- Division of Endocrinology, Diabetes and Metabolism, Icahn School of Medicine at Mount Sinai, Mount Sinai St. Luke’s and West Hospital Center, New York, NY, United States
Objective: Extrapulmonary small cell carcinoma (EPSCC) is rare and frequent metastases at presentation can complicate efforts to identify a site of origin. In particular, SCC comprises <1% of prostate cancers and has been implicated in castration resistance.
Methods: Clinical, laboratory, imaging, and pathology data are presented.
Results: A 56-year-old man with locally advanced prostate adenocarcinoma on androgen deprivation therapy presented with a clogged nephrostomy tube. Laboratory results included calcium 13.8 mg/dL (8.5–10.5 mg/dL), albumin 3.6 g/dL (3.5–5 mg/dL), and potassium 2.8 mmol/L (3.5–5.2 mmol/L). Hypercalcemia investigation revealed intact PTH 19 pg/mL (16–87 pg/mL), 25-OH vitamin D 15.7 ng/mL (>30 ng/mL), and PTH-related peptide (PTHrP) 63.4 pmol/L (<2.3 pmol/L). Workup for hypokalemia yielded aldosterone 5.3 ng/dL (<31 ng/dL), renin 0.6 ng/mL/h (0.5–4 ng/mL/h), and 6:00 a.m. cortisol 82 µg/dL (6.7–22.6 µg/dL) with ACTH 147 pg/mL (no ref. range). High-dose Dexamethasone suppression testing suggested ACTH-dependent ectopic hypercortisolism. Contrast-enhanced CT findings included masses in the liver and right renal pelvis, a heterogeneous enlarged mass in the region of the prostate invading the bladder, bilateral adrenal thickening, and lytic lesions in the pelvis and spine. Liver biopsy identified epithelioid malignancy with Ki proliferation index 98% and immunohistochemical staining positive for synaptophysin and neuron-specific enolase, compatible with high-grade small cell carcinoma. Staining for ACTH was negative; no stain for CRH was available. Two weeks after chemotherapy, 6:00 a.m. cortisol normalized and CT scans showed universal improvement.
Conclusion: Extensive literature details paraneoplastic syndromes associated with SCC, but we report the first case of EPSCC diagnosed due to onset of dual paraneoplastic syndromes.
Introduction
Extrapulmonary small cell carcinoma (EPSCC) is an uncommon presentation of SCC. Its aggressive behavior frequently results in early metastases, which can complicate attempts to identify the site of origin. Extensive literature details paraneoplastic syndromes associated with neuroendocrine neoplasms, such as SCC (1–52), but there are no reports of co-occurring ectopic hypercortisolism and humoral hypercalcemia at initial diagnosis.
Additionally, SCC is an unusual, aggressive variant of prostate carcinoma and has been implicated in castration resistance (53). SCC may be diagnosed at the time of initial diagnosis of prostate cancer or may arise as a secondary malignancy following androgen deprivation therapy (ADT) to treat the more common prostate adenocarcinoma (49, 54).
Methods
Written informed consent was obtained from the patient for the publication of this case report. Clinical, laboratory, imaging, and pathology data are presented. We performed a search of the PubMed database through October 2017 for studies addressing paraneoplastic syndromes associated with neuroendocrine tumors, with particular emphasis on prostate carcinoma, Cushing’s syndrome, and hypercalcemia. Relevant cited articles were also retrieved and non-English articles were translated when possible.
Case Report
A 56-year-old man with prostate adenocarcinoma presented to the emergency department with clogged nephrostomy tubes. He had initially been diagnosed with Gleason 3 + 4 prostate adenocarcinoma 5 years prior to admission, then was lost to follow-up for 4 years. Repeat biopsy at that time reportedly revealed Gleason 5 + 5 prostate adenocarcinoma. The full pathology is not available but to the best of our knowledge, no neuroendocrine component was reported after either biopsy. At this time, the patient had local progression of disease to the rectum and bladder and bilateral hydroureteronephrosis so he underwent channel transurethral resection of his prostate and was started on daily oral bicalutamide and monthly leuprolide injections, which he was still taking at the time of admission. The patient was also referred for external beam radiation, but did not establish care with radiation oncology.
Review of systems was positive for constipation, weight loss, anorexia, and imbalance. Physical exam was notable for cachexia. Admission laboratory evaluation showed calcium 13.8 mg/dL (8.5–10.5 mg/dL), albumin 3.6 mg/dL (3.5–5 mg/dL), and potassium 2.8 mmol/L (3.5–5.2 mmol/L). Two months prior to admission, calcium had been reported 9.1 mg/dL (8.5–10.5 mg/dL) with no albumin reported and potassium 3.9 mmol/L (3.5–5.3 mmol/L). Further biochemical testing to determine the etiology of this patient’s hypercalcemia found intact PTH 19 pg/mL (16–87 pg/mL), 25-OH vitamin D 15.7 ng/mL (>30 ng/mL), and PTH-related peptide (PTHrP) 63.4 pmol/L (<2.3 pmol/L). Pamidronate was administered. Six days later, the patient experienced transient hypocalcemia to 6.6 mg/dL (8.5–10.5 mg/dL) with albumin 3.3 mg/dL (3.5–5 mg/dL). Hypocalcemia resolved the next day and the patient has remained normocalcemic for 8 months post-treatment.
Biochemical investigation in search of the underlying cause of refractory hypokalemia as low as 2.4 mmol/L (3.2–5.2 mmol/dL) revealed aldosterone 5.3 ng/dL (<31 ng/dL), renin 0.6 ng/mL/h (0.5–4 ng/mL/h), and 6:00 a.m. cortisol 82 µg/dL (6.7–22.6 µg/dL) with ACTH 147 pg/mL (no ref. range). After high-dose Dexamethasone suppression testing with 8 mg administered at midnight, 6:00 a.m. cortisol remained 62 µg/dL (6.7–22.6 µg/dL) with ACTH 115 pg/mL (no ref. range), compatible with ectopic ACTH-dependent hypercortisolism. Ketoconazole was initiated along with Spironolactone to address refractory hypokalemia as well as resistant hypertension (HTN) already treated with Enalapril, Carvedilol, and Amlodipine. The patient’s hemoglobin A1c on admission was 5.8% (40 mmol/mol) (4.2–5.9%, 22–41 mmol/mol), signifying prediabetes. New-onset severe hyperglycemia as high as >400 mg/dL during the second week of this hospitalization was managed with Glargine and Lispro insulins.
Pituitary MRI would have been a reasonable next step but was not performed. Given this patient’s history of malignancy on hormonal therapy, subacute clinical presentation and new diagnosis of PTHrP-mediated hypercalcemia, our suspicion for an ectopic source of ACTH-dependent hypercortisolism was very high so localizing imaging was performed urgently. Contrast-enhanced CT scans of the chest, abdomen, and pelvis detected multiple hypoattenuated masses in the liver up to 3.3 cm in diameter, subcentimeter pleural nodules, multiple masses in the right renal pelvis and ureter up to 1.2 cm in diameter, necrotic lymph nodes, a heterogeneous enlarged mass in the region of the prostate invading the bladder, bilateral adrenal thickening, and lytic lesions in the pelvis and lumbar spine (see Figures 1A,B). Medical oncology was consulted when the biopsy of a liver lesion identified epithelioid malignancy with cords and nests of small to medium size cells with high N/C ratio, salt and pepper chromatin, evident mitoses and numerous apoptotic bodies, Ki proliferation index 98%, and immunohistochemistry (IHC) staining positive for synaptophysin, neuron-specific enolase, CD56, cytokeratins CAM 5.2 and AE1/AE3, and focally for CK20, consistent with high-grade small cell neuroendocrine carcinoma (see Figure 2). IHC staining was negative for PSA, PTH, and ACTH. Stains for PTHrP and CRH were not available.
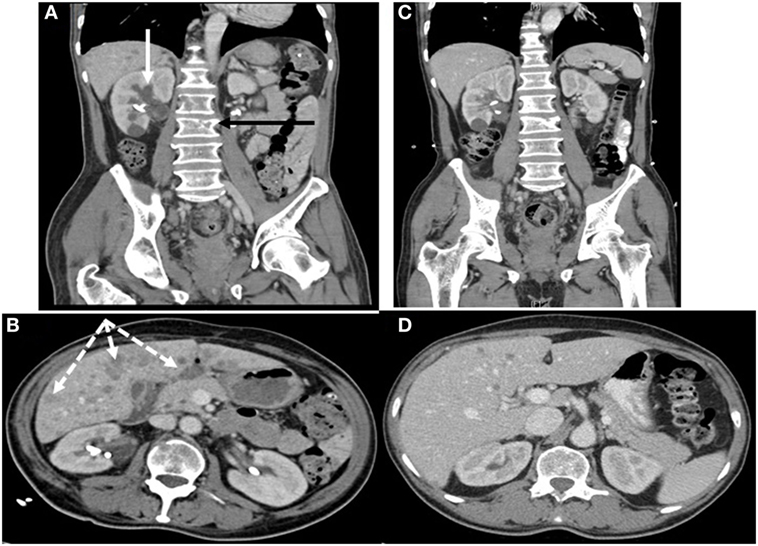
Figure 1. Contrast-enhanced CT scans of chest, abdomen, and pelvis (A,B) showing renal, hepatic, and osseous metastases before chemotherapy (C,D) 2 weeks afterward showing significant interval improvement. (A) Hypoattenuated masses in the right renal pelvis and ureter (solid white arrow) and lytic lesions in the spine (black arrow). (B) Multiple hypoattenuated hepatic masses (dashed white arrows).
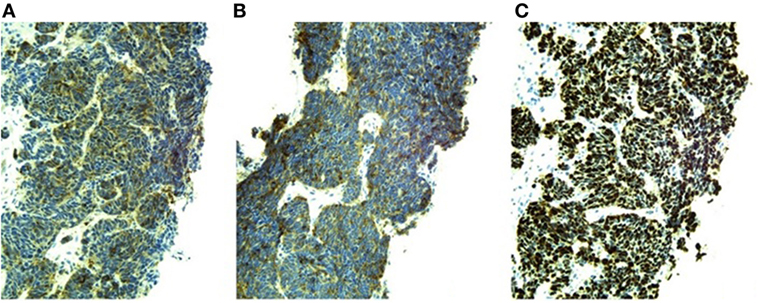
Figure 2. Positive immunohistochemical stains and Ki-67 proliferation index of liver biopsy sample (20× magnification). (A) Neuron-specific enolase. (B) Synaptophysin. (C) Ki-67 proliferation index 98%.
Five days after initiation of treatment with Carboplatin and Etoposide chemotherapy targeting EPSCC, the patient’s 6:00 a.m. cortisol decreased to 10 µg/dL (6.7–22.6 µg/dL) so Ketaconazole and Spironolactone were discontinued. CT scans obtained 2 weeks after chemotherapy initiation documented diminution or resolution of all prior findings (see Figures 1C,D). Repeated CT scans 7 months later showed an increase in number of hepatic lesions, a stable mass in the region of the prostate invading the bladder, stable pelvic lymphadenopathy, and sclerotic changes in known spinal and pelvic metastases without new bone lesions. The next month, now 8 months after chemotherapy initiation and following five more cycles of Carboplatin and Etoposide plus two cycles of Irinotecan, his potassium level was 3.5 mmol/L (3.2–5.2 mmol/dL) and calcium 9.6 mg/dL (8.5–10.5 mg/dL) with albumin 4.2 mg/dL (3.5–5 mg/dL). He was taking Lisinopril, Carvedilol, and Hydrochlorothiazide at an oncology appointment 8 months after chemotherapy initiation.
Discussion
Epidemiology of EPSCC
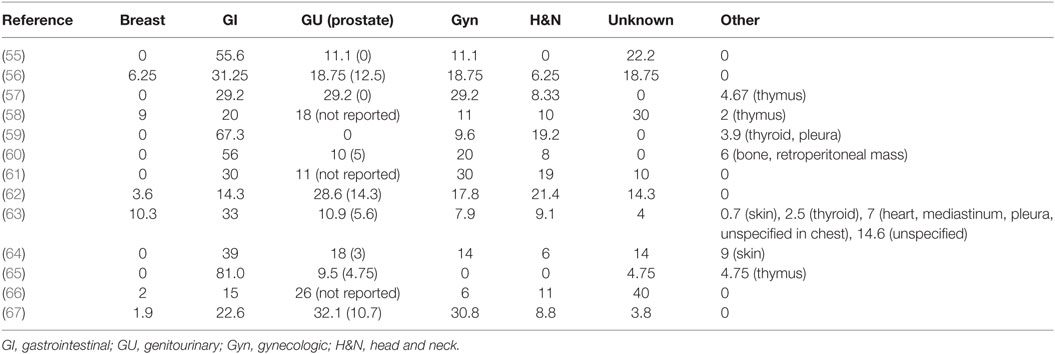
The age-adjusted incidence rate (AAIR) of EPSCC is estimated to be 3.5/1,000,000 person-years in the United States, only 1/22 that of pulmonary SCC (55). Such low incidence limits available literature. Papers from North America, Asia, and Europe report conflicting prevalence data, but agree on the overall most common categories of primary sites: gastrointestinal, genitourinary, gynecologic, and head and neck (see Table 1).
Genitourinary EPSCC includes malignancies that originate in the prostate as well as in the kidney, urinary bladder, urinary tract, and other reproductive organs. In particular, SCC has been noted to occur in patients previously diagnosed with prostate adenocarcinoma who were being treated with ADT such as our patient. For example, a retrospective chart review of 83 patients diagnosed with prostatic SCC over a 20-year period found 67% of the cohort had been previously diagnosed with prostate adenocarcinoma, while histologic diagnoses of SCC were 64% mixed SCC and adenocarcinoma rather than pure SCC (68).
Existing data regarding the clinical and laboratory characteristics of patients diagnosed with neuroendocrine prostate carcinoma is limited by low prevalence but does demonstrate some common features. Two retrospective studies of the SEER database describe the population diagnosed with prostatic SCC as being predominately older than 80 years of age and Caucasian. The majority of patients also exhibited distant metastases and low PSA level at the time of initial diagnosis (68, 69). AAIR of this entity has been estimated to be 0.582/1,000,000 person-years in 2011 (69), while SCC represented only 0.06% of all prostate cancers diagnosed between 1973 and 2008 in the United States (68).
Hypothesized Mechanisms for Post-ADT Prostatic Neuroendocrine Cancer
Postulated mechanisms of the concurrent or subsequent diagnosis of prostate adenocarcinoma and SCC include the reversal of quiescence in neuroendocrine cell nests routinely present in adenocarcinoma by ADT or independent development from shared multipotent progenitor cells (70, 71). Therapeutic targets being explored include: (1) the tyrosine kinase AURKA found to be overexpressed by prostate adenocarcinoma which includes a neuroendocrine component, (2) the oncogene MYCN, which is also overexpressed, (3) the silencing transcription factor gene REST, found to be underexpressed, (4) the gene Rb encoding a regulator of the cell cycle which is lost, (5) the metalloproteinase inhibitor TIMP-1 that is overexpressed, (6) the cell adhesion protein CD44 that is expressed only in the neuroendocrine component of mixed prostate malignancies, and (7) the aberrant fusion product of genes encoding the serine protease TMPRSS2 and the zinc finger transcription factor ERG, which are both overexpressed in neuroendocrine prostate malignancies (71–73).
Treatment of EPSCC
The North American Neuroendocrine Tumor Society issued management guidelines for EPSCC in 2010 (74). These guidelines are recommended for all cases, regardless of site of origin. The current standard of care is to treat ESPCC similarly to pulmonary SCC: attempt debulking with or without adjunctive radiation treatment for local or locoregional disease, otherwise systemic treatment with platinum-based chemotherapy and Etoposide is first-line therapy for metastatic disease at presentation. Findings vary regarding whether significant differences in response to treatment modalities, progression-free survival, and overall survival exist between pulmonary SCC and EPSCC and between EPSCC of different sites of origin, but most studies agree on improved survival in EPSCC and in younger patients with limited rather than metastatic disease (55, 65, 66, 75).
Ectopic Endocrine Syndromes Associated With Neuroendocrine Neoplasms
Neuroendocrine neoplasms, most commonly pulmonary SCC and carcinoid tumors, have been known to produce many ectopic hormones, including ACTH and CRH. These typically trigger rapid onset of objective signs of hypercortisolism without Cushingoid features on physical exam. Such features include HTN, insulin resistance and hyperglycemia, hyperpigmentation due to increased expression of the alpha-subunit of ACTH or increased precursor peptide POM-C expression, and hypokalemia due to activity at the mineralocorticoid receptor, which also results in suppressed serum renin levels. These patients typically present with cachexia, weight loss, and easy bruising due to severe catabolism and are known to be at increased risk for steroid-induced psychosis. This atypical cohort constitutes up to 20% of all cases of Cushing’s syndrome (1).
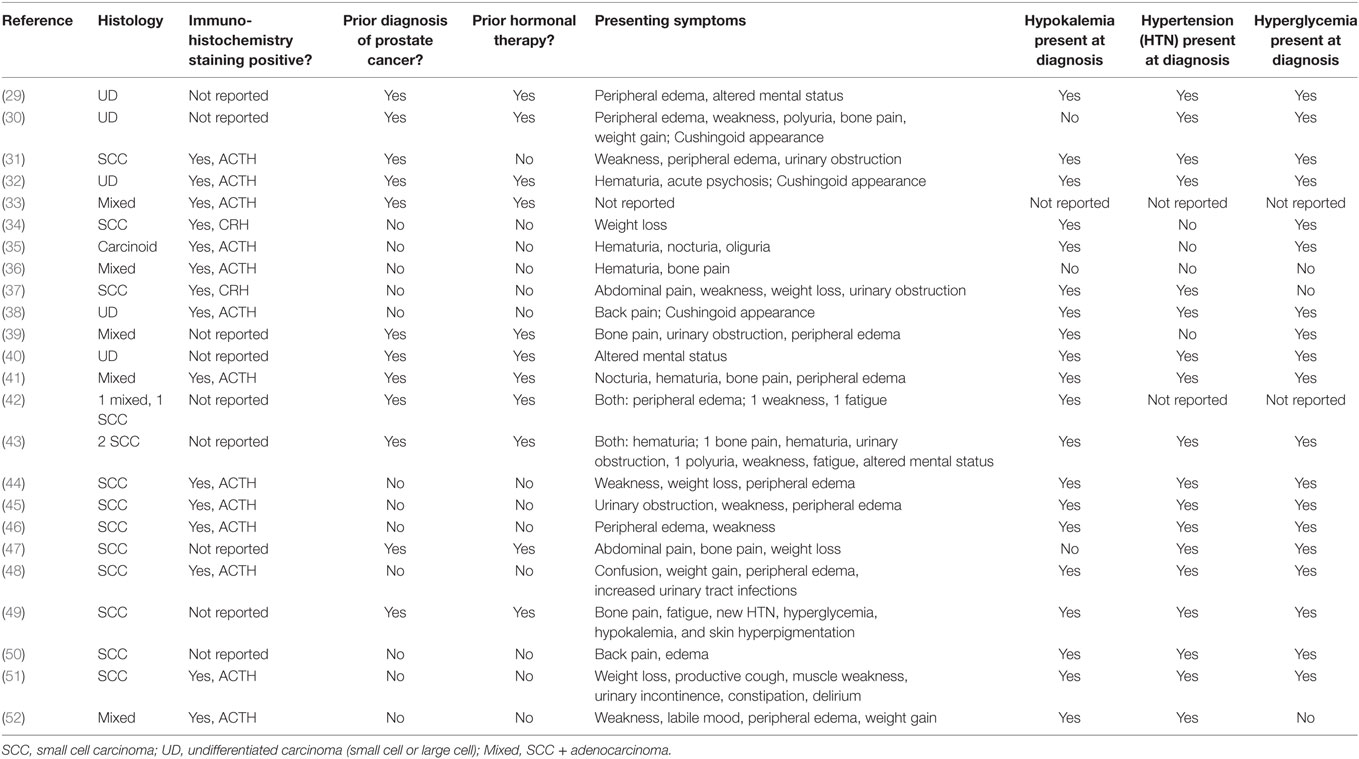
Extrapulmonary neuroendocrine neoplasms, including EPSCC, can produce a variety of ectopic hormones to cause other kinds of paraneoplastic syndromes as noted in case reports. For example, prostatic undifferentiated carcinoma has been linked to both SIADH (2, 3) and atypical myasthenia gravis due to Lambert–Eaton syndrome (4). Additional case reports support the postulated ectopic secretion of ADH with positive IHC staining in prostatic SCC (5). Paraneoplastic SIADH has also been associated with SCC of the nasal cavity and ethmoid sinuses (6–8). Neuroendocrine neoplasms of the GI tract, including the esophagus, liver, gall bladder, pancreas, and colon have likewise been linked to SIADH, humoral hypercalcemia of malignancy, and anti-Hu sensory neuropathy (9–16). Similarly, SCC of the vagina, cervix, endometrium, ovary, and breast have been documented to show IHC positivity for ACTH, the ACTH precursor peptide POM-C, PTH, and PTHrP (17–25). Case reports and series of patients with prostatic SCC have documented the occurrence of ectopic hypercortisolism among only 26 patients in 24 papers, since 1973 (see Table 2). Similarly, humoral hypercalcemia due to ectopic PTHrP has been reported in prostate adenocarcinoma (26, 27), while an in vitro study localized PTHrP expression to nests of neuroendocrine cells within prostate adenocarcinoma (28). Again, the relative rarity of these conditions limits data available beyond case series.
Although the primary site of our patient’s EPSCC could not be definitively identified, we believe it is of GU origin given the patient’s history of taking ADT and the epidemiology of EPSCC as discussed above. The decision to biopsy a hepatic lesion was made by the interventional radiologist who performed the procedure, and the consulting medical oncologist did not require further data from other sites to form a treatment plan so no additional biopsy was performed. Based on global improvement on post-treatment CT scans, it is reasonable to conclude that all lesions are the same SCC as diagnosed in the liver. We suspect our patient’s SCC liver biopsy sample tested was negative in IHC staining for ACTH because it was instead producing CRH or POM-C; however, other possible explanations cannot be ruled out. These include sampling error of biopsy leading to a false negative result, mosaicism among individual cells or metastatic sites of this malignancy, or a coexisting distinct malignancy producing ACTH.
Concluding Remarks
To the best of our knowledge, we present the first reported case of EPSCC diagnosed due to onset of dual paraneoplastic syndromes. Overall, ectopic endocrine syndromes due to neuroendocrine neoplasms are rare, but can be important markers of onset or progression of malignancy. Moreover, hormonal excess causes significant morbidity in its own right.
This particular case also highlights the risk of development of aggressive neuroendocrine carcinoma after hormonal treatment of prostate adenocarcinoma. Further research is indicated to elucidate the pathophysiology of post-ADT EPSCC and determine clinicopathologic characteristics that can predict which patients are most at risk. Such information would hopefully facilitate development of screening guidelines for this disease.
Author Contributions
JF drafted the original manuscript and created all figures and tables. NB and JA contributed to expansion of the discussion and proofread the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to acknowledge Dr. Ippolito Modica and Dr. Raza Roshan for performing immunohistochemistry staining and providing pathology images.
References
1. Jameson J, Longo DL. Paraneoplastic syndromes: endocrinologic/hematologic. 19 ed. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, editors. Harrison’s Principles of Internal Medicine. New York: McGraw-Hill (2014). 263 p.
2. Peverelli G, Grassi P. Pure small cell recurrent prostate cancer developing syndrome of inappropriate antidiuretic hormone secretion. Tumori (2017) 103:e56–9. doi:10.5301/tj.5000651
3. Sellwood RA, Spencer J, Azzopardi JG, Wapnick S, Welbourn RB, Kulatilake AE. Inappropriate secretion of antidiuretic hormone by carcinoma of the prostate. Br J Surg (1969) 56(12):933–5. doi:10.1002/bjs.1800561217
4. Têtu B, Ro JY, Ayala AG, Ordoñez NG, Logothetis CJ, von Eschenbach AC. Small cell carcinoma of prostate associated with myasthenic (Lambert-Eaton) syndrome. Urology (1989) 33(2):148–52. doi:10.1016/0090-4295(89)90017-4
5. Kawai S, Hiroshima K, Tsukamoto Y, Tobe T, Suzuki H, Ito H, et al. Small cell carcinoma of the prostate expressing prostate-specific antigen and showing syndrome of inappropriate secretion of antidiuretic hormone: an autopsy case report. Pathol Int (2003) 53:892–6. doi:10.1046/j.1440-1827.2003.01568.x
6. Bach CA, Guilleré L, Le Stanc E, Chabolle F. Small cell neuroendocrine carcinoma of the ethmoid sinus revealed by syndrome of inappropriate antidiuretic hormone secretion. Eur Ann Otorhinolaryngol Head Neck Dis (2016) 133:71–2. doi:10.1016/j.anorl.2015.05.006
7. Ma ATW, Lei KIK. Small cell neuroendocrine carcinoma of the ethmoid sinuses presenting with generalized seizure and syndrome of inappropriate antidiuretic hormone secretion: a case report and review of the literature. Am J Otolaryngol (2009) 30:54–7. doi:10.1016/j.amjoto.2007.12.005
8. Rossi P, Suissa J, Bagneres D, Martin F, Edy E, Demoux AL, et al. Syndrome of inappropriate antidiuretic hormone secretion disclosing a sinonasal neuroendocrine carcinoma: case report. Rev Med Interne (2007) 28(6):426–8. doi:10.1016/j.revmed.2007.02.006
9. Ando T, Hosokawa A, Yamawaki H, Hasumoto Y, Kajiura S, Itaya Y, et al. Esophageal small-cell carcinoma with syndrome of inappropriate secretion of antidiuretic hormone. Intern Med (2011) 50:1099–103. doi:10.2169/internalmedicine.50.4694
10. Kanno K, Hikichi T, Saito K, Watanabe K, Takagi T, Shibukawa G, et al. A case of esophageal small cell carcinoma associated with hypercalcemia causing severe acute pancreatitis. Fukushima J Med Sci (2007) 53(1):51–60. doi:10.5387/fms.53.51
11. Lortholary AH, Cadeau SD, Gertrand GM, Guerin-Meyer VI, Gamelin EC, Audrin MJ. Humoral hypercalcemia in patients with colorectal carcinoma. Report of two cases and review of the literature. Cancer (1999) 86(11):2217–21. doi:10.1002/(SICI)1097-0142(19991201)86:11<2217::AID-CNCR7>3.0.CO;2-V
12. Luh JY, Han ES, Simmons JR, Whitehead RP. Poorly differentiated colon carcinoma with neuroendocrine features presenting with hypercalcemia and cutaneous metastases. Case report and review of the literature. Am J Clin Oncol (2002) 25(2):160–3. doi:10.1097/00000421-200204000-00011
13. Saleem A. Severe hyponatremia presenting as paraneoplastic syndrome in a patient with small cell carcinoma of gallbladder. J Coll Phys Surg Pak (2016) 26(5):451–2.
14. Shah NA, Urusova IA, D’Agnolo A, Colquhon SD, Rosenbloom BE, Vener SL, et al. Primary hepatic carcinoid tumor presenting as Cushing’s syndrome. J Endocrinol Invest (2007) 30(4):327–33. doi:10.1007/BF03346308
15. Tamura T, Takeuchi K. Small cell gall bladder carcinoma complicated by syndrome of inappropriate secretion of antidiuretic hormone (SIADH) treated with mozavaptan. BMJ Case Rep (2013) 2013:bcr2013010039. doi:10.1136/bcr-2013-010039
16. Uribe-Uribe NO, Jimenez-Garduño AM, Henson DE, Albores-Saavendra J. Paraneoplastic sensory neuropathy associated with small cell carcinoma of the gallbladder. Ann Diagn Pathol (2009) 13(2):124–6. doi:10.1016/j.anndiagpath.2007.08.003
17. Abeler V, Kjørstad KE, Nesland JM. Small cell carcinoma of the ovary: a report of six cases. Int J Gynecol Pathol (1988) 7(4):315–29. doi:10.1097/00004347-198812000-00003
18. Chen L, Dinh TA, Haque A. Small cell carcinoma of the ovary with hypercalcemia and ectopic parathyroid hormone production. Arch Pathol Lab Med (2005) 129(4):531–3. doi:10.1043/1543-2165(2005)129<531:SCCOTO>2.0.CO
19. Hashi A, Yasumizu T, Yoda I, Kou T, Mizuno K, Hirata S, et al. A case of small cell carcinoma of the uterine cervix presenting Cushing’s syndrome. Gynecol Oncol (1996) 61(3):427–31. doi:10.1006/gyno.1996.0168
20. Ohira S, Itoh K, Shiozawa T, Horiuchi A, Ono K, Takeuchi H, et al. Ovarian non-small cell neuroendocrine carcinoma with paraneoplastic parathyroid hormone related hypercalcemia. Int J Gynecol Pathol (2004) 23(4):393–7. doi:10.1097/01.pgp.0000139655.18062.12
21. Pelte MF, Schwaller J, Cerrato C, Meier CA. Pro-opiomelanocortin expression in a metastatic breast carcinoma with ectopic ACTH secretion. Breast J (2004) 10(4):350–4. doi:10.1111/j.1075-122X.2004.21467.x
22. Popiolek DA, Kumar AR, Mittal K. Large cell variant of small cell carcinoma, hypercalcemic type, of primary peritoneal origin. Gynecol Oncol (2005) 96(1):249–53. doi:10.1016/j.ygyno.2004.09.040
23. Sato H, Kanai G, Kajiwara H, Itoh J, Yoshiyuki R. Small-cell carcinoma of the endometrium presenting as Cushing’s syndrome. Endocr J (2010) 57(1):31–8. doi:10.1507/endocrj.K09E-212
24. Schweiger LM, Hsiang HY. Pathological case of the month. Arch Pediatr Adolesc Med (2002) 156(1):83–4. doi:10.1001/archpedi.156.1.83
25. Young RH, Oliva E, Scully RE. Small cell carcinoma of the ovary, hypercalcemic type: a clinicopathologic analysis of 150 cases. Am J Surg Pathol (1994) 18(11):1102–16. doi:10.1097/00000478-199411000-00004
26. Hanazawa K, Higashi N, Kawachi Y, Suzuki F, Ishi K, Fujime M. Small cell carcinoma of the prostate with hypercalcemia. Int J Urol (2005) 12(1):108–10. doi:10.1111/j.1442-2042.2004.00977.x
27. Smith DC, Tucker JA, Trump DL. Hypercalcemia and neuroendocrine carcinoma of the prostate: a report of three cases and a review of the literature. J Clin Oncol (1992) 10(3):499–505. doi:10.1200/JCO.1992.10.3.499
28. Iwamura M, Wu G, Abrahamsson P-A, Di Sant’agnese PA, Cockett ATK, Deftos LJ. Parathyroid hormone-related protein is expressed by prostatic neuroendocrine cells. Urology (1994) 43(5):667–74. doi:10.1016/0090-4295(94)90182-1
29. Newmark SR, Dluhy RG, Bennett AH. Ectopic adrenocorticotropin syndrome with prostatic carcinoma. Urology (1973) 2(6):666–8. doi:10.1016/0090-4295(73)90333-6
30. Lovern WJ, Farriss BL, Wettlaufer JN, Hane S. Ectopic ACTH production in disseminated prostatic adenocarcinoma. Urology (1975) 5(6):817–20. doi:10.1016/0090-4295(75)90365-9
31. Wenk RE, Bhagavan BS, Levy R, Miller D, Weisburger W. Ectopic ACTH, prostatic oat cell carcinoma, and marked hypernatremia. Cancer (1977) 40(2):773–8. doi:10.1002/1097-0142(197708)40:2<773::AID-CNCR2820400226>3.0.CO;2-I
32. Molland EA. Prostatic adenocarcinoma with ectopic ACTH production. Br J Urol (1978) 50(5):358. doi:10.1111/j.1464-410X.1978.tb03653.x
33. Vuitch MF, Mendelsohn G. Relationship of ectopic ACTH production to tumor differentiation: a morphologic and immunohistochemical study of prostate carcinoma with Cushing’s syndrome. Cancer (1981) 47(2):296–9. doi:10.1002/1097-0142(19810115)47:2<296::AID-CNCR2820470215>3.0.CO;2-N
34. Carey RM, Varma SK, Drake CR Jr, Rhorner MO, Kovacs K, River J, et al. Ectopic secretion of corticotropin-releasing factor as a cause of Cushing’s syndrome. A clinical, morphologic, and biochemical study. N Engl J Med (1984) 311(1):13–20. doi:10.1056/NEJM198407053110103
35. Slater D. Carcinoid tumour of the prostate associated with inappropriate ACTH secretion. Br J Urol (1985) 57(5):591–2. doi:10.1111/j.1464-410X.1985.tb05878.x
36. Rojas-Corona RR, Chen LZ, Mahadevia PS. Prostatic carcinoma with endocrine features. A report of a neoplasm containing multiple immunoreactive hormonal substances. Am J Clin Pathol (1987) 88(6):759–62. doi:10.1093/ajcp/88.6.759
37. Fjellestad-Paulsen A, Abrahamsson PA, Bjartell A, Grino M, Grimelius L, Hedeland H, et al. Carcinoma of the prostate with Cushing’s syndrome: a case report with histochemical and chemical demonstration of immunoreactive corticotropin-releasing hormone in plasma and tumoral tissue. Acta Endocrinol (Copenh) (1988) 119(4):506–16.
38. Yonaha M, Tanaka M, Kawano T, Sugiyama M, Nishikawa T, Sasano K. [An autopsy case of ACTH producing prostate neoplasms with Cushing’s syndrome]. Nihon Naika Gakkai Zasshi (1992) 81(12):2005–6. doi:10.2169/naika.81.2005
39. Haukaas SA, Halvorsen OJ, Nygaard SJ, Paus E. Cushing’s syndrome in prostate cancer. An aggressive course of prostate malignancy. Urol Int (1999) 63(2):126–9. doi:10.1159/000030431
40. Rickman T, Garmany R, Doherty T, Benson D, Okusa MD. Hypokalemia, metabolic alkalosis, and hypertension: Cushing’s syndrome in a patient with metastatic prostate adenocarcinoma. Am J Kidney Dis (2001) 37(4):838–46. doi:10.1016/S0272-6386(01)80134-7
41. Kataoka K, Akasaka Y, Nakajima K, Nagao K, Hara H, Miura K, et al. Cushing syndrome associated with prostatic tumor adrenocorticotropic hormone (ACTH) expression after maximal androgen blockade therapy. Int J Urol (2007) 14:436–9. doi:10.1111/j.1442-2042.2006.01710.x
42. Nimalasena S, Freeman A, Harland S. Paraneoplastic Cushing’s syndrome in prostate cancer: a difficult management problem. BJU Int (2008) 101(4):424–7. doi:10.1111/j.1464-410X.2007.07294.x
43. Rajec J, Mego M, Sycova-Mila Z, Obertova J, Brozmanova K, Mardiak J. Paraneoplastic Cushing’s syndrome as the first sign of progression of prostate cancer. Bratisl Lek Listy (2008) 109(8):362–3.
44. Alwani RA, Neggers SJ, van der Klift M, Baggen MG, van Leenders GJ, van Aken MO, et al. Cushing’s syndrome due to ectopic ACTH production by (neuroendocrine) prostate carcinoma. Pituitary (2009) 12(3):280–3. doi:10.1007/s11102-008-0100-z
45. Alshaikh OM, Al-Mahfouz AA, Al-Hindi H, Mahfouz AB, Alzahrani AS. Unusual case of ectopic secretion of adrenocorticotropic hormone: Cushing syndrome attributable to small cell prostate cancer. Endocr Pract (2010) 16(2):249–54. doi:10.4158/EP09243.CR
46. McMahon GT, Blake M, Wu CL. Case 1-2010 – a 75-year-old man with hypertension, hyperglycemia, and edema. NEJM (2010) 362:156–66. doi:10.1056/NEJMcpc0905546
47. Alves D, Calmeiro ME, Silva R, Coelho H. Small-cell neuroendocrine cancer of the prostate: an atypical presentation of a common disease. BMJ Case Rep (2016) 2016:bcr2016216199. doi:10.1136/bcr-2016-216199
48. Balestrieri A, Magnani E, Nuzzo F. Unusual Cushing’s syndrome and hypercalcitoninemia due to a small cell prostate carcinoma. Case Rep Endocrinol (2016) 2016:6308058. doi:10.1155/2016/6308058
49. Ramalingam S, Eisenberg A, Foo WC, Freedman J, Armstrong AJ, Moss LG, et al. Treatment-related neuroendocrine prostate cancer resulting in Cushing’s syndrome. Int J Urol (2016) 23(12):1038–41. doi:10.1111/iju.13225
50. Rueda-Camino JA, Losada-Villa B, De Ancos-Aracil CL, Rodriguez-Lajusticia L, Tardio JC, Zapatero-Gaviria A. Small cell carcinoma of the prostate presenting with Cushing syndrome. A narrative review of an uncommon condition. Ann Med (2016) 48(4):293–9. doi:10.3109/07853890.2016.1168936
51. Shrosbree J, Pokorny A, Stone E, Epstein R, McCormack A, Greenfield JR. Ectopic Cushing syndrome due to neuroendocrine prostatic cancer. Intern Med J (2016) 46(5):630–2. doi:10.1111/imj.13063
52. Elston MS, Crawford VB, Swarbrick M, Dray MS, Head M, Conaglen JV. Severe Cushing’s syndrome due to small cell prostate carcinoma: a case and review of the literature. Endocr Connect (2017) 6(5):R80–6. doi:10.1530/EC-17-0081
53. Alanee S, Moore A, Nutt M, Holland B, Dynda D, El-Zawahry A, et al. Contemporary incidence and mortality rates of neuroendocrine prostate cancer. Anticancer Res (2015) 35:4145–50.
54. Lipianskaya J, Cohen A, Chen CJ, Hsia E, Squires J, Li Z, et al. Androgen-deprivation therapy-induced aggressive prostate cancer with neuroendocrine differentiation. Asian J Androl (2014) 16(4):541–4. doi:10.4103/1008-682X.123669
55. Remick SC, Hafez GR, Carbone PP. Extrapulmonary small cell carcinoma. A review of the literature with emphasis on therapy and outcome. Medicine (Baltimore) (1987) 66(6):457–71. doi:10.1097/00005792-198711000-00004
56. Sengoz M, Abacioglu U, Salepci T, Eren F, Yumuk F, Turhal S. Extrapulmonary small cell carcinoma: multimodality treatment results. Tumori (2003) 89(3):274–7.
57. Kim JH, Lee SH, Park J, Kim HY, Lee SI, Nam EM, et al. Extrapulmonary small cell carcinoma: a single-institution experience. Jpn J Clin Oncol (2004) 34(5):250–4. doi:10.1093/jjco/hyh052
58. Haider K, Shahid RK, Finch D, Sami A, Ahmad I, Yadav S, et al. Extrapulmonary small cell cancer: a Canadian province’s experience. Cancer (2006) 107(9):2262–9. doi:10.1002/cncr.22235
59. Yuan ZY, Guan ZZ, Zhou ZM, Xia Y, Huang WZ, Yang XL. Extrapulmonary small cell carcinoma in 52 patients. Chin J Cancer (2006) 25(9):1131–3.
60. Lee SS, Lee JL, Ryu MH, Chang HM, Kim TW, Lee JS, et al. Extrapulmonary small cell carcinoma: single center experience with 61patients. Acta Oncol (2007) 46(6):846–51. doi:10.1080/02841860601071893
61. Lin YL, Chung CY, Chang CS, Wu JS, Kuo KT, Kuo SH, et al. Prognostic factors in extrapulmonary small cell carcinomas: a large retrospective study. Oncology (2007) 72(3):181–7. doi:10.1159/000112804
62. Ochsenreither S, Marnitz-Schultze S, Schneider A, Koehler C, Daum S, Loddenkemper C, et al. Extrapulmonary small cell carcinoma (EPSCC): 10 years’ multi-disciplinary experience at Charité. Anticancer Res (2009) 29(8):3411–5.
63. Wong YN, Jack RH, Mak V, Henrik M, Davies EA. The epidemiology and survival of extrapulmonary small cell carcinoma in South East England, 1970-2004. BMC Cancer (2009) 9:209. doi:10.1186/1471-2407-9-209
64. Li AF, Hsu HS, Hsu CY, Li AC, Li WY, Liang WY, et al. A 20-year retrospective study of small-cell carcinomas in Taiwan. J Surg Oncol (2010) 102(5):497–502. doi:10.1002/jso.21629
65. Terashima T, Morizane C, Hiraoka N, Tsuda H, Tamura T, Shimada Y, et al. Comparison of chemotherapeutic treatment outcomes of advanced extrapulmonary neuroendocrine carcinomas and advanced small-cell lung carcinoma. Neuroendocrinology (2012) 96:324–32. doi:10.1159/000338794
66. Brammer JE, Lulia P, Lynch GR. Retrospective review of extra-pulmonary small cell carcinoma and prognostic factors. Int J Clin Oncol (2014) 19(5):822–8. doi:10.1007/s10147-013-0626-6
67. Gennatas S, Noble J, Stanway S, Gunapala R, Chowdhury R, Wotherspoon A, et al. Patterns of relapse in extrapulmonary small cell carcinoma: retrospective analysis of outcomes from two cancer centres. BMJ Open (2015) 5(1):e006640. doi:10.1136/bmjopen-2014-006440
68. Priemer DS, Montironi R, Wang L, Williamson SR, Lopez-Beltran A, Cheng L. Neuroendocrine tumors of the prostate: emerging insights from molecular data and updates to the 2016 World Health Organization Classification. Endocr Pathol (2016) 27:123–35. doi:10.1007/s12022-016-9421-z
69. Spiess PE, Pettawy CA, Vakar-Lopez F, Kassouf W, Wang X, Busby JE, et al. Treatment outcomes of small cell carcinoma of the prostate: a single-center study. Cancer (2007) 110(8):1729–37. doi:10.1002/cncr.22971
70. Dores GM, Qubaiah O, Mody A, Ghabach B, Devesa SS. A population-based study of incidence and patient survival of small cell carcinoma in the United States, 1992-2010. BMC Cancer (2015) 15:185. doi:10.1186/s12885-015-1188-y
71. Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov (2011) 1(6):487–95. doi:10.1158/2159-8290.CD-11-0130
72. Li Q, Zhang CS, Zhang Y. Molecular aspects of prostate cancer with neuroendocrine differentiation. Review. Clin J Cancer Res (2016) 28(1):122–9. doi:10.1186/1756-9966-28-122
73. Marcus DM, Goodman M, Jani AB, Osunkoya AO, Rossi PJ. A comprehensive review of incidence and survival in patients with rare histological variants of prostate cancer in the United States from 1973 to 2008. Prostate Cancer Prostatic Dis (2012) 15:283–8. doi:10.1038/pcan.2012.4
74. Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas (2010) 39(6):799–800. doi:10.1097/MPA.0b013e3181ebb56f
Keywords: ectopic Cushing’s syndrome, humoral hypercalcemia, PTH-related peptide, neuroendocrine carcinoma, androgen deprivation therapy, extrapulmonary small cell carcinoma
Citation: Feffer JB, Branis NM and Albu JB (2018) Dual Paraneoplastic Endocrine Syndromes Heralding Onset of Extrapulmonary Small Cell Carcinoma: A Case Report and Narrative Review. Front. Endocrinol. 9:170. doi: 10.3389/fendo.2018.00170
Received: 22 February 2018; Accepted: 03 April 2018;
Published: 18 April 2018
Edited by:
Antongiulio Faggiano, University of Naples Federico II, ItalyReviewed by:
Luiz Eduardo Armondi Wildemberg, Instituto Estadual do Cérebro Paulo Niemeyer, BrazilSabrina Corbetta, Università degli Studi di Milano, Italy
Copyright: © 2018 Feffer, Branis and Albu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jill B. Feffer, jbfeffer@gmail.com
 Jill B. Feffer
Jill B. Feffer Natalia M. Branis
Natalia M. Branis