Reproductive Contributions of Cardinals Are Consistent with a Hypothesis of Relaxed Selection in Urban Landscapes
- 1Cornell Lab of Ornithology and Department of Natural Resources, Cornell University, Ithaca, NY, United States
- 2Forest and Conservation Sciences, University of British Columbia, Vancouver, BC, Canada
Human activities are leading to rapid environmental change globally and may affect the eco-evolutionary dynamics of species inhabiting human-dominated landscapes. Theory suggests that increases in environmental heterogeneity should promote variation in reproductive performance among individuals. At the same time, we know that novel environments, such as our urbanizing study system, may represent more benign or predictable environments due to resource subsidies and ecological changes. We tested the hypothesis that reduced environmental heterogeneity and enhanced resource availability in cities relax selective pressures on birds by testing if urban females vary less than rural females in their demographic contributions to local populations. From 2004 to 2014, we monitored local population densities and annual reproductive output of 470 female Northern Cardinals (Cardinalis cardinalis) breeding at 14 forested sites distributed across a rural-to-urban landscape gradient in Ohio, USA. Reproductive contribution was measured as the difference between individual and site-averaged annual reproductive output across all nesting attempts, divided by the annual density at each site. We show that among-individual variation in reproductive contribution to the next year's population declined with increasing urbanization, despite similar variability in body condition across the rural-urban gradient. Thus, female cardinals that bred in urban habitats within our study area were more similar in their contribution to the next generation than rural breeders, where a pattern of winners and losers was more evident. Within-individual variation in annual reproductive contribution also declined with increasing urbanization, indicating that performance of females was also more consistent among years in urban than rural landscapes. These findings are consistent with the hypothesis that urbanized environments offer more homogeneous or predictable conditions that may buffer individuals from environmental heterogeneity and relax natural selection.
Introduction
Human activities are causing rapid and novel environmental change around the planet, highlighting a need to understand how non-human species adapt to such changes. Although, evolutionary and ecological processes are often characterized as operating on different time scales, rapid environmental change can lead to strong natural selection and rapid adaptive evolution (Thompson, 1998; Reznick and Ghalambor, 2001; Hendry et al., 2008). Examples of rapid adaptation to pollutants (Berry, 1964; McNeilly and Bradshaw, 1968; Kettlewell, 1973), pesticides (Georghiou, 1972), and harvest (Handford et al., 1977) are well-known, but more recent examples (e.g., Olsen et al., 2004; Phillips and Shine, 2004; Law and Salick, 2005) highlight the key role of eco-evolutionary dynamics on species persistence and distribution (e.g., Davis and Shaw, 2001; Stuart et al., 2014).
Perhaps nowhere is an understanding of the interplay between ecological and evolutionary processes more urgently needed than in human-altered systems, where anthropogenic disturbance can lead to strong selection on fitness traits (Stockwell et al., 2003; Kinnison and Hairston, 2007; Hendry et al., 2011). Urbanizing landscapes therefore offer excellent opportunities to study spatial and temporal heterogeneity in selection across rural to urban gradients (Siepielski et al., 2009; Cornwallis and Uller, 2010; Safran et al., 2010). Urban development alters biological (e.g., types of food), physical (e.g., temperature, light), chemical (e.g., pollutants), and ecological (e.g., densities of competitors or predators) factors known in other systems to strongly influence selection on phenotype (Reznick and Ghalambor, 2001). However, relatively few studies of eco-evolutionary dynamics in urban systems exist (Alberti, 2015). Badyaev et al. (2008) found evidence that urbanization via access to human-provided birdseed led to adaptive divergence in bill shape in urban and desert house finches (Carpodacus mexicanus). Atwell et al. (2014) report correlated changes in life history traits in an urbanizing junco population (Junco hyemalis). Likewise, Marnocha et al. (2011) provided evidence that morphological changes in brown anoles (Anolis sagrei) represented an adaptive response to human-induced habitat alteration via residential development. A recent global meta-analysis of 1,600 phenotypic changes across species, regions, and ecosystems showed that rates of phenotypic change were also greater urban than non-urban systems, consistent with an hypothesis of strong selection (Alberti et al., 2017).
In contrast, urban breeders might also be expected to experience relaxed selection pressures if urban environments are buffered from the deleterious effects of environmental variation experienced by rural breeders. For example, buffering in urban habitats might occur via increased predictability or homogenization of resources and the subsequent decoupling of urban breeders from naturally-occurring cycles of nutrients and water (Shochat et al., 2006; Buyantuyev and Wu, 2009, 2012; Groffman et al., 2014). Although, some work suggests that even abundant urban birds may reproduce less well in cities than rural areas (Meyrier et al., 2017), our prior results show that urban forests provide cardinals with more reliable and predictable food subsidies, including birdfeeders and the fruits of exotic shrubs, than those available to rural breeders (Atchison and Rodewald, 2006; Leston and Rodewald, 2006). Urban areas also offer warmer winter temperatures (Atchison and Rodewald, 2006; Shustack et al., 2009) and preferred nesting substrates (Leston and Rodewald, 2006; Rodewald et al., 2010). Overall, these factors promote high densities of cardinals in urban forests in patterns that are consistent with resource-matching (Rodewald and Shustack, 2008). However, despite nesting earlier than rural cardinals (Shustack and Rodewald, 2011) and experiencing similar rates of brood parasitism and predation on nests (Rodewald et al., 2013), fledglings (Ausprey and Rodewald, 2011), and adults (Rodewald and Shustack, 2008), urban and rural cardinals produced similar numbers of offspring each year (Rodewald and Shustack, 2008; Rodewald et al., 2013). Our prior results are also consistent with the hypothesis that abundant, accessible resources in urban habitats has relaxed selection on male coloration in Northern Cardinals (Cardinalis cardinalis) by disassociating color, condition, and reproductive performance (Rodewald et al., 2011). Overall, therefore, we expected to observe evidence of relaxed selection on breeding cardinals in urban vs. rural habitats.
Specifically, we estimated individual variation in the reproductive contributions of female cardinals to local population growth following Ezard et al. (2009) and Coulson et al. (2006). These methods allowed us to test the prediction that variation in female reproductive contributions to the next generation should be more similar in urban than rural habitats due to environmental buffering, consistent with an hypothesis of relaxed selection in urban vs. rural habitats, while accounting for variation in local population density (Coulson et al., 2006; Pelletier et al., 2007, 2009). We also tested two corollaries of the hypothesis that urban areas represent more benign selective environments than rural areas, by testing if urban females were in better condition and had greater reproductive success than rural females.
Materials and Methods
Study System
From 2004 to 2014, we studied a common synanthropic bird, the Northern Cardinal (C. cardinalis). Cardinals are year-round residents that nest in understory and midstory vegetation and defend territories during the breeding season (Halkin and Linville, 1999). Breeding densities of cardinals are highest in sites with dense understory shrubs (Leston and Rodewald, 2006), particularly Amur honeysuckle (Lonierca mackii), an exotic shrub that is preferred as a nesting substrate and can act as an ecological trap (Rodewald et al., 2010). Cardinals are multi-brooded, can quickly re-nest after failure, and often make 3–5 nest attempts annually. Nest predation is overwhelmingly the most common cause of nest failure in our system, and the nest predator community is diverse with 21 species documented to depredate cardinal nests (Rodewald and Kearns, 2011).
We studied cardinals at 14 sites located in mature riparian forests distributed across a rural-to-urban landscape in Ohio, USA (ca. 40N 00′ 83W 00′). Forest patches varied in size (mean width 163 m ± 2 SE) but were comparable in urban and rural landscapes. Building densities in our landscapes ranged from 0.1 to 7.3 buildings per ha (10–727 buildings/km2), and agriculture was the most common non-urban land use in rural landscapes. To quantify the matrix, we derived an urban index based on a principal components analysis (PCA) of landscape composition (number of buildings, percentages of agriculture, pavement, lawn, roads) within a 1-km radius area centered on each study site. We used the first principal component as an “urban index” because it explained most of the variation among sites and correlated strongly with urban land uses, being positively correlated with buildings, roads, pavement, and lawn, and negatively related to agriculture. Our measurements of landscape composition at our sites in 2001 and 2006 indicated that study sites changed little in the amount of urbanization over the course of our study.
Surveys of Avian Communities
Density of breeding cardinals within a 2-ha grid at each site was determined using spot-mapping (Bibby et al., 2000), noting the location, sex, and behavior of birds on detailed maps. This allowed us to estimate of the density of birds and number of territories in a specified area based on territorial behavior. Each grid was traversed at 50-m intervals 8–10 times from mid-April to June of each year.
Banding and Nest-Searching
To estimate annual reproduction, female cardinals were target-banded and individually-marked with a numbered aluminum metal (United States Geological Survey) and unique combination of colored plastic bands. Birds were captured and measured early in the breeding season, typically during territory establishment or nest building. Morphometric measurements (mass ± 0.5 g, wing ± 0.5 mm, and tarsus length ± 0.1 mm) were collected for each individual and used to calculate an index of body condition for each female at the time of first capture. While no single metric can fully capture body condition of an organism as is relevant to fitness, we used an approach commonly used in ornithological studies. To do so, we first used a PCA to estimate frame-size from wing and tarsus length. PC1 was positively correlated to wing and tarsus length (0.76 for each), explained 59% of variation in size (eigenvalue = 1.16), and used thereafter to indicate frame-size. We then regressed mass on frame-size and used residuals as a body condition index: females heavier or lighter than expected given frame-size were thus assumed to be in higher or lower condition than expected on average. Previous research in our system indicates that male brightness, timing of breeding, and ability to secure a preferred territory were all positively related to our condition index in rural birds (Rodewald et al., 2011), suggesting that it is linked positively to potentially influential reproductive traits. We examined relationships between urbanization and (a) body condition and (b) variance in body condition (i.e., among females breeding at the same site) separately using mixed models using site as a random factor and urban index as a fixed effect (predictor).
Field teams monitored all nesting attempts of known individuals from late March to September. Most nests were located early in the nesting stage, usually during building or egg-laying, and then checked at 1–3 day intervals. For nests that successfully fledged young, numbers of young were determined by either counting the number of nestlings immediately prior to fledging and/or by observing parents and young for extended periods near the time of fledging. Numbers of young that successfully fledged were summed across all nesting attempts for a given female in each year. Number of fledglings was then used as a response variable in a mixed model that included urban index as a fixed effect and site as a random effect.
Calculation of Reproductive Contributions of Individuals to Local Populations
Because natural selection and population dynamics are each driven by the birth and death of individuals, a demographic signature of selection can be measured on ecological time scales by examining the differential contributions of individuals to populations in a next generation (Coulson et al., 2006; Pelletier et al., 2007, 2009). Many studies of evolutionary change and selection estimate the fitness of alleles or phenotypes by measuring their representation in populations in future (Hamilton, 1964; Dawkins, 1982; Metz et al., 1992; Benton and Grant, 2000). Fitness can also be estimated as the relative reproductive performance of individuals within populations (Fisher, 1930; Lande, 1982), which is a function of population size and individual reproductive contributions to future generations (Coulson et al., 2006), and can be estimated annually to avoid complications related to among-individual variation in generation length, and helps control for the potential effects of environmental and ecological variation over an individual's lifetime.
We followed Coulson et al. (2006) to estimate individual contributions to population growth annually rather than by generation. Because our previous work showed that survival in rural and urban sites was similar in adults (φ = 0.57 + 0.04 SE; Rodewald and Shustack, 2008) and juveniles (φ = 0.44; 71 days, n = 45 birds; Ausprey and Rodewald, 2011), we simplified Coulson's equation by treating survival in each site as constant and calculated an individual's annual contribution to population growth, pt(i), as the number of offspring produced by female i in year t [ft(i)] minus the mean ft for that site and year, divided by the population density (per 10 ha) for that site and year minus one.
Reproductive contributions were calculated for 470 females in 14 study sites. In order to compare across populations of different densities, we summed the squared reproductive contributions across all females breeding within a given site and year [Σ = pt(i)2]. We then used this sum as a measure of variation in contribution and also as a response variable in a mixed model that included urban index as a fixed effect and site as a random effect. We also calculated variance in the reproductive contributions of individual females across years, and used that as a response variable in a mixed model that included urban index as a fixed effect and site as a random effect. Two post-hoc tests were used to examine the relationships between numbers of young produced (response variable) and either body condition of females or population density, using mixed models with site as a random effect variable. Sample sizes differ among the tests (see Section Results) because we did not have morphometric measurements for every banded female in every year for which we had annual reproduction, nor reproductive data for every banded female.
Results
Variation in individual contributions to local populations declined with increasing urbanization [β = −0.08 ± 0.03 SE, F(1, 90) = 5.41, P = 0.02; n = 113; Figure 1], indicating that the reproductive contributions of females to local populations were more homogenous in urban than rural sites. However, despite marked variation in the reproductive contributions of individuals, the mean number of fledglings produced by females at a site in a given year was similar across the rural-urban landscape gradient [F(1, 99) = 0.06; P = 0.81, n = 113; Table 1].
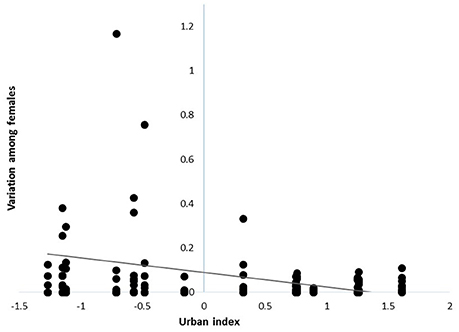
Figure 1. Variance in reproductive contributions of female cardinals breeding across a rural-to-urban landscape gradient in Ohio, USA from 2004 - 2014. Each point reflects the variation among females breeding within a given site and year (i.e., sum of squared reproductive contributions).
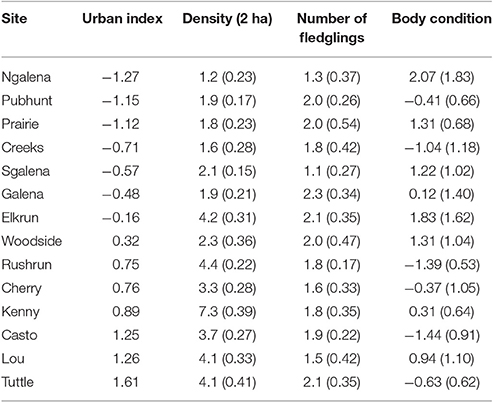
Table 1. The urban index and mean (SE) for density, mean number of fledglings per year, and body condition of female cardinals distributed across 14 forested sites in central Ohio, 2004–2014. Body condition is the residual from a regression of mass against a principle component of frame size (i.e., wing and tarsus).
Body condition declined as urbanization increased [β = −0.49 ± 0.22 SE; F(1, 317) = 5.14, P = 0.02; n = 341], but variation in body condition among females at the same site was similar across the rural to urban gradient [F(1, 64) = 0.03, P = 0.86; n = 78]. Thus, despite heterogeneity in the reproductive contributions of females across the rural-urban gradient, similar patterns did not emerge with respect to body condition. In contrast, urban females were more similar in their reproductive contributions among years than were females in rural sites [β = −0.03 ± 0.01 SE, F(1, 108) = 7.91, P = 0.01; n = 122; Figure 2]. Our two post-hoc analyses showed that (1) body condition was negatively related to number of fledglings produced in a given year [β = −0.08 + 0.03; F(1, 182) = 5.54; P = 0.02, n = 204] and (2) density at a site was not significantly related to fledgling production [F(1, 446) = 0.70; P = 0.40, n = 470].
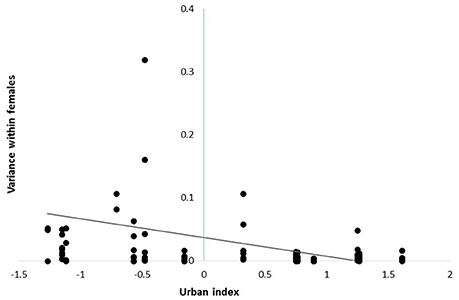
Figure 2. Within-individual variance in reproductive contributions for female cardinals across years at sites distributed along a rural-to-urban landscape gradient in Ohio, USA from 2004 - 2014. Each point represents the variance within an individual across years.
Discussion
We found that the annual reproductive contributions of female cardinals breeding in cities were less variable—both within and among individuals—than observed among rural females. The comparatively lower variation in contributions among females at urban than rural sites was observed despite similar patterns of among-individual variation in body condition. This finding implies that the higher variation in reproductive contribution in rural as compared to urban females was not a consequence of higher variation in female condition.
Theory suggests that environmental heterogeneity, either spatially or temporally, should promote variation in reproductive performance among individuals and the intensity of natural selection on phenotype (Wade and Kalisz, 1990; Byers, 2005; Siepielski et al., 2009, 2011, 2013; MacColl, 2011). Conversely, in more homogeneous or benign environments, we might expect reduced variation in the reproductive contributions of individuals and intensity of natural selection. Although, we did not measure selection directly, our results are consistent with the hypothesis that urban environments are more spatially and temporally homogeneous and/or resource-rich than rural environments and thereby buffer individuals from environmental variation and resource shortage in ways that relax natural selection. Indeed, our prior results in this system show that urban forests contained 2.5 times more fruit, 2.7 times more birdfeeders, and 2.5 times more preferred nesting substrate for cardinals than rural forests (Leston and Rodewald, 2006). High resource availability in urban as compared to rural forests is also consistent with our earlier observation that cardinal territories in urban forests were about one-third the size of those in rural forests (Rodewald and Shustack, 2008), as expected if territory size and resource abundance were negatively related (Hixon, 1980; Norton et al., 1982; Smith and Shugart, 1987). Access to urban-associated resources was also suggested to have reduced selection on male plumage color in urban vs. rural cardinals (Rodewald et al., 2011).
In contrast, we failed to support two assumptions about how resource-rich environments might affect urban populations. First, although high resource abundance or predictability in urban areas might be expected to enhance female condition, we found that female body condition declined as urbanization increased and that body condition was negatively related to number of fledglings produced. Such a pattern might arise if females in resource-rich environments invest more heavily in reproduction at the expense of future survival or reproduction in order to take advantage of temporal peaks in resource abundance (Williams, 1966; Wilson et al., 2007; Tarwater and Arcese, 2017). Indeed, many birds adjust body condition to balance the risks of food deprivation and depredation (Rogers, 1987) and when trading-off future reproduction or survival to invest in current reproduction (Arcese and Smith, 1988; Tarwater and Arcese, 2017). Whether cardinals engage in such trade-offs remains uncertain, but previous work in our system shows that urban cardinals bred earlier than rural birds, and although individuals that bred early attempted a greater number of nests, there was no measurable increase in numbers of fledglings due to the high rates of predation early in the breeding season (Rodewald et al., 2010; Shustack and Rodewald, 2011). Previous work in our system also provided evidence that cardinals distribute themselves in an ideal-free, or “resource-matching” manner, whereby resource-rich urban sites attain higher densities and support smaller territories, but perform similarly to individuals at lower density site in terms of condition, survival and reproduction (Rodewald and Shustack, 2008). If “faster” life histories are favored in predictable and/or resource-rich sites, which we have shown previously occur mainly in our urban study sites, the high variance in annual reproductive contribution observed among females in rural sites may simply reflect a wider range of reproductive tactics employed by females faced with higher spatial and temporal variation in resources (Williams, 1966; Wilson et al., 2007; Tarwater and Arcese, 2017). However, there is also the possibility that there is an advantage to being lean in urban environments. For example, in resource-rich environments, the relative benefit of fat reserves may be small compared to the potential costs of doing so via reduced agility or ability to evade predators (Rogers, 1987, 2015; Rogers and Smith, 1993).
In contrast to the assumption that resource-rich urban environments might enhance reproductive success, we found that urban and rural females had similar reproductive success. This result may be due to the fact that cardinal density increased in resource-rich urban areas, but territory size declined (Rodewald and Shustack, 2008), suggesting an ideal-free distribution of cardinal territories and reproductive success overall (Fretwell and Lucas, 1969). Similarly, the resource matching hypothesis predicts that individual fitness will not differ in rural and urban sites and is consistent with our finding no effect of site density on the number of young fledged annually. An ideal-free distribution of territories and annual reproductive success via resource matching is also consistent with our earlier results showing no difference in survival, condition, or reproductive output between urban and rural environments (Rodewald and Shustack, 2008). Similar patterns of resource matching have been demonstrated in many taxa, including birds (Harper, 1982; Recer et al., 1987; Diaz et al., 1998; Telleria and Perez-Tris, 2003), mammals (Morris, 1994), and fish (Milinski, 1984, 1988; Abrahams, 1989; Gotceitas and Colgan, 1991).
By driving change in global climate and land cover, humans create novel ecological conditions that are likely to drive evolutionary change in species capable of taking advantage of these “ecological opportunities” (Schluter, 2000; Badyaev et al., 2008; Atwell et al., 2014; Norman and Christidis, 2016). In particular, to the degree that urbanization increases environmental heterogeneity, we might expect populations occupying urban areas to experience increased variation in reproductive performance, their contributions to future generations, and the intensity of natural selection on individual phenotype. In contrast, our results are consistent with the hypothesis that urban areas represent more benign or predictable environments than rural areas due to resource subsidies and habitat homogenization, leading to a reduction in individual variance in the annual reproductive contributions among urban as compared to rural females. These and other results from urbanizing bird populations (e.g., Badyaev et al., 2008; Atwell et al., 2014; Alberti et al., 2017) suggest that comparative studies of life history and morphological evolution in populations distributed across more and less human-dominated landscapes offer outstanding opportunities to test for temporal and spatial variation in the intensity of natural selection and evolution of novel phenotypes.
Author Contributions
Both authors contributed to developing research questions, analyzing the data, interpreting the results, and writing the paper. AR oversaw the field research as part of her long term study system.
Funding
This work was supported by funding from the National Science Foundation (DEB-0340879 and DEB- 0639429), US Fish and Wildlife Service, Ohio Division of Wildlife, Ohio State University, and the Ohio Agricultural Research and Development Center.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Our deep gratitude goes to the many graduate students, especially D. Shustack, L. Kearns, I. Ausprey, M. Bakermans, K. Borgmann, L. Leston, J. Malpass, D. Narango, B. Padilla, S. Rose, L. Rowse, J. Smith-Castro, and others who have spent countless hours collecting field data. We thank Franklin County Metro Parks, Columbus Recreation, and Parks, Ohio Division of Wildlife, The Nature Conservancy, City of Bexley, Gahanna Parks, and Recreation and private landowners for access to study sites. We appreciate the thoughtful comments from reviewers of this manuscript, particularly T. Coulson. All research was conducted in accordance with approved protocol by Ohio State University's Institutional Animal Use and Care Committee (2010A0003, 2007A0015, 2004A0047, 00A0167) and banding was conducted under US Federal Bird Banding Permit 22272.
References
Abrahams, M. V. (1989). Foraging guppies and the ideal free distribution - the influence of information on patch choice. Ethology 82, 116–126. doi: 10.1111/j.1439-0310.1989.tb00492.x
Alberti, M. (2015). Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 30, 114–126. doi: 10.1016/j.tree.2014.11.007
Alberti, M., Correa, C., Marzluff, J. M., Hendry, A. P., Palkovacs, E. P., Gotanda, K. M., et al. (2017). Global urban signatures of phenotypic changes in animal and plant populations. Proc. Natl. Acad. Sci. U.S.A. doi: 10.1073/pnas.1606034114
Arcese, P., and Smith, J. N. M. (1988). The effects of supplemental food and population density on reproduction in song sparrows. J. Anim. Ecol. 57, 119–136. doi: 10.2307/4768
Atchison, K. A., and Rodewald, A. D. (2006). Do wintering birds prefer urban landscapes? Nat. Areas J. 26, 280–288. doi: 10.3375/0885-8608(2006)26[280:TVOUFT]2.0.CO;2
Atwell, J. W., Cardoso, G. C., Whittaker, D. J., Price, T. D., and Ketterson, E. D. (2014). Hormonal, behavioral, and life-history traits exhibit correlated shifts in relation to population establishment in a novel environment. Am. Nat. 184, E147–E160. doi: 10.1086/678398
Ausprey, I., and Rodewald, A. D. (2011). Post-fledging survivorship and habitat selection across a rural-to-urban landscape gradient. Auk 128, 293–302. doi: 10.1525/auk.2011.10158
Badyaev, A. V., Young, R. L., Oh, K. P., and Addison, C. (2008). Evolution on a local scale: developmental, functional, and genetic bases of divergence in bill form and associated changes in song structure between adjacent habitats. Evolution 62, 1951–1964. doi: 10.1111/j.1558-5646.2008.00428.x
Benton, T. G., and Grant, A. (2000). Evolutionary fitness in ecology: comparing measures of fitness in stochastic, density-dependent environments. Evol Ecol. Res. 2, 769–789.
Berry, R. (1964). Evolution of island population of house mouse. Evolution 18, 468–483. doi: 10.1111/j.1558-5646.1964.tb01623.x
Bibby, C. J., Burgess, N. D., Hill, D. A., and Mustoe, S. H. (2000). Bird Census Techniques, 2nd Edn. London: Academic Press.
Buyantuyev, A., and Wu, J. (2009). Urbanization alters spatiotemporal patterns of ecosystem primary production: a case study of the Phoenix metropolitan region, USA. J. Arid. Environ. 73, 512–520. doi: 10.1016/j.jaridenv.2008.12.015
Buyantuyev, A., and Wu, J. (2012). Urbanization diversifies land surface phenology in arid environments: interactions among vegetation, climatic variation, and land use pattern in the Phoenix metropolitan region, USA. Landsc. Urban Plan. 105, 149–159. doi: 10.1016/j.landurbplan.2011.12.013
Byers, D. (2005). Evolution in heterogeneous environments and the potential of maintenance of genetic variation in traits of adaptive significance. Genetica 123, 107–124. doi: 10.1007/s10709-003-2721-5
Cornwallis, C. K., and Uller, T. (2010). Towards an evolutionary ecology of sexual traits. Trends Ecol. Evol. 25, 145–152. doi: 10.1016/j.tree.2009.09.008
Coulson, T., Benton, T., Lundberg, P., Dall, S., Kendall, B., and Gaillard, J. (2006). Estimating individual contributions to population growth: evolutionary fitness in ecological time. Proc. R. Soc. B Biol. Sci. 273, 547–555. doi: 10.1098/rspb.2005.3357
Davis, M. B., and Shaw, R. G. (2001). Range shifts and adaptive responses to quaternary climate change. Science 292, 673–679. doi: 10.1126/science.292.5517.673
Diaz, M., Illera, J. C., and Atienza, J. C. (1998). Food resource matching by foraging tits Parus spp. during spring-summer in a Mediterranean mixed forest; evidence for an ideal free distribution. Ibis 140, 654–660. doi: 10.1111/j.1474-919X.1998.tb04711.x
Ezard, T. H. G., Coté, S. D., and Pelletier, F. (2009). Eco-evolutionary dynamics: disentangling phenotypic, environmental, and population fluctuations. Philos. Trans. R. Soc. 364, 1491–1498. doi: 10.1098/rstb.2009.0006
Fisher, R. A. (1930). The Genetical Theory of Natural Selection: A Complete Variorum Edition. Oxford: Oxford University Press.
Fretwell, D., and Lucas, H. L. Jr. (1969). On territorial behavior and other factors influencing habitat distribution in birds. I. Theoretical development. Acta Biotheor. 19, 16–36. doi: 10.1007/BF01601953
Georghiou, G. P. (1972). The evolution of resistance to pesticides. Annu. Rev. Ecol. Syst. 3, 133–168. doi: 10.1146/annurev.es.03.110172.001025
Gotceitas, V., and Colgan, P. (1991). Assessment of patch profitability and ideal free distribution - the significance of sampling. Behaviour 119, 65–76. doi: 10.1163/156853991X00373
Groffman, P. M., Cavender-Bares, J., Bettez, N. D., Grove, J. M., Hall, S. J., Heffernan, J. B., et al. (2014). Ecological homogenization of urban USA. Front. Ecol. Environ. 12:74. doi: 10.1890/120374
Halkin, S. L., and Linville, S. U. (1999). “Northern cardinal (Cardinalis cardinalis),” in The Birds of North America, No. 440, eds A. Poole and F. Gill (Philadelphia, PA: The Birds of North America, Inc).
Handford, P., Bell, G., and Reimchen, T. (1977). Gillnet fishery considered as an experiment in artificial selection. J. Fish. Res. Board Can. 34, 954–961.
Harper, D. G. C. (1982). Competitive foraging in Mallards - ideal free ducks. Anim. Behav. 30, 575–584. doi: 10.1016/S0003-3472(82)80071-7
Hendry, A. P., Farrugia, T. J., and Kinnison, M. T. (2008). Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 17, 20–29. doi: 10.1111/j.1365-294X.2007.03428.x
Hendry, A. P., Kinnison, M. T., Heino, M., Day, T., Smith, T. B., Fitt, G., et al. (2011). Evolutionary principles and their practical application. Evol. Appl. 4, 159–183. doi: 10.1111/j.1752-4571.2010.00165.x
Hixon, M. A. (1980). Food-production and competitor density as the determinants of feeding territory size. Am. Nat. 115, 510–530. doi: 10.1086/283577
Kinnison, M. T., and Hairston, N. G. Jr. (2007). Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct. Ecol. 21, 444–454. doi: 10.1111/j.1365-2435.2007.01278.x
Lande, R. (1982). A quantitative theory of life history evolution. Ecology 63, 607–615. doi: 10.2307/1936778
Law, W., and Salick, J. (2005). Human-induced dwarfing of Himalayan snow lotus, Saussurea laniceps (Asteraceae). Proc. Natl. Acad. Sci. U.S.A. 102, 10218–10220. doi: 10.1073/pnas.0502931102
Leston, L. F. V., and Rodewald, A. D. (2006). Are urban forests ecological traps for understory birds? An examination using Northern Cardinals. Biol. Conserv. 131, 566–574. doi: 10.1016/j.biocon.2006.03.003
MacColl, A. D. C. (2011). The ecological causes of evolution. Trends Ecol. Evol. 26, 514–522. doi: 10.1016/j.tree.2011.06.009
Marnocha, E., Pollinger, J., and Smith, T. B. (2011). Human-induced morphological shifts in an island lizard. Evol. Appl. 4, 388–396. doi: 10.1111/j.1752-4571.2010.00170.x
McNeilly, T., and Bradshaw, A. (1968). Evolutionary processes in populations of copper tolerant Agrostis tenuis, Sibth. Evolution 22, 108–118. doi: 10.1111/j.1558-5646.1968.tb03454.x
Metz, J. A., Nisbet, R. M., and Geritz, S. A. (1992). How should we define fitness for general ecological scenarios. Trends Ecol. Evol. 7, 198–202. doi: 10.1016/0169-5347(92)90073-K
Meyrier, E., Jenni, L., Bötsch, Y., Strebel, S., Erne, B., and Tablado, Z. (2017). Happy to breed in the city? Urban food resources limit reproductive output in Western Jackdaws. Ecol. Evol. 2017, 1363–1374. doi: 10.1002/ece3.2733
Milinski, M. (1984). Competitive resource sharing - an experimental test of a learning rule for ESSS. Anim. Behav. 32, 233–242. doi: 10.1016/S0003-3472(84)80342-5
Milinski, M. (1988). Games fish play - making decisions as a social forager. Trends Ecol. Evol. 3, 325–330. doi: 10.1016/0169-5347(88)90088-2
Morris, D. W. (1994). Habitat matching - alternatives and implications to populations and communities. Evol. Ecol. 8, 387–406. doi: 10.1007/BF01238190
Norman, J. A., and Christidis, L. (2016). Ecological opportunity and the evolution of habitat preferences in an arid-zone bird: implications for speciation in a climate-modified landscape. Sci. Rep. 6:19613. doi: 10.1038/srep19613
Norton, M. E., Arcese, P., and Ewald, P. W. (1982). Effect of intrusion pressure on territory size in the black-chinned hummingbird. Auk 99, 761–763.
Olsen, E., Heino, M., Lilly, G., Morgan, M., Brattey, J., Ernande, B., et al. (2004). Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature 428, 932–935. doi: 10.1038/nature02430
Pelletier, F., Clutton-Brock, T., Pemberton, J., Tuljapurkar, S., and Coulson, T. (2007). The evolutionary demography of ecological change: linking trait variation and population growth. Science 315, 1571–1574. doi: 10.1126/science.1139024
Pelletier, F., Garant, D., and Hendry, A. P. (2009). Eco-evolutionary dynamics. Philos. Trans. R. Soc. B Biol. Sci. 364, 1483–1489. doi: 10.1098/rstb.2009.0027
Phillips, B., and Shine, R. (2004). Adapting to an invasive species: Toxic cane toads induce morphological change in Australian snakes. Proc. Natl. Acad. Sci. U.S.A. 101, 17150–17155. doi: 10.1073/pnas.0406440101
Recer, G. M., Blanckenhorn, W. U., Newman, J. A., Tuttle, E. M., Withiam, M. L., and Caraco, T. (1987). Temporal resource variability and the habitat-matching rule. Evol. Ecol. 1, 363–378. doi: 10.1007/BF02071559
Reznick, D., and Ghalambor, C. (2001). The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112, 183–198. doi: 10.1023/A:1013352109042
Rodewald, A. D., and Kearns, L. J. (2011). Shifts in dominant nest predators along a rural-to-urban landscape gradient. Condor 113, 899–906. doi: 10.1525/cond.2011.100132
Rodewald, A. D., Kearns, L. J., and Shustack, D. P. (2013). Consequences of urbanizing landscapes to reproductive performance of birds in remnant forests. Biol. Conserv. 160, 32–39. doi: 10.1016/j.biocon.2012.12.034
Rodewald, A. D., and Shustack, D. P. (2008). Consumer resource matching in urbanizing landscapes: are synanthropic species over-matching? Ecology 89, 515–521. doi: 10.1890/07-0358.1
Rodewald, A. D., Shustack, D. P., and Hitchcock, L. E. (2010). Exotic shrubs as ephemeral ecological traps for nesting birds. Biol. Invasions 12, 33–39. doi: 10.1007/s10530-009-9426-3
Rodewald, A. D., Shustack, D. P., and Jones, T. M. (2011). Dynamic selective environments and evolutionary traps in human-dominated landscapes. Ecology 92, 1781–1788. doi: 10.1890/11-0022.1
Rogers, C. M. (1987). Predation risk and fasting capacity: do wintering birds maintain optimal body mass? Ecology 68, 1051–1061. doi: 10.2307/1938377
Rogers, C. M. (2015). Testing optimal body mass theory: evidence for cost of fat in wintering birds. Ecosphere 6:55. doi: 10.1890/ES14-00317.1
Rogers, C. M., and Smith, J. N. M. (1993). Life history theory in the nonbreeding period – tradeoffs in avian fat reserves. Ecology 74, 419–426. doi: 10.2307/1939303
Safran, R. J., Vitousek, M. N., Hauber, M. E., and Ghalambor, C. K. (2010). Sexual selection: a dynamic state of affairs Response to the comments of Cornwallis and Uller in the article: towards an evolutionary ecology of sexual traits. Trends Ecol. Evol. 25, 429–430. doi: 10.1016/j.tree.2010.04.004
Shochat, E., Warren, P., Faeth, S., McIntyre, N., and Hope, D. (2006). From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 21, 186–191. doi: 10.1016/j.tree.2005.11.019
Shustack, D. P., and Rodewald, A. D. (2011). Nest predation reduces benefit to early clutch initiation in an urbanizing landscape. J. Avian Biol. 42, 204–209. doi: 10.1111/j.1600-048X.2011.05231.x
Shustack, D. P., Rodewald, A. D., and Waite, T. A. (2009). Springtime in the city: exotic shrubs promote earlier green-up of urban forests. Biol. Invasions 11, 1357–1371. doi: 10.1007/s10530-008-9343-x
Siepielski, A. M., DiBattista, J. D., and Carlson, S. M. (2009). It's about time: the temporal dynamics of phenotypic selection in the wild. Ecol. Lett. 12, 1261–1276. doi: 10.1111/j.1461-0248.2009.01381.x
Siepielski, A. M., DiBattista, J. D., Evans, J. A., and Carlson, S. M. (2011). Differences in the temporal dynamics of phenotypic selection among fitness components in the wild. Proc. R. Soc. B Biol. Sci. 278, 1572–1580. doi: 10.1098/rspb.2010.1973
Siepielski, A. M., Gotanda, K. M., Morrissey, M. B., Diamond, S. E., DiBattista, J. D., and Carlson, S. M. (2013). The spatial patterns of directional phenotypic selection. Ecol. Lett. 16, 1382–1392. doi: 10.1111/ele.12174
Smith, T. M., and Shugart, H. H. (1987). Territory size variation in the ovenbird - the role of habitat structure. Ecology 68, 695–704. doi: 10.2307/1938475
Stockwell, C., Hendry, A., and Kinnison, M. (2003). Contemporary evolution meets conservation biology. Trends Ecol. Evol. 18, 94–101. doi: 10.1016/S0169-5347(02)00044-7
Stuart, Y. E., Campbell, T. S., Hohenlohe, P. A., Reynolds, R. G., Revell, L. J., and Losos, J. B. (2014). Rapid evolution of a native species following invasion by a congener. Science 346, 463–466. doi: 10.1126/science.1257008
Tarwater, C., and Arcese, P. (2017). Young individuals pay higher costs of reproduction in a short-lived bird. Behav. Ecol. 71:84. doi: 10.1007/s00265-017-2309-1
Telleria, J. L., and Perez-Tris, J. (2003). Seasonal distribution of a migratory bird: effects of local and regional resource tracking. J. Biogeogr. 30, 1583–1591. doi: 10.1046/j.1365-2699.2003.00960.x
Thompson, J. (1998). Rapid evolution as an ecological process. Trends Ecol. Evol. 13, 329–332. doi: 10.1016/S0169-5347(98)01378-0
Wade, M. J., and Kalisz, S. (1990). The causes of natural selection. Evolution 44, 1947–1955. doi: 10.1111/j.1558-5646.1990.tb04301.x
Williams, G. C. (1966). Natural selection, the costs of reproduction and a refinement of Lack's principle. Am. Nat. 100, 687–690. doi: 10.1086/282461
Keywords: birds, body condition, rural, urban, natural selection, variance in reproduction
Citation: Rodewald AD and Arcese P (2017) Reproductive Contributions of Cardinals Are Consistent with a Hypothesis of Relaxed Selection in Urban Landscapes. Front. Ecol. Evol. 5:77. doi: 10.3389/fevo.2017.00077
Received: 08 January 2017; Accepted: 29 June 2017;
Published: 18 July 2017.
Edited by:
Jordi Figuerola, Estación Biológica de Doñana (CSIC), SpainReviewed by:
Fernando Mateos-Gonzalez, Department of Collective Behaviour, MPI for Ornithology, University of Konstanz, GermanyTim Coulson, University of Oxford, United Kingdom
Copyright © 2017 Rodewald and Arcese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda D. Rodewald, arodewald@cornell.edu
 Amanda D. Rodewald
Amanda D. Rodewald Peter Arcese
Peter Arcese