15) = 1.105, p > 0.05; see Figure 2C].
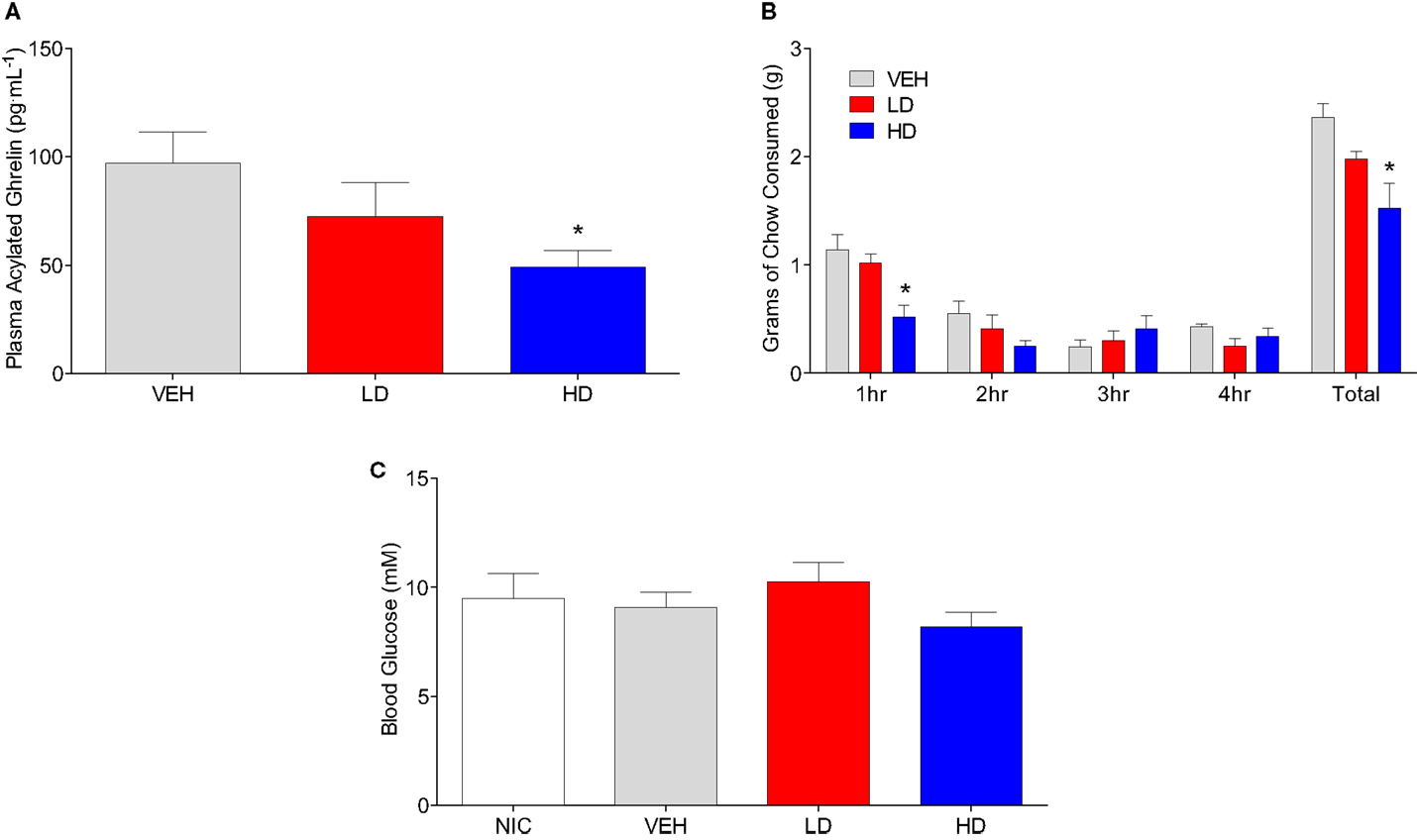
Figure 2. Animals given HD CF801 displayed reduced levels of plasma acylated ghrelin following a 24 h fast compared to vehicle-treated animals (A). Average grams of regular laboratory chow consumed following a 24-h fast upon refeeding (B), and blood glucose measured from trunk blood (C). All values are expressed as mean ± SEM. *p < 0.05 relative to VEH-injected controls.
CF801 Does Not Serve as an Effective Inhibitor of Ghrelin Octanoylation by GOAT
To test the potential that CF801’s ability to reduce acyl ghrelin secretion in SG-1 cells is due to direct inhibition of GOAT-catalyzed ghrelin octanoylation, we incubated CF801 with both the human and mouse isoforms of the GOAT enzyme under assay conditions previously described (63), with the potent GOAT inhibitor octanoyl-(Dap3)-ghrelin (1–5)–NH2 (56) examined in parallel as a positive control for GOAT inhibition. In this assay, we tested concentrations comparable with those reported for GO-CoA-Tat, which was reported to have an IC50 for GOAT of <1 μM (60). At concentrations up to 10 μM, no inhibition was seen with CF801 incubation [see Figure 3; hGOAT: F(3,8) = 1.251, p > 0.05; mGOAT: F(3,8) = 0.139, p > 0.05], while robust inhibition was seen with the control [IC50 of 20 ± 2 nM and 80 ± 10 nM with hGOAT and mGOAT, respectively; hGOAT: F(3,8) = 485.023, p < 0.05, all doses less than vehicle with p’s < 0.05 by Fisher’s LSD post hoc; mGOAT: F(3,8) = 51.476, p < 0.05, all doses less than vehicle with p’s < 0.05 by Fisher’s LSD post hoc]. This suggests that the observed effects of CF801 in cell- and animal-based studies are not caused by direct inhibition of ghrelin octanoylation by GOAT.
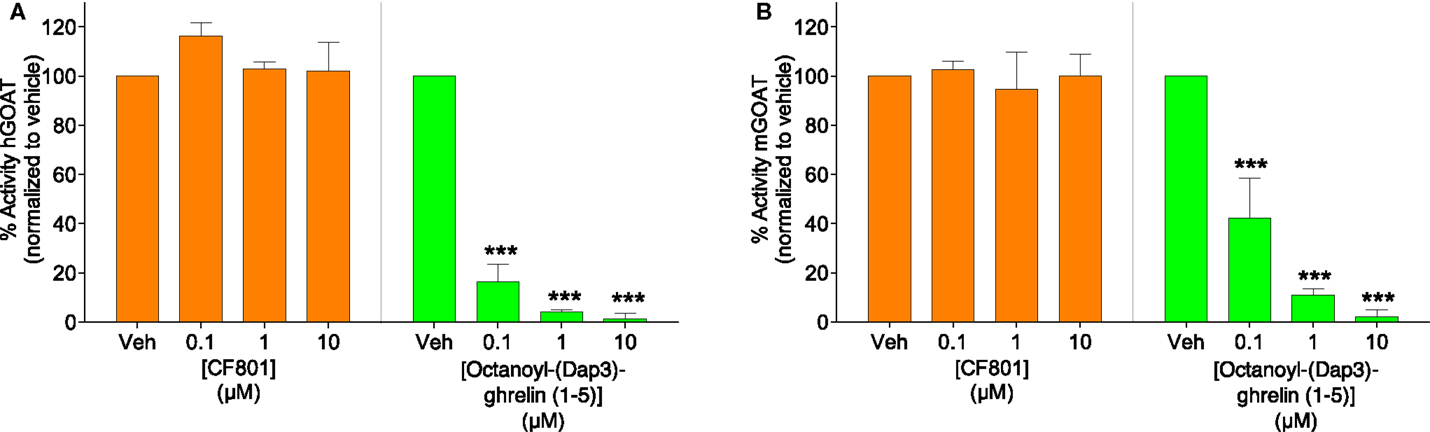
Figure 3. Percent activity of human GOAT (A) and mouse GOAT (B) (obtained from membrane fractions from Sf9 cells expressing hGOAT and mGOAT, resepectively) when exposed to CF801 and octanoyl-(Dap3)-ghrelin (1–5)–NH2 using a fluorescence-based microsomal assay. GSSFLCAcDan was used as an acylation target with fluorescence measured by reverse phase HPLC with fluorescence detection. CF801 had no effect on either hGOAT or mGOAT acylation activity, while octanoyl-(Dap3)-ghrelin (1–5)–NH2 dose-dependently reduced GOAT activity. All values are expressed as mean ± SEM. ***p < 0.001 compared to vehicle control.
CF801 Treatment Reduces High-Fat Diet Intake and Body Weight Gain without Affecting Caloric Intake
Given that CF801 decreased ghrelin concentrations and rebound feeding, we decided to examine if CF801 affected weight gain and food intake in mice that had access to a high-fat diet in addition to regular chow. As illustrated in Figure 4, there were no differences between any groups in the amount of calories consumed during the baseline period [F(1,29) = 0.586, p > 0.05], treatment period [F(1,29) = 0.783, p > 0.05], and recovery period [F(1,29) = 2.093, p > 0.05]. It was evident, however, that mice preferred the high-fat diet during the baseline period as they consumed a higher proportion of calories from this diet than the calories consumed from the regular chow diet (p < 0.05; see Figure 5). This diet preference changed during the treatment period. In particular, animals receiving high dose of CF801 consumed significantly more regular chow than animals in every other group as revealed by a one-way ANOVA followed by Fisher LSD post hoc [see Figures 5A,B; F(3,28) = 5.305, p < 0.05, partial eta2 = 0.41]. In contrast, CF801 caused an overall significant dose-dependent decrease in the intake of the high-fat diet [Figures 5C,D; F(3,29) = 6.788, p < 0.05, partial eta2 = 0.45]. There were no differences in high-fat diet consumption between NIC animals and VEH-treated animals. Animals receiving LD CF801 treatment consumed significantly less high-fat diet compared to both NIC and VEH groups (p’s < 0.05). Animals receiving HD CF801 consumed significantly less high-fat diet compared to both NIC and VEH groups (p’s < 0.05) and tended to consume less than animals receiving LD CF801 (p = 0.073). Interestingly, in correlation with decreased intake of the high calorie diet, mice treated with the high dose of CF801 showed a decrease in weight gain compared to mice treated with saline or mice that were not injected at all as determined by a one way ANOVA followed by Fisher LSD post hoc tests [Figures 5E,F; F(3,29) = 3.098, p < 0.05, partial eta2 = 0.271]. Mice given the low dose of CF801 tended to have lower weight gain than control NIC (p = 0.092) and VEH-treated mice (p = 0.065), but this difference did not attain statistical significance. Nevertheless, post hoc tests also revealed that there were no differences in weight gain between mice treated with the low dose versus mice treated with the high dose of CF801 (p > 0.05).
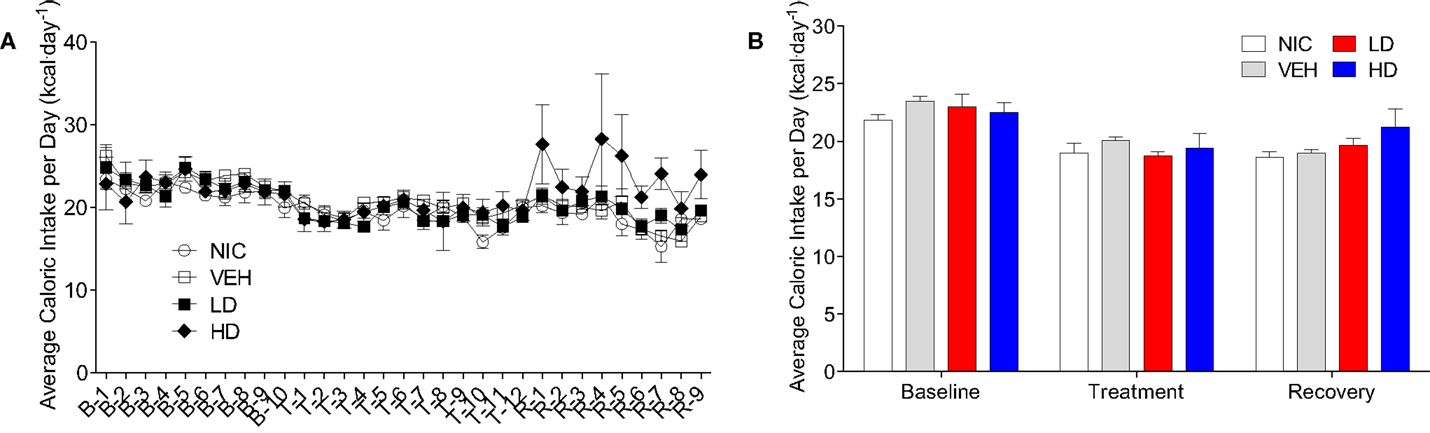
Figure 4. Average daily (A) and overall average (B) caloric intake during the baseline, treatment, and recovery period. All values are expressed as the mean ± SEM.
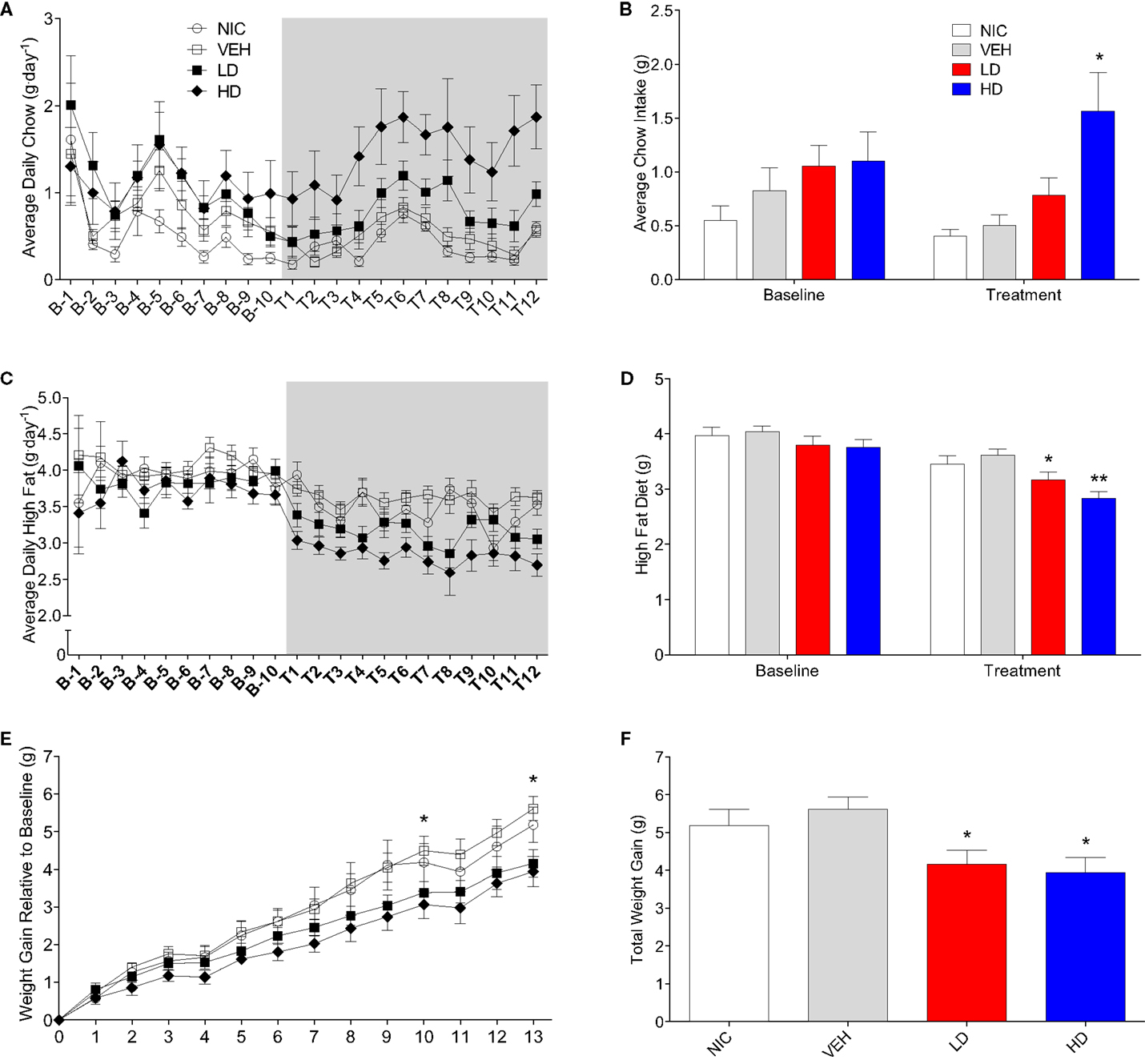
Figure 5. Average daily (A) and overall average (B) chow consumption during baseline and treatment periods. Average daily (C) and overall average (D) high-fat diet consumption during baseline and treatment periods. Weight gain since end of baseline (E) and total weight gain at the end of 13-day treatment period (F). All values are expressed as means ± SEM. *p < 0.05, **p < 0.01 compared to VEH-treated controls, (E): *p < 0.05 for HD and LD versus VEH.
CF801 Increases the Utilization of Fat as Fuel
Given that treatment with CF801 decreased weight gain without altering caloric intake, we reasoned that perhaps CF801 was exerting its effects at the metabolic level. To examine this, we examined differences in the respiratory exchange ratio (RER) of mice treated with the high dose of CF801 (n = 5) with that of saline-treated mice (n = 5) after a week of daily injections. While both groups of mice showed nearly identical RER during baseline period (p > 0.05), mice treated with CF801 displayed a significant decrease in RER during the 4-h period that followed the injection of the drug, an effect not seen in vehicle-treated mice [t(5) = 3.457, p < 0.05] (see Figure 6A).
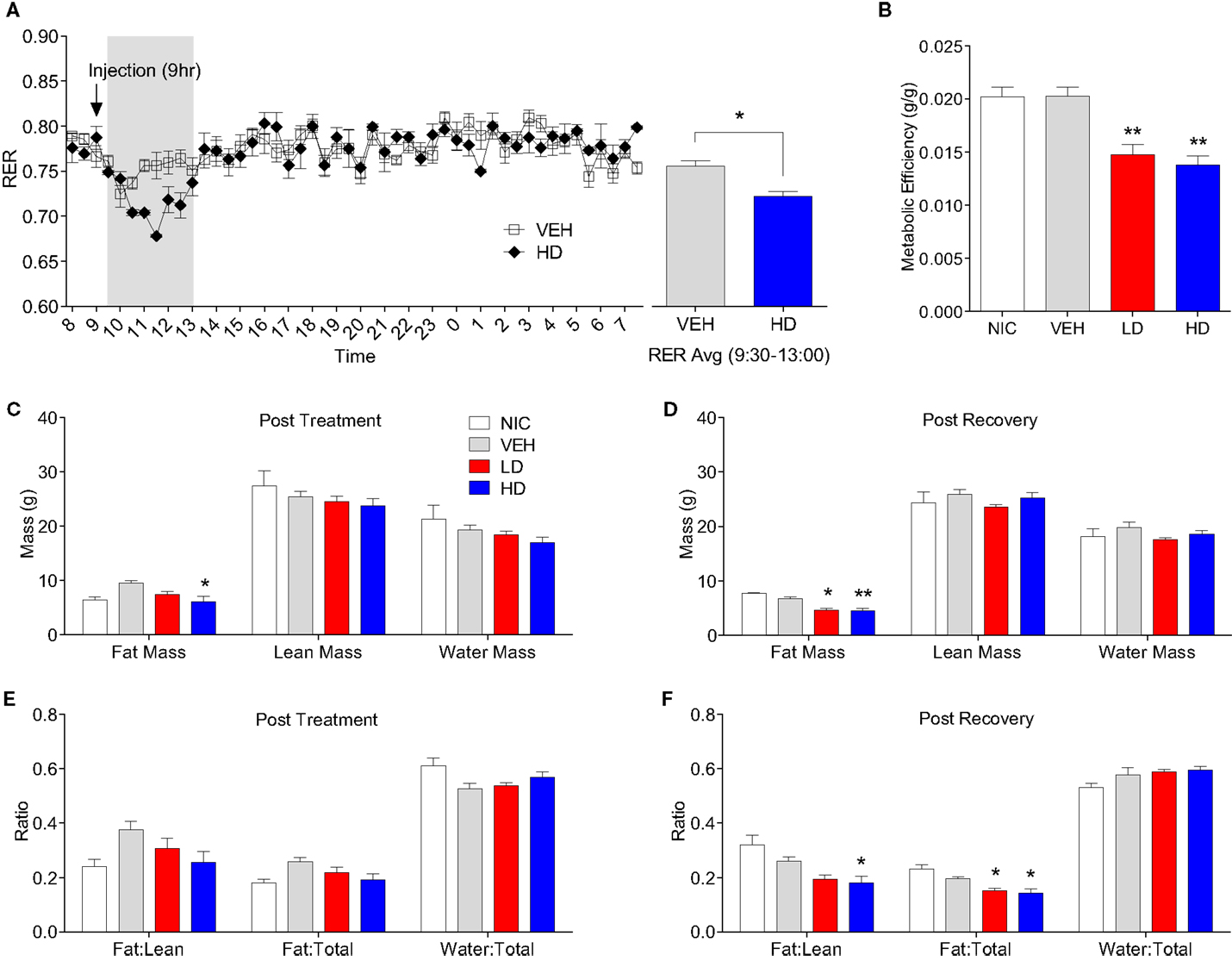
Figure 6. Twenty-four hour respiratory exchange ratio following 8 days of vehicle and CF801 (high dose) treatment [(A), left] and average RER during the 4-h period following injection at 09:00 hours [(A), right]. Metabolic efficiency (B); fat mass, lean mass, and water mass following treatment period (C) and recovery period (D); fat mass:lean mass, fat mass:total mass, and water mass:total mass following treatment period (E) and recovery period (F). All values are expressed as mean ± SEM. *p < 0.05, **p < 0.01 compared to VEH-treated animals.
CF801 Treatment Alters Body Composition
Metabolic efficiency, defined as the amount of weight gained per calorie of energy consumed during a given time period, was calculated for each mouse during the baseline and treatment period. There were no differences in metabolic efficiency between any groups during the baseline period (data not shown). As illustrated in Figure 6B, there was a significant treatment effect on metabolic efficiency throughout the treatment period [F(1,29) = 9.086, p < 0.05, partial eta2 = 0.522]. Post hoc analyses revealed that animals receiving LD CF801 had significantly lower metabolic efficiencies during the treatment period compared to both NIC (p < 0.05) and VEH treated animals (p < 0.05). Similarly, animals receiving HD CF801 showed significantly lower metabolic efficiencies during the treatment period compared with both NIC (p < 0.05) and VEH-treated animals (p < 0.05), but were no different than animals receiving LD CF801 (p > 0.05).
Given these changes in metabolic efficiency as well as the decrease in RER observed in the calorimetry experiment, we hypothesized that CF801 treatment would result in less accumulation of fat. To determine if this was the case, we conducted EchoMRI analyses on every animal post-mortem to identify differences in body composition as a function between groups. As shown in Figures 6C,D, the amount of total fat mass was significantly lower in mice treated with the high dose of CF801, compared to VEH injected animals right after the treatment period [F(1,14) = 3.54, p > 0.05; partial eta2 = 0.515] and this decrease was more evident 2 weeks after the treatment period was terminated [F(1,15) = 15.18, p < 0.05 partial eta2 = 0.805]. Mice receiving LD CF801 treatment and sacrificed at the end of the treatment period had lower fat mass compared to VEH-treated animals, but this was not statistically significant (p. > 0.05), but animals from the same group sacrificed 2 weeks after the last treatment had lower fat mass than mice in the VEH and NIC sacrificed at the same time point (p < 0.05). There were no differences between the groups in lean body mass or water mass (p > 0.05). Interestingly, there was a significant effect of treatment on fat mass:lean mass ratio following the recovery period [see Figures 6E,F; F(1,15) = 7.735, p < 0.05; partial eta2 = 0.678]. An LSD post hoc analysis revealed that animals receiving LD CF801 treatment tended to show a reduction in fat mass:lean mass ratio following the recovery period compared to VEH-treated animals (p = 0.058) and had a significantly lower fat mass:lean mass ratio compared to NIC animals (p < 0.05). Similarly, animals receiving HD CF801 showed a significantly lower fat mass:lean mass ratio compared to both NIC animals (p < 0.05) and VEH-treated animals (p < 0.05), but were no different than animals treated with LD CF801 (p > 0.05). Furthermore, there was a significant treatment effect on the fat mass:total mass ratio following the recovery period [F(1,15) = 9.245, p < 0.05; partial eta2 = 0.716]. LSD post hoc analysis revealed that both LD and HD CF801-treated animals had significantly lower fat mass:total mass ratios compared to NIC animals (p’s < 0.05) and VEH-treated animals (p’s < 0.05).
Discussion
Given that ghrelin is one of the few peripherally circulating hormones capable of stimulating food intake, ghrelin has become a target for the pharmacological control of appetite and weight gain. Many attempts have been made at interrupting the ghrelin signaling system through the inhibition of ghrelin secretion (64), neutralizing the mature ghrelin protein via vaccination (65) or spiegelmer antagonism (66–68), and/or interrupting ghrelin signaling at the level of GHSR-1a (19, 21, 29, 41, 69). An emerging approach is the development of compounds that target GOAT as a potential therapeutic agent for combating weight gain and adiposity (56, 57, 59, 60). There are several advantages of targeting GOAT for the treatment of obesity, such as the selectivity to the targeted acylated ghrelin molecule, the uninterrupted constitutive activity of GHSR-1a (presumably necessary for appropriate growth hormone secretion) and the up regulation of unacylated ghrelin (UAG). Finally, it has been suggested that UAG may itself be a viable treatment option through counteracting the activity of acylated ghrelin and improving insulin sensitivity (70, 71).
In this paper, we show that CF801, a modified decapeptide similar in structure to the ghrelin peptide, reduces weight gain and adiposity without affecting total caloric intake. This is reminiscent of studies reporting that mice with targeted mutations to the gene encoding the message for the GOAT enzyme do not gain as much weight and store less adipose tissue when fed a high-fat diet compared to WT mice (46). Similarly, GHSR-null mice that do not respond to the acylated ghrelin product of GOAT enzymatic activity have reduced respiratory quotient and reduced metabolic efficiency, consume less food, and put on less weight, particularly in the form of adipose tissue, when given a high-fat diet (72). These findings are also similar to those from studies where mice were treated with GO-CoA-Tat, a previously characterized inhibitor of GOAT activity (60). Unlike GO-CoA-Tat, the mechanism underlying these effects does not appear to involve direct inhibition of GOAT octanoylation activity although it does involve a reduction in ghrelin secretion. Furthermore, CF801 was able to blunt the usual rebound eating response to a 24-h fast, reminiscent to what had been shown previously for a known GHSR antagonist (29).
One way in which CF801 could be decreasing weight gain and adiposity is through a dietary change. Indeed, while CF801 did not alter total caloric intake, it did affect the proportion of calories mice consumed from a high-fat diet and those consumed from laboratory chow diet. Looking at the baseline period, one can observe that mice generally preferred to consume the high-fat diet, a trend that continued throughout the study in control animals. CF801 treated mice, however, reduced their intake of the high-fat diet, and increased their intake of chow diet. It is unclear if this change in preference is due to an effect of the drug on preference or palatability, but it is certainly not due to a change in overall appetite as these mice ate enough to maintain their caloric intake throughout the study. It is important to note that at the end of the treatment period, there was a small spike in caloric intake in CF801-treated animals, suggesting that appetite increases once CF801 injections stop. This may indicate that CF801 is associated with enhancing signals that promote satiety, or simply by reducing ghrelin tone, but this needs to be studied further. The latter explanation is supported by data from our first study, where we show that mice repeatedly treated with CF801 decrease rebound food intake following an overnight fast in a dose-dependent manner and in correlation with acyl ghrelin concentrations in plasma.
Another way by which CF801 decreases weight gain and adiposity without affecting total caloric intake may be through a change in metabolism. It is well known that ghrelin favors the utilization of carbohydrates as a source of fuels as demonstrated by ghrelin-induced increases in RER in mice receiving daily injections of ghrelin (3). Here, we show that repeated injections of the higher dose of CF801 led to acute decreases in RER suggesting that CF801 changes metabolism favoring the utilization of lipids as fuel. This may also explain why CF801-treated mice also showed a lower proportion of body fat with no changes in lean or water mass. This is also similar to the effects of daily injections of GO-CoA-Tat, a treatment that also resulted in a reduction of overall fat mass relative to control vehicle-treated mice (60).
As described above, CF801 produces its effects through a reduction in acyl ghrelin concentrations, but not through a direct inhibition of GOAT activity. Using an in vitro cell-based assay, we were able to detect significant reductions in acyl ghrelin secretion from SG-1 cells following 24 h of incubation in 100 μM and 300 μM of CF801. Our preliminary data indicated that at a shorter incubation time of only 6 h, CF801 had no effect on acyl ghrelin secretion (data not shown), even at the highest concentration of 300 μM. Although Tat-peptide conjugates have been shown to attain maximal penetration within the first 3 h (73), our specific combination of amino acids has not been directly examined in this regard although time frames considerably above 3 h seem unlikely. Furthermore, stability of acylated ghrelin within vesicles is not known, but does not preclude the possibility of reserve acylated ghrelin packaged within secretory vesicles. Our data suggest that CF801’s effects may not be by direct interaction with the GOAT enzyme. This is also suggested by the lack of potency demonstrated by CF801 in the in vitro microsomal GOAT activity assay that directly assesses GOAT-catalyzed octanoylation of ghrelin mimetic peptide substrates. In contrast to the SG-1 cell-based assay and the effects of CF801 in vivo, CF801 was not able to reduce GOAT activity, while the known GOAT inhibitor octanoyl-(Dap3)-ghrelin (1–5)–NH2 showed a significant reduction.
Given the high amino acid similarity between CF801 and desacyl ghrelin, it is possible that CF801 acts like an analog of desacyl ghrelin. Indeed, desacyl ghrelin and the cyclic desacyl ghrelin analog AZP531 appear to have effects that are similar to those of CF801, including the decrease in weight gain, ghrelin secretion, and adipose tissue accumulation (74, 75). Alternatively, CF801 may reduce acylated ghrelin simply by reducing GOAT mRNA, and thus acyl ghrelin levels, without altering GOAT activity at least in the way that this is tested in vitro. While the mechanisms underlying these effects are not well understood, the idea that CF801 works like desacyl ghrelin or its analogs is an interesting contention as these are currently being proposed for potential clinical treatments to curb obesity. Given that ghrelin may be a hormone that is an important hormone in attenuating the effects of stress (19, 76, 77) or those of acute insults (78), more studies are required to determine if reducing acyl ghrelin concentrations with CF801 could have unwarranted side effects. Nevertheless, decreasing acyl ghrelin concentration peripherally with CF801 or with other bona fide GOAT inhibitors, may prove to be a desirable option for the treatment of obesity without affecting the functionality of the ghrelin receptor in the CNS.
Conflict of Interest Statement
Alfonso Abizaid, Martin K. Wellman, and Zachary R. Patterson hold a patent on CF801 (WO/2015/010210). All other authors have nothing to declare.
Acknowledgments
This project was supported by funds obtained from the Canadian Institutes for Health Research (CIHR), Natural Sciences and Engineering Research Council (NSERC), and Carleton University (AA) CIHR (MOP106445), and NSERC (RGPIN341538), NSERC CGSD Scholarship (ZP and MW), Ontario Graduate Scholarship (MW and HM), the Hilda and Preston Davis Foundation Postdoctoral Fellowship in Eating Disorders Research (BM), an International Research Alliance grant with the Novo Nordisk Foundation Center for Basic Metabolic Research at the University of Copenhagen (JZ), the Foundation for Prader-Willi Research (JH), a National Science Foundation Predoctoral Fellowship (DGE-1247399) (JD), and Syracuse University (JD and JH). We thank Prof. Jef Boeke (NYU Langone Medical Center) for the gift of a plasmid encoding the mouse GOAT isoform.
References
1. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature (1999) 402:656–60. doi:10.1038/45230
2. Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature (2001) 409:194–8. doi:10.1038/35051587
3. Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature (2000) 407:908–13. doi:10.1038/35038090
4. Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, et al. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience (2009) 164:351–9. doi:10.1016/j.neuroscience.2009.08.009
5. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes (2001) 50:1714–9. doi:10.2337/diabetes.50.8.1714
6. Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology (2006) 147:23–30. doi:10.1210/en.2005-0973
7. LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci U S A (2009) 106:13582–7. doi:10.1073/pnas.0906426106
8. Broglio F, Gottero C, Benso A, Prodam F, Destefanis S, Gauna C, et al. Effects of ghrelin on the insulin and glycemic responses to glucose, arginine, or free fatty acids load in humans. J Clin Endocrinol Metab (2003) 88:4268–72. doi:10.1210/jc.2002-021940
9. Sun Y, Asnicar M, Smith RG. Central and peripheral roles of ghrelin on glucose homeostasis. Neuroendocrinology (2007) 86:215–28. doi:10.1159/000109094
10. Gualillo O, Lago F, Dieguez C. Introducing GOAT: a target for obesity and anti-diabetic drugs? Trends Pharmacol Sci (2008) 29:398–401. doi:10.1016/j.tips.2008.06.003
11. Yada T, Dezaki K, Sone H, Koizumi M, Damdindorj B, Nakata M, et al. Ghrelin regulates insulin release and glycemia: physiological role and therapeutic potential. Curr Diabetes Rev (2008) 4:18–23. doi:10.2174/157339908783502352
12. Chuang JC, Sakata I, Kohno D, Perello M, Osborne-Lawrence S, Repa JJ, et al. Ghrelin directly stimulates glucagon secretion from pancreatic α-cells. Mol Endocrinol (2011) 25:1600–11. doi:10.1210/me.2011-1001
13. McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE. Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab (2004) 89:1630–5. doi:10.1210/jc.2003-031572
14. Dezaki K, Kakei M, Yada T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes (2007) 56:2319–27. doi:10.2337/db07-0345
15. Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun (2000) 276:905–8. doi:10.1006/bbrc.2000.3568
16. Andreis PG, Malendowicz LK, Trejter M, Neri G, Spinazzi R, Rossi GP, et al. Ghrelin and growth hormone secretagogue receptor are expressed in the rat adrenal cortex: evidence that ghrelin stimulates the growth, but not the secretory activity of adrenal cells. FEBS Lett (2003) 536:173–9. doi:10.1016/S0014-5793(03)00051-6
17. Walker AK, Rivera PD, Wang Q, Chuang JC, Tran S, Osborne-Lawrence S, et al. The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mol Psychiatry (2015) 20:500–8. doi:10.1038/mp.2014.34
18. Carlini VP, Monzón ME, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, et al. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun (2002) 299:739–43. doi:10.1016/S0006-291X(02)02740-7
19. Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci (2008) 11:752–3. doi:10.1038/nn.2139
20. Patterson ZR, Ducharme R, Anisman H, Abizaid A. Altered metabolic and neurochemical responses to chronic unpredictable stressors in ghrelin receptor-deficient mice. Eur J Neurosci (2010) 32:632–9. doi:10.1111/j.1460-9568.2010.07310.x
21. Patterson ZR, Khazall R, Mackay H, Anisman H, Abizaid A. Central ghrelin signaling mediates the metabolic response of C57BL/6 male mice to chronic social defeat stress. Endocrinology (2013) 154:1080–91. doi:10.1210/en.2012-1834
22. Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, et al. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol Psychiatry (2012) 72:457–65. doi:10.1016/j.biopsych.2012.03.010
23. Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M, et al. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology (2001) 74:143–7. doi:10.1159/000054680
24. Currie PJ, Khelemsky R, Rigsbee EM, Dono LM, Coiro CD, Chapman CD, et al. Ghrelin is an orexigenic peptide and elicits anxiety-like behaviors following administration into discrete regions of the hypothalamus. Behav Brain Res (2012) 226:96–105. doi:10.1016/j.bbr.2011.08.037
25. Carlini VP, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun (2004) 313:635–41. doi:10.1016/j.bbrc.2003.11.150
26. Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest (2006) 116:3229–39. doi:10.1172/JCI29867
27. Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience (2011) 180:129–37. doi:10.1016/j.neuroscience.2011.02.016
28. King SJ, Isaacs AM, O’Farrell E, Abizaid A. Motivation to obtain preferred foods is enhanced by ghrelin in the ventral tegmental area. Horm Behav (2011) 60:572–80. doi:10.1016/j.yhbeh.2011.08.006
29. Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry (2010) 67:880–6. doi:10.1016/j.biopsych.2009.10.030
30. Currie PJ, Mirza A, Fuld R, Park D, Vasselli JR. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am J Physiol Regul Integr Comp Physiol (2005) 289:R353–8. doi:10.1152/ajpregu.00756.2004
31. Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, et al. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab (2006) 4:323–31. doi:10.1016/j.cmet.2006.09.004
32. Seoane LM, López M, Tovar S, Casanueva FF, Señarís R, Diéguez C. Agouti-related peptide, neuropeptide Y, and somatostatin-producing neurons are targets for ghrelin actions in the rat hypothalamus. Endocrinology (2003) 144:544–51. doi:10.1210/en.2002-220795
33. Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature (2001) 411:480–4. doi:10.1038/35078085
34. Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology (2000) 141:4325–8. doi:10.1210/endo.141.11.7873
35. Healy JE, Bateman JL, Ostrom CE, Florant GL. Peripheral ghrelin stimulates feeding behavior and positive energy balance in a sciurid hibernator. Horm Behav (2011) 59:512–9. doi:10.1016/j.yhbeh.2011.01.013
36. Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol (2005) 288:R716–22. doi:10.1152/ajpregu.00705.2004
37. Tang-Christensen M, Vrang N, Ortmann S, Bidlingmaier M, Horvath TL, Tschöp M. Central administration of ghrelin and agouti-related protein (83-132) increases food intake and decreases spontaneous locomotor activity in rats. Endocrinology (2004) 145:4645–52. doi:10.1210/en.2004-0529
38. Choi K, Roh SG, Hong YH, Shrestha YB, Hishikawa D, Chen C, et al. The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology (2003) 144:754–9. doi:10.1210/en.2002-220783
39. Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, et al. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest (2006) 116:1983–93. doi:10.1172/JCI25811
40. Egecioglu E, Jerlhag E, Salomé N, Skibicka KP, Haage D, Bohlooly-Y M, et al. Ghrelin increases intake of rewarding food in rodents. Addict Biol (2010) 15:304–11. doi:10.1111/j.1369-1600.2010.00216.x
41. Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, et al. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest (2011) 121:2684–92. doi:10.1172/JCI57660
42. Merkestein M, Brans MA, Luijendijk MC, de Jong JW, Egecioglu E, Dickson SL, et al. Ghrelin mediates anticipation to a palatable meal in rats. Obesity (Silver Spring) (2012) 20:963–71. doi:10.1038/oby.2011.389
43. Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science (1996) 273:974–7. doi:10.1126/science.273.5277.974
44. Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res (1997) 48:23–9. doi:10.1016/S0169-328X(97)00071-5
45. Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol (2006) 494:528–48. doi:10.1002/cne.20823
46. Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med (2009) 15:741–5. doi:10.1038/nm.1997
47. Abizaid A, Horvath TL. Brain circuits regulating energy homeostasis. Regul Pept (2008) 149:3–10. doi:10.1016/j.regpep.2007.10.006
48. Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, et al. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem (2000) 43:4370–6. doi:10.1021/jm0001727
49. Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A (2008) 105:6320–5. doi:10.1073/pnas.0800708105
50. Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell (2008) 132:387–96. doi:10.1016/j.cell.2008.01.017
51. Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci (2000) 25:111–2. doi:10.1016/S0968-0004(99)01539-X
52. Stengel A, Goebel M, Wang L, Taché Y, Sachs G, Lambrecht NW. Differential distribution of ghrelin-O-acyltransferase (GOAT) immunoreactive cells in the mouse and rat gastric oxyntic mucosa. Biochem Biophys Res Commun (2010) 392:67–71. doi:10.1016/j.bbrc.2009.12.169
53. Sakata I, Yang J, Lee CE, Osborne-Lawrence S, Rovinsky SA, Elmquist JK, et al. Colocalization of ghrelin O-acyltransferase and ghrelin in gastric mucosal cells. Am J Physiol Endocrinol Metab (2009) 297:E134–41. doi:10.1152/ajpendo.90859.2008
54. Lim CT, Kola B, Grossman A, Korbonits M. The expression of ghrelin O-acyltransferase (GOAT) in human tissues. Endocr J (2011) 58:707–10. doi:10.1507/endocrj.K11E-117
55. Darling JE, Zhao F, Loftus RJ, Patton LM, Gibbs RA, Hougland JL. Structure-activity analysis of human ghrelin O-acyltransferase reveals chemical determinants of ghrelin selectivity and acyl group recognition. Biochemistry (2015) 54:1100–10. doi:10.1021/bi5010359
56. Yang J, Zhao TJ, Goldstein JL, Brown MS. Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc Natl Acad Sci U S A (2008) 105:10750–5. doi:10.1073/pnas.0805353105
57. Garner AL, Janda KD. A small molecule antagonist of ghrelin O-acyltransferase (GOAT). Chem Commun (Camb) (2011) 47:7512–4. doi:10.1039/c1cc11817j
58. Garner AL, Janda KD. Cat-ELCCA: a robust method to monitor the fatty acid acyltransferase activity of ghrelin O-acyltransferase (GOAT). Angew Chem Int Ed (2010) 49:9630–4. doi:10.1002/anie.201003387
59. Zhao F, Darling JE, Gibbs RA, Hougland JL. A new class of ghrelin O-acyltransferase inhibitors incorporating triazole-linked lipid mimetic groups. Bioorg Med Chem Lett (2015) 25(14):2800–3. doi:10.1016/j.bmcl.2015.05.009
60. Barnett BP, Hwang Y, Taylor MS, Kirchner H, Pfluger PT, Bernard V, et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science (2010) 330:1689–92. doi:10.1126/science.1196154
61. Zhao TJ, Sakata I, Li RL, Liang G, Richardson JA, Brown MS, et al. Ghrelin secretion stimulated by β1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci U S A (2010) 107:15868–73. doi:10.1073/pnas.1011116107
62. Mani BK, Chuang JC, Kjalarsdottir L, Sakata I, Walker AK, Kuperman A, et al. Role of calcium and EPAC in norepinephrine-induced ghrelin secretion. Endocrinology (2014) 155:98–107. doi:10.1210/en.2013-1691
63. Darling JE, Prybolsky EP, Sieburg M, Hougland JL. A fluorescent peptide substrate facilitates investigation of ghrelin recognition and acylation by ghrelin O-acyltransferase. Anal Biochem (2013) 437:68–76. doi:10.1016/j.ab.2013.02.013
64. Pérez-Tilve D, González-Matías L, Alvarez-Crespo M, Leiras R, Tovar S, Diéguez C, et al. Exendin-4 potently decreases ghrelin levels in fasting rats. Diabetes (2007) 56:143–51. doi:10.2337/db05-0996
65. Zorrilla EP, Iwasaki S, Moss JA, Chang J, Otsuji J, Inoue K, et al. Vaccination against weight gain. Proc Natl Acad Sci U S A (2006) 103:13226–31. doi:10.1073/pnas.0605376103
66. Helmling S, Maasch C, Eulberg D, Buchner K, Schröder W, Lange C, et al. Inhibition of ghrelin action in vitro and in vivo by an RNA-Spiegelmer. Proc Natl Acad Sci U S A (2004) 101:13174–9. doi:10.1073/pnas.0404175101
67. Kobelt P, Helmling S, Stengel A, Wlotzka B, Andresen V, Klapp BF, et al. Anti-ghrelin spiegelmer NOX-B11 inhibits neurostimulatory and orexigenic effects of peripheral ghrelin in rats. Gut (2006) 55:788–92. doi:10.1136/gut.2004.061010
68. Shearman LP, Wang SP, Helmling S, Stribling DS, Mazur P, Ge L, et al. Ghrelin neutralization by a ribonucleic acid-SPM ameliorates obesity in diet-induced obese mice. Endocrinology (2006) 147:1517–26. doi:10.1210/en.2005-0993
69. Moulin A, Ryan J, Martinez J, Fehrentz JA. Recent developments in ghrelin receptor ligands. ChemMedChem (2007) 2:1242–59. doi:10.1002/cmdc.200700015
70. Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, Papotti M, et al. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab (2004) 89:3062–5. doi:10.1210/jc.2003-031964
71. Gauna C, Meyler FM, Janssen JA, Delhanty PJ, Abribat T, van Koetsveld P, et al. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. J Clin Endocrinol Metab (2004) 89:5035–42. doi:10.1210/jc.2004-0363
72. Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest (2005) 115:3564–72. doi:10.1172/JCI26002
73. Jones SW, Christison R, Bundell K, Voyce CJ, Brockbank SM, Newham P, et al. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br J Pharmacol (2005) 145:1093–102. doi:10.1038/sj.bjp.0706279
74. Delhanty PJ, Neggers SJ, van der Lely AJ. Mechanisms in endocrinology: ghrelin: the differences between acyl- and des-acyl ghrelin. Eur J Endocrinol (2012) 167:601–8. doi:10.1530/EJE-12-0456
75. Delhanty PJ, Huisman M, Baldeon-Rojas LY, van den Berge I, Grefhorst A, Abribat T, et al. Des-acyl ghrelin analogs prevent high-fat-diet-induced dysregulation of glucose homeostasis. FASEB J (2013) 27:1690–700. doi:10.1096/fj.12-221143
76. Abizaid A, Anisman H. Gut feelings about depression. J Psychiatry Neurosci (2014) 39:364–6. doi:10.1503/jpn.140276
77. Spencer SJ, Emmerzaal TL, Kozicz T, Andrews ZB. Ghrelin’s role in the hypothalamic-pituitary-adrenal axis stress response: implications for mood disorders. Biol Psychiatry (2015) 78:19–27. doi:10.1016/j.biopsych.2014.10.021
Keywords: ghrelin, ghrelin-O-acyltransferase, ghrelin antagonist, weight loss, appetite, energy expenditure, adiposity
Citation: Wellman MK, Patterson ZR, MacKay H, Darling JE, Mani BK, Zigman JM, Hougland JL and Abizaid A (2015) Novel regulator of acylated ghrelin, CF801, reduces weight gain, rebound feeding after a fast, and adiposity in mice. Front. Endocrinol. 6:144. doi: 10.3389/fendo.2015.00144
Received: 19 June 2015; Accepted: 01 September 2015;
Published: 25 September 2015
Edited by:
Francisco Gracia-Navarro, University of Cordoba, SpainReviewed by:
Jeff S. Davies, Swansea University, UKRiccarda Granata, University of Turin, Italy
Manuel D. Gahete, University of Cordoba, Spain
Copyright: © 2015 Wellman, Patterson, MacKay, Darling, Mani, Zigman, Hougland and Abizaid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alfonso Abizaid, Department of Neuroscience, Carleton University, 1125 Colonel By Drive, 329 Life Science Research Building, Ottawa, ON K1S 5B6, Canada, alfonso_abizaid@carleton.ca
 Martin K. Wellman
Martin K. Wellman Zachary R. Patterson
Zachary R. Patterson Harry MacKay
Harry MacKay Joseph E. Darling
Joseph E. Darling Bharath K. Mani
Bharath K. Mani Jeffrey M. Zigman
Jeffrey M. Zigman James L. Hougland
James L. Hougland Alfonso Abizaid
Alfonso Abizaid