Sub-surface Biogeochemical Characteristics and Its Effect on Arsenic Cycling in the Holocene Gray Sand Aquifers of the Lower Bengal Basin
- 1Department of Thematic Studies–Environmental Change, Linköping University, Linköping, Sweden
- 2Integrative Taxonomy and Microbial Ecology Research Group, Department of Biological Sciences, Indian Institute of Science Education and Research-Kolkata, Mohanpur, India
High arsenic (As) content in the fertile delta plains of West Bengal has been widely reported since the 1990s. The shallow gray sand aquifers (GSA) deposited during the Holocene, are more commonly used as potable water sources, but they have high As levels. The release of As into groundwater is influenced by indigenous microbial communities metabolizing different organic carbon sources present in the GSA sediments. After pre-screening the groundwater for assessing their microbial phylogenetic diversity, two 50-m deep boreholes were drilled in the GSAs, and 19 sediment samples were recovered from each core. In each of these samples, grain-size distribution, sequential extraction, and quantification of trace metals and total extractable lipids were analyzed. The aquifer sediments consisted of medium to fine micaceous sand with clay lenses in between them; a thick clay layer occurred on top of both boreholes. Arsenic concentration in these sediments varied from 1.80 to 41.0 mg/kg and was mostly associated with the oxide and silicate-rich crystalline minerals. Arsenic showed a significant correlation with Fe in all fractions, suggesting the presence of Fe-(oxy)-hydroxides bound As minerals. The diagnostic lipid biomarkers showed presence of compounds derived from higher plants (epicuticular waxes) and microbial inputs. The biomarkers were abundant in clay and silt-rich layers. The samples indicated preferential preservation of n-alkanes over other functional compounds (e.g., alcohols and fatty acids), that are more reactive, and hence subject to further degradation. Sediments recovered from the borehole indicated the presence of Eustigmatophytes and vascular plant waxes that are mostly surface-derived. The sedimentary lipids also indicated the presence of complex petroleum-derived hydrocarbons. These compounds provide organic substrates, and support the preferential survival of specific microbial communities in these sediments.
Introduction
Global arsenic (As) contamination of groundwater and its adverse effects on human health have been reported since the late 1930s (Arguello et al., 1938). The fertile deltaic plains of rivers Ganges and Brahmaputra in India and Bangladesh are well-known As “hot spots,” where high amounts of naturally occurring As has been associated with adverse health issues (Acharyya et al., 2000; Mukherjee and Fryar, 2008). This has resulted in an intensive scrutiny of different biogeochemical processes associated with As cycling, its toxicity, mobilization, and distribution pattern in sedimentary aquifers. Over the years, several processes have been proposed by scientists to explain mobilization of As from sediments such as: geothermal release (Ballantyne and Moore, 1988), alkaline desorption (Ayotte et al., 2003), reductive dissolution of ferric Fe(III)-oxyhydroxides (Bhattacharya et al., 1997; Jacks et al., 2001 and the references therein), reduction of arsenate [As(V); Oremland and Stolz, 2005], and oxidation of As-bearing sulfides (Ravenscroft et al., 2009). Amongst these processes, the most widely accepted mechanism for As mobilization in deltaic sediments is through microbial reductive dissolution of As bearing iron-oxyhydroxides and/or dissimilatory As(V) reduction (Bhattacharya et al., 1997; McArthur et al., 2004).
In general, the As affected sites in southeast Asia contain very low (<1 %) total organic carbon (TOC) in sediments (Rowland et al., 2006; Héry et al., 2010; Lawati et al., 2012). However, there could be presence of organic-rich clay or peat lenses in the sub-surface at these sites (Goodbred and Kuehl, 2000; McArthur et al., 2004; Lawati et al., 2012). These organic-rich sediments are a key factor driving As mobilization as in case of the Bengal Delta Plain (BDP) aquifers, where sub-surface conditions are reducing, and variable quantities of OM exist in the aquatic and sediment phases (Harvey et al., 2002; McArthur et al., 2004; Ghosh et al., 2015a,b). These organic-rich sediments contain relatively recalcitrant OM derived from higher vascular plant remains, and play an important role in release of As (Nickson et al., 2000; McArthur et al., 2004). However, OM in these sediments could also be introduced due to human activities involving agricultural practices, and releasing sewage wastes into surface water bodies (Ravenscroft et al., 2001; Harvey et al., 2002; Ghosh et al., 2017).
The earlier studies on characterization of OM sources in the sediments and DOC pool, had shown typical presence of thermally mature petroleum-derived hydrocarbons in addition to the various terrigenous sources (Rowland et al., 2006; Ghosh et al., 2015a). Such, hydrocarbons are complex organic carbon sources, and only specific microbial communities can metabolize them. Lawati et al. (2012) reported the presence of similar microbial communities in the Red River delta in Vietnam. Such hydrocarbon degrading microbial communities have been also detected in the As-contaminated BDP groundwater samples, but absent in safe aquifer systems (Ghosh et al., 2015a,b). It clearly indicated the complex carbon sources of BDP has a role in sustaining and supporting only specific microbial communities. Such natural selection of microbial communities in the BDP groundwaters correlate with increasing As concentration pointing toward the putative role of microbial communities in As mobilization and distribution (Ghosh et al., 2014).
The sub-surface geochemical (mainly inorganic aspects) and lithological characteristics of the BDP gray and brown sand aquifers have been investigated to divide them into As “safe” vs. contaminated aquifers (Biswas et al., 2012). The BDP gray sand aquifers (GSA) are contaminated because of the high (typically > 50 μg/L) As level in groundwater. Although gray and brown sand aquifers are often located next to each other in these deltaic environments, their biogeochemical characteristics differ. It seems very little work has been done on characterizing the OM type and/or its quality, in the BDP aquifers to understand its relationship with sub-surface microbial processes and As cycling. We hypothesize that the high As concentration in the GSAs is largely driven by the availability of OM and biogeochemical interactions involving the sub-surface microbial communities and sedimentary OM. As part of our ongoing investigations on biogeochemical characteristics in BDP aquifers, our objectives in this study are to: (1) investigate the distribution of trace metals and their trends in relation to sediment lithology, (2) characterize the different lipid fractions in sediments, and (3) investigate the role of OM driving As mobilization in GSAs. The data will help in understanding the complex relationships between sediment lithology, trace metals and OM characteristics, and how these factors influence the distribution of microbial communities and their metabolism in the sub-surface.
Study Area
The Nadia district in West Bengal, India is a well-known As “hot-spot,” and sedimentary aquifers have been frequently investigated in this district to resolve the As contamination problem (Bhattacharya et al., 2002, 2006; Mukherjee and Fryar, 2008). The deltaic sediments in Nadia district consist of Holocene micaceous gray sands and Pleistocene iron-rich brown sands (Biswas et al., 2012). These sediments are channel-fill sands and overbank clays, and its deposition have been controlled by fluvial and tidal/estuarine processes (Ahmed et al., 2004). The aquifer sediments in Karimpur indicate flood plain stratigraphy consisting of fining-upward sand to silt with clay bands in between the stratigraphic horizons deposited during channel migration (Alam, 1989; Goodbred and Kuehl, 2000; McArthur et al., 2004). There is large heterogeneity, and this makes correlation even between the similar GSAs and BSAs, and prediction of hydrochemical characteristics extremely difficult. The micaceous GSAs occasionally have peat lenses composed of decomposed OM derived from plant matter. These organic-rich layers were deposited as water-logged back-channel deposits (Donselaar et al., 2016), and they play an important role in driving sub-surface microbial processes.
In this study, sediments were collected from two boreholes installed next to the two GSA wells, referred hereafter, as wells 28 (N 23 ° 55.064′, E 088 ° 33.350′) and 204 (N 23 ° 56.352′, E 088 ° 33.814′; Ghosh et al., 2014) in Karimpur II block located in Nadia district, West Bengal (Figure 1). Arsenic concentration in these wells ranged from 64 to 131 μg/l and microbial communities in the groundwater were associated with biogeochemical cycling of As and Fe (Ghosh et al., 2014). Well 28 is located in the backyard of a farmer's house and the groundwater from this well is used as a potable supply for drinking water in the household. Well 204 is located adjacent to agricultural fields, and groundwater is frequently pumped from this well for irrigating crops (rice and seasonal vegetables).
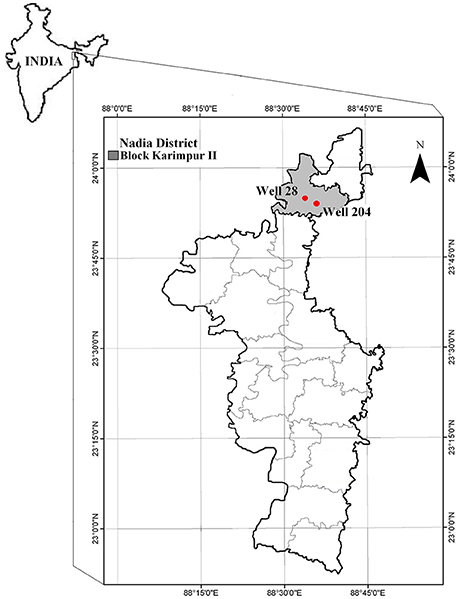
Figure 1. Location of the gray sand aquifer wells 28 and 204 in Karimpur II block, Nadia district, West Bengal (India).
Methods
Sample Collection
In April 2011, sediment samples were recovered by drilling shallow boreholes using the conventional hand percussion and reversed circulation technique. This method allows continuous recovery of drilled material, and is widely practiced for coring shallow wells in the district. Sediment samples from both boreholes were collected from the surface to 50 m depth at every 2 m interval up to 20 m, and then at every 4 m interval up to 50 m. The samples from well 28 were named as 28–1 to 28–50 (where the second numerical identifier denoted the sediment depth in m). Samples from well 204 were named in similar way as 204–1 to 204–50. The samples were stored in individual zip-lock polyethylene bags, and stored at 4 °C until further analysis.
TOC Analysis
The carbonates were removed by pre-treating the sediment samples with HCl following the method proposed by Brodie et al. (2011). The percent abundance of TOC in pre-treated sediment samples were determined in a Liquid TOC analyzer (Elementar Analysensysteme GmbH) with an IR detector by combusting the samples at 1,000 °C. The instrument was calibrated using the Canadian Stream Sediment (STSD-1).
Sedimentology
The sediments were wet-sieved through a 250 and 63 μm mesh sieves using 0.05 % of Na3PO4 solution and sonicated in a Vibracell CV334 (Sonics and Materials Inc.). The grain-size distribution in terms of % sand, silt, and clay in the suspension was determined on a SediGraph enabled with an auto-sampler Mastertech 52 (Micrometrics).
Extraction of Trace Metals
The sequential extraction BCR protocol proposed by the Standards, Measurement, and Testing Program (formerly the Community Bureau of Reference) of the European Commission (Quevauviller et al., 1997; Rauret et al., 2000; Table S1) was followed. A modified three-step extraction procedure of the original BCR method was used (Routh and Hjelmquist, 2011; Ghosh et al., 2015b). For the first fraction (Fraction 1), 20 ml of 0.11 M acetic acid was mixed with 0.5 g of freeze-dried sample and shaken for 16 h at 50 rpm. The sample was centrifuged at 3,000 rpm for 20 min and the supernatant acid phase was removed. Fraction 2 was extracted using 20 ml of 0.5 M hydroxylamine hydrochloride at pH 1.5 for 16 h at 50 rpm. The supernatant was removed after centrifuging for 20 min. Fraction 3 was extracted using 5 ml of 8.8 M hydrogen peroxide for 1 h, and then heated at 85 °C for 1 h. The volume lost due to evaporation at 85 °C was readjusted. The extract was treated with 25 ml of 1.0 M ammonium acetate at pH 2.0 and then shaken for 16 h at 50 rpm; the supernatant was removed at the end. After each step, the sample was washed with 10 ml of deionized distilled water. The residual metals were extracted by subjecting the sample to pseudo-total digestion by treating it with 10 ml of 7 M HNO3 at 100 kPa and 121 °C for 30 min (SIS, 1993). The extraction efficiency has been reported using the certified standard reference for sediments CRM-601. All the extracted fractions (named as fraction 1–3) were analyzed on a PerkinElmer NexION 300D ICP-MS. The detection limits for the instrument are detailed in Table S2.
Lipid Extraction
All the 38 samples (19 from each borehole) were freeze-dried and 10 g of each sample was used for extracting the lipids. The samples were extracted using a mixture of CH2Cl2 and CH3OH (9:1 v/v) using the Dionex ASE 300 at 100 °C for 1 h and another 1 h cycle at 140 °C. The recovery standard (n-hexatriacontane-d50) was added before extraction. The total lipid extracts (TLE) were separated into two fractions using column chromatography. The 6 mL glass columns were packed with 500 mg LC-NH2 (Kim and Salem, 1990), and eluted with DCM/isopropanol (2:1, v/v; 15 ml; neutral fraction) and 2 % acetic acid in diethyl ether (15 mL; acid fraction). The neutral fractions were further separated using column chromatography (Agilent Bond Elut® AL-N 500 mg, 3 mL) with hexane (5 mL; non-polar fraction) and DCM/MeOH (1:1 v/v; 5 mL; polar fraction). The polar fractions were dissolved in 100 μL bis(trimethylsilyl) trifluroacetamide (BSTFA) and 100 μL of pyridine, and heated (at 70 °C for 120 min) to convert alkanols into trimethylsilyl ethers. The acid fractions were derivatized with bromotriflouride (BF3) in MeOH and heated (at 70 °C for 120 min) to convert the acids into their corresponding methyl esters, and these were extracted with NaCl and hexane. To ensure that contamination was not introduced during the extraction procedure, blanks were prepared in the same way. All fractions were analyzed by gas chromatography mass spectrometry (GC–MS) after adding the necessary internal standards namely deuterated tetracosane and androstane to the neutral lipids, and deuterated eicosinoic acid to the fatty acid fraction, respectively.
Gas Chromatography–Mass Spectrometry (GC–MS)
An Agilent 6890N GC interfaced to an Agilent 5975C MSD mass spectrometer operating under electronionization mode at 40 eV and scanning from m/z 40 to 600 at 2.62 scans/s was used. The samples were dissolved in hexane prior to injection, and were injected using the split-less injection mode, and separated using a HP-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm film). The samples were run at constant flow (1.3 ml/min) with He as carrier gas. The interface temperature was set at 300 °C, the mass source at 230 °C and the MS quadrupole at 150 °C. The samples were injected at 35 °C and the oven was programmed to 130 °C at 20 °C/min and then at 6 °C/min to 320 °C, and held isothermally for 15 min. The compounds were identified by comparing the retention time and elution order in standards, and mass spectra published in the literature (Peters et al., 2005, and online libraries from NIST and the Lipid library). Detection limit in the different standards ranged from 0.1 to 1 ng/g. Reproducibility of internal standards was in the range of ±3.5–7.0 % for different compounds.
Results
Sedimentology
The sediment profile in wells 28 and 204 consisted of fining-upward stacks of coarse to fine sand, silt and clay (Figures 2, 3). A layer of clay and silty sediments (6–10 m thick) topped the boreholes. The rest of the core predominantly consisted of fine to coarse micaceous gray sand. The coarse gray sand layers contained grains of diameter up to 2 mm. Some clay lenses were found in between 15 and 19 m in well 28 and between 30 and 34 m in well 204, respectively.
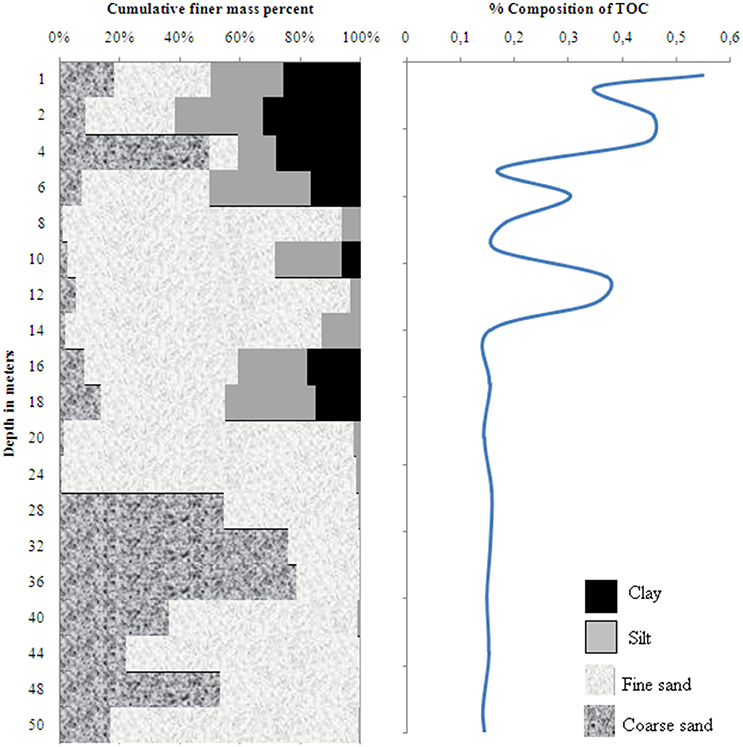
Figure 2. Sediment grain size and distribution of total organic carbon in well 28 in Karimpur II block, Nadia district, West Bengal (India).
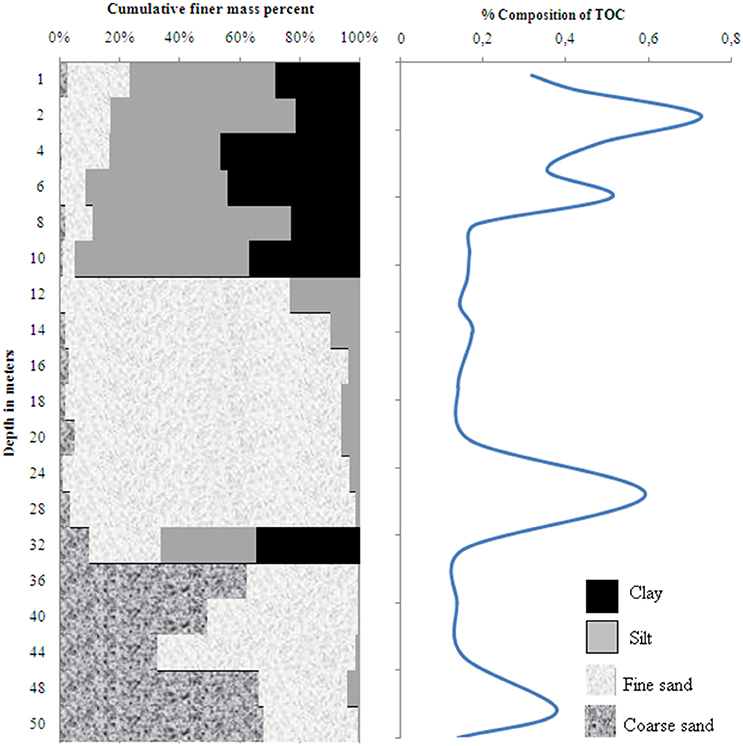
Figure 3. Distribution of sediment grain size and total organic carbon in well 204 in Karimpur II block, Nadia district, West Bengal (India).
Distribution of Trace Metals
The standard CRM-601 was used along with the BDP samples to check the reliability of the method for sequential extraction of metals. We selected six trace elements (Cd, Cr, Cu, Ni, Pb, and Zn) from each fraction and for the pseudo-total extraction as reported in the original BCR method (López-Sánchez et al., 1998). The concentration of these trace elements was detected in the different fractions in CRM-601, and compared with the half width at 95 % confidence interval of the BCR method (Table S3). It was observed that the BCR extraction scheme was only partially reliable, and similar to other studies from the BDP region (Routh and Hjelmquist, 2011; Ghosh et al., 2015b).
Samples from the top and intermittent clay-rich sediment lenses had the highest concentration for most elements (As, Fe, Mn, Ca, Mg, and P). Arsenic was mainly associated with the residual fraction in both cores. The total As concentration in sediment samples in well 28 varied between 1.88 and 29.6 mg/kg, whereas in well 204 concentrations varied between 2.04 and 41.8 mg/kg (Figure 4). Arsenic was high in fraction 2 and concentrations ranged from 0.25 to 1.56 mg/kg in well 28 and 0.24 to 2.26 mg/kg in well 204, respectively. Arsenic extracted in other fractions varied [e.g., As content ranged from 0.02 to 0.72 mg/kg in well 28 (Figure 4) and 0.01 to 1.72 mg/kg in well 204 (Figure 5)].
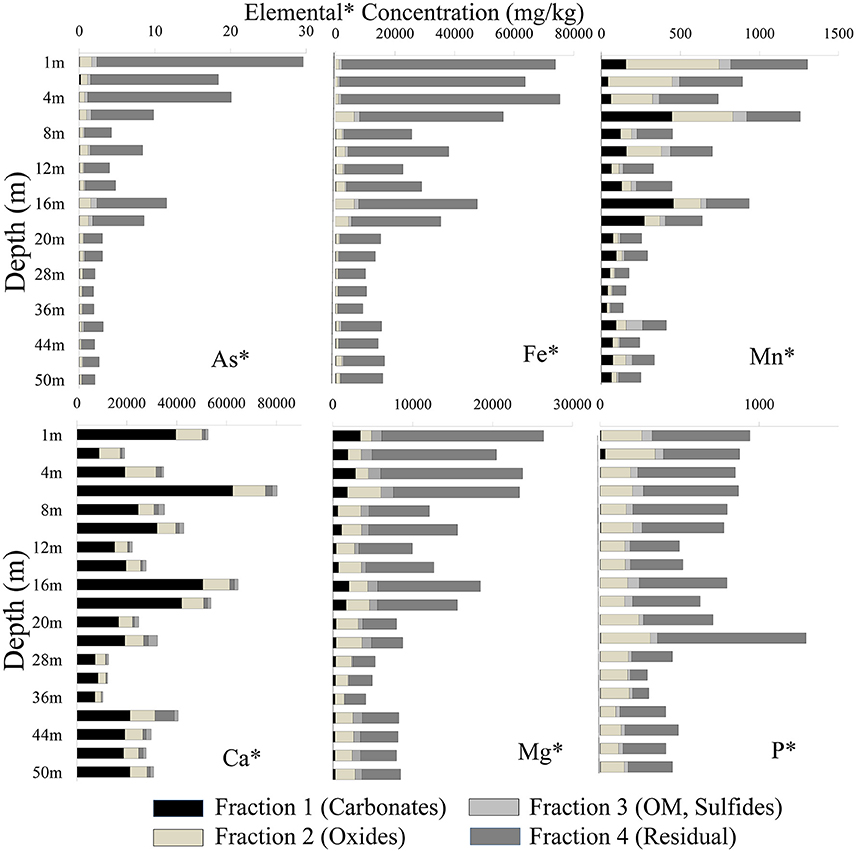
Figure 4. Distribution of different elements in four fractions using the BCR extraction protocol (Quevauviller et al., 1997) in well 28 in Karimpur II block, Nadia district, West Bengal (India).
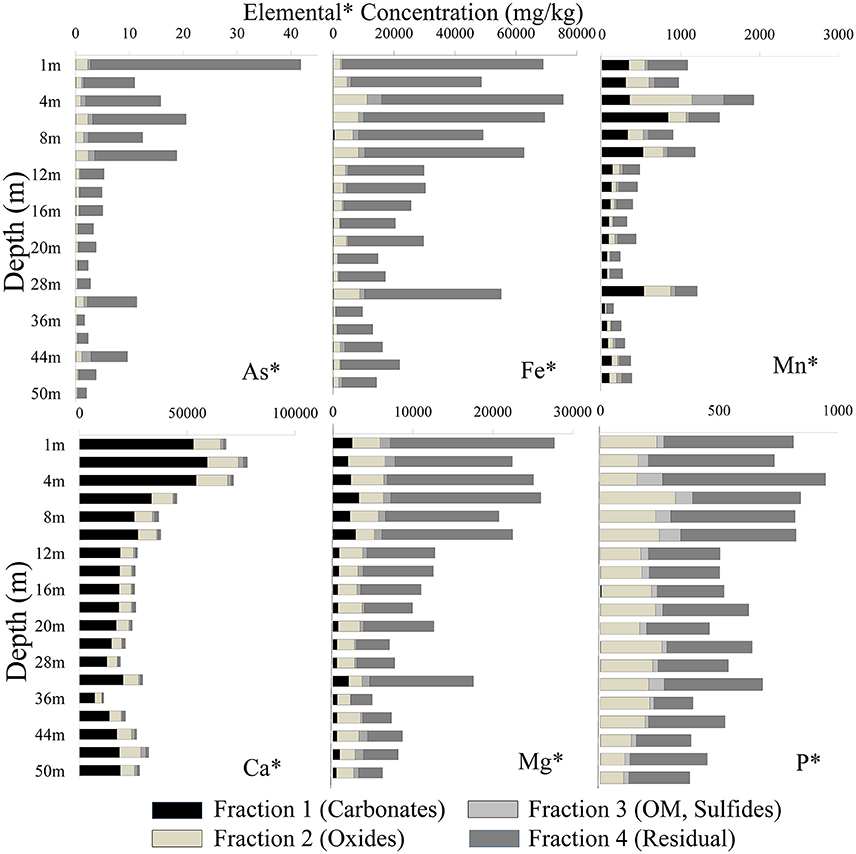
Figure 5. Distribution of different elements in four fractions using the BCR extraction protocol (Quevauviller et al., 1997) in well 204 in Karimpur II block, Nadia district, West Bengal (India).
The Fe concentration in these sediments was high. Fe was mainly associated with fractions 2 and 4. In well 28, the total Fe concentration varied from 9,188 to 75,360 mg/kg and in well 204, Fe varied from 9,675 to 75,501 mg/kg. The total Mn content varied from 1.88 and 29.6 mg/kg in well 28 and 147 to 1,924 mg/kg in well 204. Manganese primarily occurred in fractions 1 and 2. The total P concentration in sediments varied between 296 and 1,295 mg/kg (Figure 4), and mainly occurred in fractions 2 and 4. Calcium was dominant in fraction 1. The Ca content varied from 7,237 and 62,526 mg/kg in fraction 1 in well 28 and 1,759 to 59,238 mg/kg in well 204. The total Mg concentration in sediments varied between 4,130 to 26,396 mg/kg in well 28 and 4,891 to 27,691 mg/kg in well 204.
Distribution of Organic Matter
The TOC content in wells 28 and 204 varied from 0.14 to 0.73 % and 0.14 to 0.55 %, respectively; the clayey layers had higher TOC-values than the sands. OM analyses in the sediments indicated specific compound classes as discussed below.
n-Alkanes
The total n-alkane concentration in well 28 (0.16–7.58 ng/mg) is comparatively lower than well 204 (4.10–28.5 ng/mg). The n-alkane concentrations showed a steady decline with depth in well 28, but this trend was not evident in well 204 (Figure 6). In the chromatograms, a characteristic hump (rising baseline) was observed indicating the presence of an unresolved complex mixture (UCM) derived from petroleum hydrocarbons in samples (Peters et al., 2005). Based on the distribution of different n-alkanes various ratios were calculated to characterize the OM.
1) Carbon preference index (CPI) including n-C23 to n-C32 alkanes (Allan and Douglas, 1977) were reported as CPITOT (n-C13 to n-C35), CPILMW (n-C13 to n-C21), and CPIHMW (n-C23 to n-C35).
where, TOT = total, LMW = low molecular weight, HMW = high molecular weight n- alkanes.
In well 28, CPITOT ranged from 0.55 to 3.01, CPILMW ranged between 0.49 to 1.87 and CPIHMW ranged from 0.32 to 4.63. In well 204, CPITOT ranged from 0.43 to 4.19, CPILMW ranged from 0.13 to 1.26 and CPIHMW ranged from 0.22 to 5.52.
2) The Terrigenous/aquatic ratio (TAR) proposed by Bourbonniere and Meyers (1996), was used to estimate the terrigenous vs. aquatic derived OM inputs.
TAR ranged from 1.06 to 33.3 in well 28, and in well 204 TAR ranged from 0.60 to 33.3.
3) Average Chain Length (ACL) proposed by Cranwell et al. (1987) to describe the type of vegetation and environmental factor was calculated.
where n = 25 and m = 33.
The ACL in GSA wells 28 and 204 ranged from 28 to 31 and 26 to 30, respectively.
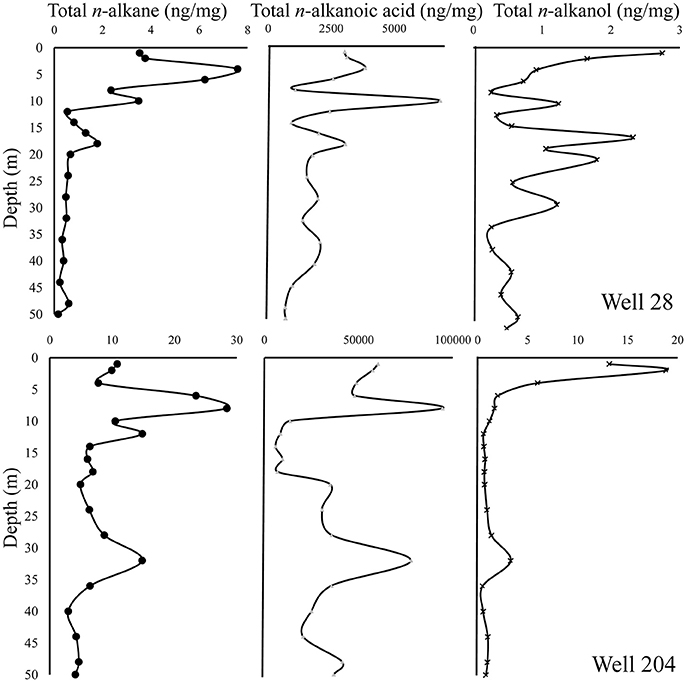
Figure 6. Distribution of n-alkanes, n-alkanols, and n-alkanoic acids in the gray sand aquifer wells 28 and 204 in Karimpur II block, Nadia district, West Bengal.
n-Alkanoic Acids
The total n-alkanoic acid concentration was >99 % of the total lipid concentration in both wells. The total n-alkanoic acid concentration in well 28 and 204 ranged from 670 to 6,823 and 5,920 to 94,535 ng/mg, respectively (Figure 6). To trace the different OM sources and its fate (i.e., degradation or preservation) in these sediments, various lipid ratios were calculated based on the different n-alkanoic acid monomers.
1) The Carbon Preference Index proposed by Matsuda and Koyama (1977) was modified by dividing this ratio into low molecular weight CPIL (C12 to C18) and high molecular weight CPIH (C22 to C32) ratios.
In well 28, CPIL ranged from 4.78 to 82.1 and CPIH ranged from 0.66 to 47.5. In well 204, CPIL ranged from 1.47 to 20.5 and CPIH ranged from 1.51 to 3.93.
2) The terrigenous: aquatic fatty acid ratio (TARFA) proposed by Meyers (1997), was calculated.
The TARFA-value in well 28 ranged from 0.05 to 1.57 and in well 204 TARFA ranged from 0.03 to 0.79.
n-Alkanol
The n-alkanol concentration in sediments from well 28 ranged from 0.23 to 2.74 ng/mg, whereas in well 204 the concentration varied from 0.16 to 18.8 ng/mg (Figure 6). To study the preservation potential of OM, the ratio of alkanes over alkanols referred to as the Higher Plant Alkane index (Westerhausen et al., 1993) was calculated.
The HPA-value in well 28 ranged up to 0.64 and in well 204 the value ranged up to 0.23.
Discussion
The GSAs and BSA sin Nadia district are randomly present in close vicinity of each other (Biswas et al., 2012). These aquifers have strong heterogeneity in their physical and chemical properties because of depositional conditions in deltaic environments, which resulted in rapid changes in marine transgression along with mechanical and chemical weathering (Goodbred and Kuehl, 2000; McArthur et al., 2004). The micaceous GSAs in Karimpur are lean in OM, but they occasionally have peat lenses in between the sediment layers. The peat lenses consist of mostly decomposed OM derived from plant matter.
Biogeochemical Characteristics of GSAs
Inorganic Geochemical Characteristics of GSAs
The total As concentration in Karimpur is within the range for BDP sediments, which varies from 0.40 to 40 mg/kg (Chakraborty et al., 2015 and references therein). However, As concentration in Karimpur is higher than the global average for unconsolidated sediments, which is around 2.00 to 7.00 mg/kg (Smedley and Kinniburgh, 2002). It is most likely that the high As concentrations in aquifers result from ongoing biogeochemical reactions in the sub-surface that mobilize As (Biswas et al., 2012; Chakraborty et al., 2015; Ghosh et al., 2015b). The total As and Fe concentrations were high in surface clay layers and in between clay plugs, but this trend steadily decreases with depth. This trend has been related to the irrigation return flow in the BDP sediments (Meharg and Rahman, 2003). It is also likely that ferric oxide and clay minerals that are abundant in surface sediments sorb As, and influence its distribution in the sub-surface (Chakraborty et al., 2015). Consistent with this, at both sites a bivariate correlation of As with several elements was observed. The statistical significance between the elements in samples from well 28 is detailed in Table 1. In fraction 1, As has significant correlation with P. In fraction 2, As has significant correlation with Fe, Mn, P, Ni, Cu, Zn, Pb, Cd, Na, Mo, Cr, and V. This relationship is expected because As correlates with various redox sensitive elements (Biswas et al., 2012). In fraction 3, As has significant correlation, particularly with Ca and Mg. These elements occur as sulfidic minerals or they are associated with OM. In fraction 4, As has significant correlation with all the elements that also have significant correlation in fraction 2.
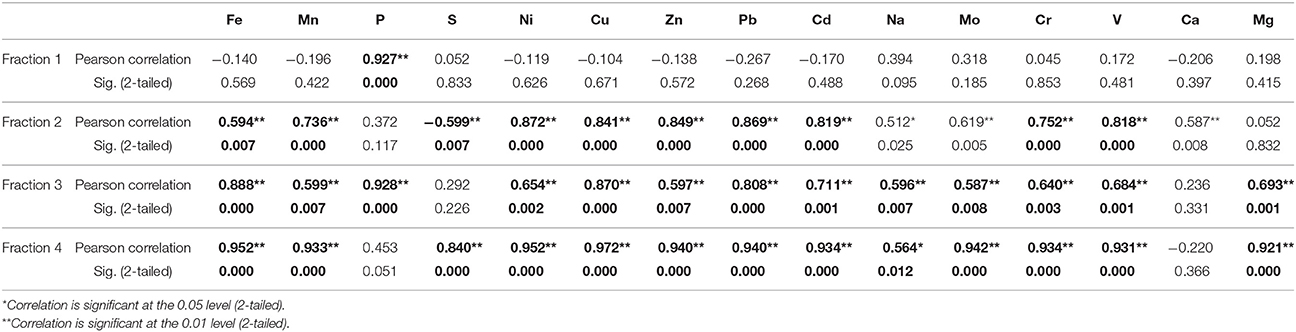
Table 1. Correlation of As with other trace elements in four different fractions from sediments in well 28 in Karimpur II block, Nadia district, West Bengal (India).
In well 204, a similar trend was observed in the distribution of various elements; significance levels for the elemental distributions are detailed in Table 2. In fraction 1, As has significant correlation with only V. In fractions 2 and 3, As has significant correlation with Fe, Mn, P, Ni, Cu, Zn, Pb, Cd, Na, Mo, Cr, and V. In fraction 4, As has significant correlation with all elements except Ca. In both wells, As has significant correlation with Fe, which suggests presence of Fe-hydroxy bound As minerals (Routh and Hjelmquist, 2011; Biswas et al., 2012; Ghosh et al., 2015b). The presence of Mn and Ca occurs mainly in fraction 1, indicating carbonate minerals to be an important component in the BDP sediments. The sequential extraction suggests that P and Mg are mainly present as carbonate and silica bound minerals, and they are suggested to play a key role in uptake of As in rice cultivated in the BDP region (Seyfferth and Fendorf, 2014).
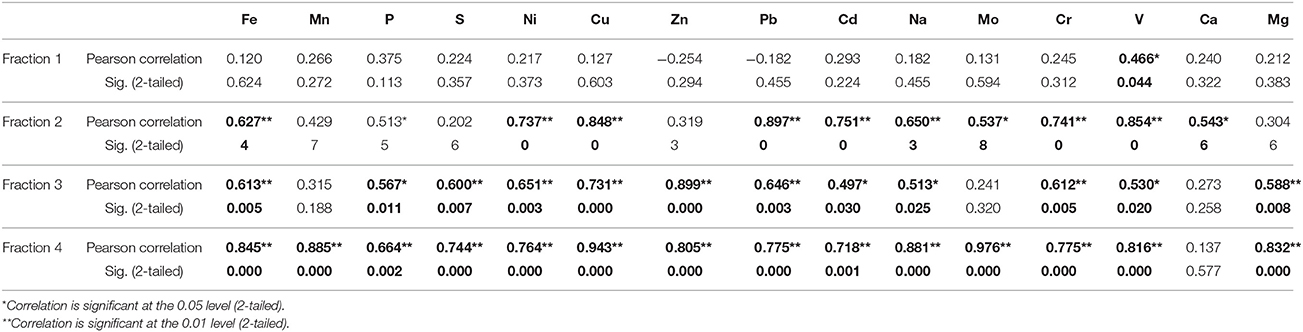
Table 2. Correlation of As with other trace elements in four different extracted fractions in sediments from well 204 in Karimpur II block, Nadia district, West Bengal (India).
Organic Geochemical Characteristics of GSAs
Distribution of different biomarkers (n-alkanes, n-alkanols, and n-alkanoic acids) in the sediment profiles show a striking correspondence with groundwater DOC characteristics in Karimpur (Ghosh et al., 2015a). The sediments indicate predominance of HMW n-alkanes that are of terrestrial origin. In addition, well 204 shows presence of various LMW n-alkanes resulting in a bimodal distribution pattern. A similar bimodal distribution pattern has been observed in the DOC fraction in this well (Ghosh et al., 2015a), suggesting input of surface derived OM and/or mixing of surface and in-situ sedimentary OM (Harvey et al., 2002; Mukherjee and Fryar, 2008). The bimodal pattern can also arise from degradation of complex OM sources in the deeper sediments. Consistent with this, both wells indicate microbial reworking of sedimentary OM like other deltaic aquifers in the BDP (Rowland et al., 2006; Héry et al., 2010) and southeast Asia (van Dongen et al., 2008; Lawati et al., 2012), and is further discussed below.
The n-alkane pattern in both wells suggests degradation of sedimentary OM (Figure 6). The abundance of LMW n-alkanes, which is less compared to the HMW n-alkanes suggests poor preservation of sedimentary OM (Rowland et al., 2006; Lawati et al., 2012) in these sediments. These LMW hydrocarbons can be transported from organic-rich clay sediments into aquifer sands as demonstrated in groundwater aquifers (Routh et al., 2001), and could support microbial processes associated with As cycling. The odd over even predominance of n-alkanes indicated by CPITOT shows there is higher plant derived input in the clay and silt-rich layers. Consistent with this, the TAR-values correspond well with the CPI trend indicating the dominance of higher plant derived input. Likewise, the ACL-value indicates there is a predominance of terrestrial higher plant derived OM input (Cranwell et al., 1987) into these sediments.
The abundance of monounsaturated n-alkanoic acids C16 and C18 in both wells is unspecific in terms of its diagnostic sources i.e., OM derived from marine/terrigenous bacteria, animals or plants. These LMW n-alkanoic acids are molecular components of microbial cell membrane (Bianchi and Canuel, 2011). In contrast, the abundance of long chain n-alkanoic acids
(C24 to C30) in sediment extracts indicates OM input derived from epicuticular leaf wax of vascular plants (Eglinton and Hamilton, 1967; Bianchi and Canuel, 2011). Consistent with this, the extracts indicate high TARFA-values implying higher plant derived input in deeper samples. The predominance of C18 unsaturated alkanoic acids suggests algal as well as higher plant inputs. Among the LMW n-alkanoic acids, the predominance of even-over-odd suggests microbial reworking of sedimentary OM in aquifer sediments (Bianchi and Canuel, 2011).
The abundance of n-C22 alkanol indicates presence of suberin (Bull et al., 2000), Eustigmatophytes (Volkman et al., 1999), and phototrophic marine and fresh water microalgae (Jaffé et al., 2001) as potential sources. The typical signatures of plant derived n-C29-alkanol is however very lean in these sediments, which implies that the abundance of n-C22-alkanol is most likely due to microbial degradation (Volkman et al., 1999). In addition, the HPA index is >1 in clay-rich samples, indicating the preferential preservation of n-alkanes over n-alkanols because of their lower reactivity (Westerhausen et al., 1993).
Previous studies have indicated the presence of petroleum-derived thermally mature hydrocarbons in GSAs (based on the presence of UCM and specific compounds; Rowland et al., 2006; Héry et al., 2010). In addition, we have indicated signature of petroleum degrading microbial communities (Ghosh et al., 2014) and microbially reworked petroleum hydrocarbons (LMW-UCM) in the DOC fraction (Ghosh et al., 2015a). These compounds are suggested to affect microbial reductive dissolution of As (Rowland et al., 2006; Paul et al., 2015). The n-alkane chromatograms from wells 28 and 204 indicate the distinct presence of UCM (Figure 7) in these samples suggesting the occurrence of petroleum-derived hydrocarbons in GSAs. It is likely that specific OM sources in GSA influence the growth of microbial communities that undertake an active role in biogeochemical cycling of As in the organic-rich clay plugs. The presence of such hydrocarbons and microbial communities are notably absent in the brown sand aquifers (Ghosh et al., 2014, 2015b). This is an important distinction between the GSA and BSA wells, and implies causality between OM sources and sub-surface microbial communities and processes associated with mobilization of As, and the safety of drinking water resources. Such biomarker signatures have also recently been reported from the other As-contaminated aquifers of SE Asia, indicating their critical role in As-mobilization (Magnone et al., 2017). This inference however, needs further investigations involving microbial degradation of OM in sediments on similar lines as established in DOC extracted from these wells (Ghosh et al., 2015a), using tracer compounds in laboratory and in situ measurements of degradation products and As levels in the microbial cultures.
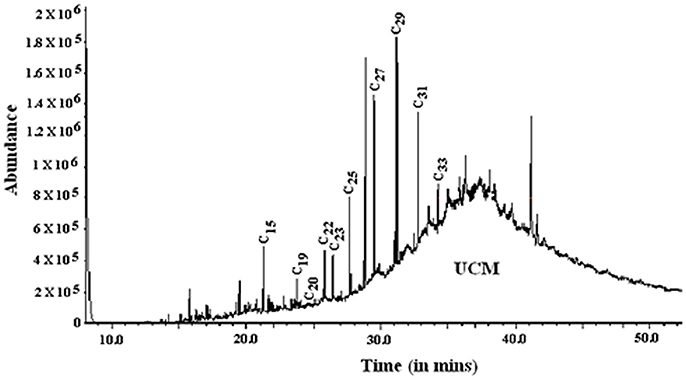
Figure 7. The ion chromatogram (m/z 57) for lipid extracts indicating presence of n-alkanes (C15-C33) and the unresolved complex mixture (UCM). UCM indicates presence of petroleum-derived hydrocarbons in the lipid extract.
Arsenite oxidizing petroleum degrading bacteria such as Polymorphum gilvum and Acinetobacter sp. are dominant in the GSA groundwater, and laboratory experiments indicate consumption of DOC by microorganisms isolated from GSA groundwater (Ghosh et al., 2014, 2015a). These trends suggest that indigenous microbial communities in GSAs are involved in As cycling, and microorganisms utilize the OM present in aquifer sediments. Likewise, Islam et al. (2004) reported Clostridium sp., which are capable of OM degradation coupled to As reduction in the BDP sediments. These studies are consistent with earlier claims by McArthur et al. (2004) that reductive dissolution of iron(oxy)-hydroxides would be readily triggered in presence of OM in aquifer sediments. They cite that elevated As levels in BDP aquifers correlate with high concentrations of metabolic by-products such as dissolved inorganic C, CH4, , and ions. Likewise, Routh and Hjelmquist (2011) claim that the unexpectedly high As(III) species in the BDP sediments is not only a result of redox conditions in the sub-surface, but is partly related to microbial reduction of As(V) in presence of OM.
Arsenic Cycling and Distribution
von Brömssen et al. (2008) and others suggested that in the Pleistocene BSAs, As levels are low because of the oxidized nature of these sediments (As is sequestered to the iron oxy- hydroxides), enhanced groundwater flushing, and the refractory nature of OM in aquifer sediments. In contrast waterlogged anoxic conditions and negligible flushing of groundwater in the GSAs promoted development of strong reducing conditions that coincided with rapid sea-level rise in the Bengal basin ca. 10–5 ka BP (Anwar et al., 2010). These conditions also facilitated formation of petroleum and natural gas deposits in these deltaic sediments (Alam, 1989; Ganguly, 1997; Milici et al., 2002). It is likely the presence of such hydrocarbon sources in GSAs play a crucial role in shaping the microbial community structure and functions. The absence of such petroleum-derived OM in sediments is a characteristic difference between the BSA (Ghosh et al., 2015b) and GSA sites (present study). Likewise, DOC samples characterized indicate differences in the presence/absence of petroleum-derived hydrocarbons in the aquatic phase (Ghosh et al., 2015a). The absence of these petroleum-derived OM sources in BSAs have perhaps kept them relatively safe besides other hydrochemical conditions (e.g., redox conditions) as suggested in previous studies (Harvey et al., 2002; McArthur et al., 2004; van Geen et al., 2014). Recently, Donselaar et al. (2016) suggested that delta fronts consisting of multiple fluvial point-bars surrounded by clay-plugs in meander bends eventually evolve into ox-bow lakes that are the loci for microbially-mediated reductive dissolution of iron oxy-hydroxides. This inference was supported by the geochemical characteristics in ox-bow lakes and their associated aquifers (Ghosh et al., 2017). The proposed geomorphologic evolution of the landscape by Donselaar et al. (2016) casts a wider net when it comes to new areas that will be vulnerable to As contamination in the near future in these rapidly changing deltaic environments.
The GSA and BSA aquifers often lie in proximity, and there is evidence for mixing of groundwater between these aquifers (Mukherjee and Fryar, 2008; Biswas et al., 2012). Thus, intensive pumping of groundwater has led to infiltration of DOC from surface (e.g., pond bottoms) to greater vertical and horizontal extents in the BDP aquifers (Mukherjee and Fryar, 2008; Lawson et al., 2013). The infiltration of shallow groundwater could lead to transfer of both the reactive OM fraction and specific microbial communities into the “As safe” BSAs, and affect remobilization of As. Since natural selection comes with adaptation mechanisms; microbes will adapt, and thereby increase the vulnerability of BSAs to As contamination in future.
Conclusions
In this study, sediment cores were retrieved from two shallow GSA wells in the Karimpur Block in Nadia district. Metal analyses involving the BCR method and different lipid fractions were investigated in the cores. The sediments indicated high As concentrations; As correlated well with Fe and other redox metals. Plant derived OM inputs dominated in both sediment cores. The clay-rich sediments had higher OM content consisting mainly of HMW n-alkanes, even-chain alkanoic acids and n-C22-alkanols. Petroleum-derived hydrocarbons were present in these sediments along with petroleum degrading microbial communities that were involved in As mobilization in the aquifers. Redox conditions along with OM and in-situ microbial communities in the GSA contribute to the high As levels in groundwater, and its elevated vulnerability to the As crisis.
Author Contributions
Fieldwork was led by JR and DG. Experimental design and lab work was led by DG and JR. DG wrote the paper with contributions from JR and PB in data interpretation and writing.
Funding
Funding was provided by the Swedish Research Link-Asia Program (Grant 2009-6470). DG thanks DST, Government of India for providing the INSPIRE fellowship.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the Public Health Engineering Department, Government of West Bengal for providing the background information on these sites. Apurba Mandal helped with drilling the boreholes. We thank the local villagers for their hospitality. We are grateful to Susanne Karlsson and Mårten Dario for helping us in the laboratory.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2017.00082/full#supplementary-material
References
Acharyya, S. K., Lahiri, S., Raymahashay, B. C., and Bhowmik, A. (2000). Arsenic toxicity of groundwater in parts of the Bengal basin in India and Bangladesh: the role of Quaternary stratigraphy and Holocene sea-level fluctuation. Environ. Geol. 39, 1127–1137. doi: 10.1007/s002540000107
Ahmed, K. M., Bhattacharya, P., Hasan, M. A., Akhter, S. H., Alam, S. M. M., Bhuyian, M. A. H., et al. (2004). Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: an overview. Appl. Geochem. 19, 181–200. doi: 10.1016/j.apgeochem.2003.09.006
Alam, M. (1989). Geology and depositional history of Cenozoic sediments of the Bengal basin of Bangladesh. Palaeogeogr. Palaeoclimatol. Palaeoecol. 69, 125–139. doi: 10.1016/0031-0182(89)90159-4
Allan, J., and Douglas, A. G. (1977). Variations in the content and distribution of n-alkanes in a series of Carboniferous vitrinites and sporinites of bituminous rock. Geochim. Cosmochim. Acta 41, 1223–1230. doi: 10.1016/0016-7037(77)90068-0
Anwar, H. M., Yoshioka, T., Konohira, E., Akai, J., Freitas, M. C., and Tareq, S. M. (2010). Sources of organic carbon and depositional environment in the Bengal delta plain sediments during the Holocene period. Limnology 11, 133–142. doi: 10.1007/s10201-009-0301-9
Arguello, R. A., Cenget, D. D., and Tello, E. E. (1938). Cancer and endemic arsenism in the Cordoba region. Rev Argent Dermatosifiorg 22, 461–487 (in Spanish).
Ayotte, J. D., Montgomery, D. L., Flanagan, S. M., and Robinson, K. W. (2003). Arsenic in groundwater in eastern New England: occurrence, controls, and human health implications. Environ. Sci. Technol. 37, 2075–2083. doi: 10.1021/es026211g
Ballantyne, J. M., and Moore, J. N. (1988). Arsenic geochemistry in geothermal systems. Geochim. Cosmochim. Acta 52, 475–483. doi: 10.1016/0016-7037(88)90102-0
Bhattacharya, P., Ahmed, K. M., Hasan, M. A., Broms, S., Fogelström, J., Jacks, G., et al. (2006). “Mobility of arsenic in groundwater in a part of Brahmanbaria district, NE Bangladesh,” in Managing Arsenic in the Environment: from Soils to Human Health, eds R. Naidu, E. Smith, G. Owens, P. Bhattacharya, and P. Nadebaum (Collingwood, VIC: CSIRO Publishing), 95–115.
Bhattacharya, P., Chatterjee, D., and Jacks, G. (1997). Occurrence of arsenic contaminated groundwater in alluvial aquifers from Delta Plains, Eastern India: options for safe drinking water supply. Int. J. Water Resour. Manage. 13, 79–82. doi: 10.1080/07900629749944
Bhattacharya, P., Jacks, G., Ahmed, K. M., Routh, J., and Khan, A. A. (2002). Arsenic in groundwater of the Bengal Delta Plain aquifers in Bangladesh. Bull. Environ. Contam. Toxicol. 69, 538–545. doi: 10.1007/s00128-002-0095-5
Bianchi, T. S., and Canuel, E. A. (2011). Chemical Biomarkers in Aquatic Ecosystem. Oxfordshire: Princeton University Press.
Biswas, A., Nath, B., Bhattacharya, P., Halder, D., Kundu, A. K., Mandal, U., et al. (2012). Hydrogeochemical contrast between brown and grey sand aquifers in shallow depth of Bengal Basin: consequences for sustainable drinking water supply. Sci. Tot. Environ. 431, 402–412. doi: 10.1016/j.scitotenv.2012.05.031
Bourbonniere, R. A., and Meyers, P. A. (1996). Sedimentary geolipid records of historical changes in the watersheds and productivities of Lakes Ontario and Erie. Limnol. Oceanogr. 41, 352–359. doi: 10.4319/lo.1996.41.2.0352
Brodie, C. R., Casford, J. S. I., Lloyd, J. M., Leng, J., Heaton, T. H. E., Kendrick, C. P., et al. (2011). Evidence for a bias in C/N, δ13C and δ15N values of bulk organic matter, and on environmental interpretation from a lake sedimentary sequence by pre-analysis acid treatment methods. Quat. Sci. Rev. 30, 3076–3087. doi: 10.1016/j.quascirev.2011.07.003
Bull, I. D., van Bergen, P. F., Noh, C. J., Poulton, P. R., and Evershed, R. P. (2000). Organic geochemical studies of soil from Rothamsted classical experiment-V. The fate of lipids in different long-term experiments. Organ. Geochem. 31, 389–408. doi: 10.1016/S0146-6380(00)00008-5
Chakraborty, M., Mukherjee, A., and Ahmed, K. M. (2015). A Review of groundwater arsenic in the Bengal Basin, Bangladesh and India: from source to sink. Curr. Pollut. Rep. 1, 220–247. doi: 10.1007/s40726-015-0022-0
Cranwell, P. A., Eglinton, G., and Robinson, N. (1987). Lipids of aquatic organisms as potential contributors to lacustrine sediments. Organ. Geochem. 11, 513–527. doi: 10.1016/0146-6380(87)90007-6
Donselaar, M. E., Bhatt, A. G., and Ghosh, A. K. (2016). On the relation between fluvio-deltaic flood basin geomorphology and the wide-spread occurrence of arsenic pollution in shallow aquifers. Sci. Tot. Environ. 574, 901–913. doi: 10.1016/j.scitotenv.2016.09.074
Eglinton, G., and Hamilton, R. J. (1967). Leaf epicuticular waxes. Science 156, 1322–1335. doi: 10.1126/science.156.3780.1322
Ganguly, S. (1997). Petroleum geology and exploration history of the Bengal Basin in India and Bangladesh. Indian J. Geol. 69, 1–25.
Ghosh, D., Bhadury, P., and Routh, J. (2014). Diversity of arsenite oxidizing bacterial communities in arsenic-rich deltaic aquifers in West Bengal, India. Front. Microbiol. 5:602. doi: 10.3389/fmicb.2014.00602
Ghosh, D., Donselaar, M. E., and Routh, J. (2017). “Role of organic carbon of oxbow lakes in making aquifers vulnerable to arsenic mobilization,” in 28th International Meeting on Organic Geochemistry (IMOG-2017) (Florence).
Ghosh, D., Routh, J., and Bhadury, P. (2015a). Characterization and microbial utilization of dissolved lipid organic fraction in arsenic impacted aquifers (India). J. Hydrol. 527, 221–233. doi: 10.1016/j.jhydrol.2015.04.051
Ghosh, D., Routh, J., Dario, M., and Bhadury, P. (2015b). Elemental and biomarker characteristics in a Pleistocene aquifer vulnerable to arsenic contamination in the Bengal Delta Plain, India. Appl. Geochem. 61, 87–98. doi: 10.1016/j.apgeochem.2015.05.007
Goodbred, S. L. Jr., and Kuehl, S. A. (2000). The significance of large sediment supply, active tectonism and eustasy on margin sequence development: late Quaternary stratigraphy and evolution of the Ganges-Brahmaputra delta. Sed. Geol. 133, 227–248. doi: 10.1016/S0037-0738(00)00041-5
Harvey, C. F., Swartz, C. H., Badruzzaman, A. B., Keon-Blute, N., Yu, W., Ashraf, A. M., et al. (2002). Arsenic mobility and groundwater extraction in Bangladesh. Science 298, 1602–1606. doi: 10.1126/science.1076978
Héry, M., Van Dongen, B. E., Gill, F., Mondal, D., Vaughan, D. J., Pancost, R. D., et al. (2010). Arsenic release and attenuation in low organic carbon aquifer sediments from West Bengal. Geobiology 8, 166–168. doi: 10.1111/j.1472-4669.2010.00233.x
Islam, F. S., Gault, A. G., Boothman, C., Polya, D. A., Charnock, J. M., Chatterjee, D., et al. (2004). Direct evidence of arsenic release from Bengal sediments mediated by indigenous metal-reducing bacteria. Nature 430, 68–71. doi: 10.1038/nature02638
Jacks, G., Bhattacharya, P., and Khan, A. A. (2001). “Groundwater arsenic contamination in the Bengal Delta Plain of Bangladesh: KTH Special Publication,” in Proceedings of the KTH-Dhaka University Seminar (Stockholm).
Jaffé, R., Mead, R., Hermandez, M. E., Peralba, M. C., and Di Guida, O. A. (2001). Origin and transport of sedimentary and organic matter in two subtropical estuaries: a comparative biomarker based study. Organ. Geochem. 32, 507–526. doi: 10.1016/S0146-6380(00)00192-3
Kim, H. Y., and Salem, N. Jr. (1990). Separation of lipid classes by solid phase extraction. J. Lipid Res. 31, 2285–2289.
Lawati, W. M. A., Rizoulis, A., Eiche, E., Boothman, C., Polya, D. A., Lloyd, J. R., et al. (2012). Characterization of organic matter and microbial communities in contrasting arsenic-rich Holocene and arsenic-poor Pleistocene aquifers, Red River Delta, Vietnam. Appl. Geochem. 27, 315–325. doi: 10.1016/j.apgeochem.2011.09.030
Lawson, M., Polya, D. A., Boyce, A. J., Bryant, C., Mondal, D., Shantz, A., et al. (2013). Pond-derived organic carbon driving changes in arsenic hazard found in Asian groundwaters. Environ. Sci. Technol. 47, 7085–7094. doi: 10.1021/es400114q
López-Sánchez, J. F., Sahuquilloa, A., Fiedlera, H. D., Rubioa, R., Raureta, G., Muntaub, H., et al. (1998). CRM 601, A stable material for its extractable content of heavy metals. Analyst 123, 1675–1677. doi: 10.1039/a802720j
Magnone, D., Richards, L. A., Polya, D. A., Bryant, C., Jones, M., and van Dongen, B. E. (2017). Biomarker-indicated extent of oxidation of plant-derived organic carbon (OC) in relation to geomorphology in an arsenic contaminated Holocene aquifer, Cambodia. Sci. Rep. 7:13093. doi: 10.1038/s41598-017-13354-8
Matsuda, H., and Koyama, T. (1977). Early diagenesis of fatty acids in lacustrine sediments – I. Identification and distribution of fatty acids in recent sediment from a freshwater lake. Geochim. Cosmochim. Acta 41, 777–783.
McArthur, J. M., Banerjee, D. M., Hudson-Edwards, K. A., Mishrab, R., Purohit, R., Ravenscroft, P., et al. (2004). Natural organic matter in sedimentary basins and its relation to arsenic in anoxic ground water: the example of West Bengal and its worldwide implications. Appl. Geochem. 19, 1255–1293. doi: 10.1016/j.apgeochem.2004.02.001
Meharg, A. A., and Rahman, M. M. (2003). Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environ. Sci. Technol. 37, 229–234. doi: 10.1021/es0259842
Meyers, P. A. (1997). Organic geochemical proxies for paleoceanographic, paleolimnologic and paleoclimatic processes. Organ. Geochem. 27, 213–250. doi: 10.1016/S0146-6380(97)00049-1
Milici, R. C., Warwick, P. D., Attansai, E., and Wandrey, C. J. (2002). To sell or not sell: assessments of Bangladesh hydrocarbons. Oil Gas J. 100, 24–28. Available online at: https://www.scopus.com/record/display.uri?eid=2-s2.0-0037132004&origin=inward&txGid=fee0b5d77b256c84131fcc533232f815
Mukherjee, A., and Fryar, A. E. (2008). Deeper groundwater chemistry and geochemical modeling of the arsenic affected western Bengal basin, West Bengal. India. Appl. Geochem. 23, 863–894. doi: 10.1016/j.apgeochem.2007.07.011
Nickson, R. T., McArthur, J. M., Ravenscroft, P., Burgess, W. B., and Ahmed, K. Z. (2000). Mechanism of arsenic release to groundwater in Bangladesh and West Bengal. Appl. Geochem. 15, 403–413. doi: 10.1016/S0883-2927(99)00086-4
Oremland, R. S., and Stolz, J. F. (2005). Arsenic, microbes and contaminated aquifers. Trends Microbiol. 13, 45–49. doi: 10.1016/j.tim.2004.12.002
Paul, D., Kazy, S. K., Gupta, A. K., Pal, T., and Sar, P. (2015). Diversity, metabolic properties and arsenic mobilization potential of indigenous bacteria in arsenic contaminated groundwater of West Bengal, India. PLoS ONE 10:e0118735. doi: 10.1371/journal.pone.0118735
Peters, K. E., Walters, C. C., and Moldowan, J. M. (2005). The Biomarker Guide, Biomarkers in Isotopes Petroleum Exploration and Earth History, Vol. 2, 2nd Edn. Cambridge: Cambridge University Press.
Quevauviller, P., Rauret, G., López-Sánchez, J. F., Rubio, R., Ure, A., and Muntau, H. (1997). Certification of trace metal extractable contents in a sediment reference material (CRM 601) following a three-step sequential extraction procedure. Sci. Tot. Environ. 205, 223–234.
Rauret, G., López-Sánchez, J. F., Sahuquillo, A., Muntau, H., and Quevauviller, P. H. (2000). Indicative values for extractable contents (mass fractions) of Cd, Cr, Cu, Ni, Pb and Zn in sediment (CRM 601) following the modified BCR-Sequential Extraction (Three-step) Procedure (Addendum to Report 17554 EN) EUR 19502 EN. European Commission BCR Information Reference Materials, Luxembourg.
Ravenscroft, P., Brammer, H., and Richards, K. (2009). Arsenic Pollution – A Global Synthesis. Chichester: Wiley-Blackwell.
Ravenscroft, P., McArthur, J. M., and Hoque, B. A. (2001). “Geochemical and palaeohydrological controls on pollution of groundwater by arsenic,” in Arsenic Exposure and Health Effects IV, eds W. R. Chappell, C. O. Abernathy, and R. Calderon (Oxford: Elsevier Science Ltd.), 53–78.
Routh, J., Grossman, E. L., Murphy, E. M., and Benner, R. (2001). Characterization and origin of dissolved organic carbon in Yegua ground water in Brazos County, Texas. Ground Water 39, 760–767. doi: 10.1111/j.1745-6584.2001.tb02367
Routh, J., and Hjelmquist, P. (2011). Distribution of arsenic and its mobility in shallow aquifer sediments from Ambikanagar, West Bengal, India. Appl. Geochem. 26, 505–515. doi: 10.1016/j.apgeochem.2011.01.009
Rowland, H. A. L., Polya, D. A., Lloyd, J. R., and Pancost, R. D. (2006). Characterization of organic matter in a shallow, reducing, arsenic-rich aquifer, West Bengal. Organ. Geochem. 37, 1101–1114. doi: 10.1016/j.orggeochem.2006.04.011
Seyfferth, A. L., and Fendorf, S. (2014). Silicate mineral impacts on the uptake and storage of arsenic and plant nutrients in rice (Oryza sativa L.). Environ. Sci. Technol. 46, 13176–13183. doi: 10.1021/es3025337
SIS (1993). Swedish Standard Method SS 02 81 50. Vattenundersökningar – Bestämning av Metaller Med Atom Absorptions Spektrometri i Flamma – Allmänna Principer och Regler (Translatedtitle – Wateranalyses – Metalanalyses by Flameatomic Absorption Spectroscopy – Principles and Methods).
Smedley, P. L., and Kinniburgh, D. G. (2002). A review of the source, behavior and distribution of arsenic in natural waters. Appl. Geochem. 17, 517–568. doi: 10.1016/S0883-2927(02)00018-5
van Dongen, B. E., Rowland, H. A. L., Gault, A. G., Polya, D. A., Bryant, C., and Pancost, R. D. (2008). Hopane, sterane and n-alkane distributions in shallow sediments hosting high arsenic groundwaters in Cambodia. Appl. Geochem. 23, 3047–3058. doi: 10.1016/j.apgeochem.2008.06.012
van Geen, A., Win, K. H., Zaw, T., Naing, W., Mey, J. L., and Mailloux, B. (2014). Confirmation of elevated arsenic levels in groundwater of Myanmar. Sci. Tot. Environ. 15, 21–24. doi: 10.1016/j.scitotenv.2014.01.073
Westerhausen, L., Poynter, J., Eglinton, G., Erlenkeuser, H., and Sarnthein, M. (1993). Marine and terrigenous origin of organic matter in modern sediments of the equatorial East Atlantic: the δ13C and molecular record. Deep Sea Res. Part I 40, 1087–1121.
Volkman, J. K., Barret, S. M., and Blackburn, S. I. (1999). Eustigmatophyte microalgae are potential sources of C29 sterol, C22-C28 n-alcohols and C28-C32 n-alkyl diols in freshwater environments. Organ. Geochem. 30, 307–318.
von Brömssen, M., Larsson, S. H., Bhattacharya, P., Hasan, M. A., Ahmed, K. M., Jakariya, M., et al. (2008). Geochemical characterization of shallow aquifer sediments of Matlab Upazila, Southeastern Bangladesh – implications for targeting low-As aquifers. J. Contam. Hydrol. 99, 137–149. doi: 10.1016/j.jconhyd.2008.05.005
Keywords: arsenic, groundwater, aquifer sediment, biomarkers, microbial communities
Citation: Ghosh D, Routh J and Bhadury P (2017) Sub-surface Biogeochemical Characteristics and Its Effect on Arsenic Cycling in the Holocene Gray Sand Aquifers of the Lower Bengal Basin. Front. Environ. Sci. 5:82. doi: 10.3389/fenvs.2017.00082
Received: 17 October 2017; Accepted: 21 November 2017;
Published: 19 December 2017.
Edited by:
Prosun Bhattacharya, Royal Institute of Technology, SwedenReviewed by:
Tanushree Bhattacharya, Birla Institute of Technology, Mesra, IndiaPankaj Kumar, United Nations University, Japan
Copyright © 2017 Ghosh, Routh and Bhadury. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Devanita Ghosh, devanita@iisc.ac.in
Joyanto Routh, joyanto.routh@liu.se
†Present Address: Devanita Ghosh, Laboratory of Biogeochem-mystery, Centre for Earth Sciences, Indian Institute of Science, Bangalore, India
 Devanita Ghosh
Devanita Ghosh Joyanto Routh
Joyanto Routh Punyasloke Bhadury
Punyasloke Bhadury