- Beijing Key Laboratory of Reproductive Endocrinology and Assisted Reproductive Technology and Key Laboratory of Assisted Reproduction, Ministry of Education, Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing, China
Kisspeptins are a family of neuropeptides that are critical for initiating puberty and regulating ovulation in sexually mature females via the central control of the hypothalamic–pituitary–gonadal axis. Recent studies have shown that kisspeptin and its receptor kisspeptin receptor (KISS1R) are expressed in the mammalian ovary. Convincing evidence indicates that kisspeptins can activate a wide variety of signals via its binding to KISS1R. Experimental data gathered recently suggest a putative role of kisspeptin signaling in the direct control of ovarian function, including follicular development, oocyte maturation, steroidogenesis, and ovulation. Dysregulation or naturally occurring mutations of the kisspeptin/KISS1R system may negatively affect the ovarian function, leading to reproductive pathology or female infertility. A comprehensive understanding of the expression, actions, and underlying molecular mechanisms of this system in the human ovary is essential for novel approaches to therapeutic and diagnostic interventions in reproductive diseases and infertility.
Introduction
Female reproduction is a highly orchestrated and regulated process controlled by the hypothalamic–pituitary–ovarian (HPO) axis. The pulsatile gonadotropin-releasing hormone (GnRH), and therefore gonadotropins (FSH and LH), secretion primarily governs the HPO axis at puberty and maintains the cyclic function in adulthood (1). This tonic GnRH/gonadotropins secretion is modulated by a negative feedback effect of serum estrogen secreted from the growing ovarian follicles (2). Apart from the pulsatile secretion of GnRH, the surge mode of GnRH release is characterized by a large amount of LH secretion, which is required for triggering ovulation in female mammals (3). During the periovulatory stage, the high serum level of estrogen exerts its positive feedback influence upon GnRH neurons to induce a GnRH surge and hence the LH surge (4). Studies related to the underlying cellular and molecular mechanisms of the negative and positive feedback effects of estrogen have been of considerable interest. Even though the critical role of GnRH in regulating female reproduction, there exist several functional limitations of the GnRH neuronal network. The major issue is that GnRH neurons do not express estrogen receptor α, the principle receptor that mediates both negative and positive estrogen feedback actions (5).
In the past decade, emerging studies have found that kisspeptin (KISS1) is the upstream regulator of pulsatile and surge GnRH release, with indispensable roles in female reproduction, including gonadotropin secretion, puberty onset, brain sex differentiation, ovulation, and metabolic regulation of fertility (6, 7). In mammals, two populations of hypothalamus kisspeptin neurons, anteroventral periventricular nucleus (AVPV) and arcuate nucleus (ARC), have been identified to play different functional roles in exerting the positive and negative feedback actions in response to estrogen (8, 9). Specifically, kisspeptins function through a G-protein-coupled receptor, kisspeptin receptor (KISS1R) to stimulate the release of GnRH (and subsequent secretion of FSH and LH) in many mammals. Notably, in humans and mice, inactivating mutations in either kisspeptin or KISS1R lead to the phenotype of hypogonadotropic hypogonadism. In addition, emerging evidence indicates the potential physiological roles of extra-hypothalamic kisspeptins in modulating the activity of diverse systems in the brain and many peripheral organs (10–14). Several studies have demonstrated that kisspeptins and their putative receptor, KISS1R are expressed across different types of tissues (15–19). Therefore, kisspeptins may exert their direct actions on various types of tissues in an autocrine/paracrine manner depending on different physiological conditions. With regard to the reproductive function, an increasing number of reports have shown that the reproductive tissues, such as ovary, female genital tract, placenta, and testis, can express functional form of kisspeptin/KISSR system among various species including humans (18, 20, 21). Furthermore, several publications have appeared in recent years documenting that locally produced kisspeptin/KISSR directly participates in a series of physiological and pathological activities in the ovary. Indeed, the extra-hypothalamic roles of kisspeptins have recently attracted special attention in fields related to reproductive biology and clinical reproductive medicine. This review will focus mainly on available literature related to the pathophysiological roles of kisspeptin/KISSR in regulating ovarian function and summarize our current understanding of the mechanisms by which kisspeptin exert its cellular actions as well as the therapeutic implications of kisspeptins in reproductive medicine.
Methods
A systematic literature search was performed using PubMed and Web of Science for all English-language articles up to November 2017. A systematic review of English-language publications was carried out using the following keywords: Kiss1, kisspeptin, metastin, KISS1R, GPR54, ovary, kisspeptin signaling, premature ovarian failure, PCOS, endometriosis, follicular development, steroidogenesis, oocyte maturation, ovulation, knockout, and therapeutic application. The goal of this review is to summarize the latest studies regarding the direct roles and physiological significance of kisspeptin/KISS1R in the ovary and discuss some molecular mechanisms and potential therapeutic targets in reproductive diseases.
Discovery of Kisspeptin and KISS1R
In 1996, kisspeptin (the 145 amino acid) and its encoding gene KISS1 were first identified as a suppressor (and a gene) of human malignant melanoma in Hershey, Pennsylvania, USA—the hometown of the famous Hershey’s kisses chocolates (22). The name of KISS1 was derived from these sweets, with the “SS” representative of “suppressor sequence.” In human, KISS1 is located on the long (q) arm of chromosome 1 at q32. KISS1 encodes an unstable and biologically inactive intermediate prepropeptide of 145 amino acids, which is further post-translationally converted to four biologically active peptides distinguished on the basis of their number of amino acid: kisspeptin-54, 14, 13, and 10 (Figure 1). All of the peptides have a C-terminal region that contains an Arg–Phe–NH2 motif characteristic of the RF-amide peptide family, which allows them to fully activate KISS1R. Based on structural similarities and their common origin as KISS1-derived peptides, the term kisspeptin was globally used to define this family (6, 23). Kisspeptin-54 was initially termed as “metastin” because of its capacity to inhibit tumor metastasis. This peptide has been considered as the major product of the human KISS1 gene (19). Whereas in rats and mice, the largest proteolytic product of the kisspeptin precursor is kisspeptin-52 (composed of 52 amino acids), and the terminal RF-amide signature is substituted by an Arg–Tyr–NH2 motif (6). Kisspeptin-54, -14, and -13 as well as a shorter peptide designated kisspeptin-10 have the same affinity and efficacy on KISS1R in both humans and rats, indicating that the C-terminal part of the peptides is responsible for the high-affinity binding and the activation of KISS1R (23).
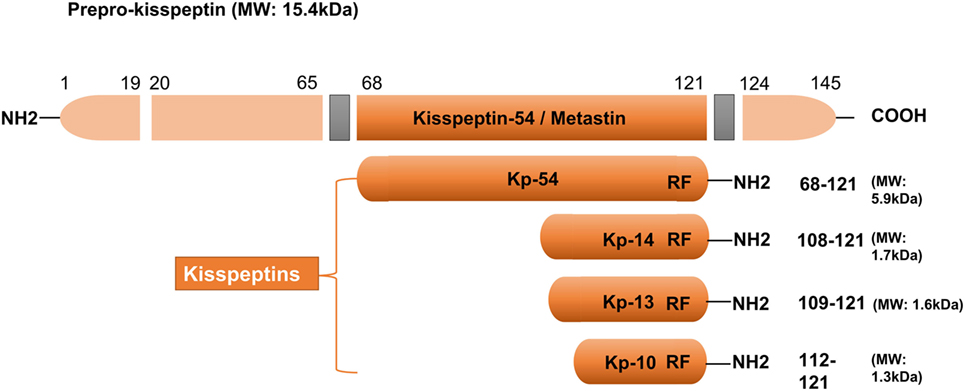
Figure 1. Major structural features of human kisspeptins, the products of the Kiss1 gene. Different kisspeptins are generated by the cleavage from a common precursor, the prepro-kisspeptin. The prepro-kisspeptin contains 145 amino acids, with a 19-amino acid signal peptide and a central 54-amino acid region, kisspeptin-54 (Kp-54; formerly termed as metastin). Further cleavage of metastin generates kisspeptins of lower molecular weight: kisspeptin-14 (Kp-14), Kp-13, and Kp-10. All kisspeptins contain the RF-amide motif that is able to bind and activate kisspeptin receptor. Modified from Ref. (6).
Kisspeptin receptor is a seven-transmembrane G-protein-coupled receptor, which was firstly identified in the rat brain as an orphan receptor with around 40% sequence similarity with the transmembrane region of galanin receptors (24). Subsequently, the human ortholog of KISS1R was cloned and cataloged as a putative receptor for KISS1-derived peptides (19, 25). Since various groups of researchers independently noted its presence or studied its physiological roles, KISS1R has been given various different names, including KISS1R, GPR54, AXOR12, hOT7T175, CPPB1, and HH8 (19, 23–25). It was not until 2003 that the physiological role of kisspeptins and their receptor, KISS1R in the neuroendocrine–reproductive axis was identified (26, 27), which thereafter revolutionized the field of reproductive physiology. These findings suggest that kisspeptins and their receptor KISS1R play a critical role as gatekeepers of sexual maturation during puberty onset and central processors for the dynamic regulation of the gonadotropic axis at adulthood.
The Kisspeptin/KISS1R System in the Ovary
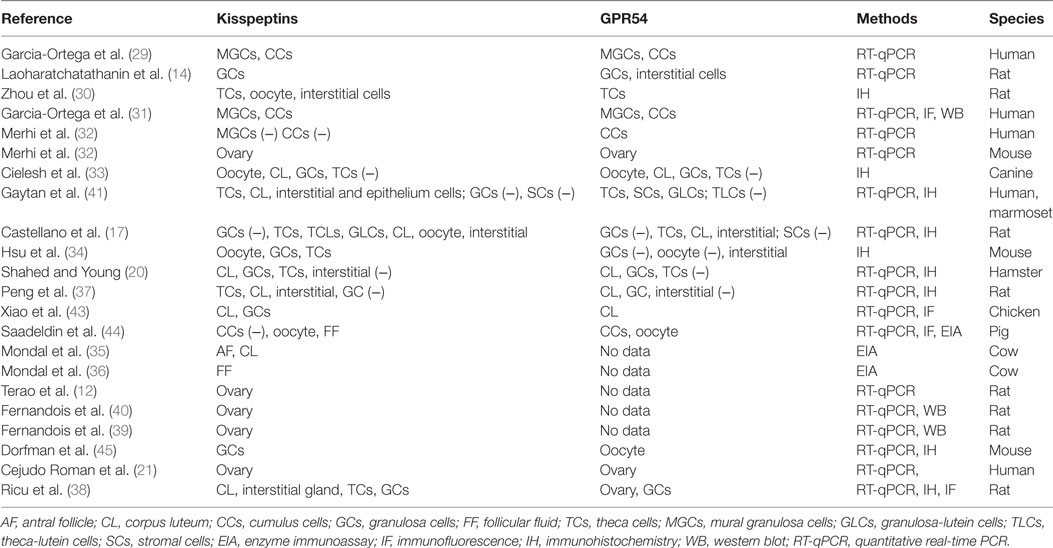
The action of kisspeptin/KISS1R in the ovary presupposes the presence of functional KISS1R and its endogenous ligand kisspeptin. Expression of both kisspeptin and KISS1R has been reported in a variety of tissues, including adipose tissue, pancreas, liver, small intestine, peripheral blood lymphocytes, testis, lymph nodes, aorta, coronary artery, and umbilical vein, female tract, and particularly abundant in placenta and the central nervous system (12, 21, 28). Terao et al. first reported that kisspeptin mRNA was prominently expressed in the rat ovary, suggesting a locally functional role of kisspeptin in this reproductive tissue (12). Specific structure or cell expression of kisspeptin and its receptor KISS1R had not been indicated until 2006, when Castellano et al. found that kisspeptin and KISS1R were both expressed in theca cells, corpora lutea, and interstitial tissues (17). While there are inconsistent results from later studies that are particular puzzling with regard to the cell expression of Kiss1/KISS1R even in the same species (14, 20, 29–36) (summarized in Table 1). For example, some studies demonstrated the absence of kisspeptin/KISS1R in granulosa cells (GCs) of rat ovary (17, 30), whereas other studies indicated the strong expression of kisspeptin or KISS1R in rat GCs (14, 37, 38). One study has identified that GC was the major site for kisspeptin synthesis in rats, as Kiss1 mRNA expression was significantly higher in the GCs compared with the theca cells and other ovarian cells (38). However, other studies showed that KISS1R mRNA was equally distributed between the GCs and other cells of the residual ovary. These inconsistencies in the expression of the kisspeptin/KISS1R system in the ovary are partly because of the different methods employed to examine their presence. In addition, the discrepancies of age and ovarian tissues and cells obtained from different estrous/menstrual cycles can significantly affect the expression patterns (17, 20, 32–35, 38–41). Similar to other species, the follicular expression of kisspeptin/KISS1R is gradually increased as the follicles grow, with a highest level at the preovulatory stage in humans (20, 35, 36), which is partly due to the stimulatory effect of the gradually increased gonadotropins on the expression of kisspeptin at this stage (17). In gene knockout models, Kiss1−/− or Kiss1R−/− mice showed small ovarian size and weight compared to the wild-type counterparts (see Table 2). Initially, no such difference in ovarian weight was detected between wild-type and Kiss1R−/− females in 3-week-old animals. Eventually, in 9-week-old and 7-month-old animals, there was a significant decrease in reproductive organ weights in the female Kiss1R−/− mice (42). These findings suggest a possible age-related physiological role for the kisspeptin/KISS1R system in ovarian physiology and that this system functions mainly at the puberty and adult stages.
Roles of the Kisspeptin/KISS1R System in Regulating Ovarian Function
Follicular Development
The population of primordial follicles decreases at variable rates until menopause in humans and infertility in rodents. During follicular development, the selection and activation of primordial follicles in the ovarian pool (ovarian reserve), established early in life, provides all growing follicles, including primary, secondary, small antral, and large antral follicles, and ovulated oocytes.
Using an implanted mini-osmotic pump containing kisspeptin or kisspeptin antagonist P234 for 28 days, Fernandois et al. evaluate the long-term effect of kisspeptin on ovarian follicular development (39). In 6- and 10-month-old rats, ovaries infused with a low dose of kisspeptin had a fewer number of antral follicles, but an increased number of preovulatory follicles and corpora lutea. On the contrary, ovaries infused with P234 had an increased number of antral follicles and a decreased number of preovulatory follicles and corpora lutea. This study also showed that kisspeptin attenuated the initial follicle recruitment (primary to secondary) by downregulating the expression of FSH receptor (FSHR). Apart from the downregulation of FSHR, kisspeptin can suppress the initial follicle recruitment through the upregulation of circulating anti-Müllerian hormone (AMH). AMH is primarily secreted by the secondary and small antral follicles and is a biomarker for ovarian reserve (57) due to its ability to inhibit the activation of primordial follicles (58). Interestingly, Fernandois et al. found that local administration of kisspeptin increased plasma AMH, whereas administration of P234 decreased plasma AMH in 6- and 10-month-old rats (39). Therefore, kisspeptin may negatively regulate preantral (including primordial, primary, and secondary follicles in this review) follicular development through upregulating AMH and downregulating FSHR expression in the ovary. However, there was an intriguing phenomenon that kisspeptin can induce an accumulation of preovulatory follicles and a decrease in the number of small antral follicles (39). We may speculate that kisspeptin inhibits the growth of preantral and small antral follicles by negatively regulating the expression of FSHR. On the other hand, kisspeptin upregulates AMH expression and promotes the maturation of large antral follicles, as the same phenotype shown in Kiss1−/− or Kiss1r−/− knockout models (54, 55). Intriguingly, low dose of LH has been shown to suppress the development of small ovarian follicles and stimulate the growth of large ovarian follicles (59–64). Collectively, these findings indicate that kisspeptin could be the downstream target of LH signals to regulate the follicular development. In line with these findings, Castellano and coworkers found that Kiss1 mRNA was significantly increased in rat ovary following the injection of hCG (17).
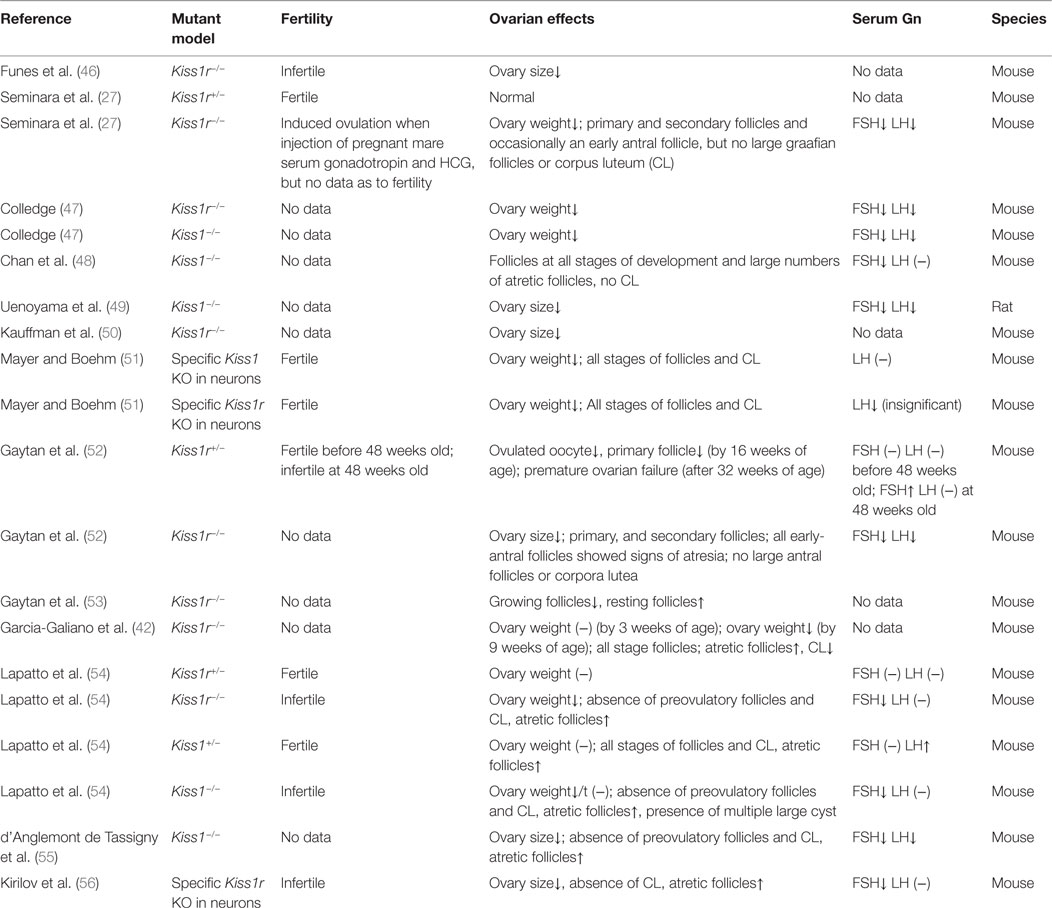
In gene knockout models, both Kiss1r−/− and Kiss1−/− mice display significantly reduced ovarian weight and size (46, 47, 49, 50), which may be resulted from the absence of large follicles (Table 2). Interestingly, the Kiss1r haploinsufficient (Kiss1r+/−) mice have significantly decreased ovarian kiss1r expression and exhibit premature ovarian failure (POF) at 32-week-old age, with a substantial loss of preantral follicles and increased percentage of atretic follicles. The depletion of these follicles seems not because of the defect in gonadotropin secretion as the exhaustion of follicular reserve cannot be rescued by gonadotropin replacement (52). Moreover, the gonadotropin levels were not significantly different between the wild-type and Kiss1r+/− mice as demonstrated in another study (54). Interestingly, the discrepancy of the number of preantral and antral follicles between Kiss1r+/− and wild-type mice is related to age. Indeed, no significant differences in preantral follicle development were observed between wild-type and Kiss1r+/− mice before puberty, but the progressively decreased number of preantral follicles were detected in Kiss1r haploinsufficient mice after puberty, and thereafter until the age of 32-week olds. Consistent with these results, immature ovaries showed low to negligible levels of Kiss1 mRNA, which were significantly enhanced by gonadotropin priming (17). Taken together, all these findings strongly suggest that functional role of kisspeptin in regulating follicular development mainly occurs after puberty, which is in consistence with the age-related expression of kisspeptin in the ovary (17, 32, 39).
Increased atretic follicles were also observed in the ovaries obtained from KISS1R−/− humans and Kiss1−/− mice (48, 54, 55). However, the increased number of atretic follicles cannot be totally attributed to the local kisspeptin/KISS1R signals, as the decreased FSH levels caused by the neural Kiss1/Kiss1r knockout and subsequently the downregulation of hypothalamic–pituitary–gonadal axis can also induce the atretic changes of the follicles (56, 65) (Table 2). Nevertheless, one study using ovarian histology showed the presence of all stages of follicular development and corpora lutea in mice with targeted ablation of Kiss1 and/or Kiss1r expressing neurons (reduced more than 90%). Interestingly, the LH levels of mutant mice were lower than the wild-type mice, without showing the FSH levels (51). These results suggested the indispensable role of the neural kisspeptin/KISS1R system in regulating follicular development. Future studies aimed at addressing the functional role of intraovarian kisspeptin/KISSR1 system in regulating follicular development using the established ovary-specific Kiss1/Kiss1r knockout model will be urgently required.
Oocyte Maturation
Evidence from two independent studies showed that kisspeptin-54 injection could trigger human oocyte maturation effectively and safely (66, 67). Given that kisspeptin-54 is able to cross the blood–brain barrier and then stimulates LH release to the peripheral circulation (44), we can expect a dramatically elevated level of LH in plasma following the kisspeptin-54 injection as reported in two studies (66, 67). Therefore, it is difficult to establish a theoretical concept that kisspeptin can directly induce oocyte maturation in the ovary because oocyte maturation can also be induced by the elevated LH level. Nevertheless, kisspeptin has been shown to enhance in vitro maturation of the oocytes of pigs (44) and sheep (68). However, these studies used an in vitro culture system of the cumulus–oophorus complex instead of the denuded oocytes. Since both cumulus cells (CCs) and oocyte can express KISS1R in pigs and mice (44, 45), it is easily confused whether the kisspeptin-induced oocyte maturation is mediated by the CCs or the oocyte itself. One interesting result derived from these studies is that during in vitro maturation of pig cumulus–oophorus complex, treatment with kisspeptin-10 resulted in temporally elevated expression of GDF9 and BMP15 in oocytes, two essential growth factors involved in regulating folliculogenesis, ovulation, luteinization, oocyte maturation, and developmental competency (69–71). Furthermore, these effects are potentially mediated via a MAPK signaling pathway in GCs (72, 73). On the other hand, administration of kisspeptin-10 also upregulated the expression of C-MOS in oocytes, which plays a crucial role in promoting the meiosis process, formation of normal spindle and chromosome, and reactivation of purified maturation promoting factor after first meiosis (74–77). Collectively, these findings suggest that kisspeptin-induced oocyte maturation is mediated by the upregulation of C-MOS, GDF9, and BMP15. To the best of our knowledge, the utilization of kisspeptin to induce in vitro maturation of human oocytes has not been reported.
Ovulation
Peripheral administration of kisspeptin has been reported to induce ovulation in rats (78) and ewes (79). Since plasma levels of gonadotropins (FSH and LH) significantly increased after the administration of kisspeptin, the stimulatory effect of kisspeptin on ovulation is most likely at the hypothalamus instead of at the ovary.
In gene knockout mice, depletion of Kiss1r can induce ovulation after standard gonadotropin priming (27), suggesting that the ovarian kisspeptin signaling is not mandatory for ovulation. In addition, in Kiss1r−/− female mice following the extended GnRH plus gonadotropin stimulation, newly formed corpora lutea could be observed in the ovaries, and cumulus–oophorus complexes could be found in the oviducts (52). Although there seems no significant difference of oocyte quality between wild-type and Kiss1r−/− female mice, null animals presented significantly fewer ovulated oocytes and corpora lutea (52), suggesting that the GnRH plus gonadotropin stimulation is not enough to reverse the functional loss due to Kiss1r knockout. Given that ovaries from nearly all Kiss1r−/− and many Kiss1−/− mice do not contain follicles past the antral follicle stage (54), and most Kiss1−/− mice and at least one Kiss1r−/− mouse exhibit multiple large cysts with no sign of ovulation, the depletion of kisspeptin/KISS1R system significantly influences the process of ovulation. In neuron-specific Kiss1 and Kiss1r knockout mice, the ovarian histology showed follicles at all developmental stages and the presence of corpora lutea. All female mutant mice can produce offspring when mated to wild-type males (51). These two independent studies strongly suggest that the locally produced kisspeptin/KISS1R system, instead of that in the neuron, may be involved in the process of ovulation and oocyte maturation. However, these findings cannot completely exclude the central role of the neural kisspeptin/KISS1R system in controlling the LH surge and the subsequent ovulation.
COX-2/prostaglandins have a crucial role in the ovulatory process (80–82). Gaytan et al. found that inhibition of COX-2 in cyclic female rats resulted in a dramatic drop of ovarian Kiss1 mRNA levels at the time of ovulation, which was fully rescued by the coadministration of prostaglandin E2 (41). In addition, injection of hCG increased the Kiss1 mRNA level in the ovary, which was completely reversed by the inhibition of COX-2 (41). These results indicate that kisspeptin is one of the downstream targets of COX-2/prostaglandins, and that kisspeptin may participate in the process of ovulation. In rat ovaries, the Kiss1 mRNA levels fluctuated in a cyclic-dependent manner, with a robust increase shortly before ovulation, suggesting a functional role of kisspeptin at the time of ovulation (17). In line with this result, pregnant mare serum gonadotropin evoked a significant increase in ovarian Kiss1 mRNA level that was further enhanced by the injection of an ovulatory dose of hCG. Furthermore, the rise of the ovarian Kiss1 mRNA level was prevented by the blockade of LH surge using an antagonist of GnRH (17). In both 6- and 10-month-old rats, ovarian infusion with kisspeptin increased the number of corpora lutea, while infusion with P234 decreased the number of corpora lutea (39), suggesting that locally increased kisspeptin may promote the process of ovulation. Interestingly, an exposure of female rats to the high-fat diets resulted in a downregulation of ovarian Kiss1 mRNA and kisspeptins, which is likely associated with the obesity-related ovulatory dysfunction (30).
Steroidogenesis
The role of kisspeptins in the regulation of endocrine system was first identified in 2001 showing that kisspeptin was highly expressed in human placenta, pituitary gland, pancreas, and spinal cord and was an endogenous stimulator of oxytocin (23). In rat ovaries, kisspeptin was intensively expressed in morphologically discernible steroidogenic luteal cells of newly formed copora lutea (17). Two reports documented the direct stimulatory effects of kisspeptin on the secretion of progesterone in chicken GCs (43) and rat luteal cells (37), respectively. The synthesis of progesterone is controlled by a series of processing enzymes, including steroidogenic acute regulatory protein (StAR), cytochrome P450 side-chain cleavage (P450scc) enzyme, and 3β-hydroxysteroid dehydrogenase (3β-HSD) enzyme (83). The mRNA levels of all these progesterone-producing enzymes in chicken GCs were significantly increased when treated with kisspeptin-10 (43). In rat luteal cells, treatment with kisspeptin alone had no significant effect on 3β-HSD mRNA level, while cotreatment with kisspeptin and hCG significantly increased the transcript level of 3β-HSD (37). In addition, treatment with kisspeptin alone increased the mRNA levels of StAR and CYP11A, and these stimulatory effects were enhanced when cotreated with hCG (37). In line with these results, hCG stimulated the expression of kisspeptin in rat GCs, and the hCG-induced increase in progesterone production was suppressed by a kisspeptin antagonist P234 (14), suggesting an indispensable role of ovarian kisspeptin in the regulation of progesterone production.
Unlike the stimulatory effect on progesterone production, kisspeptin has no effect on estrogen production in rat luteal cells (37). However, whether kisspeptin can promote the estrogen synthesis in GCs of the growing follicle during the mid- and late-proliferative phase, when the expression of kisspeptin reaches peak levels, has not been investigated. Neurokinin B (NKB) stimulate kisspeptin secretion in an autocrine and/or paracrine manner in neurons [reviewed in Skorupskaite et al. (84)]. Interestingly, NKB and its receptor were coexpressed with kisspeptin and KISS1R in human GCs and CCs (14, 29, 31). In addition, a recent study showed that NKB exerted a direct effect on stimulating estradiol production in zebrafish follicular cells and human GCs via the activation of ERK signaling (85). It is likely that kisspeptin/KISS1R system is involved in NKB-induced estrogen production, as kisspeptin acts as a downstream mediator of NKB in the hypothalamus and that kisspeptin can stimulate the activation of ERK signaling (discussed later). Further studies are required to confirm the functional role of intraovarian kisspeptin in regulating steroidogenesis in human GCs of growing follicles.
The Kisspeptin Signaling Pathway
Convincing evidence indicates that kisspeptin can activate a wide variety of signals via KISS1R. Being a G-protein-coupled receptor, KISS1R belongs to the subgroup of typical Gq/11 protein-associated receptors. After kisspeptin binds to KISS1R, the phosphorylated Gq/11 protein activates phospholipase C (PLC)-β, which leads to the activation of various second messengers, including phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis, accumulation of inositol-(1,4,5)-triphosphate (IP3), and diacylglycerol, protein kinase C (PKC) activation, and intracellular Ca2+ mobilization and release (Figure 2) (19, 23, 25, 86, 87). It has been reported that activation of PKC is required for LH-induced progesterone synthesis the preovulatory follicles of rats, hen, pigs, and quails (88–92). In addition, activation of PKC activity is required to promote the ERK signaling cascade that ultimately facilitates LH-induced progesterone production (93). Consistent with this result, Peng et al. showed that kisspeptin promoted progesterone synthesis by phosphorylating ERK1/2 (37). Therefore, the PLC–PKC–ERK signaling pathway most likely mediates progesterone synthesis in granulosa-luteal cells.
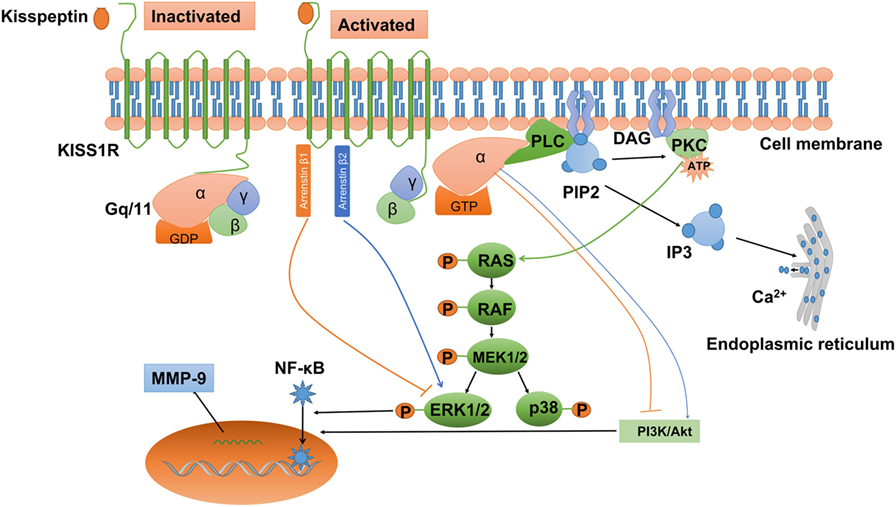
Figure 2. Kisspeptin/kisspeptin receptor (KISS1R) signaling at a glance. KISS1R is a seven-transmembrane domain, Gq/11-coupled receptor. Upon binding of kisspeptin, the intracellular portion of KISS1R phosphorylates Gq/11. The α-subunit of Gq/11 activates PLC, which cleaves PIP2 into IP3 and DAG. IP3 promotes intracellular Ca2+ release from the endoplasmic reticulum, while DAG activates a signaling cascade by phosphorylating PKC. PKC activation induces the phosphorylation of MAP kinases, such as ERK1/2 and p38. In addition, activation of KISS1R recruits arrestin-1 and -2, which downregulated and upregulated phosphorylated ERK1/2 levels, respectively. The activation of KISS1R can stimulate or inhibit the phosphorylation of PI3K/Akt, depending on the cell types, but the intermediator is not investigated. Activated KISS1R also enhances the expression of MMP-9 via PI3K/Akt/NF-κB or ERK/NF-κB signaling. DAG, diacylglycerol; ERK1/2, extracellular signal-regulated kinase; IP3, inositol 1,4,5-triphosphate; PI3K, phosphatidylinositol-3-kinase; MMP-9, matrix metalloproteinase-9; NF-κB, nuclear factor κB; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PLC, phospholipase C.
Ca2+ is initially stored in the lumen of the endoplasmic reticulum. Following the signal from IP3, Ca2+ is released through specialized channels, and therefore the free Ca2+ concentration in the cytoplasm is elevated (94). LH induces a rapid rise of intracellular Ca2+ that is released from Ca2+ stores in the cumulus layers, and subsequently an increased Ca2+ efflux into the oocyte (95). The increase of Ca2+ in the oocyte is thought to play a role in controlling either spontaneous or gonadotropin-induced oocyte maturation, possibly by modulating the intracytoplasmic cAMP concentrations via a Ca2+-sensitive adenylate cyclase (96, 97).
In addition to the PLC–PKC–Ca2+ pathway, kisspeptin also induces other intracellular transduction pathways. The activation of ERK1/2 is thought to be the most conserved kinase signal among many cell types examined. However, not all cell types with the activation of ERK1/2 show stable p38 MAPK and PI3K/Akt activation when exposed to kisspeptin (98). In Chinese hamster ovary K1 cells, treatment with kisspeptin-10 induced a strong and sustained phosphorylation of ERK1/2, while a weak phosphorylation of p38 and no phosphorylation of stress-activated protein kinase/c-Jun NH2-terminal kinase (23). Similarly, kisspeptin can activate the ERK1/2 signaling without any effect on P38 signaling in rat luteal cells (37). Intriguingly, activated KISS1R can also recruit arrestin β-1 and β-2 to the plasma membrane, which further modulates the intracellular phosphorylated ERK1/2 levels in many mammalian cells (99–101). The attenuation of MAPK signaling pathway in GCs results in cell apoptosis and the subsequent follicle atresia (102). Interestingly, Kiss1 or Kiss1r mutant animals showed much more atretic follicles than the wild-type counterparts (54, 55), suggesting that kisspeptin/KISS1R system may prevent the apoptosis of GCs. However, kisspeptin also downregulates the expression of FSHR in rat follicles, which may increase follicle atresia by inhibiting the follicular growth and inducing apoptosis of GCs (103, 104). Therefore, we may speculate that the intraovarian kisspeptin/KISS1R system modulates GC proliferation and apoptosis, oocyte maturation, ovulation, and steroidogenesis by regulating the MAPK signaling pathway.
Apart from the MAPK pathway, PI3K/Akt pathway also functions in both GCs and oocytes (105). In mammals, the PI3K/AKT signaling pathway is required for primordial follicle survival and activation, determination of the primordial follicle pool, and transition of the primordial follicle to growing follicles (106). In addition, this intra-follicular signaling modulates GC apoptosis, oocyte meiosis resumption, polar body emission, and spindle organization (107, 108). The effect of kisspeptin on the activation of PI3K/Akt signaling pathway is cell type specific. In rat luteal and thyroid cancer cells, kisspeptin cannot stimulate the phosphorylation of PI3K/Akt (37, 109), whereas kisspeptin induced the phosphorylation of PI3K/Akt in stably KISS1R-overexpressed thyroid cancer cells (110). Similarly, kisspeptin-10 was reported to inhibit the phosphorylation of PI3K/Akt in tumor cells (111–113), while kisspeptin-10 promoted the activation of PI3K/Akt in preoptic neurons (114). Whether the intraovarian kisspeptin/KISS1R system can modulate PI3K/Akt signaling pathway and further regulate GC apoptosis and oocyte maturation remains to be elucidated.
In HT-1080 cell line, overexpression with KISS1 inhibited matrix metalloproteinase-9 (MMP-9) enzyme activity via blocking nuclear factor κB (NF-κB) nuclear translocation and subsequently reducing the capacity of NF-κB binding to the MMP-9 promoter (115). This kisspeptin-induced signaling pathway has been demonstrated in several cancers, including urothelial carcinoma (116), ovarian epithelial cancers (117), and breast cancers (118). ERK and PI3K/Akt have been shown to act the upstream of NF-κB and regulate the NF-κB DNA-binding activity in melanoma cells (119–121). Interestingly, the enhanced expression of MMP-9 via PI3K/Akt/NF-κB (122) or ERK/NF-κB signaling pathway (123, 124) has also been established in other cell types. Therefore, it is most likely that ERK or PI3K/Akt signaling is involved in kisspeptin-induced downregulation of NF-κB/MMP-9.
Matrix metalloproteinase-9 is a matrix metalloproteinase that plays a critical role in tissue remodeling and follicular rupture (125). In addition, MMP-9 is involved in the mechanism by which kisspeptin prevents the tumor metastasis (112, 115, 126). In rats, intraovarian bursa administration of kisspeptin antagonist p234 resulted in the distortion of corpus luteum (14), indicating that kisspeptin can inhibit the degradation of the extracellular matrix in the ovary.
Roles of the Kisspeptin/KISS1R System in Female Reproductive Pathology
Genetic analysis in humans gave the first evidence of the indispensable role of kisspeptin/KISS1R system in the control of reproduction. Using complementary genetic approaches, KISS1R has been identified as the causative gene responsible for the consanguineous families with idiopathic hypogonadotropic hypogonadism (26, 27). In addition, an inactivating mutation of KISS1 gene has been reported as causative for idiopathic hypogonadotropic hypogonadism (127). The subsequent animal studies also confirmed that target depletion of either Kiss1 or Kiss1r had similar phenotypes of the human condition (27, 54).
Premature Ovarian Failure
A series of animal studies indicate a direct role of kisspeptin signaling in the ovary, and the defect of kisspeptin/KISS1R system precipitates a state of POF (or primary ovarian insufficiency). The haploinsufficient Kiss1r mice displayed a premature decline in ovulatory rate, progressive loss of oocytes, and antral follicles, reduced numbers of preantral follicles, and reduced fertility (52). In addition, the ovarian tissues of these precocious ovarian aging mice showed atrophic appearance without growing follicles and corpora lutea during their 48 weeks of ages (52). Furthermore, the phenotype is associated with a decreased expression level of ovarian Kiss1r mRNA. Notably, the failure of follicular development and ovulation due to the absent function of Kiss1r cannot be rescued by the replacement with gonadotropins (52). In line with these results, the loss of NTRK2 and Kiss1r receptor-mediated signaling in mouse oocytes caused POF (45). Collectively, these findings suggest a direct role of kisspeptin/KISS1r system in the ovary. Data generated from animal studies may provide a potential contribution to the evaluation or screening of isolated heterozygous mutations of KISS1R to POF in humans.
Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is a heterogenous endocrine disorder that affecting reproductive-aged women. This highly prevalent disease is characterized by hyperandrogenism, ovulatory dysfunction, and metabolic dysregulation (128). In women with PCOS, the classic neuroendocrine dysfunction leading to the ovarian phenotype includes increased LH pulsatility, decreased FSH secretion, and perturbed LH–FSH ratios, which could be due to the disrupted GnRH secretion (128). Since kisspeptin/KISS1R system is the upstream central controller for inducing GnRH (and LH) secretion, we may speculate that kisspeptin levels will be higher in women with PCOS. Indeed, a recent study showed that serum kisspeptin levels were significantly higher in women with PCOS and that serum levels of kisspeptin were negatively correlated with those of FSH (129). In line with this result, other studies demonstrated that higher serum levels of kisspeptin in women with PCOS (130, 131).
During the menstrual cycle, the increased LH pulsatility in PCOS persisted throughout the luteal phase, which resulted in the persistent stimulation of androgen production by ovarian theca cells (132). In addition, women with PCOS displayed metabolic alterations, which manifest insulin resistance and hyperinsulinemia (133). Interestingly, serum levels of kisspeptin in women with PCOS were positively correlated with those of testosterone and DHEAS (129). Studies in mice showed that administration of kisspeptin significantly increased the serum levels of testosterone (134). The metabolic dysregulation has been demonstrated to exert a suppressive effect on different levels of the gonadotropin axis in patients with PCOS (135) and an inhibitory effect on Kiss1 mRNA expression in the hypothalamus of rats (136). Furthermore, ovary-derived kisspeptins have been shown to play a role in regulating the secretion of gonadotropins (137). All these findings provide the available, albeit indirect, evidence supporting a potential link between PCOS and the kisspeptin/KISS1R system.
Endometriosis
Endometriosis is a common benign gynecologic disease defined as the ectopic presence of endometrial glandular epithelium and stroma outside the uterus (138). At present, the detailed pathogenesis of this disease remains unclear despite extensive research. Although a benign lesion, endometriosis shares several characteristics of malignancy, such as cell invasion, motility, and adhesion, which is a unique paradigm of benign metastasis (139). Several metastasis suppressor genes have been identified to suppress the metastasis at different steps of the metastatic cascade (140). KISS1 was originally identified as a human metastasis suppressor gene that is able to suppress the metastasis of melanoma and breast cancer (22). A recent study showed that the expression of kisspeptin (also known as metastin) is significantly higher in the glandular endometrium of endometriosis lesions compared with the eutopic glandular endometrium, indicating that kisspeptin is potentially implicated in the pathogenesis and maintenance of endometriosis (141). In contrast, other study did not detect the expression of kisspeptin in any endometrial tissue obtained from women with endometriosis (142). The discrepancy could be attributed to differences in study design and experimental methods. Future studies will focus on investigating the relationship between kisspeptin and endometriosis and evaluating the potential clinical application of kisspeptin as a marker for early and minimally invasive detection of endometriosis.
Future Directions and Clinical Applications
Although most research mainly focuses on the functional roles of the kisspeptin/KISS1R system in the central modulation of the H–P–G axis, the growing, albeit as yet limited, experimental data gathered recently suggest a putative role of kisspeptin signaling in the direct control of ovarian function (Figure 3). It must be stressed that our understanding of the physiological relevance, putative molecular mechanisms, and subsequent pathophysiological implications of such direct actions is still limited. However, such fragmentary evidence has supported the existence of a local kisspeptin/KISS1R system and the dysregulation of this system might contribute to several ovarian pathologies. Future study aimed at addressing the local role of kisspeptin in regulating ovarian function using a specific Kiss1 or kiss1r knockout model in the ovary will be of great interest. Functional studies using the kisspeptin antagonist P234 or other inhibition approaches will help investigate the physiological and pathophysiological roles of kisspeptin in the ovarian biology (143, 144).
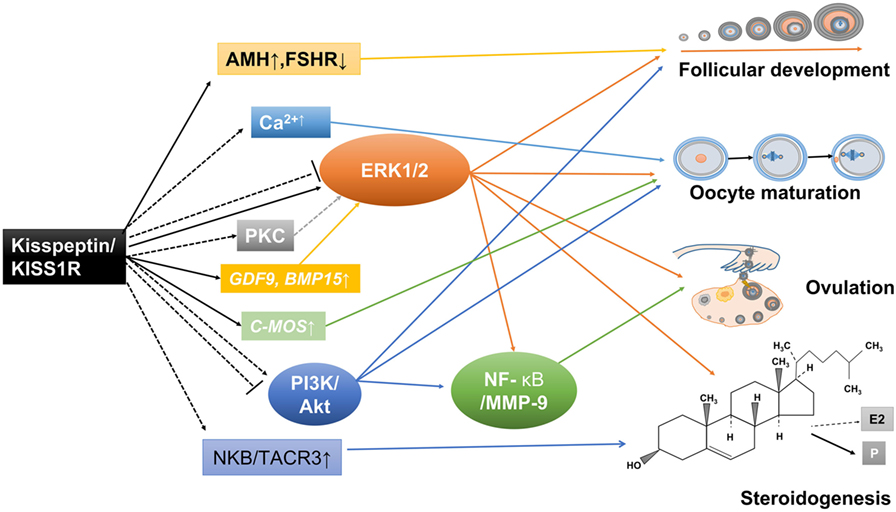
Figure 3. Potential mechanisms involved in the direct ovarian effects of the kisspeptin/kisspeptin receptor (KISS1R) system. Kisspeptin and KISS1R are expressed in ovarian cells. This locally produced kisspeptin might regulate follicular development, oocyte maturation, ovulation, and steroidogenesis in a paracrine or autocrine manner. Solid arrows stand for actions that have been clearly demonstrated in ovarian cells. Dotted arrows reflect potential pathways that could be involved in mediating the intraovarian kisspeptin/KISS1R effects, which have been proposed.
The involvement of kisspeptin/KISS1R system and its downstream signaling pathways in a range of ovarian function has led researchers to develop potential therapeutic approaches to overcome ovarian pathology and infertility. Data obtained from animal and human have indicated that the peripheral administration of kisspeptin-10 and kisspeptin-54 can initiate the LH surge (145, 146). At present, kisspeptin-54 has been used to trigger oocyte maturation effectively in women undergoing in vitro fertilization (IVF) (66). Moreover, kisspeptin-54 may be a substitute medication to trigger oocyte maturation during IVF treatment for women at high risk of developing ovarian hyperstimulation syndrome (67, 147, 148). However, safety concern should be seriously taken into consideration before its application, as kisspeptin supplementation was reported to have a harmful impact on the cultured hatched blastocysts in pig (44). Nevertheless, data from several clinical studies have shown that administration of kisspeptin in humans is safe without any observed adverse effects (149–151). More extensive clinical trials are required to investigate the safety, efficacy, and administration routes of the pharmaceutical applications of kisspeptins in humans. Animal studies have demonstrated that administration of pharmaceutical KISS1R antagonist suppresses the secretion of reproductive hormones (144), indicating a potential development of therapeutic targets for precocious puberty, endometriosis, some hormone-dependent cancers, and an alternative form of contraception.
During follicular development, kisspeptin suppressed the initial follicle recruitment through the upregulation of circulating AMH, which is able to inhibit the activation of primordial follicles (58). We may expect the clinical application of kisspeptin as a potential biomarker for ovarian reserve and an indicator for ovulation induction during IVF treatment. Studies in mice demonstrated that the haploinsufficient Kiss1r mice displayed a phenotype of POF (52). Such data will remind us a more careful evaluation of the possible attribution of certain heterozygous gene mutations in KISS1R to POF in humans and provide a useful screening method for these genetic variants.
Conclusion
In the past decade, research regarding locally produced kisspeptin in the ovary has been of considerable interest. Emerging evidence indicates that the intraovarian kisspeptin/KISS1R system is of great importance in controlling female reproduction, including follicular development, oocyte maturation, steroidogenesis, and ovulation. Any abnormality or dysregulation of kisspeptin signaling may negatively affect the ovarian function, leading to reproductive pathology or female infertility. In this review, we provided a concise overview of the available, mainly indirect, evidence suggesting the local effects of the kisspeptin/KISS1R system in regulating ovarian function and the potential underlying molecular mechanisms. The conclusive demonstration of the physiological and pathophysiological roles of kisspeptin signaling in the ovary is still pending, and additional studies are required to better characterize the kisspeptin/KISS1R system in reproductive biology and pathology. Expanding our understanding of the expression, actions, and molecular mechanisms of this system in the human ovary is essential for determining whether therapeutic interventions targeting kisspeptin signaling can ameliorate several reproductive pathology and infertility.
Author Contributions
K-LH collected the information, designed the pictures, wrote the manuscript, and manuscript submission. HZ collected the information and joined critical discussion. H-MC critically revised the manuscript and contributed to the conception of design. YY collected the information, designed the pictures, critically revised the manuscript, and contributed to the conception of design. JQ contributed to the conception of design and critical discussion.
Conflict of Interest Statement
We declare that all authors have no conflict of interest with the contents of this manuscript.
Funding
This work was supported in part by “Reproductive health and major birth defects prevention and control research” Key Special Fund (2016YFC1000601), the National Natural Science Funds for general program (31371521, 81571400, 31501201), and Beijing Nova Program (xxjh2015011).
References
1. Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science (1980) 207:1371–3. doi:10.1126/science.6766566
2. Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev (1998) 19:302–30. doi:10.1210/edrv.19.3.0332
3. Moenter SM, Brand RC, Karsch FJ. Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology (1992) 130:2978–84. doi:10.1210/endo.130.5.1572305
4. Goodman RL. The site of the positive feedback action of estradiol in the rat. Endocrinology (1978) 102:151–9. doi:10.1210/endo-102-1-151
5. Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol (2001) 22:292–308. doi:10.1006/frne.2001.0219
6. Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev (2012) 92:1235–316. doi:10.1152/physrev.00037.2010
7. Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol (2008) 29:48–69. doi:10.1016/j.yfrne.2007.07.002
8. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology (2005) 146:3686–92. doi:10.1210/en.2005-0488
9. Tomikawa J, Uenoyama Y, Ozawa M, Fukanuma T, Takase K, Goto T, et al. Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proc Natl Acad Sci U S A (2012) 109:E1294–301. doi:10.1073/pnas.1114245109
10. Uenoyama Y, Pheng V, Tsukamura H, Maeda KI. The roles of kisspeptin revisited: inside and outside the hypothalamus. J Reprod Dev (2016) 62:537–45. doi:10.1262/jrd.2016-083
11. Comninos AN, Anastasovska J, Sahuri-Arisoylu M, Li X, Li S, Hu M, et al. Kisspeptin signaling in the amygdala modulates reproductive hormone secretion. Brain Struct Funct (2016) 221:2035–47. doi:10.1007/s00429-015-1024-9
12. Terao Y, Kumano S, Takatsu Y, Hattori M, Nishimura A, Ohtaki T, et al. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta (2004) 1678:102–10. doi:10.1016/j.bbaexp.2004.02.005
13. Pinto FM, Cejudo-Roman A, Ravina CG, Fernandez-Sanchez M, Martin-Lozano D, Illanes M, et al. Characterization of the kisspeptin system in human spermatozoa. Int J Androl (2012) 35:63–73. doi:10.1111/j.1365-2605.2011.01177.x
14. Laoharatchatathanin T, Terashima R, Yonezawa T, Kurusu S, Kawaminami M. Augmentation of metastin/kisspeptin mRNA expression by the proestrous luteinizing hormone surge in granulosa cells of rats: implications for luteinization. Biol Reprod (2015) 93:15. doi:10.1095/biolreprod.115.127902
15. Xu Z, Kaga S, Mochiduki A, Tsubomizu J, Adachi S, Sakai T, et al. Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocr J (2012) 59:161–71. doi:10.1507/endocrj.EJ11-0193
16. Arai AC, Xia YF, Suzuki E, Kessler M, Civelli O, Nothacker HP. Cancer metastasis-suppressing peptide metastin upregulates excitatory synaptic transmission in hippocampal dentate granule cells. J Neurophysiol (2005) 94:3648–52. doi:10.1152/jn.00590.2005
17. Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, et al. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology (2006) 147:4852–62. doi:10.1210/en.2006-0117
18. Zhang P, Tang M, Zhong T, Lin Y, Zong T, Zhong C, et al. Expression and function of kisspeptin during mouse decidualization. PLoS One (2014) 9:e97647. doi:10.1371/journal.pone.0097647
19. Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature (2001) 411:613–7. doi:10.1038/35079135
20. Shahed A, Young KA. Differential ovarian expression of KiSS-1 and GPR-54 during the estrous cycle and photoperiod induced recrudescence in Siberian hamsters (Phodopus sungorus). Mol Reprod Dev (2009) 76:444–52. doi:10.1002/mrd.20972
21. Cejudo Roman A, Pinto FM, Dorta I, Almeida TA, Hernandez M, Illanes M, et al. Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract. Fertil Steril (2012) 97:1213–9. doi:10.1016/j.fertnstert.2012.02.021
22. Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst (1996) 88:1731–7. doi:10.1093/jnci/88.23.1731
23. Kotani M, Detheux M, Vandenbbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem (2001) 276:34631–6. doi:10.1074/jbc.M104847200
24. Lee DK, Nguyen T, O’Neill GP, Cheng R, Liu Y, Howard AD, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett (1999) 446:103–7. doi:10.1016/S0014-5793(99)00009-5
25. Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem (2001) 276:28969–75. doi:10.1074/jbc.M102743200
26. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A (2003) 100:10972–6. doi:10.1073/pnas.1834399100
27. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med (2003) 349:1614–27. doi:10.1056/NEJMoa035322
28. Hussain MA, Song WJ, Wolfe A. There is kisspeptin – and then there is kisspeptin. Trends Endocrinol Metab (2015) 26:564–72. doi:10.1016/j.tem.2015.07.008
29. Garcia-Ortega J, Pinto FM, Prados N, Bello AR, Almeida TA, Fernandez-Sanchez M, et al. Expression of tachykinins and tachykinin receptors and interaction with kisspeptin in human granulosa and cumulus cells. Biol Reprod (2016) 94:124. doi:10.1095/biolreprod.116.139881
30. Zhou Q, Chen H, Yang S, Li Y, Wang B, Chen Y, et al. High-fat diet decreases the expression of Kiss1 mRNA and kisspeptin in the ovary, and increases ovulatory dysfunction in postpubertal female rats. Reprod Biol Endocrinol (2014) 12:127. doi:10.1186/1477-7827-12-127
31. Garcia-Ortega J, Pinto FM, Fernandez-Sanchez M, Prados N, Cejudo-Roman A, Almeida TA, et al. Expression of neurokinin B/NK3 receptor and kisspeptin/KISS1 receptor in human granulosa cells. Hum Reprod (2014) 29:2736–46. doi:10.1093/humrep/deu247
32. Merhi Z, Thornton K, Bonney E, Cipolla MJ, Charron MJ, Buyuk E. Ovarian kisspeptin expression is related to age and to monocyte chemoattractant protein-1. J Assist Reprod Genet (2016) 33:535–43. doi:10.1007/s10815-016-0672-x
33. Cielesh ME, McGrath BM, Scott CJ, Norman ST, Stephen CP. The localization of kisspeptin and kisspeptin receptor in the canine ovary during different stages of the reproductive cycle. Reprod Domest Anim (2017) 52(Suppl 2):24–8. doi:10.1111/rda.12841
34. Hsu MC, Wang JY, Lee YJ, Jong DS, Tsui KH, Chiu CH. Kisspeptin modulates fertilization capacity of mouse spermatozoa. Reproduction (2014) 147:835–45. doi:10.1530/REP-13-0368
35. Mondal M, Baruah KK, Prakash BS. Determination of plasma kisspeptin concentrations during reproductive cycle and different phases of pregnancy in crossbred cows using bovine specific enzyme immunoassay. Gen Comp Endocrinol (2015) 224:168–75. doi:10.1016/j.ygcen.2015.08.014
36. Mondal M, Karunakaran M, Baruah KK. Development and validation of a sensitive enzymeimmunoassay for determination of plasma metastin in mithun (Bos frontalis). J Immunoassay Immunochem (2016) 37:201–16. doi:10.1080/15321819.2015.1120745
37. Peng J, Tang M, Zhang BP, Zhang P, Zhong T, Zong T, et al. Kisspeptin stimulates progesterone secretion via the Erk1/2 mitogen-activated protein kinase signaling pathway in rat luteal cells. Fertil Steril (2013) 99:1436–43.e1. doi:10.1016/j.fertnstert.2012.12.008
38. Ricu MA, Ramirez VD, Paredes AH, Lara HE. Evidence for a celiac ganglion-ovarian kisspeptin neural network in the rat: intraovarian anti-kisspeptin delays vaginal opening and alters estrous cyclicity. Endocrinology (2012) 153:4966–77. doi:10.1210/en.2012-1279
39. Fernandois D, Na E, Cuevas F, Cruz G, Lara HE, Paredes AH. Kisspeptin is involved in ovarian follicular development during aging in rats. J Endocrinol (2016) 228:161–70. doi:10.1530/JOE-15-0429
40. Fernandois D, Cruz G, Na EK, Lara HE, Paredes AH. Kisspeptin level in the aging ovary is regulated by the sympathetic nervous system. J Endocrinol (2017) 232:97–105. doi:10.1530/JOE-16-0181
41. Gaytan F, Gaytan M, Castellano JM, Romero M, Roa J, Aparicio B, et al. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab (2009) 296:E520–31. doi:10.1152/ajpendo.90895.2008
42. Garcia-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology (2012) 153:316–28. doi:10.1210/en.2011-1260
43. Xiao Y, Ni Y, Huang Y, Wu J, Grossmann R, Zhao R. Effects of kisspeptin-10 on progesterone secretion in cultured chicken ovarian granulosa cells from preovulatory (F1-F3) follicles. Peptides (2011) 32:2091–7. doi:10.1016/j.peptides.2011.09.001
44. Saadeldin IM, Koo OJ, Kang JT, Kwon DK, Park SJ, Kim SJ, et al. Paradoxical effects of kisspeptin: it enhances oocyte in vitro maturation but has an adverse impact on hatched blastocysts during in vitro culture. Reprod Fertil Dev (2012) 24:656–68. doi:10.1071/RD11118
45. Dorfman MD, Garcia-Rudaz C, Alderman Z, Kerr B, Lomniczi A, Dissen GA, et al. Loss of Ntrk2/Kiss1r signaling in oocytes causes premature ovarian failure. Endocrinology (2014) 155:3098–111. doi:10.1210/en.2014-1111
46. Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun (2003) 312:1357–63. doi:10.1016/j.bbrc.2003.11.066
47. Colledge WH. Transgenic mouse models to study Gpr54/kisspeptin physiology. Peptides (2009) 30:34–41. doi:10.1016/j.peptides.2008.05.006
48. Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol (2009) 21:1015–23. doi:10.1111/j.1365-2826.2009.01926.x
49. Uenoyama Y, Nakamura S, Hayakawa Y, Ikegami K, Watanabe Y, Deura C, et al. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J Neuroendocrinol (2015) 27:187–97. doi:10.1111/jne.12257
50. Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, et al. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci (2007) 27:8826–35. doi:10.1523/JNEUROSCI.2099-07.2007
51. Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci (2011) 14:704–10. doi:10.1038/nn.2818
52. Gaytan F, Garcia-Galiano D, Dorfman MD, Manfredi-Lozano M, Castellano JM, Dissen GA, et al. Kisspeptin receptor haplo-insufficiency causes premature ovarian failure despite preserved gonadotropin secretion. Endocrinology (2014) 155:3088–97. doi:10.1210/en.2014-1110
53. Gaytan F, Morales C, Leon S, Garcia-Galiano D, Roa J, Tena-Sempere M. Crowding and follicular fate: spatial determinants of follicular reserve and activation of follicular growth in the mammalian ovary. PLoS One (2015) 10:e0144099. doi:10.1371/journal.pone.0144099
54. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, et al. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology (2007) 148:4927–36. doi:10.1210/en.2007-0078
55. d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A (2007) 104:10714–9. doi:10.1073/pnas.0704114104
56. Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, et al. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun (2013) 4:2492. doi:10.1038/ncomms3492
57. Peluso C, Fonseca FL, Rodart IF, Cavalcanti V, Gastaldo G, Christofolini DM, et al. AMH: an ovarian reserve biomarker in assisted reproduction. Clin Chim Acta (2014) 437:175–82. doi:10.1016/j.cca.2014.07.029
58. Yang MY, Cushman RA, Fortune JE. Anti-Mullerian hormone inhibits activation and growth of bovine ovarian follicles in vitro and is localized to growing follicles. Mol Hum Reprod (2017) 23:282–91. doi:10.1093/molehr/gax010
59. Filicori M, Cognigni GE, Samara A, Melappioni S, Perri T, Cantelli B, et al. The use of LH activity to drive folliculogenesis: exploring uncharted territories in ovulation induction. Hum Reprod Update (2002) 8:543–57. doi:10.1093/humupd/8.6.543
60. Filicori M, Cognigni GE, Taraborrelli S, Parmegiani L, Bernardi S, Ciampaglia W. Intracytoplasmic sperm injection pregnancy after low-dose human chorionic gonadotropin alone to support ovarian folliculogenesis. Fertil Steril (2002) 78:414–6. doi:10.1016/S0015-0282(02)03243-0
61. Loumaye E, Engrand P, Shoham Z, Hillier SG, Baird DT. Clinical evidence for an LH ‘ceiling’ effect induced by administration of recombinant human LH during the late follicular phase of stimulated cycles in World Health Organization type I and type II anovulation. Hum Reprod (2003) 18:314–22. doi:10.1093/humrep/deg066
62. Filicori M, Cognigni GE, Gamberini E, Parmegiani L, Troilo E, Roset B. Efficacy of low-dose human chorionic gonadotropin alone to complete controlled ovarian stimulation. Fertil Steril (2005) 84:394–401. doi:10.1016/j.fertnstert.2005.02.036
63. Filicori M, Cognigni GE, Tabarelli C, Pocognoli P, Taraborrelli S, Spettoli D, et al. Stimulation and growth of antral ovarian follicles by selective LH activity administration in women. J Clin Endocrinol Metab (2002) 87:1156–61. doi:10.1210/jcem.87.3.8322
64. Sullivan MW, Stewart-Akers A, Krasnow JS, Berga SL, Zeleznik AJ. Ovarian responses in women to recombinant follicle-stimulating hormone and luteinizing hormone (LH): a role for LH in the final stages of follicular maturation. J Clin Endocrinol Metab (1999) 84:228–32. doi:10.1210/jcem.84.1.5389
65. Dupont J, Scaramuzzi RJ. Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle. Biochem J (2016) 473:1483–501. doi:10.1042/BCJ20160124
66. Jayasena CN, Abbara A, Comninos AN, Nijher GMK, Christopoulos G, Narayanaswamy S, et al. Kisspeptin-54 triggers egg maturation in women undergoing in vitro fertilization. J Clin Invest (2014) 124:3667–77. doi:10.1172/JCI75730
67. Abbara A, Jayasena CN, Christopoulos G, Narayanaswamy S, Izzi-Engbeaya C, Nijher GMK, et al. Efficacy of kisspeptin-54 to trigger oocyte maturation in women at high risk of ovarian hyperstimulation syndrome (OHSS) during in vitro fertilization (IVF) therapy. J Clin Endocrinol Metab (2015) 100:3322–31. doi:10.1210/jc.2015-2332
68. Byri P, Gangineni A, Reddy KR, Raghavender KBP. Effect of kisspeptin on in vitro maturation of sheep oocytes. Vet World (2017) 10:276–80. doi:10.14202/vetworld.2017.276-280
69. Persani L, Rossetti R, Di Pasquale E, Cacciatore C, Fabre S. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum Reprod Update (2014) 20:869–83. doi:10.1093/humupd/dmu036
70. McNatty KP, Juengel JL, Reader KL, Lun S, Myllymaa S, Lawrence SB, et al. Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function. Reproduction (2005) 129:473–80. doi:10.1530/rep.1.0511
71. Chang HM, Qiao J, Leung PC. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update (2016) 23:1–18. doi:10.1093/humupd/dmw039
72. Reader KL, Heath DA, Lun S, McIntosh CJ, Western AH, Littlejohn RP, et al. Signalling pathways involved in the cooperative effects of ovine and murine GDF9+BMP15-stimulated thymidine uptake by rat granulosa cells. Reproduction (2011) 142:123–31. doi:10.1530/REP-10-0490
73. Mottershead DG, Ritter LJ, Gilchrist RB. Signalling pathways mediating specific synergistic interactions between GDF9 and BMP15. Mol Hum Reprod (2012) 18:121–8. doi:10.1093/molehr/gar056
74. Hashiba Y, Asada Y, Heikinheimo O, Lanzendorf SE, Mizutani S. Microinjection of antisense c-mos oligonucleotides prevents the progression of meiosis in human and hamster oocytes. Fertil Steril (2001) 76:143–7. doi:10.1016/S0015-0282(01)01821-0
75. Araki K, Naito K, Haraguchi S, Suzuki R, Yokoyama M, Inoue M, et al. Meiotic abnormalities of c-mos knockout mouse oocytes: activation after first meiosis or entrance into third meiotic metaphase. Biol Reprod (1996) 55:1315–24. doi:10.1095/biolreprod55.6.1315
76. Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, et al. The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci U S A (1996) 93:7032–5. doi:10.1073/pnas.93.14.7032
77. O’Keefe SJ, Wolfes H, Kiessling AA, Cooper GM. Microinjection of antisense c-mos oligonucleotides prevents meiosis II in the maturing mouse egg. Proc Natl Acad Sci U S A (1989) 86:7038–42. doi:10.1073/pnas.86.18.7038
78. Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun (2004) 320:383–8. doi:10.1016/j.bbrc.2004.05.185
79. Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, et al. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology (2007) 148:5258–67. doi:10.1210/en.2007-0554
80. Sirois J, Sayasith K, Brown KA, Stock AE, Bouchard N, Dore M. Cyclooxygenase-2 and its role in ovulation: a 2004 account. Hum Reprod Update (2004) 10:373–85. doi:10.1093/humupd/dmh032
81. Duffy DM, Stouffer RL. The ovulatory gonadotrophin surge stimulates cyclooxygenase expression and prostaglandin production by the monkey follicle. Mol Hum Reprod (2001) 7:731–9. doi:10.1093/molehr/7.8.731
82. Ben-Ami I, Freimann S, Armon L, Dantes A, Strassburger D, Friedler S, et al. PGE2 up-regulates EGF-like growth factor biosynthesis in human granulosa cells: new insights into the coordination between PGE2 and LH in ovulation. Mol Hum Reprod (2006) 12:593–9. doi:10.1093/molehr/gal068
83. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev (2011) 32:81–151. doi:10.1210/er.2010-0013
84. Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update (2014) 20:485–500. doi:10.1093/humupd/dmu009
85. Qi X, Salem M, Zhou W, Sato-Shimizu M, Ye G, Smitz J, et al. Neurokinin B exerts direct effects on the ovary to stimulate estradiol production. Endocrinology (2016) 157:3355–65. doi:10.1210/en.2016-1354
86. Jiang Y, Berk M, Singh LS, Tan H, Yin L, Powell CT, et al. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin Exp Metastasis (2005) 22:369–76. doi:10.1007/s10585-005-8186-4
87. Stafford LJ, Xia C, Ma W, Cai Y, Liu M. Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res (2002) 62:5399–404.
88. Yuan W, Connor ML. Protein kinase C activity and its effect on progesterone production by large and small porcine luteal cells. Proc Soc Exp Biol Med (1997) 216:86–92. doi:10.3181/00379727-216-44160
89. Ciereszko RE, Petroff BK, Ottobre AC, Guan Z, Stokes BT, Ottobre JS. Assessment of the mechanism by which prolactin stimulates progesterone production by early corpora lutea of pigs. J Endocrinol (1998) 159:201–9. doi:10.1677/joe.0.1590201
90. Jamaluddin M, Molnar M, Marrone BL, Hertelendy F. Signal transduction in avian granulosa cells: effects of protein kinase C inhibitors. Gen Comp Endocrinol (1994) 93:471–9. doi:10.1006/gcen.1994.1051
91. Morris JK, Richards JS. Luteinizing hormone induces prostaglandin endoperoxide synthase-2 and luteinization in vitro by A-kinase and C-kinase pathways. Endocrinology (1995) 136:1549–58. doi:10.1210/endo.136.4.7895665
92. Sasanami T, Mori M. Effects of oestradiol-17beta and testosterone on progesterone production in the cultured granulosa cells of Japanese quail. Br Poult Sci (1999) 40:536–40. doi:10.1080/00071669987322
93. Woods DC, Johnson AL. Protein kinase C activity mediates LH-induced ErbB/Erk signaling in differentiated hen granulosa cells. Reproduction (2007) 133:733–41. doi:10.1530/REP-06-0261
94. Wang CM, Machaty Z. Calcium influx in mammalian eggs. Reproduction (2013) 145:R97–105. doi:10.1530/REP-12-0496
95. Mattioli M, Gioia L, Barboni B. Calcium elevation in sheep cumulus-oocyte complexes after luteinising hormone stimulation. Mol Reprod Dev (1998) 50:361–9. doi:10.1002/(SICI)1098-2795(199807)50:3<361::AID-MRD13>3.0.CO;2-7
96. Deguchi R, Takeda N, Stricker SA. Calcium signals and oocyte maturation in marine invertebrates. Int J Dev Biol (2015) 59:271–80. doi:10.1387/ijdb.150239ss
97. Silvestre F, Boni R, Fissore RA, Tosti E. Ca2+ signaling during maturation of cumulus-oocyte complex in mammals. Mol Reprod Dev (2011) 78:744–56. doi:10.1002/mrd.21332
98. Castano JP, Martinez-Fuentes AJ, Gutierrez-Pascual E, Vaudry H, Tena-Sempere M, Malagon MM. Intracellular signaling pathways activated by kisspeptins through GPR54: do multiple signals underlie function diversity? Peptides (2009) 30:10–5. doi:10.1016/j.peptides.2008.07.025
99. Goertzen CG, Dragan M, Turley E, Babwah AV, Bhattacharya M. KISS1R signaling promotes invadopodia formation in human breast cancer cell via beta-arrestin2/ERK. Cell Signal (2016) 28:165–76. doi:10.1016/j.cellsig.2015.12.010
100. Pampillo M, Camuso N, Taylor JE, Szereszewski JM, Ahow MR, Zajac M, et al. Regulation of GPR54 signaling by GRK2 and {beta}-arrestin. Mol Endocrinol (2009) 23:2060–74. doi:10.1210/me.2009-0013
101. Szereszewski JM, Pampillo M, Ahow MR, Offermanns S, Bhattacharya M, Babwah AV. GPR54 regulates ERK1/2 activity and hypothalamic gene expression in a Galpha(q/11) and beta-arrestin-dependent manner. PLoS One (2010) 5:e12964. doi:10.1371/journal.pone.0012964
102. Peter AT, Dhanasekaran N. Apoptosis of granulosa cells: a review on the role of MAPK-signalling modules. Reprod Domest Anim (2003) 38:209–13. doi:10.1046/j.1439-0531.2003.00438.x
103. Meduri G, Bachelot A, Cocca MP, Vasseur C, Rodien P, Kuttenn F, et al. Molecular pathology of the FSH receptor: new insights into FSH physiology. Mol Cell Endocrinol (2008) 282:130–42. doi:10.1016/j.mce.2007.11.027
104. Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJW. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology (1996) 137:1447–56. doi:10.1210/endo.137.4.8625923
105. Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update (2015) 21:779–86. doi:10.1093/humupd/dmv037
106. Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science (2008) 319:611–3. doi:10.1126/science.1152257
107. Zheng W, Nagaraju G, Liu Z, Liu K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol (2012) 356:24–30. doi:10.1016/j.mce.2011.05.027
108. Cecconi S, Mauro A, Cellini V, Patacchiola F. The role of Akt signalling in the mammalian ovary. Int J Dev Biol (2012) 56:809–17. doi:10.1387/ijdb.120146sc
109. Ringel MD, Hardy E, Bernet VJ, Burch HB, Schuppert F, Burman KD, et al. Metastin receptor is overexpressed in papillary thyroid cancer and activates MAP kinase in thyroid cancer cells. J Clin Endocrinol Metab (2002) 87:2399. doi:10.1210/jcem.87.5.8626
110. Stathatos N, Bourdeau I, Espinosa AV, Saji M, Vasko VV, Burman KD, et al. KiSS-1/G protein-coupled receptor 54 metastasis suppressor pathway increases myocyte-enriched calcineurin interacting protein 1 expression and chronically inhibits calcineurin activity. J Clin Endocrinol Metab (2005) 90:5432–40. doi:10.1210/jc.2005-0963
111. Navenot JM, Wang Z, Chopin M, Fujii N, Peiper SC. Kisspeptin-10-induced signaling of GPR54 negatively regulates chemotactic responses mediated by CXCR4: a potential mechanism for the metastasis suppressor activity of kisspeptins. Cancer Res (2005) 65:10450–6. doi:10.1158/0008-5472.CAN-05-1757
112. Chen S, Chen W, Zhang X, Lin S, Chen Z. Overexpression of KiSS-1 reduces colorectal cancer cell invasion by downregulating MMP-9 via blocking PI3K/Akt/NF-kappaB signal pathway. Int J Oncol (2016) 48:1391–8. doi:10.3892/ijo.2016.3368
113. Navenot JM, Fujii N, Peiper SC. KiSS1 metastasis suppressor gene product induces suppression of tyrosine kinase receptor signaling to Akt, tumor necrosis factor family ligand expression, and apoptosis. Mol Pharmacol (2009) 75:1074–83. doi:10.1124/mol.108.054270
114. Hanchate NK, Parkash J, Bellefontaine N, Mazur D, Colledge WH, d’Anglemont de Tassigny X, et al. Kisspeptin-GPR54 signaling in mouse NO-synthesizing neurons participates in the hypothalamic control of ovulation. J Neurosci (2012) 32:932–45. doi:10.1523/JNEUROSCI.4765-11.2012
115. Yan C, Wang H, Boyd DD. KiSS-1 represses 92-kDa type IV collagenase expression by down-regulating NF-kappa B binding to the promoter as a consequence of Ikappa Balpha-induced block of p65/p50 nuclear translocation. J Biol Chem (2001) 276:1164–72. doi:10.1074/jbc.M008681200
116. Takeda T, Kikuchi E, Mikami S, Suzuki E, Matsumoto K, Miyajima A, et al. Prognostic role of KiSS-1 and possibility of therapeutic modality of metastin, the final peptide of the KiSS-1 gene, in urothelial carcinoma. Mol Cancer Ther (2012) 11:853–63. doi:10.1158/1535-7163.MCT-11-0521
117. Gao GL, Liu LD, Zou XS, Chen WX. [Expression of KiSS-1, matrix metalloproteinase-9, nuclear factor-kappaBp65 in ovarian tumour]. Zhonghua Fu Chan Ke Za Zhi (2007) 42:34–8. doi:10.3760/j.issn:0529-567x.2007.01.011
118. Cho SG, Li D, Stafford LJ, Luo J, Rodriguez-Villanueva M, Wang Y, et al. KiSS1 suppresses TNFalpha-induced breast cancer cell invasion via an inhibition of RhoA-mediated NF-kappaB activation. J Cell Biochem (2009) 107:1139–49. doi:10.1002/jcb.22216
119. Bai D, Ueno L, Vogt PK. Akt-mediated regulation of NF kappa B and the essentialness of NF kappa B for the oncogenicity of PI3K and Akt. Int J Cancer (2009) 125:2863–70. doi:10.1002/ijc.24748
120. Dhawan P, Richmond A. A novel NF-kappa B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B activity in melanoma cells. J Biol Chem (2002) 277:7920–8. doi:10.1074/jbc.M112210200
121. Lee KB, Byun HJ, Park SH, Park CY, Lee SH, Rho SB. CYR61 controls p53 and NF-kappa B expression through PI3K/Akt/mTOR pathways in carboplatin-induced ovarian cancer cells. Cancer Lett (2012) 315:86–95. doi:10.1016/j.canlet.2011.10.016
122. Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene (2006) 25:7009–18. doi:10.1038/sj.onc.1209706
123. Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL, Kim JS, et al. Metastatic function of BMP-2 in gastric cancer cells: the role of PI3K/AKT, MAPK, the NF-kappaB pathway, and MMP-9 expression. Exp Cell Res (2011) 317:1746–62. doi:10.1016/j.yexcr.2011.04.006
124. Birkenkamp KU, Tuyt LM, Lummen C, Wierenga AT, Kruijer W, Vellenga E. The p38 MAP kinase inhibitor SB203580 enhances nuclear factor-kappa B transcriptional activity by a non-specific effect upon the ERK pathway. Br J Pharmacol (2000) 131:99–107. doi:10.1038/sj.bjp.0703534
125. Curry TE Jr, Song L, Wheeler SE. Cellular localization of gelatinases and tissue inhibitors of metalloproteinases during follicular growth, ovulation, and early luteal formation in the rat. Biol Reprod (2001) 65:855–65. doi:10.1095/biolreprod65.3.855
126. Lee KH, Kim JR. Kiss-1 suppresses MMP-9 expression by activating p38 MAP kinase in human stomach cancer. Oncol Res (2009) 18:107–16. doi:10.3727/096504009789954591
127. Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med (2012) 366:629–35. doi:10.1056/NEJMoa1111184
128. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet (2007) 370:685–97. doi:10.1016/S0140-6736(07)61345-2
129. Gorkem U, Togrul C, Arslan E, Sargin Oruc A, Buyukkayaci Duman N. Is there a role for kisspeptin in pathogenesis of polycystic ovary syndrome? Gynecol Endocrinol (2017):1–4. doi:10.1080/09513590.2017.1379499
130. Chen X, Mo Y, Li L, Chen Y, Li Y, Yang D. Increased plasma metastin levels in adolescent women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol (2010) 149:72–6. doi:10.1016/j.ejogrb.2009.11.018
131. Jeon YE, Lee KE, Jung JA, Yim SY, Kim H, Seo SK, et al. Kisspeptin, leptin, and retinol-binding protein 4 in women with polycystic ovary syndrome. Gynecol Obstet Invest (2013) 75:268–74. doi:10.1159/000350217
132. Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF Jr. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab (1988) 66:165–72. doi:10.1210/jcem-66-1-165
133. Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod (2013) 28:777–84. doi:10.1093/humrep/des463
134. Mikkelsen JD, Bentsen AH, Ansel L, Simonneaux V, Juul A. Comparison of the effects of peripherally administered kisspeptins. Regul Pept (2009) 152:95–100. doi:10.1016/j.regpep.2008.10.001
135. Tena-Sempere M. Ghrelin as a pleotrophic modulator of gonadal function and reproduction. Nat Clin Pract Endocrinol Metab (2008) 4:666–74. doi:10.1038/ncpendmet1003
136. Forbes S, Li XF, Kinsey-Jones J, O’Byrne K. Effects of ghrelin on kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett (2009) 460:143–7. doi:10.1016/j.neulet.2009.05.060
137. Balasch J, Fabregues F, Carmona F, Casamitjana R, Tena-Sempere M. Ovarian luteinizing hormone priming preceding follicle-stimulating hormone stimulation: clinical and endocrine effects in women with long-term hypogonadotropic hypogonadism. J Clin Endocrinol Metab (2009) 94:2367–73. doi:10.1210/jc.2009-0262
138. Brosens IA, Brosens JJ. Redefining endometriosis – is deep endometriosis a progressive disease? Hum Reprod (2000) 15:1–3. doi:10.1093/humrep/15.1.1
139. Starzinski-Powitz A, Handrow-Metzmacher H, Kotzian S. The putative role of cell adhesion molecules in endometriosis: can we learn from tumour metastasis? Mol Med Today (1999) 5:304–9. doi:10.1016/S1357-4310(99)01497-5
140. Horak CE, Lee JH, Marshall JC, Shreeve SM, Steeg PS. The role of metastasis suppressor genes in metastatic dormancy. APMIS (2008) 116:586–601. doi:10.1111/j.1600-0463.2008.01213.x
141. Timologou A, Zafrakas M, Grimbizis G, Miliaras D, Kotronis K, Stamatopoulos P, et al. Immunohistochemical expression pattern of metastasis suppressors KAI1 and KISS1 in endometriosis and normal endometrium. Eur J Obstet Gynecol Reprod Biol (2016) 199:110–5. doi:10.1016/j.ejogrb.2016.02.004
142. Makri A, Msaouel P, Petraki C, Milingos D, Protopapas A, Liapi A, et al. KISS1/KISS1R expression in eutopic and ectopic endometrium of women suffering from endometriosis. In Vivo (2012) 26:119–27.
143. Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, et al. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology (2010) 151:722–30. doi:10.1210/en.2009-0803
144. Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci (2009) 29:3920–9. doi:10.1523/JNEUROSCI.5740-08.2009
145. Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol (2004) 16:850–8. doi:10.1111/j.1365-2826.2004.01240.x
146. Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab (2007) 92:3958–66. doi:10.1210/jc.2007-1116
147. Abbara A, Clarke S, Islam R, Prague JK, Comninos AN, Narayanaswamy S, et al. A second dose of kisspeptin-54 improves oocyte maturation in women at high risk of ovarian hyperstimulation syndrome: a phase 2 randomized controlled trial. Hum Reprod (2017) 32:1915–24. doi:10.1093/humrep/dex253
148. Zhai J, Liu J, Zhao S, Zhao H, Chen Z, Du Y, et al. Kisspeptin-10 inhibits OHSS by suppressing VEGF secretion. Reproduction (2017) 154:355–62. doi:10.1530/rep-17-0268
149. Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab (2005) 90:6609–15. doi:10.1210/jc.2005-1468
150. Chan YM, Broder-Fingert S, Paraschos S, Lapatto R, Au M, Hughes V, et al. GnRH-deficient phenotypes in humans and mice with heterozygous variants in KISS1/Kiss1. J Clin Endocrinol Metab (2011) 96:E1771–81. doi:10.1210/jc.2011-0518
Keywords: kisspeptin, kisspeptin receptor, ovarian function, follicular development, oocyte maturation, ovulation, steroidogenesis, kisspeptin signaling
Citation: Hu K-L, Zhao H, Chang H-M, Yu Y and Qiao J (2018) Kisspeptin/Kisspeptin Receptor System in the Ovary. Front. Endocrinol. 8:365. doi: 10.3389/fendo.2017.00365
Received: 16 November 2017; Accepted: 13 December 2017;
Published: 04 January 2018
Edited by:
Marc Yeste, University of Girona, SpainReviewed by:
Alejandro S. Mechaly, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaIlpo Huhtaniemi, Imperial College London, United Kingdom
Copyright: © 2018 Hu, Zhao, Chang, Yu and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongcui Zhao, rainbowandsun@163.com;
Yang Yu, yuyang5012@hotmail.com
 Kai-Lun Hu
Kai-Lun Hu Hongcui Zhao*
Hongcui Zhao*