The Right Tools for the Job: Cooperative Breeding Theory and an Evaluation of the Methodological Approaches to Understanding the Evolution and Maintenance of Sociality
- 1Centre for Sustainable Ecosystems Solutions, School of Biological Sciences, University of Wollongong, Wollongong, NSW, Australia
- 2Fish Ecology Laboratory, School of the Life Sciences, University of Technology Sydney, Sydney, NSW, Australia
- 3Centre for Medical and Molecular Bioscience, School of Biological Sciences, University of Wollongong, Wollongong, NSW, Australia
Why do we observe so many examples in nature in which individuals routinely delay or completely forgo their own reproductive opportunities in order to join and remain within a group? Cooperative breeding theory provides a rich framework with which to study the factors that may influence the costs and benefits of remaining philopatric as a non-breeder. This is often viewed as an initial step in the development of costly helping behavior provided by non-breeding subordinates. Despite many excellent empirical studies testing key concepts of the theory, there is still debate regarding the relative importance of various evolutionary forces, suggesting that there may not be a general explanation but rather a dynamic and taxonomically varied combination of factors influencing the evolution and maintenance of sociality. Here, we explore two potential improvements in the study of sociality that could aid in the progress of this field. The first addresses the fact that empirical studies of social evolution are typically conducted using either comparative, observational or manipulative methodologies. Instead, we suggest a holistic approach, whereby observational and experimental studies are designed with the explicit view of advancing comparative analyses of sociality for the taxon, and in tandem, where comparative work informs targeted research effort on specific (usually understudied) species within the lineage. A second improvement relates to the broadening of tests of cooperative breeding theory to include taxa where subordinates do not necessarily provide active cooperation within the group. The original bias toward “helpful subordinates” arose from a focus on terrestrial taxa. However, recent consideration of other taxa, especially marine taxa, is slowly revealing that the theory can and should encompass a continuum of cooperative social systems, including those where subordinates do not actively help. This review summarizes the major hypotheses of cooperative breeding theory, one of the dominant frameworks to examine social evolution, and highlights the potential benefits that a combined methodological approach and a broader application could provide to the study of sociality.
Introduction
The animal kingdom contains many examples of species, including our own, which form surprisingly complex social structures (Munday et al., 1998; Purcell, 2011; Grueter et al., 2012; Chapais, 2013; Johnson et al., 2013; Taborsky and Wong, 2017). The size, structure and composition of these groups can vary both within and between species, from pair-bonding monogamous partners (Kleiman, 2011; Servedio et al., 2013) to large and highly complex societies exhibiting social hierarchies and division of labor (Duffy and Macdonald, 2010; Nandi et al., 2013). Such variation in social structure is intriguing as it suggests that there may be a great diversity of underlying social, ecological or life history factors that influence the evolution of stable groups and their maintenance over many generations.
One of the most fascinating cases within the broad spectrum of sociality is the formation of groups where individuals delay or forgo their own reproductive opportunities (Clutton-Brock et al., 2001; Buston, 2003b; Faulkes and Bennett, 2013; Margraf and Cockburn, 2013). Subordinate members of such groups often but not always, provide help in raising the offspring of dominant breeders. When this alloparental care is present in the group the social system is often referred to as “cooperative breeding.” Delayed dispersal is widely believed to be the first step in the evolution of cooperative breeding (Emlen, 1982a; Brown, 1987). Importantly, the factors influencing an individual's decision to delay its dispersal and breeding are often the same as the factors that select for the evolution of subsequent cooperative actions, such as alloparental care, territory defense or nest maintenance. For example, high predation pressure can act as a constraint on dispersal, driving group formation (as shown experimentally by Heg et al., 2004a). This same pressure may then select for individuals who contribute to the collective defense of the group by increasing their individual chances of survival and future reproduction (e.g., Heg and Taborsky, 2010; Groenewoud et al., 2016).
Besides explaining the evolution of group-living and helpful cooperation in groups, we propose that cooperative breeding theory can also be applied to explain the evolution and maintenance of group living even for species where there is no helpful cooperation. In such groups subordinate group members may exhibit behaviors that offset or avoid inflicting costs on dominants (Kokko et al., 2002; Buston and Balshine, 2007; Wong et al., 2007) such that their overall effect on dominant fitness is neutral (termed “peaceful cooperation”; Wong et al., 2007). While such actions may not increase dominant fitness, it still represents a cost to a subordinate who must assess this against the benefits gained from remaining within the group. That is, subordinates in groups, whether or not they actively cooperate must weigh the costs and benefits of group membership. It is these costs and benefits that the hypotheses that make up cooperative breeding theory focus on. Thus, studies investigating the determinants of group living need not be restricted to applying cooperative breeding theory only to species where helping actively occurs.
Notwithstanding the excellent empirical and theoretical work conducted in this field (e.g., Emlen, 1994; Cockburn, 1996; Arnold and Owens, 1998; Hatchwell and Komdeur, 2000; Pen and Weissing, 2000; Buston and Balshine, 2007), the relative importance of the evolutionary forces at play which influence the decision of non-breeders to forego their own reproductive opportunities and remain within a group are still the subject of much discussion. Advances in understanding have so far been made through either comparative studies, focusing on a broad group of taxa, or through more narrowly focused observational or manipulative work on a more restricted subset of species in a generally piecemeal fashion. Each methodology provides important insights into the study system, but they also have their own unique limitations. A combination of methodologies will address many of these limitations and give a more general understanding of the system (Brown, 1974). Indeed, comparative studies often use data from focused observational and experimental studies and many researchers have combined observational and manipulative methodologies to provide powerful results. However, we contend that combining all three methodologies under a single framework provides the most comprehensive approach to studying the evolution of sociality. The fresh water cichlid Neolamprologus pulcher provides an excellent example of how many comparative, observational and experimental studies have provided an extremely robust view of social evolution and maintenance and challenged terrestrially derived theories, such as kinship based mechanisms, in being involved in social evolution (e.g., Wong and Balshine, 2011). But what does the evolution of sociality in N. pulcher, tell us about sociality in the (roughly) 50 other species in the Neolamprologus genus? Can these results be generalized to all social freshwater fishes or indeed all vertebrates? Interspecies comparative analyses are the only way that we can answer such broad evolutionary questions. Obviously, gathering the observational and experimental data for comparative analysis of 50 species would represent an extremely time consuming and costly process. Carefully coordinated collaborations between research groups could help to spread the research effort. In order to maximize the impact of any individual piece of research, focused observational and experimental work should be targeted toward species within the given lineage which are lacking in data and designed with the express view of contributing to future comparative work. Mapping sociality and traits of interest onto a phylogeny for the lineage would help to identify suitable species and can be used to study questions about the evolutionary origins of sociality and how those traits might have contributed. Manipulative studies should then be undertaken for the purpose of assigning causality to the findings of the comparative work. This approach will allow the comparison of multiple traits across a lineage and will allow researchers to provide robust answers to broad evolutionary questions about sociality.
The great variation in factors contributing to social evolution is likely to differ among species. For this reason it is imperative that research effort is spread across a large number of species in order to gain a truly comprehensive understanding of the role that these factors play in the evolution of sociality. Comparisons across multiple species would be best performed when focused observational or experimental data has been gathered under the same theoretical framework. The majority of studies of social group living have so far focused on species of birds, mammals and insects with comparatively little attention given to ectothermic vertebrates with the exception of one notable family of freshwater fishes (Elgar, 2015; Figure 1). Inclusion of understudied animal groups is important for our ability to assess the universality of frameworks of social evolution and to gain novel insights as a result, especially when these species display uncommon traits or unconventional life-histories. For instance, the ability of many social marine fishes to change sex may have interesting implications for hypotheses regarding an individual's ability to acquire a mate and hence on its decision disperse or remain within a group. Likewise, comparisons of long-lived social reptiles and avian lineages could lend support to hypotheses examining the role that longevity plays in the evolution of sociality. In this review, we assess the major theoretical framework in this field, highlight the advantages and disadvantages of the different methodologies used to test existing theory, and discuss developments made in less-well studied social systems with the aim of galvanizing a more holistic integration of multiple techniques and taxa into future studies of social evolution.
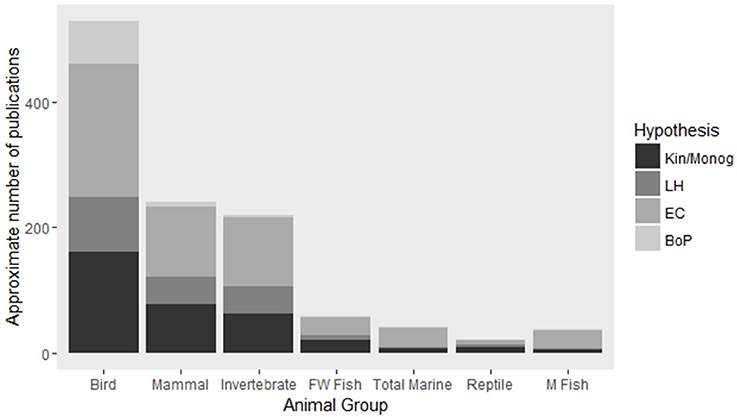
Figure 1. Approximate number of articles published on major animal groups focusing on four key hypotheses of cooperative breeding. Abbreviations are: Kin, Kinship; Monog, Monogamy; LH, Life-history; EC, Ecological Constraints; BoP, Benefits of Philopatry; FW, Freshwater; M, Marine. Search parameters are available in Supplementary Table S1. Numbers presented here are intended as approximations only as search parameters were not completely mutually exclusive or exhaustive.
Theoretical Framework
Group Living as a Major Transition
The evolution of sociality in animals may be considered as one of the most recent evolutionary transitions according to Szathmáry and Smith's (1995) major evolutionary transitions theory. This theory examines the idea that major evolutionary transitions occur when groups of “individuals” come together to form more complex forms of life. This theory explains the evolution of all life from individual biological molecules through to colonies of eusocial multicellular animals (Bourke, 2011). The evolution of cooperation was a necessary step along the path toward eusociality. There is a continuum of cooperation among group members in animal societies and the degree of cooperation displayed is likely to depend on a range of life-history, social and ecological factors (Kokko et al., 2002; Buston and Balshine, 2007).
Reproductive Skew Theory
Reproductive skew theory offers a potential general theory for social evolution through competitive effects and conflict resolution. Reproductive skew theory views reproduction as a limited resource and focuses on the distribution of reproductive shares within the group (Emlen, 1982b; Reeve and Ratnieks, 1993; Johnstone, 2000). Groups with one or a few dominant breeders fall at the “highly skewed” end of the spectrum while aggregations where any individual can breed with any other individual would be considered to have “low skew.” In this review we will restrict our discussion to groups with dominant breeders and one or more non-breeding subordinates, i.e., high reproductive skew societies.
Cooperative Breeding Theory
Cooperative breeding theory (Brown, 1974) is derived from Hamilton's rule (Hamilton, 1964; Grafen, 1982; Bourke, 2014) and describes the evolution of social systems in which reproductively mature individuals delay their own independent breeding in order to remain within a group as non-breeding subordinates and help to raise the offspring of dominant breeders. Cooperative breeding groups are generally characterized by high reproductive skew. Offspring of such groups often, but not always remain on the natal territory and many groups are therefore comprised of subordinates related to the dominant breeders, in which case relatedness is high (in Hamilton's rule) and the likelihood of cooperative actions being selectively favored is raised (Bourke, 2014). However, a growing number of studies have revealed social systems where non-breeding subordinates disperse to other groups and are unrelated to the dominant breeders (Double and Cockburn, 2003; Gardner et al., 2003; Awata et al., 2005; Dierkes et al., 2005; Wong, 2010; Riehl, 2013). In these cases, cooperative rearing of young may still take place as well as other forms of cooperative behavior in order to avoid conflict and maintain a stable group structure (Gardner et al., 2003; Wong et al., 2007). While these latter groups may not strictly fit the definition of a cooperatively breeding group if they do not provide alloparental care, cooperative breeding theory forms a rich framework with which to assess the circumstances that could lead to an individuals' decision to forgo its own reproductive opportunities and remain in a group as a non-breeding subordinate (Emlen et al., 1991; Koenig et al., 1992).
Cooperative breeding theory encompasses several non-mutually exclusive hypotheses for the evolution of sociality (Table 1). Cooperative breeding theory can be applied to two broad areas of social behavior—the evolution of group living and the evolution of cooperation (Koenig et al., 1992). This review will focus primarily on those studies addressing the evolution of group living so as to incorporate studies where subordinate individuals remain in groups but do not provide any active forms of help to dominant breeders (e.g., Eden, 1987; Gardner et al., 2003; Wong and Buston, 2013; Buston and Wong, 2014; Drobniak et al., 2015). In groups where subordinates do not provide active help, dominant group members may still tolerate their presence. Actions such as regulation of growth may facilitate group stability in groups where active subordinate help is absent (e.g., Wong et al., 2007). Whether or not help is later provided, the first step of this evolutionary strategy is an individuals' decision of whether to disperse and pursue its own breeding opportunities or to delay such opportunities in order to obtain the benefits of group living (Emlen, 1982a). Furthermore, the factors involved in the evolution and maintenance of sociality and in the development of helping behavior are often the same (e.g., Groenewoud et al., 2016). The hypotheses comprised within cooperative breeding theory may therefore be useful to study social systems in which non-breeding subordinate members cooperate in some form regardless of relatedness or whether active help is provided in the care of offspring.
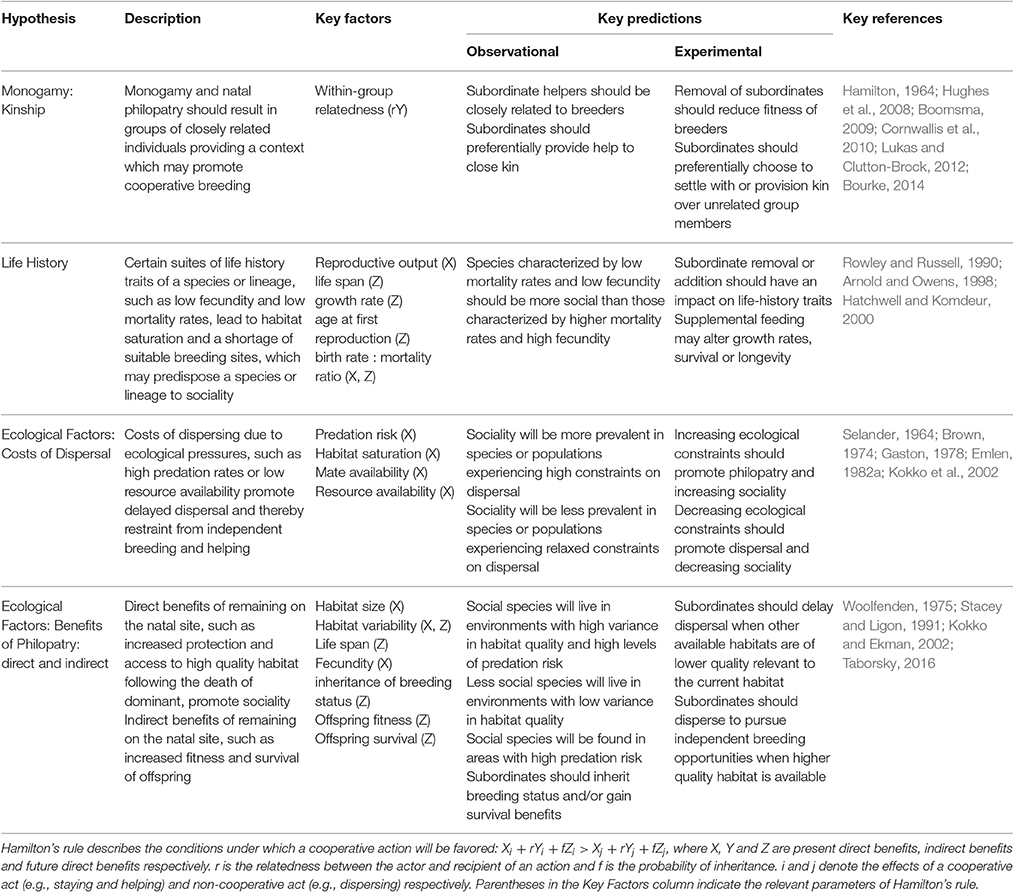
Table 1. Four of the major hypotheses of Cooperative Breeding Theory and the respective key factors proposed to influence the evolution of sociality.
Methodological Approaches
Many studies have focused on testing four key hypotheses of cooperative breeding theory (Table 1) using broad comparisons of relevant ecological, social and life history variables across multiple species of birds, mammals and insects (Cockburn, 1996; Arnold and Owens, 1998; Johnson et al., 2002; Purcell, 2011). Essentially, these studies have investigated the evolution of sociality by phylogenetic comparative analysis, comparing differences in key variables between multiple social and asocial species within a given lineage. While such contrasts enable broad generalizations to be made, they fall short of identifying causality of effects. In contrast to this methodology, studies that have tested these hypotheses through refined experimental manipulation of characteristics associated with the evolution of sociality (Komdeur, 1992; Baglione et al., 2002; Wong, 2010) do demonstrate causality, but their necessary focus on just one or a few species greatly reduces the ability to draw general conclusions. Therefore, it is through using a combination of these approaches for a given lineage that holds the potential to provide an insight into the generality and causality of sociality across a broad range of species (Figures 1, 2). While many studies do combine observational and experimental methodologies (e.g., Komdeur, 1992; Stiver et al., 2005) we suggest that great advances could be made by following such work with comparative studies. This would work most efficiently if the observational and experimental studies were specifically designed with comparative analysis in mind.
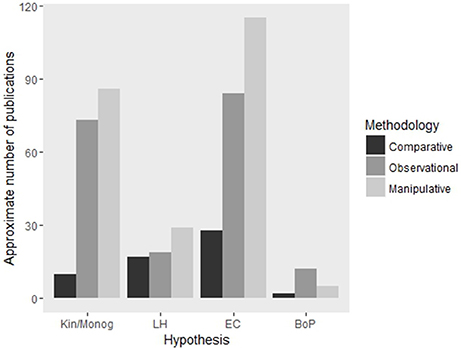
Figure 2. Approximate number of articles published on each of the major hypotheses using comparative, observational and experimental methodologies. Abbreviations are: Kin, Kinship; Monog, Monogamy; LH, Life-history; EC, Ecological Constraints; BoP, Benefits of Philopatry. Search parameters are available in Supplementary Table S1. Numbers presented here are intended as approximations only as search parameters were not completely mutually exclusive or exhaustive.
Comparative Analyses and Syntheses
Comparative analyses are used to compare traits across multiple taxa or populations across multiple geographic locations and may range in taxonomic scale from studies within a genus to studies across phyla (e.g., Blumstein and Armitage, 1999; Boomsma, 2009; Jetz and Rubenstein, 2011). They may draw upon the findings of other observational and/or manipulative studies (Cockburn, 2006) or they may make use of novel data (Du Plessis et al., 1995). Combining this comparative approach with phylogenetic information is arguably one of the most powerful methods with which to examine broad evolutionary trends and patterns (Arnold and Owens, 1998; Briga et al., 2012). Comparing ecological, life-history, morphological and/or behavioral traits across multiple taxa in a molecular phylogenetic context may allow researchers to examine the evolutionary history of many different attributes and identify ecological, social, morphological and behavioral differences between social and non-social species (Ford et al., 1988; Pagel and Harvey, 1988; Arnold and Owens, 1998; Cornwallis et al., 2010). In turn, the differences that are detected may provide an insight to the conditions under which sociality (or other traits) may have evolved. In this way, future observational and experimental studies could be targeted at specific sets of species within the lineage showing variation in sociality and traits of interest. Understanding the causes of these variations (only achievable through experimental manipulations) could provide specific mechanisms that have caused the observed social systems in these socially contrasting species.
One issue arising from the comparison of a trait across multiple taxa within a given lineage is that the individual species are part of a hierarchical structure. That is, they are related by a common ancestor and therefore not independent. Felsenstein (1985) discussed this issue and proposed a method to overcome the non-independence of species which he terms “phylogenetically independent contrasts.” Essentially, while the species themselves may not be independent, the contrast (or difference) between pairs of species in the trait being measured is independent. This method requires a fully resolved phylogeny of the lineage and a model of evolutionary change. Other authors have since improved upon this method to enable the use of partially resolved phylogenies (Garland et al., 1992; Pagel, 1999; Freckleton et al., 2002). For this reason, comparative analyses are particularly well suited to taxa with well-studied phylogenies or for which genetic material can be easily obtained. Thus far, the majority of comparative studies have focused on terrestrial taxa which has resulted in many great advancements in the field (Brown, 1974; Arnold and Owens, 1998; Boomsma, 2009; Riehl, 2013). However, marine organisms are relatively understudied in terms of comparative work, which is unfortunate as they offer a rich diversity of social organization and varied ecological niches and life-history strategies with which to explore the various hypotheses of social evolution and maintenance (McLaren, 1967; Gowans et al., 2001; Duffy and Macdonald, 2010; Wong and Buston, 2013). Given the great variety of social organization displayed in these organisms, there is clearly enormous potential to test and challenge terrestrially derived cooperative breeding hypotheses under novel conditions.
A variety of studies have so far demonstrated the increasing availability and ease of phylogenetic analyses as a powerful tool to conduct comparisons across multiple taxa. Arnold and Owens (1998) employed this technique in a comparative analysis of 9,672 bird species representing 139 families to demonstrate that cooperative breeding was not randomly distributed amongst avian taxa, and in fact showed an uneven geographic distribution of “hotspots” of cooperatively breeding species which the authors considered could infer some common biological predisposition to this system. Similarly, Edwards and Naeem (1993) found that cooperative breeding in birds was not randomly distributed among taxa in a meta-analysis of avian cooperative breeding including phylogenetic simulations of ancestral states. Most recently, this non-random phylogenetic distribution of cooperative breeding amongst avian taxa has been confirmed in a comprehensive review of modes of parental care amongst the avian phylogeny (Cockburn, 2006). Another phylogenetic comparison of 44 species of mammals found that there was a strong phylogenetic signal for allomaternal care (multiple females assisting a dominant female in maternal care duties), in other words, that cooperative breeding in the form of allomaternal care was strongly clustered (Briga et al., 2012). This finding is similar to the non-random phylogenetic distribution of cooperative breeding observed in birds (Edwards and Naeem, 1993; Arnold and Owens, 1998; Cockburn, 2006) suggesting that cooperative breeding is strongly clustered in birds and mammals. These studies demonstrate the effectiveness of phylogenetic comparative analyses for uncovering broad trends across multiple species. With molecular genetic techniques becoming increasingly available, it is more feasible for researchers to conduct phylogenetic comparative studies and to incorporate them into a research program alongside observational and experimental studies. The piecemeal approach widely used at the time of writing, while highly effective at advancing our knowledge of the evolution of sociality, could be made more efficient if finer scale observations and experiments were specifically designed around planned comparative work. This comparative work can then be used to more effectively target research effort on sets of species which contrast in their degree of sociality and in other traits of interest.
Monogamy and Kinship
Studies of the relationship between monogamy, kinship and sociality have championed the use of phylogenetic comparative analysis to test entrenched theory. In particular, the idea that monogamous breeding systems lead to high levels of relatedness amongst subordinates which in turn promotes sociality has been suggested comparatively for insect, bird and mammalian societies (Hughes et al., 2008; Boomsma, 2009; Cornwallis et al., 2010; Lukas and Clutton-Brock, 2012). For example, Cornwallis et al. (2010) conducted a comparative analysis of 267 birds and showed that species displaying high levels of promiscuity (i.e., polygamous species) were less likely to transition to cooperatively breeding systems. Furthermore, this study showed that lineages that evolved cooperative breeding systems and subsequently reverted to independent breeding systems had more promiscuous ancestors (Cornwallis et al., 2010). Similarly, Hughes et al. (2008) concluded that monogamy was critical in the evolution of eusociality in a comparative analysis of 267 species of eusocial bees, ants and wasps. Boomsma (2009) later reviewed monogamy and eusociality in insects and found that all of the evidence at the time of writing indicated that eusocial insect societies with sterile worker castes only arose in lineages with monogamous parents.
High levels of kinship due to monogamous associations may certainly predispose a species to cooperative breeding, but the emerging number of cases of cooperative breeding amongst unrelated group members suggests that direct benefits from group living and cooperation must be considered (Riehl, 2013; Bourke, 2014). In a review of 213 cooperatively breeding birds, Riehl (2013) suggested that as much as 15% of these species nest with unrelated individuals. These individuals are clearly not gaining inclusive fitness benefits and must therefore be accruing sufficient direct benefits, either presently or in the future, to offset the costs associated with group living. However, the majority of species in this study did nest with related individuals. Therefore, monogamy and kinship likely played a significant role in the evolution of cooperative breeding in these species. It should also be noted that living in groups of close kin may involve costs due to deleterious inbreeding effects and many group living species have developed behaviors to avoid this (costs of inbreeding are discussed in Lubin and Bilde, 2007). Thus far little comparative work has taken place to examine the evolution of sociality amongst groups of unrelated individuals (but see Groenewoud et al., 2016 for an intraspecific comparative analysis). Social marine species with a pelagic larval stage present an excellent avenue for future comparative work in this area as the mixing of larvae in the water column makes settlement in family groups highly unlikely.
Comparative studies have substantially contributed to our understanding of the role that kinship and monogamy has played in promoting the evolution of sociality. However, there is a bias toward terrestrial taxa in the comparative literature which confines our understanding of the factors involved in the evolution of group living to relatively conventional breeding strategies (Figure 1). Social marine fishes are particularly interesting as many species undergo a pelagic stage in their life-cycles, whereby larvae are mixed in the water column and eventually settle onto a habitat. This mixing of larvae means that social groups formed by these species are unlikely to consist of related individuals (Avise and Shapiro, 1986; Buston et al., 2007, 2009). Cooperative rearing of young does not appear to occur in the species studied to date which supports the idea that kinship is a major factor in the evolution of helping but may be less influential in the development of group living. Direct fitness benefits however, likely play a greater role in social group formation and maintenance in these species (Wong and Buston, 2013; Buston and Wong, 2014) and there is a need for more comparative studies focusing on these benefits and their role in the evolution of sociality. In any case, such examples of non-kin social groups are the minority in terrestrial systems which have typically shown strong support for monogamy and kin selection as key factors in the evolution of group living and cooperative breeding.
Life-History Hypothesis
Akin to the reasoning that monogamy creates the necessary conditions for cooperative breeding to evolve through kinship based mechanisms, life-history traits such as longevity are thought to promote favorable ecological conditions for the evolution of sociality. Comparative work in this field has informed much of the debate surrounding the life-history hypothesis. Based on their comparative analysis, Arnold and Owens (1998) proposed that low annual mortality was the main factor predisposing avian species to cooperative breeding—a key prediction of the life-history hypothesis (Table 1). This proposition was questioned by Blumstein and Moller (2008) based on their comparative study of 257 North American birds. Their study controlled for body mass, sampling effort, latitude, mortality rate, migration distance and age at first reproduction (factors which Arnold and Owens, 1998; had not accounted for), and found no association between sociality and increased longevity per se. Blumstein and Moller (2008) note however, that longevity and sociality are often confounded with other life-history factors, such as reproductive suppression, delayed breeding, increased parental care and survival, suggesting the need for further comparative research into these factors. Similarly, a more recent comparative meta-analysis of mammalian phylogenies found no support for longevity playing a part in the transition from independent breeding to cooperative breeding in mammals (Lukas and Clutton-Brock, 2012). Instead, they found that cooperative breeding only occurred in mammalian lineages displaying monogamy and polytocy (multiple offspring per birth). However, using Australian Scincid lizards (genus Egernia) as model species, Chapple (2003) demonstrated that several species of this genus were shown to exhibit life-history traits (increased longevity and age at maturity) associated with similar levels of sociality to those found in avian taxa, suggesting that life history traits could still play a role in some vertebrate groups. From these varied results it seems clear that the role that life-history plays in the evolution of group living is likely to be taxonomically specific which highlights the need to assess life-history factors and sociality across a broad range of taxa and to incorporate species which display unusual life-history strategies.
Besides the latter example, there appears to be relatively little support for the life history hypothesis, at least from comparative studies. However, the majority of comparative analyses have focused on the relationship between longevity and sociality, a single case among a myriad of potential life-history traits that could have influenced the evolution of sociality (Blumstein and Moller, 2008). Given the potential role that life-history traits may have played in setting the stage for the evolution of sociality, phylogenetically independent contrasts across multiple species combined with more focused observational and experimental (where possible) studies would be a useful method for future research in this area. Also, species with less conventional life-history strategies, such as small body size, high mortality rates, sex change and indeterminate growth, all traits exhibited by a range of marine fishes (Munday and Jones, 1998; Munday et al., 1998; Wong et al., 2005; Depczynski and Bellwood, 2006), have thus far received little attention (Figure 1). To this end, social habitat specialist fishes would make particularly good study species for comparative analysis, especially given that several groups have already well resolved phylogenies (e.g., Herler et al., 2009; Thacker and Roje, 2011; Duchene et al., 2013).
Ecological Factors
While monogamy and life-history traits may create ideal conditions for social evolution, ecological factors may ultimately determine which species display social behavior. Comparative analyses are ideal for the study of large scale environmental influences on the evolution of sociality since their very aim is to compare patterns across multiple taxa or within a single species over large geographic areas. Such analyses have demonstrated that there is a non-random geographic distribution of sociality in a variety of taxa (Jetz and Rubenstein, 2011; Purcell, 2011). For example, Purcell (2011) conducted an extensive review of the literature pertaining to arthropod sociality along latitudinal and altitudinal gradients, and reanalyzed five previous case studies of social spiders and four ant subfamilies. It was found that climatic factors were correlated with variation in colony size, with social arthropod species occurring more frequently at lower latitudes. Such geographic hot-spotting of cooperative breeding was also recognized by Jetz and Rubenstein (2011), who conducted a global comparative analysis of sociality for 95% of the world's bird species. They found that temporal (among-year) variability in precipitation was a major predictor of the occurrence of cooperative breeding. Together, these studies demonstrate the effectiveness of comparative analyses in identifying likely environmental factors involved in the evolution of sociality, suggesting that broad scale environmental characteristics, such as rainfall, temperature, predator abundance and the size and availability of food resources may be important in the evolution of sociality in a diverse range of animals.
Some comparative studies have also shown that cooperative breeders are more likely to occur in temporally variable (unstable) environments (Rowley, 1968; Grimes, 1976; Jetz and Rubenstein, 2011). In contrast, other studies have shown a greater occurrence of cooperatively breeding species in less temporally variable (stable) environments (Brown, 1974; Ricklefs, 1975; Woolfenden, 1975; Ford et al., 1988). Emlen (1982a) sought to reconcile this discrepancy in ecological observations of cooperatively breeding birds with his ecological constraints model. The ecological constraints hypothesis focuses on the ecological characteristics of a species' environment that may prevent group members from dispersing (Emlen, 1982a). Emlen (1982a) proposed that the common thread in these opposing observations was that individuals were faced with the decision of either dispersing to pursue independent breeding opportunities or to remain at the nest as a non-breeding subordinate. Either environmental condition (stable or unstable) could sufficiently restrict an individual's success in dispersing and pursuing independent breeding opportunities and thus “force” them to remain at the nest. For example, in stable environments, populations of animals may expand and preferable breeding habitat could quickly become saturated (e.g., Schradin and Pillay, 2005). In this situation, dispersal due to limited opportunities for successful independent breeding options is constrained. Alternatively, in unstable environments, the benefits of remaining at the nest may be greater than dispersing and rearing young independently, which is what Stacey and Ligon (1991) subsequently coined as the benefits of philopatry hypothesis.
Environmental variability is likely linked to the availability of food resources which has also been shown to be a constraint on dispersal and hence a factor of interest in the evolution of cooperative breeding (Rubenstein and Lovette, 2007). A comparative analysis conducted by Du Plessis et al. (1995) investigated 217 South African birds comprising 175 non-cooperative breeding species and 25 obligate and 17 facultative cooperative breeding species. Based on the findings of their study, Du Plessis et al. (1995) proposed that obligate and facultative cooperative breeding systems had evolved independently under different ecological circumstances. Obligate cooperative breeders tended to live in predictable habitats where year-round food availability was sufficient to sustain permanent groups and benefited by increasing survival from predation. Facultative cooperative breeders, on the other hand, lived in less predictable environments where food limitations negated the formation of stable groups, with cooperative breeding occurring in years of higher food availability, suggesting that benefits gained were predominantly related to reproduction rather than survival.
Many of the comparative analyses discussed thus far have focused on broad scale environmental patterns, and the availability of resources. One area that appears to be distinctly lacking is risks of dispersal. One notable exception to this observation is an intraspecific comparative analysis of the African cichlid Neolamprologus pulcher by Groenewoud et al. (2016) which examined predation risk and its interaction with other ecological factors such as shelter availability and population density across eight populations. This study concluded that predation risk was a significant driver of group formation and the evolution of complex social behavior. Comparative analyses appear to be well suited to examine risks of dispersal as a mechanism of ecological constraint on dispersal. For example, one might expect that dispersal would be more risky in arid environments where foraging success is enhanced by group size, as predicted in the aridity food-distribution hypothesis (Faulkes et al., 1997; Spinks et al., 2000; Ebensperger, 2001). Aridity is a large-scale environmental factor linked to precipitation which, as previously discussed, has been well studied through comparative analyses. While the paucity of comparative studies specifically addressing dispersal risk appears to be a significant gap in the literature, it should be noted that some comparative analyses may touch on risks of dispersal through other mechanisms such as increased benefits of philopatry gained through predator defense (e.g., Ebensperger, 2001). Such constraints on dispersal may also increase the benefits of remaining philopatric through increased survival.
Benefits of philopatry can be gained through either direct fitness benefits (e.g., survival, growth, predator detection, dilution or competitive advantage) or indirect benefits (e.g., increased fitness and survival of offspring). While many comparative analyses have examined the ecological factors that constrain dispersal and hence promote natal philopatry (Emlen, 1994; Hatchwell and Komdeur, 2000; Lucia et al., 2008), none have explicitly focused on the benefits of philopatry hypothesis on its own. Instead, discussion of benefit based mechanisms of social evolution and maintenance are from studies examining the effects of multiple ecological factors. Much support for benefit based models has come from the mammalian literature, especially rodents, particularly in relation to the thermoregulatory benefits of huddling (Hayes, 2000; Ebensperger, 2001; Solomon, 2003). Ebensperger (2001) suggested that comparative methods should be used for future studies of the evolution of rodent sociality and that they should simultaneously weigh the constraints and benefits associated with group living. The concept that these hypotheses are not mutually exclusive led Hayes (2000) to propose a “pup defense—animal density hypothesis” in a review of communal nesting in rodents. This hypothesis explores the idea that the benefit of pup defense generally increases with group size (Manning et al., 1995), but this benefit must be weighed against the potential constraint of the increased probability of infanticide by conspecifics locating the nest, which is more likely at higher animal densities (Wolff, 1997).
It is clear that ecological factors are influential in determining the costs and benefits of remaining philopatric and hence group-living, though much debate remains over which particular ecological factors provide sufficient benefits or constraints for sociality and subsequent cooperative breeding to evolve and be maintained. Comparative analyses have proven a useful tool with which to identify these benefits and constraints as cooperative breeding species likely share similar benefits or occupy similar ecological niches.
Other Hypotheses
Much of the work discussed thus far has focused on the roles of kinship, life-history and ecological factors. While these factors tend to dominate the literature (Figure 2), there are alternative hypotheses such as group augmentation (Kokko et al., 2001), which examines the benefits conferred to breeders in the group from maintaining a number of subordinate helpers at the nest (i.e., breeders actively recruit or even kidnap subordinate group members) rather than focusing on constraints placed upon subordinate dispersal from a group, or the ecologically-associated benefits conferred to subordinates of remaining at the nest. Such alternative hypotheses should also be considered when examining mechanisms of social evolution. Thus far, no comparative analyses have explicitly addressed group augmentation as a mechanism of social evolution and maintenance, but the hypothesis was the subject of a review by Kingma et al. (2014) who formalized a clear conceptual framework to guide future empirical work in the area. Several comparative studies have also alluded to group augmentation effects such as increased survival (and hence greater lifetime reproductive output) through group defense or predator detection (the “many eyes hypothesis”) (Ebensperger, 2001; Ridley and van den Heuvel, 2012).
Multiple Factors
Although the ecological constraints, benefits of philopatry and life-history hypotheses have so far been dealt with separately in this review, it is important to note, as many of these studies have done, that ecological and life-history factors are not mutually exclusive and often act in concert and alongside other evolutionary selective forces. Multiple factors likely have varying influences on different species. It is therefore paramount that these factors are studied in concert across a range of lineages if we are to gain a truly representative view of how sociality evolved and is maintained. Comparative analyses and syntheses are well placed to advance the study of social evolution in this way.
For example, the comparative analysis conducted by Arnold and Owens (1998) suggested that while life-history traits such as longevity predisposed avian lineages to cooperative breeding, ecological constraints might then determine which species would benefit from cooperative breeding behavior (and hence determine whether cooperative breeding was actually expressed in a given lineage). While this explanation accounted for the patchy phylogenetic distribution of cooperative breeding, it did not fully explain why species within the same lineages varied so markedly in their social behavior. Hatchwell and Komdeur (2000) coined a “broad constraints hypothesis,” whereby life-history traits and ecological constraints acted together causing a broad constraint on the turn-over of breeding opportunities of a species, a concept originally proposed by Ricklefs (1975). This broad constraints approach was also echoed by Solomon (2003) in a review of factors influencing philopatry in rodents. These studies show that broad constraints on breeding opportunities explain the variation in cooperative behavior observed in species exhibiting similar life histories and inhabiting similar ecological niches. Blumstein and Armitage (1999) argued that ecological factors such as harsh environmental conditions, and food availability drove life-history characteristics such as growth rates and age of maturation. They found that marmots living in harsh environments delayed dispersal past a reproductive maturity index which resulted in the formation of extended family groups, further highlighting the link between ecological factors and life-history in the formation of family groups.
Although these examples demonstrate the effectiveness of comparative analyses in studying multiple factors of social evolution, to date they have only focused on the interplay of ecological and life-history traits. There is clearly a need for more comparative studies focusing on integrating additional factors as well, such as kinship and group augmentation (Figure 2). Furthermore, comparative studies are not capable of showing causation. To gain this level of understanding researchers should aim to follow comparative work with manipulative experiments. The comparative analysis can be used to target these experiments at sets of species contrasting in sociality.
Observational Studies
Observational studies covered in this section refer to those that are correlative and focus on a small subset of species, often a single species, as opposed to the comparative analyses which examine broad scale patterns across multiple taxa or manipulative experiments which are capable of demonstrating causality. For these reasons, observational studies should be augmented by comparative and experimental work to gain a holistic view of social evolution. Observational studies are particularly well suited to investigating animals which live in groups on discrete habitat patches or well defined territories (e.g., Nam et al., 2010; Marino et al., 2012). Similar to comparative analyses, there is a pervading taxonomic bias in observational studies leaning toward terrestrial taxa (Figure 1). Species with less conventional life-histories, often seen in marine taxa, are relatively understudied yet could shed new light on the evolution and maintenance of sociality. Habitat specialist fish are particularly well suited to observational work as they are widely distributed on coral reefs, display a wide variety of social organization and live on discrete habitat patches (Buston, 2003b; Wong et al., 2005). Additionally, many are demersal spawners and as such provide a convenient measure of fecundity through egg counts (Herler et al., 2011).
Finer scale observational studies are useful for examining intraspecific variation in cooperative breeding behavior, which may be overlooked in comparative analyses (Schradin and Pillay, 2005; Sorato et al., 2012). Additionally, the comparative analyses discussed above often rely on the data provided by finer scale observational studies. For example, Cockburn's (1996) breeding data was compiled from 20 different studies in order to compare ecological correlates of cooperative breeding in a group of Australian birds (Table 1 in Cockburn, 1996). Alternatively, other studies such as Ridley and van den Heuvel (2012) have used comparative methods to identify a trend to focus on and subsequently conduct finer scale observational analyses. In both methodologies, detailed observational data from a subset of species has played a key role in informing discussion on the evolution of sociality. Furthermore, many observational studies can be performed over large temporal scales (e.g., Rubenstein, 2011; Marino et al., 2012), which is often logistically impractical for experimental manipulations and typically outside of the aims of such research (though multi-generational experimental manipulations may be an option for researchers wishing to demonstrate evolutionary mechanisms). Such long-term data is extremely important in the study of sociality, especially when species are subjected to seasonal or other temporal fluctuations in their ecology or behavior.
Monogamy and Kinship
Kinship based models of cooperative breeding propose that helpers should maximize their indirect genetic benefits by preferentially helping descendent or close kin. Testing this hypothesis requires knowledge of the relatedness of individuals in a population. This can be achieved through observation of group history of the study population or by inferring relatedness by comparing genetic markers. Microsatellite markers have thus far tended to be the preferred tool for genetic inference of relatedness. However, more recently, single nucleotide polymorphisms (SNP's) have emerged as a potential alternative as the markers tend to be cheaper and easier to develop than microsatellites (Weinman et al., 2015). Genetic inference of pedigree is not always straightforward, especially when researchers have difficulty in determining the relative frequency of kin/non-kin in the population, which is often the case in wild populations in which the dispersal or settlement of offspring cannot be directly observed (e.g., fish with a pelagic larval phase). Combining observations of group history with genetic inference is an effective method of determining relatedness and many studies have used this approach (e.g., Wright et al., 1999; Legge, 2000; Clutton-Brock et al., 2001; Dierkes et al., 2005). However, when such observational data is not available, researchers must rely on genetic tools alone (e.g., Buston et al., 2009). A number of estimators of pair-wise relatedness have been proposed (Lynch and Ritland, 1999; Van De Casteele et al., 2001), but these estimators still rely on sound knowledge of the true frequency distribution of relationship in the population in order to determine the likelihood that two individuals are indeed related (Buston et al., 2009, Supplementary Material). If this requirement can be fulfilled, genetic inference of relatedness is a powerful method for studying the effects of kinship on the evolution of sociality.
These methods have been used to demonstrate preferential provisioning of close kin in many species such as long-tailed tits (Nam et al., 2010), carrion crows (Canestrari et al., 2005), and gray mouse lemurs (Eberle and Kappeler, 2006). However, there is some ambiguity as to whether related individuals actively choose to remain philopatric and provision care to related young in order to maximize indirect benefits, or whether family groups form due to some direct benefit of remaining at the natal habitat and the provision of help to close kin is merely a result of nesting in family groups. Observational studies have played a key role in informing this debate. For example, Nam et al. (2010) examined the investment of helpers of the cooperatively breeding long-tailed tit, Aegithalos caudatus, using group history pedigrees and microsatellite genotypes from a 14 year field study to show that investment by helpers increased with relatedness. Likewise, Bruintjes et al. (2011) found that subordinate cichlids, Neolamprologus pulcher, raised their levels of helping behavior when they had bred successfully and their offspring were present in the clutch. In another observational study, Canestrari et al. (2005) found that among a cooperative breeding population of carrion crows, Corvus corone corone, genetic parents fed chicks at greater rates than helpers with no parentage. However, the nests often contained the offspring of several breeding individuals, and the amount of feeding was not proportional to the number of offspring in the nest. This may indicate that carrion crows do not have a mechanism to recognize close kin and/or that costs associated with provisioning unrelated chicks may be low.
Conversely, in mammalian lineages, cooperative breeding in the form of allosuckling represents a high energetic investment to mothers. Eberle and Kappeler (2006) documented this behavior during a long-term observational study of a population of gray mouse lemurs (Microcebus murinus). Microsatellite analyses showed that groups consisted of related individuals and their observations showed a high mortality rate of both adults and juveniles in this species. Eberle and Kappeler's (2006) study indicated that female gray mouse lemurs possess a kin recognition mechanism, regularly discriminating their own offspring over the offspring of other females in communal nests, but provisioned allomaternal care and in some instances, adopted the young of other related individuals in the case of their mother's death. The provision of care however, was more often directed toward direct descendent pups and pups suckled more at their own mothers. Eberle and Kappeler (2006) concluded that kin selection was most likely the main selective force behind this cooperative breeding system which provided “family insurance” in the face of high mortality risk in this species.
In contrast, other observational studies have found little evidence to support a relationship between relatedness and helping behavior (Wright et al., 1999; Legge, 2000; Clutton-Brock et al., 2001; Wong et al., 2012). For example, in a six year observational study of meerkats (Suricata suricatta), Clutton-Brock et al. (2001) assessed the individual contributions of helpers toward relatives. They found that individual variation in the amount of food that helpers gave to pups was related to individual foraging success, sex and age rather than relatedness to the pups. Similarly, in a population of Arabian babblers, Turdiodes squamiceps, Wright et al. (1999) found little effect of relatedness on feeding rates or load sizes using three different measures of relatedness. Cooperatively breeding kookaburras (Dacelo novaeguineae) also did not invest in higher provisioning or incubation at nests of related individuals (Legge, 2000). Instead, individuals in larger groups provisioned less food to chicks regardless of relatedness. Since food provision represents a high energetic cost in this species, Legge (2000) believed that larger groups of kookaburras may gain direct fitness benefits through higher survival and hence greater life-time reproduction by “load lightening” when more helpers were available rather than indirect genetic benefits via kin selection. Similarly, Wong et al. (2012) found that while helpers were indeed more related to breeders in monogamous than polygynous mating systems, they did not provide more help in the cooperatively breeding cichlid, Neolamprologus pulcher. However, Stiver et al. (2005) found that other factors acted alongside kinship effects to determine helping behavior in the same species. They showed that relatedness, although not the only driver in helping behavior, still plays a role in the amount of help provided by subordinate N. pulcher.
It is evident from these studies and others that the evolution of sociality through kinship based processes is likely to be species specific. However, the true specificity of these processes cannot be determined unless subsequent comparative work is undertaken. Furthermore, the question of whether the provisioning of close kin is a cause or a consequence of kinship based group formation can only be disentangled using manipulative experiments. Nevertheless, these observational studies highlight the importance of the relationship between genetic relatedness and helping behavior, uncovered using either group history information, genetic inference or both, to examine whether kinship might have been a driver of sociality in these species.
Life-History Hypothesis
The importance of longevity in the evolution of cooperative breeding has been demonstrated by Rowley and Russell (1990) in a long term observational study of Splendid Fairy-Wrens (Malurus splendens). In this study, Rowley and Russell (1990) monitored color banded groups of Splendid Fairy-wrens (long-lived cooperative breeders) between 1973 and 1987. Rowley and Russell (1990) pointed out that the available habitat tends to become saturated in longer lived species which restricts independent breeding opportunities. In a study conducted on Australian skinks, Egernia stokesii, Duffield and Bull (2002) highlighted the similarity in life-history characteristics and group formation in cooperatively breeding birds and mammals. Duffield and Bull (2002) considered that the longevity of these skinks caused the finite number of available rock crevices to become saturated, constraining dispersal and promoting group living. Kent and Simpson (1992) also describe eusociality in a particularly long lived beetle, Autroplatypus incompertus, though it is not clear whether this longevity is a cause of the social structure.
Theoretically, the rate of development may also influence the evolution of sociality through delayed dispersal as animals exhibiting slower development and lower growth rates likely require extended parental care (Solomon, 2003). While there is some support for this hypothesis (Burda, 1990), several observational studies of growth rates in mammals tend to view this life-history trait as a consequence of sociality rather than a potential cause (Oli and Armitage, 2003; Hodge, 2005). Nevertheless, these studies show the usefulness of observational methodology in informing discussions surrounding the role that life-history traits may or may not have played in the evolution of sociality. However, as observational studies are not able to show causality, it is difficult to determine whether changes in life-history are a cause or a consequence of sociality. This limitation may be mitigated if the observational work is later tested with experimental manipulations. Supplemental feeding or food restriction experiments (e.g., Wong et al., 2008b; Bruintjes et al., 2010 respectively) may be capable of altering growth rates or overall body condition and hence longevity in some species and as such may be capable of disentangling cause from consequence especially if it is possible to maintain over multiple generations. The relative ease with which observational studies can be conducted over long periods makes them a valuable method to use to study the role of life-history traits in the evolution of group living and complex social behavior.
Ecological Factors
Finer scale observational studies are also excellent for examining ecological correlates of sociality such as predation risk and habitat saturation. Since such studies usually occur in situ, they are valuable for providing a view of the relationship between sociality and ecology under natural conditions. Sorato et al. (2012) investigated the effects of predation risk on foraging behavior and group size in the chestnut-crowned babbler, Pomatostomus ruficeps, and found that larger groups were less likely to be attacked by a predator. Sorato et al. (2012) proposed that predation was therefore likely to be a key factor promoting the evolution of group living in Pomatostomus ruficeps. Curry (1989) examined patterns of sociality and habitat availability amongst four species of allopatric Galapagos mockingbirds (Nesomimus spp.) and found that species constrained by a lack of available habitat maintained cooperatively breeding social groups. Similarly, Schradin and Pillay (2005) found that group formation in arid populations of the African striped mouse, Rhabdomys pumilio, was likely caused by habitat saturation. They also suggest that group living benefits such as increased vigilance against predators and thermoregulation could be important factors in promoting philopatric behavior.
As is the case for comparative analyses, there appear to be fewer observational studies examining the effects of dispersal risk on delayed dispersal in terrestrial taxa. Waser et al. (1994) pointed out the absence of a parameter estimating the probability of dispersing successfully in the cooperative breeding literature. However, the authors believe that estimates of the survival rate of emigrants and philopatric animals could be calculated from existing census data and behavioral observations to estimate such a parameter. Waser et al. (1994) demonstrated the effectiveness of this approach using data from a number of observational studies on dwarf mongooses, Helogale parvula (Rood, 1983, 1987, 1990; Creel and Waser, 1994). This study showed that in this species, older and more experienced individuals had greater dispersal success. Surprisingly, given that dwarf mongooses are monomorphic, the study also showed that males had greater survival after dispersing than females indicating a lower dispersal risk for males than for females. Therefore, census and behavioral observation data will certainly be vital for continued advancement in this field, as risks of dispersal are likely to play a role in group formation in a range of taxa.
Habitat specialist fishes for example, provide an excellent opportunity to test such hypotheses under novel circumstances as many of these species are sequential or bi-directional hermaphrodites (Nakashima et al., 1996; Buston 2004b) and few congregate in groups of related individuals. Such a varied life-history, rarely observed in terrestrial taxa, means that indirect genetic benefits are unlikely to be key factors in the evolution and maintenance of sociality in these species. As such, these species provide a novel system in which to explore the enhanced role that ecological constraints and direct benefits could contribute to the evolution and maintenance of sociality. For example, a recent observational study by Groenewoud et al. (2016) showed that predation risk was a significant constraint on dispersal in the cooperatively breeding cichlid Neolamprologus pulcher. A lack of available habitat to disperse to may also pose a substantial risk to a subordinate considering dispersal. Buston (2003a) showed that dominant clown anemonefish (Amphiprion percula) strictly regulate the number of subordinates in their group. A subordinate considering dispersal from the group would therefore need to gauge its likelihood of being allowed entry to a new group. Buston (2004b) further showed that subordinate A. percula form a perfect queue for a breeding position in the group and stand to inherit the breeding territory. In this species and likely other habitat specialist fish which form dominance hierarchies, the benefits of remaining philopatric (territory inheritance) may help to explain the evolution of group formation, especially when there are substantial risks of dispersal (Buston 2004b; Wong, 2011; but see Mitchell, 2005). The ability of many species of habitat specialist fish to change sex could be a key element in the development of social queuing and increase the benefit of remaining in the group in these species because once a breeding position is obtained, the previously subordinate individual can change to the appropriate sex to facilitate breeding. This ability may also mitigate the risk of dispersing and not finding a mate of the opposite sex. The effects of sex changing ability on the costs and benefits of dispersal are largely untested and these habitat specialist marine fishes represent exciting opportunities for future studies (Munday et al., 1998). Furthermore, these species also tend to congregate on discrete habitat patches enabling long term observation of social behavior (Herler et al., 2011; Wong and Buston, 2013).
The benefits of philopatry hypothesis provides an excellent example of how the combination of many smaller scale observational studies have significantly advanced our understanding of this particular hypothesis of cooperative breeding theory. Notably, Stacey and Ligon (1991) initially conceived this hypothesis by drawing upon observational data from several long term studies of acorn woodpeckers (Stacey, 1979a,b; Stacey and Ligon, 1987), green woodhoopoes (Ligon and Ligon, 1978, 1990) and mountain chickadee (McCallum, 1988). Support for this theory has gained momentum through observational studies of mammalian species. Marino et al. (2012) conducted a long term observational study in Ethiopian wolves (Canis simensis) which form large packs in areas of high prey abundance, but are only found in pairs in areas where prey was limited. While this observation may be characteristic of an ecological constraint, groups of wolves gained benefits through defense of high quality habitat against neighboring packs. Additionally, Marino et al. (2012) found that even when habitat saturation was relaxed following an outbreak of rabies in the population, subordinate individuals remained philopatric, taking advantage of the enhanced foraging success of the group. This indicates that subordinate individuals are not likely to be constrained by ecological factors in this species, but are in fact receiving direct benefits (increased foraging success) related to remaining philopatric. Marino et al.'s (2012) study highlights the importance of long term observational studies in providing evidence to tease apart different hypotheses of cooperative breeding theory.
Other Hypotheses
Other observational studies have questioned the life-history and ecological constraints hypotheses as explanations for delayed dispersal. Doerr and Doerr (2006) investigated two sympatric species of treecreepers (Climacteris picumnus and Cormobates leucophaea) and suggested that the life-history and ecological constraints hypotheses did not explain why some bird species remain at the nest while others adopt a range of “floater strategies.” Instead, Doerr and Doerr (2006) proposed an “anti-predator tactics” hypothesis based upon their findings to explain the divergence between group and solitary living in these species. Group augmentation, where advantages are positively related to group size, has also been raised as a mechanism promoting the formation of social groups (reviewed in Kingma et al., 2014). Few observational studies have specifically focused on this mechanism, although several have mentioned its effects whilst focusing on alternative cooperative breeding hypotheses (Clutton-Brock et al., 1999; Wright et al., 1999; Balshine et al., 2001; Marino et al., 2012) or allee effects (Courchamp and Macdonald, 2001; Heg et al., 2005).
Manipulative Experiments
While the literature discussed so far has been extremely important in supporting debate regarding a number of social, life-history and ecological correlates of sociality, we must keep in mind that these comparative and observational methods are not able to provide causal explanations of sociality. Brockmann (1997) pointed out an apparent lack of data with which to study the ecological constraints model at the time, deeming the majority of evidence to be correlational. This finding may have changed since Brockmann (1997) wrote her review, with manipulative studies leading observational and comparative studies in publication numbers in the last five years (Figure 3). While experimental manipulation is an extremely powerful tool for examining factors of social evolution, it must be considered that the time and expense involved in altering aspects of an individuals' social or ecological environment may be prohibitive to long term study. It is no surprise then that the majority of manipulative studies are “snap-shots” and care should be taken in the interpretation of results in an evolutionary timeframe. Because of the logistical constraints of manipulative experiments, many studies have focused on smaller species which are more easily housed or species with habitats that can be easily manipulated in situ. Social marine or freshwater fish make excellent study species for this methodology as they tend to congregate on discrete habitat patches which can be easily picked up and moved or simulated in aquaria, making experimental manipulations of ecological factors highly feasible (Wong, 2010). Many are also demersal spawners which provides a convenient measure of reproductive success and fecundity (Buston 2004a; Wong et al., 2008a, 2012). Recent experimental manipulations on these fish are pushing the boundaries of our understanding of the evolution and maintenance of sociality (Wong, 2011; reviewed in Wong and Buston, 2013; Buston and Wong, 2014).
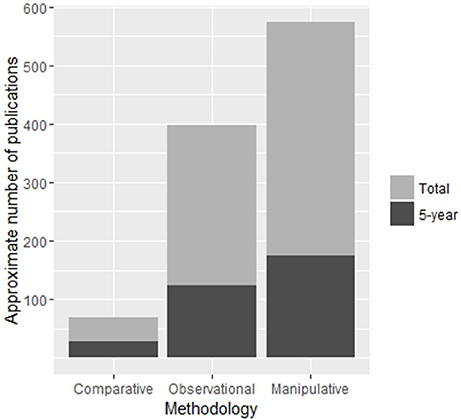
Figure 3. Approximate number of publications on cooperative breeding for each methodology. The number articles published in the last 5 years is shown in dark gray and is included in the total count. Search parameters are available in Supplementary Table S1. Numbers presented here are intended as approximations only as search parameters were not completely mutually exclusive or exhaustive.
Monogamy and Kinship
Monogamy is thought to be directly related to the formation of close family groups and hence sets the stage for cooperative breeding to occur (Boomsma, 2009; Cornwallis et al., 2010). In these close family groups, individuals are expected to increase their indirect genetic benefits by provisioning close kin. While much support for kin selection models has been gained through observational and comparative studies, several experimental studies have questioned kin selection as a mechanism driving sociality. Clutton-Brock et al. (2001) conducted a supplemental feeding manipulation in a population of meerkats (Suricata suricatta) to test whether relatedness of helpers to a litter predicted the amount of food they provisioned to the litter. They found that the provision of food to the litter was related to the foraging success of the individual helpers, regardless of relatedness to the litter. Similarly, Riehl and Strong (2015) cross-fostered broods of nestlings between pairs of nests ensuring that none of the broods were related to the provisioning adults. Feeding rates did not differ at cross-fostered nests compared to those of sham-manipulated control nests (where nestlings were removed and then returned to their original nests), suggesting that provisioning was not influenced by relatedness. Furthermore, Carter and Wilkinson (2013) demonstrated that food sharing in vampire bats, Desmodus rotundus, was predicted more by reciprocation than relatedness (that is, food donors were more likely to share food with a recipient if the recipient had previously donated food, regardless of relatedness).
A similar lack of kinship effect has also been demonstrated in three independent experiments of artificially formed groups of African cichlids, Neolamprologus pulcher (Stiver et al., 2005; Le Vin et al., 2011; Zöttl et al., 2013). All three studies compared groups of cichlids under laboratory conditions where helpers were either related or unrelated to an adult pair and showed that kinship was not related to the amount or type of help that subordinates performed. While these findings may appear to contradict kin selection based models, it is possible that related and unrelated helpers are provisioning help for different reasons. Le Vin et al. (2011) Stiver et al. (2005) and Zöttl et al. (2013) all pointed out that related helpers may help their relatives in order to receive indirect genetic benefits while unrelated helpers may have to “pay to stay” (i.e., provide help to avoid eviction) in order to enjoy the direct fitness benefits of group living (see Quiñones et al., 2016 for a model based on this species showing that negotiations in a pay to stay scenario can result in higher levels of cooperation than relatedness). These studies highlight the importance of using experimental studies to demonstrate causality of effects described using observational data.
Life-History
While much support for the life-history hypothesis has been gained from observational and comparative analyses, life-history traits are generally difficult or in some cases impossible to manipulate experimentally. It is not surprising therefore that the majority of manipulative experiments designed to examine the evolution of sociality, have focused on manipulating ecological and social variables. However, Heg et al. (2011) performed a series of manipulative experiments on Neolamprologus pulcher, and concluded that although ecological and social factors were responsible for the extent of cooperative breeding, a life-history approach could best integrate the environmental and social factors that influenced an individual's decision of whether to join a group as a subordinate helper or disperse to pursue independent breeding opportunities. Despite this, there is clearly a distinct lack of experimental studies focused on the life-history hypothesis. Manipulating sociality by subordinate removal or addition for example, could be an effective way of determining whether measurable life-history traits, such as longevity or growth rates, are a consequence rather than cause of sociality. While not specifically designed to test this hypothesis, growth rate adjustment has been experimentally induced by breeder or helper removal or replacement experiments in a species of African cichlid (Neolamprologus pulcher; synonymous with N. brichardi, Duftner et al., 2007) and in a social marine fish (Amphiprion percula) (Taborsky, 1984; Buston, 2003b; Heg et al., 2004b; Bergmüller et al., 2005 respectively). However, to specifically test the life-history hypothesis experiments would necessitate considerably long time scales and the arrival or premature departure of subordinates would need to be tightly controlled. Such experiments would therefore be best suited to fast growing, short lived species or animals which could be easily housed in a captive setting. Several studies have used supplemental feeding which has resulted in altered growth rates and increased survival of subordinates (Cole and Batzli, 1978; Boland et al., 1997; Wong et al., 2008b). While not designed to test the life-history hypothesis, these short-term experiments have coincidentally changed life-history factors and this method may be worthy of investigation for future experimentation in this field. There is also a need for long-term experimentation in order to detect changes in sociality over the temporal scale of the life-history trait in question. Habitat specialist marine fishes would make good study species as they display a variety of life-history traits such as indeterminate growth rates and sex-change, a life-history trait rarely observed in terrestrial taxa and many species are short lived and have rapid growth rates (Munday and Jones, 1998; Munday et al., 1998; Wong et al., 2005; Depczynski and Bellwood, 2006).
Ecological Factors
Ecological variables such as rainfall, and temperature can vary substantially with latitude (Tewksbury et al., 2008). Reciprocal transplant experiments over large latitudinal gradients are therefore useful for assessing the role that ecological factors could play in promoting sociality in broadly distributed taxa. For example, Baglione et al. (2002) demonstrated a clear link between sociality and environmental factors in carrion crows, Corvus corone corone, via a transplant experiment where eggs from asocial nests in Switzerland were moved to social nests in Spain. Offspring of non-cooperative crows which were reared in the cooperative population in Spain displayed cooperative behavior and delayed dispersal. Although Baglione et al. (2002) suspected that habitat saturation was not a factor contributing to cooperative breeding in crows, habitat saturation as a constraint on dispersal has been well supported in many species through experimental manipulation (Curry, 1989; Schradin and Pillay, 2005). In contrast, Riechert and Jones (2008) found that a species of spider, Anelosimus studiosus, which is only social at high latitudes, maintained its social structure regardless of location when transplanted between social and asocial nests, demonstrating that sociality in this species does not change in response to ecological factors.
Experimental studies can be used to tease apart the relative effects of individual benefits and constraints, or examine their interactions. Indeed, many experimental studies have examined the combined effects of ecological constraints on dispersal and benefits of philopatry, similar to comparative and observational analyses of this hypothesis. For example, Heg et al. (2011) examined the effects of habitat saturation, benefits of philopatry and kin-selection on the extent of helping in the cichlid, Neolamprologus pulcher. They found that habitat saturation and benefits of philopatry were responsible for helping but contrary to the kin selection model, found that individuals preferred to settle with unrelated fish in an absence of dispersal constraints. Previous experimental studies in freshwater fish have also supported the idea that ecological constraints and benefits are responsible for delayed dispersal in cooperatively breeding cichlids (Heg et al., 2004a, 2008; Bergmüller et al., 2005; Jungwirth et al., 2015). Predation risk in particular has been shown to be a crucial ecological constraint on dispersal in these species (Taborsky, 1984; reviewed in Taborsky, 2016). Komdeur (1992) showed that habitat saturation and benefits of philopatry were important factors in the dispersal of Seychelles warblers by experimentally introducing individuals to unoccupied islands. Two years after the initial introduction of warblers, all of the high quality territory was occupied, and yearlings born on these territories began to stay and help instead of pursuing independent breeding opportunities on still vacant lower quality habitat. Komdeur's (1992) results showed that while habitat saturation constrained young birds from leaving high quality habitat, the benefits of remaining at a high quality nest resulted in higher life-time reproductive success. Similarly, Wong (2010) used field and laboratory experiments to demonstrate that subordinate dispersal in a coral reef fish, Paragobiodon xanthosomus, was affected by a combination of ecological constraints (habitat saturation and risk of movement) and benefits of philopatry (coral size—a proxy for habitat quality in this species), but not by social factors (social rank and forcible eviction). Ligon et al. (1991) also tested the effects of several ecological factors on cooperative breeding in groups of superb fairy wrens, Malurus cyaneus. They examined the effects of mate availability, habitat saturation and group augmentation. Ligon et al. (1991) found that their study population of M. cyaneus was not constrained by a lack of breeding partners, or by limitations of available breeding habitat and that subordinate presence was not related to reproductive success. Ligon et al. (1991) concluded that benefits of remaining on a higher quality habitat were responsible for natal philopatry in male M. cyaneus. These examples demonstrate the power of experimental manipulation in identifying multiple factors which may have affected the evolution and maintenance of sociality.
Experimental work, both on larger and smaller scales, has been extremely important in identifying species which have evolved sociality in response to ecological factors. These studies have demonstrated that ecological factors relating to sociality have proven relatively amenable to manipulation, either in the field or the laboratory, for a range of species. It is clear from these examples that the role that ecological factors have played in the evolution of sociality is species specific, and that other factors are likely to play a role in determining whether a species exhibits social behavior. The relative effects and causality of these factors can only be teased apart using robust manipulative experiments.
Combining Methods
The discussion so far has highlighted the benefits and pitfalls of each individual methodology. We suggest that a combination of these methodologies will provide an efficient and comprehensive view of social evolution. This combination should start with sourcing or building a phylogeny for the taxa. Building the phylogeny would involve collection of genetic material from all of the species within the lineage. Observational data on sociality and associated ecological, life-history and behavioral factors could also be collected at the same time. This data can then be mapped onto the phylogeny and correlations between sociality and these factors can be determined. This mapping can then be used to target experimental work on sets of species varying in sociality and other factors of interest to determine whether causality can be assigned to any particular factors.
In many cases, phylogenies will already be fully resolved and relevant social observational work may have been undertaken for some species. In such cases research effort should be directed to “filling in the blanks” for any species lacking in data. Research effort is often directed at a “popular” subset of species within a lineage because field techniques have been well established. While such research is valuable for examining sociality at the species level, the results are less meaningful at higher taxonomic levels. For this reason, we encourage researchers to design observational and experimental studies with the express view of contributing to future interspecific comparative work. Observations and manipulative experiments should be conducted using similar methods to previous work so that meaningful comparisons can be made.
Conclusion
In this review, we explored the factors influencing the evolution of social systems containing non-breeding subordinates, from the perspective of the methodological approaches that have been used to test multiple hypotheses. Great advances in the field have been made through comparative work (Brockmann, 1997; Arnold and Owens, 1998; Clutton-Brock, 2002; Taborsky and Wong, 2017) and fine scale observational (Rowley and Russell, 1990; Schradin and Pillay, 2005) and manipulative studies (Komdeur, 1992; Riechert and Jones, 2008; Heg et al., 2011), although each method has its limitations and taxonomic biases. Comparative analyses have proven useful for studying evolutionary questions especially when combined with molecular phylogenetic tools as they are able to reveal patterns across multiple species and lineages (e.g., Edwards and Naeem, 1993; Arnold and Owens, 1998; Cockburn, 2006). Intraspecific comparisons across ecologically diverging populations have also proven extremely valuable for testing ecological hypotheses (e.g., Groenewoud et al., 2016). However, these broad patterns may overlook contrasting patterns in smaller sets of species or within any given population, and are currently taxonomically restricted to terrestrial species and a handful of freshwater fish species. Smaller-scale observational studies have been effectively used to investigate the relationships between life-history, ecology and sociality, especially over the long-term. However, observational studies are not able to show causality between these factors and sociality and are limited in impact as only a few species can be investigated. Manipulative experiments may save the day by demonstrating causality in many cases, but they too by necessity focus on smaller sets of species and short-term manipulations which limits the generality of their conclusions. There is also a need for more comparative and observational studies on the effects of dispersal risk on delayed dispersal and additional manipulative work on the life-history hypothesis in order to give a well-balanced perspective of social evolution and maintenance. We suggest that combining these approaches under a single framework would provide a comprehensive method of studying the evolution of sociality across a broad range of taxa, though few studies have attempted to do so.
Additionally, different animal groups have proven to be more amenable to particular methodologies. For example, birds, insects and mammals have been well studied through comparative analyses due to their well-defined phylogenies and long history of observation. On the other hand, habitat specialist fish, because of their small body size and site attachment, are extending the boundaries of our understanding of sociality through amenability to experimental manipulation. Overall, hypotheses for social evolution have been less extensively studied in marine taxa. While cooperative rearing of young has not been observed in marine fish, there are group living species which are typically comprised of unrelated individuals and often a monogamous breeding pair with a number of non-breeding subordinates (Taborsky and Wong, 2017). These groups bear many resemblances to cooperative breeding birds and mammals and cooperative breeding theories have proven successful in explaining the evolution and maintenance of these social systems (Buston and Balshine, 2007; Wong, 2010; Wong and Buston, 2013). Unconventional life-history strategies, such as bi-directional sex-change, and amenability to experimental manipulation and observation present further opportunities to challenge hypotheses of social evolution under novel conditions. For example, the ability to change sex may alter the costs and benefits associated with dispersal from the group. Additionally, indeterminate growth as observed in social marine fishes presents opportunities for exploring the life-history hypothesis which is currently lacking experimental testing. Combining multiple methodological approaches with investigations of novel taxa are now clearly required to gain a truly general understanding of the evolution of sociality.
Author Contributions
MH and MW formulated the idea for the manuscript. MH drafted and revised the manuscript. MW provided substantial feedback on drafts of the manuscript. OK and MD critically reviewed and provided comment on the manuscript.
Funding
This research has been conducted with the support of the Hermon Slade Foundation (HSF13/8) and the Australian Government Research Training Program Scholarship.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank P. Buston, for comments on the manuscript and in depth discussion on the technicalities of determining relatedness. We would also like to thank Dr. Taborsky, Dr. Stiver, one anonymous reviewer and the editor, Dr. Bilde for their helpful comments on the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fevo.2017.00100/full#supplementary-material
References
Arnold, K. E., and Owens, I. P. F. (1998). Cooperative breeding in birds: a comparative test of the life history hypothesis. Proc. R. Soc. B Biol. Sci. 265, 739–745. doi: 10.1098/rspb.1998.0355
Avise, J. C., and Shapiro, D. Y. (1986). Evaluating kinship of newly settled juveniles within social groups of the coral reef fish Anthias squamipinnis. Evolution 40, 1051–1059. doi: 10.1111/j.1558-5646.1986.tb00572.x
Awata, S., Munehara, H., and Kohda, M. (2005). Social system and reproduction of helpers in a cooperatively breeding cichlid fish (Julidochromis ornatus) in Lake Tanganyika: field observations and parentage analyses. Behav. Ecol. Sociobiol. 58, 506–516. doi: 10.1007/s00265-005-0934-6
Baglione, V., Canestrari, D., Marcos, J. M., Griesser, M., and Ekman, J. (2002). History, environment and social behaviour: experimentally induced cooperative breeding in the carrion crow. Proc. R. Soc. B Biol. Sci. 269, 1247–1251. doi: 10.1098/rspb.2002.2016
Balshine, S., Leach, B., Neat, F., Reid, H., Taborsky, M., and Werner, N. (2001). Correlates of group size in a cooperatively breeding cichlid fish (Neolamprologus pulcher). Behav. Ecol. Sociobiol. 50, 134–140. doi: 10.1007/s002650100343
Bergmüller, R., Heg, D., and Taborsky, M. (2005). Helpers in a cooperatively breeding cichlid stay and pay or disperse and breed, depending on ecological constraints. Proc. R. Soc. B Biol. Sci. 272, 325–331. doi: 10.1098/rspb.2004.2960
Blumstein, D. T., and Armitage, K. B. (1999). Cooperative breeding in marmots. Oikos 84, 369–382. doi: 10.2307/3546418
Blumstein, D. T., and Moller, A. P. (2008). Is sociality associated with high longevity in North American birds? Biol. Lett. 4, 146–148. doi: 10.1098/rsbl.2007.0606
Boland, C. R. J., Heinsohn, R., and Cockburn, A. (1997). Experimental manipulation of brood reduction and parental care in cooperatively breeding white-winged choughs. J. Anim. Ecol. 66, 683–691. doi: 10.2307/5921
Boomsma, J. J. (2009). Lifetime monogamy and the evolution of eusociality. Philos. Trans. R. Soci. B Biol. Sci. 364, 3191–3207. doi: 10.1098/rstb.2009.0101
Bourke, A. F. G. (2014). Hamilton's rule and the causes of social evolution. Philos. Trans. R. Soc. B Biol. Sci. 369, 10. doi: 10.1098/rstb.2013.0362
Briga, M., Pen, I., and Wright, J. (2012). Care for kin: within-group relatedness and allomaternal care are positively correlated and conserved throughout the mammalian phylogeny. Biol. Lett. 8, 533–536. doi: 10.1098/rsbl.2012.0159
Brockmann, H. J. (1997). “Cooperative breeding in wasps and vertebrates: the role of ecological constraints,” in The Evolution of Social Behavior in Insects and Arachnids, eds J. C. Choe and B. J. Crespi (Cambridge, UK: Cambridge University Press), 347–371.
Brown, J. L. (1974). Alternate routes to sociality in jays—with a theory for the evolution of altruism and communal breeding. Am. Zool. 14, 63–80. doi: 10.1093/icb/14.1.63
Brown, J. L. (1987). Helping and Communal Breeding in Birds. Princeton, NJ: Princeton University Press.
Bruintjes, R., Bonfils, D., Heg, D., and Taborsky, M. (2011). Paternity of subordinates raises cooperative effort in cichlids. PLoS ONE 6:e25673. doi: 10.1371/journal.pone.0025673
Bruintjes, R., Hekman, R., and Taborsky, M. (2010). Experimental global food reduction raises resource acquisition costs of brood care helpers and reduces their helping effort. Funct. Ecol. 24, 1054–1063. doi: 10.1111/j.1365-2435.2010.01715.x
Burda, H. (1990). Constraints of pregnancy and evolution of sociality in mole-rats - with special reference to reproductive and social patterns in Cryptomys hottentotus (Bathyergidae, Rodentia). Z. Zool. Syst. Evol. 28, 26–39. doi: 10.1111/j.1439-0469.1990.tb00362.x
Buston, P. (2003a). Forcible eviction and prevention of recruitment in the clown anemonefish. Behav. Ecol. 14, 576–582. doi: 10.1093/beheco/arg036
Buston, P. (2003b). Social hierarchies: size and growth modification in clownfish. Nature 424, 145–146. doi: 10.1038/424145a
Buston, P. M. (2004a). Does the presence of non-breeders enhance the fitness of breeders? An experimental analysis in the clown anemonefish Amphiprion percula. Behav. Ecol. Sociobiol. 57, 23–31. doi: 10.1007/s00265-004-0833-2
Buston, P. M. (2004b). Territory inheritance in clownfish. Proc. R. Soc. B Biol. Sci. 271, S252–S254. doi: 10.1098/rsbl.2003.0156
Buston, P. M., and Balshine, S. (2007). Cooperating in the face of uncertainty: a consistent framework for understanding the evolution of cooperation. Behav. Processes 76, 152–159. doi: 10.1016/j.beproc.2007.01.020
Buston, P. M., Bogdanowicz, S. M., Wong, A., and Harrison, R. G. (2007). Are clownfish groups composed of close relatives? An analysis of microsatellite DNA variation in Amphiprion percula. Mol. Ecol. 16, 3671–3678. doi: 10.1111/j.1365-294X.2007.03421.x
Buston, P. M., Fauvelot, C., Wong, M. Y. L., and Planes, S. (2009). Genetic relatedness in groups of the humbug damselfish Dascyllus aruanus: small, similar-sized individuals may be close kin. Mol. Ecol. 18, 4707–4715. doi: 10.1111/j.1365-294X.2009.04383.x
Buston, P. M., and Wong, M. Y. L. (2014). Why Some Animals Forgo Reproduction in Complex Societies Behaviors of coral reef fishes provide strong support for some major new ideas about the evolution of cooperation. Am. Sci. 102, 290–297. doi: 10.1511/2014.109.290
Canestrari, D., Marcos, J. M., and Baglione, V. (2005). Effect of parentage and relatedness on the individual contribution to cooperative chick care in carrion crows Corvus corone corone. Behav. Ecol. Sociobiol. 57, 422–428. doi: 10.1007/s00265-004-0879-1
Carter, G. G., and Wilkinson, G. S. (2013). Food sharing in vampire bats: reciprocal help predicts donations more than relatedness or harassment. Proc. R. Soc. B Biol. Sci. 280:1753. doi: 10.1098/rspb.2012.2573
Chapais, B. (2013). Monogamy, strongly bonded groups, and the evolution of human social structure. Evol. Anthropol. 22, 52–65. doi: 10.1002/evan.21345
Chapple, D. G. (2003). Ecology, life-history, and behavior in the Australian Scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetol. Monogr. 17, 145–180. doi: 10.1655/0733-1347(2003)017[0145:ELABIT]2.0.CO;2
Clutton-Brock, T. (2002). Behavioral ecology - breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72. doi: 10.1126/science.296.5565.69
Clutton-Brock, T. H., Brotherton, P. N. M., O'Riain, M. J., Griffin, A. S., Gaynor, D., Kansky, R., et al. (2001). Contributions to cooperative rearing in meerkats. Anim. Behav. 61, 705–710. doi: 10.1006/anbe.2000.1631
Clutton-Brock, T. H., Gaynor, D., McIlrath, G. M., Maccoll, A. D. C., Kansky, R., Chadwick, P., et al. (1999). Predation, group size and mortality in a cooperative mongoose, Suricata suricatta. J. Anim. Ecol. 68, 672–683. doi: 10.1046/j.1365-2656.1999.00317.x
Cockburn, A. (1996). “Why do so many australian birds cooperate: social evolution in the corvida?” in Frontiers of Population Ecology, eds R. B. Floyd, A. W. Sheppard, and P. J. Debarro (Canberra: CSIRO Publishing, Melbourne), 451–472.
Cockburn, A. (2006). Prevalence of different modes of parental care in birds. Proc. R. Soc. B Biol. Sci. 273, 1375–1383. doi: 10.1098/rspb.2005.3458
Cole, F. R., and Batzli, G. O. (1978). Influence of supplemental feeding on a vole population. J. Mammal. 59, 809–819. doi: 10.2307/1380145
Cornwallis, C. K., West, S. A., Davis, K. E., and Griffin, A. S. (2010). Promiscuity and the evolutionary transition to complex societies. Nature 466, 969–U91. doi: 10.1038/nature09335
Courchamp, F., and Macdonald, D. W. (2001). Crucial importance of pack size in the African wild dog Lycaon pictus. Anim. Conserv. 4, 169–174. doi: 10.1017/S1367943001001196
Creel, S. R., and Waser, P. M. (1994). Inclusive fitness and reproductive strategies in dwarf mongooses. Behav. Ecol. 5, 339–348. doi: 10.1093/beheco/5.3.339
Curry, R. L. (1989). Geographic-variation in social-organization of galapagos mockingbirds - ecological correlates of group territoriality and cooperative breeding. Behav. Ecol. Sociobiol. 25, 147–160. doi: 10.1007/BF00302932
Depczynski, M., and Bellwood, D. R. (2006). Extremes, plasticity, and invariance in vertebrate life history traits: insights from coral reef fishes. Ecology 87, 3119–3127. doi: 10.1890/0012-9658(2006)87[3119:EPAIIV]2.0.CO;2
Dierkes, P., Heg, D., Taborsky, M., Skubic, E., and Achmann, R. (2005). Genetic relatedness in groups is sex-specific and declines with age of helpers in a cooperatively breeding cichlid. Ecol. Lett. 8, 968–975. doi: 10.1111/j.1461-0248.2005.00801.x
Doerr, E. D., and Doerr, V. A. J. (2006). Comparative demography of treecreepers: evaluating hypotheses for the evolution and maintenance of cooperative breeding. Anim. Behav. 72, 147–159. doi: 10.1016/j.anbehav.2005.10.017
Double, M. C., and Cockburn, A. (2003). Subordinate superb fairy-wrens (Malurus cyaneus) parasitize the reproductive success of attractive dominant males. Proc. R. Soc. Lond. B Biol. Sci. 270, 379–384. doi: 10.1098/rspb.2002.2261
Drobniak, S. M., Wagner, G., Mourocq, E., and Griesser, M. (2015). Family living: an overlooked but pivotal social system to understand the evolution of cooperative breeding. Behav. Ecol. 26, 805–811. doi: 10.1093/beheco/arv015
Duchene, D., Klanten, S. O., Munday, P. L., Herler, J., and van Herwerden, L. (2013). Phylogenetic evidence for recent diversification of obligate coral-dwelling gobies compared with their host corals. Mol. Phylogenet. Evol. 69, 123–132. doi: 10.1016/j.ympev.2013.04.033
Duffield, G. A., and Bull, C. M. (2002). Stable social aggregations in an Australian lizard, Egernia stokesii. Naturwissenschaften 89, 424–427. doi: 10.1007/s00114-002-0346-7
Duffy, J. E., and Macdonald, K. S. (2010). Kin structure, ecology and the evolution of social organization in shrimp: a comparative analysis. Proc. R. Soc. B Biol. Sci. 277, 575–584. doi: 10.1098/rspb.2009.1483
Duftner, N., Sefc, K. M., Koblmüller, S., Salzburger, W., Taborsky, M., and Sturmbauer, C. (2007). Parallel evolution of facial stripe patterns in the Neolamprologus brichardi/pulcher species complex endemic to Lake Tanganyika. Mol. Phylogenet. Evol. 45, 706–715. doi: 10.1016/j.ympev.2007.08.001
Du Plessis, M. A., Siegfried, W. R., and Armstrong, A. J. (1995). Ecological and life-history correlates af cooperative breeding in south-african birds. Oecologia 102, 180–188. doi: 10.1007/BF00333250
Ebensperger, L. A. (2001). A review of the evolutionary causes of rodent group-living. Acta Theriol. 46, 115–144. doi: 10.4098/AT.arch.01-16
Eberle, M., and Kappeler, P. M. (2006). Family insurance: kin selection and cooperative breeding in a solitary primate (Microcebus murinus). Behav. Ecol. Sociobiol. 60, 582–588. doi: 10.1007/s00265-006-0203-3
Eden, S. F. (1987). When do helpers help? Food availability and helping in the moorhen, Gallinula chloropus. Behav. Ecol. Sociobiol. 21, 191–195. doi: 10.1007/BF00303210
Edwards, S. V., and Naeem, S. (1993). The phylogenetic component of cooperative breeding in perching birds. Am. Nat. 141, 754–789. doi: 10.1086/285504
Elgar, M. A. (2015). Challenges for social evolution: integrating insights across diverse taxa. Front. Ecol. Evol. 3:124. doi: 10.3389/fevo.2015.00124
Emlen, S. T. (1982a). The evolution of helping 1. An ecological constraints model. Am. Nat. 119, 29–39. doi: 10.1086/283888
Emlen, S. T. (1982b). The evolution of helping. II. The role of behavioral conflict. Am. Nat. 119, 40–53. doi: 10.1086/283889
Emlen, S. T. (1994). Benefits, constraints and the evolution of the family. Trends Ecol. Evol. 9, 282–285. doi: 10.1016/0169-5347(94)90030-2
Emlen, S. T., Reeve, H. K., Sherman, P. W., Wrege, P. H., Ratnieks, F. L. W., and Shellmanreeve, J. (1991). Adaptive versus nonadaptive explanations of behavior - the case of alloparental helping. Am. Nat. 138, 259–270. doi: 10.1086/285216
Faulkes, C. G., and Bennett, N. C. (2013). Plasticity and constraints on social evolution in African mole-rats: ultimate and proximate factors. Philos. Trans. R. Soc. B Biol. Sci. 368:20120347. doi: 10.1098/rstb.2012.0347
Faulkes, C. G., Bennett, N. C., Bruford, M. W., Obrien, H. P., Aguilar, G. H., and Jarvis, J. U. M. (1997). Ecological constraints drive social evolution in the African mole-rats. Proc. R. Soc. B Biol. Sci. 264, 1619–1627. doi: 10.1098/rspb.1997.0226
Felsenstein, J. (1985). Phylogenies and the comparative method. Am. Nat. 125, 1–15. doi: 10.1086/284325
Ford, H. A., Bell, H., Nias, R., and Noske, R. (1988). The relationship between ecology and the incidence of cooperative breeding in australian birds. Behav. Ecol. Sociobiol. 22, 239–249. doi: 10.1007/BF00299838
Freckleton, R. P., Harvey, P. H., and Pagel, M. (2002). Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726. doi: 10.1086/343873
Gardner, J. L., Magrath, R. D., and Kokko, H. (2003). Stepping stones of life: natal dispersal in the group-living but noncooperative speckled warbler. Anim. Behav. 66, 521–530. doi: 10.1006/anbe.2003.2206
Garland, T., Harvey, P. H., and Ives, A. R. (1992). Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 41, 18–32. doi: 10.1093/sysbio/41.1.18
Gaston, A. J. (1978). The evolution of group territorial behavior and cooperative breeding. Am. Nat. 112, 1091–1100. doi: 10.1086/283348
Gowans, S., Whitehead, H., and Hooker, S. K. (2001). Social organization in northern bottlenose whales, Hyperoodon ampullatus: not driven by deep-water foraging? Anim. Behav. 62, 369–377. doi: 10.1006/anbe.2001.1756
Grimes, L. (1976). The occurrence of cooperative breeding behaviour in African birds. Ostrich 47, 1–15. doi: 10.1080/00306525.1976.9639530
Groenewoud, F., Frommen, J. G., Josi, D., Tanaka, H., Jungwirth, A., and Taborsky, M. (2016). Predation risk drives social complexity in cooperative breeders. Proc. Natl. Acad. Sci. U.S.A. 113, 4104–4109. doi: 10.1073/pnas.1524178113
Grueter, C. C., Chapais, B., and Zinner, D. (2012). Evolution of multilevel social systems in nonhuman primates and humans. Int. J. Primatol. 33, 1002–1037. doi: 10.1007/s10764-012-9618-z
Hamilton, W. D. (1964). The genetical evolution of social behaviour. II. J. Theor. Biol. 7, 17–52. doi: 10.1016/0022-5193(64)90039-6
Hatchwell, B. J., and Komdeur, J. (2000). Ecological constraints, life history traits and the evolution of cooperative breeding. Anim. Behav. 59, 1079–1086. doi: 10.1006/anbe.2000.1394
Hayes, L. D. (2000). To nest communally or not to nest communally: a review of rodent communal nesting and nursing. Anim. Behav. 59, 677–688. doi: 10.1006/anbe.1999.1390
Heg, D., Bachar, Z., Brouwer, L., and Taborsky, M. (2004a). Predation risk is an ecological constraint for helper dispersal in a cooperatively breeding cichlid. Proc. R. Soc. B Biol. Sci. 271, 2367–2374. doi: 10.1098/rspb.2004.2855
Heg, D., Bender, N., and Hamilton, I. (2004b). Strategic growth decisions in helper cichlids. Proc. R. Soc. B Biol. Sci. 27(Suppl. 6), S505–S508. doi: 10.1098/rsbl.2004.0232
Heg, D., Brouwer, L., Bachar, Z., and Taborsky, M. (2005). Large group size yields group stability in the cooperatively breeding cichlid Neolamprologus pulcher. Behaviour 142, 1615–1641. doi: 10.1163/156853905774831891
Heg, D., Heg-Bachar, Z., Brouwer, L., and Taborsky, M. (2008). Experimentally induced helper dispersal in colonially breeding cooperative cichlids. Environ. Biol. Fishes 83, 191–206. doi: 10.1007/s10641-007-9317-3
Heg, D., Rothenberger, S., and Schurch, R. (2011). Habitat saturation, benefits of philopatry, relatedness, and the extent of co-operative breeding in a cichlid. Behav. Ecol. 22, 82–92. doi: 10.1093/beheco/arq170
Heg, D., and Taborsky, M. (2010). Helper response to experimentally manipulated predation risk in the cooperatively breeding cichlid Neolamprologus pulcher. PLoS ONE 5:e10784. doi: 10.1371/journal.pone.0010784
Herler, J., Koblmuller, S., and Sturmbauer, C. (2009). Phylogenetic relationships of coral-associated gobies (Teleostei, Gobiidae) from the Red Sea based on mitochondrial DNA data. Mar. Biol. 156, 725–739. doi: 10.1007/s00227-008-1124-7
Herler, J., Munday, P. L., and Hernaman, V. (2011). “Gobies on coral reefs,” in The Biology of Gobies, eds R. A. Patzner, J. L. Van Tassel, M. Kovačić, and B. G. Kapoor (Jersey; Enfield, NH: Science Publishers), 493–529.
Hodge, S. J. (2005). Helpers benefit offspring in both the short and long-term in the cooperatively breeding banded mongoose. Proc. R. Soc. B Biol. Sci. 272, 2479–2484. doi: 10.1098/rspb.2005.3255
Hughes, W. O. H., Oldroyd, B. P., Beekman, M., and Ratnieks, F. L. W. (2008). Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 320, 1213–1216. doi: 10.1126/science.1156108
Jetz, W., and Rubenstein, D. R. (2011). Environmental uncertainty and the global biogeography of cooperative breeding in birds (vol 21, pg 72, 2011). Curr. Biol. 21, 438–438. doi: 10.1016/j.cub.2011.02.025
Johnson, D. D. P., Jetz, W., and Macdonald, D. W. (2002). Environmental correlates of badger social spacing across Europe. J. Biogeogr. 29, 411–425. doi: 10.1046/j.1365-2699.2002.00680.x
Johnson, J. S., Kropczynski, J. N., and Lacki, M. J. (2013). Social network analysis and the study of sociality in bats. Acta Chiropterol. 15, 1–17. doi: 10.3161/150811013X667821
Johnstone, R. A. (2000). Models of reproductive skew: a review and synthesis. Ethology 106, 5–26. doi: 10.1046/j.1439-0310.2000.00529.x
Jungwirth, A., Walker, J., and Taborsky, M. (2015). Prospecting precedes dispersal and increases survival chances in cooperatively breeding cichlids. Anim. Behav. 106, 107–114. doi: 10.1016/j.anbehav.2015.05.005
Kent, D. S., and Simpson, J. A. (1992). Eusociality in the beetle Austroplatypus incompertus (Coleoptera: Curculionidae). Naturwissenschaften 79, 86–87. doi: 10.1007/BF01131810
Kingma, S. A., Santema, P., Taborsky, M., and Komdeur, J. (2014). Group augmentation and the evolution of cooperation. Trends Ecol. Evol. 29, 476–484. doi: 10.1016/j.tree.2014.05.013
Kleiman, D. G. (2011). Canid mating systems, social behavior, parental care and ontogeny: are they flexible? Behav. Genet. 41, 803–809. doi: 10.1007/s10519-011-9459-0
Koenig, W. D., Pitelka, F. A., Carmen, W. J., Mumme, R. L., and Stanback, M. T. (1992). The evolution of delayed dispersal in cooperative breeders. Q. Rev. Biol. 67, 111–150. doi: 10.1086/417552
Kokko, H., and Ekman, J. (2002). Delayed dispersal as a route to breeding: territorial inheritance, safe havens, and ecological constraints. Am. Nat. 160, 468–484. doi: 10.1086/342074
Kokko, H., Johnstone, R. A., and Clutton-Brock, T. H. (2001). The evolution of cooperative breeding through group augmentation. Proc. R. Soc. B Biol. Sci. 268, 187–196. doi: 10.1098/rspb.2000.1349
Kokko, H., Johnstone, R. A., and Wright, J. (2002). The evolution of parental and alloparental effort in cooperatively breeding groups: when should helpers pay to stay? Behav. Ecol. 13, 291–300. doi: 10.1093/beheco/13.3.291
Komdeur, J. (1992). Importance of habitat saturation and territory quality for evolution of cooperative breeding in the seychelles warbler. Nature 358, 493–495. doi: 10.1038/358493a0
Legge, S. (2000). Helper contributions in the cooperatively breeding laughing kookaburra: feeding young is no laughing matter. Anim. Behav. 59, 1009–1018. doi: 10.1006/anbe.2000.1382
Le Vin, A. L., Mable, B. K., Taborsky, M., Heg, D., and Arnold, K. E. (2011). Individual variation in helping in a cooperative breeder: relatedness versus behavioural type. Anim. Behav. 82, 467–477. doi: 10.1016/j.anbehav.2011.05.021
Ligon, J. D., and Ligon, S. H. (1978). The communal social system of the green woodhoopoe in Kenya. Living Bird 17, 159–197.
Ligon, J. D., and Ligon, S. H. (1990). “Green woodhoopoes: life history traits and sociality,” in Cooperative Breeding in Birds, eds P. B. Stacey and W. D. Koenig (Cambridge, UK: Cambridge University Press), 31–66
Ligon, J. D., Ligon, S. H., and Ford, H. A. (1991). An experimental-study of the bases of male philopatry in the cooperatively breeding superb fairy-wren Malurus cyaneus. Ethology 87, 134–148. doi: 10.1111/j.1439-0310.1991.tb01195.x
Lubin, Y., and Bilde, T. (2007). The evolution of sociality in spiders. Adv. Study Behav. 37, 83–145. doi: 10.1016/S0065-3454(07)37003-4
Lucia, K. E., Keane, B., Hayes, L. D., Lin, Y. K., Schaefer, R. L., and Solomon, N. G. (2008). Philopatry in prairie voles: an evaluation of the habitat saturation hypothesis. Behav. Ecol. 19, 774–783. doi: 10.1093/beheco/arn028
Lukas, D., and Clutton-Brock, T. (2012). Life histories and the evolution of cooperative breeding in mammals. Proc. R. Soc. B Biol. Sci. 279, 4065–4070. doi: 10.1098/rspb.2012.1433
Lynch, M., and Ritland, K. (1999). Estimation of pairwise relatedness with molecular markers. Genetics, 152, 1753–1766.
Manning, C. J., Dewsbury, D. A., Wakeland, E. K., and Potts, W. K. (1995). Communal nesting and communal nursing in house mice, Mus-Musculus Domesticus. Anim. Behav. 50, 741–751. doi: 10.1016/0003-3472(95)80134-0
Margraf, N., and Cockburn, A. (2013). Helping behaviour and parental care in fairy-wrens (Malurus). Emu 113, 294–301. doi: 10.1071/MU13001
Marino, J., Sillero-Zubiri, C., Johnson, P. J., and Macdonald, D. W. (2012). Ecological bases of philopatry and cooperation in Ethiopian wolves. Behav. Ecol. Sociobiol. 66, 1005–1015. doi: 10.1007/s00265-012-1348-x
McCallum, D. A. (1988). Alternative Emigration Strategies and the Adaptive Significance of Natal Dispersal in a Population of Mountain Chickadees (Parus gambeli). Ph.D., dissertation, University of New Mexico, Albuquerque.
Mitchell, J. (2005). Queue selection and switching by false clown anernonefish, Amphiprion ocellaris. Anim. Behav. 69, 643–652. doi: 10.1016/j.anbehav.2004.05.017
Munday, P. L., Caley, M. J., and Jones, G. P. (1998). Bi-directional sex change in a coral-dwelling goby. Behav. Ecol. Sociobiol. 43, 371–377. doi: 10.1007/s002650050504
Munday, P. L., and Jones, G. P. (1998). The ecological implications of small body size among coral-reef fishes. Oceanogr. Mar. Biol. 36, 373–411.
Nakashima, Y., Kuwamura, T., and Yogo, Y. (1996). Both-ways sex change in monogamous coral gobies, Gobiodon spp. Environ. Biol. Fishes 46, 281–288. doi: 10.1007/BF00005004
Nam, K. B., Simeoni, M., Sharp, S. P., and Hatchwell, B. J. (2010). Kinship affects investment by helpers in a cooperatively breeding bird. Proc. R. Soc. B Biol. Sci. 277, 3299–3306. doi: 10.1098/rspb.2010.0737
Nandi, A. K., Bhadra, A., Sumana, A., Deshpande, S. A., and Gadagkar, R. (2013). The evolution of complexity in social organization-A model using dominance-subordinate behavior in two social wasp species. J. Theor. Biol. 327, 34–44. doi: 10.1016/j.jtbi.2013.01.010
Oli, M. K., and Armitage, K. B. (2003). Sociality and individual fitness in yellow-bellied marmots: insights from a long-term study (1962-2001). Oecologia 136, 543–550. doi: 10.1007/s00442-003-1291-7
Pagel, M. (1999). Inferring the historical patterns of biological evolution. Nature 401, 877–884. doi: 10.1038/44766
Pagel, M. D., and Harvey, P. H. (1988). Recent developments in the analysis of comparative data. Q. Rev. Biol. 63, 413–440. doi: 10.1086/416027
Pen, I., and Weissing, F. J. (2000). Towards a unified theory of cooperative breeding: the role of ecology and life history re-examined. Proc. R. Soc. B Biol. Sci. 267, 2411–2418. doi: 10.1098/rspb.2000.1299
Purcell, J. (2011). Geographic patterns in the distribution of social systems in terrestrial arthropods. Biol. Rev. 86, 475–491. doi: 10.1111/j.1469-185X.2010.00156.x
Quiñones, A. E., Van Doorn, G. S., Pen, I., Weissing, F. J., and Taborsky, M. (2016). Negotiation and appeasement can be more effective drivers of sociality than kin selection. Philos. Trans. R. Soc. B Biol. Sci. 371:20150089. doi: 10.1098/rstb.2015.0089
Reeve, H. K., and Ratnieks, F. L. W. (1993). Queen-Queen Conflicts in Polygynous Societies: Mutual Tolerance and Reproductive Skew. Queen Number and Sociality in Insects. Oxford Oxford: University Press, 45–85.
Ricklefs, R. E. (1975). Evolution of cooperative breeding in birds. Ibis 117, 531–534. doi: 10.1111/j.1474-919X.1975.tb04252.x
Ridley, A. R., and van den Heuvel, I. M. (2012). Is there a difference in reproductive performance between cooperative and non-cooperative species? A southern African comparison. Behaviour 149, 821–848. doi: 10.1163/1568539X-00003005
Riechert, S. E., and Jones, T. C. (2008). Phenotypic variation in the social behaviour of the spider Anelosimus studiosus along a latitudinal gradient. Anim. Behav. 75, 1893–1902. doi: 10.1016/j.anbehav.2007.10.033
Riehl, C. (2013). Evolutionary routes to non-kin cooperative breeding in birds. Proc. R. Soc. B Biol. Sci. 280:7. doi: 10.1098/rspb.2013.2245
Riehl, C., and Strong, M. J. (2015). Social living without kin discrimination: experimental evidence from a communally breeding bird. Behav. Ecol. Sociobiol. 69, 1293–1299. doi: 10.1007/s00265-015-1942-9
Rood, J. P. (1983). “The social system of the dwarf mongoose,” in Advances in the Study of Mammalian Behavior, eds J. F. Eisenberg and D. G. Kleiman (Shippensburg, PA: American Society of Mammalogists), 454–488.
Rood, J. P. (1987). “Dispersal and intergroup transfer in the dwarf mongoose,” in Mammalian Dispersal Patterns: The Effects of Social Structure on Population Genetics, eds B. D. Chepko-Sade and Z. T. Halpin (Chicago: University of Chicago Press), 85–103.
Rood, J. P. (1990). Group-size, survival, reproduction, and routes to breeding in dwarf mongooses. Anim. Behav. 39, 566–572. doi: 10.1016/S0003-3472(05)80423-3
Rowley, I., and Russell, E. (1990). “Splendid fairy-wrens: demonstrating the importance of longevity,” in Cooperative Breeding in Birds: Long-Term Studies of Ecology And Behavior, ed P. K. Stacey (Cambridge: Cambridge University Press), 3–30.
Rubenstein, D. R. (2011). Spatiotemporal environmental variation, risk aversion, and the evolution of cooperative breeding as a bet-hedging strategy. Proc. Natl. Acad. Sci. U.S.A. 108, 10816–10822. doi: 10.1073/pnas.1100303108
Rubenstein, D. R., and Lovette, I. J. (2007). Temporal environmental variability drives the evolution of cooperative breeding in birds. Curr. Biol. 17, 1414–1419. doi: 10.1016/j.cub.2007.07.032
Schradin, C., and Pillay, N. (2005). Intraspecific variation in the spatial and social organization of the African striped mouse. J. Mammal. 86, 99–107. doi: 10.1644/1545-1542(2005)086<0099:IVITSA>2.0.CO;2
Selander, R. K. (1964). Speciation in Wrens of the genus Campylorhynchus. Berkeley, CA: University of California Publications in Zoology.
Servedio, M. R., Price, T. D., and Lande, R. (2013). Evolution of displays within the pair bond. Proc. R. Soc. B Biol. Sci. 280:1757. doi: 10.1098/rspb.2012.3020
Solomon, N. G. (2003). A reexamination of factors influencing philopatry in rodents. J. Mammal. 84, 1182–1197. doi: 10.1644/BLe-013
Sorato, E., Gullett, P. R., Griffith, S. C., and Russell, A. F. (2012). Effects of predation risk on foraging behaviour and group size: adaptations in a social cooperative species. Anim. Behav. 84, 823–834. doi: 10.1016/j.anbehav.2012.07.003
Spinks, A. C., Jarvis, J. U. M., and Bennett, N. C. (2000). Comparative patterns of philopatry and dispersal in two common mole-rat populations: implications for the evolution of mole-rat sociality. J. Anim. Ecol. 69, 224–234. doi: 10.1046/j.1365-2656.2000.00388.x
Stacey, P. (1979a). Kinship, promiscuity, and communal breeding in the acorn woodpecker. Behav. Ecol. Sociobiol. 6, 53–66. doi: 10.1007/BF00293245
Stacey, P. B. (1979b). Habitat saturation and communal breeding in the acorn woodpecker. Anim. Behav. 27, 1153–1166. doi: 10.1016/0003-3472(79)90063-0
Stacey, P. B., and Ligon, J. D. (1987). Territory quality and dispersal options in the acorn woodpecker, and a challenge to the habitat-saturation model of cooperative breeding. Am. Nat. 130, 654–676. doi: 10.1086/284737
Stacey, P. B., and Ligon, J. D. (1991). The benefits-of-philopatry hypothesis for the evolution of cooperative breeding - variation in territory quality and group-size effects. Am. Nat. 137, 831–846. doi: 10.1086/285196
Stiver, K. A., Dierkes, P., Taborsky, M., Gibbs, H. L., and Balshine, S. (2005). Relatedness and helping in fish: examining the theoretical predictions. Proc. R. Soc. B Biol. Sci. 272, 1593–1599. doi: 10.1098/rspb.2005.3123
Szathmáry, E., and Smith, J. M. (1995). The major evolutionary transitions. Nature 374, 227–232. doi: 10.1038/374227a0
Taborsky, M. (1984). Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Anim. Behav. 32, 1236–1252. doi: 10.1016/S0003-3472(84)80241-9
Taborsky, M. (2016). “Cichlid fishes: a model for the integrative study of social behavior,” in Cooperative Breeding in Vertebrates: Studies of Ecology, Evolution and Behavior, eds W. D. Koenig and J. L. Dickinson (Cambridge: Cambridge University Press), 354–389.
Taborsky, M., and Wong, M. (2017). “Sociality in fishes,” in Comparative Social Evolution, eds D. I. Rubenstein and P. Abbot (Cambridge: Cambridge University Press).
Tewksbury, J. J., Huey, R. B., and Deutsch, C. A. (2008). Ecology - putting the heat on tropical animals. Science 320, 1296–1297. doi: 10.1126/science.1159328
Thacker, C. E., and Roje, D. M. (2011). Phylogeny of Gobiidae and identification of gobiid lineages. System. Biodivers. 9, 329–347. doi: 10.1080/14772000.2011.629011
Van De Casteele, T., Galbusera, P., and Matthysen, E. (2001). A comparison of microsatellite-based pairwise relatedness estimators. Mol. Ecol. 10, 1539–1549. doi: 10.1046/j.1365-294X.2001.01288.x
Waser, P. M., Creel, S. R., and Lucas, J. R. (1994). Death and disappearance - estimating mortality risks associated with philopatry and dispersal. Behav. Ecol. 5, 135–141. doi: 10.1093/beheco/5.2.135
Weinman, L. R., Solomon, J. W., and Rubenstein, D. R. (2015). A comparison of single nucleotide polymorphism and microsatellite markers for analysis of parentage and kinship in a cooperatively breeding bird. Mol. Ecol. Resour. 15, 502–511. doi: 10.1111/1755-0998.12330
Wolff, J. O. (1997). Population regulation in mammals: an evolutionary perspective. J. Anim.l Ecol. 66, 1–13. doi: 10.2307/5959
Wong, M., and Balshine, S. (2011). The evolution of cooperative breeding in the African cichlid fish, Neolamprologus pulcher. Biol. Rev. 86, 511–530. doi: 10.1111/j.1469-185X.2010.00158.x
Wong, M. Y. L. (2010). Ecological constraints and benefits of philopatry promote group-living in a social but non-cooperatively breeding fish. Proc. R. Soc. B Biol. Sci. 277, 353–358. doi: 10.1098/rspb.2009.1453
Wong, M. Y. L. (2011). Group size in animal societies: the potential role of social and ecological limitations in the group-living fish, Paragobiodon xanthosomus. Ethology 117, 638–644. doi: 10.1111/j.1439-0310.2011.01913.x
Wong, M. Y. L., and Buston, P. M. (2013). Social systems in habitat-specialist reef fishes: key concepts in evolutionary ecology. Bioscience 63, 453–463. doi: 10.1525/bio.2013.63.6.7
Wong, M. Y. L., Buston, P. M., Munday, P. L., and Jones, G. P. (2007). The threat of punishment enforces peaceful cooperation and stabilizes queues in a coral-reef fish. Proc. R. Soc. B Biol. Scie. 274, 1093–1099. doi: 10.1098/rspb.2006.0284
Wong, M. Y. L., Jordan, L. A., Marsh-Rollo, S., St-Cyr, S., Reynolds, J. O., Stiver, K. A., et al. (2012). Mating systems in cooperative breeders: the roles of resource dispersion and conflict mitigation. Behav. Ecol. 23, 521–530. doi: 10.1093/beheco/arr218
Wong, M. Y. L., Munday, P. L., Buston, P. M., and Jones, G. P. (2008a). Monogamy when there is potential for polygyny: tests of multiple hypotheses in a group-living fish. Behav. Ecol. 19, 353–361. doi: 10.1093/beheco/arm141
Wong, M. Y. L., Munday, P. L., Buston, P. M., and Jones, G. R. (2008b). Fasting or feasting in a fish social hierarchy. Curr. Biol. 18, R372–R373. doi: 10.1016/j.cub.2008.02.063
Wong, M. Y. L., Munday, P. L., and Jones, G. P. (2005). Habitat patch size, facultative monogamy and sex change in a coral-dwelling fish, Caracanthus unipinna. Environ. Biol. Fishes 74, 141–150. doi: 10.1007/s10641-005-6715-2
Wright, J., Parker, P. G., and Lundy, K. J. (1999). Relatedness and chick-feeding effort in the cooperatively breeding Arabian babbler. Anim. Behav. 58, 779–785. doi: 10.1006/anbe.1999.1204
Keywords: cooperative breeding theory, evolution of sociality, methodology, kinship, ecological constraints, benefits of philopatry, life-history hypothesis, fish
Citation: Hing ML, Klanten OS, Dowton M and Wong MYL (2017) The Right Tools for the Job: Cooperative Breeding Theory and an Evaluation of the Methodological Approaches to Understanding the Evolution and Maintenance of Sociality. Front. Ecol. Evol. 5:100. doi: 10.3389/fevo.2017.00100
Received: 23 October 2016; Accepted: 10 August 2017;
Published: 31 August 2017.
Edited by:
Trine Bilde, Aarhus University, DenmarkReviewed by:
Michael Taborsky, University of Bern, SwitzerlandKelly Anne Stiver, Southern Connecticut State University, United States
Copyright © 2017 Hing, Klanten, Dowton and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin L. Hing, mlh913@uowmail.edu.au
Marian Y. L. Wong, marianw@uow.edu.au
 Martin L. Hing
Martin L. Hing O. Selma Klanten
O. Selma Klanten Mark Dowton
Mark Dowton Marian Y. L. Wong
Marian Y. L. Wong