Varying importance of cuticular hydrocarbons and iridoids in the species-specific mate recognition pheromones of three closely related Leptopilina species
- 1Institute for Zoology, University of Regensburg, Regensburg, Germany
- 2Department of Chemistry, Kenyon College, Gambier, OH, USA
Finding a suitable mate for reproduction is one of the most important tasks for almost all animals. In insects this task is often facilitated by pheromone-mediated communication. While insect pheromones in general show enormous chemical diversity, closely related species often use structurally similar compounds in their pheromones. Despite this similarity, pheromones of congeneric species living in sympatry need to be species specific. We investigated the pheromone-mediated mate recognition by males of three closely related species of Leptopilina, a genus of parasitoid wasps that utilize the larvae of Drosophila as hosts. The study species, L. heterotoma, L. boulardi, and L. victoriae, occur sympatrically and have a similar ecology and life history. We have found that mate recognition is species specific in all three species. This species specificity is achieved by a differing importance of cuticular hydrocarbons (CHCs) and iridoids in the female mate recognition pheromones. In L. heterotoma the iridoids are of major importance while CHCs play a negligible role. In L. boulardi, however, the CHCs are as important as the iridoids, while in L. victoriae, the CHCs alone elicit a full behavioral response of males. Our results provide novel insights into pheromone evolution in insects by showing that selection on two completely different classes of chemical compounds may generate conditions where compounds from both classes contribute to a varying degree to the chemical communication of closely related species and that this variation also generates the species specificity of the signals.
1. Introduction
For successful reproduction sexual organisms need to find a suitable mate. This process can be divided into three distinct steps (Ruther, 2013): (1) mate attraction at long range, (2) mate recognition at short range, and (3) courtship and elicitation of receptiveness. Signals perceived by any sensory modality can be involved in all three steps, but in insects it is often sex pheromones that are crucial for these steps. Mate recognition requires high species specificity to ensure that individuals do not erroneously invest resources in courtship without potential for successful reproduction.
Species-specific sex pheromones enable insects to recognize conspecifics with a high reliability and to date, over 1500 chemical compounds used as sex pheromones are known (El-Sayed, 2014). This large number of compounds is accompanied by an impressive diversity in pheromone composition, which ranges from a single compound to a dozen or more pheromone components (Wyatt, 2014), and includes compounds from many different chemical classes. However, species from the same genus typically use structurally similar compounds in their pheromone communication (Wyatt, 2014), as has been found in numerous studies for instance in Lepidoptera, Coleoptera, and Hymenoptera (Hardie and Minks, 1999; Ando et al., 2004).
Species-specific sex pheromones enable individuals to recognize conspecifics with high reliability, even if closely related heterospecifics occur within the same time and location, which can easily happen in sympatric species with similar ecology. In parasitoid wasps, species from the same genus may share the same host genus if not host species (e.g., Leptopilina; Nordlander, 1980) and in microsympatry virgin males and females of different species might even emerge from the very same host (e.g., Nasonia; Grillenberger et al., 2009). In the jewel wasp genus Nasonia, the female cuticular hydrocarbon (CHC) profile is attractive to males (Steiner et al., 2006; Buellesbach et al., 2013). Buellesbach et al. showed by multivariate statistical methods that the female CHC profiles are chemically distinguishable in all four Nasonia species. Pre-zygotic reproductive isolation, however, is incomplete in Nasonia as interspecific matings can be regularly observed (Buellesbach et al., 2014). This is surprising, as pre-zygotic reproductive isolation would probably prevent fitness losses due to very effective post-zygotic reproductive isolation caused by Wolbachia (Saul, 1961; Breeuwer and Werren, 1990). Females that mate with a heterospecific male can only produce sons, but no daughters, because of the haplodiploid sex determination in Hymenoptera (Cook, 1993; Heimpel and de Boer, 2008). Wolbachia infection frequently occurs in insects (Hilgenboecker et al., 2008) and has been described in several parasitic hymenopterans (Trichogramma: Pintureau et al., 1999, 2002; Nasonia: Breeuwer and Werren, 1990; Bordenstein and Werren, 2007; Leptopilina: Fleury et al., 2000; Gueguen et al., 2012). The resulting post-zygotic reproductive isolation combined with the fact that most (solitary) parasitoid hymenopterans are monandrous (Ridley, 1993), should drive the evolution of a strong pre-zygotic reproductive isolation. Thus, hymenopteran parasitoids are excellent model organisms to study how pre-zygotic reproductive isolation through highly specific sex pheromones evolves and is maintained in sympatric species with similar ecologies.
In a recent study (Weiss et al., 2013), we have identified the female sex pheromone responsible for mate attraction in Leptopilina heterotoma, a larval parasitoid of Drosophila. The pheromone consists of five iridoid compounds: (−)-iridomyrmecin, (+)-isoiridomyrmecin, a third stereoisomer of iridomyrmecin and two iridodials. (−)-iridomyrmecin, the major component of the pheromone, is also used for chemical defense in L. heterotoma (Stökl et al., 2012), and seems likely to have been the starting point for the evolution of the sex pheromone (Weiss et al., 2013). Apart from iridoids, we also considered CHCs as candidate pheromone components in L. heterotoma. CHCs did not attract males of L. heterotoma in y-tube experiments (Weiss et al., 2013), but a possible role in courtship is yet to be investigated. The genus Leptopilina comprises 30 described species and has a worldwide distribution (Nordlander, 1980; Quinlan, 1988; Nordlander and Grijpma, 1991; Allemand et al., 2002; Novkovic et al., 2011; Forshage et al., 2013). All Leptopilina species investigated so far parasitize larvae of Drosophila and have a similar ecology and life history (Nordlander, 1980; Allemand et al., 2002; Novkovic et al., 2011). In our previous study (Weiss et al., 2013), we have shown, that males of L. heterotoma were also attracted by female-derived extracts of the sympatric species L. boulardi. At close range and upon contact, however, L. heterotoma males did not respond to iridoid extracts from L. boulardi females, while those from conspecific females elicited courtship behavior. The species specificity of the mate recognition pheromone in L. boulardi males, however, has not yet been investigated. Leptopilina heterotoma, L. boulardi, and a third closely related species, L. victoriae, have overlapping distribution (Allemand et al., 2002; Novkovic et al., 2011) and thus it is reasonable to expect species-specific mate recognition pheromones in these species. Male courtship in Leptopilina consists of several distinct behaviors that can be easily identified (van den Assem, 1968). Males typically start to show wing-fanning, a high-frequency vibration of the wings, as soon as they recognize an attractive female. Females are then followed and touched with the antennae, which is followed by mounting. After mounting the female, males start antennal stroking, moving their antennae in a circular pattern, thereby bringing their own proximal antennomeres into contact with the female's distal antennomeres in a rhythmical fashion. Wing fanning is usually maintained throughout courtship and stops only when copulation occurs or courtship is abandoned (van den Assem, 1968).
Due to their similar ecology, we expected all three species to produce iridoids and employ these in mate recognition. To ensure species specificity, however, it stands to reason that the composition of the iridoid profiles should differ significantly between the species or iridoid signals should be modulated by interaction with other pheromone chemicals such as CHCs.
In this study, we compare the sex pheromones responsible for mate recognition in L. heterotoma, L. boulardi, and L. victoriae. In an approach that combines chemical analysis and behavioral assays, we asked the following questions:
1. Is mate recognition species-specific in the three species?
2. Are iridoids produced by and used in mate recognition in L. boulardi and L. victoriae?
3. Do CHCs play a role in mate recognition in the three species?
2. Materials and Methods
2.1. Insects
We reared all three wasp species on Drosophila melanogaster hosts. The flies were reared on a corn-based diet (504 ml water, 66 g sugar, 6 g baker's yeast, 2.3 g agar, 52 g cornmeal, 1.3 ml propanoic acid, 0.8 g nipagin). Both flies and wasps were kept at 25°C, about 75 % humidity and a 16:8 h L:D cycle. For each rearing, about 30 flies of both sexes were put into a jar containing the freshly prepared diet. After 48 h, the flies were removed from the jar and about 10 mated females of either L. heterotoma, L. boulardi or L. victoriae were introduced to parasitize the fly larvae. Several days before emergence (about 3 weeks after oviposition), parasitized fly pupae were identified by their dark coloration and removed from the jars and put singly into 1.5 ml microcentrifuge tubes. The isolated pupae were screened daily for emerged wasps. In this way, unmated and naive wasps of known age were obtained. Emerged wasps were kept individually in the microcentrifuge tubes until they were used in an experiment. Each individual wasp was used for a single experiment only.
2.2. Species Specificity of Courtship
To determine the species specificity of the male courtship behavior, 1-d-old naive females of each species were presented to 1-d-old naive males of each species. For each trial, a female was carefully placed into a glass arena (15 mm diameter, 2 mm height). Shortly thereafter, a single male was introduced into the arena, which was then covered with a glass lid. Male behavior was recorded as digital video for 2 min and afterwards the total wing fanning duration of responding males was determined with the video module of the scientific observation software The Observer XT 11.0 (Noldus, Wageningen, The Netherlands). Wing fanning in Leptopilina consists of both continuous sequences as well as intermittent bouts of wing fanning (personal observation). Thus, high frequency wing vibrations of any length were classified as wing fanning. The duration of the experiment was chosen according to our previous wing fanning experiments, which lasted 5 min and in which wing fanning was only rarely observed after 2 min. After each replicate the used arena was rinsed with ethanol and left to dry at room temperature. Each combination of species was tested 20 times.
2.3. Pheromone Extraction and Fractionation
To test whether the courtship behavior is elicited by pheromones and to disentangle the contribution of iridoids and CHCs to the pheromone function, we extracted female wasps of either species for 10 min in 5 μl dichloromethane (DCM) per wasp. To separate iridoid compounds from CHCs, we fractionated the extract either by solid-phase extraction (SPE; samples from L. heterotoma and L. boulardi) or size-exclusion chromatography (SEC; samples from L. victoriae), following the method of Kühbandner et al. (2012). Both SPE and SEC resulted in the same fractionation, i.e., an iridoid fraction and a CHC fraction. We switched from SPE to SEC to avoid the additional step of drying and redissolving the hexane fraction for bioassays (see below), which was required after the SPE fractionation.
Prior to SPE, the raw extracts were dried under a stream of nitrogen, and the samples were then redissolved in 50 μl hexane. Cyanopropyl-bonded silica gel columns (50 mg, DSC-CN, Sigma-Aldrich, Taufkirchen, Germany) were pre-conditioned by rinsing them with 2 ml each of DCM and hexane. Then, the samples were applied to the column and eluted with 300 μl hexane followed by 300 μl DCM. Between elution with hexane and elution with DCM, the column was flushed with additional 300 μl hexane. The hexane fractions contained the CHCs and the DCM fraction contained the iridoids. For bioassays, hexane fractions were carefully dried under nitrogen and then redissolved in DCM.
Prior to SEC, raw extracts were reduced to about 25 μl under nitrogen. The samples were then injected onto a PLgel SEC column (300 mm × 7.5 mm, particle size 5 μm, pore size 100 Å, Agilent Technologies, Waldbronn, Germany) using a Rheodyne model 7125 HPLC injector equipped with a 25 μl sample loop (Rheodyne, Cotati, CA, USA). The column was connected to an LC-20 AD HPLC pump (Shimadzu Europe, Duisburg, Germany) with DCM as mobile phase at a flow rate of 1.00 ml min−1. Two fractions were collected: fraction SEC 1, eluting between 6.75 and 7.17 min, and fraction SEC 2, eluting between 7.50 and 8.00 min. SEC 1 contained the CHCs and SEC 2 contained the iridoids.
The composition of all fractions was analyzed by GC-MS (see below). The concentration of fractions and extracts was adjusted to 1 female equivalent per 5 μl for chemical analysis and to 1 female equivalent per 20 μl for behavioral experiments.
2.4. Chemical Analysis
Extracts and fractions were analyzed on a GC2010 gas chromatograph (GC) connected to a QP2010 plus mass spectrometer (MS; both Shimadzu, Duisburg, Germany). The GC was equipped with a non-polar capillary column (BPX-5, 30 m length, 0.25 mm inner diameter (i.d.), 0.25 μm film thickness; SGE Analytical Sciences, Milton Keynes, UK). Helium was used as carrier gas with a constant linear velocity of 50 cm s−1. The temperature of the GC oven started at 80°C and was raised by 5°C min−1 to 280°C, where it was kept for 20 min. The MS was run in electron impact (EI) mode at 70 eV and set to a scan range from 35–600 mz−1. Sample volumes of 1 μl were injected splitless at an injector temperature of 280°C. For the enantioselective analysis of iridoids, the GC was equipped with a chiral β-cyclodextrin column (Beta DEX 225, 30 m length, 0.25 mm i.d., 0.25 μm film thickness; Sigma-Aldrich, Taufkirchen, Germany). For these analyses, the GC oven temperature started at 80°C and was raised by 6°C min−1 to 200°C, where it was kept for 20 min. The MS settings were as described above. Sample volumes of 1 μl were injected splitless at an injector temperature of 200°C.
2.4.1. Iridoids
The iridoids produced by female L. heterotoma have been identified in previous studies (Stökl et al., 2012; Weiss et al., 2013). These iridoids are (−)-iridomyrmecin, (+)-isoiridomyrmecin, a third iridomyrmecin of unknown absolute configuration and two stereoisomers of iridodial, with (−)-iridomyrmecin making up about 80 % of the pheromone. Leptopilina boulardi females also possess these iridoids, albeit in different ratios (Weiss et al., 2013). Iridoids in L. victoriae were identified by comparing mass spectra and retention indices on both the non-polar and the cyclodextrin column to those of the L. heterotoma iridoids. Additionally, (+)-iridomyrmecin and (−)-iridomyrmecin as well as (+)-isoiridomyrmecin and (−)-isoiridomyrmecin were used as synthetic references (Fischman et al., 2013). Compounds that contributed less than 0.5 % to the total amount of iridoids were not considered.
2.4.2. Cuticular Hydrocarbons
The n-alkanes in females of all three Leptopilina species were identified by comparing mass spectra and retention indices to those of synthetic reference compounds. Methyl-branched hydrocarbons were identified by interpretation of diagnostic ions resulting from the favored fragmentation at the branching points (Nelson, 1993) and comparison of linear retention indices with literature data (Carlson et al., 1998). Double bond positions of unsaturated compounds were identified by derivatization with dimethyl disulfide (DMDS; Carlson, 1989). Derivatized samples were analyzed on the non-polar column as described above with a modified temperature program (final temperature 300°C for 178 min) and scan range 35–800 m/z. Compounds that contributed less than 0.5 % to the total amount of CHCs were not considered.
2.4.3. Quantification
For quantification of both iridoids and CHCs, single females were extracted in 15 μl DCM, containing 5 ng μl−1 methyl decanoate as an internal standard. GC-MS analyses were carried out with the non-polar column, as described above. A separate calibration curve (1–50 ng each) was established for iridoids and CHCs assuming that response factors would differ little within each structural class. For iridoids, we established a calibration curve using (+)-iridomyrmecin as the standard. Hydrocarbons were quantified using n-tricosane as the standard. Quantification of the iridoids and CHCs was based on 10 individuals from each species.
2.5. Pheromone Bioassays
Extracts from females and fractions thereof were tested for their ability to elicit wing fanning in conspecific males. For this purpose, 2 μl of extracts, fractions (equivalent to one 10th of a female) or the pure solvent control were applied to a small disc (5 mm diameter) of filter paper. The filter paper was then placed in an arena [dimensions as described above for L. boulardi and L. victoriae; 55 mm diameter, 8.5 mm height for L. heterotoma (we used a bigger arena for L. heterotoma because only few L. heterotoma males had responded in preliminary pheromone bioassays in the smaller arena)] and the solvent was allowed to evaporate for 1 min. After solvent evaporation, a naive 1-d-old male was introduced into the arena which was then covered with a glass lid. The male's behavior was recorded as digital video for 2 min. After each replicate the used arena was rinsed with ethanol and left to dry at room temperature. Afterwards, the video files were analyzed with the video module of The Observer XT 11.0 to measure the wing fanning duration of responding males (n = 20 for L. boulardi and L. victoriae; n = 25 for L. heterotoma; sample size for L. heterotoma was increased because males had responded less frequently in the preliminary pheromone bioassays).
2.6. Statistical Analysis
Wing fanning duration was analyzed using the Kruskal-Wallis test, followed by pairwise comparisons with the Mann-Whitney U-test with Bonferroni-Holm correction. Wing fanning duration was only compared within a species but not between species. All statistical analyses were performed using R 3.1.0 (R Core Team, 2014).
3. Results
3.1. Species Specificity of Courtship
In all three Leptopilina species male courtship behavior elicitation was shown to be species specific as demonstrated by significantly increased wing fanning durations toward conspecific females (Figure 1). Statistical details are given in Table 1. Although males of all three species showed short wing fanning bouts toward heterospecific females, males very rarely tried to copulate and no interspecific matings were observed. In intraspecific trials, matings were regularly observed.
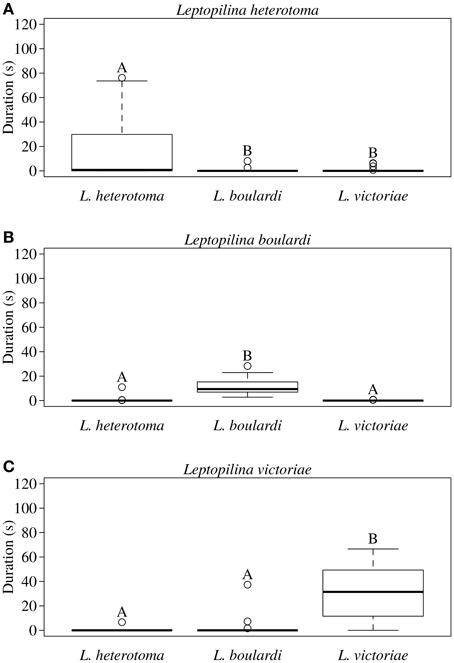
Figure 1. Total duration of wing fanning displayed by males of (A) L. heterotoma, (B) L. boulardi, and (C) L. victoriae toward con- and heterospecific females (n = 20). Different letters indicate significant differences between median values at p < 0.05 (Mann-Whitney U-test with Bonferroni-Holm correction); comparisons were only made within but not between male species.
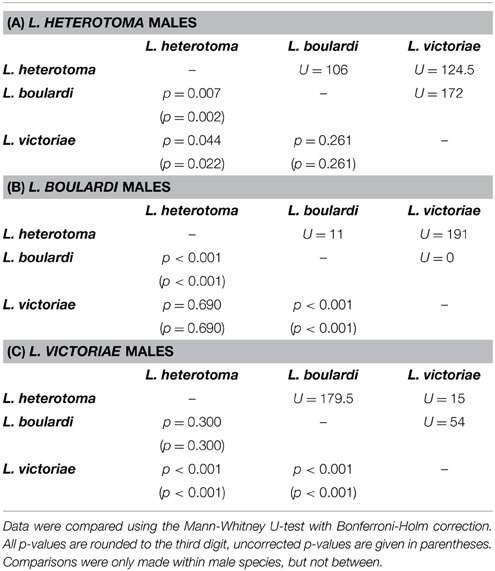
Table 1. Statistical details for the pairwise comparisons of courtship duration displayed by (A) L. heterotoma, (B) L. boulardi, and (C) L. victoriae males toward conspecific and heterospecific females.
3.2. Fractionation
Fractions obtained from SPE and SEC, respectively, were analyzed with GC-MS to ensure that the fractions contained only the expected compounds. The analyses confirmed that the hexane and DCM fractions from the SPE fractionation contained the CHCs and iridoids, respectively. For SEC fractions, the analyses showed that the SEC1 fraction contained the CHCs and that the SEC2 fraction contained the iridoids. For simplification, the DCM fraction (SPE) and the SEC2 fraction will be referred to as “iridoid fraction,” and the hexane fraction (SPE) and the SEC1 fraction will be referred to as “CHC fraction.”
3.3. Chemical Analysis
3.3.1. Iridoids
The major iridoid compound found in L. heterotoma and L. boulardi females is (−)-iridomyrmecin, whereas extracts from L. victoriae females contained (+)-iridomyrmecin as the major compound (Table 2). Extracts from all three species contained (+)-isoiridomyrmecin, two stereoisomers of iridodial, and a third iridomyrmecin stereoisomer of unknown absolute configuration (for more details on the structure of the latter three compounds see Weiss et al., 2013). In addition to the mentioned iridoids, extracts from L. boulardi and L. victoriae females contained some additional putative iridoids. The total amount of iridoids is lower in L. victoriae than in L. heterotoma and L. boulardi (Table 2). Overall, the iridoid profiles differ both qualitatively and quantitatively between the three species.
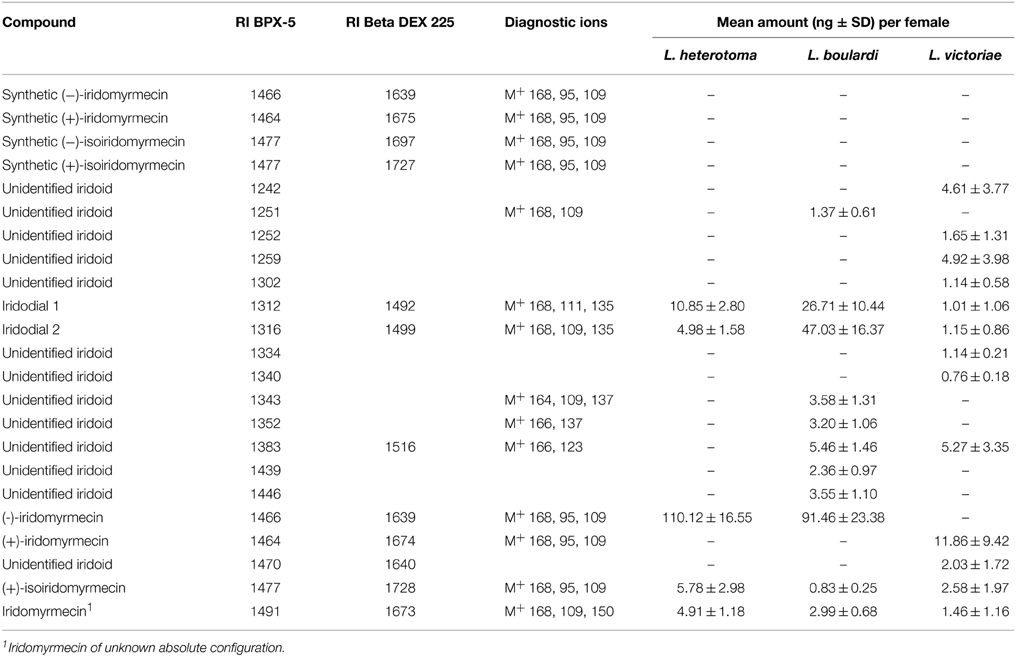
Table 2. Iridoid compounds and amounts thereof found in females of L. heterotoma, L. boulardi, and L. victoriae.
3.3.2. Cuticular Hydrocarbons
The CHCs found in all Leptopilina species were mainly methyl-branched and mono- or di-unsaturated alkenes. The n-alkanes were found only in low amounts. While all three species share a number of n-alkanes, 4-methyl alkanes, and mono-unsaturated n-alkenes, each species was characterized by a number of species-specific CHCs (Table 3).
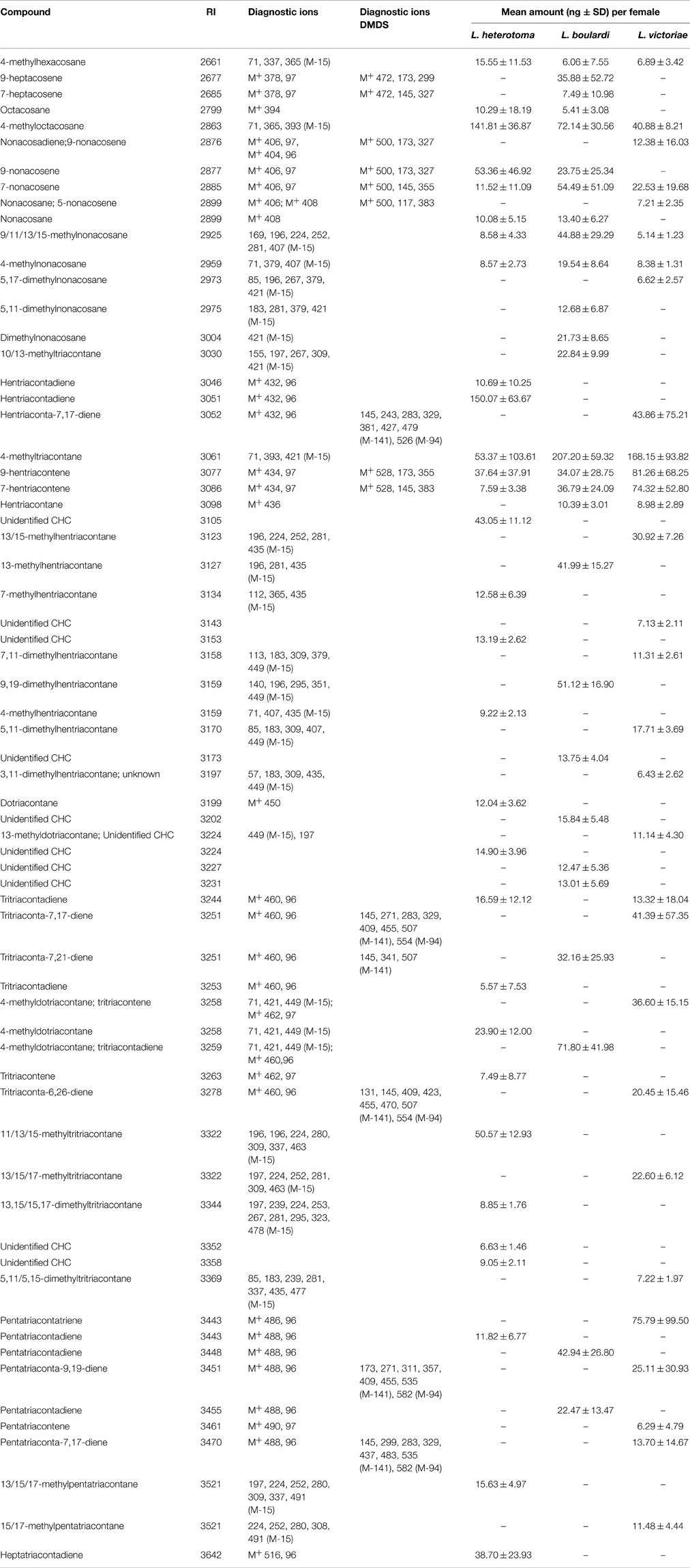
Table 3. Cuticular hydrocarbons and amounts thereof found in females of L. heterotoma, L. boulardi, and L. victoriae.
3.4. Pheromone Bioassays
Raw extracts from conspecific females elicited wing fanning behavior in males from all three Leptopilina species, indicating the presence of courtship eliciting mate recognition pheromones. The relative contribution of iridoids and CHCs to the behavioral activity of the extracts, however, differed significantly between the three species.
In L. heterotoma the iridoids elicited the same degree of wing fanning as the raw extract. In contrast, CHCs were only slightly attractive and elicited significantly less intense wing fanning than the raw extract and the iridoids, respectively (Figure 2A; statistical details in Table 4).
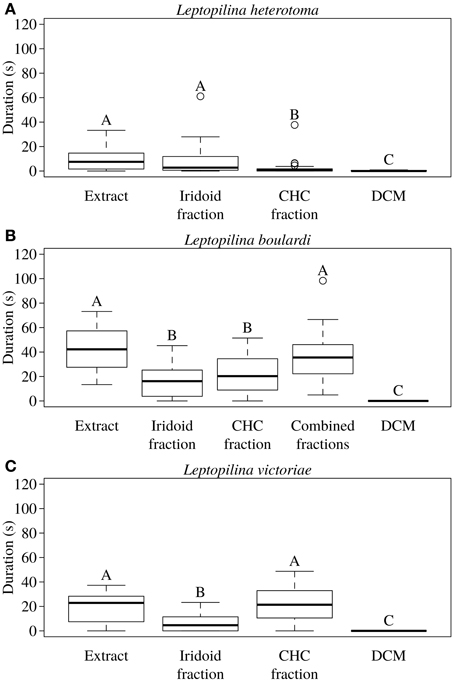
Figure 2. Total duration of wing fanning displayed by males of (A) L. heterotoma (n = 25), (B) L. boulardi (n = 20), and (C) L. victoriae (n = 20) toward raw extract from conspecific females, iridoid and CHC fractions thereof, the combined fractions (only for L. boulardi), and solvent (control). Different letters indicate significant differences between median values at p < 0.05 (Mann-Whitney U-test with Bonferroni-Holm correction).
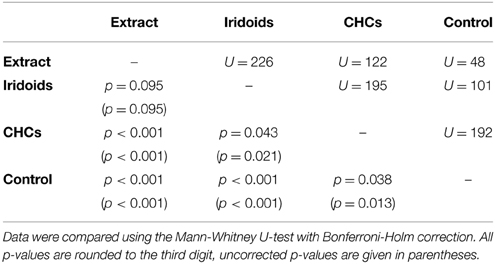
Table 4. Statistical details for the pairwise comparisons of courtship duration toward extract, fractions, and control for L. heterotoma.
In L. boulardi, significant wing fanning responses were elicited in males by both iridoids and CHCs, which did not differ in their behavioral activity. Both fractions alone, however, were less active than the raw extract, while the recombined fractions were as active as the raw extract (Figure 2B; statistical details in Table 5).
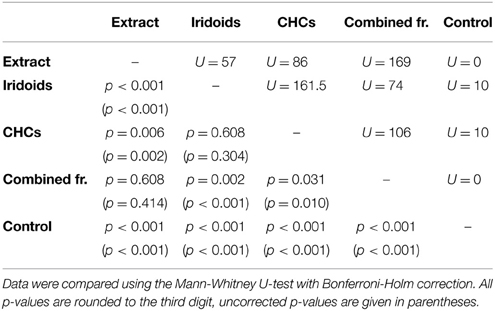
Table 5. Statistical details for the pairwise comparisons of courtship duration toward extract, fractions, combined fractions, and control for L. boulardi.
Both fractions elicited wing fanning behavior also in males of L. victoriae but the response to CHCs was significantly stronger in this species. When compared to the raw extract, CHCs elicited the same degree of wing fanning in males while iridoids were significantly less active (Figure 2C; statistical details in Table 6).
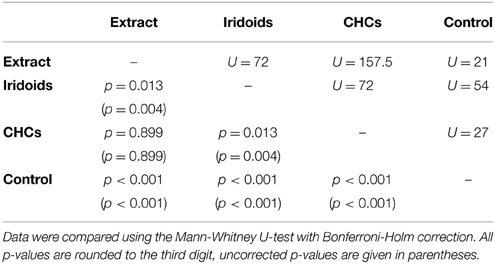
Table 6. Statistical details for the pairwise comparisons of courtship duration toward extract, fractions, and control for L. victoriae.
4. Discussion
4.1. Species Specificity of Courtship
Our results show that male courtship behavior in L. boulardi, L. heterotoma, and L. victoriae is species specific and that this specificity is accompanied by a high chemical diversity of the female courtship-eliciting pheromone.
Females of most (solitary) parasitoid wasps mate only once (Ridley, 1993) while males can mate multiple times. This is also true for Leptopilina, and although heterospecific matings probably do not occur, hybridization of species would still be prevented by Wolbachia-mediated cytoplasmatic incompatibility (Fleury et al., 2000; Gueguen et al., 2012). Females that mate with a heterospecific male are still able to produce male offspring, because of the haplo-diploid sex determination in Hymenoptera (Cook, 1993; Heimpel and de Boer, 2008), but nevertheless experience a massive fitness loss. This fitness loss is even greater in species with (partial) local mate competition (which includes Leptopilina Debout et al., 2002), which usually produce female-biased sex ratios (Hamilton, 1967). For males the sperm transferred to a heterospecific female is lost without any reward. It stands to reason that accurate conspecific mating represents a fitness advantage and our courtship elicitation experiments with both conspecific and heterospecific females indicate, that species recognition is indeed highly accurate. Even though heterospecific females elicited short bouts of wing fanning in some males, conspecific females were courted significantly longer. Some compounds (iridoids and CHCs) were identified in the extracts of females from all three investigated species. This overlap in the chemical profiles may very well explain the observed heterospecific courtship elicitation. However, we cannot exclude that other than chemical cues or signals, like visual or tactile, elicited the short courtship of males toward heterospecific females.
All males that courted heterospecific females failed to elicit female receptiveness and no heterospecific matings were observed. Most likely, a species-specific male aphrodisiac pheromone ensures that females do not accept heterospecific males as a mate (Isidoro et al., 1999). Leptopilina heterotoma, L. boulardi, and L. victoriae occur sympatrically and even share a common host, D. melanogaster. Thus, the putative species-specific male aphrodisiac pheromone is of great importance as it provides a second species border beyond the highly, but not completely, species-specific female courtship pheromone.
Females of the jewel wasp Nasonia, a parasitoid of fly pupae, also show a high rejection rate of heterospecific mates, while males showed very little discrimination against heterospecific mates (Buellesbach et al., 2014). But in contrast to Leptopilina, heterospecific matings do occur in Nasonia, despite the presence of a male aphrodisiac pheromone which is necessary to elicit female receptiveness (van den Assem et al., 1980). This indicates that the male aphrodisiac pheromone of Nasonia might not be species-specific. On the contrary, preliminary results of our own group suggest that the male aphrodisiac pheromones in L. boulardi and other Leptopilina species are indeed species specific. The phenomenon of male aphrodisiac pheromones is widespread in hymenopteran parasitoids (e.g., van den Assem et al., 1980; Isidoro and Bin, 1995; Bin et al., 1999; Isidoro et al., 1999; Romani et al., 2005), however, to date, no such putative pheromone has been fully identified and more work is needed to understand the evolutionary background of those male pheromones.
Overall, we showed that male courtship is highly species specific in the three investigated Leptopilina species. We assume that the chemical profiles of the females alone are sufficient for males to distinguish con- and heterospecific females but further studies are needed to identify the exact cues used for species recognition in Leptopilina.
4.2. Composition of Courtship Pheromone
The results of the pheromone bioassays indicate that the three investigated Leptopilina species possess different courtship eliciting female sex pheromones. While this result was indicated by the species specific courtship of the males, it is surprising, how much the female sex pheromones of the three species differ in their chemical composition. All three species have the same two classes of chemical compounds, iridoids and CHCs, available, but use them to very different extents in their mate recognition pheromone. In L. heterotoma, the iridoids elicited full wing fanning and thus are of major importance, while the CHCs elicited almost no wing fanning and contribute only marginally to the sexual signal in this species. This is in concordance with previous results from y-tube experiments, in which L. heterotoma males were attracted by the female-derived iridoids, but not by the female CHCs (Weiss et al., 2013). In L. boulardi the relative importance of iridoids and CHCs is different from L. heterotoma. Both iridoids and CHCs elicited significant wing fanning responses in males, but significantly less than the crude extract. Only the combination of iridoids and CHCs elicited the full behavioral response in L. boulardi males. This means, that in L. boulardi, iridoids and CHCs both convey important information in sexual communication. The picture is yet different in L. victoriae: CHCs alone elicited the full wing fanning response in males; hence, the importance of CHCs in sexual communication is even greater than in L. boulardi. In L. victoriae, the iridoids elicited a weak but significant wing fanning response when presented alone, but unlike in L. boulardi, the iridoids are not required for the full courtship response.
To date, the female courtship eliciting pheromones of about a dozen parasitoid wasp species have been chemically identified (Ruther, 2013; Stökl et al., 2014). Those consists of both CHCs and non-CHC compounds, but a combination of both, like in L. boulardi, has only been found in Lariophagus distinguendus (CHCs and triacylglycerides, Kühbandner et al., 2012) and in Asobara tabida [Methyl 6-methyl salicylate, fatty alcohol acetates and CHCs, Stökl et al. (2014)]. Although in A. tabida CHCs elicit courtship behavior, they are not necessary for a full response of the males.
CHCs are commonly used in the chemical communications of insects (Howard, 1993) and approximately half of the parasitoid wasps with identified female sex pheromones rely on CHCs for their sexual communication (Ruther, 2013). Iridoids are far less common than CHCs, but have been described in the defensive secretion of several ants, beetles and parasitoid wasps (e.g., Huth and Dettner, 1990; Völkl et al., 1994; Do Nascimento et al., 1998; Stökl et al., 2012), and are also used as sex pheromone components by some species [e.g., aphids, Stewart-Jones et al. (2007)]. However, in parasitoid wasps iridoids have so far only been found in the genera Alloxysta and Leptopilina. Species from both genera use the iridoids for defense (Völkl et al., 1994; Stökl et al., 2012), but their use as sex pheromone has so far only been demonstrated for L. heterotoma (Weiss et al., 2013).
The reasons why one collection of available compounds is selected over another to compose a pheromone is one of the big questions in pheromone research. In a previous study we demonstrated, that in L. heterotoma (−)-iridomyrmecin most likely evolved primarily as a defensive compound against predators and later gained a second role in communication as sex pheromone (Weiss et al., 2013). At the moment we can only speculate about the reasons and evolutionary constraints, why L. boulardi and L. victoriae do not use iridoids for mate recognition to the same extent as L. heterotoma. It is interesting to note, that the observed low importance of iridoids in mate recognition in L. victoriae is correlated with a lower total amount of iridoids in L. victoriae compared to the other two species. Perhaps a lower investment into defense led to the selection of more abundant and therefore more reliable compounds, the CHCs, as the mate recognition pheromone in L. victoriae.
Molecular analyses of the genus Leptopilina have shown that L. heterotoma and L. victoriae are closely related, while L. boulardi is placed in a more distantly related species group (Allemand et al., 2002; Novkovic et al., 2011). This means that the two most closely related species use the most divergent sex pheromones while the distantly related L. boulardi uses a hybrid of the signals from the other two species. Therefore, it seems unlikely that there was a gradual evolution from only CHCs as sex pheromone to a pheromone consisting solely of iridoids, or vice versa. Future studies that elucidate the chemical composition of the pheromone of more species could be coupled with a more reliable phylogeny of the genus to test this hypothesis.
Vast differences in pheromones of sibling species can also be explained by saltational evolution [reviewed in Symonds and Elgar (2008)]. Pheromones that are under strong stabilizing selection, and thus cannot evolve gradually, such as sex pheromones, might undergo quite drastic changes in a rather short time, leading to clearly different signals in sibling species. Examples for saltational evolution include aggregation pheromones in bark beetles (Symonds and Elgar, 2004) and sex pheromones in Yponomeuta moths (Löfstedt et al., 1991). Buellesbach et al. (2013) showed that male CHC profiles in Nasonia correlated with the Nasonia phylogeny. The female CHC profiles, however, are highly divergent and not correlated with the phylogeny. This is in strict accordance with the concept of gradual and saltational evolution: the male CHC profiles are not under strong stabilizing selection and can thus evolve gradually, leading to similar profiles in related species, while the female CHC profiles, with their role in sexual signaling, are under strong stabilizing selection and can thus only evolve through major shifts to establish reproductive isolation. Preliminary results show qualitative and quantitative differences in both the iridoid and the CHC profiles of males and females in most species of Leptopilina. Therefore, a comparative analysis might also be a useful approach to investigate the evolution of iridoids and CHCs in the chemical communication of Leptopilina. However, the currently available molecular phylogenies do not provide sufficient resolution and statistical support for such an analysis.
Our analysis of the iridoids found in the three species showed, to our surprise, that females of L. victoriae possess (+)-iridomyrmecin instead of (−)-iridomyrmecin. (−)-iridomyrmecin has been found in four species of Leptopilina [L. heterotoma and L. boulardi, Stökl et al. (2012) and Weiss et al. (2013); L. guineaensis and L. clavipes, unpublished data], and therefore seems to represent the ancestral state. Weiss et al. (2013) demonstrated, that males of L. heterotoma are able to discriminate between (−)-iridomyrmecin and (+)-iridomyrmecin, and thus males of L. victoriae probably can do so as well. And although the biosynthetic pathway of iridomyrmecin has not been investigated in detail so far, it is plausible to assume that the modification of a single enzyme in the biosynthetic pathway can lead to the production of (+)-iridomyrmecin instead of (−)-iridomyrmecin. The shift from (−)-iridomyrmecin to (+)-iridomyrmecin in L. victoriae females could thus be an example of such a saltational evolution in sex pheromones. A detailed analysis of the biosynthetic pathways of the different iridoids is required to clarify how the shift from one enantiomer to the other may have happened.
It is furthermore noteworthy, that (−)-iridomyrmecin proved to be a more potent repellent than (+)-iridomyrmecin in bioassays with ants (Stökl et al., 2012). This and the finding that L. victoriae produces lower amounts of iridoids compared to L. heterotoma and L. boulardi, leads us to the conclusion, that chemical defense might be of less importance for L. victoriae. This is surprising, as all three species have a very similar ecology and detailed studies will be needed to better understand the differences in the chemical ecology of Leptopilina species.
Wing fanning is a courtship element commonly found in parasitic Hymenoptera (van den Assem, 1968, 1986). In several species, the male wing fanning performance has been found to be correlated with the outcome of the male courtship. For example males of Lysiphlebus testaceipes that produced high-frequency wing fanning had a higher mating success than males that fanned at a lower frequency (Benelli et al., 2015). Similarly, in males of Lariophagus distinguendus the frequency of wing fanning observed before successful courtship has been found to be significantly higher than the frequency before unsuccessful courtship (Benelli et al., 2013). Thus, male wing fanning may be an indicator of male fitness in parasitic Hymenoptera. The videos recorded in this study do not allow to determine wing fanning characteristics such as frequency or amplitude. It would, however, be interesting to compare these features between the studied species and correlate the features with the outcome of the courtship, especially since L. boulardi males seem to elicit female receptiveness more often than males from the other species (personal observation).
Drosophila species, the host species of Leptopilina, are usually no pests. As a consequence, predators and parasitoids of Drosophila had not been investigated regarding their potential application to control Drosophila populations. This situation has changed with the appearance of Drosophila suzukii, a pest species that originates from Asia (Cini et al., 2012) and only recently emerged in Europe and North America (Hauser, 2011; Calabria et al., 2012). Ovipositing D. suzukii females frequently damage fruit and thereby ruin crops (Cini et al., 2012); the appearance of D. suzukii has thus led to first initial research into the application of Drosophila predators (Cuthbertson et al., 2014) and parasitoids (Chabert et al., 2012) to control D. suzukii populations. Chabert et al. (2012) reported that L. heterotoma developed only very rarely in D. suzukii larvae. In a more recent article, however, Kasuya et al. (2013) reported that several larval parasitoids, including Leptopilina japonica, successfully parasitize D. suzukii in the field. The potential role of Leptopilina in controlling the emerging pest species D. suzukii stresses the importance of a profound understanding of the parasitoid's ecology, especially aspects regarding the efficient rearing and deployment of parasitoids.
Coming back to our introductory questions, we conclude that (1), mate recognition is species specific in L. heterotoma, L. boulardi, and L. victoriae; (2), iridoids, including iridomyrmecin, are produced by females of all three species, but contribute to a different extent to the sex pheromone; and (3) CHCs are used as sex pheromones by L. boulardi and L. victoriae. However, further comparative studies including more Leptopilina species are necessary to generalize our findings and to understand the selective forces acting on iridoids and CHCs which create the unexpectedly high pheromone diversity within the genus Leptopilina.
Author Contributions
IW, JH, JR, and JS designed the experiments. IW conducted the experiments. IW analyzed the data. JH synthesized the compounds. IW, JH, JR, and JS wrote the manuscript.
Funding
This study was funded by the German Research Council (Deutsche Forschungsgemeinschaft, DFG; grant STO 966/1-1 to JS).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Thomas Hoffmeister (University of Bremen), Roland Allemand (Université Claude Bernard Lyon 1), and Leo W. Beukeboom (University of Groningen) for sending us a starter culture of L. heterotoma, L. boulardi, and L. victoriae, respectively, and Michael Brummer for rearing the insects.
References
R Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Allemand, R., Lemaître, C., Frey, F., Boulétreau, M., Vavre, F., Nordlander, G., et al. (2002). Phylogeny of six African Leptopilina species (Hymenoptera: Cynipoidea, Figitidae), parasitoids of Drosophila, with description of three new species. Ann. Soc. Entomol. Fr. 38, 319–332. doi: 10.1080/00379271.2002.10697346
Ando, T., Inomata, S.-I., and Yamamoto, M. (2004). “Lepidopteran sex pheromones,” in The Chemistry of Pheromones and Other Semiochemicals I Vol. 239 of Topics in Current Chemistry, ed S. Schulz (Berlin; Heidelberg: Springer), 51–96.
Benelli, G., Bonsignorie, G., Stefanini, C., Dario, P., and Canale, A. (2013). Male wing fanning performance during successful and unsuccessful mating in the parasitic wasp Lariophagus distinguendus Förster (Hymenoptera: Pteromalidae). J. Insect Behav. 26, 228–237. doi: 10.1007/s10905-012-9356-2
Benelli, G., Kavallieratos, N. G., Donati, E., Giunti, G., Stefanini, C., and Canale, A. (2015). Singing on the wings! Male wing fanning performances affect female willingness to copulate in the aphid parasitoid Lysiphlebus testaceipes (Hymenoptera: Braconidae: Aphidiinae). Insect Sci. doi: 10.1111/1744-7917.12201. [Epub ahead of print].
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bin, F., Wäckers, F., Romani, R., and Isidoro, N. (1999). Tyloids in Pimpla turionellae (L.) are release structures of male antennal glands involved in courtship behaviour (Hymenoptera: Ichneumonidae). Int. J. Insect Morphol. Embryol. 28, 61–68. doi: 10.1016/S0020-7322(99)00015-X
Bordenstein, S. R., and Werren, J. H. (2007). Bidirectional incompatibility among divergent Wolbachia and incompatibility level differences among closely related Wolbachia in Nasonia. Heredity 99, 278–287. doi: 10.1038/sj.hdy.6800994
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Breeuwer, J. A. J., and Werren, J. H. (1990). Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature 346, 558–560. doi: 10.1038/346558a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Buellesbach, J., Gadau, J., Beukeboom, L. W., Echinger, F., Raychoudhury, R., Werren, J. H., et al. (2013). Cuticular hydrocarbon divergence in the jewel wasp Nasonia: evolutionary shifts in chemical communication channels? J. Evol. Biol. 26, 2467–2478. doi: 10.1111/jeb.12242
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Buellesbach, J., Greim, C., Raychoudhury, R., and Schmitt, T. (2014). Asymmetric assortative mating behaviour reflects incomplete pre-zygotic isolation in the Nasonia species complex. Ethology 120, 834–843. doi: 10.1111/eth.12250
Calabria, G., Máca, J., Bächli, G., Serra, L., and Pascual, M. (2012). First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J. Appl. Entomol. 136, 139–147. doi: 10.1111/j.1439-0418.2010.01583.x
Carlson, D. A., Bernier, U. R., and Sutton, B. D. (1998). Elution patterns from capillary GC for methyl-branched-alkanes. J. Chem. Ecol. 24, 1845–1865. doi: 10.1023/A:1022311701355
Carlson, D. A. (1989). Dimethyl disulfide derivatives of long chain alkenes, alkadienes, and alkatrienes for gas chromatography/mass spectrometry. Anal. Chem. 61, 1564–1571. doi: 10.1021/ac00189a019
Chabert, S., Allemand, R., Poyet, M., Eslin, P., and Gilbert, P. (2012). Ability of European parasitoids (Hymenoptera) to control a new invasive Asiatic pest, Drosophila suzukii. Biol. Control 31, 40–47. doi: 10.1016/j.biocontrol.2012.05.005
Cini, A., Ioratti, C., and Anfora, G. (2012). A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectol. 65, 149–160. Available online at: http://hdl.handle.net/10449/21029
Cook, J. M. (1993). Sex determination in the Hymenoptera: a review of models and evidence. Heredity 71, 421–435. doi: 10.1038/hdy.1993.157
Cuthbertson, A. G. S., Blackburn, L. F., and Audsley, N. (2014). Efficacy of commercially available invertebrate predators against Drosophila suzukii. Insects 5, 952–960. doi: 10.3390/insects5040952
Debout, G., Fauvergue, X., and Fleury, F. (2002). The effect of foundress number on sex ratio under partial local mate competition. Ecol. Entomol. 27, 242–246. doi: 10.1046/j.1365-2311.2002.00402.x
Do Nascimento, R. R., Billen, J., Sant'ana, A. E. G., Morgan, E. D., and Harada, A. Y. (1998). Pygidial gland of Azteca nr. bicolor and Azteca chartifex: morphology and chemical identification of volatile components. J. Chem. Ecol. 24, 1629–1637. doi: 10.1023/A:1020864427854
El-Sayed, A. M. (2014). The Pherobase: Database of Pheromones and Semiochemicals. Available online at: http://www.pherobase.com
Fischman, C. J., Adler, S., and Hofferberth, J. E. (2013). Divergent diastereoselective synthesis of iridomyrmecin, isoiridomyrmecin, teucrimulactone, and dolicholactone from citronellol. J. Organ. Chem. 78, 7318–7323. doi: 10.1021/jo400884g
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fleury, F., Vavre, F., Ris, N., Fouillet, P., and Boulétrau, M. (2000). Physiological cost induced by the maternally-transmitted endosymbiont Wolbachia in the Drosophila parasitoid Leptopilina heterotoma. Parasitology 121, 493–500. doi: 10.1017/S0031182099006599
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Forshage, M., Nordlander, G., and Buffington, M. L. (2013). Eucoilinae of North America: a revised catalog of genera and described species. Proc. Entomol. Soc. Wash. 115, 225–255. doi: 10.4289/0013-8797.115.3.225
Grillenberger, B. K., van de Zande, L., Bijlsma, R., Gadau, J., and Beukeboom, L. W. (2009). Reproductive strategies under multiparasitism in natural populations of the parasitoid wasp Nasonia (Hymenoptera). J. Evol. Biol. 22, 460–470. doi: 10.1111/j.1420-9101.2008.01677.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gueguen, G., Onemola, B., and Govind, S. (2012). Association of a new Wolbachia strain with, and its effects on, Leptopilina victoriae, a virulent waps parasitic to Drosophila spp. Appl. Environ. Microbiol. 78, 5962–5966. doi: 10.1128/AEM.01058-12
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hamilton, W. D. (1967). Extraordinary sex ratios. Science 156, 477–488. doi: 10.1126/science.156.3774.477
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hardie, J., and Minks, A. K. (eds.). (1999). Pheromones of Non-Lepidopteran Insects Associated with Agricultural Plants. Wallingford, UK: CABI Publishing.
Hauser, M. (2011). A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag. Sci. 67, 1352–1357. doi: 10.1002/ps.2265
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Heimpel, G. E., and de Boer, J. G. (2008). Sex determination in the Hymenoptera. Annu. Rev. Entomol. 53, 209–230. doi: 10.1146/annurev.ento.53.103106.093441
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hilgenboecker, K., Hammerstein, P., Schlattmann, P., Telschow, A., and Werren, J. H. (2008). How many species are infected with Wolbachia? – a statistical analysis of current data. FEMS Microbiol. Lett. 281, 215–220. doi: 10.1111/j.1574-6968.2008.01110.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Howard, R. W. (1993). “Cuticular hydrocarbons and chemical communication,” in Insect Lipids, eds D. W. Stanley and D. R. Nelson (Lincoln, NE: University of Nebraska Press), 179–226.
Huth, A., and Dettner, K. (1990). Defense chemicals from abdominal glands of 13 rove beetle species of subtribe staphylinina (Coleoptera: Staphylinidae, Staphylininae). J. Chem. Ecol. 16, 2691–2711. doi: 10.1007/BF00988079
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Isidoro, N., and Bin, F. (1995). Male antennal gland of Amitus spiniferus (Brethes) (Hymenoptera: Platygastridae), likely involved in courtship behavior. Int. J. Insect Morphol. 24, 365–373. doi: 10.1016/0020-7322(95)00014-U
Isidoro, N., Bin, F., Romani, R., Pujade-Villar, J., and Palmira, R.-F. (1999). Diversity and function of male antennal glands in Cynipoidea (Hymenoptera). Zool. Scr. 28, 165–174. doi: 10.1046/j.1463-6409.1999.00013.x
Kühbandner, S., Sperling, S., Mori, K., and Ruther, J. (2012). Deciphering the signature of cuticular lipids with contact sex pheromone function in a parasitic wasp. J. Exp. Biol. 215, 2471–2478. doi: 10.1242/jeb.071217
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kasuya, N., Mitsui, H., Shinsuke, I., Watada, M., and Kimura, M. T. (2013). Ecological, morphological and molecular studies on Ganaspis individuals (Hymenoptera: Figitidae) attacking Drosophila suzukii (Diptera: Drosophilidae). Appl. Entomol. Zool. 48, 87–92. doi: 10.1007/s13355-012-0156-0
Löfstedt, C., Herrebout, W. M., and Menken, S. B. J. (1991). Sex pheromones and their potential role in the evolution of reproductive isolation in smale ermine moths (Yponomeutidae). Chemoecology 2, 20–28. doi: 10.1007/BF01240662
Nelson, D. R. (1993). “Methyl-branched lipids in insects,” in Insect Lipids, eds D. W. Stanley and D. R. Nelson (Lincoln, NE: University of Nebraska Press), 271–316.
Nordlander, G., and Grijpma, P. (1991). Systematics and biology of Rhoptromeris strobigena sp. n., a parasitoid of chloropids inhabiting conifer cones (Hymenoptera: Cynipoidea: Eucoilidae). Entomol. Scand. 22, 209–218. doi: 10.1163/187631291X00084
Nordlander, G. (1980). Revision of the genus Leptopilina Förster, 1869, with notes on the status of some other genera (Hymenoptera, Cynipoidea: Eucoilidae). Entomol. Scand. 11, 428–453.
Novkovic, B., Mitsui, H., Suwito, A., and Kimura, M. T. (2011). Taxonomy and phylogeny of Leptopilina species (Hymenoptera: Cynipoidea: Figitidae) attacking frugivorous drosophilid flies in Japan, with description of three new species. Entomol. Sci. 14, 333–346. doi: 10.1111/j.1479-8298.2011.00459.x
Pintureau, B., Chapelle, L., and Delobel, B. (1999). Effects of repeated thermic and antibiotic treatments on a Trichogramma (Hym., Trichogrammatidae) symbiont. J. Appl. Entomol. 123, 473–483. doi: 10.1046/j.1439-0418.1999.00412.x
Pintureau, B., Lassablière, F., Daumal, J., and Grenier, S. (2002). Does a cyclic natural thermal cure occur in Wolbachia-infected Trichogramma species? Ecol. Entomol. 27, 466–372. doi: 10.1046/j.1365-2311.2002.00416.x
Quinlan, J. (1988). A revision of some aftrotropical genera of Eucoilidae (Hymenoptera). Bull. Br. Mus. Nat. Hist. 56, 171–229.
Ridley, M. (1993). Clutch size and mating frequency in parasitic Hymenoptera. Am. Nat. 142, 893–910. doi: 10.1086/285579
Romani, R., Isidoro, N., Riolo, P., Bin, F., Fortunato, A., Turillazzi, S., et al. (2005). A new role for antennation in paper wasps (Hymenoptera, Vespidae): antennal courtship and sex dimorphic glands in antennomeres. Insectes Soc. 52, 92–102. doi: 10.1007/s00040-004-0780-y
Ruther, J. (2013). “Novel insights into pheromone-mediated communication in parasitic hymenopterans,” in Chemical Ecology of Insect Parasitoids, eds E. Wajnberg and S. Colazza (Hoboken, NJ: Wiley-Blackwell), 112–144.
Saul, G. B. II. (1961). An analysis of non-reciprocal cross incompatibility in Mormoniella vitripennis (Walker). Z. Vererbungsl. 92, 28–33. doi: 10.1007/BF01854097
Stökl, J., Hofferberth, J., Pritschet, M., Brummer, M., and Ruther, J. (2012). Stereoselective chemical defense in the Drosophila parasitoid Leptopilina heterotoma is mediated by (−)-iridomymrecin and (+)-isoiridomyrmecin. J. Chem. Ecol. 38, 331–339. doi: 10.1007/s10886-012-0103-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stökl, J., Dandekar, A.-T., and Ruther, J. (2014). High chemical diversity in a wasp pheromone: a blend of methyl 6-methylsalicylate, fatty alcohol acetates and cuticular hydrocarbons releases courtship behavior in the Drosophila parasitoid Asobara tabida. J. Chem. Ecol. 40, 159–168. doi: 10.1007/s10886-014-0378-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Steiner, S., Hermann, N., and Ruther, J. (2006). Characterization of a female-produced courtship pheromone in the parasitoid Nasonia vitripennis. J. Chem. Ecol. 32, 1687–1702. doi: 10.1007/s10886-006-9102-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stewart-Jones, A., Dewhirst, S. Y., Durrant, L., Fitzgerald, J. D., Hardie, J., Hooper, A. M., et al. (2007). Structure, ratio and patterns of release in the sex pheromone of an aphid, Dysaphis plantaginea. J. Exp. Biol. 210, 4335–4344. doi: 10.1242/jeb.009944
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Symonds, M. R. E., and Elgar, M. A. (2004). The mode of pheromone evolution: evidence from bark beetles. Proc. Biol. Sci. 271, 839–846. doi: 10.1098/rspb.2003.2647
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Symonds, M. R. E., and Elgar, M. A. (2008). The evolution of pheromone diversity. Trends Ecol. Evol. 23, 220–228. doi: 10.1016/j.tree.2007.11.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Völkl, W., Hübner, G., and Dettner, K. (1994). Interactions between Alloxysta brevis (Hymenoptera, Cynipoidea, Alloxystidae) and honeydew-collecting ants: how an aphid hyperparasitoid overcomes ant aggression by chemical defense. J. Chem. Ecol. 20, 2901–2915. doi: 10.1007/BF02098397
van den Assem, J., Jachmann, F., and Simbolotti, P. (1980). Courtship behaviour of Nasonia vitripennis (Hym., Pteromalidae): Some qualitative, experimental evidence for the role of pheromones. Behaviour 75, 301–307. doi: 10.1163/156853980X00456
van den Assem, J. (1968). Reproductive behaviour of Pseudeucoila bochei (Hymenoptera: Cynipidae). Neth. J. Zool. 19, 641–649. doi: 10.1163/002829669X00080
van den Assem, J. (1986). Insect Parasitoids Chapter Mating Behaviour in Parasitic Wasps. London: Academic Press. 137–167.
Weiss, I., Rössler, T., Hofferberth, J., Brummer, M., Ruther, J., and Stökl, J. (2013). A nonspecific defensive compound evolves into a competition avoidance cue and a female sex pheromone. Nat. Commun. 4:2767. doi: 10.1038/ncomms3767
Keywords: iridomyrmecin, Drosophila, parasitoid wasp, chemical diversity, saltational evolution
Citation: Weiss I, Hofferberth J, Ruther J and Stökl J (2015) Varying importance of cuticular hydrocarbons and iridoids in the species-specific mate recognition pheromones of three closely related Leptopilina species. Front. Ecol. Evol. 3:19. doi: 10.3389/fevo.2015.00019
Received: 19 December 2014; Accepted: 17 February 2015;
Published: 16 March 2015.
Edited by:
Sebastien Lebreton, Swedish University of Agricultural Sciences, SwedenReviewed by:
Giovanni Benelli, University of Pisa, ItalyStefano Colazza, University of Palermo, Italy
Copyright © 2015 Weiss, Hofferberth, Ruther and Stökl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johannes Stökl, Institute for Zoology, University of Regensburg, Universitätsstraße 31, Regensburg 93053, Germany johannes.stoekl@ur.de
 Ingmar Weiss
Ingmar Weiss John Hofferberth
John Hofferberth Joachim Ruther
Joachim Ruther Johannes Stökl
Johannes Stökl