Chemicals and chemoreceptors: ecologically relevant signals driving behavior in Drosophila
- 1Centre National de la Recherche Scientifique, UMR6265 Centre des Sciences du Goût et de l'Alimentation, Dijon, France
- 2INRA, UMR1324 Centre des Sciences du Goût et de l'Alimentation, Dijon, France
- 3Université de Bourgogne, UMR Centre des Sciences du Goût et de l'Alimentation, Dijon, France
Insects encounter a vast repertoire of chemicals in their natural environment, which can signal positive stimuli like the presence of a food source, a potential mate, or a suitable oviposition site as well as negative stimuli such as competitors, predators, or toxic substances reflecting danger. The presence of specialized chemoreceptors like taste and olfactory receptors allows animals to detect chemicals at short and long distances and accordingly, trigger proper behaviors toward these stimuli. Since the first description of olfactory and taste receptors in Drosophila melanogaster 15 years ago, our knowledge on the identity, properties, and function of specific chemoreceptors has increased exponentially. In the last years, multidisciplinary approaches combining genetic tools with electrophysiological techniques, behavioral recording, evolutionary analysis, and chemical ecology studies are shedding light on our understanding on the ecological relevance of specific chemoreceptors for the survival of Drosophila in their natural environment. In this review we discuss the current knowledge on chemoreceptors of both the olfactory and taste systems of the fruitfly. We focus on the relevance of particular receptors for the detection of ecologically relevant cues such as pheromones, food sources, and toxic compounds, and we comment on the behavioral changes that the detection of these chemicals induce in the fly. In particular, we give an updated outlook of the chemical communication displayed during one of the most important behaviors for fly survival, the courtship behavior. Finally, the ecological relevance of specific chemicals can vary depending on the niche occupied by the individual. In that regard, in this review we also highlight the contrast between adult and larval systems and we propose that these differences could reflect distinctive requirements depending on the change of ecological niche occupied by Drosophila along its life cycle.
Introduction
Chemoreception is defined as the physiological response to a chemical stimulus. Depending on the spatial scale, a classical division exists between olfaction and taste chemoreception. Olfaction is involved in the detection of volatile molecules coming from long distances, while taste is a contact sense that allows detection of molecules at a short distance. Highly volatile hydrophobic molecules can be rapidly transported by air and, once they reach the living organism, activate olfactory receptors. On the contrary, hydrophilic molecules are less volatile and they most likely activate taste receptors when presented at a short distance. This definition might not be suitable for aquatic environments where solubility instead of volatility is the determinant factor for long-distance transport of molecules (Mollo et al., 2014).
One of the favorite model organisms for the study of olfaction and taste perception is the fly Drosophila melanogaster. In the last two decades, and due to its amazing repertoire of genetic tools, Drosophila has been at the leading front in the discovery of chemoreceptors and chemoreceptive neuronal pathways that account for the behavioral responses toward ecologically relevant chemicals. Even more, the extensive work done in Drosophila helps us to better understand the chemoreceptive systems of insects relevant for human's health, such as the mosquitos Anopheles gambiae and Aedes aegypti, dangerously efficient vectors of malaria and Dengue hemorrhagic fever.
Flies are able to perceive relevant chemical cues present in their food, in host plant, and those produced by conspecific. Attractive odors and tastants in the food can induce feeding, while toxic compounds present in food or produced by host plants trigger avoidance. Before activating the oviposition motor program, female flies carefully analyze the chemical composition of the substrate. Also, conspecific chemical cues are essential for aggregation, aggression, and courtship. All of these effects depend on proper detection of chemical cues at the level of olfactory and gustatory receptors present in dedicated structures.
Here we will review the extensive recent research focused on detection of ecologically relevant chemicals in flies and its behavioral consequences. Firstly, we will very briefly outline the olfactory and gustatory system of fly adults and larvae, giving more emphasis to the description of the different families of chemoreceptors. Secondly, we will present several examples of chemoreceptors involved in the detection of chemical signals that impact on behaviors relevant for fly survival, such as feeding, toxic compounds avoidance, and oviposition site and sexual partner selection. Finally, we will review and discuss the ecological relevance of specific chemicals and chemoreceptors in the context of the particular requirements of two stages of Drosophila life cycle, the larva and the adult fly.
Olfactory and Gustatory Chemoreceptors in Flies: Several Receptors Distributed in Several Families
The olfactory organs of the adult fly are located on the third antennal segment (also known as funiculus) and on the maxillary palps, where three different types of sensilla, the basiconic, the trichoid, and the coeloconic, harbor the olfactory sensory neurons (OSNs) (Figure 1A). In the OSNs, olfactory receptors directly contact their specific ligands. From the periphery, OSNs send axonal projections to specific glomeruli in the antennal lobe, the first olfactory relay center in the brain. In the antennal lobe, the odor signals are processed by local interneurons and projection neurons. Local interneurons connect different glomeruli mainly triggering later inhibition (Silbering and Galizia, 2007), and projection neurons transmit the olfactory trace to higher centers in the lateral horn and mushroom bodies (reviewed in Stocker, 1994; Laissue and Vosshall, 2008). Careful anatomical description of the olfactory system allowed building a near complete map of OSN's connectivity. OSNs expressing the same olfactory receptor project into the same unique glomerulus in the antennal lobe (Couto et al., 2005; Fishilevich and Vosshall, 2005; Goldman et al., 2005). In addition, OSNs harbored in different type of sensilla project into distinct regions of the antennal lobe, highlighting the level of topographic organization of the olfactory system (Couto et al., 2005).
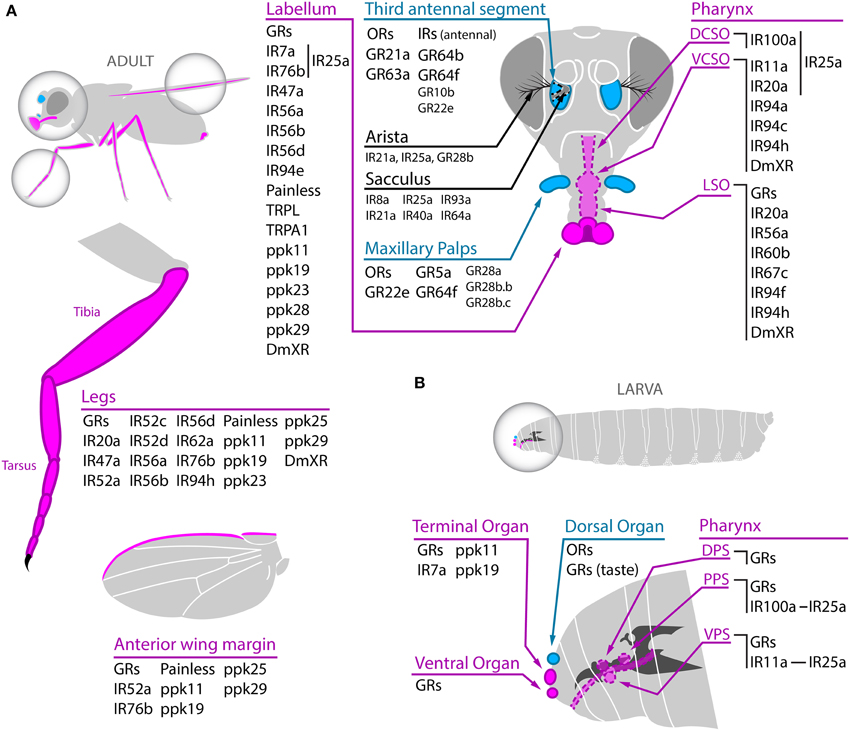
Figure 1. Chemoreceptors expressed in adult (A) and larval (B) olfactory and gustatory organs. (A) Adult taste organs (magenta) are located on the proboscis's labellum, the tarsus and tibia of the leg, the anterior wing margin, the female genitalia (not shown), and in internal taste structures in the pharynx (DCSO, VCSO, and LSO). Within those taste organs, GRs, several members of the IR family, some members of the TRP family (Painless, TRPL and TRPA1), ppk channels (ppk11, ppk19, ppk23, ppk25, ppk28, and ppk29), and the insect orphan receptor DmXR work as chemoreceptors (although some of them have not been confirmed as bona fide receptors yet). At least IR7a, IR76b in the labellum and IR11a, IR20a, and IR100a in internal taste organs are coexpressed with IR25a. The third antennal segment and the maxillary palps are the adult olfactory organs (cyan) and they mainly harbor ORs and IRs, although expression of 7 GRs (GR10b, GR22e, the CO2 receptors GR21a and GR63a, and the sugar receptors GR5a, GR64b, and GR64f) has also been detected. Plus, GR28a, GR28b.b, and GR28b.c show expression in maxillary palp neurons of unknown function. IRs and GRs expressed in the arista and the sacculus and with unknown function are also displayed in the antenna scheme. (B) In the larva taste receptors, mainly GRs and some IRs, are localized in the terminal, ventral, and to a lesser extent dorsal organs as well as in several internal taste organs (DPS, PPS, and VPS). As in adults, IR11a and IR100a are expressed in gustatory receptor neurons coexpressing IR25a. The dorsal organ harbors ORs involved in olfactory responses. Dotted line indicates internal or deeper structures.
In contraposition to olfactory organs, taste organs are widely distributed in the adult body, with external gustatory centers on the proboscis's labellum, legs, wings, and female genitalia, and internal taste structures in the pharynx (Figure 1A). The labellum is the principal taste organ of the adult fly and it harbors two major types of sensilla, the taste bristles and taste pegs, wherein gustatory receptors expressed in gustatory receptor neurons (GRNs) directly detect tastant. In the pharynx, the labral sense organ (LSO), the ventral and dorsal cibarial sense organs (VCSO and DCSO), and a ventral and a dorsal row of “fish-trap” bristles allow taste detection after ingestion (reviewed in Stocker, 1994; Montell, 2009). From the taste organs located in the mouth parts, proboscis, and legs, GRNs transmit directly or through activation of interneurons the gustatory information to the subesophageal ganglion (SOG), a dedicated taste center in the brain (Wang et al., 2004). Some taste-like sensilla are also present on the genitalia and on the wing margin, but their precise role is still under investigation (Boll and Noll, 2002; Yanagawa et al., 2014). In the SOG, axonal projections coming from different peripheral tissues are segregated even if they contain the same receptor (Wang et al., 2004). Even more, bitter and sugar sensing neurons clearly segregate in the SOG, demonstrating that the first gustatory relay center displays a topographic and functional organization (Thorne et al., 2004; Wang et al., 2004).
The external chemosensory organs of the larvae are all located in the cephalic lobe with the exception of some putative taste organs in thoracic and abdominal segments (Dambly-chaudière and Ghysen, 1986; Scott et al., 2001) (Figure 1B). Larval olfactory structures are located in the dorsal organ, while external gustatory structures are mainly distributed between the terminal and ventral organs, and to a lesser extent, the dorsal organ. In addition, three internal pharyngeal organs, the dorsal, ventral, and posterior pharyngeal sense organs (DPS, VPS, and PPS, respectively) include mainly taste sensilla (Stocker, 2008). Similar to the case of the adult gustatory system, the larval SOG shows a certain topographic and functional organization although in the larvae there is no complete segregation between external and internal GRNs axonal projections (Colomb et al., 2007; Kwon et al., 2011). The dorsal organ is composed of the central “dome” that harbors the dendrites of the 21 larval OSNs and a few putative taste sensilla (Gerber and Stocker, 2007; Stocker, 2008). This small number of OSNs contrasts with the around 1300 OSNs that are present in adults. Despite these numeric differences, the adult and larval olfactory pathways share the same design (Stocker, 2009). Nonetheless, the larval olfactory system is not just a reduced version of the adult system because some olfactory receptors are only expressed in the larval stage (Stocker, 2008). Even more, the larval local interneurons in the antennal lobe do not keep resemblance with their adult counterparts and in the larva they tightly connect gustatory and olfactory centers (Thum et al., 2011).
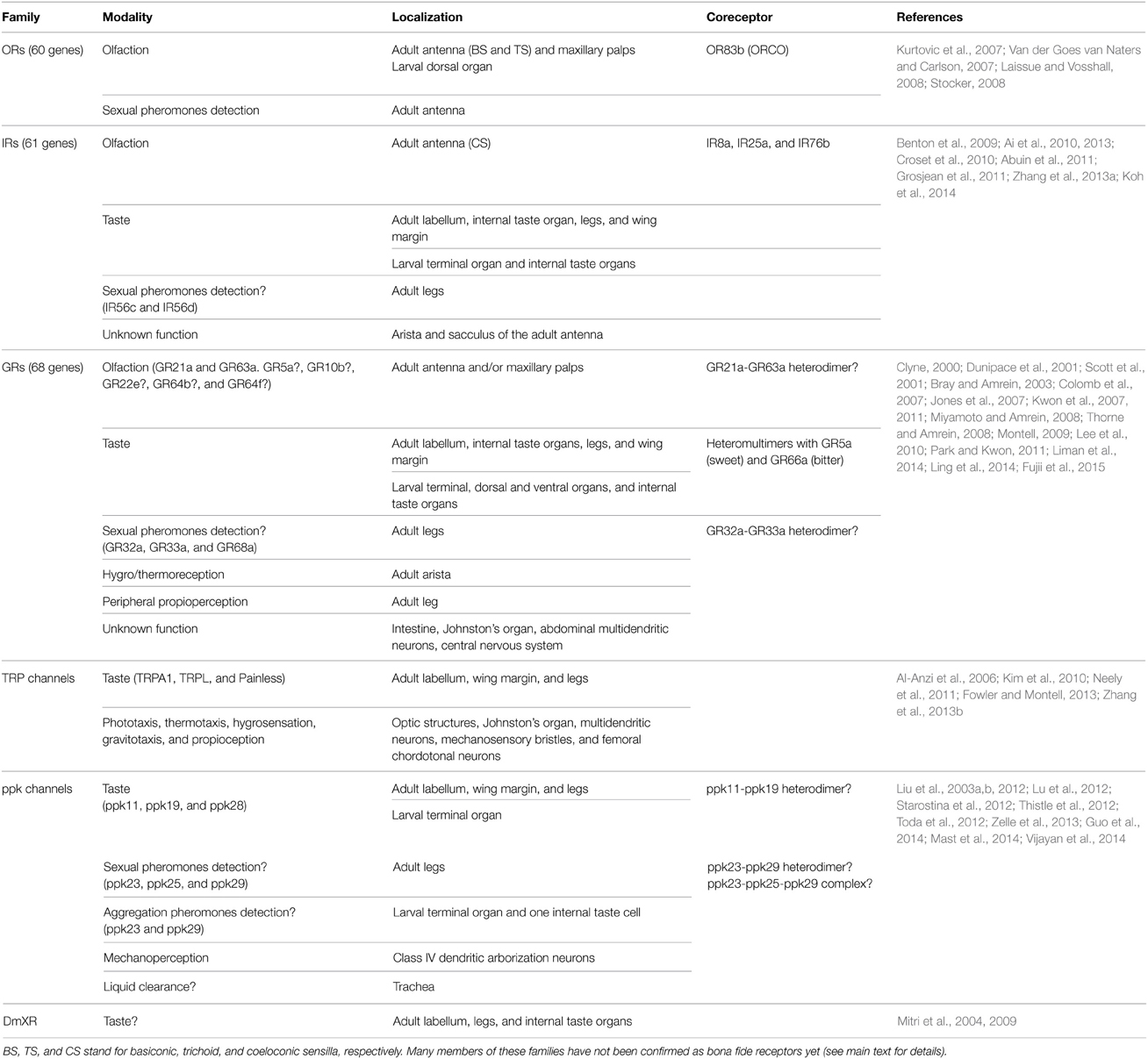
Regarding the chemoreceptors, in flies more than 150 receptors are distributed in three principal families, the gustatory receptors (GRs), the odorant receptors (ORs), and the ionotropic receptors (IRs). In addition, some members of the TRP family and degenerin/epithelial sodium channel/pickpocket (ppk) channels as well as the insect orphan G-protein-coupled DmXR are either bona fide chemoreceptors or they are tightly involved in chemoreception in flies (Table 1).
In mammals, most chemoreceptors are classic seven-transmembrane G-protein-coupled receptors (GPCRs) (Chandrashekar et al., 2006; Spehr and Munger, 2009). In insects, GRs and ORs are also seven transmembrane domain proteins with a no amino acid sequence homology compared to mammalian ORs and GRs (Vosshall et al., 1999; Clyne, 2000; Scott et al., 2001); however, insect ORs have a topology opposite to mammalian GPCRs, with cytoplasmic N-termini and extracellular C-termini (Benton et al., 2006). A phylogenetic analysis indicated that insect OR family is an expanded lineage within the ancestral insect GR family (Robertson et al., 2003), highlighting a common evolutionary origin. In addition, insect IRs are ligand-gated ion channels involved in chemoreception and they belong to the superfamily of ionotropic glutamate receptors (Benton et al., 2009). Interestingly, a subgroup of IRs are expressed in the antenna and, contrary to what is seen in most of the ORs, they are highly conserved within insects, both in sequence and expression pattern, suggesting that antennal IRs might represent the ancestral olfactory receptor family in insects (Croset et al., 2010). Below, we will describe in more detail some characteristics of these families of chemoreceptors, separating between those involved in olfaction and the ones dedicated to taste perception.
Chemoreceptors and Olfactory Detection
The first family of chemoreceptors described in Drosophila was that of odorant receptors (ORs) comprising 60 genes expressed in subpopulations of OSNs (Clyne et al., 1999; Vosshall et al., 1999) mainly in basiconic and trichoid sensilla. The 60 OR genes give rise through alternative splicing to 62 proteins, and of those expressed in the adult system some are exclusively expressed in the antenna, and some others in the maxillary palps (Laissue and Vosshall, 2008). In addition, 13 ORs are only detectable in olfactory organs of the larvae (Couto et al., 2005; Fishilevich et al., 2005; Kreher et al., 2005) (Figure 1 and Table 1). OR83b (also known as ORCO) is expressed in all adult maxillary palp OSNs, around 75% of the antennal OSNs, and in all the larval OSNs. ORCO forms heterodimeric complexes with other OR protein (Larsson et al., 2004; Neuhaus et al., 2005) and, with a few exceptions, only one pair “ORCO-conventional OR” is expressed per OSN (Couto et al., 2005). Furthermore, ORCO's expression is necessary both in adults and in larvae for electrophysiological and behavioral responses to several odorants, demonstrating that ORCO is an essential coreceptor for all the ORs (Larsson et al., 2004).
By using a mutant antennal neuron that lacks its endogenous chemoreceptors (the “empty neuron” system; Dobritsa et al., 2003), the group of Carlson performed extensive electrophysiological characterizations of ORs responsiveness toward relevant food-derived odorants, both in the adult antenna and the larval olfactory system (Hallem et al., 2004; Hallem and Carlson, 2006; Kreher et al., 2008; Mathew et al., 2013). Individual receptors range along a continuum from narrowly to broadly tuned although, in general, reducing odor concentration reduces the number of ORs activated (Hallem and Carlson, 2006). In contraposition to the extensive analysis of electrophysiological responses at the periphery, little is known about the relevance of specific ORs in driving behavior. In larvae, some odors weakly activate ORs but trigger strong behavioral responses and, on the contrary, other odors can strongly activate ORs but elicit weak behavioral responses (Mathew et al., 2013; Grewal et al., 2014); this highlights that odor coding in higher-olfactory centers is a relevant process that modulates odor-trigger behaviors.
Drosophila olfactory responses also rely on the activity of IRs (Benton et al., 2009). Of the 61 members of the IR family, 18 are normally expressed in the adult antenna in coeloconic sensilla, the sacculus, or the arista, while no expression has been described so far in olfactory organs of the larvae (Benton et al., 2009; Croset et al., 2010) (Figure 1 and Table 1). In the adult olfactory system, IRs act in combination of up to three subunits with IR8a or IR25a and maybe other IRs serving as general coreceptors for odor-specific IRs (Abuin et al., 2011). A comparative electrophysiological analysis of the two olfactory subsystems, ORs and IRs, in the adult antenna revealed some differences in ligand specificity. In general, IR8a+ OSNs respond to carboxylic acids and some aldehydes, whereas IR25+ OSNs are preferentially activated by amines, and OR+ OSNs are more dedicated to the detection of esters, alcohols, and ketones (Silbering et al., 2011).
In addition to ORs and IRs, pioneer expression analysis indicated that four gustatory receptors, GR21a, GR63a, GR10b, and GR22e, are expressed in the adult antenna (Dunipace et al., 2001; Scott et al., 2001), and at least GR21a and GR63a are bona fide olfactory receptors (Jones et al., 2007; Kwon et al., 2007). Plus, a very recent study demonstrated the expression of three sugar GRs, GR5a, GR64a, and GR64f, in adult olfactory organs although their function in these cells has not been determined yet (Fujii et al., 2015) (Figure 1 and Table 1).
Chemoreceptors and Gustatory Detection
At least three families of chemoreceptors or channels are involved in gustatory responses: GRs, IRs, and TRPs channels. Of those, GRs were the first to be characterized as contact receptors and, up to now, the majority of taste and pheromones receptors found in flies belong to this extensive family of 68 members expressed differentially in all the taste organs of the adult and the larva (Clyne, 2000; Dunipace et al., 2001; Scott et al., 2001). At least 38 GRs are expressed in the labellum (Weiss et al., 2011) and 28 GRs in the leg (Ling et al., 2014). A minimum of 39 GRs is present in larval taste organs, and most of them are presumed to be bitter receptors (Colomb et al., 2007; Kwon et al., 2011) (Figure 1 and Table 1). In the labellum, two GRs, GR5a and GR66a, are extensively expressed in non-overlapping GRNs populations. GR5a+ neurons respond to sugar and elicit feeding behavior, while bitter compounds activate GR66a+ neurons and trigger avoidance behavior (Dahanukar et al., 2001; Chyb et al., 2003; Thorne et al., 2004; Marella et al., 2006).
In contrast to the simple heterodimers of ORs and antennal IRs, GRs seem to act as heteromultimeric complexes. Eight GRs, among them GR5a, are expressed in a combinatorial manner giving rise to a minimum of eight sets of sweet-sensing neurons in adult taste organs (Fujii et al., 2015). GR66a, GR93a, and GR33a appear to be coexpressed in most if not all bitter-sensing GRNs in the labellum (Lee et al., 2009; Moon et al., 2009); plus, GR33a is necessary in those GRNs for the response to all bitter compounds tested (Moon et al., 2009). Moreover, in larvae GRs are expressed combinatorially and up to 17 subunits could be present in a single GRN (Kwon et al., 2011).
In addition to GRs, evidence from expression profile analysis and loss of function studies point to other proteins as taste receptors or at least as essential components for certain taste modalities. Several IR members are expressed in taste organs where they could act as taste receptors. Both in adults and in larvae, IR25a is coexpressed with IR7a, IR11a, and IR100a in taste organs (Croset et al., 2010). In adults, IR76b is located in L-type sensilla in the labellum where it acts as a low-salt detector, and additional expression is also seen in GRNs in the leg tarsi and wing margins (Zhang et al., 2013a). Very recently, several members of the non-antennal IRs were found in almost all the taste organs of the adult fly (Koh et al., 2014) (Figure 1 and Table 1). In addition, at least three members of the TRP channels, TRP1, Painless, and TRPL are expressed in bitter neurons in the labellum where they are involved in detection of aversive compounds (Al-Anzi et al., 2006; Kim et al., 2010; Zhang et al., 2013b). Also, several ppk channels are expressed in taste neurons where they are required for relevant taste modalities such as low-salt detection (Liu et al., 2003b) and intraspecific chemical communication in larvae (Mast et al., 2014), and water perception (Cameron et al., 2010) and chemical communication during courtship in adults (Liu et al., 2012; Lu et al., 2012; Starostina et al., 2012; Thistle et al., 2012; Toda et al., 2012; Vijayan et al., 2014). Finally, DmXR, a receptor homologous to metabotropic glutamate receptors that has lost the ability to bind glutamate (Mitri et al., 2004), may also act as a taste receptor. It is expressed in GRNs in the labellum, the leg, and internal taste organs (LSO and VCSO) and it was originally described as a L-canavanine amino acid receptor, even if its exact role on chemoreception is still under debate (Mitri et al., 2009; Lee et al., 2012) (Figure 1 and Table 1).
Ecological Relevance of Specific Chemoreceptors
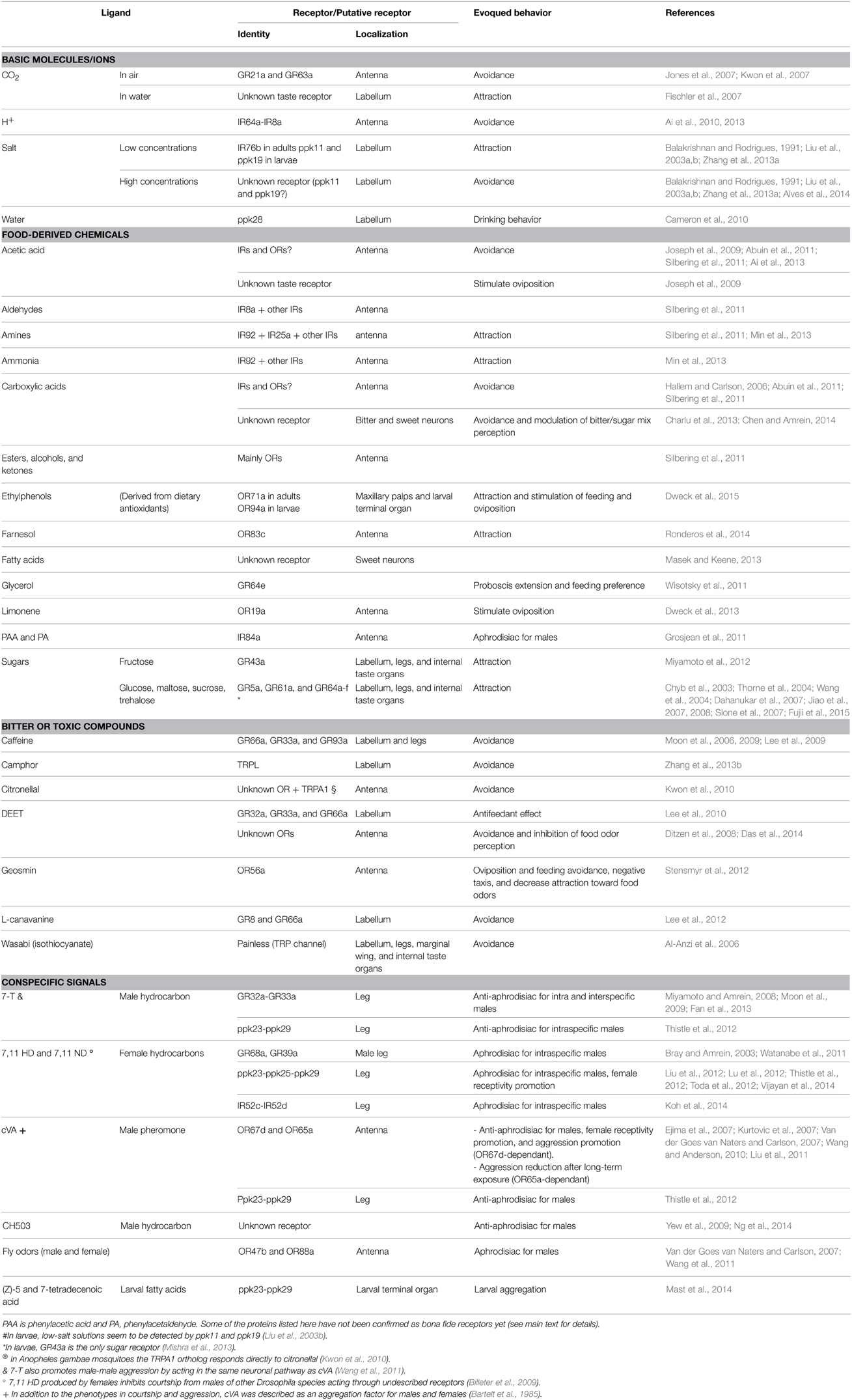
Genomic comparative studies highlight the rapid evolution of chemoreceptors both in number and identity (Robertson et al., 2003; Croset et al., 2010). This feature led to the hypothesis that changes at the level of chemosensory systems contribute to the diversification of behaviors (Cande et al., 2013). Evidence in favor of this hypothesis comes mainly from comparative studies of closely related Drosophila species with different behaviors, as it is the case of D. melanogaster and D. sechellia. D. melanogaster is a generalist species that can survive in several fruit substrates, while D. sechellia is a host-plant specialist. Interestingly, D. melanogaster has a complex olfactory system that allows detection of hundreds of fruit-derived odors; D. sechellia, on the contrary, has lost many chemoreceptors that are not relevant for its very specialized ecology (Stensmyr et al., 2003; Cande et al., 2013). Although a very provocative hypothesis, it is difficult to prove that mutations in chemoreceptor gene loci are important driving forces behind behavioral change. Nonetheless, there is no doubt that the current chemoreceptors allow detection of ecologically relevant chemicals present in the fruitfly's environment. In this section we will discuss the ecological relevance of specific chemoreceptors related to behaviors such as food searching and the analysis of its composition, avoidance of toxic or bitter compounds, oviposition site selection, and the search for a sexual partner (Table 2).
Chemoreceptors Involved in Food Sources Searching and Food Composition Analysis
During larval stage, Drosophila individuals increase their size in about 200 times in 4 days. Such high growth rate requires an immense amount of energy, and to obtain it larvae have to eat constantly (Tennessen and Thummel, 2011). The group of Vosshall studied the relevance of general odor detection for survival during this critical period when larvae are foraging for food. In a situation of excess food, anosmic foraging larvae show a survival rate comparable to that of larvae with an intact olfactory system. However, under limited food conditions or high competition, larvae need their sense of olfaction to localize a new food source (Asahina et al., 2008). Thus, the evolutionary advantage of an olfactory system tuned to food odors is reasonably evident. The importance of olfaction detection is also evident under mixed-age high-density laboratory cultures when younger larvae could turn toward cannibalism. In that scenario, chemosensory cues released from victim's injuries during the first attack could be relevant to induce aggregation and further collective cannibalistic behavior (Vijendravarma et al., 2013).
Larvae show general attraction toward a big range of odors of varied chemical characteristics, such as acids, alcohols, ketones, aldehydes, esters, and to a lesser extent, some terpenes and aromatics (Fishilevich et al., 2005; Khurana and Siddiqi, 2013). Among these odorants, some are present in common tropical fruits where Drosophila flies are naturally found (Khurana and Siddiqi, 2013). Interestingly, these odorants elicit stronger attractive responses than odors produced by non-fruit substrates, including flowers, leaves, and bark (Khurana and Siddiqi, 2013). However, not only the chemical identity of the odorant but also its concentration constitutes relevant information coded by the olfactory system. Depending on the concentration, some odorants could trigger responses that range from indifference to attraction or in some cases, even repulsion (Stensmyr, 2003). The dose-responses curves could be different even for odorants with related chemical structure, so each odorant should be analyzed individually (Khurana and Siddiqi, 2013). On the other hand, in the taste system, the concentration of the tastant is also a relevant cue. For example, both larvae and adult flies prefer low and reject high concentration of salts (Miyakawa, 1981; Zhang et al., 2013a). In this sense, the concentration of the chemical must be taking into account when analyzing the effects on the olfaction and taste systems.
In adult flies, food-derived odors also trigger attraction. At long distances, the presence of vinegar, or even acetic acid alone, is sufficient to trigger upwind flight attraction in starved flies (Becher et al., 2010; Lebreton et al., 2012). At short distances, fly odors together with food odors elicit attraction (Ruebenbauer et al., 2008; Lebreton et al., 2012). Some food-derived odors activate several olfactory receptors while others target only few or just one receptor (Hallem and Carlson, 2006). For example, OR83c receptor is essential for the detection of farnesol, a compound found in citrus fruit peel that triggers attraction in adult flies (Ronderos et al., 2014). A very recent study demonstrated that flies are attracted to antioxidants supplemented food thanks to their detection through olfactory cues. Polyphenol antioxidants normally present in fly food are converted by yeast into ethylphenols, and these strongly activate OR71a in adults and OR94a in larvae, leading to attraction in both stages and promoting feeding and oviposition in adults (Dweck et al., 2015). Dietary antioxidants offer protection against oxidative stress in flies (Jimenez-Del-Rio et al., 2010), so an olfactory pathway dedicated to the detection of antioxidant-supplemented food may, most likely, increase D. melanogaster fitness. In addition, IR92+ neurons detect ammonia and several different amines and activate a specific neuronal pathway dedicated to attractive behavior (Min et al., 2013). Interestingly, ammonia and amines are highly attractive for both flies and mosquito, although the ecological context in which they find them is different; flies may perceive ammonia and amines produced by fruit decomposition, while mosquito are attracted to the same compounds but emanated from animal hosts (Meijerink et al., 2001; Min et al., 2013). Anyway, in both species, a specific receptor to ammonia and amines appears to be important for the detection of a food source.
Another interesting case of chemoreception of ecologically relevant signals is that of CO2 detection. CO2 is a complex signal for the fly since it is a component of the aversive Drosophila stress odorant (Suh et al., 2004) and also an indicator of food source suitability (Faucher et al., 2006). It is sensed through GR21a and GR63a in the olfactory system and mediates avoidance behavior both in adult and in larvae (Jones et al., 2007; Kwon et al., 2007). This aversive olfactory effect is also mediated by IR64a via the solubilization of CO2 in the antennal hemolymph leading to the production of H+ (Ai et al., 2010). The aversive response of atmospheric CO2 depends on life stage, sex, and olfactory context (Faucher et al., 2006). Furthermore, adult flies also perceive CO2 in solution (carbonated water) through unknown taste receptors, and the taste of carbonated water mediates acceptance behavior (Fischler et al., 2007). Direct orthologs of GR21a and GR63a are present in mosquitos like A. aegypti and A. gambiae, and these are also dedicated to CO2 perception. However, the underlying neuronal circuits do not seem to be conserved because in Drosophila CO2 perception triggers avoidance behavior while in mosquitos it elicits attraction toward the host (Robertson and Kent, 2009; McMeniman et al., 2014). Interestingly, the “domestic” form of A. aegypti has evolved host specificity toward humans, and the olfactory coreceptor ORCO is crucial to discriminate human from non-human hosts (DeGennaro et al., 2013). Moreover, this human preference correlates with antennal expression of OR4a, a receptor for the human odorant sulcatone (McBride et al., 2014).
As most animals, flies ingest sugar for nutrition purposes; therefore the ability to taste sweet substances ensures the ingestion of these vital compounds. In the case of the adult D. melanogaster, contrary to the larvae, the arrangement of chemoreceptors involved in sugar detection is complex. Several GRs are coexpressed in the same sugar-responding neuron in the labellum and the leg (Fujii et al., 2015). In particular, GR5a expressed in taste neurons detects trehalose, the principal sugar found in the insect's hemolymph (Chyb et al., 2003), and triggers attraction in adults (Thorne et al., 2004; Wang et al., 2004). GR5a, GR61a, and GR64a-f mediate responses to sucrose, maltose, and several other sugars (Dahanukar et al., 2007; Jiao et al., 2007, 2008; Slone et al., 2007; Fujii et al., 2015). GR43a is a fructose receptor in adults but is also necessary for the detection of multiple sugars in larvae (Miyamoto et al., 2012; Mishra et al., 2013). We will discuss in depth the possible rationales behind the complexity of sugar detection in adult flies as well as the differences with the simpler larval system in the Section Chemoreceptors along the Life Cycle: Adult vs. Larvae Dimorphism in Receptors, Structures, and Elicited Behaviors.
In addition to the five canonical taste modalities (sweet, bitter, salt, sour, and umami or the taste of amino acids), flies can detect a range of fatty acids through taste and concomitantly elicit feeding behavior; this represents a clear advantage since fatty acids are a potent energy source for animals. The chemoreceptor dedicated to fatty acids detection in flies remains unknown but fatty acids tasting requires intact phospholipase C signal specifically in sweet-sensing neurons (Masek and Keene, 2013).
Chemoreceptors Involved in Toxic/Bitter Compounds Avoidance
Plants produce a diverse variety of unpalatable compounds as defense mechanisms toward herbivores. These compounds are generally sensed as bitter in the animal taste system and produce an aversive behavior that represents a clear advantage for the plant. Flies, on their behalf, also benefit from this avoidance behavior since many bitter compounds are not very nutritive and are even toxic. Bellow, we will present several examples of toxic/bitter compounds produced by plants (natural insect repellents) that trigger avoidance in flies. In addition, we will also consider the case of DEET, since it is the most widely used synthetic insect repellent nowadays.
One of the first described and most studied receptor for plant bitter compounds in Drosophila is that for caffeine. Detection and avoidance of caffeine requires a multimeric receptor including at least GR66a, GR33a, and GR93a subunits (Moon et al., 2006, 2009; Lee et al., 2009). Another plant bitter compound is isothiocyanate, the spicy ingredient of wasabi. Isothiocyanate triggers aversive responses in flies and this repellent behavior depend on the TRP channel Painless (Al-Anzi et al., 2006). Interestingly, this same channel is required for the fructose avoidance behavior that occurs in the change of food attraction to aversion during the wandering stage of larvae (Xu et al., 2008). Drosophila flies detect and avoid citronellal, an insect repellent produced by plants, through undescribed olfactory receptors in the antenna. TRPA1 channel is required for this avoidance behavior, and in A. gambiae mosquitoes the TRPA1 ortholog responds directly to citronellal (Kwon et al., 2010). Furthermore, many plants can accumulate in their seeds L-canavanine, a toxic amino acid. In flies, L-canavanine triggers strong aversion through the detection by bitter neurons (Mitri et al., 2009). The insect orphan G-protein-coupled DmXR was first identified as the L-canavanine receptor in flies (Mitri et al., 2009), although a later study determined instead GR8a and GR66a to be the chemoreceptors responsible for L-canavanine detection (Lee et al., 2012).
Natural insect repellents are also produced by harmful microorganisms such as Penicillium fungal molds and Streptomyces soil bacteria. Thanks to the specific olfactory receptor OR56a, flies can detect in the food very small quantities of geosmin, an indicator of contamination with these toxic microorganisms, and avoid the contact with toxic substrates. Through the activation of a dedicated olfactory pathway, geosmin triggers a strong aversive response that includes, oviposition and feeding avoidance, negative taxis, and decreases the attraction toward food odors (Stensmyr et al., 2012).
Although unpleasant, not all of these compounds produced by plants are actually toxic for the flies. An interesting example is the case of camphor, an unpalatable but nontoxic tastant that triggers aversion in adult flies. Very recently, it was demonstrated that pre-exposure to a camphor-rich diet attenuates camphor rejection through reduction of the TRPL receptor expression in the proboscis. In this sense, such desensitization mechanism reduces an unnecessary avoidance of a bitter but non-toxic compound, and this allows the use of camphor rich medium as a nutritional source in the absence of more appealing food sources. Interestingly, when returned to a camphor-free medium flies restore the strong rejection to camphor, suggesting that taste biases could be regulated depending on the quality of available food (Zhang et al., 2013b).
In addition to repulsive chemicals produced by plants, synthetic compounds can also trigger avoidance in flies. In the last 50 years, DEET has been the most widely used synthetic insect repellent. Although it proved to be effective in the control of several insect pests, its mechanisms of action are still under debate. In flies, DEET is detected by GR32a, GR33a, GR66a, and possibly other receptors expressed in GRNs, which mediate the antifeedant effects of the insect repellent (Lee et al., 2010). In addition, DEET inhibits odor-evoked activation of a subset of insect ORs, thereby inhibiting the perception of food odors (Ditzen et al., 2008). In the mosquito A. aegypti, DEET acts as an insect repellent at long distances through the activation of ORs. The olfactory detection of DEET not only triggers an immediate aversive response but can also form a short-term aversive memory. Thus, relevant odorants can induce plastic changes in the system, allowing flies to learn to avoid specific substrates (Das et al., 2014).
Finally, sour taste, evoked by low pH and carboxylic acids, is also generally associated with harmful conditions and triggers avoidance. Adult flies generally prefer slightly acid mediums while they reject extremely acid foods (Fuyama, 1976; Ai et al., 2010). While the detection of specific carboxylic acids seems complex and still under debate (see Section Chemoreceptors along the Life Cycle: Adult vs. Larvae Dimorphism in Receptors, Structures, and Elicited Behaviors), adult flies have a simple system to detect protons. IR64 acting together with IR8a form an olfactory receptor to sense acidity in the antenna. The olfactory detection of low pH solutions by the complex IR64a-IR8a activates a dedicated neuronal circuit that leads to avoidance behavior (Ai et al., 2010, 2013).
Chemoreceptors Involved in Oviposition Site Selection
In order to select the proper oviposition site, female flies evaluate the composition of the medium through gustatory receptors present in their ovipositor and proboscis (Yang et al., 2008) as well as olfactory receptors in the antenna (Stensmyr et al., 2012; Dweck et al., 2013). It is believed that in this search, females have to evaluate, according to the presence and concentration of specific chemicals, if larvae will be able to survive or not in this medium. Small quantities of geosmin, an indicator of the presence of harmful microorganisms in a substrate, are detected by OR56a and are sufficient to repel flies from lying eggs on this medium (Stensmyr et al., 2012). In the absence of harmful microorganisms, other chemicals can also prevent fly egg-laying. For instance, Drosophila females avoid egg laying in substrates with high sugar concentration, although this decision seems to be highly context dependent (Yang et al., 2008; Schwartz et al., 2012).
The presence of particular chemicals in the substrate can induce oviposition in flies. In this regard, the case of acetic acid is an interesting example. Although both females and males avoid 5% acetic acid solutions (i.e., the concentration present in vinegar), females choose acetic acid supplemented mediums to lay their eggs. The positional avoidance appears to be mediated by olfactory receptors present in the antenna, while the attraction to oviposit depends on gustatory perception (Joseph et al., 2009). In addition, it has been recently demonstrated that terpenes produced by citrus peels, in particular limonene, stimulate oviposition in Drosophila through the activation of the OR19a receptor. Interestingly, wasps which parasite Drosophila show a strong aversion to these same terpenes, suggesting that oviposition preference on citrus substrate could confer protection against these endoparasitoids (Dweck et al., 2013).
The presence of dedicated olfactory receptors to detect geosmin and limonene allows flies to avoid to oviposit in harmful substrates while promoting oviposition in citrus substrates that will guarantee the absence of wasps parasites. This confers a clear adaptive advantage for Drosophila flies, and it suggests an adaptation of the olfactory system to the different substrates present in their natural environment. In contraposition, it remains still unclear why flies prefer to lay eggs in low sugar or acetic acid complemented medium, although some hypotheses have been formulated (Parsons, 1980; Joseph et al., 2009; Schwartz et al., 2012).
Chemoreceptors Involved in Sexual Behavior
Male courtship is a complex and relatively stereotyped behavior that compromises multimodal sensory signals. Males use visual cues to orientate and chase the female, produce auditory signals (known as the “mate song”) by wing vibrations, and emit, and perceive through dedicated olfactory and gustatory receptors, many chemical cues (Ziegler et al., 2013). These chemical cues are principally sexual pheromones (Gomez-Diaz and Benton, 2013) although recently it has been demonstrated that food-derived odors can modulate courtship as well (Grosjean et al., 2011). Members of ppk (Liu et al., 2012; Lu et al., 2012; Thistle et al., 2012; Toda et al., 2012; Vijayan et al., 2014), OR (Kurtovic et al., 2007; Van der Goes van Naters and Carlson, 2007; Wang et al., 2011), IR (Koh et al., 2014), and GR (Bray and Amrein, 2003; Miyamoto and Amrein, 2008; Moon et al., 2009; Koganezawa et al., 2010) receptor families have been identified or proposed as sexual pheromones receptors; plus, IR84 is involved in the food-odor-mediated modulation of courtship (Grosjean et al., 2011). In the last few years important advances have been made on the field of chemoreception in sexual behavior, hence in the next section we will present an updated view of the relevance of specific pheromone and food odor receptors involved in courtship. In addition, in the context of chemoreceptors implicated in sexual behavior, we will introduce several examples of sexual dimorphism in olfactory and gustatory circuits in Drosophila. Although we do not intend to go into detail on the differences between male and female chemosensory structures, we hope these examples will serve to illustrate how flies achieve sexual dimorphic behaviors in response to aphrodisiac/anti-aphrodisiac stimuli. Readers interested on sexual dimorphism in Drosophila could revise the bibliography proposed in the next section, of which the review of Yamamoto and Koganezawa (2013) is highly recommendable.
Chemoreceptors and Sexual Behavior: Relevance of Pheromone and Food Odor Receptors
OSNs expressing either OR67d, OR47b, or IR84 are the only three OSNs that express a sex-specific transcript of the gene fruitless (FruM) (Stockinger et al., 2005; Grosjean et al., 2011), and this suggests their involvement in sex-specific behaviors such as courtship. These OSNs project respectively to DA1, VA1v, and VL2a glomeruli, which are significantly larger in males. From these glomeruli, Fru+ projection neurons connect with the lateral horn (Kondoh et al., 2003; Stockinger et al., 2005; Grosjean et al., 2011). Regarding taste structures, males harbor more gustatory receptors in their legs compared to females, and sex determination factors like fruitless and doublesex are responsible for sexually dimorphic axonal pattern in these sensory neurons (Possidente and Murphey, 1989; Mellert et al., 2012; Yamamoto and Koganezawa, 2013; Koh et al., 2014) (Figure 2A). Fru+ gustatory neurons are present mainly in the dorsal labellum and foreleg tarsi (Stockinger et al., 2005). With the exception of IR84a that has been confirmed to respond to food odors (Grosjean et al., 2011), the rest of these sexually dimorphic receptors are annotated or predicted to detect sexual pheromones.
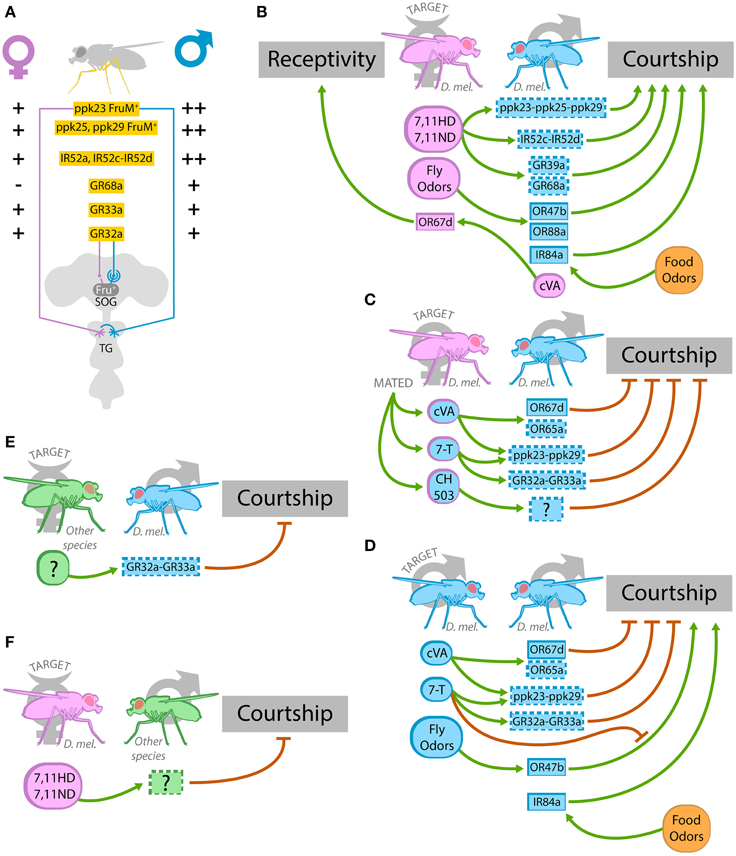
Figure 2. Chemoreceptors involved in sexual behavior. (A) Sexual dimorphism in chemoreceptor's expression and in neuronal morphology of the chemosensory system of the leg. Male legs express more ppk23+, ppk25+, ppk29+ FruM+, and IR52a+, IR52c-IR52d+ sensory neurons than female legs. In males, axons of ppk23+ neurons cross the ventral nerve cord midline in the thoracic ganglion (TG), whereas female axons do not. GR68a is only expressed in male legs, while GR32a and GR33a are expressed in the same number of leg sensory neurons in both sexes. Sexual dimorphism is observed in the dendritic arbor of the GR32a postsynaptic neurons expressing Fru in the SOG. (B–F) Illustrations of olfactory and gustatory cues (sexual pheromones or food odors) and the chemoreceptors involved in sexual behavior during an encounter between a virgin female and a male (B), a mated female and a male (C), a male and another male (D), and interspecific heterosexual encounters (E,F). (B) Female hydrocarbons 7,11 HD and 7,11 ND activate most likely IR52c-IR52d, GR68a, GR39a, and the complex ppk23-ppk25-ppk29 in the male leg, and this induce courtship. Undetermined fly odors activate OR47b and OR88a, and at least the activation of OR47b leads to increase courtship in males. cVA produced by males stimulates OR67d receptors in female antenna and increase female receptivity. In addition to the signals produced by flies, food odors, in particular phenylacetic acid and phenylacetaldehyde, activate IR84a and promote courtship in males. (C) During courtship, males temporarily pass some 7-T hydrocarbon on to females, and cVA and CH503 are transferred from males to females during mating. 7-T deposited on courted females apparently activates GR32a-GR33a and ppk23-ppk29+ neurons in the male leg and concomitantly reduces courtship by other males. cVA transferred to mated females activates OR67d and presumably OR65a in the male antenna, and probably ppk23-ppk29+ neurons in the male leg, leading to courtship inhibition. CH503 acts through unknown receptors and also leads to a reduction of courtship. (D) In a male-male encounter, cVA and 7-T inhibit homosexual courtship acting through OR67d, OR65a in the antenna and presumably ppk23-ppk29, GR32a-GR33a complexes in the leg. The courtship-promoting signal that follows the activation of OR47b by fly odors is inhibited by 7-T. Food odors activate IR84a and promote male-male courtship. To simplify, only the signals emitted by one of the males are shown in the drawing but the reciprocal ones are also present in the encounter. (E) Unidentified cuticular hydrocarbons of females of other Drosophila species (D. simulans, D. yakuba, and D. virilis) are most likely sensed by GR32a-Gr33a in D. melanogaster males and this prevents interspecific courtship. (F) The female hydrocarbons 7,11 HD and 7,11 ND act through unknown chemoreceptors in males of other Drosophila species (D. simulans, D. yakuba, and D. erecta) and inhibit courtship. In panels (B–F), pink stands for female, blue, male, and green, male or female of another Drosophila species. In the boxes, a full-lined frame indicates that the protein is a bona fide chemoreceptor, while a dotted-line means that there is still no clear demonstration of the protein's role as chemoreceptor.
A sexual pheromone is defined as a chemical signal produced by the organism involved in the control of sexual behaviors. In Drosophila, the principal known sexual pheromones are the volatile cis-vaccenyl acetate (cVA) and the cuticular hydrocarbons 7-tricosene (7-T), 7,11-heptacosadiene (7,11-HD), and 7,11 nonacosadiene (7,11-ND). Briefly, cVA and 7-T are produced by males and they act as anti-aphrodisiac for other males (although cVA has several additional roles; see below) while 7,11-HD and 7,11-ND are female pheromones that promote courtship (Fernández and Kravitz, 2013; Gomez-Diaz and Benton, 2013). Anyhow, more recent and highly sensible methods of detection have demonstrated that all of these four pheromones are present in virgin socially naïve individuals of both sexes but in different quantities (Yew et al., 2009). cVA produced and stored in the ejaculatory bulb of males (Butterworth, 1969; Brieger and Butterworth, 1970) is transferred to females during copulation (Butterworth, 1969; Ejima et al., 2007). Together with cVA, an acetylated hydrocarbon named CH503 is also transferred to females during copulation leading to a prolonged inhibition of male courtship acting through an unknown sensory receptor (Yew et al., 2009) (Figures 2B–D and Table 2).
In males, cVA acts as an anti-aphrodisiac that reduces courtship toward mated females or other males. cVA also modulates male-male aggression while increasing receptivity in females (Jallon, 1984; Ejima et al., 2007; Kurtovic et al., 2007; Wang and Anderson, 2010; Liu et al., 2011). At long ranges, cVA is described to function as an aggregation factor for males and females (Bartelt et al., 1985). Electrophysiological studies demonstrated that cVA is sensed through OSNs expressing OR67d and to a lesser extent, OR65a (Ha and Smith, 2006; Kurtovic et al., 2007; Van der Goes van Naters and Carlson, 2007). Although some discrepancies have been observed in different studies, the role of cVA on sexual behaviors seems to be mediated by OR67d activation (Ejima et al., 2007; Kurtovic et al., 2007). While acute promotion of aggression depends on OR67d, chronic exposure to cVA reduces aggression through OR65a activation (Wang and Anderson, 2010; Liu et al., 2011). Interestingly, both females and males express OR67d and OR65a, and these receptors respond equally to cVA in both sexes (Kurtovic et al., 2007; Van der Goes van Naters and Carlson, 2007). However, the neuronal circuit underlying OR67d is sexually dimorphic and, consequently, different neuronal cluster are activated in males and females (Datta et al., 2008; Ruta et al., 2010; Kohl et al., 2013). This sexual dimorphism in the neuronal circuit downstream of OR67d could be responsible for the different behaviors elicited by cVA in both sexes. In addition to the activation of OR67d and OR65a by cVA, uncharacterized fly odors activate OR47b and OR88a both in males and females, suggesting the presence of other volatile pheromones (Van der Goes van Naters and Carlson, 2007) (Figures 2B–D and Table 2).
7-T is a male hydrocarbon that inhibits courtship in other males (Antony and Jallon, 1982; Lacaille et al., 2007) and promotes male-male aggression by acting in the same neuronal pathway as cVA (Wang et al., 2011). Several receptors have been proposed for 7-T, in particular GR32a and GR33a (Miyamoto and Amrein, 2008; Moon et al., 2009). Males lacking GR32a display high courtship toward males and mated females, suggesting that GR32a could sense an anti-aphrodisiac signal produced by males and transferred to females during courtship (Miyamoto and Amrein, 2008) (Figures 2B–D). Moreover, GR32a prevents males to court with individuals from other species, contributing to the isolation barrier within the Drosophila genus (Fan et al., 2013) (Figure 2E). GR32a is present in the labellum and in the leg, but only in the leg GR32+ GRNs are surrounded by cells expressing OBP57, an odorant-binding protein implicated in the carrying of pheromones (Koganezawa et al., 2010). Although no sexual dimorphism is observed in GR32a sensory neurons, they seem to contact Fru+ neurons in the SOG that display sexually dimorphic dendritic arbors (Koganezawa et al., 2010; Fan et al., 2013) (Figure 2A). It would be interesting to study if these differences in the dendritic arbor determine different postsynaptic neuronal clusters that could activate different motor programs in males and females in response to GR32a activation. At the same time, GR33a, a key receptor in the detection of several aversive compounds, is also required to inhibit male-male courtship (Moon et al., 2009) and it is essential for the male preference for younger virgin females (Hu et al., 2015). GR33a and GR32a seem to be expressed in the same GRNs in the leg, suggesting that they might be part of the same heterodimeric receptor (Moon et al., 2009). In addition, 7-T appears to inhibit a male-male courtship-promoting-signaling pathway that is OR47b dependent (Wang et al., 2011) (Figures 2B–E and Table 2).
The production of 7,11 HD in females serves as an aphrodisiac for males of the same species (Antony and Jallon, 1982; Antony et al., 1985) and acts as a barrier to prevent interspecific courtship (Billeter et al., 2009). Males sense female pheromones, probably 7,11-HD, through GR68a expressed in their forelegs (Figures 2B,F and Table 2). GR68a is exclusively expressed in male forelegs (Figure 2A), and its expression depends on the sex determination factor doublesex (Bray and Amrein, 2003). In addition, GR39a may also be involved in female pheromones perception since male mutants for GR39a display reduced courtship toward wild-type females (Watanabe et al., 2011).
In the last few years, several studies have demonstrated the relevance of ppk channels, notably ppk23, ppk25, and ppk29, in sexual behavior. These 3 channels are expressed in Fru+ gustatory neurons of both sexes although males have around double the amount of ppk+ cells in the leg compared to females (Liu et al., 2012; Lu et al., 2012; Vijayan et al., 2014) (Figure 2A). Different subpopulations of ppk23+-FruM+ neurons in the leg respond to male and female pheromones, and both ppk23 and ppk29 are required for the pheromone-evoked effects on courtship (Lu et al., 2012; Thistle et al., 2012; Toda et al., 2012). Those responding to female pheromones express also ppk25, and this channel is necessary for the 7,11-HD effects of promoting courtship toward virgin females as well as for the stimulation of courtship by pheromones present on immature males and for normal female receptivity (Vijayan et al., 2014) (Figures 2B–D and Table 2). ppk25 is also expressed in olfactory neurons, but this expression is not relevant for courtship control (Starostina et al., 2012). Interestingly, similar responses at the level of ppk+ cells activation in response to male and female compounds were observed in both sexes (Thistle et al., 2012; Vijayan et al., 2014). This suggests, once again, that sexual dimorphism downstream of the activation of receptor neurons might be responsible for the different behaviors triggered in male and females in response to sexual pheromones. In effect, at least ppk23+ neurons display sexually dimorphic axonal projections, although the physiological consequences of this sexual dimorphism have not been studied (Lu et al., 2012; Thistle et al., 2012) (Figure 2A).
The studies presented here demonstrate the relevance of ppk23, ppk25, and ppk29 channels in sexual behavior, but a clear demonstration of their role as chemoreceptors is still lacking. Attempts to prove the direct requirement of ppk23 and ppk29 as pheromone receptors failed, suggesting that additional subunits may be required (Thistle et al., 2012). Consistent with this idea is the fact that ppk29 (also known as NOPE) forms a complex with ppk25 (Liu et al., 2012). Alternatively, ppk channels could be playing a fundamental role on pheromone-evoked responses, not as direct receptors but as a unique cellular component of gustatory neurons expressing a yet unknown chemoreceptor (Pikielny, 2012). Strikingly, a recent study demonstrated that ppk23 and ppk29 are also essential for the detection of a novel aggregation pheromone in D. melanogaster larvae, the (Z)-5 and (Z)-7-tetradecenoic acid (Mast et al., 2014). In this study the authors clearly demonstrate the relevance of ppk23 and ppk29 in the detection of the aggregation pheromone but, again, no direct proof of their role as chemoreceptors has been provided. Taking into account that ppk23-ppk29 are essential for the detection of signals of very different structure (long-chain fatty acids in the case of larval aggregation and hydrocarbons in the case of sexual behavior), it seems more reasonable that they don't act as direct chemoreceptors. Anyhow, more suitable experiments like analysis of response to pheromones using in vitro or in vivo ectopic expression of these proteins will help to clarify this matter.
In addition to ORs, GRs, and ppk channels, IRs play a relevant role in the control of sexual behaviors in Drosophila. IR52a, IR52c, and IR52d are present in the foreleg of both sexes although they are expressed in more cells in males (Figure 2A). IR52c and IR52d show complete, or nearly complete, coexpression in the foreleg suggesting that they are part of the same complex. IR52c+ neurons are activated by female compounds and form putative synapses with Fru+ neurons in the prothoracic ganglia. Interestingly, ectopic activation of IR52c+ neurons increase courtship while mutants lacking IR52c and IR52d display reduced courtship behavior and higher latency to copulate, suggesting a possible role on sexual pheromone detection (Koh et al., 2014) (Figure 2B and Table 2).
Last but not least, food odors, notably phenylacetaldehyde and phenylacetic acid, can also promote male courtship through IR84a-FruM+ OSNs. The VL2a FruM+ glomerulus is activated downstream of IR84a+ OSNs, and from this glomerulus, projection neurons send olfactory information to a pheromone-processing region of the lateral horn (Grosjean et al., 2011) (Figures 2B,D and Table 2). In this regard, we now understand that not only sexual pheromones but also compounds present in the environment, at least in the fly food, directly modulate sexual behavior in Drosophila, highlighting the impact of external cues in a key behavior for species survival.
Chemoreceptors Along the Life Cycle: Adult vs. Larvae Dimorphism in Receptors, Structures, and Elicited Behaviors
When we compare a Drosophila larva with an adult, differences become much more obvious than similarities. Although sharing the same genome and developmental program, the larval and adult stages of holometabolous insects contrast strikingly in regards to general anatomy, behaviors displayed, and lifestyles or niches occupied. In the nervous system, the differences between larvae and adults rise as a consequence of the extensive apoptosis and neuronal remodeling occurring during the metamorphosis (Truman, 1990). The case of chemoreceptive structures is not an exception. The external taste organs of the larva, the terminal and ventral organs, undergo apoptosis during the metamorphosis and are then completely replaced by adult structures. While the main olfactory organ, the dorsal organ, does not disappear during metamorphosis, the olfactory system undergoes critical neuronal changes, e.g., neuronal migration, proliferation, and development of progenitor cells, dendritic pruning and extension, and axonal remodeling, among other processes (Gerber and Stocker, 2007; Rodrigues and Hummel, 2008). In this regard, taking into account the extensive remodeling of the chemoreceptive structures along the life cycle of the fly, it does not come as a surprise that larvae and adults can trigger very different behaviors in response to the same stimuli or that they may even use different receptors to detect the same compounds. Nonetheless, one can still wonder why invest so much energy in developing two strikingly different chemoreception systems in such a short time. Are these differences a consequence of developmental constrains or do they reflect an adaptation to different niches occupied along the life? Below, we will present the cases of sugar, carboxylic acid, and salt detection in adult and larvae as interesting examples of stage-specific chemoreceptors involved in the responses to ecologically relevant compounds. We will further discuss how these differences in the system and the behavior could be interpreted in the context of stage-specific needs.
Sugar detection is an interesting example of the differences between larval and adult taste systems. In adults, responses to common sugars, like glucose, sucrose, and maltose, may include up to eight gustatory receptors, i.e., GR5a, GR61a, and GR64a-f (Dahanukar et al., 2007; Jiao et al., 2007, 2008; Slone et al., 2007; Fujii et al., 2015). Fructose is detected by GR43a, a narrowly tuned receptor expressed in taste organs as well as in the central nervous system (Table 2 and Figure 3A). After a sugar-rich diet, the levels of glucose and trehalose in hemolymph do not increase significantly while fructose levels increase between 3 and 10 times. In line with this, GR43a located in the brain acts as a nutrient sensor, assessing the levels of fructose in the hemolymph (Miyamoto et al., 2012). Even though larvae detect and behaviorally respond to sugar, none of the sweet receptors GR5a, GR61a, and GR64a-f are expressed in this stage (Kwon et al., 2011). Alternatively, larvae only express GR43a in internal taste neurons and in the brain. Larvae display an immediate attraction to fructose and sucrose (disaccharide of fructose and glucose) and a delayed preference toward glucose and trehalose (disaccharide of glucose). Surprisingly, all of these attractive responses depend on GR43a; fructose's attraction depends on GR43a expressed in internal taste neurons while glucose's attraction requires GR43a in the brain (Mishra et al., 2013). These differences in dynamics and receptor localization, suggest that larvae sense fructose or fructose-containing disaccharides directly on internal pharyngeal taste neurons while detection of non-fructose sugars relies on their conversion to fructose post-ingestion, elevation of fructose levels in the hemolymph, and subsequent sensing in the brain (Mishra et al., 2013) (Table 2 and Figure 3A). This apparently inefficient sugar sensing setup provides a simple system that satisfies the larval needs. In order to grow, larvae need to constantly incorporate nutrients and, since their mobility is reduced, looking for the perfect sugar-source could result in great energy costs. It is the adult female fly who carefully analyzes the composition of the medium before choosing an oviposition site and, in doing so, it seems to look for a suitable substrate that will provide with the minimal nutritional requirements for the larvae to grow (Joseph et al., 2009; Schwartz et al., 2012). In addition, fructose and sucrose are present in most fruits, suggesting that having only a rapid fructose detection system may be sufficient on most ecologically relevant substrates. In this sense, larvae generally don't need to search for sugars but they can simply start eating and then evaluate the nutritional content by fast activation of pharyngeal receptors or slower activation of brain receptors. Adult flies, on the contrary, display more complex behaviors that involve the exploration of more heterogeneous environments. In these new environments, flies not only need to evaluate the substrates for the presence of sugars but also the quality of those sugars, since non-fructose sugars may be present in higher proportion. In this sense, a more complex taste system allowing rapid detection of a huge variety of sugars appears as a more suitable setup than the simple version of the larvae.
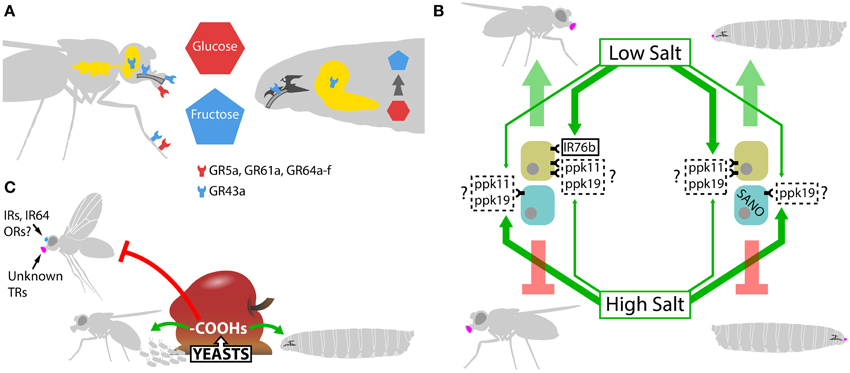
Figure 3. Adult vs. larvae dimorphism. (A) Sugar detection mechanisms in adult and Drosophila larvae. In adults, glucose, sucrose, and other common sugars are sensed in the periphery by GR5a, GR61a, and GR64a-f, while fructose activates specifically GR43a in the labellum, legs, internal taste organs, and in the brain. GR43a located in the brain acts as a nutrient sensor, assessing the levels of fructose in the hemolymph. In larvae, only GR43a is expressed. This sugar receptor is present in internal taste organs where it is responsible for fructose or fructose-containing disaccharides detection in the feeding medium, and in the brain where it assesses internal fructose levels. Non-fructose sugars are not detected in the periphery, their detection depends on their internal conversion to fructose and subsequent sensing of internal fructose levels by GR43a in the brain. (B). Carboxylic acid (-COOHs) perception. Rotten fruits present moderate concentrations of different carboxylic acids product of yeast and bacteria fermentation. The presence of these carboxylic acids induces oviposition and, at the same time, triggers positional avoidance in adults. On the contrary, larvae are attracted by carboxylic acid rich media. The chemoreceptors implied in these responses are still not well defined, but at least in adults, IRs, ORs, and the H+ sensor IR64a in the olfactory system and unknown taste receptors (TR) are responsible for carboxylic acid perception. (C) Salt detection in adult and Drosophila larva. Both adults and larvae are attracted to low salt and repulsed by high-salt solutions. In adults, IR76b is a Na+-permeable channel essential for low-salt responses; plus, ppk11 and ppk19 might be involved in the attraction toward low-salt solutions. IR76b is expressed in a subset of GRNs, which respond strongly to low salt, while a different subpopulation of GRNs is strongly activated by high-salt solutions. In larvae, ppk11 and ppk19 are most likely the taste receptors responsible for attractive responses to low-salt concentrations. The chemoreceptors for high-salt solutions remain unknown, but ppk11 and ppk19 may contribute to the responses. At least in larvae, the cytoplasmic protein SANO expressed in bitter neurons is essential for the behavioral aversion to high-salt concentrations. The thick green arrows indicate strong activation while the thin ones, weak activation. In the boxes, a full-lined frame indicates that the protein is a bona fide chemoreceptor, while a dotted-line means that there is still no clear demonstration of the protein's role as chemoreceptor.
Another interesting example of chemoreception dimorphism in the fly's life cycle is the case of carboxylic acids perception. While adult flies are strongly repulsed by acidity or high carboxylic acid concentrations (Fuyama, 1976; Ai et al., 2010), larvae display clear attraction (Monte et al., 1989; Kreher et al., 2008; Khurana and Siddiqi, 2013). The chemoreceptors relevant for the attractive responses in larvae have not been identified yet, and only weak activation of some ORs in response to carboxylic acids has been observed (Kreher et al., 2005, 2008). In the case of adults, carboxylic acids are detected through olfaction and taste. In the olfactory system, protons are directly detected by the complex IR64-IR8a (Ai et al., 2010, 2013), and different carboxylic acids trigger strong electrophysiological responses in IR8a+ neurons and mild responses in OR neurons (Hallem and Carlson, 2006; Abuin et al., 2011; Silbering et al., 2011). Nevertheless, efforts to elucidate the role of IR8a on carboxylic acid-triggered behavioral avoidance produced contradictory results (Silbering et al., 2011; Ai et al., 2013). In the gustatory system, it was recently shown that high concentrations of carboxylic acids activate a subset of bitter neurons while they inhibit the activity of sweet neurons (Charlu et al., 2013). At the same time, another study demonstrated that carboxylic acids suppress bitter neuron activity when presented in dietary relevant concentrations (Chen and Amrein, 2014). Interestingly, normally aversive bitter/sugar mixtures are rendered more appealing with the addition of moderate concentrations of carboxylic acids (Chen and Amrein, 2014). The identity of the carboxylic acid receptors in the taste organ has not been revealed yet, but they seem to be different from the H+ sensor IR64a and the bitter receptors GR33a and Painless (Charlu et al., 2013) (Table 2 and Figure 3B).
Several carboxylic acids are normally present in fly food as fermentation products of yeast and bacteria (Bridges and Mattice, 1939; Idstein et al., 1985; Moat et al., 2002) so, in addition to a simple pH indicator, detection of high concentration of carboxylic acids may serve also as indication of rotten fruit and of the presence of yeasts. This would be important because a previously processed substrate such as rotten fruit could be easier for larvae to feed on; plus, yeasts are the typical source of important nutrients for the larvae, such as proteins and some carbohydrates (Lee et al., 2008; Schwarz et al., 2014). On the other hand, extremely acid solutions are very toxic for adult flies and can result in high mortality in the population (Chakir et al., 1993). The resistance to high concentration of carboxylic acids observed in larvae could represent a tolerance product of the long exposure to low pH media. Consistent with this idea is the fact that female flies normally lay eggs in rotting fruit (Atkinson and Shorrocks, 1977; Markow, 1988), suggesting that larvae are exposed to low pH media throughout their development (Figure 3B). In adults, tolerance to high acetic acid concentrations has already been described for a geographic population. Although still unclear, this increased tolerance could be a consequence of a more efficient detoxification system (Chakir et al., 1993). It would be interesting to test if larvae also have a more efficient detoxification system that allows them to tolerate long exposures to high concentrations of carboxylic acids present in their environment.
Animals in general present bimodal responses to salts: low concentrations of salt trigger attraction while high concentrations, repulsion. This feature reflects the dual effect of salt in the organism: moderate levels of salt are necessary to control electrolyte homeostasis, neuronal activity, and muscle contraction while high levels have deleterious effects as dehydration and hypertension (Liman et al., 2014). In Drosophila, larvae and adult also display the same bimodal responses to salts (Miyakawa, 1981; Balakrishnan and Rodrigues, 1991) but they detect salt apparently through different mechanisms. Attractive responses to low-salt concentrations in larvae require the ENac channels ppk11 and ppk19 (Liu et al., 2003b) in taste neurons. Interestingly, ENac channels are also involved in the low-salt responses in mammals (Chandrashekar et al., 2010), albeit there is no consistent proof that ppk11 and ppk19 in fly larvae or ENac channels in mammals are direct receptors for low-salt solutions (Liu et al., 2003b; Chandrashekar et al., 2010). In adult flies, a recent paper described IR76b as a Na+-permeable channel essential for low-salt responses. Furthermore, the authors have clearly demonstrated the existence of two populations of GRNs, one displaying a stronger response to high-salt concentrations and the other one, expressing IR76b, displaying a stronger response to low-salt concentrations. IR76b+ taste neurons constitute a new class of GRN specifically tuned to low-salt detection (Zhang et al., 2013a). Interestingly, IR76b is also present in some adult antennal coeloconic OSNs where it might act as a coreceptor (Silbering et al., 2011). In addition to IR76b, ppk11 and ppk19 might also play a role in low-salt detection in adults (Liu et al., 2003b). Coimmunostaining analysis would help to elucidate if ppk11, ppk19, and IR76b are all part of the same detection system or if they constitute two parallel pathways. Moreover, future experiments should analyze whether IR76b is also required in larvae for low-salt detection (Table 2 and Figure 3C).
Regarding high-salt detection, the receptors are still elusive, but ppk11 and ppk19 may contribute to the aversive responses in both adults and larvae (Liu et al., 2003b; Alves et al., 2014). A recent study identified Serrano (SANO), a cytoplasmic protein expressed in bitter neurons, as an essential molecule for the behavioral aversion to high-salt concentrations in larvae. Moreover, inactivation of SANO+ bitter neurons triggers an attractive response to high-salt concentrations in larvae (Alves et al., 2014). This strongly suggests that, as it is the case in adults, two neuronal groups are simultaneously activated in response to both low and high-salt concentration, but it is the outcome between these two populations what will determine if there is attraction or repulsion (Figure 3C). Again, the requirement of SANO for high-salt detection in adults was not analyzed. Complementary studies are needed to clearly define whether larvae and adult salt-detection systems are conserved or not.
Perspectives
Our current knowledge of chemosensory perception in D. melanogaster is growing very fast with the identification and ongoing characterization of the different receptor families. However, there is still a lot to do to clearly understand how chemicals are detected, and how this information is processed at the periphery and in the brain to lead to a specific behavioral response. For example, most studies focus on identifying potential ligands for a specific chemoreceptor by using single odor stimulation, a case far removed from the complexity of the natural environmental conditions to which flies are normally exposed. In the natural environment odors are generally present in complex mixtures and it is from these blends that flies need to extract the most relevant signals to behave accordingly. Some putative mechanisms for how the olfactory system decodes relatively complex odorant mixtures have been proposed (Silbering and Galizia, 2007) but this still remains a very important open question.
The complexity of sensing and decoding chemical mixtures is also true for taste perception. It has been shown in a recent study (Chen and Amrein, 2014) that the presence of carboxylic acids in a mixture can modulate bitter and sweet perception. Several questions arise from this observation, could the activation of a specific neuron sensitive to acids potentiate the activity of the neighboring sugar sensing neurons in the peripheral nervous system? Is this possible mechanism shared by all chemosensory neurons? Or could it be specific to some neurons and sensory modalities? Moreover, could acids also inhibit bitter sensing neurons as suggested by this recent work (Chen and Amrein, 2014)?
Concerning the integration of the chemosensory stimuli in the brain the picture is still incomplete. Even though the olfactory system is better described than the gustatory system their neuronal networks are still under characterization. What are the exact connections between the different centers in the brain? Moreover, the precise and complete network from the detection of a chemical at the periphery to the muscle cells that lead to a behavioral output is still partially described. Some recent studies on cVA detection have started to decipher this network (Kohl et al., 2013), and have shown that this cVA circuit seems to be interconnected with other sensory modalities such as hearing (Zhou et al., 2014) which highlights the importance of the connectivity between modalities.
D. melanogaster is a powerful genetic model and we owe it most of our current knowledge on the molecular basis of chemoreception in insects. Nonetheless, it would be interesting to compare how chemical perception is processed in other Drosophila species that have a highly specialized living substrate and to analyze differences and similarities between them. Through these comparative studies we could follow evolutionary traces and study if specific sensory systems have been selected to ensure species survival. The comparison with the chemosensory systems of more distant insects such as mosquitos and bees would also be of great value for the management of species that impact deeply on human health and agriculture.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique, and by an ERC starting Grant (GliSFCo, 311403).
References
Abuin, L., Bargeton, B., Ulbrich, M. H., Isacoff, E. Y., Kellenberger, S., and Benton, R. (2011). Functional architecture of olfactory ionotropic glutamate receptors. Neuron 69, 44–60. doi: 10.1016/j.neuron.2010.11.042
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ai, M., Blais, S., Park, J. Y., Min, S., Neubert, T. A., and Suh, G. S. (2013). Ionotropic glutamate receptors IR64a and IR8a form a functional odorant receptor complex in vivo in Drosophila. J. Neurosci. 33, 10741–10749. doi: 10.1523/JNEUROSCI.5419-12.2013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ai, M., Min, S., Grosjean, Y., Leblanc, C., Bell, R., Benton, R., et al. (2010). Acid sensing by the Drosophila olfactory system. Nature 468, 691–695. doi: 10.1038/nature09537
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Al-Anzi, B., Tracey, W. D., and Benzer, S. (2006). Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr. Biol. 16, 1034–1040. doi: 10.1016/j.cub.2006.04.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Alves, G., Sallé, J., Chaudy, S., Dupas, S., and Manière, G. (2014). High-NaCl perception in Drosophila melanogaster. J. Neurosci. 34, 10884–10891. doi: 10.1523/JNEUROSCI.4795-13.2014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Antony, C., and Jallon, J. (1982). The chemical basis for sex recognition in Drosophila melanogaster. J. Insect Physiol. 28, 873–880. doi: 10.1016/0022-1910(82)90101-9
Antony, C., Davis, T., Carlson, D., Pechine, J., and Jallon, J. (1985). Compared behavioral responses of male Drosophila melanogaster (Canton S) to natural and synthetic aphrodisiacs. J. Chem. Ecol. 11, 1617–1629. doi: 10.1007/BF01012116
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Asahina, K., Pavlenkovich, V., and Vosshall, L. B. (2008). The survival advantage of olfaction in a competitive environment. Curr. Biol. 18, 1153–1155. doi: 10.1016/j.cub.2008.06.075
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Atkinson, W., and Shorrocks, B. (1977). Oecologia in the domestic species of Drosophila. Oecologia 29, 223–232. doi: 10.1007/BF00345697
Balakrishnan, B. Y. R., and Rodrigues, V. (1991). The shaker and shaking-B genes specify elements in the processing of gustatory information in Drosophila melanogaster. J. Exp. Biol. 157, 161–181.
Bartelt, R. J., Schaner, A. M., and Jackson, L. L. (1985). cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J. Chem. Ecol. 11, 1747–1756. doi: 10.1007/BF01012124
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Becher, P. G., Bengtsson, M., Hansson, B. S., and Witzgall, P. (2010). Flying the fly: long-range flight behavior of Drosophila melanogaster to attractive odors. J. Chem. Ecol. 36, 599–607. doi: 10.1007/s10886-010-9794-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Benton, R., Sachse, S., Michnick, S. W., and Vosshall, L. B. (2006). Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4:e20. doi: 10.1371/journal.pbio.0040020
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Benton, R., Vannice, K. S., Gomez-diaz, C., and Vosshall, L. B. (2009). Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 5–7. doi: 10.1016/j.cell.2008.12.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Billeter, J.-C., Atallah, J., Krupp, J. J., Millar, J. G., and Levine, J. D. (2009). Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461, 987–991. doi: 10.1038/nature08495
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Boll, W., and Noll, M. (2002). The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development 129, 5667–5681. doi: 10.1242/dev.00157
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bray, S., and Amrein, H. (2003). A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron 39, 1019–1029. doi: 10.1016/S0896-6273(03)00542-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bridges, M. A., and Mattice, M. R. (1939). Over two thousand estimations of the ph of representative foods. Am. J. Dig. Dis. 6, 440–449. doi: 10.1007/BF02996505
Brieger, G., and Butterworth, F. M. (1970). Drosophila melanogaster: identity of male lipid in reproductive system. Science 167:1262. doi: 10.1126/science.167.3922.1262
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Butterworth, F. M. (1969). Lipids of Drosophila: a newly detected lipid in the male. Science 163, 1356–1357. doi: 10.1126/science.163.3873.1356
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cameron, P., Hiroi, M., Ngai, J., and Scott, K. (2010). The molecular basis for water taste in Drosophila. Nature 465, 91–95. doi: 10.1038/nature09011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cande, J., Prud'homme, B., and Gompel, N. (2013). Smells like evolution: the role of chemoreceptor evolution in behavioral change. Curr. Opin. Neurobiol. 23, 152–158. doi: 10.1016/j.conb.2012.07.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chakir, M., Peridy, O., Capy, P., Pla, E., and David, J. R. (1993). Adaptation to alcoholic fermentation in Drosophila: a parallel selection imposed by environmental ethanol and acetic acid. Proc. Natl. Acad. Sci. U.S.A. 90, 3621–3625. doi: 10.1073/pnas.90.8.3621
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chandrashekar, J., Hoon, M. A., Ryba, N. J. P., and Zuker, C. S. (2006). The receptors and cells for mammalian taste. Nature 444, 288–294. doi: 10.1038/nature05401
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chandrashekar, J., Kuhn, C., Oka, Y., Yarmolinsky, D. A., Hummler, E., Ryba, N. J., et al. (2010). The cells and peripheral representation of sodium taste in mice. Nature 464, 297–301. doi: 10.1038/nature08783
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Charlu, S., Wisotsky, Z., Medina, A., and Dahanukar, A. (2013). Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nat. Commun. 4:2042. doi: 10.1038/ncomms3042
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chen, Y., and Amrein, H. (2014). Enhancing perception of contaminated food through acid-mediated modulation of taste neuron responses. Curr. Biol. 24, 1969–1977. doi: 10.1016/j.cub.2014.07.069
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chyb, S., Dahanukar, A., Wickens, A., and Carlson, J. R. (2003). Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc. Natl. Acad. Sci. U.S.A. 100(Suppl.), 14526–14530. doi: 10.1073/pnas.2135339100
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Clyne, P. J. (2000). Candidate Taste Receptors in Drosophila. Science. 287, 1830–1834. doi: 10.1126/science.287.5459.1830
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Clyne, P. J., Warr, C. G., Freeman, M. R., Lessing, D., Kim, J., and Carlson, J. R. (1999). A Novel family of Divergent Seven-Transmembrane proteins?: candidate odorant receptors in Drosophila. Neuron 22, 327–338. doi: 10.1016/S0896-6273(00)81093-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Colomb, J., Grillenzoni, N., Ramaekers, A., and Stocker, R. F. (2007). Architecture of the primary taste center of Drosophila melanogaster larvae. J. Comp. Neurol. 847, 834–847. doi: 10.1002/cne.21312
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Couto, A., Alenius, M., and Dickson, B. J. (2005). Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 15, 1535–1547. doi: 10.1016/j.cub.2005.07.034
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Croset, V., Rytz, R., Cummins, S. F., Budd, A., Brawand, D., Kaessmann, H., et al. (2010). Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 6:e1001064. doi: 10.1371/journal.pgen.1001064
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dahanukar, A., Foster, K., van der Goes van Naters, W. M., and Carlson, J. R. (2001). A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat. Neurosci. 4, 1182–1186. doi: 10.1038/nn765
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dahanukar, A., Lei, Y.-T., Kwon, J. Y., and Carlson, J. R. (2007). Two Gr genes underlie sugar reception in Drosophila Anupama. Neuron 56, 503–516. doi: 10.1016/j.neuron.2007.10.024
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dambly-chaudière, C., and Ghysen, A. (1986). The sense organs in the Drosophila larva and their relation to the embryonic pattern of sensory neurons. Roux's Arch. Dev. Biol. 195, 222–228. doi: 10.1007/BF02438954
Das, G., Klappenbach, M., Vrontou, E., Perisse, E., Clark, C. M., Burke, C. J., et al. (2014). Drosophila learn opposing components of a compound food stimulus. Curr. Biol. 24, 1723–1730. doi: 10.1016/j.cub.2014.05.078
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Datta, S. R., Vasconcelos, M. L., Ruta, V., Luo, S., Wong, A., Demir, E., et al. (2008). The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature 452, 473–477. doi: 10.1038/nature06808
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
DeGennaro, M., McBride, C. S., Seeholzer, L., Nakagawa, T., Dennis, E. J., Goldman, C., et al. (2013). Orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498, 487–491. doi: 10.1038/nature12206
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ditzen, M., Pellegrino, M., and Vosshall, L. B. (2008). Insect odorant receptors are molecular targets of the insect repellent DEET. Science 319, 1838–1842. doi: 10.1126/science.1153121
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dobritsa, A. A., Van der Goes van Nater, W., Warr, C. G., Steinbrecht, R. A., and Carlson, J. R. (2003). Integrating the molecular and cellular basis of Odor coding in the Drosophila Antenna. Neuron 37, 827–841. doi: 10.1016/S0896-6273(03)00094-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dunipace, L., Meister, S., McNealy, C., and Amrein, H. (2001). Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 11, 822–835. doi: 10.1016/S0960-9822(01)00258-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dweck, H. K. M., Ebrahim, S. A. M., Farhan, A., Hansson, B. S., and Stensmyr, M. C. (2015). Olfactory proxy detection of dietary antioxidants in Drosophila. Curr. Biol. 25, 455–466. doi: 10.1016/j.cub.2014.11.062
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dweck, H. K. M., Ebrahim, S. A. M., Kromann, S., Bown, D., Hillbur, Y., Sachse, S., et al. (2013). Olfactory preference for egg laying on citrus substrates in Drosophila. Curr. Biol. 23, 2472–2480. doi: 10.1016/j.cub.2013.10.047
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ejima, A., Smith, B. P. C., Lucas, C., van der Goes van Naters, W., Miller, C. J., Carlson, J. R., et al. (2007). Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr. Biol. 17, 599–605. doi: 10.1016/j.cub.2007.01.053
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fan, P., Manoli, D. S., Ahmed, O. M., Chen, Y., Agarwal, N., Kwong, S., et al. (2013). Genetic and neural mechanisms that inhibit Drosophila from mating with other species. Cell 154, 89–102. doi: 10.1016/j.cell.2013.06.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Faucher, C., Forstreuter, M., Hilker, M., and de Bruyne, M. (2006). Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J. Exp. Biol. 209, 2739–2748. doi: 10.1242/jeb.02297
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fernández, M. P., and Kravitz, E. A. (2013). Aggression and courtship in Drosophila: pheromonal communication and sex recognition. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 199, 1065–1076. doi: 10.1007/s00359-013-0851-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fischler, W., Kong, P., Marella, S., and Scott, K. (2007). The detection of carbonation by the Drosophila gustatory system. Nature 448, 1054–1057. doi: 10.1038/nature06101
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fishilevich, E., and Vosshall, L. B. (2005). Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 15, 1548–1553. doi: 10.1016/j.cub.2005.07.066
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fishilevich, E., Domingos, A. I., Asahina, K., Naef, F., Vosshall, L. B., Louis, M., et al. (2005). Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr. Biol. 15, 2086–2096. doi: 10.1016/j.cub.2005.11.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fowler, M. A., and Montell, C. (2013). Drosophila TRP channels and animal behavior. Life Sci. 92, 394–403. doi: 10.1016/j.lfs.2012.07.029
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fujii, S., Yavuz, A., Slone, J., Jagge, C., Song, X., Amrein, H., et al. (2015). Drosophila sugar receptors in sweet taste perception, olfaction, and internal nutrient sensing. Curr. Biol. 25, 621–627. doi: 10.1016/j.cub.2014.12.058
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fuyama, Y. (1976). Behavior genetics of olfactory responses in Drosophila. I. Oifactometry and strain differences in Drosophila melanogaster. Behav. Genet. 6, 406–420. doi: 10.1007/BF01065698
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gerber, B., and Stocker, R. F. (2007). The Drosophila larva as a model for studying chemosensation and chemosensory learning: a review. Chem. Senses 32, 65–89. doi: 10.1093/chemse/bjl030
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Goldman, A. L., Van der Goes van Naters, W., Lessing, D., Warr, C. G., and Carlson, J. R. (2005). Coexpression of two functional odor receptors in one neuron. Neuron 45, 661–666. doi: 10.1016/j.neuron.2005.01.025
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gomez-Diaz, C., and Benton, R. (2013). The joy of sex pheromones. EMBO Rep. 14, 874–883. doi: 10.1038/embor.2013.140
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grewal, J. S., Nguyen, C., Robles, R., Cho, C., Kir, K., Fledderman, N., et al. (2014). Complex and non-redundant signals from individual odor receptors that underlie chemotaxis behavior in Drosophila melanogaster larvae. Biol. Open 3, 947–957. doi: 10.1242/bio.20148573
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grosjean, Y., Rytz, R., Farine, J.-P., Abuin, L., Cortot, J., Jefferis, G. S., et al. (2011). An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature 478, 236–240. doi: 10.1038/nature10428
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Guo, Y., Wang, Y., Wang, Q., and Wang, Z. (2014). The Role of PPK26 in Drosophila Larval Mechanical Nociception. Cell Rep. 9, 1183–1190. doi: 10.1016/j.celrep.2014.10.020
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ha, T. S., and Smith, D. P. (2006). A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J. Neurosci. 26, 8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hallem, E. A., Ho, M. G., Carlson, J. R., and Haven, N. (2004). The molecular basis of odor coding in the Drosophila Antenna. Cell 117, 965–979. doi: 10.1016/j.cell.2004.05.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hallem, E. A., and Carlson, J. R. (2006). Coding of odors by a receptor repertoire. Cell 125, 143–160. doi: 10.1016/j.cell.2006.01.050
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hu, Y., Han, Y., Shao, Y., Wang, X., Ma, Y., Ling, E., et al. (2015). Gr33a modulates Drosophila male courtship preference. Sci. Rep. 5:7777. doi: 10.1038/srep07777
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Idstein, H., Bauer, C., and Schreier, P. (1985). Volatile acids in tropical fruits: cherimoya (Annona cherimolia, Mill.), guava (psidium guajava, L.), mango (Mangifera indica, L., var. Alphonso), papaya (Carica papaya, L.). Z. Lebensm. Unters. Forsch. 180, 394–397. doi: 10.1007/BF01027773
Jallon, J. (1984). A few chemical words exchanged by Drosophila during courtship and mating. Behav. Genet. 14, 441–476. doi: 10.1007/BF01065444
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jiao, Y., Moon, S. J., and Montell, C. (2007). A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc. Natl. Acad. Sci. U.S.A. 104, 14110–14115. doi: 10.1073/pnas.0702421104
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jiao, Y., Moon, S. J., Wang, X., Ren, Q., and Montell, C. (2008). Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr. Biol. 18, 1797–1801. doi: 10.1016/j.cub.2008.10.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jimenez-Del-Rio, M., Guzman-Martinez, C., and Velez-Pardo, C. (2010). The effects of polyphenols on survival and locomotor activity in Drosophila melanogaster exposed to iron and paraquat. Neurochem. Res. 35, 227–238. doi: 10.1007/s11064-009-0046-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jones, W. D., Cayirlioglu, P., Kadow, I. G., and Vosshall, L. B. (2007). Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 445, 86–90. doi: 10.1038/nature05466
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Joseph, R. M., Devineni, A. V., King, I. F. G., and Heberlein, U. (2009). Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 106, 11352–11357. doi: 10.1073/pnas.0901419106
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Khurana, S., and Siddiqi, O. (2013). Olfactory responses of Drosophila larvae. Chem. Senses 38, 315–323. doi: 10.1093/chemse/bjs144
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, S. H., Lee, Y., Akitake, B., Woodward, O. M., Guggino, W. B., Montell, C., et al. (2010). Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl. Acad. Sci. U.S.A. 107, 8440–8445. doi: 10.1073/pnas.1001425107
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Koganezawa, M., Haba, D., Matsuo, T., and Yamamoto, D. (2010). The shaping of male courtship posture by lateralized gustatory inputs to male-specific interneurons. Curr. Biol. 20, 1–8. doi: 10.1016/j.cub.2009.11.038
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Koh, T.-W., He, Z., Gorur-Shandilya, S., Menuz, K., Larter, N. K., Stewart, S., et al. (2014). The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron 83, 850–865. doi: 10.1016/j.neuron.2014.07.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kohl, J., Ostrovsky, A. D., Frechter, S., and Jefferis, G. S. (2013). A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell 155, 1610–1623. doi: 10.1016/j.cell.2013.11.025
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kondoh, Y., Kaneshiro, K. Y., Kimura, K., and Yamamoto, D. (2003). Evolution of sexual dimorphism in the olfactory brain of Hawaiian Drosophila. Proc. Biol. Sci. 270, 1005–1013. doi: 10.1098/rspb.2003.2331
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kreher, S. A., Kwon, J. Y., and Carlson, J. R. (2005). The molecular basis of odor coding in the Drosophila larva. Neuron 46, 445–456. doi: 10.1016/j.neuron.2005.04.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kreher, S. A., Mathew, D., Kim, J., and Carlson, J. R. (2008). Translation of sensory input into behavioral output via an olfactory system. Neuron 59, 110–124. doi: 10.1016/j.neuron.2008.06.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kurtovic, A., Widmer, A., and Dickson, B. J. (2007). A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542–546. doi: 10.1038/nature05672
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kwon, J. Y., Dahanukar, A., Weiss, L. A., and Carlson, J. R. (2007). The molecular basis of CO2 reception in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 104, 3574–3578. doi: 10.1073/pnas.0700079104
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kwon, J. Y., Dahanukar, A., Weiss, L. A., and Carlson, J. R. (2011). Molecular and cellular organization of the taste system in the Drosophila larva. J. Neurosci. 31, 15300–15309. doi: 10.1523/JNEUROSCI.3363-11.2011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kwon, Y., Kim, S. H., Ronderos, D. S., Lee, Y., Woodward, O. M., Guggino, W. B., et al. (2010). Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr. Biol. 20, 1672–1678. doi: 10.1016/j.cub.2010.08.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lacaille, F., Hiroi, M., Twele, R., Inoshita, T., Umemoto, D., Manière, G., et al. (2007). An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS ONE 2:e661. doi: 10.1371/journal.pone.0000661
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Laissue, P. P., and Vosshall, L. B. (2008). “The olfactory sensory map in Drosophila,” in Brain Development in Drosophila melanogaster, ed G. M. Technau (Austin, TX: Landes Bioscience and Springer Science), 102–114. doi: 10.1007/978-0-387-78261-4_7
Larsson, M. C., Domingos, A. I., Jones, W. D., Chiappe, M. E., Amrein, H., Vosshall, L. B., et al. (2004). Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714. doi: 10.1016/j.neuron.2004.08.019
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lebreton, S., Becher, P. G., Hansson, B. S., and Witzgall, P. (2012). Attraction of Drosophila melanogaster males to food-related and fly odours. J. Insect Physiol. 58, 125–129. doi: 10.1016/j.jinsphys.2011.10.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, K. P., Simpson, S. J., Clissold, F. J., Brooks, R., Ballard, J. W. O., Taylor, P. W., et al. (2008). Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl. Acad. Sci. U.S.A. 105, 2498–2503. doi: 10.1073/pnas.0710787105
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, Y., Jun, S., and Montell, C. (2009). Multiple gustatory receptors required for the caffeine response in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 106, 4495–4500. doi: 10.1073/pnas.0811744106
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, Y., Kang, M. J., Shim, J., Cheong, C. U., Moon, S. J., Montell, C., et al. (2012). Gustatory receptors required for avoiding the insecticide L-canavanine. J. Neurosci. 32, 1429–1435. doi: 10.1523/JNEUROSCI.4630-11.2012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, Y., Kim, S. H., and Montell, C. (2010). Avoiding DEET through insect gustatory receptors. Neuron 67, 555–561. doi: 10.1016/j.neuron.2010.07.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liman, E. R., Zhang, Y. V., and Montell, C. (2014). Peripheral coding of taste. Neuron 81, 984–1000. doi: 10.1016/j.neuron.2014.02.022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ling, F., Dahanukar, A., Weiss, L. A., Kwon, J. Y., and Carlson, J. R. (2014). The molecular and cellular basis of taste coding in the legs of Drosophila. J. Neurosci. 34, 7148–7164. doi: 10.1523/JNEUROSCI.0649-14.2014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, L., Johnson, W. A., and Welsh, M. J. (2003a). Drosophila DEG/ENaC pickpocket genes are expressed in the tracheal system, where they may be involved in liquid clearance. Proc. Natl. Acad. Sci. U.S.A. 100, 2128–2133. doi: 10.1073/pnas.252785099
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, L., Leonard, A. S., Motto, D. G., Feller, M. A., Price, M. P., Johnson, W. A., et al. (2003b). Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron 39, 133–146. doi: 10.1016/S0896-6273(03)00394-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, T., Starostina, E., Vijayan, V., and Pikielny, C. W. (2012). Two Drosophila DEG/ENaC channel subunits have distinct functions in gustatory neurons that activate male courtship. J. Neurosci. 32, 11879–11889. doi: 10.1523/JNEUROSCI.1376-12.2012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, W., Liang, X., Gong, J., Yang, Z., Zhang, Y.-H., Zhang, J.-X., et al. (2011). Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat. Neurosci. 14, 896–902. doi: 10.1038/nn.2836
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lu, B., LaMora, A., Sun, Y., Welsh, M. J., and Ben-Shahar, Y. (2012). ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 8:e1002587. doi: 10.1371/journal.pgen.1002587
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Marella, S., Fischler, W., Kong, P., Asgarian, S., Rueckert, E., and Scott, K. (2006). Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron 49, 285–295. doi: 10.1016/j.neuron.2005.11.037
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Markow, T. A. (1988). Reproductive Behavior of Drosophila melanogaster and D. nigrospiracula in the field and in the laboratory. J. Comp. Psychol. 102, 169–173. doi: 10.1037/0735-7036.102.2.169
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Masek, P., and Keene, A. C. (2013). Drosophila fatty acid taste signals through the PLC pathway in sugar-sensing neurons. PLoS Genet. 9:e1003710. doi: 10.1371/journal.pgen.1003710
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mast, J. D., De Moraes, C. M., Alborn, H. T., Lavis, L. D., and Stern, D. L. (2014). Evolved differences in larval social behavior mediated by novel pheromones. Elife 3, 1–21. doi: 10.7554/eLife.04205
Mathew, D., Martelli, C., Kelley-Swift, E., Brusalis, C., Gershow, M., Samuel, A. D. T., et al. (2013). Functional diversity among sensory receptors in a Drosophila olfactory circuit. Proc. Natl. Acad. Sci. U.S.A. 110, E2134–E2143. doi: 10.1073/pnas.1306976110
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McBride, C. S., Baier, F., Omondi, A. B., Spitzer, S. A., Lutomiah, J., Sang, R., et al. (2014). Evolution of mosquito preference for humans linked to an odorant receptor. Nature 515, 222–227. doi: 10.1038/nature13964
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McMeniman, C. J., Corfas, R. A., Matthews, B. J., Ritchie, S. A., and Vosshall, L. B. (2014). Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060–1071. doi: 10.1016/j.cell.2013.12.044
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Meijerink, J., Braks, M. A., and Van Loon, J. J. (2001). Olfactory receptors on the antennae of the malaria mosquito Anopheles gambiae are sensitive to ammonia and other sweat-borne components. J. Insect Physiol. 47, 455–464. doi: 10.1016/S0022-1910(00)00136-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mellert, D. J., Robinett, C. C., and Baker, B. S. (2012). Doublesex functions early and late in gustatory sense organ development. PLoS ONE 7:e51489. doi: 10.1371/journal.pone.0051489
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Min, S., Ai, M., Shin, S. A., and Suh, G. S. B. (2013). Dedicated olfactory neurons mediating attraction behavior to ammonia and amines in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 110, E1321–E1329. doi: 10.1073/pnas.1215680110
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mishra, D., Miyamoto, T., Rezenom, Y. H., Broussard, A., Yavuz, A., Slone, J., et al. (2013). The molecular basis of sugar sensing in Drosophila larvae. Curr. Biol. 23, 1466–1471. doi: 10.1016/j.cub.2013.06.028
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mitri, C., Parmentier, M.-L., Pin, J.-P., Bockaert, J., and Grau, Y. (2004). Divergent evolution in metabotropic glutamate receptors. A new receptor activated by an endogenous ligand different from glutamate in insects. J. Biol. Chem. 279, 9313–9320. doi: 10.1074/jbc.M310878200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mitri, C., Soustelle, L., Framery, B., Bockaert, J., Parmentier, M.-L., Grau, Y., et al. (2009). Plant insecticide L-canavanine repels Drosophila via the insect orphan GPCR DmX. PLoS Biol. 7:e1000147. doi: 10.1371/journal.pbio.1000147
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Miyakawa, Y. (1981). Bimodal response in a chemotactic behaviour of Drosophila larvae to monovalent salts. J. Insect Physiol. 27, 387–392. doi: 10.1016/0022-1910(81)90016-0
Miyamoto, T., and Amrein, H. (2008). Suppression of male courtship by a Drosophila pheromone receptor. Nat. Neurosci. 11, 874–876. doi: 10.1038/nn.2161
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Miyamoto, T., Slone, J., Song, X., and Amrein, H. (2012). A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell 151, 1113–1125. doi: 10.1016/j.cell.2012.10.024
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moat, A. G., Foster, J. W., and Spector, M. P. (eds.). (2002). “Fermentation pathways,” in Microbial Physiology (Hoboken, NJ: Wiley-Liss), 412–433. doi: 10.1002/0471223867.ch11
Mollo, E., Fontana, A., Roussis, V., Polese, G., Amodeo, P., Ghiselin, M. T., et al. (2014). Sensing marine biomolecules: smell, taste, and the evolutionary transition from aquatic to terrestrial life. Front. Chem. 2:92. doi: 10.3389/fchem.2014.00092
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Monte, P., Woodard, C., Ayer, R., Lilly, M., Sun, H., Carlson, J., et al. (1989). Characterization of the Larval Olfactory Response in Drosophila and Its Genetic Basis. Behav. Genet. 19, 267–283. doi: 10.1007/BF01065910
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Montell, C. (2009). A taste of the Drosophila gustatory receptors. Curr. Opin. Neurobiol. 19, 345–353. doi: 10.1016/j.conb.2009.07.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moon, S. J., Köttgen, M., Jiao, Y., Xu, H., and Montell, C. (2006). A taste receptor required for the caffeine response in vivo. Curr. Biol. 16, 1812–1817. doi: 10.1016/j.cub.2006.07.024
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moon, S. J., Lee, Y., Jiao, Y., and Montell, C. (2009). A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr. Biol. 19, 1623–1627. doi: 10.1016/j.cub.2009.07.061
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Neely, G. G., Keene, A. C., Duchek, P., Chang, E. C., Wang, Q.-P., Aksoy, Y. A., et al. (2011). TrpA1 regulates thermal nociception in Drosophila. PLoS ONE 6:e24343. doi: 10.1371/journal.pone.0024343
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Neuhaus, E. M., Gisselmann, G., Zhang, W., Dooley, R., Störtkuhl, K., Hatt, H., et al. (2005). Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat. Neurosci. 8, 15–17. doi: 10.1038/nn1371
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ng, S. H., Shankar, S., Shikichi, Y., Akasaka, K., Mori, K., Yew, J. Y., et al. (2014). Pheromone evolution and sexual behavior in Drosophila are shaped by male sensory exploitation of other males. Proc. Natl. Acad. Sci. U.S.A. 111, 3056–3061. doi: 10.1073/pnas.1313615111
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Park, J.-H., and Kwon, J. Y. (2011). Heterogeneous expression of Drosophila gustatory receptors in enteroendocrine cells. PLoS ONE 6:e29022. doi: 10.1371/journal.pone.0029022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Parsons, P. A. (1980). Acetic acid vapour as a resource: threshold differences among three Drosophila species. Experientia 36, 1363. doi: 10.1007/BF01960099
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pikielny, C. W. (2012). Sexy DEG/ENaC channels involved in gustatory detection of fruit fly pheromones. Sci. Signal. 5:pe48. doi: 10.1126/scisignal.2003555
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Possidente, D. R., and Murphey, R. K. (1989). Genetic control of sexually dimorphic axon morphology in Drosophila sensory neurons. Dev. Biol. 132, 448–457. doi: 10.1016/0012-1606(89)90241-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Robertson, H. M., and Kent, L. B. (2009). Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J. Insect Sci. 9, 19. doi: 10.1673/031.009.1901
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Robertson, H. M., Warr, C. G., and Carlson, J. R. (2003). Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 100(Suppl.), 14537–14542. doi: 10.1073/pnas.2335847100
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rodrigues, V., and Hummel, T. (2008). “Development of the Drosophila olfactory system,” in Brain Development in Drosophila melanogaster, ed G. M. Technau (Austin, TX: Landes Bioscience and Springer Science), 82–101. doi: 10.1007/978-0-387-78261-4_6
Ronderos, D. S., Lin, C.-C., Potter, C. J., and Smith, D. P. (2014). Farnesol-detecting olfactory neurons in Drosophila. J. Neurosci. 34, 3959–3968. doi: 10.1523/JNEUROSCI.4582-13.2014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ruebenbauer, A., Schlyter, F., Hansson, B. S., Löfstedt, C., and Larsson, M. C. (2008). Genetic variability and robustness of host odor preference in Drosophila melanogaster. Curr. Biol. 18, 1438–1443. doi: 10.1016/j.cub.2008.08.062
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ruta, V., Datta, S. R., Vasconcelos, M. L., Freeland, J., Looger, L. L., Axel, R., et al. (2010). A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature 468, 686–690. doi: 10.1038/nature09554
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schwartz, N. U., Zhong, L., Bellemer, A., and Tracey, W. D. (2012). Egg laying decisions in Drosophila are consistent with foraging costs of larval progeny. PLoS ONE 7:e37910. doi: 10.1371/journal.pone.0037910
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schwarz, S., Durisko, Z., and Dukas, R. (2014). Food selection in larval fruit flies: dynamics and effects on larval development. Naturwissenschaften 101, 61–68. doi: 10.1007/s00114-013-1129-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Scott, K., Brady, R., Cravchik, A., Morozov, P., Rzhetsky, A., Zuker, C., et al. (2001). A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 104, 661–673. doi: 10.1016/S0092-8674(01)00263-X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Silbering, A. F., and Galizia, C. G. (2007). Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J. Neurosci. 27, 11966–11977. doi: 10.1523/JNEUROSCI.3099-07.2007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Silbering, A. F., Rytz, R., Grosjean, Y., Abuin, L., Ramdya, P., Jefferis, G. S., et al. (2011). Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 31, 13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Slone, J., Daniels, J., and Amrein, H. (2007). Sugar receptors in Drosophila Jesse. Curr. Biol. 17, 1809–1816. doi: 10.1016/j.cub.2007.09.027
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Spehr, M., and Munger, S. D. (2009). Olfactory receptors: G protein-coupled receptors and beyond. J. Neurochem. 109, 1570–1583. doi: 10.1111/j.1471-4159.2009.06085.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Starostina, E., Liu, T., Vijayan, V., Zheng, Z., Siwicki, K. K., Pikielny, C. W., et al. (2012). A Drosophila DEG/ENaC subunit functions specifically in gustatory neurons required for male courtship behavior. J. Neurosci. 32, 4665–4674. doi: 10.1523/JNEUROSCI.6178-11.2012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stensmyr, M. C. (2003). Novel natural ligands for Drosophila olfactory receptor neurones. J. Exp. Biol. 206, 715–724. doi: 10.1242/jeb.00143
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stensmyr, M. C., Dekker, T., and Hansson, B. S. (2003). Evolution of the olfactory code in the Drosophila melanogaster subgroup. Proc. Biol. Sci. 270, 2333–2340. doi: 10.1098/rspb.2003.2512
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stensmyr, M. C., Dweck, H. K. M., Farhan, A., Ibba, I., Strutz, A., Mukunda, L., et al. (2012). A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151, 1345–1357. doi: 10.1016/j.cell.2012.09.046
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stocker, R. F. (1994). The organization of the chemosensory system in Drosophila melanogaster: a rewiew. Cell Tissue Res. 275, 3–26. doi: 10.1007/BF00305372
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stocker, R. F. (2008). “Design ofthe larval chemosensory system,” in Brain Development in Drosophila melanogaster, ed G. M. Technau (Austin, TX: Landes Bioscience and Springer Science), 69–81. doi: 10.1007/978-0-387-78261-4_5
Stocker, R. F. (2009). The olfactory pathway of adult and larval Drosophila: conservation or adaptation to stage-specific needs? Ann. N.Y. Acad. Sci. 1170, 482–486. doi: 10.1111/j.1749-6632.2009.03896.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stockinger, P., Kvitsiani, D., Rotkopf, S., Tirián, L., and Dickson, B. J. (2005). Neural circuitry that governs Drosophila male courtship behavior. Cell 121, 795–807. doi: 10.1016/j.cell.2005.04.026
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Suh, G. S. B., Wong, A. M., Hergarden, A. C., and Wang, J. W. (2004). A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431, 854–858. doi: 10.1038/nature02980
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tennessen, J. M., and Thummel, C. S. (2011). Coordinating growth and maturation - insights from Drosophila. Curr. Biol. 21, R750–R757. doi: 10.1016/j.cub.2011.06.033
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thistle, R., Cameron, P., Ghorayshi, A., Dennison, L., and Scott, K. (2012). Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell 149, 1140–1151. doi: 10.1016/j.cell.2012.03.045
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thorne, N., and Amrein, H. (2008). Atypical expression of Drosophila gustatory receptor genes in sensory. J. Comp. Neurol. 568, 548–568. doi: 10.1002/cne.21547
Thorne, N., Chromey, C., Bray, S., and Amrein, H. (2004). Taste perception and coding in drosophila. Curr. Biol. 14, 1065–1079. doi: 10.1016/j.cub.2004.05.019
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thum, A. S., Leisibach, B., Gendre, N., Selcho, M., and Stocker, R. F. (2011). Diversity, variability, and suboesophageal connectivity of antennal lobe neurons in D. melanogaster larvae. J. Comp. Neurol. 519, 3415–3432. doi: 10.1002/cne.22713
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Toda, H., Zhao, X., and Dickson, B. J. (2012). The Drosophila female aphrodisiac pheromone activates ppk23(+) sensory neurons to elicit male courtship behavior. Cell Rep. 1, 599–607. doi: 10.1016/j.celrep.2012.05.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Truman, J. W. (1990). Metamorphosis of the central nervous system of Drosophila. J. Neurobiol. 21, 1072–1084. doi: 10.1002/neu.480210711
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Van der Goes van Naters, W., and Carlson, J. R. (2007). Receptors and neurons for fly odors in Drosophila. Curr. Biol. 17, 606–612. doi: 10.1016/j.cub.2007.02.043
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vijayan, V., Thistle, R., Liu, T., Starostina, E., and Pikielny, C. W. (2014). Drosophila pheromone-sensing neurons expressing the ppk25 ion channel subunit stimulate male courtship and female receptivity. PLoS Genet. 10:e1004238. doi: 10.1371/journal.pgen.1004238
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vijendravarma, R. K., Narasimha, S., and Kawecki, T. J. (2013). Predatory cannibalism in Drosophila melanogaster larvae. Nat. Commun. 4, 1789. doi: 10.1038/ncomms2744
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vosshall, L. B., Amrein, H., Morozov, P. S., Rzhetsky, A., and Axel, R. (1999). A Spatial Map of Olfactory Receptor Expression in the Drosophila Antenna. Cell 96, 725–736. doi: 10.1016/S0092-8674(00)80582-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, L., and Anderson, D. J. (2010). Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463, 227–231. doi: 10.1038/nature08678
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, L., Han, X., Mehren, J., Hiroi, M., Billeter, J.-C., Miyamoto, T., et al. (2011). Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat. Neurosci. 14, 757–762. doi: 10.1038/nn.2800
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, Z., Singhvi, A., Kong, P., and Scott, K. (2004). Taste representations in the Drosophila brain. Cell 117, 981–991. doi: 10.1016/j.cell.2004.06.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Watanabe, K., Toba, G., Koganezawa, M., and Yamamoto, D. (2011). Gr39a, a highly diversified gustatory receptor in Drosophila, has a role in sexual behavior. Behav. Genet. 41, 746–753. doi: 10.1007/s10519-011-9461-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Weiss, L. A., Dahanukar, A., Kwon, J. Y., Banerjee, D., and Carlson, J. R. (2011). The Molecular and cellular basis of bitter taste in Drosophila Linnea. Neuron 69, 258–272. doi: 10.1016/j.neuron.2011.01.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wisotsky, Z., Medina, A., Freeman, E., and Dahanukar, A. (2011). Evolutionary differences in food preference rely on Gr64e, a receptor for glycerol. Nat. Neurosci. 14, 1534–1541. doi: 10.1038/nn.2944
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Xu, J., Sornborger, A. T., Lee, J. K., and Shen, P. (2008). Drosophila TRPA channel modulates sugar-stimulated neural excitation, avoidance and social response. Nat. Neurosci. 11, 676–682. doi: 10.1038/nn.2119
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yamamoto, D., and Koganezawa, M. (2013). Genes and circuits of courtship behaviour in Drosophila males. Nat. Rev. Neurosci. 14, 681–692. doi: 10.1038/nrn3567
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yanagawa, A., Guigue, A. M., and Marion-Poll, F. (2014). Hygienic grooming is induced by contact chemicals in Drosophila melanogaster. Front. Behav. Neurosci. 8:254. doi: 10.3389/fnbeh.2014.00254
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yang, C.-H., Belawat, P., Hafen, E., Jan, L. Y., and Jan, Y.-N. (2008). Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319, 1679–1683. doi: 10.1126/science.1151842
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yew, J. Y., Dreisewerd, K., Luftmann, H., Müthing, J., Pohlentz, G., Kravitz, E. A., et al. (2009). A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr. Biol. 19, 1245–1254. doi: 10.1016/j.cub.2009.06.037
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zelle, K. M., Lu, B., Pyfrom, S. C., and Ben-Shahar, Y. (2013). The genetic architecture of degenerin/epithelial sodium channels in Drosophila. G3 (Bethesda) 3, 441–450. doi: 10.1534/g3.112.005272
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, Y. V., Ni, J., and Montell, C. (2013a). The molecular basis for attractive salt-taste coding in Drosophila. Science 340, 1334–1338. doi: 10.1126/science.1234133
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, Y. V., Raghuwanshi, R. P., Shen, W. L., and Montell, C. (2013b). Food experience – induced taste desensitization modulated by the Drosophila TRPL channel. Nat. Neurosci. 16, 1468–1476. doi: 10.1038/nn.3513
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhou, C., Pan, Y., Robinett, C. C., Meissner, G. W., and Baker, B. S. (2014). Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron 83, 149–163. doi: 10.1016/j.neuron.2014.05.038
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ziegler, A. B., Berthelot-Grosjean, M., and Grosjean, Y. (2013). The smell of love in Drosophila. Front. Physiol. 4:72. doi: 10.3389/fphys.2013.00072
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: Olfaction, taste, receptor, Drosophila, attraction, repulsion, ecological niche
Citation: Depetris-Chauvin A, Galagovsky D and Grosjean Y (2015) Chemicals and chemoreceptors: ecologically relevant signals driving behavior in Drosophila. Front. Ecol. Evol. 3:41. doi: 10.3389/fevo.2015.00041
Received: 30 January 2015; Accepted: 31 March 2015;
Published: 24 April 2015.
Edited by:
Emmanuelle Jacquin-Joly, Institut National de la Recherche Agronomique, FranceReviewed by:
Ewald Grosse-Wilde, Max Planck Institute for Chemical Ecology, GermanyAlisha Anderson, The Commonwealth Scientific and Industrial Research Organisation, Australia
Copyright © 2015 Depetris-Chauvin, Galagovsky and Grosjean. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yael Grosjean, UMR 6265, Centre des Sciences du Gût et de l'Alimentation, Centre National de la Recherche Scientifique, UMR 1324-INRA, Université de Bourgogne, 6 boulevard Gabriel, F-21000 Dijon, France yael.grosjean@u-bourgogne.fr
 Ana Depetris-Chauvin1,2,3
Ana Depetris-Chauvin1,2,3  Yael Grosjean
Yael Grosjean