Information transfer beyond the waggle dance: observational learning in bees and flies
- 1Centre de Recherches sur la Cognition Animale, Université Toulouse III, Université Paul Sabatier, Toulouse, France
- 2Centre National de la Recherche Scientifique, Centre de Recherches sur la Cognition Animale, Toulouse, France
Social information transfer is part of the success of animal societies and has been documented in a variety of taxa, from slime molds to humans. In invertebrates, the historical research focus has been on the specialized signals shaped by selection to convey information, such as the honeybee waggle dance. However, growing evidence shows that invertebrates also commonly glean critical information about their environment by observing others. For instance, a bumblebee's choice between novel flower species is influenced by the observation of the foraging choices of more experienced conspecifics. Recent studies suggest that these seemingly complex learning abilities can be explained in terms of simple associative learning, whereby individuals learn to associate social cues (conditioned stimuli) to reward cues (unconditioned stimuli). Here, we review the behavioral evidence of observational learning both in bees and Drosophila. We discuss the validity of associative accounts of observational learning and the potential neural circuits mediating visual social learning in these model species to define future research avenues for studying the neurobiology of social cognition in miniature brains.
Introduction
The waggle dance of the honey bee constitutes one of the most sophisticated systems known for information transfer about profitable food sources (Von Frisch, 1967). A successful forager performs a stereotyped behavior within the hive, originally described as a “dance,” which conveys information about the direction and distance from the hive of an exploited food source (Riley et al., 2005; Grüter and Farina, 2009; Seeley, 2010). This dance “language” is a case of true communication (Markl, 1985) whereby the sender explicitly transfers information to the receivers in order to modify their behavior. Yet, many animals use social information to learn about their environment simply by attending the behavior of others (Danchin et al., 2004; Galef and Laland, 2005). This type of social learning differs from true communication in that the demonstrator does not explicitly attempt to modify the receiver's behavior. In this review we will focus on social learning based on visual observation of a demonstrator's behavior (Zentall, 2012).
Invertebrates provide paradigmatic examples of observational learning. For instance, Octopus observers that are allowed to watch conditioned Octopus demonstrators choosing one of two different colored objects presented simultaneously, consistently select the same object as the demonstrators did (Fiorito and Scotto, 1992). Other fascinating cases are found among insects. These animals exhibit developed learning capacities and accessible miniature nervous systems, thereby constituting ideal organisms for dissecting the neural and molecular bases of learning (Giurfa, 2013). Model species, such as the honey bee Apis mellifera (Menzel, 1999; Giurfa, 2007; Galizia et al., 2012), and the fruit fly Drosophila melanogaster (Heisenberg, 2003; Davis, 2005; Guven-Ozkan and Davis, 2014) have been extremely useful for pioneer studies on the mechanisms of learning and memory.
Here we focus on social bees (Hymenoptera) and Drosophila, in which observational learning has been documented. Our goal, beyond various excellent reviews on the topic of social learning in insects (e.g., Leadbeater and Chittka, 2007b; Dukas, 2008; Grüter and Leadbeater, 2014; Leadbeater, 2015) is to provide a mechanistic view of these complex behaviors. It has recently been suggested that social learning can emerge from simple associations between a relevant stimulus (unconditioned stimulus, US), such as a food reward or a predator threat, and a conspecific's presence or behavior (conditioned stimulus, CS), which is not different from individual learning of non-social cues (Leadbeater and Chittka, 2007b; Avarguès-Weber et al., 2011; Heyes, 2011; Giurfa, 2012). Using this idea, we discuss the nature of learning associations and the neural circuits potentially involved in insect observational learning.
Observational Learning in Bees
Behavioral Evidences
During their foraging activities, bees need to exploit multiple floral resources whose reward levels change rapidly and unpredictably (Heinrich, 1979, 2004; Goulson, 2010; Lihoreau et al., 2012b). A forager's choice of plant species is guided by unlearned preferences and learned information about current reward levels gained through individual sampling (Raine et al., 2006). As many pollinators often work concurrently in a meadow, information acquired individually can be complemented by social information (Grüter and Leadbeater, 2014), but also by information gained inside the nest through communication and food exchange (Biesmeijer and Seeley, 2005; Arenas et al., 2008). It has long been known that during foraging, bees are attracted to visibly occupied flowers [e.g., bumblebees (Brian, 1957); stingless bees (Slaa et al., 2003); honey bees (Von Frisch, 1967), suggesting that they learn to exploit food resources by copying the choices of other bees (Romanes, 1884)]. Recent studies with bumblebees have shown that individuals can indeed glean information from watching other foragers, and change accordingly their floral choices (Leadbeater and Chittka, 2005, 2007a; Worden and Papaj, 2005; Kawaguchi et al., 2007; Baude et al., 2011; Avarguès-Weber and Chittka, 2014a,b), their choice of location (Leadbeater and Chittka, 2005, 2009; Kawaguchi et al., 2006; Baude et al., 2008; Dawson and Chittka, 2012; Plowright et al., 2013) and their handling strategies (Leadbeater and Chittka, 2008; Goulson et al., 2013; Mirwan and Kevan, 2013).
In particular, when bees observe the floral choices of conspecific demonstrators from behind a transparent screen (Figure 1A), they land more often on the flower type chosen by demonstrators in tests where the demonstrators are absent, than compared to non-observing controls (conspecifics separated from demonstrators by an opaque screen) (Worden and Papaj, 2005). Similar results are obtained with artificial demonstrators (inanimate model bees made of resin), thus indicating that visual cues associated with the presence of conspecifics are sufficient to promote social acquisition of flower preferences (Worden and Papaj, 2005; Dawson et al., 2013; Avarguès-Weber and Chittka, 2014b).
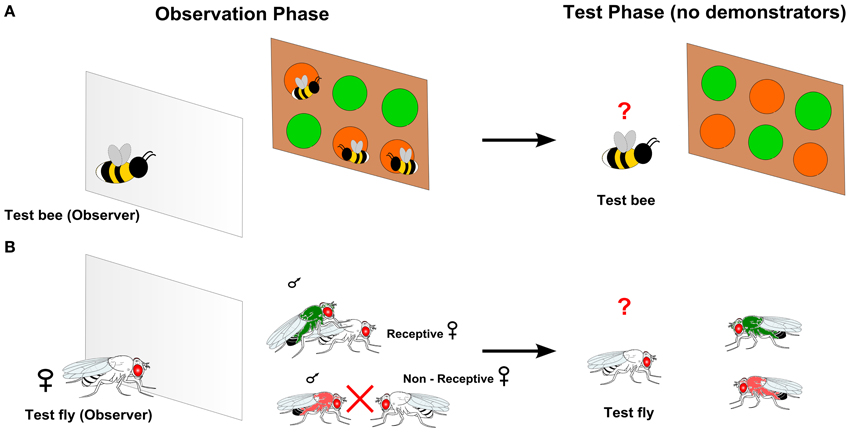
Figure 1. Experimental set-ups used to study observational learning in insects. (A) An observer bumblebee can observe a vertical array of two unfamiliar flower types (e.g., green or orange) through a transparent screen. Demonstrators (e.g., living bees, dead bees or model bees) are associated to one flower type (e.g., green). Observational learning is successful if the observer bee preferentially visits flowers of the same type as the demonstrators did, when subsequently tested alone (Worden and Papaj, 2005; Avarguès-Weber and Chittka, 2014b). (B) An observer Drosophila (virgin female) can observe interactions between two pairs of demonstrator males (dusted with color pigments) and females through a screen. The observer is first presented a colored male (e.g., green) mating with a sexually receptive female. The pair of demonstrators is then replaced by a male of a different color (e.g., pink) and a non-sexually receptive female. Observational learning is successful if the observer preferentially copulates with the male of the same color as the demonstrator did, when subsequently given the choice between new males of each color (Mery et al., 2009).
An Associative Account
The fact that bees acquire flower preferences by observing foragers through a screen (Worden and Papaj, 2005; Dawson et al., 2013; Avarguès-Weber and Chittka, 2014b) is incompatible with a simple associative hypothesis in which foragers associate profitable flowers (US) with the presence of the conspecifics (CS). In this case, the demonstrators are no longer present when the observer makes its choice, which implies that the positive value of conspecifics has been transferred to the associated flowers themselves. An explanation based on associative learning is still possible but in the form of a phenomenon termed second-order conditioning (Pavlov, 1927). Under second-order conditioning, bees learn a positive association between a conspecific (CS1) and a food reward (US), due to past-shared foraging experience on the same resources. When observing a conspecific feeding from an unknown flower, the close association between the CS1 (conspecifics) and the flower induces the bee to consider flower cues themselves as indicative of reward (CS2). Such a mechanism would lead to a socially acquired preference for all flowers sharing the same characteristics and not only for the occupied flowers (Leadbeater and Chittka, 2007b; Dawson et al., 2013).
If observational learning for new flower preferences is the consequence of a second-order conditioning, then impeding or modifying the first association should alter flower preference. In bumblebees, this hypothesis is supported by the fact that naive bees with no previous social foraging experience tend to ignore the choices of conspecifics in their foraging decision (Dawson et al., 2013; Avarguès-Weber and Chittka, 2014a), suggesting that there is a decisive role of prior associations between social cues and a reward. Additionally, the preference for socially demonstrated flowers can be reversed into avoidance if the tested bees are allowed to form an association between the conspecifics and a bitter aversive solution beforehand (Dawson et al., 2013).
The associative learning hypothesis also predicts that non-social cues should promote social-like learning behavior given that they have been previously associated with rewarding flowers. However, bumblebees follow different flower choice strategies when social cues (model bees) or non-social cues (wooden white blocks) are used as indicators of reward (Avarguès-Weber and Chittka, 2014a). If they have learned that the white blocks are present on a rewarded flower color, they will choose afterwards a different color, only if it displays the presence of the blocks. Unoccupied flowers presenting that same color will not be chosen. This behavior can be explained in simple associative terms as the blocks were previously associated with reward. The situation is different if bees have learned to forage on a flower color on which bees were present. In this case, they will choose afterwards a novel color if dummy bees are present on it but, in addition, they will extend their choice to unoccupied flowers with that same color. Thus, in the latter case, the bees' choice is not restricted to the flowers occupied by a model bee but includes all flowers presenting the same characteristics (Avarguès-Weber and Chittka, 2014a). The difference between these two scenarios may reside in the fact that foragers gather experience in the field in which conspecifics, contrary to wooden blocks, are not only predictive of reward but also mobile. This mobility may allow transferring the choice form the occupied flower to the unoccupied flower as long as both share the same color. This strategy may be advantageous: in many typical flower species, it might not be adaptive for a pollinator to visit a flower that is currently being drained by another visitor. Instead, it would be more useful to steer toward unvisited flowers of the same plant species where the visitation activities of others indicate that the flower type is profitable. Therefore, specific mechanisms might have evolved to promote efficient social information use in flower foraging, suggesting that social and asocial learning are dissociated.
It is, however, also possible that there is no special role of conspecifics in the flower generalization pattern observed, specifically when demonstrators indicate rewarding flowers. Bees that never got the chance to see live conspecifics within or outside the nest and were only familiarized with model static bees pinned on flowers show a pattern of choices similar to that of bees familiarized and tested with non-social cues (Avarguès-Weber and Chittka, 2014a). By contrast, bees exposed to live bees experienced that the socially indicated flower species will sometimes be occupied by demonstrators and sometimes not, occasionally in rapid succession, and the situation might change while the observer is on the flower. Thus, observers will get exposure to mobile demonstrators physically dissociated from the flowers, and this in turn may favor future generalization to unoccupied but socially indicated flowers. The possibility that non-social moving objects could generate a social-like flower choice pattern remains to be tested. Alternatively, social learning specificity may require a familiarization phase with live conspecifics to learn to associate the conspecific's chemical signature acquired within the nest (Krasnec and Breed, 2012), and with the species visual characteristics.
Relying on the choices of others is not always an adaptive strategy (Giraldeau et al., 2002; Laland, 2004; Rieucau and Giraldeau, 2011; Grüter and Leadbeater, 2014). In the most extreme case of a population always favoring social learning over individual sampling, an ecological dead end would be quickly reached with some resources being overexploited while others are left unexplored. From the colony perspective, keeping enough individual information acquisition is essential for social learning behavior to remain beneficial (Giraldeau et al., 2002; Rieucau and Giraldeau, 2011; Grüter and Leadbeater, 2014). Bumblebees present restrictions in the use of social over individual learning that are consistent with the theory. Indeed, the response to social cues is flexible, depending on the context of the observation (Leadbeater and Chittka, 2005; Kawaguchi et al., 2007; Baude et al., 2011). In a field study, B. diversus foragers were given a choice between two inflorescences attached to a stick (“interview bouquet”), one of which was occupied by a conspecific (freshly killed bee pinned on flowers). While bees preferred occupied inflorescences when they were presented two unfamiliar flower species, they avoided conspecifics when confronted with flower species found in their daily environment (Kawaguchi et al., 2007). Presumably, this conditional use of social information enables bees to maximize their foraging efficiency when searching for novel food items while minimizing the costs of competition when they know resource locations (Laland, 2004; Dall et al., 2005). Competition level is also reduced by another flexible usage of social information as B. terrestris foragers do not follow the preferences of demonstrators when the conspecifics density on the flowers patch is too high (Baude et al., 2011; Plowright et al., 2013).
All these results suggest that observational social learning in bumblebees is the consequence of simple associative processes and specific enhanced attention toward conspecifics as cue providers (stimulus enhancement) and/or places where these conspecifics can be seen (local enhancement) (Zentall, 2006; Leadbeater, 2015). An intricate interplay between evolutionary adaptation to attend to conspecific cues, individual experience with such cues and their contingencies with salient aspects of the environment is probably at hand to generate the observed complexity of observational social learning.
Observational Learning in Drosophila
Behavioral Evidence
Although considerable knowledge on insect observational learning comes from research on bumblebees, visual social learning has also been described in a non-social species, the fruit fly D. melanogaster. In this species, females learn the quality of potential mating partners by observing their success with other females (Mery et al., 2009) (Figure 1B). This capacity was shown in experiments in which two artificial male phenotypes were produced by dusting flies with green or pink pigments (Mery et al., 2009). An observer (virgin) female was placed in a glass tube where she could see demonstrator males and females through a colorless screen. In the first observation phase, the demonstrator male (e.g., green) successfully mated with the demonstrator female. In the second phase, a male of another color (e.g., pink) was paired with a non-receptive female, thus leading to unsuccessful copulation attempts by the male. When the observer female was later presented with two males (green and pink) simultaneously, she preferentially mated with the male of the color that was associated with a successful copulation (e.g., green) (Figure 1B). This effect disappeared when the observers could not directly observe the demonstrator flies (Mery et al., 2009). This example shows that observational learning is not restricted to social insects. Rather, it seems to be a general capacity issued from the insects' faculty to learn associations in their environment. Observational learning in Drosophila could also be interpreted as a special case of associative visual learning. It is possible that the vision of a female copulating with a male acts as a biologically relevant reinforcement to be associated with the male color (CS). Under this hypothesis, observer flies should learn to associate a male color phenotype with a successful mating signal. Later, when confronted with males of different phenotypes, observers would preferentially choose the learned color based on a simple associative memory. Visual associative learning has been extensively documented in Drosophila in an individual context (Heisenberg et al., 2001; Foucaud et al., 2010; Schnaitmann et al., 2010; Ofstad et al., 2011; Vogt et al., 2014) so that transferring this capacity to a mating, observational context is plausible.
Genetic and Molecular Basis
The discovery of mate choice copying in a main model organism holds considerable promises to unravel the genetic and molecular substrates of observational learning in insects, an approach that is currently not possible in bees. While such analysis has not been conducted yet, recent studies have begun to identify the neural substrates of Drosophila visual learning that may also be involved in observational learning in particular if the associative learning hypothesis is verified.
Different forms of visual learning are mediated by the central complex (CX). This neuropil is located between the protocerebral brain hemispheres and comprises four interconnected regions: the fan-shaped body, the ellipsoid body, the protocerebral bridge and the paired noduli (Figure 2). It receives information from visual processing neuropils (lamina, lobula, medulla) connected to each compound eye, and whose learning-dependent plasticity has not been explored until now. The implication of the CX in visual recognition was first demonstrated using a flight simulator, in which a fly whose head is attached to a torque meter controls the position of visual patterns on the walls of a circular arena with its flight direction (Heisenberg et al., 2001). Using this approach, flies can be trained to learn to avoid visual cues (such as colors and geometric forms, CS) due to their association with an aversive stimulus (a heat beam, US). The sequence of CS and US stimuli can either be controlled by the fly itself (operant training) or by the experimenter (Pavlovian training) (Brembs and Heisenberg, 2000). Memory mutants lacking the Rutabaga (Rut) protein—a type 1 Ca2+/Calmodulin-dependent adenylyl cyclase that produces cAMP—display impaired operant and Pavlovian visual learning, indicating that Rut plays a decisive role in the US/CS association, probably as a coincidence detector of the visual CS and the heat US (Liu et al., 2006). By using the UAS/GAL4 system to differentially express Rut in specific subsets of cerebral neurons, it has been shown that the discrimination of visual patterns of different elevations or orientations requires two different groups of neurons extending branches in the fan-shaped body, respectively the F5 and F1 neurons (Liu et al., 2006). Another subset of large field neurons located in the ellipsoid body (the ring neurons R2 and R4m) are also involved in recognition of several pattern features through excitatory and inhibitory visual subfields (Pan et al., 2009; Seelig and Jayaraman, 2013) (Figure 2). Taken together, these results demonstrate the implication of the CX in visual learning and memory through dynamic interactions between the ellipsoid-body and the fan-shaped body.
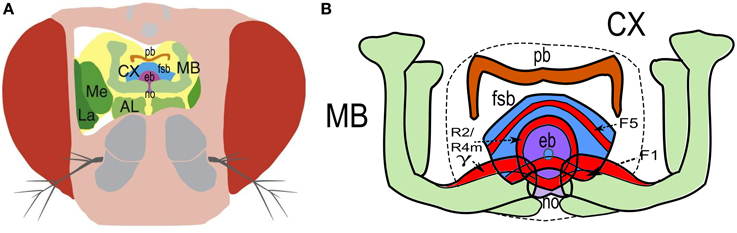
Figure 2. Neurobiological structures involved in visual learning in the Drosophila brain. (A) A schematic diagram of the head of D. melanogaster revealing several major neuropiles: the lamina (La) and medulla (Me) involved in visual processing, the antennal lobes (AL) involved in olfactory processing and the mushroom bodies (MB) and the central complex (CX) involved, among other functions, in visual learning. Subdivisions of the central complex: the protocerebral bridge (pb; orange), the fan-shaped body (fsb; blue), the ellipsoid body (eb; magenta) and the noduli (no; purple). Adapted from Niven (2010) with permission. (B) Enlargement of the central part of the brain showing the neuropiles and their substructures involved in visual learning (highlighted in red), as the F1 and F5 neurons extending branches in the fan-shaped body, the R2 and R4m ring neurons located in the ellipsoid body, and the MBs gamma-neurons.
Recent studies also point toward a contribution of the mushroom bodies (MBs) in visual memories. The MBs are central brain structures involved in olfactory learning and memory (Davis, 2005), courtship (McBride et al., 1999), locomotion (Martin et al., 1998), and sleep (Joiner et al., 2006; Pitman et al., 2006), among others. Despite the absence of obvious anatomical connections between the optic lobes to the MBs (Barth and Heisenberg, 1997; Otsuna and Ito, 2006; Mu et al., 2012), the volume of the MB calyces (dendrites) changes with light regime, suggesting that MBs are involved in visual information processing (Barth and Heisenberg, 1997). Indeed, it has been shown that MBs are required in visual context generalization (Liu et al., 1999) and could stabilize visual memories against context changes (Brembs and Wiener, 2006). Interestingly, the MBs (γ neurons) seem also necessary for the memorization of simple associations between color stimuli and a sugar reward or with an electric shock (Vogt et al., 2014). Presumably, the implication of the CX or the MBs might be dependent of the locomotion state (flying vs. walking) as flies were trained in a flight simulator in one case (Liu et al., 2006; Pan et al., 2009) vs. a walking plate in the other (Vogt et al., 2014). Locomotor activity is known to affect the activity of octopamine neurons and the behavioral response to CO2 (Suver et al., 2012; Wasserman et al., 2013), and thus possibly modifies neural pathways involved in visual information memorization (Kottler and Van Swinderen, 2014; Vogt et al., 2014). Additionally, walking activity has no direct effect on the activity of ring neurons of the CX while flying activity significantly decreases their responses to visual stimuli (Seelig and Jayaraman, 2013).
Importantly, the MBs and their associate dopaminergic signaling are also involved in visual attention in the form of visual tracking of a moving bar (Xi et al., 2008; Van Swinderen et al., 2009). They may consequently mediate the specific attentional state elicited by social visual cues during an observational learning task.
Conclusion
Placing social learning within the conceptual framework of associative learning is an appealing approach for explaining seemingly complex behavior in insects with pinhead-sized brains. However, bumblebee studies are beginning to suggest that observational learning by insects does not only reflect visual associative learning but also involves attentional processing of social cues as information providers.
In parallel, the neurogenetic approaches well mastered in Drosophila hold considerable promises in revealing the neural basis of such complex behavior. Future investigations may target the CX and MBs as the potential neuronal structures involved, given their implication in visual learning and attention.
The popularity of bees and fruit flies as models for visual cognition research associated with the abundance of genomic information available make them ideal study systems to explore the genetic, molecular, neuronal, and behavioral basis of visual social learning, a major challenge on the way of understanding the evolutionary relationships between animal brains, cognitive capacities and their social environment (Lihoreau et al., 2012a).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
M.G. thanks the Institut Universitaire de France, the Human Frontier Science Program (HFSP) and the French National Research Agency (ANR) for generous support. M.L. was funded by an IDEX—University of Toulouse Starting Grant and a Research Grant from the Foundation Fyssen. A.A-W. thanks the l'Oréal Foundation for support. All authors acknowledge the support of the French Research Council (CNRS) and the University of Toulouse.
References
Arenas, A., Fernández, V. M., and Farina, W. M. (2008). Floral scents experienced within the colony affect long-term foraging preferences in honeybees. Apidologie 39, 714–722. doi: 10.1051/apido:2008053
Avarguès-Weber, A., and Chittka, L. (2014a). Local enhancement or stimulus enhancement? Bumblebee social learning results in a specific pattern of flower preference. Anim. Behav. 97, 185–191. doi: 10.1016/j.anbehav.2014.09.020
Avarguès-Weber, A., and Chittka, L. (2014b). Observational conditioning in flower choice copying by bumblebees Bombus terrestris: influence of observer distance and demonstrator movement. PLoS ONE 9:e88415. doi: 10.1371/journal.pone.0088415
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Avarguès-Weber, A., Deisig, N., and Giurfa, M. (2011). Visual cognition in social insects. Annu. Rev. Entomol. 56, 423–443. doi: 10.1146/annurev-ento-120709-144855
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Barth, M., and Heisenberg, M. (1997). Vision affects mushroom bodies and central complex in Drosophila melanogaster. Learn. Mem. 4, 219–229. doi: 10.1101/lm.4.2.219
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baude, M., Dajoz, I., and Danchin, E. (2008). Inadvertent social information in foraging bumblebees: effects of flower distribution and implications for pollination. Anim. Behav. 76, 1863–1873. doi: 10.1016/j.anbehav.2008.08.010
Baude, M., Danchin, É., Mugabo, M., and Dajoz, I. (2011). Conspecifics as informers and competitors: an experimental study in foraging bumble-bees. Proc. Biol. Sci. 278, 2806–2813. doi: 10.1098/rspb.2010.2659
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Biesmeijer, J., and Seeley, T. (2005). The use of waggle dance information by honey bees throughout their foraging careers. Behav. Ecol. Sociobiol. 59, 133–142. doi: 10.1007/s00265-005-0019-6
Brembs, B., and Heisenberg, M. (2000). The operant and the classical in conditioned orientation of Drosophila melanogaster at the flight simulator. Learn. Mem. 7, 104–115. doi: 10.1101/lm.7.2.104
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brembs, B., and Wiener, J. (2006). Context and occasion setting in Drosophila visual learning. Learn. Mem. 13, 618–628. doi: 10.1101/lm.318606
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brian, A. D. (1957). Differences in the flowers visited by four species of bumble-bees and their causes. J. Anim. Ecol. 26, 71–98. doi: 10.2307/1782
Dall, S. R. X., Giraldeau, L.-A., Olsson, O., McNamara, J. M., and Stephens, D. W. (2005). Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193. doi: 10.1016/j.tree.2005.01.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Danchin, E., Giraldeau, L.-A., Valone, T. J., and Wagner, R. H. (2004). Public information: from nosy neighbors to cultural evolution. Science 305, 487–491. doi: 10.1126/science.1098254
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Davis, R. L. (2005). Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu. Rev. Neurosci. 28, 275–302. doi: 10.1146/annurev.neuro.28.061604.135651
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dawson, E. H., Avarguès-Weber, A., Leadbeater, E., and Chittka, L. (2013). Learning by observation emerges from simple associations in an insect model. Curr. Biol. 23, 727–730. doi: 10.1016/j.cub.2013.03.035
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dawson, E. H., and Chittka, L. (2012). Conspecific and heterospecific information use in bumblebees. PLoS ONE 7:e31444. doi: 10.1371/journal.pone.0031444
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dukas, R. (2008). Evolutionary biology of insect learning. Annu. Rev. Entomol. 53, 145–160. doi: 10.1146/annurev.ento.53.103106.093343
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fiorito, G., and Scotto, P. (1992). Observational learning in Octopus vulgaris. Science 256, 545–547. doi: 10.1126/science.256.5056.545
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Foucaud, J., Burns, J. G., and Mery, F. (2010). Use of spatial information and search strategies in a water maze analog in Drosophila melanogaster. PLoS ONE 5:e15231. doi: 10.1371/journal.pone.0015231
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Galef, B. G., and Laland, K. N. (2005). Social learning in animals: empirical studies and theoretical models. Bioscience 55, 489–499. doi: 10.1641/0006-3568(2005)055[0489:SLIAES]2.0.CO;2
Galizia, C. G., Eisenhardt, D., and Giurfa, M. (2012). Honeybee Neurobiology and Behaviour. Dordrecht; Heidelberg; London, NY: Springer. doi: 10.1007/978-94-007-2099-2
Giraldeau, L. A., Valone, T. J., and Templeton, J. J. (2002). Potential disadvantages of using socially acquired information. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 1559–1566. doi: 10.1098/rstb.2002.1065
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Giurfa, M. (2007). Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 193, 801–824. doi: 10.1007/s00359-007-0235-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Giurfa, M. (2012). Social learning in insects: a higher-order capacity? Front. Behav. Neurosci. 6:57. doi: 10.3389/fnbeh.2012.00057
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Giurfa, M. (2013). Cognition with few neurons: higher-order learning in insects. Trends Neurosci. 36, 285–294. doi: 10.1016/j.tins.2012.12.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Goulson, D., Park, K. J., Tinsley, M. C., Bussière, L. F., and Vallejo-Marin, M. (2013). Social learning drives handedness in nectar-robbing bumblebees. Behav. Ecol. Sociobiol. 67, 1141–1150. doi: 10.1007/s00265-013-1539-0
Grüter, C., and Farina, W. M. (2009). The honeybee waggle dance: can we follow the steps? Trends Ecol. Evol. 24, 242–247. doi: 10.1016/j.tree.2008.12.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grüter, C., and Leadbeater, E. (2014). Insights from insects about adaptive social information use. Trends Ecol. Evol. 29, 177–184. doi: 10.1016/j.tree.2014.01.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Guven-Ozkan, T., and Davis, R. L. (2014). Functional neuroanatomy of Drosophila olfactory memory formation. Learn. Mem. 21, 519–526. doi: 10.1101/lm.034363.114
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Heinrich, B. (1979). Resource heterogeneity and patterns of movement in foraging bumblebees. Oecologia 40, 235–245. doi: 10.1007/BF00345321
Heisenberg, M. (2003). Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4, 266–275. doi: 10.1038/nrn1074
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Heisenberg, M., Wolf, R., and Brembs, B. (2001). Flexibility in a single behavioral variable of Drosophila. Learn. Mem. 8, 1–10. doi: 10.1101/lm.8.1.1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Heyes, C. M. (2011). What's social about social learning? J. Comp. Psychol. 126, 193–202. doi: 10.1037/a0025180
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Joiner, W. J., Crocker, A., White, B. H., and Sehgal, A. (2006). Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441, 757–760. doi: 10.1038/nature04811
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kawaguchi, L. G., Ohashi, K., and Toquenaga, Y. (2006). Do bumble bees save time when choosing novel flowers by following conspecifics? Funct. Ecol. 20, 239–244. doi: 10.1111/j.1365-2435.2006.01086.x
Kawaguchi, L. G., Ohashi, K., and Toquenaga, Y. (2007). Contrasting responses of bumble bees to feeding conspecifics on their familiar and unfamiliar flowers. Proc. Biol. Sci. 274, 2661–2667. doi: 10.1098/rspb.2007.0860
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kottler, B., and Van Swinderen, B. (2014). Taking a new look at how flies learn. Elife 3:e03978. doi: 10.7554/eLife.03978
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Krasnec, M. O., and Breed, M. (2012). “Eusocial evolution and the recognition systems in social insects,” in Sensing in Nature, ed C. Lopez-Larrea (New York, NY: Landes Bioscience & Springer Science), 78–92.
Laland, K. N. (2004). Social learning strategies. Learn. Behav. 32, 4–14. doi: 10.3758/BF03196002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leadbeater, E. (2015). What evolves in the evolution of social learning? J. Zool. 295, 4–11. doi: 10.1111/jzo.12197
Leadbeater, E., and Chittka, L. (2005). A new mode of information transfer in foraging bumblebees? Curr. Biol. 15, R447–R448. doi: 10.1016/j.cub.2005.06.011
Leadbeater, E., and Chittka, L. (2007a). The dynamics of social learning in an insect model, the bumblebee (Bombus terrestris). Behav. Ecol. Sociobiol. 61, 1789–1796. doi: 10.1007/s00265-007-0412-4
Leadbeater, E., and Chittka, L. (2007b). Social learning in insects—from miniature brains to consensus building. Curr. Biol. 17, R703–R713. doi: 10.1016/j.cub.2007.06.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leadbeater, E., and Chittka, L. (2008). Social transmission of nectar-robbing behaviour in bumble-bees. Proc. Biol. Sci. 275, 1669–1674. doi: 10.1098/rspb.2008.0270
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leadbeater, E., and Chittka, L. (2009). Bumble-bees learn the value of social cues through experience. Biol. Lett. 5, 310–312. doi: 10.1098/rsbl.2008.0692
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lihoreau, M., Latty, T., and Chittka, L. (2012a). An exploration of the social brain hypothesis in insects. Front. Physiol. 3:442. doi: 10.3389/fphys.2012.00442
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lihoreau, M., Raine, N. E., Reynolds, A. M., Stelzer, R. J., Lim, K. S., Smith, A. D., et al. (2012b). Radar tracking and motion-sensitive cameras on flowers reveal the development of pollinator multi-destination routes over large spatial scales. PLoS Biol. 10:e1001392. doi: 10.1371/journal.pbio.1001392
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, G., Seiler, H., Wen, A., Zars, T., Ito, K., Wolf, R., et al. (2006). Distinct memory traces for two visual features in the Drosophila brain. Nature 439, 551–556. doi: 10.1038/nature04381
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, L., Wolf, R., Ernst, R., and Heisenberg, M. (1999). Context generalization in Drosophila visual learning requires the mushroom bodies. Nature 400, 753–756. doi: 10.1038/22919
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Markl, H. (1985). Manipulation, modulation, information, cognition: some of the riddles of communication. Exp. Behav. Ecol. Sociobiol. 31, 163–194.
Martin, J.-R., Ernst, R., and Heisenberg, M. (1998). Mushroom bodies suppress locomotor activity in Drosophila melanogaster. Learn. Mem. 5, 179–191.
McBride, S. M., Giuliani, G., Choi, C., Krause, P., Correale, D., Watson, K., et al. (1999). Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron 24, 967–977. doi: 10.1016/S0896-6273(00)81043-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Menzel, R. (1999). Memory dynamics in the honeybee. J. Comp. Physiol. A 185, 323–340. doi: 10.1007/s003590050392
Mery, F., Varela, S. A. M., Danchin, É., Blanchet, S., Parejo, D., Coolen, I., et al. (2009). Public versus personal information for mate copying in an invertebrate. Curr. Biol. 19, 730–734. doi: 10.1016/j.cub.2009.02.064
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mirwan, H., and Kevan, P. G. (2013). Social learning in bumblebees (Bombus impatiens): worker bumblebees learn to manipulate and forage at artificial flowers by observation and communication within the colony. Psyche 2013:768108. doi: 10.1155/2013/768108
Mu, L., Ito, K., Bacon, J. P., and Strausfeld, N. J. (2012). Optic glomeruli and their inputs in Drosophila share an organizational ground pattern with the antennal lobes. J. Neurosci. 32, 6061–6071. doi: 10.1523/JNEUROSCI.0221-12.2012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Niven, J. E. (2010). Visuomotor control: Drosophila bridges the gap. Curr. Biol. 20, R309–R311. doi: 10.1016/j.cub.2010.02.028
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ofstad, T. A., Zuker, C. S., and Reiser, M. B. (2011). Visual place learning in Drosophila melanogaster. Nature 474, 204–207. doi: 10.1038/nature10131
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Otsuna, H., and Ito, K. (2006). Systematic analysis of the visual projection neurons of Drosophila melanogaster. I. Lobula-specific pathways. J. Comp. Neurol. 497, 928–958. doi: 10.1002/cne.21015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pan, Y., Zhou, Y., Guo, C., Gong, H., Gong, Z., and Liu, L. (2009). Differential roles of the fan-shaped body and the ellipsoid body in Drosophila visual pattern memory. Learn. Mem. 16, 289–295. doi: 10.1101/lm.1331809
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pavlov, I. P. (1927). Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. New York, NY: Dover.
Pitman, J. L., McGill, J. J., Keegan, K. P., and Allada, R. (2006). A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 441, 753–756. doi: 10.1038/nature04739
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Plowright, C. M. S., Ferguson, K. A., Jellen, S. L., Xu, V., Service, E. W., and Dookie, A. L. (2013). Bumblebees occupy: when foragers do and do not use the presence of others to first find food. Insectes Soc. 60, 517–524. doi: 10.1007/s00040-013-0318-2
Raine, N. E., Ings, T. C., Dornhaus, A., Saleh, N., and Chittka, L. (2006). Adaptation, genetic drift, pleiotropy, and history in the evolution of bee foraging behavior. Adv. Stud. Behav. 36, 305–354. doi: 10.1016/S0065-3454(06)36007-X
Rieucau, G., and Giraldeau, L.-A. (2011). Exploring the costs and benefits of social information use: an appraisal of current experimental evidence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 949–957. doi: 10.1098/rstb.2010.0325
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Riley, J. R., Greggers, U., Smith, A. D., Reynolds, D. R., and Menzel, R. (2005). The flight paths of honeybees recruited by the waggle dance. Nature 435, 205–207. doi: 10.1038/nature03526
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schnaitmann, C., Vogt, K., Triphan, T., and Tanimoto, H. (2010). Appetitive and aversive visual learning in freely moving Drosophila. Front. Behav. Neurosci. 4:10. doi: 10.3389/fnbeh.2010.00010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Seelig, J. D., and Jayaraman, V. (2013). Feature detection and orientation tuning in the Drosophila central complex. Nature 503, 262–266. doi: 10.1038/nature12601
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Slaa, E. J., Wassenberg, J., and Biesmeijer, J. C. (2003). The use of field–based social information in eusocial foragers: local enhancement among nestmates and heterospecifics in stingless bees. Ecol. Entomol. 28, 369–379. doi: 10.1046/j.1365-2311.2003.00512.x
Suver, M. P., Mamiya, A., and Dickinson, M. H. (2012). Octopamine neurons mediate flight-induced modulation of visual processing in Drosophila. Curr. Biol. 22, 2294–2302. doi: 10.1016/j.cub.2012.10.034
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Van Swinderen, B., McCartney, A., Kauffman, S., Flores, K., Agrawal, K., Wagner, J., et al. (2009). Shared visual attention and memory systems in the Drosophila brain. PLoS ONE 4:e5989. doi: 10.1371/journal.pone.0005989
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vogt, K., Schnaitmann, C., Dylla, K. V., Knapek, S., Aso, Y., Rubin, G. M., et al. (2014). Shared mushroom body circuits underlie visual and olfactory memories in Drosophila. Elife 3:e02395. doi: 10.7554/eLife.02395
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Von Frisch, K. (1967). The Dance Language and Orientation of Bees. Cambridge: Harvard University Press.
Wasserman, S., Salomon, A., and Frye, M. A. (2013). Drosophila tracks carbon dioxide in flight. Curr. Biol. 23, 301–306. doi: 10.1016/j.cub.2012.12.038
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Worden, B. D., and Papaj, D. R. (2005). Flower choice copying in bumblebees. Biol. Lett. 1, 504–507. doi: 10.1098/rsbl.2005.0368
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Xi, W., Peng, Y., Guo, J., Ye, Y., Zhang, K., Yu, F., et al. (2008). Mushroom bodies modulate salience-based selective fixation behavior in Drosophila. Eur. J. Neurosci. 27, 1441–1451. doi: 10.1111/j.1460-9568.2008.06114.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zentall, T. R. (2006). Imitation: definitions, evidence, and mechanisms. Anim. Cogn. 9, 335–353. doi: 10.1007/s10071-006-0039-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zentall, T. R. (2012). Perspectives on observational learning in animals. J. Comp. Psychol. 126, 114–128. doi: 10.1037/a0025381
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: bumblebees, Drosophila, social cognition, social learning, visual cognition
Citation: Avarguès-Weber A, Lihoreau M, Isabel G and Giurfa M (2015) Information transfer beyond the waggle dance: observational learning in bees and flies. Front. Ecol. Evol. 3:24. doi: 10.3389/fevo.2015.00024
Received: 06 December 2014; Accepted: 24 February 2015;
Published: 12 March 2015.
Edited by:
Madeleine Beekman, The University of Sydney, AustraliaReviewed by:
Nancy R. Kohn, The College of New Jersey, USAChristoph Grüter, University of Lausanne, Switzerland
Copyright © 2015 Avarguès-Weber, Lihoreau, Isabel and Giurfa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Giurfa, Centre de Recherches sur le Cognition Animale, Université Toulouse III, Université Paul Sabatier, 118 Route de Narbonne, 31062 Toulouse Cedex 9, France martin.giurfa@univ-tlse3.fr
†These authors have contributed equally to this work.
 Aurore Avarguès-Weber
Aurore Avarguès-Weber Mathieu Lihoreau
Mathieu Lihoreau Guillaume Isabel
Guillaume Isabel Martin Giurfa
Martin Giurfa