Species-Dependent Effects of the Urban Environment on Fatty Acid Composition and Oxidative Stress in Birds
- Department of Biology, Lund University, Lund, Sweden
Ecological impacts of urbanization include the loss of biodiversity and changes in species composition and population densities. However, how the urban environment affects fundamental physiological parameters is largely unknown. Here, we investigated physiological components related to health and nutrition, namely, plasma fatty acids (FA) and lipid peroxidation at inter-habitat and interspecific levels. Specifically, we compared four passerine bird species—the great tit (Parus major), the blue tit (Cyanistes caeruleus), the house sparrow (Passer domesticus), and the tree sparrow (P. montanus)—from urban and rural environments. Significant interactions between species and habitat were revealed for the majority of the FAs. Interestingly, the observed inter-habitat variation in FAs was frequently in opposite directions when comparing species from the two families (tits, Paridae; sparrows, Passeridae). These patterns suggest that sparrows and tits feed on different food sources, or modulate their FA metabolism differently, across the urban-rural gradient. By using canonical discriminant analyses (CDA), we further demonstrated species-specific signals in FA composition, with misclassification of species being <1% within habitats and <7% between habitats. Finally, the urban-rural FA differences between species and families were manifested in two indices of health. Firstly, urban blue tits had a higher total ω-6/ω-3 polyunsaturated FA ratio than rural conspecifics, which is believed to increase inflammatory responses. Secondly, urban sparrows of both species showed higher lipid peroxidation indices (indicating a higher susceptibility to lipid peroxidation if exposed to pro-oxidants), and consequently, a higher level of lipid peroxidation compared to their rural conspecifics. Collectively, the species- and habitat-specific differences in plasma FA composition, which are linked to nutrition and metabolism, suggest that the urban environment affect tits and sparrows primarily via two different pathways—inflammation and oxidative stress, respectively,—with potential consequences for the health of urban populations.
Introduction
Many urban-dwelling birds are declining, despite the suggested benefits of high abundance of anthropogenic food and artificial nesting holes in urban environments (Jokimäki et al., 1996; Jokimäki, 1999; Marzluff et al., 2001; Crick et al., 2002, 2003; McKinney, 2002; Beckerman et al., 2007; Grimm et al., 2008). Species that seemingly do well in urban environments often share a range of traits, such as a high degree of feeding innovation, high storage of dietary antioxidants, high annual fecundity, high adult survival, and large breeding ranges (Møller, 2009; Møller et al., 2010). Despite such similarities, the consequences of inhabiting an urban environment are likely to differ between species, such as between those classified as “urban adapters” vs. “urban exploiters” (McKinney, 2002). The urban adapter species refer to those that take advantage of human-provided resources, but do not depend on them. Urban exploiters, on the other hand, are considered to depend on human-provided resources in order not to decline. Nevertheless, the house sparrow (Passer domesticus), an urban exploiter species that is so closely associated with human settlements that its original niche is unknown (Diamond, 1986), has declined with 60% in urban environments over the last 30 years (Crick et al., 2002, 2003). While there is a considerable number of species that exploit human-provided resources in urban habitats, little is known about the associated physiological modulations, and their consequences for urban life (Diamond, 1986; Isaksson, 2015). This is unfortunate, because physiological data, such as biomarkers for health and nutritional status, may provide critical information on the underlying causations for why a species thrives in, or simply just copes with, the urban environment (Isaksson, 2015).
Stress from the urban environment, such as air-, light-, and noise pollution, put city dwellers under considerable stress. This has repeatedly been shown to result in changes in traits with potential fitness links, e.g., behavior, morphology, and reproductive investment (Gorissen et al., 2005; Isaksson et al., 2005; Fuller et al., 2007; Kempenaers et al., 2010). Likewise, several molecular and physiological parameters are also affected by the urban environment, including altered gene expression, endocrine changes, increased oxidative stress, and accelerated telomere attrition (Partecke et al., 2006; Isaksson, 2010; Atwell et al., 2012; Dominoni et al., 2013; Salmón et al., 2016; Watson et al., 2017). Such changes to blood- and cell chemistry may be exacerbated by a suboptimal diet in urban populations (Romieu et al., 2008; Isaksson et al., 2011). For example, while species in their natural environments have evolved unique feeding niches, interspecific dietary differences tend to converge in urban areas due to superabundance of human-provided food items (e.g., sunflower seeds, peanuts, bread, tallow, coconut fat, and leftovers; Jones and Reynolds, 2008). Such food items have a different, potentially poorer, nutrient composition compared to natural food sources (Schoech and Bowman, 2003; Schoech et al., 2004; Jones and Reynolds, 2008; Andersson et al., 2015). Reliance on (exploiters), or high intake of (adapters), human-provided food may therefore further reduce individual health and reproductive success in urban birds. Moreover, natural food sources in urban areas can also be of poorer quality than those in rural areas, which contributes to the lower availability of important macro- and micro-nutrients in cities (Isaksson and Andersson, 2007; Møller et al., 2010; Andersson et al., 2015; Isaksson et al., 2015).
The composition of fatty acids (FAs) has been suggested to differ between anthropogenic and natural food sources (Andersson et al., 2015), but FA variation has rarely been investigated in this context. FAs are obtained via dietary sources, or by de novo biosynthesis. However, two polyunsaturated fatty acids (PUFAs)—the omega (ω)-3 PUFA α-linolenic acid (α-LNA) and the ω-6 PUFA linoleic acid (LA)—are strictly dietary (i.e., essential) for all birds. These FAs can be elongated to long-chained, highly unsaturated PUFAs. These are especially relevant to study in the urbanization context due to their involvement in the regulation of physiological processes, such as cell membrane fluidity and transmembrane molecular movements, cardiac function, brain development, and immune responses (e.g., Hazel, 1995; Larsson et al., 2004; Pierce et al., 2005; Ben-Hamo et al., 2011; Hulbert and Abbott, 2012). Indeed, a recent study on great tits (Parus major) demonstrated that genes encoding enzymes responsible for biosynthesis of long-chain PUFAs were differentially expressed between urban and rural individuals, although the factors underlying this habitat-specific difference, and the resulting physiological consequences, were not determined (Watson et al., 2017). Moreover, in birds, the dietary FA composition can affect several aspects of performance (Twining et al., 2016). From a health perspective, the composition of ω-6 and ω-3 PUFAs is particularly interesting, given their generally opposing effects on inflammatory responses and oxidative stress (Larsson et al., 2004; Romieu et al., 2008; Isaksson, 2015). Accordingly, a high total ω-6/ω-3 PUFA ratio is associated with increased sensitivity to antigens by promoting inflammation (e.g., Calder, 2007, 2009; Romieu et al., 2008). In mammals, a ratio above 3 is considered to induce not only inflammation, but also pro-oxidant production, thereby potentially further increasing oxidative stress in urban-dwelling animals (Simopoulos, 2002; Kiecolt-Glasera et al., 2013). Because the immune system responds to pollution (Romieu et al., 2008; Isaksson, 2015, and references therein), the dietary FA composition, and resultant FA compositions of blood and tissues (Austin, 1993; Pierce et al., 2005; McCue et al., 2009; Ben-Hamo et al., 2011), can play a significant role on birds' health in the urban environment. In addition, long-chain PUFAs are themselves sensitive to oxidative stress by being susceptible to lipid peroxidation.
The present study has two main aims. Firstly, we compared the plasma FA profiles of four common species, which falls into the “urban adapter” (great tit, P. major; blue tit, Cyanistes caeruleus) and “urban exploiter” (house sparrow, Passer domesticus; tree sparrow, P. montanus) categories, in urban and rural habitats. We predicted (1) that intraspecific FA profiles would differ between urban- and rural-dwelling individuals due to differences in diet composition. We also predicted (2) that the FA composition would show distinct family signals given pronounced diet differences between the exploiters and adapters, but that these signals would converge in the urban habitat due to a more homogenous diet availability. Secondly, we investigated whether biomarkers of health, viz. the total ω-6/ω-3 PUFA ratio, lipid peroxidation susceptibility index (peroxidation index, PI; Pamplona et al., 1998; Kang et al., 2004), and lipid peroxidation (malondialdehyde, MDA), differed between the urban and rural individuals of the four species (Gutteridge, 1995). We predicted (3) that urban birds should have a lower overall proportion of both ω-6 and ω-3 PUFAs, but due to the even lower availability of ω-3 PUFAs (compared to ω-6 PUFAs) in the urban habitat (Andersson et al., 2015; Toledo et al., 2016), we expected to find an increase in the ω-6/ω-3 PUFA ratio in urban birds. We also predicted (4) that the adapter species would show a more pronounced difference between the urban and rural environments, given that the exploiter species rely on human-provided foods also in the rural habitat. In addition, we predicted (5) that urban birds should have higher MDA due to higher environmentally induced oxidative stress (Isaksson, 2010). Furthermore, we predicted (6) that if a high ω-6/ω-3 PUFA ratio produces pro-oxidants via pro-inflammatory responses, a positive association between the ω-6/ω-3 PUFA ratio and MDA would be revealed in the urban, but not in the rural, environment. Similarly, we predicted (7) a positive association between the PI and the concentration of MDA in the pro-oxidative urban environment, but not in the rural environment.
Materials and Methods
Study Species
All four species feed predominantly on nuts and seeds in winter. However, blue- and great tits maintain a proportion of arthropods (e.g., overwintering insects and spiders) in their winter diet, whereas tree- and house sparrows are largely granivorous at this time of the year. To varying degrees, all species exploit human-provided food sources, such as sunflower seeds, peanuts and bread (Jokimäki and Suhonen, 1998). For practical reasons, it was not possible to monitor individual or species-specific diet composition in the present study.
Field Sites and Sampling
The study was approved by the Malmö/Lund Ethical Committee for Animal Research under the Swedish Board of Agriculture (permit no. M454 12:1), and capture was performed under license from the Swedish Museum of Natural History (to CI, license no. 681). Fieldwork was performed in the province of Scania in southern Sweden from 15-Feb-2013 to 6-April-2013. Urban birds were caught at four locations within the city limits of Malmö, the largest city in southernmost Sweden with approximately 300,000 inhabitants (3,600 inhabitants/ km2; study areas centered at 55°35′ N, 12°59′ E). Samples were collected either in city parks and recreational areas (tree sparrows, blue tits, great tits; two sites), on ruderal land with rich deciduous secondary growth (tree sparrows, blue tits, great tits; one site), or along hedgerows adjacent to granaries (house sparrows; one site). Rural birds were caught at seven locations 15–45 km ENE to NNE of Malmö (with most activity centered on 55°40′ N, 13°31′ E). Sampling was undertaken in deciduous forest stands on grassland (blue tits, great tits, tree sparrows; two sites), along hedgerows on rural farms (all four species; five sites), and in granaries on the same farms (house sparrows, tree sparrows; four of five sites). The rural areas were sparsely inhabited by humans (< 5 inhabitants per km2). Sampling took place throughout the day, from 6 a.m. to 7 p.m., at both urban and rural sites.
All species were caught with mist nets (ntot. = 243 birds), but not all individuals could be measured for all physiological assays due to limited plasma volumes. The assay- and model-specific sample sizes are provided in Table 1, and in figure legends when relevant. All birds were individually ringed and (when plumage characteristics permitted) sexed (great tits, blue tits, house sparrows) according to Svensson (1992). Tree sparrows, which are sexually monomorphic, were molecularly sexed using the primers P2 and P8 (Griffiths et al., 1998). A blood sample (100–125 μl) was collected from the jugular vein with a heparinized syringe, and kept on ice until centrifugation (1,800 rpm for 10 min) and separation of blood plasma 0–1 h later. All samples were stored at −80°C until biochemical analyses.
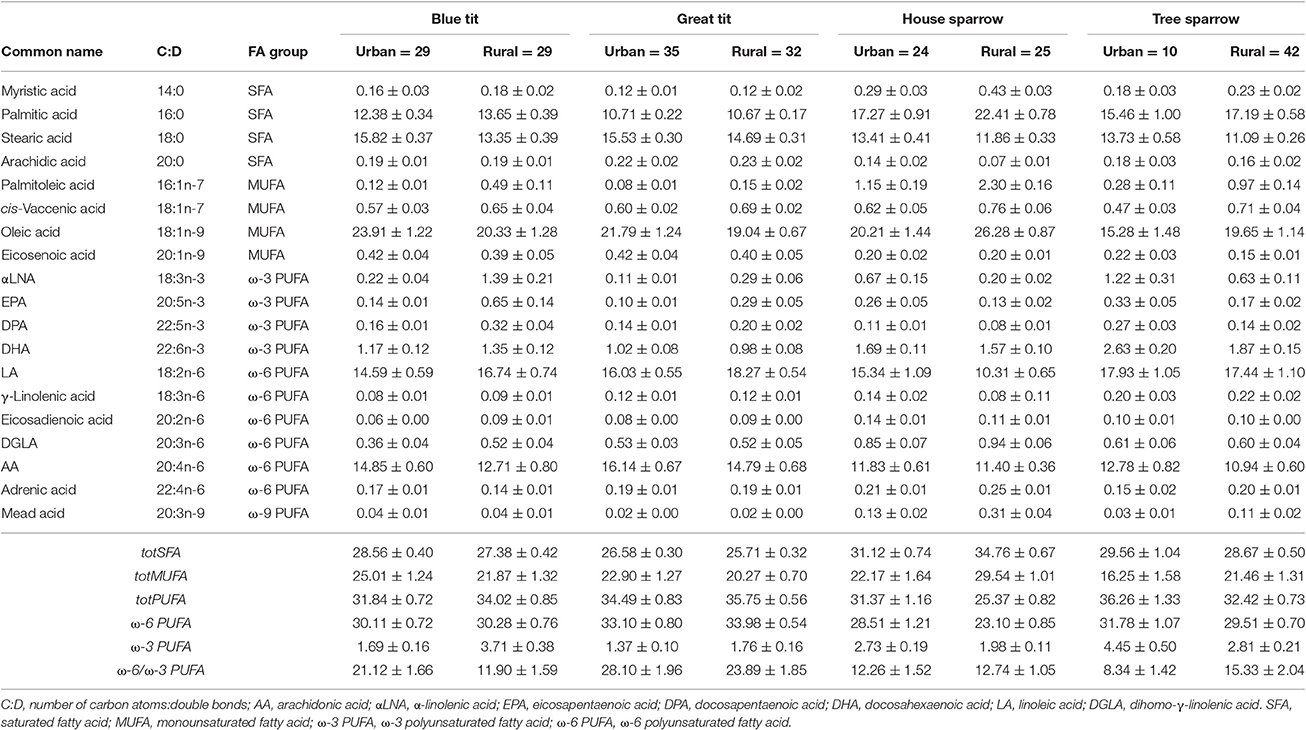
Table 1. Relative abundance of plasma fatty acids (% of total fatty acid content) in four passerine species from urban and rural habitats (mean values ± SEM).
Fatty Acid Extraction and Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
Fatty acids were extracted following the protocol described in Andersson et al. (2015). Briefly, a total lipid extraction of 5 μl plasma was performed for 1 h at room temperature using 50 μl chloroform:methanol (2:1 v/v). The solvent was then evaporated, and the lipid extracts were subjected to base methanolysis for conversion into corresponding fatty acid methyl esters (FAMEs), which were then extracted in n-hexane. Extracts were washed with H2O and then dried over anhydrous sodium sulfate.
The FAME extracts were analyzed using an Agilent 5975 MS coupled to an Agilent 6890 GC equipped with an HP-88 capillary column [(88%-Cyanopropy)aryl-polysiloxane; 30 m, 0.25 mm id, df 0.20 μm; Agilent, CA, USA]. The oven temperature was set to 80°C for 1 min, then increased by 10°C/min to 230°C and held for 20 min. Helium was used as carrier gas at a constant flow of 1 ml/min. FAMEs present in plasma were identified by comparing their mass spectra and retention times with those of synthetic standards (Supelco 37-Component FAME Mix, Sigma-Aldrich, Stockholm, Sweden).
Lipid Peroxidation Quantification
We quantified malondialdehyde (MDA), the most frequently used biomarker of overall lipid peroxidation, following the protocol described in Eikenaar et al. (2016). Briefly, 15 μl plasma was mixed with 50 μl O– (2, 3, 4, 5, 6-pentafluorbenzyl) solution (1 mM in sodium acetate buffer, pH 5.0) and the micro-reaction was conducted for 1 h at room temperature. The resulting MDA-bis-(PFB-oxime) derivatives were extracted in 300 μl n-hexane containing 1.65 pg/μl of 3-bromofluorobenzene as internal standard, and analyzed using an Agilent 5975 MS coupled to an Agilent 6890 GC. A non-polar capillary column, HP-5MS (30 m, 0.25 mm id, df 0.25 μm; J&W Scientific, USA), was used for GC/MS analysis, and the characteristic ion at m/z 250 was measured under selected ion monitoring mode to quantify the target derivatives of MDA. The GC oven was programmed to 60°C for 1 min, followed by 15°C/min to 150°C, and then 10°C/min to 270°C, which was held for 5 min. All chemicals were purchased from Sigma-Aldrich (Stockholm, Sweden).
Data Handling and Statistical Analyses
In total, 19 FAs were identified and quantified in all species (Table 1). The proportion of each FA was calculated by dividing each peak area with the sum of all FA peak areas in each individual. All FA proportions were logit-transformed (log(y/[1-y])), prior to statistical analyses (Warton and Hui, 2011). Individual saturated fatty acids, SFAs (i.e., ∑tot[SFA] = 14:0 + 16:0 + 18:0 + 20:0) were pooled, because they are all mainly used as metabolic fuel, in contrast to mono- and polyunsaturated FAs (i.e., MUFAs and PUFAs) that typically have specific biological functions.
Firstly, general linear models (GLMs) were performed on nineteen variables; fifteen individual FAs, totSFA, total ω-6/ω-3 PUFA ratio, peroxidation index (PI), and MDA. The PI was calculated as PI = [(% monoenoic × 0.025) + (% dienoic × 1) + (% trienoic × 2) + (% tetraenoic × 4) + (% pentaenoic × 6) + (% hexaenoic × 8)] (Pamplona et al., 1998; Kang et al., 2004). PI takes into account that the sensitivity to peroxidation increases as a power function of the number of double bonds in each FA molecule (Pamplona et al., 2000); in other words, the PI provides an estimate of oxidative stress susceptibility in terms of lipid peroxidation. Apart from the GLMs for MDA (see below), all models included two fixed factors: “species” (four levels) and “habitat” (two levels), along with their interaction (species × habitat). In addition, our previous work on great tits has shown that sampling hour affects the proportion of FAs (Isaksson et al., 2015). Therefore, “sampling time” was always included as a covariate in these GLMs. Furthermore, when the interaction between species and habitat was significant in the above model, we proceeded by fitting species-specific GLMs, including time as a covariate. When the main effect of species or habitat was significant, but the interaction between them non-significant, we proceeded by traditional post-hoc analyses (Student's t-test), and removed the interaction from the final model (Supplementary Material 1). There were no sex differences among any of the FAs (ANOVAs: F = 0.0002–2.237, p = 0.14–0.96). Accordingly, sex was excluded from all FA models.
Secondly, multivariate canonical discriminant analysis (CDA) was performed to complement the GLMs for the individual FAs. Here, the proportions of each unsaturated FA along with the proportions of totSFA were included, and the analysis performed both for each habitat separately, and with habitats combined, with species as a categorical variable. These analyses allowed us to test whether the FA profiles showed distinct species- and family (exploiter vs. adapter) signals, and whether these signals were less pronounced in the urban environment due to expected dietary convergence (i.e., reliance on human-provided food sources). The CDA predicts an individual's classification in a category, i.e., species/family, based on observed values of several continuous variables (here, the FAs). A quadratic discriminant method was used, because the covariances between FAs were not expected to be equal across species.
Lastly, MDA was log10-transformed to obtain a Gaussian distribution. The GLM with MDA included the three fixed factors “species,” “habitat,” and “sex,” and the three covariates “sampling hour,” “PI” and the “total ω-6/ω-3 PUFA ratio.” In addition, we included all possible two-way interactions. For MDA, we derived final models using backward-elimination of non-significant (P > 0.05) interactions and covariates, starting with the least significant interaction followed by the covariates.
All analyses were performed in JMP Pro 11.0 (2013 SAS Institute Inc.). For clarity, we only present significant results in the Results Section [see Supplementary Materials 1–3 for all GLMs and species-specific models for FA, FA-derived indices (PI and total ω-6/ω-3 PUFA ratio), and MDA].
Results
Habitat Differences in FA Profiles
Total SFA (totSFA) and MUFA
There was a significant interaction between habitat and species for totSFA [F(3, 217) = 8.37, P < 0.001]. Specifically, urban house sparrows had lower proportions of totSFA than their rural conspecifics [F(1, 46) = 12.39, P = 0.001], but totSFA did not differ between urban and rural populations of the other species (Figure 1A). Oleic acid was by far the most abundant MUFA in all species (Table 1, Figure 1B), although proportions differed for the species in the different habitats [F(3, 217) = 6.28, P < 0.001]. Urban blue tits had a higher proportion of oleic acid compared to their rural conspecifics [F(1, 55) = 6.20, P = 0.016], whereas the opposite pattern was true for house sparrows [F(1, 46) = 13.97, P < 0.001] and tree sparrows [F(1, 51) = 5.46, P = 0.024]. Habitat-wise differences in oleic acid for great tits tended to follow those of blue tits (P = 0.057; Figure 1B and Supplementary Materials 1, 2). Two other MUFAs showed significant habitat effects [palmitoleic acid: F(1, 220) = 46.36, P < 0.001; and cis-vaccenic acid: F(1, 220) = 21.81, P < 0.001; Figures 1C,D], whereas habitat was non-significant for eicosenoic acid (Figure 1E). Across all species, palmitoleic- and cis-vaccenic acid were both higher in the rural habitat than in the urban habitat (P < 0.05 in all cases; Figures 1C,D).
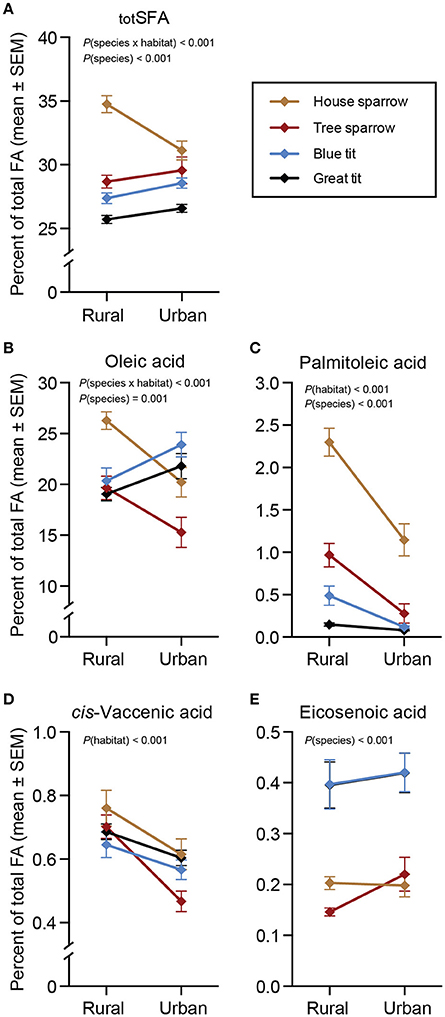
Figure 1. Plasma saturated- and monounsaturated fatty acids (%) in four species of passerines from urban and rural environments. (A) Total saturated fatty acids (totSFA), (B–E) four monounsaturated fatty acids (MUFA). Data are presented as means ± standard error of the means (SEM). Note the different scales on the y-axes. Significant effects of species, habitat and/or the habitat × species interaction are indicated by the P-values. For n-values see Table 1.
ω-3 PUFA
All statistical models for ω-3 PUFAs revealed significant interactions between habitat and species [α-LNA: F(3, 217) = 18.58, P < 0.001; eicosapentaenoic acid (EPA): F(3, 217) = 13.59, P < 0.001; docosapentaenoic acid (DPA): F(3, 216) = 13.88, P < 0.001; docosahexaenoic acid [DHA]: F(3, 217) = 4.69, P = 0.003; Figures 2A–D]. Species-specific habitat models revealed that all ω-3 PUFAs, except DHA, in blue- and great tits were higher in the rural compared to the urban habitat [blue tit: α-LNA: F(1, 57) = 41.56, P < 0.001; EPA: F(1, 57) = 24.73, P < 0.001; DPA: F(1, 57) = 15.49, P < 0.001; great tit: α-LNA: F(1, 66) = 5.68, P = 0.020; EPA: F(1, 66) = 19.97, P < 0.001; DPA: F(1, 66) = 9.96, P = 0.002]. Both sparrow species showed the opposite pattern, with higher proportions of ω-3 PUFAs in the urban compared to the rural habitat [house sparrow: α-LNA: F(1, 48) = 11.84, P = 0.001; DPA: F(1, 48) = 4.24, P = 0.045; tree sparrow: α-LNA: F(1, 51) = 6.67, P = 0.013; EPA: F(1, 51) = 5.22; P = 0.027; DPA: F(1, 51) = 7.88, P = 0.007; DHA: F(1, 51) = 5.04, P = 0.029].
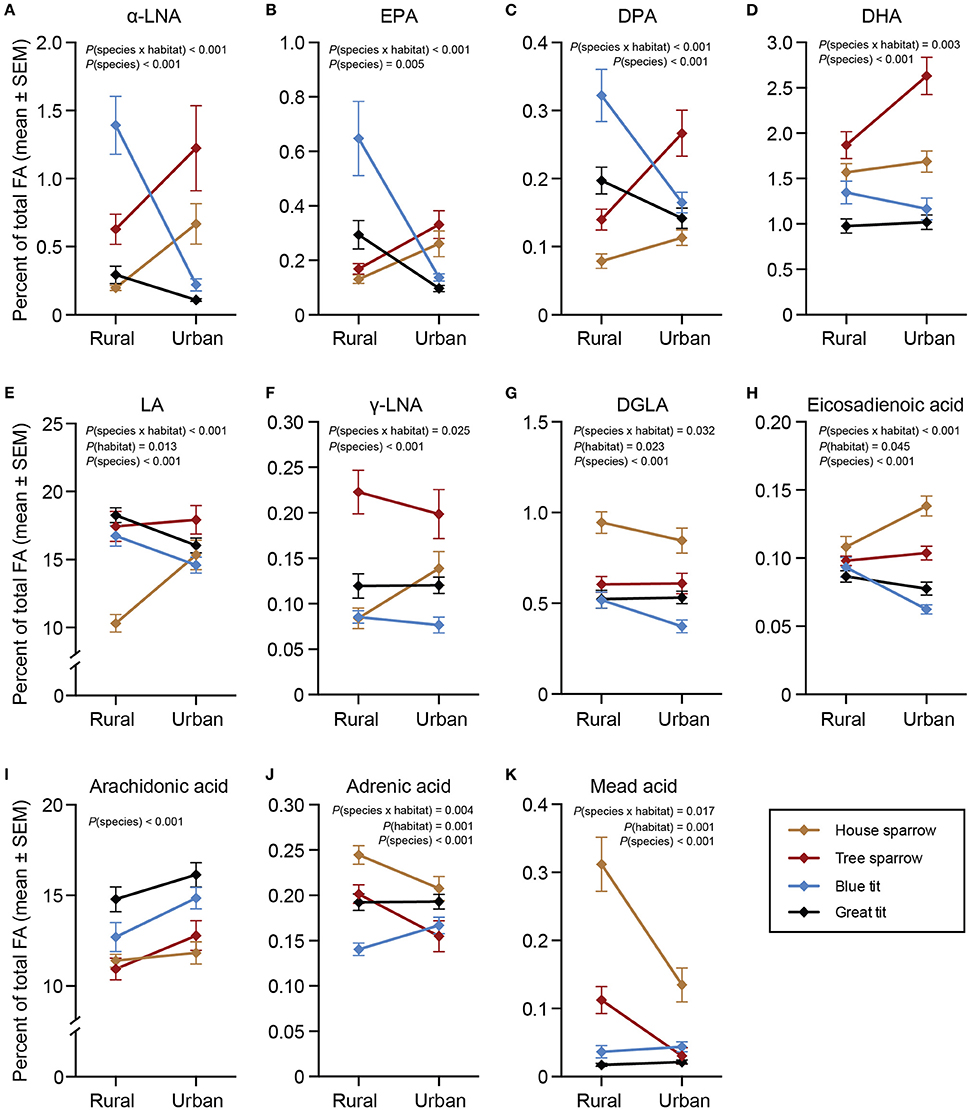
Figure 2. Plasma polyunsaturated fatty acids (%) in four species of passerines from urban and rural environments. (A–D) ω-3 polyunsaturated fatty acids (PUFAs), (E–J) ω-6 PUFAs, and (K) ω-9 PUFA. Data are plotted as means ± standard error of the means (SEM). Note the different scales on the y-axes. Significant effects of species, habitat and/or the habitat × species interaction are indicated by the P-values. For n-values see Table 1. One urban blue tit did not have detectable levels of DGLA, thus the sample size was reduced from 29 to 28. α-LNA, α-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid, DHA, docosahexaenoic acid; LA, linoleic acid; γ-LNA, γ-linolenic acid; DGLA, dihomo-γ- linolenic acid.
ω-6 PUFA
There were significant interactions between habitat and species for all ω-6 PUFAs, except arachidonic acid [LA: F(3, 217) = 7.97, P < 0.001; γ-linolenic acid (γ-LNA): F(3, 217) = 3.18, P = 0.025; eicosadienoic acid: F(3, 217) = 13.71, P < 0.001; dihomo-γ-linolenic acid (DGLA): F(3, 217) = 3.00, P = 0.032; adrenic acid: F(3, 217) = 4.54, P = 0.004; Figures 2E–J]. Urban blue tits had lower eicosadienoic acid [F(1, 57) = 20.90, P < 0.001] and DGLA [F(1, 57) = 5.73, P = 0.020], and urban great tits had lower LA [F(1, 66) = 4.52, P = 0.037] and eicosadienoic acid [F(1, 66) = 8.00, P = 0.006] compared to their rural conspecifics. Interestingly, house sparrows showed the opposite result with urban individuals having higher LA [F(1, 48) = 20.77, P = 0.001], γ-LNA [F(1, 48) = 5.71, P = 0.021], and eicosadienoic acid [F(1, 48) = 8.72, P = 0.005]. Similar to urban blue tits, however, urban house sparrows had lower DGLA [F(1, 48) = 6.27, P = 0.016] than the rural ones. Finally, urban house- and tree sparrows had lower proportions of adrenic acid [house sparrows: F(1, 48) = 11.33, P = 0.002; tree sparrows: F(1, 51) = 12.99, P < 0.001] compared to their rural conspecifics.
ω-9 PUFA
There was a significant interaction between species and habitat for mead acid [F(3, 217) = 3.45, P = 0.017; Figure 2K]. The species-specific models revealed that urban sparrows had lower mead acid proportions than rural sparrows [house sparrow: F(1, 48) = 13.99, P < 0.001; tree sparrow: F(1, 51) = 4.74, P = 0.034].
Species Differences in FA Profiles
The CDA analyses revealed that the first three discriminant functions significantly discriminated the four species in both urban and rural habitats [urban: P < 0.001, eigenvalues: Canonical 1 (C1) = 10.325, C2 = 1.091, C3 = 0.611; Figure 3A; rural: P < 0.001, eigenvalues: C1 = 11.808, C2 = 1.103, C3 = 0.743; Figure 3B]. In the urban habitat, C1 accounted for 85.85%, C2 for 9.07%, and C3 for 5.08% of the variation [Wilk's lambda: 0.026, F(48, 233) = 11.65, P < 0.001]. In the rural habitat, C1 accounted for 86.47%, C2 for 8.083% and C3 for 5.45% of the variation [Wilk's lambda: 0.021, F(48, 325) = 17.93, P < 0.0001]. 98.97 and 99.22% of the individuals were correctly assigned to the pre-determined groups (species) in the urban and rural habitat, respectively. When birds from the two habitats were analyzed together the correct species assignment was reduced to 93.34%, but the model was still highly significant [Wilk's lambda: 0.044, F(48, 613) = 23.80, P < 0.001; Figure 3C]. Most misclassified individuals were still assigned to the correct family, with only one blue tit misclassified as a tree sparrow. Similarly, there was a clear distinction between the two families (adapters vs. exploiters), despite the significant species signals [Wilk's lambda: 0.118, F(16, 208) = 97.02, P < 0.001].
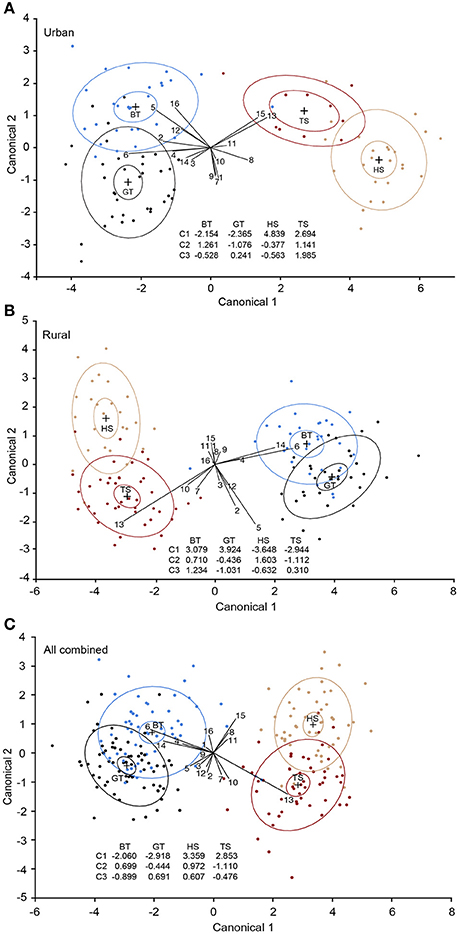
Figure 3. Interspecific comparison of overall plasma fatty acid compositions in four passerine species. (A) Urban birds, (B) rural birds, and (C) urban and rural birds combined. The plots are based on the first (C1) and the second (C2) discriminant functions estimated from canonical discriminant analyses (CDA) using 15 individual fatty acids (FAs) along with total saturated fatty acids. Although the figure is two-dimensional, the CDA model is based on FA loadings of three functions, hence all three discriminant functions are presented. The ellipses represent 95% of the data clouds of each species. BT, blue tit (blue); GT, great tit (black); HS, house sparrow (tan); TS, tree sparrow (dark red). 1, palmitoleic acid; 2, oleic acid; 3, cis-vaccenic acid; 4, eicosenoic acid; 5, linoleic acid; 6, arachidonic acid; 7, γ-linolenic acid; 8, eicosadienoic acid; 9, dihomo-γ- linolenic acid; 10, adrenic acid; 11, α-linolenic acid; 12, eicosapentaenoic acid; 13, docosahexaenoic acid; 14, docosapentaenoic acid; 15, mead acid; 16, total saturated fatty acids.
In the full-species GLMs, there was a clear effect of the main factor “species” for two of the MUFAs: palmitoleic acid [F(3, 220) = 54.11, P < 0.001] and eicosenoic acid [F(3, 220) = 25.55, P < 0.001]. The post-hoc analyses revealed that specifically palmitoleic acid was significantly higher in house sparrows than in any of the other species, and eicosenoic acid was significantly higher in the two tit species than in the sparrows (P < 0.05 in all cases; Figures 1C,E). Finally, the ω-6 PUFA, arachidonic acid, differed significantly between species [F(3, 220) = 35.90, P < 0.001], with the two tit species having higher proportions than the two sparrow species (P < 0.05 in all cases; Figure 2I).
Habitat Differences in Health Markers
ω-6/ω-3 PUFA Ratio
The total ω-6/ω-3 PUFA ratio showed a significant habitat by species interaction [F(3, 217) = 6.04, P < 0.001; Figure 4A]. Species-specific analyses revealed that only the blue tit differed between the urban and rural habitats, with rural birds having a lower ratio compared to urban birds [F(1, 57) = 13.84, P < 0.001].
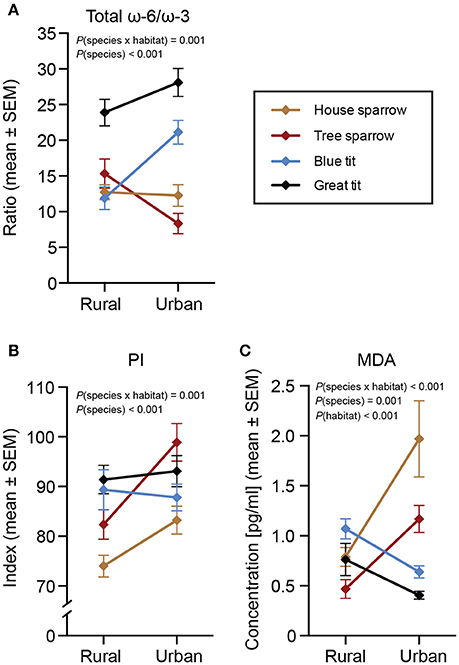
Figure 4. Biomarkers of health in four species of passerines from urban and rural environments. (A) Total ω-6/ω-3 polyunsaturated fatty acids (PUFA) ratio, (B) peroxidation index (PI), and (C) lipid peroxidation (MDA). Data are plotted as means ± standard error of the means (SEM). Note the different scales on the y-axes. Significant effects of species, habitat and/or the habitat × species interaction are indicated by the P-values. The n-values for (A,B) are presented in Table 1. The n-values for MDA (C) were: house sparrowurban = 24, house sparrowrural = 24; tree sparrowurban = 12, tree sparrowrural = 44; blue titurban = 32, blue titrural = 30; great titurban = 34, great titrural = 29.
Peroxidation Index (PI)
The interaction between habitat and species was significant also for PI [F(3, 217) = 5.54, P = 0.001; Figure 4B]. Specifically, the PI was higher for urban house sparrows [F(1, 48) = 4.80, P = 0.034] and tree sparrows [F(1, 51) = 6.86, P = 0.012] than for their rural conspecifics, whereas there was no significant inter-habitat variation in the two tit species.
Malondialdehyde (MDA)
There was a significant interaction between habitat and species also for MDA [F(3, 205) = 26.29, P < 0.001; Figure 4C]. Urban exploiters (i.e., sparrows) of both species had higher MDA in the city compared to conspecifics in the rural habitats [house sparrow: F(1, 47) = 12.27, P = 0.001; tree sparrow: F(1, 55) = 13.42, P < 0.001], whereas urban blue tits had lower MDA than their rural conspecifics [F(1, 61) = 11.97, P = 0.001; note that great tits showed a similar tendency: F(1, 62) = 3.60, P = 0.062]. In addition, MDA was affected by the interaction between PI and species [F(3, 205) = 13.32, P < 0.001], suggesting a negative association between MDA and peroxidizable FAs in tree sparrows, whereas no association was found for the other species. Likewise, we found no support for our prediction about habitat-specific relationships between MDA, PI, and the ω-6/ω-3 PUFA ratio (Supplementary Material 3). Finally, males had higher MDA than females across species [F(1, 205) = 6.44, P = 0.012].
Discussion
In line with our predictions, we found large inter-habitat and inter-specific differences in several FAs, often varying in opposing directions for adapters and exploiters. Secondly, the discriminant analyses revealed species- and family-specific FA profiles, suggesting that FA compositions could be informative when investigating inter- and intraspecific variation in nutrition and lipid metabolism. However, in contrast to our prediction, there was no consistent convergence of FA profiles of the four species in the urban habitat (i.e., due to expected dietary similarity). Thirdly, significant interactions between habitat and species were revealed for all three health indices. Urban blue tits had higher total ω-6/ω-3 PUFA ratio than their rural conspecifics, which follows our prediction about the lower availability of ω-3 PUFA in the urban habitat. MDA was higher in the urban than in rural individuals for the two exploiter species, whereas the adapter species showed the opposite pattern. In the exploiters, the peroxidation index (PI) increased from rural to urban, but this was not the case for the adapters. Taken together, the effects of the urban environment on health indices largely followed our general predictions. However, the opposing effects between the adapter and exploiter species were striking and surprising, which we discuss below.
Habitat Differences in Fatty Acid Profiles
The exploiter and adapter species often showed opposite differences in plasma FA composition between the urban and rural environments (Figures 1, 2). For instance, urban blue tits had higher proportions of the main MUFA oleic acid (great tits showed a similar tendency), whereas both urban house- and tree sparrows had lower oleic acid than their rural counterparts. For totSFA, a habitat difference was only found in house sparrows, with urban individuals having lower totSFA than the rural conspecifics. Since both SFAs and MUFAs can be biosynthesized by birds, their relative levels can be controlled to a larger extent than the levels of PUFAs. Thus, the results for totSFA and MUFA are likely due to a combination of metabolic regulation, and habitat- and species-specific diets.
Moreover, urban exploiters had lower proportions of several ω-3 PUFAs, whereas urban adapters had higher proportions than their rural conspecifics. Differences between the habitats in plasma levels of the essential ω-3 PUFA α-LNA should be a direct reflection of dietary differences. This could be a result of either different food items being eaten/or available across the habitats, or habitat-specific differences in fatty acid composition of the same food items. Our previous diet experiments on great tits showed that the proportion of all ω-3 PUFA in plasma is highly affected by availability in diet, with plasma amounts rapidly declining (within a few days) after restricting ω-3 PUFAs in the diet (Andersson et al., unpublished). Thus, the results also for the other ω-3 PUFAs in the present study are likely to be at least partly affected by dietary differences across the habitats. In addition, it is also possible that there are species/family-specific effects on metabolic conversion across habitats (Jing et al., 2013; Watson et al., 2017). Regardless, the lower ω-3 PUFA of rural house- and tree sparrows compared to urban ones indicates a “poorer” nutritional status at the rural farms, rather than in the urban habitat. In support of this argument, mead acid—a suggested biomarker of malnutrition or deficiency of essential FAs (Mead and Slaton, 1956; Smit et al., 2004)—was higher in the rural house- and tree sparrows than in the urban conspecifics. Furthermore, the strictly dietary ω-6 PUFA LA was lower for urban great tits, but higher for urban house sparrows compared to their rural conspecifics. Such a contrasting effect of habitat was also evident for eicosadienoic acid, with both adapters having lower levels in the urban habitat, whereas urban house sparrows had higher levels than rural ones. Whether these interspecific differences have any adaptive values in the urban-rural context requires further investigation.
Species and Family Differences in Fatty Acid Profiles
The present study is the first to investigate interspecific differences in FA profiles among terrestrial bird species. Previously, a similar approach for FA profiles was used on pelagic seabirds in relation to their feeding ecology (Iverson et al., 2007). In that study, a captive feeding experiment was conducted along with sampling of adipose tissue in four species of seabirds and their natural prey. Distinct species-specific discrimination of FA signatures was evident, which could be explained by interspecific differences in diet (Iverson et al., 2007). It was suggested that species FA signatures could be a complement or even an alternative to stable isotope analyses, because FA profiles can provide more information about the diet composition and not simply indicate trophic levels. Although the current study cannot link the plasma FA content with the FA profile of specific food items, it supports the previous conclusions given the clear species and family distinction in FA profiles.
More specifically, our CDA revealed that the two bird families (or the adapter vs. exploiter categorization) were separated by the first canonical function (C1) (Figure 3). The separation between adapters and exploiters could be a combined result of shared evolutionary history, as well as similar life-styles and diets among the two species within each group. Unfortunately, the relative contribution of each of these factors could not be determined. However, despite the resemblance between the species of the same taxonomic family, and the variation in FAs in birds across the habitats, the four species were also discriminated by the FA loadings on C2 and C3 both within each habitat and across habitats. Overall, 99% of the birds were successfully assigned into the correct species within each habitat, and although the correctness of species classification was reduced when birds from the two habitats were combined, it was still high (93%). Interestingly, both α-LNA and mead acid signified house sparrows with a decrease in α-LNA and an increase in mead acid likely indicating malnutrition or nutrient deficiency (Mead and Slaton, 1956; Smit et al., 2004). The effect of mead acid was only present in the rural habitat, which is in line with the suggestion that the rural house sparrows are more malnourished than urban conspecifics (see above).
Species and Habitat Differences in Health Markers
Despite the interspecific differences across the urban-rural habitats in several ω-3 and ω-6 PUFAs, only the blue tit differed in the total ω-6/ω-3 ratio between the habitats. As predicted, the urban blue tits had a higher ratio than the rural blue tits, suggesting that urban birds have a diet-induced increased susceptibility to inflammation when exposed to antigens, such as traffic-generated particulate matter and pathogens (Ezenwa, 2004; Romieu et al., 2008; Isaksson, 2015). Since non-pathogenic inflammatory agents are more common in the urban environment, future studies should investigate whether urban blue tits have a higher inflammatory response than their rural conspecifics, and whether this could be explained by their higher ω-6/ω-3 PUFA ratio. However, at the species level, great tits had the highest ratio of all species across habitats (Figure 4A). Thus, also great tits are likely to suffer from increased, dietary induced, negative effects, if exposed to pro-inflammatory agents, such as urban air pollution.
MDA is a commonly used biomarker for lipid peroxidation (i.e., oxidative damage) that is generated by pro-oxidant exposure (e.g., Del Rio et al., 2005; Lykkesfeldt, 2007). The two exploiter species followed our prediction, with higher MDA in the urban habitat as compared to the rural habitat. Surprisingly, blue tits had lower MDA in the urban compared to the rural environment. Previous studies on lipid peroxidation in birds have not detected a difference between urban and rural populations (great tits in Gothenburg: Isaksson et al., 2009; house sparrows in Madrid: Herrera-Dueñas et al., 2014). Possibly, our more sensitive quantification of MDA using GC/MS, compared to the absorbance measurements used in the previous studies (thiobarbituric acid [TBARS] assays), is able to more accurately detect smaller differences in lipid peroxidation (Del Rio et al., 2005). Alternatively, lipid peroxidation may be more context-, nutrition-, and species-specific than previously appreciated, particularly since it exhibited the opposite patterns in the two bird families. In support of this argument, both urban exploiter species also showed higher susceptibility to lipid peroxidation, as indicated by the higher PI, than rural birds. This result might be a consequence of population differences in dietary intake or biosynthesis of the long-chain, peroxidation prone, PUFAs, especially the highly unsaturated DHA.
Moreover, due to high levels of traffic-generated air pollution (acting as pro-oxidants) in the urban habitat, we predicted that MDA levels in urban birds would be positively associated with the ω-6/ω-3 PUFA ratio (via increased ROS production during a pro-inflammatory response), and/or the PI (via increased susceptibility to FA peroxidation). However, in contrast to our predictions, there was no significant interaction between habitat and either of the two FA-derived estimates. Instead, there was an interaction between species and PI, revealing a negative association between PI and MDA in tree sparrows, but not in the other species. The direction of this association in tree sparrows is in contrast to our prediction, and also to the results at the population level (where both MDA and PI were higher in the urban compared to the rural environment). Possibly, individual tree sparrows with a high PI have a corresponding increase in dietary antioxidants (Eikenaar et al., 2016, 2017). This could prevent lipid peroxidation to some degree, but not enough to override the population/habitat effect. Alternatively, individuals with a high peroxidation rate, independent of habitat (e.g., due to other unknown factors such as age and life history), might reduce their plasma PUFA levels, thus reducing the PI.
Conclusions
The present study reveals interspecific and inter-habitat differences in FA profiles and health markers associated with FA intake and physiology (cf. Fokidis et al., 2008). These differences could make the two urban adapters (blue tits and great tits) more prone to be negatively affected by inflammatory responses via their higher ω-6/ω-3 ratio, whereas the urban exploiters (house- and tree sparrows) are likely to be more affected by oxidative stress, at least in terms of lipid peroxidation.
Moreover, multivariate analyses of FA profiles can provide important information about species-, population-, and individual-level variation in foraging behaviors and feeding ecology, which can be of particular value in the urban context where human-provided foods are abundant. These food sources can reduce winter mortality caused by starvation and possibly predation (Jansson et al., 1981; Hole et al., 2002; Perkins et al., 2007). However, artificial food might also impact fitness negatively by causing deficiency of essential micro-and macro-nutrients (Isaksson and Andersson, 2007; Plummer et al., 2013; Isaksson, 2015). This could be exacerbated by the presumably poorer quality of natural food in the urban habitat. Disentangling the mechanistic underpinnings of how, and when, the negative effects of anthropogenic food outweigh the positive effects will be important for understanding its short- and long-term impacts on wild birds.
Ethics Statement
The present study was carried out in accordance with a license (Dnr M454 12:1) from the Swedish Board of Agriculture, and capture was performed under C.Isaksson's ringing license (ID: 681) from the Natural History Museum, Sweden.
Author Contributions
CI, AN, and MP designed the study. CI, AN, and MP performed fieldwork. CI, MA, and HW performed the laboratory analyses. CI, MA, and AN drafted the manuscript. MP and HW edited the manuscript. All authors reviewed and approved the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer CH and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
We are grateful to Christer Löfstedt for hosting MA and HW. We thank Amparo Herrera-Dueñas for molecular sexing of tree sparrows. We also acknowledge Sydvatten AB and the City of Malmö for access to field localities, and Lennart Blomquist for help with field work in Malmö. CI was funded by a Marie Curie re-integration grant (FP7-CIG), Carl Trygger's Foundation, the Royal Physiographic Society in Lund, and the Crafoord Foundation. AN was supported by the Swedish Research Council (grant no. 637-2013-7442).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fevo.2017.00044/full#supplementary-material
References
Andersson, M. N., Wang, H.-L., Nord, A., Salmon, P., and Isaksson, C. (2015). Composition of physiologically important fatty acids in great tits differs between urban and rural populations on a seasonal basis. Front. Ecol. Evol. 3:93. doi: 10.3389/fevo.2015.00093
Atwell, J. W., Cardoso, G. C., Whittaker, D. J., Campbell-Nelson, S., Robertson, K. W., and Ketterson, E. D. (2012). Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav. Ecol. 23, 960–969. doi: 10.1093/beheco/ars059
Austin, J. E. (1993). Fatty acid composition of fat depots in wintering Canada geese. Wilson Bull. 105, 339–347.
Beckerman, A. P., Boots, M., and Gaston, K. J. (2007). Urban bird declines and the fear of cats. Anim. Conserv. 320, 320–325. doi: 10.1111/j.1469-1795.2007.00115.x
Ben-Hamo, M., McCue, M. D., McWilliams, S. R., and Pinshow, B. (2011). Dietary fatty acid composition influences tissue lipid profiles and regulation of body temperature in Japanese quail. J. Comp. Physiol. B. Biochem. Syst. Environ. Physiol. 181, 807–816. doi: 10.1007/s00360-011-0558-2
Calder, P. C. (2007). Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 77, 327–335. doi: 10.1016/j.plefa.2007.10.015
Calder, P. C. (2009). Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie 91, 791–795. doi: 10.1016/j.biochi.2009.01.008
Crick, H. P. Q., Marchant, J. H., Noble, D. G., Baillie, S. R., Balmer, D. E., Beaven, L. P., et al. (2003). Breeding Birds in the Wider Countryside: their Conservation Status 2003 – Trends in Numbers and Breeding Performance for UK Birds. Thetford: British Trust for Ornithology.
Crick, H. P. Q., Robertson, R. A., Appleton, G. F., Clark, N. A., and Rickard, A. D. (2002). Investigation into the Causes of the Decline of Starlings and House Sparrows in Great Britain. A Report to the Department for Environment, Food and Rural Affairs by a consortium led by the British Trust for Ornithology, DEFRA, Bristol.
Del Rio, D., Stewart, A. J., and Pellegrini, N. (2005). A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovas. 15, 316–328. doi: 10.1016/j.numecd.2005.05.003
Dominoni, D., Quetting, M., and Partecke, J. (2013). Artificial light at night advances avian reproductive physiology. Proc. R. Soc. 280:20123017. doi: 10.1098/rspb.2012.3017
Eikenaar, C., Jönsson, J., Fritzsch, A., and Wang, H-L., Isaksson, C. (2016). Migratory refueling affects non-enzymatic antioxidant capacity, but does not increase lipid peroxidation. Physiol. Behav. 158, 26–32. doi: 10.1016/j.physbeh.2016.02.033
Eikenaar, C., Källstig, E., Andersson, M. N., Herrera-Dueñas, A., and Isaksson, C. (2017). Oxidative challenges of avian migration: a comparative field study on a partial migrant. Physiol. Biochem. Zool. 90, 223–229. doi: 10.1086/689191
Ezenwa, V. O. (2004). Interaction among host diet, nutritional status and gastrointestinal parasite infection in wild bovids. Int. J. Parasitol. 34, 535–542. doi: 10.1016/j.ijpara.2003.11.012
Fokidis, H. B., Orchinik, M., and Deviche, P. (2008). Corticosterone and corticosteroid binding globulin in birds: relation to urbanization in a desert city. Gen. Comp. Endocrin. 160, 259–270. doi: 10.1016/j.ygcen.2008.12.005
Fuller, R. A., Warren, P. H., and Gaston, K. J. (2007). Day-time noise predicts nocturnal singings in urban robins. Biol. Lett. 3, 386–370. doi: 10.1098/rsbl.2007.0134
Gorissen, L., Snoeijs, T., Van Duyse, E., and Eens, M. (2005). Heavy metal pollution affects dawn singing behaviour in a small passerine bird. Oecologia 145, 504–509. doi: 10.1007/s00442-005-0091-7
Griffiths, R., Double, M. C., Orr, K., and Dawson, R. J. (1998). A DNA test to sex most birds. Mol. Ecol. 7, 1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x
Grimm, N. B., Faeth, S. H., Golubiewski, N. E., Redman, C. L., Wu, J., Bai, X., et al. (2008). Global change and the ecology of cities. Science 319, 756–760. doi: 10.1126/science.1150195
Gutteridge, J. M. (1995). Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 41, 1819–1828.
Hazel, J. R. (1995). Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 57, 19–42. doi: 10.1146/annurev.ph.57.030195.000315
Herrera-Dueñas, A., Pineda, J., Antonio, M. T., and Aguirrea, J. I. (2014). Oxidative stress of house sparrow as bioindicator of urban pollution. Ecol. Indic. 42, 6–9. doi: 10.1016/j.ecolind.2013.08.014
Hole, D. G., Whittingham, M. J., Bradbury, R. B., Anderson, G. Q. A., Lee, P. L. M., Wilson, J. D., et al. (2002). Agriculture: widespread local house-sparrow extinctions. Nature 418, 931–932. doi: 10.1038/418931a
Hulbert, A., and Abbott, S. K. (2012). Nutritional ecology of essential fatty acids: an evolutionary perspective. Aust. J. Zool. 59, 369–379. doi: 10.1071/ZO11064
Isaksson, C. (2010). Pollution and its impact on wild animals: a meta-analysis on oxidative stress physiology. Ecohealth 7, 342–350. doi: 10.1007/s10393-010-0345-7
Isaksson, C. (2015). Urbanisation, oxidative stress and inflammation: a question of evolving, acclimatizing or coping with urban environmental stress. Funct. Ecol. 29, 913–923. doi: 10.1111/1365-2435.12477
Isaksson, C., and Andersson, S. (2007). Carotenoid diet and nestling provisioning in urban and rural great tits, Parus major. J. Avian Biol. 38, 564–572. doi: 10.1111/j.0908-8857.2007.04030.x
Isaksson, C., Hanson, M. A., and Burdge, G. C. (2015). The effects of spatial and temporal ecological variation on fatty acid compositions of wild great tits (Parus major). J. Avian Biol. 46, 245–253. doi: 10.1111/jav.00409
Isaksson, C., Örnborg, J., Stephensen, E., and Andersson, S. (2005). Plasma glutathione and carotenoid coloration as potential biomarkers of environmental stress in great tits. Ecohealth 2, 138–146. doi: 10.1007/s10393-005-3869-5
Isaksson, C., Sheldon, B. C., and Uller, T. (2011). The challenges of integrating oxidative stress into life history biology. Bioscience 60, 194–202. doi: 10.1525/bio.2011.61.3.5
Isaksson, C., Sturve, J., Almrot, B. C., and Andersson, S. (2009). The impact of urban environment on oxidative damage (TBARS) and enzymatic and non-enzymatic defence system in lungs and liver of great tits, Parus major. Environ. Res. 109, 46–50. doi: 10.1016/j.envres.2008.10.006
Iverson, S. J., Springer, A. M., and Kitaysky, A. S. (2007). Seabirds as indicators of food web structure and ecosystem variability: qualitative and quantitative diet analyses using fatty acids. Mar. Ecol. Prog. Ser. 352, 235–244. doi: 10.3354/meps07073
Jansson, C., Ekman, J., and von Brömssen, A. (1981). Winter mortality and food supply in tits Parus spp. Oikos 37, 313–322. doi: 10.2307/3544122
Jing, M., Gakhar, N., Gibson, R. A., and House, J. D. (2013). Dietary and ontogenic regulation of fatty acid desaturase and elongase expression in broiler chickens. Prostaglandins Leukot. Essent. Fatty Acids 89, 107–113. doi: 10.1016/j.plefa.2013.05.006
Jokimäki, J. (1999). Occurrence of breeding bird species in urban parks: effects of park structure and broad-scale variables. Urban Ecosyst. 3, 21–34. doi: 10.1023/A:1009505418327
Jokimäki, J., and Suhonen, J. (1998). Distribution and habitat selection of wintering birds in urban environments. Landsc. Urban Plan. 39, 253–263. doi: 10.1016/S0169-2046(97)00089-3
Jokimäki, J., Suhonen, J., Inki, K., and Jokinen, S. (1996). Biogeographical comparison of winter bird assemblages in urban environments in Finland. J. Biogeogr. 23, 379–386. doi: 10.1046/j.1365-2699.1996.00033.x
Jones, D. N., and Reynolds, S. J. (2008). Feeding birds in our towns and cities: a global research opportunity. J. Avian Biol. 39, 265–271. doi: 10.1111/j.0908-8857.2008.04271.x
Kang, M. J., Lee, E. K., and Lee, S. S. (2004). Effects of two P/S ratios with same peroxidizability index value and antioxidants supplementation on serum lipid concentration and hepatic enzyme activities of rats. Clin. Chim. Acta 350, 79–87. doi: 10.1016/j.cccn.2004.07.005
Kempenaers, B., Börgstroem, P., Loes, P., Schlicht, E., and Valcu, M. (2010). Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. doi: 10.1016/j.cub.2010.08.028
Kiecolt-Glasera, J. K., Epel, E. S., Belury, M. A., Andridge, R., Lin, J., Glaser, R., et al. (2013). Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: a randomized controlled trial. Brain Behav. Immun. 28, 16–24. doi: 10.1016/j.bbi.2012.09.004
Larsson, S. C., Kumlin, M., Ingelman-Sundberg, M., and Wolk, A. (2004). Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am. J. Clin. Nutr. 79, 935–945.
Lykkesfeldt, J. (2007). Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin. Chim. Acta 380, 50–58. doi: 10.1016/j.cca.2007.01.028
Marzluff, J. M., Bowman, R., and Dennelly, R. (Eds.). (2001). Avian Ecology and Conservation in an Urbanizing World. Boston, MA: Kluwer Academic Publishers.
McCue, M. D., Amitai, O., Khozin-Goldberg, I., McWilliams, S. R., and Pinshow, B. (2009). Effect of dietary fatty acid composition on fatty acid profiles of polar and neutral lipid tissue fractions in zebra finches, Taeniopygia guttata. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 154, 165–172. doi: 10.1016/j.cbpa.2009.06.002
McKinney, M. L. (2002). Urbanization, biodiversity, and conservation. Bioscience 52, 883–890. doi: 10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2
Mead, J. F., and Slaton, W. H. Jr. (1956). Metabolism of essential fatty acids. III Isolation of 5,8,11-eicosatrienoic acid from fat-deficient rats. J. Biol. Chem. 219, 705–709.
Møller, A. P. (2009). Successful city dwellers: a comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 159, 849–858. doi: 10.1007/s00442-008-1259-8
Møller, A. P., Erritzøe, J., and Karadas, F. (2010). Level of antioxidant in rural and urban birds and their consequences. Oecologia 163, 35–45. doi: 10.1007/s00442-009-1525-4
Pamplona, R., Portero-Otín, M., Riba, D., Requena, J. R., Thorpe, S. R., López-Torres, M., et al. (2000). Low fatty acid unsaturation: A mechanism for lowered lipoperoxidative modification of tissue proteins in mammalian species with long life spans. J. Gerontol. 55A, B286–B291. doi: 10.1093/gerona/55.6.b286
Pamplona, R., Portero-Otín, M., Riba, D., Ruiz, C., Prat, J., Bellmunt, M. J., et al. (1998). Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammals. J. Lipid Res. 39, 1989–1994.
Partecke, J., Schwabl, I., and Gwinner, E. (2006). Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology 87, 1945–1952. doi: 10.1890/0012-9658(2006)87[1945:SATCUA]2.0.CO;2
Perkins, A. J., Anderson, G., and Wilson, J. D. (2007). Seed food preferences of granivorous farmland passerines. Bird Study 54, 46–53. doi: 10.1080/00063650709461455
Pierce, B. J., McWilliams, S. R., O'Connor, T. P., Place, A. R., and Guglielmo, C. G. (2005). Effect of dietary fatty acid composition on depot fat and exercise performance in a migrating songbird, the red-eyed vireo. J. Exp. Biol. 208, 1277–1285. doi: 10.1242/jeb.01493
Plummer, K. E., Bearhop, S., Leech, D. I., Chamberlain, D. E., and Blount, J. D. (2013). Fat provisioning in winter impairs egg production during the following spring: a landscape-scale study of blue tits. J. Anim. Ecol. 82, 673–682. doi: 10.1111/1365-2656.12025
Romieu, I., Castro-Giner, F., Kunzli, N., and Sunyer, J. (2008). Air pollution, oxidative stress and dietary supplementation: a review. Eur. Respir. J. 31, 179–196. doi: 10.1183/09031936.00128106
Salmón, P., Nilsson, J., Nord, A., Bensch, S., and Isaksson, C. (2016). Urban environment shortens telomere length in nestling great tits, Parus major. Biol. Lett. 12:20160155. doi: 10.1098/rsbl.2016.0155
Schoech, S. J., and Bowman, R. (2003). Does differential access to protein influence differences in timing of breeding of Florida scrub-jays (Aphelocoma coerulsecens) in suburban and wildland habitats. Auk 120, 1114–1127. doi: 10.1642/0004-8038(2003)120[1114:DDATPI]2.0.CO;2
Schoech, S. J., Bowman, R., and Reynolds, S. J. (2004). Food supplementation and possible mechanisms underlying early breeding in the Florida scrub-jay (Aphelocoma coerulsecens). Horm. Behav. 46, 565–573. doi: 10.1016/j.yhbeh.2004.06.005
Simopoulos, A. P. (2002). The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 56, 365–379. doi: 10.1016/S0753-3322(02)00253-6
Smit, E. N., Muskiet, F. A. J., and Boersma, E. R. (2004). The possible role of essential fatty acids in the pathophysiology of malnutrition: a review. Prostaglandins Leukot. Essent. Fatty Acids 71, 241–250. doi: 10.1016/j.plefa.2004.03.019
Toledo, A., Andersson, M. N., Wang, H.-L., Salmón, P., Watson, H., Burdge, G. C., et al. (2016). Fatty acid profiles of great tit (Parus major) eggs differ between urban and rural habitats, but not between coniferous and deciduous forests. Sci. Nat. 103, 55. doi: 10.1007/s00114-016-1381-0
Twining, C. W., Brenna, J. T., Lawrence, P., Shipley, J. R., Tollefson, T. N., and Winkler, D. W. (2016). Omega-3 long-chain polyunsaturated fatty acids support aerial insectivore performance more than food quantity. Proc. Natl. Acad. Sci. U.S.A. 113, 10920–10925. doi: 10.1073/pnas.1603998113
Warton, D. I., and Hui, F. K. C. (2011). The arcsine is asinine: the analysis of proportions in ecology. Ecology 92, 3–10. doi: 10.1890/10-0340.1
Keywords: diet, lipid peroxidation, omega-3, omega-6, Paridae, Passeridae, polyunsaturated fatty acid, urbanization
Citation: Isaksson C, Andersson MN, Nord A, von Post M and Wang H-L (2017) Species-Dependent Effects of the Urban Environment on Fatty Acid Composition and Oxidative Stress in Birds. Front. Ecol. Evol. 5:44. doi: 10.3389/fevo.2017.00044
Received: 14 February 2017; Accepted: 25 April 2017;
Published: 12 May 2017.
Edited by:
François Criscuolo, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Pierre J. Deviche, Arizona State University, USACaroline Habold, Centre National de la Recherche Scientifique (CNRS), France
Copyright © 2017 Isaksson, Andersson, Nord, von Post and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caroline Isaksson, caroline.isaksson@biol.lu.se
†Present Address: Andreas Nord, Department of Arctic and Marine Biology, Arctic Animal Physiology, University of Tromsø, Tromsø, Norway
 Caroline Isaksson
Caroline Isaksson Martin N. Andersson
Martin N. Andersson Andreas Nord
Andreas Nord Maria von Post
Maria von Post  Hong-Lei Wang
Hong-Lei Wang