Status Specific Tailoring of Sperm Behavior in an External Fertilizer
- 1Faculty of Biosciences and Aquaculture, Nord University, Bodø, Norway
- 2Department of Arctic and Marine Biology, UIT—The Arctic University, Tromsø, Norway
Why dominant males experiencing intense sperm competition sometimes show low investments in sperm production is not always obvious. One well-documented example is that of the external fertilizing teleost, the Arctic charr (Salvelinus alpinus), where individuals becoming dominant reduce sperm production and sperm swimming speed in water compared to subordinates. Here, we report how ovarian fluid differentially influences sperm velocity of dominant and subordinate male Arctic charr. That is, sperm from dominant males increase their velocity in water diluted ovarian fluid compared to that observed in water, while sperm from subordinates, on the other hand, decrease velocity in ovarian fluid compared to that observed in water. Thus, subordinates, who invest more resources in their sperm and usually show the highest sperm velocity in water, have lower gains from their investment than dominant males when sperm are swimming in ovarian fluid. In sum, our result suggests that ovarian fluid increase sperm velocity more in dominant males than in subordinate males. Although this finding could partly be caused by cryptic female choice exerted by the ovarian fluid for sperm from dominant males, an alternative and more parsimonious explanation is that sperm from dominant males may simply be better designed for swimming in ovarian fluid compared to sperm from subordinate males. Thus, sperm production in the two reproductive roles seems to be adaptively tailored to different external environments.
Introduction
Polyandry leads to conflict between males over fertilizations resulting in both pre- and post-copulatory male adaptations (Birkhead and Møller, 1992, 1998; Andersson, 1994; Andersson and Iwasa, 1996; Eberhard, 1996; Alonzo and Warner, 2000; Simmons, 2001; Chapman et al., 2003). This is easily seen in species with external fertilization where adorned dominant males gain fitness benefits by spawning in synchrony with the female and close to her eggs after courting and aggressive mate guarding. Less competitive males, on the other hand, are often forced by the dominant male to spawn out of synchrony with the female and further away from the eggs (Taborsky, 1998). This behavior often results in sperm competition where sperm from two or more males co-occur at the site of fertilization (Parker, 1970; Simmons, 2005). When there is risk of sperm competition, males may produce more sperm, larger sperm or sperm that have higher velocity than would be required to fertilize the eggs in absence of competition, at least in theory (Parker, 1970, 1998; Ball and Parker, 1996). Recent empirical studies have, in line with theory, also shown that increased risk of sperm competition leads to a higher investment in sperm velocity (Burness et al., 2004; Rudolfsen et al., 2006) and that such sperm velocity increases may be important for fertilization success (Levitan, 2000; Al-Qarawi et al., 2002; Kupriyanova and Havenhand, 2002; Gage et al., 2004; Liljedal, 2005; Schulte-Hostedde and Burness, 2005; Egeland et al., 2015).
Inference about the importance of sperm velocity for fertilization in external fertilizers stems in general from evaluations of sperm velocity measurements obtained from activation in water (Lahnsteiner et al., 1998; Levitan, 2000; Gage et al., 2004; Liljedal, 2005). However, eggs of external fertilizers are embedded in ovarian fluid, and in certain species the amount of ovarian fluid released together with the eggs is up to 30% of the total egg volume (Lahnsteiner et al., 1999). Ovarian fluid is suggested to compensate for the sub-optimal environmental conditions for the sperm in water (Lahnsteiner, 2002), and has been shown to enhance overall sperm longevity and velocity compared to that of water (Hayakawa and Munehara, 1998; Lahnsteiner, 2002; Turner and Montgomerie, 2002). Thus, the characteristics of ovarian fluid in external fertilizing species is likely to have evolved, at least partly, to increase the probability of fertilizing the eggs (Lahnsteiner, 2002).
Females of external fertilizers which experience strong sperm competition are expected to evolve mechanisms to enhance paternity of favorable males at the cost of unfavorable males, and should not be regarded as only providing an arena for sperm competition (Thornhill, 1983; Eberhard, 1996; Olsson et al., 1996; Zeh and Zeh, 1996; Birkhead, 1998). Ovarian fluid has been shown to favor swimming speed of sperm from certain males over others, suggesting that ovarian fluid may act as a medium where female-mediated cryptic selection processes can occur (Urbach et al., 2005; Nordeide, 2007; Dietrich et al., 2008; Rosengrave et al., 2008; Alonzo et al., 2016). However, disentangling the separate effects of varying quality of sperm and differing ovarian fluids on fertilization success and offspring quality under sperm competition is challenging. Some authors have demonstrated positive effects of ovarian fluid on sperm velocity (Gasparini and Pilastro, 2011; Evans et al., 2012; Oliver and Evans, 2014; Alonzo et al., 2016; Rosengrave et al., 2016), while Lumley et al. (2016) revealed no effect of ovarian fluid on relative offspring fitness. Moreover, the only published intraspecific study exchanging ovarian fluid between eggs from different females documented no overall effect of ovarian fluid on paternity success under sperm competition and no evidence for male-female interactions (Evans et al., 2013).
The Arctic charr (Salvelinus alpinus) has external fertilization with males aggregating annually at specific spawning areas. Dominant males attract and guard arriving females, yet spawning can hardly occur isolated from other males as the spawning area offer no form of protection from sneakers (Sigurjonsdottir and Gunnarsson, 1989; Sørum et al., 2011; http://naturweb.uit.no/amb/evolution/). Moreover, males show high plasticity in reproductive behaviors, and social status seems to be conditional depending on other interacting males (Fabricius and Gustafson, 1954; Sigurjonsdottir and Gunnarsson, 1989; Cutts et al., 2001). Observational studies of reproductively active male charr show, in accordance with that predicted from theoretical models (Parker, 1990; Parker et al., 2013), that social status is negatively related to sperm velocity (Figenschou et al., 2013). Additionally, males experiencing a change in mating roles have repeatedly been found to rapidly adjust sperm production. That is, compared to males in subordinate mating roles, males attaining dominance reduce sperm production and velocity of sperm cells in their ejaculate within 4 days in their new mating role (Liljedal and Folstad, 2003; Rudolfsen et al., 2006; Vaz Serrano et al., 2006; Haugland et al., 2008). Additionally, this difference in sperm velocity between dominant and subordinate individuals is most predominant among the fastest sperm cells—those most likely to fertilize the eggs (Vaz Serrano et al., 2006; Haugland et al., 2008). Moreover, sperm velocity is documented to be of major importance for fertilization success under sperm competition in Arctic charr (Liljedal, 2005; Egeland et al., 2015) and carefully controlled in vitro sperm competition trials, including a realistic time-lag to subordinates ejaculation, have shown that subordinate males may fully compensate for disadvantages in their unfavorable mating role (i.e., ejaculating out of synchrony with the female) by having more and faster sperm than dominants (Egeland et al., 2015).
So, why do males becoming dominant reduce sperm numbers and sperm velocity in their ejaculates when they have large fitness benefits under sperm competition by maintaining high sperm production and high sperm velocity (see Figure 3 in Egeland et al., 2015)? In the present study, we reanalyze data from Egeland et al. (2015) (See first paragraph in Material and Methods) in order to evaluate the potential modulating effect from ovarian fluid on sperm velocity from dominant and subordinate male charr. Dominant and subordinate Arctic charr have different sperm velocity when measured in water, but whether this difference in velocity is maintained when sperm is swimming under the influence of ovarian fluid is not known.
Materials and Methods
The data used in this study have partly been analyzed and presented for other purposes in Egeland et al. (2015). In the former publication, we used eight pairs of males and females to test the effect of spawning asynchrony, sperm quantity, and sperm quality on paternity. To increase the sample size in the present study we use those eight pairs in addition to eight more pairs of males and females (i.e., in total 16 pairs, 32 males, and 32 females) caught and analyzed during the same spawning season in 2008.
Fish Sampling and Handling
During mid-September 2008, in Lake Fjellfrøsvatn northern Norway (69° 4′ N, 19° 20′ E), we gill netted reproductively active charr at one spawning ground (i.e., males and females came from one naturally interbreeding population; see Figenschou et al., 2004). To minimize stress the fish were continuously removed from the gill nets. The 32 males included in the experiment were transported to the field laboratory where they were anesthetized using benzocaine. The length was measured (29.7 cm ± 2.1, mean ± SD) and the males were then stripped for all available milt before id tagging (see Egeland et al., 2015). Thereafter the males were size-matched and caged in pairs, with a maximum length difference of 5 mm within each of the 16 pairs. Rudolfsen et al. (2006) showed, using the exact same procedures that males entering a dominant position in pair-wise interactions do not initially differ in ejaculate characteristics, size or ornamental development from males taking up a subordinate position. The cages (made of chicken wire, 40 × 60 × 90 cm) were placed 2–3 m apart at about 1.5 m depth and left undisturbed for 24 h before the first behavioral observation started (see below). After 4 days, the fish were again anesthetized and stripped for all available milt produced during social interactions as either dominant or subordinate. The collected milt was stored on ice for further analysis (See Sperm Analysis). Females were caught on the fourth day and stored separately from the males before they were anesthetized and stripped for all their eggs and ovarian fluid. Ovarian fluid was separated from the eggs using a pipette and stored at lake temperature (6°C). Troms County Governor's environment department gave permission to catch the fish (see Haugland et al., 2011 and Egeland et al., 2015 for more details about capture and handling methods). At the time of commencement, ethical approval was not required for this study as per the legislation in Norway.
Social Position
Although the social rank between males is highly dynamic at the spawning site over the nearly 1 month long spawning period, the status roles have never changed during our behavioral observations. That is, when status roles are established (after 1 day) they are maintained the next 3 days (see Liljedal and Folstad (2003) for more information). On day 2 we started the observation period in order to determine dominance. We observed the pairs twice a day during the last 3 days of the 4-day caging period. Observation periods lasted for 5 min. For observing the individual number of aggressive acts (e.g., an initiation of a chase) we used Bathyscope underwater viewers and the males performing most aggressive acts were considered dominants. Subordinate individuals are usually stationary at the bottom of the cage and are hardly seen conducting aggressive acts at all. Dominant males, on the other hand, roam around in the cage, and sometimes initiate interactions. The average number of aggressive acts for subordinates and dominants was, respectively, 0.1 ± 0.2 (mean ± SD) and 6.1 ± 6.1 (mean ± SD) during the 5 min long observation periods. Liljedal and Folstad (2003) found that the presence of an observer does not significantly alter fish activity or the within pair hierarchical position under such experimental conditions.
Evaluating Sperm Behavior
All sperm sampling was done by one skilled person and the measurements were done as fast as possible and randomized without the experimenter knowing the fish's social position. For each male in a pair we quantified sperm motility and velocity in water and in water diluted ovarian fluid (1:2, OF:water) from the same two females. The ovarian fluid:water ratio was chosen under the assumption that the sperm of salmonids are only able to swim around half the circumference of the egg (Billard and Cosson, 1992) and that males must therefore shed sperm in the immediate proximity of the eggs where the ovarian fluid concentration is likely to be high. We evaluated sperm in ovarian fluid solutions from two females per male pair. For measurements of sperm motility and velocity, we placed <0.12 μl of sperm on a pre-cooled chamber and initiated motility by adding 4.5 μl of either water or ovarian fluid dilution (termed “ovarian fluid” throughout). Measurement were taken 10, 20, 30, and 40 s following activation and lasted 0.5 s. Measurements of sperm behavior, including curvilinear velocity (VCL), were later analyzed using CASA (HTM-CEROS v.12) using the methods described in Vaz Serrano et al. (2006).
Data Analysis
For statistical analyses, we used R (version 3.3.1, R Development Core Team, 2016). To make the results easier to interpret we ran four different linear mixed models, based on model simplification, using four different subsets. Model fitting and estimates were obtained with the linear mixed-effects package lme4 (version 1.1–12, Bates et al., 2016). In all four models sperm velocity was entered as the response variable, and male pair and female ID were included as random factors, with female ID nested in male pair (i.e., 16 pairs of males, 2 females per pair, and 2 replicates per pair × female combination). To assess if the change in sperm velocity over time depended on activation medium we entered time and activation medium as fixed factors (Table 1, Model 1). In order to test the effect of status on sperm velocity we ran two separate models, one model for sperm velocity in water and another model for sperm velocity in ovarian fluid. We ran the two models with status and time as fixed factors (Table 1, Model 2 and 3). To assess the effect of activation medium on sperm velocity for the dominant and subordinate male we used data from 10 s and entered status and activation medium as fixed factors (Table 1, Model 4). The formula ( = the variance of the random intercept, = the variance of the residuals) were used to calculate interclass correlation coefficients. To visualize the results we used the ggplot2 package (version 2.1.0, Hadley and Winston, 2016). We checked the model fit using visual examination of normal probability plots and residual plots, the qq plot showed no marked deviations from linearity.
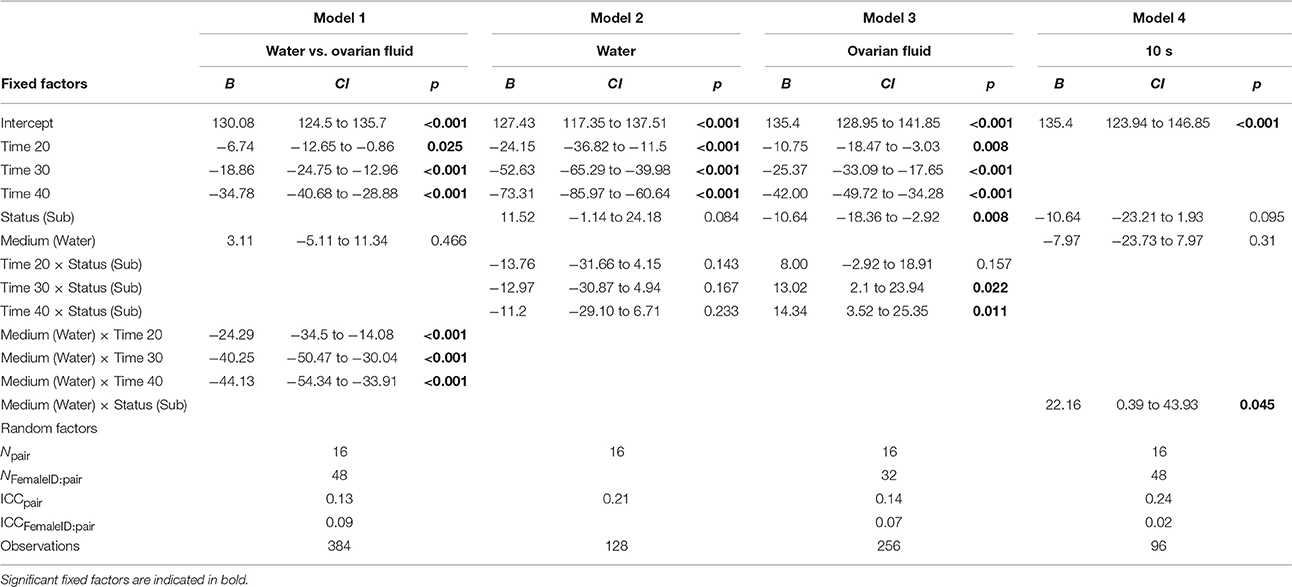
Table 1. The four models fixed factors including estimate (B), 95% confidence intervals (CI), p-values (p), random factors including the model's group count (N), intraclass correlation coefficient (ICC), and observations.
Results
Sperm Velocity in Water Vs. Ovarian Fluid
There was a significant main effect of activation medium. That is, sperm swim in general faster in ovarian fluid than in water (Table 1, Figure 1). Furthermore, the decrease in sperm velocity from 10 to 40 s after activation was highly significant (Table 1), but the velocity decrease was much larger in water than in ovarian fluid (Table 1).
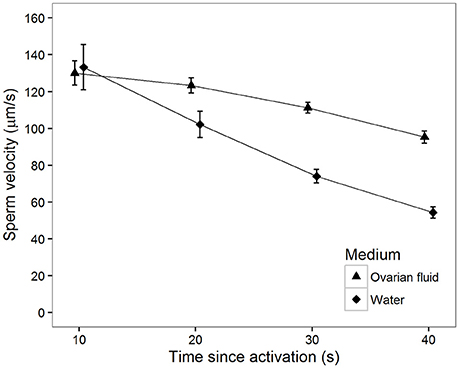
Figure 1. Mean sperm velocity (VCL) in water (squares) and ovarian fluid (triangles) measured at different times (s) after activation. Vertical bars are 95% confidence intervals.
Sperm Velocity in Water
There was a significant decline in sperm velocity over time (Table 1, Figure 2). Although the effect of male status on sperm velocity in water did not reach significance at this sample size (Table 1), the general pattern of a higher sperm velocity among subordinates in the initial period after activation was also apparent in this sample (see Haugland et al. (2008) for a meta-analysis of previous data). Additionally, there was no significant status-specific decline in sperm velocity over time (Table 1).
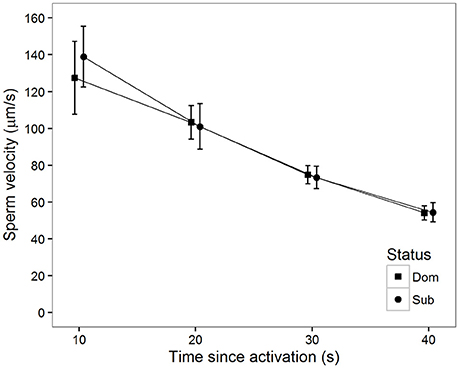
Figure 2. Mean sperm velocity (VCL) in water after social status was established among subordinate (n = 16, circles) and dominant (n = 16, squares) males measured at different time (s) after activation. Vertical bars are 95% confidence intervals.
Sperm Velocity in Ovarian Fluid
There was also a significant decline in sperm velocity over time in ovarian fluid (Table 1, Figure 3). Additionally, sperm from dominant males swam faster than sperm from subordinate males at 10 s (Figure 3). Contrary to what was observed in water, there was a tendency for a status-specific decline in sperm velocity with a larger velocity decrease for the dominant than for the subordinate males (Table 1, Figure 3). That is, sperm from dominant males show a significantly more rapid velocity decline in the latter part of our 40 s long observation period compared to subordinates.
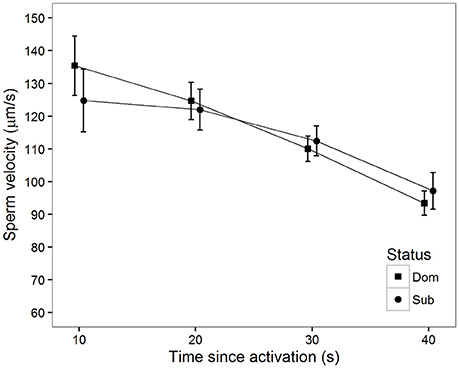
Figure 3. Mean sperm velocity (VCL) in ovarian fluid after social status was established among subordinate (n = 16, circles) and dominant (n = 16, squares) males measured at different time (s) after activation. Vertical bars are 95% confidence intervals.
Sperm Velocity in the Two Media
Ten seconds after activation there was a significant interaction between activation medium and social status (Table 1, Figure 4). That is, sperm from dominant males increase their velocity in water diluted ovarian fluid compared to that observed in water, while sperm from subordinates, on the other hand, decrease velocity in ovarian fluid compared to that observed in water. There were no significant interactions between activation medium and social status at any other time after activation (20 s: B = 0.4, p = 0.93, 30 s: B = −3.8, p = 0.31 and 40 s: B = −3.5, p = 0.36).
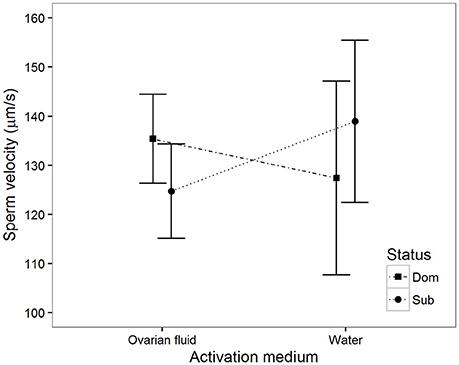
Figure 4. Mean sperm velocity (VCL) 10 s after activation among subordinate (n = 16, circles) and dominant (n = 16, squares) males measured in ovarian fluid and in water. Vertical bars are 95% confidence intervals.
Discussion
In accordance with our previous reporting, we show that male Arctic charr occupying a subordinate social position produce sperm that initially tend to swim faster than sperm from dominant males in water, a status specific adjustment. Yet, more important for our present reporting, ovarian fluid seems to have a status specific effect on enhancement of sperm velocity favoring sperm originating from dominant males. That is, sperm from dominant males increase their velocity in ovarian fluid compared to that observed in water while sperm from subordinate decrease velocity in ovarian fluid compared to that observed in water. Additionally the dominant males show the most rapid decrease in sperm speed in ovarian fluid through our 40 s observational period.
In accordance with theoretical models (Parker, 1970, 1998; Ball and Parker, 1996; Parker et al., 2013), sperm from males mating in disfavored roles tend to have higher velocity in water than the sperm from males mating in favored reproductive roles. This difference in sperm velocity between dominant and subordinate males is mainly manifested in the initial period after activation and in water only. Additionally, there is no status specific difference in the velocity decline through our observation period. These results are similar to that previously well-documented in Arctic charr (Rudolfsen et al., 2006; Vaz Serrano et al., 2006; Haugland et al., 2008) and also in Bluegill (Lepomis macrochirus; Burness et al., 2004). ATP stored in spermatozoa prior to ejaculation provides the necessary chemical energy to sustain sperm motility (Jeulin and Soufir, 1992), and in Bluegills, sperm from subordinates have about 1.5 times more ATP than sperm from dominants (Burness et al., 2004). Further, sperm ATP is positively associated with sperm velocity (Burness et al., 2004; Figenschou et al., 2013) and could be the proximate explanation for the differences in sperm velocity observed previously between males in the two mating roles when activated in water (see Haugland et al., 2008). Moreover, as sperm velocity in water has been found to predict fertilization under sperm competition (Gage et al., 2004; Liljedal, 2005; Schulte-Hostedde and Burness, 2005, see also Egeland et al., 2015), this investment could compensate for mating in a disfavored mating role when ejaculating out of synchrony and further away from the egg releasing female and the dominant male (Sørum et al., 2011; Egeland et al., 2015). That is, unlike dominant's that may spawn directly into the stream of released gonadal products of the female, subordinate's ejaculate is met by an environment more dominated by water and the adaptation to high velocity in water among subordinates thus seems reasonable. In this context it should be noted that the distance covered by self-propulsion of sperm cells represent approximately half the circumference of the egg (Billard and Cosson, 1992) while the ejected distance of gonadal products often exceed 10 cm (own observations from videos of spawning events). Thus, the ability of subordinates to eject the sperm correctly into the gonadal products released from females must be paramount.
Recent studies have shown that there can be considerable female-male interaction in offspring survival among external fertilizing species (Welch et al., 1998; Wedekind et al., 2001; Welch, 2003; Rudolfsen et al., 2005; Evans et al., 2007), suggesting that there might be larger fitness benefits from female choice than the 5–10% increase suggested from estimating variance in fitness and comparing selected and unselected populations (see Burt, 1995). Thus, the female's role in determining which sperm fertilize her eggs, either through her own preferential mate selection or through her cryptic choice, may be important. In accordance with this contention, we found that sperm velocity was influenced by ovarian fluid in charr. This is not surprising as the ovarian fluid of Arctic charr contains a variety of compounds for the sperm to metabolize (Lahnsteiner et al., 1995) and the fluid is also known to increase sperm velocity (Turner and Montgomerie, 2002) depending on individual male-females interaction (Urbach et al., 2005). Yet, the results from current intraspecific studies on the importance of ovarian fluid as a medium for cryptic female choice in external fertilizers are not unambiguous (See Introduction). However, our present documentation of a status dependent modulation of sperm activity, increasing the sperm speed of dominant males while reducing the speed of sperm from subordinates compared to that seen in water, suggest that ovarian fluid could act as a medium for cryptic female choice. That is, as dominant males have less ATP in their sperm cells than subordinates (Figenschou et al., 2013), ovarian fluid seems selectively promoting swimming of sperm from dominant males. Yet, if it were a general tendency for ovarian fluid to “prefer” sperm from dominant males, one would probably not predict a more rapid decline in sperm velocity for sperm from dominant males. Sperm from dominant males show, however, a significantly more rapid velocity decline in the latter part of our 40 s long observation period compared to sperm from subordinates. This suggests that the higher sperm velocity in ovarian fluid of dominants, compared to subordinates, is a male adaption rather than an effect of cryptic female choice. Alonzo et al. (2016) suggested something similar: “The differences between the male types in sperm characteristics and the effect of ovarian fluid on male sperm characteristics are likely the result of male adaptation to selection arising from the environment provided by the female's ovarian fluid during sperm competition.” Thus, both the Alonzo et al. (2016) study and our study indicate that increased velocity of sperm in ovarian fluid observed among males mating in a favored mating role must involve a male adaption. That is, there must be something with the gonadal products from dominants that separate them from gonadal products from subordinates. This difference must be a prerequisite for any female medium that should manage to influence sperm from dominant and subordinate males differently. If there had been no difference in sperm from dominants and subordinates, ovarian fluid would have nothing to act upon. Yet, cryptic female choice might still occur in ovarian fluid, adaptively promoting swimming speed of sperm from dominant males, but this additional rationale is not needed for explaining our results. Thus, status specific tailoring of sperm behavior is the most parsimonious explanation for our observation (Beck, 1943). On the other hand, our study is a retrospective study, and it was not designed to disentangle the importance of the two models of male and female adaptations. We can, consequently, not exclude that cryptic female choice may also be operating in ovarian fluid (see also Simmons et al. (2008) for an example within Anuran).
Recent evidence suggests that when dominant and subordinate charr compete in pairwise sperm competitions over fertilizing eggs embedded in ovarian fluid, subordinates seem to be fully able to compensate for their delayed ejaculation by increasing sperm numbers and sperm speed (Egeland et al., 2015). However, as the authors of the latter study also wrote: The “.. experiment mimicked the situation with ejaculations from one dominant and one subordinate male given an equal distance to the eggs. The proximity of the female to the male during spawning may also be of large importance for the outcome of reproductive activities and our study is, consequently, not a complete description of all factors influencing reproductive success under sperm competition in charr.” If sperm from dominant males had been given the advantage of entering the ovarian fluid influenced environment immediately after ejaculation, something that under natural spawning normally would occur for dominant males (when gametes are released in synchrony and in close proximity to the released female spawning products), a different outcome might have been produced. Thus, a better mimicking of a natural spawning with an immediate mix of ovarian fluid and sperm following ejaculation might have given sperm from dominant males an immediate access to the environment to which they were better adapted and produced different results to those of Egeland et al. (2015).
So, why do dominant males reduce sperm production? We believe that the benefits observed by tailoring sperm production to a specific fertilization environment combined with a synchronized spawning and positional effects might compensate for low sperm numbers and low energy content of sperm throughout the annual spawning season. Our results suggest that future sperm competition experiments should be very sensitive to positional effects as sperm production may be adapted to different fertilization environments. In charr, sperm competition does not seem to be a “fair raffle.”
Ethics Statement
The fieldwork was carried out in 2008 in accordance with the ethical guidelines stated by the Norwegian Ministry of Agriculture through the Animal Welfare Act from 1996. All fish used in this study were released back into the lake.
Author Contributions
TE, GR, JN, and IF have contributed to the design of the work, sampling of the data, labwork, and statistics. TE, GR, JN, and IF have worked on the manuscript and have approved the submitted version. TE, GR, JN, and IF have all agreed to be accountable for all aspects of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank two referees and Lars Figenschou, Vidar Sørum, and Sissel Kaino for help during field and laboratory work.
References
Alonzo, S. H., Stiver, K. A., and Marsh-Rollo, S. E. (2016). Ovarian fluid allows directional cryptic female choice despite external fertilization. Nat. Commun. 7:12452. doi: 10.1038/ncomms12452
Alonzo, S. H., and Warner, R. R. (2000). Allocation to mate guarding or increased sperm production in a Mediterranean wrasse. Am. Nat. 156, 266–275. doi: 10.1086/303391
Al-Qarawi, A. A., Abdel-Rahman, H. A., El-Mougy, S. A., and El-Belely, M. S. (2002). Use of a new computerized system for evaluation of spermatozoal motility and velocity characteristics in relation to fertility levels in dromedary bulls. Anim. Reprod. Sci. 74, 1–9. doi: 10.1016/S0378-4320(02)00163-X
Andersson, M., and Iwasa, Y. (1996). Sexual selection. Trends Ecol. Evol. 11, 53–58. doi: 10.1016/0169-5347(96)81042-1
Ball, M. A., and Parker, G. A. (1996). Sperm competition games: external fertilization and “adaptive” infertility. J. Theor. Biol. 180, 141–150. doi: 10.1006/jtbi.1996.0090
Bates, D. M., Maechler, M., Bolker, B., and Walker, S. (2016). lme4: Linear Mixed-effects Models Using Eigen and S4. R package version 1, 1–12. Available online at: http://CRAN.R-project.org/package=lme4
Beck, L. W. (1943). The principle of parsimony in empirical science. J. Philos. 40, 617–633. doi: 10.2307/2019692
Billard, R., and Cosson, M. P. (1992). Some problems related to the assessment of sperm motility in fresh-water fish. J. Exp. Zool. 261, 122–131. doi: 10.1002/jez.1402610203
Birkhead, T. R. (1998). Cryptic female choice: criteria for establishing female sperm choice. Evolution 52, 1212–1218. doi: 10.2307/2411251
Birkhead, T. R., and Møller, A. P. (1992). Sperm Competition in Birds: Evolutionary Causes and Consequences. San Diego, CA: Academic Press.
Birkhead, T. R., and Møller, A. P. (1998). Sperm Competition and Sexual Selection. San Diego, CA: Academic Press.
Burness, G., Casselman, S. J., Schulte-Hostedde, A. I., Moyes, C. D., and Montgomerie, R. (2004). Sperm swimming speed and energetics vary with sperm competition risk in bluegill (Lepomis macrochirus). Behav. Ecol. Sociobiol. 56, 65–70. doi: 10.1007/s00265-003-0752-7
Chapman, T., Arnqvist, G., Bangham, J., and Rowe, L. (2003). Sexual conflict. Trends Ecol. Evol. 18, 41–47. doi: 10.1016/S0169-5347(02)00004-6
Cutts, C. J., Adams, C. E., and Campbell, A. (2001). Stability of physiological and behavioural determinants of performance in Arctic char (Salvelinus alpinus). Can. J. Fish. Aquat. Sci. 58, 961–968. doi: 10.1139/f01-050
Dietrich, G. J., Wojtczak, M., Slowinska, M., Dobosz, S., Kuzminski, H., and Ciereszko, A. (2008). Effects of ovarian fluid on motility characteristics of rainbow trout (Oncorhynchus mykiss Walbaum) spermatozoa. J. Appl. Ichthyol. 24, 503–507. doi: 10.1111/j.1439-0426.2006.01130.x
Eberhard, W. G. (1996). Female Control: Sexual Selection by Cryptic Female Choice. Princeton, NJ: Princeton University Press.
Egeland, T. B., Rudolfsen, G., Nordeide, J. T., and Folstad, I. (2015). On the relative effect of spawning asynchrony, sperm quantity, and sperm quality on paternity under sperm competition in an external fertilizer. Front. Ecol. Evol. 3:77. doi: 10.3389/fevo.2015.00077
Evans, J. P., Garcia-Gonzàlez, F., and Marshall, D. J. (2007). Sources of genetic and phenotypic variance in fertilization rates and larval traits in a sea urchin. Evolution 61, 2832–2838. doi: 10.1111/j.1558-5646.2007.00227.x
Evans, J. P., Garzia-Gonzales, F., Almbro, M., Robinson, O., and Fitzpatrick, J. L. (2012). Assessing the potential for egg chemoattractants to mediate sexual selection in a broadcast spawning marine invertebrate. Proc. Biol. Sci. 279, 2855–2861. doi: 10.1098/rspb.2012.0181
Evans, J. P., Rosengrave, P., Gasparini, C., and Gemmell, N. J. (2013). Delineating the roles of males and females in sperm competition. Proc. Biol. Sci. 280:20132047. doi: 10.1098/rspb.2013.2047
Fabricius, E., and Gustafson, K. (1954). Further Aquarium Observations on The Spawning Behaviour of The Char, Salmo Alpinus. Drottingholm: Institute of Freshwater Research Report.
Figenschou, L., Folstad, I., and Liljedal, S. (2004). Lek fidelity of male Arctic charr. Can. J. Zool. 82, 1278–1284. doi: 10.1139/z04-106
Figenschou, L., Folstad, I., Rudolfsen, G., Hanssen, S. A., Kortet, R., Skau, P. A., et al. (2013). The relative effect of parasites and social status on sperm traits in Arctic charr. Behav. Ecol. 24, 497–504. doi: 10.1093/beheco/ars190
Gage, M. J., Macfarlane, C. P., Yeates, S., Ward, R. G., Searle, J. B., and Parker, G. A. (2004). Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 14, 44–47. doi: 10.1016/s0960-9822(03)00939-4
Gasparini, C., and Pilastro, A. (2011). Cryptic female female preference for genetically unrelated males is mediated by ovarian fluid in the guppy. Proc. Biol. Sci. 278, 2495–2501. doi: 10.1098/rspb.2010.2369
Hadley, W., and Winston, C. (2016). ggplot2: An Implementation of The Grammar of Graphics. R package version 2.1.0. Available online at: http://CRAN.R-project.org/package=ggplot2.
Haugland, T., Rudolfsen, G., Figenschou, L., and Folstad, I. (2008). Sperm velocity and its relation to social status in Arctic charr (Salvelinus alpinus). Anim. Reprod. Sci. 115, 231–237. doi: 10.1016/j.anireprosci.2008.11.004
Haugland, T., Rudolfsen, G., Figenschou, L., and Folstad, I. (2011). Is the adipose fin and the lower jaw (kype) related to social dominance in male Arctic charr Salvelinus alpinus? J. Fish Biol. 70, 1076–1083. doi: 10.1111/j.1095-8649.2011.03087.x
Hayakawa, Y., and Munehara, H. (1998). Fertilization environment of the non-copulating marine sculpin, Hemilepidotus gilberti. Environ. Biol. Fish. 52, 181–186. doi: 10.1023/A:1007432322099
Jeulin, C., and Soufir, J. C. (1992). Reversible intracellular ATP changes in intact rat spermatozoa and effects on flagellar sperm movement. Cell Mot. Cytoskeleton 21, 210–222. doi: 10.1002/cm.970210305
Kupriyanova, E., and Havenhand, J. N. (2002). Variation in sperm swimming behaviour and its effect on fertilization success in the serpulid polychaete Galeolaria caespitosa. Invert. Reprod. Dev. 41, 21–26. doi: 10.1080/07924259.2002.9652731
Lahnsteiner, F. (2002). The influence of ovarian fluid on the gamete physiology in the Salmonidae. Fish Physiol. Biochem. 27, 49–59. doi: 10.1023/B:FISH.0000021792.97913.2e
Lahnsteiner, F., Berger, B., Weismann, T., and Patzner, R. A. (1998). Determination of semen quality of the rainbow trout, Oncorhynchus mykiss, by sperm motility, seminal plasma parameters, and spermatozoal metabolism. Aquaculture 163, 163–181. doi: 10.1016/S0044-8486(98)00243-9
Lahnsteiner, F., Weismann, T., and Patzner, R. A. (1995). Composition of the ovarian fluid in 4 salmonid species - Oncorhynchus-mykiss, Salmo-trutta F Lacustris, Salvelinus- alpinus and Hucho-hucho. Reprod. Nutr. Dev. 35, 465–474. doi: 10.1051/rnd:19950501
Lahnsteiner, F., Weismann, T., and Patzner, R. A. (1999). Physiological and biochemical parameters for egg quality determination in lake trout, Salmo trutta Lacustris. Fish Physiol. Biochem. 20, 375–388. doi: 10.1023/A:1007715621550
Levitan, D. R. (2000). Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proc. Biol. Sci. 267, 531–534. doi: 10.1098/rspb.2000.1032
Liljedal, S. (2005). Factors Influencing Sperm Production, Sperm Competition and Male Fertilization Success in The Arctic Charr, Salvelinus Alpinus. Ph.D. thesis, University of Tromsø, Tromsø.
Liljedal, S., and Folstad, I. (2003). Milt quality, parasites, and immune function in dominant and subordinate Arctic charr. Can. J. Zool. 81, 221–227. doi: 10.1139/z02-244
Lumley, A. J., Diamond, S. E., Einum, S., Yeates, S. E., Peruffo, D., Emerson, B. C., et al. (2016). Post-copulatory opportunities for sperm competition and cryptic females choice provide no offspring fitness benefits in externally fertilizing salmon. R. Soc. Open Sci. 3:150709. doi: 10.1098/rsos.150709
Nordeide, J. T. (2007). Is there more in “gamete quality” than quality of the gametes? A review of effects of female mate choice and genetic compatibility on offspring quality. Aquat. Res. 38, 1–16. doi: 10.1111/j.1365-2109.2006.01635.x
Oliver, M., and Evans, J. P. (2014). Chemically moderated gamete preferences predict offspring fitness in a broadcast spawning invertebrate Proc. Biol. Sci. 281:20140148. doi: 10.1098/rspb.2014.0148
Olsson, M., Shine, R., Madsen, T., Gullberg, A., and Tegelstrom, H. (1996). Sperm selection by females. Nature 383:585. doi: 10.1038/383585a0
Parker, G. A. (1970). Sperm competition and its evolutionary consequences in insects. Biol. Rev. 45, 525–567. doi: 10.1111/j.1469-185X.1970.tb01176.x
Parker, G. A. (1990). Sperm competition games: raffles and roles. Proc. R. Soc. Lond. B 242, 120–126. doi: 10.1098/rspb.1990.0114
Parker, G. A. (1998). “Sperm competition and the evolution of ejaculates: towards a theory base,” in Sperm Competition and Sexual selection, eds T. R. Birkhead and A. P. Møller (London: Academic Press), 3–54.
Parker, G. A., Lessells, C. M., and Simmons, L. W. (2013). Sperm competition games: a general model for precopulatory male-male competition. Evolution 67, 95–109. doi: 10.1111/j.1558-5646.2012.01741.x
R Development Core Team (2016). R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Rosengrave, P., Gemmel, N. J., Metcalf, V., McBride, K., and Montgomerie, R. (2008). A mechanism for cryptic female choice in chinook salmon. Behav. Ecol. 19, 1179–1185. doi: 10.1093/beheco/arn089
Rosengrave, P., Montgomerie, R., and Gemmell, N. (2016). Cryptic female choice enhances fertilization success and embryo survival in chinook salmon. Proc. Biol. Sci. 283:20160001. doi: 10.1098/rspb.2016.0001
Rudolfsen, G., Figenschou, L., Folstad, I., Nordeide, J. T., and Søreng, E. (2005). Potential fitness benefits from mate selection in the Atlantic cod (Gadus morhua). J. Evol. Biol. 18, 172–179. doi: 10.1111/j.1420-9101.2004.00778.x
Rudolfsen, G., Figenschou, L., Folstad, I., Tveiten, H., and Figenschou, M. (2006). Rapid adjustments of sperm characteristics in relation to social status. Proc. Biol. Sci. 273, 325–332. doi: 10.1098/rspb.2005.3305
Schulte-Hostedde, A. I., and Burness, G. (2005). Fertilization dynamics of sperm from different male mating tactics in bluegill (Lepomis macrochirus). Can. J. Zool. 83, 1638–1642. doi: 10.1139/z05-164
Sigurjonsdottir, H., and Gunnarsson, K. (1989). Alternative mating tactics of arctic charr, Salvelinus-Alpinus, in Thingvallavatn, Iceland. Environ. Biol. Fish 26, 159–176. doi: 10.1007/BF00004814
Simmons, L. W. (2001). Sperm Competition and its Evolutionary Consequences in the Insects. Princeton, NJ: Princeton University Press.
Simmons, L. W. (2005). The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu. Rev. Ecol. Syst. 36, 125–146. doi: 10.1146/annurev.ecolsys.36.102403.112501
Simmons, L. W., Roberts, J. D., and Dziminski, M. A. (2008). Egg jelly influences sperm motility in the externally fertilizing frog, Crinia Georgiana. J. Evol. Biol. 22, 225–229. doi: 10.1111/j.1420-9101.2008.01628.x
Sørum, V., Figenschou, L., Rudolfsen, G., and Folstad, I. (2011). Spawning behaviour of Arctic charr (Salvelinus alpinus): risk of sperm competition and timing of milt release for sneaker and dominant males. Behaviour 148, 1157–1172. doi: 10.1163/000579511X596615
Taborsky, M. (1998). Sperm competition in fish: ‘bourgeois’ males and parasitic spawning. Trends Ecol. Evol. (Amst) 13, 222–227. doi: 10.1016/S0169-5347(97)01318-9
Thornhill, R. (1983). Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. Am. Nat. 122, 765–788. doi: 10.1086/284170
Turner, E., and Montgomerie, R. (2002). Ovarian fluid enhances sperm movement in Arctic charr. J. Fish Biol. 60, 1570–1579. doi: 10.1111/j.1095-8649.2002.tb02449.x
Urbach, D., Folstad, I., and Rudolfsen, G. (2005). Effects of ovarian fluid on sperm velocity in Arctic charr (Salvelinus alpinus). Behav. Ecol. Sociobiol. 57, 438–444. doi: 10.1007/s00265-004-0876-4
Vaz Serrano, J., Folstad, I., Rudolfsen, G., and Figenschou, L. (2006). Do the fastest sperm within an ejaculate swim faster in subordinate than in dominant males of Arctic char? Can. J. Zool. 84, 1019–1024. doi: 10.1139/Z06-097
Wedekind, C., Muller, R., and Spicher, H. (2001). Potential genetic benefits of mate selection in whitefish. J. Evol. Biol. 14, 980–986. doi: 10.1046/j.1420-9101.2001.00349.x
Welch, A. M. (2003). Genetic benefits of a female mating preference in gray tree frogs are context-dependent. Evolution 57, 883–893. doi: 10.1111/j.0014-3820.2003.tb00299.x
Welch, A. M., Semlitsch, R. D., and Gerhardt, H. C. (1998). Call Duration as an Indicator of Genetic Quality in Male Gray Tree Frogs. Science 280, 1928–1930. doi: 10.1126/science.280.5371.1928
Keywords: sexual selection, cryptic female choice, sperm competition, sperm selection, sperm velocity, ovarian fluid
Citation: Egeland TB, Rudolfsen G, Nordeide JT and Folstad I (2016) Status Specific Tailoring of Sperm Behavior in an External Fertilizer. Front. Ecol. Evol. 4:135. doi: 10.3389/fevo.2016.00135
Received: 25 July 2016; Accepted: 09 November 2016;
Published: 24 November 2016.
Edited by:
Michaela Hau, Max Planck Institute for Ornithology, GermanyReviewed by:
Francisco Garcia-Gonzalez, Spanish Research Council (CSIC), SpainJon Paul Evans, University of Western Australia, Australia
Copyright © 2016 Egeland, Rudolfsen, Nordeide and Folstad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Torvald B. Egeland, torvald.b.egeland@nord.no
 Torvald B. Egeland
Torvald B. Egeland Geir Rudolfsen
Geir Rudolfsen Jarle T. Nordeide
Jarle T. Nordeide Ivar Folstad
Ivar Folstad