- Department of Endocrinology; Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China
Graves’ disease (GD) is one of the most common endocrine diseases. Antithyroid drugs (ATDs) treatment is frequently used as the first-choice therapy for GD patients in most countries due to the superiority in safety and tolerance. However, GD patients treated with ATD have a relatively high recurrence rate after drug withdrawal, which is a main limitation for ATD treatment. It is of great importance to identify some predictors of the higher recurrence risk for GD patients, which may facilitate an appropriate therapeutic approach for a given patient at the time of GD diagnosis. The genetic factor was widely believed to be an important pathogenesis for GD. Increasing studies were conducted to investigate the relationship between gene polymorphisms and the recurrence risk in GD patients. In this article, we updated the current literatures to highlight the predictive value of gene polymorphisms on recurrence risk in GD patients after ATD withdrawal. Some gene polymorphisms, such as CTLA4 rs231775, human leukocyte antigen polymorphisms (DRB1*03, DQA1*05, and DQB1*02) might be associated with the high recurrence risk in GD patients. Further prospective studies on patients of different ethnicities, especially studies with large sample sizes, and long-term follow-up, should be conducted to confirm the predictive roles of gene polymorphism.
Introduction
Hyperthyroidism is a common endocrine disease caused by increased synthesis and secretion of thyroid hormone (1). Graves’ disease (GD) is an autoimmune thyroid disease that results from excessive stimulation of the thyroid by circulating TSH receptor antibodies (TRAb), which is the most common cause of hyperthyroidism (1). As antithyroid drugs (ATDs) treatment avoids radiation exposure and has a low risk of hypothyroidism, it is frequently considered as the first-choice therapy for GD patients (2). However, GD patients treated with ATD have a relatively high recurrence rate after drug withdrawal (3, 4). The persistent or recurrent hyperthyroidism results in increased medical expenses and a wide spectrum of complications, such as atrial fibrillation, heart failure, and osteoporosis, even a long-term and negative impact on the quality of life (5, 6). Thus, it is of great importance to identify some predictors, prior to ATD treatment, which can indicate a higher risk of recurrence. If the likelihood of recurrence after ATD treatment is high, radioiodine therapy or thyroidectomy might be more preferable. This strategy would facilitate an appropriate therapeutic approach for a given patient at the time of GD diagnosis.
The genetic factor was widely believed to be an important pathogenesis for GD (7). Increasing studies were conducted to investigate the relationship between gene polymorphisms and the recurrence risk in GD patients after ATD withdrawal (8–13). In this article, we updated the current literatures to highlight the predictive value of gene polymorphisms on recurrence risk in GD patients after ATD withdrawal.
The High Recurrence Risk in GD Patients after ATD Withdrawal
There are three treatment options for GD patients, including ATD, radioiodine therapy, and thyroidectomy (1). ATD treatment is frequently used as the first-choice therapy for GD patients in most countries (2). However, the recurrence rate of ATD treatment is approximately 50–60%, which is relatively higher as compared with radioiodine therapy or thyroidectomy (3, 4). The previous studies have demonstrated that the recurrence risk varies in GD patients with different clinical characteristics (4, 9, 14, 15). Therefore, the risk predictors for recurrence should be taken into account when choosing an appropriate therapeutic approach for every GD patient. Previous studies have shown that young age, high concentrations of TRAb, large goiter size, and severe thyrotoxicosis were associated with higher recurrence risk (4, 9, 14, 15). Unfortunately, the predicting ability of these factors is unsatisfactory.
The Relationship Between Gene Polymorphisms and Recurrence Risk of GD Patients
Several studies have found that the occurrence of GD appeared in family aggregation (16). The evidence from further genetic studies showed that genetic factors are related to the pathogenesis of GD (7). Some immune-regulatory genes, including human leukocyte antigen (HLA), CD40, cytotoxic T-lymphocyte-associated factor 4 (CTLA4), protein tyrosine phosphatase, non-receptor type 22 (PTPN22), and Fc receptor-like protein 3 (FCRL3), have been observed to be involved in the development of GD (7). In addition, thyroid autoantigen genes are also related to the development of GD (7). Thus, researchers want to find some genetic predictors for the recurrence risk in GD patients after ATD withdrawal.
Recently, the relationship between gene polymorphisms and the recurrence risk in GD patients after ATD withdrawal have been investigated (8–13). We carried out a computerized literature search in PubMed, EMBASE, Web of Science, Cochrane database, and reference lists of relevant studies up to July 20, 2017. The keywords of retrieval were (“polymorphism” or “variant” or “mutation” or “gene” or “genotype”) and (“antithyroid drug” or “antithyroid medicine” or “antithyroid agent” or “methimazole” or “propylthiouracil” or “carbimazole”), in combination with (“hyperthyroidism” or “Graves’ disease” or “thyrotoxicosis”), without language or region restriction. In general, the ATD treatment duration is recommended as 12–18 months, so the recurrence of GD was defined as the recurrence of hyperthyroidism after at least 12 months of ATD treatment (17). The eligible studies were observational cohort or case-control studies and were identified according to the following criteria: (1) studies that investigated the association between gene polymorphism and the recurrence in GD patients after ATD withdrawal; (2) the duration of ATD therapy was 12 months or more; (3) TRAb was negative when stopping ATD treatment; (4) the follow-up duration was at least 12 months after ATD withdrawal. The exclusion criteria included: (1) duplicated studies; (2) case, reviews, letter, or books; and (3) unavailable data.
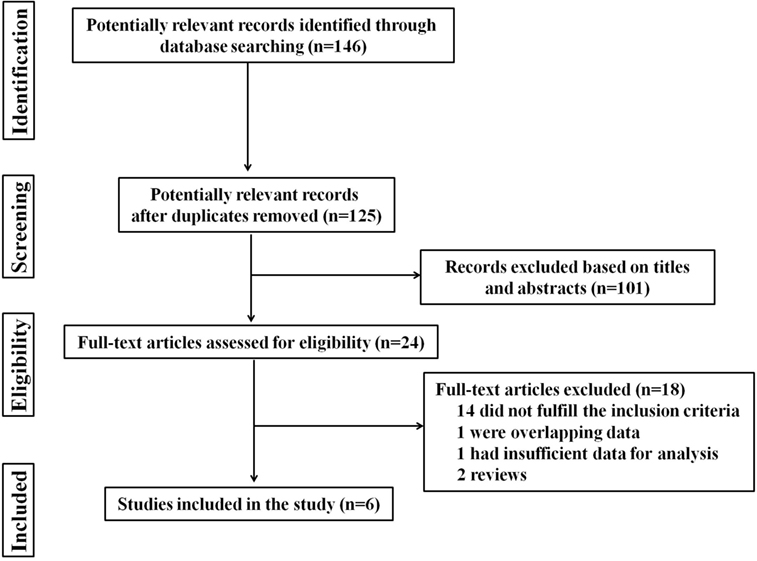
A total of 146 studies were identified through database searching. However, after manually screening the titles and abstracts, 24 studies were chosen after excluding duplicates and irrelevant papers. The remaining 24 studies were reviewed in detail. Fourteen of these were removed because they did not fulfill the inclusion criteria, and four were excluded due to overlapping data, insufficient data for analysis, or other reasons. The flowchart, including study identification, inclusion, and exclusion factors is shown in Figure 1. Finally, only 6 studies, including 4 observational cohort studies and 2 case-control studies, with a total of 1,398 GD patients, were included (8–13).
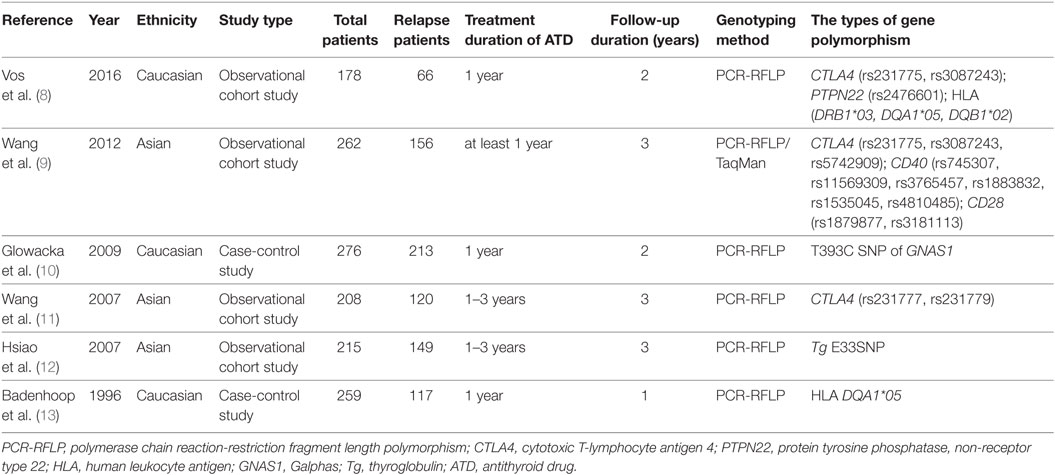
The main characteristics of the included studies are shown in Table 1. Of the six studies, three were conducted in Asian populations, and three in Caucasian populations. The types of gene polymorphism included: CTLA4 polymorphisms (rs231775, rs3087243, rs5742909, rs231777, and rs231779), PTPN22 polymorphism (rs2476601), HLA polymorphisms (DRB1*03, DQA1*05, and DQB1*02), CD40 polymorphisms (rs745307, rs11569309, rs3765457, rs1883832, rs1535045, and rs4810485), CD28 polymorphisms (rs1879877 and rs3181113), T393C SNP of Galphas gene (GNAS1), and E33SNP of thyroglobulin (Tg) (Tg E33SNP). The follow-up duration was 12–36 months after withdrawing therapy. Among these studies, two evaluated CTLA4 rs231775 (8, 9), two evaluated CTLA4 rs3087243 (8, 9), two evaluated HLA DQA1*05 (8, 13). Only one study evaluated the rest of the gene polymorphisms. Among these gene polymorphisms, CTLA4 rs231775, HLA polymorphisms (DRB1*03, DQA1*05, and DQB1*02), CD40 polymorphisms (rs745307, rs11569309, and rs3765457), PTPN22 polymorphism (rs2476601), T393C SNP of GNAS1 and Tg E33SNP were observed to be associated with the high-recurrence risk in GD patients after ATD withdrawal (8–13).
CTLA4
T-cell abnormality is associated with the pathogenesis of GD (1). The CTLA4 is a main negative-regulatory factor of T-cell-mediated immune responses (18, 19). CTLA4 inhibited T-cell activation and mediated antigen-specific apoptosis of T cells (19). Several studies have revealed that CTLA4 polymorphisms including rs231775, rs231779, and rs3087243 were likely the susceptibility variants for GD (20–22). A recent observational cohort study showed that there is no association between recurrence risk and the CTLA4 polymorphism (rs231775, rs3087243) in Caucasians patients with GD (8). However, Wang et al.’ studies showed that CTLA4 rs231775 was significantly associated with the recurrence of hyperthyroidism in Asians patients with GD (9). On the one hand, the inconsistent results for the association between CTLA4 polymorphisms and the recurrence in GD patients might be related to the ethnic differences. On the other hand, these two studies were small-scale, which probably also contributed to the divergent results. Then, the follow-up duration was different in the included studies. The recurrence cases will increase if the follow-up duration extends, which might further influence the association between gene polymorphism and the recurrence risk in GD patients.
Human Leukocyte Antigen
Human leukocyte antigen region contains many immune response genes, which linked HLA to some autoimmune diseases, such as GD. Several studies have demonstrated that HLA DRB1*03, DQB1*02, and DQA1*05 displayed strong associations with GD in Caucasians (23, 24). Recently, some Asian studies also showed the association between HLA polymorphism (DPB1*05:01) and the susceptibility of GD (25, 26). A recent study from Caucasians showed that HLA DRB1*03, DQA1*05, and DQB1*02 polymorphisms were significantly related to the high recurrence risk after ATD treatment in GD patients (8). However, another Caucasians study did not observe the association between recurrence risk and HLA DQA1*05 (13). So far, the study regarding the association between recurrence risk of GD and HLA polymorphism is still absent in Asians populations. The inconsistent results in Caucasians might be involved in the small sizes of included studies and different duration of follow-up.
CD40
CD40 is mainly expressed on antigen-presenting cells and B cells, as well as on other types of cells, such as thyroid follicular cells (27). CD40 not only interacts with the CD40 ligand (CD40L) on the T cells but also promotes B-cell proliferation and antibody secretion (27, 28). In spite of some inconsistent results, a meta-analysis still confirmed the association between CD40 polymorphism (rs1883832) and GD (29). So far, only a recent study evaluated the association between CD40 polymorphism and recurrence risk of GD and showed that CD40 polymorphisms (rs745307, rs11569309, and rs3765457) were associated with the high recurrence risk of GD patients (9).
PTPN22
PTPN22 is related to the activity of lymphoid tyrosine phosphatase, which a negative regulator of T-cell activation (30). The association between PTPN22 polymorphism (rs2476601) and GD has been shown in many studies among Caucasians (30, 31). And a recent Caucasians study has shown that this polymorphism is associated with recurrence risk in GD patients as well (8). Interestingly, PTPN22 polymorphism (rs2476601) occurs very rarely in Asians and Africans, and no association between PTPN22 polymorphism (rs2476601) and the onset or recurrence risk of GD was observed in Asians and Africans (32).
GNAS1
GNAS1 is a gene that encodes α-subunit of G proteins (33). The T393C SNP of GNAS1 has been significantly associated with the clinical course in a variety of cancers (33). Interestingly, although not directly involved in the development of GD, T393C SNP of GNAS1 was shown to be related to the relapse of hyperthyroidism in GD patients after ATD withdrawal (10). The TT genotype of T393C SNP of GNAS1 was considered to be associated with the increased expression of Gαs mRNA (33). TSH receptor belongs to the G-protein coupled receptor superfamily (1). So, T393C SNP of GNAS1 might be related to the relapse after ATD withdrawal in GD patients by modulating TSH receptor.
Thyroglobulin
Thyroglobulin is a main autoantigen of autoimmune thyroid diseases, including both GD and Hashimoto’s thyroiditis (34). Some studies in patients with autoimmune thyroid disease have shown that anti-Tg antibodies are specific toward a restricted number of epitopes on Tg, thus, Tg is important in the pathogenesis of GD due to its specific features (35). In several studies, Tg gene polymorphism in exon 33 (Tg E33SNP) was related to the increased susceptibility for GD (35, 36). And a recent study also showed the association between Tg E33SNP and the recurrence risk of GD (12).
Based on a limited number of studies, CTLA4 polymorphism (rs231775), HLA polymorphisms (DRB1*03, DQA1*05, and DQB1*02), CD40 polymorphisms (rs745307, rs11569309, and rs3765457), PTPN22 polymorphism (rs2476601), T393C SNP of GNAS1, and Tg E33SNP might be associated with the high recurrence risk in GD patients after ATD withdrawal (8–13). However, there is still some query about the predictive value of gene polymorphisms for the high recurrence risk in GD patients. First, the studies evaluating the association between gene polymorphisms and the recurrence in GD patients after ATD withdrawal were relatively small. Each gene polymorphism was investigated only by one or two studies. Furthermore, most of the included studies were small scale. Then, the quality of the included studies was relatively poor. Further prospective studies with large sample sizes, and long-term follow-up, should be conducted to confirm the predictive roles of gene polymorphism. Despite these conflicting results, one implication is that gene polymorphisms are supposed to an important risk predictor for the recurrence risk in GD patients. In addition, it is worth noting that the etiology of GD is related to genetic and environmental factors, so it is difficult for any single factor to well predict the recurrence risk for a given patients. Thus, a prediction model based on both genetic and environmental risk factors for GD recurrence might better predict the recurrence risk for a given patient.
Conclusion
Antithyroid drug is a common choice for a new diagnosed GD patient, and some genetic factors may greatly influence therapeutic outcome for GD patients. Based on a limited number of studies, CTLA4 polymorphism (rs231775), HLA polymorphisms (DRB1*03, DQA1*05, and DQB1*02), CD40 polymorphisms (rs745307, rs11569309, and rs3765457), PTPN22 polymorphism (rs2476601), T393C SNP of GNAS1 and Tg E33SNP might be associated with the high recurrence risk in GD patients after ATD withdrawal. Further, prospective studies on patients of different ethnicities, especially studies with large sample sizes, and long-term follow-up, should be conducted to confirm the predictive roles of gene polymorphism.
Author Contributions
JL and GW conceived and designed the review; JL, JF, YD, and GW performed the review; JL, JF, and YD analyzed the data; JL and GW wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by grants from the Capital Clinical Research Foundation of Beijing Municipal Commission of Science and Technology (No. Z161100000516069) to GW, and the Chinese National Natural Science Foundation (No. 81600657) and Beijing Municipal Administration of Hospitals’ Youth Programme (QML20150308) to JL.
References
1. De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet (2016) 388:906–18. doi:10.1016/S0140-6736(16)00278-6
2. Burch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves’ disease. J Clin Endocrinol Metab (2012) 97:4549–58. doi:10.1210/jc.2012-2802
3. Sundaresh V, Brito JP, Wang Z, Prokop LJ, Stan MN, Murad MH, et al. Comparative effectiveness of therapies for Graves’ hyperthyroidism: a systematic review and network meta-analysis. J Clin Endocrinol Metab (2013) 98:3671–7. doi:10.1210/jc.2013-1954
4. Abraham P, Avenell A, McGeoch SC, Clark LF, Bevan JS. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst Rev (2010) 1:CD003420. doi:10.1002/14651858.CD003420.pub4
5. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med (2001) 344:501–9. doi:10.1056/NEJM200102153440707
6. Abraham-Nordling M, Torring O, Hamberger B, Lundell G, Tallstedt L, Calissendorff J, et al. Graves’ disease: a long-term quality-of-life follow up of patients randomized to treatment with antithyroid drugs, radioiodine, or surgery. Thyroid (2005) 15:1279–86. doi:10.1089/thy.2005.15.1279
7. Marino M, Latrofa F, Menconi F, Chiovato L, Vitti P. Role of genetic and non-genetic factors in the etiology of Graves’ disease. J Endocrinol Invest (2015) 38:283–94. doi:10.1007/s40618-014-0214-2
8. Vos XG, Endert E, Zwinderman AH, Tijssen JG, Wiersinga WM. Predicting the risk of recurrence before the start of antithyroid drug therapy in patients with Graves’ hyperthyroidism. J Clin Endocrinol Metab (2016) 101:1381–9. doi:10.1210/jc.2015-3644
9. Wang PW, Chen IY, Juo SH, Hsi E, Liu RT, Hsieh CJ. Genotype and phenotype predictors of relapse of Graves’ disease after antithyroid drug withdrawal. Eur Thyroid J (2013) 1:251–8. doi:10.1159/000342621
10. Glowacka D, Loesch C, Johnson KT, Mann K, Esser J, Morgenthaler NG, et al. The T393C polymorphism of the Galphas gene (GNAS1) is associated with the course of Graves’ disease. Horm Metab Res (2009) 41:430–5. doi:10.1055/s-0029-1220902
11. Wang PW, Chen IY, Liu RT, Hsieh CJ, Hsi E, Juo SH. Cytotoxic T lymphocyte-associated molecule-4 gene polymorphism and hyperthyroid Graves’ disease relapse after antithyroid drug withdrawal: a follow-up study. J Clin Endocrinol Metab (2007) 92:2513–8. doi:10.1210/jc.2006-2761
12. Hsiao JY, Hsieh MC, Tien KJ, Hsu SC, Shin SJ, Lin SR. Association between a C/T polymorphism in exon 33 of the thyroglobulin gene is associated with relapse of Graves’ hyperthyroidism after antithyroid withdrawal in Taiwanese. J Clin Endocrinol Metab (2007) 92:3197–201. doi:10.1210/jc.2007-0675
13. Badenhoop K, Donner H, Braun J, Siegmund T, Rau H, Usadel KH. Genetic markers in diagnosis and prediction of relapse in Graves’ disease. Exp Clin Endocrinol Diabetes (1996) 104:98–100. doi:10.1055/s-0029-1211712
14. Allahabadia A, Daykin J, Holder RL, Sheppard MC, Gough SC, Franklyn JA. Age and gender predict the outcome of treatment for Graves’ hyperthyroidism. J Clin Endocrinol Metab (2000) 85:1038–42. doi:10.1210/jc.85.3.1038
15. Piantanida E, Lai A, Sassi L, Gallo D, Spreafico E, Tanda ML, et al. Outcome prediction of treatment of Graves’ hyperthyroidism with antithyroid drugs. Horm Metab Res (2015) 47:767–72. doi:10.1055/s-0035-1555759
16. Laurberg P, Krejbjerg A, Andersen SL. Relapse following antithyroid drug therapy for Graves’ hyperthyroidism. Curr Opin Endocrinol Diabetes Obes (2014) 21:415–21. doi:10.1097/MED.0000000000000088
17. Liu J, Fu J, Xu Y, Wang G. Antithyroid drug therapy for Graves’ disease and implications for recurrence. Int J Endocrinol (2017) 2017:3813540. doi:10.1155/2017/3813540
18. Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood (2005) 105:13–21. doi:10.1182/blood-2004-04-1596
19. Gribben JG, Freeman GJ, Boussiotis VA, Rennert P, Jellis CL, Greenfield E, et al. CTLA4 mediates antigen-specific apoptosis of human T cells. Proc Natl Acad Sci U S A (1995) 92:811–5. doi:10.1073/pnas.92.3.811
20. Ting WH, Chien MN, Lo FS, Wang CH, Huang CY, Lin CL, et al. Association of cytotoxic T-lymphocyte-associated protein 4 (CTLA4) gene polymorphisms with autoimmune thyroid disease in children and adults: case-control study. PLoS One (2016) 11:e0154394. doi:10.1371/journal.pone.0154394
21. Patel H, Mansuri MS, Singh M, Begum R, Shastri M, Misra A. Association of cytotoxic T-lymphocyte antigen 4 (CTLA4) and thyroglobulin (TG) genetic variants with autoimmune hypothyroidism. PLoS One (2016) 11:e0149441. doi:10.1371/journal.pone.0149441
22. Hou HF, Jin X, Sun T, Li C, Jiang BF, Li QW. Cytotoxic T lymphocyte-associated antigen 4 gene polymorphisms and autoimmune thyroid diseases: an updated systematic review and cumulative meta-analysis. Int J Endocrinol (2015) 2015:747816. doi:10.1155/2015/747816
23. Yanagawa T, Mangklabruks A, Chang YB, Okamoto Y, Fisfalen ME, Curran PG, et al. Human histocompatibility leukocyte antigen-DQA1*0501 allele associated with genetic susceptibility to Graves’ disease in a Caucasian population. J Clin Endocrinol Metab (1993) 76:1569–74. doi:10.1210/jcem.76.6.8501164
24. Zamani M, Spaepen M, Bex M, Bouillon R, Cassiman JJ. Primary role of the HLA class II DRB1*0301 allele in Graves disease. Am J Med Genet (2000) 95:432–7. doi:10.1002/1096-8628(20001218)95:5<432::AID-AJMG5>3.0.CO;2-7
25. Chen PL, Fann CS, Chang CC, Wu IL, Chiu WY, Lin CY, et al. Linkage of Graves’ disease to the human leucocyte antigen region in the Chinese-Han population in Taiwan. Clin Endocrinol (Oxf) (2007) 66:646–51. doi:10.1111/j.1365-2265.2007.02787.x
26. Chen PL, Fann CS, Chu CC, Chang CC, Chang SW, Hsieh HY, et al. Comprehensive genotyping in two homogeneous Graves’ disease samples reveals major and novel HLA association alleles. PLoS One (2011) 6:e16635. doi:10.1371/journal.pone.0016635
27. Metcalfe RA, McIntosh RS, Marelli-Berg F, Lombardi G, Lechler R, Weetman AP. Detection of CD40 on human thyroid follicular cells: analysis of expression and function. J Clin Endocrinol Metab (1998) 83:1268–74. doi:10.1210/jcem.83.4.4732
28. Dittmar M, Kahaly GJ. Immunoregulatory and susceptibility genes in thyroid and polyglandular autoimmunity. Thyroid (2005) 15:239–50. doi:10.1089/thy.2005.15.239
29. Kurylowicz A, Kula D, Ploski R, Skorka A, Jurecka-Lubieniecka B, Zebracka J, et al. Association of CD40 gene polymorphism (C-1T) with susceptibility and phenotype of Graves’ disease. Thyroid (2005) 15:1119–24. doi:10.1089/thy.2005.15.1119
30. Skorka A, Bednarczuk T, Bar-Andziak E, Nauman J, Ploski R. Lymphoid tyrosine phosphatase (PTPN22/LYP) variant and Graves’ disease in a Polish population: association and gene dose-dependent correlation with age of onset. Clin Endocrinol (Oxf) (2005) 62:679–82. doi:10.1111/j.1365-2265.2005.02279.x
31. Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, Donaldson PT, et al. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves’ disease. J Clin Endocrinol Metab (2004) 89:5862–5. doi:10.1210/jc.2004-1108
32. Ploski R, Szymanski K, Bednarczuk T. The genetic basis of Graves’ disease. Curr Genomics (2011) 12:542–63. doi:10.2174/138920211798120772
33. Frey UH, Eisenhardt A, Lummen G, Rubben H, Jockel KH, Schmid KW, et al. The T393C polymorphism of the G alpha s gene (GNAS1) is a novel prognostic marker in bladder cancer. Cancer Epidemiol Biomarkers Prev (2005) 14:871–7. doi:10.1158/1055-9965.EPI-04-0720
34. Salvi M, Fukazawa H, Bernard N, Hiromatsu Y, How J, Wall JR. Role of autoantibodies in the pathogenesis and association of endocrine autoimmune disorders. Endocr Rev (1988) 9:450–66. doi:10.1210/edrv-9-4-450
35. Hsiao JY, Hsieh MC, Hsiao CT, Weng HH, Ke DS. Association of CD40 and thyroglobulin genes with later-onset Graves’ disease in Taiwanese patients. Eur J Endocrinol (2008) 59:617–21. doi:10.1530/EJE-08-0410
Keywords: gene polymorphism, Graves’ disease, antithyroid drugs, recurrence, hyperthyroidism
Citation: Liu J, Fu J, Duan Y and Wang G (2017) Predictive Value of Gene Polymorphisms on Recurrence after the Withdrawal of Antithyroid Drugs in Patients with Graves’ Disease. Front. Endocrinol. 8:258. doi: 10.3389/fendo.2017.00258
Received: 02 July 2017; Accepted: 19 September 2017;
Published: 29 September 2017
Edited by:
Noriyuki Koibuchi, Gunma University, JapanReviewed by:
Akira Hishinuma, Dokkyo Medical University, JapanEijun Nishihara, Kuma Hospital, Japan
Rohit Anthony Sinha, Duke-NUS Medical School, Singapore
Copyright: © 2017 Liu, Fu, Duan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Wang, drwg6688@126.com
 Jia Liu
Jia Liu Jing Fu
Jing Fu Yan Duan
Yan Duan Guang Wang
Guang Wang