Behavioral Differences among Eastern Bluebird Populations Could Be a Consequence of Tree Swallow Presence: A Pilot Study
- Biology Department, Appalachian State University, Boone, NC, United States
Aggressive interference competition for limited resources is frequently observed among animals. However, these behavioral interactions within (intraspecific) and between (interspecific) species are costly as they can be energetically expensive and cause injury or death. To avoid these agonistic interactions, numerous species alter their behaviors and resource requirements. Spatial variation in nest site competition allows for investigation of concurrent variation in territorial defense behavior. Further, among species with bi-parental nest defense, behavioral similarity in territorial defense may benefit pairs. Here, we studied territorial aggression between two eastern bluebird (Sialia sialis) populations (North Carolina and Alabama, USA) that differ in avian community structure; those in North Carolina have recently begun to experience strong interspecific competition for nesting cavities by tree swallows (Tachycineta bicolor), a competitive pressure that is relatively new for North Carolina bluebirds (~35–40 years) and is absent in Alabama populations. We found that bluebirds in North Carolina are more aggressive to simulated territorial intrusions compared to their Alabama counterparts. Behavioral similarity (here, in aggression) between partners is strong and similar in both populations. These results suggest that bluebirds in North Carolina may have to maintain higher baseline aggression during territory establishment and nest construction to co-occur with highly aggressive tree swallows, but that, in both populations, behavioral similarity between partners may be adaptive. Finally, we acknowledge the preliminary nature of this study and the need for expanding the behavioral studies to other sites in the southeastern United States. Greater regional coverage would exclude the possibility of alternative drivers of the observed behavioral differences between the North Carolina and Alabama populations.
Introduction
Competition is a fundamental component in ecology and population biology, and for decades, researchers have investigated how inter- and intraspecific competition influences ecological niches and community dynamics (reviewed in Alley, 1982). The competitive exclusion principle argues that interspecific competition will eventually lead to one species out-competing the other or there will be a behavioral shift toward an ecological niche to allow co-existence (Hardin, 1960; reviewed in Mooney and Cleland, 2001). Additionally, competitive pressures within a population fluctuate as resource abundance and populations change (reviewed in Alley, 1982), which may reduce fitness.
Population dynamics can be further altered during invasions as nonnative species colonize (reviewed in Shea and Chesson, 2002). When nonnative species are introduced, native species experience novel competitive, dominant, and/or aggressive interactions (Freeman and Byers, 2006; Strauss et al., 2006). Individuals may cope with increased agonistic interspecific interactions via alteration of morphology (Freeman and Byers, 2006; Langkilde, 2009), physiology (Phillips and Shine, 2006), and/or behavior (Langkilde, 2009). However, not all individuals possess the ability to respond quickly and effectively to a rapid change in community composition. For example, aggressive house sparrows (Passer domesticus) that were suddenly introduced to West Mexico quickly outcompeted local avian species for food and habitat and now dominate cities (MacGregor-Fors et al., 2010). House sparrows are just one of many examples of nonnative selection pressures that displace resident species through aggressive competition. Indeed, during most agonistic interactions the “winner” is often more aggressive. Among range expanding western bluebirds (Sialia mexicana), individuals with more aggressive phenotypes are able to obtain and keep territories in novel environments; however, when breeding environments are no longer novel, aggressive individuals experience lower fitness (Duckworth and Badyaev, 2007).
For species with bi-parental care, behavioral compatibility within breeding partners can benefit reproductive success (Spoon et al., 2007; Schuett et al., 2010). A key component to mate compatibility is partners that are behavioral similar, which is the tendency for two individuals to behave like each other (Spoon et al., 2007; Schuett et al., 2010). For example, partners that are similar in nest defense and feeding strategies can achieve high fitness (Spoon et al., 2007; reviewed in Schuett et al., 2010, 2011a). Indeed, partner similarity positively influences the reproductive success of zebra finches (Taeniopygia guttata; Schuett et al., 2011a) and great tits (Parus major; Both et al., 2005). Aggression of both parents may be especially important for cavity nesting birds because both male and female defend the nest (reviewed in Schuett et al., 2010).
Here, we aimed to understand how a natural range expansion of an interspecific competitor (tree swallow, Tachycineta bicolor) affects the behavior of a native species (eastern bluebird, Sialia sialis). We compared bluebird aggression and similarity in aggression of mated partners between two breeding populations: in North Carolina, where bluebirds experience interspecific competition with tree swallows (a locally novel competitor) and in Alabama, where tree swallows have not yet colonized. Additionally, because population dynamics can be influenced by environmental factors like habitat, we also investigated land use type at each site (e.g., open habitat, developed, etc.). We hypothesized that the presence of tree swallows would influence bluebird behavior. We predicted that bluebirds in North Carolina would defend territories more aggressively than those in Alabama because tree swallows are intense competitors for nest boxes and have negative effects on eastern and western bluebird species' reproductive success (Duckworth, 2006; Harris and Siefferman, 2014). Additionally, because Harris and Siefferman (2014) demonstrated fitness benefits of similarity in partner territorial defense behavior (in both aggressive and nonaggressive partners) at locations of high competition with tree swallows in North Carolina, we predicted that partner similarity might be more pronounced in the North Carolina population.
Materials and Methods
Study Species
Eastern bluebirds and tree swallows are obligate secondary cavity nesting passerines that readily use nest boxes and the nest cavity is the limiting resource that the two species agonistically compete over. Both species exhibit bi-parental care of young and territorial defense (reviewed in Lambrechts et al., 2010). Eastern bluebirds weigh ~30 g and breed throughout eastern and central United States. Both sexes defend the nest cavity and territory (75 m radius around box) throughout the season (pairs can produce two to three broods per year) and forage on terrestrial arthropods (Gowaty and Plissner, 2015). Individual eastern bluebirds exhibit repeatable aggressive behavior (Burtka and Grindstaff, 2013; Harris and Siefferman, 2014) and behavioral similarity of partners in nest defense has been demonstrated (Harris and Siefferman, 2014; Burtka and Grindstaff, 2015). Tree swallows weigh ~20 g and have recently expanded their breeding range to the southeastern United States (~40 years; Lee, 1993) and often outcompete bluebirds for nesting cavities (45% usurped in 2015, pers. obs.; Harris and Siefferman, 2014). Tree swallows are semi-colonial nesters that forage on emergent aquatic insects and feed within a 300 m radius of their nest (McCarty and Winkler, 1999). Although bluebirds and tree swallows co-occur in the northeastern North America, for southeastern breeding bluebirds, tree swallows represent a relatively new interspecific competitor, thus allowing for the unique opportunity to investigate the effects of an invasive-like competitor on a native species.
Field Sites
We studied eastern bluebirds at two sites during the early breeding season: Lee Co., Alabama (32.5934 N 85.4952 W) from March 8 to 14, 2015 and Watauga Co., North Carolina (32.2996 N 81.6765 W) from March 21 to April 9, 2015. Although bluebird pairs can produce multiple successful nests per season, we only monitored first nests. There is little interspecific competition (i.e., no tree swallows) at our Alabama field site; although other species occupy nest boxes in Alabama, bluebirds are the dominant competitors, and thus intraspecific competition likely plays a larger role than interspecific. Site occupancy for bluebirds is ~49% of nest boxes per breeding season (65/134 boxes used) and the average distance between nearest intraspecific neighbor is 154 m. Site occupancy of other species combined in Alabama is 8% (11/134 boxes). At the North Carolina site, the bluebird population has recently experienced the arrival of tree swallows (pers. obs.; Lee, 1993). Site occupancy for bluebirds is ~32% of nest boxes per breeding season (52/159 boxes used) and the average distance between nearest intraspecific neighbor is 281 m. Tree swallows occupy ~31% of the nest boxes per breeding season (50/159) and 22% of the boxes used by bluebirds are occupied sequentially by bluebirds and then tree swallows (11/50). Also, tree swallows often harass bluebirds, cause nest failure and then build nests in adjacent nest boxes. In 2015 at the North Carolina site, we monitored bluebird nests throughout the breeding season, and calculated success/fail rates. If bluebird pairs successfully fledged offspring, the nest was considered a success. However, if a bluebird pair had eggs that did not survive to fledge, the nest was considered a failure. Nest were considered usurped by tree swallows if the eggs were either abandoned, found punctured in the nest, or found punctured on the ground just below the nest, combined with either (1) observation of tree swallows harassing the bluebird pair prior to nest failure or (2) evidence that tree swallow commenced building a nest with 10 days of the bluebird nest failure.
Behavioral Trials
At both sites, we conducted simulated territorial intrusions (STIs) with an intraspecific playback (bluebird chatter) to measure aggressive behavior in the early breeding season (defined as nest building to the day the first egg was laid). We chose to use early breeding season because bluebirds compete against each other for territories in the early part of the season and most strongly against tree swallows (Harris and Siefferman, 2014; pers. obs.). Moreover, we monitored behavior for only a subset of the earliest breeding pairs at each site due to logistical limitations. Tree swallows initiate clutches ~1 month later than bluebirds and typically produce only 1 brood per season. However, they defend cavities much earlier than nest initiation (pers. obs.), overlapping temporally when bluebirds initiate their first brood. After identifying an active pair of bluebirds at a nest box, we placed a CD player with speaker (Memorex® MP8806) directly under the box and broadcasted bluebird chatter at a preset high volume for 10 min. Each bird experienced the same chatter recording, which was obtained from subsampling multiple bird vocalizations from Macaulay Library at Cornell Lab of Ornithology. We quantified male and female latency to approach the nest box (within ~1 m radius of box) and the number of dives at the speaker. We used an intraspecific playback to ensure it was a stimulus the bluebirds were familiar with between both populations (North Carolina and Alabama). We chose to broadcast chatter instead of song for multiple reasons: song can be influenced by dialect while call notes are less influenced by geography (Lemon, 1975), and both sexes use and respond to chatter during territory defense and anti-predator interactions. Finally, chatter tends to elicit quicker responses from bluebirds (L. Siefferman, pers. obs.). We recognize the limitation of using a single exemplar of vocalization allows for the possibility that individual or population level responses may have been driven by some characteristic of the recording itself.
Habitat Analysis
Land use/land cover (LULC) was characterized using ArcGIS v 10.2 (ESRI 2013). We obtained LULC data from the United States Geological Survey (USGS) National Land Cover Dataset (2011) for North Carolina and Alabama, USA at 30 × 30 m resolution, and were projected using the Universal Transverse Mercator (16S for Alabama and 17S for North Carolina) coordinate system. We created a 75 m radius foraging/territory buffer around each nest box to assess LULC within each buffer. Because bluebirds forage within a 75 m radius of the box (Gowaty and Plissner, 2015), this buffer size is most likely biologically and ecologically important for survival and reproductive success. Previous research has shown that bluebirds preferentially settle in open habitat (Jones et al., 2014; Gowaty and Plissner, 2015). The USGS LULC data provides multiple land cover parameters that are intercorrelated. Therefore, we classified developed-open, barren land, hay/pasture, and cultivated crops as one “open habitat” variable.
Statistical Analysis
Statistical analyses were performed using SPSS v. 23 (IBM, 2015). Shapiro-Wilk tests demonstrated that all behavioral and habitat data deviated significantly from a normal distribution (p < 0.05), except Alabama percent openness (p = 0.41). Additionally, North Carolina territories had greater percent openness than those in Al, but there was no significant correlation between percent open and behavior (i.e., latency to approach, number of dives, LULC). Therefore, we did not control for openness.
We investigated habitat variation and behavioral differences between sexes and between sites using Mann Whitney U-test. Additionally, in Alabama, because only two bluebird pairs dove, we did not analyze number of dives. We utilized a cross-tab chi-square test to determine if males or females in North Carolina were more likely to dive at the speaker. Partner similarity in behavior was quantified by subtracting male from female latency to approach and creating a new dependent variable (based on methods of Burtka and Grindstaff, 2015).
Ethics Statement
This study was carried out in accordance with the recommendations for the Care and Use of Animals for Research, Teaching, or Demonstrations provided by Appalachian State University (#12-09) and Auburn University (#12-68) through Institutional Animal Care and Use Committee (IACUC) under USFWS Master Banding Permit #23563. All animals were handled in such a way to reduce stress and avoid physical harm. All adults were released in their home territory.
Results
Alabama
We found evidence for partner similarity in response to STIs. Male [median (md); md = 56.50 s; n = 19] and female latency (md = 75.00 s; n = 19) were significantly positively correlated (rs = 0.81, p < 0.001, n = 19; Figure 1). Moreover, males and females did not differ in latency to approach the nest boxes (U = 147.00, Z = −0.73, p = 0.48, n = 38).
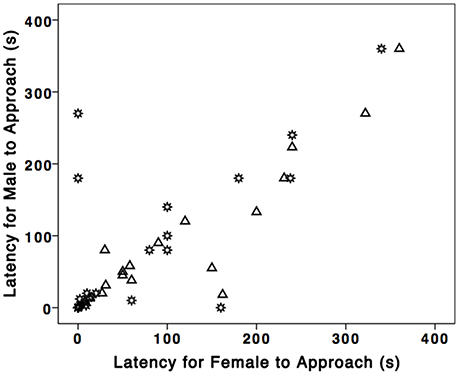
Figure 1. Male and female partner similarity in North Carolina and Alabama breeding eastern bluebirds. Each symbol represents a mated pair of bluebirds. Gear symbol represents NC and triangles represent AL.
North Carolina
Bluebird partners behaved similarly in response to STIs: female (md = 10.00 s; n = 29) and male latency (md = 7.00 s; n = 29) to approach the nest box were significantly positively correlated (rs = 0.66, p < 0.001, n = 29; Figure 1) as were female (md = 3) and male (md = 5) number of dives (rs = 0.62, p < 0.001, n = 29). Males and females did not differ significantly in either latency to approach the nest box (U = 397, Z = –0.38, p = 0.71, n = 58) or likelihood of diving at speaker (Males 86%, Females 80%; χ2 = 1.10, p = 0.50, n = 58). Additionally, 23/29 early bluebird nests failed, and 13 of those were directly attributed to tree swallows.
Differences between Field Sites
Alabama females (n = 19) were slower to approach the nest box (U = 131.0, Z = –3.07, p = 0.002; Figure 2) and dove less often (U = 104.5, Z = –3.8, p < 0.001) compared to North Carolina females (n = 29). Alabama males (n = 19) took longer to approach nest box (U = 163.5, Z = –2.37, p = 0.01; Figure 2) and dove less often (U = 92.5, Z = –3.92, p < 0.001) compared to North Carolina (n = 29) males. There were no differences in the extent to which partners showed similar behavior (i.e., female latency subtracted from male latency, described in section Materials and Methods) between sites (U = 216.5, Z = –1.36, p = 0.175, n = 48).
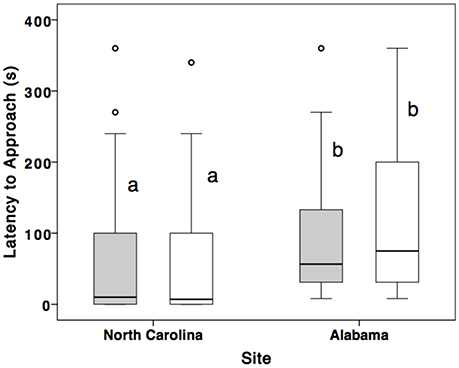
Figure 2. Comparison of male and female latency to approach nest box (within 1 m) during STI in Alabama and North Carolina breeding eastern bluebirds. Males are represented by shading and females are clear. Middle line represents median, bottom and top hinges represent 25 and 75th percentile, respectively. The whiskers are 1.5x hinges and points represent 1 SD above the interquartile range.
We found no significant correlations between land use, nesting stage, age, or bluebird behavior at either site (all p ≥ 0.09). However, North Carolina territories had higher percent openness than Alabama (U = 55.50, Z = –4.78, p < 0.001, n = 49). When we compared latency to respond to the STI with land use (data not split by site), openness did not predict female (rs = –0.198, p = 0.20, n = 44) or male latency to approach (rs = –0.230, p = 0.12, n = 48).
Discussion
Bluebirds in North Carolina responded more aggressively to STIs than did the bluebirds in Alabama despite statistically similar habitat cover types, thus suggesting that the presence of tree swallows may affect bluebird behavior. At both sites, mated partners behaved similarly and behavioral phenotype did not differ between the sexes. Finally, we found no relationship between habitat quality and behavior at either site.
Among secondary cavity nesting species, cavities are a highly contested limited resource. Therefore, when an intense competitor is introduced, aggressive individuals should have a selective advantage (see DeWitt et al., 1998) because they should be better able to obtain and retain nest cavities. There should be strong selection for bluebirds in North Carolina to respond aggressively to territorial intrusions; in 2015, 44% of bluebird nest sites were usurped by tree swallows. Bluebirds in North Carolina may be more aggressive than those in Alabama because (1) either they are behaviorally flexible and responding to frequent harassment by tree swallows or (2) North Carolina birds have more aggressive behavioral genotypes than do bluebirds in Alabama. These two explanations, however, are not mutually exclusive. We think genetic differences are likely because (1) strong heritability for aggression has been shown in western bluebirds (Duckworth and Kruuk, 2009) and (2) aggression is highly repeatable in both the North Carolina and Alabama populations of eastern bluebirds (Harris and Siefferman, 2014, L. Siefferman, unpublished data), but is not related to adult age in either population, and does not vary with tree swallow density in North Carolina (Harris and Siefferman, 2014). Nonetheless, it is also possible that agonistic interactions with tree swallows may increase bluebird aggression during the early breeding season in North Carolina. Indeed, bluebirds may be effectively responding to repeated social challenges from tree swallows via physiological mechanisms associated with social priming (reviewed in Rosvall and Peterson, 2014). However, further experimentation is necessary to understand the proximate cause of higher aggression in North Carolina. Further, we recognize important limitations of this dataset. First, a better approach would be to collect data from multiple sites that cover a larger geographic range, some with and without tree swallows. Second, pairing behavioral data with fitness data would better help us understand the adaptive significance of bluebird behavior in the presence and absence of tree swallows.
Bluebird partners showed behavioral similarity (both aggressive and nonaggressive phenotypes) at both sites studied here (Harris and Siefferman, 2014) and at an Oklahoma population (Burtka and Grindstaff, 2015). If there is selection to mate similarly, then there should be fitness benefits (see Snekser et al., 2009). The benefits of partner similarity have been shown in the North Carolina population and in the OK population but in this Alabama population partner similarity does not influence reproductive success (Siefferman, unpublished data). In Oklahoma, eastern bluebird partners that are both aggressive have more successful fledglings than dissimilar partners (Burtka and Grindstaff, 2015). In North Carolina, it is not necessarily the aggressive bluebird partners that have higher reproductive success in areas of high tree swallow competition, but rather it is those that pair with a behaviorally similar individual (i.e., both aggressive and nonaggressive partners have equal reproductive success in areas of high interspecific competition; Harris and Siefferman, 2014). However, whether mate behavior is consistent and influences mate selection (e.g., zebra finches, T. guttata, Schuett et al., 2011b) or whether behavior is flexible and behavioral similarity is achieved post pairing (Laubu et al., 2016) is unknown. For example, in western bluebirds, individuals modify their aggression to match their mate to a limited extent (Duckworth and Kruuk, 2009). Among species that provide biparental care and share territorial defense roles, aggressive individuals may be high-quality mates. Because behavioral similarity within partners is beneficial to bluebirds at the North Carolina site, they may be under pressure to both behave aggressively to secure a nesting box and behave similarly to their mate.
Within field sites, we found no relationship between land use and behavior. We assume that more open territories represent a higher-quality bluebird habitat; bluebirds settle in open habitat because open space likely allows them to better visualize and capture insects (Gowaty and Plissner, 2015). North Carolina territories used by bluebirds tended to be more open than those used in AL. It may be that bluebirds in North Carolina are particularly likely to avoid nesting in poor quality habitats. Indeed, NC bluebirds are more likely to settle in open habitat when they breed among high densities of tree swallows (Jones et al., 2014). There may be less selection pressure to avoid less open habitats in Alabama. It is also possible that our habitat parameter (i.e., percent openness) may be too coarse to identify relationships with behavior.
We acknowledge the preliminary nature of this study and the need for expanding the behavioral studies to other sites in the southeastern United States. Greater regional coverage would exclude the possibility of alternative drivers of the observed behavioral differences between the North Carolina and Alabama populations (e.g., finer-scale habitat variation, climate, etc.). Nonetheless, currently, there is a unique opportunity to follow North Carolina bluebirds to investigate (1) potential changes in population dynamics, (2) if the bluebirds will be successful against tree swallows, and (3) the adaptations and selection pressures of partner similarity. To monitor population effects and fluctuations, future research should aim to investigate population responses to competitive pressures and nonnative species colonization across a spatial gradient.
Author Contributions
AA designed the project, collected the data, analyzed the data, and wrote the paper. JJ contributed to the design, data collection, analysis, and writing of the paper. LS contributed to the design, analysis, and writing of the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Wendy Hood for logistical assistance at the Alabama population. We thank members of the LS and Gangloff labs for providing helpful comments to early drafts of this manuscript and the extensive review from Michelle Beck and three reviewers. Additionally, field work would not have been possible without the help of Daniel Mason. Research was supported by Appalachian State University, Office of Student Research.
References
Alley, T. (1982). Competition theory, evolution, and the concept of an ecological niche Acta Biotheor. 31, 165–179. doi: 10.1007/BF01857239
Both, C., Dingemanse, N. J., Drent, P. J., and Tinbergen, J. M. (2005). Pairs of extreme avian personalities have highest reproductive success. J. Anim. Ecol. 74, 667–674. doi: 10.1111/j.1365-2656.2005.00962.x
Burtka, J. L., and Grindstaff, J. L. (2013). Repeatable nest defense behavior in a wild population of Eastern bluebirds (Sialia sialis) as evidence of personality. Acta Ethol. 16, 135–146. doi: 10.1007/s10211-013-0143-7
Burtka, J. L., and Grindstaff, J. L. (2015). Similar nest defence strategies within pairs increase reproductive success in the Eastern bluebird, Sialia sialis. Anim. Behav. 100, 174–182. doi: 10.1016/j.anbehav.2014.12.004
DeWitt, T. J., Sih, A., and Wilson, D. S. (1998). Costs and limits of phenotypic plasticity. TREE 13, 77–81. doi: 10.1016/S0169-5347(97)01274-3
Duckworth, R. A. (2006). Behavioral correlations across breeding contexts provide a mechanism for a cost of aggression. Behav. Ecol. 17, 1011–1019. doi: 10.1093/beheco/arl035
Duckworth, R. A., and Badyaev, A. V. (2007). Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc. Natl. Acad. Sci. U.S.A. 104, 15017–15022. doi: 10.1073/pnas.0706174104
Duckworth, R. A., and Kruuk, L. E. (2009). Evolution of genetic integration between dispersal and colonization ability in a bird. Evolution 63, 968–977. doi: 10.1111/j.1558-5646.2009.00625.x
Freeman, A. S., and Byers, J. E. (2006). Divergent induced responses to an invasive predator in marine mussel populations. Science 313, 831–833. doi: 10.1126/science.1125485
Gowaty, P. A., and Plissner, J. H. (2015). Eastern bluebird (Sialia sialis). The birds of North America Online (A. Poole, Ed.). Ithaca: Cornell Lab of Ornithology; Retrieved from the Birds of North America Available Online at: http://bna.birds.cornell.edu/bna/species/381.
Hardin, G. (1960). The competitive exclusion principle. Science 131, 1292–1297. doi: 10.1126/science.131.3409.1292
Harris, M. R., and Siefferman, L. (2014). Interspecific competition influences fitness benefits of assortative mating for territorial aggression in Eastern bluebirds (Sialia sialis). PLoS ONE 9:e88668. doi: 10.1371/journal.pone.0088668
Jones, J. A., Harris, M. R., and Siefferman, L. (2014). Physical habitat quality and interspecific competition interact to influence territory settlement and reproductive success in a cavity nesting bird. Front. Ecol. Evol. 2:71. doi: 10.3389/fevo.2014.00071
Lambrechts, M. M., Adriaense, F., Ardia, D. R., Artemyev, A. V., Atiénzar, F., Bańbura, J., et al. (2010). The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol. 45, 1–26. doi: 10.3161/000164510X516047
Langkilde, T. (2009). Invasive fire ants alter behavior and morphology of native lizards. Ecology 90, 208–217. doi: 10.1890/08-0355.1
Laubu, C., Dechaume-Moncharmont, F. X., Motreuil, S., and Schweitzer, C. (2016). Mismatched partners that achieve postpairing behavioral similarity improve their reproductive success. Sci. Adv. 2:1501013. doi: 10.1126/sciadv.1501013
Lee, D. S. (1993). Range expansion of the tree swallow, Tachycineta bicolor (Passeriformes: Hirundinidae), in the Southeastern United States. Brimleyana 18, 103–113.
MacGregor-Fors, I., Morales-Pérez, L., Quesada, J., and Schondube, J. E. (2010). Relationship between the presence of house sparrows (Passer domesticus) and neotropical bird community structure and diversity. Biol. Invas. 12, 87–96. doi: 10.1007/s10530-009-9432-5
McCarty, J. P., and Winkler, D. W. (1999). Foraging ecology and diet selectivity of tree swallows feeding nestlings. Condor 101, 246–254. doi: 10.2307/1369987
Mooney, H. A., and Cleland, E. E. (2001). The evolutionary impact of invasive species. Proc. Natl. Acad. Sci. U.S.A. 98, 5446–5451. doi: 10.1073/pnas.091093398
Phillips, B. L., and Shine, R. (2006). An invasive species induces rapid adaptive change in a native predator: cane toads and black snakes in Australia. Proc. R. Soc. B. 273, 1545–1550. doi: 10.1098/rspb.2006.3479
Rosvall, K. A., and Peterson, M. P. (2014). Behavioral effects of social challenges and genomic mechanisms of social priming: what's testosterone got to do with it? Curr. Zool. 60, 791–803. doi: 10.1093/czoolo/60.6.791
Schuett, W., Dall, S. R. X., and Royle, N. J. (2011a). Pairs of zebra finches with similar “personalities” make better parents. Anim. Behav. 81, 609–618. doi: 10.1016/j.anbehav.2010.12.006
Schuett, W., Godin, J. G. J., and Dall, S. R. (2011b). Do female zebra finches, Taeniopygia guttata, choose their mates based on their ‘personality’? Ethology 117, 908–917. doi: 10.1111/j.1439-0310.2011.01945.x
Schuett, W., Tregenza, T., and Dall, S. R. (2010). Sexual selection and animal personality. Biol. Rev. 85, 217–246. doi: 10.1111/j.1469-185X.2009.00101.x
Shea, K., and Chesson, P. (2002). Community ecology theory as a framework for biological invasions. TREE 17, 170–176. doi: 10.1016/S0169-5347(02)02495-3
Snekser, J. L., Leese, J., Ganim, A., and Itzkowitz, M. (2009). Caribbean damselfish with varying territory quality: correlated behaviors but not a syndrome. Behav. Ecol. 20, 124–130. doi: 10.1093/beheco/arn123
Spoon, T. R., Millam, J. R., and Owings, D. H. (2007). Behavioural compatibility, extrapair copulation and mate switching in a socially monogamous parrot. Anim. Behav. 73, 815–824. doi: 10.1016/j.anbehav.2006.10.010
Keywords: interspecific competition, invasion, nonnative species, aggression, assortative mating
Citation: Albers AN, Jones JA and Siefferman L (2017) Behavioral Differences among Eastern Bluebird Populations Could Be a Consequence of Tree Swallow Presence: A Pilot Study. Front. Ecol. Evol. 5:116. doi: 10.3389/fevo.2017.00116
Received: 02 February 2017; Accepted: 12 September 2017;
Published: 26 September 2017.
Edited by:
Jordi Figuerola, Estación Biológica de Doñana (CSIC), SpainReviewed by:
Maren N. Vitousek, Cornell University, United StatesMichaela Hau, Max Planck Institute for Ornithology, Germany
Copyright © 2017 Albers, Jones and Siefferman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lynn Siefferman, sieffermanlm@appstate.edu
†Present Address: John Anthony Jones, Department of Ecology and Evolutionary Biology, Tulane University, New Orleans, LA, United States
 Alexandria N. Albers
Alexandria N. Albers  John Anthony Jones
John Anthony Jones Lynn Siefferman
Lynn Siefferman