Behavioral Correlations Associated with Fear of Humans Differ between Rural and Urban Burrowing Owls
- 1Department of Physical, Chemical and Natural Systems, Universidad Pablo de Olavide, Sevilla, Spain
- 2Department of Conservation Biology, Estación Biológica de Doñana (CSIC), Sevilla, Spain
Behavioral studies are fundamental to understanding how animal populations face global change. Although much research has centered upon the idea that individuals can adaptively modify their behaviors to cope with environmental changes, recent evidence supports the existence of individual differences in suites of correlated behaviors. However, little is known about how selection can change these behavioral structures in populations subject to different environmental constraints. The colonization of urban environments by birds has been related to their inter-individual variability in their fear of humans, measured as their flight initiation distance to an approaching human, such that urban life would select for fearless individuals. This behavior has been demonstrated to be heritable and highly consistent throughout the adult lifespan of burrowing owls (Athene cunicularia). Here, we experimentally assessed, in field conditions, whether urban life involves changes in other behaviors such as exploration and antipredatory response through their correlation with fear of humans. Breeding urban birds were more fearless toward humans and were quicker to explore a new food resource and defend their nests from predators than their rural counterparts. However, while fear of humans positively correlated with exploration and antipredatory response in the rural population, it only correlated with exploration in the urban one. Predator release in urban environments could relax—and even counterselect—antipredator behaviors, thus dismantling the behavioral correlation existent in natural populations. Altogether, our results suggest that rural and urban animals may differ in some behavioral aspects, may be as a consequence of the selection processes acting during the colonization of urban areas as well as the different ecological environments encountered by individuals.
Introduction
Behavioral studies are fundamental to our understanding of how animals respond to environmental changes (Sih et al., 2011). Over the past four decades, much research has centered upon the proposition that individuals can alter their behavioral phenotype to cope adaptively with environmental conditions that change within their lifetime (Piersma and Drent, 2003; Réale and Dingemanse, 2010). Indeed, a meta-analysis of more than 3,000 rates of recent phenotypic change suggested that most of the phenotypic changes associated with rapid human-induced environmental changes involve phenotypic plasticity rather than immediate genetic evolution (Hendry et al., 2008). Despite this flexible nature, however, there is also a tendency for individuals to behave consistently through time, and there is a growing body of evidence indicating that behavioral variation among individuals within populations sometimes exceeds the variation expressed by individuals over time or across contexts. Such stable interindividual variation is referred to as “animal personality” (Dall et al., 2004), “temperament” (Réale et al., 2007), and “coping style” (Koolhaas et al., 1999), and several reviews have emphasized its potential evolutionary causes and functions under current global change (e.g., McDougall et al., 2006; Réale et al., 2007; Smith and Blumstein, 2008; Sih et al., 2011).
Urbanization is one of the most prevailing and lasting forms of rapid human-induced habitat change occurring worldwide, and is causing the loss of biodiversity through local extinction processes (McKinney, 2006; Sol et al., 2014). However, the response of species are greatly variable, and although most of them are unable to occupy these new habitats, others persist or even reach higher densities in urban than in rural areas (Stracey and Robinson, 2012; Sol et al., 2013; Rodriguez-Martínez et al., 2014; Tella et al., 2014). Thus, a growing number of studies have explored ecological and life history traits that allow some species to thrive in urban environments (Bonier et al., 2007; Sol et al., 2014). Recently, it has been proposed that the ability of certain species to colonize urban habitats is related to their interindividual variability in the fear of humans, measured as the distance between an approaching human and a focal animal at which the latter flees (i.e., flight initiation distance; Carrete and Tella, 2011). Although differences in fear of humans among populations subject to different degrees of human disturbance have been traditionally interpreted as indicative of habituation (e.g., Blumstein et al., 2003; Martínez-Abrain et al., 2008; Rodríguez-Prieto et al., 2009), recent work demonstrated that this behavior is highly repeatable within an individual's adulthood (Carrete and Tella, 2010, 2013) and heritable (Møller, 2014; Carrete et al., 2016), leaving a small margin for behavioral flexibility (Vincze et al., 2016). Thus, urban invasion seems to be the result of tame individuals from species with high interindividual variability in their fear of humans crossing the disturbance frontier, supporting the disturbance-induced habitat selection hypothesis (Carrete and Tella, 2010).
Recent studies have shown that individuals within a population can be defined as bold or aggressive across a wide range of situations (i.e., territorial, feeding, parental, or antipredator behavior), while others are consistently nonaggressive or shy. The existence of these suites of correlated behaviors (Sih et al., 2004) suggests that behaviors should be evaluated together rather than as isolated units due to their potential consequences on individual fitness and to understand potential trade-offs or conflicts. The idea that urban areas (and humanized environments in a more broad sense) can act to select individuals with tolerant behaviors toward people (Arroyo et al., 2017) opens the question of whether they can also induce changes in other behaviors because of genetic correlations (constraint hypothesis) or through pressures acting in a similar direction (adaptive hypothesis; Bell, 2005, 2007; Dingemanse et al., 2007). In this sense, previous studies have correlated flight initiation distance with exploration or aggression toward conspecifics (Garamszegi et al., 2009; Evans et al., 2010), suggesting that other behaviors in addition to fear of humans could also change when birds enter urban areas.
Here, we test whether urbanization influences the distribution and diversity of individual behaviors, selecting for populations with particular behavioral structures. For this purpose, we focused on the burrowing owl, a bird species largely studied as a model of recent urban colonization (Carrete and Tella, 2010, 2011, 2013; Rodriguez-Martínez et al., 2014; Rebolo-Ifrán et al., 2015; Carrete et al., 2016). We experimentally show that rural and urban individuals differ in their fear of humans and in its association with antipredatory and exploratory behaviors. This suggests that behavioral correlations in this species are the result of selective pressures acting on the different behaviors and supporting their adaptive nature.
Materials and Methods
Study Species, Area and Population Monitoring
The burrowing owl (Athene cunicularia) is a small owl found across American open landscapes, showing diurnal activity and nesting in burrows excavated by themselves or by mammals (del Hoyo et al., 1999). Pairs are territorial and highly conspicuous in the daytime during the breeding season (from October to early February in the study area), and are easily located usually within 50m of their nests. Sexual differences in coloration and plumage patterns (del Hoyo et al., 1999) allow experienced observers to sex breeding adults at a distance using binoculars (Carrete and Tella, 2010).
During the 2009–2010 breeding season, we GPS-located 192 active nests of burrowing owls in a 3,500 km2 area, comprising the city of Bahía Blanca (Argentina) and its surrounding rural areas. Urban birds excavated their own nests in private and public gardens and in spaces among houses in urbanized residential areas, usually within 10–100 m of inhabited buildings, but also on curbs of streets and even on large avenues in the city. Rural birds, however, breed in the surrounding large expanses of natural grasslands and pastures devoted to wide-ranging livestock and low-intensive cereal crops, where owls excavate their own nests but can also occupy burrows of fossorial mammals (Carrete and Tella, 2011). There, human presence and activities are extremely low (Carrete and Tella, 2011). There is not a clear gradient neither habitat barriers (rivers, mountains, forests, etc.) between urban and rural habitats, as the city is immediately surrounded by large and flat rural expanses.
Territories were regularly visited to monitor breeding success, capture birds, and perform behavioral tests. Breeding birds were captured using bow nets and ribbon carpets placed at the entrance of their nests, and marked by using a plastic ring with an individual alphanumeric code readable at a distance.
Behavioral Experiments
From November 2009 to January 2010, we visited all territories to establish the experimental conditions needed to characterize behavioral traits in different ecological situations, namely: (1) presence of a new food source (exploration), (2) presence of predators (aggression toward predators) and (3) fear of humans (risk taking). To homogenize the underlying state of the individuals as much as possible, we only performed behavioral tests in those territories where breeders where rearing chicks, excluding those with fledglings or unsuccessful nests (e.g., nests where chicks were predated). Behavioral tests were performed sequentially (exploration, antipredatory behavior toward a terrestrial predator, and antipredatory behavior toward an aerial predator) to facilitate comparison between individuals, except for the fear of humans, which was measured throughout the study period. We are not aware of any lasting harm (e.g., nest failure, territory abandonment, individual injury) caused by the experimental approaches performed in this study.
Risk Taking
Fear of humans is indicative of the risk that individuals are willing to take in our presence, and has been shown to be key to understanding avian urban invasion (Carrete and Tella, 2011). We measured it as the distance at which a bird flees when approached by a human, using the standard procedure of walking toward undisturbed focal individuals (perched on the ground or on small poles close to their nests, Figure 1A), following a direct trajectory at a constant speed of 0.5 m/s, with no obstacles between the bird and the observer. Distances at which birds fled were measured using a laser telemeter (Leica Geovid, range: 10–1,300 m) or counting paces for distances of less than 10 m (Carrete and Tella, 2010, 2011, 2013). FIDs were measured during the day, when owls were easily located at a distance, given the bare ground and short vegetation surrounding their nests. Due to the high within-individual repeatability of FID in urban and rural owls, both within (r = 0.84–0.92; Carrete and Tella, 2010) and across breeding seasons covering the lifespan of individuals (r = 0.90–0.96; Carrete and Tella, 2013), we only used one measure per individual for analysis (average values when more than one measure was obtained from a single individual).

Figure 1. Breeding burrowing owls were systematically approached by a researcher to measure their flight initiation distances when they were perching close to their nests (A), and then were sequentially exposed to a trap with a white laboratory mouse kept within a small cage (B), and polyester reproductions of a Peregrine Falcon Falco peregrinus (C) and a Pampa Fox Pseudalopex gymnocercus (D) placed close to the entrance of their nests.
Exploration/Avoidance
We tested differences in an individual's behavior when facing a novel food source by presenting birds with an unfamiliar food item placed in an unfamiliar object close (1 m) to the entrance of their nests. The novel food used was a white laboratory mouse kept within a small cage in a metallic trap (Figure 1B). Thus, the exploration/avoidance experiment coincided with the capturing sessions. Owls can prey on house mice Mus musculus and other rondentines, which are mainly brownish, so we inferred that birds had not previously exploited such a novel (white mouse) but potential food source. Moreover, the non-camouflaged cage in which the mice were presented also changes the way mice are usually encountered. Therefore, we assumed that the responses of individuals would reflect how they cope (exploration or avoidance) with altered foraging opportunities. For each bird, we measured time (in minutes) to approach the trap (i.e., perching close ≤ 1 m- to the trap with hunting attitude) as a measure of its willingness to explore the new food. As trapability can be affected by aspects other than the interest of the animal in the mouse, we did not take into account the final result of the capture session (captured or not). Observations were recorded from a vehicle using binoculars (10 × 40) and telescopes (20–60×) at a minimum distance of twice the FID of the most shy individual of the pair to avoid interfering in the activity of the birds. The experiment was completed after a variable time depending on an individual's behaviors.
Antipredatory Behavior
After the exploration/avoidance experiment, we quantified the antipredatory behavior of birds toward a terrestrial (Pampa Fox Pseudalopex gymnocercus) and an aerial (peregrine falcon Falco peregrinus) predator, both of which are native to the study area. Predator models (Figures 1C,D) were sequentially exposed to the same nests (firstly the fox, and secondly the falcon) during the period in which breeders were rearing their offspring, with a lag of ca. 10 days between them.
During the experiments, we placed a polyester reproduction of the predator close (1 m) to the entrance of the nests for 15 min to minimize disturbance. We measured the aggressive reaction toward the predator as the time (in minutes) to approach it (i.e., when the individual perched close to the predator, performing displays, and was ready to attack). As in the previous experiments, observations were recorded from a vehicle using binoculars and telescopes at a distance to avoid interfering with the activity of the birds.
We estimated the repeatability of antipredatory behavior using a Bayesian Markov chain Monte Carlo technique implemented in the MCMCglmm package in R (Hadfield, 2010), modeling the latency to approach a predator (log-transformed to reach normality) as dependent variable, including predator species as a fixed effect and individual as a random term. Models were run with priors for the random variances set to 1, and a degree of belief n = 2. We used a “cengaussian” distribution as latencies were right-censored. Estimates were insensitive to the choice of priors (prior variances range 0.01–100). Parameter expansion was used to avoid poor mixing if variance component estimates were close to zero. All models were run for 100,000 iterations, preceded by a burn-in of 10,000 iterations. Estimates of parameters were stored every 25th iteration to reduce autocorrelation. We tested the statistical support of the fixed effect by evaluating whether their posterior distributions (95% credible interval) overlapped zero. Repeatability (r) in latency to approach a predator was obtained separately for urban and rural birds as r = σindividual/(σindividual+ σunits). Latencies to approach predators resulted repeatable within individuals (rural birds: r = 0.55; 95% CI = 0.35–0.74; urban birds: r = 0.33; 95% CI = 0.19–0.50), independently of the predator species used (95% CI for the effect of the species of predator considered: rural birds: −0.37–0.16, urban birds: −0.18–0.30), so we used one randomly selected measure per individual for further analysis (see below).
Analytical Procedures
We first compared FID, and latencies to approach the new food source and the predators across birds from different habitats (i.e., urban vs. rural). Differences in FID (log-transformed) between urban and rural birds were assessed using Generalized Linear Models (normal error distribution, identity link function). Because latencies were right truncated at different times for those birds that did not approach the new food or the predators, we applied survival analyses to handle such censored data appropriately, using the package survival in R. Latency in each test was analyzed as a function of habitat using survival curves estimated through Kaplan-Meier methods, which allowed us to not assume underlying probability distributions and compare between two groups (urban and rural birds). Survival curves for urban and rural birds were compared using log-rank tests.
We then investigated behavioral correlations in urban and rural birds, separately. As we had censored data (i.e., minimum latencies) for both predator and food approaches, we were not able to perform classical Structural Equation Models (Dingemanse et al., 2010) without turning to imputation. Due to the large number of missing values, this procedure can reduce our ability to detect relationships among behaviors. Thus, as we prefer to avoid this last procedure, we calculated the covariances and correlations between behaviors using multivariate MCMCglmm, which are ultimately the main parameters indicative of the existence of a relationship between two behaviors. Although both statistics deal with the relationship between two variables, covariance indicates whether two variables change in tandem while correlation measures how strongly this relationship is. Moreover, while in the first case values are highly sensitive to the scale of measurement, correlations are calculated as the covariance between two behaviors divided by the product of their variances, so they can be viewed as the scaled form of covariance. Since the experiments were designed to measure the covariances between behaviors, we fitted completely parameterized covariance matrices during modeling (us()). An uninformative prior for these models was an improper prior with V = diag(x) and nu = dim(x+1), where x is the number of dependent variables (Hadfield, 2010). Support for the presence of relationships between behaviors was based on the posterior distribution of the estimated covariances and the corresponding 95% credible intervals.
Results
We measured fear of humans (FID) in 357 breeding burrowing owls. The number of individuals included in successive behavioral tests decreased, given the logistic limitations imposed by the duration of the nestling period and the fact that some offspring fledged or were predated before we could complete all the tests. Therefore, exploration was measured in 253 individuals, and latency in approaching a predator in 165 individuals, respectively.
Rural birds did not respond to the stimulus as fast as the urban birds and thus, censored data were rather common among the former, skewing the distribution of the data and providing some infinite values for the mean and the 95% credible intervals. However, Kaplan-Meier analyses allowed us to deal with this, showing that flight initiation distance, latency in approaching the new food, and latency in approaching a predator significantly differed between urban and rural birds (Table 1; Figure 2). Urban birds were more fearless in the face of humans and approached both the new food resource and the predator more quickly than their rural counterparts.
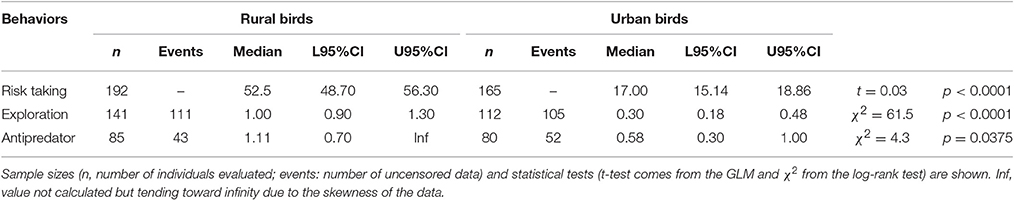
Table 1. Median log-transformed values (and 95% credible intervals: L95%CI: lower tail, U95%CI: upper tail) obtained for risk taking (measured as FID, in m), exploration (measured as latency in approaching a new food source, in minutes) and aggression toward predators (measured as latency in approaching a predator, in minutes) in rural and urban burrowing owls Athene cunicularia.
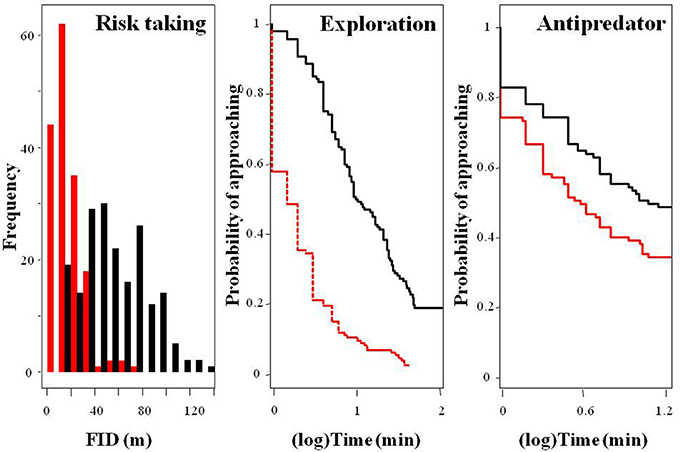
Figure 2. Frequency distribution of fear of humans (risk taking behavior, measured as FID) and survival curves showing the probability of approaching a new feeding source (exploration) or a predator (antipredator) over time among urban (red) and rural (black) burrowing owls.
Moreover, urban and rural birds differed in their expression of behavioral correlations (Table 2; Figure 3). Among rural birds, which can be considered as the reference group, we found significant positive relationships between risk taking and the other two behaviors tested. Although covariances and correlations were rather low, they were all significantly higher than 0, meaning that rural individuals with large flight initiation distances when facing a human also show large latencies in approaching a predator and a new food item. It should be noted that the credible interval for the correlation between risk-taking and antipredatory behavior slightly overlap 0, while the bulk of the distribution is highly skewed toward positives values. This means that we can assume that the probability of having a correlation between these behaviors equal or lower than zero is negligible. Finally, the 95% credible interval for the correlation between exploration and response toward predators widely overlapped with zero (Table 2), suggesting that these 2 traits could be measuring independent aspects of the individual phenotype. All together, differences between these covariances and correlations suggest that latencies in the presence of a new food are manifestations of a behavioral trait related to the exploration/avoidance axis, which is not completely independent of the bold/shy axis represented by the latency to approach a predator and individual's fear of humans.
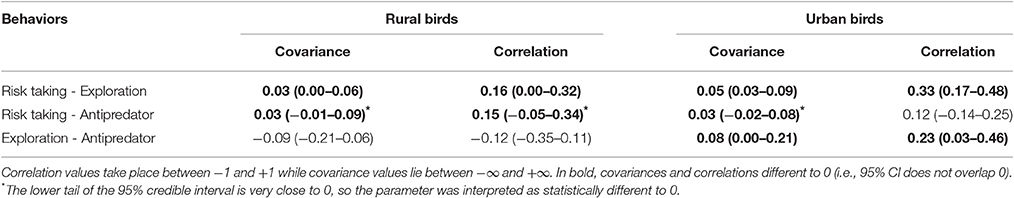
Table 2. Mean covariances and correlations, with their 95% credible intervals in brackets, obtained for risk taking (measured as FID), exploration (measured as latency in approaching a new food source) and antipredatory behavior (measured as latency in approaching a predator) in rural and urban burrowing owls Athene cunicularia.
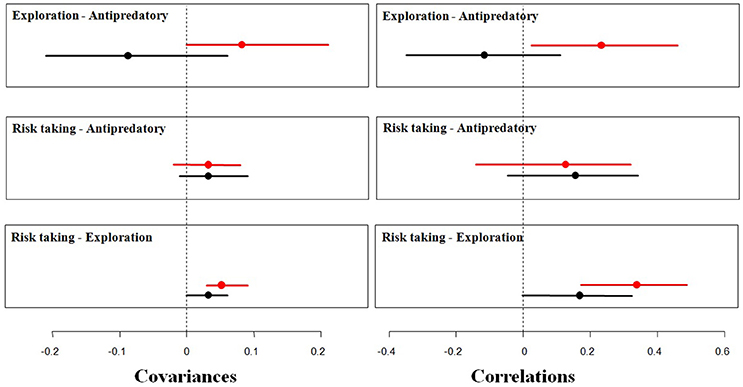
Figure 3. Mean covariances and correlations (points), with their 95% credible intervals (segments), between fear of humans (risk taking behavior, measured as FID), antipredatory response and exploration among urban (red) and rural (black) burrowing owls. The vertical, dashed line represent 0 covariance and correlation.
When considering the same relationships between behaviors among urban birds, however, all of them change. On the one side, the relationship between risk-taking and exploration became stronger in the urban population, as shown by the increase in the correlation coefficient, while exploration and latency to approach a predator coupled (positive correlation and 95% CI that does not overlap 0). Risk-taking and antipredatory behavior slightly covaried, but the correlation is not significantly different to 0 (95% CI widely overlap 0). All this means that urban birds with shorter flight distances approach a new food resource more rapidly while more explorative birds also approach more quickly the predator (Table 2).
Discussion
We found differences in three main behaviors between urban and rural burrowing owls, namely exploration, aggression toward predators and risk taking, in particular fear of humans. Our results show that rural birds were, in general, less fearless when facing humans, less explorative and less aggressive toward predators than their urban counterparts. Moreover, these behavioral traits did not vary independently of each other among these birds, as shown by the significant and positive correlations between them. Thus, rural individuals with larger flight initiation distances when facing a human also showed longer latencies in approaching a predator and a new food item. However, when the same relationships were investigated among urban birds, we found a significant covariance between fear of humans and exploration and between latency to approach a predator and a new feeding source, suggesting that selection pressures acting during urban invasion and while living in the city may be dismantling the behavioral correlations existent among individuals occupying more natural environments.
Two main hypotheses have been proposed to explain the existence of behavioral correlations. The constraint hypothesis assumes that a shared proximate link between personality traits, e.g., physiological or genetic factors (Ketterson and Nolan, 1999; van Oers et al., 2005), is responsible for the behavioral correlations. Conversely, the adaptive hypothesis states that correlations between personality traits emerge when selection favors correlated behaviors in particular environments, this correlation being adaptive itself (Bell, 2005). Here, we show how behavioral syndromes detected among rural birds changed when considering their urban counterparts, supporting the adaptive nature of these behavioral correlations (Scales et al., 2011; Bókony et al., 2012).
In the most natural scenario, burrowing owls that tolerate humans at closer distances were also more reactive toward predators, and approached new feeding sources sooner, the last two behavior remaining independent. However, in urban environments, burrowing owls face different ecological pressures compared to those present in rural ones. On the one hand, they are constantly interacting with people (Carrete and Tella, 2011), which may represent an important selective factor. Indeed, differences in the fear of humans between urban and rural populations seem to arise as a consequence of selective pressures precluding frightened individuals from colonizing urban areas and/or favoring their emigration/mortality in urbanized areas (Evans et al., 2010; Møller, 2010; Carrete and Tella, 2011; Atwell et al., 2012). At the same time, predation pressure also differs between urban and rural areas, with predators being much less abundant and diverse in the former than in the latter (Rebolo-Ifrán et al., in press). Avoidance of predation is an important determinant of fitness in many animals (Godin, 1997; Ruxton et al., 2004). However, when isolated from predators, costly or no longer functional antipredator behaviors can be selected against or their effectiveness cannot be selected any more, as occurs in species occupying islands (Blumstein and Daniel, 2002) or individuals bred in captivity (Carrete and Tella, 2015). Thus, urban individuals living in a predator-free area where humans are constantly present can lose their antipredator behavior while breaking its links to the fear of humans. Moreover, urban birds can take advantage of the predator release effect by gaining higher breeding success than their rural counterparts (Rebolo-Ifrán et al., in press), such that the heritabilities of the fear of humans (Carrete et al., 2016) and antipredator behavior (Bize et al., 2012) can subsequently reinforce the behavioral differences between urban and rural populations initially resulting from selection. Whatever the mechanism causing the decoupling between fear of humans and antipredator behaviors in urban individuals, our results—in line with those obtained by Myers and Hyman (2016)- challenge previous interpretations of flight initiation distances (FID). While FID has been frequently used as an experimental measure of the response of individuals facing a predator (Díaz et al., 2013), our results suggest that FID actually measures their response toward humans which, in some but not in all cases (i.e., urban populations), correlates with responses to predators. Therefore, FID could be interpreted as a measure of fear of humans rather than a broader measure of response when facing predators.
Exploration positively correlated with fear of humans, more strongly among rural than among urban birds. However, exploration and antipredatory behavior were positively related among urban birds, but not among their rural counterparts. Exploration and fear of humans have been shown to be positively correlated in domestic red junglefowls (Agnvall et al., 2012), although studies relating exploration and antipredator behavior yielded inconclusive results (Jones and Godin, 2010; Couchoux and Cresswell, 2011). Explorative behaviors can be advantageous when invading novel habitats such as urban areas (Martin and Fitzgerald, 2005), neophilic/explorative phenotypes having advantages in exploiting novel food resources or food available in novel contexts (Tryjanowski et al., 2016). Recently, it has been shown that exploration is repeatable and heritable (Dingemanse et al., 2002; Mazué et al., 2015), with permanent environmental (maternal) effects explaining most of the resemblance between parents and offspring (Schuett et al., 2013). Thus, bold and explorative individuals that successfully raise chicks in both urban and rural sites may transmit these behaviors to their progeny, even increasing the correlation between both behavioral traits in urban areas.
A major complication of assessing the consequences of human disturbance on wildlife is that those consequences are not always directly visible. For instance, even if seemingly unaffected (i.e., behaviorally calm), animals might undergo profound physiological changes in response to anthropogenic disturbances, or even to the mere presence of human observers. Our results show that fear of humans, a key behavior during urban invasion, correlated with other behaviors (antipredatory and exploratory behaviors) in birds living in natural environments. However, changes in selection pressures faced by urban individuals change these relationships, maintaining only those that are adaptive themselves (Bell, 2005). These results support the idea that differences among populations in the strength or direction of a behavioral correlation imply that these correlations can change during the evolutionary divergence of populations and are mainly due to the adaptive nature of each behavioral trait involved, demonstrating how human disturbances have the potential to contribute to population differentiation.
Ethics Statement
This study was carried out in accordance with the recommendations of Argentinean wildlife agencies and the owners of private properties. The protocol was approved by the Ethic committee of CSIC.
Author Contributions
MC and JT conceived the idea. JT and MC conducted field work. MC analyzed the data. JT and MC wrote the paper and discussed the results and commented on the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
M. Santillán helped with field work. Field work was conducted under permits from Argentinean wildlife agencies and the owners of private properties, and was funded by Canal Sur TV, Fundación Repsol and Projects RYC-2009-04860, CGL2012-31888 and CGL2015-71378-P from MINECO (Spain). D. Blumstein and an reviewer greatly helped to improve the paper.
References
Agnvall, B., Jöngren, M., Strandberg, E., and Jensen, P. (2012). Heritability and genetic correlations of fear-related behaviour in red junglefowl–possible implications for early domestication. PLoS ONE 7:e35162. doi: 10.1371/journal.pone.0035162
Arroyo, B., Mougeot, F., and Bretagnolle, V. (2017). Individual variation in behavioural responsiveness to humans leads to differences in breeding success and long-term population phenotypic changes. Ecol. Lett. 20, 317–325. doi: 10.1111/ele.12729
Atwell, J. W., Cardoso, G. C., Whittaker, D. J., Campbell-Nelson, S., Robertson, K. W., and Ketterson, E. D. (2012). Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav. Ecol. 23, 960–969. doi: 10.1093/beheco/ars059
Bell, A. M. (2005). Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 18, 464–473. doi: 10.1111/j.1420-9101.2004.00817.x
Bell, A. M. (2007). Future directions in behavioural syndromes research. Proc. R. Soc. B Biol. Sci. 274, 755–761. doi: 10.1098/rspb.2006.0199
Bize, P., Diaz, C., and Lindström, J. (2012). Experimental evidence that adult antipredator behaviour is heritable and not influenced by behavioural copying in a wild bird. Proc. R. Soc. B Biol. Sci. 279, 1380–1388. doi: 10.1098/rspb.2011.1789
Blumstein, D. T., Anthony, L. L., Harcourt, R., and Ross, G. (2003). Testing a key assumption of wildlife buffer zones: is flight initiation distance a species-specific trait? Biol. Conserv. 110, 97–100. doi: 10.1016/S0006-3207(02)00180-5
Blumstein, D. T., and Daniel, J. C. (2002). Isolation from mammalian predators differentially affects two congeners. Behav. Ecol. 13, 657–663. doi: 10.1093/beheco/13.5.657
Bókony, V., Kulcsár, A., Tóth, Z., and Liker, A. (2012). Personality traits and behavioral syndromes in differently urbanized populations of house sparrows (Passer domesticus). PLoS ONE 7:e36639. doi: 10.1371/journal.pone.0036639
Bonier, F., Martin, P. R., and Wingfield, J. C. (2007). Urban birds have broader environmental tolerance. Biol. Lett. 3, 670–673. doi: 10.1098/rsbl.2007.0349
Carrete, M., Martínez-Padilla, J., Rodríguez-Martínez, S., Rebolo-Ifrán, N., Palma, A., and Tella, J. L. (2016). Heritability of fear of humans in urban and rural populations of a bird species. Sci. Rep. 6:31060. doi: 10.1038/srep31060
Carrete, M., and Tella, J. L. (2010). Individual consistency in flight initiation distances in burrowing owls: a new hypothesis on disturbance-induced habitat selection. Biol. Lett. 6, 167–170. doi: 10.1098/rsbl.2009.0739
Carrete, M., and Tella, J. L. (2011). Inter-individual variability in fear of humans and relative brain size of the species are related to contemporary urban invasion in birds. PLoS ONE 6:e18859. doi: 10.1371/journal.pone.0018859
Carrete, M., and Tella, J. L. (2013). High individual consistency in fear of humans throughout the adult lifespan of rural and urban burrowing owls. Sci. Rep. 3:3524. doi: 10.1038/srep03524
Carrete, M., and Tella, J. L. (2015). Rapid loss of antipredatory behaviour in captive-bred birds is linked to current avian invasions. Sci. Rep. 5:e18274. doi: 10.1038/srep18274
Couchoux, C., and Cresswell, W. (2011). Personality constraints versus flexible antipredation behaviors: how important is boldness in risk management of redshanks (Tringa totanus) foraging in a natural system? Behav. Ecol. 23, 290–301. doi: 10.1093/beheco/arr185
Dall, S. R. X., Houston, A. I., and McNamara, J. M. (2004). The behavioral ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739. doi: 10.1111/j.1461-0248.2004.00618.x
del Hoyo, J., Elliot, A., and Sargatal, J. (1999). Handbook of the Birds of the World. Barcelona: Lynx Editions.
Díaz, M., Møller, A. P., Flensted-Jensen, E., Grim, T., Ibáñez-Álamo, J. D., Markó, G., et al. (2013). The geography of fear: a latitudinal gradient in anti-predator escape distances of birds across Europe. PLoS ONE 8:e64634. doi: 10.1371/journal.pone.0064634
Dingemanse, N. J., Both, C., Drent, P. J., Van Oers, K., and Van Noordwijk, A. J. (2002). Repeatability and heritability of exploratory behavior in great tits from the wild. Anim. Behav. 64, 929–937. doi: 10.1006/anbe.2002.2006
Dingemanse, N. J., Dochtermann, N., and Wright, J. (2010). A method for exploring the structure of behavioural syndromes to allow formal comparison within and between data sets. Anim. Behav. 79, 439–450. doi: 10.1016/j.anbehav.2009.11.024
Dingemanse, N. J., Wright, J., Kazem, A. J. N., Thomas, D. K., Hickling, R., and Dawnay, N. (2007). Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138. doi: 10.1111/j.1365-2656.2007.01284.x
Evans, J., Boudreau, K., and Hyman, J. (2010). Behavioural syndromes in urban and rural populations of song sparrows. Ethology 116, 588–595. doi: 10.1111/j.1439-0310.2010.01771.x
Garamszegi, L. Z., Eens, M., and Török, J. (2009). Behavioral syndromes and trappability in free-living collared flycatchers, Ficedula albicollis. Anim. Behav. 77, 803–812. doi: 10.1016/j.anbehav.2008.12.012
Godin, G. J. (1997). “Evading predators,” in Behavioural Ecology of Teleost Fishes, ed J. G. J. Godin (Oxford: Oxford University Press), 191–236.
Hadfield, J. D. (2010). MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Software 33, 1–22. doi: 10.18637/jss.v033.i02
Hendry, A. P., Farrugia, T. J., and Kinnison, M. T. (2008). Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 17, 20–29. doi: 10.1111/j.1365-294X.2007.03428.x
Jones, K. A., and Godin, J. G. J. (2010). Are fast explorers slow reactors? Linking personality type and anti-predator behaviour. Proc. R. Soc. B Biol. Sci. 277, 625–632. doi: 10.1098/rspb.2009.1607
Ketterson, E. D., and Nolan, V. (1999). Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 153, S4–S25. doi: 10.1086/303280
Koolhaas, J. M., Korte, S. M., de Boer, S. F., van der Vegt, B. J., van Reenen, C. G., et al. (1999). Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav. Rev. 23, 925–935. doi: 10.1016/S0149-7634(99)00026-3
Martínez-Abrain, A., Oro, D., Conesa, D., and Jiménez, J. (2008). Compromise between seabird enjoyment and disturbance: the role of observed and observers. Environ. Conserv. 35, 104–108. doi: 10.1017/S0376892908004748
Martin, L. B., and Fitzgerald, L. (2005). A taste for novelty in invading house sparrows, Passer domesticus. Behav. Ecol. 16, 702–707. doi: 10.1093/beheco/ari044
Mazué, G. P. F., Dechaume-Moncharmont, F. X., and Godin, J. G. J. (2015). Boldness–exploration behavioral syndrome: interfamily variability and repeatability of personality traits in the young of the convict cichlid (Amatitlania siquia). Behav. Ecol. 26, 900–908. doi: 10.1093/beheco/arv030
McDougall, P. T., Réale, D., Sol, D., and Reader, S. M. (2006). Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced, and wild populations. Anim. Conserv. 9, 39–48. doi: 10.1111/j.1469-1795.2005.00004.x
McKinney, M. L. (2006). Urbanization as a major cause of biotic homogenization. Biol. Conserv. 127, 247–260. doi: 10.1016/j.biocon.2005.09.005
Møller, A. P. (2010). Interspecific variation in fear responses predicts urbanization in birds. Behav. Ecol. 21, 365–371. doi: 10.1093/beheco/arp199
Møller, A. P. (2014). Life history, predation and flight initiation distance in a migratory bird. J. Evol. Biol. 27, 1105–1113. doi: 10.1111/jeb.12399
Myers, R. E., and Hyman, J. (2016). Differences in measures of boldness even when underlying behavioral syndromes are present in two populations of the song sparrow (Melospiza melodia). J. Ethol. 34, 197–206. doi: 10.1007/s10164-016-0465-9
Piersma, T., and Drent, J. (2003). Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 18, 228–233. doi: 10.1016/S0169-5347(03)00036-3
Réale, D., and Dingemanse, N. J. (2010). “Personality and individual social specialization,” in Social Behavior: Genes, Ecology and Evolution, eds T. Szekely, A. J. Moore, and J. Komdeur (Cambridge: Cambridge University Press), 417–441.
Réale, D., Reader, S. M., Sol, D., Mcdougall, P. T., and Dingemanse, N. J. (2007). Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. doi: 10.1111/j.1469-185X.2007.00010.x
Rebolo-Ifrán, N., Carrete, M., Sanz-Aguilar, A., Rodríguez-Martínez, S., Cabezas, S., Marchant, T. A., et al. (2015). Links between fear of humans, stress and survival support a non-random distribution of birds among urban and rural habitats. Sci. Rep. 5:13723. doi: 10.1038/srep13723
Rebolo-Ifrán, N., Tella, J. L., and Carrete, M. (in press). Urban conservation hotspots: predation release allows the grassland-specialist burrowing owl to perform better in the city. Sci. Rep.
Rodriguez-Martínez, S., Carrete, M., Roques, S., Rebolo, N., and Tella, J. L. (2014). High urban breeding densities do not disrupt genetic monogamy in a bird species. PLoS ONE 9:e91314. doi: 10.1371/journal.pone.0091314
Rodríguez-Prieto, I., Fernández-Juricic, E., Martín, J., and Regis, Y. (2009). Antipredator behavior in blackbirds: habituation complements risk allocation. Behav. Ecol. 20, 371–377. doi: 10.1093/beheco/arn151
Ruxton, G. D., Sherratt, T. N., and Speed, M. P. (2004). Avoiding attack: the evolutionary ecology of crypsis, warning signals, and mimicry. Oxford: Oxford University Press. doi: 10.1093/acprof:oso/9780198528609.001.0001
Scales, J., Hyman, J., and Hughes, M. (2011). Behavioral syndromes break down in urban song sparrow populations. Ethology 117, 887–895. doi: 10.1111/j.1439-0310.2011.01943.x
Schuett, W., Dall, S. R., Wilson, A. J., and Royle, N. J. (2013). Environmental transmission of a personality trait: foster parent exploration behaviour predicts offspring exploration behaviour in zebra finches. Biol. Lett. 9:20130120. doi: 10.1098/rsbl.2013.0120
Sih, A., Bell, A., and Johnson, J. C. (2004). Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. doi: 10.1016/j.tree.2004.04.009
Sih, A., Ferrari, M. C. O., and Harris, D. J. (2011). Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387. doi: 10.1111/j.1752-4571.2010.00166.x
Smith, B. R., and Blumstein, D. T. (2008). Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455. doi: 10.1093/beheco/arm144
Sol, D., Gonzalez-Lagos, C., Moreira, D., and Maspons, J. (2014). Urbanisation tolerance and the loss of avian diversity. Ecol. Lett. 17, 942–950. doi: 10.1111/ele.12297
Sol, D., Lapiedra, O., and González-Lagos, C. (2013). Behavioural adjustments for a life in the city. Anim. Behav. 85, 1101–1112. doi: 10.1016/j.anbehav.2013.01.023
Stracey, C. M., and Robinson, S. K. (2012). Are urban habitats ecological traps for a native songbird? Season-long productivity, apparent survival, and site fidelity in urban and rural habitats. J. Avian Biol. 43, 50–60. doi: 10.1111/j.1600-048X.2011.05520.x
Tella, J. L., Canale, A., Carrete, M., Petracci, P., and Zalba, S. M. (2014). Anthropogenic nesting sites allow urban breeding in burrowing parrots Cyanoliseus patagonus. Ardeola 61, 311–321. doi: 10.13157/arla.61.2.2014.311
Tryjanowski, P., Møller, A. P., Morelli, F., Biaduń, W., Brauze, T., Ciach, M., et al. (2016). Urbanization affects neophilia and risk-taking at bird-feeders. Sci. Rep. 6:28575. doi: 10.1038/srep28575
van Oers, K., de Jong, G., van Noordwijk, A. J., Kempenaers, B., and Drent, P. J. (2005). Contribution of genetics to the study of animal personalities: a review of case studies. Behaviour 142, 1191–1212. doi: 10.1163/156853905774539364
Keywords: antipredator behavior, exploration behavior, flight initiation distance, neophily, personalities, risk-taking
Citation: Carrete M and Tella JL (2017) Behavioral Correlations Associated with Fear of Humans Differ between Rural and Urban Burrowing Owls. Front. Ecol. Evol. 5:54. doi: 10.3389/fevo.2017.00054
Received: 13 February 2017; Accepted: 10 May 2017;
Published: 31 May 2017.
Edited by:
Amanda D. Rodewald, Cornell University, United StatesReviewed by:
Jeremy Hyman, Western Carolina University, United StatesDaniel T. Blumstein, University of California, Los Angeles, United States
Copyright © 2017 Carrete and Tella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martina Carrete, mcarrete@upo.es
 Martina Carrete
Martina Carrete José L. Tella
José L. Tella