Female song rates in response to simulated intruder are positively related to reproductive success
- Evolution, Ecology and Genetics, Research School of Biology, The Australian National University, Canberra, ACT, Australia
Bird song is well studied in males as a sexually selected behavior. However, although song is also common among females, it is infrequently examined and poorly understood. Research suggests that song is often used as a resource defense behavior and is important in female-female competition for limited resources, e.g., mates and territories. If so, song should be positively related to fitness and related to other resource defense behaviors, but this possibility has rarely been explored. Here we examine fitness estimates in relation to spontaneous song rates and song rates in response to a simulated intruder (playback), in the superb fairy-wren (Malurus cyaneus), a cooperatively breeding songbird. We also determine how song rates relate to other territorial defense behaviors. Song rate in response to playback, but not spontaneous song rate, was positively related to nest success and the number of fledglings produced by successful females. Further, response song rate was also correlated with other territorial defense behaviors (latency to respond and flights). This evidence supports the hypothesis that female song may be used in the context of female-female competition to improve access to limited reproductive resources, and suggests that song may provide direct fitness benefits.
Introduction
Territorial song is a classic example of a sexually selected trait in males, and usually functions in the contexts of male-male competition and female mate choice (Catchpole and Slater, 1995). However, females may also be territorial, and recent comparative work has shown that female song is both phylogenetically widespread and ancestral, suggesting that song may also serve critical functions in females (Price, 2009; Odom et al., 2014). Researchers have argued that female song may be an important signal used by females in intrasexual competition when critical resources are limited (e.g., social partners or territories; Langmore, 1998; Price et al., 2009; Odom et al., 2014). If so, variation in the expression of song should be positively related to resource acquisition and fitness. Further, if song is used in competitive scenarios, it should be positively associated with other defense behaviors, e.g., attacks. However, there are scant data addressing these possibilities (Eens and Pinxten, 1998; Langmore, 1998; Illes and Yunes-Jimenez, 2009; Illes, 2014).
Here, we begin to address these questions by examining the relationships between female fitness, song, and other territorial defense behaviors in the superb fairy-wren (Malurus cyaneus). Superb fairy-wrens are socially monogamous, though >70% of young are sired by extra-group males in our study population (Dunn et al., 2001). They also are cooperative breeders: sons from previous broods may remain on the natal territory as subordinate helpers (Cockburn et al., 2003). However, daughters disperse and must acquire and defend a territory or die; there are no floater females (Cockburn et al., 2008). Multiple lines of evidence suggest that female fairy-wrens appear to use song to defend breeding resources. Females sing year-round, and at rates similar to males (excluding the dawn chorus; Cooney and Cockburn, 1995). Female song rates peak during the transition from winter to pre-breeding, during initial territory establishment (Cooney and Cockburn, 1995), and song rates increase slightly across date within the breeding season (Cain and Langmore, 2015). Further, females, but not males, increase song rates in response to a simulated female intruder, i.e., playback (Cain and Langmore, 2015).
To test the hypothesis that song rate is important in territory defense and has important consequences for female fitness we examined the relationship between reproductive success and song in two contexts: spontaneous song rates and song in response to a simulated female intruder (playback). We also examined how song rates relate to other territorial defense behaviors (flights and latency to respond). We predicted that if female song rate is important for acquiring and maintaining a quality breeding territory or other reproductive resources, then reproductive success would be positively related to song rates. Further, if female song functions in territorial defense, we predicted that song rates in response to playback would be positively related to other response behaviors.
Methods
Study System
This study was conducted in the Australian National Botanic Gardens (BG), ACT, Australia (35°16′S, 149°06′E). All adults were uniquely color-banded, and group composition and all nesting attempts were monitored from 27 August to 2 March. The first egg was laid on 16 September and the last brood fledged on 5 February (maximum of three successful broods per season). Previous research found age differences in success between first-year females and older females (Cockburn et al., 2008), thus we classified females as young (first year only, n = 8) or old (beyond first year, n = 21) using previous banding records. The same study found that high quality territories accumulate more subordinates, and thus are more likely to be occupied by group breeding females, so we coded females as group or pair-breeding.
Understanding the selective advantages of behavior requires the use of quality fitness estimates, and the benefits of female competition are often poorly captured by immediate fitness proxies, e.g., number of eggs (Clutton-Brock, 2009; Tobias et al., 2012; Cain and Rosvall, 2014). We use three fitness proxies that integrate longer time periods and better estimates of realized fitness. First, nest success is a critical selective period for passerines and is perhaps the most important fitness component for breeding females (Martin, 1995, 2015). Females were categorized as successful if they fledged at least one offspring (n = 19 successful, 10 unsuccessful). The number of fledglings may provide nuanced information about relative fitness; therefore we also examined total annual fledgling production. Finally, because predation can also be high just after fledging, we also examine relationships with number of independent young, previously defined as 4 weeks post fledgling (Cockburn et al., 2008).
Song Rates and Territorial Behaviors
We quantified female song rates using a standardized behavioral paradigm that allowed us to determine both spontaneous song rates, and song rates in response to a simulated intruder (Cain and Langmore, 2015). Spontaneous song rates (songs per min) were quantified by passively observing focal females for 10 min and counting songs produced. After the observation period we simulated a foreign female intruding on the resident female's territory using playback of unfamiliar female song and quantified female response: number of songs, flights greater than 1 m, and latency to respond with a flight or song. We constructed 18 unique stimuli from female fairy-wrens at least 5 km away. Each playback consisted of two unique songs taken from the same female repeated six times in alternate order (for more details see Cain and Langmore, 2015). All focal females were paired, but did not yet have eggs in the nest. Territorial behavior and pairing begins in August (Cockburn et al., 2009). Trials occurred between 15 September and 15 November 2012, between 7:00 and 13:00. The Australian National University Animal Experimental Ethics Committee (protocol A2012/54) and the Australian Capital Territory Municipal Services (license LT 2012559) approved all procedures.
Statistical Analysis
To test how spontaneous and response song rates relate to other territorial defense behaviors, we determined the correlation coefficients between pre-trial song-rate, response song rate, number of flights, closest approach, and latency to respond. Because superb fairy-wren song rates are related to date (Cain and Langmore, 2015), we regressed both measures of song rate on date to calculate date-adjusted song rates for further analysis, regressions for spontaneous, and response song rates were done separately. To examine the relationship between fitness estimates and female response, we built three generalized linear models, using different fitness estimates as the dependent variable and spontaneous song rates (date adjusted), response song rates (date adjusted), age, and age by song rate interactions as potential predictors variables. The first model examined the relationships between nest success (binomial, successful, or failed); the second and third models focused on the subset of females that produced at least one successful nest, and examined the relationships between number of fledglings and the number of independent young (normal error distributions). We used stepwise backward procedures to remove non-significant variables (P > 0.1 to remove), starting with interactions.
Results
Song and Territorial Behavior
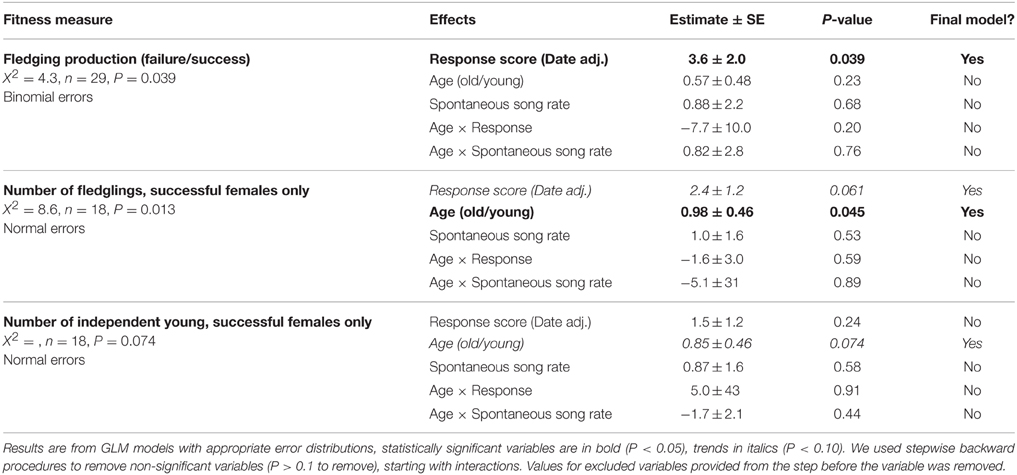
Behaviors in response to playback of novel female song (songs, flights, latency to response) were significantly inter-correlated; i.e., birds that responded strongly in one measure tended to respond strongly in other measures (Figure 1, Table 1). Closest approach was unrelated to song rates, but negatively related to the number of flights (birds that approached closer also had more dives), however the relationship was a trend. Pre-trial song rates were unrelated to any response variables during the playback trial period (all P < 0.15).
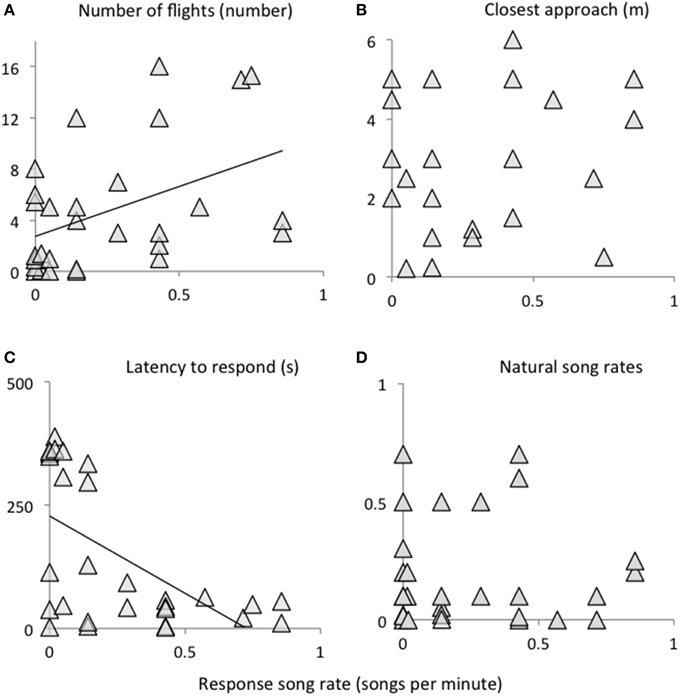
Figure 1. Scatterplots illustrating the relationships between response song rate and defense behaviors: (A) number of flights, (B) closest approach, (C) latency to respond, and (D) natural song rate.
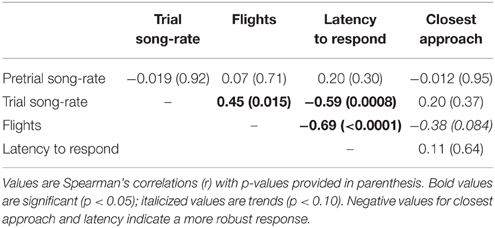
Table 1. Correlation matrix for pre-trial song rates, and behaviors in response to simulated intrusion using female playback.
Song Rates and Reproductive Success
Individuals with higher response song rates were more likely to have at least one successful nesting attempt (Figure 2, Table 2). Among successful females, the total number of fledglings produced across the breeding season was also positively related to response song rate, although it was not statistically significant (Figure 3, Table 2). Spontaneous song rates were unrelated to any fitness estimate (all P > 0.15).
Figure 2. Relationship between female response song rate and nest success. Response song rates were plotted according to whether the female was successful (produced at least one fledgling) or failed in all attempts; overlapping points are jittered for clarity. Line is a logistic regression estimating the probability that a female with a given response score would be successful.
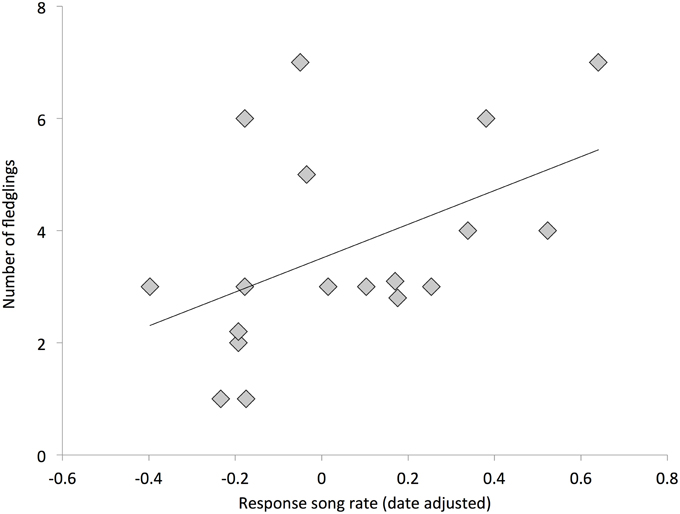
Figure 3. Relationship between female response song rates and total fledglings produced (among successful females); overlapping points are jittered for clarity. Line is from a linear regression.
Discussion
Our results show that female song is a common element of territorial response to a simulated intruder (playback) and reveal a positive relationship between territorial song rates (response) and two critical components of fitness, nest success, and number of fledglings. However, among successful females, individual differences in response song rates were unrelated to the number of young that survived 4 weeks after fledging, and spontaneous song-rates were unrelated to any fitness estimate.
We found strong relationships between response song rates and other territorial behaviors, replicating earlier findings (Cooney and Cockburn, 1995). These findings echo other research on female song. In European starlings, spontaneous song rate was positively related to number of aggressive interactions (Pavlova et al., 2007). In New Zealand bellbirds (Anthornis melanura), females counter-sing more to neighbors than strangers and also approach the speaker more closely for neighbors (Brunton et al., 2008), and in stripe-headed sparrows (Aimophila r. ruficauda) singing behavior in response to female playback showed similar patterns to approaches, latencies, and flights (Illes and Yunes-Jimenez, 2009). Vocalizations in female dunnocks (Prunella modularis) were associated with physical attacks (Langmore and Davies, 1997). Female purple-crowned fairy-wren (Malurus coronatus), increased song rates and rapidly approached the speaker in response to playback (Hall and Peters, 2008). Taken together, these results suggest that song is an important component of resource defense behavior for female songbirds (Langmore, 1998). However, it is still unclear whether any aspect of song deters or repels potential rivals, or whether any aspect of song is directly related to resource acquisition (e.g., territory quality). This possibility is an important avenue for future research.
Nest success sets an upper limit on female reproductive success (Martin, 1995, 2015). Thus, the positive relationship between nest success and female response song rates indicates an important fitness advantage. Further, the positive relationship between fledgling number and response song rates suggests that even among successful females, females that sing more frequently in response to novel female song experience a continued advantage. However, caution is warranted in interpreting these results given that this pattern had eroded by 4 weeks post fledgling, the approximate time of independence (Cockburn et al., 2008). This lack of relationship may be a statistical limitation, i.e., low detection power due to small sample. However, the lack of relationship may also be a product of real costs for strong responders, or due to stochastic processes. For instance, predation pressure can be very strong in the first week after fledging, which might undermine a portion of the advantages strongly responding females experienced. This possibility is supported by research in other passerines that reported negative relationships between some measure of maternal care and aggression (Dunn et al., 2001; Rosvall, 2011a; Cain and Ketterson, 2013).
There are a number of potential mechanisms that may underlie the positive relationship between response song rates and two of our critical fitness estimates, nest success, and fledgling number. First, response song rates may reflect individual competitive ability or quality, i.e., females capable of a high response song rate may be more likely to acquire high-quality territories (Cockburn et al., 2003; Rosvall, 2008; Cain and Ketterson, 2012). A second possibility is that females occupying high quality territories defend them more robustly than females on low quality territories (Enquist and Leimar, 1987; Cooney and Cockburn, 1995). In either case, we would expect territory quality to be positively related to response song rates. We were unable to test this directly, however, past research in this population of fairy-wrens found that territories with high nest success accumulate more subordinates, suggesting that high-quality territories are more likely to have subordinates (Cooney and Cockburn, 1995; Cockburn et al., 2008). Thus, if response song rates predicted territory quality, or if territory quality drove female response song rates, we would expect that response song rates would be higher in group-breeding females. However, previous research found no difference in response song rates scores between group and pair-breeding females (Cain and Langmore, 2015). However, this is a coarse measure and differences in territory quality may yet be an important mechanism underlying the positive relationship within pair-breeding females. Thus, this is an important direction for future research.
Finally, the positive relationship between response song rates and reproductive success might occur because female response song rate is related to a third, unmeasured variable that positively influences reproductive success. For example, in other female songbirds, response to a simulated intruder has been positively related to predator defense behavior (Clutton-Brock, 2009; Cain et al., 2011; Tobias et al., 2012; Cain and Rosvall, 2014), body size (Langston et al., 1990; Martin, 1995; Cain and Ketterson, 2012), and maternal care (Rosvall, 2011a; Cain and Ketterson, 2013). Further, a robust response might also be a reflection of overall activity level. Female aggression and territorial defense behaviors have been related to testosterone levels in other passerines and high testosterone levels can increase activity (Langmore et al., 2002; Zysling et al., 2006; Cain and Ketterson, 2012; Rosvall, 2013). Further research is needed before we can conclude whether selection is acting directly on female song rates or some other aspect of female phenotype.
In contrast to the positive relationships between response song rates to a playback of a novel female and reproductive success, we found no relationships between spontaneous song rates and fitness estimates. This lack of relationship may be a by-product of our experimental protocol; the observation period was 10 min, which may not be sufficient to capture important variation in spontaneous song rates. Alternatively, it may be that variation in spontaneous song rates is less important for defending a territory, or is unrelated to the quality of the territory or female. Support for this possibility comes from previous research on female fairy-wren song, which found that spontaneous song rates are highest when a female has recently established a new territory, suggesting song serves a territorial function, but is not maintained at a high level once ownership is established (Cooney and Cockburn, 1995). A final possibility is that it is some aspect of the song itself, rather than the number of songs that is important. Research in female European starlings (Sturnus vulgaris) has shown that song complexity and performance are repeatable, and that repertoire changes with age, suggesting that song traits may be quality indicators (Pavlova et al., 2010). Similarly, older female alpine acceptors (Prunella collaris) sang more complex songs, and laid larger clutches (Langmore et al., 1996).
Conclusion
Females in a variety of species appear to use ornaments, weapons, and same-sex aggression to attract and defend mates (Amundsen, 2000; Weiss, 2006), or compete with other females for critical reproductive resources (Robinson and Kruuk, 2007; Rosvall, 2008; Watson and Simmons, 2010). The results presented here join a growing body of work that reports positive relationships between fitness estimates and competitive trait expression in females (Rosvall, 2008; Sinn et al., 2008; Watson and Simmons, 2010; Cain and Ketterson, 2012, 2013), and suggests that the persistence of female competitive traits are in many cases the product of direct selection favoring exaggerated traits when female-female competition is strong (Rosvall, 2011b; Tobias et al., 2012; Stockley and Campbell, 2013; Cain and Rosvall, 2014).
To our knowledge, this is the first study to examine the relationship between fitness and female song. Future work should determine the mechanisms of this relationship and to examine how song rates relate to other fitness components, which would add critical insights regarding the costs and benefits of trait expression (Cain and Rosvall, 2014). Finally, examining the relationship between female competitive traits and fitness in different contexts, such as when resource availability differs, will be essential to our understanding how selection shapes trait expression (Cain and Rosvall, 2014).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank W. Dimond and H. Osmond for coordinating the study sites, as well as K. Hehn, J. Pink, F. Spagopoulou, S. Vulling, P. Costa, and U. Kail, for help in the field, and S. Hoobler for support. KC was supported by Australian Endeavor and America-Australia Association post-doctoral research fellowships and a grant from the Canberra Ornithologist Group; NL and AC were supported by research grants and fellowships from the Australian Research Council. We'd also like to thank Frontiers in Ecology and Evolution for hosting this special issue, Behavior 2015 for hosting the symposia, and M. Hall for the invitation to contribute. NEL was supported by The Australian Research Council (DP110101966) and National Geographic (9221-12).
References
Amundsen, T. T. (2000). Why are female birds ornamented? Trends Ecol. Evol. (Amst). 15, 149–155. doi: 10.1016/S0169-5347(99)01800-5
Brunton, D. H., Evans, B., Cope, T., and Ji, W. (2008). A test of the dear enemy hypothesis in female New Zealand bellbirds (Anthornis melanura): female neighbors as threats. Behav. Ecol. 19, 791–798. doi: 10.1093/beheco/arn027
Cain, K. E., and Ketterson, E. D. (2012). Competitive females are successful females; phenotype, mechanism and selection in a common songbird. Behav. Ecol. Sociobiol. 66, 241–252. doi: 10.1007/s00265-011-1272-5
Cain, K. E., and Ketterson, E. D. (2013). Costs and benefits of competitive traits in females: aggression, maternal care and reproductive success. PLoS ONE 8:e77816. doi: 10.1371/journal.pone.0077816
Cain, K. E., and Langmore, N. E. (2015). Female and male song rates across breeding stage: testing for sexual and nonsexual functions of female song. Anim. Behav. 109, 65–71. doi: 10.1016/j.anbehav.2015.07.034
Cain, K. E., Rich, M. S., Ainsworth, K., and Ketterson, E. D. (2011). Two sides of the same coin? Consistency in aggression to conspecifics and predators in a female songbird. Ethology 117, 786–795. doi: 10.1111/j.1439-0310.2011.01932.x
Cain, K. E., and Rosvall, K. A. (2014). Next steps for understanding the selective relevance of female-female competition. Front. Ecol. Evol. 2:32. doi: 10.3389/fevo.2014.00032
Catchpole, C. K., and Slater, P. (1995). Bird Song: Biological Themes and Variations. Cambridge, UK: Cambridge University Press.
Clutton-Brock, T. H. (2009). Sexual selection in females. Anim. Behav. 77, 3–11. doi: 10.1016/j.anbehav.2008.08.026
Cockburn, A., Dalziell, A. H., Blackmore, C. J., Double, M. C., Kokko, H., Osmond, H. L., et al. (2009). Superb fairy-wren males aggregate into hidden leks to solicit extragroup fertilizations before dawn. Behav. Ecol. 20, 501–510. doi: 10.1093/beheco/arp024
Cockburn, A., Osmond, H. L., Mulder, R. A., Green, D. J., and Double, M. C. (2003). Divorce, dispersal and incest avoidance in the cooperatively breeding superb fairy−wren Malurus cyaneus. J. Anim. Ecol. 72, 189–202. doi: 10.1046/j.1365-2656.2003.00694.x
Cockburn, A., Sims, R. A., Osmond, H. L., Green, D. J., Double, M. C., and Mulder, R. A. (2008). Can we measure the benefits of help in cooperatively breeding birds: the case of superb fairy-wrens Malurus cyaneus? J. Anim. Ecol. 77, 430–438. doi: 10.1111/j.1365-2656.2007.01351.x
Cooney, R., and Cockburn, A. (1995). Territorial defence is the major function of female song in the superb fairy-wren, Malurus cyaneus. Anim. Behav. 49, 1635–1647. doi: 10.1016/0003-3472(95)90086-1
Dunn, P. O., Whittingham, L. A., and Pitcher, T. E. (2001). Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution 55, 161–175. doi: 10.1111/j.0014-3820.2001.tb01281.x
Eens, M., and Pinxten, R. (1998). Female song for mate attraction: an overlooked phenomenon? Trends Ecol. Evol. 13, 322–323. doi: 10.1016/S0169-5347(98)01410-4
Enquist, M., and Leimar, O. (1987). Evolution of fighting behaviour: the effect of variation in resource value. J. Theor. Biol. 127, 187–205. doi: 10.1016/S0022-5193(87)80130-3
Hall, M. L., and Peters, A. (2008). Coordination between the sexes for territorial defence in a duetting fairy-wren. Anim. Behav. 76, 65–73. doi: 10.1016/j.anbehav.2008.01.010
Illes, A. E. (2014). Context of female bias in song repertoire size, singing effort, and singing independence in a cooperatively breeding songbird. Behav. Ecol. Sociobiol. 69, 139–150. doi: 10.1007/s00265-014-1827-3
Illes, A. E., and Yunes-Jimenez, L. (2009). A female songbird out-sings male conspecifics during simulated territorial intrusions. Proc. Biol. Sci. 276, 981–986. doi: 10.1098/rspb.2008.1445
Langmore, N. E. (1998). Functions of duet and solo songs of female birds. Trends Ecol. Evol. 13, 136–140. doi: 10.1016/S0169-5347(97)01241-X
Langmore, N. E., Cockrem, J. F., and Candy, E. J. (2002). Competition for male reproductive investment elevates testosterone levels in female dunnocks, Prunella modularis. Proc. Biol. Sci. 269, 2473–2478. doi: 10.1098/rspb.2002.2167
Langmore, N. E., and Davies, N. B. (1997). Female dunnocks use vocalizations to compete for males. Anim. Behav. 53, 881–890. doi: 10.1006/anbe.1996.0306
Langmore, N. E., Davies, N. B., Hatchwell, B. J., and Hartley, I. R. (1996). Female song attracts males in the alpine accentor Prunella collaris. Proc. Biol. Sci. 263, 141–146. doi: 10.1098/rspb.1996.0022
Langston, N. E., Freeman, S., Rohwer, S., and Gori, D. (1990). The evolution of female body size in red-winged blackbirds: the effects of timing of breeding, social competition, and reproductive energetics. Evolution 44, 1764–1779. doi: 10.2307/2409505
Martin, T. E. (1995). Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 65, 101–127. doi: 10.2307/2937160
Martin, T. E. (2015). Age-related mortality explains life history strategies of tropical and temperate songbirds. Science 349, 966–970. doi: 10.1126/science.aad1173
Odom, K. J., Hall, M. L., Riebel, K., Omland, K. E., and Langmore, N. E. (2014). Female song is widespread and ancestral in songbirds. Nat. Commun. 5:3379. doi: 10.1038/ncomms4379
Pavlova, D. Z., Pinxten, R., and Eens, M. (2007). Seasonal singing patterns and individual consistency in song activity in female European starlings (Sturnus vulgaris). Behaviour 144, 663–680. doi: 10.1163/156853907781347835
Pavlova, D. Z., Pinxten, R., and Eens, M. (2010). Age-related changes of song traits in female European Starlings (Sturnus vulgaris). Anim. Biol. 60, 43–59. doi: 10.1163/157075610X12610595764138
Price, J. J. (2009). Evolution and life-history correlates of female song in the New World blackbirds. Behav. Ecol. 20, 967–977. doi: 10.1093/beheco/arp085
Price, J. J., Lanyon, S. M., and Omland, K. E. (2009). Losses of female song with changes from tropical to temperate breeding in the New World blackbirds. Proc. Biol. Sci. 276, 1971–1980. doi: 10.1098/rspb.2008.1626
Robinson, M. R., and Kruuk, L. E. B. (2007). Function of weaponry in females: the use of horns in intrasexual competition for resources in female Soay sheep. Biol. Lett. 3, 651–654. doi: 10.1098/rsbl.2007.0278
Rosvall, K. A. (2008). Sexual selection on aggressiveness in females: evidence from an experimental test with tree swallows. Anim. Behav. 75, 1603–1610. doi: 10.1016/j.anbehav.2007.09.038
Rosvall, K. A. (2011a). Cost of female intrasexual aggression in terms of offspring quality: a cross-fostering study. Ethology 117, 332–344. doi: 10.1111/j.1439-0310.2011.01881.x
Rosvall, K. A. (2011b). Intrasexual competition in females: evidence for sexual selection? Behav. Ecol. 22, 1131–1140. doi: 10.1093/beheco/arr106
Rosvall, K. A. (2013). Life history trade-offs and behavioral sensitivity to testosterone: an experimental test when female aggression and maternal care co-occur. PLoS ONE 8:e54120. doi: 10.1371/journal.pone.0054120
Sinn, D. L., While, G. M., and Wapstra, E. (2008). Maternal care in a social lizard: links between female aggression and offspring fitness. Anim. Behav. 76, 1249–1257. doi: 10.1016/j.anbehav.2008.06.009
Stockley, P., and Campbell, A. (2013). Female competition and aggression: interdisciplinary perspectives. Philos. Trans. R. Soc. B 368:20130073. doi: 10.1098/rstb.2013.0073
Tobias, J. A., Montgomerie, R., and Lyon, B. E. (2012). The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Philos. Trans. R. Soc. B 367, 2274–2293. doi: 10.1098/rstb.2011.0280
Watson, N. L., and Simmons, L. W. (2010). Reproductive competition promotes the evolution of female weaponry. Proc. Biol. Sci. 277, 2035–2040. doi: 10.1098/rspb.2009.2335
Weiss, S. L. (2006). Female-specific color is a signal of quality in the striped plateau lizard (Sceloporus virgatus). Behav. Ecol. 17, 726–732. doi: 10.1093/beheco/arl001
Zysling, D. A., Greives, T. J., Breuner, C. W., Casto, J. M., Demas, G. E., and Ketterson, E. D. (2006). Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos (Junco hyemalis carolinensis). Horm. Behav. 50, 200–207. doi: 10.1016/j.yhbeh.2006.03.004
Keywords: female aggression, female song, female competition, competitive traits, social selection, reproductive success
Citation: Cain KE, Cockburn A and Langmore NE (2015) Female song rates in response to simulated intruder are positively related to reproductive success. Front. Ecol. Evol. 3:119. doi: 10.3389/fevo.2015.00119
Received: 26 July 2015; Accepted: 08 October 2015;
Published: 27 October 2015.
Edited by:
Michaela Hau, Max Planck Institute for Ornithology, GermanyReviewed by:
Scott MacDougall-Shackleton, University of Western Ontario, CanadaKeith Tarvin, Oberlin College, USA
Silke Kipper, Technische Universität München, Germany
Copyright © 2015 Cain, Cockburn and Langmore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristal E. Cain, kristalcain@gmail.com
 Kristal E. Cain
Kristal E. Cain Andrew Cockburn
Andrew Cockburn Naomi E. Langmore
Naomi E. Langmore