Two Neural Measures Differ between Urban and Rural Song Sparrows after Conspecific Song Playback
- Department of Biological Sciences, Virginia Tech, Blacksburg, VA, USA
Urbanization is a critical form of environmental change that can affect the physiology and behavior of wild animals and, notably, birds. One behavioral difference between birds living in urban and rural habitats is that urban males show elevated boldness or territorial aggression in response to simulated social challenge. This pattern has been described in several populations of song sparrow, Melospiza melodia. Such behavioral differences must be underpinned by differences in the brain, yet little work has explored how urbanization and neural function may be interrelated. We explored the relationship between urbanization and neural activation within a network of brain regions, collectively called the social behavior network, which contributes to the regulation of territorial aggression. Specifically, we captured free-living, territorial male song sparrows by playing them conspecific songs for 6–11 min, and then collected their brains. We estimated recent neural activation, as indicated by the immediate early gene FOS, and measured levels of a neuropeptide, arginine vasotocin (AVT), which is involved in the regulation of social behavior. Based on previous studies we expected urban males, which are generally more territorially aggressive, to have lower FOS expression in a node of the social behavior network implicated in regulating territoriality, the lateral septum (LS). Additionally, we expected urban males to have lower AVT expression in a brain region involved in the regulation of sociality, the medial bed nucleus of the stria terminalis (BSTm). We found that, compared to rural males, urban male song sparrows did have lower FOS expression in the LS. This pattern suggests that lower neural activation in the LS could contribute to behavioral adjustments to urbanization in male song sparrows. Additionally, counter to our predictions, urban male song sparrows had higher AVT-like immunoreactivity in the BSTm. Future work building upon these findings is needed to determine the causal role of such neural differences across rural and urban habitats. Understanding the mechanisms impacted by urbanization will inform our understanding of the reversibility and consequences of this form of habitat change.
Introduction
Anthropogenic habitat disturbance is now recognized as impacting the phenotypes of wild animals and is a particular concern for wild birds (Crick, 2004; Both et al., 2006; Caro, 2007; Visser, 2008; Wingfield, 2008; Bonier, 2012; Sol et al., 2013; Wong and Candolin, 2015). Though some species are threatened by anthropogenic habitat disturbance, many animals adjust their behavior and physiology through phenotypic plasticity to cope with such environmental change (Vitousek et al., 1997; Wingfield, 2008; Bonier, 2012; Sol et al., 2013; Wong and Candolin, 2015). Endocrine mechanisms are a major link between organisms' perception of environmental conditions and behavioral and physiological responses, and thus play a central role in mediating phenotypic plasticity or acclimation to urbanization (Lessells, 2008). There is an urgent need to understand the endocrine and neuroendocrine mechanisms that permit animals to cope with changing environments because elucidating how animals adjust their physiology to urbanization will shed light on why some species persist and others decline when faced with a changing environment (Cockrem, 2005; Visser, 2008; Wingfield, 2008; Whitman and Agrawal, 2009; Engel et al., 2011; Hoffmann and Sgrò, 2011; Wong and Candolin, 2015). Reciprocally, determining how novel urban environments alter neuroendocrine and behavioral phenotypes provides insight into the function and evolution of these traits.
Several species of birds and mammals living in urban habitats are more bold or aggressive toward conspecifics than those living in undisturbed habitats (Warren et al., 2006; Parker and Nilon, 2008; Evans et al., 2010; Fokidis et al., 2011; Atwell et al., 2012). Song sparrows are an excellent illustration of this, as multiple research groups have shown that urban male song sparrows are more aggressive toward conspecifics than their rural counterparts (Evans et al., 2010; Foltz et al., 2015b; Davies and Sewall, 2016). Further, male song sparrows living in urban habitats are more bold in response to heterospecific alarm calls and human approach than are rural males, though only response to alarm calls is correlated with conspecific aggression (Evans et al., 2010; Scales et al., 2011; Myers and Hyman, 2016). Understanding the origins of such differences across habitat types requires addressing both ultimate explanations and proximate mechanisms of behavior. Increased boldness or aggression in urban male song sparrows could be a response to resource availability (Foltz et al., 2015b) or differences in conspecific density (though see Davies and Sewall, 2016). However, regardless of the fitness benefit of the behavior, proximate mechanisms must mediate behavioral differences. Despite repeated demonstrations of behavioral differences in song sparrows living along urban-rural gradients, the neural and physiological basis of differences in aggression are not fully understood (Evans et al., 2010; Foltz et al., 2015a; Davies and Sewall, 2016).
Understanding the mechanisms underlying reliable differences in conspecific territorial aggression across habitats is important for predicting the reversibility and fitness consequences of behavioral responses to urbanization. A first step in this process is to compare measures of the physiological processes that underlie behavior in animals living in different habitats to determine which traits vary and therefore could regulate behavioral changes. Prior studies have failed to find reliable differences in levels of the avian stress hormone, corticosterone, between rural and urban songbirds despite predictions that urban habitats could impact stress reactivity (Partecke et al., 2006; Bonier et al., 2007; Schoech et al., 2007; Fokidis et al., 2009, 2011; Atwell et al., 2012; Bonier, 2012; Foltz et al., 2015a). Nor have consistent differences been reported in levels of testosterone, a hormone traditionally thought to promote aggression in vertebrates (Partecke et al., 2005; Fokidis et al., 2011; Deviche and Davies, 2013; Atwell et al., 2014; Davies and Sewall, 2016; Davies et al., 2016). Failure to find differences in circulating hormone levels suggest that reliable behavioral differences may be underpinned by deeper brain mechanisms, yet little work has explored how urbanization and neural function may be interrelated. Therefore, we explored possible relationships between urbanization and neural activation within brain regions involved in the regulation of social behaviors, namely territoriality and aggression, to identify mechanisms that could be impacted by, or reflect behavioral adjustment to, urbanization.
To identify brain mechanisms that could be impacted by urbanization, or underpin adjustments to novel urban environments, we first measured the expression of the immediate early gene (IEG) FOS, a rapidly inducible transcription factor that is a proxy for recent neural activity (Clayton, 2000), within regions of the brain social behavior network. The social behavior network is a taxonomically conserved, reciprocally connected, network of brain regions that play a central role in regulating patterns of social behavior, including regulating male territorial behavior and aggression, across a wide range of taxa (Newman, 1999; Goodson, 2005; O'Connell and Hofmann, 2011). Despite the central role of the social behavior network in regulating conspecific territorial aggression, it remains unclear whether activation of these brain regions in response to social challenge differs across urban and rural habitats. Therefore, we compared FOS expression in the social behavior network of male song sparrows from urban and rural habitats exposed to conspecific song playback. This commonly employed experimental approach (Jarvis et al., 1997; Clayton, 2000) is a first step toward identifying the brain regions underpinning behavioral adjustments to urbanization. Based on previous studies examining territorial aggression and FOS expression in male song sparrows, we expected to find lower FOS-ir in the lateral septum (LS) and paraventricular nucleus (PVN) of urban sparrows (Goodson et al., 2005b).
Additionally, the neuropeptide arginine vasotocin (AVT), the avian homolog of vasopressin (AVP), plays a central role in the modulation of sociosexual behaviors across taxa (Goodson and Bass, 2001; Insel and Young, 2000). Although the AVT and AVP systems have a range of homeostatic functions, including stress responsiveness and osmoregulation (Simon-Oppermann et al., 1980; Madison et al., 2008), this peptide acts within several nodes of the social behavior network to regulate sex and species-typical behaviors (Santangelo and Bass, 2006; Goodson et al., 2009a; Kabelik et al., 2009). In birds, AVT expressing cell bodies in the bed nucleus of the stria terminalis (BSTm) are selectively responsive to social stimuli and are implicated in regulating male territorial aggression, while AVT in the PVN is involved in homeostatic function (Kiss et al., 1987; Panzica et al., 1999; Plumari et al., 2004; Goodson and Wang, 2006; Goodson et al., 2009b; Fokidis and Deviche, 2012). Therefore, we also examined whether AVT expression differed within the BSTm and PVN of male song sparrows living in rural and urban habitats.
Collectively, our approach was designed to determine how neural activation within the brain related to urbanization and thus which regions could be impacted by habitat. Future work can then assess how activity within these brain regions may be involved in regulating persistent differences in territorial aggression between urban and rural male song sparrows (Evans et al., 2010; Foltz et al., 2015b; Davies and Sewall, 2016). Additionally, this work evaluated whether AVT could be a mechanism impacted by urbanization, as it is known to contribute to the regulation of aggression. Based on previous studies, we expected urban birds to have different patterns of neural expression in the social behavior network compared to rural birds, particularly within the BSTm and lateral septum (LS), regions centrally involved in conspecific aggression and territoriality (Kollack-Walker et al., 1997; Goodson, 1998; Goodson et al., 2009b; Motta et al., 2009; Goodson and Kingsbury, 2011). Additionally, based on previous studies, we expected rural birds to have less AVT within the BSTm, but not the PVN (Fokidis and Deviche, 2012). This work moves us toward understanding how “urban adapters” are able to cope with human-impacted habitats and why some species may be limited in such physiological or neural acclimation.
Materials and Methods
Subjects
Permission to conduct the procedures described in this study was granted by the US Fish and Wildlife Service (permit MB08005B-0), the US Department of the Interior (permit 23818), the State of Virginia's Department of Game and Inland Fisheries (permit 053668), and Virginia Tech's Institutional Animal Care and Use Committee (protocol 13-074).
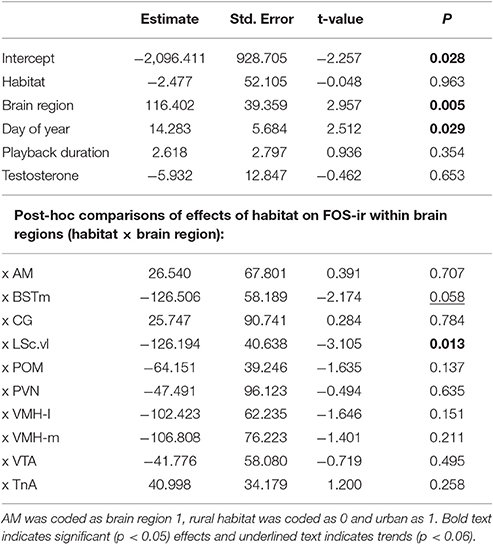
Male song sparrows in breeding condition were captured in the wild from one urban (N = 9) and one rural (N = 7) site within a group of 9 field sites near Blacksburg, VA that are along a rural urban gradient. We calculated urbanization scores using a technique validated by Seress and colleagues, which uses aerial images to quantify land-cover in a 1 km2 area around each study site (Seress et al., 2014; Davies and Sewall, 2016; Figure 1). Larger PC scores from this analysis indicate higher abundance of buildings and paved surfaces, and lower abundance of vegetation. Though the limited number of sites constrains our ability to generalize our findings, it was necessary to collect birds from only two sites and limit impact on the other long-term study populations (each population consists of ~20–30 breeding pairs). Males from these urban sites are reliably more territorial throughout the breeding season compared to rural male song sparrows (Davies and Sewall, 2016) and this pattern has been found in other rural and urban populations (Evans et al., 2010; Scales et al., 2011). Males were captured between 11 June and 19 June 2014 by placing a speaker (Micro II; JBL, Northbridge, CA, USA) and mist net in the center of a focal male's territory and playing one of 16 conspecific song playback stimuli to simulate a social challenge to the territory holder. Each playback track consisted of two song types recorded from one of 16 males from a population in Durham, NC, USA. Songs were presented at a rate of 1 song per 10 s at an amplitude of 80 dB, 1 m from the speaker. An average of 8.5 (±2) min of playback was required to capture each male; we did not present longer playback to avoid habituation and to maximize our chance of capturing the territory holder. We were unable to collect behavioral data because of insufficient field assistance, but collected blood samples within 3 min. of capture, permitting us to later quantify plasma testosterone. After collecting blood, we held birds in darkness and silence until we collected brains (ca. 40 min) to allow time for FOS protein translation, while minimizing the contribution of additional stimuli to patterns of neural expression (Herdegen and Leah, 1998). We sacrificed males by deeply anesthetizing them with isoflurane before rapidly decapitating them and removing the brain from the skull. We collected brains ca. 50 min from the start of playback, which is shorter than the experimental timeline of previous studies in song sparrows (e.g., 90 min; Goodson et al., 2005b) but longer than the half life of FOS protein (45 min; Herdegen and Leah, 1998). Thus, though the FOS expression we quantified was at least partially induced by hearing and responding to conspecific song, comparison with other FOS studies in song sparrows may be limited. We fixed the brains by immersion in acrolein for 4 h, saturated them in sucrose, flash froze them on dry ice, and stored them at −80°C until sectioning and immunohistochemistry (IHC) was carried out.
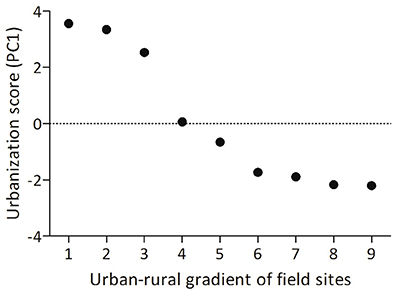
Figure 1. Birds in this study were collected from one urban site (number 1) and one rural site (number 9, above) along an urban rural gradient within 15 km of Blacksburg, VA, USA. The urbanization index for each site was calculated following Seress et al. (2014); larger PC scores on the y-axis indicate higher abundance of buildings and paved surfaces, and lower abundance of vegetation.
Plasma Testosterone
We collected blood samples by veinipuncture of the alar wing vein and stored them on ice until they were centrifuged, the plasma separated, and frozen at –80°C later the same day. We measured plasma testosterone using enzyme-linked immunoassay kits (ADI-900-065, Enzo Life Sciences, Farmingdale, NY, USA), which we have optimized and validated for use with song sparrow plasma (Davies and Sewall, 2016). Briefly, we diluted samples 30 times, added steroid displacement reagent at 0.5% of plasma volume, randomly assigned samples to assay plates (N = 3 plates), and assayed all samples in duplicate. The average assay sensitivity was 1.1 pg/mL. The average intra- and inter-assay coefficients of variation were 8.1 and 5.7%, respectively.
Histology
We coronally sectioned brains at a thickness of 40 μm using a cryostat at −21°C and divided them into three series, which we stored as floating sections in cryoprotectant at −20°C. We immuno-stained two series of brain sections separately, one for FOS and one for AVT, in two runs per antigen, with subjects randomly assigned to runs. For both AVT and FOS, following washes in 1 × phosphate buffered saline (PBS), we incubated in 1% sodium borohydride for 30 min to unmask antigens. In the case of AVT, but not FOS, we then incubated three times, each for 5 min, in freshly boiled citrate buffer (10 mM citric acid, 0.05% tween 20, pH 6.0). We then incubated both AVT and FOS sections in 0.3% hydrogen peroxide for 30 min to quench endogenous peroxidase activity, and then 5% normal goat serum for 1 h to block background immunoreactivity. We then incubated sections for ~24 h at 4°C in either rabbit anti-FOS (Santa Cruz Biotechnology, Dallas, TX, USA, cat. # sc-253 at 1:5,000) or guinea pig anti-AVP (Penisula Laboratories International, San Carlos, CA, USA, cat. # T-5048 at 1:5,000, previously distributed by Bachem, Torrence, CA). Following the primary incubation, we blocked endogenous aviden and biotin by incubating for 15 min in avidin/biotin blocking reagent (Vector Laboratories, Burlingame, CA, USA), then incubated in biotinylated secondary antibody for 1 h (FOS: biotinylated goat anti-rabbit at 1:500; AVT: goat anti-guinea pig at 1:250; Vector Laboratories, Burlingame, CA, USA). We next incubated sections for 1 h in avidin-biotin complex (ABC Vectastain Elite kit at 1:200; Vector Laboratories, Burlingame, CA, USA), then visualized labeling by incubating for 1 min in 3, 3–diaminobenzidine chromagen (Vector Laboratories, Burlingame, CA, USA). Between each incubation described above, we washed in PBS. After mounting on glass microscope slides, we allowed immunolabeled sections to dry at room temperature for 24 h before dehydrating through a graded ethanol series, clearing in xylenes, and affixing coverslips using Permount mounting medium (Fisher Scientific, Pittsburg, PA, USA).
Imaging and Quantification
All quantification of immunoreactivity (ir) for FOS and AVT was carried out by research assistants (T. Breeding and A. Wells) blind to the experimental condition of each subject. We captured gray scale images of each brain region using an AxioCam MR camera attached to a Zeiss Axioimager microscope (Zeiss, USA). The brain regions of interest included the anterior hypothalamus (AH); medial preoptic area (POM); medial extended amygdala, which includes the medial bed nucleus of the stria terminalis (BSTm) and nucleus taeniae (TnA); lateral septum, specifically the caudal ventrolateral portion (LSc.vl; Goodson et al., 2004); ventral tegmental area (VTA); central gray (CG); ventromedial hypothalamus (VMH), which consists of both a lateral and medial portion (Goodson, 2005; Maney et al., 2008); and the paraventricular nucleus of the hypothalamus (PVN). To quantify FOS immunoreactivity (ir) we imaged all brain regions using the 20 × objective (200 × total magnification).
We located the social behavior network regions following Maney et al. (2008) and references therein. Specifically, we located the AH following Kuenzel and van Tienhoven (1982) and quantified FOS-ir in the region dorsal to the dorsal supraoptic decussation. We located the POM with reference to Alger and Riters (2006) and quantified the region medial to the septomesencephalic tract. To identify the BSTm, we followed Aste et al. (1998) and Maney et al. (2008) and we placed the counting circle dorsal to the anterior commissure. We used Cheng et al. (1999) and Stokes et al. (1974) to locate the TnA. For the caudal ventrolateral portion of the LS, we referenced Goodson et al. (2004) and placed the counting circle medial to the lateral ventricle and beginning rostrally at the level of the anterior commissure. To identify the VTA and CG, we followed Heimovics and Riters (2005) and LeBlanc et al. (2007). For the VTA, we measured the region lateral to the rostral extent of the oculomotor nerve, and for the CG we measured FOS-ir within this region when it resembled the shape of a dolphin's tail. We identified the lateral and medial portions of the VMH at the level of the median eminence following Goodson et al. (2005a) and Maney et al. (2008). We quantified FOS-ir in the PVN starting rostrally from the ventral supraoptic decussation and caudally until the anterior commissure and occipitomesencephalic tract fuse following Davies et al. (2015). We made cell counts within a 0.20 mm2 circle placed within the AM, BSTm, VTA, and PVN; a 0.15 mm2 circle in CG and TnA; a 0.13 mm2 circle inside VMH-m and VMH-l; a 0.05 mm2 circle placed within the LSc.vl; and a 0.03 mm2 circle placed in POM. We made counts in the medial most tissue sections of each brain region of interest for a total of 2 sections in AM; 3 sections in CG, POM, VTA, VMH-l, and VMH-m; 4 sections in LSc.vl; 5 sections in BSTm and TnA; and 6 sections in PVN. We used Image J software (ver. 3.1, National Institutes of Health) to view and manually count immunoreactive cells that were visible within the counting frame. All FOS data were calculated as the total number of immunoreactive cells per mm2.
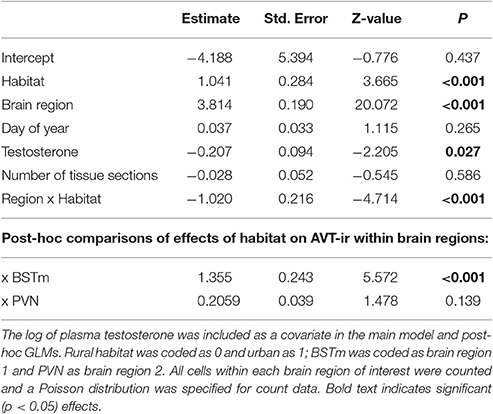
To quantify AVT-containing cells we counted every immunopositive cell in the BSTm and the PVN, after locating and imaging these brain regions as described above. These data are reported as total cell counts per brain region for each bird. The number of sections in which the brain region of interest was observed is included in analyses (see below) because the value differed across subjects. Though the antibody we used has been validated in avian species (e.g., Kabelik et al., 2010; Kelly and Goodson, 2014) staining in the BSTm was light (Figure 2). This could be because of how we prepared the tissue, the ephemeral nature of this cluster of AVT neurons (reviewed in Goodson and Kingsbury, 2011), or cross-reactivity with other non-apeptides. To be conservative, we refer to this hereafter as AVT-like immunoreactivity.
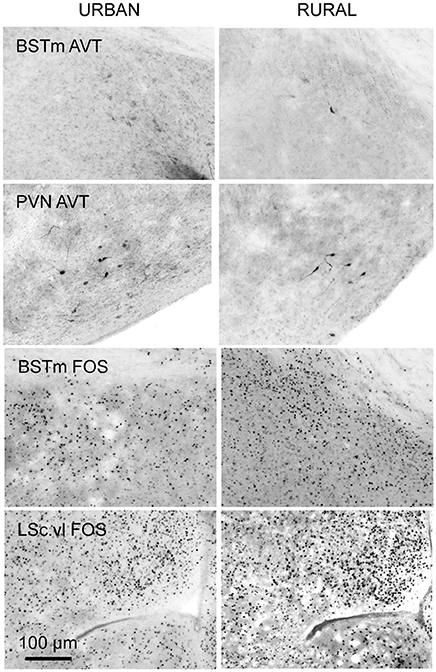
Figure 2. Coronal sections showing examples of AVT-like (top two rows) and FOS induction (bottom two rows) in the BSTm, PVN, and LSc.vl from urban (left column) and rural (right column) subjects. Urban birds had greater AVT-like staining in the BSTm than rural birds but less FOS-ir in the BSTm and LSc.vl than rural birds.
Statistics
We used R statistical software (R Development Core Team, 2008) for all analyses. First, to assess the relationship between habitat type and plasma testosterone we ran a general linear model (GLM) with habitat as a factor and playback duration and the day of year as covariates. We also examined possible relationships between testosterone and AVT cell numbers in the BSTm and PVN using two separate Pearson's correlations.
Because our data are hierarchically structured and to minimize the number of comparisons, we ran one general linear mixed model (GLMM) and one generalized linear mixed model using the lme and lmer package in R (Bates et al., 2015). To examine habitat differences in FOS-ir within the social behavior network we ran a GLMM with habitat type and brain region as fixed factors. We included individual as a random factor to account for the non-independence of samples and nested the number of sections measured per brain region within individual because different numbers of sections were quantified for different brain regions. We used a default Gaussian distribution. The covariates in this model were the day of year, plasma testosterone, and playback duration because reproductive condition, exposure to playback, and hormonal status can influence IEG response (Goodson and Evans, 2004; Goodson et al., 2005b; Maney et al., 2008).
We ran a generalized linear mixed model for AVT in which we tested whether total counts of all AVT cells within a brain region of interest were explained by habitat type, brain region, or the interaction between the two. We specified individual as a random factor to account for non-independence of cell counts. We specified a Poisson distribution for count data (which was supported by reduced residual deviance; Crawley, 2007). We included plasma testosterone and the day of the year as covariates because of the interaction between the testosterone and AVT systems and the effects of breeding state on AVT expression (Panzica et al., 2001). Additionally, we coded the number of tissue sections in which the brain region of interested was found as a covariate because this value differed both between subjects and across brain regions and therefore was not hierarchically structured.
Lastly, we ran separate general or generalized linear models as post-hoc tests for each neural marker and brain region of interest, specifying the same covariates and distributions as the main models. We report only main effects from initial models and the main effects of habitat type on measures within each brain region in Tables 1, 2 but include all model outputs in the Supplementary Materials.
Results
Plasma Testosterone
Though there was a trend for the day of sampling to impact plasma testosterone such that testosterone was counter-intuitively higher later in the season (GLM, effect of day of year, t = 2.093, P = 0.058), there were no overall differences in testosterone levels as a function of habitat type when day of year was taken into account (mean ± SEM: rural 2.36 ± 0.717 ng/mL; urban 1.35 ± 0.198 ng/mL; GLM effect of habitat, t = −0.892, P = 0.389). Nor was testosterone correlated with the number of cells showing AVT-like immunoreactivity in either the BSTm (R = −0.306, p = 0.287) or PVN (R = −0.325, p = 0.279).
FOS Immunoreactivity
We found a significant effect of habitat type on FOS-ir in the LSc.vl (GLMM, habitat x LSc.vl, t = −3.105, P = 0.013) and, nearly, in the BSTm (GLMM, habitat x BSTm, t = −2.174, P = 0.058; Table 1; Figure 3). Relative to urban birds, rural birds had more FOS-ir neurons in the LSc.vl and a trend of more in the BSTm. Though there was no overall effect of habitat on the number of FOS-ir neurons, the date of the sampling contributed to FOS-ir throughout the brain such that birds collected later in the season had higher FOS-ir (GLMM, effect of day of year, t = 2.512, P = 0.029; Table 1) and there were overall differences in expression across brain regions (GLMM, effect of brain region, t = 2.957, P = 0.005; Table 1). Neither the duration of playback nor plasma testosterone levels significantly explained the number of FOS-ir neurons in the main model or post-hoc tests (Table 1; Supplemental Materials).
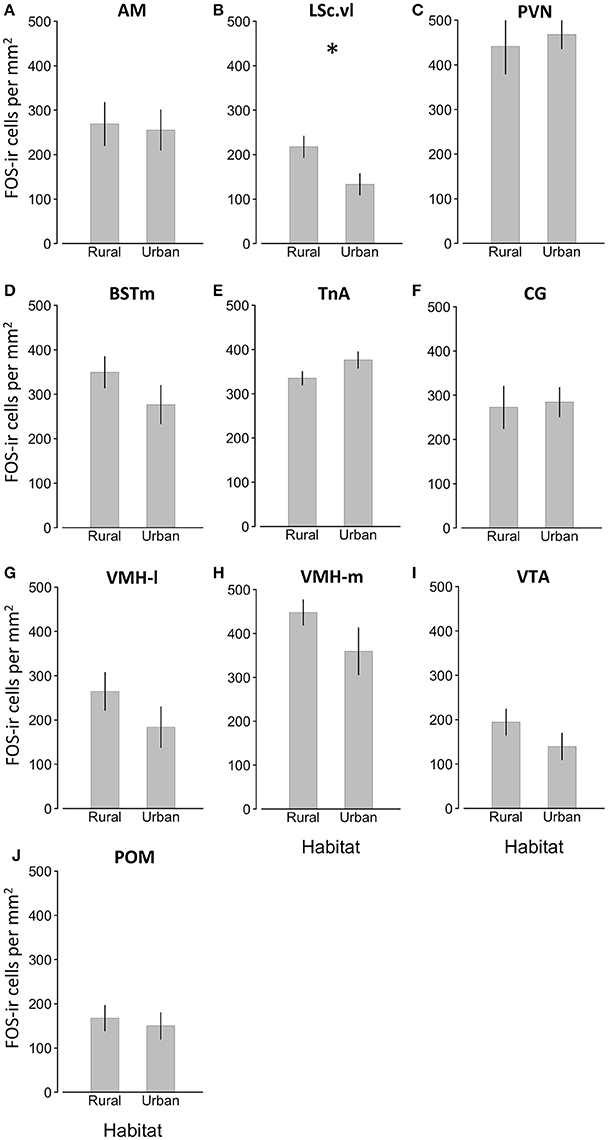
Figure 3. Main effects of habitat type on FOS-ir ±SEM within nine brain regions of interest (A–J) Effects of day of year, duration of playback, relationship with plasma testosterone, and controls for the number of tissue sections in which we found the brain regions of interest were included in the model, but are not represented. Asterisk denotes significant difference between groups.
AVT Immunoreactivity
We found a significant effect of habitat on AVT-like immunoreactivity (GLMM, effect of habitat, z = 3.665, p < 0.001; Table 2). Additionally, AVT-like immunoreactivity differed across brain regions (GLMM, effect of brain region, z = 20.072, p < 0.001), with far higher cell numbers in the PVN than the BSTm (Figure 4). Of most importance, there was an interaction between habitat and brain region such that urban male song sparrows had more cells showing AVT-like immunoreactivity in BSTm than rural males (GLMM, habitat x BSTm, z = 5.572, p < 0.001) though this did not hold in the PVN (Table 2; Figure 4). Finally, testosterone levels contributed to differences in AVT-like immunoreactivity (GLMM, effect of testosterone, z = −2.205, p = 0.027).
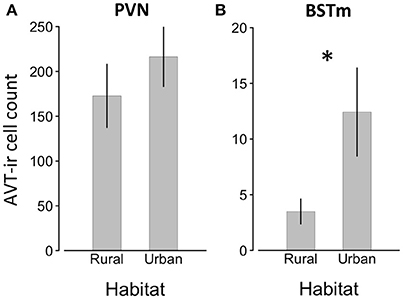
Figure 4. Number of AVT positive cells ±SEM in the PVN (A) and BSTSm (B) in male song sparrows collected in rural and urban habitats. Asterisk denotes significant difference between groups.
Discussion
We found that male song sparrows living in urban habitats of Blacksburg, VA have different patterns of immediate early gene and neuropeptide levels in their brains in response to hearing conspecific song, compared to birds living in rural habitats. This work demonstrates that urbanization has impacts on neural processes, and is an important first step toward understanding how habitat change and neural function are interrelated; it is among only a handful of studies exploring how urbanization impacts the brains of wild birds (Maklakov et al., 2011; Fokidis and Deviche, 2012; Davies et al., 2016). The present findings raise two specific hypotheses about how urbanization and neural processes may be interrelated. First, associations between FOS expression and urbanization implicate the social behavior network in the regulation of persistent differences in territorial behavior between urban and rural male song sparrows (Evans et al., 2010; Foltz et al., 2015b; Davies and Sewall, 2016). Second, the finding of elevated AVT-like immunoreactivity in the BSTm of urban male song sparrows supports the hypothesis that these socially sensitive AVT neurons (Goodson et al., 2009b) could also contribute to maintaining behavioral differences between urban and rural song sparrow populations.
Habitat Differences in Neural Response to Conspecific Song
Patterns of IEG expression in the social behavioral network are associated with both perception of social stimuli and behavioral response to those stimuli, with the result that measures differ across social contexts and also as a function of a focal animals' phenotype (Goodson and Wang, 2006; Maney et al., 2008; Kabelik et al., 2009; Goodson and Kingsbury, 2011). Previous studies have found that simulated territory intrusions increase IEG expression in the BSTm, LS, PVN, AH, VMH, CG, and VTA of territorial birds (Maney and Ball, 2003; Goodson and Evans, 2004; Goodson et al., 2005b). However, aggressive response to social challenge is negatively correlated with IEG responses in the AH, PVN, and multiples zones of the LS (Goodson et al., 2005b; Goodson and Kingsbury, 2011) in territorial male song sparrows both during and outside of the breeding season, implicating these regions in the maintenance of persistent differences in territorial behavior (Goodson and Evans, 2004; Goodson et al., 2005b; Goodson and Kingsbury, 2011).
The primary finding in the present study was that urban male song sparrows, who are reliably more territorially aggressive (Evans et al., 2010; Foltz et al., 2015b; Davies and Sewall, 2016), had fewer FOS-ir neurons in the LSc.vl than their rural counterparts (Table 1; Figure 4). Thus, our finding is consistent with patterns of lower FOS expression in the LSc.vl of animals that show elevated territorial aggression and suggest this region is involved in behavioral adjustments to living in urban areas. Though we also expected to find this pattern of FOS expression across other nodes of the social behavior network (e.g., the AH and PVN; Goodson et al., 2005b) we may not have seen this pattern because our playback was shorter than prior studies in song sparrows (10 min instead of 30 min; Goodson and Evans, 2004; Goodson et al., 2005b; though see Maney and Ball, 2003 who also used 10 min) or because capturing males in the wild using playback may unavoidably select the most aggressive birds from each population, minimizing our chances of detecting differences (though thoroughly sampling only two populations should have minimized this risk). Future studies should test the hypothesis that neural activity in the LSc.vl contributes to the modulation of territoriality across rural and urban habitats by comparing FOS expression within this brain region in passively caught subjects from each habitat, as well as by correlating expression with experimental subjects' behavioral responses to song challenge.
While FOS-ir in the LSc.vl is associated with differences in aggressive behavior, FOS-ir in the BSTm may reflect the perception of social challenge, but not the regulation of behavioral responses. It is difficult to disentangle neural responses induced by the perception of a social challenge from those induced by behavioral response to challenge, but previous studies in song sparrows have found correlations between IEG expression in the BSTm and exposure to song playback, but not behavioral response to challenge (Goodson and Evans, 2004; Goodson et al., 2005b). Working from the hypothesis that FOS-ir in the BSTm is positively associated with the perception of social challenge, our results suggest that rural and urban birds perceived playback differently. Specifically, the duration of playback did not impact FOS-ir in the BSTm (Table 1; Supplemental materials), yet there was a trend of elevated FOS-ir in the BSTm of rural birds relative to urban birds. This could be interpreted as rural birds perceiving the playback as a greater social challenge than did urban birds. This hypothesis could be tested by presenting birds from different habitats with social challenges of varying intensity such as familiar and unfamiliar conspecifics (Stoddard et al., 1990).
Habitat Differences in AVT-Like Immunoreactivity
Like FOS, the relationship between AVT within the BSTm and aggression can depend upon context and phenotype (Goodson et al., 2009a,b; Kabelik et al., 2009). However, generally, elevated AVT is associated with decreased territorial aggression in breeding, territorial male songbirds. Septal infusions of AVT reduce male-male aggression (Goodson, 1998; Kabelik et al., 2009). Further, studies using double labeling of cells with IEGs and AVT show that AVT neurons in the BSTm are activated by positive social stimuli, such as a potential mate (Goodson and Wang, 2006; Goodson et al., 2009a; Goodson and Kingsbury, 2011). Here, we found that urban birds, which are persistently more territorially aggressive, had more AVT-like immunoreactive neurons in the BSTm, but not the PVN. While AVT levels in the BSTm are associated with territoriality and aggression, AVT in the PVN is involved in maintaining homeostasis (Simon-Oppermann et al., 1980; Madison et al., 2008; Goodson et al., 2009b; Fokidis and Deviche, 2012). Thus, our finding is seemingly counter to studies that used septal manipulations of AVT and found decreased aggression (Goodson, 1998). However, immunoreactivity can reflect elevated production and secretion of the neuropeptide, or it can indicate an accumulation of peptide due to reduced secretion, making it difficult to interpret the functional significance of greater staining (Panzica et al., 2001; Goodson and Kabelik, 2009; Sewall et al., 2010). One interpretation is that urban male song sparrows were sequestering AVT within cell bodies in the BSTm, reducing bioavailable AVT, which would be consistent with lower AVT levels increasing territorial aggression. However, the present finding is counter to that of Fokidis and Deviche (2012), who reported that urban curve-billed thrashers, Toxostoma curvirostre, had significantly less AVT staining in the BSTm than desert thrashers and that AVT staining was also inversely related to territorial aggression. Overall, greater AVT-like staining in the BSTm of urban birds is counter to our predictions and the findings of a prior study, so resolving the contribution of the AVT system to behavioral differences associated with urbanization will require double-labeling cells for AVT and IEGs. The present results implicate AVT within socially-sensitive neurons of the BSTm in regulating well-described differences in territorial behavior between rural and urban male song sparrows, but more work is needed to resolve the direction of this relationship. Moreover, additional mechanisms, such as serotonin could contribute to the regulation of territorial aggression (Nelson and Trainor, 2007). Future work is needed to understand how multiple regulatory pathways are integrated to yield differences in territorial aggression in urban and rural song sparrows (Cohen et al., 2012). Further, given that species differ in their behavioral responses to urbanization and multiple physiological pathways could yield similar behavioral outcomes, our understanding of the proximate mechanisms that permit animals to cope with changing habitats is only in its infancy.
Conclusions
Urbanization impacts the physiology and behavior of free-living animals and is a particular concern for birds (Wingfield, 2008; Bonier, 2012; Sol et al., 2013; Wong and Candolin, 2015). Determining the mechanistic basis of behavioral responses to urbanization is essential to predicting the consequences and limitations of organismal reactions to habitat disturbance and will ultimately help us understand why some species adjust and others decline in the face of environmental change. Here, we found that, compared to rural male song sparrows, urban song sparrows had reduced expression of the IEG FOS within a node of the social behavior network of the brain (the LS), and also more AVT-like immunoreactive neurons within the BSTm. This study adds to the growing evidence that urbanization impacts the brain to influence behavior. We expect future work in this area will identify a vast number of behavioral responses to urbanization and also considerable diversity in the proximate mechanisms that permit such adjustments.
Ethics Statement
This study was carried out in accordance with the recommendations of the US Geological Service's bird banding lab and the National Research Council's Guide for the care and use of laboratory animals. The protocol was approved by the Virginia Tech's Institutional Animal Care and Use Committee (protocol 13-074), the US Fish and Wildlife Service (permit MB08005B-0), the US Department of the Interior (permit 23818), the State of Virginia's Department of Game and Inland Fisheries (permit 053668).
Author Contributions
KS conducted field work, sample collection, data analysis, and wrote the majority of the paper. SD completed all histology, enzyme immunoassay, immunohistochemistry, quantification, and assisted with editing the paper.
Funding
This work was supported by Start up funds from Virginia Tech and a Jeffress Memorial Trust award to KS.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to T. Breeding and A. Wells for assistance with data collection, and Virginia Tech campus and StREAM lab for access to field sites.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fevo.2017.00046/full#supplementary-material
References
Alger, S. J., and Riters, L. V. (2006). Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris). Behav. Neurosci. 120, 1326–1336. doi: 10.1037/0735-7044.120.6.1326
Aste, N., Balthazart, J., Absil, P., Grossmann, R., Mülhbauer, E., Viglietti-Panzica, C., et al. (1998). Anatomical and neurochemical definition of the nucleus of the stria terminalis in Japanese quail (Coturnix japonica). J. Comp. Neurol. 396, 141–157. doi: 10.1002/(SICI)1096-9861(19980629)396:2<141::AID-CNE1>3.0.CO;2-0
Atwell, J. W., Cardoso, G. C., Whittaker, D. J., Campbell-Nelson, S., Robertson, K. W., and Ketterson, E. D. (2012). Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav. Ecol. 23, 960–969. doi: 10.1093/beheco/ars059
Atwell, J. W., Cardoso, G. C., Whittaker, D. J., Price, T. D., Ketterson, E. D., Williams, A. E. T. D., et al. (2014). Hormonal, behavioral, and life-history traits exhibit correlated shifts in relation to population establishment in a novel environment. Am. Nat. 184, E147–E160. doi: 10.1086/678398
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bonier, F. (2012). Hormones in the city: endocrine ecology of urban birds. Horm. Behav. 61, 763–772. doi: 10.1016/j.yhbeh.2012.03.016
Bonier, F., Martin, P. R., Sheldon, K. S., Jensen, J. P., Foltz, S. L., and Wingfield, J. C. (2007). Sex-specific consequences of life in the city. Behav. Ecol. 18, 121–129. doi: 10.1093/beheco/arl050
Both, C., Bouwhuis, S., Lessells, C. M., and Visser, M. E. (2006). Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83. doi: 10.1038/nature04539
Caro, T. (2007). Behavior and conservation: a bridge too far? Trends Ecol. Evol. 22, 394–400. doi: 10.1016/j.tree.2007.06.003
Cheng, M.-F., Chaiken, M., Zuo, M., and Miller, H. (1999). Nucleus taenia of the amygdala of virds: anatomical and functional studies in ring Doves (Streptopelia risoria) and European Starlings (Sturnus vulgaris). Brain Behav. Evol. 53, 243–270. doi: 10.1159/000006597
Clayton, D. F. (2000). The genomic action potential. Neurobiol. Learn. Mem. 74, 185–216. doi: 10.1006/nlme.2000.3967
Cockrem, J. F. (2005). Conservation and behavioral neuroendocrinology. Horm. Behav. 48, 492–501. doi: 10.1016/j.yhbeh.2005.03.008
Cohen, A. A., Martin, L. B., Wingfield, J. C., McWilliams, S. R., and Dunne, J. A. (2012). Physiological regulatory networks: ecological roles and evolutionary constraints. Trends Ecol. Evol. 27, 428–435. doi: 10.1016/j.tree.2012.04.008
Crick, H. Q. P. (2004). The impact of climate change on birds. Ibis 146, 48–56. doi: 10.1111/j.1474-919X.2004.00327.x
Davies, S., and Sewall, K. B. (2016). Agonistic urban birds: elevated territorial aggression of urban song sparrows is individually consistent within a breeding period. Biol. Lett. 12:20160315. doi: 10.1098/rsbl.2016.0315
Davies, S., Cros, T., Richard, D., Meddle, S. L., Tsutsui, K., and Deviche, P. (2015). Food availability, energetic constraints and reproductive development in a wild seasonally breeding songbird. Funct. Ecol. 29, 1421–1434. doi: 10.1111/1365-2435.12448
Davies, S., Lane, S., Meddle, S. L., Tsutsui, K., and Deviche, P. (2016). The ecological and physiological bases of variation in the phenology of gonad growth in an urban and desert songbird. Gen. Comp. Endocrinol. 230–231, 17–25. doi: 10.1016/j.ygcen.2016.03.013
Deviche, P., and Davies, S. (2013). “Reproductive phenology of urban birds – environmental cues and mechanisms,” in Avian Urban Ecology, eds D. Gil and H. Brumm (Oxford, UK: Oxford University Press), 98–115.
Engel, K., Tollrian, R., and Jeschke, J. M. (2011). Integrating biological invasions, climate change and phenotypic plasticity. Commun. Integr. Biol. 4, 247–250. doi: 10.4161/cib.4.3.14885
Evans, J., Boudreau, K., and Hyman, J. (2010). Behavioural syndromes in urban and rural populations of song sparrows. Ethology 116, 588–595. doi: 10.1111/j.1439-0310.2010.01771.x
Fokidis, H. B., and Deviche, P. (2012). Brain Arginine vasotocin immunoreactivity differs between urban and desert curve-billed thrashers, Toxostoma curvirostre: relationships with territoriality and stress physiology. Brain Behav. Evol. 79, 84–97. doi: 10.1159/000332766
Fokidis, H. B., Orchinik, M., and Deviche, P. (2009). Corticosterone and corticosteroid binding globulin in birds: relation to urbanization in a desert city. Gen. Comp. Endocrinol. 160, 259–270. doi: 10.1016/j.ygcen.2008.12.005
Fokidis, H. B., Orchinik, M., and Deviche, P. (2011). Context-specific territorial behavior in urban birds: no evidence for involvement of testosterone or corticosterone. Horm. Behav. 59, 133–143. doi: 10.1016/j.yhbeh.2010.11.002
Foltz, S. L., Davis, J. E., Battle, K. E., Greene, V. W., Laing, B. T., Rock, R. P., et al. (2015a). Across time and space: effects of urbanization on corticosterone and body condition vary over multiple years in song sparrows (Melospiza melodia). J. Exp. Zool. 323, 109–120. doi: 10.1002/jez.1906
Foltz, S. L., Ross, A. E., Laing, B. T., Rock, R. P., Battle, K. E., and Moore, I. T. (2015b). Get off my lawn: increased aggression in urban song sparrows is related to resource availability. Behav. Ecol. 26, 1548–1557. doi: 10.1093/beheco/arv111
Goodson, J. L. (1998). Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla). Horm. Behav. 34, 67–77. doi: 10.1006/hbeh.1998.1467
Goodson, J. L. (2005). The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 48, 11–22. doi: 10.1016/j.yhbeh.2005.02.003
Goodson, J. L., and Bass, A. H. (2001). Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res. Rev. 35, 246–265. doi: 10.1016/S0165-0173(01)00043-1
Goodson, J. L., and Evans, A. K. (2004). Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm. Behav. 46, 371–381. doi: 10.1016/j.yhbeh.2004.02.008
Goodson, J. L., and Kabelik, D. (2009). Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front. Neuroendocrinol. 30, 429–441. doi: 10.1016/j.yfrne.2009.05.007
Goodson, J. L., and Kingsbury, M. A. (2011). Nonapeptides and the evolution of social group sizes in birds. Front. Neuroanat. 5:13. doi: 10.3389/fnana.2011.00013
Goodson, J. L., and Wang, Y. (2006). Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc. Natl. Acad. Sci. U.S.A. 103, 17013–17017. doi: 10.1073/pnas.0606278103
Goodson, J. L., Evans, A. K., and Lindberg, L. (2004). Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J. Comp. Neurol. 473, 293–314. doi: 10.1002/cne.20061
Goodson, J. L., Evans, A. K., and Soma, K. K. (2005b). Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. Neuroreport 16, 1719–1723. doi: 10.1097/01.wnr.0000183898.47160.15
Goodson, J. L., Evans, A. K., Lindberg, L., and Allen, C. D. (2005a). Neuro–evolutionary patterning of sociality. Proc. R. Soc. Lond. B Biol. Sci. 272, 227–235. doi: 10.1098/rspb.2004.2892
Goodson, J. L., Kabelik, D., and Schrock, S. E. (2009a). Dynamic neuromodulation of aggression by vasotocin: influence of social context and social phenotype in territorial songbirds. Biol. Lett. 5, 554–556. doi: 10.1098/rsbl.2009.0316
Goodson, J. L., Rinaldi, J., and Kelly, A. M. (2009b). Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm. Behav. 55, 197–202. doi: 10.1016/j.yhbeh.2008.10.007
Heimovics, S. A., and Riters, L. V. (2005). Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J. Neurobiol. 65, 207–224. doi: 10.1002/neu.20181
Herdegen, T., and Leah, J. D. (1998). Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Rev. 28, 370–490. doi: 10.1016/S0165-0173(98)00018-6
Hoffmann, A. A., and Sgrò, C. M. (2011). Climate change and evolutionary adaptation. Nature 470, 479–485. doi: 10.1038/nature09670
Insel, T. R., and Young, L. J. (2000). Neuropeptides and the evolution of social behavior. Curr. Opin. Neurobiol. 10, 784–789. doi: 10.1016/S0959-4388(00)00146-X
Jarvis, E. D., Schwabl, H., Ribeiro, S., and Mello, C. V. (1997). Brain gene regulation by territorial singing behavior in freely ranging songbirds. Neuroreport 8, 2073–2077. doi: 10.1097/00001756-199705260-00052
Kabelik, D., Kelly, A. M., and Goodson, J. L. (2010). Dopaminergic regulation of mate competition aggression and aromatase-Fos colocalization in vasotocin neurons. Neuropharmacology 58, 117–125. doi: 10.1016/j.neuropharm.2009.06.009
Kabelik, D., Klatt, J. D., Kingsbury, M. A., and Goodson, J. L. (2009). Endogenous vasotocin exerts context-dependent behavioral effects in a semi-naturalistic colony environment. Horm. Behav. 56, 101–107. doi: 10.1016/j.yhbeh.2009.03.017
Kelly, A. M., and Goodson, J. L. (2014). Personality is tightly coupled to vasopressin-oxytocin neuron activity in a gregarious finch. Front. Behav. Neurosci. 8:55. doi: 10.3389/fnbeh.2014.00055
Kiss, J. Z., Voorhuis, T. A. M., van Eekelen, J. A. M., de Kloet, E. R., and de Wied, D. (1987). Organization of vasotocin-immunoreactive cells and fibers in the canary brain. J. Comp. Neurol. 263, 347–364. doi: 10.1002/cne.902630304
Kollack-Walker, S., Watson, S. J., and Akil, H. (1997). Social stress in hamsters: defeat activates specific neurocircuits within the brain. J. Neurosci. 17, 8842–8855.
Kuenzel, W. J., and van Tienhoven, A. (1982). Nomenclature and location of avian hypothalamic nuclei and associated circumventricular organs. J. Comp. Neurol. 206, 293–313. doi: 10.1002/cne.902060309
LeBlanc, M. M., Goode, C. T., MacDougall-Shackleton, E. A., and Maney, D. L. (2007). Estradiol modulates brainstem catecholaminergic cell groups and projections to the auditory forebrain in a female songbird. Brain Res. 1171, 93–103. doi: 10.1016/j.brainres.2007.06.086
Lessells, C. M. (2008). Neuroendocrine control of life histories: what do we need to know to understand the evolution of phenotypic plasticity? Philos. Trans. R. Soc. B Biol. Sci. 363, 1589–1598. doi: 10.1098/rstb.2007.0008
Madison, F. N., Jurkevich, A., and Kuenzel, W. J. (2008). Sex differences in plasma corticosterone release in undisturbed chickens (Gallus gallus) in response to arginine vasotocin and corticotropin releasing hormone. Gen. Comp. Endocrinol. 155, 566–573. doi: 10.1016/j.ygcen.2007.08.014
Maklakov, A. A., Immler, S., Gonzalez-Voyer, A., Rönn, J., and Kolm, N. (2011). Brains and the city: big-brained passerine birds succeed in urban environments. Biol. Lett. 7, 730–732. doi: 10.1098/rsbl.2011.0341
Maney, D. L., and Ball, G. F. (2003). Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J. Neurobiol. 56, 163–170. doi: 10.1002/neu.10227
Maney, D. L., Goode, C. T., Lange, H. S., Sanford, S. E., and Solomon, B. L. (2008). Estradiol modulates neural responses to song in a seasonal songbird. J. Comp. Neurol. 511, 173–186. doi: 10.1002/cne.21830
Motta, S. C., Goto, M., Gouveia, F. V., Baldo, M. V. C., Canteras, N. S., and Swanson, L. W. (2009). Dissecting the brain's fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc. Natl. Acad. Sci. U.S.A. 106, 4870–4875. doi: 10.1073/pnas.0900939106
Myers, R. E., and Hyman, J. (2016). Differences in measures of boldness even when underlying behavioral syndromes are present in two populations of the song sparrow (Melospiza melodia). J. Ethol. 34, 197–206. doi: 10.1007/s10164-016-0465-9
Nelson, R. J., and Trainor, B. C. (2007). Neural mechanisms of aggression. Nat. Rev. Neurosci. 8, 536–546. doi: 10.1038/nrn2174
Newman, S. W. (1999). The medial extended amygdala in male reproductive behavior a node in the mammalian social behavior network. Ann. N.Y. Acad. Sci. 877, 242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x
O'Connell, L. A., and Hofmann, H. A. (2011). The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599–3639. doi: 10.1002/cne.22735
Panzica, G. C., Aste, N., Castagna, C., Viglietti-Panzica, C., and Balthazart, J. (2001). Steroid-induced plasticity in the sexually dimorphic vasotocinergic innervation of the avian brain: behavioral implications. Brain Res. Rev. 37, 178–200. doi: 10.1016/S0165-0173(01)00118-7
Panzica, G. C., Plumari, L., García-Ojeda, E., and Deviche, P. (1999). Central vasotocin-immunoreactive system in a male passerine bird (Junco hyemalis). J. Comp. Neurol. 409, 105–117.
Parker, T. S., and Nilon, C. H. (2008). Gray squirrel density, habitat suitability, and behavior in urban parks. Urban Ecosyst. 11, 243–255. doi: 10.1007/s11252-008-0060-0
Partecke, J., Van't Hof, T., and Gwinner, E. (2005). Underlying physiological control of reproduction in urban and forest-dwelling European blackbirds Turdus merula. J. Avian Biol. 36, 295–305. doi: 10.1111/j.0908-8857.2005.03344.x
Partecke, J., Schwabl, I., and Gwinner, E. (2006). Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology 87, 1945–1952. doi: 10.1890/0012-9658(2006)87[1945:SATCUA]2.0.CO;2
Plumari, L., Plateroti, S., Deviche, P., and Panzica, G. C. (2004). Region-specific testosterone modulation of the vasotocin-immunoreactive system in male dark-eyed junco, Junco hyemalis. Brain Res. 999, 1–8. doi: 10.1016/j.brainres.2003.10.037
R Development Core Team (2008). R: A Language and Environment for Statistical Computing. Available online at: https://www.r-project.org/
Santangelo, N., and Bass, A. H. (2006). New insights into neuropeptide modulation of aggression: field studies of arginine vasotocin in a territorial tropical damselfish. Proc. R. Soc. London B. Biol. Sci. 273, 3085–3092. doi: 10.1098/rspb.2006.3683
Scales, J., Hyman, J., and Hughes, M. (2011). Behavioral syndromes break down in urban song sparrow populations. Ethology 117, 887–895. doi: 10.1111/j.1439-0310.2011.01943.x
Schoech, S. J., Bowman, R., Bridge, E. S., and Boughton, R. K. (2007). Baseline and acute levels of corticosterone in Florida Scrub-Jays (Aphelocoma coerulescens): effects of food supplementation, suburban habitat, and year. Gen. Comp. Endocrinol. 154, 150–160. doi: 10.1016/j.ygcen.2007.05.027
Seress, G., Lipovits, Á., Bókony, V., and Czúni, L. (2014). Quantifying the urban gradient: a practical method for broad measurements. Landsc. Urban Plan. 131, 42–50. doi: 10.1016/j.landurbplan.2014.07.010
Sewall, K. B., Dankoski, E. C., and Sockman, K. W. (2010). Song environment affects singing effort and vasotocin immunoreactivity in the forebrain of male Lincoln's sparrows. Horm. Behav. 58, 544–553. doi: 10.1016/j.yhbeh.2010.04.002
Simon-Oppermann, C., Simon, E., Deutsch, H., Möhring, J., and Schoun, J. (1980). Serum arginine-vasotocin (AVT) and afferent and central control of osmoregulation in conscious Pekin ducks. Pflugers Arch. 387, 99–106. doi: 10.1007/BF00584259
Sol, D., Lapiedra, O., and González-Lagos, C. (2013). Behavioural adjustments for a life in the city. Anim. Behav. 85, 1101–1112. doi: 10.1016/j.anbehav.2013.01.023
Stoddard, P. K., Beecher, M. D., Horning, C. L., and Willis, M. S. (1990). Strong neighbor-stranger discrimination in song sparrows. Condor 92, 1051–1056. doi: 10.2307/1368741
Stokes, T. M., Leonard, C. M., and Nottebohm, F. (1974). The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J. Comp. Neurol. 156, 337–374. doi: 10.1002/cne.901560305
Visser, M. E. (2008). Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. Lond. B Biol. Sci. 275, 649–659. doi: 10.1098/rspb.2007.0997
Vitousek, P. M., Mooney, H. A., Lubchenco, J., and Melillo, J. M. (1997). Human domination of earth's ecosystems. Science 277, 494–499. doi: 10.1126/science.277.5325.494
Warren, P., Tripler, C., Bolger, D., Faeth, S., Huntly, N., Lepczyk, C., et al. (2006). Urban food webs: predators, prey, and the people who feed them. Bull. Ecol. Soc. Am. 87, 387–393. doi: 10.1890/0012-9623(2006)87[387:UFWPPA]2.0.CO;2
Whitman, D. W., and Agrawal, A. A. (2009). “What is phenotypic plasticity and why is it important?,” in Phenotypic Plasticity of Insects: Mechanisms and Consequences, eds D. W. Whitman and T. N. Ananthakrishnan (Enfield: Science Publishers), 1–63.
Wingfield, J. C. (2008). Comparative endocrinology, environment and global change. Gen. Comp. Endocrinol. 157, 207–216. doi: 10.1016/j.ygcen.2008.04.017
Keywords: urbanization, social behavior network, arginine vasotocin, immediate early gene, territorial aggression, song sparrow
Citation: Sewall KB and Davies S (2017) Two Neural Measures Differ between Urban and Rural Song Sparrows after Conspecific Song Playback. Front. Ecol. Evol. 5:46. doi: 10.3389/fevo.2017.00046
Received: 06 February 2017; Accepted: 28 April 2017;
Published: 17 May 2017.
Edited by:
Diego Gil, Consejo Superior de Investigaciones Científicas, SpainReviewed by:
Naomi Ondrasek, University of California, Davis, USAKirill Tokarev, Hunter College (CUNY), USA
Copyright © 2017 Sewall and Davies. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kendra B. Sewall, ksewall@vt.edu
 Kendra B. Sewall
Kendra B. Sewall Scott Davies
Scott Davies