- 1Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, USA
- 2Endocrine Research Laboratory, Aurora St. Luke’s Medical Center, Aurora Research Institute, Milwaukee, WI, USA
Stress-induced increases in neonatal corticosterone demonstrate a unique shift from ACTH independence to ACTH dependence between postnatal day 2 (PD2) and day 8 (PD8) in newborn rats. This shift could be due to the binding of a bioactive, non-immunoreactive plasma ligand to the adrenocortical melanocortin 2 receptor (MC2R) (ACTH receptor). A potent MC2R antagonist would be useful to evaluate this phenomenon in the neonate. Therefore, we investigated the acute corticosterone response to ACTH(1–39) injection in rat pups pretreated with newly developed MC2R antagonists (GPS1573 and GPS1574), which have not been tested in vivo. The doses used in vivo were based on their in vitro potency, with GP1573 being more potent than GPS1574. GPS1573 (PD2 and PD8), GPS1574 (PD2 only), or vehicle were injected intraperitoneally (ip) 10 min before baseline sampling. Then, 0.001 mg/kg of ACTH(1–39) was injected ip, and subsequent blood samples obtained for the measurement of plasma corticosterone. Pretreatment of PD2 pups with GPS1573 demonstrated augmentation, rather than inhibition, of the corticosterone response to ACTH. In PD8 pups, pretreatment with 0.1 mg/kg GPS1573, but not 4 mg/kg, augmented the corticosterone response to ACTH. Pretreatment with GPS1574 attenuated the plasma corticosterone response to ACTH at 30 min in PD2 pups. The activity of these two compounds in vivo do not match their potency in vitro, with GPS1573 leading to a small augmentation of the corticosterone response to ACTH in vivo while GPS1574 resulted in inhibition.
Introduction
Development of the neonatal hypothalamic–pituitary–adrenal (HPA) axis is critical for normal maturation of the lung, closing of the patent ductus arteriosus, and improving vasoconstrictor responses to catecholamines, as well as for stress-induced increases in blood glucose and blood pressure (1–3). In light of the increasing rate of premature births in the United States, it is important to understand the mechanisms of steroidogenesis and HPA axis maturation in the premature and full-term neonate (4). The neonatal rat model of human prematurity serves as a useful tool in studying the development of the HPA axis because the rat is an altricial animal. The full-term, newborn rat is immature compared to a full-term human neonate (5, 6). The primary glucocorticoid in the neonatal rat, corticosterone, is secreted from the zona fasciculata of the adrenal cortex in response to ACTH binding and activation of the melanocortin 2 receptor (MC2R) (7, 8). The binding of ACTH to MC2R leads to an increase in intracellular cyclic adenosine monophosphate (cAMP), causing activation of protein kinase A (PKA), and subsequent increase in movement of free cholesterol across the mitochondrial membrane into the cytosol (7–10). This transport of free cholesterol, mediated by the steroidogenic acute regulatory protein (StAR), is the rate-limiting step of steroidogenesis (11).
We have previously shown that on postnatal day 2 (PD2), rat pups exposed to hypoxic stress demonstrate an increase in corticosterone without an appreciable increase in immunoassayable ACTH and adrenal cAMP (12, 13). This phenomenon could be due to larger posttranslational products of POMC activating the MC2R receptor (14), which are not necessarily detected in our ACTH immunoassay. Although difficult to study without completely eliminating potential confounders, there are data suggesting that premature infants born at <32 weeks can mount a cortisol response without the large increase in plasma ACTH found in infants born >32 weeks gestational age (15). As stated earlier, the newborn rat is useful as a model of human prematurity (5, 6).
By postnatal day 8 (PD8), pups show an increase in corticosterone with the classic increase in immunoassayable ACTH and adrenal cAMP (12, 13, 16). The ability of the PD2 adrenal gland to respond to stress without a detectable increase in immunoreactive ACTH could be due to a bioactive, non-immunoreactive form of ACTH or another POMC product that can bind to and activate the adrenocortical MC2R. If the corticosterone response to stress in PD2 pups can be blocked by antagonizing the MC2R, it would suggest that a bioactive form of ACTH (not measured by immunoassay) is working through the MC2R in PD2 pups.
GPS1573 (Nle-P-f-R-w-F-K-A-V-G-K-K-R-R NH2) and GPS1574 [Nle-(E-f-R-w-F-K)-A-V-G-K-K-R-R NH2] are newly described, potent (IC50 = 66 ± 23 and 260 ± 1 nM, respectively), and dose-dependent antagonists of ACTH-stimulated MC2R activity in vitro (17). Note that the structures of the two compounds are similar except that GPS1574 has a ring structure. However, they have not been studied in vivo. The primary goal of the present study is to investigate the effect of GPS1573 and GPS1574 in vitro (adrenal cells) and in vivo (neonatal rats) in order to set the stage for its use in evaluating the role of endogenous ACTH in the neonatal adrenal stress response. We hypothesize that these MC2R antagonists are effective in adrenal cells from neonatal rats in vitro. Furthermore, we hypothesize that in the neonatal rat, GPS1573 and GPS1574, when given at a 100- to 4000-fold higher dose and 4000- to 8000-fold higher dose, respectively, than exogenous ACTH(1–39), will attenuate the adrenocortical corticosterone response to ACTH.
Materials and Methods
Animal Treatment and Experimental Protocol
The animal protocol was approved by the Institutional Animal Care and Use Committee of Aurora Health Care. Timed-pregnant Sprague-Dawley rats at 14–17 gestational days (N = 45) were obtained from Harlan Sprague Dawley (Indianapolis, IN, USA), maintained on a standard diet, and had water available ad libitum in a controlled environment (0600–1800 hours lights on). Dams were allowed to deliver and care for their pups without interference until experimentation. The MC2R antagonists GPS1573 and GPS1574 were synthesized by Genepep (St. Jean de Védas, France) and reconstituted, as described previously (17).
Adrenal Cell Preparation and GPS1573 and GPS1574 In Vitro
Adrenal cells from rats (N = 11 adults, 46 PD2 pups, and 26 PD8 pups) were dispersed and studied as previously described (18, 19). Briefly, rats were killed by decapitation, and both adrenal glands were removed and cleaned of surrounding fat. Only adult adrenal glands were decapsulated prior to cell dispersion. After a 90-min type I collagenase treatment and washing, viable cells were plated in a 96-well microtiter plate (10,000 cells/well) and pretreated for 1 h with (a) no antagonist (b) 750 nM GPS1573, or (c) 750-nM GPS1574 at 37°C in 10% CO2 (balance room air). After pretreatment, rat ACTH(1–39) (Bachem) was added to cell suspensions at appropriate concentrations and incubated for 1 h. Medium was removed and immediately assayed for corticosterone (20).
GPS1573 and GPS1574 Studies in the Neonatal Rat In Vivo
Rat pups were randomly assigned to experimental groups on the morning of the experiment. Then, rat pups were removed from the dams and placed in a small cage with adequate bedding, where they were allowed free range of motion and room to huddle. A variable control heating pad (Moore Medical, Farmington, CT, USA) was placed beneath the bedding and kept at the lowest setting required to maintain body temperature in normoxic rats at these ages (13, 21). After 10 min, rat pups were removed from the cage and quickly weighed.
In the GPS1573 studies, pups (both sexes; N = 252) were studied at PD2 (N = 96) and PD8 (N = 156) because these ages span the critical time during which the neonatal rat’s adrenal response to hypoxia shifts from immunoreactive ACTH independent to ACTH dependent (13, 16). Pups were injected intraperitoneally (ip) with either vehicle (10 μl/kg body wt of isotonic saline) or GPS1573 (diluted in isotonic saline) in low or high dose (0.1 or 4.0 mg/kg body wt, respectively). We chose to give GPS1573 ip because (a) it is a small peptide amenable to proper absorption by this route, (b) ACTH injections given ip are effective, and (c) subcutaneous injection was ineffective (data not shown).
In the GPS1574 studies, pups (both sexes, N = 42) were only studied at PD2, with the 60-min time point omitted because of drug’s limited supply, the data from the 60-min time point with GPS1573 described above, higher necessary dose based on the in vitro studies [current experiments and Ref. (17)], and its expense. GPS1574 was given at a dose of 4 or 8 mg/kg ip. Otherwise, the experiments were performed as described for GPS1573.
Ten minutes after GPS1573 or GPS1574 injection, a subset of pups was quickly decapitated and trunk blood was collected (baseline, time 0). Immediately after the baseline collection, 1 μg/kg (0.001 mg/kg) of porcine ACTH (Sigma Chemical, St. Louis, MO, USA) was injected ip, as described (22–24). Subsets of pups were decapitated at 15, 30, or 60 min (GPS1573 only) post-injection. In another group of pups (N = 163), the vehicle for ACTH injection (isotonic saline) was injected ip 10 min after GPS1573 or GPS1574 administration, and blood collected as described above. Trunk blood was collected in EDTA tubes (1 pup/sample), processed to plasma, and stored frozen (−20°C). Plasma corticosterone was measured by immunoassay, as described previously (MP Biomedicals, Orangeburg, NJ, USA) (25).
Statistical Analyses
Corticosterone data were analyzed by two-way ANOVA. Post hoc analysis was performed by Holm–Sidak multiple range test (P < 0.05) (SigmaPlot 11.0). Data are presented as mean ± SEM.
Results
GPS1573 and GPS1574 In Vitro
Figure 1 shows the corticosterone response to ACTH in dispersed adrenal cells of rat pups (PD2 and PD8) and adults treated with GPS1573 and GPS1574. The cells from adult adrenal glands showed significant inhibition of the corticosterone response to ACTH in vitro, with GPS1573 being more potent than GPS1574. A similar response was observed in PD2 pup adrenal cells, as previously described for adult rats (17). In PD8 pup adrenal cells, however, there was equivalent inhibition between GPS1573 and GPS1574, compared to vehicle control.
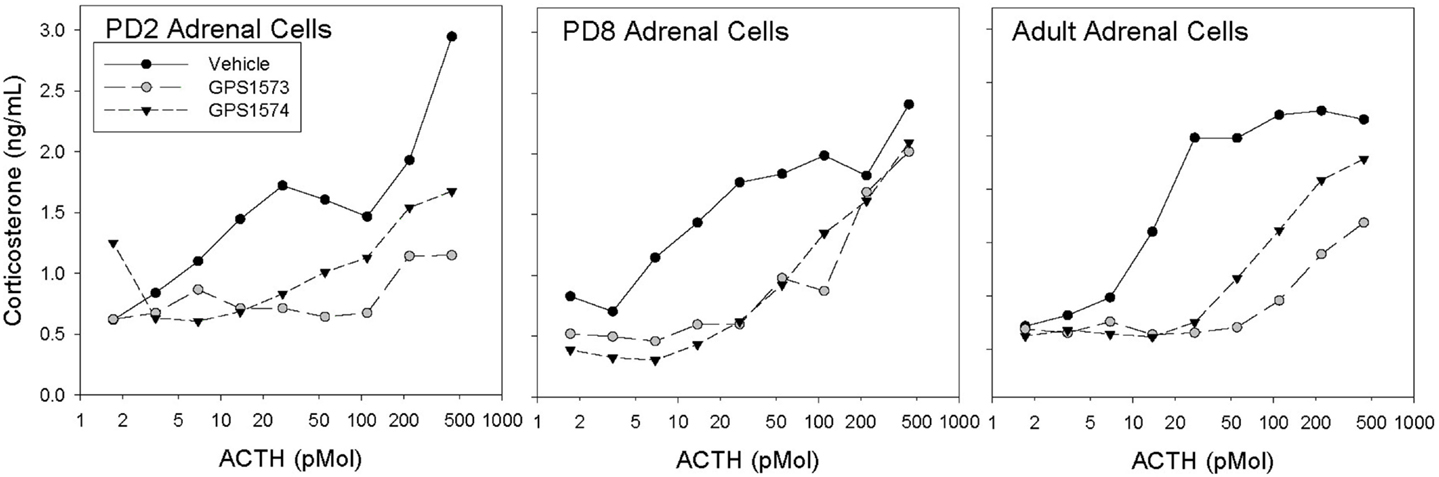
Figure 1. Corticosterone responses to ACTH in vitro in adrenal cells from rats of different ages. Cells were pretreated with vehicle, GPS1573, or GPS1574 (750 nM). Each point is the mean of two replicates.
GPS1573 In Vivo
The plasma corticosterone responses to exogenous ACTH in PD2 pups pretreated with either vehicle (for GPS1573) or the low or high dose of GPS1573 are shown in Figure 2. Baseline plasma corticosterone responses (10 min after injection of GPS1573) ranged from 30.4 ± 3.7 to 45.7 ± 8.7 ng/ml and were not different from our previously published baseline data without an injection 10 min before sampling (23, 24). PD2 pups pretreated with vehicle did not show an increase in corticosterone in response to ACTH at 15 min but did have an increase in corticosterone at 30 min (166.0 ± 17.1 ng/ml) and 60 min (137.2 ± 43.5 ng/ml). After pretreatment with the low dose of GPS1573, compared to vehicle, the PD2 pups showed a significantly augmented plasma corticosterone response at 15 min (67.8 ± 7.9 ng/ml) and 60 min (200.0 ± 23.6 ng/ml). When pretreated with the high dose of GPS1573, PD2 pups demonstrate an even greater augmentation of the corticosterone response at 15 min (115.5 ± 11.0 ng/ml), when compared to pretreatment with low dose or vehicle.
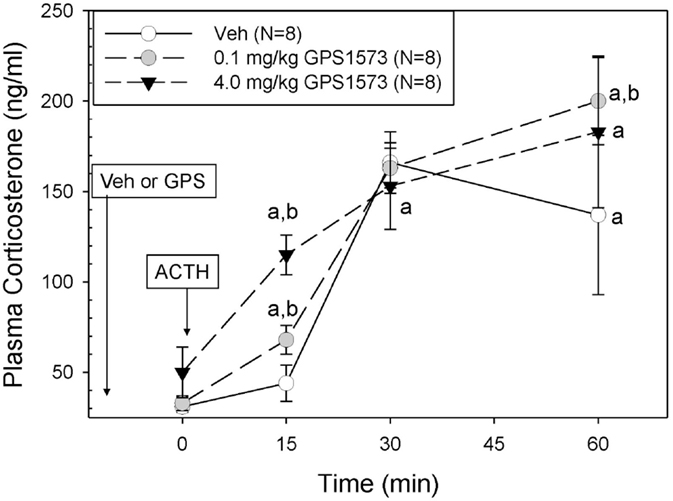
Figure 2. Plasma corticosterone response to ACTH injection in postnatal 2 (PD2) rats pretreated with the low and high doses of GPS1573. PD2 pups were injected with vehicle and low or high dose of GPS1573 (0.1 or 4.0 mg/kg body wt ip, respectively). Ten minutes later, baseline samples were obtained, and then porcine ACTH (0.001 mg/kg) was injected ip. aSignificantly different from baseline (0 min). bSignificantly different from vehicle (P < 0.05). Data are presented as means ± SE. N values (number of pups) for each mean are shown in the figure label.
The plasma corticosterone responses to exogenous ACTH in PD8 pups pretreated with either vehicle (for GPS1573) or the low or high dose of GPS1573 are shown in Figure 3. Baseline plasma corticosterone responses (10 min after injection of GPS1573) ranged from 22.8 ± 3.7 to 25.7 ± 2.9 ng/ml and were not different from our previously published baseline data, without an injection 10 min before sampling (23, 24). The plasma corticosterone response to ACTH in PD8 pups was less than PD2 pups. In fact, there was no significant increase in corticosterone in response to ACTH in PD8 pups. Compared to vehicle, PD8 pups pretreated with the low dose of GPS1573 showed an augmentation of the plasma corticosterone response to ACTH at 15 min (45.8 ± 2.6 ng/ml), 30 min (54.5 ± 3.7 ng/ml), and 60 min (50.4 ± 6.5 ng/ml). The corticosterone response to ACTH in PD8 pups pretreated with the high dose of GPS1573 was not different than vehicle at 15 min (34.3 ± 4.5 ng/ml) and 30 min (36.0 ± 6.0 ng/ml) but was less than the low dose of GPS1573 at 60 min (40.4 ± 5.9 ng/ml).
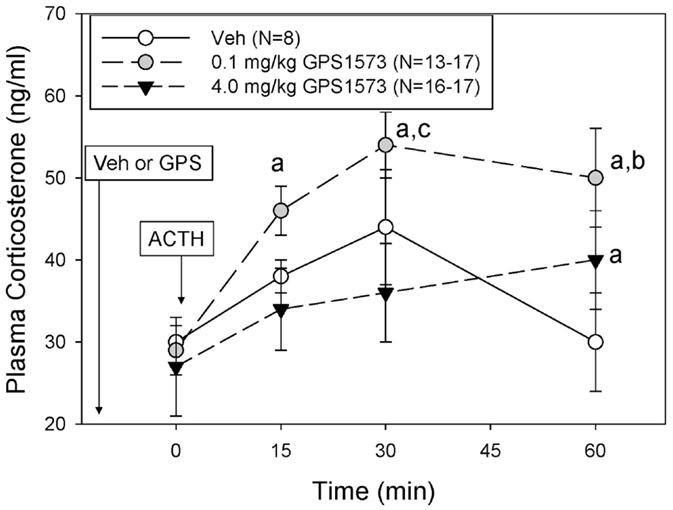
Figure 3. Plasma corticosterone response to ACTH injection in postnatal 8 (PD8) rats pretreated with the low and high doses of GPS1573. PD8 pups were injected with vehicle and low or high dose of GPS1573 (0.1 or 4.0 mg/kg body wt ip, respectively). Ten minutes later, baseline samples were obtained, and then porcine ACTH (0.001 mg/kg) was injected ip. aSignificantly different from baseline (0 min). bSignificantly different from vehicle (P < 0.05). cSignificantly different from 4.0 mg/kg. Data are presented as means ± SE. Note the y-axis range is lower than Figure 2 (PD2 pups). N values (number of pups) for each mean are shown in the figure label.
We also evaluated a time control in which pups were pretreated with GPS1573 and then injected with vehicle for ACTH (Table 1). For the most part, there were no statistically significant changes in plasma corticosterone. It is important to note that baseline plasma corticosterone concentrations were similar to those in our prior studies without ip injection prior to baseline (23, 24). However, there was a small increase in plasma corticosterone at 15 min in PD2 pups pretreated with the high dose of GPS1573 and at 30 min in PD8 pups pretreated with the low dose of GPS1573.
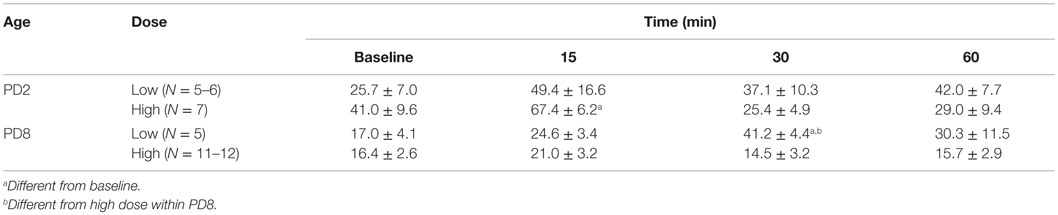
Table 1. Plasma corticosterone (nanogram per milliliter) response to vehicle (for ACTH injections) after pretreatment of PD2 and PD8 pups with the low dose (0.1 μg/kg) or high dose (4 μg/kg) of GPS1573.
GPS1574 In Vivo
Although we originally planned to only evaluate GPS1573 because of its higher potency in vitro (17), which was confirmed by our in vitro studies, the lack of inhibition with GPS1573 described above led us to do a limited number of new in vivo experiments with the apparently less potent GPS1574. We limited the GPS1574 study to PD2 (an age we are most interested in) through 30 min because of the need to use a higher dose of this very expensive drug, which was in limited supply and because the peak response to ACTH injection was at 30 min. As Figure 4 demonstrates, there was no inhibition at 15 min after ACTH administration, but a dose-dependent inhibition at 30 min was seen after ACTH injection. The time control for GPS1574 (saline injection rather than ACTH) did not reveal any changes in corticosterone (data not shown) and had baseline serum corticosterone concentrations similar to our previous studies without injection before baseline sampling.
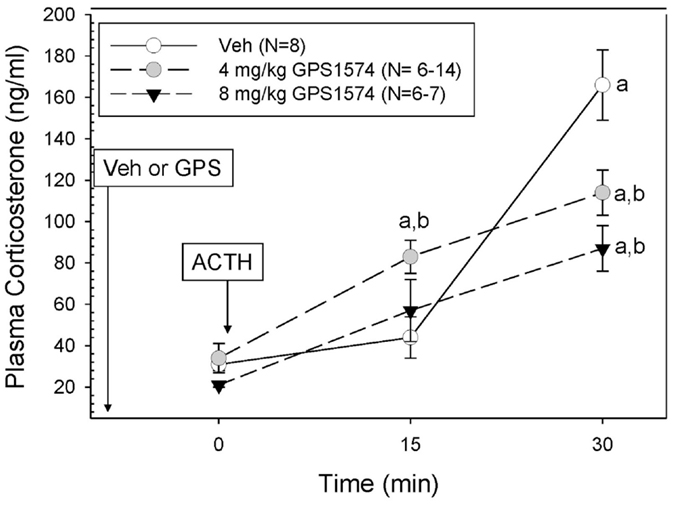
Figure 4. Plasma corticosterone response to ACTH injection in postnatal 2 (PD2) rats pretreated with GPS1574. Pups were injected with vehicle or GPS1574 (4 or 8 mg/kg body wt ip, respectively). Ten minutes later, baseline samples were obtained, and then porcine ACTH (0.001 mg/kg) was injected ip. aSignificantly different from baseline (0 min). bSignificantly different from vehicle (P < 0.05). Data are presented as means ± SE. N values (number of pups) for each mean are shown in the figure label.
Discussion
This study evaluated the corticosterone response to exogenous ACTH injection in neonatal rats pretreated with potent, in vitro MC2R antagonists, which have not previously been tested in vivo (17). We hypothesized that these compounds would attenuate the adrenocortical MC2R, thereby resulting in a lower corticosterone response to ACTH in PD2 and PD8 rats. If these antagonists were effective in vivo, it would allow us to evaluate the possibility that stress-induced increases in corticosterone at PD2 that are independent of increases in immunoreactive plasma ACTH could be explained by binding of a non-immunoreactive corticotrophic ligand to the adrenocortical MC2R (12, 13, 16, 21). We chose to study neonatal rats based on previous studies (23, 24). Additionally, we are currently unable to study these compounds in adult rats because of the very large quantities of the GPS compounds needed, the compounds’ expense, and their limited supply.
Although the limited GPS1574 in vivo studies were performed after the responses to GPS1573 were ascertained, we will first discuss the GPS1574 data. GPS1574 resulted in an attenuated corticosterone response at 30 min after ACTH injection. We studied GPS1574 at a dose 4000- and 8000-fold higher than the exogenous ACTH dose. We were unable to use an even higher dose because of the limited supply of this compound. With the assumption that these GPS compounds are absorbed in a manner similar to ACTH, we calculated the plasma concentration of the GPS compounds to be at least 10- to 20-fold higher than the peak plasma ACTH concentration after injection. It is possible that these drugs are not as well absorbed as ACTH(1–39) and/or that they are metabolized much more readily than ACTH(1–39), perhaps requiring even higher doses ip. These preliminary results show promise for a more comprehensive analysis of this compound at much higher doses.
GPS1573, given in vivo at a 4000-fold higher dose of exogenous ACTH, did not antagonize the adrenal response to ACTH at either age. Rather, we demonstrated that pretreatment with GPS1573 augmented the corticosterone response to ACTH stimulation in vivo. In PD2 pups, the low dose of GPS1573 significantly augmented the corticosterone response to ACTH at 15 and 60 min compared to vehicle while the high dose demonstrated an even greater augmentation at 15 min compared to vehicle and low dose. In PD8 pups, pretreatment with the low dose of GPS1573 showed significant augmentation compared to vehicle at 60 min while pretreatment with the high dose showed no significant increase in corticosterone at any time point. GPS1573 did not consistently act as an agonist per se, with only small increases in corticosterone in response to ACTH vehicle (saline) at two time points.
This leads us to conclude that although GPS1573 acts as a competitive antagonist in vitro in adrenal cells from PD2, PD8, and adult cells, pretreatment with it in vivo results in an augmentation of the response to the natural ligand for the MC2R (ACTH). A similar phenomenon has been shown for nuclear (intracellular) receptors (26).
What could explain the effect demonstrated for a G-protein coupled receptor like the MC2R? First, it could be that the IC50 of GPS1573 is too high to be effective in vivo, despite the fact that we gave it at a 4000-fold higher dose than ACTH. The data from GPS1574 do not corroborate this notion since it has a higher IC50 in vitro but was effective in vivo. It is also possible that GPS1573 acts as an antagonist in vitro but is a biasing agonist in vivo. That is, in vivo, rather than blocking the receptor, it actually augments G-protein coupled transduction when ACTH binds to the MC2R (27, 28). This phenomenon could also be attributable to GPS1573 triggering a non-specific sympathetic nervous system-induced increase in the sensitivity of the adrenal cortex to ACTH (22, 29–36). Another possibility is that GPS1573 is inactivated shortly after being injected or that it has a very short half-life in vivo.
Potential drawbacks of our study design include the route of administration of GPS1573 and GPS1574. We chose ip administration a priori, since these antagonists are small compounds, and we have previously shown that ip ACTH injection is effective in stimulating corticosteronogenesis (22–24). We performed a few pilot studies with subcutaneous injection, which were ineffective (data not shown). However, an alternate route of administration (e.g., intravenous or intramuscular) may be more effective. It is also possible that these compounds would have been more effective in vivo in postpubertal rats. Alternate controllers of neonatal steroidogenesis, including postganglionic sympathetic input described above, have been proposed; however, the current study avoids these confounders by injecting ACTH rather than using stress as a stimulus to ACTH (22–24).
In conclusion, it appears that high dose of GPS1574 has potential as a competitive antagonist of ACTH in vivo. However, GPS1573 seems to act like a biasing agonist in vivo when given before an ACTH injection even though it is not consistently an agonist when given alone. The differences in behavior between these two compounds in vivo may be related to the ring structure of GPS1574.
Author Contributions
NN, JB, AG, and HR all contributed substantially to the design and performance of the experiments and assays, writing and editing of the manuscript, approval of the final version, and agreement to account for all aspects of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Barbara Jankowski and Brian Hoeynck for their expert technical assistance. This study was funded, in part, by the Aurora Research Institute and by the NHLBI T35 Summer Research Training Grant.
References
1. Watterberg KL, Gerdes JS, Gifford KL, Lin HM. Prophylaxis against early adrenal insufficiency to prevent chronic lung disease in premature infants. Pediatrics (1999) 104(6):1258–63. doi: 10.1542/peds.104.6.1258
2. Morales P, Rastogi A, Bez ML, Akintorin SM, Pyati S, Andes SM, et al. Effect of dexamethasone therapy on the neonatal ductus arteriosus. Pediatr Cardiol (1998) 19(3):225–9. doi:10.1007/s002469900290
3. Deruelle P, Houfflin-Debarge V, Magnenant E, Jaillard S, Riou Y, Puech F, et al. Effects of antenatal glucocorticoids on pulmonary vascular reactivity in the ovine fetus. Am J Obstet Gynecol (2003) 189(1):208–15. doi:10.1067/mob.2003.444
4. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: final data for 2005. Natl Vital Stat Rep (2007) 56(6):1–103.
5. Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev (1991) 26(1):61–7. doi:10.1016/0378-3782(91)90044-4
6. Sheldon RA, Chuai J, Ferriero DM. A rat model for hypoxic-ischemic brain damage in very premature infants. Biol Neonate (1996) 69(5):327–41. doi:10.1159/000244327
7. Gallo-Payet N, Payet MD. Mechanism of action of ACTH: beyond cAMP. Microsc Res Tech (2003) 61(3):275–87. doi:10.1002/jemt.10337
8. Gallo-Payet N, Battista MC. Steroidogenesis-adrenal cell signal transduction. Compr Physiol (2014) 4(3):889–964. doi:10.1002/cphy.c130050
9. Raff H, Findling JW. A physiologic approach to diagnosis of the Cushing syndrome. Ann Intern Med (2003) 138(12):980–91. doi:10.7326/0003-4819-138-12-200306170-00010
10. Richards JS. New signaling pathways for hormones and cyclic adenosine 3’,5’-monophosphate action in endocrine cells. Mol Endocrinol (2001) 15(2):209–18. doi:10.1210/mend.15.2.0606
11. Stocco DM, Clark BJ. The role of the steroidogenic acute regulatory protein in steroidogenesis. Steroids (1997) 62(1):29–36. doi:10.1016/S0039-128X(96)00155-9
12. Chintamaneni K, Bruder ED, Raff H. Effects of age on ACTH, corticosterone, glucose, insulin, and mRNA levels during intermittent hypoxia in the neonatal rat. Am J Physiol Regul Integr Comp Physiol (2013) 304(9):R782–9. doi:10.1152/ajpregu.00073.2013
13. Bruder ED, Kamer KJ, Guenther MA, Raff H. Adrenocorticotropic hormone and corticosterone responses to acute hypoxia in the neonatal rat: effects of body temperature maintenance. Am J Physiol Regul Integr Comp Physiol (2011) 300(3):R708–15. doi:10.1152/ajpregu.00708.2010
14. Karpac J, Ostwald D, Bui S, Hunnewell P, Shankar M, Hochgeschwender U. Development, maintenance, and function of the adrenal gland in early postnatal proopiomelanocortin-null mutant mice. Endocrinology (2005) 146(6):2555–62. doi:10.1210/en.2004-1290
15. Bagnoli F, Mori A, Fommei C, Coriolani G, Badii S, Tomasini B. ACTH and cortisol cord plasma concentrations in preterm and term infants. J Perinatol (2013) 33(7):520–4. doi:10.1038/jp.2012.165
16. Bruder ED, Taylor JK, Kamer KJ, Raff H. Development of the ACTH and corticosterone response to acute hypoxia in the neonatal rat. Am J Physiol Regul Integr Comp Physiol (2008) 295(4):R1195–203. doi:10.1152/ajpregu.90400.2008
17. Bouw E, Huisman M, Neggers SJ, Themmen AP, van der Lely AJ, Delhanty PJ. Development of potent selective competitive-antagonists of the melanocortin type 2 receptor. Mol Cell Endocrinol (2014) 394(1–2):99–104. doi:10.1016/j.mce.2014.07.003
18. Jasper MS, Engeland WC. Splanchnicotomy increases adrenal sensitivity to ACTH in nonstressed rats. Am J Physiol (1997) 273(2 Pt 1):E363–8.
19. Bruder ED, Nagler AK, Raff H. Oxygen-dependence of ACTH-stimulated aldosterone and corticosterone synthesis in the rat adrenal cortex: developmental aspects. J Endocrinol (2002) 172(3):595–604. doi:10.1677/joe.0.1720595
20. Wilkinson CW, Shinsako J, Dallman MF. Daily rhythms in adrenal responsiveness to adrenocorticotropin are determined primarily by the time of feeding in the rat. Endocrinology (1979) 104(2):350–9. doi:10.1210/endo-104-2-350
21. Guenther MA, Bruder ED, Raff H. Effects of body temperature maintenance on glucose, insulin, and corticosterone responses to acute hypoxia in the neonatal rat. Am J Physiol Regul Integr Comp Physiol (2012) 302(5):R627–33. doi:10.1152/ajpregu.00503.2011
22. Raff H, Lee JJ, Widmaier EP, Oaks MK, Engeland WC. Basal and adrenocorticotropin-stimulated corticosterone in the neonatal rat exposed to hypoxia from birth: modulation by chemical sympathectomy. Endocrinology (2004) 145(1):79–86. doi:10.1210/en.2003-1130
23. Bodager J, Gessert T, Bruder ED, Gehrand A, Raff H. Adrenocortical sensitivity to ACTH in neonatal rats: correlation of corticosterone responses and adrenal cAMP content. Am J Physiol Regul Integr Comp Physiol (2014) 307(3):R347–53. doi:10.1152/ajpregu.00125.2014
24. Johnson K, Bruder ED, Raff H. Adrenocortical control in the neonatal rat. Physiol Rep (2013) 1(3):e00054. doi:10.1002/phy2.54
25. Raff H, Jacobson L, Cullinan WE. Elevated corticosterone and inhibition of ACTH responses to CRH and ether in the neonatal rat: effect of hypoxia from birth. Am J Physiol Regul Integr Comp Physiol (2003) 285(5):R1224–30. doi:10.1152/ajpregu.00259.2003
26. Wardell SE, Kazmin D, McDonnell DP. Research resource: transcriptional profiling in a cellular model of breast cancer reveals functional and mechanistic differences between clinically relevant SERM and between SERM/estrogen complexes. Mol Endocrinol (2012) 26(7):1235–48. doi:10.1210/me.2012-1031
27. Reversi A, Rimoldi V, Marrocco T, Cassoni P, Bussolati G, Parenti M, et al. The oxytocin receptor antagonist atosiban inhibits cell growth via a “biased agonist” mechanism. J Biol Chem (2005) 280(16):16311–8. doi:10.1074/jbc.M409945200
28. Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ. Recent developments in biased agonism. Curr Opin Cell Biol (2014) 27:18–24. doi:10.1016/j.ceb.2013.10.008
29. Ehrhart-Bornstein M, Bornstein SR, Gonzalez-Hernandez J, Holst JJ, Waterman MR, Scherbaum WA. Sympathoadrenal regulation of adrenocortical steroidogenesis. Endocr Res (1995) 21(1–2):13–24. doi:10.3109/07435809509030417
30. Ehrhart-Bornstein M, Haidan A, Alesci S, Bornstein SR. Neurotransmitters and neuropeptides in the differential regulation of steroidogenesis in adrenocortical-chromaffin co-cultures. Endocr Res (2000) 26(4):833–42. doi:10.3109/07435800009048606
31. Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev (1998) 19(2):101–43. doi:10.1210/edrv.19.2.0326
32. Bruder ED, Henderson LM, Raff H. Adrenal lipid profiles of chemically sympathectomized normoxic and hypoxic neonatal rats. Horm Metab Res (2006) 38(12):807–11. doi:10.1055/s-2006-956183
33. Engeland WC. Functional innervation of the adrenal cortex by the splanchnic nerve. Horm Metab Res (1998) 30(6–7):311–4. doi:10.1055/s-2007-978890
34. Schinner S, Bornstein SR. Cortical-chromaffin cell interactions in the adrenal gland. Endocr Pathol (2005) 16(2):91–8. doi:10.1385/EP:16:2:091
35. Seidler FJ, Slotkin TA. Adrenomedullary function in the neonatal rat: responses to acute hypoxia. J Physiol (1985) 358:1–16. doi:10.1113/jphysiol.1985.sp015536
Keywords: ACTH, MC2R, corticosterone, adrenal cortex, antagonist
Citation: Nensey NK, Bodager J, Gehrand AL and Raff H (2016) Effect of Novel Melanocortin Type 2 Receptor Antagonists on the Corticosterone Response to ACTH in the Neonatal Rat Adrenal Gland In Vivo and In Vitro. Front. Endocrinol. 7:23. doi: 10.3389/fendo.2016.00023
Received: 12 November 2015; Accepted: 07 March 2016;
Published: 21 March 2016
Edited by:
Nicole Gallo-Payet, University of Sherbrooke, CanadaReviewed by:
Gábor B. Makara, Hungarian Academy of Sciences, HungaryDominique Walker, Douglas Hospital Research Center, Canada
Robert Dores, University of Minnesota, USA
Copyright: © 2016 Nensey, Bodager, Gehrand and Raff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hershel Raff, hraff@mcw.edu
 Nasha K. Nensey1,2
Nasha K. Nensey1,2 Ashley L. Gehrand
Ashley L. Gehrand Hershel Raff
Hershel Raff