Phylogenetic and Functional Diversity of Fleshy-Fruited Plants Are Positively Associated with Seedling Diversity in a Tropical Montane Forest
- 1Senckenberg Biodiversity and Climate Research Centre (BiK-F), Frankfurt am Main, Germany
- 2Department of Biological Sciences, Goethe Universität, Frankfurt am Main, Germany
- 3Department of Evolutionary Biology and Animal Ecology, Faculty of Biology, University of Freiburg, Freiburg, Germany
Mutualistic interactions between plants and animals can affect both plant and animal communities, and potentially leave imprints on plant demography. Yet, no study has simultaneously tested how trait variation in plant resources shapes the diversity of animal consumers, and how these interactions influence seedling recruitment. Here, we analyzed whether (i) phylogenetic diversity and functional diversity of fruiting plants were correlated with the corresponding diversity of frugivorous birds, and (ii) whether phylogenetic diversity and functional identity of plant and bird communities influenced the corresponding diversity and identity of seedling communities. We recorded mutualistic interactions between fleshy-fruited plants and frugivorous birds and seedling communities in 10 plots along an elevational gradient in the Colombian Andes. We built a phylogeny for plants/seedlings and birds and measured relevant morphological plant and bird traits that influence plant-bird interactions and seedling recruitment. We found that phylogenetic diversity and functional diversity of frugivorous birds were positively associated with the corresponding diversities of fruiting plants, consistent with a bottom-up effect of plants on birds. Moreover, the phylogenetic diversity of seedlings was related to the phylogenetic diversity of plants, but was unrelated to the phylogenetic diversity of frugivorous birds, suggesting that top-down effects of animals on seedlings were weak. Mean seed mass of seedling communities was positively associated with the mean fruit mass of plants, but was not associated with the mean avian body mass in the frugivore communities. Our study shows that variation in the traits of fleshy-fruited plants was associated with the diversity of frugivorous birds and affected the future trajectory of seedling recruitment, whereas the morphological traits of animal seed dispersers were unrelated to the phylogenetic and functional structure of seedling communities. These findings suggest that bottom-up effects are more important than top-down effects for seed-dispersal interactions and seedling recruitment in diverse tropical communities. Data available from the BiK-F Data & Metadata Repository: https://doi.org/10.12761/SGN.2017.10191.
Introduction
Plant-animal interactions structure ecological communities in terrestrial ecosystems (Price, 2002). For instance, mutualistic interactions between plants and animals promote the establishment of plant individuals or populations, contributing to the stability of ecological communities (Lortie et al., 2004; Bascompte and Jordano, 2007). Plants are an essential component of local food webs by offering food resources to a wide range of animal consumers (Power, 1992). In turn, the foraging behavior of mutualistic animals influences plant demography at the community level (Yang et al., 2008). Plant-animal interactions are mediated by morphological traits, i.e., physically measurable properties of individuals, that modify the intensity of interactions across trophic levels (Bello et al., 2010; Maglianesi et al., 2014). Therefore, trait variation among the plant species involved in the interactions affects ecological processes and ecosystem functioning (Díaz and Cabido, 2001). So far, only few studies have investigated the influence of trait variation among plant resources on the phylogenetic and functional diversity of animal consumers involved in mutualistic interactions (Chamberlain et al., 2014; Dehling et al., 2014a).
Phylogenetic diversity (PD) and functional diversity (FD) are powerful quantitative measures of biodiversity that may help to elucidate how biodiversity is related to ecosystem functioning (Petchey and Gaston, 2006; Cadotte et al., 2011). PD accounts for the evolutionary history of co-occurring species and can be used as a proxy for quantifying the diversity of species' ecological roles in a community. It assumes that distantly related species fulfill more distinct functional roles than closely related species and, thus, the metric increases if co-occurring species have distant, rather than recent common ancestors (Cadotte et al., 2008). Functional diversity (FD) comprises the range of functional traits present in the co-occurring organisms and measures the distinctiveness of a community in terms of functional traits (Flynn et al., 2011). For instance, it represents the diversity of Eltonian species' niches or roles in a community (Villéger et al., 2008) determined by species morphology (Dehling et al., 2016). An additional component of functional diversity is functional identity, which reflects the functional composition of a particular trait in a community and influences trophic interactions among species and ecosystem processes (Mokany et al., 2008; Gagic et al., 2015). PD and FD provide more information than only species richness or abundance (Díaz and Cabido, 2001; Cadotte et al., 2011) as they reflect ecological variation among species that is potentially associated with species' contributions to ecosystem functioning (Hooper et al., 2005). While measures of FD account for the functional traits that are relevant for a specific ecological function, e.g., a set of morphological traits for frugivory (Dehling et al., 2016), PD additionally accounts for unmeasured traits, such as behavioral, life history or physiological traits, that are often associated with the phylogenetic history of species (Cavender-Bares et al., 2009). Thus, studying both PD and FD is needed for a deeper understanding of how mutualistic plant-animal interactions structure ecological communities and their associated ecosystem functions.
Seed dispersal by frugivores is a key mutualistic interaction that provides an important ecosystem function for plant communities (Farwig and Berens, 2012). Fleshy-fruited plants depend on frugivorous animals to disperse their seeds. Indeed, fruit removal and the ingestion and digestion of fruits by frugivores enable seed movement away from the parental tree and usually facilitate seedling recruitment (Herrera, 2002; Muñoz et al., 2017b). Seed dispersal by animals is constrained by trait matching between the phenotypic features of plant and animal species in mutualistic interactions (Schleuning et al., 2015), e.g. between fruit or seed size and avian gape width (Wheelwright, 1985; Dehling et al., 2014b) or between plant crop size and avian body mass (Dehling et al., 2014b). Trait matching between plants and animals structures mutualistic interaction networks, but can also be detected in corresponding patterns of plant and animal diversity across spatial gradients (Kissling et al., 2007; Dehling et al., 2014b). The relationship between plant and animal FD is expected to be particularly close if the degree of trait matching in plant-animal interactions is high (Dehling et al., 2014b). However, it is yet less clear how plant and animal diversity are related to the associated ecosystem function of seedling recruitment.
An important trait that affects seedling recruitment is seed size (Muñoz et al., 2017a). Seed size influences seedling survival and growth, and is related to a plant's life history strategy (Westoby et al., 1996; Rees et al., 2001). For instance, large-seeded plant species can grow and survive in shaded conditions, whereas small-seeded species usually depend on a high light availability for establishment (Rees et al., 2001). Given the trait matching between plants and animals in mutualistic networks, it is expected that a diverse animal community will be able to disperse seeds with a wide range of plant traits, such as seed size, whereas the range of dispersed seeds should be reduced in communities of low animal diversity (Vanthomme et al., 2010). Top-down effects of animals on seedlings are expected to be most pronounced if different animal species provide complementary contributions to seed dispersal (Jordano et al., 2007; Schleuning et al., 2015). In contrast, a high degree of animal redundancy, and a low degree of trait matching, would dilute the signal of frugivore traits on seedling diversity, e.g., because many large frugivores are able to feed on different fruit sizes (Bender et al., 2017; Muñoz et al., 2017a). It has been shown that mutualistic plant-animal interactions are influenced by bottom-up effects of plants on animal diversity (e.g., Albrecht et al., 2014), whereas top-down effects on these interactions are usually weaker (Schleuning et al., 2016). If bottom-up effects are more pronounced than top-down effects, one might expect an association between the diversity of plant and seedling communities even in a scenario where species traits have little effect on mutualistic interactions. This is because the abundances of plants and seedlings should be related if there is little trait filtering during the seed-dispersal process (Díaz et al., 2007). To our knowledge, there has been so far no attempt to relate the PD and FD of plant and animal communities to the corresponding diversity of seedling communities. Such studies can shed light on the relevance of top-down vs. bottom-up effects in these communities and are lacking for natural communities from highly diverse ecosystems, such as those present in the tropical Andes.
Here, we use PD and FD of plants and animals to quantify the variability in functional roles in a diverse seed-dispersal system. We ask how PD and FD of fleshy-fruited plants are associated with the respective measures of the diversity of frugivorous birds. Moreover, we test how PD of plants and birds are linked to the respective diversity of seedling communities. First, we hypothesize that a high PD and FD of fleshy-fruited plants are positively associated with those of frugivorous birds, which corresponds to a bottom-up effect of plants on animals. Second, we hypothesize that the PD of seedlings is positively related to the PD of frugivores, due to top-down effects of fruit removal by frugivores on seedling recruitment. Third, we hypothesize that the functional identity of fleshy-fruited plant and frugivorous bird communities (measured as mean fruit and body mass) is related to the functional identity of the seedling community (measured as mean seed mass). Alternatively, only plant and seedling diversity should be related if top-down effects of seed dispersal on seedling recruitment are weak. We tested these hypotheses by studying fleshy-fruited plants, frugivorous birds and seedling communities in a montane forest of the Colombian Andes.
Methods
Study Area and Design
We conducted our study along an elevational gradient in the Colombian Andes, at the western slope of the central Andean range (Cordillera Central). The gradient covers continuous forest, dominated by cloud forest, and is located in two protected areas, the National Park Santuario de Fauna y Flora Otún Quimbaya (4°43′ N, 75°34′W, 489 ha, elevational range 1,750–2,276 m), and the Regional Park Ucumarí (4°42′ N, 75°29′W, 3,986 ha, elevational range 1,850–2,700 m). The annual rainfall varies along the gradient between 2,000 and 4,000 mm, and there are two seasonal peaks of precipitation in April-May and October-November. Mean annual temperature along the gradient varies between 12 and 18°C (Londoño, 1994). The forest vegetation in the plots comprises mainly late secondary forests (>50 years old) with a mean canopy height between 15 and 25 m (Doumenge et al., 1995).
Frugivorous Birds and Avian Morphological Traits
We established 10 plots that were located between 1,800 m and 2,700 m a.s.l., with neighboring plots placed 100 m of elevation apart. Each plot had a size of 100 × 20 m. We recorded all frugivorous birds feeding on fleshy fruited plants in each plot. To detect frugivorous birds, we conducted four intensive surveys in 2012, covering twice the rainy season (May and November) and the dry season (February and July). Our surveys were based on direct observations of fruiting plants in the morning (6:30–12:30 h). In total, we conducted five visits per plot and survey (30 h per plot) and repeated the surveys four times, yielding a total of 120 h of observation per plot across the year. We recorded all birds swallowing or carrying away fruits and excluded bird species from the analyses that destroyed the seeds (i.e., parrot species). Birds were identified to species level and unidentified bird individuals were removed from the analysis (<2% of the observations). We did not include mammal seed dispersers because they play a minor role as seed dispersers in the study area (Muñoz et al., 2017b). Based on the field observations, we quantified the frequency of visits of each bird species to each fleshy-fruited plant species on each plot.
We measured the morphological traits of all bird species recorded during our observations in three natural history museums in Colombia (i.e., Instituto de Ciencias Naturales in Bogotá, Museo de la Universidad del Valle in Cali and Museo de Ciencias Naturales Federico Carlos Lehman in Cali). We measured three important morphological traits that are related to fruit removal, i.e., bill width, Kipp's index and body mass. Bill width restricts the size of the fruits a bird can swallow, Kipp's index is related to the mobility and preferred forest stratum, and body mass is associated with the energetic demands of a bird species (Moermond and Denslow, 1985; Dehling et al., 2014b). Bill width was measured as the external distance between the two commissural points of the beak and corresponds to gape width. We estimated Kipp's index by dividing Kipp's distance (i.e., the difference between the tip of the first secondary feather and the tip of the longest primary feather) by wing length (wing length equals the distance between the bend of the wing and the tip of the longest primary). We measured four specimens per species, two females and two males, and chose the sub-species present in our study area. We excluded one species from the analysis because it was not available in the regional museum collections (Vireo flavifrons, Vireonidade). In addition, we compiled information on avian body mass using Dunning (2007). We calculated the specie, mean of all three bird traits and log-transformed body mass and bill width before the analysis.
Fleshy-Fruited Plants and Morphological Traits
In each plot, we marked each plant individual bearing fleshy fruits and identified all plants to species level that were consumed by frugivorous birds during our surveys. To quantify the density of adults, we also counted all adult plants independent of their fruiting stage, i.e., we recorded all shrubs with a DBH ≥ 3 cm and all trees with a DBH ≥ 20 cm in each plot. For each of these groups, we measured three morphological traits that affect the foraging behavior of frugivores and correspond to the respective bird traits for trait matching: fruit size, plant height and crop mass (Dehling et al., 2014b; Muñoz et al., 2017a). Fruits contain the seeds of a plant species. Fruit size (i.e., length and width) was measured for at least two individuals per plant species in the laboratory (i.e., 20 or 30 fruits per species). Due to the high correlation between fruit length and width (n = 47 species, r = 0.8), we used fruit length as a proxy for fruit size in the analysis. For each plant individual with ripe fruits, we recorded plant height (i.e., the maximum height of the infructescence) and estimated crop size by counting the number of fruits for a subset of branches and the total number of branches with fruits (i.e., the number of ripe fruits on each fruiting plant), for details see Muñoz et al. (2017b). We additionally recorded the fruit mass per plant species (i.e., the dry weight of a whole fruit) and estimated the mean crop mass per species (i.e., mean crop size multiplied by mean fruit mass). For the analysis, we log-transformed the specie's means of fruit length, fruit mass, plant height and crop mass.
Seedling Establishment and Morphological Traits
In order to record seedling recruitment of fleshy-fruited plants along the elevational gradient, we counted the total number of seedlings per species and plot. In each plot, we randomly set up 60 sub-plots (1 m2 in size), yielding a total of 600 sub-plots across all plots. We counted seedlings recruited in two surveys, the first survey was in March 2012 and the second in February 2013. We only recorded seedlings of animal-dispersed plants with fleshy fruits (excluding epiphytic plants) assuming that birds were the primary dispersers of these species in the study area. We marked all seedlings (i.e., individuals ≤ 20 cm in height) with a unique code and identified them to species level (in most of the cases) or to genus level with the help of an expert botanist. Seedlings that we could not identify in the sub-plots were compared to a reference collection of seedlings grown in a greenhouse. We determined the abundance of each seedling species (i.e., seedling density) in each plot. We obtained the abundance of established seedlings by counting the number of seedlings present in the first and second surveys, i.e., seedlings that had survived 1 year plus the new seedlings that had recruited in the meantime. For each seedling species, we recorded the mass of the individual seed. To this end, we made a reference collection of seeds that were collected from at least three individual plants per species. The seeds of each individual were oven-dried at 50°C for seven days to weigh the dry seeds (i.e., dry seed mass). We calculated the mean seed mass for each plant species. We log-transformed seed mass before the analysis.
Phylogenetic Diversity
We constructed phylogenies in order to estimate the PD of each group in this study, i.e., fleshy-fruited plants/seedlings and frugivorous birds. For each group and study plot, we estimated PD following the definition given by Faith (1992), in which PD represents the sum of all branch lengths contained by the minimum spanning tree that links all species in a local assemblage within the regional pool phylogeny, including the root node. For the subset of fleshy-fruited plant species, we built a dated phylogeny at genus and species level by pruning the consensus tree (mega-tree) provided by the software Phylomatic, version 3.0 (Webb and Donoghue, 2005). We adjusted the branch lengths producing a dated phylogeny of the fleshy-fruited plants with the software Phylocom 4.2 (Webb et al., 2008). The phylogenetic tree for the fleshy-fruited plants had some polytomies at genus level (e.g., Miconia and Palicourea). Unsolved polytomies were held because congeneric species have similar phylogenetic distances and, therefore, this inaccuracy should not greatly affect the comparison of community-wide PD among plots. We calculated the PD of fleshy-fruited plants for each plot with the dated phylogeny of the plants and the presence-absence matrix of adult plants for each plot using the R package “Picante” (Kembel et al., 2010). We used the same sources and protocol to build a dated phylogeny for seedlings. We calculated the PD of seedlings per plot with the dated phylogeny and a presence-absence matrix of seedlings species for each plot.
For the frugivorous bird species that were observed feeding on fruits in the study area, we obtained 1,000 dated phylogenies from BirdTree.org (Jetz et al., 2012). We created a majority-rule consensus tree (25% burn-in removed, 95% maximum clade credibility) with a Bayesian approach (Markov chain Monte Carlo) using the package “TreeAnnotator” in BEAST (Drummond et al., 2012). We calculated the PD of frugivorous birds in each plot with the consensus tree and the presence-absence matrix of frugivorous birds for each plot.
Functional Diversity and Functional Identity
We estimated the functional diversity (FD) of frugivorous bird and fleshy-fruited plant communities in each plot. We calculated functional dispersion (FDis) as a measure of functional diversity because it quantifies species' dissimilarity independent of species richness (Laliberté and Legendre, 2010). Specifically, functional dispersion is the mean distance of each species in a community to the community centroid across all species in a multidimensional trait space (Laliberté and Legendre, 2010). We calculated FDis per plot based on three morphological traits that promote trait matching between fleshy-fruited plants and frugivorous birds (i.e., bill width, Kipp's index and body mass for birds; fruit length, plant height and crop mass for plants). To account for trait collinearity, trait spaces of birds and plants were defined by a Principal Coordinate Analysis (PCoA) on the Euclidean trait distances between bird and plant species, respectively. Prior to the PCoA, traits were standardized to zero mean and unit variance. Consistent with the analysis of PD, community matrices were unweighted and we only recorded the presence or absence of a species on a specific plot. We did not calculate FDis for the seedling community as we only measured a single functional trait for the seedlings (i.e., seed mass).
We additionally calculated the functional identity of plant and bird communities and of the recruited seedlings as the community weighted mean (CWM) of specific traits in the community (Laliberté and Legendre, 2010). For plants, we estimated the CWM of fruit mass, weighted by the species' density of adult plants recorded in each plot. For birds, we calculated the CWM of the avian body mass for each plot, weighted by the total frequency of interactions of each bird species on all fruiting plants in each plot. For the seedlings, we calculated the CWM of seed mass for each plot, weighted by the species' abundance of seedlings corresponding to the observed seedling density in each plot. We used the R package “FD” to estimate metrics of functional diversity and functional identity for the respective groups (Laliberté et al., 2015).
Statistical Analysis
To describe how PD and FD of plants, birds and seedlings were associated, we fitted linear regression models. First, we tested the relationships between PD of plants and PD of birds and between FD of plants and FD of birds in two separate regression models. Second, we analyzed how PD of seedlings was related to PD of plants and PD of birds in two separate models. Third, we examined in two separate models whether the functional identity of frugivorous birds (avian body mass) and fleshy-fruited plants (fruit mass) corresponded to the functional identity of the seedling communities (seed mass). All analyses were performed with R (R version 3.1.1, R Core Team, 2014).
Results
We recorded 67 frugivorous bird species and 47 fleshy-fruited plant species across all plots. The minimum and maximum number of frugivorous bird species recorded in the plots was 11 and 30 species, respectively (mean = 17, standard deviation SD = 5.8). The recorded bird species covered a wide range of morphological sizes and shapes, e.g., the smallest frugivore was Euphonia xanthogaster (Fringillidae, 13 g) and the largest Aburria aburri (Cracidae, 1,407 g). Among the fleshy-fruited plant species, we recorded 22 tree species, 13 shrub species, and 12 species of epiphytic plants. The minimum and maximum number of fleshy-fruited adult plants recorded in the plots was 8 and 15 species, respectively (mean = 11, SD = 2.31).
In total, we recorded 2,265 seedlings of 112 fleshy-fruited plant species (trees and shrubs) along the elevational gradient across the study year. The minimum and maximum numbers of seedlings recorded in the plots were 12 and 45 species, respectively (mean = 28, SD = 11.4). There was a high variation in the density of seedlings per species. Several species were only recorded once, while we recorded a total number of 253 individuals of Aniba muca (Lauraceae). For each studied group, the spatial turnover of species identities along the elevational gradient was high, i.e., the mean Sørensen dissimilarity between plots was 0.66 for birds, 0.59 for plants and 0.65 for seedlings.
Consistent with our first hypothesis, PD and FD of frugivorous birds were significantly related to those of fleshy-fruited plants (Figure 1). The PD of frugivorous birds increased with the PD of fleshy-fruited adult plants (Figure 1A). Similarly, there was a positive relationship between the FD of fleshy-fruited plants and the FD of frugivorous birds (Figure 1B). In line with our second hypothesis, PD of seedlings significantly increased with the PD of fleshy-fruited plants (Figure 2A). However, PD of seedlings was not significantly related to that of frugivorous birds (Figure 2B). According to our third hypothesis, the functional identity of seedlings communities (i.e., the CWM of seed mass) was positively associated with the CWM fruit mass of the fleshy-fruited plant communities (Figure 3A). However, CWM seed mass was unrelated to the CWM body mass of the frugivorous bird communities (Figure 3B).
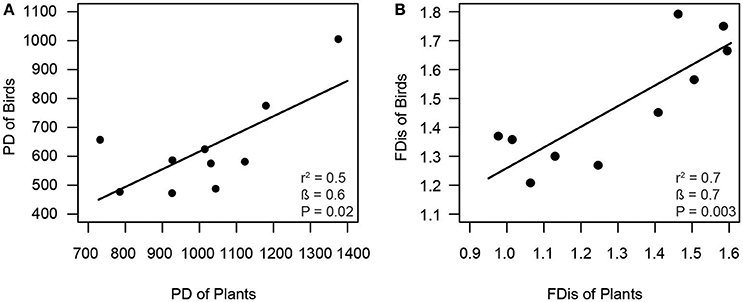
Figure 1. Positive relationship between (A) phylogenetic diversity (PD) and (B) functional dispersion (FDis) of fleshy-fruited adult plant and frugivorous bird communities across ten study plots in the Colombian Andes (elevational range: 1,800–2700 m a.s.l.). Shown are results from linear regression models. Given are the overall model fit (r2) and the estimated slope (β) with its associated P-value; solid lines indicate the predicted trend lines.
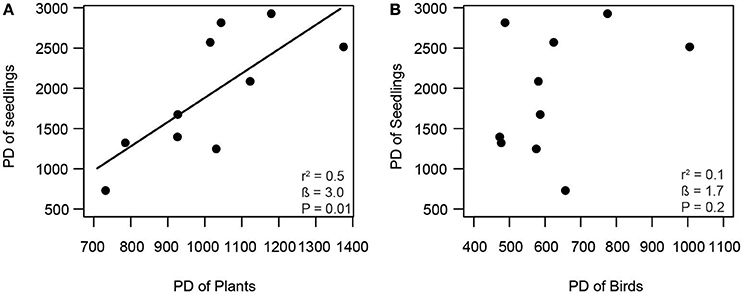
Figure 2. Positive relationship between (A) phylogenetic diversity (PD) of fleshy-fruited plants and PD of seedlings, and lack of a relationship (B) between PD of birds and PD of seedlings across 10 study plots in the Colombian Andes (elevational range: 1,800 m–2,700 m a.s.l.). Shown are results from linear regression models. Given are the overall model fit (r2) and the estimated slope (β) with its associated P-value; the solid line indicates a significant trend.
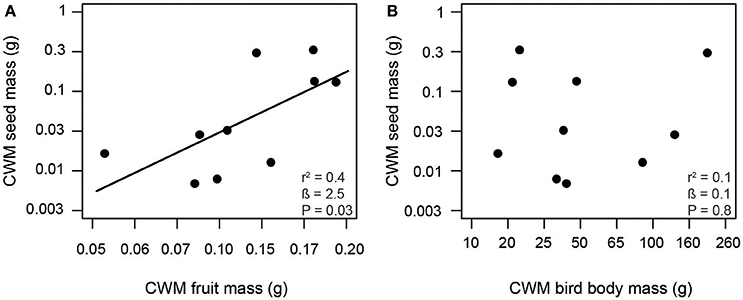
Figure 3. Positive relationship between (A) fruit mass of adult plants and seed mass of recruited seedlings, and a lack of a relationship (B) between avian body mass and seed mass across 10 study plots in the Colombian Andes (elevational range: 1,800 m to 2,700 m a.s.l.). Trait values correspond to the community-weighted means (CWM) of the respective communities. Shown are results from linear regression models. Given are the overall model fit (r2) and the estimated slope (β) with its associated P-value; the solid line indicates a significant trend.
Discussion
This empirical study in a tropical montane forest shows that the PD and FD of fleshy-fruited plants is related to the corresponding diversity of frugivorous birds, suggesting that an increase in resource diversity leads to a corresponding increase in the diversity of consumer species (bottom-up effect of resources on consumers). Although seed dispersal is a service provided by frugivorous birds to plants, we could not detect a relationship between the PD or functional identity of frugivorous birds and those of seedling communities (no top-down effects of seed dispersers on seedling communities). Instead, the PD and functional identity of seedlings was related to those of adult plant communities. Our results suggest that trait variation in fleshy-fruited plant communities has a community-wide impact on communities of frugivores and seedlings. Thus, morphological plant traits structure the communities of their animal partners and affect the future trajectory of plant recruitment.
Plant and Bird Communities
Consistent with our first hypothesis, we found that the PD and FD of frugivorous birds were strongly associated with those of fleshy-fruited plants. This relationship suggests that an increase in resource diversity increases the number of feeding niches of frugivorous birds (Dehling et al., 2016), consistent with a bottom-up control of mutualistic plant-animal interactions (Albrecht et al., 2014). Frugivorous birds have the ability to track fruits and respond directly to the spatial-temporal availability of fruits (Hampe, 2008; García et al., 2011). As a consequence, a diverse community of fruiting plants can attract a morphologically diverse bird community, which can effectively exploit the whole spectrum of available fruits. This finding is also corroborated by previous studies that have shown that birds migrate along elevational gradients or show seasonal displacement among habitats in response to spatially and temporally fluctuating fruit availability (Wheelwright, 1983; Mulwa et al., 2013). However, this is one of the first studies that shows that differences in the type of resources offered by fruiting plants influence the PD and FD of frugivorous birds at the community level (see also Dehling et al., 2014a). Thus, our results show that phylogenetic and morphological variation in plant resources are an important element structuring the PD and FD of fruit consumers in tropical mountain forests.
The finding that PD and FD of fleshy-fruited plants are correlated to corresponding measures of bird diversity is consistent with trait matching between plants and birds and with an ecological fitting between resource and consumer communities (Janzen, 1985). Interactions between fleshy-fruited plants and frugivorous birds are influenced by morphological traits of birds and plants, which promote or constrain fruit consumption (Dehling et al., 2014b; Muñoz et al., 2017a). Here we estimated the FD of birds and plants by corresponding bird and plant traits such as bill width and fruit size, wing shape and foraging stratum and body mass and crop size (Dehling et al., 2016). In fact, the relationship between FD of plants and birds was slightly stronger than the respective relationship of PD, indicating that FD comparisons are particularly valuable when the functional traits involved in an interaction have been previously identified. Although PD and FD of plant and bird communities were generally positively correlated (n = 10 plots; plants: r = 0.7, P = 0.03; birds: r = 0.6, P = 0.06), this could suggest that morphological trait matching was more important for structuring the interacting communities than associations in other types of plant and animal traits. Such other species traits that are likely conserved along the phylogenetic tree, and for instance, relate to the physiological demands of consumers or the nutritional content present in fruit resources (Lavin et al., 2008; Valido et al., 2011; Blendinger et al., 2016) may be less important than phenotypic matching traits. This is also corroborated by a recent study that shows that fruit nutritional content had a weaker effect than crop and fruit size on fruit removal by animals (Muñoz et al., 2017b). This suggests that seed-dispersal interactions in a community are primarily constrained by the phenotypes of species, and that morphological trait matching seems to directly influence the assemblage structure of frugivorous birds, due to ecological fitting between plant and animal communities. These findings suggest that bottom-up effects of plants on animals are important for structuring ecological communities in the tropical Andes.
Seedling Communities
We did not detect an effect of PD and functional identity of frugivorous birds on seedling communities, consistent with a lack of top-down effects of birds on the phylogenetic and functional structure of seedling communities. Instead, we found that PD and functional identity of seedlings were only affected by corresponding measures of fleshy-fruited adult plants. The lack of a top-down effect of birds on seedlings could be due to a high degree of redundancy in the seed-dispersal functions provided by the bird community. Especially large frugivorous birds are able to feed on a wide range of fleshy-fruited plants (Muñoz et al., 2017a), which could contribute to a decoupling between bird and seedling traits. In the study area, large frugivorous birds were present in all plots. For example, the green toucanets (Aulacorhynchus prasinus, Aulacorhynchus haematopygus) were important seed dispersers at the lowest elevation, whereas the mountain toucans (Andigena hypoglauca, Andigena nigrirostris) fulfilled a similar role at the highest elevations. Similarly, different species of guans (Cracidae) occurred throughout the elevational gradient. Hence, it is likely that the importance of large generalist frugivores in the study area weakened the relationship between animal and seedling traits and suggests that trait filtering by seed-dispersal processes is relatively weak in natural forests in the tropical Andes. In contrast, other studies from disturbed tropical forests have shown that a reduction in the trait diversity of animal seed dispersers, especially the absence of large-bodied frugivores, leads to a reduced diversity of seedling communities (Terborgh et al., 2008; Galetti et al., 2013). Hence, the local extirpation of large frugivores is usually not buffered by compensation effects from other animal species and seems to have important top-down effects on plant recruitment.
Since top-down effects of animals on seedlings were weak, a positive relationship between adult plant and seedling communities is expected because abundant plants should recruit more seedlings than rare plants in the absence of trait filters. The association of the PD of seedlings with that of adult plants may be further strengthened by abiotic filters, such as species-specific habitat requirements. Establishment limitation may filter the type of seedlings that are able to recruit and can override effects of seed dispersal and arrival (Nathan and Muller-Landau, 2000; Beckman and Rogers, 2013). For instance, many pioneer species have a high fruit removal rate by frugivorous birds (Kessler-Rios and Kattan, 2012). However, seedling recruitment of these species is restricted to early successional habitats (Dalling et al., 2002). Similarly, the habitat filtering hypothesis states that specific life history strategies are selected by specific environmental conditions (Webb et al., 2002). Environmental heterogeneity, e.g. associated with light or nutrients, may favor the co-existence of adults and seedlings in many tropical forests (Webb and Peart, 2000; Engelbrecht et al., 2007). Such effects of abiotic conditions on plant recruitment may be particularly pronounced along the elevational gradient studied here, due to the topographic variation and environmental heterogeneity in tropical mountains (Lippok et al., 2014). In addition to these abiotic filters, it is expected that more seedlings recruit near parental plants if seed dispersal is spatially constrained (Nathan and Muller-Landau, 2000). Thus, plant life-history characteristics in interaction with local environmental conditions along the elevational gradient may leave stronger imprints on seedling communities than animal seed dispersers. Since we generally found a higher PD of seedlings than plants, our results further suggest that there is a local increase in PD during seed dispersal, probably due to the immigration of seeds to new localities. The reduction in PD from the seedling to the adult stage could then be the consequence of abiotic filters or competition that constrain survival and growth from the seedling to the adult stage.
We found a positive correlation between mean fruit mass of adult plants and mean seed mass of recruited seedlings, whereas the mean body mass and seed mass of birds and seedlings were unrelated. These findings underscore that trait filtering by animals was generally weak. Our results, however, suggest that morphological plant traits, such as seed size, influence the recruitment probability of seedlings, which appears to result in a match between the functional identities of current and future plant communities. This is also consistent with the generally positive relationship between fruit and seed size in plants (Wright et al., 2007). In our study system, communities that were dominated by large-fruited species, such as those from the Lauraceae or Arecaceae families, were also characterized by seedling communities with a large mean seed mass. Seedlings with large seed mass may have an advantage in forest regeneration because these species establish and compete more successfully than small-seeded species, particularly in late successional, closed forests (Rees et al., 2001; Muñoz et al., 2017a). Hence, habitat conditions are likely to play an important role by filtering seed traits prior to seedling recruitment. Our finding is also consistent with the mass ratio hypothesis (Grime, 1998) which states that traits of the most productive species in terms of biomass are the most important determinants of ecosystem properties, such as seedling establishment. Thus, our findings corroborate that the functional diversity and identity of fleshy-fruited adult plants may influence the future composition of montane forests and that these bottom-up effects on seedling recruitment appear to be more important than top-down effects mediated by animal seed dispersers. Our results also warn against inferring information on plant population and community dynamics from studies on seed-dispersal interactions that do not account for bottom-up effects of current plant communities.
Conclusion
We show that the PD and FD of frugivorous birds closely correspond to the respective diversity of fleshy-fruited plants. The positive association between resource and consumer diversity underpins the relevance of bottom-up effects in ecological communities. One important implication of this finding is that the loss of plant diversity, e.g., due to forest conversion, selective logging or climate change, could have cascading effects on the associated animal communities (Brodie et al., 2014; Schleuning et al., 2016). In contrast to the bottom-up effects of plants on animals, the PD and functional identity of bird dispersers were unrelated to the PD and functional identity of seedlings. This finding demonstrates that top-down effects of animals on the phylogenetic and functional structure of plant communities are comparatively weak although animal seed dispersers can have important quantitative effects on the establishment of specific plant species (Cavallero et al., 2013; Martínez and Garcıa, 2016) and entire plant communities (Muñoz et al., 2017b). Finally, we found that the PD and functional identity of seedlings was contingent on that of the adult plants. Hence, the diversity of fleshy-fruited plant communities plays a fundamental role for the diversity of consumer species and future forest composition in this tropical mountain forests.
Author Contributions
MM, HS, KB, and MS conceived and designed the research; MM conducted field work, analyzed the data with input from EN and MS, and wrote the first draft of the manuscript. All authors contributed to data interpretation and edited the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to two referees for their constructive comments on the manuscript. We thank Teresa Cano, Yadi Toro, Manuela Calderón, Manuel Sanchez, Julio Cesar Bermudez, Cesar Andres Arango, and Sebastian Duque for field assistance. We thank William Vargas for plant identification. MM got a grant from Rufford Small Grant for Nature Conservation (ref: # 11042-1) for conducting the fieldwork, and was funded by COLCIENCIAS (Departamento Administrativo de Ciencia, Tecnología e Innovación Republica de Colombia) and the Graduate Student Scholarship “Francisco José de Caldas.” This study was also supported by the research funding programme ‘LOEWE–Landes-Off ensive zur Entwicklung wissenschaftlich- ökonomischer Exzellenz’ of Hesse's Ministry of Higher Education, Research, and the Arts. We would like to thank National Parks System of Colombia (Parques Nacionales Naturales de Colombia) for facilitating our work at Santuario de Flora y Fauna Otún Quimbaya and the research permit (No. PIDB-DTAO 015-11). We extend our thanks to CARDER (Corporación Autónoma Regional de Risaralda) for logistic support and the research permit to work at Ucumari Regional Park.
References
Albrecht, J., Berens, D. G., Jaroszewicz, B., Selva, N., Brandl, R., and Farwig, N. (2014). Correlated loss of ecosystem services in coupled mutualistic networks. Nat. Commun. 5:3810. doi: 10.1038/ncomms4810
Bascompte, J., and Jordano, P. (2007). Plant-animal mutualistic networks: the architecture of biodiversity. Annu. Rev. Ecol. Evol. Syst. 38, 567–593. doi: 10.1146/annurev.ecolsys.38.091206.095818
Beckman, N. G., and Rogers, H. S. (2013). Consequences of seed dispersal for plant recruitment in tropical forests: interactions within the seedscape. Biotropica 45, 666–681. doi: 10.1111/btp.12071
Bello, F., Lavorel, S., Díaz, S., Harrington, R., Cornelissen, J. H. C., Bardgett, R. D., et al. (2010). Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 19, 2873–2893. doi: 10.1007/s10531-010-9850-9
Bender, I. M. A., Kissling, W. D., Böhning-Gaese, K., Hensen, I., Kühn, I., Wiegand, T., et al. (2017). Functionally specialised birds respond flexibly to seasonal changes in fruit availability. J. Anim. Ecol. 86, 800–811. doi: 10.1111/1365-2656.12683
Blendinger, P. G., Martín, E., Osinaga Acosta, O., Ruggera, R. A., and Aráoz, E. (2016). Fruit selection by Andean forest birds: influence of fruit functional traits and their temporal variation. Biotropica 48, 677–686. doi: 10.1111/btp.12329
Brodie, J. F., Aslan, C. E., Rogers, H. S., Redford, K. H., Maron, J. L., Bronstein, J. L., et al. (2014). Secondary extinctions of biodiversity. Trends Ecol. Evol. 29, 664–672. doi: 10.1016/j.tree.2014.09.012
Cadotte, M. W., Cardinale, B. J., and Oakley, T. H. (2008). Evolutionary history and the effect of biodiversity on plant productivity. Proc. Natl. Acad. Sci. U.S.A. 105, 17012–17017. doi: 10.1073/pnas.0805962105
Cadotte, M. W., Carscadden, K., and Mirotchnick, N. (2011). Beyond species: functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 48, 1079–1087. doi: 10.1111/j.1365-2664.2011.02048.x
Cavallero, L., Raffaele, E., and Aizen, M. A. (2013). Birds as mediators of passive restoration during early post-fire recovery. Biol. Conserv. 158, 342–350. doi: 10.1016/j.biocon.2012.10.004
Cavender-Bares, J., Kozak, K. H., Fine, P. V., and Kembel, S. W. (2009). The merging of community ecology and phylogenetic biology. Ecol. Lett. 12, 693–715. doi: 10.1111/j.1461-0248.2009.01314.x
Chamberlain, S. A., Cartar, R. V., Worley, A. C., Semmler, S. J., Gielens, G., Elwell, S., et al. (2014). Traits and phylogenetic history contribute to network structure across Canadian plant – pollinator communities. Oecologia 176, 545–556. doi: 10.1007/s00442-014-3035-2
Dalling, J. W., Muller-Landau, H. C., Wright, S. J., and Hubbell, S. P. (2002). Role of dispersal in the recruitment limitation of neotropical pioneer species. J. Ecol. 90, 714–727. doi: 10.1046/j.1365-2745.2002.00706.x
Dehling, D. M., Fritz, S. A., Töpfer, T., Päckert, M., Estler, P., Böhning-Gaese, K., et al. (2014a). Functional and phylogenetic diversity and assemblage structure of frugivorous birds along an elevational gradient in the tropical Andes. Ecography 37, 1047–1055. doi: 10.1111/ecog.00623
Dehling, D. M., Jordano, P., Schaefer, H. M., Böhning-Gaese, K., and Schleuning, M. (2016). Morphology predicts species' functional roles and their degree of specialization in plant-frugivore interactions. Proc. B 283:20152444. doi: 10.1098/rspb.2015.2444
Dehling, D. M., Töpfer, T., Schaefer, H. M., Jordano, P., Böhning-Gaese, K., and Schleuning, M. (2014b). Functional relationships beyond species richness patterns: trait matching in plant-bird mutualisms across scales. Glob. Ecol. Biogeogr. 23, 1085–1093. doi: 10.1111/geb.12193
Díaz, S., and Cabido, M. (2001). Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 16, 646–655. doi: 10.1016/S0169-5347(01)02283-2
Díaz, S., Lavorel, S., de Bello, F., Quétier, F., Grigulis, K., and Robson, T. M. (2007). Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. U.S.A. 104, 20684–20689. doi: 10.1073/pnas.0704716104
Doumenge, C., Gilmour, D., Ruíz Pérez, M., and Blockhus, J. (1995). “Tropical montane cloud forests: conservation status and management issues,” in Tropical Montane Cloud Forest, eds S. H. Lawrence, J. O. Juvik, and F. N. Scatena (New York, NY: Springer-Verlag), 24–37.
Drummond, A. J., Suchard, M. A., Xie, D., and Rambaut, A. (2012). Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973. doi: 10.1093/molbev/mss075
Dunning, J. B. (2007). CRC Handbook of Avian Body Masses, 2nd Edn. Boca Raton, FL: Taylor and Francis.
Engelbrecht, B. M., Comita, L. S., Condit, R., Kursar, T. A., Tyree, M. T., Turner, B. L., et al. (2007). Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447, 80–82. doi: 10.1038/nature05747
Faith, D. P. (1992). Systematics and conservation: on predicting the feature diversity of subsets of taxa. Cladistic 8, 361–373. doi: 10.1111/j.1096-0031
Farwig, N., and Berens, D. G. (2012). Imagine a world without seed dispersers: a review of threats, consequences and future directions. Basic Appl. Ecol. 13, 109–115. doi: 10.1016/j.baae.2012.02.006
Flynn, D. F. B., Mirotchnick, N., Jain, M., Palmer, M. I., and Naeem, S. (2011). Functional and phylogenetic diversity as predictors of biodiversity-ecosystem-function relationships. Ecology 92, 1573–1581. doi: 10.1890/10-1245.1
Gagic, V., Bartomeus, I., Jonsson, T., Taylor, A., Winqvist, C., Fischer, C., et al. (2015). Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc. R. Soc. B 282:20142620. doi: 10.1098/rspb.2014.2620
Galetti, M., Guevara, R., Côrtes, M. C., Fadini, R., Von Matter, S., Leite, A. B., et al. (2013). Functional extinction of birds drives rapid evolutionary changes in seed size. Science 340, 1086–1090. doi: 10.1126/science.1233774
García, D., Zamora, R., and Amico, G. C. (2011). The spatial scale of plant – animal interactions: effects of resource availability and habitat structure. Ecol. Monogr. 81, 103–121. doi: 10.1890/10-0470.1
Grime, J. P. (1998). Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J. Ecol. 86, 902–910.
Hampe, A. (2008). Fruit tracking, frugivore satiation, and their consequences. Oecologia 156, 137–145. doi: 10.1007/s00442-008-0979-0
Herrera, C. M. (2002). “Seed dispersal by vertebrates” in Plant–Animal Interactions: An Evolutionary Approach, eds C. Herrera and O. Pellmyr (Padstow: Blackwell Scientific Publications), 185–208.
Hooper, D. U., Chapin, F. S. I., Ewel, J., Hector, A., Inchausti, P., Lavorel, S., et al. (2005). Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. doi: 10.1890/04-0922
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K., and Moores, A. O. (2012). The global diversity of birds in space and time. Nature 491, 444–448. doi: 10.1038/nature11631
Jordano, P., García, C., Godoy, J. A., and García-Castaño, J. L. (2007). Differential contribution of frugivores to complex seed dispersal patterns. Proc. Natl. Acad. Sci. U.S.A. 104, 3278–3282. doi: 10.1073/pnas.0606793104
Kembel, S. W., Cowan, P. D., Helmus, M. R., Cornwell, W. K., Morlon, H., Ackerly, D. D., et al. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. doi: 10.1093/bioinformatics/btq166
Kessler-Rios, M. M., and Kattan, G. H. (2012). Fruits of Melastomataceae: phenology in Andean forest and role as a food resource for birds. J. Trop. Ecol. 28, 11–21. doi: 10.1017/S0266467411000642
Kissling, W. D., Rahbek, C., and Böhning-Gaese, K. (2007). Food plant diversity as broad-scale determinant of avian frugivore richness. Proc. Biol. Sci. B 274, 799–808. doi: 10.1098/rspb.2006.0311
Laliberté, A. E., Legendre, P., Shipley, B., and Laliberté, M. E. (2015). Measuring Functional Diversity (FD) from Multiple Traits, and Other Tools For Functional Ecology. R package version 1.0–12.
Laliberté, E., and Legendre, P. (2010). A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305. doi: 10.1890/08-2244.1
Lavin, S. R., Karasov, W. H., Ives, A. R., Middleton, K. M., and Garland, T. Jr. (2008). Morphometrics of the avian small intestine compared with that of nonflying mammals: a phylogenetic approach. Physiol. Biochem. Zool. 81, 526–550. doi: 10.1086/590395
Lippok, D., Beck, S. G., Renison, D., Hensen, I., Apaza, A. E., and Schleuning, M. (2014). Topography and edge effects are more important than elevation as drivers of vegetation patterns in a neotropical montane forest. J. Veg. Sci. 25, 724–733. doi: 10.1111/jvs.12132
Londoño, E. (1994). “Parque Regional Natural Ucumarí. Un vistazo histórico,” in Ucumarí: Un caso Típico de la Diversidad Biotica Andina, ed J. O. Rangel (Pereira: Corporación Autónoma Regional de Risaralda), 25–35.
Lortie, C. J., Brooker, R. W., Choler, P., Kikvidze, Z., Pugnaire, F. I., Callaway, R. M., et al. (2004). Rethinking plant community theory. Oikos 107, 433–438. doi: 10.1111/j.0030-1299.2004.13250.x
Maglianesi, M. A., Blüthgen, N., Böhning-Gaese, K., and Schleuning, M. (2014). Morphological traits determine specialization and resource use in plant-hummingbird networks in the neotropics. Ecology 95, 3325–3334. doi: 10.1890/13-2261.1
Martínez, D., and Garcıa, D. (2016). Role of avian seed dispersers in tree recruitment in woodland pastures. Ecosystems 20, 616–629. doi: 10.1007/s10021-016-0043-6
Moermond, T. C., and Denslow, J. S. (1985). Neotropical avian frugivores: patterns of behavior, morphology, and nutrition, with consequences for fruit selection. Ornithol. Monogr. 36, 865–897.
Mokany, K., Ash, J., and Roxburgh, S. (2008). Functional identity is more important than diversity in influencing ecosystem processes in a temperate native grassland. J. Ecol. 96, 884–893. doi: 10.1111/j.1365-2745.2008.01395.x
Mulwa, R. K., Neuschulz, E. L., Böhning-Gaese, K., and Schleuning, M. (2013). Seasonal fluctuations of resource abundance and avian feeding guilds across forest-farmland boundaries in tropical Africa. Oikos 122, 524–532. doi: 10.1111/j.1600-0706.2012.20640.x
Muñoz, M. C., Schaefer, H. M., Böhning-Gaese, K., and Schleuning, M. (2017a). Importance of animal and plant traits for fruit removal and seedling recruitment in a tropical forest. Oikos 126, 823–832. doi: 10.1111/oik.03547
Muñoz, M. C., Schaefer, H. M., Böhning-Gaese, K., and Schleuning, M. (2017b). Positive relationship between fruit removal by animals and seedling recruitment in a tropical forest. Basic Appl. Ecol. 20, 31–39. doi: 10.1016/j.baae.2017.03.001
Nathan, R., and Muller-Landau, H. (2000). Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 15, 278–285. doi: 10.1016/S0169-5347(00)01874-7
Petchey, O. L., and Gaston, K. J. (2006). Functional diversity: back to basics and looking forward. Ecol. Lett. 9, 741–758. doi: 10.1111/j.1461-0248.2006.00924.x
Power, M. E. (1992). Top-down and bottom-up forces in food webs: do plants have primacy. Ecology 73, 733–746.
Price, W. P. (2002). “Species interactions and the evolution of biodiversity” in Plant-Animal Interactions an Evolutionary Approach, eds C. M. Herrera and O. Pellmyr (Padstow: Blackwell Scientific Publications), 3–25.
R Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org/
Rees, M., Condit, R., Crawley, M., Pacala, S., and Tilman, D. (2001). Long-term studies of vegetation dynamics. Science 293, 650–655. doi: 10.1126/science.1062586
Schleuning, M., Fründ, J., and García, D. (2015). Predicting ecosystem functions from biodiversity and mutualistic networks: an extension of trait-based concepts to plant–animal interactions. Ecography 38, 380–392. doi: 10.1111/ecog.00983
Schleuning, M., Fründ, J., Schweiger, O., Welk, E., Albrecht, J., Albrecht, M., et al. (2016). Ecological networks are more sensitive to plant than to animal extinction under climate change. Nat. Commun. 7:13965. doi: 10.1038/ncomms13965
Terborgh, J., Nunez-Iturri, G., Pitman, N. C., Cornejo Valverde, F. H., Alvarez, P., Swamy, V., et al. (2008). Tree recruitment in an empty forest. Ecology 89, 1757–1768. doi: 10.1890/07-0479.1
Vanthomme, H., Belle, B., and Forget, P.-M. (2010). Bushmeat hunting alters recruitment of large-seeded plant species in Central Africa. Biotropica 42, 672–679. doi: 10.1111/j.1744-7429.2010.00630.x
Valido, A., Schaefer, H. M., and Jordano, P. (2011). Colour, design and reward: phenotypic integration of fleshy fruit displays. J. Evol. Biol. 24, 751–760. doi: 10.1111/j.1420-9101.2010.02206.x
Villéger, S., Mason, N. W. H., and Mouillot, D. (2008). New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301. doi: 10.1890/07-1206.1
Webb, C., Ackerly, D. D., Mcpeek, M. A., and Donoghue, M. J. (2002). Phylogenies and community. Annu. Rev. Ecol. Syst. 33, 475–505. doi: 10.1146/annurev.ecolsys.33.010802.150448
Webb, C. O., Ackerly, D. D., and Kembel, S. W. (2008). Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24, 2098–2100. doi: 10.1093/bioinformatics/btn358
Webb, C. O., and Donoghue, M. J. (2005). Phylomatic: tree assembly for applied phylogenetics. Mol. Ecol. Notes 5, 181–183. doi: 10.1111/j.1471-8286.2004.00829.x
Webb, C., and Peart, D. R. (2000). Habitat associations of trees and seedlings in a Bornean rain forest. J. Ecol. 88, 464–478. doi: 10.1046/j.1365-2745.2000.00462.x
Westoby, M., Leishman, M., Lord, J., Poorter, H., and Schoen, D. J. (1996). Comparative ecology of seed size and dispersal. Philos. Trans. R. Soc. B Biol. Sci. 351, 1309–1318. doi: 10.1098/rstb.1996.0114
Wheelwright, N. T. (1985). Fruit-size, gape width, and the diets of fruit-eating birds. Ecology 66, 808–818.
Wright, I. J., Ackerly, D. D., Bongers, F., Harms, K. E., Ibarra-Manriquez, G., Martinez-Ramos, M., et al. (2007). Relationships among ecologically important dimensions of plant trait variation in seven neotropical forests. Ann. Bot. 99, 1003–1015. doi: 10.1093/aob/mcl066
Keywords: frugivorous bird communities, functional identity, plant-animal mutualism, functional traits, seedling communities, Colombian Andes
Citation: Muñoz MC, Schaefer HM, Böhning-Gaese K, Neuschulz EL and Schleuning M (2017) Phylogenetic and Functional Diversity of Fleshy-Fruited Plants Are Positively Associated with Seedling Diversity in a Tropical Montane Forest. Front. Ecol. Evol. 5:93. doi: 10.3389/fevo.2017.00093
Received: 24 January 2017; Accepted: 26 July 2017;
Published: 14 August 2017.
Edited by:
Tomás A. Carlo, Pennsylvania State University, United StatesReviewed by:
Daniel García, Departamento de Biología de Organismos y Sistemas - Universidad de Oviedo, SpainDonald Drake, Hawaii University, United States
Copyright © 2017 Muñoz, Schaefer, Böhning-Gaese, Neuschulz and Schleuning. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcia C. Muñoz, marcarmu@gmail.com
 Marcia C. Muñoz
Marcia C. Muñoz H. Martin Schaefer
H. Martin Schaefer Katrin Böhning-Gaese1,2
Katrin Böhning-Gaese1,2  Eike Lena Neuschulz
Eike Lena Neuschulz Matthias Schleuning
Matthias Schleuning