- 1Endocrinology Unit, Department of Medicine (DIMED), University of Padova, Padova, Italy
- 2Surgical Pathology and Cytopathology Unit, Pathology Unit, Department of Medicine (DIMED), University of Padova, Padova, Italy
- 3Endocrine Surgery Unit, Department of Surgical, Oncological and Gastroenterological Sciences (DiSCOG), University of Padova, Padova, Italy
- 4Department of Radiotherapy, Istituto Oncologico Veneto-IRCCS, Padova, Italy
Purpose: The management of thyroid nodules of indeterminate cytology is controversial. Our study aimed to establish the frequency and significance of H-,K-,N-RAS, TERT promoter, and BRAF gene mutations in thyroid nodes of indeterminate cytology and to assess their potential usefulness in clinical practice.
Methods: H-,K-,N-RAS, TERT promoter and BRAF gene mutations were examined in a series of 199 consecutive nodes of indeterminate cytology referred for surgical excision.
Results: 69/199 (35%) were malignant on histopathological review. RAS mutations were detected in 36/199 (18%), and 19/36 cases (53%) were malignant on histological diagnosis. TERT promoter mutations were detected in 7/199 (4%) nodules, which were all malignant lesions. BRAF mutations were detected in 15/199 (8%), and a BRAF K601E mutation was identified in 2 follicular adenomas and 1 noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Altogether, this panel was able to identify 48% of the malignant lesions, achieving a specificity, positive predictive value, and negative predictive value for malignancy of 85, 62, and 75%, respectively.
Conclusion: The residual malignancy risk in mutation-negative nodes is 25%. These nodes still need to be resected, but mutation analysis could help to orient the appropriate surgical strategy.
Introduction
Fine-needle aspiration (FNA) cytology is the gold standard for the nonsurgical assessment of thyroid nodules, but it is unable to exclude cancer in one in four cases, which are labeled as “indeterminate” (1). According to the Italian Society of Anatomic Pathology and Cytology (SIAPEC) Consensus Statement, this “indeterminate” category is very heterogeneous, including cytological patterns that range from follicular types and Hürtle cell lesions to scant atypical features. The cancer risk varies, reportedly averaging around 26% in the largest series (2, 3). A proposed updated classification divides atypical cellular lesions into two categories, as explained in the SIAPEC 2014 Consensus Statement (4). Indeterminate lesions are separated into TIR 3A and TIR 3B, with an estimated malignancy risk below 10% for the former, and around 15–30% for the latter. The studies published to date are not enough to confirm these malignancy risk predictions, so there is still uncertainty regarding the clinical approach to such nodules. To orient patient management, reference might be made to the malignancy risk of the extensively studied Bethesda classification. Any comparisons drawn between the indeterminate categories of the Bethesda and SIAPEC classifications should be considered with caution, however, for several reasons. For instance, atypia and follicular lesions of undetermined significance (AUS/FLUS) also includes cytological/nuclear atypia in the Bethesda system, which would convert a TIR 3A into a TIR 3B in the SIAPEC system (5). Such an unclear picture sometimes makes it difficult to manage patients with thyroid nodules of indeterminate cytology and to choose between surveillance and diagnostic surgery. In fact, most of such patients undergo diagnostic surgery, but only a minority of their surgically resected nodules prove to be malignant (6). Thyroid lobectomy can be performed for solitary nodules, but then patients need a second surgical procedure to complete the thyroidectomy if histology identifies them as malignant. A more accurate preoperative diagnosis of cancer in thyroid nodules would enable such unnecessary or two-step surgery to be avoided.
In this clinical setting, molecular profiling has been suggested as a way to diagnose nodules of indeterminate cytology more accurately (7), and rule cancer in or out in the light of the test’s positive predictive value (PPV) or negative predictive value (NPV), respectively.
Among the various molecular testing approaches, searching for BRAF and H-, K-, and N-RAS point mutations is currently the most widely used for thyroid nodules of indeterminate cytology. BRAF point mutations are the most common gene mutations in thyroid cancer, occurring in 45% of papillary thyroid carcinomas (PTC), 20–40% of poorly-differentiated thyroid carcinomas (PDTC), and 30–40% of anaplastic thyroid carcinomas (ATC) (8, 9), while they are rarely seen in follicular thyroid carcinomas (FTC). BRAF V600E mutation testing has a high specificity for malignancy, but its sensitivity is too low to rule out cancer reliably (10). Mutational analysis therefore needs to consider other mutations too, such as H-RAS, K-RAS, and N-RAS point mutations. RAS mutations occur in 10–20% of PTC (11), 36–48% of FTC, and 20–40% of PDTC and ATC (10), but they are not specific for thyroid malignancy and they also occur in follicular adenoma (FA).
Telomerase activation is considered a hallmark of cancer because it is frequently activated in many human cancers, but not expressed in most normal tissues. TERT promoter mutations have recently been described in thyroid cancers deriving from follicular cells (12), occurring in 11% of PTC, 17% of FTC, 43% of PDTC, and 40% of ATC (13). Thyroid tumors carrying TERT promoter mutations generally have more aggressive characteristics and higher tumor recurrence and mortality rates (13). TERT promoter mutations therefore have potential as novel diagnostic and prognostic markers in the setting of thyroid cancer.
Our study aimed (1) to establish the frequency and significance of RAS, TERT promoter, and BRAF gene mutations in a large series of patients undergoing thyroid surgery for a nodule of indeterminate cytology and (2) to examine the potential utility of these mutations in clinical practice.
Materials and Methods
FNA Samples and FNA Procedures
The study involved 199 FNA samples of indeterminate cytology obtained from thyroid nodules in 199 consecutive patients referred for surgical excision from December 2012 to May 2016. At our institution, BRAF and H-, K-, and N-RAS mutation analysis in FNA samples is standard procedure for single thyroid nodules and/or those with suspect features on ultrasonography. Molecular analysis for somatic mutations of TERT promoter was performed retrospectively. All studies were performed in accordance with the guidelines proposed in the Declaration of Helsinki. The present study was notified to our Local Ethical Committee (Azienda Ospedaliera di Padova, code number: 42109) and all patients (including the parent/guardian on behalf of the minors) gave their written informed consent to the use of their thyroid cytology findings for research purposes. In this sample of patients, 165/199 (83%) underwent total thyroidectomy, 25/199 (13%) had a lobectomy, and 9/199 (4%) a two-step thyroidectomy.
Of the 199 cases collected, the first 88 were classified according to the SIAPEC 2007 classification, and the remainder (111 cases) according to the revised version published during the course of the study (SIAPEC 2014) (4).
BRAF and RAS Mutation Analysis
DNA was isolated from FNA samples using the QIAamp DNA Micro kit (Qiagen, Italy) according to the manufacturer’s protocol. The BRAF (NM_004333.4) status of exon 15 was assessed by direct sequencing. The primers and PCR reaction protocol have been described elsewhere (14). Exons 2 and 3 of H-RAS (NM_005343.2), K-RAS (NM_033360.2), and N-RAS (NM_002524.3) were analyzed by direct sequencing, as described elsewhere (15, 16).
TERT Promoter Analysis
The TERT proximal promoter (NM_198253.2) was amplified from FNA samples using the amplification conditions described elsewhere (17).
Statistics
Sensitivity, specificity, PPV, and NPV were calculated for each mutation, and for combined methods, considering the histological diagnosis as the gold standard. Group comparisons of categorical variables were performed using the chi-square test. A p-value of <0.05 was considered significant in all tests. All statistical analyses were performed using MedCalc for Windows, version 17.6.
Results
Patient Statistics
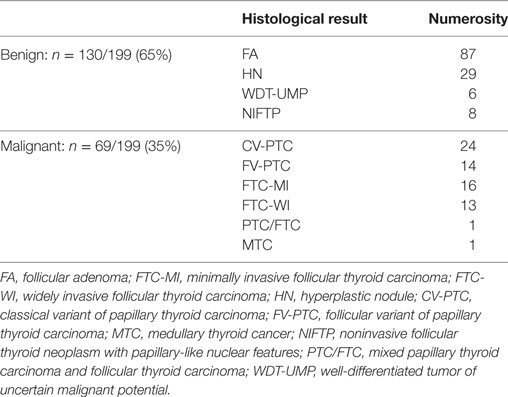
Of the 199 patients included in our study, 155 (78%) were female and 44 (22%) were male. The mean age of the patients was 50 years (median 49 years). Among the 199 indeterminate fine-needle aspirates, 69 (35%) were classified as malignant on histopathological review (final histologies are given in Table 1). In the group of 111 patients classified according to the SIAPEC 2014 system, 63 were TIR 3A and 48 were TIR 3B. Fourteen out of 63 cases (22%) in the TIR 3A group, and 28/48 (58%) in the TIR 3B group proved malignant at histology. A significant correlation emerged between malignancy and a TIR 3B diagnosis (p: 0.004 by chi-square analysis).
RAS Mutation Testing
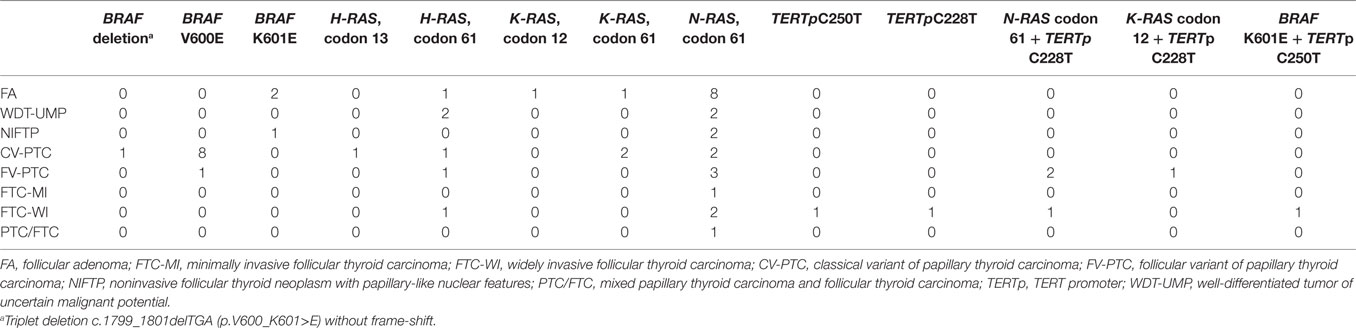
RAS mutations were the most common, detected in 36/199 (18%) patients, and occurring in 28% (19/69) of the malignant nodules. Six nodules revealed an H-RAS mutation at codon 61, and one at codon 13. Three nodules carried a K-RAS mutation at codon 61, and two at codon 12. There were also 24 nodules with an N-RAS mutation at codon 61 (Table 2). Among the 36 patients exhibiting a somatic RAS mutation, 19/36 (53%) were malignant at histology (Table 3). Table 3 shows the sensitivity, specificity, PPV, and NPV for malignancy of these RAS mutations.
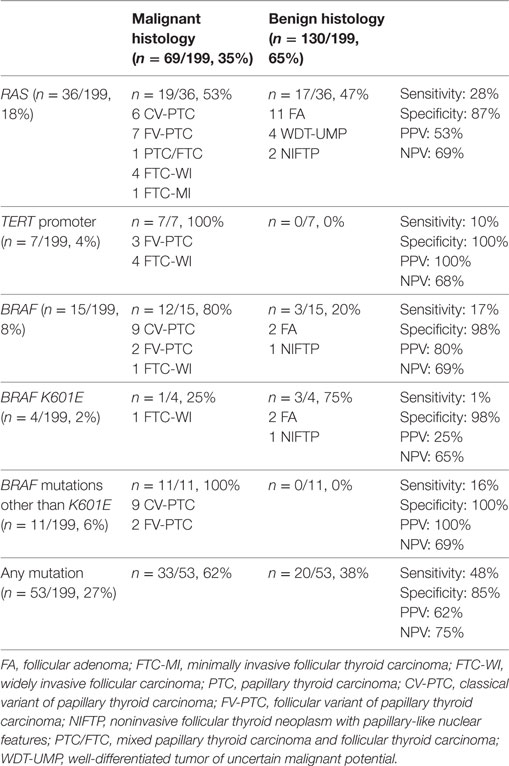
Table 3. Frequency, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and histological correlations of H-,K-,N-RAS, TERT promoter, and BRAF mutations.
BRAF Mutation Testing
BRAF mutations were detected in 15/199 indeterminate thyroid nodules (8%), and in 12/69 (17%) thyroid malignancies. The classic c.1799T>A (p.V600E) mutation was found in 10/15 indeterminate cases (67%). Two rare BRAF mutations were identified: (i) a point c.1801A>G mutation (p.K601E) in 4 patients; and (ii) an in-frame triplet deletion c.1799_1801delTGA (p.V600_K601>E) with no frame-shift in 1 patient (Table 2). The sensitivity, specificity, PPV, and NPV for malignancy of these BRAF mutations are given in Table 3.
TERT Promoter Mutation Testing
TERT promoter mutations were detected in 7/199 (4%) nodules. There was no overlap between C228T and C250T mutations, the former being more prevalent than the latter. Only 2/7 patients carried an isolated somatic TERT promoter mutation, one a C228T and the other a C250T (both FTC-WI). In 5/7 nodules, the TERT promoter mutation was associated with another mutation in the H-, K-, N-RAS, or BRAF oncogenes (Table 2). No TERT promoter mutations were found in non-cancerous lesions, so their specificity and PPV for malignancy was 100%, while their sensitivity and NPV were 10 and 68%, respectively (Table 3).
Combined Molecular Panel
Fine-needle aspiration samples were positive for gene mutations in at least one analysis in 53/199 cases (27%) (Table 3), and 33/53 (62%) of these mutated nodules were malignant. Thirty-three of the 69 malignant nodules carried a mutation, so the panel was able to identify 48% of the malignant lesions. The panel thus achieved a sensitivity, specificity, PPV, and NPV for malignancy of 48, 85, 62, and 75%, respectively, giving a posttest probability of cancer of 62% (PPV) in indeterminate nodules found positive for gene mutations. This corresponds to a 27% improvement in the posttest over the pretest prediction of the cancer risk. For indeterminate nodules proving negative for mutations, on the other hand, the predicted cancer risk decreased from 35% pretest to 25% posttest (Table 3). A distinction between TIR 3A and TIR 3B was available for 111 nodules: in the presence of any gene mutation, the panel revealed a 50% sensitivity, 78% specificity, 37% PPV, and 84% NPV for predicting the cancer risk in the TIR 3A category, and percentages of 39, 85, 79, and 50%, respectively, in the TIR 3B category.
Discussion
Our objectives in this study were to examine the frequency and significance of H-, K-, and N-RAS, TERT promoter and BRAF gene mutations in a large monocentric series of cytologically indeterminate thyroid nodules and to explore the utility (if any) of such a combined gene mutation analysis in terms of improving indeterminate thyroid nodule characterization prior to surgery.
To date, this is the largest monocentric European series of RAS-mutated cytologically indeterminate thyroid nodules with a histological diagnosis. RAS mutations were identified in 18% of the indeterminate nodules in our series, and proved to be the most common mutations. This percentage is higher than hitherto reported in patients undergoing thyroid surgery, which has been about 12% in other European series (18, 19), and as low as 6% in American series (7). On the other hand, it was 11.5% when Nikiforov et al. restricted their assessment to patients who underwent surgery with an AUS/FLUS, or with a follicular neoplasm or suspected follicular neoplasm (FN/SFN) cytology (7). Cancer was found in 53% of the RAS-mutated nodules in our series, a considerably lower proportion than the 85% reported in American series (7), but much higher than the 18% identified in another recent Italian report (20) involving only 11 RAS-mutated patients who underwent surgery, and whose cytologies were mostly benign. On the other hand, the cancer occurrence rate in our RAS-mutated nodules was comparable with that of another European series (18), in which the significance of the mutation was analyzed in a similar patient setting.
As for the frequency and significance of BRAF mutations in cytologically indeterminate thyroid nodules, the percentage of mutated nodules in our series was 8%, which is slightly higher than reported elsewhere in the literature for indeterminate nodules, i.e., 5% in a recent meta-analysis on nine studies, and 6% in a large individual study published after the meta-analysis (18, 21). So, BRAF testing alone has a low sensitivity (17%) in this setting. In addition, the BRAF K601E mutation needs to be considered separately because of its low probability of being associated with cancer: 3/4 BRAF K601E-mutated nodules in our series were benign. Two of the 3 patients harboring this mutation with a benign histology revealed FA with a microfollicular growth pattern, while the third had a noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). These three patients’ slides were reviewed to confirm their histopathological diagnosis. They were not the first cases to be reported of FA associated with a BRAF K601E mutation (22, 23), but—to our knowledge—ours is the first report of a BRAF K601E mutation occurring in a NIFTP.
This is also the largest monocentric study to have investigated the frequency and significance of TERT promoter mutations in indeterminate thyroid FNA samples. With the exception of ThyroSeq. v2 (validated in the setting of FN/SFN cytologies), commercially available molecular panels for thyroid nodules found indeterminate on FNA do not include TERT promoter analysis (24). We aimed to ascertain the value of testing TERT promoter mutation analysis in a large collection of indeterminate nodules (including cytologies with less aggressive features than those in the FN/SFN category) to see if it might be useful and cost-effective to include it in such commercial panels. In our series, TERT mutations were found in 7/199 (4%) FNA samples, and in 7/69 (10%) histologically confirmed malignant nodules. All but two cancers found positive for TERT promoter mutations (5/7) also harbored another RAS or BRAF gene mutation. TERT promoter mutation testing alone thus revealed a low diagnostic sensitivity in indeterminate FNA samples, but a 100% specificity. We only found TERT promoter mutations in follicular-derived thyroid cancer with a follicular growth pattern—4/7 cases were widely invasive FTC (FTC-WI), and 3/7 were follicular variant of PTC (FV-PTC)—while none of the classical PTC in our series revealed any TERT mutations. All the FTCs harboring TERT promoter mutations were widely invasive, consistently with the literature correlating these mutations with a more aggressive disease and a worse prognosis. It is worth noting that one patient harboring a TERT promoter C250T point mutation also carried a BRAF K601E mutation: the association between TERT promoter and BRAF V600E mutations has been amply described in the literature (13), but ours is the first report (to our knowledge) of an association between TERT promoter and BRAF K601E mutations. This case seems particularly interesting because it is only the fifth case of FTC associated with a BRAF K601E mutation to be reported in the literature (22, 25–27). Three of the five known FTCs with the BRAF K601 mutation had minimal capsular and vascular invasion, consistent with the tendency of this mutation to be associated with low-grade cancers (22, 26, 27). The fourth (25) also harbored a PIK3CA mutation and the patient had extensive angio-invasion. Our case is therefore the second in which FTC harboring a BRAF K601E mutation is associated with extensive vascular invasion. We surmise that the BRAF K601E and TERT C250T mutations worked in synergy in the pathogenesis of our patient’s FTC: this tumor’s tendency to have more aggressive phenotypic characteristics may be due not only to the presence of the TERT promoter mutation, but also to its association with the BRAF K601E mutation. Given the strong association between TERT promoter and RAS mutations, and the case of the former being combined with a BRAF K601E mutation, our TERT promoter mutation analysis was able to identify only two additional cases of cancer in our series, so it was of little use for cancer identification purposes, in this class of cytologies at least. On the other hand, RAS and K601E mutations alone carry a low-to-moderate cancer risk (53% for the former and 25% for the latter), so the identification of a TERT promoter mutation might still be useful to the clinician in the setting of indeterminate nodules.
Returning to the usefulness of combined molecular panels, their use raised the cancer risk for indeterminate thyroid nodules found positive for genetic mutations to 62% (up 27% with respect to the pretest level), which is similar to the risk associated with TIR 4 lesions. Our PPV of 62% is lower than in an American series (in which it ranged from 87 to 95%), but comparable with the percentage seen in other European series. This disparity may be due to the higher percentage of cancer documented in American RAS-mutated indeterminate nodules, possibly because the Bethesda and SIAPEC classifications do not perfectly overlap (7, 18), and/or because our panel did not include RET/PTC and PAX8/PPARG rearrangement analysis. The introduction of the new histopathologic nomenclature of NIFTP may have played a part too in reducing the PPV of molecular panels for indeterminate lesions, as expected (28). Differences in detection methods could have played a role as well. Histological correlation demonstrated that applying our panel to indeterminate thyroid nodules prompted the identification of 48% of the malignant lesions, an efficiency in identifying cancer that resembles the results achieved by Eszlinger et al. in their monocentric European study (18).
Our study has some limitations to consider. First, it was conducted on a consecutive series of patients referred for surgery, which is probably why their pretest probability of cancer was rather higher than expected (35% vs 25–30%), giving rise to a clinical setting in which “rule-in” molecular tests might be particularly effective. This is certainly not the first series of cytologically indeterminate thyroid nodules with such a high percentage of cancers (29), however. At our institution, FNA cytology is only performed for isolated nodules and those with suspect features on ultrasound, and it is well known that the presence of even one suspicious sonographic feature raises the cancer risk in indeterminate nodules (30). In addition, not all the patients in our series were classified according to the latest SIAPEC classification, and this prevented us from thoroughly examining the utility of molecular analysis in nearly half of our sample, though there was evidence of a markedly higher NPV for cancer in TIR3A than in TIR 3B patients (84 vs 50%). In other words, the utility of the gene mutation panel may be greater when the cancer rate is better defined.
In conclusion, we feel that the outcome of molecular testing, as done in our large series of cytologically indeterminate thyroid nodules, can orient physicians’ and surgeons’ patient management in clinical practice. In the light of our findings, 92 patients could have been spared total thyroidectomy and treated adequately with a lobectomy, and 4 total thyroidectomies would have been performed in one instead of two steps, possibly with fewer side effects. On the other hand, five unnecessary total thyroidectomies would have been performed in patients revealing genetic mutations associated with a benign histology. It is worth adding that there is currently a move toward novel approaches for the management of thyroid nodules in an effort to limit their over-diagnosis and over-treatment. According to the new American Thyroid Association guidelines, thyroid lobectomy suffices for malignant nodules smaller than 4 cm, with no extrathyroidal extension or clinical evidence of lymph node metastases (Recommendation 35) (1). Prospective and controlled studies resulting from the application of these guidelines are still lacking, however. Meanwhile, molecular testing may be useful for stratifying cytologically indeterminate categories for the purpose of deciding on their surgical treatment. The sensitivity of TERT promoter mutation for the purpose of predicting cancer risk is low in the indeterminate category, so testing for this mutation may not be cost-effective. In such a particular setting, however, where it remains mandatory to recognize rare cancers with an aggressive behavior, testing for TERT promoter mutations might still prove its worth, particularly in the preoperative setting.
Author Contributions
SC: study concept and design, substantial contributions to data acquisition, analysis and interpretation, drafting of the manuscript, final approval of the version to be published, and agreement with all aspects of the work; EC: study concept and design, substantial contributions to data acquisition and analysis, drafting of the manuscript, final approval of the version to be published, and agreement with all aspects of the work; SW-F, MI, EI, and FV: substantial contributions to data acquisition and interpretation, critical revision of the manuscript, final approval of the version to be published, and agreement with all aspects of the work; LB, SB, and PL: substantial contributions to data acquisition, analysis and interpretation, critical revision of the manuscript, final approval of the version to be published, and agreement with all aspects of the work; DN: study concept and design, substantial contributions to data acquisition and analysis, final approval of the version to be published, and agreement with all aspects of the work; FG, GP, and AF: substantial contributions to data acquisition, critical revision of the manuscript, final approval of the version to be published, and agreement with all aspects of the work; CM: study concept and design, drafting of the manuscript, critical revision of the manuscript, final approval of the version to be published, and agreement with all aspects of the work, study supervision.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Laura Zambonin for her excellent technical support.
References
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid (2015) 26:1–133. doi:10.1089/thy.2015.002
2. Fadda F, Basolo A, Bondi G, Bussolati G, Crescenzi A, Nappi O, et al. SIAPEC-IAP Italian Consensus Working Group: cytological classification of thyroid nodules. Proposal of the SIAPEC-IAP Italian Consensus Working Group. Pathologica (2010) 102:405–6.
3. Piana S, Frasoldati A, Ferrari M, Valcavi R, Froio E, Barbieri V, et al. Is a five-category reporting scheme for thyroid fine needle aspiration cytology accurate? Experience of over 18,000 FNAs reported at the same institution during 1998–2007. Cytopathology (2011) 22:164–73. doi:10.1111/j.1365-2303.2010.00777.x
4. Nardi F, Basolo F, Crescenzi A, Fadda G, Frasoldati A, Orlandi F, et al. Italian consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest (2014) 37:593–9. doi:10.1007/s40618-014-0062-0
5. Poller DN, Baloch ZW, Fadda G, Johnson SJ, Bongiovanni M, Pontecorvi A, et al. Thyroid FNA: new classifications and new interpretations. Cancer Cytopathol (2016) 124:457–66. doi:10.1002/cncy.21703
6. Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol (2002) 26:41–4. doi:10.1002/dc.10043
7. Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab (2011) 26:3390–7. doi:10.1210/jc.2011-1469
8. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res (2003) 63:1454–7.
9. Cohen Y, Xing M, Mambo EJ, Guo Z, Wu G, Trink B, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst (2003) 95:625–7. doi:10.1093/jnci/95.8.625
10. Nikiforov YE, Nikiforova NM. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol (2011) 7:569–80. doi:10.1038/nrendo.2011.142
11. Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol (2008) 21(Suppl 2):S37–43. doi:10.1038/modpathol.2008.10
12. Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer (2013) 20:603–10. doi:10.1530/ERC-13-0210
13. Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer (2016) 23:R143–55. doi:10.1530/ERC-15-0533
14. Barollo S, Pezzani R, Cristiani A, Redaelli M, Zambonin L, Rubin B, et al. Prevalence, tumorigenic role, and biochemical implications of rare BRAF alterations. Thyroid (2014) 24:809–19. doi:10.1089/thy.2013.0403
15. Sadow PM, Heinrich MC, Corless CL, Fletcher JA, Nosè V. Absence of BRAF, NRAS, KRAS, HRAS mutations and RET/PTC gene rearrangements distinguishes dominant nodules in Hashimoto thyroiditis from papillary thyroid carcinomas. Endocr Pathol (2010) 21:73–9. doi:10.1007/s12022-009-9101-3
16. Xu X, Quiros RM, Gattuso P, Ain KB, Prinz RA. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res (2003) 63:4561–7.
17. Muzza M, Colombo C, Rossi S, Tosi D, Cirello V, Perrino M, et al. Telomerase in differentiated thyroid cancer: promoter mutations, expression and localization. Mol Cell Endocrinol (2015) 399:288–95. doi:10.1016/j.mce.2014.10.019
18. Eszlinger M, Piana S, Moll A, Bösenberg E, Bisagni A, Ciarrocchi A, et al. Molecular testing of thyroid fine-needle aspirations improves presurgical diagnosis and supports the histologic identification of minimally invasive follicular thyroid carcinomas. Thyroid (2015) 25:401–9. doi:10.1089/thy.2014.0362
19. De Napoli L, Bakkar S, Ambrosini CE, Materazzi G, Proletti A, Macerola E, et al. Indeterminate single thyroid nodule: synergistic impact of mutational markers and sonographic features in triaging patients to appropriate surgery. Thyroid (2016) 26:390–4. doi:10.1089/thy.2015.0311
20. Rossi M, Buratto M, Tagliati F, Rossi R, Lupo S, Trasforini G, et al. Relevance of BRAF(V600E) mutation testing versus RAS point mutations and RET/PTC rearrangements evaluation in the diagnosis of thyroid cancer. Thyroid (2015) 25:221–8. doi:10.1089/thy.2014.0338
21. Trimboli P, Treglia G, Condorelli E, Romanelli F, Crescenzi A, Bongiovanni M, et al. BRAF-mutated carcinomas among thyroid nodules with prior indeterminate FNA report: a systematic review and meta-analysis. Clin Endocrinol (Oxf) (2016) 84:315–20. doi:10.1111/cen.12806
22. Afkhami M, Karunamurthy A, Chiosea S, Nikiforova MN, Seethala R, Nikiforov YE, et al. Histopathologic and clinical characterization of thyroid tumors carrying the BRAF(K601E) mutation. Thyroid (2016) 26:242–7. doi:10.1089/thy.2015.0227
23. Trovisco V, Soares P, Preto A, De Castro IV, Lima J, Castro P, et al. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients’ age but not with tumour aggressiveness. Virchows Arch (2005) 446:589–95. doi:10.1007/s00428-005-1236-0
24. Nikiforov YE, Carty SE, Chiosea SI, Coyne C, Duvvuri U, Ferris WE, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer (2014) 120:3627–34. doi:10.1002/cncr.29038
25. Pennelli G, Vianello F, Barollo S, Pezzani R, Merante Boschin I, Pelizzo MR, et al. BRAF(K601E) mutation in a patient with a follicular thyroid carcinoma. Thyroid (2011) 21:1393–6. doi:10.1089/thy.2011.0120
26. Schulten HJ, Salama S, Al-Mansouri Z, Alotibi R, Al-Ghamdi K, Al-Hamour OA, et al. BRAF mutations in thyroid tumors from an ethnically diverse group. Hered Cancer Clin Pract (2012) 10:10. doi:10.1186/1897-4287-10-10
27. Cho U, Oh WJ, Bae JS, Lee S, Lee YS, Parj GS, et al. Clinicopathological features of rare BRAF mutations in Korean thyroid cancer patients. J Korean Med Sci (2014) 29:1054–60. doi:10.1186/1897-4287-10-10
28. Haugen BR, Sawka AM, Alexander EK, Bible KC, Caturegli P, Doherty GM, et al. American thyroid association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid (2017) 27:481–3. doi:10.1089/thy.2016.0628
29. Ohori NP, Schoedel KE. Variability in the atypia of undetermined significance/follicular lesion of undetermined significance diagnosis in the Bethesda system for reporting thyroid cytopathology: sources and recommendations. Acta Cytol (2011) 55:492–8. doi:10.1159/000334218
Keywords: thyroid nodules, indeterminate thyroid cytology, TERT promoter mutations, H-,K-,N-RAS mutations, BRAF mutations
Citation: Censi S, Cavedon E, Bertazza L, Galuppini F, Watutantrige-Fernando S, De Lazzari P, Nacamulli D, Pennelli G, Fassina A, Iacobone M, Casal Ide E, Vianello F, Barollo S and Mian C (2017) Frequency and Significance of Ras, Tert Promoter, and Braf Mutations in Cytologically Indeterminate Thyroid Nodules: A Monocentric Case Series at a Tertiary-Level Endocrinology Unit. Front. Endocrinol. 8:273. doi: 10.3389/fendo.2017.00273
Received: 06 July 2017; Accepted: 27 September 2017;
Published: 16 October 2017
Edited by:
Gabriella Pellegriti, Università degli Studi di Catania, ItalyReviewed by:
Dario Giuffrida, Istituto oncologico del mediterraneo, ItaliaMaria Chiara Zatelli, University of Ferrara, Italy
Copyright: © 2017 Censi, Cavedon, Bertazza, Galuppini, Watutantrige-Fernando, De Lazzari, Nacamulli, Pennelli, Fassina, Iacobone, Casal Ide, Vianello, Barollo and Mian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caterina Mian, caterina.mian@unipd.it
 Simona Censi1
Simona Censi1 Susi Barollo
Susi Barollo Caterina Mian
Caterina Mian