- 1Bioorganic Research Institute, Suntory Foundation for Life Sciences, Kyoto, Japan
- 2Faculty of Science, Department of Biological Sciences, Nara Women’s University, Nara, Japan
Gonadotropin-releasing hormones (GnRHs) play pivotal roles in reproductive functions via the hypothalamus, pituitary, and gonad axis, namely, HPG axis in vertebrates. GnRHs and their receptors (GnRHRs) are likely to be conserved in invertebrate deuterostomes and lophotrochozoans. All vertebrate and urochordate GnRHs are composed of 10 amino acids, whereas protostome, echinoderm, and amphioxus GnRH-like peptides are 11- or 12-residue peptide containing two amino acids after an N-terminal pyro-Glu. In urochordates, Halocynthia roretzi GnRH gene encodes two GnRH peptide sequences, whereas two GnRH genes encode three different GnRH peptides in Ciona intestinalis. These findings indicate the species-specific diversification of GnRHs. Intriguingly, the major signaling pathway for GnRHRs is intracellular Ca2+ mobilization in chordates, echinoderms, and protostomes, whereas Ciona GnRHRs (Ci-GnRHRs) are endowed with multiple GnRHergic cAMP production pathways in a ligand-selective manner. Moreover, the ligand-specific modulation of signal transduction via heterodimerization among Ci-GnRHR paralogs suggests the species-specific development of fine-tuning of gonadal functions in ascidians. Echinoderm GnRH-like peptides show high sequence differences compared to those of protostome counterparts, leading to the difficulty in classification of peptides and receptors. These findings also show both the diversity and conservation of GnRH signaling systems in invertebrates. The lack of the HPG axis in invertebrates indicates that biological functions of GnRHs are not release of gonadotropins in current invertebrates and common ancestors of vertebrates and invertebrates. To date, authentic or putative GnRHRs have been characterized from various echinoderms and protostomes as well as chordates and the mRNAs have been found to be distributed not only reproductive organs but also other tissues. Collectively, these findings further support the notion that invertebrate GnRHs have biological roles other than the regulation of reproductive functions. Moreover, recent molecular phylogenetic analysis suggests that adipokinetic hormone (AKH), corazonin (CRZ), and AKH/CRZ-related peptide (ACP) belong to the GnRH superfamily but has led to the different classifications of these peptides and receptors using different datasets including the number of sequences and structural domains. In this review, we provide current knowledge of, and perspectives in, molecular basis and evolutionary aspects of the GnRH, AKH, CRZ, and ACP.
Introduction
Discovery of gonadotropin-releasing hormones (GnRHs) as a hypothalamic releasing factor for luteinizing hormone (LH) by Andrew V. Schally and Roger Guillemin in 1971 paved the way for investigation of basal endocrine reproductive systems (1, 2). This is also the origin of long and wide exploration of the GnRH kingdom. Over the past 20 years, GnRH and its related peptides have been identified in the central nervous system of not only non-mammalian vertebrates but also invertebrates such as ascidians, amphioxus, echinoderms, annelids, and mollusks (3–6). Invertebrates lack orthologs of gonadotropin hormones and pituitary glands, indicating that invertebrate GnRHs cannot serve as “gonadotropin-releasing hormones” in the hypothalamus, pituitary, and gonad axis (HPG axis) but rather function as neuropeptides that directly regulate target tissues. The expression of GnRH receptors (GnRHRs) in various tissues also supports non-hypothalamic functions of invertebrate GnRHs.
Various neuropeptides structurally related to GnRHs (Figure 1), such as adipokinetic hormone (AKH), corazonin (CRZ), and AKH/CRZ-related peptide (ACP), have also been identified in diverse invertebrates (3–11). As shown in Figure 1, these peptides share the N-terminal pyro-Glu residue and C-terminal amide and conserve Phe, Trp, or Tyr residue in position 3, Ser or Thr in position 4, and Trp or Tyr in position 7 with a vertebrate GnRH2 (pQHWSHGWYPGa). Furthermore, molecular phylogenetic and phylogenomic analyses of peptide genes have led to the presumption that GnRH, AKH, CRZ, and ACP originated from common ancestors of the Bilateria (3, 7–9), whereas the four peptides have been shown to exhibit distinct physiological functions including activation of lipid-mobilization by AKHs, stimulation of heart rate by CRZs (10), and down-regulation of oocyte proliferation and elevation of total hemolymph lipids by ACP (11) in arthropods. The cognate receptors for these peptides have also been identified in a wide invertebrate species, revealing that all of these receptors belong to the Class A G protein-coupled receptor (GPCR) family (5, 8, 12). Furthermore, sequence comparison and molecular phylogenetic analysis of these receptors have proposed several evolutionary scenarios for hundred million years (Figure 2), leading to the presumption that GnRH, AKH, CRZ, and ACP and their receptors constitute a superfamily (3, 5, 9, 13).
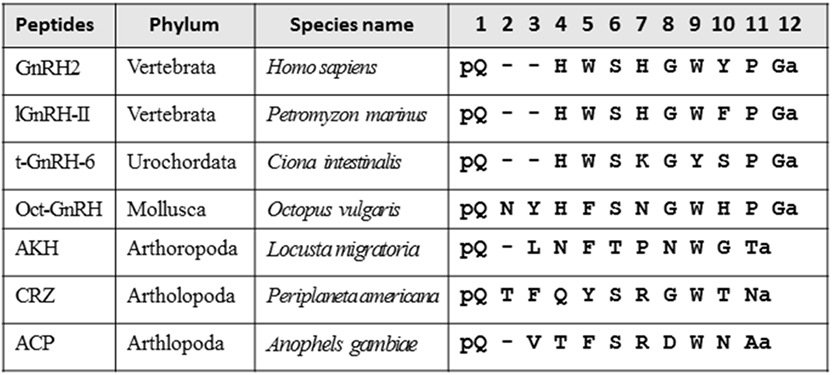
Figure 1. Amino acid sequence alignment of vertebrate gonadotropin-releasing hormones (GnRHs), urochordate GnRH, molluscan GnRH, adipokinetic hormone (AKH), corazonin (CRZ), and AKH/CRZ-related peptide (ACP).
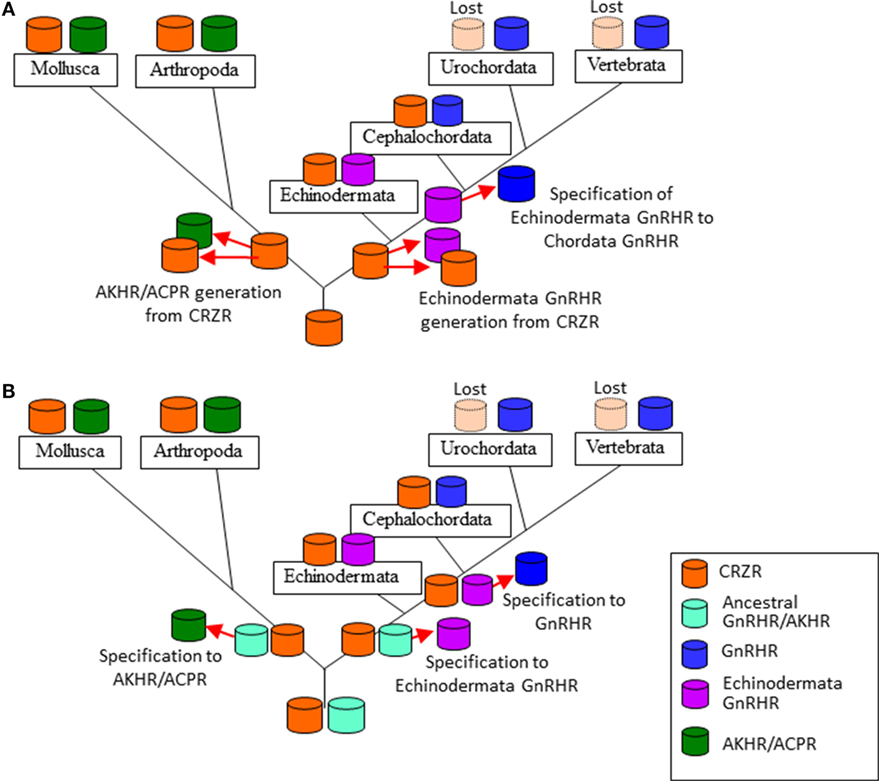
Figure 2. Two evolutionary scenarios of the formation of the gonadotropin-releasing hormone receptor (GnRHR) superfamily. (A) an ancestral corazonin receptor (CRZR), which has been conserved in the Echinodermata and Hemichordata, and the Annelida and the Mollusca, generated two lineages; (1) leading to CRZR and adipokinetic hormone receptor (AKHR), subsequently AKHR generated artholopod ACPR, (2) leading to GnRHR in the Chordata. CRZR was lost after evolution of the Urochordata in deuterostomes. (B) GnRHR and CRZR might have been arisen from an ancestral peptide receptor by gene duplication in a common ancestor of the Bilateria and a second gene duplication of GnRHR gave rise to the AKHR and ACPR in the Protostomia. CRZR has been preserved in all phyla except the Urochortada and the Vertebrata.
In this review, we provide basic and the latest knowledge regarding primary sequences, signal transductions, biological activities of GnRH, AKH, CRZ, and ACP and their receptors, and an overview of molecular evolution of these peptides and receptors.
Gonadotropin-Releasing Hormones
Vertebrate GnRHs
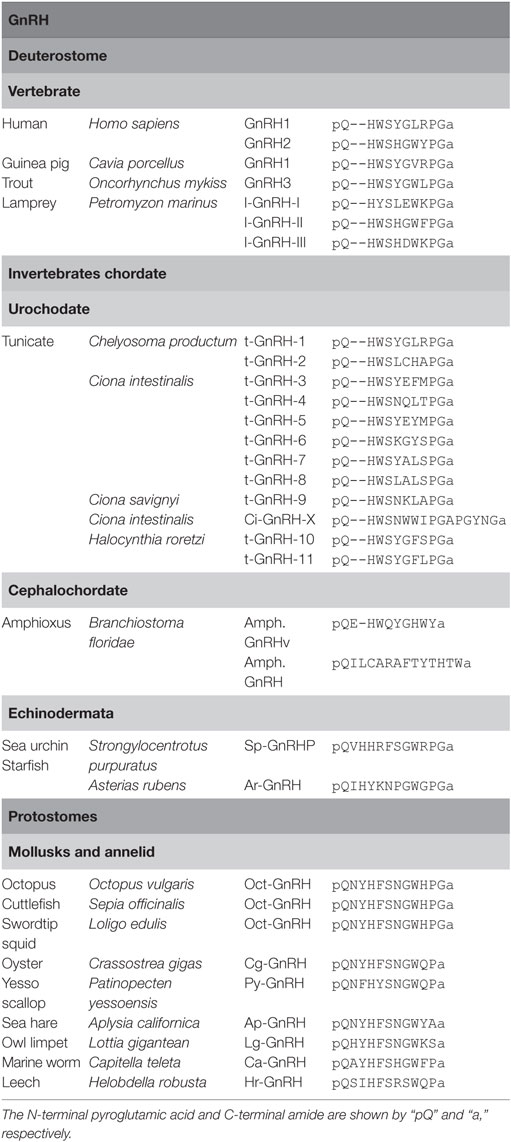
Gonadotropin-releasing hormones are composed of 10 amino acids with consensus sequences of pyro-Glu1-His2-Trp3-Ser4 and Pro9-Gly10-amide and play pivotal roles in reproduction as releasing factor of gonadotropins in vertebrates (4). As shown in Table 1, two types of GnRHs (GnRH1 or Type 1 GnRH and GnRH2 or Type 2 GnRH) have been characterized in most vertebrates, whereas the third subtype was found in teleost and lamprey (14–16). Molecular phylogenetic tree and phylogenetic genomic analyses suggest that these subtypes have been generated by gene duplications within the species (15, 16). In other words, teleost GnRH3 and lamprey GnRH-III are specific paralogs to the respective species.
Urochordate GnRHs
To date, 12 GnRH peptides have been identified in ascidians (Table 1). t-GnRH-1 and -2 were originally identified within the neural extract of an ascidian, Chelyosoma productum (17). Subsequently, ascidian GnRHs were isolated from other ascidians, Ciona intestinalis and Ciona savignyi (18). The former ascidian produces t-GnRH-3 to -8, and the latter generates t-GnRH-5 to -9 (18). In Halocynthia roretzi, t-GnRH-10 and -11 were characterized (19). All of these ascidian GnRHs conserve the consensus sequences of pyro-Glu1-His2-Trp3-Ser4 and Pro9-Gly10-amide of vertebrate GnRHs. Furthermore, a unique GnRH-related peptide, Ci-GnRH-X, was isolated from the neural tissue of C. intestinalis and was found to be composed of 16 amino acids harboring the consensus sequence of pyro-Glu1-His2-Trp3-Ser4 and Pro9-Gly10 and C-terminal Gly-amide (4, 20). The striking feature of ascidian GnRHs is multicopies of GnRH sequences in a single precursor, unlike vertebrate and non-ascidian invertebrate GnRH genes that encode a single GnRH sequence (4, 21). For instance, ci-gnrh-1 encodes t-GnRH-3, -5, and -6, whereas t-GnRH-4, -7, and -8 sequences are found in another gene, ci-gnrh-2 (18). Likewise, the H. roretzi GnRH gene encodes t-GnRH-10 and -11 (19). These findings indicate conservation and species-specific diversification of GnRHs in urochordates.
Cephalochordate and Echinoderm GnRH-Like Peptides
In the cephalochordate (amphioxus), Branchiostoma floridae, a GnRH-like peptide, Amph.GnRHv (pQEHWQYGHWYa, Table 1) was identified (12). Recently, GnRH-like peptides, SpGnRHP and ArGnRH (Table 1), were identified in the echinoderms, the sea urchin, Strongylocentrotus purpuratus (22) and the starfish, Asterias rubens (8), respectively. Unlike vertebrate and ascidian GnRHs, SpGnRHP and ArGnRH are 12-residue peptides containing a Val2-His3 or Ile2-His3 sequence, respectively (Table 1). These peptides share several amino acids with urochordate and vertebrate GnRHs and protostome GnRH-like peptides, including the N-terminal pGlu, His4 (corresponding His2 in chordate GnRHs), Gly8 (corresponding Gly6 in vertebrate GnRHs), Trp9 (corresponding Trp7 in vertebrate GnRHs), and C-terminal Pro-Gly-amide, whereas the GnRH N-terminal consensus motif displays quite low sequence homology (Table 1). Thus, categorization of the echinoderm peptides as the authentic GnRH family may remain to be concluded.
Protostome GnRH-Like Peptides
Over the past 15 years, GnRH-like peptides have been identified in protostomes including mollusks and annelids (4) (Table 1): an octopus, Octopus vulgaris; a cuttlefish, Sepia officinalis; a pacific oyster, Crassostrea gigas; a sea hare, Aplysia californica; a marine worm, Capitella teleta; a leech, Helobdella robusta; a scallop, Patinopecten yessoensis. Noteworthily, two-amino acid insertion after position 1 is found in all protostome GnRH-like peptides (Table 1). Collectively, these GnRH sequences indicate that 10-amino acid sequence length is conserved within ascidians and vertebrates, whereas protostome and non-chordate invertebrate GnRHs are featured by 2-amino acid insertion. In other words, ancestral GnRHs might have harbored such two amino acids after pyro-Glu, which might have been lost during the chordate evolutionary process.
The C-terminal Pro-Gly-amide of ascidian and vertebrate GnRHs is found in oct-GnRH of cephalopods and echinoderms but not in GnRH-like peptides of gastropods, bivalves, and annelids (Table 1), suggesting that cephalopods and echinoderms might have conserved the C-terminal Pro-Gly during their evolutionary processes. Furthermore, all known protostome GnRH-like peptides and SP-GnRHP share the Ser in position 6 or 7, while the Gly8-Trp9 sequence is conserved in cephalopod GnRH, echinoderm GnRHs, and l-GnRH-II but not in ascidian GnRHs and other vertebrate GnRHs except l-GnRH-II (Table 1). Additionally, substitution of Trp3 in the N-terminal consensus motif with Phe was found in most protostome GnRHs (Table 1). Altogether, these sequences led to the presumption that the ancestral GnRHs might have been composed of pQ-H(F/W)S-GW-PGa or pQ-H(F/W)S-GW-a, and thereafter, chordate GnRHs might have diverged via various substitution and deletion of the two N-terminal amino acids in the evolutionary process of each species.
Vertebrate GnRHRs
Gonadotropin-releasing hormone receptors belong to the Class A GPCR family (4, 14). In most vertebrates, two or three molecular forms of GnRHRs are present (14). Molecular phylogenetic analyses have provided evidence that vertebrate GnRHRs are classified into three groups, type-I, -II, and -III. The type-I GnRHRs were characterized from a wide range of vertebrate species such as teleost, amphibians, reptiles, birds, and mammals (23). Mammalian type-I GnRHRs completely lack the C-terminal tail region, which is present in its non-mammalian receptors (14). The type-II gnrhr gene is found in the genome of amphibians, reptiles, aves, and mammals (23). Most mammalian type-II gnrhr is non-functional due to the deletion of functional domains or interruption of full-length translation by the presence of a stop codon. In contrast, type-II GnRHRs of several monkeys, pigs, and other non-mammalian vertebrates were shown to be functional (23). Type-I GnRHRs show high affinity for both GnRH1 and 2, whereas type-II GnRHRs are specifically responsive to GnRH2 (14). Type-III GnRHRs were identified in non-mammalian vertebrates (24). In chicken, type-III GnRHR exhibits a 35-fold higher affinity for GnRH2 than for GnRH1 (24). GnRHRs are in general coupled with Gq protein and activate a typical phospholipase C (PLC)–inositol triphosphate (IP)–intracellular calcium mobilization signaling cascade, occasionally leading to phosphorylation of mitogen-activated protein kinase (MAPK) including ERK1/2 (4, 25, 26), while some GnRHRs are also found to trigger or suppress cAMP production via coupling with Gs or Gi protein (21, 25–28).
Invertebrate GnRHRs
Ascidian GnRHRs
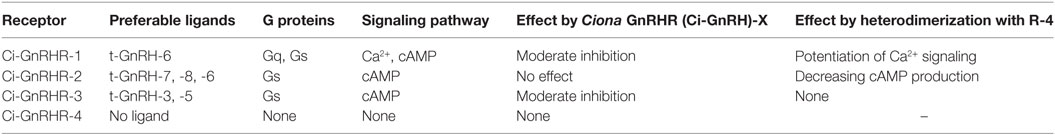
In C. intestinalis, four GnRHRs, Ciona GnRHR (Ci-GnRHR)-1 to -4, have been identified and shown to regulate exceptionally complicated signaling pathways involving ligand-receptor selectivity, coupling with multiple G-protein subtypes, and receptor heterodimerization (Table 2).
Ciona GnRHR-1, -2, and -3 sequences were found to harbor a long C-terminal tail, whereas a short tail is present in the C-terminus of Ci-GnRHR-4 (27). Ci-GnRHR mRNAs are distributed in the neural complex, heart, intestine, endostyle, branchia sac, and ovary, although biological roles of GnRHs largely remain unclear (26–28). Notably, the elevation of intracellular calcium, which is a typical response of GnRHR activation, was observed only in the t-GnRH-6 and Ci-GnRHR-1 pair (27). t-GnRH-6 also induces cAMP production via Ci-GnRHR-1 (27). Ci-GnRHR-2 exclusively stimulates cAMP production in response to t-GnRH-7, -8, and -6 in this order of potency (27). Ci-GnRHR-3 triggers cAMP production in the presence of t-GnRH-3 and -5 to a similar extent in a ligand-specific fashion. Ci-GnRHR-4 exhibited neither elevation of intracellular calcium nor production of cAMP (27). Induction of intracellular mobilization only by t-GnRH-6 and Ci-GnRHR-1 pair is attributed to the conservation of Gly6 essential for adoption of the tertiary structure for coupling with Gq (14) exclusively in t-GnRH-6 (Table 1). Such signaling profiles indicate that a major Ciona GnRH signaling is a cAMP production. Additionally, Ciona 16-amino acid GnRH-structurally related peptide, Ci-GnRH-X, was shown to exhibit moderately inhibit activation of Ci-GnRHR-1 and -3 (20). Also of particular interest in Ci-GnRHR signaling is that Ci-GnRHR-4 heterodimerizes with Ci-GnRHR-1 and then potentiates the elevation of intracellular calcium via both calcium-dependent and -independent protein kinase C subtypes and ERK phosphorylation in a ligand-selective fashion (26). Ci-GnRHR-4 was also found to heterodimerize with Ci-GnRHR-2 (28). The Ci-GnRHR-2/-4 heterodimer decreased cAMP production by 50% in a non-ligand selective manner by shifting of activation from Gs protein to Gi protein by Ci-GnRHR-2, compared to the Ci-GnRHR-2 monomer/homodimer (28). These findings verify that Ci-GnRHR-4 serves as a protomer of GPCR heterodimers rather than a ligand-binding receptor (4, 21, 29). In addition, molecular phylogenetic analysis demonstrated that Ci-GnRHRs are included in vertebrate GnRHR clades but form an independent cluster in chordate GnRHRs, suggesting that these receptors have evolved within the Ciona species (4, 21, 27, 29, 30). Collectively, these findings indicate ascidian-specific molecular and functional diversity of ascidian GnRH signaling systems.
Amphioxus GnRHRs
Four amphioxus receptors have been identified in the amphioxus, B. floridae. Amphioxus GnRHR-1 and -2 were activated only by vertebrate GnRHs but not by Amph.GnRHv, a putative B. floridae endogenous GnRH-like peptide that displays the highest sequence similarity to other species GnRHs (Table 1), whereas GnRHR-3 was activated exclusively by another amphioxus GnRH- and CRZ-like peptide (Table 2), oct-GnRH, and AKH at physiological concentrations (12, 31), indicating that amphioxus GnRHR-3 exhibits extensive ligand selectivity for GnRH superfamily peptides. Unlike Ci-GnRHRs, B. floridae GnRHR-1 to -3 were shown to stimulate only intracellular IP accumulation (12). In contrast, no ligands induced IP accumulation or cAMP stimulation via amphioxus GnRHR-4 (12, 31). It should be noted that the Amph.GnRHv failed to activate any of the four GnRHRs (12). Molecular phylogenetic analysis demonstrated that amphioxus GnRHR-1 and -2 are included in the vertebrate GnRHR clade, while amphioxus GnRHR-3 and -4 are likely to belong to the CRZR/GnRHR clade, as described later. Consequently, the authors presumed that the sequence of the neuropeptide might reflect ancestral sequence of CRZ/GnRH or the transition state between CRZ and GnRH (12). Moreover, of keen interest is the identification of authentic (endogenous) ligands for amphioxus GnRHR-1 and -2. Thus, the elucidation of authentic amphioxus GnRH–receptor pairs requires further investigation. Such difficulty may be attributed to some mismatch between amphioxus GnRHRs and cultured cells employed for heterologous functional analysis because of unsuccessful translation of the receptor mRNA or degradation of the receptor protein in heterologous expression systems (32).
Echinoderm GnRHRs
Tian et al. (8) demonstrated that Ar-GnRH (Table 1) specifically activated intracellular Ca2+ mobilization of a cognate receptor, ArGnRHR in the starfish, A. rubens. Four GnRH/CRZ-type receptors have also been identified in the sea urchin, S. purpuratus using in silico screening (22). However, no functional analysis of these receptors has been reported. Additionally, these echinoderm receptors are included in the invertebrate CRZ/GnRHR clade (12, 31).
Protostome GnRHRs
The first protostome GnRHR was identified in an octopus, O. vulgaris. The octopus GnRHR, oct-GnRHR, activates intracellular Ca2+ mobilization by oct-GnRH but not vertebrate GnRHs (33). Notably, an oct-GnRH synthetic analog with Asn2-Tyr3 deletion abolished the ability to activate the Ca2+ pathway via oct-GnRHR, whereas a chicken GnRH-II synthetic analog with an Asn-Tyr insertion after position 1 exhibited weak activation (33). These findings verify that Asn2-Tyr3 is required for the activation of oct-GnRHR, suggesting that the two amino acids after position 1 in non-chordate GnRHs are responsible for activating the protostomian GnRHR. Oct-gnrhr is expressed in the central nervous system, digestive tissues, aorta, heart, salivary gland, branchia, radula retractor muscle, egg, and genital organs in the common octopus (33). In another mollusk, gastropod (a sea hare) A. californica GnRHR, ap-GnRHR, was also cloned and was found to be expressed in the abdominal, cerebral, and buccal ganglia of the central nervous system and a few peripheral tissues including the chemosensory organ, small hermaphroditic duct, and ovotestis (13). ap-GnRH was shown to increase the IP accumulation but not cAMP production in ap-GnRHR-expressing Drosophila S2 cells in a ligand-specific manner (13). Phylogenetic analysis suggests that ap-GnRHR is clustered with several molluscan GnRHRs including oct-GnRHR, amphioxus GnRHR-3 and -4, and multiple insect CRZRs (13).
Biological Functions
In vertebrates, GnRH is synthesized in the hypothalamus, transported to the pituitary and triggers release of follicle-stimulating hormone (FSH) and LH from the pituitary, eventually regulating reproductive functions via the HPG axis. GnRH also serves as a peripheral bioactive peptide including induction of the synthesis and release of sex steroids in vertebrate reproductive tissues (14). The HPG axis-directed endocrine systems were acquired during the vertebrate evolutionary process, and thus, invertebrate GnRHs are likely to have prototypic or species-specific biological roles.
In ascidians, GnRHs were found to increase water flow and then induce the release of eggs and sperm by injection into the gonaducts, ovary, stomach, and posterior body cavity of C. intestinalis (18, 34). All four Ci-gnrhr genes were shown to be expressed in the brain of the larva of C. intestinalis (30). Ci-gnrhr-1 and -2 genes are expressed in muscle cells, while Ci-gnrhr-3 gene is expressed in notochord cells in the larval tail, which is rapidly resorbed during metamorphosis (30). Intriguingly, Kamiya et al. (35) demonstrated that tGnRH-3 and -5 suppressed the growth of adult organs by arresting cell cycle progression and the promotion of tail absorption. These results indicate that t-GnRHs play a pivotal role in the development and/or metamorphosis.
oct-GnRH induced contraction of the oviduct (36) and releases sex steroids, including testosterone-, progesterone-, and 17β-Estradiol-like steroids from the follicle and spermatozoa in octopus (33). In another mollusk, the yesso scallop (Patinopecten yessoesis), py-GnRH induces testicular cell proliferation (37). These findings suggest that molluscan GnRHs directly activate the gonadal organs as a bioactive peptide. In contrast, injection of the cognate ap-GnRH into sexually mature and immature sea hares exhibited no effects on ovotestis mass, reproductive tract mass, egg-laying, or penile eversion, altering oocyte growth and egg-laying hormone accumulation and secretion (38). Instead, ap-GnRH exerted stimulation of the parapodial opening, inhibition of feeding, and promotion of substrate attachment (38). These findings, combined with distribution of GnRHR mRNAs in various tissues, suggest that invertebrate GnRHs regulate not only reproductive responses but also other various biological behaviors. Indeed, oct-GnRH induced contraction of the radula retractor muscle expressing oct-gnrhr (33).
Adipokinetic Hormones
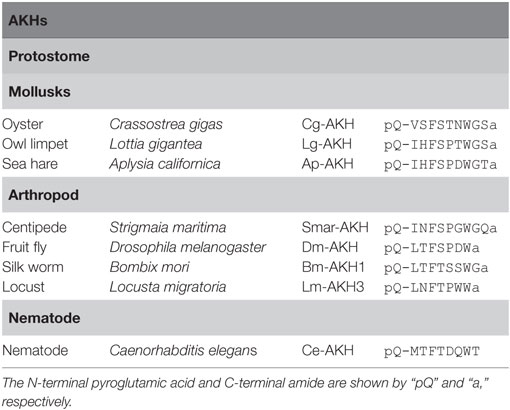
Adipokinetic hormone was originally identified in the migratory locust, Locusta migratoria as a lipid mobilizing factor (39). To date, AKHs have been isolated from insects, mollusks, and nematode (Figure 1; Table 3). AKHs are composed of 8–10 residues, harboring pGlu in position 1, an aliphatic or aromatic amino acid residue at position 2, Phe-Ser, Phe-Thr, or Tyr-Ser residues at positions 4 and 5, Trp at position 8, and Trp-amide, Trp-Gly-amide, or Trp-Gly-X-amide (where “X” is variable) at the C terminus (5). Li et al. have proposed to classify these peptides in the Protostomia as follows: authentic AKHs that fulfill the above hallmarks, AKH-like peptides that 10 amino acid residues have Trp-X-Gly-amide or Trp-X-Pro-amide at the C terminus, and proto-AKHs that are longer than 10 amino acid residues but have only 2–4 of the AKH hallmarks (40).
AKH Receptors
Adipokinetic hormone receptors belong to the Class A GPCR family identified in protostomes. Zhu et al. demonstrated that AKH activates both cAMP accumulation and Ca2+ mobilization via AKHR of the silkworm moth, Bombix moli (41). Recently, Li et al. demonstrated that AKHR of the oyster Crassostrea gigas was activated by oyster AKH at physiological concentrations (40). Moreover, Nagasawa et al. detected expression of Py-AKHR mRNAs in the nerve ganglia, lip, foot, CPG, mantle, testis, and ovary in Yesso scallop, Patinopecten yessoensis. The differential expression profile of Py-AKHR mRNA in the gonad during gonadal maturation stages suggests their reproductive function (42).
Biological Functions
Adipokinetic hormones have so far been shown to stimulate the fat body, resulting in lipid and carbohydrate mobilization into the hemolymph in insects and crustaceans. Furthermore, a homolog of AKH in the northern shrimp, Paudalus borealis, red pigment concentrating hormone, influenced the concentration of pigment chromatophore, causing its body color change (43). Notably, AKHs also showed a reduction in oocyte protein and carbohydrate content in the crickets, Gryllus bimaculatus, and a reduction in vitellogenin of oocytes in L. migratoria (44), indicating a regulatory role for AKHs in insect reproduction. AKH-deficient flies displayed the opposite phenotype in which hemolymph trehalose levels decreased and storage lipid in the fat body accumulated (45). An AKH receptor-deficient strain showed a similar phenotype to AKH-deficient flies (46). In the cricket G. bimaculatus, AKH receptor knockdown by RNAi increased feeding frequency and reduced locomotor activity (47).
Corazonins
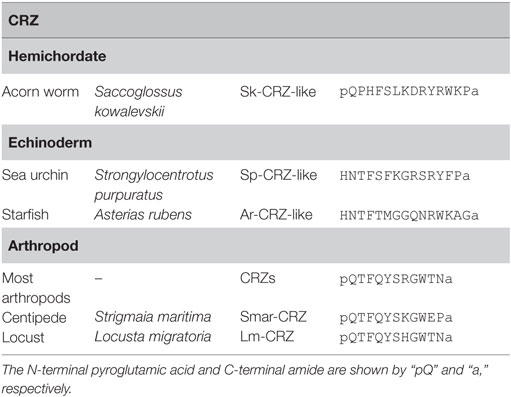
Corazonins were originally characterized as 11-amino acid arthropod neuropeptides from the cockroach, Periplaneta americana (10). A striking feature is the highest conservation of sequence similarity of CRZs in the Arthropoda regardless of diverse functions throughout a variety of species (Table 4). [Arg7]-CRZ (pQTFQYSRGWTN-amide) is the most typical CRZ peptide, and only a few homologs such as [His7]-CRZ, [Gln10]-CRZ, and [His4-Gln7]-CRZ have been found in several insects (5). Recently, however, neuropeptides weakly similar to CRZ have been identified in starfish (HNTFTMGGQNRWKAG-amide), sea urchin (HNTFSFKGRSRYFP – amide), and acorn worm (pQPHFSLKDRYRWK-amide) (Table 4), and the starfish peptide was shown to be responsive to the cognate CRZ-type receptor, leading to the presumption that these peptides are invertebrate deuterostome CRZs as putative CRZ-type receptor ligands (8).
CRZ Receptors (CRZRs)
Corazonin receptors are class A family GPCRs. The first CRZR was identified in Drosophila melanogaster, and then orthologous receptors have been cloned from moths, mosquitoes, honey bee, and other insects (48) (Figure 3). CRZR of the silkworm moth Bombix mori induces cAMP accumulation, Ca2+ mobilization, and ERK1/2 phosphorylation via the Gq- and Gs-coupled signaling pathways in response to CRZ (49). Various molecular phylogenetic analyses indicate that the annelid and molluscan GnRHRs are clustered with the CRZRs and the annelid and molluscan GnRHRs have been recognized as the members of CRZR/GnRHR clade (5, 12, 13). In the starfish, Asterias rubens, CRZ-like peptide (HNTFTMGGQNRWKAG-amide) was identified and also found to activate the cognate receptor (8). Likewise, GnRHR-type receptor was identified and found to be activated specifically by the cognate GnRH-like peptide (pQIHYKNPGWGPG-amide) in a ligand-specific manner (8). Collectively, these results suggest that echinoderms, at least A. rubens, may be endowed with the GnRH- and CRZ-directed signaling systems (8).
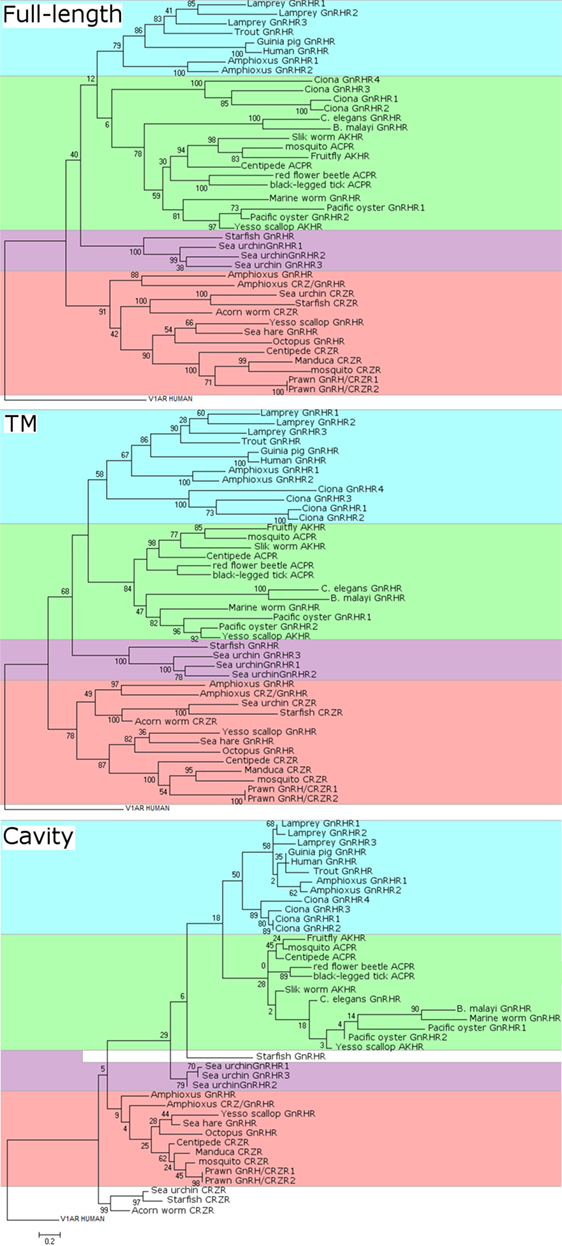
Figure 3. Molecular phylogenetic analysis of full-length (top), transmembrane (TM) domain (middle), and cavity (bottom) sequences of gonadotropin-releasing hormone receptors (GnRHRs), AKHRs, corazonin receptors (CRZRs), and ACPRs. The sequence alignments were constructed using MUSCLE in MEGA version 7 and GPCRalign (61) for full-length alignments and TM alignments, respectively. GPCRalign is a PSSM-based alignment algorism and output total 201-length gapless alignments corresponding to TM region. The output TM sequences are listed in Supplementary Material 1–7. The cavity amino acid positions in TM alignment were extracted according to previous report (60). The cavity sequences are listed in Supplementary Material 8. A phylogenic tree of GnRHRs was constructed by the maximum likelihood method based on the JTT matrix-based model. For full-length phylogenetic tree, all positions containing gaps and missing data were eliminated. The scale bar indicates the evolutionary distance of 0.2 amino acid substitutions per protein. The number at each branch node represents percentage given by 100 bootstrap replicates. Evolutionary analyses were conducted in MEGA version 7. The sequences used were as follows: human GnRHR (GNRHR_HUMAN); guinea pig GnRHR (GNRHR_CAVPO); marine worm GnRHR (R7U4C9_CAPTE); sea urchin GnRHR-1 (B2BF80_STRPU); sea urchin GnRHR-2 (B2BF81_STRPU); sea urchin GnRHR-3 (B2BF82_STRPU); tunicate GnRHR-1 (Q869J2_CIOIN); tunicate GnRHR-2 (Q869J1_CIOIN); tunicate GnRHR-3 (D2KZ68_CIOIN); tunicate GnRHR-4 (D2KZ69_CIOIN); trout GnRHR (Q9I986_ONCMY); lamprey GnRHR-1 (A9XCD3_PETMA); lamprey GnRHR-2 (A9XCD4_PETMA); lamprey GnRHR-3 (A9XCD5_PETMA); octopus GnRHR (GNRHR_OCTVU); amphioxus GnRHR-1b (A9XCD1_BRAFL); amphioxus GnRHR-2b (A9XCD2_BRAFL); amphioxus GnRHR-3 (C0IP22_BRAFL); amphioxus GnRHR-4 (C4N9P5_BRAFL); pacific oyster GnRHR-2 (B1GVI7_CRAGI); nematode GnRHR (O44731_CAEEL); sea hare GnRHR (Refseqid:AHE78444); filarial nematode worm GnRHR (A8PVQ9_BRUMA); starfish GnRHR (A0A1B0YGS0_ASTRU); yesso scallop GnRHR (Refseqid: BAX08608); pacific oyster AKHr1b (B1GVI4_CRAGI); fruit fly AKHR (Q71EB3_DROME); silk worm AKHR (Q8T6U9_BOMMO); yesso scallop AKHR (Refseqid: BAX08609); centipede ACPR (Refseqid: AFFK01020326); red flour beetle ACPR (D5FFV2_TRICA); black-legged tick ACPR (A0A0 × 7YC79_IXOSC); honeybee CRZR (B7ZKE3_APIME); tobacco hawk moth CRZR (Q6UJG5_MANSE); sea urchin CRZR (Refseqid: XP_011680711); starfish CRZR (A0A1B0YGT7_ASTRU); centipede CRZR (Refseqid: AFFK01019957); and acorn worm CRZR (Refseqid: XP_006820827).
Notably, as stated earlier, B. floridae (amphioxus) GnRHR-3 and -4 are highly homologous to the protostome CRZR/GnRHR receptor family and GnRHR-3 was activated by the amphioxus GnRH-like peptide (pQILCARAFTYTHTW-amide), oct-GnRH (pQNYHFSNGWHPG-amide), and AKH (pQLTFTSSW-amide) at physiological concentrations, indicating that B. floridae GnRHR-3 exhibits extensive ligand selectivity for GnRH superfamily peptides. The CRZ/CRZR signaling system has been lost in urochordates, vertebrates, nematodes, and some insects (50).
Biological Functions
Corazonins have a number of physiological roles associated with control of heartbeat, ecdysis behavior initiation, and cuticle coloration in the Artholopoda (5, 48). Recently, its regulatory functions on insulin producing cells in the brain of D. melanogaster (51) and on larval–pupal transition and pupariation behavior have been found in the fruit fly, Bactrocera dorsalis (52). Intriguingly, CRZs also show reproductive activities in invertebrates. In male flies, CRZs act on its receptor in a small cluster of posterior serotoninergic neurons to control activity of the accessory glands and sperm ejaculation during mating (53). In the giant freshwater prawn Macrobrachium rosenbergii, CRZs inhibit testicular development and spermatogenesis and androgenic gland secretion (54). Ablation of CRZ-GAL4 neurons increased locomotion and dopamine level in male files, D. melanogaster. Furthermore, silencing of CRZR-GAL4 neurons in male flies elicits infertility and blocks sperm and seminal fluid ejaculation (53). In B. mori, dsRNA-mediated knockdown of BmCrzR indicated a role of CRZ signaling in the regulation of silkworm growth and silk production (49).
AKH/CRZ-Related Peptides
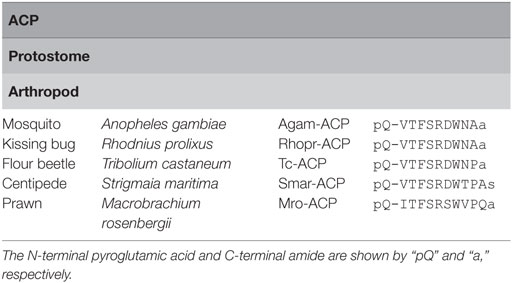
Adipokinetic hormone/CRZ-related peptide is a10–11-amino acid arthropod peptide originally identified from the malaria mosquito, Anopheles gambiae (55). In contrast to AKHs, sequences of ACPs, in particular, the N-terminal sequence “QXTFSRXW” (where “X” is variable) and C-terminal amidation, are well conserved in arthropods (Table 5), which is reminiscent of an intermediate between AKHs and CRZs (5).
ACP Receptors
Adipokinetic hormone/CRZ-related peptide receptors are Class A family GPCRs identified only in insects (Figure 3). Hansen et al. showed that the A. gambiae ACP receptor transfected into mammalian cells stably expressing the human G-protein G16, a universal G protein adapter, was activated specifically by the cognate ligand (55). Zandawala et al. characterized three splice variants encoding ACP receptors in the kissing bug Rhodnius prolixus; Rhopr-ACPR-A has only five transmembrane (TM) domains, and Rhopr-ACPR-B and C have seven TM domains. All Rhopr-ACPR-A, -B, and -C were activated by Rhopr-ACP but neither Rhopr-AKH nor Rhopr-CRZ with different sensitivities on mammalian cells stably expressing the G-protein G16, whereas Rhopr-ACPR-B and -C indicated coupling with Gq when expressed in CHO-K1-aeq cells (56).
Biological Functions
To date, the ACP signaling system has been found only in arthropods and its major biological roles are still unclear. However, recently, ACP was shown to decrease germ cell proliferation and increases in total hemolymph lipids were found by administration of the peptide in female prawn, M. rosenbergii (11). The expression of MroACP mRNA in the eyestalk, central nervous system, thoracic ganglia, and MroACPR mRNA in the neural tissues and the ovary throughout different stages of ovarian maturation indicated a neuronal regulation of ACP signaling in reproduction (11).
Proposed Evolutionary Scenarios of GnRH, AKH, CRZ, ACP, and Their Receptors
Based on the aforementioned sequence homology and molecular phylogeny, several studies suggested that GnRH, AKH, CRZ, and ACP constitute a superfamily and originated from a common ancestor (57, 58). However, marked sequence diversity in GnRH, AKH, CRZ, and ACP has led to difficulty in accurate or conclusive classification. For example, Lindemans et al. suggested that GnRH signaling might have been arisen before the divergence of protostomes and deuterostomes on the basis of the presence of the AKH-GnRH signaling system in the nematode Caenorhabditis elegans and its biological function in the egg-laying behavior (59). However, molecular phylogenetic analysis led to another presumption that the C. elegans AKH-GnRH-like peptide and its receptor belong to the authentic AKH system (5, 50, 59).
As described early, authentic GnRHRs are conserved at least in the Cephalochordata, Urochordata, and the Vertebrata. AKHRs have been identified in the Mollusca, Annelida, and Arthropoda, while ACPRs have been found only in the Arthropoda. Authentic or putative CRZRs are present in all invertebrates except the Urochordata. Molecular phylogenetic analysis (3, 5, 8, 13, 50) has thus far provided two scenarios of their evolutionary processes. The first one is that an ancestral CRZR, which has been conserved in the Ambulacraria (the Echinodermata and the Hemichordata) and the Lophotrochozoa (the Annelida and the Mollusca), generated two lineages: (1) leading to CRZR and AKHR, subsequently AKHR generated artholopod ACPR in the Ecdysozoa and (2) leading to GnRH in the Chordata. CRZR was lost during the evolution of the Urochordata and Vertebrata in deuterostomes (Figure 2A). The second one is that GnRHR and CRZR might have been arisen via gene duplication in a common ancestor of the Bilateria, and a second gene duplication of GnRHR might have generated AKHR and ACPR during the divergence of the Lophotrochozoa and Ecdysozoa (the Arthropoda and the Nematoda). CRZR has been conserved in all phyla except the Urochordata and Vertebrata (Figure 2B). Notably, these receptors were categorized as different clusters by respective research groups, e.g., GnRHR/AKHR/ACPR and CRZ (8); GnRHR, AKHR/ACPR, and CRZR (13); GnRHR, AKHR/ACPR, and CRZR/protostome GnRHR (3); and GnRHR, AKHR, ACPR, CRZR/protostome GnRHR, and CRZR (5). Furthermore, based on the results of phylogenomic analyses with 36 whole genome sequences and no functional connection of protostome GnRH signaling system to the releasing of gonadotropins because of the lack of the HPG axis in protostomes, Plachetzki et al. classified protostome GnRHs as CRZ-like (or ACP/AKH-like) peptides and categorized the receptors of GnRH superfamily as GnRHRs and CRZR (or ACPR/AKHR) (9).
Such data are mainly attributed to difference in the number, length, and domain of sequences employed for molecular phylogenetic analysis. Figure 3 shows our reanalysis of the molecular phylogeny of full-length, TM domain, and ligand-binding cavity (60) sequences of 42 receptors including GnRHRs, AKHRs, CRZRs, and ACPRs. Full-length sequences of these receptors were aligned with CLUSTALW using BLOSUM62 substitution matrix. Amino acid sequences of the TM and the cavity were individually aligned with GPCRalign (61). All of molecular phylogenetic tree analyses of the full-length sequences (Figure 3) demonstrate that these receptors are classified into the following four major clusters: (1) vertebrate GnRHRs and amphioxus GnRHR-1 and -2 (highlighted in blue), (2) invertebrate GnRHRs/AKHRs/ACPRs including the urochordate GnRHRs, Ci-GnRHRs (highlighted in green), (3) echinoderm GnRHRs (highlighted in purple), and (4) protostome GnRHRs/amphioxus GnRHR-3 and 4/CRZRs (highlighted in orange). Of note, molecular phylogenetic analyses of the TM (Figure 3, middle) and cavity (Figure 3, bottom) sequences of these receptors indicate that Ci-GnRHRs and amphioxus GnRHR-1 and -2 are included in the clade of vertebrate GnRHRs, although many bootstraps in the molecular phylogenetic tree of the cavity are very low due to much smaller information of cavity sequences (30–40 amino acids) than that of full-length and TM, suggesting extremely weak evolutionary correlations. In contrast, Ci-GnRHRs are included in the AKHR/ACPR cluster in a molecular phylogenetic tree of the full-length sequences (Figure 3, top). Moreover, the molecular phylogenetic trees of the TM and the cavity regions indicate that echinoderm “GnRHRs” form a monophyletic clade and display closer homology to the CRZR family than the GnRHR family. This molecular phylogenetic tree is consistent with species-specific sequences of echinoderm GnRH-like peptides (Table 1), suggesting species-specific diversification of echinoderm GnRH and GnRHR lineages. In combination, conservation of partial consensus motifs and molecular phylogenetic analyses are not sufficient for substantiating the evolutionary process of the “GnRH/AKH/CRZ/ACP superfamily,” which may mislead us to an incorrect conclusion.
Conclusion and Perspetives
In the Vertebrata, GnRHs play pivotal roles in reproductive function as a releasing factor for gonadotropin in the HPG axis and a neuropeptide that directly regulate target tissues. In contrast, reproductive functions of invertebrate GnRHs have not been demonstrated. Instead, there has been a growing body of reports of reproductive functions of invertebrate GnRH-related peptides, AKH, CRZ, and ACP. These findings suggest that, if GnRH, AKH, CRZ, and ACP constitute a superfamily, the superfamily peptides might have been endowed with both common and species-specific reproductive functions as well as other physiological functions. In this regard, of particular interest are biological roles of GnRHs or GnRH-like peptides in protostomes, echinoderms, cephalochordates, and urochordates, which lack the HPG axis.
Gonadotropin-releasing hormone, AKH, CRZ, and ACP bear approximately 10 amino acids, and the respective “consensus motifs” are frequently diverged among species. Furthermore, the nested clusters (Figure 3) within GnRHRs, AKHRs, and CRZRs in molecular phylogenetic trees of the TM and the cavity imply that a small number of amino acid substitutions in these regions can change their ligand selectivity. Therefore, only standard homology-based analysis may lead to insufficient data for understanding the evolutionary process of these peptides and receptors. Consequently, integration of multiple molecular phylogenetic analyses of much more sequence information of these peptides and receptors in other invertebrates with biological roles of these signaling systems in various invertebrate species will enable us to elucidate their biological significance and true evolutionary processes.
Author Contributions
TS and HS conducted manuscript preparation. TS, TK, SM, MA and HS investigated background literatures. TS, AS and HS wrote manuscripts. AS and HS analyzed data.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by the Japan Society for the Promotion of Science to HS (16K07430).
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fendo.2017.00217/full#supplementary-material.
References
1. Amoss M, Burgus R, Blackwell R, Vale W, Fellows R, Guillemin R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun (1971) 44:205–10. doi:10.1016/S0006-291X(71)80179-1
2. Schally AV, Arimura A, Baba Y, Nair RMG, Matsuo H, Redding TW, et al. Isolation and properties of the FSH and LH-releasing hormone. Biochem Biophys Res Commun (1971) 43:393–9. doi:10.1016/0006-291X(71)90766-2
3. Roch GJ, Busby ER, Sherwood NM. Evolution of GnRH: diving deeper. Gen Comp Endocrinol (2011) 171:1–16. doi:10.1016/j.ygcen.2010.12.014
4. Kawada T, Aoyama M, Sakai T, Satake H. Structure, function, and evolutionary aspects of invertebrate GnRHs and their receptors. In: Scott-Sills E, editor. Gonadotropin-Releasing Hormone (GnRH): Production, Structure and Functions. New York: Nova Science Publishers, Inc. (2013). p. 1–15.
5. Hauser F, Grimmelikhuijzen CJP. Evolution of the AKH/corazonin/ACP/GnRH receptor superfamily and their ligands in the Protostomia. Gen Comp Endocrinol (2014) 209:35–49. doi:10.1016/j.ygcen.2014.07.009
6. Semmens DC, Elphick MR. The evolution of neuropeptides signalling: insights from echinoderms. Brief Funct Genomics (2017):1–11. doi:10.1093/bfgp/elx005
7. Mirabeau O, Joly J-S. Molecular evolution of peptidergic signaling systems in bilaterians. Proc Natl Acad Sci U S A (2013) 110:E2028–37. doi:10.1073/pnas.1219956110
8. Tian S, Zandawala M, Beets I, Baytemur E, Slade SE, Scrivens JH, et al. Urbilaterian origin of paralogous GnRH and corazonin neuropeptide signalling pathways. Sci Rep (2016) 6:28788. doi:10.1038/srep28788
9. Plachetzki DC, Tsai PS, Kavanaugh SI, Sower SA. Ancient origins of metazoan gonadotropin-releasing hormone and their receptors revealed by phylogenomic analyses. Gen Comp Endocrinol (2016) 234:10–9. doi:10.1016/j.ygcen.2016.06.007
10. Veenstra JA. Isolation and structure of corazonin, a cardioactive peptide from the American cockroach. FEBS Lett (1989) 250:231–4. doi:10.1016/0014-5793(89)80727-6
11. Suwansa-ard S, Zhao M, Thongbuakaew T, Chansela P, Ventura T, Cummins SF, et al. Gonadotropin-releasing hormone and adipokinetic hormone/corazonin-related peptide in the female prawn. Gen Comp Endocrinol (2016) 236:70–82. doi:10.1016/j.ygcen.2016.07.008
12. Roch GJ, Tello JA, Sherwood NM. At the transition from invertebrates to vertebrates, a novel GnRH-like peptide emerges in amphioxus. Mol Biol Evol (2014) 31:765–78. doi:10.1093/molbev/mst269
13. Kavanaugh SI, Tsai PS. Functional authentication of a novel gastropod gonadotropin-releasing hormone receptor reveals unusual features and evolutionary insight. PLoS One (2016) 11:e0160292. doi:10.1371/journal.pone.0160292
14. Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev (2004) 25:235–75. doi:10.1210/er.2003-0002
15. Kavanaugh SI, Nozaki M, Sower SA. Origins of gonadotropin-releasing hormone (GnRH) in vertebrates: identification of a novel GnRH in a basal vertebrate, the sea lamprey. Endocrinology (2008) 149:3860–9. doi:10.1210/en.2008-0184
16. Decatur WA, Hall JA, Smith JJ, Li W, Sower SA. Insight from the lamprey genome: glimpsing early vertebrate development via neuroendocrine-associated genes and shared synteny of gonadotropin-releasing hormone (GnRH). Gen Comp Endocrinol (2013) 192:237–45. doi:10.1016/j.ygcen.2013.05.020
17. Powell JF, Reska-Skinner SM, Prakash MO, Fischer WH, Park M, Rivier JE, et al. Two new forms of gonadotropin-releasing hormone in a protochordate and the evolutionary implications. Proc Natl Acad Sci U S A (1996) 93:10461–4. doi:10.1073/pnas.93.19.10461
18. Adams BA, Tello JA, Erchegyi J, Warby C, Hong DJ, Akinsanya KO, et al. Six novel gonadotropin-releasing hormones are encoded as triplets on each of two genes in the protochordate, Ciona intestinalis. Endocrinology (2003) 144:1907–19. doi:10.1210/en.2002-0216
19. Hasunuma I, Terakado K. Two novel gonadotropin-releasing hormones (GnRHs) from the urochordate ascidian, Halocynthia roretzi: implications for the origin of vertebrate GnRH isoforms. Zoolog Sci (2013) 30:311–8. doi:10.2108/zsj.30.311
20. Kawada T, Aoyama M, Okada I, Sakai T, Sekiguchi T, Ogasawara M, et al. A novel inhibitory gonadotropin-releasing hormone-related neuropeptide in the ascidian, Ciona intestinalis. Peptides (2009) 30:2200–5. doi:10.1016/j.peptides.2009.08.014
21. Matsubara S, Kawada T, Sakai T, Aoyama M, Osugi T, Shiraishi A, et al. The significance of Ciona intestinalis as a stem organism in integrative studies of functional evolution of the chordate endocrine, neuroendocrine, and nervous systems. Gen Comp Endocrinol (2016) 227:101–8. doi:10.1016/j.ygcen.2015.05.010
22. Rowe ML, Elphick MR. The neuropeptide transcriptome of a model echinoderm, the sea urchin Strongylocentrotus purpuratus. Gen Comp Endocrinol (2012) 179:331–44. doi:10.1016/j.ygcen.2012.09.009
23. Kah O, Lethimonier C, Somoza G, Guilgur LG, Vaillant C, Lareyre JJ. GnRH and GnRH receptors in metazoa: a historical, comparative, and evolutive perspective. Gen Comp Endocrinol (2007) 153:346–64. doi:10.1016/j.ygcen.2007.01.030
24. Joseph NT, Aquilina-Beck A, MacDonald C, Decatur WA, Hall JA, Kavanaugh SI, et al. Molecular cloning and pharmacological characterization of two novel GnRH receptors in the lamprey (Petromyzon marinus). Endocrinology (2012) 153:3345–56. doi:10.1210/en.2012-1217
25. Morgan K, Stewart AJ, Miller N, Mullen P, Muir M, Dodds M, et al. Gonadotropin-releasing hormone receptor levels and cell context affect tumor cell responses to agonist in vitro and in vivo. Cancer Res (2008) 68:6331–40. doi:10.1158/0008-5472.CAN-08-0197
26. Sakai T, Aoyama M, Kusakabe T, Tsuda M, Satake H. Functional diversity of signaling pathways through G protein-coupled receptor heterodimerization with a species-specific orphan receptor subtype. Mol Biol Evol (2010) 27:1097–106. doi:10.1093/molbev/msp319
27. Tello JA, Rivier JE, Sherwood NM. Tunicate GnRH peptides selectively activate Ciona intestinalis GnRH receptors and the green monkey type II GnRH receptor. Endocrinology (2005) 146:4061–73. doi:10.1210/en.2004-1558
28. Sakai T, Aoyama M, Kawada T, Kusakabe T, Tsuda M, Satake H. Evidence for differential regulation of GnRH signaling via heterodimerization among GnRH receptor paralogs in the protochordate, Ciona intestinalis. Endocrinology (2012) 153:1841–9. doi:10.1210/en.2011-1668
29. Satake H, Matsubara S, Aoyama M, Kawada T, Sakai T. GPCR heterodimerization in the reproductive system: functional regulation and implication for biodiversity. Front Endocrinol (2013) 4:100. doi:10.3389/fendo.2013.00100
30. Kusakabe TG, Sakai T, Aoyama M, Kitajima Y, Miyamoto Y, Takigawa T, et al. A conserved non-reproductive GnRH system in chordates. PLoS One (2012) 7:e41955. doi:10.1371/journal.pone.0041955
31. Tello JA, Sherwood NM. Amphioxus: beginning of vertebrate and end of invertebrate type GnRH receptor lineage. Endocrinology (2009) 150:2847–56. doi:10.1210/en.2009-0028
32. Sekiguchi T, Suzuki N, Fujiwara N, Aoyama M, Kawada T, Sugase K, et al. Calcitonin in a protochordate, Ciona intestinalis – the prototype of the vertebrate calcitonin/calcitonin gene-related peptide superfamily. FEBS J (2009) 276:4437–47. doi:10.1111/j.1742-4658.2009.07151.x
33. Kanda A, Takahashi T, Satake H, Minakata H. Molecular and functional characterization of a novel gonadotropin-releasing-hormone receptor isolated from the common octopus (Octopus vulgaris). Biochem J (2006) 395:125–35. doi:10.1042/BJ20051615
34. Terakado K. Induction of gamete release by gonadotropin-releasing hormone in a protochordate, Ciona intestinalis. Gen Comp Endocrinol (2001) 124:277–84. doi:10.1006/gcen.2001.7728
35. Kamiya C, Ohta N, Ogura Y, Yoshida K, Horie T, Kusakabe TG, et al. Nonreproductive role of gonadotropin-releasing hormone in the control of ascidian metamorphosis. Dev Dyn (2014) 243:1524–35. doi:10.1002/dvdy.24176
36. Iwakoshi-Ukena E, Ukena K, Takuwa-Kuroda K, Kanda A, Tsutsui K, Minakata H. Expression and distribution of octopus gonadotropin-releasing hormone in the central nervous system and peripheral organs of the octopus (Octopus vulgaris) by in situ hybridization and immunohistochemistry. J Comp Neurol (2004) 477:310–23. doi:10.1002/cne.20260
37. Treen N, Itoh N, Miura H, Kikuchi I, Ueda T, Takahashi KG, et al. Mollusc gonadotropin-releasing hormone directly regulates gonadal functions: a primitive endocrine system controlling reproduction. Gen Comp Endocrinol (2012) 176:167–72. doi:10.1016/j.ygcen.2012.01.008
38. Tsai PS, Sun B, Rochester JR, Wayne NL. Gonadotropin-releasing hormone-like molecule is not an acute reproductive activator in the gastropod, Aplysia californica. Gen Comp Endocrinol (2010) 166:280–8. doi:10.1016/j.ygcen.2009.09.009
39. Mayer R, Candy D. Control of hemolymph lipid concentration during locust flight: an adipokinetic hormone from the corpora cardiaca. J Insect Physiol (1969) 15:611–20. doi:10.1016/0022-1910(69)90259-5
40. Li S, Hauser F, Skadborg SK, Nielsen SV, Kirketerp-møller N, Grimmelikhuijzen CJP. Adipokinetic hormones and their G protein-coupled receptors emerged in Lophotrochozoa. Sci Rep (2016) 6:32789. doi:10.1038/srep32789
41. Zhu C, Huang H, Hua R, Li G, Yang D, Luo J, et al. Molecular and functional characterization of adipokinetic hormone receptor and its peptide ligands in Bombyx mori. FEBS Lett (2009) 583:1463–8. doi:10.1016/j.febslet.2009.03.060
42. Nagasawa K, Muroi K, Thitiphuree T, Minegishi Y, Itoh N, Osada M. Cloning of invertebrate gonadotropin-releasing hormone receptor (GnRHR)-like gene in Yesso scallop, Patinopecten yessoensis. Agri Gene (2017) 3:46–56. doi:10.1016/j.aggene.2016.11.005
43. Josefsson J. Invertebrate neuropeptide hormones. Int J Pept Protein Res (1983) 21:459–70. doi:10.1111/j.1399-3011.1983.tb02672.x
44. Lorenz MW, Gäde G, Elphick MR. Hormonal regulation of energy metabolism in insects as a driving force for performance. Integr Comp Biol (2009) 49:380–92. doi:10.1093/icb/icp019
45. Isabel G, Martin J-R, Chidami S, Veenstra JA, Rosay P. AKH-producing neuroendocrine cell ablation decreases trehalose and induces behavioral changes in Drosophila. Am J Physiol Regul Integr Comp Physiol (2005) 288:R531–8. doi:10.1152/ajpregu.00158.2004
46. Grönke S, Muller G, Hirsch J, Fellert S, Andreou A, Haase T, et al. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol (2007) 5:e137. doi:10.1371/journal.pbio.0050137
47. Konuma T, Morooka N, Nagasawa H, Nagata S. Knockdown of the adipokinetic hormone receptor increases feeding frequency in the two-spotted cricket Gryllus bimaculatus. Endocrinology (2012) 153:3111–22. doi:10.1210/en.2011-1533
48. Veenstra JA. Does corazonin signal nutritional stress in insects? Insect Biochem Mol Biol (2009) 39:755–62. doi:10.1016/j.ibmb.2009.09.008
49. Yang J, Huang H, Yang H, He X, Jiang X, Shi Y, et al. Specific activation of the G protein-coupled receptor BNGR-A21 by the neuropeptide corazonin from the silkworm, Bombyx mori, dually couples to the Gq and Gs signaling cascades. J Biol Chem (2013) 288:11662–75. doi:10.1074/jbc.M112.441675
50. Zandawala M, Tian S, Elphick MR. The evolution and nomenclature of GnRH-type and corazonin-type neuropeptide signaling systems. Gen Comp Endocrinol (2017). doi:10.1016/j.ygcen.2017.06.007
51. Nässel DR, Kubrak OI, Liu Y, Luo J, Lushchak OV. Factors that regulate insulin producing cells and their output in Drosophila. Front Physiol (2013) 4:252. doi:10.3389/fphys.2013.00252
52. Hou Q-L, Jiang H-B, Gui S-H, Chen E-H, Wei D-D, Li H-M, et al. A role of corazonin receptor in larval-pupal transition and pupariation in the oriental fruit fly Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Front Physiol (2017) 8:77. doi:10.3389/fphys.2017.00077
53. Tayler TD, Pacheco DA, Hergarden AC, Murthy M, Anderson DJ. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proc Natl Acad Sci U S A (2012) 109:20697–702. doi:10.1073/pnas.1218246109
54. Siangcham T, Tinikul Y, Poljaroen J, Sroyraya M, Changklungmoa N, Phoungpetchara I, et al. The effects of serotonin, dopamine, gonadotropin-releasing hormones, and corazonin, on the androgenic gland of the giant freshwater prawn, Macrobrachium rosenbergii. Gen Comp Endocrinol (2013) 193:10–8. doi:10.1016/j.ygcen.2013.06.028
55. Hansen KK, Stafflinger E, Schneider M, Hauser F, Cazzamali G, Williamson M, et al. Discovery of a novel insect neuropeptide signaling system closely related to the insect adipokinetic hormone and corazonin hormonal systems. J Biol Chem (2010) 285:10736–47. doi:10.1074/jbc.M109.045369
56. Zandawala M, Haddad AS, Hamoudi Z, Orchard I. Identification and characterization of the adipokinetic hormone/corazonin-related peptide signaling system in Rhodnius prolixus. FEBS J (2015) 282:3603–17. doi:10.1111/febs.13366
57. Derst C, Dircksen H, Meusemann K, Zhou X, Liu S, Predel R. Evolution of neuropeptides in non-pterygote hexapods. BMC Evol Biol (2016) 16:51. doi:10.1186/s12862-016-0621-4
58. Jekely G. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc Natl Acad Sci U S A (2013) 110:8702–7. doi:10.1073/Pnas.1221833110
59. Lindemans M, Liu F, Janssen T, Husson SJ, Mertens I, Gade G, et al. Adipokinetic hormone signaling through the gonadotropin-releasing hormone receptor modulates egg-laying in Caenorhabditis elegans. Proc Natl Acad Sci U S A (2009) 106:1642–7. doi:10.1073/pnas.0809881106
60. Surgand JS, Rodrigo J, Kellenberger E, Rognan D. A chemogenomic analysis of the transmembrane binding cavity of human G-protein-coupled receptors. Proteins (2006) 62:509–38. doi:10.1002/prot.20768
Keywords: gonadotropin-releasing hormone, adipokinetic hormone, corazoin, receptor, invertebrate
Citation: Sakai T, Shiraishi A, Kawada T, Matsubara S, Aoyama M and Satake H (2017) Invertebrate Gonadotropin-Releasing Hormone-Related Peptides and Their Receptors: An Update. Front. Endocrinol. 8:217. doi: 10.3389/fendo.2017.00217
Received: 23 June 2017; Accepted: 14 August 2017;
Published: 06 September 2017
Edited by:
Ivana Bjelobaba, University of Belgrade, SerbiaReviewed by:
Makoto Osada, Tohoku University, JapanStacia A. Sower, University of New Hampshire, United States
Copyright: © 2017 Sakai, Shiraishi, Kawada, Matsubara, Aoyama and Satake. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Honoo Satake, satake@sunbor.or.jp
 Tsubasa Sakai
Tsubasa Sakai Akira Shiraishi
Akira Shiraishi Tsuyoshi Kawada
Tsuyoshi Kawada Shin Matsubara
Shin Matsubara Masato Aoyama
Masato Aoyama Honoo Satake
Honoo Satake