On the relative effect of spawning asynchrony, sperm quantity, and sperm quality on paternity under sperm competition in an external fertilizer
- 1Faculty of Bioscience, Fishery and Economy, University of Tromsø, Tromsø, Norway
- 2Faculty of Biosciences and Aquaculture, University of Nordland, Bodø, Norway
How much of a fitness benefit is obtained by dominant males of external fertilizers from releasing ejaculates in synchrony with female egg-release when engaging in sperm competition, and what is the most important sperm trait for paternity in these situations? The Arctic charr (Salvelinus alpinus) is an external fertilizer experiencing intense male-male competition over reproductive opportunities including sperm competition. To compensate for their disadvantage the sneaker males, which often spawn out of synchrony with the female, produce more and faster sperm than the guarding males. We used controlled in vitro fertilization trials with experimentally produced dominant and subordinate, sneaker males to test what effect relative synchrony in gamete release, sperm quality (i.e., motility and velocity) and sperm quantity have on a male's fertilization success in pair-wise sperm competitions. When the sneaker males released ejaculates after the guarding male there was no overall difference in fertilization success. The quality (i.e., motility and velocity) of a male's sperm relative to that of the competing male was the best predictor of male fertilization success regardless of their mating tactic and spawning synchrony. The relative number of sperm cells also had an effect on fertilization success, but mainly when the dominant and sneaker male ejaculated synchronously. Our close imitation of natural sperm competition in charr shows that the sneaker males of external fertilizing species may fully compensate for their disadvantaged mating role by producing ejaculates of higher quality—an adjustment strangely not met by dominants.
Introduction
Sperm competition occurs when spermatozoa of two or more males have the opportunity to fertilize the same ovum (Parker, 1970) and it occurs amongst species practicing both internal- and external-fertilization (Birkhead and Møller, 1998). Among species with internal fertilization sperm competition may occur when more than one male inseminates a female during a single fertile period, while for external fertilizers, ejaculates from several males may interact in the external environment before the spawned eggs are fertilized. Sperm competition allows the male–male competition to continue after ejaculation.
For many external fertilizers the eggs and sperm are viable for a rather short period (Billard et al., 1986) and for species without nests gametes can quickly be scattered out in a large area (Pennington, 1985; Denny and Shibata, 1989; Yund, 1990; Levitan et al., 1992; Babcock et al., 1994; Levitan, 2005). Thus, it is important that the release of gametes for males and females occur at the same site. This may, in non-sessile species, lead to an intensive pre-spawning site competition between males, often of various reproductive tactics, for a position close to the egg releasing female (see e.g., Dominey, 1984; Kodric Brown, 1986; Taborsky, 1994; Alonzo and Warner, 2000; Oliveira et al., 2001; Neff et al., 2003). Furthermore, following the pre-spawning site competition, males must synchronize ejaculation with female egg release in order to reduce the effect of sperm competition. This is documented in Atlantic salmon (Salmo salar), where a 2 s delay in sperm release under sperm competition decreased paternity by approximately 40% (Yeates et al., 2007). Moreover, among sneaker male Japanese medaka (Oryzias latipes) paternity dropped from 41 to 20% when spawning out of synchrony rather than in synchrony with the dominant male and the female. The latter findings made the authors (Koya et al., 2013) suggest that the reproductive success in medaka was primarily determined by the timing of sperm release corresponding to egg release, something certainly possible given ejaculates of equal sperm numbers and a “fair raffle” (Parker, 1990).
However, ejaculates in competition are seldom composed of equal sperm numbers and the “raffle” is often “loaded” (Parker, 1990). That is, natural adjustments of both sperm quantity and sperm quality (i.e., motility, viability, longevity, velocity, and size) have been documented important for an ejaculate's competitive ability. The importance of sperm numbers for reproductive success is shown in several external fertilizers (Lahnsteiner et al., 1998; Rurangwa et al., 2001; Neff et al., 2003; Ottesen et al., 2009). For example, in the walleye (Sander vitreus) the number of sperm cells in the ejaculate is positively related to fertilization success under sperm competition (Casselman et al., 2006). The importance of sperm motility, on the other hand, is illustrated in the Atlantic halibut (Hippoglossus hippoglossus L.) where the male with the highest proportion of motile sperm cells under multi-male sperm competitions has the highest fertilization success (Ottesen et al., 2009). Studies on the importance of sperm velocity have been conducted in several species (Gage et al., 2004; Casselman et al., 2006; Rudolfsen et al., 2008; Evans et al., 2013) producing equivocal results. For example, in the myobatrachid frog (Crinia georgiana) the fertilization success of the focal male increases as his relative sperm swimming speed decreases (Dziminski et al., 2009), while for the walleye sperm velocity is positively related to fertilization success (Casselman et al., 2006). Yet, in sperm competition it intuitively seems best not just to ejaculate many sperm cells close to and in synchrony with the spawning female, but also to maximize sperm velocity and the number of motile sperm cells—at least for a “Darwinian demon” (Law, 1979).
The arctic charr (Salvelinus alpinus) is an external fertilizer where neither males nor females provide any form of parental care after spawning (Fabricius, 1953; Sørum et al., 2011). Free-living charr are easily observed during spawning activity and thus represent a suitable model species for studying pre- and post-copulatory (i.e., spawning) competition among males. Males compete intensely throughout the approximately one-month long spawning season and their social status can easily be identified (Sigurjonsdottir and Gunnarsson, 1989; Liljedal and Folstad, 2003; Sørum et al., 2011; http://naturweb.uit.no/amb/evolution/). Large dominant males use aggressive behaviors toward smaller subordinate males (i.e., chase them away) when trying to guard the females (Sigurjonsdottir and Gunnarsson, 1989). Yet, the spawning area provides no physical protection for the spawning pair and when the female releases eggs the dominant males often spawn in competition with the subordinate males (Sigurjonsdottir and Gunnarsson, 1989; Sørum et al., 2011). Since the subordinate males often employ a sneaking behavior they experience a higher risk of sperm competition, spawn out of synchrony with the female and further away from the released eggs than the dominant males (Sørum et al., 2011). In our study population sneaker males on average ejaculate approximately 0.7 s after the guarding males, 76.5% of the ejaculates experience sperm competition and the mean number of males releasing milt (i.e., the ejaculate of a fish) in each competition is 2.6 (Sørum et al., 2011). Thus, sperm competition is common and in order to compensate for their disadvantages the subordinate males increase their investments in sperm production and sperm velocity (Liljedal and Folstad, 2003; Rudolfsen et al., 2006; Vaz Serrano et al., 2006; Haugland et al., 2011). Sperm velocity and sperm density have also been shown to influence fertilization success under sperm competition in our studied charr population (Liljedal et al., 2008).
Previous studies within the salmonida (Oncorhynchus tshawytscha) have shown that ejaculates from sneakers may outcompete ejaculates from dominant males under synchronized fertilizations (Young et al., 2013). Yet, can natural ejaculate adjustments make subordinates ejaculates successful under sperm competition also when released after the synchronized spawning between the dominant male and the female? In the present study we closely mimic natural spawning in charr and use in vitro fertilizations to evaluate the hypothesis that naturally occurring adjustments within an ejaculate allow males adopting a disfavored spawning strategy to successfully compete with the spawning strategy of males trying to monopolize access to the spawning females. That is, we investigate, for the first time, the relative importance of asynchrony in gamete release, sperm number, and sperm motility for the reproductive success of subordinate and dominant males under sperm competition. Will the allocation of resources to larger sperm numbers with higher velocity, as previously observed among subordinate charr, be sufficient to compensate for their asynchronous spawning under sperm competition from dominants?
Methods
Study Site, Fish Sampling, and Tagging
We carried out the fieldwork in our study population at Lake Fjellfrøsvatn at 69°08′N 19°34′E in northern Norway from the 8th to the 27th of September 2008. The gametes used in this experiment came from reproductively active fish (16 males and 16 females) caught with gill nets from the same spawning ground (see Figenschou et al., 2004 for details on spawning grounds). Individuals were continuously removed from the nets in order to minimize stress. Males included in the experiment were transported to the field laboratory where they were anesthetized using benzocaine. Then, they were stripped for all available milt and length was measured (29.6 cm ± 2.2, mean ± SD) before each male was id tagged in the dorsal fin with similar sized, yet easily distinguishable plastic tags (approximately 0.5 cm2), attached with Floy's elastic vinyl filament. Thereafter the males were size-matched and caged in pairs with a maximum length difference within each of the 8 pairs established of 4 mm (minimum 2 mm). Previous studies have shown that males entering a dominant position under these experimental conditions do not initially differ in ejaculate characteristics, size or ornamental development from males taking up a subordinate strategy (Rudolfsen et al., 2006). The cages (40 × 60 × 90 cm, made of chicken wire) were placed at about 1.5 m depth, 2–3 m apart and the fish were left undisturbed for 24 h before our behavioral observations started (see below). On day four, the fish were again anesthetized, and stripped for all available milt which had been produced during social interactions as either dominant or subordinate. The milt volume was measured to the nearest 0.1 ml (overall: 0.70 ± 0.34, dom: 0.78 ± 0.37, sub: 0.62 ± 0.31, mean ± SD, respectively) and then stored on ice for further analyses (see Sperm Analysis) and for fertilizations (see Fertilizations). Females were caught immediately prior to our planned fertilizations and stored in plastic containers before they were brought to the field-laboratory, anesthetized and stripped of their eggs. A small amount of ovarian fluid, later used for measurements of sperm velocity in ovarian fluid, was separated from eggs using a pipette and stored at lake water temperature (6°C). All fish used in this study were released back into the lake (see Haugland et al., 2011 for a more detailed description of capture and caging methods).
Behavioral Observations
Status roles are highly dynamic in charr and individuals employing a subordinate spawning strategy at natural leks, readily takes up dominant strategies during experimental trials lasting less than 1 day (own observations). In order to determine social rank, we recorded male behavior twice a day for 5 min during the last 3 days of the four-day caging period. Bathyscope underwater viewers were used for observing the individual number of aggressive acts (e.g., an initiation of a chase) and the male performing most aggressive acts within a pair was considered the dominant. The presence of an observer during such behavioral observations does neither significantly alter fish activity nor the within pair hierarchical position (see Liljedal and Folstad, 2003 for details).
Sperm Analysis
To minimize handling time all the measurements were conducted as fast as possible by skilled personnel and at temperatures similar to lake temperatures. The handling of males was also conducted randomly with respect to the male's social position and without knowledge about the individual's social position. Spermatocrit (i.e., the percentage of the ejaculate consisting of sperm cells) was measured by centrifuging 10 μl homogeneous milt (i.e., milt gently shaken in an Eppendorf tube) in a capillary tube for 195 s at 11 500 rpm (Compur-electronic Gmbh, Munich, Germany) and used as a measure of sperm number (overall: 14.5% ± 9.3, dom: 8.17% ± 0.82, sub: 20.86% ± 9.64, mean ± SD, respectively). Thereafter, the entire milt sample produced during the 4 days was split in 20 subsamples (average volume 0.032 ml). Each subsample of milt is thus represented with a volume giving an ejaculation frequency of 5 per day, a frequency within the range of that observed in our free-living population (Sørum et al., 2011). Of these, 16 subsamples were used for fertilizations and 4 for evaluations of sperm quality. That is, for each male we quantified sperm motility and velocity in water and in water diluted ovarian fluid (1:2) from the focal female used in each fertilization. Measurements of sperm behavior were taken 10 s following activation of the sperm cells and later analyzed using CASA (HTM-CEROS v.12). Each motility measurement lasted 0.5 s and the parameters assessed were mean curvilinear velocity (VCL) (water overall: 140.0 μm/s ± 31.6, and VCL ovarian fluid overall: 139.3 μm/s ± 23.0, mean ± SD, respectively) and percentage motile cells (water overall 89.3 ± 14.7, and ovarian fluid overall 81.2 ± 16.9, mean ± SD, respectively) using the methods described in Vaz Serrano et al. (2006).
Our statistical model (see Statistics) is only valid over the range of values in our pair-wise comparisons of velocity, motility and spermatocrit observed in our eight pairs of dominant and subordinate males. That is, sperm velocity estimates in water range from dominants having velocities 24.3 μm/s faster than subordinates and subordinates having velocities 15.5 μm/s faster than dominants (average difference 2.9 μm/s, SD ± 24.3 μm/s). Sperm velocities in ovarian fluid range from dominants having sperm swimming 58.4 μm/s faster than subordinates to subordinates having sperm swimming 52.2 μm/s faster than dominants (average difference is 10.8 μm/s, SD ± 25.9 μm/s). Motility range in water from dominants having 27% more motile sperm than subordinates to subordinates having 2.5% more motile sperm than dominants (average difference is 6.4%, SD ± 10%). Motility in ovarian fluid range from dominants having 54% more motile sperm cells than subordinates to subordinates having 27.5% more motile sperm cells than dominants (average difference is 6.4%, SD ± 24.8%). Spermatocrit values range from subordinates having 25–3% more sperm in their ejaculates than dominants (average 12.7%, SD ± 9.6%). Differences in spermatocrit between dominants and subordinates were also controlled for in our experimental design (see Fertilization).
Fertilization
We examined the relative paternity of dominant and subordinate males competing to fertilize eggs in in vitro fertilization trials using the following approach: An approximately equal numbers of mature eggs (20–30) from each female were distributed in marked plastic beakers and fertilizations were conducted by manually “ejaculating” [from pipettes aimed with the same angle toward the eggs and located at same distance (3 cm) from the eggs] sperm from the two competing males into the beakers immediately after adding 50 ml water to the eggs. The amount of water added to the eggs before “ejaculating” the sperm was just enough to cover the eggs, giving the dominant male low sperm dilutions when ejaculating before the subordinate. To investigate the effect of sperm numbers on paternity we used two kinds of sperm competition trials: One where the two competing males (i.e., ejaculates) had different sperm numbers (i.e., not controlling for initial differences in sperm cell density by adjusting ejaculate volume) and one where they had a similar number of sperm cells (i.e., after controlling for initial differences in sperm cell density by adjusting ejaculate volume). The average ejaculate volumes used in these to different trials were the same (18.5 μl), yet the standard deviation naturally differed (± 9.1 and 3.9 μl, respectively). Nested within each of these two types of sperm competition we either added the sperm from the two males synchronously or with a time delay to the subordinates “ejaculation” (i.e., asynchronous fertilizations). As the asynchrony in gamete release between dominant and subordinate male in natural spawning from the same population is on average 0.68 s (Sørum et al., 2011), we used that time as a reference to perform the asynchronous fertilizations. In order to “ejaculate” the sperm from the pipettes at the right time, we used a metronome guiding the “ejaculator” (TBE) and we also video recorded the actual “ejaculation” of milt from the pipettes. The latter enabled an a posteriori analysis of the timing of milt release to the nearest 0.01 s, showing that subordinate “ejaculations” in our fertilizations were 0.67 ± 0.04 s (mean ± SD, n = 64) after the dominant. Investigations of sperm-egg interactions were enabled by letting every pair of caged males fertilize eggs from two different females. Every treatment was done with replications, giving 16 independent fertilizations for each combination of 2 males × 2 females. After ejaculation the beakers with the mixture of eggs, water and milt were gently stirred and approximately 15 s after ejaculation 0.5 l of water was slowly added to dilute the sperm densities (to avoid possible polyspermy), which also occur under natural spawning.
Hatchery
The fertilized eggs were stored at 6°C over night and then transported carefully to the hatchery. The hatchery contained six 600 l tanks with 6°C water continuously flowing through. The eggs, which are demersal, were randomly distributed into plastic cups (4 cm3) with a bottom made of nylon net with water flowing through each cup (family). Unfertilized, infected and dead eggs, dead eyelings and dead fry were removed every week to avoid fungus growth. After 120 days, living fry were anesthetized and killed, using benzocaine, and stored on ethanol. The mean survival through the experimental period was 64% and (because of monetary limitations) 1255 of the 5688 fry were included in paternity analysis representing 128 families (i.e., an average of 9.8 fry per family, SE ± 0.11). Our experiments conform to the relevant regulatory standards in Norway.
DNA-extractions
We obtained tissue for DNA extraction by cutting the caudal fin of the larvae and a small part of the dorsal fin for the adults. The tissue samples were then stored in 96-well PCR plates. DNA was isolated using a modified procedure of Miller et al. (1988). Cell Lysis Buffer and Proteinase K were added in a relationship 3:2 (i.e., 22.5 μl Cell Lysis Buffer and 15 μl Proteinase K) using a Gilson Pipetman Concept multichannel (Gilson, Middleton, WI, USA). After incubating the plates overnight, at 55°C and 150 rpm, they were heated to 80°C for 15 min using an Eppendorf Mastercycler (Eppendorf HQ, Hamburg, Germany) followed by a short centrifugation, using a Labofuge 400 R (Heraeus, Buckinghamshire, England) and then stored in a freezer at −20°C.
PCR
Paternity was examined using microsatellites and polymerase chain reactions (PCR) on an Eppendorf Mastercycler (Eppendorf HQ, Hamburg, Germany) and a C1000 Thermal Cycler (BIO-RAD HQ, Hercules, CA, USA). The PCR's were carried out in 10 μl reaction volumes containing: 0.4 μl (50–100 ng) Arctic charr genomic DNA, 0.2 μl (0.2 μM) of each of the forward and reverse fluorescently marked primers, 0.2 μl (0.05 mM) dNTP, 1.0 μl (2.5 mM) buffer, 0.08 μl (0.04 units/μl LaTaq) enzyme and 7.92 μl distilled H2O. The PCR profile was: 94°C for 2 min, followed by 30 cycles of 94°C for 15 s, 59°C for 30 s, 72°C for 25 s with a final 72°C extension for 7 min. Later analysis of the PCR-products was carried out at the DNA-Sequencing Laboratory at the University of Tromsø on an Applied Biosystems (ABI) 3130×l Genetic Analyzer with ROX 500 and HiDi Formamide from ABI. Allele size was examined with the software GeneMarker v. 1.6 (SoftGenetics, PA, USA). Paternity was unambiguously assigned manually. We used the primers Smm_22 and Smm_24, which were isolated and characterized from other salmonid species by Crane et al. (2004). We chose these primers based on a previous study from the same population, which demonstrated high polymorphism at these loci (Table 1 in Westgaard et al., 2004). This analysis of the 32 parents gave 16 and 13 different alleles at Smm_22 and Smm_24, respectively, whereas the numbers of different alleles counted in males only were 15 and 12 at the two loci, respectively. Smm_22 and Smm_24 from all potential parents gave consistent allele sizes when examined twice.
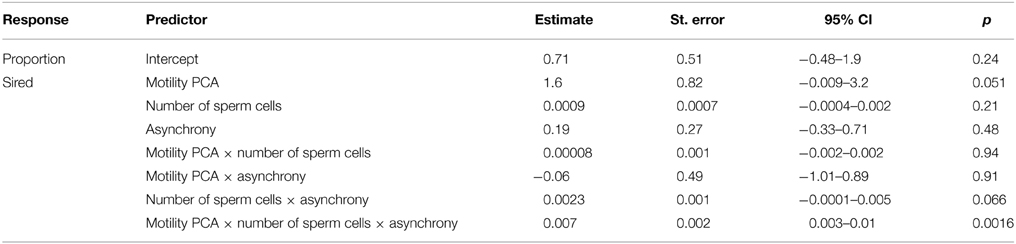
Table 1. Parameter estimates (SE, 95% CI and p-values) for the effects of sperm velocity and motility in water and ovarian fluid (i.e., motility PCA), sperm quantity (i.e., number of sperm cells), ejaculate release delay (i.e., asynchrony) and the interactions on proportion of larvae sired (n = 128).
Statistics
As sperm velocity and percentage of motile sperm cells in water and ovarian fluid were correlated (0.27 < r < 0.62), we quantified the parameters of sperm behavior (i.e., quality) by using a principal component analysis. This reduced the four variables to one statistically independent component (from now on termed motility PCA, Eigenvalue = 2.63, variance explained = 66%, the PCA's respective r−values to motility in water and ovarian fluid were 0.9 and 0.5, and the corresponding numbers for the velocity in water and ovarian fluid were 0.9 and 0.5). In order to test what effects sperm motility, sperm number and synchrony of gamete release have on the fertilization success we ran a mixed-effects model with the proportion of eggs fertilized by the focal male as the response variable (using the cbind function in R, see Crawley, 2013 p. 628 for further details). Since the response variable is a proportion, we ran the model with a binomial distribution. As fixed factors we entered sperm motility PCA and the two manipulated traits (sperm number and synchrony). Batch of eggs from the two females per male pair and female ID were included as random factors, with female ID nested in batch (i.e., 8 pairs of males, 2 females per pair, a 2 × 2 full factorial design, 2 replicates per pair × female combination). Motility PCA and number of sperm cells were entered as relative measures, which are the measures for the focal male minus the measures of the competing male in the pair. Asynchrony was entered as the relative time difference in milt release between the two competing ejaculates. For example, if the focal male's ejaculate was released 0.67 s before the competing male's ejaculate it was entered as 0.67 s. Model fitting and estimates were obtained with the linear mixed-effects (lmer) package lme4 (Bates et al., 2014) in R (version 3.1.3, R Development Core Team, 2015) using restricted maximum likelihood estimates (REML). Model fit and significance were tested using Akaike's Information Criterion corrected for sample size (AICc) (d'Auvergne and Gooley, 2003) and log-likelihood ratio statistics (LLR λ 2) (Bates, 2005). Finally, the model fit was checked using visual examination of normal probability plots and residual plots. The three-way interaction between all predictors was included in the final model, thus as higher order interactions were significant the lower order interactions and main factors also had to be included. In order to visualize results we used plots from the R libraries languageR (Baayen, 2013), hexbin (Carr, 2014), akima (Akima, 2013), and latticeExtra (Sarkar and Andrews, 2013). The parameter estimates in the plots are back-transformed to proportional scale for better interpretation and visualization.
Results
No association was apparent between the proportion of eggs surviving and the relative paternity of the males as revealed by microsatellites (rs = 0.068, p = 0.44, df = 128, Spearman). This suggests that it is unlikely that the actual fertilization success we measure was caused by differential mortality or differential ability to develop by the embryos sired by the two males (for a detailed discussion see García-González, 2008). The finding is in agreement with results previously reported using individuals from the same population, similar experimental design, the same rearing equipment and housing, and the same time period for embryonic development as in the present study (Liljedal et al., 2008).
Increased motility PCA (i.e., the percentage of motile cells and sperm velocity in water and water diluted ovarian fluid) had the strongest independent positive effect on the proportion of larvae sired under sperm competition (Table 1). Increased sperm cell numbers, on the other hand, had no independent effect on paternity and there was also no obvious independent effect of ejaculation asynchrony (Table 1). That is, whether the ejaculate of the focal male was released before, after or in synchrony with the competing male had no overall effect on proportion of larvae sired.
There was however, a positive interaction effect between synchrony in gamete release and number of sperm cells in the ejaculate on the proportion of larvae sired (p = 0.066, Table 1). That is, the relative number of sperm cells seem to influence the proportion of larvae sired more for a male that ejaculated “last” (i.e., the subordinate) than for a male that ejaculated “first” (i.e., the dominant, see Figure 1). Yet, there was no significant interaction between the relative motility PCA and relative number of sperm cells on the proportion of larvae sired (p = 0.94, Table 1). Additionally, there was no significant interaction effect between relative motility PCA and gamete release synchrony on paternity (p = 0.91, Table 1).
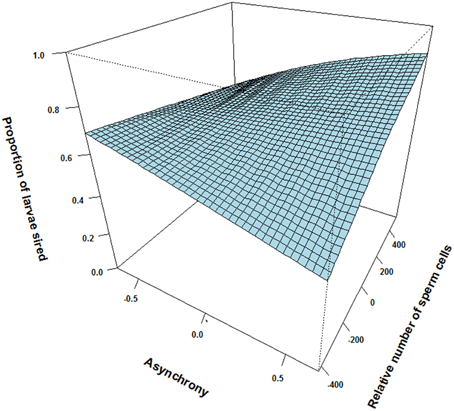
Figure 1. The interactive effect of relative number of sperm cells and ejaculation asynchrony on proportion of larvae sired between pairs of males in sperm competition. An asynchrony value of—0.67 indicates that the male releases milt 0.67 s before the competing male and 0.67 indicates that the male release milt 0.67 s after the competing male. In our experiment dominant males either released their ejaculate before or in synchrony with the subordinate male, while subordinates either released their ejaculate in synchrony or after the dominant male. Although, a subordinate male may ejaculate later than a dominant male, he might compensate for the delay by increasing sperm numbers.
There was however a highly significant positive three-way interaction involving relative motility PCA, relative amount of sperm cells and asynchrony (Table 1). That is, when ejaculating in synchrony both high motility PCA and high sperm numbers are important for paternity (Figure 2). Yet, when there is a time delay in the gamete release between the two mating tactics (the subordinate is always last) the fertilization success is much more dependent on a high motility PCA for the dominant male (Figure 3) than for the subordinate male (Figure 4). Figures 2–4 also illustrate how the effect of the motility PCA varies depending on whether the males spawn first, in synchrony or last.
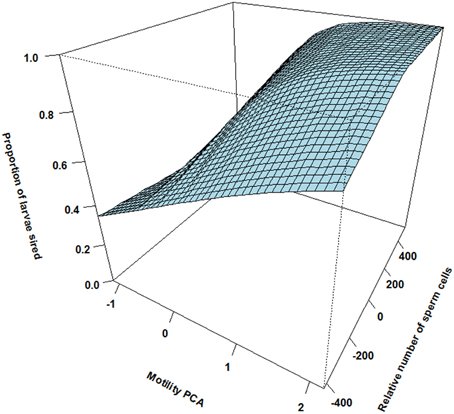
Figure 2. The interaction between relative motility PCA (i.e., per cent motile sperm cells and sperm velocity in water and ovarian fluid) and relative number of sperm cells on proportion of larvae sired for the males that ejaculated in synchrony (simultaneously). When the two males have similar sperm numbers there is a sharp increase in paternity with increasing difference in motility PCA. At similar motility PCA there is also a slight increase in paternity with increasing difference in sperm numbers.
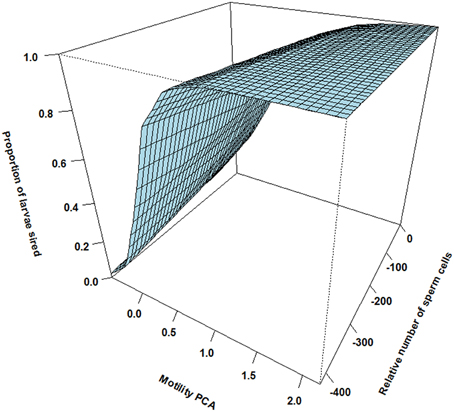
Figure 3. The effect of relative motility PCA (i.e., per cent motile sperm cells and sperm velocity in water and ovarian fluid) and the relative number of sperm cells on proportion of larvae sired for dominant males ejaculating before the competing subordinate male (asynchrony). Although dominant males show a sharp increase in paternity with increasing sperm motility PCA independent of sperm numbers, dominants show low sperm velocity compared to subordinates under natural reproduction.
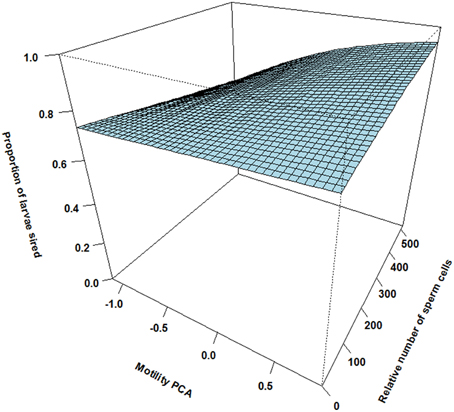
Figure 4. The effect of relative motility PCA (i.e., per cent motile sperm cells and sperm velocity in ovarian fluid) and relative number of sperm cells on proportion of larvae sired for subdominant males ejaculating after the competing dominant male (asynchrony). Here, the effect of increased motility and increased sperm numbers is weak.
Discussion
This is the first study to disentangle the effects of naturally occurring adjustments in sperm quality, sperm quantity and spawning synchrony and their interactions for paternity in sperm competition among subordinate and dominant individuals of an external fertilizing species. Our results show that sperm motility and number of sperm cells are the overall most important variables influencing paternity under our experimental conditions. Whether the ejaculate of the subordinate is released after or in synchrony with the competing male, showed no overall effect on paternity. However, ejaculate characteristics in synchronized and asynchronized spawning affect paternity of the dominant and subordinate males differently. That is, dominants ejaculating in synchrony with the female egg release and (on average 0.67 s) before the subordinate male, show a rapid increase in paternity with increasing sperm motilities—a relationship not much influenced by relative sperm numbers. Subordinates, ejaculating out of synchrony (0.67 s after the dominant male), show a slow increase in paternity with increasing sperm motility and need high sperm numbers to outcompete the dominant male.
This experiment mimicked the situation with ejaculations from one dominant and one subordinate male given an equal distance to the eggs. The proximity of the female to the male during spawning may also be of large importance for the outcome of reproductive activities and our study is, consequently, not a complete description of all factors influencing reproductive success under sperm competition in charr. The spawning in our population of charr has been studied in some detail showing a large variance in the behavioral repertoire (Sørum et al., 2011). From one to nine males may release ejaculates from very close positions to the released eggs within 1.9 s after spawning between a female and the dominant male. Dominant males and subordinates may also spawn with the female without sperm competition (Sørum et al., 2011). Additionally, as the spawning site provide no protection for the dominant male and the female, there is an increase in the density of males positioning themselves at the spawning site before the actual spawning occur between the dominant male and the female. That is, the positional advantage for the dominant male during spawning is in our population somewhat unclear, and our mimic is well within the behavioral repertoire observed (i.e., it is not a mimic of a constructed artificial situation). To get a complete understanding of all factors influencing male reproductive success in our population, female proximity and more intense sperm competition (more than two males) should also be experimentally evaluated.
In bluegill (Lepomis macrochirus), sneakers on average release their sperm approximately 0.46 s after parental (Stoltz and Neff, 2006a). Yet, when mimicking this delay in in vitro sperm competition trials, ejaculates from sneaker males outcompete those from parentals (Stoltz and Neff, 2006b). Although sperm numbers influence the outcome, the advantage for sneakers is larger than that accounted for by differences in sperm numbers. The authors conclude that some other aspect than flagellum length, curvilinear speed and path linearity, the three quality measures of sperm included in the study, must contribute to the increased competitiveness of sperm from sneakers (Stoltz and Neff, 2006b). Thus, for bluegills the cause for the “loaded raffle” seems still unclear.
Our overall results are, on the other hand, in line with earlier studies revealing sperm velocity and sperm motility as good predictors of a male's fertilization success both in the absence (Lahnsteiner et al., 1998; Froman et al., 1999; Rurangwa et al., 2001; Kupriyanova and Havenhand, 2002; Gomendio et al., 2007) and in the presence of sperm competition (Birkhead et al., 1999). These results are also in line with previous findings in charr (Liljedal et al., 2008) and in closely related species like salmon (S. salar) (Gage et al., 2004) and trout (Salmo trutta) (Lahnsteiner et al., 1998). Salmonids are known to have one of the briefest fertile windows among fish and the period of sperm survival is short when observed in water (Vladic and Järvi, 1997, own observations). Yet, the ovarian fluid represents a protective environment (Litvak and Trippel, 1998) and Arctic charr ejaculates have a higher percentage of motile sperm cells in ovarian fluid than in water (Turner and Montgomerie, 2002). Moreover, the ovarian fluid seems to represent a selective environment for sperm (Yeates et al., 2013), and there is a strong interaction effect of ovarian fluid on sperm swimming speed in charr with certain female fluids stimulating swimming speed of sperm from some males over others (Urbach et al., 2005). Eggs are fertilized after a sperm cell enters the micropyle, which is barely wide enough to allow entry of one sperm cell (Ginsburg, 1963; Kobayashi and Yamamoto, 1981; Yanagimachi et al., 1992). Thus, in salmonids, where up to 80% of the eggs can be fertilized within the first 5 s of egg and sperm interactions (Hoysak and Liley, 2001), the first sperm cell to enter the micropyle fertilizes the egg (Kobayashi and Yamamoto, 1981; Yanagimachi et al., 1992). These female evolved characteristics may help enforce cryptic choice and may explain why sperm motility, in this study also measured in ovarian fluid, is so important for the observed fertilization success under sperm competition in Arctic charr.
In previous studies where the dominance status of males is experimentally manipulated, individual male Arctic charr becoming subordinates increase average sperm velocity compared to males becoming dominant (Liljedal and Folstad, 2003; Rudolfsen et al., 2006). Ejaculates of subordinates also contain larger fractions of fast sperm cells, those most likely to fertilize the eggs, than ejaculates of dominants (Vaz Serrano et al., 2006; Haugland et al., 2008). Subordinate males becoming dominant, on the other hand, reduce their sperm velocity compared to levels previously held as subordinates (Rudolfsen et al., 2006). This velocity reduction among males becoming dominants is puzzling given the large importance of sperm motility for number of offspring sired among these individuals. Yet, the velocity reduction is in accordance with Parker's (1990) theoretical model of ejaculate investments under sperm competition which suggest that “..if mating order is non-random, the favored male should expend less on sperm.” One might speculate that the metabolic resources for sperm production and sperm velocity, are traded-off differently in dominant and subordinate males over the entire spawning season (Jeulin and Soufir, 1992; Burness et al., 2004, but see Burness et al., 2005) and that dominants in need of more energy for guarding activities, potentially resulting in positional advantages under synchronized spawning, might have to reduce energy investments in sperm. This explanation, which relies heavily on energy being a limited resource for reproductively active males, fits the observations that ATP-levels in sperm of charr is positively related to sperm velocity and negatively related to measures of high social status (i.e., dominance) (Figenschou et al., 2013). An explanation based on energy limitations also correspond with the recent suggestion that the adipose fin may have evolved as a signal of energy stores in salmonids (Haugland et al., 2011). That is, adipose fin size may actually be indicative of the energy available for reproductive activities in salmonids.
The overall positive relationship between relative sperm number and fertilization success in the present study is also in agreement with empirical evidence from other external fertilizers experiencing sperm competition (Dziminski et al., 2009; Ottesen et al., 2009). It also corresponds with results showing an inter-specific relationship between the intensity of sperm competition and sperm production in external fertilizers (Byrne et al., 2002). While the ejected distance of an ejaculate may be longer then 20 cm (own observations), sperm cells from charr are only able to swim about half of the circumference of the egg, i.e., 0.5 cm (Billard and Cosson, 1992). Thus, given a large difference in sperm numbers between the two competing males, the male with most sperm cells can scatter his sperm in a larger area and therefore reach the spawned and widespread eggs before the competing male. Moreover, the generally held assumption that a gradual dilution of seminal fluid in general is initiating sperm activity seem to be violated in charr, as activity of sperm cells here are only influenced by individual specific dilution of own seminal fluid in water and unaffected by the presence of seminal fluids from other males (Rudolfsen et al., submitted). This latter finding clearly illustrates the fine-tuned adaptions to sperm competition in charr.
Our close experimental imitation of sperm competition under natural spawning asynchrony, natural variation in sperm number and natural variation in sperm velocity show that a high degree of synchrony in gamete release between the female and the dominant male may result in a high paternity share of the dominant male. Yet, this benefit might to a large extent be offset by a rapid (within 4 days) increase in sperm cell production and sperm motility among subordinate, sneaker males. The latter is however dependent upon a rapid response to female egg release by subordinate males. In our studied population the number of subordinate males at the spawning site increases through the 0.25 s period elapsing right before egg release (Sørum et al., 2011), suggesting that subordinates may perceive the forthcoming spawning. Yet, although the first sneaker males are ejaculating on average 0.68 s after the guarding male, subordinates are also seen releasing milt as late as 1.9 s after female egg release (Sørum et al., 2011).
Although natural spawning in charr includes a large range of spawning behaviors including a high frequency of highly synchronized spawning between the female and a dominant male, our close natural mimic adds parameter estimates to the outcomes most commonly observed (Sørum et al., 2011). At this intensity of sperm competition, i.e., when approximately two males compete, allocation of resources to sperm production should be at its most intense (Parker et al., 1996) rendering our system ideal for studies on behavioral and physiological adaptations to sperm competition. We are however currently unable to quantify the fitness effects of resource investments in sperm quantity and quality for the different spawning tactics throughout the 1-month long spawning period. Is it possible that the relative low investment in sperm quantity and sperm quality observed in dominant males compared to that of subordinates under such long-term scenario prove beneficial because of benefits from synchronized spawning and positional effects? Additionally, how does ejaculate investments trade-off against the obvious costs of mate guarding and courting? Our results suggest that constraints on investments in sperm number and motility among dominant guarding males should be considerable given the large potential fitness benefits from such investments when ejaculating in synchrony with the female—and before the subordinates.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank two anonymous referees whose comments improved our manuscript and Lars Figenschou, Vidar Sørum, and Sissel Kaino for help during field and laboratory work. Marion Jom helped with microsatellite analyses.
References
Akima, H. (2013). Interpolation of Irregularly Spaced Data. R package version 0.5-11. Available online at: http://cran.r-project.org/web/packages/akima/index.html
Alonzo, S. H., and Warner, R. R. (2000). Allocation to mate guarding or increased sperm production in a Mediterranean wrasse. Am. Nat. 156, 266–275. doi: 10.1086/303391
Baayen, R. H. (2013). Data Sets and Functions with “Analyzing Linguistic Data: A Practical Introduction to Statistics.” R package version 1.4.1. Available online at: http://CRAN.R-project.org/package=languageR
Babcock, R. C., Mundy, C. N., and Whitehead, D. (1994). Sperm diffusion-models and in situ confirmation of long-distance fertilization in the free-spawning asteroid Acanthaster planci. Biol. Bull. 186, 17–28. doi: 10.2307/1542033
Bates, D. M. (2005). Fitting linear mixed models in R. R News 5, 27–30. Available online at: http://cran.r-project.org/doc/Rnews/Rnews_2005-1.pdf
Bates, D. M., Maechler, M., Bolker, B., and Walker, S. (2014). lme4: Linear Mixed-effects Models Using Eigen and S4. R package version 1.1-7. Available online at: http://CRAN.R-project.org/package=lme4
Billard, R., Christen, R., Cosson, M. P., Gatty, J. L., Letellier, L., Renard, P., et al. (1986). Biology of the gametes of some teleost species. Fish Physiol. Biochem. 2, 115–120. doi: 10.1007/BF02264079
Billard, R., and Cosson, M. P. (1992). Some problems related to the assessment of sperm motility in fresh-water fish. J Exp. Zool. 261, 122–131. doi: 10.1002/jez.1402610203
Birkhead, T. R., Martinez, J. G., Burke, T., and Froman, D. P. (1999). Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc. R. Soc. Lond. B 266, 1759–1764. doi: 10.1098/rspb.1999.0843
Birkhead, T. R., and Møller, A. P. (1998). Sperm Competition and Sexual Selection. San Diego, CA: Academic Press.
Burness, G., Casselman, S. J., Schulte-Hostedde, A. I., Moyes, C. D., and Montgomerie, R. (2004). Sperm swimming speed and energetics vary with sperm competition risk in bluegill (Lepomis macrochirus). Behav. Ecol. Sociobiol. 56, 65–70. doi: 10.1007/s00265-003-0752-7
Burness, G., Moyes, C. D., and Montgomerie, R. (2005). Motility, ATP levels and metabolic enzyme activity of sperm from bluegill (Lepomis macrochirus). Comp. Biochem. Physiol. Part A 140, 11–17. doi: 10.1016/j.cbpb.2004.09.021
Byrne, P. G., Roberts, J. D., and Simmons, L. W. (2002). Sperm competition selects for increased testes mass in Australian frogs. J. Evol. Biol. 15, 347–355. doi: 10.1046/j.1420-9101.2002.00409.x
Carr, D. (2014). Hexagonal Binning Routines. R package version 1.27.0. Available online at: http://CRAN.R-project.org/package=hexbin
Casselman, S. J., Schulte-Hostedde, A. I., and Montgomerie, R. (2006). Sperm quality influences male fertilization success in walleye (Sander vitreus). Can. J. Fish. Aquat. Sci. 63, 2119–2125. doi: 10.1139/f06-108
Crane, P. A., Lewis, C. J., Kretschmer, E. J., Miller, S. J., Spearman, W. J., Decicco, A. L., et al. (2004). Characterization and inheritance of seven microsatellite loci from Dolly Varden, Salvelinus malma, and cross-species amplification in Arctic char, S-alpinus. Conserv. Genet. 5, 737–741. doi: 10.1007/s10592-004-1853-1
d'Auvergne, E. J., and Gooley, P. R. (2003). The use of model selection in the model-free analysis of protein dynamics. J. Biomol. NMR 25, 25–39. doi: 10.1023/A:1021902006114
Denny, M. W., and Shibata, M. F. (1989). Consequences of surf-zone turbulence for settlement and external fertilization. Am. Nat. 134, 859–889. doi: 10.1086/285018
Dominey, W. J. (1984). Alternative mating tactics and evolutionarily stable strategies. Am. Zool. 24, 385–396. doi: 10.1093/icb/24.2.385
Dziminski, M. A., Roberts, J. D., Beveridge, M., and Simmons, L. W. (2009). Sperm competitiveness in frogs: slow and steady wins the race. Proc. R. Soc. Lond. B 276, 3955–3961. doi: 10.1098/rspb.2009.1334
Evans, J. P., Rosengrave, P., Gasparini, C., and Gemmell, N. J. (2013). Delineating the roles of males and females in sperm competition. Proc. R. Soc. Lond. B 280, 20132047. doi: 10.1098/rspb.2013.2047
Fabricius, E. (1953). Aquarium Observations on the Spawning Behaviour of the Char, Salmo Alpinus. Institute of Freshwater Research Report, Drottingholm.
Figenschou, L., Folstad, I., and Liljedal, S. (2004). Lek fidelity of male Arctic charr. Can. J. Zool. 82, 1278–1284. doi: 10.1139/z04-106
Figenschou, L., Folstad, I., Rudolfsen, G., Hanssen, S. A., Kortet, R., Skau, P. A., et al. (2013). The relative effect of parasites and social status on sperm traits in Arctic charr. Behav. Ecol. 24, 497–504. doi: 10.1093/beheco/ars190
Froman, D. P., Feltmann, A. J., Rhoads, M. L., and Kirby, J. D. (1999). Sperm mobility: a primary determinant of fertility in the domestic fowl (Gallus domesticus). Biol. Reprod. 61, 400–405. doi: 10.1095/biolreprod61.2.400
Gage, M. J. G., Macfarlane, C. P., Yeates, S., Ward, R. G., Searle, J. B., and Parker, G. A. (2004). Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 14, 44–47. doi: 10.1016/s0960-9822(03)00939-4
García-González, F. (2008). Male genetic quality and the inequality between paternity success and fertilization success: consequences for studies of sperm competition and the evolution of polyandry. Evolution 62, 1653–1665. doi: 10.1111/j.1558-5646.2008.00362.x
Ginsburg, A. S. (1963). Sperm-egg association and its relationship to activation of egg in salmonid fishes. J. Embryol. Exp. Morphol. 11, 13–33.
Gomendio, M., Malo, A. F., Garde, J., and Roldan, E. R. S. (2007). Sperm traits and male fertility in natural populations. Reproduction 134, 19–29. doi: 10.1530/REP-07-0143
Haugland, T., Rudolfsen, G., Figenschou, L., and Folstad, I. (2008). Sperm velocity and its relation to social status in Arctic charr (Salvelinus alpinus). Anim. Reprod. Sci. 115, 231–237. doi: 10.1016/j.anireprosci.2008.11.004
Haugland, T., Rudolfsen, G., Figenschou, L., and Folstad, I. (2011). Is the adipose fin and the lower jaw (kype) related to social dominance in male Arctic charr Salvelinus alpinus? J. Fish Biol. 79, 1076–1083. doi: 10.1111/j.1095-8649.2011.03087.x
Hoysak, D. J., and Liley, N. R. (2001). Fertilization dynamics in sockeye salmon and a comparison of sperm from alternative male phenotypes. J. Fish Biol. 58, 1286–1300. doi: 10.1111/j.1095-8649.2001.tb02286.x
Jeulin, C., and Soufir, J. C. (1992). Reversible intracellular ATP changes in intact rat spermatozoa and effects on flagellar sperm movement. Cell Motil. Cytoskeleton 21, 210–222. doi: 10.1002/cm.970210305
Kobayashi, W., and Yamamoto, T. S. (1981). Fine-structure of the micropylar apparatus of the Chum salmon egg, with a discussion of the mechanism for blocking polyspermy. J. Exp. Zool. 217, 265–275. doi: 10.1002/jez.1402170213
Kodric Brown, A. (1986). Satellites and sneakers - opportunistic male breeding tactics in puppfish (Cyprinodon pecosensis). Behav. Ecol. Sociobiol. 19, 425–432. doi: 10.1007/BF00300545
Koya, Y., Koike, Y., Onchi, R., and Munehara, H. (2013). Two patterns of parasitic male mating behaviors and their reproductive success in Japanese medaka, Oryzias latipes. Zool. Sci. 30, 76–82. doi: 10.2108/zsj.30.76
Kupriyanova, E., and Havenhand, J. N. (2002). Variation in sperm swimming behaviour and its effect on fertilization success in the Serpulid polychaete Galeolaria caespitose. Inv. Reprod. Dev. 41, 21–26. doi: 10.1080/07924259.2002.9652731
Lahnsteiner, F., Berger, B., Weismann, T., and Patzner, R. A. (1998). Determination of semen quality of the Rainbow trout, Oncorhynchus mykiss, by sperm motility, seminal plasma parameters, and spermatozoal metabolism. Aquaculture 163, 163–181. doi: 10.1016/S0044-8486(98)00243-9
Law, R. (1979). Optimal life histories under age-specific predation. Am. Nat. 114, 399–417. doi: 10.1086/283488
Levitan, D. R. (2005). Sex-specific spawning behavior and its consequences in an external fertilizer. Am. Nat. 165, 682–694. doi: 10.1086/429733
Levitan, D. R., Sewell, M. A., and Chia, F. S. (1992). How distribution and abundance influence fertilization success in the Sea urchin Strongylocentrotus franciscanus. Ecology 73, 248–254. doi: 10.2307/1938736
Liljedal, S., and Folstad, I. (2003). Milt quality, parasites, and immune function in dominant and subordinate Arctic charr. Can. J. Zool. 81, 221–227. doi: 10.1139/z02-244
Liljedal, S., Rudolfsen, G., and Folstad, I. (2008). Factors predicting male fertilization success in an external fertilizer. Behav. Ecol. Sociobiol. 62, 1805–1811. doi: 10.1007/s00265-008-0609-1
Litvak, M. K., and Trippel, E. A. (1998). Sperm motility patterns of Atlantic cod (Gadus morhua) in relation to salinity: effects of ovarian fluid and egg presence. Can. J. Fish. Aquat. Sci. 55, 1871–1877. doi: 10.1139/f98-093
Miller, S. A., Dykes, D. D., and Polesky, H. F. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16, 1215. doi: 10.1093/nar/16.3.1215
Neff, B. D., Fu, P., and Gross, M. R. (2003). Sperm investment and alternative mating tactics in Bluegill sunfish (Lepomis macrochirus). Behav. Ecol. 14, 634–641. doi: 10.1093/beheco/arg032
Oliveira, R. F., Canario, A. V. M., Grober, M. S., and Santos, R. S. (2001). Endocrine correlates of male polymorphism and alternative reproductive tactics in the Azorean rock-pool blenny, Parablennius sanguinolentus parvicornis. Gen. Comp. Endocrinol. 121, 278–288. doi: 10.1006/gcen.2001.7596
Ottesen, O. H., Babiak, I., and Dahle, G. (2009). Sperm competition and fertilization success of Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 286, 240–245. doi: 10.1016/j.aquaculture.2008.09.018
Parker, G. A. (1970). Sperm competition and its evolutionary consequences in insects. Biol. Rev. 45, 525–567. doi: 10.1111/j.1469-185X.1970.tb01176.x
Parker, G. A. (1990). Sperm competition games: raffles and roles. Proc. R. Soc. Lond. B 242, 120–126. doi: 10.1098/rspb.1990.0114
Parker, G. A., Ball, M. A., Stockley, P., and Gage, M. J. G. (1996). Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. Lond. B 263, 1291–1297. doi: 10.1098/rspb.1996.0189
Pennington, J. T. (1985). The ecology of fertilization of echinoid eggs - the consequences of sperm dilution, adult aggregation, and synchronous spawning. Biol. Bull. 169, 417–430. doi: 10.2307/1541492
R Development Core Team. (2015). R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available online at: http://R-project.org (Accessed July 3, 2015).
Rudolfsen, G., Figenschou, L., Folstad, I., and Kleven, O. (2008). Sperm velocity influence paternity in the Atlantic cod (Gadus morhua L.). Aquaculture Res. 39, 212–216. doi: 10.1111/j.1365-2109.2007.01863.x
Rudolfsen, G., Figenschou, L., Folstad, I., Tveiten, H., and Figenschou, M. (2006). Rapid adjustments of sperm characteristics in relation to social status. Proc. R. Soc. Lond. B 273, 325–332. doi: 10.1098/rspb.2005.3305
Rurangwa, E., Volckaert, F. M., Huyskens, G., Kime, D. E., and Ollevier, F. (2001). Quality control of refrigerated and cryopreserved semen using computer-assisted sperm analysis (CASA), viable staining and standardized fertilization in African catfish (Clarias gariepinus). Theriogenology 55, 751–769. doi: 10.1016/S0093-691X(01)00441-1
Sarkar, D., and Andrews, F. (2013). Extra Graphical Utilities Based on Lattice. R package version 06-26. Available online at: http://CRAN.R-project.org/package=latticeExtra
Sigurjonsdottir, H., and Gunnarsson, K. (1989). Alternative mating tactics of Arctic charr, Salvelinus alpinus, in Thingvallavatn, Iceland. Environ. Biol. Fish. 26, 159–176. doi: 10.1007/BF00004814
Sørum, V., Figenschou, L., Rudolfsen, G., and Folstad, I. (2011). Spawning behaviour of Arctic charr (Salvelinus alpinus): risk of sperm competition and timing of milt release for sneaker and dominant males. Behaviour 148, 1157–1172. doi: 10.1163/000579511X596615
Stoltz, J. A., and Neff, B. D. (2006a). Male size and mating tactic influence proximity to females during sperm competition in bluegill sunfish. Behav. Ecol. Sociobiol. 59, 811–818. doi: 10.1007/s00265-005-0127-3
Stoltz, J. A., and Neff, B. D. (2006b). Sperm competition in a fish with external fertilization: the contribution of sperm number, speed and length. J. Evol. Biol. 19, 1873–1881. doi: 10.1111/j.1420-9101.2006.01165.x
Taborsky, M. (1994). Sneakers, satellites, and helpers - parasitic and cooperative behavior in fish reproduction. Adv. Study Behav. 23, 1–100. doi: 10.1016/S0065-3454(08)60351-4
Turner, E., and Montgomerie, R. (2002). Ovarian fluid enhances sperm movement in Arctic charr. J. Fish Biol. 60, 1570–1579. doi: 10.1111/j.1095-8649.2002.tb02449.x
Urbach, D., Folstad, I., and Rudolfsen, G. (2005). Effects of ovarian fluid on sperm velocity in Arctic charr (Salvelinus alpinus). Behav. Ecol. Sociobiol. 57, 438–444. doi: 10.1007/s00265-004-0876-4
Vaz Serrano, J. V., Folstad, I., Rudolfsen, G., and Figenschou, L. (2006). Do the fastest sperm within an ejaculate swim faster in subordinate than in dominant males of Arctic char? Can. J. Zool. 84, 1019–1024. doi: 10.1139/Z06-097
Vladic, T., and Järvi, T. (1997). Sperm motility and fertilization time span in Atlantic salmon and brown trout - the effect of water temperature. J. Fish Biol. 70, 1088–1093.
Westgaard, J. I., Klemetsen, A., and Knudsen, R. (2004). Genetic differences between two sympatric morphs of Arctic charr confirmed by microsatellite DNA. J. Fish Biol. 65, 1185–1191. doi: 10.1111/j.0022-1112.2004.00524.x
Yanagimachi, R., Cherr, G. N., Pillai, M. C., and Baldwin, J. D. (1992). Factors controlling sperm entry into the micropyles of salmonid and herring eggs. Dev. Growth Diff. 34, 447–461. doi: 10.1111/j.1440-169X.1992.00447.x
Yeates, S. E., Diamond, S. E., Einum, S., Emerson, B. C., Holt, W. V., and Gage, M. J. G. (2013). Cryptic choice of conspecific sperm controlled by the impact of ovarian fluid on sperm swimming behaviour. Evolution 67, 3523–3536. doi: 10.1111/evo.12208
Yeates, S. E., Searle, J., Ward, R. G., and Gage, M. J. (2007). A two-second delay confers first-male fertilization precedence within in vitro sperm competition experiments in Atlantic salmon. J. Fish Biol. 70, 318–322. doi: 10.1111/j.1095-8649.2006.01294.x
Young, B., Conti, D. V., and Dean, M. D. (2013). Sneaker “jack” males outcompete dominant “hooknose” males under sperm competition in Chinook salmon (Oncorhynchus tshawytscha). Ecol. Evol. 3, 4987–4997. doi: 10.1002/ece3.869
Keywords: sperm competition, costly sperm production, delayed ejaculation, loaded raffle, reproductive behavior, ejaculate characteristics
Citation: Egeland TB, Rudolfsen G, Nordeide JT and Folstad I (2015) On the relative effect of spawning asynchrony, sperm quantity, and sperm quality on paternity under sperm competition in an external fertilizer. Front. Ecol. Evol. 3:77. doi: 10.3389/fevo.2015.00077
Received: 29 April 2015; Accepted: 30 June 2015;
Published: 14 July 2015.
Edited by:
Devi Meian Stuart-Fox, University of Melbourne, AustraliaReviewed by:
Ryan Calsbeek, Dartmouth College, USAFrancisco Garcia-Gonzalez, Doñana Biological Station-Spanish Research Council CSIC, Spain
Copyright © 2015 Egeland, Rudolfsen, Nordeide and Folstad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ivar Folstad, Faculty for Bioscience, Fisheries and Economy, University of Tromsø, Dramsveien 201, Tromsø, Norway, ivar.folstad@uit.no
 Torvald B. Egeland
Torvald B. Egeland Geir Rudolfsen
Geir Rudolfsen Jarle T. Nordeide
Jarle T. Nordeide Ivar Folstad
Ivar Folstad