Social carry-over effects on non-social behavioral variation: mechanisms and consequences
- 1Department Biologie II, Ludwig-Maximilians University of Munich, Planegg-Martinsried, Germany
- 2Evolutionary Ecology of Variation Group, Max Planck Institute for Ornithology, Seewiesen, Germany
The field of animal personality is interested in decomposing behaviors into different levels of variation, with its present focus on the ecological and evolutionary causes and consequences of expressed variation. Recently the role of the social environment, i.e., social partners, has been suggested to affect behavioral variation and induce selection on animal personality. Social partner effects exist because characters of social partners (e.g., size, behavior), affect the behavioral expression of a focal individual. Here, we (1) first review the proximate mechanisms underlying the social partner effects on behavioral expression and the timescales at which such effects might take place. We then (2) discuss how within- and among-individual variation in single behaviors and covariation between multiple behaviors, caused by social partners, can carry-over to non-social behaviors expressed outside the social context. Finally, we (3) highlight evolutionary consequences of social carry-over effects to non-social behaviors and (4) suggest study designs and statistical approaches which can be applied to study the nature and evolutionary consequences of social carry-over effects on non-social behaviors. Acknowledging the proximate mechanisms underpinning the social partner effects is important since it opens a door to understand in depth how social environments can affect behavioral variation and covariation at multiple levels, and the evolution of non-social behaviors (i.e., exploration, activity, boldness) that are affected by social interactions.
Introduction
Phenotypes vary at multiple levels and research in the field of animal personality has highlighted the importance of distinguishing between behavioral variation that occurs among individuals (“personality”) vs. within individuals (“plasticity”) (Dingemanse et al., 2010; Dingemanse and Dochtermann, 2013). Targeting within-individual level variation has been the more traditional approach in behavioral ecology (for social contexts: Maynard-Smith, 1982) and its evolutionary significance lies in the ability of plastic individuals to adapt to changing environments in order to maximize fitness in any given environment (Piersma and Drent, 2003; Ghalambor et al., 2007). Even though within-individual variation in phenotypic traits can be present in several different timescales across an individual's lifespan (Piersma and Drent, 2003), the costs and limits of phenotypic plasticity may restrict the optimal response to any single confronted environment (DeWitt et al., 1998; Auld et al., 2010). Among-individual level variation has more recently come to the foreground in behavioral ecology and, whilst not necessarily predicted by traditional adaptive theory (Dall et al., 2004), it is widespread across the animal kingdom (Sih et al., 2004; Dingemanse and Wolf, 2010; Wolf and Weissing, 2010; Dall et al., 2012), and has major ecological and evolutionary consequences (Wolf and Weissing, 2012). Internal features of the individual (states: metabolic rate, body size, assets) have been widely used to explain the existence of among-individual variation in behaviors (Wolf et al., 2007; Biro and Stamps, 2008; Careau et al., 2008; Luttbeg and Sih, 2010), while they also contribute to variation at the within-individual level due to the fluctuating nature of these features (Wolf and Weissing, 2010). Recently, external features like the social environment, i.e., other individuals, have been suggested as possible factors modifying behavioral variation at both within- and among-individual levels (Bergmüller and Taborsky, 2010; Montiglio et al., 2013; Dingemanse and Araya-Ajoy, 2015). However, almost without exception these levels of variation are neglected in studies of social partner effects (however for within-individual level variation within social context see Wilson et al., 2011a, 2013) and studies of carry-over effects to non-social behaviors expressed outside the social interaction are absent (however see Laskowski and Pruitt, 2014 for group level effects). The proximate mechanisms through which partner effects act can define the temporal patterns of social carry-over effects to non-social behaviors expressed after social interactions. Therefore, understanding the proximate mechanisms gives information about when social carry-over effects affect within- vs. among-individual level of variation and about the strength and temporal patterns of the potential evolutionary effects of carry-overs to non-social behaviors.
Individuals might express behaviors differently in the presence vs. absence of conspecifics (reviewed in Webster and Ward, 2011). A classic example of social interaction is direct contest behavior, usually measured between competing males (Hsu et al., 2006). However, behaviors such as mating, mate attraction, communication and different forms of co-operation are also well studied examples of social behaviors (Bradbury and Vehrencamp, 1998; Griffin and West, 2003; Wright et al., 2010; Lyon and Montgomerie, 2012). Social partners can affect the up- or down regulation of different state variables of a focal individual, like hormonal profiles, the level of energy, or body size, and thus affect the expressed behaviors of a focal individual through these proximate mechanisms (Hsu et al., 2006; Sachser et al., 2013; Wilson et al., 2013). The temporal consistency of the social partner effect on a state variable may define the temporal consistency of the focal individuals' behaviors driven by the given state (Hsu et al., 2006; cf. Wolf and Weissing, 2010). Short term social partner effects are likely based on fast-changing states, like hormonal profiles, while long term social partner effects on behaviors might be mediated through slow-changing states like body mass, neurobiological features or other physiological mechanisms.
Traditionally, social partner effects are explored mainly within the social contexts, and only at the phenotypic mean level, so that carry-over effects have been studied from one social interaction to another, i.e., how long winning vs. losing in one social interaction affects the outcome of the next social interaction (Hsu et al., 2006). However, since behaviors can covary through shared proximate mechanisms across contexts (Ketterson and Nolan, 1999; Sih et al., 2004; Sih and Bell, 2008; Garamszegi et al., 2012), social partner effects have the potential to carry over indirectly to behaviors expressed after social interactions in non-social contexts, if the underpinning proximate mechanisms persist long enough. Such non-social behaviors can be, for example, exploration, activity, and boldness, expressed after the social interaction. Statistically, social partner effects are present when the identity of the social partner explains a significant amount of variance in the behavior of a focal individual (the individual on which the phenotype is measured), either at the within- or among-individual level of variation (Wilson et al., 2011a, 2013; Dingemanse and Araya-Ajoy, 2015). However, this kind of variance partitioning models have not been applied (nor studied) to the carry-over effects on non-social behaviors expressed outside social interactions (see however Laskowski and Pruitt, 2014 for group level effects). Including a variance partitioning approach in studies of social carry-over effects, together with the knowledge of the underpinning proximate mechanisms, helps to understand the temporal patterns of social carry-over effects, and whether the repeatable or plastic part (or both) of non-social behaviors in focal individuals is affected by the social interactions. Furthermore, studying social carry-over effects in depth is not only mechanistically, but also evolutionary important. Social partners can induce indirect genetic effects (i.e., IGEs), which are present when trait expression is not only affected directly by the genes of a focal individual (i.e., DGEs), but also by the genes of its social partner (Moore et al., 1997; Wolf et al., 1999). IGEs can slow down or speed up the rate of evolution of traits associated directly (or indirectly) with social interactions (Moore et al., 1997; Wolf et al., 1999). Since the temporal patterns of the carry-over effects may depend on the nature of the underpinning proximate mechanism, the underpinning mechanism might also define the strength of the carry-over effect on the evolution of non-social behavioral traits.
Here, we introduce a framework to clarify how the proximate mechanisms might define the temporal patterns of social carry-over effects on behavioral variation at different levels in non-social behaviors like exploration, activity or boldness, expressed after social interactions. We (1) review types of proximate mechanisms causing social partner effects over short and long time periods and explain how temporal patterns of social carry-over effects might depend on these mechanisms. We then discuss (2) how within- and among-individual behavioral variance in non-social behaviors and covariance between non-social and social behaviors are affected by social partners depending on the nature of the proximate mechanisms underpinning the social carry-over effects. We also (3) highlight the evolutionary implications and fitness consequences of the carry-over effects and (4) give suggestions on how to empirically study and statistically analyze the existence, temporal persistency (using variance partitioning tools), and evolutionary consequences of the carry-over effects on non-social behaviors. Our framework highlights the importance of carry-over effects on non-social behaviors and helps to (i) predict how far temporally the social carry-over effects have the potential to affect the different variance components (i.e., plasticity and personality) in these behaviors and (ii) learn about consequent evolutionary effects of these carry-overs on non-social behaviors.
Proximate Mechanisms for Short- and Long-Term Social Partner Effects
Behavior is often assumed to be “state-dependent” (McNamara and Houston, 1996; Houston and McNamara, 1999), with “state” being anything that affects the costs and benefits of expressed behaviors: environmental, physiological, neurobiological, or morphological features (McNamara and Houston, 1996; Houston and McNamara, 1999; Wolf et al., 2011). Below, we review fast- and slow-changing state variables underpinning the social partner effects on behavioral expression in general and discuss how they can cause carry-over effects outside the social interaction at various temporal scales.
Social Carry-Over Effects on Behavioral Expression through Fast-Changing State Variables
Generally, social carry-over effects which decay quickly might have a basis in hormone- or neurotransmitter levels, short term memory, level of energy, blood pressure or other fast-changing states which affect the behavioral expression of the focal individual accordingly (Hsu et al., 2006; Briffa and Sneddon, 2007; Coppens et al., 2010; Earley et al., 2013). Since fast-changing states are relatively easily affected by external factors (Wolf and Weissing, 2010), they are also sensitive to social partner effects. Because carry-over effects through fast-changing states are measurable only shortly after social interactions, they can carry-over to non-social behaviors, but only over short temporal scales (red line in Figure 1). In extreme cases, hormonal profiles return back to the baseline level almost immediately after the social interaction giving no room for social carry-overs. In the Field cricket, Gryllus bimaculatus, the level of octopamine in haemolymph after aggressive male-male interactions or female-male courtship interactions dropped back to baseline within a few minutes after the interaction (Adamo et al., 1995), suggesting absence of carry-over effects due to hormonal mechanisms. In the pumpkinseed sunfish, Lepomis gibbosus, winner effects are only detectable between 15 and 60 minutes after winning (Chase et al., 1994). This means that, in this species, behaviors measured in non-social contexts shortly after winning a contest might be affected by social partners. Since social interactions are also energetically costly (Hsu et al., 2006; Briffa and Sneddon, 2007), other fast-changing states like amount of energy reserves may play a role in carry-over effects. In the salamander, Desmognathus ochrophaeus, oxygen consumption and lactic acid formation significantly increased during both male-male aggressive encounters and male-female courtship (Bennett and Houck, 1983). Energy consumption during social interactions may cause short term physical exhaustion or depletion of resources (Briffa and Sneddon, 2007), and affect non-social behaviors measured shortly after the social interaction accordingly. The temporal consistency of social carry-over effects based on fast-changing states might depend on the length of the social interaction. For example, in the Sierra dome spider, Neriene litigiosa, energetic costs of male-male fights increase with the temporal consistency of the fights from 3.5 to 11.5 times compared to that of resting metabolic rate (DeCarvalho et al., 2004). Moreover, in house crickets, Acheta domesticus, the energy expenditure increases with the escalation level of a fight, with the oxygen consumed in high escalation stages being up to 40 times more than the baseline level (Hack, 1997). Thus, the magnitude of the social carry-over effects on non-social behaviors of the focal individuals might correlate positively with the length of the social interaction.
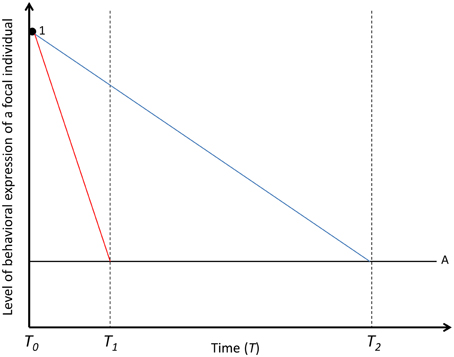
Figure 1. Decay of the social carry-over effect on behavioral expression of a focal individual in time. The solid black line represents the focal individual's (A) baseline level of behavioral expression, while red, and blue lines represent the behavioral expression of a focal individual under social carry-over effects by social partner (round black dot; 1). Carry-over effects are underpinned by either fast-changing (red line) or slow-changing (blue line) state variables. The social partner effect is decayed in T1 if underpinned by fast-changing mechanisms and in T2 if underpinned by slow-changing mechanisms. The social interaction is terminated in T0. For simplicity, the partner effects, driven by fast- and slow-changing states are assumed to be the same magnitude in T0.
Social Carry-Over Effects on Behavioral Expression through Slow-Changing State Variables
If social partner effects are underpinned by slow-changing state variables they can also effectively explain long term carry-over effects in non-social behaviors (blue line in Figure 1). However, slow-changing states, like body mass, organ size, or neurobiological features take a long time to change (Wolf and Weissing, 2010), and might not be very sensitive to social partner effects. Nevertheless, slow-changing states may be affected by social interactions if the interaction is prolonged (Jacobs et al., 2011 and refs. therein) or repeated (Wilson et al., 2013). Relatively long term male-female social interactions during the breeding season can modify several slow-changing physiological and morphological features like organ size or amount of lipid storage (Jacobs et al., 2011 and refs. therein) and affect non-social behaviors during the entire time period of the breeding season accordingly. Social interactions can define the amount of resources to which individuals have access to (reviewed in Hsu et al., 2006; Wilson et al., 2013) and lead to changes in body size (Wilson et al., 2013) or any other resource based states. Individuals with high resource inputs may become larger in size or differ otherwise in morphology or physiology compared to individuals with low resource inputs (Stearns, 1992). In the sheepshead swordtail, Xiphophorus birchmanni, individuals that are consistently more aggressive and dominant in repeated pairwise social interactions have access to larger amounts of resources and have higher growth rates, irrespective of the initial body size, compared to individuals expressing lower aggression and dominance in these social interactions (Wilson et al., 2013). Slow-changing states of a focal individual, like body size and morphology, can affect several different behaviors across different ecological contexts (Dall et al., 2004; McElreath and Strimling, 2006; Luttbeg and Sih, 2010). For example, large body size can be positively related to boldness if large body size protects individuals from predation and enables individuals to act boldly in a feeding context (McElreath and Strimling, 2006; Luttbeg and Sih, 2010).
In reality, there are multiple fast- and slow-changing state variables affecting behaviors simultaneously and these mechanisms may also act in concert. For example, repeated winner-loser effects, based on fast-changing hormonal profiles, may enable individuals to gain or prevent access to resources, respectively, for a period of time that eventually enables modification of the slow-changing features, like morphology, physiology or neurobiology. Different state variables might also work sequentially: after a fast social interaction, fast-changing hormonal profiles are responsible for carry-over effects, while after a longer interaction, depleted energy resources might define the nature of this carry-over. Therefore, the temporal consistency of the partner effect might generally increase with the length of the social interaction.
Social Carry-Over Effects on Variation and Covariation in Non-Social Behaviors
Carry Over Effects on Variation in Non-Social Behaviors
Just as focal individuals are repeatable in their behaviors, social partners can consistently differ in the behavioral responses they elicit in others (Wilson et al., 2011a, 2013; Dingemanse and Araya-Ajoy, 2015). This means, for example, that some partners always elicit higher (or lower) aggressiveness in focal individuals compared to other partners, i.e., they make the focal individuals consistently deviate from their average phenotype. Such “social partner repeatability” represents the proportion of phenotypic variance in the focal individuals' behavior explained by the social partner identity. Social partners will affect the within-individual variance in the focal individual phenotype (Wilson et al., 2011a, 2013; Dingemanse and Araya-Ajoy, 2015) as a result of adaptive behavioral plasticity of the focal individual to differences in the social environment (social responsiveness) (Webster and Ward, 2011; Taborsky and Oliveira, 2012; Wolf and McNamara, 2013; Wolf and Krause, 2014). Importantly, social interactions can also generate true among-individual variation or covariation between behaviors if social carry-over effects induce permanent environmental effects on focal individuals and if those effects vary among focal individuals. If social partners are not repeatable in the behavioral responses they elicit in focal individuals, they do not modify behavioral variation of focal individuals in a predictable manner. Therefore, we assume here that social partner repeatability exists, which has been the case in empirical research studying this variation within social contexts (Wilson et al., 2011a, 2013).
Within-Individual Variance: Plasticity
When carry-over effects are underpinned by fast-changing states, like hormonal profiles or energy levels (Hsu et al., 2006; Coppens et al., 2010), and when focal individuals do not differ in the confronted social environment, social partners will affect the within-individual component of behavioral variance in non-social behaviors, expressed after social interactions (Figure 2). For example, in male green swordtails (Xiphophorus helleri), the focal individuals' within-individual variance in aggressiveness against conspecifics (social behavior) is partly explained by the social partner identity (Wilson et al., 2011a). Because fast-changing states are easily reversed to the normal level (Wolf and Weissing, 2010), the temporary effect of social partners on non-social behaviors of focal individuals, like exploration, activity or boldness, does not have the potential to carry-over to long temporal scales, but decays steeply after the social interaction (red lines in Figure 3). Therefore, the sensitivity of a state to social partner effects is negatively correlated with the temporal consistency of the carry-over effect on non-social behaviors. This means that in short social encounters, like rapid contest situations, the social partner effect might be driven by fast-changing states and therefore be detected only within, or temporally very close to the social interaction (red lines in Figure 3). However, the carry-over effect might increase with the length of the social interaction even if the states are fast-changing. For example, increased energy expenditure with increased escalation in aggressive encounters in Sierra dome spiders and House crickets (Hack, 1997; DeCarvalho et al., 2004) indicates that the recovery time after a fight, and thus time for the decay of social partner effect, might increase with the increased time spent in the social interaction.
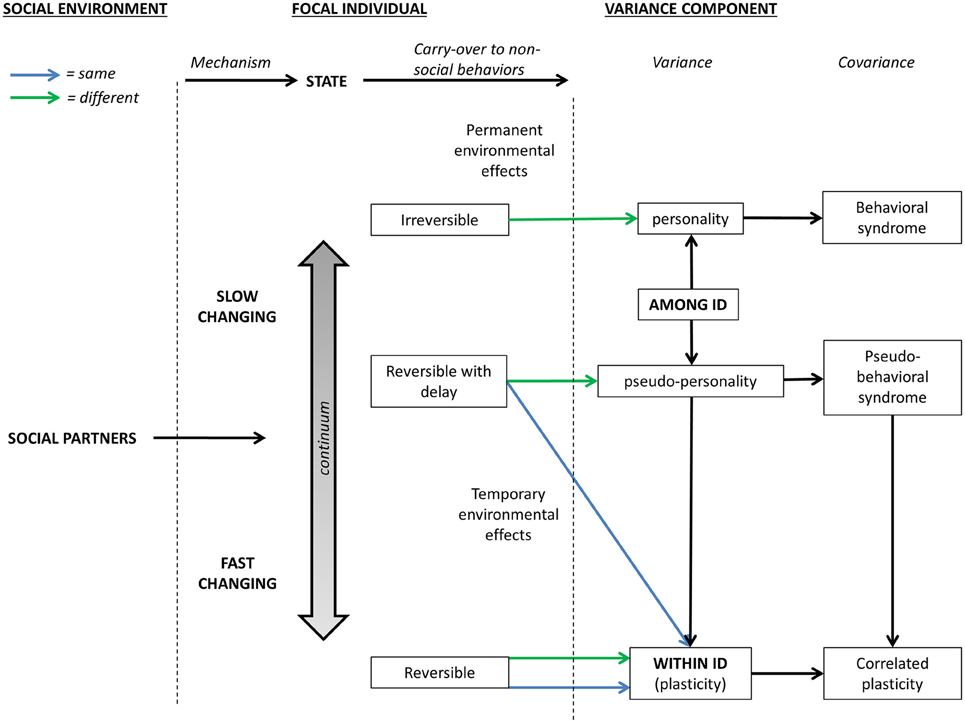
Figure 2. Schematic presentation of how the social-carry-over effects affect within- and among-individual variance components in behaviors expressed in non-social contexts through state variables. Green and blue lines represent social environments/carry-over effects that differ and do not differ among-individuals, respectively.
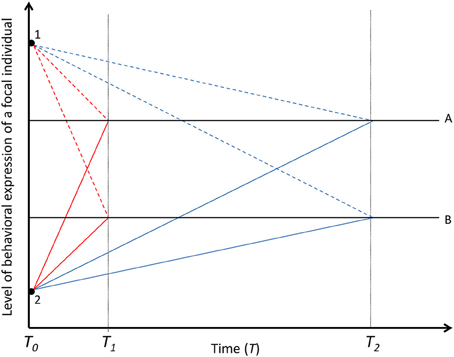
Figure 3. Decay of within-individual variation in time. Solid black lines (A and B) represent two focal individuals with different mean baseline levels of behavioral expression. Colored lines represent the behavioral expression of the two focal individuals (A and B) under the influence of two different social partners (not at the same time) [Black dots; dashed lines for the effect of partner 1 and solid lines for the effect of partner 2, underpinned by fast-changing (red lines, decayed in T1) or slow-changing (blue lines, decayed in T2) state in focal individuals]. If social carry-over effects are underpinned by fast-changing states, the social partner (1 and 2) explains within-individual variation in the behavior of the focal individuals only over short temporal scales. If social carry-over effects are underpinned by slow-changing states, the social partners explain within-individual variation in the behaviors of the focal individuals over long temporal scales. The social interaction is terminated in T0. While we acknowledge that among-individual variation for phenotypic plasticity exist (Nussey et al., 2007), for simplicity, the partner effects are assumed to be the same magnitude in T0 for both focal individuals and for the fast- and slow-changing states.
Slow-changing states within a focal individual might have the potential to explain longer term carry-over effects on within-individual variation in non-social behaviors of focal individuals (Figure 2, blue lines in Figure 3). However, since slow-changing states are not very sensitive to partner effects they may need long (or repeated) interactions in order to respond to social environments. For example, repeated contests might enable long term carry-over effects on a focal individual's slow-changing states, like body mass in Sheepshead swordtails (Xiphophorus birchmanni) (Wilson et al., 2013), to emerge. Since slow-changing state variables are not very sensitive to social carry-over effects, i.e., repeatedly confronted social partners do not induce large changes in slow-changing states, they should explain only low amounts of within-individual variance in non-social behaviors of focal individuals compared to fast-changing states.
Among-Individual Variance: Pseudo-Personality vs. True Personality
Social partners can also explain among-individual variance in non-social behaviors of focal individuals, i.e., animal personality, if social partners are confronted non-randomly among focal individuals. However, even though carry-over effects caused by reversible fast- and slow-changing states can explain such variation over different timescales (see above), the measured variance does not represent real animal personality, but rather “pseudo-personality” (Figures 2, 4) (Westneat et al., 2011; Dingemanse and Dochtermann, 2013). Pseudo-personality exists when among-individual variation in a trait is created by among-individual variation in experienced environments and the effect is not permanent, but focal individuals change their behaviors if they are moved to another environment, i.e., focal individuals express plasticity (e.g., Westneat et al., 2011; Dingemanse and Dochtermann, 2013; Dingemanse and Araya-Ajoy, 2015; Niemelä et al., 2015). For example, pairing for the whole reproductive season might cause long term carry-over effects on focal individuals' slow-changing states (Jacobs et al., 2011 and refs. therein) and potentially create among-individual variation for all behaviors expressed during the mating season, since focal individuals differ within (but not necessarily between) the reproductive season with whom they mate with. However, if in the next season the individual would pair with another individual (or if it would be experimentally swapped with another individual), the among- individual variance would disappear and the behaviors expressed under social carry-over effect would be expression of within-individual level variation.
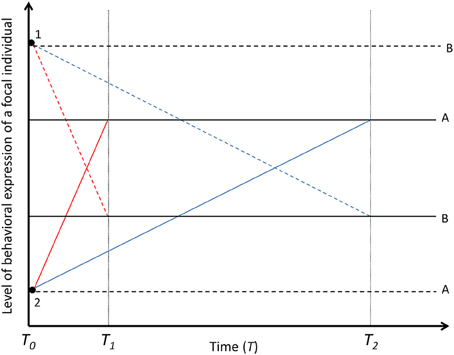
Figure 4. Decay of the among-individual variation in time. Solid black lines (A and B) represent two focal individuals with different mean baseline levels of behavioral expression. Colored lines represent the behavioral expression of two focal individuals (A and B) under the influence of social carry-over effects [Black dots; dashed lines for the effect of partner 1 and solid lines for the effect of partner 2, underpinned by fast-changing (red lines, decayed in T1) or slow-changing (blue lines, decayed in T2) states in focal individuals]. If social carry-over effects are underpinned by fast-changing states, the social environment (1 and 2) explains among-individual variation in the behavior of the focal individuals only over short temporal scales. If social carry-over effects are underpinned by slow-changing states, the social partners explain among-individual variation in the behaviors of the focal individuals over long temporal scales. Black dashed lines for focal individuals A and B represent the permanent social carry-over effects, i.e., true animal personality created by social environment. The social interaction is terminated in T0. For simplicity, the partner effects are assumed to be the same magnitude in T0 for the fast- and slow-changing states.
Social carry-over effects have the potential to create true among-individual variation in non-social behaviors if the proximate mechanisms are irreversible and thus act as permanent environmental effects (Figures 2, 4). In the wild, this kind of environmentally induced permanent among-individual level variation might be quite common due to long term assortative selection of social partners (Crespi, 1989; Wilson and Dugatkin, 1997; Croft et al., 2005), due to early social interactions like maternal effects (reviewed in Sachser et al., 2013), that both differ among focal individuals and affect state variables permanently, or by feedback loops induced by dominance interactions (Wilson et al., 2013). If there is long term among-individual variation in the confronted social environment or experienced carry-over effects between focal individuals in general, it might cause permanent environment effects on states. Such states might be, for example, any morphological, physiological or neurophysiological feature (McNamara and Houston, 1996; Houston and McNamara, 1999; Wolf and Weissing, 2010) that takes a lot of time or energy to change, or becomes genuinely irreversible. It is important to distinguish between temporary and permanent environmental carry-over effects on among-individual variation in behaviors, since true personality or behavioral syndromes exist only in the latter case (given that social interactions affect among-individual level variation: see above). One of the potential problems in separating temporary and permanent environmental effects from each other is that if temporary environmental effects are underpinned by slow-changing reversible states rather than irreversible permanent states, they may change with delay in a new environment and might be undetected. However, if the change eventually happens after individuals are translocated across environments, it means that the among-individual variation caused by the environment is instead undetected within-individual variance, i.e., plasticity, and the expressed personality or behavioral syndrome (see below) reflects environmental repeatability.
Early life-history stages might be more sensitive to environmental effects compared to adult stages (Stamps and Groothuis, 2010; Sachser et al., 2013), meaning that the same partner effect might cause carry-over of higher magnitude for the same focal individual at the juvenile stage compared to the adult stage. Therefore, permanent social environmental effects on focal individuals' states and behaviors might be triggered by early social interactions like maternal effects or other social interactions during ontogeny (Stamps and Groothuis, 2010; Runcie et al., 2013; Sachser et al., 2013). For example, in a Field cricket (Gryllus integer), the social rearing environment during ontogeny affects a suite of state variables like cellular immune defense efficiency and body mass measured later in adult stage (Niemelä et al., 2012). In principle, social partner effects are present at any life-history stage (Montiglio et al., 2013; Runcie et al., 2013; Sachser et al., 2013), but some life-history stages might be more sensitive to these environmental effects than others (Stamps and Groothuis, 2010; Sachser et al., 2013). For example, social interactions during ontogeny may have larger effects on slow-changing state variables, like body size, compared to social interactions after maturation, while effects on fast-changing states, like hormonal concentrations or energy levels, are present over an individual's lifetime.
Carry-Over Effects on Covariation between Behaviors
Behavioral syndromes, i.e., among-individual correlations between two or more repeatable behaviors, are of great interest to behavioral ecologists (Dingemanse et al., 2012; Garamszegi et al., 2012). Syndrome structure is important since the existence of a behavioral syndrome in a population suggests that behaviors might not be independent from each other, but their independent evolution can instead be constrained (Dochtermann and Dingemanse, 2013). Generally, it is important to partition the phenotypic correlation in among- and within-individual correlations in order to avoid false interpretations on the existence of behavioral syndromes (Dingemanse and Dochtermann, 2013; Brommer et al., 2014; Niemelä et al., 2015). In the field of animal personality, the common assumption is that social and non-social behaviors like aggression, boldness, exploration and activity can be correlated with each other at the among-individual level forming behavioral syndromes (e.g., Garamszegi et al., 2012). However, social carry-over effects are not taken into account when behavioral syndromes are quantified across social and non-social contexts even though the social partners might partly affect the syndrome structure via carry-over effects on within- and between-individual covariance components.
Within-Individual Covariance: Correlated Plasticity
In the same way that social partners can affect within-individual variance in one behavior, they can affect within-individual level covariance between behaviors of focal individuals, when they affect a state(s) underpinning more than one behavior and if there is no variation between focal individuals in experienced social environments (Figures 2, 5). This within-individual level correlation between focal individual's behaviors is present due to cross-context correlations of partner effects, and integrated plasticity within focal individuals, i.e., correlation of residuals (Figures 2, 5), either between social and non-social behaviors, or between non-social behaviors expressed outside the social context. Like for the within-individual variance in one behavior (see above), the within-individual level correlation between two or more behaviors of focal individuals, caused by social carry-over effects, might be absent if the time lag between the expression of social and non-social behaviors of focal individuals is longer than the decay of the underlying fast-changing state. If social carry-over effects are underpinned by slow-changing states, social partners can explain long term within-individual level covariation in focal individuals' social and non-social behaviors under specific circumstances. Generally, slow-changing states are not sensitive to the environment (Wolf and Weissing, 2010), like social partner effects. Thus, the within-individual level covariation caused by social carry-over effects and underpinned by slow-changing states is generally lower compared to those underpinned by fast-changing states, unless the social interactions are long lasting.
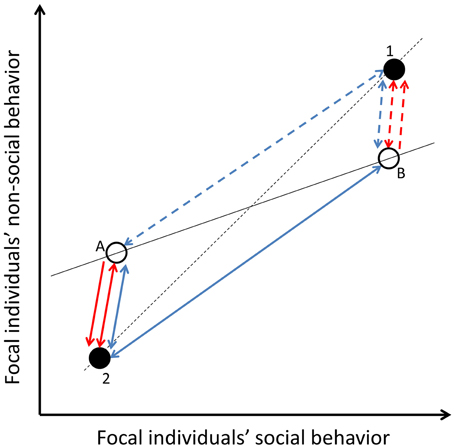
Figure 5. Covariation between focal individuals' social- and non-social behaviors. Open circles (A and B) represent two different focal individuals, while filled circles represent two different social environments or carry-over effects (1 and 2). If there is no among-individual variation in confronted social environments or carry-over effects (i.e., both focal individuals confront both partners), they explain within-individual level correlation between the behaviors of the focal individuals (two-directional blue arrows: dashed lines for carry-over effects from social environment 1 and solid arrows for carry-over effects from environment 2). The temporal consistency of the carry-over effects depends on the temporal consistency of the state underpinning the carry-over effect. If focal individuals differ in the confronted social environment or carry-over effects (i.e., focal individual A confronts environment 2 and focal individual B environment 1), social carry-overs create among-individual level correlation between the behaviors of the focal individuals (red arrows). If this among-individual correlation is underpinned by temporal environmental effects, it is reversible and thus represents plasticity (two-directional red arrows). However, social carry-over effects can also create true behavioral syndromes via permanent environmental effects (one-directional red arrows: dashed lines for carry-over effects from social environment 1 and solid arrows for carry-over effects from environment 2). Two directional arrows refer to temporary environmental effects (i.e., the reversible nature of the carry-over effect) while one directional arrows refer to permanent environmental effects. For simplicity, the social partner effects for different focal individuals (blue arrows) are assumed to be the same magnitude.
Among-Individual Covariance: Pseudo-Behavioral Syndrome vs. True Behavioral Syndrome
If focal-individuals vary consistently in the confronted social environments, social carry-over effects can also explain among-individual level covariance in behaviors, i.e., behavioral syndromes. Among-individual level covariance caused by social carry-over effects can be present at different timescales depending on the nature of the state that underpins the covariance (see above). For example, among-individual level correlations caused by social carry-over effects can be present if some social partners elicit higher hormonal concentrations or greater depletion of resources or growth rates compared to other partners and the focal individuals confront different (sets of) partners repeatedly. However, in this case the among-individual level covariation does not necessarily represent a true behavioral syndrome, but rather “pseudo-syndrome” triggered by non-permanent environmental correlations (Figures 2, 5). “Pseudo-syndromes” caused by social carry-overs occur when the effect of the social environment on the mean level of both behaviors in focal individuals changes when the social environment changes, i.e., the behavioral syndrome is actually caused by integration of plasticity which remains undetected if different focal individuals are always measured in their respective social environments.
Social carry-over effects can also create true behavioral syndromes, i.e., permanent environmental correlations (e.g., Dingemanse and Dochtermann, 2013). Permanent environmental correlations can be created by social carry-over effects when the among-individual variation in confronted social environments is present and when the social partners elicit permanent changes in focal individuals' states that carry-over time and across different contexts also to behaviors expressed in non-social situations (Figures 2, 5) (see discussion in the Section: Among-Individual Variance: Pseudo-Personality vs. True Personality).
Evolutionary Consequences of Social Carry-Over Effects
Studying the social partner effects and social carry-overs is important to fully understand the evolution of associated non-social behavioral traits. Crucially, phenotypes of social partners are expressions of their genotypes, and thus, social environments can evolve (Moore et al., 1997; Wolf et al., 1999). When social partner effects are repeatable, and these effects are heritable (i.e., due to additive genetic variance), they are known as indirect genetic effects, IGEs. For example, in a fruit fly (Drosophila melanogaster), IGEs are present on body size of focal individuals (Wolf, 2003). In other words, the genes of the social partners partly define the body size of focal individuals (Wolf, 2003). The first important evolutionary consequence of IGEs is that social partners can speed up or slow down the rate of evolution in traits expressed in, or tightly related to, social interactions if these traits are associated with fitness (Moore et al., 1997; Wolf et al., 1999). This happens when the additive genetic variance in the trait of social partner correlates positively or negatively with the additive genetic variance in the trait of a focal individual, respectively increasing or decreasing the rate of evolution (Moore et al., 1997; Brommer and Rattiste, 2008; Wilson et al., 2009) or if there is a functional integration between traits (Westneat, 2012). For example, in the fruit fly study mentioned above, the IGEs on the body size of focal individuals were negatively correlated with the direct genetic effects (DGEs), constraining the response to selection on body size of the focal individuals (Wolf, 2003). In deer mice (Peromyscus maniculatus), the covariance between IGEs and DGEs on agonistic behavior (social behavior) was positive, indicating a more rapid evolution of these behaviors compared to the case if only DGEs would have been taken into account (Wilson et al., 2009). The second evolutionary consequence of IGEs is that the additive genetic variation in social traits of focal individuals might not constrain the phenotypic evolution of those traits since they are also affected by selection through IGEs due to social interactions, i.e., genes of the social partners (Moore et al., 1997; Wolf et al., 1999). Thus, if social partner effects can carry-over to non-social behaviors like exploration, activity or boldness, they may have evolutionary consequences also on these behaviors: the evolutionary speed (rate of evolution) and evolutionary potential (additive genetic variation not restricting the trait evolution) of these non-social behaviors does not only depend on the genes of focal individuals but also on the genes of confronted social partners (Moore et al., 1997; Wilson, 2014). Such effects can be overlooked when the social environment is ignored as a source of variation acting on state variables underlying behaviors expressed outside an immediate social context. IGEs might affect the evolutionary potential of the non-social traits more through slow-changing mechanisms than through fast-changing mechanisms. This is because carry-over effects, and thus the potential IGEs, fade away quickly when they are underpinned by fast changing states. Selection has higher potential to act on non-social behaviors underpinned by slow-changing states since in such cases the carry-over effects persist over longer time periods. Interestingly, if social environments generate true personality, i.e., permanent environmental effects, the IGEs have the highest potential to affect the evolution of non-social behaviors of a focal since the effect of the social environment, causing IGEs, is permanent.
Carry-over effects might also have fitness consequences. According to the social niche hypothesis, individuals adapt their behavior to their own specific social niches in order to achieve maximal fitness (Bergmüller and Taborsky, 2010). Deviations from the optimal strategy should lead to suboptimal behavior for the social niche that the particular focal individual is occupying and potentially lowered fitness. Moreover, learning a specific behavioral strategy may increase its efficiency and increase the costs of switching to an alternative strategy (Rosenzweig and Bennett, 1996; Wolf et al., 2008; Morand-Ferron and Giraldeau, 2010). Therefore, the higher the deviation from the optimal behavioral strategy, due to the effect of social carry-overs, the higher the negative fitness effects should be. In this case, carry-overs caused by fast- and slow-changing proximate mechanisms most likely also differ in the magnitude of their fitness effects. Fast-changing state variables return quickly to the normal (and this case supposedly optimal) level of behavioral expression of a focal individual, while carry-overs caused by slow-changing state variables might take a long time to return to the normal level. Carry-overs caused by slow-changing mechanisms might thus have higher impacts on a focal individual's fitness if the non-social behaviors of a focal deviates from its “optimum” for its social niche for longer time periods.
Experimental Designs and Corresponding Statistical Analyses to Study the Social Carry-Over Effects
In this section, we focus on the key questions to be answered in order to better understand the mechanisms and consequences of social carry over effects on variation of behaviors expressed outside social interactions. Understanding the mechanisms helps us to target, using a variance partitioning approach (i.e., through mixed models), the different levels of behavioral variation on which the social carry-over effects are acting. Social partner effects on behavioral variation have been briefly studied in behaviors expressed within a social context, mainly in aggression and reproductive behaviors (e.g., Brommer and Rattiste, 2008; Wilson et al., 2011a, 2013) and the statistical tools for estimating social partner effects on different levels of variation are currently being introduced to the field of animal personality (Wilson et al., 2011a, 2013; Montiglio et al., 2013; Dingemanse and Araya-Ajoy, 2015).
If one is interested in the social partner effects on behavioral variation of focal individuals non-social behaviors at multiple levels, the general experimental setup requires the collection of repeated measurements of behaviors expressed in the non-social context after social interactions. Focal individuals should be tested against multiple social partners, with social partners interacting with multiple focal individuals. The mixed model should include, in addition to the focal individual's identity, also the social partner's identity as a random effect. This enables the decomposition of total phenotypic variance into variance attributable to the focal individual, the social partner, and residual variance. Adding the social partner identity captures previously unexplained within-individual variation (repeatability of the partner effect: the proportion of total phenotypic variance in the focal individual behavior explained by the social partner identity) (Wilson et al., 2011a, 2013; Montiglio et al., 2013; Dingemanse and Araya-Ajoy, 2015) or among-individual variation in the focal individuals' social and non-social behaviors. While the experimental setups (below) can test firm hypotheses about which level of variation the social partners affect in focal individuals' non-social behaviors, one can also compare the models with and without social partner identity as random factor. Comparing models would enable one to test exactly how among- or within-focal individual variance components, or both, are affected by the social partners, i.e., if the removal of partner identity increases one of these components. The same statistical approaches can be extended to multivariate models when studying carry-over effects on covariation between social and non-social behaviors of the focal individuals.
Exciting topics to be explored are, (1) do the temporal patterns of expressed within-individual variance (in non-social behaviors) due to carry-over effects depend on the length of the social interaction? and (2) whether social carry-over effects can create among-individual variation in non-social behaviors and whether this variation persists in time, i.e., temporary vs. permanent environmental effects. A more evolution-oriented study problem is (3) whether IGEs affect the rate of evolution of non-social traits, i.e., whether IGEs on non-social behaviors are present and if they correlate positively or negatively with DGEs (genes of a focal individual).
Temporal patterns of carry-over effects on focal individuals' behaviors might depend on the duration of the social interaction (first study problem). This is especially important if the social partner effects are underpinned by slow-changing states: they are not sensitive to social interaction unless the interaction lasts long enough. One straightforward way to study this is to construct two social treatments, where focal individuals spend short or long amounts of time with several different social partners repeatedly (the identities of the confronted social partners do not differ among focal individuals) and where non-social behaviors, like exploration or activity, are measured after a fixed amount of time (i.e., hours) after each social interaction in both treatments. If longer social interactions allow the social partner to affect slow-changing states in focal individuals, the within-focal individual variation in measured non-social behaviors explained by the social partner should be higher in the prolonged social interactions treatment. If the proximate mechanisms like hormonal level or body mass are measured simultaneously with the behaviors, one could connect the decay in behavioral variation firmly to the decay of the underlying mechanism. Statistically, this can be analyzed by applying a bivariate mixed effect model with the non-social behaviors of focal individuals measured after social interactions as two dependent variables (short and long interaction treatment) and with focal individual and social partner identity as random effects. To test if variance components differ between treatments, the within-individual variances for the two traits are restricted to be the same and the fit of the restricted model is compared to the fit of unconstrained model. Statistical significance of the model can be assessed by, for example, comparing the Log likelihood-values between the constrained and unconstrained model using Chi2-statistics (Meyer, 1992; Wilson et al., 2010).
The second study question, i.e., the social partner effects on among-individual variation, can be addressed by staging repeated social interactions so that focal individuals differ in their social environments. Among-focal individual variation in confronted social environments can be created by building groups of social partners that are all similar to each other within a group, but differ among groups in a trait value that is hypothesized to cause carry-over effects. These traits can be, for example, body size or aggression of social partners. Each focal individual would thus be tested in repeated interactions, but always with social partners belonging to the same group (i.e., with individuals that have the same mean trait value). A simple experimental design would be to measure first the non-social behaviors of interest before any social interactions (i.e., baseline, T0: T0, T1, and T2 in here are not related to the ones in figures), then soon after each social interaction (T1) and lastly some days after the social treatments have been decomposed (T2). This would allow one to quantify (i) whether among-individual variation in non-social behaviors of focal individuals increases after social interactions compared to the baseline variation due to among-individual variation in social environments (comparing T0 with T1) and (ii) if these carry-over effects on among-individual variation are temporary (i.e., pseudo-personality) or permanent (i.e., true personality) (comparing T0with T2). If social environments create among-individual variation in the focal individuals' behaviors, the among-individual variation should be higher in non-social behaviors measured directly after social interactions (T1), compared to the baseline among-individual variation (T0). One can study the temporary vs. permanent nature of carry-over effects on among-individual variation by comparing the baseline among-individual variance (T0) to the among-individual variance measured few days after the decomposition of social treatments (T2). If among-individual variance in behaviors is higher when measured a few days after treatment decomposition compared to the baseline, it would suggest the existence of permanent environmental effects. Statistically, this can be analyzed by applying multivariate mixed effect models as in the first study question (see above), where behaviors in T0, T1, and T2 are fitted as three dependent variables, and focal individual and social partner identity as random effects. One can then test if the variance components differ significantly from each other between time points (T0, T1, and T2) of interest by constraining the among-focal individual variances to be the same and by comparing the fit of the restricted model to the fit of unconstrained model. Statistical significance of model comparing can be done, for example, by comparing the Log likelihood-values between the constrained and unconstrained model using Chi2-statistics (Meyer, 1992; Wilson et al., 2010).
For the third study question, one needs to have a pedigreed population or experimental breeding design that allows the estimation of quantitative genetic parameters from the collected data (e.g., Moore et al., 1997; Wolf et al., 1999). This kind of design allows the estimation of DGEs, IGEs and the covariance between these genetic components from the data collected from repeated social interactions (for details: Wolf, 2003; Brommer and Rattiste, 2008; Dochtermann and Roff, 2010; Wilson et al., 2011b). As stated earlier, the positive covariance between DGEs and IGEs suggests a potential increase in the rate of evolution for measured non-social traits (affected by carry-overs) while a negative covariance suggest restriction for the rate of evolution (Wolf, 2003).
Conclusion
Here we introduced a conceptual framework about the potential proximate mechanisms behind social carry-over effects on behavioral variation in non-social behaviors. One of the main goals in the field of animal personality is to study the ecological and evolutionary causes and consequences of expressed behavioral variation at different levels. In our paper, we clarified how social partners have the potential to affect the expressed within- and among-individual level behavioral variation and covariation of focal individuals outside social contexts over various temporal scales. Moreover, our framework gives new insights on the evolutionary potential of carry-over effects, which can also be extended to non-social behaviors. It is important to acknowledge the social carry-over effects on behavioral (co)variation of behaviors expressed outside social interactions since they may allow us to make predictions about the patterns of variation generated by social environments and the evolution of non-social behavioral traits.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
PTN is supported by the Alexander von Humboldt foundation (3.3-7121-FIN/1151177) and DFG (German National Research Foundation, NI 1539/1-1) and FS by the Max Planck Society. FS is part of the International Max Planck Research School for Organismal Biology. We thank Niels J. Dingemanse, Robin Abbey-Lee, Yimen Araya-Ajoy and two referees for constructive comments on this manuscript.
References
Adamo, S. A., Linn, C. E., and Hoy, R. R. (1995). The role hormonal octopamine during ‘fight or flight’ behaviour in the field cricket Gryllus bimaculatus. J. Exp. Biol. 198, 1691–1700.
Auld, J. R., Agrawal, A. A., and Relyea, R. A. (2010). Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. Biol. Sci. 277, 503–511. doi: 10.1098/rspb.2009.1355
Bennett, A. F., and Houck, L. D. (1983). The energetic cost of courtship and aggression in a plethodontid salamander. Ecology 64, 979–983. doi: 10.2307/1937804
Bergmüller, R., and Taborsky, M. (2010). Animal personality due to social niche specialization. Trends Ecol. Evol. 25, 504–511. doi: 10.1016/j.tree.2010.06.012
Biro, P., and Stamps, J. (2008). Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368. doi: 10.1016/j.tree.2008.04.003
Bradbury, J. W., and Vehrencamp, S. L. (1998). Principles of Animal Communication. Sunderland: Sinauer Associates.
Briffa, M., and Sneddon, L. U. (2007). Physiological constraints on contest behaviour. Funct. Ecol. 21, 627–637. doi: 10.1111/j.1365-2435.2006.01188.x
Brommer, J. E., Karell, P., Ahola, K., and Karstinen, T. (2014). Residual correlations, and not individual properties, determine a nest defense boldness syndrome. Behav. Ecol. 25, 802–812. doi: 10.1093/beheco/aru057
Brommer, J. E., and Rattiste, K. (2008). “Hidden” reproductive conflict between mates in a wild bird population. Evolution 62, 2326–2333. doi: 10.1111/j.1558-5646.2008.00451.x
Careau, V., Thomas, D., Humphries, M. M., and Réale, D. (2008). Energy metabolism and animal personality. Oikos 117, 641–653. doi: 10.1111/j.0030-1299.2008.16513.x
Chase, I. D., Bartolomeo, C., and Dugatkin, L. A. (1994). Aggressive interactions and inter-contest interval: how long do winners keep winning? Anim. Behav. 48, 393–400. doi: 10.1006/anbe.1994.1253
Coppens, C. M., de Boer, S. F., and Koolhaas, J. M. (2010). Coping styles and behavioural flexibility: towards underlying mechanisms. Philos. Trans. R. Soc. B. 365, 4021–4028. doi: 10.1098/rstb.2010.0217
Crespi, B. J. (1989). Causes of assortative mating in arthropods. Anim. Behav. 38, 980–1000. doi: 10.1016/S0003-3472(89)80138-1
Croft, D. P., James, R., Ward, A. J. W., Botham, M. S., Mawdsley, D., and Krause, J. (2005). Assortative interactions and social networks in fish. Ooecologia 143, 211–219. doi: 10.1007/s00442-004-1796-8
Dall, S. R. X., Alison, A. M., Bolnick, D. I., and Ratnieks, F. L. W. (2012). An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198. doi: 10.1111/j.1461-0248.2012.01846.x
Dall, S. R. X., Houston, A. L., and McNamara, J. M. (2004). The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739. doi: 10.1111/j.1461-0248.2004.00618.x
DeCarvalho, T. N., Watson, P. J., and Field, S. A. (2004). Costs increase as ritualized fighting progresses within and between phases in the sierra dome spider, Neriene litigiosa. Anim. Behav. 68, 473–482. doi: 10.1016/j.anbehav.2003.08.033
DeWitt, T. J., Sih, A., and Wilson, D. S. (1998). Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. doi: 10.1016/S0169-5347(97)01274-3
Dingemanse, N. J., and Araya-Ajoy, Y. G. (2015). Interacting personalities: behavioural ecology meets quantitative genetics. Trends Ecol. Evol. 30, 88–97. doi: 10.1016/j.tree.2014.12.002
Dingemanse, N. J., and Dochtermann, N. A. (2013). Quantifying individual variation in behavior: mixed-effect modelling approaches. J. Anim Ecol. 82, 39–54. doi: 10.1111/1365-2656.12013
Dingemanse, N. J., Dochtermann, N. A., and Nakagawa, S. (2012). Defining behavioural syndromes and the role of “syndrome deviation” to study its evolution. Behav. Ecol. Sociobiol. 66, 1543–1548. doi: 10.1007/s00265-012-1416-2
Dingemanse, N. J., Kazem, A. J. N., Réale, D., and Wright, J. (2010). Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. doi: 10.1016/j.tree.2009.07.013
Dingemanse, N. J., and Wolf, M. (2010). Recent models for adaptive personality differences: a review. Philos. Trans. R. Soc. B. 365, 3947–3958. doi: 10.1098/rstb.2010.0221
Dochtermann, N. A., and Dingemanse, N. J. (2013). Behavioral syndromes as evolutionary constrains. Behav. Ecol. 24, 806–811. doi: 10.1093/beheco/art002
Dochtermann, N. A., and Roff, D. A. (2010). Applying quantitative genetics framework to behavioral syndrome reseach. Philos. Trans. R. Soc. B. 365, 4013–4020. doi: 10.1098/rstb.2010.0129
Earley, R. L., Lu, C.-K., Lee, I.-H., Wong, S. C., and Hsu, Y. (2013). Winner and loser effects are modulated by hormonal states. Front. Zool. 10:6. doi: 10.1186/1742-9994-10-6
Garamszegi, L. Z., Markó, G., and Herczeg, G. (2012). A meta-analysis of correlated behaviours with implications for behavioural syndromes: mean effect size, publication bias, phylogenetic effect and the role of mediator variables. Evol. Ecol. 26, 1213–1235. doi: 10.1007/s10682-012-9589-8
Ghalambor, C. K., McKay, J. K., Carroll, S. P., and Reznick, D. N. (2007). Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. doi: 10.1111/j.1365-2435.2007.01283.x
Griffin, A. S., and West, S. A. (2003). Kin discrimination and the benefits of helping in cooperatively breeding vertebrates. Science 302, 634–636. doi: 10.1126/science.1089402
Hack, M. P. (1997). The energetic costs of fighting in the house cricket, Acheta domesticus L. Behav. Ecol. 8, 28–36. doi: 10.1093/beheco/8.1.28
Houston, A. I., and McNamara, J. M. (1999). Models of Adaptive Behaviour. Cambridge, UK: Cambridge University Press.
Hsu, Y., Earley, R. L., and Wolf, L. L. (2006). Modulation of aggressive behavior by fighting experience: mechanisms and contest outcomes. Biol. Rev. Camb. Philos. Soc. 81, 33–74. doi: 10.1017/S146479310500686X
Jacobs, S. R., Edwards, D. B., Ringrose, J., Elliot, K. H., Weber, J.-M., and Gaston, A. J. (2011). Changes in body composition during breeding: reproductive strategies of three species of seabirds under poor environmental conditions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 158, 77–82. doi: 10.1016/j.cbpb.2010.09.011
Ketterson, E. D., and Nolan, V. Jr. (1999). Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 152, S4–S25. doi: 10.1086/303280
Laskowski, K. L., and Pruitt, J. N. (2014). Evidence of social niche construction: persistent and repeated social interactions generate stronger personalities in a social spider. Proc. R. Soc. B. 281, 20133166. doi: 10.1098/rspb.2013.3166
Luttbeg, B., and Sih, A. (2010). Risk, resources and state-dependent adaptive behavioral syndromes. Philos. Trans. R. Soc. B. 365, 3977–3990. doi: 10.1098/rstb.2010.0207
Lyon, B. E., and Montgomerie, R. (2012). Sexual selection is a form of social selection. Philos. Trans. R. Soc. B. 367, 2266–2273. doi: 10.1098/rstb.2012.0012
Maynard-Smith, J. (1982). Evolution and the Theory of Games. Cambridge, UK: Cambridge University Press. doi: 10.1017/CBO9780511806292
McElreath, R., and Strimling, P. (2006). How noisy information and individual asymmetries can make ‘personality’ an adaptation: a simple model. Anim. Behav. 72, 1135–1139. doi: 10.1016/j.anbehav.2006.04.001
McNamara, J. M., and Houston, A. I. (1996). State-dependent life histories. Nature 380, 215–221. doi: 10.1038/380215a0
Meyer, K. (1992). Variance components due to direct and maternal effects for growth traits of australian beef cattle. Livest. Prod. Sci. 31, 179–204. doi: 10.1016/0301-6226(92)90017-X
Montiglio, P.-E., Ferrari, C., and Réale, D. (2013). Social niche specialization under constraints: personality, social integration and environmental heterogeneity. Philos. Trans. R. Soc. B. 368, 20120343. doi: 10.1098/rstb.2012.0343
Moore, A. J., Brodie, E. D., and Wolf, J. B. (1997). Interacting phenotypes and the evolutionary process: I. direct and indirect genetic effects of social interactions. Evolution 51, 1352–1362. doi: 10.2307/2411187
Morand-Ferron, J., and Giraldeau, L. A. (2010). Learning behaviorally stable solutions to producer-scrounger games. Behav. Ecol. 21, 343–348. doi: 10.1093/beheco/arp195
Niemelä, P. T., Lattenkamp, E. Z., and Dingemanse, N. D. (2015). Personality-related survival and sampling bias in wild cricket nymphs. Behav. Ecol. doi: 10.1093/beheco/arv036. (in press).
Niemelä, P. T., Vainikka, A., Lahdenperä, S., and Kortet, R. (2012). Nymphal density, behavioral development, and life history in a field cricket. Behav. Ecol. Sociobiol. 66, 645–652. doi: 10.1007/s00265-011-1312-1
Nussey, D. H., Wilson, A. J., and Brommer, J. E. (2007). The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844. doi: 10.1111/j.1420-9101.2007.01300.x
Piersma, T., and Drent, J. (2003). Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 18, 228–233. doi: 10.1016/S0169-5347(03)00036-3
Rosenzweig, M. R., and Bennett, E. L. (1996). Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav. Brain Res. 78, 57–65. doi: 10.1016/0166-4328(95)00216-2
Runcie, D. E., Wiedmann, R. T., Archie, E. A., Altmann, J., Wray, G. A., and Alberts, S. C. (2013). Social environment influence the relationship between genotype and gene expression in wild baboons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120345. doi: 10.1098/rstb.2012.0345
Sachser, N., Kaiser, S., and Hennessy, M. B. (2013). Behavioural profiles are shaped by social experience: when, how and why. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120344. doi: 10.1098/rstb.2012.0344
Sih, A., Bell, A., and Johnson, J. C. (2004). Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. doi: 10.1016/j.tree.2004.04.009
Sih, A., and Bell, A. M. (2008). Insights for behavioral ecology from behavioral syndromes. Adv. Stud. Behav. 38, 227–281. doi: 10.1016/S0065-3454(08)00005-3
Stamps, J., and Groothuis, T. G. G. (2010). The development of animal personality: relevance, concepts and perspectives. Biol. Rev. 85, 301–325. doi: 10.1111/j.1469-185X.2009.00103.x
Taborsky, B., and Oliveira, R. F. (2012). Social competence: an evolutionary approach. Trends Ecol. Evol. 27, 679–688. doi: 10.1016/j.tree.2012.09.003
Webster, M. M., and Ward, A. J. W. (2011). Personality and social context. Biol. Rev. 86, 759–773. doi: 10.1111/j.1469-185X.2010.00169.x
Westneat, D. F. (2012). Evolution in response to social selection: the importance of interactive effects of traits on fitness. Evolution 66, 890–895. doi: 10.1111/j.1558-5646.2011.01490.x
Westneat, D. F., Hatch, M. I., Wetzel, D. P., and Ensminger, A. L. (2011). Individual variation in parental care reaction norms: integration of personality and plasticity. Am. Nat. 178, 652–667. doi: 10.1086/662173
Wilson, A. J. (2014). Competition as a source of constraint on life history evolution in natural populations. Heredity 112, 70–78. doi: 10.1038/hdy.2013.7
Wilson, A. J., de Boer, M., Arnott, G., and Grimmer, A. (2011a). Integrating personality research and animal contest theory: aggressiveness on green swordtail Xiphophorus helleri. PLoS ONE 6:e28024. doi: 10.1371/journal.pone.0028024
Wilson, A. J., Gelin, U., Perron, M.-C., and Réale, D. (2009). Indirect genetic effects and the evolution of aggression in a vertebrate system. Proc. Biol. Sci. 276, 533–541. doi: 10.1098/rspb.2008.1193
Wilson, A. J., Grimmer, A., and Rosenthal, G. G. (2013). Causes and consequences of contest outcome: aggressiveness, dominance and growth in the sheepshead swordtail, Xiphophorus birchmanni. Behav. Ecol. Sociobiol. 67, 1151–1161. doi: 10.1007/s00265-013-1540-7
Wilson, A. J., Morrissey, M. B., Adams, M. J., Walling, C. A., Guinness, F. E., Pemberton, J. M., et al. (2011b). Indirect genetics effects and evolutionary constraint: an analysis of social dominance in red deer, Cervus elaphus. J. Evol. Biol. 24, 772–783. doi: 10.1111/j.1420-9101.2010.02212.x
Wilson, A. J., Réale, D., Clements, M. N., Morrissey, M. M., Postma, E., Walling, C. A., et al. (2010). An ecologist's guide to the animal model. J. Anim. Ecol. 79, 13–26. doi: 10.1111/j.1365-2656.2009.01639.x
Wilson, D. A., and Dugatkin, L. A. (1997). Group selection and assortative interactions. Am. Nat. 149, 336–351. doi: 10.1086/285993
Wolf, J. B. (2003). Genetic architecture and evolutionary constraint when the environment contains genes. Proc. Natl. Acad. Sci. U.S.A. 100, 4655–4660. doi: 10.1073/pnas.0635741100
Wolf, J. B., Brodie, E. D. III, and Moore, A. J. (1999). Interacting phenotypes and the evolutionary process. II. Selection resulting from social interactions. Am. Nat. 153, 254–266. doi: 10.1086/303168
Wolf, M., and Krause, J. (2014). Why personality matters for social functioning and social structure. Trends Ecol. Evol. 29, 306–308. doi: 10.1016/j.tree.2014.03.008
Wolf, M., and McNamara, J. M. (2013). Adaptive between-individual differences in social competence. Trends Ecol. Evol. 28, 253–254. doi: 10.1016/j.tree.2013.01.006
Wolf, M., van Doorn, G. S., Leimar, O., and Weissing, F. J. (2007). Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. doi: 10.1038/nature05835
Wolf, M., van Doorn, G. S., and Weissing, F. J. (2008). Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl. Acad. Sci. U.S.A. 105, 15825–15830. doi: 10.1073/pnas.0805473105
Wolf, M., van Doorn, G. S., and Weissing, F. J. (2011). On the coevolution of social responsiveness and behavioural consistency. Proc. Biol. Sci. 278, 440–448. doi: 10.1098/rspb.2010.1051
Wolf, M., and Weissing, F. J. (2010). An explanatory framework for adaptive personality differences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 3959–3968. doi: 10.1098/rstb.2010.0215
Wolf, M., and Weissing, F. J. (2012). Animal personalities: consequences for ecology and evolution. Trends Ecol. Evol. 27, 452–461. doi: 10.1016/j.tree.2012.05.001
Keywords: behavioral plasticity, behavioral variation, animal personality, behavioral syndrome, social evolution, social environment, social carry-over
Citation: Niemelä PT and Santostefano F (2015) Social carry-over effects on non-social behavioral variation: mechanisms and consequences. Front. Ecol. Evol. 3:49. doi: 10.3389/fevo.2015.00049
Received: 24 January 2015; Accepted: 01 May 2015;
Published: 22 May 2015.
Edited by:
Devi Meian Stuart-Fox, The University of Melbourne, AustraliaReviewed by:
Michelle L. Hall, The University of Melbourne, AustraliaDevi Meian Stuart-Fox, The University of Melbourne, Australia
Copyright © 2015 Niemelä and Santostefano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petri T. Niemelä, Department Biologie II, Ludwig-Maximilians University of Munich, GroßhadernerStr. 2, 82152 Planegg-Martinsried, Munich, Germany, niemela@bio.lmu.de
 Petri T. Niemelä
Petri T. Niemelä Francesca Santostefano
Francesca Santostefano