Forest tent caterpillar, Malacosoma disstria (Lepidoptera: Lasiocampidae), mate-finding behavior is greatest at intermediate population densities: implications for interpretation of moth capture in pheromone-baited traps
- Department of Biological Sciences, University of Alberta, Edmonton, AB, Canada
The forest tent caterpillar, Malacosoma disstria Hübner (Lepidoptera: Lasiocampidae) is a native forest defoliator with a broad geographic range in North America. Forest tent caterpillars experience cyclical population changes and at high densities, repeated defoliation can cause reduced tree growth and tree mortality. Pheromone-based monitoring of forest tent caterpillar moths can provide information on spatial and temporal patterns of incipient outbreaks. Pheromone-baited trap capture of male moths correlates to the number of eggs and pupae in a population but this relationship breaks down at high population densities, when moth trap capture declines. The objective of the current study is to understand the mechanisms that reduce trap capture at high population densities. We tested two different hypotheses: (1) at high population densities, male moth orientation to pheromone sources is reduced due to competition for pheromone plumes; and (2) moths from high density populations will be in poor condition and less likely to conduct mate-finding behaviors than moths from low density populations. A field study showed non-linear effects of density on male moth capture in female-baited traps. The number of males captured increased up to an intermediate density level and declined at the highest densities. Field cage studies showed that female moth density affected male moth orientation to female-baited traps, as more males were recaptured at low than high female densities. There was no effect of male density on the proportion of males that oriented to female-baited traps. Moth condition was manipulated by varying larval food quantity. Although feeding regimes affected the moth condition (size), there was no evidence of an effect of condition on mate finding or close range mating behavior. In the field, it is likely that competition for pheromone plumes at high female densities during population outbreaks reduces the efficacy of pheromone-baited monitoring traps.
Introduction
The most common use of insect pheromones in Integrated Pest Management (IPM) is to bait traps to detect or delimit populations of the target insect pest (Witzgall et al., 2010). In some instances, capture of adult insects in pheromone-baited traps can predict reproductive capacity of the population (Contarini et al., 2009), subsequent densities of immatures (Evenden et al., 1995) and crop damage (Mori et al., 2014). Despite the extensive interest in and application of pheromone monitoring in IPM, relatively few studies examine how the effectiveness of pheromone-baited traps may vary with population density of the target pest and therefore, the population estimates obtained (McNeil, 1991). As population density increases, the number of individuals releasing pheromone in the managed ecosystem will also increase. If natural pheromone plumes are as or more attractive than those from synthetic lures, high insect densities could cause synthetic pheromone-baited traps to be less efficient at high than low population densities (Unnithan and Saxena, 1991; Thorpe et al., 1993; Delisle et al., 1998), and result in population estimates that are biased downwards at high density (Asaro and Berisford, 2001; Beroza et al., 1974; Jones et al., 2009). This problem would be predicted to be most acute for species that experience cyclical changes in population density, like many lepidopteran defoliators of forests.
Pheromone-baited traps have been developed as a tool to detect invasions and predict population densities for several forest defoliators. Male spruce budworm moths, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae), captured in pheromone-baited traps positioned in Ontario, Canada annually over a 21-year period were strongly correlated with late-instar larval population densities in each subsequent year (Sanders, 1988). As a result, sex pheromone-based monitoring is used operationally for spruce budworm in eastern North America. The number of male gypsy moths, Lymantria dispar L. (Lepidoptera: Erebidae), captured in traps baited with either 1 or 500 μg of the gypsy moth sex pheromone, (+)-disparlure, was correlated with subsequent egg mass density at the same sites (Thorpe et al., 1993). Male moth capture in traps baited with low but not high doses of the sex pheromone of the western hemlock looper, Lambdina fiscellaria lugubrosa (Hulst) (Lepidoptera: Geometridae), was correlated with samples of larval and pupal population density within the same generation and significantly predicted the number of eggs sampled at the same sites in the subsequent generation. This relationship was best, however, at the beginning of the flight season before peak flight (Evenden et al., 1995). In several studies, however, pheromone-baited trap capture of forest defoliators does not accurately reflect population densities and/or pheromone traps are less efficient at high population densities (Delisle et al., 1998). Western spruce budworm, Choristoneura occidentalis (Walsingham) (Lepidoptera: Tortricidae), season-long capture was not correlated to larval numbers sampled in the mid-crown of 50 trees at the same sites (Sweeney et al., 1990). Trap capture of Douglas-fir tussock moths, Orgyia pseudotsugata (McDunnough) (Lepidoptera: Erebidae), in traps baited with various pheromone doses did not predict numbers of egg masses or defoliation in the following generation (Shepherd et al., 1985).
The forest tent caterpillar is a native forest defoliator with a broad geographic range in North America (Stehr and Cook, 1968). Although broadly polyphagous, the primary host of the forest tent caterpillar in its northern range is trembling aspen, Populus tremuloides Michenaux (Salicaceae). Forest tent caterpillars experience cyclical changes in population density (Daniels and Myers, 1995). At high densities, repeated defoliation of aspen can cause reduced tree growth (Hildahl and Reeks, 1960; Churchill et al., 1964) and tree mortality (Candau et al., 2002). The commercial value of trembling aspen has increased in recent years (Hogg et al., 2002; Brandt et al., 2003) and tools are needed to monitor forest tent caterpillar populations and accurately predict incipient outbreaks. Males search for mates in response to a female-produced sex pheromone (Struble, 1970) and orient to synthetic pheromone positioned in non-saturating, high volume traps (Schmidt et al., 2003). Trap capture of male forest tent caterpillar moths in synthetic pheromone-baited traps increases with moth density to an intermediate level but declines at the highest population densities tested (Jones et al., 2009).
There are several potential mechanisms that could be driving the reduced trap capture of male forest tent caterpillars in synthetic pheromone-baited traps at high population densities. Competition for pheromone sources could occur at high population densities due to the high number of female moths releasing pheromone. It is known that male forest tent caterpillars compete for mates at high population densities, as late-arriving males will attempt to separate pairs already in copula (Bieman, 1982; Bieman and Witter, 1983). This competition may be enhanced in established outbreak populations that exhibit a male-biased sex ratio in the adult forest tent caterpillar population (Witter, 1979). A high density of calling females in stands with high population densities could create a natural mating disruption effect. Although mating success of female spruce budworm moths increases with population density, there is evidence for competition for pheromone sources at high population densities that negatively affects the capacity of females to attract a mate (Régnière et al., 2013). Mating disruption using synthetic pheromone sources results in reduced trap capture of male forest tent caterpillars in synthetic pheromone-baited traps positioned in treated aspen stands (Palaniswamy et al., 1983) but the potential for natural mating disruption at high population densities has not been tested in this species.
Another potential impact of high population density on mate finding behavior is the effect of mating on subsequent pheromone response. At high population densities, due to close proximity of females in the area, male moths may quickly secure a mate. Following copulation, there may be physiological changes in male moths that alter their ability to subsequently respond to pheromone. Mating is known to reduce pheromone production in female moths (Raina et al., 1994; Ramaswamy et al., 1996) and pheromone response by male moths (Barrozo et al., 2010). Adult forest tent caterpillar moths have a short 5–10 day lifespan (Fitzgerald, 1995) and even a short post-mating refractory period could significantly impact the likelihood that a mated male moth would orient to pheromone and be captured in a pheromone-baited monitoring trap. The effect of mating on subsequent capture in pheromone-baited traps would be expected to be highest at high population densities.
Moth quality can be reduced at high population densities due to larval competition for nutrition and increased incidence of disease. The size of male moths affects flight behavior in several outbreaking forest lepidopteran species. Large male C. conflictana fly farther than small moths (Elliott and Evenden, 2009) and male Epirrita autumnata (Lepidoptera: Geometridae) from low-density populations fly farther than those from high-density populations despite higher wing loading of individuals at low densities (Ruohomäki, 1992). The forest tent caterpillar is a capital-breeding moth that obtains all nutrients for adult traits through larval feeding (Fitzgerald, 1995). Nutritional stress during the larval stage results in prolonged larval development in this species (Jones and Despland, 2006) and moths that emerge late in the season have smaller absolute wing area than early emerging adults (Jones and Evenden, 2008). Larval feeding by forest tent caterpillars results in both rapid- and delayed-induced responses in aspen that change the quality of the food source and negatively affects pupal mass (Parry et al., 2003). Male flight requirements to find females will likely vary with population density and by extension with availability of larval nutrition whereby less food may be available at higher densities. Recent research (Evenden et al., 2015) showed that flight capacity was not influenced by wing loading of male forest tent caterpillar moths collected from populations at different densities but energy use in flight increased with distance flown. At low population densities, males may have to fly considerable distances to encounter a receptive female (Elkinton and Cardé, 1980; Sanders, 1983). The effect of moth quality on mate location is not known for the forest tent caterpillar but large male E. autumnata are more successful in mate location by flight than small males (Tammaru et al., 1996). The effect of moth size on mate location of E. autumnata is more apparent at low than high population densities where males would need to fly greater distances to successfully locate a mate (Tammaru et al., 1996).
The purpose of this study is to determine how population density affects orientation of male forest tent caterpillar moths to pheromone sources. We test male moth response to calling females at different densities in the field. We subsequently use field cage bioassays to assess the effects of density and sex ratio on male orientation and mating success. Based on previous findings (Jones et al., 2009) in field bioassays, we predict that competition for pheromone plumes occurs at high population densities. Our experiments are also designed to determine if moth quality (size) and mating status influence mate finding and mating behavior in this species.
Materials and Methods
Field Study
Twenty aspen-dominated field sites were selected on the basis of forest tent caterpillar density near Rocky Mountain House, Alberta (latitude 52°22′N longitude 114°55′W) in 2002. Sites were located at least 1 km from each other in forest stands at least one hectare in size. The density of forest tent caterpillars was estimated at each site using 15-min timed-pupal collections. The density of healthy moths at each site was then determined from the number of moths that successfully eclosed from the collected pupae.
Female moths were collected for use in the field experiment as pupae in mid-July of 2002 and 2003 from other sites in the Rocky Mountain House area. Silk was removed from cocoons and the pupae were separated by sex. Female pupae were housed individually in 150 ml transparent plastic cups under a 18:6 LD, 30°C photoregime to encourage eclosion. Female moths were held at 10°C until transport to the field in refrigerated containers, 1–4 days post-eclosion.
A transect of four female-baited cages was erected at each of the 20 sites in 2002 and 16 sites in 2003. Cages were placed 20 meters into the forest from the edge and were separated by 40 m. Cages were made from clear plastic to provide visual detection of females by males in addition to the release of the pheromone plume. Cages were constructed from clear 2-liter plastic bottles with the top third of the bottle removed, inverted, and reattached in order to create a funnel. The funnel was modified with mesh to allow pheromone dispersion from the trap. A trembling aspen twig was provided inside the cage for each female to rest on and to call. Cages were hung from a branch approximately 2 m from the ground. Cages were left in the same locations each night and were checked each day until the females' death. The ability of females to attract mates was measured by the presence or absence of males in the trap over each female's life. Females were replaced at each site as possible, two (2002) and three (2003) times in each season.
Multiple logistic regression analysis was used to investigate the relationship between population density and mating attempts at each site. Date and proportion aspen at each site were included as additional independent terms, as was the interaction between date:density. Linear and non-linear effects of population density on the ability of females to attract mates were modeled using generalized linear models (GLM) (McCullagh and Nelder, 1989) and generalized additive models (GAM) (Hastie and Tibshirani, 1990), respectively, both using R (R Core Development Team, 2013). Non-linear relationships were explored through a non-parametric spline fit GAM as opposed to a parametric non-linear fit because there was no a priori expectation of the shape of the response.
Field Cage Studies
Forest tent caterpillars were collected as either eggs or pupae from infestations near Fort McMurray, Alberta (56°43′N, 111°22′W) or Lac LaBiche (54°84′N, 111°97′W) in 2008 and 2009, respectively. Egg masses were stored in paper bags at 4°C until use. Larvae reared from egg masses were maintained on artificial diet (Addy, 1969) at room temperature under natural lighting conditions beginning in May of each year. Cocoons of lab-reared and field-collected pupae were removed using a 3% sodium hypochlorite solution (Grisdale, 1975). Pupae were stored individually under the same rearing conditions and checked at 1–2 day intervals for moth eclosion. Adults were separated by sex and males and females were stored in separate chambers at 15°C until use in field cage studies. Only moths < 3 days old were used in experiments and both males and females were evenly distributed by age between treatment and control cages.
Three field cage studies were designed to test the hypothesis that female forest tent caterpillar density affects male moth orientation to pheromone sources through competition. The moth densities reflected those that would occur under natural outbreak conditions (Hodson, 1941; Batzer et al., 1995). All experiments were conducted at the University of Alberta, South Campus Farm in early July to mid-August in both years. A paired cage design was used, with one cage serving as a control and the second cage housing the various population density treatments (Table 1). Field cages (1.8 × 1.8 × 1.8 m) were separated by at least 50 m. Moth orientation was measured by male response to a female-baited sentinel trap. The sentinel trap consisted of a white Delta trap (Contech Inc., Delta, BC) fitted with a sticky insert and baited with two mesh pouches each containing a newly emerged virgin female moth. The trap was positioned on a wooden stake 1 m above the ground in the center of each field cage. All trials were set up in the late afternoon and male orientation to pheromone was measured as the number of males captured in female-baited traps the following morning.

Table 1. Experimental design of treatment and control field cages in experiments 1-5, conducted in 2008 and 2009.
Experiments 1 and 2 (Table 1) tested the impact of background pheromone from evenly distributed calling females on the disruption of orientation of males to female-produced pheromone plumes emitting from the sentinel trap. In the treatment cage, 35 (Experiment 1) and 50 (Experiment 2) mesh pouches (10 × 10 cm), each containing one virgin female, were evenly distributed on strips of green flagging tape suspended from the top of the cage. In the control cage a similar number of pouches were deployed but they remained empty. In each replicate (Table 1), 30 (Experiment 1) and 50 (Experiment 2) virgin, free-flying male forest tent caterpillar moths were introduced into the cages. The cages were left overnight and the number of male moths captured in the sentinel trap was recorded the next morning. Each replicate was established with new moths on each of seven (Experiment 1) and six (Experiment 2) nights (Table 1).
Experiment 3 followed the same protocol as the first two experiments but allowed for the sex ratio of moths in the treated cage to differ (Table 1). A male-biased sex ratio of approximately 2:1 occurs in established forest tent caterpillar outbreaks (Witter, 1979). This experiment consisted of three treatments: (1) A control cage with only the sentinel trap and 30 mesh pouches (10 × 10 cm) evenly distributed on strips of green flagging tape suspended from the top of the cage to provide perching locations for males but no female moths were placed in these bags. Eighteen male moths were released into the control cage in each replicate. (2) The first treatment cage (low density) was set up exactly as the control cage but 18 of the 30 mesh bags contained virgin females resulting in a 1:1 male to female ratio. (3) The second treatment cage (high density) was set up like the low density treatment cage but twice the number of male moths (36) was released per replicate resulting in a 2:1 male to female ratio. Nine replicates were conducted using new moths in each replicate (Table 1).
A fourth field cage experiment (Table 1) tested the hypothesis that at high population densities, a greater proportion of male forest tent caterpillars will mate and mated males will be subsequently less responsive to pheromone-baited traps. In addition to the sentinel trap, treated cages received 30 free-flying virgin females that could mate with males and the control cages did not receive females. Thirty, free-flying males were introduced into both cages and the number of males captured in the sentinel trap after one night was recorded. Prior to introduction to the treated cage, females were dusted with a small amount of fluorescent powder (DayGlo, Cleaveland OH) and all males were recovered at the end of each trial and observed for powder transferred to the abdomen under ultraviolet light as an indication of “mating.” Differently colored fluorescent powders were used in each of eight replicates (Table 1) of the experiment to avoid potential contamination.
A final field cage experiment (Experiment 5) tested the hypothesis that male moth condition (size) would impact the mate finding ability of male forest tent caterpillar moths. Thirty mesh pouches (10 × 10 cm) were evenly distributed on strips of green flagging tape suspended from the top of each cage to provide perching locations for males but no female moths were placed in these bags. “Big” males (pupal mass > 250 mg) were released into one of the two field cages at a density of 6–20 moths per replicate (Table 1). In the second cage, “small” males (pupal mass < 250 mg) were released at densities of 9–28 per replicate. The cages were left overnight and the proportion of released male moths captured in the sentinel trap was recorded the next morning. Ten replicates (Table 1) were conducted using new moths in each replicate.
For Experiments 1–4, to determine if moth density treatments affected the number of males captured in female-baited traps separate generalized linear models were conducted with a Poisson error distribution and night of the experiment specified as a block. If no males were captured during the night, the replicate was removed from the analysis (Table 1). To determine if moth size affected the proportion of males captured in female-baited traps in Experiment 5, a generalized linear mixed-effects model with a binomial distribution was used.
Mating Cage Study
A mating cage experiment was designed to test the hypothesis that moth condition would influence mating behavior of the forest tent caterpillar. Moths of varying condition were obtained by rearing larvae from eggs collected in Lac La Biche, AB under different nutrient regimes. Ten third instar larvae from each egg mass were separated into three different feeding regimens so that genetic effects among treatments could be minimized. The three feeding regimens included: (1) well-fed larvae fed 2 (1 × 1 cm) cubes of artificial diet 3 days per week; (2) medium fed larvae fed 2 cubes of artificial diet 2 days per week and two cubes of agar 1 day per week; (3) starved larvae fed 2 cubes of artificial diet 1 day per week and two cubes of agar two times per week. The survival and time to pupation were monitored in each feeding treatment. Pupae of surviving insects in each treatment were weighed. Pupae were observed at 1–2 day intervals for moth eclosion and the resulting moths were separated by sex. Individual moth pairs were placed in small cages (60 × 60 × 60 cm) in one of nine pairing treatments: (1) well-fed females × well-fed males (N = 30); (2) well-fed females × medium-fed males (N = 20); (3) well-fed females × starved males (N = 5); (4) medium-fed females × medium-fed males (N = 41); (5) medium-fed females × well-fed males (N = 19); (6) medium-fed females × starved males (N = 7); (7) starved females × starved males (N = 5); (8) starved females × well-fed males (N = 7); (9) starved females × medium-fed males (N = 5). Nine cages were positioned within field cages (1.8 × 1.8 × 1.8 m) at the University of Alberta south campus farm. Moth pairs were established in the late afternoon and provided with a fresh aspen branch as an oviposition substrate. Moths were observed at the onset of scotophase (10 pm) each night and mating behavior was recorded. Moths remained in cages until the female died or an egg mass was laid. Moths were considered to have mated if they were observed mating or if females laid a robust egg mass. To verify female mating status, females were subsequently dissected to confirm transfer of a spermatophore to the bursa copulatrix.
Kruskal-Wallis tests were conducted on each sex separately to determine if days to pupation and pupal mass varied with feeding regime. A Bonferroni correction procedure was used for multiple comparisons. Finally, χ2-tests were used to determine if feeding treatment influenced the number of matings obtained for moths of each sex.
The distribution of residuals was assessed to determine the model fit in all analyses.
Results
Field Study
Density positively affected the capacity of female forest tent caterpillar moths to attract a mate in the field (Coeff = 0.009, F = 3.69, df = 1, P = 0.05, Figure 1), and over-all success of mate attraction differed among the five dates (F = 11.46, df = 4, P < 0.001). There was no significant interaction between date and density (F = 1.25, df = 4, P = 0.29) suggesting that the effect of population density was similar among dates. The percentage of aspen in the forest canopy had no effect on mate finding success (Coeff = 0.009, F = 0.46, df = 1, P = 0.50). A linear fit accounted for 31% of the variation in success at mate attraction (Figure 1).
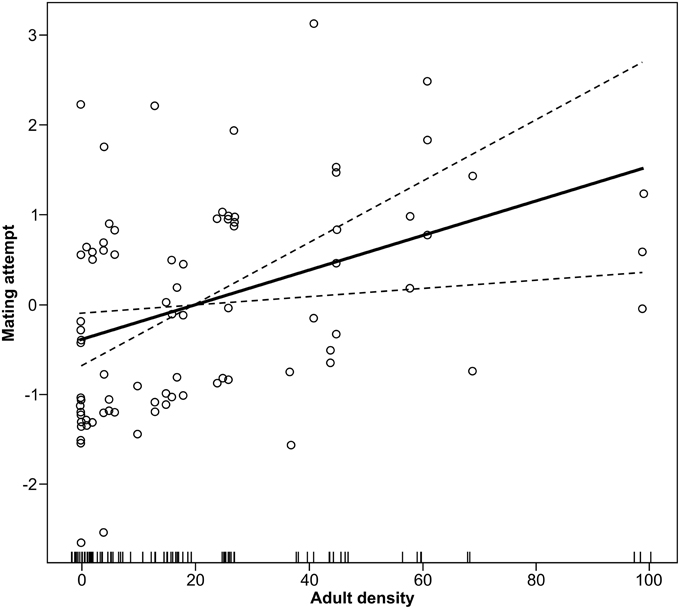
Figure 1. Linear effect of moth density on mating attempts in the forest tent caterpillar. Mating attempt is measured by ability of a female to attract a male. The Y-axis is the added effect of density on mate attraction, with the mean rate of attempts as a logit of the proportion successfully attracted centered on zero (GLM, P = 0.05).
There was a significant non-linear effect of population density on mate attraction as detected using the non-parametric spline fit GAM (χ23 = 12.42, P = 0.002). This model indicates that mate attraction increases with density up to an intermediate density followed by a decrease at the highest population levels (Figure 2). Because aspen content and interactions between density and date were found to be non-significant in preliminary analyses these variables were not included in the GAM. The overall fit for the non-linear model accounted for about 35% of the variation.
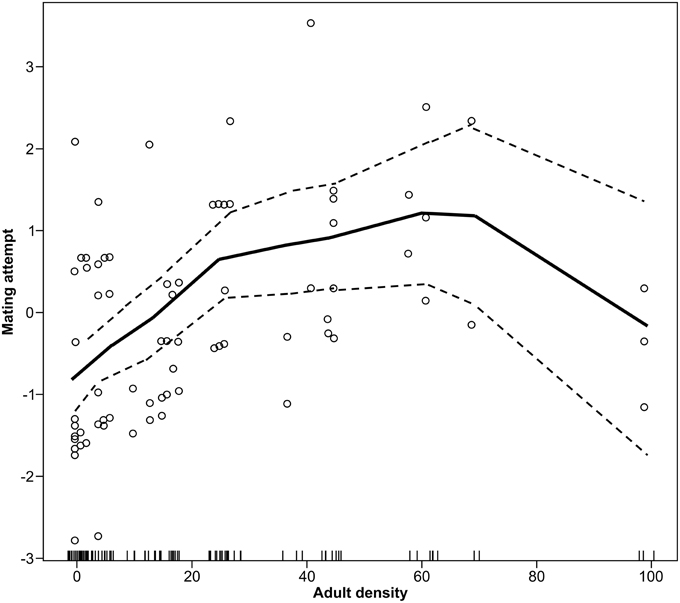
Figure 2. Non-linear effects of moth density on mating attempts by male forest tent caterpillar moths shown using a non-parametric spline fit GAM (P = 0.02). Plot is the fitted response to density with the residuals from the full model added. The Y-axis is the added effect of density on mate attraction, with the mean rate of attempts as a logit of the proportion successfully attracted centered on zero.
Field Cage Studies
Experiments 1 and 2 (Figure 3) both showed that the presence of calling females evenly distributed in the field cages negatively impacted the ability of male forest tent caterpillars to find a mate, as indicated by trap capture in the female-baited sentinel traps. There was a significant reduction in the number of males captured in the sentinel trap in the cages treated with 35 (χ21, = 3.85, P = 0.0496) and 50 (χ21 = 6.93, P = 0.0085) calling females as compared to the clean-air control cages in both experiments (Figures 3A,B). Further, there appears to be an effect of density on this interference as some males were captured in the trap located in the treated cage in Experiment 1 when there were 35 females in the background and no males were captured in the treated cage in Experiment 2 when there were 50 females in the background (Figure 3B). In Experiment 3, in which the density of male moths was varied to alter the moth sex ratio in the cage, there was no effect of male moth density on the number of released males captured in female-baited sentinel traps (χ22 = 2.53, P = 0.2821). Interestingly, this experiment showed no effect of the presence of calling females in the background on male orientation to sentinel females at a density of 18 females per cage.
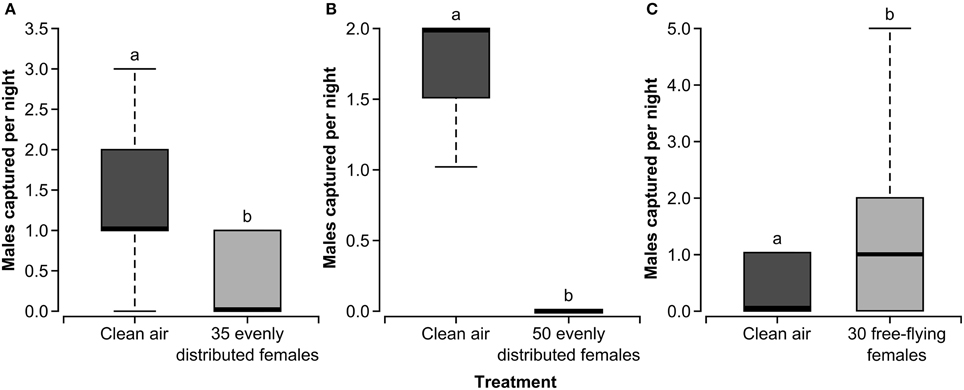
Figure 3. Box and whisker plots of male forest tent caterpillar moths captured per night in a sentinel trap baited with two calling females in field cages with clean air or 35 (A) or 50 (B) calling females evenly distributed in mesh bags throughout the cage or 30 free-flying females (C). The first and third quartiles are represented by the top and bottom of the box, respectively. The midline indicates the median and the vertical lines extending from the box (whiskers) represent the maximum and minimum values. Data are based on the release of 30 (A,C) and fifty (B) males in each cage for each replicate. Within a panel, bars with different letters are significantly different (GLM, P < 0.05).
In Experiment 4, both the effect of increased pheromone and the effect of mating with females on subsequent mate finding were factors as females were free flying in the treated cage. Females were marked with fluorescent powder to track the number of males that mated (transferred powder to males). Interestingly, in the cage that contained free-flying females, more males were captured in sentinel female-baited traps than in the control cage without free-flying females. (Figure 3C) (χ21 = 3.85, P = 0.0496). Approximately 41% of males collected from the treated cage at the end of each replicate in Experiment 4 had fluorescent powder indicating abdominal contact with females. Some males captured in the sentinel trap were marked indicating that males can mate and pursue another female in the same night.
There was no effect of moth size on the proportion of released males that were captured in sentinel female-baited traps in Experiment 5 (χ21 = 1.50, P = 0.2212).
Mating Cage Studies
There was a significant effect of nutrient regime on the time to pupation for both male (χ22 = 35.05 P < 0.0001) and female χ22 = 45.95, P < 0.0001) forest tent caterpillars. Development time was fastest for well-fed insects, intermediate for medium-fed insects and slowest for starved insects (Figure 4). Pupal weight was also affected by nutrient regime presented to the larvae for both males (χ22, = 22.06, P < 0.0001) and females (χ22 = 31.03, P < 0.0001). As would be expected, medium-fed larvae resulted in pupae with a mass that was intermediate between the heavy pupae from well-fed larvae and the light pupae from starved larvae (Figure 4). There was no effect of the condition of adult moths generated from the different nutrient regimes on the number of matings acquired by males (χ22 = 3.81, P = 0.1489) or females (χ22 = 4.45, P = 0.1083).
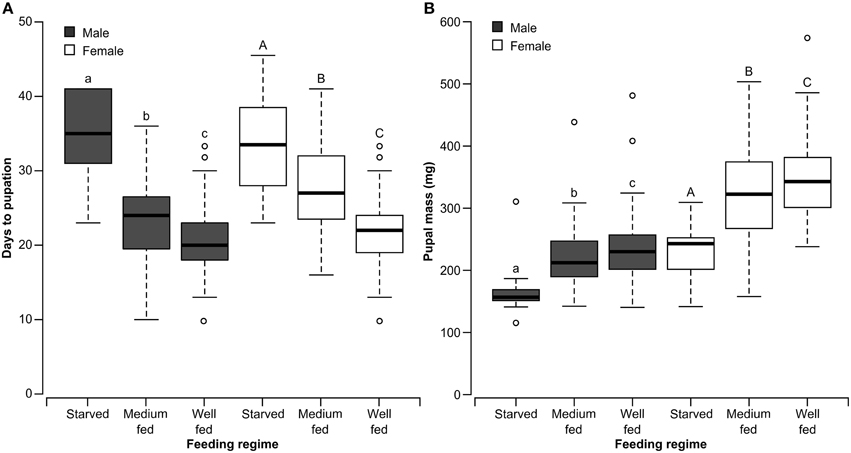
Figure 4. Box and whisker plots of the days to pupation (A) and pupal mass (B) of male and female forest tent caterpillars reared on different quantities of artificial diet. The first and third quartiles are represented by the top and bottom of the box, respectively. The midline indicates the median and the vertical lines extending from the box (whiskers) represent the maximum and minimum values. Within each panel and sex, bars with different letters are significantly different (Kruskal-Wallis Test, P < 0.05).
Discussion
The results of this study show that density of forest tent caterpillar populations influences the capacity of male moths to orient to the pheromonal cues released by females. In general, female forest tent caterpillars were more successful at attracting a mate in aspen stands with high than low population densities. These data support the presence of a mate-finding Allee effect (Gascoigne et al., 2009) similar to that which has been illustrated in other forest defoliators that undergo cyclical changes in population density (Contarini et al., 2009; Régnière et al., 2013). In gypsy moth populations in Virginia, West Virginia (Sharov et al., 1995) and Wisconsin (Tcheslavskaia et al., 2002; Contarini et al., 2009), the probability of females mating increases exponentially with male moth density, as measured by moth capture in pheromone-baited traps. The relationship between mating success of female spruce budworm moths and population density is non-linear and mating levels plateau before reaching 100%, even at the highest population densities examined (Régnière et al., 2013). In the current study, non-linear models reveal that, for the forest tent caterpillar, an intermediate density promotes the highest levels of orientation to pheromone and subsequent mate finding. Similarly, trap capture of male forest tent caterpillar moths in synthetic pheromone-baited traps is highest at intermediate population densities (Jones et al., 2009). This suggests that competition for the chemical communication channel exists in dense populations of forest tent caterpillars and interferes with pheromone orientation and mate location.
Competition for pheromone sources is implicated in the reduced efficiency of pheromone-baited traps at high population densities in some forest defoliators (Delisle et al., 1998) but not in others (Östrand et al., 2007). Delisle et al. (1998) directly compare the efficiency of pheromone-baited vs. light traps at different densities of the eastern hemlock looper, Lambdina fiscellaria (Lepidoptera: Geometridae). At low population densities, pheromone-baited traps capture more moths than light traps, whereas, light traps are more efficient than pheromone traps at high population densities (Delisle et al., 1998). Although several studies suggest that competition for natural pheromone sources can reduce the efficacy of pheromone-baited traps at high population densities (Campbell et al., 1992; Delisle et al., 1998), few studies directly manipulate moth density to test this assumption. In the current study, we manipulate female and male moth density in field cages and illustrate that increased female density results in higher competition for pheromone sources and reduced orientation capacity of males to sentinel females. This can be considered as a natural mating disruption effect as orientation interference occurs when females are evenly distributed throughout the field cage space and is greater at high than low female densities. Recent application of theoretical research (Miller et al., 2006a) to empirical studies of mating disruption of moths using synthetic pheromone in various managed ecosystems (Miller et al., 2006b) shows that competition for pheromone sources is the main mechanism by which mating disruption interferes with male moth orientation to females in pheromone-treated crops. This is true for pheromone-based disruption of mating of the gypsy moth in which interference of mate location increases with the number of synthetic pheromone dispensers positioned in the study area (Tcheslavskaia et al., 2005). In our study, competitive attraction among female-produced pheromone sources appears to be driving the disruption of orientation of male forest tent caterpillar moths to sentinel females because alteration of male density in the field cages does not affect the number of male moths captured. These data provide a mechanistic understanding for the lower capture of male forest tent caterpillar moths in synthetic pheromone-baited traps at high compared to low population densities (Jones et al., 2009).
Interestingly, competition for pheromone sources at high female densities interfered with male forest tent caterpillar moth orientation to sentinel pheromone sources only when the females were evenly distributed throughout the field cages. When the females were flying freely within the cage, their presence appeared to stimulate rather than interfere with male orientation capacity to the sentinel female. Females released into the cage tended to be clumped in distribution as they alighted on the mesh covering of the cage surface. It is likely that natural mating disruption by competitive attraction does not occur when females are clumped in distribution because much of the caged area would contain clean air and allow for males to orient to the distinct pheromone signal of the sentinel females. Enhanced orientation to the sentinel female in cages containing free-flying females as compared to the control cages without females could be explained by increased activity or sensitivity of males as a result of stimulation by female pheromone. In a few instances, exposure to pheromone appears to sensitize male moths so that subsequent response to pheromone is enhanced (Anderson et al., 2003, 2007; Stelinski et al., 2004). Further research is required to measure the neurophysiological response of male forest tent caterpillars to pheromone exposure to determine if it enhances subsequent upwind orientation to pheromonal cues.
Although moth condition is known to influence mate finding behavior in other forest defoliators (Tammaru et al., 1996), we could find no evidence that moth size or mating status influence subsequent mate finding behavior in the forest tent caterpillar. Some of the male moths captured in female-baited traps in the field cage were marked with fluorescent powder indicating that they had come into contact and potentially mated with the free-flying females in the cage prior to orientation to the calling sentinel female. Male forest tent caterpillar moths are known to mate multiple times (Stehr and Cook, 1968) but this study provides evidence that males can mate more than once in a single scotophase. Mating duration varies with population density in forest tent caterpillar moths with copulations lasting an average of 85 and 187 min at low and high densities, respectively (Bieman, 1980). It is more likely that males would pursue a second mate within the same scotophase at low than high population density as most matings are restricted to a three and a half hour period commencing 2 h before sunset in the northern parts of its range (Shepherd, 1979; Bieman, 1980). This finding suggests that mated male forest tent caterpillar moths are physiologically capable of orientation to pheromone and reduction of male moth trap capture at high population densities (Jones et al., 2009) is not likely due to differential attraction of virgin male moths to pheromone sources.
The quality of nutrition obtained by forest tent caterpillar larvae is known to affect larval development (Jones and Despland, 2006; Noseworthy and Despland, 2006) and the traits of adult moths (Colasurdo et al., 2009). Both rapid- and delayed-induced responses to forest tent caterpillar feeding occur in aspen that change the quality of the food source and negatively affect pupal mass and subsequent fecundity (Parry et al., 2003). Less is known about the effect of nutrient quantity on the development and mating behavior of forest tent caterpillars, despite the implication of larval competition for food in the collapse of population outbreaks (Witter et al., 1972; Witter, 1979; Smith and Goyer, 1986). In the current study, the quantity of food provided to larvae was manipulated and well-fed larvae developed the fastest and had the highest pupal weights. There was, however, no effect of male moth body size on the proportion of released moths captured in sentinel female-baited traps. Flight capacity of male forest tent caterpillar moths is not influenced directly by wing loading but is limited by energy use (Evenden et al., 2015). As the field cage limited the dispersal distance of released moths in the current study, energy use is likely not a factor in male moth orientation to females within the cage. Under field conditions, copulation is more likely to occur between male and female forest tent caterpillar moths of similar size (Miller, 2006) which suggests that short range mate acceptance behavior occurs in this species. In the current study, individual pairings of moths of varying quality did not reveal an effect of moth quality on mating success. A future bioassay in which moths of different quality have a choice of mates might provide further insight into the occurrence of size-based assortative mating in this species.
Conclusions
The cyclical changes in population density of the forest tent caterpillar make it a good system to examine the impact of moth density on mate location using pheromones. The work has further implications to understanding the population dynamics of this species and the use of pheromone-baited traps to accurately monitor populations. Females positioned in traps in the field were more likely to attract male moths at sites with increasing moth density. This finding suggests that there is a mate-finding Allee effect that may limit population establishment at low population densities, as has been reported in other forest defoliators (Contarini et al., 2009; Régnière et al., 2013). Interestingly, mate attraction was greatest at intermediate population densities as the capacity of male moths to orient to females declined at sites with the highest densities. This is similar to the curvilinear relationship reported for male forest tent caterpillar moths captured in synthetic pheromone-baited traps at sites with different population densities (Jones et al., 2009). This suggests that population estimates from pheromone-baited traps are less reliable at high population densities and monitoring efforts should be adjusted to incorporate approaches that are less affected by density such as the use of light traps (Delisle et al., 1998). Closer examination of male orientation to females in field cages in which female density was manipulated revealed that the most likely mechanism for reduced mate finding at high densities was mating disruption by competitive attraction to many natural pheromone sources. Fewer male moths were attracted to sentinel females in cages with females evenly distributed throughout the field cage. Male moths spend time and energy orienting to female-produced plumes and are less likely to orient to sentinel females. This study also provides preliminary evidence for a stimulatory effect of pheromone exposure on male moth mate searching, as males in cages with free-flying females were more likely to orient to sentinel females than in cages without the presence of females. There was no evidence from our studies that lower moth condition as a result of nutrient stress from larval competition influenced mate finding or close range mating behaviors. Further experimentation is necessary to assess any potential effect of moth condition on long and short distance mating behaviors under varying population densities and field conditions.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jeremiah Bolstad for assistance with field work. This work was supported by funds from the Spray Efficacy Research Group (SERG) International to ME and the National Science and Engineering Research Council (NSERC) to ME and JR.
References
Addy, N. D. (1969). Rearing the forest tent caterpillar on an artificial diet. J. Econ. Entomol. 62, 270–271. doi: 10.1093/jee/62.1.270
Anderson, P., Hansson, B. S., Nilsson, U., Han, Q., Sjoholm, M., Skals, N., et al. (2007). Increased behavioral and neuronal sensititivity to sex pheromone after brief odor experience in a moth. Chem. Senses 32, 483–491. doi: 10.1093/chemse/bjm017
Anderson, P., Sadek, M. M., and Hansson, B. S. (2003). Pre-exposure modulates attraction to sex pheromone in a moth. Chem. Senses 28, 285–291. doi: 10.1093/chemse/28.4.285
Asaro, C., and Berisford, C. W. (2001). Predicting infestation levels of the nantucket pine tip moth (Lepidoptera: Tortricidae) using pheromone traps. Environ. Entomol. 30, 776–784. doi: 10.1603/0046-225X-30.4.776
Barrozo, R. B., Gadenne, C., and Anton, S. (2010). Switching attraction to inhibition: mating induced reversed role of sex pheromone in an insect. J. Exp. Biol. 213, 2933–2939. doi: 10.1242/jeb.043430
Batzer, H. O., Martin, M. P., Mattson, W. J., and Miller, W. E. (1995). The forest tent caterpillar in aspen stands: distribution and density estimation of four life stages in four vegetation strata. For. Sci. 41, 99–121.
Beroza, M., Hood, C. S., Trefrey, D., Leonard, D. E., Knipling, E. F., Klassen, W., et al. (1974). Large field trial with microencapsulated sex pheromone to prevent mating of the gypsy moth. J. Econ. Entomol. 67, 659–664. doi: 10.1093/jee/67.5.659
Bieman, D. N. (1980). An Evolutionary Study of Mating of Malacosoma Americanum (Fabricius) and Malacosoma Disstria Hübner (Lepidoptera: Lasiocampidae). M.S. thesis, University of Michigan, Ann Arbor.
Bieman, D. N. (1982). Mating wounds in Malacosoma: an insight into bed bug mating behaviour. Florida Entomol. 65, 377–378.
Bieman, D. N., and Witter, J. A. (1983). Mating behaviour of Malacosoma disstria at two levels of mate competition. Florida Entomol. 66, 272–279.
Brandt, J. P., Cerezke, H. F., Mallett, K. I., Volney, W. J. A., and Weber, J. D. (2003). Factors affecting trembling aspen (Populus tremuloides Michx.) health in the boreal forest of Alberta, Saskatchewan, and Manitoba, Canada. For. Ecol. Manage. 178, 287–300. doi: 10.1016/S0378-1127(02)00479-6
Campbell, C. D., Walgenbach, J. F., and Kennedy, G. G. (1992). Comparison of black light and pheromone traps for monitoring Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in tomato. J. Agri. Entomol. 9, 17–24.
Candau, J.-N., Abt, V., and Keatley, L. (2002). Bioclimatic Analysis of Declining Aspen Stands in Northeastern Ontario. Forest Research Report, No. 154. Ontario Forest Research Institute, Sault Ste. Marie, Ontario.
Churchill, G. B., John, H. H., Duncan, D. P., and Hodson, A. C. (1964). Long-term effects of defoliation of aspen by the forest tent caterpillar. Ecology 45, 630–633.
Colasurdo, N., Gélinas, Y., and Despland, E. (2009). Larval nutrition affects life history traits in a capital breeding moth. J. Exp. Biol. 212, 1794–1800. doi: 10.1242/jeb.027417
Contarini, M., Onufrieva, K. S., Thorpe, K. W., Raffa, K. F., and Tobin, P. C. (2009). Mate-finding failure as an important cause of Allee effects along the leading edge of an invading insect population. Entomol. Exp. Appl. 133, 307–314. doi: 10.1111/j.15707458.2009.00930x
Daniels, C. J., and Myers, J. H. (1995). Climate and outbreaks of the forest tent caterpillar. Ecography 18, 353–362.
Delisle, J., West, R. J., and Bowers, W. W. (1998). The relative performance of pheromone and light traps in monitoring the seasonal activity of the eastern hemlock looper, Lambdina fiscellaria fiscellaria. Entomol. Exp. Appl. 89, 87–98. doi: 10.1046/j.1570-7458.1998.00385.x
Elkinton, J. S., and Cardé, R. T. (1980). Distribution, dispersal and apparent survival of male gypsy moths as determined by capture in pheromone-baited traps. Environ. Entomol. 9, 729–737. doi: 10.1093/ee/9.6.729
Elliott, C. G., and Evenden, M. L. (2009). Factors influencing flight potential of Choristoneura conflictana. Physiol. Entomol. 34, 71–78. doi: 10.1111/j.1365-3032.2008.00654.x
Evenden, M. L., Borden, J. H., and van Sickle, G. A. (1995). Predictive capabilities of a pheromone-based monitoring system for western hemlock looper (Lepidoptera: Geometridae). Environ. Entomol. 24, 933–943. doi: 10.1093/ee/24.4.933
Evenden, M. L., Whitehouse, C. M., and Jones, B. C. (2015). Resource allocation to flight in an outbreaking forest defoliator, the forest tent caterpillar, Malacosoma disstria. Environ. Entomol. 44, 835–845. doi: 10.1093/ee/nvv055
Gascoigne, J., Berec, L., Gregory, S., and Courchamp, F. (2009). Dangerously few liaisons: a review of mate-finding Allee effects. Pop. Ecol. 51, 355–372. doi: 10.1007/s10144-009-0146-4
Grisdale, D. G. (1975). A simple method for removing pupae from cooons: Malacosoma disstria and Orgyia spp. Can. For. Ser. Bi-mon. Res. Notes 31, 9.
Hastie, T. J., and Tibshirani, R. J. (1990). Generalized Additive Models. New York, NY: Chapman and Hall.
Hildahl, V., and Reeks, W. A. (1960). Outbreaks of the forest tent caterpillar, Malacosoma disstria Hbn., and their effects on stands of trembling aspen in Manitoba and Saskatchewan. Can. Entomol. 92, 199–209. doi: 10.4039/Ent92199-3
Hodson, A. C. (1941). An ecological study of the forest tent caterpillar, Malacosoma disstria Hbn., in northern Minnesota. Univ. Minn. Agric. Exp. Stn. Bull. 148, 1–55.
Hogg, E. H., Brandt, J. P., and Kochtubajda, B. (2002). Growth and dieback of aspen forests in northwestern Alberta, Canada, in relation to climate and insects. Can. J. For. Res. 32, 23–832.
Jones, B. C., and Despland, E. (2006). Effects of synchronization with host plant phenology occur early in the larval development of a spring folivore. Can. J. Zool. 84, 628–633. doi: 10.1139/Z06-025
Jones, B. C., and Evenden, M. L. (2008). Ecological applications of pheromone trapping for Malacosoma disstria and Choristoneura conflictana. Can. Entomol. 140, 573–581. doi: 10.4039/n08-013
Jones, B. C., Roland, J., and Evenden, M. L. (2009). Development of a combined sex pheromone based monitoring system for Malacosoma disstria (Lepidoptera: Lasiocampidae) and Choristoneura conflictana (Lepidoptera: Tortricidae). Environ. Entomol. 38, 459–447. doi: 10.1603/022.038.0220
McCullagh, P., and Nelder, J. A. (1989). Generalized Linear Models. 2nd Edn. New York, NY: Chapman and Hall.
McNeil, J. N. (1991). Behavioral ecology of pheromone-mediated communication in moths and its importance in the use of pheromone traps. Annu. Rev. Entomol. 36, 407–430.
Miller, J. R., Gut, L. J., de Lamé, F. M., and Stelinski, L. L. (2006a). Differentiation of competitive vs. non-competitive mechanisms mediating disruption of moth sexual communication by point sources of sex pheromone: (Part 1) Theory. J. Chem. Ecol. 32, 2089–2114. doi: 10.1007/s10886-006-9134-8
Miller, J. R., Gut, L. J., de Lamé, F. M., and Stelinski, L. L. (2006b). Differentiation of competitive vs. non-competitive mechanisms mediating disruption of moth sexual communication by point sources of sex pheromone: (Part 2) Case studies. J. Chem. Ecol. 32, 2115–2143. doi: 10.1007/s10886-006-9136-6
Miller, W. E. (2006). Forest tent caterpillar: mating, oviposition, and adult congregation at town lights during a northern Minnesota outbreak. J. Lepid. Soc. 30, 156–160.
Mori, B. A., Yoder, C., Otani, J., and Evenden, M. L. (2014). Relationships among male Coleophora deauratella (Lepidoptera: Coleophoridae) pheromone-baited trap capture, larval abundance, damage and flight phenology. Agric. For. Entomol. 16, 207–215. doi: 10.1111/afe.12050
Noseworthy, M. K., and Despland, E. (2006). How do primary nutrients affect the performance and preference of forest tent caterpillars on trembling aspen? Can. Entomol. 138, 367–375. doi: 10.4039/n05-076
Östrand, F., Elek, J. A., and Steinbauer, M. J. (2007). Monitoring autumn gum moth (Mnesampela privata): relationships between pheromone and light trap catches and oviposition in eucalypt plantations. Aust. For. 70, 185–191. doi: 10.1080/00049158.2007.10675019
Palaniswamy, P., Chislhom, M. D., Underhill, E. W., Reed, D. W., and Peesker, S. J. (1983). Disruption of forest tent caterpillar (Lepidoptera: Lasiocampidae) orientation to baited traps in aspen groves by air permeation with (5Z, 7E)-5,7-dodecadienal. J. Econ. Entomol. 76, 1159–1163. doi: 10.1093/jee/76.5.1159
Parry, D., Herms, D. A., and Mattson, W. J. (2003). Responses of an insect folivore and its parasitoids to multiyear experimental defoliation of aspen. Ecology 84, 1768–1783. doi: 10.1890/0012-9658(2003)084[1768:ROAIFA]2.0.CO;2
Raina, A. K., Kingan, T. G., and Giebultowicz, J. M. (1994). Mating-induced loss of sex pheromone and sexual receptivity in insects with emphasis on Helicoverpa zea and Lymantria dispar. Arch. Insect. Biochem. Physiol. 25, 317–327. doi: 10.1002/arch.940250407
Ramaswamy, S. B., Qiu, Y., and Park, Y. I. (1996). Neuronal control of post-coital pheromone production in the moth Heliothis virescens. J. Exp. Zool. 274, 255–268. doi: 10.1002/(SICI)1097-010X(19960301)274:4<255::AID_JEZ6>3.0.CO;2-O
R Core Development Team. (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Régnière, J., Delisle, J., Pureswaran, D. S., and Trudel, R. (2013). Mate-finding allee effect in spruce budworm population dynamics. Entomol. Exp. Appl. 146, 112–122. doi: 10.1111/eea.12019
Ruohomäki, K. (1992). Wing size variation in Epirrita autumnata (Lep., Geometridae) in relation to larval density. Oikos 63, 260–266.
Sanders, C. J. (1983). Local dispersal of male spruce budworm (Lepidoptera: Torticidae) moths determined by mark, release, and recapture. Can. Entomol. 115, 1065–1070. doi: 10.4039/Ent1151065-9
Sanders, C. J. (1988). Monitoring spruce budworm population density with sex pheromone traps. Can. Entomol. 120, 175–184. doi: 10.4039/Ent120175-2
Schmidt, B. C., Roland, J., and Wakarchuk, D. (2003). Evaluation of synthetic pheromones for monitoring forest tent caterpillar (Lepidoptera: Lasiocampidae) populations. Environ. Entomol. 32, 214–219. doi: 10.1603/0046-225X-32.1.214
Sharov, A. A., Liebhold, A. M., and Ravlin, F. W. (1995). Prediction of gypsy moth (Lepidoptera: Lymantriidae) mating success from pheromone trap counts. Environ. Entomol. 24, 1239–1244. doi: 10.1093/ee/24.5.1239
Shepherd, R. F. (1979). Comparison of the daily cycle of adult behaviour of five forest lepidoptera from Western Canada, and their response to pheromone traps. Mitt. Sch. Entomol. Ges. 52, 157–168.
Shepherd, R. F., Gray, T. G., Chorney, R. J., and Daterman, G. E. (1985). Pest management of Douglas-fir tussock moth, Orgyia pseudotsugata (Lepidoptera: Lymantriidae): monitoring endemic populations with pheromone traps to detect incipient outbreaks. Can. Entomol. 117, 839–848. doi: 10.4039/Ent117839-7
Smith, J. D., and Goyer, R. A. (1986). Population fluctuations and causes of mortality for the forest tent caterpillar, Malacosoma disstria (Lepidoptera: Lasiocampidae), on three different sites in southern Louisiana. Environ. Entomol. 15, 1184–1188. doi: 10.1093/ee/15.6.1184
Stehr, F. W., and Cook, E. F. (1968). A Revision of the Genus Malacosoma Hübner in North America (Lepidoptera: Lasiocampidae): Systematics, Biology, Immatures, and Parasites. U.S. National Museum Bulletin 276. Washington, DC: Smithsonian Institution Press.
Stelinski, L. L., Gut, L. J., Vogel, K. J., and Miller, J. R. (2004). Behaviors of naive vs. pheromone-exposed leafroller moths in plumes from high-dosage pheromone dispensers in a sustained-flight wind tunnel: implications for mating disruption of these species. J. Insect Behav. 17, 533–554. doi: 10.1023/B:JOIR.0000042540.09188.eb
Struble, D. L. (1970). A sex pheromone in the forest tent caterpillar. J. Econ. Entomol. 63, 295–296. doi: 10.1093/jee/63.1.295
Sweeney, J. D., McLean, J. A., and Shepherd, R. F. (1990). Factors affecting catch in pheromone traps for monitoring the western spruce budworm Choristoneura occidentalis Freeman. Can. Entomol. 122, 1119–1130. doi: 10.4039/Ent1221119-11
Tammaru, T., Ruohomäki, K., and Saikkonen, K. (1996). Components of male fitness in relation to body size in Epirrita autumnata (Lepidoptera, Geometridae). Ecol. Entomol. 21, 185–192.
Tcheslavskaia, K., Brewster, C. C., and Sharov, A. A. (2002). Mating success of gypsy moth (Lepidoptera: Lymantriidae) females in southern Wisconsin. Great Lakes Entomol. 35, 1–7.
Tcheslavskaia, K. S., Thorpe, K. W., Brewster, C. C., Sharov, A. A., Leonard, D. S., Reardon, R. C., et al. (2005). Optimization of pheromone dosage for gypsy moth mating disruption. Entomol. Exp. Appl. 115, 355–361. doi: 10.1111/j.1570-7458.2005.00266.x
Thorpe, K. W., Ridgway, R. L., and Leonhardt, B. A. (1993). Relationship between gypsy moth (Lepidoptera: Lymantriidae) pheromone trap catch and population density: comparison of traps baited with 1 and 500 μg (+)-Disparlure lures. J. Econ. Entomol. 86, 86–92. doi: 10.1093/jee/89.1.86
Unnithan, G. C., and Saxena, K. N. (1991). Pheromonal trapping of Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae) moths in relation to male population density and competition with females. Appl. Entomol. Zool. 26, 17–28.
Witter, J. A. (1979). The forest tent caterpillar (Lepidoptera: Lasiocampidae) in Minnesota: a case history review. Great Lakes Entomol. 12, 191–197.
Witter, J. A., Kulman, H. M., and Hodson, A. C. (1972). Life tables for the forest tent caterpillar. Ann. Entomol. Soc. Am. 65, 25–31. doi: 10.1093/aesa/65.1.25
Keywords: population dynamics, pheromone monitoring, forest defoliators, Allee effect, traps, mate-finding
Citation: Evenden ML, Mori BA, Sjostrom KD and Roland J (2015) Forest tent caterpillar, Malacosoma disstria (Lepidoptera: Lasiocampidae), mate-finding behavior is greatest at intermediate population densities: implications for interpretation of moth capture in pheromone-baited traps. Front. Ecol. Evol. 3:78. doi: 10.3389/fevo.2015.00078
Received: 28 April 2015; Accepted: 07 July 2015;
Published: 22 July 2015.
Edited by:
Lukasz Lech Stelinski, University of Florida, USAReviewed by:
Caroline Marie Nieberding, Université catholique de Louvain, BelgiumMartin James Steinbauer, La Trobe University, Australia
Copyright © 2015 Evenden, Mori, Sjostrom and Roland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maya L. Evenden, Department of Biological Sciences, University of Alberta, CW405, Biological Sciences Building, Edmonton, AB T6G 2E9, Canada, mevenden@ualberta.ca
 Maya L. Evenden
Maya L. Evenden Boyd A. Mori
Boyd A. Mori K. Dana Sjostrom
K. Dana Sjostrom  Jens Roland
Jens Roland