- 1Department of Biomedical Sciences, University of Missouri, Columbia, MO, United States
- 2Department of Medical Pharmacology and Physiology, University of Missouri, Columbia, MO, United States
- 3Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, MO, United States
- 4Dalton Cardiovascular Research Center, University of Missouri, Columbia, MO, United States
Physical inactivity is a primary contributor to diseases such as obesity, cardiovascular disease, and type 2 diabetes. Accelerometry data suggest that a majority of US adults fail to perform substantial levels of physical activity needed to improve health. Thus, understanding the molecular factors that stimulate physical activity, and physical inactivity, is imperative for the development of strategies to reduce sedentary behavior and in turn prevent chronic disease. Despite many of the well-known health benefits of physical activity being described, little is known about genetic and biological factors that may influence this complex behavior. The mesolimbic dopamine system regulates motivating and rewarding behavior as well as motor movement. Here, we present data supporting the hypothesis that obesity may mechanistically lower voluntary physical activity levels via dopamine dysregulation. In doing so, we review data that suggest mesolimbic dopamine activity is a strong contributor to voluntary physical activity behavior. We also summarize findings suggesting that obesity leads to central dopaminergic dysfunction, which in turn contributes to reductions in physical activity that often accompany obesity. Additionally, we highlight examples in which central leptin activity influences physical activity levels in a dopamine-dependent manner. Future elucidation of these mechanisms will help support strategies to increase physical activity levels in obese patients and prevent diseases caused by physical inactivity.
Introduction
Physical inactivity presents a major public health problem. Predictions by Lee et al. (1) estimated that physical inactivity accounts for between 6 and 10% of type 2 diabetes (T2D) and coronary heart disease prevalence, with this percentage further elevated for specific diseases (30% for ischemic heart disease) (2). Moreover, the World Health Organization declared physical inactivity as the fourth leading risk factor for death worldwide, responsible for ~6% of the deaths worldwide in 2008 (1, 2). Accelerometry measurements by Troiano et al. (3) reported that less than 5% of adults met the US guidelines for physical activity, while questionnaire data collected globally in 2009 suggested that 31% of the world’s population did not attain minimum recommended levels of physical activity (4). Given the deleterious effects of physical inactivity, understanding molecular mechanisms that influence physical activity adherence is needed. Here, we summarize current knowledge suggesting the mesolimbic dopamine system regulates physical activity, obesity-induced impairments in dopamine signaling may cause physical inactivity, and central leptin resistance in obesity and T2D may alter physical activity in a dopamine-dependent manner. Specifically, our discussion focuses on motivated and self-rewarding (i.e., voluntary wheel running), rather than spontaneous (i.e., cage activity, tremors, etc.), forms of physical activity.
Genetic Control of Physical Activity
In 1953, Mayer, a leader who helped clarify the natures of hunger and of obesity, demonstrated that physical activity behavior has a biological basis (5). Mayer noted that obese, hyperglycemic mice were far less active than non-obese littermates. However, when the obese mice were bred against mice with a so-called “waltzing gene” physical activity increased to sufficiently prevent the development of obesity. Since Mayer’s original speculation of an uncharacterized “waltzing gene,” studies in animals and humans have estimated the genetic component for physical inactivity to be between 20 and 80% (6–12). Analysis of 772 same-sex twin pairs concluded that 31% of the variance in daily sedentary time was explained by heritable factors (13). Of these heritable factors, associations between dopamine and motivated physical activity are well established, as discussed below. However, other neuromodulators such as endocannabinoids (14, 15), opioids (16), and brain-derived neurotrophic factor (17) also influence physical activity behavior. Furthermore, interactions between these neuromodulatory systems imply that biological networks control voluntary physical activity (18). Evolutionary perspectives also argue that while selection did not operate to cope with the detrimental effects of long-term physical inactivity, humans adapted to avoid unnecessary exertion due to limited energy supply (19). Additionally, gene–environment interactions influence physical activity. Rowland (20) proposed that through components related to energy balance control an “activity-stat” may regulate the propensity for physical activity. Furthermore, obesity was speculated to be a critical negative influencer of the “activity-stat” (21). Collectively, these findings suggest that physical activity levels have strong genetic control.
Dopaminergic Control of Physical Activity
Although detailed mechanisms describing the neurobiology of wheel running are incomplete, substantial evidence suggests that the mesolimbic dopamine pathway, specifically the ventral striatum and nucleus accumbens (NAc), plays an important role in determining voluntary running behavior (22–24). A detailed review of the mesolimbic dopamine system is beyond the scope of this review; however, a brief overview is provided next [please see Ref. (25, 26) for more detailed review]. In the mesolimbic dopamine system, dopaminergic neurons originating in the ventral tegmental area (VTA) project to various limbic nuclei, including the NAc, and changes in dopamine transmission play central roles in modulating information flow through the limbic system (27–30). These nuclei, through interconnections via dopaminergic neurons, have implications in reward, motivation, learning, and motor movement (31). Importantly, the NAc acts as a “filter” and/or “amplifier” of information passing between various limbic, cortical, and motor areas of the brain, suggesting the NAc is instrumental in orchestrating behavioral processes related to motivation (25). Several reports have demonstrated that other mesolimbic structures, such as the VTA and prefrontal cortex, contribute to reward derived from physical activity, potentially through their interactions with the NAc (32–34).
Disruption of dopaminergic transmission and/or dopamine receptor expression in the NAc and ventral striatum can strongly influence voluntary physical activity. The depletion of NAc dopamine by 6-hydroxydopamine decreased wheel running ~40% (35). Knab et al. (22) suggested that differences in dopamine 1-like (D1-like) receptors and tyrosine hydroxylase (Th) mRNA, the rate-limiting enzyme in dopamine synthesis, in the NAc influence different running distances between mouse strains.
Selective breeding studies have provided ample insight into voluntary physical activity regulation. Mice bred by Garland et al for high voluntary running distance displayed dysfunctional dopaminergic profiles in the NAc (36, 37) and increased dopamine receptor 2 (Drd2) and dopamine receptor 4 (Drd4) mRNA ~20% in the hippocampus (38), compared to control mice. Furthermore, agonism (24) and antagonism (37) of D1-like receptors in the NAc paradoxically both decreased wheel running in high-running mice to a greater extent than in control mice. Similar findings from our group using rat lines selectively bred for high (HVR) and low (LVR) wheel-running suggested rats predisposed to run high nightly distances may quickly develop a rewarding response to exercise due to optimal D1-like receptor signaling in the NAc (39). Collectively, these data suggest the following: (1) dopamine signaling is optimally primed to achieve reward associated with running in high-running rats, (2) dopamine is at least partially required for wheel-running behavior, and (3) animals run to achieve the rewarding effects of dopamine but do not want to run when dopamine signaling is artificially activated. Dopamine receptors 1 (Drd1), Drd2, and dopamine receptor 5 (Drd5) mRNA were also inherently 50 to 85% higher in the NAc of HVR compared to LVR (16). Similarly, inherent ~1.3-fold increases in NAc Drd1 mRNA and ~1.8-fold greater dopaminergic activity were speculated to mediate increased wheel running in rats selectively bred for high, compared to low, aerobic capacity, suggesting that aerobic capacity may influence physical activity levels through alterations in mesolimbic dopamine activity (40, 41). Furthermore, the loss of dopamine receptors or reduced dopamine release in the brain was associated with age-related declines in physical activity across many species (42) and was hypothesized to influence age-related physical activity reductions in humans (43). Single nucleotide polymorphism (SNP) analysis suggested that the DRD2 gene associated with physical activity levels in women (44) and that individuals with the CC homozygous variant in rs1800955 of the DRD4 gene were more prone to sport-specific sensation seeking (45). Similarly, Wilkinson et al. (46) found associations between SNPs in two dopamine pathway genes, angiotensin I converting enzyme (ACE) and synaptosomal-associated protein 25 (SNAP25), and decreased likelihood for physical activity in youth.
However, whether alterations in the dopamine system are the result or driver of differences in voluntary physical activity is unknown. For example, previous reports show that voluntary wheel running is rewarding, and over time, able to alter behavior and affect the neuroplasticity of the mesolimbic reward pathway (34). Furthermore, endurance exercise training increased central dopamine concentrations up to 1.5-fold (47). Thus, physical activity, itself, could function in a feed-forward mechanism to further elevate physical activity.
Obesity and Dopaminergic Dysregulation
In the past three decades, obesity prevalence in the US has risen from below 20 to 36.5% (48). Additionally, physical inactivity levels and excessive food intake have increased over a similar period, directly contributing to increases in obesity and T2D (1) (Figure 1). Increases in unadjusted food intake from ~1980 to 1994 were associated with initial rapid increases in obesity, but not T2D, prevalence. Furthermore, beginning in ~1998 to 2000, physical activity levels rapidly dropped and sedentary time rapidly increased. This decrease in physical activity and increase in physical inactivity corresponded with increases in both obesity and T2D prevalence, despite food intake staying relatively constant during the same period. In our opinion, more recent increases in obesity are thus better associated with physical inactivity increases as caloric intakes were unchanged. Importantly, while declining physical activity levels contribute to obesity development, obesity contributed to reductions in physical activity in humans, even after controlling for baseline differences in physical activity (49). This interaction may promote the development of self-perpetuating vicious cycles whereby physical inactivity and obesity promote each other’s development (50).
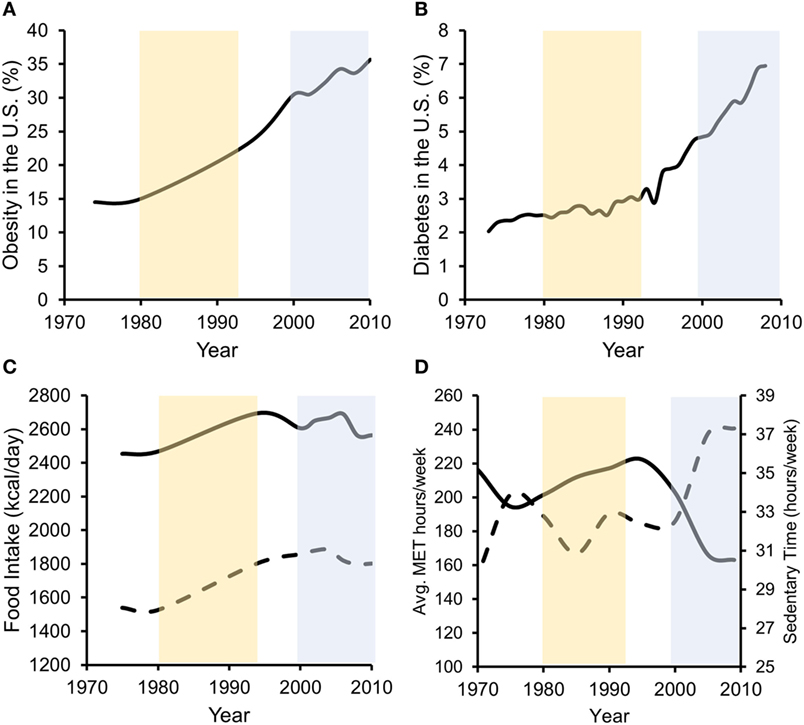
Figure 1. Data suggest that both increases in energy intake and reductions in energy expenditure associate with increased obesity prevalence, while in later years, decreased energy expenditure more strongly associates with T2D prevalence. Percentage of US adults with obesity (A) or diagnosed with type 2 diabetes (B) over the past ~40 years. (C) Unadjusted food intake for male (solid line) and female (dashed line) adults in the US during the same time frame. (D) Physical activity (solid line/left axis) [average metabolic equivalent (MET) hours per week] and physical inactivity (dashed line/right axis) (hours per week of sedentary time) performed by US adults. Obesity data redrawn from Ref. (48, 51), diabetes data from the CDC (52), food intake data from Ref. (53), and physical activity data from Ref. (54).
The effects of obesity on the mesolimbic dopamine system are well studied, and hypotheses suggesting “reward dysfunction” in obesity have developed given findings that obesity is associated with alterations in striatal dopamine signaling (55). For example, reduced dopamine function, particularly DRD2 signaling, is associated with obesity development in rodents (56–59) and humans (60–62). However, these studies associated hyperphagia with obesity development and did not assess physical activity. Similarly, using positron emission tomography (PET) Guo et al. (63) observed a negative relationship between D2-like receptor binding in the ventral striatum and body mass index (BMI), suggesting that BMI could influence rewarding and effort-based actions. Similar measurements associating D1-like receptor neuron activity with obesity in humans are lacking, although several animal studies found that Drd1 mRNA is reduced up to ninefold in the NAc of obese rats (64, 65). High-fat diet consumption for 12 weeks decreased tonic dopamine and Drd1 and Drd2 mRNA expression ~50% in the NAc of mice (66). Interestingly, following a 4-week recovery from high-fat diet, NAc Drd1 and Drd2 mRNA expressions were normalized in female, but not male, mice (66). Similarly, PET studies in humans show that DRD2 binding is not recovered (67) or partially recovered (68) following Roux-en-Y gastric bypass surgery. Collectively, these data suggest that reductions in dopamine function accompanying obesity could persist following weight loss. This notion is consistent with findings that physical inactivity levels remained high in obese humans months after weight loss (69–71), raising the question whether “physical activity resistance” exists temporarily/permanently after weight loss.
Interestingly, animal studies also suggest that high-fat diet exposure, rather than weight gain, may be more predictive of changes in striatal dopamine signaling. Isocaloric high-fat diet feeding in rats resulted in ~40% lower DRD2 in the NAc (72). Furthermore, chronic ad libitum high-fat diet reduced dopamine turnover 3.5-fold in the NAc of rats, although similar reductions were observed following isocaloric high-fat diet (73). Additionally, animal studies suggest that longer-term high-fat diet exposure can suppress dopamine synthesis, release, or turnover, ultimately reducing motivated behaviors not limited to motivation for food, such as physical activity (74). Despite considerable variability in experimental outcomes, we conclude that decreased dopamine signaling, particularly decreased D2-type function, could be particularly relevant to obesity.
Obesity and Physical Inactivity
Obesity is strongly associated with physical inactivity (75, 76). While sparsely studied, several studies suggest that diet-induced dopaminergic alterations accompanying obesity may promote physical inactivity. Friend et al. (77) noted that diet-induced obesity in mice reduced D2-type receptor binding in the striatum that associated with decreased voluntary physical activity. Furthermore, in the same study the deletion of the Drd2 gene, specifically in inhibitory medium spiny neurons (iMSNs), decreased wheel revolutions compared to littermate controls, although these mice were surprisingly not more vulnerable to diet-induced weight gain (77). Finally, the restoration of iMSN signaling reversed deficits in wheel running (77). Collectively, these data support the notion that D2-type receptor dysregulation contributes to obesity-induced physical inactivity, but that physical inactivity may be a consequence, rather than effector, of obesity.
Similarly, comparisons between mice bred for excessive exercise or obesity revealed that NAc dopamine content was increased in high running compared to obese and control mice, while Drd1, Drd2, and adenylate cyclase 5 (Adcy5) mRNAs were downregulated 92, 80, and 91%, respectively, in obese compared to control mice (78). Nonetheless, the authors hypothesized that modifications in the dopaminergic system may contribute to the differences in voluntary exercise between the high-running and obese mice (78). Analysis of obesity-resistant, compared to obesity-prone, rats also suggested that reduced physical activity levels in obesity-prone rats may stem from decreased action of hypothalamic orexin on dopamine neurons in the striatum and substantia nigra (79, 80). Finally, lower striatal dopaminergic activity may have contributed to low wheel running activity in rats with low aerobic capacity, who also had greater body weight and metabolic disease risk (40).
A recent study found that decreased DRD2 signaling in the striatum influences obesity development via reductions in physical activity rather than increases in food intake. Using Drd2 knockdown mice, Beeler et al. (81) observed that when presented with voluntary exercise in an enriched environment, Drd2 knockdown mice were dramatically less active than wild-type mice. Importantly, in the same study reduced voluntary exercise by Drd2 knockdown mice promoted an obese phenotype despite no differences in food intake (81). These intriguing observations not only suggest a direct link between reduced dopamine function and decreased physical activity, but that the decreases DRD2 signaling can contribute to obesity via reduced energy expenditure rather than the initiation of compulsive overeating. Furthermore, obesity-induced reduction in DRD2 signaling could initiate the following feedback mechanism to further amplify obesity and physical inactivity: obesity → ↓ DRD2 signaling → ↑ physical inactivity → ↑ obesity → futile cycle. On the contrary, separate experiments show that dietary restriction increased wheel running (82) and dopamine overflow and receptor expression in the NAc (83, 84), suggesting that obesity and dietary restriction may have opposing effects on dopamine signaling and, in turn, voluntary physical activity. However, future research is needed to dissect causal and consequential relationships between obesity, dopamine, and physical inactivity.
Central Leptin Action and Physical Activity
Relationships between leptin and physical activity are well established. Central leptin resistance is a hallmark of obesity (85, 86), and leptin resistance in the VTA following diet-induced obesity has been noted previously (87). Normal leptin signaling in VTA dopaminergic neurons is well characterized, with a general consensus being that leptin receptor (LEPR) signaling inhibits dopamine activity (88–90). Correspondingly, associations between select DRD2 and LEPR allelic gene variations have been associated with the development of severe obesity (91).
Leptin suppressed the rewarding effects of wheel running in mice via activation of signal transducer and activator of transcription-3 (STAT3) signaling in VTA dopamine neurons, an effect which likely influenced dopamine overflow and function in the NAc and suggested that leptin may influence the motivational and rewarding effects of wheel running (92). Additional studies show that dopamine overflow in the NAc is reduced by leptin deficiency (88) and diet-induced obesity (57). In mice bred by Garland et al for high voluntary wheel running, which display dysfunctional dopaminergic profiles in the NAc as described above (36, 37), intraperitoneal leptin injection increased running by 17%, while control mice were unaffected (93). Paradoxically, in the same study high-fat feeding increased wheel running 20% in high-running mice, an effect speculated to be mediated by leptin (93). Intracerebroventricular injection of a recombinant adeno-associated virus (rAAV) overexpressing a mutant of leptin, which produces a protein that acts as a LEPR antagonist, decreased wheel running 25 and 40% in rats fed either a standard chow or high-fat diet, respectively, while rAAV overexpression of functional leptin increased wheel running ~2-fold Matheny et al. (94). However, changes in voluntary physical activity in the Matheny et al. study could be secondary to changes in adiposity following rAAV injection. Collectively, a hypothesis describing the interaction between obesity, dopamine, leptin, and physical inactivity is presented in Figure 2.
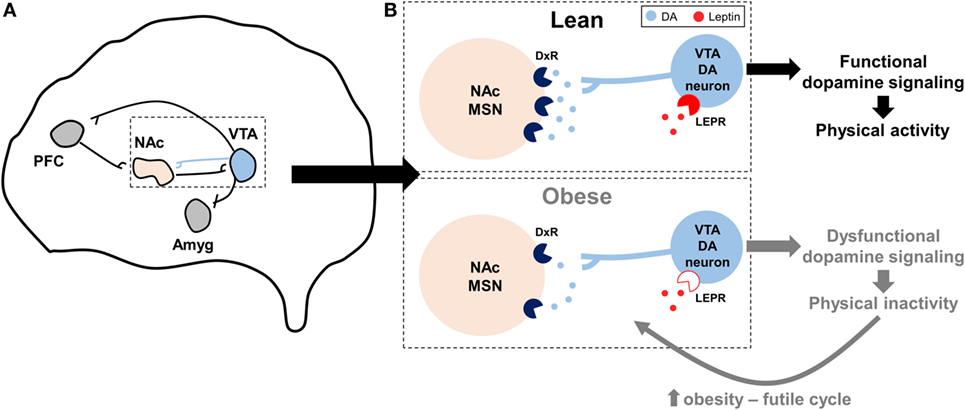
Figure 2. Hypothesized model by which impaired dopaminergic signaling promotes physical inactivity in obesity. (A) Summary of the reward circuitry in the brain; originally established by Robison and Nestler (95). The blue projection illustrates dopaminergic projections from the ventral tegmental area (VTA) that release dopamine (DA) onto post-synaptic neurons in the nucleus accumbens (NAc). (B) Expanded, but simplified, illustration of this dopaminergic VTA to NAc projection as it is hypothesized to relate to physical inactivity in lean and obese individuals. In obesity, dopamine receptor (DxR), particularly dopamine receptor 2, expression is decreased in NAc medium spiny neurons (MSNs). Similarly, mechanisms controlling DA production and release are reduced with obesity, leading to less DA in the synapse. Central leptin resistance in obesity [denoted by open leptin receptor (LEPR) symbol] may influence LEPR signaling in VTA DA neurons, in turn further diminishing downstream DA function. Collectively, these obesity-induced impairments in dopaminergic signaling may lead to exacerbated levels of physical inactivity, which may in turn lead to a futile cycle of increased obesity, dopaminergic dysregulation, and physical inactivity. Other abbreviations: Amyg, amygdala; PFC, prefrontal cortex.
Further suggesting that leptin may impact the motivational and rewarding effects of running are observations that high serum leptin levels inversely correlated with low marathon run times after BMI adjustment (96), and with running performance (time and speed) in mice bred for high voluntary running (97). Leptin deficiency has also been shown to influence physical activity humans, whereas acute leptin increased locomotor activity in leptin-deficient patients during the fed state (98, 99). Similarly, leptin-deficient ob/ob mice increased wheel running 3.5-fold during the fed state following acute subcutaneous leptin injection, while no effect was observed in wild-type mice (100). Collectively, these studies highlight the important role of leptin as an effector of voluntary physical activity, potentially through alternations in dopamine signaling.
Conclusion
Physical inactivity and obesity have reached pandemic levels (101). The abovementioned studies strongly suggest that dopaminergic function influences physical inactivity levels. Similarly, obesity-induced suppression of dopamine signaling may contribute to the high prevalence of physical inactivity observed in obese people. Additional understanding of mechanisms by which dopaminergic dysfunction contributes to obesity, physical inactivity, or their interactions may reveal novel approaches for increasing physically activity in obese populations.
Author Contributions
GR and FB conceived the idea, wrote, and edited this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors disclose no conflicts of interest. No funding sources were acquired for this project.
References
1. Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet (2012) 380:219–29. doi: 10.1016/S0140-6736(12)61031-9
2. World Health Organization. Global Recommendations on Physical Activity for Health. World Health Organization Press (2010).
3. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, Mcdowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc (2008) 40:181–8. doi:10.1249/mss.0b013e31815a51b3
4. Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet (2012) 380:247–57. doi:10.1016/S0140-6736(12)60646-1
5. Mayer J. Decreased activity and energy balance in the hereditary obesity-diabetes syndrome of mice. Science (1953) 117:504–5. doi:10.1126/science.117.3045.504
6. Festing MF. Wheel activity in 26 strains of mouse. Lab Anim (1977) 11:257–8. doi:10.1258/002367777780936530
7. Kaprio J, Koskenvuo M, Sarna S. Cigarette smoking, use of alcohol, and leisure-time physical activity among same-sexed adult male twins. Prog Clin Biol Res (1981) 69(Pt C):37–46.
8. Lauderdale DS, Fabsitz R, Meyer JM, Sholinsky P, Ramakrishnan V, Goldberg J. Familial determinants of moderate and intense physical activity: a twin study. Med Sci Sports Exerc (1997) 29:1062–8. doi:10.1097/00005768-199708000-00012
9. Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, et al. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol (1985) (2002) 92:2245–55. doi:10.1152/japplphysiol.01045.2001
10. Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiol Genomics (2004) 19:270–6. doi:10.1152/physiolgenomics.00125.2004
11. Lightfoot JT, Turner MJ, Pomp D, Kleeberger SR, Leamy LJ. Quantitative trait loci for physical activity traits in mice. Physiol Genomics (2008) 32:401–8. doi:10.1152/physiolgenomics.00241.2007
12. Kelly SA, Pomp D. Genetic determinants of voluntary exercise. Trends Genet (2013) 29:348–57. doi:10.1016/j.tig.2012.12.007
13. den Hoed M, Brage S, Zhao JH, Westgate K, Nessa A, Ekelund U, et al. Heritability of objectively assessed daily physical activity and sedentary behavior. Am J Clin Nutr (2013) 98:1317–25. doi:10.3945/ajcn.113.069849
14. Keeney BK, Raichlen DA, Meek TH, Wijeratne RS, Middleton KM, Gerdeman GL, et al. Differential response to a selective cannabinoid receptor antagonist (SR141716: rimonabant) in female mice from lines selectively bred for high voluntary wheel-running behaviour. Behav Pharmacol (2008) 19:812–20. doi:10.1097/FBP.0b013e32831c3b6b
15. Fuss J, Steinle J, Bindila L, Auer MK, Kirchherr H, Lutz B, et al. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci U S A (2015) 112:13105–8. doi:10.1073/pnas.1514996112
16. Ruegsegger GN, Toedebusch RG, Will MJ, Booth FW. Mu opioid receptor modulation in the nucleus accumbens lowers voluntary wheel running in rats bred for high running motivation. Neuropharmacology (2015) 97:171–81. doi:10.1016/j.neuropharm.2015.05.022
17. Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res (2003) 974:228–35. doi:10.1016/S0006-8993(03)02584-8
18. Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, et al. Neurobiology of exercise. Obesity (Silver Spring) (2006) 14:345–56. doi:10.1038/oby.2006.46
19. Lieberman DE. Is exercise really medicine? An evolutionary perspective. Curr Sports Med Rep (2015) 14:313–9. doi:10.1249/JSR.0000000000000168
20. Rowland TW. The biological basis of physical activity. Med Sci Sports Exerc (1998) 30:392–9. doi:10.1097/00005768-199803000-00009
21. Li G, Kohorst JJ, Zhang W, Laritsky E, Kunde-Ramamoorthy G, Baker MS, et al. Early postnatal nutrition determines adult physical activity and energy expenditure in female mice. Diabetes (2013) 62:2773–83. doi:10.2337/db12-1306
22. Knab AM, Bowen RS, Hamilton AT, Gulledge AA, Lightfoot JT. Altered dopaminergic profiles: implications for the regulation of voluntary physical activity. Behav Brain Res (2009) 204:147–52. doi:10.1016/j.bbr.2009.05.034
23. Knab AM, Lightfoot JT. Does the difference between physically active and couch potato lie in the dopamine system? Int J Biol Sci (2010) 6:133–50. doi:10.7150/ijbs.6.133
24. Knab AM, Bowen RS, Hamilton AT, Lightfoot JT. Pharmacological manipulation of the dopaminergic system affects wheel-running activity in differentially active mice. J Biol Regul Homeost Agents (2012) 26:119–29.
25. Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron (2012) 76:470–85. doi:10.1016/j.neuron.2012.10.021
26. Salamone JD, Pardo M, Yohn SE, Lopez-Cruz L, Sanmiguel N, Correa M. Mesolimbic dopamine and the regulation of motivated behavior. Curr Top Behav Neurosci (2016) 27:231–57. doi:10.1007/7854_2015_383
27. Napier TC, Maslowski-Cobuzzi RJ. Electrophysiological verification of the presence of D1 and D2 dopamine receptors within the ventral pallidum. Synapse (1994) 17:160–6. doi:10.1002/syn.890170304
28. Carr DB, O’Donnell P, Card JP, Sesack SR. Dopamine terminals in the rat prefrontal cortex synapse on pyramidal cells that project to the nucleus accumbens. J Neurosci (1999) 19:11049–60.
29. Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol (1999) 9:223–7. doi:10.1016/S0959-4388(99)80031-2
30. Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci (2003) 1003:36–52. doi:10.1196/annals.1300.066
31. Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res (2009) 196:155–67. doi:10.1016/j.bbr.2008.09.038
32. Isobe Y, Nishino H. Circadian rhythm of drinking and running-wheel activity in rats with 6-hydroxydopamine lesions of the ventral tegmental area. Brain Res (2001) 899:187–92. doi:10.1016/S0006-8993(01)02223-5
33. Rhodes JS, Gammie SC, Garland T Jr. Neurobiology of mice selected for high voluntary wheel-running activity. Integr Comp Biol (2005) 45:438–55. doi:10.1093/icb/45.3.438
34. Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res (2011) 217:354–62. doi:10.1016/j.bbr.2010.11.005
35. Robbins TW, Koob GF. Selective disruption of displacement behaviour by lesions of the mesolimbic dopamine system. Nature (1980) 285:409–12. doi:10.1038/285409a0
36. Rhodes JS, Hosack GR, Girard I, Kelley AE, Mitchell GS, Garland T Jr. Differential sensitivity to acute administration of cocaine, GBR 12909, and fluoxetine in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology (Berl) (2001) 158:120–31. doi:10.1007/s002130100857
37. Rhodes JS, Garland T. Differential sensitivity to acute administration of Ritalin, apomorphine, SCH 23390, but not raclopride in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology (Berl) (2003) 167:242–50. doi:10.1007/s00213-003-1399-9
38. Bronikowski AM, Rhodes JS, Garland T Jr, Prolla TA, Awad TA, Gammie SC. The evolution of gene expression in mouse hippocampus in response to selective breeding for increased locomotor activity. Evolution (2004) 58:2079–86. doi:10.1111/j.0014-3820.2004.tb00491.x
39. Roberts MD, Gilpin L, Parker KE, Childs TE, Will MJ, Booth FW. Dopamine D1 receptor modulation in nucleus accumbens lowers voluntary wheel running in rats bred to run high distances. Physiol Behav (2012) 105:661–8. doi:10.1016/j.physbeh.2011.09.024
40. Waters RP, Renner KJ, Pringle RB, Summers CH, Britton SL, Koch LG, et al. Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiol Behav (2008) 93:1044–54. doi:10.1016/j.physbeh.2008.01.013
41. Park YM, Kanaley JA, Padilla J, Zidon T, Welly RJ, Will MJ, et al. Effects of intrinsic aerobic capacity and ovariectomy on voluntary wheel running and nucleus accumbens dopamine receptor gene expression. Physiol Behav (2016) 164:383–9. doi:10.1016/j.physbeh.2016.06.006
42. Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc (2000) 32:1623–9. doi:10.1097/00005768-200009000-00016
43. Sallis JF. Age-related decline in physical activity: a synthesis of human and animal studies. Med Sci Sports Exerc (2000) 32:1598–600. doi:10.1097/00005768-200009000-00012
44. Simonen RL, Rankinen T, Perusse L, Leon AS, Skinner JS, Wilmore JH, et al. A dopamine D2 receptor gene polymorphism and physical activity in two family studies. Physiol Behav (2003) 78:751–7. doi:10.1016/S0031-9384(03)00084-2
45. Thomson CJ, Hanna CW, Carlson SR, Rupert JL. The 521 C/T variant in the dopamine-4-receptor gene (DRD4) is associated with skiing and snowboarding behavior. Scand J Med Sci Sports (2013) 23:e108–13. doi:10.1111/sms.12031
46. Wilkinson AV, Gabriel KP, Wang J, Bondy ML, Dong Q, Wu X, et al. Sensation-seeking genes and physical activity in youth. Genes Brain Behav (2013) 12:181–8. doi:10.1111/gbb.12006
47. Meeusen R, Smolders I, Sarre S, De Meirleir K, Keizer H, Serneels M, et al. Endurance training effects on neurotransmitter release in rat striatum: an in vivo microdialysis study. Acta Physiol Scand (1997) 159:335–41. doi:10.1046/j.1365-201X.1997.00118.x
48. Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief (2015) 219:1–8.
49. Tucker JM, Tucker LA, Lecheminant J, Bailey B. Obesity increases risk of declining physical activity over time in women: a prospective cohort study. Obesity (Silver Spring) (2013) 21:E715–20. doi:10.1002/oby.20415
50. Pietilainen KH, Kaprio J, Borg P, Plasqui G, Yki-Jarvinen H, Kujala UM, et al. Physical inactivity and obesity: a vicious circle. Obesity (Silver Spring) (2008) 16:409–14. doi:10.1038/oby.2007.72
51. Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology (2007) 132:2087–102. doi:10.1053/j.gastro.2007.03.052
52. Centers for Disease Control and Prevention. Long-Term Trends in Diagnosed Diabetes. CDC Division of Diabetes Translation (2011).
53. Ford ES, Dietz WH. Trends in energy intake among adults in the United States: findings from NHANES. Am J Clin Nutr (2013) 97:848–53. doi:10.3945/ajcn.112.052662
54. Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev (2012) 13:659–80. doi:10.1111/j.1467-789X.2011.00982.x
55. Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron (2011) 69:664–79. doi:10.1016/j.neuron.2011.02.016
56. Huang XF, Zavitsanou K, Huang X, Yu Y, Wang H, Chen F, et al. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav Brain Res (2006) 175:415–9. doi:10.1016/j.bbr.2006.08.034
57. Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, et al. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J (2008) 22:2740–6. doi:10.1096/fj.08-110759
58. Geiger B, Haburcak M, Avena N, Moyer M, Hoebel B, Pothos E. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience (2009) 159:1193–9. doi:10.1016/j.neuroscience.2009.02.007
59. Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci (2010) 13:635–41. doi:10.1038/nn.2519
60. Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet (2001) 357:354–7. doi:10.1016/S0140-6736(00)03643-6
61. Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science (2008) 322:449–52. doi:10.1126/science.1161550
62. van de Giessen E, Celik F, Schweitzer DH, Van Den Brink W, Booij J. Dopamine D2/3 receptor availability and amphetamine-induced dopamine release in obesity. J Psychopharmacol (2014) 28:866–73. doi:10.1177/0269881114531664
63. Guo J, Simmons WK, Herscovitch P, Martin A, Hall KD. Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol Psychiatry (2014) 19:1078–84. doi:10.1038/mp.2014.102
64. Vucetic Z, Carlin JL, Totoki K, Reyes TM. Epigenetic dysregulation of the dopamine system in diet-induced obesity. J Neurochem (2012) 120:891–8. doi:10.1111/j.1471-4159.2012.07649.x
65. Zhang C, Wei NL, Wang Y, Wang X, Zhang JG, Zhang K. Deep brain stimulation of the nucleus accumbens shell induces anti-obesity effects in obese rats with alteration of dopamine neurotransmission. Neurosci Lett (2015) 589:1–6. doi:10.1016/j.neulet.2015.01.019
66. Carlin J, Hill-Smith TE, Lucki I, Reyes TM. Reversal of dopamine system dysfunction in response to high-fat diet. Obesity (Silver Spring) (2013) 21:2513–21. doi:10.1002/oby.20374
67. Dunn JP, Cowan RL, Volkow ND, Feurer ID, Li R, Williams DB, et al. Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings. Brain Res (2010) 1350:123–30. doi:10.1016/j.brainres.2010.03.064
68. Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg (2010) 20:369–74. doi:10.1007/s11695-009-0015-4
69. Bond DS, Jakicic JM, Unick JL, Vithiananthan S, Pohl D, Roye GD, et al. Pre- to postoperative physical activity changes in bariatric surgery patients: self report vs. objective measures. Obesity (Silver Spring) (2010) 18:2395–7. doi:10.1038/oby.2010.88
70. Berglind D, Willmer M, Eriksson U, Thorell A, Sundbom M, Udden J, et al. Longitudinal assessment of physical activity in women undergoing Roux-en-Y gastric bypass. Obes Surg (2015) 25:119–25. doi:10.1007/s11695-014-1331-x
71. Berglind D, Willmer M, Tynelius P, Ghaderi A, Naslund E, Rasmussen F. Accelerometer-measured versus self-reported physical activity levels and sedentary behavior in women before and 9 months after Roux-en-Y gastric bypass. Obes Surg (2016) 26:1463–70. doi:10.1007/s11695-015-1971-5
72. Adams WK, Sussman JL, Kaur S, D’Souza AM, Kieffer TJ, Winstanley CA. Long-term, calorie-restricted intake of a high-fat diet in rats reduces impulse control and ventral striatal D2 receptor signalling – two markers of addiction vulnerability. Eur J Neurosci (2015) 42:3095–104. doi:10.1111/ejn.13117
73. Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, et al. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci (2008) 122:1257–63. doi:10.1037/a0013111
74. Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev (2013) 37:2047–58. doi:10.1016/j.neubiorev.2012.12.001
75. Brownson RC, Boehmer TK, Luke DA. Declining rates of physical activity in the United States: what are the contributors? Annu Rev Public Health (2005) 26:421–43. doi:10.1146/annurev.publhealth.26.021304.144437
76. Ekkekakis P, Vazou S, Bixby WR, Georgiadis E. The mysterious case of the public health guideline that is (almost) entirely ignored: call for a research agenda on the causes of the extreme avoidance of physical activity in obesity. Obes Rev (2016) 17:313–29. doi:10.1111/obr.12369
77. Friend DM, Devarakonda K, O’Neal TJ, Skirzewski M, Papazoglou I, Kaplan AR, et al. Basal ganglia dysfunction contributes to physical inactivity in obesity. Cell Metab (2017) 25:312–21. doi:10.1016/j.cmet.2016.12.001
78. Mathes WF, Nehrenberg DL, Gordon R, Hua K, Garland T Jr, Pomp D. Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav Brain Res (2010) 210:155–63. doi:10.1016/j.bbr.2010.02.016
79. Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol (2006) 291:R889–99. doi:10.1152/ajpregu.00536.2005
80. Teske JA, Billington CJ, Kotz CM. Mechanisms underlying obesity resistance associated with high spontaneous physical activity. Neuroscience (2014) 256:91–100. doi:10.1016/j.neuroscience.2013.10.028
81. Beeler JA, Faust RP, Turkson S, Ye H, Zhuang X. Low dopamine D2 receptor increases vulnerability to obesity via reduced physical activity, not increased appetitive motivation. Biol Psychiatry (2016) 79:887–97. doi:10.1016/j.biopsych.2015.07.009
82. Ruegsegger GN, Speichinger KR, Manier JB, Younger KM, Childs TE, Booth FW. Hypothalamic Npy mRNA is correlated with increased wheel running and decreased body fat in calorie-restricted rats. Neurosci Lett (2016) 618:83–8. doi:10.1016/j.neulet.2016.02.037
83. Levin P, Janda JK, Joseph JA, Ingram DK, Roth GS. Dietary restriction retards the age-associated loss of rat striatal dopaminergic receptors. Science (1981) 214:561–2. doi:10.1126/science.7291993
84. Diao LH, Bickford PC, Stevens JO, Cline EJ, Gerhardt GA. Caloric restriction enhances evoked DA overflow in striatum and nucleus accumbens of aged Fischer 344 rats. Brain Res (1997) 763:276–80. doi:10.1016/S0006-8993(97)00494-0
85. El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest (2000) 105:1827–32. doi:10.1172/JCI9842
86. Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol (2008) 70:537–56. doi:10.1146/annurev.physiol.70.113006.100707
87. Matheny M, Shapiro A, Tumer N, Scarpace PJ. Region-specific diet-induced and leptin-induced cellular leptin resistance includes the ventral tegmental area in rats. Neuropharmacology (2011) 60:480–7. doi:10.1016/j.neuropharm.2010.11.002
88. Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron (2006) 51:811–22. doi:10.1016/j.neuron.2006.09.006
89. Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron (2006) 51:801–10. doi:10.1016/j.neuron.2006.08.023
90. Domingos AI, Vaynshteyn J, Voss HU, Ren X, Gradinaru V, Zang F, et al. Leptin regulates the reward value of nutrient. Nat Neurosci (2011) 14:1562–8. doi:10.1038/nn.2977
91. Carpenter CL, Wong AM, Li Z, Noble EP, Heber D. Association of dopamine D2 receptor and leptin receptor genes with clinically severe obesity. Obesity (Silver Spring) (2013) 21:E467–73. doi:10.1002/oby.20202
92. Fernandes MF, Matthys D, Hryhorczuk C, Sharma S, Mogra S, Alquier T, et al. Leptin suppresses the rewarding effects of running via STAT3 signaling in dopamine neurons. Cell Metab (2015) 22:741–9. doi:10.1016/j.cmet.2015.08.003
93. Meek TH, Dlugosz EM, Vu KT, Garland T Jr. Effects of leptin treatment and Western diet on wheel running in selectively bred high runner mice. Physiol Behav (2012) 106:252–8. doi:10.1016/j.physbeh.2012.02.012
94. Matheny M, Zhang Y, Shapiro A, Tumer N, Scarpace PJ. Central overexpression of leptin antagonist reduces wheel running and underscores importance of endogenous leptin receptor activity in energy homeostasis. Am J Physiol Regul Integr Comp Physiol (2009) 297:R1254–61. doi:10.1152/ajpregu.90449.2008
95. Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci (2011) 12:623–37. doi:10.1038/nrn3111
96. Bobbert T, Mai K, Brechtel L, Schulte HM, Weger B, Pfeiffer AF, et al. Leptin and endocrine parameters in marathon runners. Int J Sports Med (2012) 33:244–8. doi:10.1055/s-0031-1291251
97. Girard I, Rezende EL, Garland T Jr. Leptin levels and body composition of mice selectively bred for high voluntary locomotor activity. Physiol Biochem Zool (2007) 80:568–79. doi:10.1086/521086
98. Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med (1999) 341:879–84. doi:10.1056/NEJM199909163411204
99. Licinio J, Caglayan S, Ozata M, Yildiz BO, De Miranda PB, O’Kirwan F, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A (2004) 101:4531–6. doi:10.1073/pnas.0308767101
100. Morton GJ, Kaiyala KJ, Fisher JD, Ogimoto K, Schwartz MW, Wisse BE. Identification of a physiological role for leptin in the regulation of ambulatory activity and wheel running in mice. Am J Physiol Endocrinol Metab (2011) 300:E392–401. doi:10.1152/ajpendo.00546.2010
Keywords: physical activity, physical inactivity, motivation, dopamine, obesity, leptin
Citation: Ruegsegger GN and Booth FW (2017) Running from Disease: Molecular Mechanisms Associating Dopamine and Leptin Signaling in the Brain with Physical Inactivity, Obesity, and Type 2 Diabetes. Front. Endocrinol. 8:109. doi: 10.3389/fendo.2017.00109
Received: 15 March 2017; Accepted: 05 May 2017;
Published: 23 May 2017
Edited by:
Jonathan Peter Little, University of British Columbia, CanadaReviewed by:
Catherine M. Kotz, University of Minnesota, United StatesLindsay Spielman, University of British Columbia Okanagan, Canada
Copyright: © 2017 Ruegsegger and Booth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frank W. Booth, boothf@missouri.edu
 Gregory N. Ruegsegger
Gregory N. Ruegsegger Frank W. Booth
Frank W. Booth