Mating Status Influences Cold Tolerance and Subsequent Reproduction in the Invasive Ladybird Harmonia axyridis
- 1Institut National de la Recherche Agronomique, UMR PVBMT, Saint-Pierre, Réunion, France
- 2CBGP, Institut National de la Recherche Agronomique, CIRAD, IRD, Montpellier SupAgro, University of Montpellier, Montpellier, France
- 3Department of Bioagricultural Science and Pest Management, Graduate Degree Program in Ecology, Colorado State University, Fort Collins, CO, United States
Among arthropods, ability to survive cold conditions may be instrumental for species invading temperate or colder climatic zones. Cold tolerance can be influenced by multiple environmental and physiological factors. We experimentally investigated the effects of mating status (unmated, mated, or mated, and reproductive) on cold tolerance and subsequent reproduction of the invasive harlequin ladybird Harmonia axyridis. We found that unmated adults survived cold better than mated ones. Among mated individuals, those that had not reproduced survived better than those that had reproduced. After cold stress, formerly unmated females were mated, and we evaluated their ability to reproduce. Females that reproduced prior to cold stress were less likely to reproduce after cold stress than females from the other treatments. We discuss what these results mean for the proportion of unmated females in H. axyridis aggregates at overwintering sites. This study highlights the importance of physiological status on cold tolerance of invasive arthropods.
Introduction
Temperature constitutes one of the main environmental factors explaining the geographic distribution of ectotherm species (Andrewartha and Birch, 1954). Noticeably, species from temperate and polar regions have to cope with cold conditions during winter. Insects have evolved several mechanisms allowing the persistence of populations including diapause, dispersal toward protective microclimate locations or production of antifreeze compounds (Graham et al., 2000; Denlinger, 2002; Hahn and Denlinger, 2011; Lee and Leskey, 2015).
The ability to pass through cold conditions may be instrumental for the establishment of species introduced into a temperate or polar region (Dalin et al., 2010). Indeed, the more individuals able to overwinter, the more quickly a population can build-up the following spring. More genetic variation is also likely to be maintained, potentially facilitating future invasion success. Several studies have shown that ability to survive cold conditions in invasive species evolves to better match novel environmental conditions in the introduced areas (Hsiao, 1985; Sadakiyo and Ishihara, 2011; Lehmann et al., 2014).
Cold tolerance may be influenced by the physiological status of an organism. In particular, factors such as age, sex, or nutritional status can impact cold tolerance within a given species (Bowler and Terblanche, 2008; Jeno and Brokordt, 2014; Plantamp et al., 2016). Mating can induce profound behavioral and physiological changes in organisms that can be either beneficial or detrimental. For instance, in some ant queens, mating can decrease immune responses (Baer et al., 2006). Similarly, sperm from multiple males reduces female hibernation success in bumble bees (Baer and Schmid-Hempel, 2005). In contrast, mated flies are more resistant to starvation than unmated ones (Goenaga et al., 2012). The effect of mating status on cold tolerance is up to now largely unknown (but see Boulétreau-Merle and Fouillet, 2002; Ryan et al., 2016).
In this study, we thus explored experimentally the effect of mating status on cold tolerance and subsequent reproductive capacity in the invasive harlequin ladybird Harmonia axyridis (Coleoptera: Coccinellidae). Native to Asia, H. axyridis has been widely used as a biological control agent against aphids, particularly in North America and Europe (Koch, 2003). It since has become invasive in four different continents (North and South America, Europe, and Africa) with several negative side effects: reducing biodiversity through non-target prey and intraguild predation, overwintering in large numbers in houses, where it is a nuisance and causes allergies, and decreasing the taste and quality in fruit products, especially wine (Koch, 2003; van Lenteren et al., 2008; De Clercq and Bale, 2011). It is considered to be bivoltine but up to five generations per year have been observed (Bazzocchi et al., 2004; Nedved and Honek, 2012). In winter months, the species aggregates in concealed and sheltered locations that provide a protective microclimate (Berkvens et al., 2010; Wang et al., 2011; Durieux et al., 2012; Raak-van den Berg et al., 2012). It might be advantageous for a female to be fertilized before leaving these overwintering aggregation sites, as this would allow females to found populations even without males (Awad et al., 2013). One may ask which strategy, mating before or after the cold period leading to a dormancy state, provides the highest fitness. Although it is usually believed that diapausing coccinellids mate at the end of hibernation, before aggregations disperse, some mating may also occur before diapause (Hodek, 1973; Awad et al., 2013). To better understand the consequence of mating on overwintering individuals, we manipulated mating status under controlled conditions and tested whether it impacted survival of cold conditions and subsequent reproduction.
Materials and Methods
Biological Material and Rearing Conditions
We sampled over 100 H. axyridis individuals in November 2012 from a European population located in Biot, Southern France (43°40′44″ North; 07°02′26″ East). These individuals were in the process of initiating overwintering. Individuals were sampled from building walls and nearby Albizzia trees in an area covering several 100 m2. Before the experiments started, this natural invasive population was reared under controlled environmental conditions for two generations (around 800 individuals per generation) to standardize maternal environmental effects. All individuals were reared at 24°C, with 14:10 L:D and fed with an excess of ionized Ephestia kuehniella eggs (Lepidoptera: Pyralidae). We used pieces of black cardboard, folded above the food, as oviposition supports. We produced 720 G2 individuals (1:1 sex ratio) for the cold tolerance experiments. Males and females were immediately separated after emergence to prevent mating. They were maintained in the same environmental conditions for 10 days to insure their reproductive maturity at the beginning of the experiments.
Experimental Protocol
The 720 individuals were randomly distributed into equal density groups between three treatments: unmated, mated, and reproductive. For the unmated group, 120 males and 120 females were kept in unisex groups of 20 individuals per box (length: 26 cm, width: 13 cm, height: 8 cm) for 10 days prior to the cold stress event. To create the mated group, 120 males and 120 females were kept in unisex groups of 20 for 8 days, and then for the final 2 days, males and females were mixed (10 males and 10 females per box) to mate at will just prior to the cold stress event. This timing insured that all females were mated at least once (Laugier et al., 2013), but that they did not have time to lay eggs before the cold stress. To create the reproductive group, 120 males, and 120 females were kept in mixed groups of 20 (10 males and 10 females) for the full 10 days prior to the cold stress event. The reproductive treatment allowed individuals to mate and reproduce. We confirmed that all females of this reproductive group laid eggs prior to cold stress.
As strictly replicating the natural conditions of diapause in the lab (progressive shifts of temperature, photoperiod, duration of cold temperature,…etc.) is very difficult, if not impossible, we decided to plan a cold-stress experiment (see for instance Boulétreau-Merle and Fouillet, 2002; Baer and Schmid-Hempel, 2005). For the cold stress, individuals of age 10 days were distributed in boxes (length: 26 cm, width: 13 cm, height: 8 cm), with 10 females and 10 males by box according to mating status (hence a total of 12 boxes per treatment group). These boxes were first placed at 13°C during 2 days as an intermediate low temperature step, and then either at −1.5 or −5°C during 10 days. During the 13°C step, ladybirds keep still in such a way that no copulation happens. One box of each mating status was placed at 13°C for 10 days to serve as control. To end the cold stress event, all the boxes were placed at 13°C for 2 days and then transferred back to 24°C. We then counted the number of dead males and females per box on the fourth day at 24°C.
Subsequently, we evaluated the ability of females that survived cold stress at −1.5°C to reproduce. After 10 days at 24°C, we paired individuals (one male and one female) within each mating status in cylindric boxes (height: 2.5 cm, diameter: 5 cm). We then counted the number of females that laid eggs during the 2 following weeks. Without cold stress, females can produce on average about 4,000 eggs over the course of 15 weeks in our laboratory conditions (Tayeh et al., 2015). Thus, it is important to note that the few clutches laid in the reproductive treatment prior to cold stress would not have depleted egg load, and whether or not any eggs were laid over 2 weeks after cold stress was thus a function of general physiological status, rather than egg load.
Statistical Analyses
All statistical analyses were conducted using the JMP Pro 9 package (SAS Institute 2009). To study survival, we analyzed the proportion of surviving individuals using a general linear model (GLM) with a binomial probability distribution and a logit link function. The model included four factors: temperature (−1.5 or −5°C), mating status (unmated, mated and reproductive), box nested in mating status, and sex. It also included the interactions between temperature and mating status, sex, and mating status and between sex and temperature.
To study the reproductive capacity following cold stress, we analyzed the proportion of females that laid eggs after the cold stress at −1.5°C using a GLM with a binomial probability distribution and a logit link function. We tested only the factor mating status.
Results
All individuals in the control boxes at 13°C survived. Survival rates differed significantly among the two tested temperatures (P < 0.0001) with an average proportional survival rate of 0.89 at −1.5°C and 0.26 at −5°C (Figure 1, Table 1). There was also a pronounced effect of mating status on survival rates (P < 0.0001). Unmated individuals survived better (overall mean survival rate = 0.77) than mated or mated and reproductive individuals (P < 0.00001). Mated but not reproductive individuals survived at a higher rate than those that had reproduced prior to cold stress (0.53 vs. 0.42, respectively; P = 0.0004). Sex, box as well as all the two-way interactions were not significant (see Table 1).
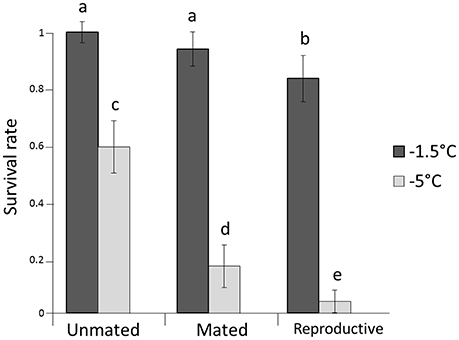
Figure 1. Survival rates for females after exposed to −1.5 and −5°C. Error bars represent the 95% confidence interval around estimates of the mean. Lower case letters represent significant differences.
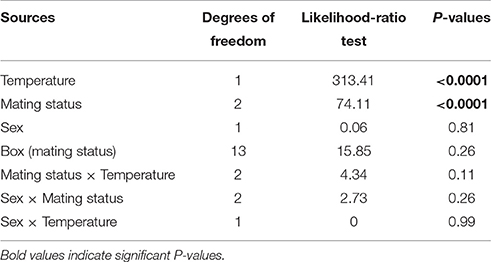
Table 1. Effects of temperature, mating status, sex, box, and the interactions on the hatching rate proportion of surviving individuals after a cold stress event at two different sub-zero temperatures (i.e., −1.5 and −5°C).
Mating status had a significant effect on subsequent reproductive capacity (P = 0.045; Figure 2). Females from the reproductive group had lower reproductive capacity (proportion of females laying eggs = 0.53) than females from both other groups (mated group 0.75; unmated group 0.76; P = 0.038 and 0.021, respectively). There was no significant difference between females from the mated group and females from the unmated group (P = 0.97).
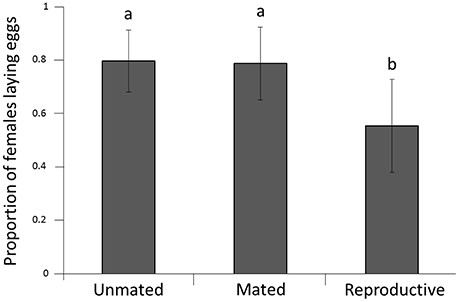
Figure 2. Proportion of females ovipositing after cold stress at −1.5°C. Error bars represent the 95% confidence interval around estimates of the mean. Lower case letters represent significant differences.
Discussion
In this study, we tested the prediction that mating experience would impact cold tolerance and subsequent reproduction in the invasive ladybird H. axyridis. We measured survival and subsequent ability of individuals exposed to a sub-zero temperature stress to reproduce, and evaluated whether survival and reproduction depended upon mating status.
Our study clearly demonstrates that mating experience influences adult survival of H. axyridis at the two tested temperatures (−1.5 and −5°C). Unmated males and females survive cold stress better than mated ones, demonstrating that mating is costly for both sexes in H. axyridis. This result fits well with the findings of Himuro and Fujisaki (2010), who show that mating experience weakens starvation tolerance for both sexes in the seed bug Togo hemipterus (Heteroptera: Lygaeidae). The present study provides new evidence that mating is not cost-free, even for males (Perry and Tse, 2013). Indeed, reproductive costs for males may be as high as those for females (Scharf et al., 2013).
Moreover we found that, among mated H. axyridis individuals, those that were not yet reproductive before the cold stress survived better than those that were already reproductive. For females, this difference can be easily explained by the egg-laying costs (Rönn et al., 2006). In the reproductive group, males, which could mate multiple times, also had decreased survival with cold stress in a similar way. Investment in reproduction prior to cold stress reduced fecundity of females after cold stress. In contrast, there was no cost of mating but not investing in reproduction. Reproductive females could also have experienced more mating events during the 10 days before the cold stress than females mated only 2 days before. By definition, reproductive females had laid eggs. However, the eggs produced during the first few days of reproduction represent little compared to the 4,000 eggs produced over the reproductive lifetime (Tayeh et al., 2015). It seems likely that the decrease of their subsequent ability to lay any eggs was linked to physiological changes that these reproductive females go through, changes that evidently decreased their ability to reproduce after cold stress compared to the two other groups of females (Chapman et al., 1998).
Although our cold-stress experiment is a little remote of the natural overwintering conditions (Sakurai et al., 1992), it is interesting to evaluate the situation in the field in light of the effect of mating regime that we identified in controlled-conditions. The percentage of overwintering females that are fertilized is quite variable between field samples. Nalepa et al. (1996) found that only 12% of females were fertilized in samples collected in USA. In France, four sites have been sampled with 10, 17, 37, and 53% of females fertilized (Iperti and Bertrand, 2001). Within the native range, the percentage of fertilized females varies between 20 and 60% (Nedvědová et al., 2013). These field estimates are congruent with our results, which give rise to the prediction that most females should not be fertilized. The variability across field samples may be due to different timing of those particular collections. Samples collected through time from the same localities could be used to properly test whether the percentage of fertilized females decreases throughout the winter due to differential mortality. Another interesting prospect could be to test whether the percentage of overwintering fertilized females has evolved during the course of the invasion using samples from native and invasive localities, including locations at different latitudes within both ranges.
The effect of mating status on cold tolerance may be a general pattern, occurring in a large number of species. According to Hodek (1973), diapausing coccinellids usually mate at the end of hibernation, before aggregations disperse. For instance, only unmated females were found in winter collections of Semiadalia undecimnotata (Coleoptera: Coccinellidae) (Hodek and Landa, 1971). Unmated females seem also to be the majority at winter sites in the migrating monarch butterfly with only 1/3 of the overwintering females containing sperm from summer males within their spermatheca (Leong et al., 2012). It has also been shown that common fruit flies with longer preoviposition duration survive longer under cold than flies with short-retention phenotypes (Boulétreau-Merle and Fouillet, 2002). In contrast, no effect of mating status on the overwintering survival has been found in the social wasp Vespula maculifrons (Hymenoptera: Vespidae) (Kovacs and Goodisman, 2012) and in the invasive fruit fly Drosophila suzukii (Diptera: Drosophilidae) (Ryan et al., 2016).
Author Contributions
Conceived study: BF, AE, and AT. Collected data: BF and AT. Statistical analyses: AT. Wrote the manuscript: BF. Improved the manuscript: RH and JF.
Funding
This study was supported by the GDR 3647 “Invasions biologiques,” funds from INRA and a grant from the ERA-Net BiodivERsA, with the national funders ANR (France), DFG (Germany), and BELSPO (Belgium), as part of the 2012–2013 BiodivERsA call for research proposals. RH and AE acknowledge financial support by the French Agropolis Foundation and the Labex Agro-Montpellier through the AAP “International Mobility” (CfP 2015-02), the labex CEMEB through the AAP “invited scientist 2016,” and the French programme investissement d'avenir.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Andrewartha, H. G., and Birch, L. C. (1954). The Distribution and Abundance of Animals. Chicago, IL: Chicago University Press.
Awad, M., Kalushkov, P., Nedvědová, T., and Nedvěd, O. (2013). Fecundity and fertility of ladybird beetle Harmonia axyridis after a prolonged cold storage. BioControl 58, 657–666. doi: 10.1007/s10526-013-9512-4
Baer, B., and Schmid-Hempel, P. (2005). Sperm influences female hibernation success, survival and fitness in the bumble-bee Bombus terrestris. Proc. R. Soc. Lond. B 272, 319–323. doi: 10.1098/rspb.2004.2958
Baer, B., Armitage, S. A. O., and Boomsma, J. J. (2006). Sperm storage induces an immunity cost in ants. Nature 441, 872–875. doi: 10.1038/nature04698
Bazzocchi, G. G., Lanzoni, A., Accinelli, G., and Burgio, G. (2004). Overwintering, phenology and fecundity of Harmonia axyridis in comparison with native coccinellid species in Italy. Biocontrol 49, 245–260. doi: 10.1023/B:BICO.0000025382.07841.b4
Berkvens, N., Bale, J. S., Berkvens, D., Tirry, L., and De Clercq, P. (2010). Cold tolerance of the harlequin ladybird Harmonia axyridis in Europe. J. Insect Physiol. 56, 438–444. doi: 10.1016/j.jinsphys.2009.11.019
Boulétreau-Merle, J., and Fouillet, P. (2002). How to overwinter and be a founder: egg-retention phenotypes and mating status in Drosophila melanogaster. Evol. Ecol. 16, 309–332. doi: 10.1023/A:1020216230976
Bowler, K., and Terblanche, J. S. (2008). Insect thermal tolerance: what is the role of ontogeny, ageing and senescence? Biol. Rev. 83, 339–355. doi: 10.1111/j.1469-185X.2008.00046.x
Chapman, T., Miyatake, T., Smith, H., and Partridge, L. (1998). Interactions of mating, egg production and death rates in females of the Mediterranean fruit fly, Ceratitis capitata. Proc. R. Soc. Lond. B. 265, 1879–1894. doi: 10.1098/rspb.1998.0516
Dalin, P., Bean, D. W., Dudley, T. L., Carney, V. A., Eberts, D., Gardner, K. T., et al. (2010). Seasonal adaptations to day length in ecotypes of Diorhabda spp. (Coleoptera: Chrysomelidae) inform selection of agents against saltcedars (Tamarix spp.). Environ. Entomol. 39, 1666–1675. doi: 10.1603/EN09270
De Clercq, P., and Bale, J. S. (2011). “Risks of invertebrate biological control agents—Harmonia axyridis as a case study,” in Regulation of Biological Control Agents, ed R. U. Ehlers (Dordrecht: Springer), 243–255.
Denlinger, D. L. (2002). Regulation of diapause. Annu. Rev. Entomol. 47, 93–122. doi: 10.1146/annurev.ento.47.091201.145137
Durieux, D., Fischer, C., Brostaux, Y., Sloggett, J. J., Deneubourg, J.-L., Vandereycken, A., et al. (2012). Role of long-chain hydrocarbons in the aggregation behavior of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). J. Insect Physiol. 58, 801–807. doi: 10.1016/j.jinsphys.2012.03.006
Goenaga, J., Mensch, J., Fanara, J. J., and Hasson, E. (2012). The effect of mating on starvation resistance in natural populations of Drosophila melanogaster. Evol. Ecol. 26, 813–823. doi: 10.1007/s10682-011-9540-4
Graham, L. A., Walker, V. K., and Davies, P. L. (2000). Developmental and environmental regulation of antifreeze proteins in the mealworm beetle Tenebrio molitor. Eur. J. Biochem. 267, 6452–6458. doi: 10.1046/j.1432-1327.2000.01734.x
Hahn, D. A., and Denlinger, D. L. (2011). Energetics of insect diapause. Annu. Rev. Entomol. 56, 103–121. doi: 10.1146/annurev-ento-112408-085436
Himuro, C., and Fujisaki, K. (2010). Mating experience weakens starvation tolerance in the seed bug Togo hemipterus (Heteroptera: Lygaeidae). Physiol. Entomol. 35, 128–133. doi: 10.1111/j.1365-3032.2009.00719.x
Hodek, I. (1973). Biology of Coccinellidae. Prague: Academia Publishing House of the Czechoslovak, Academy of Sciences.
Hodek, I., and Landa, V. (1971). Anatomical and histological changes during dormancy in two Coccinellidae. BioControl 16, 239–251.
Hsiao, T. H. (1985). “Ecophysiological and genetic aspects of geographic variations of the Colorado potato beetle,” in Proceedings of the Symposium on the Colorado Potato Beetle, XVIIth International Congress of Entomology, eds D. N. Ferro and R. H. Voss (Hamburg) 63–77.
Iperti, G., and Bertrand, E. (2001). Hibernation of Harmonia axyridis (Coleoptera: Coccinellidae) in Southern France. Acta Soc. Zool. Bohem. 65, 207–210.
Jeno, K., and Brokordt, K. (2014). Nutritional status affects the capacity of the snail Concholepas concholepas to synthesize Hsp70 when exposed to stressors associated with tidal regimes in the intertidal zone. Mar. Biol. 161, 1039–1049. doi: 10.1007/s00227-014-2397-7
Koch, R. L. (2003). The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control and non-target impacts. J. Insect Sci. 3, 1–16. doi: 10.1673/031.003.3201
Kovacs, J. L., and Goodisman, M. A. D. (2012). Effects of size, shape, genotype, and mating status on queen overwintering survival in the social wasp Vespula maculifrons. Environ. Entomol. 41, 1612–1620. doi: 10.1603/EN12023
Laugier, G. J. M., Le Moguédec, G., Tayeh, A., Loiseau, A., Osawa, N., Estoup, A., et al. (2013). Increase in male reproductive success and female reproductive investment in invasive populations of the harlequin ladybird Harmonia axyridis. PLoS ONE 8:e77083. doi: 10.1371/journal.pone.0077083
Lee, D. H., and Leskey, T. C. (2015). Flight behavior of foraging and overwintering brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). B. Entomol. Res. 105, 566–573. doi: 10.1017/S0007485315000462
Lehmann, P., Lyytinen, A., Piiroinen, S., and Lindström, L. (2014). Northward range expansion requires synchronization of both overwintering behaviour and physiology with photoperiod in the invasive Colorado potato beetle (Leptinotarsa decemlineata). Oecologia 176, 57–68. doi: 10.1007/s00442-014-3009-4
Leong, K. H. L., Yoshimura, M. A., and Williams, C. (2012). Adaptive significance of previously mated monarch butterfly females (Danaus plexippus (Linneaus)) overwintering at a California site. J. Lepid. Soc. 66, 205–210. doi: 10.18473/lepi.v66i4.a3
Nalepa, C. A., Kidd, K. A., and Ahlstrom, K. R. (1996). Biology of Harmonia axyridis (Coleoptera: Coccinellidae) in winter aggregations. Ann. Entomol. Soc. Am. 89, 681–685. doi: 10.1093/aesa/89.5.681
Nedved, O., and Honek, A. (2012). “Life history and development,” in Ecology and Behaviour of the Ladybird Beetles (Coccinellidae), eds I. Hodek, H. F. van Emden, and A. Honek (Oxford, UK: Wiley-Blackwell), 54–109.
Nedvědová, T., Awad, M. A. E. E., Ungerová, D., and Nedvěd, O. (2013). Characteristics of the ladybird Harmonia axyridis during autumn migration. IOBC-WPRS Bull. 94, 117–122.
Perry, J. C., and Tse, C. T. (2013). Extreme costs of mating for male two-spot ladybird beetles. PLoS ONE 8:e81934. doi: 10.1371/journal.pone.0081934
Plantamp, C., Salort, K., Gibert, P., Dumet, A., Mialdea, G., Mondy, N., et al. (2016). All or nothing: survival, reproduction and oxidative balance in spotted wing Drosophila (Drosophila suzukii) in response to cold. J. Insect Physiol. 89, 28–36. doi: 10.1016/j.jinsphys.2016.03.009
Raak-van den Berg, C. L., Hemerik, L., de Jong, P. W., and van Lenteren, J. C. (2012). Mode of overwintering of invasive Harmonia axyridis in the Netherlands. BioControl 57, 71–84. doi: 10.1007/s10526-011-9394-2
Rönn, J., Katvala, M., and Arnqvist, G. (2006). The costs of mating and egg production in Callosobruchus seed beetles. Anim. Behav. 72, 335–342. doi: 10.1016/j.anbehav.2005.10.024
Ryan, G. D., Emiljanowicz, L., Wilkinson, F., Kornya, M., and Newman, J. A. (2016). Thermal tolerance of the spotted-wing Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 109, 746–752. doi: 10.1093/jee/tow006
Sadakiyo, S., and Ishihara, M. (2011). Rapid seasonal adaptation of an alien bruchid after introduction: geographic variation in life cycle synchronization and critical photoperiod for diapause induction. Entomol. Exp. Appl. 140, 69–76. doi: 10.1111/j.1570-7458.2011.01136.x
Sakurai, H., Kawai, T., and Takeda, S. (1992). Physiological changes related to diapause of the lady beetle, Harmonia axyridis (Coleoptera: Coccinellidae). Appl. Entomol. Zool. 27, 479–487.
Scharf, I., Peter, F., and Martin, O. Y. (2013). Reproductive trade-offs and direct costs for males in arthropods. Evol. Biol. 40, 169–184. doi: 10.1007/s11692-012-9213-4
Tayeh, A., Hufbauer, R., Estoup, A., Ravigné, V., Frachon, L., and Facon, B. (2015). Biological invasion and biological control select for different life histories. Nat. Commun. 6:8268. doi: 10.1038/ncomms8268
van Lenteren, J. C., Loomans, A., Babendreier, D., and Bigler, F. (2008). Harmonia axyridis: an environmental risk assessment for northwest Europe. BioControl 53, 37–54. doi: 10.1007/s10526-007-9120-2
Keywords: cold tolerance, mating status, invasive species, overwintering, Harmonia axyridis
Citation: Facon B, Estoup A, Hufbauer RA, Foucaud J and Tayeh A (2017) Mating Status Influences Cold Tolerance and Subsequent Reproduction in the Invasive Ladybird Harmonia axyridis. Front. Ecol. Evol. 5:108. doi: 10.3389/fevo.2017.00108
Received: 09 June 2017; Accepted: 29 August 2017;
Published: 09 October 2017.
Edited by:
Eric W. Riddick, Agricultural Research Service (USDA), United StatesReviewed by:
Su Wang, Beijing Academy of Agricultural and Forestry Sciences, ChinaPeter Schausberger, University of Vienna, Austria
Copyright © 2017 Facon, Estoup, Hufbauer, Foucaud and Tayeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benoit Facon, benoit.facon@inra.fr
 Benoit Facon
Benoit Facon Arnaud Estoup
Arnaud Estoup Ruth A. Hufbauer2,3
Ruth A. Hufbauer2,3  Julien Foucaud
Julien Foucaud