Impacts of Nitrogen and Phosphorus: From Genomes to Natural Ecosystems and Agriculture
- 1Comparative Plant and Fungal Biology Department, Royal Botanic Gardens, Kew, Richmond, United Kingdom
- 2Organismal Biology, School of Biological and Chemical Sciences, Queen Mary University of London, London, United Kingdom
- 3Department of Biology, Institute for Evolution and Biodiversity, University of Münster, Münster, Germany
- 4School of Life Sciences, Arizona State University, Tempe, AZ, United States
- 5Flathead Lake Biological Station, University of Montana, Polson, MT, United States
- 6Centre of Ecological and Evolutionary Synthesis, University of Oslo, Oslo, Norway
- 7Department of Integrative Biology, Oklahoma State University, Stillwater, OK, United States
- 8Department of Biology, University of Iowa, Iowa City, IA, United States
- 9CSIRO Agriculture and Food, Canberra, ACT, Australia
- 10Florida Museum of Natural History, University of Florida, Gainesville, FL, United States
- 11Department of Biology, University of Florida, Gainesville, FL, United States
- 12Lancaster Environment Centre, University of Lancaster, Lancaster, United Kingdom
- 13Department of Biology, University of Oklahoma, Norman, OK, United States
- 14Comparative Plant and Fungal Biology Department, Royal Botanic Gardens, Kew, London, United Kingdom
Nitrogen (N) and/or phosphorus (P) availability can limit growth of primary producers across most of the world's aquatic and terrestrial ecosystems. These constraints are commonly overcome in agriculture by applying fertilizers to improve yields. However, excessive anthropogenic N and P inputs impact natural environments and have far-reaching ecological and evolutionary consequences, from individual species up to entire ecosystems. The extent to which global N and P cycles have been perturbed over the past century can be seen as a global fertilization experiment with significant redistribution of nutrients across different ecosystems. Here we explore the effects of N and P availability on stoichiometry and genomic traits of organisms, which, in turn, can influence: (i) plant and animal abundances; (ii) trophic interactions and population dynamics; and (iii) ecosystem dynamics and productivity of agricultural crops. We articulate research priorities for a deeper understanding of how bioavailable N and P move through the environment and exert their ultimate impacts on biodiversity and ecosystem services.
Introduction
Fertilizers are central to the “green revolution,” which has seen about half of the world's land converted to agriculture (Kareiva et al., 2007). Nitrogen (N) and phosphorus (P) are the dominant rate-limiting nutrients in most natural systems and the major constituents of agrochemical fertilizers. The consequences of N and P losses from agricultural land, e.g., through runoff and leaching, can span multiple organizational levels and scales in time and space, threatening essential ecosystem services (Smith et al., 1999; Elser, 2012; Fowler et al., 2013). In nature excessive loadings of N and P are extrinsic drivers that (i) often reduce biodiversity directly (Chapin et al., 2000; Erisman et al., 2008; Lambers et al., 2010), and have indirect effects through (ii) increased local extinction via dominance of a few competitive species, leading to (iii) altered plant community structure (Rohr et al., 2016), (iv) reduced functional trait diversity of communities and ecosystems (Tilman and Lehman, 2001; Díaz et al., 2006), and (v) ultimately reshaping ecosystem services (Millennium Ecosystem Assessment., 2005; Figure 1). Examples of such services include the provision of clean water for human needs and leisure, maintaining and regulating soil fertility, and supporting services such as nutrient cycling and the transfer of nutrients through trophic levels (Millennium Ecosystem Assessment., 2005; Bommarco et al., 2013; Harrison et al., 2014).
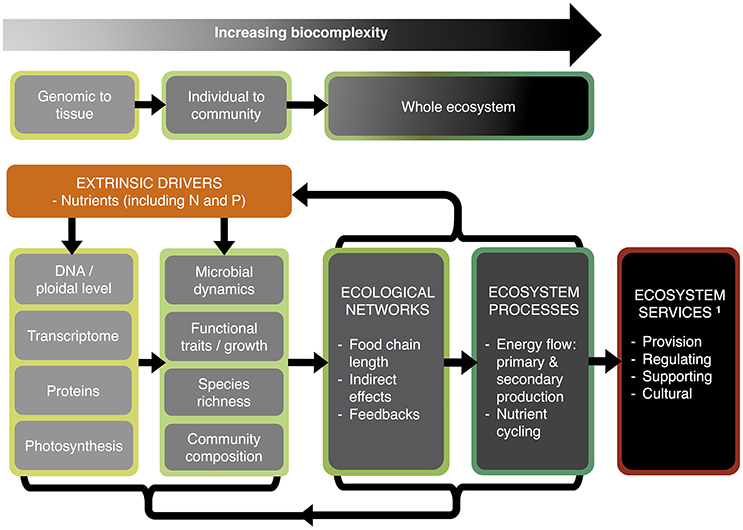
Figure 1. Effects of N and P availability—from genomes to ecosystems. Inputs of nutrients, in particular nitrogen (N) and phosphorus (P), act as extrinsic drivers affecting biological dynamics at the genomic to the ecosystem level, and which in turn feed-back on these drivers. An essential component of the ecosystem is the cycling of nutrients through the food web and back to the atmosphere and soil in inorganic forms. Such ecosystems provide services of benefit to humans. 1Services as defined by the Millennium Ecosystem Assessment. (2005).
At the organism level, N and P availability is known to have powerful influences on functional traits and growth rates, but we are only just beginning to understand that genome structure (e.g., genome size, ploidal level) can also play an important role (e.g., Neiman et al., 2009, 2013b; Šmarda et al., 2013; Guignard et al., 2016). At the genomic level, environmental nutrient limitation may constrain cellular processes (e.g., photosynthesis, transcriptomes) and over time may result in divergence of genes and the proteins they encode (Acquisti et al., 2009a,b; Elser et al., 2011; Seward and Kelly, 2016; Figure 1).
Thus, the fluxes, feedbacks, and availability of N and P fundamentally impact biota at all levels from genes and genomes to ecosystems, reshaping ecological and ultimately ecosystem processes. It is therefore crucial to understand how environmental N and P impact all levels of biological organization, from genome dynamics and cell metabolism, to the structure and functioning of multispecies systems (Figure 1). Such research could dramatically improve both the efficacy of biodiversity conservation and the development of more sustainable farming systems with lower N and P demands.
This paper evaluates the roles of N and P (1) in the environment, (2) within organisms, (3) in multispecies systems, and (4) in meeting human needs in the context of rising to the dual challenges of increasing food production and maintaining functional biodiversity to underpin the delivery of essential ecosystem services. It also proposes research priorities in these areas.
(1) N and P in the Environment
Nitrogen and P both underpin photosynthetic processes, cell growth, metabolism, and protein synthesis (Chapin et al., 2011), but their natural sources and rates of supply are very different: in principle, N availability is unlimited as an atmospheric gas, whereas P comes from rock phosphate, renewed with the uplift of continental rock. N and P co-limitation is common across the Earth's ecosystems in all the major biomes (Elser et al., 2007a). Today the primary sources of N and P originate from the massive anthropogenic inputs of fertilizers onto agricultural land (Fowler et al., 2013; Figure 2). For instance, industrial N production via the Haber–Bosch process surpassed natural fixation of dinitrogen gas more than 50 years ago. It affects vast areas by increased deposition of oxidized and reduced N with increased runoff of N to freshwater and coastal areas (Galloway et al., 2013).
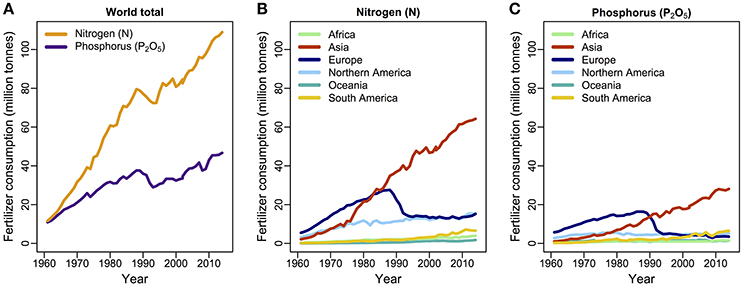
Figure 2. Consumption of N and P fertilizer by year (A) globally, and (B,C) by world regions, from 1961 to 2014. Data are available from the Food and Agriculture Organization of the United Nations (FAO, 2016).
Anthropogenic N-deposition also originates from nitrogen oxides created during fossil fuel combustion, from nitrogen fixation of cultivated legumes (Ciais et al., 2014), and ammonia produced from animal wastes (Sutton et al., 2013). This elevated N-deposition may shift ecosystems from N to P limitation, with subsequent detrimental ecosystem impacts (Elser et al., 2009, 2010). Conversely, the deposition of P from P-rich dust from sand and agricultural soils may shift some ecosystems from P to N limitation (e.g., mountain lakes: Camarero and Catalan, 2012; Brahney et al., 2015).
In ecosystems, N and P are bound within waste organic products and dead organic matter (e.g., in nucleic acids). They must first be remineralized to release inorganic orthophosphates or dissolved and reduced to inorganic nitrate and ammonia before either element can be absorbed by primary producers (i.e., autotrophic bacteria, algae, plants). The mineralization of organic to inorganic forms of N and P is completed primarily by microorganisms as they metabolize carbon (Spohn and Kuzyakov, 2013). These microorganisms also require N investment to synthesize the alkaline phosphatase enzymes that release P. Because organic N concentrations are higher than those of P, this investment is balanced by N availability and the corresponding gain in P. In terrestrial systems, P cycling is closely linked to mycorrhizal fungi in plant roots that release extracellular phosphatases. If N is applied above a certain threshold, P cycles faster in response to greater N availability for producing phosphatase enzymes. This in turn results in faster rates of P removal and increased P limitation (Vitousek et al., 2010). This implies that elemental imbalances generated at the molecular level can influence elemental stoichiometry in the ecosystem.
In agriculture, nutrients are also lost from ecosystems when crop products are harvested, leaving less plant litter to decompose and fewer nutrients to be returned to the soil. Consequently, fertilizers are added to build and maintain soil fertility. When applied at excessive levels, however, N and P may be lost via leaching, runoff, and erosion (e.g., globally, an estimated 15 million tons of P are lost annually from crop fields due to erosion; Smil, 2000), and an estimated 8 million tons of P are lost in runoff from arable land annually (Cordell et al., 2009). Nitrogen is additionally removed from an ecosystem as N2 and N2O gases derived from microbial denitrification (when bacteria use nitrate as a source of oxygen) and anaerobic ammonium oxidation (or anammox, when the oxidation of ammonium is coupled with the reduction of nitrite). With entry into aquatic systems, N and P will trigger eutrophication before being cycled or buried as sediments.
Future Research Priorities
Frequently, the stores, fluxes, and cycling of N and P have been considered separately, partly because of the relative ease of tracing N cycles compared with P. A unified interdisciplinary approach is needed to fully understand macronutrient fate and transport through the ecosystem. It needs to incorporate both terrestrial and aquatic components of the landscape as well as how different macronutrient cycles interact (Grimm et al., 2003; Guenet et al., 2010; Soininen et al., 2015). A better understanding of how N:P supply ratios affect microbial activity (e.g., nitrogen-fixing bacteria and mycorrhizal fungi), and subsequently the flow of these nutrients to and between organisms (e.g., Cherif and Loreau, 2009), may be obtained via controlled experiments. This may help to develop a clearer understanding of the impact of fertilizers on soil and water health and a more informed and environmentally sensitive approach to how we use fertilizers. The urgent need for a more comprehensive understanding is highlighted by the rising concern of a potential global scarcity of P (Cordell et al., 2009).
(2) N and P Usage within Organisms and Genome Structure
Nucleic acids are approximately 39% N and nearly 9% P by mass (Sterner and Elser, 2002), making them among the most N- and P-demanding biomolecules of the cell. Accordingly, the nucleus of eukaryotes represents a substantial sink for N and P. How this nucleic acid “sink” influences N and P stoichiometry remains unclear, yet these connections are potentially profound: genome size is one of the most variable of all organismal traits, varying 4-fold in mammals, 187-fold in insects, 378-fold in bony fishes, 460-fold in crustaceans (Gregory, 2016), and 2,400-fold in angiosperms (Pellicer et al., 2010). These differences in genome size reflect underlying genomic processes such as (retro)transposon amplification and deletion and whole-genome duplication (polyploidy). All of these processes can influence molecular evolution, gene expression, and organismal phenotype (Neiman et al., 2009, 2013a; Gerstein, 2013; Mayfield-Jones et al., 2013; Ramsey and Ramsey, 2014; Dodsworth et al., 2015; Selmecki et al., 2015; Soltis and Soltis, 2016). Polyploidy in particular has been implicated in the remarkably successful radiations of angiosperms (Soltis et al., 2009; Jiao et al., 2011; Albert et al., 2013; Tank et al., 2015; Van de Peer et al., 2017) and teleost fish (Van de Peer et al., 2009; Braasch and Postlethwait, 2012). Even so, many groups of eukaryotes are characterized by relatively small genomes, arising from genome-streamlining processes such as unequal and illegitimate recombination and chromosomal rearrangements triggered following polyploidy (Leitch and Bennett, 2004; Hessen et al., 2010; Dodsworth et al., 2016). Furthermore, the limited availability of environmental N and P and the expense of building and maintaining nucleic acids and associated proteins necessary to maintain a larger genome and hence cell may, under certain circumstances, act as a selection pressure driving the evolution of smaller genomes.
Selection on N and P use may also have an impact on genome composition. A comparative genomics approach on a set of animal and plant model organisms has shown N-conservation in the transcriptomes of wild plant taxa relative to both crop plants, which have a history of fertilizer application, and animals, which harvest N in organic form from other organisms (Acquisti et al., 2009a,b). Similarly, bias toward lower numbers of N atoms is reported in the highly expressed proteins of bacteria and yeast (Li et al., 2009), and in bacterial and eukaryotic parasites with low-N diets (Seward and Kelly, 2016).
The biochemical link between N and P in growth processes might underpin the broadly convergent ratios reported in many eukaryotic groups, such as the classic Redfield ratio, which states that marine plankton exhibit a mean C:N:P of 106:16:1 (Redfield, 1934; Klausmeier et al., 2004). Most eukaryotes, including microbes (Cleveland and Liptzin, 2007), maintain a certain degree of C, N, and P homeostasis. However, ratios vary with species growth rate (Hillebrand et al., 2013), trophic level, and environmental parameters. For example, N:P ratios range from 21:1 in broadleaved forests to 43:1 in tropical forests (McGroddy et al., 2004), while a more general ratio of 28:1 has been attributed to vascular plants (Chapin et al., 2011). Thus, while general ratios have been described, there is considerable variation due to fluctuations in environmental nutrient availability (Güsewell, 2004), phylogeny, and geography.
Biotic stoichiometric ratios are especially good reflections of nutrient availability at the base of the food web, where the scope for plasticity is greatest: proxy measures associated with latitudinal gradients have been described for both terrestrial plants (Reich and Oleksyn, 2004; Kerkhoff et al., 2006) and marine phytoplankton (Martiny et al., 2013). For example, older tropical soils are richer in N than P, whereas soils of recently glaciated regions at higher latitudes show the opposite pattern, implying reduced N:P ratios in colder areas (Reich and Oleksyn, 2004). Latitudinal and temperature-related changes in N:P likely reflect a higher demand for ribosomes (and thus P) to maintain sufficient protein synthesis at lower temperatures (Toseland et al., 2013; Thrane et al., 2017). This apparent link between temperature and stoichiometry in primary producers is perhaps unsurprising given that temperature drives the rates of many biological processes, including photosynthesis (C gain) and N and P uptake from the environment. At the genomic level, transcription rates in plants also increase with temperature (Sidaway-Lee et al., 2014). The higher demands for ribosomes at lower temperatures may occur to maintain protein synthesis or arise from the increased number of stored immature ribosomes in the nucleolus (Leitch et al., 1995), which can also translate into higher P-demands (and lower N:P) (Woods et al., 2003; Toseland et al., 2013; Thrane et al., 2017).
Future Research Priorities
At an organismal level, functional traits influence fitness via their effects on growth rate, reproduction, and survival (Violle et al., 2007). A stoichiometric perspective leads us to speculate that genome size and ploidal level are important but often ignored functional traits. For example, very large genomes in plants could be selected against in many ecosystems due to their higher demands for N and P. In animals, there may also be selection against larger genomes due to the reduced fitness associated with relatively low developmental rates and the relatively high demand for P invested in RNA (as much as 50–80% of cellular P) for protein synthesis (Hessen and Persson, 2009; Neiman et al., 2013a, but see Larkin et al., 2016). A definitive answer to the question of the extent to which larger genome sizes and higher ploidal levels translate into N and P costs will require characterization of organism-level consequences. For example, the increased N and P demands could be offset by the lower number of cells that are sometimes, but not always, associated with larger genomes (Neiman et al., 2017). Demands may also be offset by more efficient allocation of cellular P to RNA. Indeed polyploidy can rapidly induce a diversity of genetic and epigenetic responses, which can lead to highly variable total transcriptome volumes (Grover et al., 2012), a trait upon which selection can act.
Another key unanswered question is the nature of trade-offs between the genome, transcriptome, proteome, and metabolites for N and P usage, under differing N and P stress, at the cellular, tissue, and organismal levels and in organisms with different genome sizes. Controlled growth experiments under differing nutrient regimes, combined with biochemical, DNA/RNA, and genome analyses, are needed. Understanding the associations between C:N:P ratios and how these elements are partitioned for ribosomal synthesis vs., for example, histone synthesis may not only lead to important insights for organisms within ecosystems, but also provide novel medical insights into cancer dynamics (Elser et al., 2003, 2007b).
(3) The Roles of N and P and Genome Sizes in Species Assemblies
As we move beyond the organism and population levels, we can begin to elucidate some of the roles and consequences of these phenomena on multispecies systems such as communities, food webs, and entire ecosystems. Polyploidy is a genomic trait that may have cascading effects on food webs. For instance, in marine ecosystems, polyploidy may influence the composition of zooplankton communities. Polyploid zooplankton are more common in the Arctic, where a time constraint is imposed by the short growing season (Van Geest et al., 2010). The differential responses between taxa of different ploidal levels could shift the balance of power in both horizontal (competitive) and vertical (consumer-resource) interactions within ecological networks of interacting taxa.
The functional traits of species can influence energy and nutrient fluxes and the resilience of ecosystems to environmental disturbances. Recent evidence suggests that genome structure (i.e., genome size, ploidal level) and nutrient availability can influence plant distributions, community composition, and biomass production in grasslands (Šmarda et al., 2013; Guignard et al., 2016; Segraves, 2017). This possibility is bolstered by large-scale comparative analyses using the Plant DNA C-values database (Bennett and Leitch, 2012) which suggest that plants with large genomes are at greater risk of extinction and are less tolerant of polluted soils and extreme environmental conditions (Vinogradov, 2003; Knight et al., 2005; Greilhuber and Leitch, 2013). Although genome size effects can depend on ploidal level, these data clearly demonstrate that genome structure has ecological consequences that can shape the distribution and persistence of biodiversity.
Variation in DNA and RNA usage in primary producers may also cascade upwards to higher trophic levels. For example, higher ploidal-level representatives of aquatic animals fare better (biomass as well as amount of N and P in their tissues) than their lower ploidal-level congeners, when their diets (composed of primary producers) have relatively high nutrient content. The reverse holds true in low-nutrient conditions (Neiman et al., 2013b; Jeyasingh et al., 2015). Radiotracer assays have also revealed that polyploid Daphnia incorporated significantly more 33P and excreted significantly less 33P compared with diploids (Jeyasingh et al., 2015), indicating potentially strong effects of ploidal level on key population and, by extension, community parameters related to consumer-resource interactions. In addition to these “green pathways” that link autochthonous producers to herbivorous consumers, there is clearly the potential for the “brown pathways” that transfer energy and nutrients via detrital feeding links to also be affected by N and P and genome structure. For instance, terrestrial leaf litter fuels the base of many freshwater food webs and the main determinants of its consumption are its C:N:P stoichiometry, which is shaped by the taxonomic and functional attributes of the plants as well as environmental nutrient conditions (e.g., Hladyz et al., 2009; Woodward et al., 2012). Consequently, if the C:N:P stoichiometry of terrestrial plants is itself linked to genome size, genome attributes of these plants have clear potential to shape ecosystem-level processes and the trophic basis of production of the higher trophic levels in the food web. How, and to what extent, these influences are manifested in natural ecosystems also could depend on the extent of nutrient enrichment from agriculture within the surrounding landscape.
Future Research Priorities
Polyploids and taxa with larger genome sizes should be more common where nutrients are more abundant (Lewis, 1985; Leitch and Bennett, 2004; Leitch and Leitch, 2008; Hessen et al., 2010; Neiman et al., 2013a; Leitch et al., 2014). Support for these predictions has come from recent studies of freshwater snails (Neiman et al., 2013b) and angiosperms (Šmarda et al., 2013; Guignard et al., 2016). Further data collection on, and empirical tests of, the associations between N and P limitation, genome size, and ploidal-level variation in diverse habitat types and biomes are clearly needed to determine how far such predictions hold across different ecosystems and larger, continental scales. Such investigations may take advantage of geographical information systems and niche modeling approaches.
Organisms are frequently linked in stoichiometric feedback loops (Sterner, 1990; Gruner et al., 2008) within wider elemental cycles. Even so, and perhaps due to the sheer complexity of ecological systems, research is most often focused on top-down (consumer-directed) vs. bottom-up (resource-based) effects within only a small part of the food web. A broader system-level approach that can also include indirect effects or reciprocity is needed. Elemental availability can also play a large role in predator-prey interactions, including nutrient cycling by predators, which, in turn, influences what elements are available to prey (Grover, 2003; Andersen et al., 2004; Sardans et al., 2012). With respect to macro-organisms, plants form the base of the food web; the diversity of plant structures is hypothesized to influence multiple trophic levels via effects on differential nutrient requirements, intake, growth rates, and, thus, food quality for higher trophic levels, either as an autochthonous resource that is processed via the food web's green pathways or as detritus within the brown pathways.
Plants are affected by their environment but they can also modify this environment via shifts in microbial communities that have short- and long-term effects (Putten et al., 2013; Van Nuland et al., 2016). Such ecological feedbacks occur when interactions at one time determine the performance or interactions of organisms at another (Hendry, 2016). The impact of nutrient availability has already been demonstrated to alter eco-evolutionary dynamics in plants (Wooliver et al., 2016) and in fish due to increased eutrophication, resulting in changes to parasite load and individual feeding ecology (Anaya-Rojas et al., 2016; Brunner et al., 2017). Future research will thus not only have to focus on direct effects of N and P on species but also on how they impact the interactions between species at the ecosystem level.
(4) N and P and Genomes—toward Sustainable Agriculture
The world's consumption of N- and P-based fertilizers has increased substantially since the 1960's although that rise is now largely driven by agriculture across Asia (FAO, 2016; Figure 2). As discussed above, N and P availability may influence the productivity of plant taxa differentially, depending on genome structure: when nutrients are in excess, polyploid plants tend to increase more in biomass production and competitiveness than diploids. This increased yield may be one reason why most crops are polyploids (Leitch and Leitch, 2008). The rapid rate of biomass production for which crops are typically selected is associated with high soil nutrient demands. Indeed this high demand for nutrients has essentially been “designed” into our current agricultural systems. That in turn has led to a high dependence for fertilizer inputs, whereby agricultural crops generally display high critical nutrient requirements (N and P) for optimal growth with high product removal. This is not only economically expensive but also biologically inefficient and environmentally destabilizing, with increased potential for collateral damage to aquatic ecosystems via eutrophication. For example, P inputs are two to five times greater than the amount exported in the final product (Simpson et al., 2011), and crops take up only 30–40% of applied N (Kant et al., 2011). Nutrient-use efficiency in crops can be improved by, for example, selection of morphological and physiological traits that maximize nutrient uptake (Richardson et al., 2011), optimizing traits that increase the efficiency of ribosomes (Kreps et al., 2002; Kant et al., 2011; Veneklaas et al., 2012), and reducing the carbon costs of nutrient uptake (Lynch and Ho, 2005). Crops are high in P content with a 15:1 N:P ratio (Veneklaas et al., 2012), in contrast to the 28:1 ratio across vascular plants as a whole. Moreover, high concentrations of P in grain crops are undesirable because P is predominantly stored as phytate, which is indigestible, and which reduces absorption of other nutrients in non-ruminant animals, including humans (Veneklaas et al., 2012). The indigestibility of this form of P means it ends up in our sewage and waterways, and one solution may be to reduce P uptake and/or P concentrations in seeds and grains using genomic approaches (Raboy, 2001; Yamaji et al., 2017).
Future Research Priorities
Many engineering approaches are being considered to help improve the management of N and P in the environment. One key goal is to apply less fertilizer while maintaining or even enhancing agricultural yields. A key part of this process will be to revisit our polyploid crops. To date, much plant breeding has exploited polyploids, where genic diversity is fixed and favorable characters can be selected (e.g., allopolyploids: wheat, cotton, tobacco, sugarcane; autopolyploids: strawberry, alfalfa, banana) (Udall and Wendel, 2006; Renny-Byfield and Wendel, 2014). Yet these crops have been developed in a context of high inputs of N, P, and other nutrients. By contrast, the wild relatives of these crop species typically grow in relatively infertile habitats. By targeting inbred introgressed lines and applying high-throughput sequencing in combination with marker-assisted or genomic selection approaches (Heffner et al., 2009; Xu et al., 2014; Jan et al., 2016; Lv et al., 2016), it may be possible to reduce nutrient requirements and/or improve nutrient-use efficiency (higher yield per unit used of fertilizer) of crop plants. There are significant commercial gains to be made from reducing our dependency on N- and P-containing fertilizers. For example, increasing N use efficiency by 1% alone could lead to estimated annual savings of $1.1 billion (Kant et al., 2011). Additional reductions in fertilizer use could also come from harnessing the microbiome in plant selection, especially under limiting N and P. Future avenues include the use of bacteria and fungi to increase a plant's uptake of nutrients, in particular P (reviewed in Owen et al., 2015), and to exploit new technologies aiming to inhibit P loss and increase fertilizer recovery (Withers et al., 2015).
Conclusion
In defining a “safe-operating space” for humanity in the Anthropocene, Rockström et al. (2009a,b) identified nine planetary boundaries and thresholds for anthropogenic activities to remain globally sustainable. They argued that some of these have already been surpassed, including a proposed boundary of 35 million tonnes (Tg) per year of N2 removed from the atmosphere, far below the actual annual rate of 121 Tg (Rockström et al., 2009b). We are very close to the proposed boundary of 11 Tg per year of P flowing into oceans, currently at c. 9 Tg per year (Rockström et al., 2009b). However, if freshwater systems are also taken into account, we have also surpassed that P boundary (Carpenter and Bennett, 2011). Because N and P are linked to life systems ranging from global ecosystems (e.g., oceans) to genomes, a more complete understanding and an incorporation of stoichiometric analysis at all levels of biological organization are needed. This is indeed an urgent goal, as it will enable us to maintain or enhance agricultural productivity whilst simultaneously conserving and enriching the biodiversity that is essential for the continued provision of ecosystem services across the globe.
Author Contributions
MG, AL, CA, CE, JE, DH, PJ, MN, AR, PS, DS, CS, MT, GW, LW and IL wrote, revised, and evaluated the manuscript, led by MG, AL, and IL.
Funding
This project received grants from the Research Council of Norway (“Genome” project, no. 196468), awarded to DH. MT and MG benefitted from funding by the Natural Environment Research Council (NE/J012106/1). CE acknowledges funding from the German Research Foundation (DFG, EIZ841/4-1, and EIZ841/6-1). LW was funded through the U.S. National Science Foundation (NSF-IOS-OEI) grant no. 1256881 during the manuscript preparation stage. The U.S. National Science Foundation, grant no. 1439461, provided support for graduate students and post-doctoral researchers to attend the conference. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This paper arose out of a Theo Murphy discussion meeting coordinated by AL, DH, PJ, MN, LW, and IL and held at the Kavli Royal Society Centre, Chicheley Hall, UK, on 1st and 2nd June 2015. The discussions are available online. We thank the Royal Society for sponsoring and hosting this meeting.
References
Acquisti, C., Elser, J. J., and Kumar, S. (2009a). Ecological nitrogen limitation shapes the DNA composition of plant genomes. Mol. Biol. Evol. 26, 953–956. doi: 10.1093/molbev/msp038
Acquisti, C., Kumar, S., and Elser, J. J. (2009b). Signatures of nitrogen limitation in the elemental composition of the proteins involved in the metabolic apparatus. Proc. R. Soc. London B Biol. Sci. 276, 2605–2610. doi: 10.1098/rspb.2008.1960
Albert, V. A., Barbazuk, W. B., Der, J. P., Leebens-Mack, J., Ma, H., Palmer, J. D., et al. (2013). The Amborella genome and the evolution of flowering plants. Science 342:1241089. doi: 10.1126/science.1241089
Anaya-Rojas, J. M., Brunner, F. S., Sommer, N., Seehausen, O., Eizaguirre, C., and Matthews, B. (2016). The association of feeding behaviour with the resistance and tolerance to parasites in recently diverged sticklebacks. J. Evol. Biol. 29, 2157–2167. doi: 10.1111/jeb.12934
Andersen, T., Elser, J. J., and Hessen, D. O. (2004). Stoichiometry and population dynamics. Ecol. Lett. 7, 884–900. doi: 10.1111/j.1461-0248.2004.00646.x
Bennett, M. D., and Leitch, I. J. (2012). Plant DNA C-Values Database (Release 6.0, Dec. 2012). Available online at: http://www.kew.org/cvalues/
Bommarco, R., Kleijn, D., and Potts, S. G. (2013). Ecological intensification: harnessing ecosystem services for food security. Trends Ecol. Evol. 28, 230–238. doi: 10.1016/j.tree.2012.10.012
Braasch, I., and Postlethwait, J. H. (2012). “Polyploidy in fish and the teleost genome duplication,” in Polyploidy and Genome Evolution, eds P. S. Soltis and D. E. Soltis (Berlin; Heidelberg: Springer), 341–383.
Brahney, J., Mahowald, N., Ward, D. S., Ballantyne, A. P., and Neff, J. C. (2015). Is atmospheric phosphorus pollution altering global alpine lake stoichiometry? Global Biogeochem. Cycles 29, 1369–1383. doi: 10.1002/2015GB005137
Brunner, F. S., Anaya-Rojas, J. M., Matthews, B., and Eizaguirre, C. (2017). Experimental evidence that parasites drive eco-evolutionary feedbacks. Proc. Natl. Acad. Sci. U.S.A. 114, 3678–3683. doi: 10.1073/pnas.1619147114
Camarero, L., and Catalan, J. (2012). Atmospheric phosphorus deposition may cause lakes to revert from phosphorus limitation back to nitrogen limitation. Nat. Commun. 3:1118. doi: 10.1038/ncomms2125
Carpenter, S. R., and Bennett, E. M. (2011). Reconsideration of the planetary boundary for phosphorus. Environ. Res. Lett. 6:14009. doi: 10.1088/1748-9326/6/1/014009
Chapin, F. S. III., Matson, P. A., and Vitousek, P. (2011). Principles of Terrestrial Ecosystem Ecology. New York, NY: Springer Science & Business Media.
Chapin, F. S. III., Zavaleta, E. S., Eviner, V. T., Naylor, R. L., Vitousek, P. M., Reynolds, H. L., et al. (2000). Consequences of changing biodiversity. Nature 405, 234–242. doi: 10.1038/35012241
Cherif, M., and Loreau, M. (2009). When microbes and consumers determine the limiting nutrient of autotrophs: a theoretical analysis. Proc. R. Soc. London B Biol. Sci. 276, 487–497. doi: 10.1098/rspb.2008.0560
Ciais, P., Sabine, C., Bala, G., Bopp, L., Brovkin, V., Canadell, J., et al. (2014). “Carbon and other biogeochemical cycles,” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge; New York, NY: Cambridge University Press), 465–570.
Cleveland, C. C., and Liptzin, D. (2007). C: N: P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85, 235–252. doi: 10.1007/s10533-007-9132-0
Cordell, D., Drangert, J.-O., and White, S. (2009). The story of phosphorus: global food security and food for thought. Glob. Environ. Chang. 19, 292–305. doi: 10.1016/j.gloenvcha.2008.10.009
Díaz, S., Fargione, J., Chapin, F. S. III., and Tilman, D. (2006). Biodiversity loss threatens human well-being. PLoS Biol. 4:e277. doi: 10.1371/journal.pbio.0040277
Dodsworth, S., Chase, M. W., and Leitch, A. R. (2016). Is post-polyploidization diploidization the key to the evolutionary success of angiosperms? Bot. J. Linn. Soc. 180, 1–5. doi: 10.1111/boj.12357
Dodsworth, S., Leitch, A. R., and Leitch, I. J. (2015). Genome size diversity in angiosperms and its influence on gene space. Curr. Opin. Genet. Dev. 35, 73–78. doi: 10.1016/j.gde.2015.10.006
Elser, J. J. (2012). Phosphorus: a limiting nutrient for humanity? Curr. Opin. Biotechnol. 23, 833–838. doi: 10.1016/j.copbio.2012.03.001
Elser, J. J., Acquisti, C., and Kumar, S. (2011). Stoichiogenomics: the evolutionary ecology of macromolecular elemental composition. Trends Ecol. Evol. 26, 38–44. doi: 10.1016/j.tree.2010.10.006
Elser, J. J., Andersen, T., Baron, J. S., Bergström, A.-K., Jansson, M., Kyle, M., et al. (2009). Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326, 835–837. doi: 10.1126/science.1176199
Elser, J. J., Bracken, M. E. S., Cleland, E. E., Gruner, D. S., Harpole, W. S., Hillebrand, H., et al. (2007a). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x
Elser, J. J., Kyle, M. M., Smith, M. S., and Nagy, J. D. (2007b). Biological stoichiometry in human cancer. PLoS ONE 2:e1028. doi: 10.1371/journal.pone.0001028
Elser, J. J., Nagy, J. D., and Kuang, Y. (2003). Biological stoichiometry: an ecological perspective on tumor dynamics. Bioscience 53, 1112–1120. doi: 10.1641/0006-3568(2003)053[1112:BSAEPO]2.0.CO;2
Elser, J. J., Peace, A. L., Kyle, M., Wojewodzic, M., McCrackin, M. L., Andersen, T., et al. (2010). Atmospheric nitrogen deposition is associated with elevated phosphorus limitation of lake zooplankton. Ecol. Lett. 13, 1256–1261. doi: 10.1111/j.1461-0248.2010.01519.x
Erisman, J. W., Sutton, M. A., Galloway, J., Klimont, Z., and Winiwarter, W. (2008). How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636–639. doi: 10.1038/ngeo325
FAO (2016). FAOSTAT Statistics Database. Food and Agriculture Organization of the United Nations Statistics Database. 453. Available online at: http://www.fao.org/faostat/en/#data/RF
Fowler, D., Coyle, M., Skiba, U., Sutton, M. A., Cape, J. N., Reis, S., et al. (2013). The global nitrogen cycle in the twenty-first century. Philos. Trans R. Soc. Lond. B Biol. Sci. 368:20130164. doi: 10.1098/rstb.2013.0164
Galloway, J. N., Leach, A. M., Bleeker, A., and Erisman, J. W. (2013). A chronology of human understanding of the nitrogen cycle. Philos. Trans. R. Soc. B Biol. Sci. 368:20130120. doi: 10.1098/rstb.2013.0120
Gerstein, A. C. (2013). Mutational effects depend on ploidy level: all else is not equal. Biol. Lett. 9:20120614. doi: 10.1098/rsbl.2012.0614
Gregory, T. R. (2016). Animal Genome Size Database. Available online at: http://www.genomesize.com
Greilhuber, J., and Leitch, I. J. (2013). “Genome size and the phenotype,” in Plant Genome Diversity Vol. 2: Physical Structure, Behaviour and Evolution of Plant Genomes, eds I. Leitch, J. Greilhuber, J. Dolezel, and J. F. Wendel (Vienna: Springer), 323–344.
Grimm, N. B., Gergel, S. E., McDowell, W. H., Boyer, E. W., Dent, C. L., Groffman, P., et al. (2003). Merging aquatic and terrestrial perspectives of nutrient biogeochemistry. Oecologia 137, 485–501. doi: 10.1007/s00442-003-1382-5
Grover, C. E., Gallagher, J. P., Szadkowski, E. P., Yoo, M. J., Flagel, L. E., and Wendel, J. F. (2012). Homoeolog expression bias and expression level dominance in allopolyploids. New Phytol. 196, 966–971. doi: 10.1111/j.1469-8137.2012.04365.x
Grover, J. P. (2003). The impact of variable stoichiometry on predator-prey interactions: a multinutrient approach. Am. Nat. 162, 29–43. doi: 10.1086/376577
Gruner, D. S., Smith, J. E., Seabloom, E. W., Sandin, S. A., Ngai, J. T., Hillebrand, H., et al. (2008). A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecol. Lett. 11, 740–755. doi: 10.1111/j.1461-0248.2008.01192.x
Guenet, B., Danger, M., Abbadie, L., and Lacroix, G. (2010). Priming effect: bridging the gap between terrestrial and aquatic ecology. Ecology 91, 2850–2861. doi: 10.1890/09-1968.1
Guignard, M. S., Nichols, R. A., Knell, R. J., Macdonald, A., Romila, C., Trimmer, M., et al. (2016). Genome size and ploidy influence angiosperm species' biomass under nitrogen and phosphorus limitation. New Phytol. 210, 1195–1206. doi: 10.1111/nph.13881
Güsewell, S. (2004). N: P ratios in terrestrial plants: variation and functional significance. New Phytol. 164, 243–266. doi: 10.1111/j.1469-8137.2004.01192.x
Harrison, P. A., Berry, P. M., Simpson, G., Haslett, J. R., Blicharska, M., Bucur, M., et al. (2014). Linkages between biodiversity attributes and ecosystem services: a systematic review. Ecosyst. Serv. 9, 191–203. doi: 10.1016/j.ecoser.2014.05.006
Heffner, E. L., Sorrells, M. E., and Jannink, J.-L. (2009). Genomic selection for crop improvement. Crop Sci. 49, 1–12. doi: 10.2135/cropsci2008.08.0512
Hessen, D. O., Jeyasingh, P. D., Neiman, M., and Weider, L. J. (2010). Genome streamlining and the elemental costs of growth. Trends Ecol. Evol. 25, 75–80. doi: 10.1016/j.tree.2009.08.004
Hessen, D. O., and Persson, J. (2009). Genome size as a determinant of growth and life-history traits in crustaceans. Biol. J. Linn. Soc. 98, 393–399. doi: 10.1111/j.1095-8312.2009.01285.x
Hillebrand, H., Steinert, G., Boersma, M., Malzahn, A., Meunier, C. L., Plum, C., et al. (2013). Goldman revisited: faster-growing phytoplankton has lower N: P and lower stoichiometric flexibility. Limnol. Ocean. 58, 2076–2088. doi: 10.4319/lo.2013.58.6.2076
Hladyz, S., Gessner, M., Giller, P., Pozo, J., and Woodward, G. (2009). Resource quality and stoichiometric constraints on stream ecosystem functioning. Freshw. Biol. 54, 957–970. doi: 10.1111/j.1365-2427.2008.02138.x
Jan, H. U., Abbadi, A., Lücke, S., Nichols, R. A., and Snowdon, R. J. (2016). Genomic prediction of testcross performance in canola (Brassica napus). PLoS ONE 11:e0147769. doi: 10.1371/journal.pone.0147769
Jeyasingh, P. D., Chowdhury, P. R., Wojewodzic, M. W., Frisch, D., Hessen, D. O., and Weider, L. J. (2015). Phosphorus use and excretion varies with ploidy level in Daphnia. J. Plankton Res. 37, 1210–1217. doi: 10.1093/plankt/fbv095
Jiao, Y., Wickett, N. J., Ayyampalayam, S., Chanderbali, A. S., Landherr, L., Ralph, P. E., et al. (2011). Ancestral polyploidy in seed plants and angiosperms. Nature 473, 97–100. doi: 10.1038/nature09916
Kant, S., Bi, Y.-M., and Rothstein, S. J. (2011). Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 62, 1499–1509. doi: 10.1093/jxb/erq297
Kareiva, P., Watts, S., McDonald, R., and Boucher, T. (2007). Domesticated nature: shaping landscapes and ecosystems for human welfare. Science 316, 1866–1869. doi: 10.1126/science.1140170
Kerkhoff, A. J., Fagan, W. F., Elser, J. J., and Enquist, B. J. (2006). Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 168, E103–E122. doi: 10.1086/507879
Klausmeier, C. A., Litchman, E., Daufresne, T., and Levin, S. A. (2004). Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 429, 171–174. doi: 10.1038/nature02454
Knight, C. A., Molinari, N. A., and Petrov, D. A. (2005). The large genome constraint hypothesis: evolution, ecology and phenotype. Ann. Bot. 95, 177–190. doi: 10.1093/aob/mci011
Kreps, J. A., Wu, Y., Chang, H.-S., Zhu, T., Wang, X., and Harper, J. F. (2002). Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 130, 2129–2141. doi: 10.1104/pp.008532
Lambers, H., Brundrett, M. C., Raven, J. A., and Hopper, S. D. (2010). Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 334, 11–31. doi: 10.1007/s11104-010-0444-9
Larkin, K., Tucci, C., and Neiman, M. (2016). Effects of polyploidy and reproductive mode on life history trait expression. Ecol. Evol. 6, 765–778. doi: 10.1002/ece3.1934
Leitch, A. R., Glyn, M., Kingham, K., Aragon-Alcaide, A., Somasekaram, A., and Duckett, J. (1995). “The dynamic organization of interphase nuclei during cell differentiation and changing cell activity,” in Kew Chromosome Conference IV, eds P. Brandham and M. Bennett (Kew: Royal Botanic Gardens), 83–94.
Leitch, A. R., and Leitch, I. J. (2008). Genomic plasticity and the diversity of polyploid plants. Science 320, 481–483. doi: 10.1126/science.1153585
Leitch, A. R., Leitch, I. J., Trimmer, M., Guignard, M. S., and Woodward, G. (2014). Impact of genomic diversity in river ecosystems. Trends Plant Sci. 19, 361–366. doi: 10.1016/j.tplants.2013.12.005
Leitch, I. J., and Bennett, M. D. (2004). Genome downsizing in polyploid plants. Biol. J. Linn. Soc. 82, 651–663. doi: 10.1111/j.1095-8312.2004.00349.x
Lewis, W. M. Jr. (1985). Nutrient scarcity as an evolutionary cause of haploidy. Am. Nat. 125, 692–701. doi: 10.1086/284372
Li, N., Lv, J., and Niu, D.-K. (2009). Low contents of carbon and nitrogen in highly abundant proteins: evidence of selection for the economy of atomic composition. J. Mol. Evol. 68, 248–255. doi: 10.1007/s00239-009-9199-4
Lv, Y., Liang, Z., Ge, M., Qi, W., Zhang, T., Lin, F., et al. (2016). Genome-wide identification and functional prediction of nitrogen-responsive intergenic and intronic long non-coding RNAs in maize (Zea mays L.). BMC Genomics 17:350. doi: 10.1186/s12864-016-2650-1
Lynch, J. P., and Ho, M. D. (2005). Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269, 45–56. doi: 10.1007/s11104-004-1096-4
Martiny, A. C., Pham, C. T. A., Primeau, F. W., Vrugt, J. A., Moore, J. K., Levin, S. A., et al. (2013). Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat. Geosci. 6, 279–283. doi: 10.1038/ngeo1757
Mayfield-Jones, D., Washburn, J. D., Arias, T., Edger, P. P., Pires, J. C., and Conant, G. C. (2013). Watching the grin fade: tracing the effects of polyploidy on different evolutionary time scales. Semin. Cell Dev. Biol. 24, 320–331. doi: 10.1016/j.semcdb.2013.02.002
McGroddy, M. E., Daufresne, T., and Hedin, L. O. (2004). Scaling of C: N: P stoichiometry in forests worldwide: implications of terrestrial redfield-type ratios. Ecology 85, 2390–2401. doi: 10.1890/03-0351
Millennium Ecosystem Assessment. (2005). Ecosystems and Human Well-being: Synthesis. Washington, DC: Island Press.
Neiman, M., Beaton, M. J., Hessen, D. O., Jeyasingh, P. D., and Weider, L. J. (2017). Endopolyploidy as a potential driver of animal ecology and evolution. Biol. Rev. 92, 234–247. doi: 10.1111/brv.12226
Neiman, M., Kay, A. D., and Krist, A. C. (2013a). Can resource costs of polyploidy provide an advantage to sex? Heredity 110, 152–159. doi: 10.1038/hdy.2012.78
Neiman, M., Kay, A. D., and Krist, A. C. (2013b). Sensitivity to phosphorus limitation increases with ploidy level in a New Zealand snail. Evolution 67, 1511–1517. doi: 10.1111/evo.12026
Neiman, M., Theisen, K. M., Mayry, M. E., and Kay, A. D. (2009). Can phosphorus limitation contribute to the maintenance of sex? A test of a key assumption. J. Evol. Biol. 22, 1359–1363. doi: 10.1111/j.1420-9101.2009.01748.x
Owen, D., Williams, A. P., Griffith, G. W., and Withers, P. J. A. (2015). Use of commercial bio-inoculants to increase agricultural production through improved phosphorus acquisition. Agric. Ecosyst. Environ. Appl. Soil Ecol. 86, 41–54. doi: 10.1016/j.apsoil.2014.09.012
Pellicer, J., Fay, M. F., and Leitch, I. J. (2010). The largest eukaryotic genome of them all? Bot. J. Linn. Soc. 164, 10–15. doi: 10.1111/j.1095-8339.2010.01072.x
Putten, W. H., Bardgett, R. D., Bever, J. D., Bezemer, T. M., Casper, B. B., Fukami, T., et al. (2013). Plant–soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265–276. doi: 10.1111/1365-2745.12054
Raboy, V. (2001). Seeds for a better future:“low phytate”grains help to overcome malnutrition and reduce pollution. Trends Plant Sci. 6, 458–462. doi: 10.1016/S1360-1385(01)02104-5
Ramsey, J., and Ramsey, T. S. (2014). Ecological studies of polyploidy in the 100 years following its discovery. Philos. Trans. R. Soc. B Biol. Sci. 369:20130352. doi: 10.1098/rstb.2013.0352
Redfield, A. C. (1934). On the Proportions of Organic Derivatives in Sea Water and their Relation to the Composition of Plankton. Liverpool: Liverpool University Press.
Reich, P. B., and Oleksyn, J. (2004). Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. U.S.A. 101, 11001–11006. doi: 10.1073/pnas.0403588101
Renny-Byfield, S., and Wendel, J. F. (2014). Doubling down on genomes: polyploidy and crop plants. Am. J. Bot. 101, 1711–1725. doi: 10.3732/ajb.1400119
Richardson, A. E., Lynch, J. P., Ryan, P. R., Delhaize, E., Smith, F. A., Smith, S. E., et al. (2011). Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349, 121–156. doi: 10.1007/s11104-011-0950-4
Rockström, J., Steffen, W. L., Noone, K., Persson, Å., Chapin, F. S. III., Lambin, E., et al. (2009a). Planetary boundaries: exploring the safe operating space for humanity. Ecol. Soc. 14:32.
Rockström, J., Steffen, W., Noone, K., Persson, Å., Chapin, F. S., Lambin, E. F., et al. (2009b). A safe operating space for humanity. Nature 461, 472–475. doi: 10.1038/461472a
Rohr, R. P., Saavedra, S., Peralta, G., Frost, C. M., Bersier, L.-F., Bascompte, J., et al. (2016). Persist or produce: a community trade-off tuned by species evenness. Am. Nat. 188, 411–422. doi: 10.1086/688046
Sardans, J., Rivas-Ubach, A., and Penuelas, J. (2012). The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: a review and perspectives. Biogeochemistry 111, 1–39. doi: 10.1007/s10533-011-9640-9
Segraves, K. A. (2017). The effects of genome duplications in a community context. New Phytol. 215, 57–69. doi: 10.1111/nph.14564
Selmecki, A. M., Maruvka, Y. E., Richmond, P. A., Guillet, M., Shoresh, N., Sorenson, A. L., et al. (2015). Polyploidy can drive rapid adaptation in yeast. Nature 519, 349–352. doi: 10.1038/nature14187
Seward, E. A., and Kelly, S. (2016). Dietary nitrogen alters codon bias and genome composition in parasitic microorganisms. Genome Biol. 17:226. doi: 10.1186/s13059-016-1087-9
Sidaway-Lee, K., Costa, M. J., Rand, D. A., Finkenstadt, B., and Penfield, S. (2014). Direct measurement of transcription rates reveals multiple mechanisms for configuration of the Arabidopsis ambient temperature response. Genome Biol. 15:R45. doi: 10.1186/gb-2014-15-3-r45
Simpson, R. J., Oberson, A., Culvenor, R. A., Ryan, M. H., Veneklaas, E. J., Lambers, H., et al. (2011). Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. Plant Soil 349, 89–120. doi: 10.1007/s11104-011-0880-1
Šmarda, P., Hejcman, M., Brezinová, A., Horová, L., Steigerová, H., Zedek, F., et al. (2013). Effect of phosphorus availability on the selection of species with different ploidy levels and genome sizes in a long-term grassland fertilization experiment. New Phytol. 200, 911–921. doi: 10.1111/nph.12399
Smil, V. (2000). Phosphorus in the environment: natural flows and human interferences. Annu. Rev. Energy Environ. 25, 53–88. doi: 10.1146/annurev.energy.25.1.53
Smith, V. H., Tilman, G. D., and Nekola, J. C. (1999). Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 100, 179–196. doi: 10.1016/S0269-7491(99)00091-3
Soininen, J., Bartels, P., Heino, J., Luoto, M., and Hillebrand, H. (2015). Toward more integrated ecosystem research in aquatic and terrestrial environments. Bioscience 65, 174–182. doi: 10.1093/biosci/biu216
Soltis, D. E., Albert, V. A., Leebens-Mack, J., Bell, C. D., Paterson, A. H., Zheng, C., et al. (2009). Polyploidy and angiosperm diversification. Am. J. Bot. 96, 336–348. doi: 10.3732/ajb.0800079
Soltis, P. S., and Soltis, D. E. (2016). Ancient WGD events as drivers of key innovations in angiosperms. Curr. Opin. Plant Biol. 30, 159–165. doi: 10.1016/j.pbi.2016.03.015
Spohn, M., and Kuzyakov, Y. (2013). Phosphorus mineralization can be driven by microbial need for carbon. Soil Biol. Biochem. 61, 69–75. doi: 10.1016/j.soilbio.2013.02.013
Sterner, R. W. (1990). The ratio of nitrogen to phosphorus resupplied by herbivores: zooplankton and the algal competitive arena. Am. Nat. 136, 209–229. doi: 10.1086/285092
Sterner, R. W., and Elser, J. J. (2002). Ecological Stoichiometry. The Biology of Elements from Molecules to the Biosphere. Princeton, NJ: Princeton University Press.
Sutton, M. A., Reis, S., Riddick, S. N., Dragosits, U., Nemitz, E., Theobald, M. R., et al. (2013). Towards a climate-dependent paradigm of ammonia emission and deposition. Philos. Trans. R. Soc. B Biol. Sci. 368:20130166. doi: 10.1098/rstb.2013.0166
Tank, D. C., Eastman, J. M., Pennell, M. W., Soltis, P. S., Soltis, D. E., Hinchliff, C. E., et al. (2015). Nested radiations and the pulse of angiosperm diversification: increased diversification rates often follow whole genome duplications. New Phytol. 207, 454–467. doi: 10.1111/nph.13491
Thrane, J., Hessen, D. O., and Andersen, T. (2017). Plasticity in algal stoichiometry: experimental evidence of a temperature-induced shift in optimal supply N: P ratio. Limnol. Oceanogr. doi: 10.1002/lno.10500. [Epub ahead of print].
Tilman, D., and Lehman, C. (2001). Human-caused environmental change: impacts on plant diversity and evolution. Proc. Natl. Acad. Sci. U.S.A. 98, 5433–5440. doi: 10.1073/pnas.091093198
Toseland, A., Daines, S. J., Clark, J. R., Kirkham, A., Strauss, J., Uhlig, C., et al. (2013). The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat. Clim. Chang. 3, 979–984. doi: 10.1038/nclimate1989
Udall, J. A., and Wendel, J. F. (2006). Polyploidy and crop improvement. Crop Sci. 46, S-3. doi: 10.2135/cropsci2006.07.0489tpg
Van de Peer, Y., Maere, S., and Meyer, A. (2009). The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 10, 725–732. doi: 10.1038/nrg2600
Van de Peer, Y., Mizrachi, E., and Marchal, K. (2017). The evolutionary significance of polyploidy. Nat. Rev. Genet. 18, 411–424. doi: 10.1038/nrg.2017.26
Van Geest, G. J., Sachse, R., Brehm, M., Van Donk, E., and Hessen, D. O. (2010). Maximizing growth rate at low temperatures: RNA: DNA allocation strategies and life history traits of Arctic and temperate Daphnia. Polar Biol. 33, 1255–1262. doi: 10.1007/s00300-010-0814-z
Van Nuland, M. E., Wooliver, R. C., Pfennigwerth, A. A., Read, Q. D., Ware, I. M., Mueller, L., et al. (2016). Plant–soil feedbacks: connecting ecosystem ecology and evolution. Funct. Ecol. 30, 1032–1042. doi: 10.1111/1365-2435.12690
Veneklaas, E. J., Lambers, H., Bragg, J., Finnegan, P. M., Lovelock, C. E., Plaxton, W. C., et al. (2012). Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 195, 306–320. doi: 10.1111/j.1469-8137.2012.04190.x
Vinogradov, A. E. (2003). Selfish DNA is maladaptive: evidence from the plant Red List. Trends Genet. 19, 609–614. doi: 10.1016/j.tig.2003.09.010
Violle, C., Navas, M., Vile, D., Kazakou, E., Fortunel, C., Hummel, I., et al. (2007). Let the concept of trait be functional! Oikos 116, 882–892. doi: 10.1111/j.0030-1299.2007.15559.x
Vitousek, P. M., Porder, S., Houlton, B. Z., and Chadwick, O. A. (2010). Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 20, 5–15. doi: 10.1890/08-0127.1
Withers, P. J. A., Elser, J. J., Hilton, J., Ohtake, H., Schipper, W. J., and Van Dijk, K. C. (2015). Greening the global phosphorus cycle: how green chemistry can help achieve planetary P sustainability. Green Chem. 17, 2087–2099. doi: 10.1039/C4GC02445A
Woods, H. A., Makino, W., Cotner, J. B., Hobbie, S. E., Harrison, J. F., Acharya, K., et al. (2003). Temperature and the chemical composition of poikilothermic organisms. Funct. Ecol. 17, 237–245. doi: 10.1046/j.1365-2435.2003.00724.x
Woodward, G., Gessner, M. O., Giller, P. S., Gulis, V., Hladyz, S., Lecerf, A., et al. (2012). Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336, 1438–1440. doi: 10.1126/science.1219534
Wooliver, R., Pfennigwerth, A. A., Bailey, J. K., and Schweitzer, J. A. (2016). Plant functional constraints guide macroevolutionary trade-offs in competitive and conservative growth responses to nitrogen. Funct. Ecol. 30, 1099–1108. doi: 10.1111/1365-2435.12648
Xu, Y., Wang, R., Tong, Y., Zhao, H., Xie, Q., Liu, D., et al. (2014). Mapping QTLs for yield and nitrogen-related traits in wheat: influence of nitrogen and phosphorus fertilization on QTL expression. Theor. Appl. Genet. 127, 59–72. doi: 10.1007/s00122-013-2201-y
Keywords: crops, genome size, nitrogen, nutrients, phosphorus, polyploidy, stoichiometry
Citation: Guignard MS, Leitch AR, Acquisti C, Eizaguirre C, Elser JJ, Hessen DO, Jeyasingh PD, Neiman M, Richardson AE, Soltis PS, Soltis DE, Stevens CJ, Trimmer M, Weider LJ, Woodward G and Leitch IJ (2017) Impacts of Nitrogen and Phosphorus: From Genomes to Natural Ecosystems and Agriculture. Front. Ecol. Evol. 5:70. doi: 10.3389/fevo.2017.00070
Received: 03 March 2017; Accepted: 19 June 2017;
Published: 06 July 2017.
Edited by:
Peter Schausberger, University of Vienna, AustriaReviewed by:
Valeria Souza, National Autonomous University of Mexico, MexicoBotir Khaitov, Tashkent State Agrarian University, Uzbekistan
Copyright © 2017 Guignard, Leitch, Acquisti, Eizaguirre, Elser, Hessen, Jeyasingh, Neiman, Richardson, Soltis, Soltis, Stevens, Trimmer, Weider, Woodward and Leitch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ilia J. Leitch, i.leitch@kew.org
 Maïté S. Guignard
Maïté S. Guignard Andrew R. Leitch
Andrew R. Leitch Claudia Acquisti
Claudia Acquisti Christophe Eizaguirre
Christophe Eizaguirre James J. Elser
James J. Elser Dag O. Hessen
Dag O. Hessen Punidan D. Jeyasingh
Punidan D. Jeyasingh Maurine Neiman
Maurine Neiman Alan E. Richardson
Alan E. Richardson Pamela S. Soltis
Pamela S. Soltis Douglas E. Soltis10,11
Douglas E. Soltis10,11  Mark Trimmer
Mark Trimmer Lawrence J. Weider
Lawrence J. Weider Guy Woodward
Guy Woodward Ilia J. Leitch
Ilia J. Leitch