Urban Bird Feeders Dominated by a Few Species and Individuals
- 1Auckland Museum, Auckland, New Zealand
- 2Centre for Biodiversity and Biosecurity, School of Biological Sciences, University of Auckland, Auckland, New Zealand
- 3Environmental Futures Research Institute, Griffith University, Nathan, QLD, Australia
- 4Institute of Fundamental Sciences, Massey University, Palmerston North, New Zealand
The practice of garden bird feeding is a global phenomenon, involving millions of people and vast quantities of food annually. Many people engage in the practice of feeding assuming that birds gain some benefit from the food they provide, yet recent studies have revealed the potential for detrimental impacts as well. However, there is still a paucity of information on the impacts of feeding, including the ubiquity of these impacts among and within feeder-visiting species. Consistency in feeder use among birds is likely an important determinant of this. Individual birds and species that make frequent use of feeders are more likely to experience both the benefits and detrimental impacts of supplementary food. We investigated patterns of feeder use by garden birds visiting experimental feeding stations in Auckland, New Zealand, with the specific aim of determining whether use of supplementary food was consistent or variable among individuals and species. We used camera traps as well as Radio Frequency Identification (RFID) technology to examine intra- and interspecific feeder visitation patterns and to discern species associations. Eleven bird species were detected using feeding stations, however, two introduced species (house sparrow Passer domesticus and spotted dove Streptopelia chinensis) dominated visitation events. These species were present at feeders most frequently, with the largest conspecific group sizes. Significant associations were detected among a number of species, suggesting interspecific interactions are important in determining feeder use. We also found within-species differences in feeder use for all focal species, with individual variation greatest in house sparrows. Furthermore, season had an important influence on most visitation parameters. The observed individual and species-specific differences in supplementary food resource use imply that the impacts of garden bird feeding are not universal. Crucially, particularly given the avifaunal context in New Zealand, resource dominance by introduced species could have potential negative outcomes for native species conservation in cities.
Introduction
Garden bird feeding is a phenomenally popular activity in many parts of the world, including in New Zealand, Australia, the UK, Europe, and the USA (Jones, 2017). Participation rates for engagement in bird feeding are consistently estimated at between one- to two-thirds of households (Jones and Reynolds, 2008; Galbraith et al., 2014; Orros and Fellowes, 2015b). In recent decades bird feeding has shifted from a predominantly winter-only activity to a pastime commonly practiced year-round (Jones and Reynolds, 2008; Horn and Johansen, 2013; Galbraith et al., 2014). Effectively a massive ecosystem-scale intervention, bird feeding has numerous potential implications for the biology and ecology of feeder-visiting birds (Jones, 2011), as well as the wider faunal community (e.g., Bonnington et al., 2014; Orros et al., 2015). Although studies of bird-feeding impacts in urban habitats are rare, there is mounting evidence to confirm that garden bird feeding can be profoundly influential for urban-dwelling bird communities (Amrhein, 2014). For example, feeding can alter body condition, reproductive outputs, adult survival, disease dynamics, community assemblages, and migration (Robb et al., 2008; Jokimäki and Kaisanlahti-Jokimäki, 2012; Galbraith et al., 2015, 2017; Orros and Fellowes, 2015a; Plummer et al., 2015; Wilcoxen et al., 2015). The vast body of scientific literature on the influence of supplementary feeding on a wide range of species and non-urban habitats corroborates many of these findings (e.g., Boutin, 1990; Clout et al., 2002; Ilarri et al., 2008; Schoech, 2009; Ruffino et al., 2014).
Whether the impacts of supplementary feeding are universal among and within feeder-visiting species remains largely unstudied. Certainly, food availability is an important factor acting to limit bird populations (Newton, 1980), affecting reproductive success and survival of many bird species in different systems (Martin, 1987). Despite the additional deliberate (i.e., bird feeding) and unintentional (e.g., refuse) food resources available to birds in urban systems, demand for food can be high due to high bird densities reducing the per capita amount of food available (Seress and Liker, 2015). This demand is illustrated by an experimental study in Arizona, USA, that found supplementary food in urban areas was depleted much faster than equivalent amounts provided in natural habitats (Shochat et al., 2004). Thus, competition for food resources in urban systems, including supplementary food, may be high. The competitive ability of animals, in discovering and dominating a resource, plays an important role in the structuring of numerous faunal assemblages (e.g., in ants; Parr and Gibb, 2012; Bertelsmeier et al., 2015). Intraspecific and interspecific asymmetries in competitive abilities commonly give rise to dominance hierarchies (Holway, 1999), dictating resource access and consequently determining which individuals or species gain benefits from the resource (French and Smith, 2005). Dominant competitors may displace others via interference (physical exclusion from a resource via aggression) or exploitative (rapid discovery and removal of a resource) competition (Bertelsmeier et al., 2015). Agonistic interactions are certainly common at bird feeders, with body size found to be a critical factor in determining outcomes (Tamm, 1985; Wojczulanis-Jakubas et al., 2015). Speed of novel food discovery (Tryjanowski et al., 2015) and bird densities at feeding locales (Galbraith et al., 2015) can also vary by species. Furthermore, in natural habitats, different foraging strategies are frequently used by different individuals within a species to optimize foraging efficiency (Gustafsson, 1988). Consequently, it is unlikely that the ability to exploit supplementary food is consistent among all feeder-visiting birds, both within and between species.
Intraspecific and interspecific differences in use of supplementary food could have important implications for population- and community-level impacts of urban bird feeding, potentially determining the mechanisms by which changes to survival, reproduction, migratory patterns, and community organization occur (Newton, 1980; Robb et al., 2008). Few studies, though, have looked at individual variation in supplementary food use or how species associations at feeders affect resource access in an urban context specifically (but see Cowie and Hinsley, 1988; Crates et al., 2016; Jack, 2016). Bird populations in urban habitats are subject to different pressures than those in more natural environments, frequently resulting in differences in ecology, behavior, and life history (Chace and Walsh, 2006; Chamberlain et al., 2009; Seress and Liker, 2015; Garcia et al., 2017; Lepczyk et al., 2017). Furthermore, urban areas, particularly in New Zealand, are hotspots for introduced (i.e., nonnative/exotic/alien) bird species (Day, 1995; Duncan et al., 2003; van Heezik et al., 2008; Spurr, 2012; Davis et al., 2014), with bird feeding implicated in the success of some of these species (Strubbe and Matthysen, 2007; Peck et al., 2014; Orros and Fellowes, 2015b). Thus, it is immensely important to study supplementary food use in situ, in urban areas where most bird feeding occurs, to gain a realistic understanding of the demand for these resources, the urban-specific competitive interactions which may be occurring, and the wider implications for urban birds.
Here we explore avian visitation patterns at experimental bird-feeding stations established in the gardens of volunteer households in Auckland, New Zealand. Via this experiment we have also investigated the impacts of bird feeding on avian disease dynamics (Galbraith et al., 2017) and avian community structure (Galbraith et al., 2015). We know from the latter that our feeding regime significantly altered bird communities at feeding locales, prompting a shift toward communities heavily dominated by introduced birds, primarily house sparrows (Passer domesticus) and spotted doves (Streptopelia chinensis). This study investigates whether there were intraspecific and interspecific asymmetries in feeder use that could be indicative of resource dominance, giving insight into the community-level observations of Galbraith et al. (2015). We also consider whether supplementary food use is modified by interspecific interactions or seasonality. The demand for supplementary food resources, and the associated competitive interactions at feeders, are likely to vary across seasons (e.g., Ottoni et al., 2009; Cox et al., 2016) due to fluctuations in natural food availability and physiological (thermoregulatory as well as reproductive) demands on birds. We used camera traps at feeding stations to identify species-level patterns of feeder use and examine species associations, and Radio Frequency Identification (hereafter “RFID”) technology to explore the feeder-visitation patterns of individuals. Specifically, the objectives of our study were to: (1) examine whether feeder use varies among and within species to determine whether birds exploit supplementary food equally; (2) determine whether individuals and species are consistent in their use of supplementary food over time or if use varies seasonally; and (3) explore the associations between species at feeders that may modify access to supplementary food.
Methods
Experimental Feeding Stations
Experimental feeding stations were established at 11 urban residential properties in northern Auckland, New Zealand, as part of a wider study of typical bird feeding practices (Galbraith et al., 2015, 2017). The study area is largely suburban residential, with a population density of 1,600/km2 in 2006 (New Zealand Census data, www.stats.govt.nz). Properties representative of the study area were selected from a pool of 42 volunteered properties. Feeding stations (a low feeding table (40 × 80 cm, 17-cm high), a seed feeder, and a mesh bread-tube; Supplementary Figure 1A) were active for 18 months from March 2012 to September 2013, and householders were responsible for provisioning them on a daily basis for the duration of the study. The feeding regime consisted of 4–5 slices of bread and 1 metric cup of birdseed (white millet, Hungarian millet, hulled oats, and canary seed blend) per day; householders were asked to put the food out between 0700 and 0800 h NZST. This regime and the design of the feeding stations reflected typical feeding practices of the New Zealand public (Galbraith et al., 2014). The majority (75%) of feeding participants in New Zealand throw food directly onto the ground, rather than using structures or containers (Galbraith et al., 2014); however, for the present study it was necessary to use a fixed structure and food containers to standardise experimental feeding among properties and to enable data collection. Properties were a minimum of 900 m apart (Supplementary Figure 2), with no detections of banded birds at properties other than their place of capture. For full details of the experimental setup see Galbraith et al. (2015).
Species Visitation and Association Data
The visitation patterns of feeder-visiting species and species associations were examined using camera traps. Camera trap data were collected at all feeding stations (n = 11) over four sampling periods: Austral winter 2012 (June–July; non-breeding season), spring 2012 (October–November; early breeding season), summer 2013 (February; late breeding season), and winter 2013 (June–July). In each sampling period, three cameras (ScoutGuard SG570V, HCO Outdoors, USA; 1.5 m from the feeding station, at a height of 0.5 m) were rotated around the feeding stations over a 4-week timeframe, and operated for 4–6 nights at each feeding station depending on weather. Three full days of recordings were scored for analysis. The cameras were programmed to record upon motion-activation, with a 10-min delay period following a recording event to increase independence of observations. Three consecutive photographs, stamped with the date and time, were taken at 2-s intervals upon motion activation. These photo sets were considered as one observation event.
We used co-occurrence at feeding stations to identify associations among species. For scoring, we counted the maximum number of individuals of a species observed simultaneously in an event. For analysis we included only those recordings with at least one “feeder visitor,” henceforth referred to as a “visitation event.” Birds on the feeding station structure itself (i.e., on the feeding table or feeders) were considered feeder visitors; birds on the ground or elsewhere nearby were disregarded. We also noted the level of food remaining at the time of the visitation event: bread, seed, or both food types remaining (visible in the containers) or none (no food visible, negligible quantities available).
Individual Visitation Data
To investigate individual patterns of feeder use we used RFID and Passive Integrated Transponder (hereafter “PIT”) tag technology. At three study properties birds were captured via mistnetting as part of our wider study of feeding impacts (for full details see Galbraith et al., 2017). We PIT-tagged a subset of captured individuals, and fitted RFID antennae to feeding stations (Supplementary Figures 1A, 2). The RFID reader setup (Microchips Australia Pty Ltd) consisted of a 30 × 25 cm (inner dimensions) coil antenna attached via an RS232 serial cable to a LID-650N decoder (Trovan Ltd, UK) mounted on nearby posts that also supported a 20 W solar panel (Supplementary Figure 1B). RFID readers were powered with a 26 A h, 12 V sealed lead acid battery (HAZE Solar Gel, USA) in weatherproof housing. Acrylic walls supported antennae and encouraged birds to pass through the antenna to reach the food containers (Supplementary Figure 1A). Antennae were intentionally designed to allow multiple birds access to the feeding stations simultaneously, reflecting a typical bird-feeding situation. Readers recorded the individual code (ID) of all PIT-tagged feeder-visitors and the time and date of the visit, with a 1-s read-delay between consecutive reads of the same ID, and were active between 0600 and 2100 h NZST from 22 September 2012 until the end of the study (13 September 2013). Readers did not distinguish between arrival/departure movements.
We PIT-tagged five species (Supplementary Table 1) under the conditions of our animal ethics permit (University of Auckland Animal Ethics Committee Permit R921). Birds were tagged over three capture rounds conducted at 6-monthly intervals, with the monitoring duration (number of days from PIT-tag implantation to the end of the experiment) among returning individuals varying between 134 and 377 days (mean = 291.5 d ± 10.7 SE). Sample sizes depended largely on capture rates of those species. PIT-tagging took place on only those days where two experienced researchers were present in the field. Only adult birds, without apparent injuries or clinical signs of illness, were PIT-tagged. We used Trovan Unique ID100 implantable PIT tags (2.12 × 11.5 mm; 0.1 g; Trovan Ltd, UK) with a unique 5-byte code. These were injected subcutaneously in the back of birds above the scapula following the methods of Nicolaus et al. (2008). The tags are pre-sterilized by the manufacturer, and come ready to use with a disposable needle. During each procedure, one person cleaned the insertion site and injected the PIT tag, while a second person held the bird and gently pulled up the skin at the top of the back to facilitate injection. The perforation of the skin at the insertion site was closed by applying a small quantity of surgical adhesive (Vetbond, 3M, St. Paul, MN, USA). This method has been used on small passerines without obvious negative effects on the birds (Nicolaus et al., 2008; Nomano et al., 2014). Five PIT-tagged individuals were recaptured during subsequent days or capture rounds and we found all tags to be in place with no visible problems for these individuals (Supplementary Figure 1C).
For analysis of PIT-tag data we omitted readings from the same individual that occurred within 2 s of each other, to obtain a more conservative estimate of feeder visitation rates. Two key parameters were calculated for analyses: the total number of reads per day for each individual, and the presence/absence of each individual at the feeder for each day that individual was monitored.
Statistical Analyses
We used basic descriptive and multivariate statistics to examine feeder visitation parameters. These visitation parameters may be considered a proxy for food resource consumption, though our data do not allow for the exact relationship between consumption and visitation to be explored. Note for logistical reasons data collection periods for camera trap and PIT-tag data differed, hence in the analyses treatment of season differed for each. Specifically, because collection of camera trap data encompassed two winters, seasonal period (season ID) was used for analysis of this dataset to enable variation between winters to be examined (in the absence of sufficient data to include year as a fixed effect). Means are shown with their standard errors (SEs) (x ± SEM), and the critical α level was 0.05 for all tests.
Species Visitation and Co-occurrence (Camera Trap Data)
To explore species associations at feeders (camera trap data) we initially used a probabilistic model approach (Veech, 2013) to test for overall patterns of co-occurrence between species pairs (across all sites and seasons). This approach, implemented in the cooccur package (Griffith et al., 2015) in R 3.4.0 (R Core Team, 2017), uses presence/absence data to calculate an expected frequency of co-occurrence between species pairs if they were distributed independently of one another across sites, or observations in this case. The model then calculates the probability that the observed co-occurrence frequency is greater than the expected frequency (a positive co-occurrence association), less than the expected frequency (negative association), or random. Here the model tests the probability of co-occurrence at the level of the visitation event. For this analysis we used feeder-visiting species whose occupancy across study properties was >50% (Supplementary Table 2; determined via bird surveys conducted over the study duration; Galbraith et al., 2015).
We then examined the composition of and variation in bird assemblages visiting feeding stations simultaneously using the PERMANOVA+ add-on to PRIMER (Anderson et al., 2008). We calculated Bray-Curtis distances on fourth-root-transformed data as there were large differences in the baseline abundance of species. We then performed a non-metric multidimensional scaling (NMDS; Kruskal, 1964) on the distance-matrix which is displayed as a reduced space plot where each point represents the bird species composition at each feeding site and time point. To visualise species associations we superimposed vector lines, the length of which represents how much weight a species carried in determining the position of the points in the plot. The degree to which two lines are aligned with each other show the extent to which two species are correlated, with lines pointing in opposite directions meaning negative correlation. To explore the impact of seasonal period and food availability on species composition at feeding stations, a permutational analysis of variance (PERMANOVA) was used. Initially we fitted a PERMANOVA model with four factors: season ID (winter 2012, spring 2012, summer 2013, and winter 2013), food remaining (bread + seed remaining, bread remaining, seed remaining, no food remaining), property identity (ID), and observation day (1, 2, or 3). Observation day was found to be non-significant with minimal variance and was removed from the final model. Property ID was treated as a random factor while season ID and food availability were treated as fixed factors, and all factors were crossed.
To investigate predictors of feeder use at the species level we used a mixed model approach, focusing on the six most frequent feeder-visiting species in the camera trap data (Supplementary Table 3). Generalized Linear Mixed Models (GLMMs) implemented in R were used, accounting for the repeated measures structure of the data. Two feeder-use parameters, abundance at feeders and number of daily visitation events (daily visitation rate), were modelled as the response variables for each species. Abundance models tested whether the number of conspecifics (individuals of the same species) during a visitation event was influenced by the abundance of other visiting species and/or varied among seasonal periods. Visitation rate models tested whether the number of daily visitation events for a focal species varied among seasonal periods. In abundance models, we included season ID, food remaining, the abundances of each of the other five focal species in the visitation event, minimum daily temperature (°C), and rainfall (mm) as fixed effects. In visitation rate models, we included season ID, duration food available (min day−1), minimum daily temperature, and rainfall as fixed effects. Temperature and rainfall data were obtained from the NIWA National Climate Database (for the Albany, North Shore, Auckland weather station; https://cliflo.niwa.co.nz/, accessed 15 November 2016). While avian foraging patterns typically vary with time of day, food was not consistently available throughout the day in our study due to depletion (reflecting the typical feeding practices of the public). As such, feeder visitation across the day was likely to reflect food levels remaining rather than daily patterns of foraging activity. We accounted for this in abundance models by including the “food remaining” parameter, with minutes after sunrise also included as a fixed effect to account for expected variation in foraging activity over the course of the day. Similarly, for visitation rate models we calculated the duration that food was available each day, and included it as a fixed effect to account for its likely influence on daily visitation rates. We included property ID in all models as a random effect, to account for variation between sites. In initial models we also included day length (this was correlated with season ID and so was removed), as well as observation day in the random effects structure (the effect was negligible so data were pooled across observation days for final models).
Prior to model fitting we assessed the distribution of each response variable using the fitdistrplus package (Delignette-Muller and Dutang, 2015) in R; the best-fitting distributions were used in the corresponding models. We estimated the parameters using negative binomial GLMMs, fitted using a Laplace approximation of maximum likelihood in the glmmADMB package (Skaug et al., 2015), in all cases [with the exception of the Eurasian blackbird (Turdus merula) abundance model where a Poisson error structure was the better fit]. For initial analyses, both ordinary and zero-inflated models were fitted. We used Pearson residual plots and Akaike's Information Criterion (AIC) to compare model fits, retaining the best fitting model for interpretation of effects. Additionally, we checked for overdispersion by dividing the sum of squared Pearson residuals by the residual degrees of freedom and comparing this to a χ2 distribution (Venables and Ripley, 2002). Zero-inflation improved model fit for the house sparrow abundance model only. In initial model fitting, the blackbird, common starling (Sturnus vulgaris), and silvereye (Zosterops lateralis) abundance models all had large SEs for the “seed” level of the “food remaining” parameter, as did the silvereye abundance and visitation rate models for the “summer 2013” level of the “season ID” parameter. This was due to separation in the data due to cells with zero frequencies within the contingency table of response variable × food available (and × season for silvereye). To yield sensible parameter SEs, we added a dummy row to the dataset that added a small non-negative constant (a count of 1) to the cells with zero counts (Agresti, 2002; Jones et al., 2012) with other cells containing mean values. We then refitted these models and checked parameter SEs. For the final models we assessed the significance of whole model terms using likelihood ratio tests (LRTs) implemented with the “drop1” function in R. Post hoc pairwise comparisons among levels of season ID were conducted using the multcomp package (Hothorn et al., 2008).
Individual Visitation (PIT-Tag Data)
We used GLMMs implemented in R to explore feeder visitation patterns of individuals (PIT-tag data). Two feeder-use parameters, daily presence at feeders (binomial; present = 1, absent = 0) and daily visitation rate (count data; total reads per day active at feeder), were modelled as the response variables. Daily presence data were modelled with a binomial error structure (logit link) using the lme4 package (Bates et al., 2014). Daily visitation rate data were modelled using a negative binomial error structure using the glmmADMB package, after assessing the distribution using the fitdistrplus package. For both response variables we fitted initial models that included species as a fixed effect and individual ID as a random effect, to confirm differences among species in feeder use. This also allowed us to estimate the relative contribution of among-individual variability (i.e., individual heterogeneity) and among-species variability to the overall variation in feeder visitation data. We calculated the marginal R2 (proportion of variance explained by the fixed effects) and the conditional R2 (proportion of variance explained by fixed and random effects combined) using the “r.squaredGLMM” function in the R package MuMIn (Bartoń, 2015), which implements the methods of Nakagawa and Schielzeth (2013). - gives the random effect component of the variance, . is analogous to repeatability (Nakagawa and Schielzeth, 2010; Crates et al., 2016). Note, estimating the variance components of fixed vs. random effects for negative binomial models required refitting models using the “glmmPQL” function of the MASS package (which uses penalized quasilikelihood for parameter estimation; Venables and Ripley, 2002), as glmmADMB objects cannot be passed to “r.squaredGLMM.” Parameter estimates were comparable between “glmmPQL” and “glmmadmb” fitted models (see Supplementary Tables 4, 5); coefficients of the latter are presented in the results for consistency.
We then fitted separate GLMMs for each species for each response variable to examine the effects of season on feeder-use parameters. For both daily presence and daily visitation rate models, we included season (winter, spring, summer, and autumn), minimum daily temperature, rainfall, and day (number of days since feeding started) as fixed effects. Individual ID was included in the models as a random effect to account for repeated measures from the same individuals and to determine the contribution of individual heterogeneity to model variance. For all species except house sparrow, there were too few individuals at each property to adequately estimate among-property variation, thus data were pooled across properties. For house sparrow data, larger sample sizes at each property enabled initial models to be fitted with property ID (Feeder 1, 2, and 3) included as a fixed effect. As property ID did not contribute significantly to model fit for either response variable (see Supplementary Table 6), we pooled data across properties for final analyses as per the other species. Significance of whole model terms was assessed using LRTs.
Results
Camera Trap Visitation Events
A total of 3066 visitation events were captured by the camera traps over 132 trap days. We recorded 725 visitation events in winter 2012, 1006 in spring 2012, 581 in summer 2013, and 754 in winter 2013. There were 723 visitation events made when bread and seed were both available, representing the time period immediately following food provision. There were fewest observations when only seed remained (n = 31); typically this food type was depleted fastest, leaving only bread remaining (n = 839 observations). The other visitation events occurred when there was no food remaining (n = 1473), although a small amount may have still been available on the table.
Structure of Bird Assemblage at Feeding Stations
Eleven bird species were recorded visiting the feeding stations (Supplementary Table 3). House sparrows and spotted doves were the most frequently observed species over all visitation events, present in 64.9 and 58.0% of recordings, respectively. Silvereyes were the only native species, present in only 4.6% of visitation events. Most visitation events had only a single species present (57.3%), with two species present in 33.5% of visitation events. Only 9.2% of visitation events had three or more species present. Mean total abundance per visitation event was 4.9 ± 1.53 birds, with a mean species richness of 1.5 ± 0.01 species. A maximum of five species were observed together on the feeding station in one visitation event. Mean conspecific group size (number of same-species individuals feeding simultaneously) was highest in house sparrow with 5.2 ± 0.12 individuals per visitation event, and a maximum of 45 sparrows recorded in one visitation event. In contrast, Eurasian blackbirds, song thrushes (Turdus philomelos), and chaffinches (Fringilla coelebs) were typically observed without conspecifics present (Supplementary Table 3).
Species Visitation Patterns
House sparrows and spotted doves had the highest daily visitation rates (number of visitation events recorded by camera traps) to feeding stations (mean no. visitation events day−1 = 15.08 ± 0.71 and 13.46 ± 0.63, respectively), well above that for any other species (Supplementary Table 3, Figure 1A). Abundances of all six focal species (the most frequent feeder-visitors) at feeding stations were significantly influenced by food levels remaining (GLMM analyses; Table 1). In particular, abundances tended to be lowest when no food remained in comparison to when bread and seed remained (Wald-Z < −3.01, p < 0.003 in all cases). Furthermore, the speed at which food was depleted varied significantly among seasons (GLMM LRT: χ2 = 28.2, d.f. = 3, p < 0.001); food was available for longer in winter 2012 (mean min available day−1 = 247 min ± 27) and spring 2012 (290 min ± 30) compared to summer 2013 (134 min ± 17), when food was depleted fastest, and winter 2013 (170 min ± 18). This duration of food availability significantly affected the number of daily visitation events by Eurasian blackbird and common myna, and marginally improved model fit for spotted dove and silvereye daily visitation rate models (Table 2). Nevertheless, after accounting for this variation in food availability, abundances at feeding stations and daily visitation rates for all species varied significantly among seasonal periods (except the spotted dove abundance model; Tables 1, 2 and Figures 1A,B). This seasonality was most striking for silvereyes, with no feeder visitations recorded for the summer period at all (Figure 1). House sparrow abundance at feeders was higher in winter 2013 compared to winter 2012, whereas both daily visitation rate and abundance were lower in the second winter for Eurasian blackbirds (Figure 1, Supplementary Table 7). The remaining species showed no significant difference between winters for either visitation parameter (Figure 1, Supplementary Table 7).
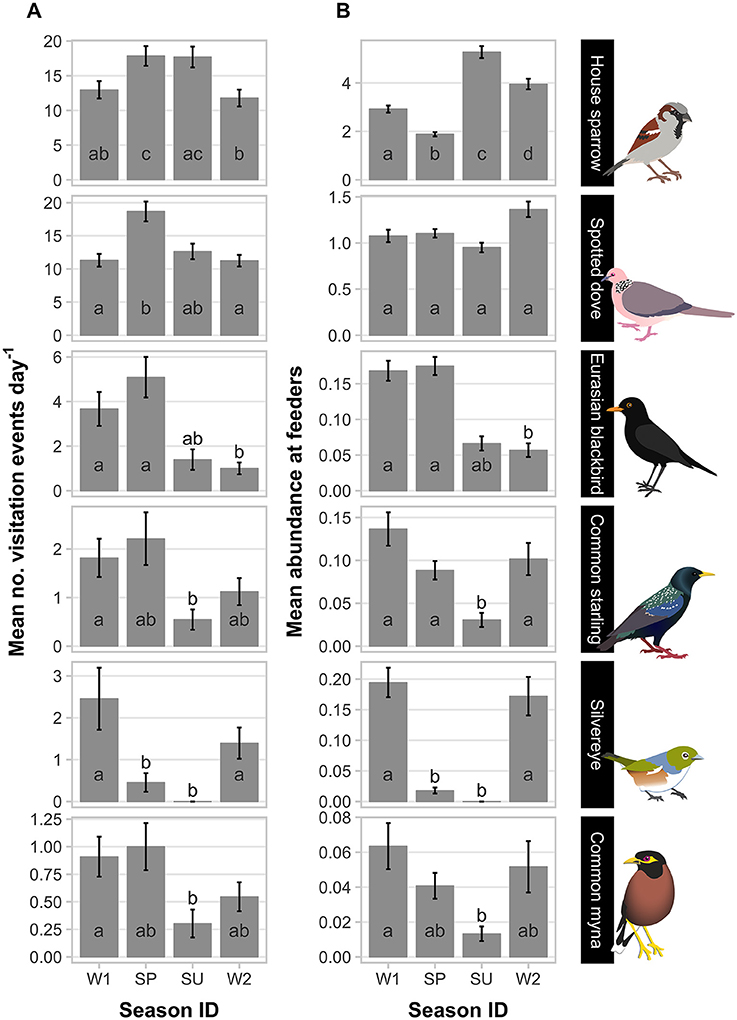
Figure 1. Patterns of feeder visitation of the top six avian visitors captured in camera traps at experimental feeding stations at urban study properties in northern Auckland, New Zealand. Mean count data (± SEM) are presented for two visitation parameters scored from camera trap data: (A) daily visitation rate (number of daily visitation events recorded); and (B) abundance (number of individuals in a visitation event). In each panel, means not sharing the same letter are significantly different in pairwise comparisons (Tukey's HSD; p < 0.05; Supplementary Table 7). Sampling season ID: W1, winter 2012; SP, spring 2012; SU, summer 2013; W2, winter 2013. Note y-axis scale varies with species.
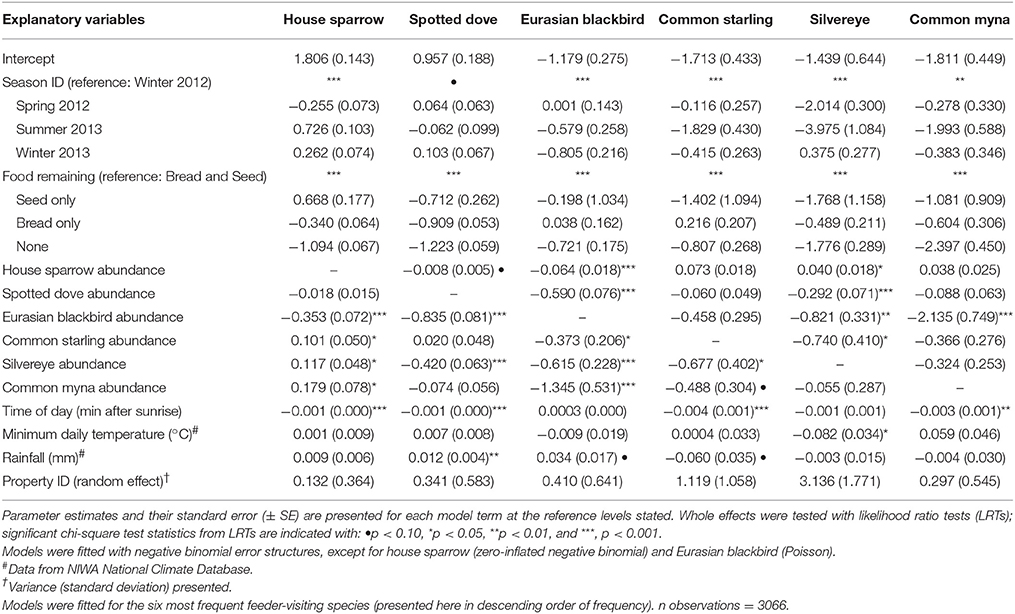
Table 1. Results of Generalized Linear Mixed Models (GLMMs) examining factors affecting the abundance of garden birds captured in camera traps at experimental feeding stations in northern Auckland, New Zealand.
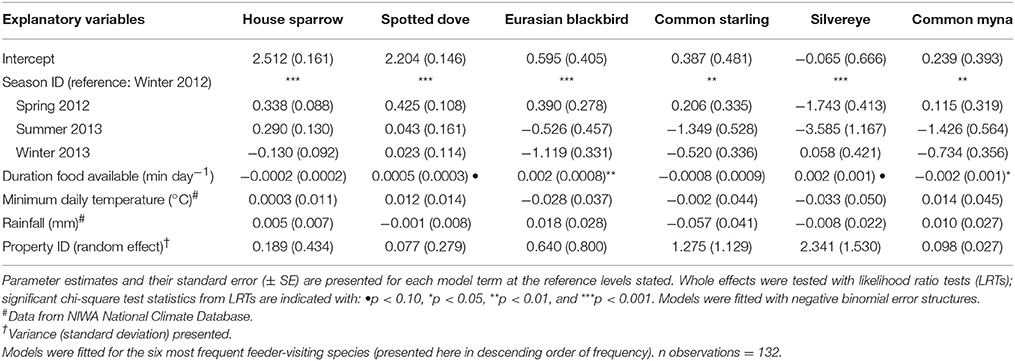
Table 2. Results of Generalized Linear Mixed Models (GLMMs) testing whether the number of visitation events per day captured by camera traps varied among seasons.
Species Associations
A number of significant associations in overall species co-occurrence patterns at feeding stations were detected with the probabilistic modelling (Figure 2). Of the 36 species pairs, three were positive (8.3%) with the two species co-occurring at feeders significantly more frequently than expected, 10 were negative (27.8%) with species co-occurring significantly less frequently than expected, and 23 were random (63.9%). House sparrow had the highest number of significant associations with other species (n = 6), and the highest proportion of positive associations (37.5% of pairings). Eurasian blackbirds had five significant associations with other species, while spotted doves had four, with all of these being negative.
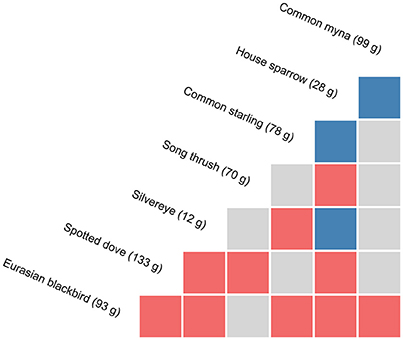
Figure 2. Pairwise associations between species at experimental feeding stations at urban study properties in northern Auckland, New Zealand, determined through probabilistic modelling of species co-occurrences (Veech, 2013). Species that co-occur more frequently than by chance (α threshold = 0.05) are considered to have a positive association (in blue), those that co-occur less frequently than by chance are considered to have a negative association (in red), with random associations shown in grey. Note chaffinch and greenfinch pairings are not shown; no significant associations were found for these species. Species masses are means from birds captured in this study.
Analysis at the community level also indicated there were patterns in the assemblages of birds feeding concurrently at the feeding stations. Species composition was dominated by three species, house sparrows, spotted doves, and Eurasian blackbirds, with strong negative correlations between the abundances of these species (Figure 3); blackbirds were present at feeding stations when there were less spotted doves and house sparrows. Species composition at feeding stations varied significantly with seasonal period and food availability, and was influenced by property ID (PERMANOVA: F = 2.38, d.f. = 54, P = 0.001).
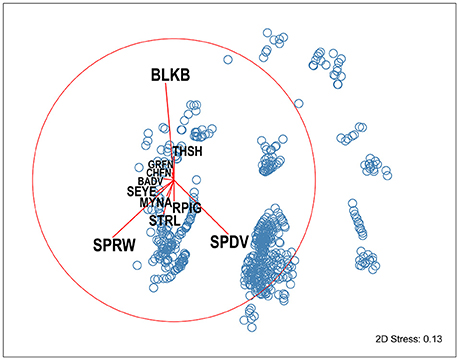
Figure 3. NMDS ordination of avian community composition at experimental feeding stations at urban study properties in northern Auckland, New Zealand. Each point (blue circles) represents the avian assemblage recorded in a single visitation event. The overlaid species vectors (red lines) illustrate that three bird species, Eurasian blackbirds (BLKB), house sparrows (SPRW), and spotted doves (SPDV) dominate community composition (as they have the longest vectors), and that there are strong negative correlations between the abundances of these species. BADV, Barbary dove; CHFN, chaffinch; GRFN, greenfinch; MYNA, common myna; RPIG, rock pigeon; SEYE, silvereye; STRL, common starling; THSH, song thrush.
GLMM analyses provided further evidence of species associations, with the abundance of all focal species during visitation events influenced by the abundance of at least one other top feeder-visitor (Table 1). Significant effects were generally negative, with the exception of the house sparrow and silvereye models. With all other factors held constant, house sparrow abundance increased with common starling, silvereye, and common myna abundances, and silvereye abundance increased with house sparrow abundance. Effect sizes for season ID and food remaining were typically larger than for co-occurring species predictors though.
Individual Visitation Patterns
We PIT-tagged a total of 110 individuals from five feeder-visiting species (Supplementary Table 1). Numbers tagged were equivalent at each feeding station (Feeder 1: n = 37; Feeder 2: n = 37; Feeder 3: n = 36). The redetection rates varied among species (Supplementary Table 1), with 70 individuals (63.6%) overall redetected by RFID readers at feeding stations on at least one occasion after initial capture. In total 83265 reads were recorded (with duplicate reads removed) for these 70 individuals.
There were obvious differences among individuals in the consistency of feeder visitation over time, with some individuals returning almost daily while others only visited sporadically (Supplementary Figure 3). Daily presence at feeding stations differed significantly among species (i.e., the proportion of days birds returned to the feeder out of the total days monitored; GLMM LRT: χ2 = 18.9, d.f. = 4, P < 0.001; Figure 4; Supplementary Table 4). The variation among individuals explained a greater proportion of the variation in daily presence data than among-species variation (48.4 vs. 14.8%, respectively). Considering each species separately (except for common starling for which there were too few individuals for valid comparisons), GLMM analyses indicated that the daily likelihood of individuals visiting feeders varied seasonally for all species except house sparrow (Table 3). House sparrows showed the greatest variability among individuals, with individual heterogeneity accounting for 63.2% of the variance in the model [Eurasian blackbirds 52.1%, spotted doves 38.4%, Barbary doves (Streptopelia roseogrisea) 14.3%; Table 3].
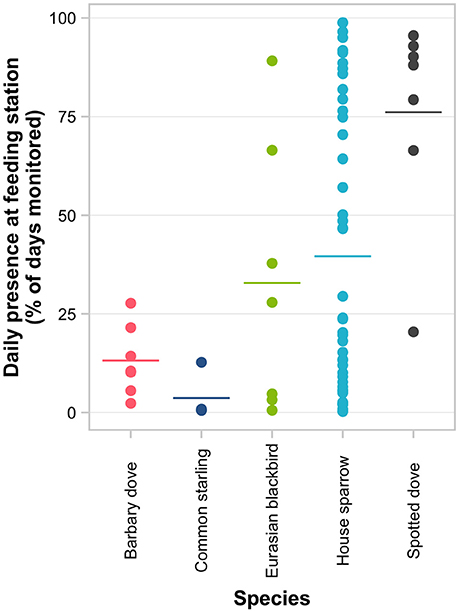
Figure 4. Consistency of feeder visitation of 70 individually PIT-tagged birds at experimental feeding stations in northern Auckland, New Zealand. Points represent the percentage of days an individual was active at a feeding station out of all days monitored for that individual. Horizontal lines represent the group means for each species.
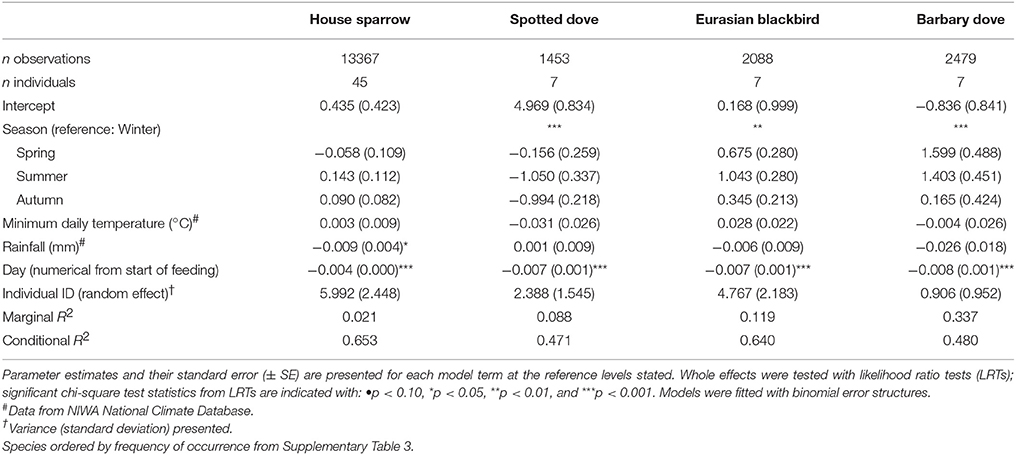
Table 3. Results of Generalized Linear Mixed Models (GLMMs) testing the effect of season on daily visitation (presence/absence) of individually PIT-tagged birds to experimental feeding stations.
Mean daily visitation rates varied significantly between species (GLMM LRT: χ2 = 17.6, d.f. = 4, P < 0.01; Supplementary Table 4). However, heterogeneity among individuals explained a greater proportion of the variance in daily visitation rate than among-species variation (19.6 vs. 4.9%, respectively). Three house sparrow individuals had the highest visitation rates per active day, with means of 32.7 (± 14.5) to 42.8 (± 8.2) reads per day (Figure 5). Analysed separately (again except for common starling), daily visitation rates varied significantly with season for all species (Table 4). Individual heterogeneity in daily visitation rate was highest for house sparrows and blackbirds, accounting for 31.1 and 28.4% of explained variance in the models, respectively (Table 4).
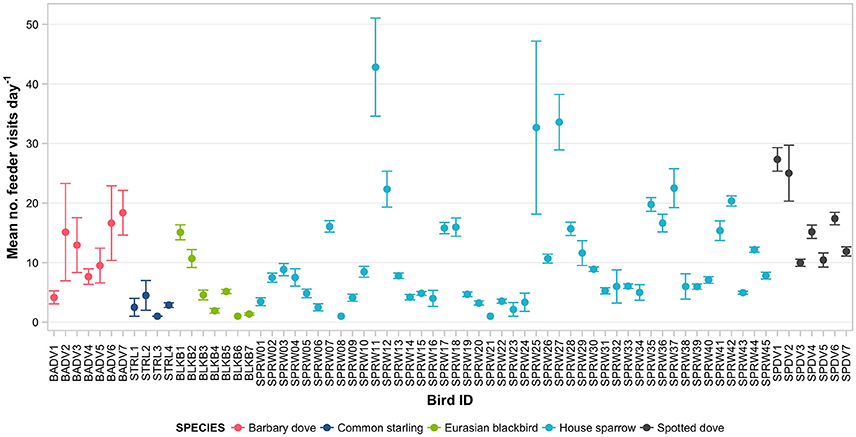
Figure 5. Daily rates of feeder visitation (number of visitation events per day actively using the feeder) of 70 individually PIT-tagged birds at experimental feeding stations in northern Auckland, New Zealand. Data are raw means ± SEM.
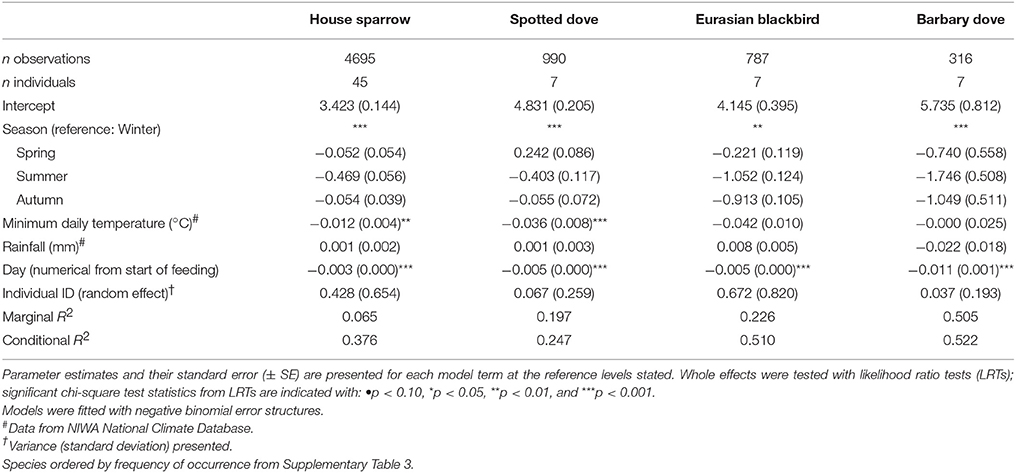
Table 4. Results of Generalized Linear Mixed Models (GLMMs) testing the effect of season on daily visitation rate (no. visits per active day) of individually PIT-tagged birds at experimental feeding stations.
Discussion
Summary of Findings
Our results demonstrate considerable among- and within-species variation in the use of supplementary food resources by birds in situ in urban habitats, confirming that feeder-visiting birds do not exploit supplementary food resources equally. The species using feeding stations in our study were predominantly introduced; this was predicted because of the grain-based feeding regime used by householders (in our study and more generally; Galbraith et al., 2014), and the division in dietary preferences of native vs. introduced birds in our study area (Galbraith et al., 2015). Two species in particular dominated resource use: house sparrow and spotted doves. Our study corroborates the findings of two other recent experimental studies, one focusing on great tits (Parus major) and blue tits (Cyanistes caeruleus) in woodland habitat in Oxford, UK, during winter (Crates et al., 2016) and the other examining visitation patterns of six species at a university campus in Cornwall, UK (Jack, 2016). These studies both reported individual and interspecific variation in use of supplementary food at experimental feeders over a comparatively shorter timeframe (c. 3 months). The findings from our longer-term study also demonstrate that season has an important influence on a number of visitation parameters both at the individual and species levels. Relatedly, seasonal differences in movement of birds among networks of urban feeders have been found in great tits and blue tits (Cox et al., 2016). We acknowledge, though, that in our study there are limitations to interpreting the results from those PIT-tagged species with small sample sizes. Nevertheless, significant seasonal variation in feeder use was evident across most species and visitation parameters we assessed.
Visitation Patterns
The interspecific differences in feeder use observed in our study reinforce the concern that typical feeding practices in New Zealand do not benefit native species, but instead support introduced species (Galbraith et al., 2015). Feeding grain-based food types (e.g., bread, seed) is the prevalent practice in New Zealand (Galbraith et al., 2014), yet most native garden birds here are frugivores, nectarivores, or insectivores (Heather and Robertson, 1996). Thus, unsurprisingly, native species were almost entirely absent from our experimental feeding stations, with the exception of the silvereye which primarily visited in winter. Of the 11 recorded feeder-visiting species, two largely granivorous introduced species—house sparrow and spotted dove—dominated the majority of visitation events. They were also the two most abundant species at feeders, both in terms of mean and maximum conspecific group size using the feeder simultaneously. These results reflect the findings of bird counts conducted at study properties over the duration of the experiment, in which house sparrow and spotted dove showed dramatic increases in abundance at feeding locales in response to the feeding treatment, contributing to a shift in community composition (Galbraith et al., 2015). This is concerning for urban ecosystems in New Zealand, where introduced birds are already the dominant component of avifaunal assemblages, and increasingly relevant globally, as supplementary feeding is implicated as a contributing factor in the spread of other invasive bird species, such as monk parakeets (Myiopsitta monachus) in the northern USA (Davis et al., 2014) and ring-necked parakeets (Psittacula krameri) in the UK and mainland Europe (Strubbe and Matthysen, 2007; Peck et al., 2014; Orros and Fellowes, 2015b). This concern is not limited to introduced birds, however, as native species may also become numerically dominant as a result of food subsidies and displace other birds.
The prevalence of house sparrows and spotted doves in visitation events and their distinct positive numerical responses to the feeding treatment (Galbraith et al., 2015) suggests both species were successfully competing for access to the food resource. However, there were differences between house sparrows and spotted doves in visitation patterns which may be indicative of distinct, but equally successful, competitive behaviours. Spotted dove feeder visitors appear to consist of a core-group of individuals that are highly consistent in their feeder use (i.e., show high fidelity to the resource once they discover it), whereas house sparrow feeder visitors are highly numerous but also highly variable in their feeder use.
Our results provide multiple lines of evidence (both camera trap and PIT-tag data), that seasonality has an important effect on feeder visitation patterns. Unsurprisingly, the level of food remaining had a significant impact on feeder visitation parameters–when there was more food left, bird abundance at feeders was higher. Nevertheless, after accounting for the effect of food availability, species abundance and species daily visitation rate varied significantly with seasonal period for most focal species. We also found that the daily presence and visitation rates of individuals varied significantly among seasons for all focal species (though we could not account for food availability here). There were, however, two exceptions: first, the number of spotted doves feeding at the same time was consistent among seasonal periods. This may well reflect the maximum capacity of the feeding station structure itself—ultimately the number of birds, particularly large-bodied species, such as spotted doves, feeding simultaneously will be restricted by physical space at feeders. Second, no seasonal effect on the likelihood of individuals being present each day was evident for house sparrow—individual heterogeneity was more influential compared to season for this species.
The physiological demands on birds vary seasonally with changes in environmental conditions, natural food availability, and behavioural activity, thereby influencing the demand for supplementary food. Here our proxy for demand was the speed of food depletion, which indicated that demand for supplementary food resources did fluctuate seasonally in our study. Demand was highest in summer rather than winter, which is contrary to the common public perception that birds should be fed in the winter when their struggle for survival is greatest (Jones, 2017). However, in our study region winter conditions are fairly mild, so the physiological demand is likely less compared to many regions. Instead, the energetic costs of breeding activities (e.g., increased energy required for reproduction, and/or reduced foraging time due to incubation and chick provisioning activities; Martin, 1987) may drive the increased demand for readily available supplementary food in summer. Additionally, it is crucial to note that our study period encompassed a pronounced, New-Zealand-wide drought event over the summer of 2012–2013 (Porteous and Mullan, 2013). This event would probably have had significant impacts on natural food availability, and may well have contributed to food demand. The differences observed between winters may also reflect follow-on impacts of the drought event, if, for instance, natural food resource availability had not recovered. However, visitation patterns of the only native feeder visitor provide a notable contrast to the overall seasonal pattern of demand. Silvereyes were scarce at feeders in spring and entirely absent in summer, suggesting that natural food resources exploited by this species were more abundant (or more profitable) during these seasons and that supplementary food was unnecessary to meet energy requirements.
Species Associations
We found evidence that access to supplementary food was influenced by species associations at feeding stations. Feeder activity typically peaked in the morning following food provisioning. Although multiple species were typically present in the vicinity of feeders, single-species visitation events were more common than those with multiple species at the feeder simultaneously. It is likely, then, that some species excluded others from access to feeders and some avoided using feeders when heterospecifics were present. Multi-species visitation events, however, still comprised a substantial proportion of our observations (42.7% of visitation events).
A number of significant interspecific associations were identified from the probabilistic modelling of the likelihood of co-occurrence between species pairs, which generally agreed with findings from GLMM analyses of predictors of species abundance at feeders. Body size frequently determines, or contributes to, the development of dominance hierarchies, outcomes of agonistic interactions, and subsequent resource access (Forrester, 1991; Wojczulanis-Jakubas et al., 2015; Miller et al., 2017). However, there were no obvious patterns in the species co-occurrence matrix to indicate that negative associations were more common between species closest in body size, or that positive associations were more common between species of greater size disparity. Thus, potentially, behavioural differences among species (i.e., in foraging strategies, territoriality, and sociality), rather than morphological differences alone, are driving co-occurrence patterns in this system.
The known ecology of the focal species provides some insight here. For instance, house sparrow are a highly gregarious species, typically foraging, roosting, and nesting in conspecific flocks, and frequently foraging with other species (Higgins et al., 2006a). Consistent with this apparent tolerance of foraging with heterospecifics, house sparrow had the highest number of positive associations with other feeder-visitors. In contrast, Eurasian blackbirds are highly territorial, typically forage singly or in pairs, with agonistic interspecific interactions occurring where they have been observed foraging with other species (Higgins et al., 2006a). This was reflected in our observed mean conspecific group size for blackbirds at feeders (1.04 birds) and the predominantly negative associations we found between blackbirds and other feeder-visiting species. Thus, blackbirds were apparently intolerant of using feeders in the presence of other species or could not gain access to them. They may have avoided using the feeder when other birds were present, actively excluded other species, been excluded by other species, or a combination of these. Detailed behavioural analysis of camera trap videos would help elucidate the direction of these interspecific interactions.
Underlying Foraging Mechanisms
Although we did not explicitly investigate which competitive mechanisms controlled feeder access, it seems probable that both interference and exploitative competition were at play. Agonistic interactions at feeding stations were regularly observed in this study (between species but more frequently within species; JAG pers. obs.) and are frequent among feeder-visiting birds in other systems (e.g., Wojczulanis-Jakubas et al., 2015), indicating that interference competition certainly influences feeder access to some degree. However, in our study system exploitative competition is also likely to be a critical mechanism. For example, house sparrows and spotted doves typically arrived at feeding stations in the greatest numbers and consumed food quickly, which are important indicators of exploitative ability (Holway, 1999; Bertelsmeier et al., 2015). Additionally, house sparrows forage in localised areas, typically moving through slowly (Higgins et al., 2006b); spending longer in a particular patch adds to efficiency in resource exploitation (Holway, 1999; Shochat et al., 2004). The exploitative ability of the spotted dove may also be enhanced by the reduced food handling time and food intake rates associated with consuming seeds whole (Shochat et al., 2004).
Individual Variability
Individual variation or “specialisation” is widespread in many taxa, yet conspecific individuals are treated as ecologically equivalent in the majority of studies concerning resource use (Bolnick et al., 2003; Araújo et al., 2011). Here we found convincing evidence that, within species, individuals varied considerably in their feeder use. Individual heterogeneity was highest in the house sparrow, in terms of both feeder visitation rates per day and likelihood of daily presence at feeders, with these individual differences highly repeatable. This high level of individual variation may result from a number of behavioural mechanisms. For instance, it may be indicative of an intraspecific dominance hierarchy dictated by the competitive ability of individuals (Richner, 1989; Rat et al., 2015). Alternatively, individual variation may reflect behavioural flexibility, a trait which has been linked to successful urban exploiter and invasive species (Sol et al., 2002; Peck et al., 2014), especially in regard to flexible foraging strategies. Distinctive individual foraging strategies have been found, for example, within white storks (Ciconia ciconia) in southwestern Spain which show both consistent (i.e., specialist) and flexible (i.e., generalist) use of landfills vs. rice fields (Sanz-Aguilar et al., 2015). These differences in foraging strategy within a species may arise from phenotypic, physiological, or competitive discrepancies among individuals (Araújo et al., 2011; Sanz-Aguilar et al., 2015). Individual-level variation in feeder use may also be indicative of differences in personality (Aplin et al., 2014). Among Eurasian blackbird individuals the high variation in feeder use may reflect territorial behaviour. Expectedly, territory owners would use feeders more frequently and with greater consistency, whereas territory intruders would face a greater challenge accessing feeders due to territorial defence behaviour of the territory owner, and so be less frequent and/or less consistent feeder visitors. Studies in territorial calliope hummingbirds (Selasphorus calliope; Tamm, 1985) and great tits (Ydenberg, 1984) have found that individual birds that have been provided with supplementary food show increased display and/or defence behaviours, thus making it more difficult for others to access the resource.
Individual heterogeneity in feeder use has important implications at both the individual-and population-level. Among other impacts, individuals that heavily rely on supplementary food may have altered body condition, reproductive success, home range size, and/or survival likelihood (Annett and Pierotti, 1999; Oro et al., 2013). The collective impacts of supplementary feeding on individuals determines the overall effect on population dynamics, including changes in population size, distribution, and migratory patterns (Robb et al., 2008; Oro et al., 2013; Amrhein, 2014), and will depend on both the proportion of the population using feeders and individual heterogeneity in feeder use, particularly where feeder users are a nonrandom subset of the population (Sanz-Aguilar et al., 2015). Furthermore, individuals which follow a high-use, consistent foraging strategy at feeders are more likely to pose a disease risk than sporadic users, as exposure to pathogens and parasites increases as feeder visitation increases (Adelman et al., 2015).
Conclusions
Our study highlights that individual and species-specific differences in feeder use are present within feeder-visiting bird communities, importantly demonstrating this across seasons within an urban system. These intraspecific and interspecific asymmetries support the likelihood of competitive interactions operating to regulate access to food, and suggest that the effects of supplementary feeding are unlikely to be equivalent across all birds within communities of feeder visitors. In New Zealand resource dominance by introduced species is particularly important, with negative outcomes for native species conservation in cities possible. Individual differences in feeder use observed here are likely to affect the population-level impacts of bird feeding, and consequently should be considered in future studies of garden bird feeding.
Author Contributions
JG was the lead author and investigator on the study, leading the experimental design, data collection, and data analysis. DJ, JB, and MS helped develop the experimental design, assisted with logistics and data collection, and reviewed the manuscript. KP assisted with statistical analyses and reviewed the manuscript. All authors reviewed and approved the final version of the manuscript.
Funding
This study was funded by the University of Auckland and the Auckland Council.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Our thanks to the volunteer householders involved in the study and our many field volunteers, including the Galbraith Family, Jo Peace, Ellery McNaughton, Cheryl Krull, Megan Young, and Auckland Zoo staff. Particular thanks to Oliver Hannaford for statistical support, and Doug Black from Microchips Australia Pty Ltd for his technical support setting up RFID readers. EcoStock donated bread for the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fevo.2017.00081/full#supplementary-material
References
Adelman, J. S., Moyers, S. C., Farine, D. R., and Hawley, D. M. (2015). Feeder use predicts both acquisition and transmission of a contagious pathogen in a North American songbird. Proc. R. Soc. B 282:20151429. doi: 10.1098/rspb.2015.1429
Amrhein, V. (2014). “Wild bird feeding (probably) affects avian urban ecology,” in Avian Urban Ecology: Behavioural and Physiological Adaptations, eds D. Gil and H. Brumm (Oxford: Oxford University Press), 29–37.
Anderson, M., Gorley, R., and Clarke, K. (2008). PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. Plymouth: PRIMER-E Ltd.
Annett, C. A., and Pierotti, R. (1999). Long-term reproductive output in western gulls: consequences of alternate tactics in diet choice. Ecology 80, 288–297. doi: 10.1890/0012-9658(1999)080[0288:LTROIW]2.0.CO;2
Aplin, L. M., Farine, D. R., Mann, R. P., and Sheldon, B. C. (2014). Individual-level personality influences social foraging and collective behaviour in wild birds. Proc. R. Soc. B Biol. Sci. 281:20141016. doi: 10.1098/rspb.2014.1016
Araújo, M. S., Bolnick, D. I., and Layman, C. A. (2011). The ecological causes of individual specialisation. Ecol. Lett. 14, 948–958. doi: 10.1111/j.1461-0248.2011.01662.x
Bartoń, K. (2015). MuMIn: Multi-Model Inference. R package version 1.13.4. Available online at: http://CRAN.R-project.org/package=MuMIn
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2014). lme4: Linear Mixed-Effects Models Using Eigen and S4. R package version 1.1-5 Available online at: http://cran.r-project.org/package=lme4
Bertelsmeier, C., Avril, A., Blight, O., Confais, A., Diez, L., Jourdan, H., et al. (2015). Different behavioural strategies among seven highly invasive ant species. Biol. Invasions 17, 2491–2503. doi: 10.1007/s10530-015-0892-5
Bolnick, D. I., Svanbäck, R., Fordyce, J. A., Yang, L. H., Davis, J. M., Hulsey, C. D., et al. (2003). The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28. doi: 10.1086/343878
Bonnington, C., Gaston, K. J., and Evans, K. L. (2014). Relative roles of grey squirrels, supplementary feeding, and habitat in shaping urban bird assemblages. PLoS ONE 9:e109397. doi: 10.1371/journal.pone.0109397
Boutin, S. (1990). Food supplementation experiments with terrestrial vertebrates: patterns, problems, and the future. Can. J. Zool. 68, 203–220. doi: 10.1139/z90-031
Chace, J. F., and Walsh, J. J. (2006). Urban effects on native avifauna: a review. Landsc. Urban Plan. 74, 46–69. doi: 10.1016/j.landurbplan.2004.08.007
Chamberlain, D. E., Cannon, A. R., Toms, M. P., Leech, D. I., Hatchwell, B. J., and Gaston, K. J. (2009). Avian productivity in urban landscapes: a review and meta-analysis. Ibis 151, 1–18. doi: 10.1111/j.1474-919X.2008.00899.x
Clout, M. N., Elliott, G. P., and Robertson, B. C. (2002). Effects of supplementary feeding on the offspring sex ratio of kakapo: a dilemma for the conservation of a polygynous parrot. Biol. Conserv. 107, 13–18. doi: 10.1016/S0006-3207(01)00267-1
Cowie, R. J., and Hinsley, S. A. (1988). The provision of food and the use of bird feeders in suburban gardens. Bird Study 35, 163–168. doi: 10.1080/00063658809476985
Cox, D. T. C., Inger, R., Hancock, S., Anderson, K., and Gaston, K. J. (2016). Movement of feeder-using songbirds: the influence of urban features. Sci. Rep. 6:37669. doi: 10.1038/srep37669
Crates, R. A., Firth, J. A., Farine, D. R., Garroway, C. J., Kidd, L. R., Aplin, L. M., et al. (2016). Individual variation in winter supplementary food consumption and its consequences for reproduction in wild birds. J. Avian Biol. 47, 678–689. doi: 10.1111/jav.00936
Davis, A. Y., Malas, N., and Minor, E. S. (2014). Substitutable habitats? The biophysical and anthropogenic drivers of an exotic bird's distribution. Biol. Invasions 16, 415–427. doi: 10.1007/s10530-013-0530-z
Day, T. D. (1995). Bird species composition and abundance in relation to native plants in urban gardens, Hamilton, New Zealand. Notornis 42, 172–186.
Delignette-Muller, M. L., and Dutang, C. (2015). fitdistrplus: an R package for fitting distributions. J. Stat. Softw. 64, 1–34. doi: 10.18637/jss.v064.i04
Duncan, R. P., Blackburn, T. M., and Sol, D. (2003). The ecology of bird introductions. Annu. Rev. Ecol. Evol. Syst. 34, 71–98. doi: 10.1146/annurev.ecolsys.34.011802.132353
Forrester, G. E. (1991). Social rank, individual size and group composition as determinants of food consumption by humbug damselfish, Dascyllus aruanus. Anim. Behav. 42, 701–711. doi: 10.1016/S0003-3472(05)80116-2
French, A. R., and Smith, T. B. (2005). Importance of body size in determining dominance hierarchies among diverse tropical frugivores. Biotropica 37, 96–101. doi: 10.1111/j.1744-7429.2005.04051.x
Galbraith, J. A., Beggs, J. R., Jones, D. N., McNaughton, E. J., Krull, C. R., and Stanley, M. C. (2014). Risks and drivers of wild bird feeding in urban areas of New Zealand. Biol. Conserv. 180, 64–74. doi: 10.1016/j.biocon.2014.09.038
Galbraith, J. A., Beggs, J. R., Jones, D. N., and Stanley, M. C. (2015). Supplementary feeding restructures urban bird communities. Proc. Natl. Acad. Sci. U.S.A. 112, E2648–E2657. doi: 10.1073/pnas.1501489112
Galbraith, J. A., Stanley, M. C., Jones, D. N., and Beggs, J. R. (2017). Experimental feeding regime influences urban bird disease dynamics. J. Avian Biol. 48, 700–713. doi: 10.1111/jav.01076
Garcia, C. M., Suárez-Rodríguez, M., and López-Rull, I. (2017). “Becoming citizens: avian adaptations to urban life,” in Ecology and Conservation of Birds in Urban Environments, eds E. Murgui and M. Hedblom (Cham Springer International Publishing), 91–112.
Griffith, D. M., Veech, J. A., and Marsh, C. J. (2015). Cooccur: probabilistic species co-occurrence analysis in R. J. Stat. Softw. 69, 1–17. doi: 10.18637/jss.v069.c02
Gustafsson, L. (1988). Foraging behaviour of individual coal tits, Parus ater, in relation to their age, sex and morphology. Anim. Behav. 36, 696–704. doi: 10.1016/S0003-3472(88)80152-0
Heather, B., and Robertson, H. (1996). The Field Guide to the Birds of New Zealand. Auckland: Viking.
Higgins, P. J., Peter, J. M., and Cowling, S. J. (eds.). (2006a). “Handbook of Australian, New Zealand and Antarctic Birds,” in Boatbill to Starlings: Part 7A, Boatbill to Larks, Vol. 7 (Melbourne, VIC: Oxford University Press).
Higgins, P. J., Peter, J. M., and Cowling, S. J. (eds.). (2006b). “Handbook of Australian, New Zealand and Antarctic Birds,” in Boatbill to Starlings: Part 7B, Dunnock to Starlings, Vol. 7 (Melbourne, VIC: Oxford University Press).
Holway, D. A. (1999). Competitive mechanisms underlying the displacement of native ants by the invasive Argentine ant. Ecology 80, 238–251. doi: 10.1890/0012-9658(1999)080[0238:CMUTDO2.0.CO;2]
Horn, D. J., and Johansen, S. M. (2013). A comparison of bird-feeding practices in the United States and Canada. Wildl. Soc. Bull. 37, 293–300. doi: 10.1002/wsb.281
Hothorn, T., Bretz, F., and Westfall, P. (2008). Simultaneous inference in general parametric models. Biom. J. 50, 346–363. doi: 10.1002/bimj.200810425
Ilarri, M. D. I., Souza, A. T. d., Medeiros, P. R. d., Grempel, R. G., and Rosa, I. M. d. L. (2008). Effects of tourist visitation and supplementary feeding on fish assemblage composition on a tropical reef in the Southwestern Atlantic. Neotrop. Ichthyol. 6, 651–656. doi: 10.1590/S1679-62252008000400014
Jack, S. L. (2016). The Use of Supplementary Food Sources by Bird Communities and Individuals. MSc, University of Exeter.
Jokimäki, J., and Kaisanlahti-Jokimäki, M.-L. (2012). Residential areas support overwintering possibilities of most bird species. Ann. Zool. Fenn. 49, 240–256. doi: 10.5735/086.049.0404
Jones, C. M., Sanou, A., Guelbeogo, W. M., Sagnon, N., Johnson, P., and Ranson, H. (2012). Aging partially restores the efficacy of malaria vector control in insecticide-resistant populations of Anopheles gambiae s.l. from Burkina Faso. Malar. J. 11, 10.1186. doi: 10.1186/1475-2875-11-24
Jones, D. N. (2011). An appetite for connection: why we need to understand the effect and value of feeding wild birds. Emu 111, i–vii. doi: 10.1071/muv111n2_ed
Jones, D. N., and Reynolds, S. J. (2008). Feeding birds in our towns and cities: a global research opportunity. J. Avian Biol. 39, 265–271. doi: 10.1111/j.0908-8857.2008.04271.x
Kruskal, J. B. (1964). Nonmetric multidimensional scaling: a numerical method. Psychometrika 29, 115–129. doi: 10.1007/BF02289694
Lepczyk, C. A., La Sorte, F. A., Aronson, M. F. J., Goddard, M. A., MacGregor-Fors, I., Nilon, C. H., et al. (2017). “Global patterns and drivers of urban bird diversity,” in Ecology and Conservation of Birds in Urban Environments, eds E. Murgui and M. Hedblom (Cham: Springer International Publishing), 13–33.
Martin, T. E. (1987). Food as a limit on breeding birds: a life-history perspective. Annu. Rev. Ecol. Syst. 18, 453–487. doi: 10.1146/annurev.es.18.110187.002321
Miller, E. T., Bonter, D. N., Eldermire, C., Freeman, B. G., Greig, E. I., Harmon, L. J., et al. (2017). Fighting over food unites the birds of North America in a continental dominance hierarchy. bioRxiv. doi: 10.1101/104133
Nakagawa, S., and Schielzeth, H. (2010). Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. doi: 10.1111/j.1469-185x.2010.00141.x
Nakagawa, S., and Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. doi: 10.1111/j.2041-210x.2012.00261.x
Nicolaus, M., Bouwman, K. M., and Dingemanse, N. J. (2008). Effect of PIT tags on the survival and recruitment of great tits Parus major. Ardea 96, 286–292. doi: 10.5253/078.096.0215
Nomano, F. Y., Browning, L. E., Nakagawa, S., Griffith, S. C., and Russell, A. F. (2014). Validation of an automated data collection method for quantifying social networks in collective behaviours. Behav. Ecol. Sociobiol. 68, 1379–1391. doi: 10.1007/s00265-014-1757-0
Oro, D., Genovart, M., Tavecchia, G., Fowler, M. S., and Martínez-Abraín, A. (2013). Ecological and evolutionary implications of food subsidies from humans. Ecol. Lett. 16, 1501–1514. doi: 10.1111/ele.12187
Orros, M. E., and Fellowes, M. D. E. (2015a). Widespread supplementary feeding in domestic gardens explains the return of reintroduced Red Kites Milvus milvus to an urban area. Ibis 157, 230–238. doi: 10.1111/ibi.12237
Orros, M. E., and Fellowes, M. D. E. (2015b). Wild bird feeding in an urban area: intensity, economics and numbers of individuals supported. Acta Ornithol. 50, 43–58. doi: 10.3161/00016454AO2015.50.1.006
Orros, M. E., Thomas, R. L., Holloway, G. J., and Fellowes, M. D. E. (2015). Supplementary feeding of wild birds indirectly affects ground beetle populations in suburban gardens. Urban Ecosyst. 18, 465–475. doi: 10.1007/s11252-014-0404-x
Ottoni, I., de Oliveira, F. F. R., and Young, R. J. (2009). Estimating the diet of urban birds: the problems of anthropogenic food and food digestibility. Appl. Anim. Behav. Sci. 117, 42–46. doi: 10.1016/j.applanim.2008.11.002
Parr, C. L., and Gibb, H. (2012). The discovery–dominance trade-off is the exception, rather than the rule. J. Anim. Ecol. 81, 233–241. doi: 10.1111/j.1365-2656.2011.01899.x
Peck, H. L., Pringle, H. E., Marshall, H. H., Owens, I. P., and Lord, A. M. (2014). Experimental evidence of impacts of an invasive parakeet on foraging behavior of native birds. Behav. Ecol. 25, 582–590. doi: 10.1093/beheco/aru025
Plummer, K. E., Siriwardena, G. M., Conway, G. J., Risely, K., and Toms, M. P. (2015). Is supplementary feeding in gardens a driver of evolutionary change in a migratory bird species? Glob. Change Biol. 21, 4353–4363. doi: 10.1111/gcb.13070
Porteous, A., and Mullan, B. (2013). The 2012–13 Drought: An Assessment and Historical Perspective. MPI Technical Paper No: 2012/18. New Zealand: Ministry for Primary Industries.
Rat, M., van Dijk, R. E., Covas, R., and Doutrelant, C. (2015). Dominance hierarchies and associated signalling in a cooperative passerine. Behav. Ecol. Sociobiol. 69, 437–448. doi: 10.1007/s00265-014-1856-y
R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Richner, H. (1989). Phenotypic correlates of dominance in carrion crows and their effects on access to food. Anim. Behav. 38, 606–612. doi: 10.1016/S0003-3472(89)80005-3
Robb, G. N., McDonald, R. A., Chamberlain, D. E., and Bearhop, S. (2008). Food for thought: supplementary feeding as a driver of ecological change in avian populations. Front. Ecol. Environ. 6, 476–484. doi: 10.1890/060152
Ruffino, L., Salo, P., Koivisto, E., Banks, P. B., and Korpimäki, E. (2014). Reproductive responses of birds to experimental food supplementation: a meta-analysis. Front. Zool. 11:80. doi: 10.1186/s12983-014-0080-y
Sanz-Aguilar, A., Jovani, R., Melián, C. J., Pradel, R., and Tella, J. L. (2015). Multi-event capture–recapture analysis reveals individual foraging specialization in a generalist species. Ecology 96, 1650–1660. doi: 10.1890/14-0437.1
Schoech, S. J. (2009). Food supplementation experiments: a tool to reveal mechanisms that mediate timing of reproduction. Integr. Comp. Biol. 49, 480–492. doi: 10.1093/icb/icp005
Seress, G., and Liker, A. (2015). Habitat urbanization and its effects on birds. Acta Zool. Academ. Sci. Hung. 61, 373–408. doi: 10.17109/AZH.61.4.373.2015
Shochat, E., Lerman, S. B., Katti, M., and Lewis, D. B. (2004). Linking optimal foraging behavior to bird community structure in an urban-desert landscape: field experiments with artificial food patches. Am. Nat. 164, 232–243. doi: 10.1086/422222
Skaug, H., Fournier, D., Bolker, B., Magnusson, A., and Nielsen, A. (2015). glmmADMB: Generalized Linear Mixed Models using AD Model Builder. R package version 0.8.1. Available online at: http://glmmadmb.r-forge.r-project.org/
Sol, D., Timmermans, S., and Lefebvre, L. (2002). Behavioural flexibility and invasion success in birds. Anim. Behav. 63, 495–502. doi: 10.1006/anbe.2001.1953
Spurr, E. B. (2012). New Zealand garden bird survey–analysis of the first four years. N. Z. J. Ecol. 36, 1A–5A.
Strubbe, D., and Matthysen, E. (2007). Invasive ring-necked parakeets Psittacula krameri in Belgium: habitat selection and impact on native birds. Ecography 30, 578–588. doi: 10.1111/j.0906-7590.2007.05096.x
Tamm, S. (1985). Breeding territory quality and agonistic behavior: effects of energy availability and intruder pressure in hummingbirds. Behav. Ecol. Sociobiol. 16, 203–207. doi: 10.1007/BF00310982
Tryjanowski, P., Morelli, F., Skórka, P., Goławski, A., Indykiewicz, P., Møller, A. P., et al. (2015). Who started first? Bird species visiting novel birdfeeders. Sci. Rep. 5:11858. doi: 10.1038/srep11858
van Heezik, Y., Smyth, A., and Mathieu, R. (2008). Diversity of native and exotic birds across an urban gradient in a New Zealand city. Landsc. Urban Plan. 87, 223–232. doi: 10.1016/j.landurbplan.2008.06.004
Veech, J. A. (2013). A probabilistic model for analysing species co-occurrence. Glob. Ecol. Biogeogr. 22, 252–260. doi: 10.1111/j.1466-8238.2012.00789.x
Venables, W. N., and Ripley, B. D. (2002). Modern Applied Statistics with S. 4th Edn. New York, NY,: Springer.
Wilcoxen, T. E., Horn, D. J., Hogan, B. M., Hubble, C. N., Huber, S. J., Flamm, J., et al. (2015). Effects of bird-feeding activities on the health of wild birds. Conserv. Physiol. 3:cov058. doi: 10.1093/conphys/cov058
Wojczulanis-Jakubas, K., Kulpińska, M., and Minias, P. (2015). Who bullies whom at a garden feeder? Interspecific agonistic interactions of small passerines during a cold winter. J. Ethol. 33, 159–163. doi: 10.1007/s10164-015-0424-x
Keywords: competition, individual variation, interspecific interactions, resource use, supplementary feeding, urban wildlife
Citation: Galbraith JA, Jones DN, Beggs JR, Parry K and Stanley MC (2017) Urban Bird Feeders Dominated by a Few Species and Individuals. Front. Ecol. Evol. 5:81. doi: 10.3389/fevo.2017.00081
Received: 01 March 2017; Accepted: 11 July 2017;
Published: 02 August 2017.
Edited by:
Diego Gil, Consejo Superior de Investigaciones Científicas (CSIC), SpainReviewed by:
Daniel Thomas Carr Cox, University of Exeter, United KingdomKarl L. Evans, University of Sheffield, United Kingdom
Copyright © 2017 Galbraith, Jones, Beggs, Parry and Stanley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Margaret C. Stanley, mc.stanley@auckland.ac.nz
 Josie A. Galbraith
Josie A. Galbraith Darryl N. Jones
Darryl N. Jones Jacqueline R. Beggs2
Jacqueline R. Beggs2  Katharina Parry
Katharina Parry Margaret C. Stanley
Margaret C. Stanley