Spiny Prey, Fortunate Prey. Dorsal Spines Are an Asset in Intraguild Interactions among Lady Beetles
- 1Plant Protection and Ecotoxicology Unit, Life Sciences Department, Walloon Agricultural Research Centre, Gembloux, Belgium
- 2Biological Control and Spatial Ecology, Université Libre de Bruxelles, Bruxelles, Belgium
- 3Invasive Species Unit, Département de l'Etude du Milieu Naturel et Agricole, Namur, Belgium
The Multicolored Asian Ladybird, Harmonia axyridis, is an extremely successful invasive species. Here we suggest that, in addition to many other traits, the dorsal spines of its larvae contribute to their success, as suggested by behavioral observations of agonistic interactions between H. axyridis and European coccinellids. In coccinellids, the role of dorsal spines in these interactions has been poorly studied and they could be a physical protection against intraguild predators. Dorsal spines of second instar H. axyridis larvae were removed with micro-scissors, which resulted in spineless larvae after molting (spineless group). These larvae were then exposed to starved Coccinella septempunctata larvae. Two control categories were also submitted to interactions: H. axyridis larvae with all their spines (control group) and with their spines, but injured by pin stings (injured group). Spine removal at the second instar did not hamper H. axyridis development. The bite rate by C. septempunctata was significantly higher on the spineless H. axyridis and more dorsally located compared to the control and injured groups, while no bite rate difference was observed between the injured and the control group. Our results suggest that in addition to behavioral and chemical defenses, the dorsal spines play a significant protective role against bites. Therefore, spines in ladybirds could be considered as a morphological defense against intraguild predation. In H. axyridis, these defenses might contribute to its success in food resources already exploited by other guild members and thus further facilitate the invasion of new areas.
Introduction
The Multicolored Asian Ladybird, Harmonia axyridis Pallas, is an extremely successful invasive species (Koch et al., 2006; Brown et al., 2011; Roy et al., 2016). Here we suggest that, in addition to many other traits like phenotypic plasticity (Grill et al., 1997), omnivory (Berkvens et al., 2008), and strong dispersion capacity (Hemptinne et al., 2012), the dorsal spines of its larvae contribute to their success. In the animal kingdom, three different categories of defenses have been developed against natural enemies: chemical (Braekman et al., 1998), behavioral (Lima and Dill, 1990), and morphological (Edmunds, 1974).
The Coccinellidae are well-known to have developed chemical defenses. They produce various alkaloids and external feeding deterrents (Pasteels et al., 1973; Daloze et al., 1994; Glisan King and Meinwald, 1996; Hemptinne et al., 2000; Laurent et al., 2005). Behavioral defenses are also observed in larvae (running away; dropping to the ground), in pupae (abdominal flipping), or in adults (flight or clamping down—thanatosis; Hodek and Honek, 1996; Sato et al., 2003, 2005; Majerus et al., 2007). The selection of molting, pupation, or oviposition sites has also been considered as behavioral defenses (Schellhorn and Andow, 1999; Lucas et al., 2000). In addition to these strategies, morphological defenses against predation have also been described. The relatively hard exoskeleton of coccinellid pupae and the hard exoskeleton of adults can display aposematic visual signals often linked to chemical defense (Majerus, 1994). Various protections were developed by different species to compensate the softness of the larval exoskeleton. For example, Scymnus spp. larvae produce wax, ineffective against coccinellids, but which protect them against predation by carabids, syrphid larvae, and ants (Völkl and Vohland, 1996; Agarwala and Yasuda, 2001; Schwartzberg et al., 2010). In other species the dorsal part of the exoskeleton is covered by protuberances of different lengths and forms (Gage, 1920; Hodek, 1973) which, as assumed by several authors (Richards, 1980; Dixon, 2000; Yasuda et al., 2001; Ware and Majerus, 2008), might provide protection against predation and more specifically against intraguild predation. However, this form of protection has only been tested with Curinus coeruleus Mulsant (Michaud and Grant, 2003). They observed that the dorsal spines of this species have a minor defensive value against coccinellids and chrysopids.
All larval instars of H. axyridis are covered with impressive dorsal cuticular spines (Figure 1). Larvae of this invasive species have been reported to behave as intraguild predators of ladybirds (Gagnon et al., 2011; Hautier et al., 2011) but they are rarely mentioned as intraguild prey of ladybirds. Yasuda et al. (2001) noted that only second and third instar H. axyridis larvae were eaten by older Coccinella septempunctata L. larvae, while Ware and Majerus (2008) observed frequent predation on fourth instar H. axyridis larvae, mostly by larvae of the large ladybird Anatis ocellata (L.) but also, to a lesser extent, by Harmonia quadripunctata (Pontoppidan) and Calvia quatuordecimguttata (L.) larvae. Moser and Obrycki (2009) reported that H. axyridis larvae have greater ability to develop in presence of competitors than others coccinellid species. In the field, In addition, H. axyridis migrates later in the season than other coccinellids (e.g., C. septempunctata) to aphid resources (Takahashi, 1989; Hironori and Katsuhiro, 1997; Jansen and Hautier, 2008) and thus is faced with the risk of prey scarcity, increasing the occurrence of intraguild predation. Furthermore, oviposition by H. axyridis is not inhibited by oviposition-deterring pheromones from heterospecific larval tracks but is deterred by conspecific tracks (Yasuda et al., 2000; Agarwala et al., 2003). Therefore, to survive in food resources already exploited by other guild members, H. axyridis should have superior defensive strategies in comparison to other coccinellids. Two defensive alkaloids, harmonine, and 3-hydroxypiperidine-2-one were identified in this species (Sloggett et al., 2009; Kajita et al., 2010). Behavioral observations of agonistic events between H. axyridis and European coccinellids suggest that these dorsal spines protect their bearers from predation (Hautier, 2003; Ware and Majerus, 2008). The dorsal spines of H. axyridis could thus supplement chemical and behavioral defenses to protect the beetles during intraguild interactions.

Figure 1. Dorsal face of a fourth instar H. axyridis larva. On the right, at the base of a spine, a drop of hemolymph produced by reflex bleeding.
In the present study, we experimentally tested this hypothesis. We removed the dorsal spines of second instar H. axyridis larvae to obtain spineless third instar larvae after molting (“spineless” group), and we confronted these larvae to an intraguild predator (IG-predator). To take into account of possible negative effects of spines cutting, a treatment (pricking intact larvae with a needle) was added to reproduce the woundings and hemolymph losses created by spine ablation (“injured” group). A last batch of whole and unwounded H. axyridis larvae were used as control (“control” group).
The agonistic behaviors of fourth instar C. septempunctata larvae toward spineless, intact, or injured H. axyridis third instar larvae were recorded and the survival of both species under these different conditions was assessed. We choose to confront third H. axyridis larval instars and fourth C. septempunctata instars to provide these latter with an advantage in terms of body size (Wissinger, 1992; Snyder and Hurd, 1995; Lucas et al., 1998; Woodward and Hildrew, 2002). This unbalanced situation is not unrealistic as it occurs under field conditions because of the later arrival of H. axyridis, generating in this latter species an obvious need for maximal defenses against older IG-predators.
Materials and Methods
Insect Cultures
Ladybirds and aphids were reared in climatic rooms at 20 ± 2°C, 16L:8D photoperiod and 60–90% relative humidity. Pea aphids, Acyrthosiphon pisum (Harris), were reared on horse bean plants, Vicia faba minor L. Adults of H. axyridis and C. septempunctata were kept in plastic boxes (40 × 30 × 20 cm) with living aphids and honeybee pollen which were renewed three times a week. At the same occasion, egg batches laid on absorbent paper in these boxes were removed and placed separately in Petri dishes (90 mm in diameter). After hatching, the first instar larvae were isolated in 55 mm Petri dishes and fed ad libitum with aphids. Larval development was followed each day and the exact stage was established by counting the exuviae.
Spine Removal and Pin Injuries
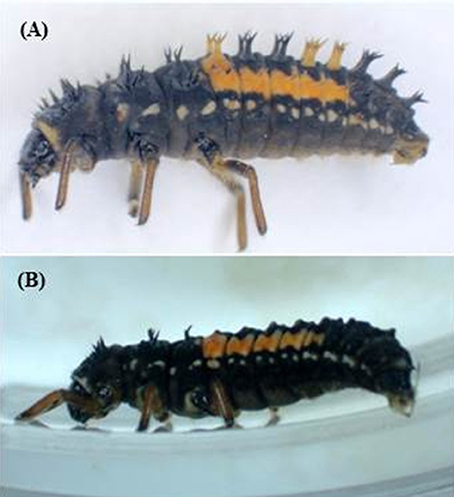
Second instar H. axyridis larvae were anesthetized by contact with an ice pack (ca. 6°C) for about 2 min. They were then submitted to spine removal or pin injuries under a binocular lens (Nikon SMZ-U, Zoom 1:10, 3.75x−37.5x). The abdominal dorsal spines were cut with micro-scissors (Moria MC19/B Pascheff-Wolf Spring Scissors−3.0 mm Blades, Fine Science Tools, Germany). Larvae were maintained on their ventral face. First, the abdominal top spines were removed (Supplementary Video 1). Next, the spines on each lateral side were cut. Spined and spineless H. axyridis larvae are illustrated in Figure 2. The dorsal injuries were done with an entomological pin N° 00. Twenty punctures were made on the whole dorsal face of the abdomen. Afterwards, all the treated H. axyridis larvae were kept in Petri dishes with fresh aphids, and their development was followed.
Effect of Spine Removal and Pin Injuries on Development and Survival
A preliminary experiment was made to assess possible impacts of anesthesia with ice pack and surgery on larval development. Four treatments were applied to second instar larvae (20 replicates): (1) anesthesia only (“cold control”); (2) anesthesia and spines removal (“spineless”); (3) anesthesia and dorsal injuries (“injured”), and (4) no anesthesia and no dorsal injuries (“control”).
First instar H. axyridis were isolated in single Petri dishes (55 mm) and, when reaching second instar, they were weighed and submitted to one of the treatments. The larvae were weighed daily with a 0.01 mg accuracy on an analytical balance (Sartorius LE225D) and the instars were noted. All larvae were fed ad libitum with A. pisum and kept in climatic rooms as described above.
Predation Experiments
These experiments consisted of' pairing tests where one “prey” larva was kept together with one “predator” larva in a Petri dish (55 mm) without extraguild prey. All experiments were made with the fourth C. septempunctata larval instar (as IG-predator) and third H. axyridis larval instar (as IG-prey). Before starting the experiments C. septempunctata was starved for 24 h to standardize their hunger level while H. axyridis was not starved. The following combinations of larvae were made: (1) spined H. axyridis & C. septempunctata; (2) spineless H. axyridis & C. septempunctata; (3) injured H. axyridis & C. septempunctata. A minimum of 25 replicates of each combination were observed. To avoid a confounding effect of size, all larvae in the IG-prey and the IG-predator groups, were weighed before the tests to select similar weights for all treatment. The IG-predator behavior was recorded for 30 min according to the ethogram of Yasuda et al. (2001) represented in Figure 3. When a contact occurs between a predator and a prey, the predator can either: run away, ignore, or attack the prey. When an attack occurs, the prey can either escape or be caught. If the prey is caught, the prey can either be bitten or not. If bitten, the location on the body of the prey can be either dorsal, ventral, lateral thorax or abdomen. Each event was counted, and several rates were calculated: (1) attack rate: number of individuals attacked/number of individuals contacted; (2) escape rate: number of individuals escaped/number of individuals attacked; (3) bite rate: number of individuals bitten/number of individuals caught; (4) dorsal bite rate: number of individuals bitten on the dorsal face/number of individuals bitten. The bite rate is based on biting injuries, which are characterized by the shedding of haemolymph. In most cases, biting does not lead to prey death.
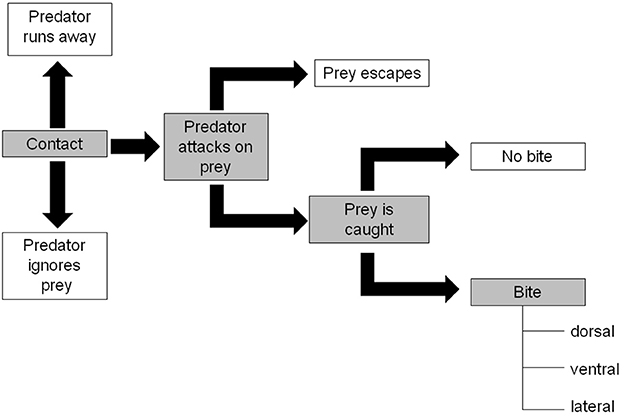
Figure 3. IG-predator behavior description adapted from Yasuda et al. (2001). Gray shaded, interactions leading to a bite.
The survival time of the IG-prey in the experimental arena was followed by time lapse photography with a 1 min interval between pictures, using a Nikon D2h digital camera (35 mm, f2.0). The death of the prey was determined by the absence of reaction for several minutes or the consumption of the prey body. All IG-predators and IG-prey were used only once.
Data Analysis
Effect of Treatment on Development and Survival
The development time of the four groups was compared with a normal linear model (ANOVA) and Tukey's range tests. Survival time was analyzed with a Weibull survival analysis model with censored data. The age at death or at the end of the experiment was used as the dependent variable along with the status (alive or dead) and the treatment was included as nominal explanatory variable.
The change of weight across time in the four treatments before and after treatment up to pupation (prepupal stage) was analyzed with a linear mixed model. Weight was used as a dependent variable and time (continuous), treatment (4 nominal levels) and “surgery” (before/after) were used as fixed explanatory variables along with all their interactions (first and second level). The larva identifier (ID) was used as random effect to take into account the within-larva residual correlation. No temporal autocorrelation was observed (Autocorrelation Function graph, comparison of models with different autoregressive residuals structures) and it was therefore not modeled. Weights were square-root transformed and time was squared to achieve linearity and homoscedasticity. As we are primarily interested to test for differences in weight and growth rate after treatment, the time variable was centered on the day following the treatment.
The global effects of the explanatory variables and their interactions were tested via Likelihood Ratio Test of models estimated via Maximum Likelihood. The inferences on the model parameters were performed via Markov Chain Monte Carlo simulations as provided by the mcmcsamp function from the lme4 R package (Bates et al., 2011).
Predation Experiments
Linear model and generalized linear models (GLM) were used to analyze all the predation experiments results, with treatment (control, injured, and spineless) as a nominal explanatory variable. The attack, escape, bite, and dorsal bite rates were analyzed with binomial GLMs. No over-dispersion was observed in these models. A binary binomial GLM was used to analyze mortality after 30 min and a simple linear model with normal residuals (ANOVA) was used to analyze the log transformed survival time in the behavioral experiments. Global significance of the treatment effect was tested with Likelihood Ratio (LR) tests for the GLMs and with F-tests for the linear model. All pairwise comparisons were then performed with multiplicity adjusted P-values based on the underlying multivariate normal distribution, thus accounting for the correlation between the tests (multcomp R package; Bretz et al., 2010).
All analyses were performed with R (R Development Core Team, 2012). Model assumptions and simulation convergence were checked graphically.
Results
Effect of Spine Removal and Pin Injuries
Spine removal on second instar H. axyridis larvae is a critical operation. The day following surgery, 40% of the larvae in the spineless group died whilst one larva died in the injured group and no larva died in the cold control and the control groups (Figure 4). According to the Weibull survival model, the spineless group is the only group with a lower survival rate than that of the control group (model parameter = −3.037, se = 1.545, Z = −1.96, P = 0.049). However, after this period, none of the larvae that survived surgery died during the course of the experiment and all reached the adult stage.
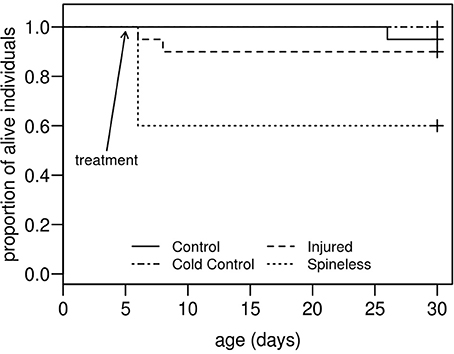
Figure 4. Survival curves for H. axyridis larvae submitted, at the second instar, one of these treatments: (1) no treatment (Control), (2) anesthesia with ice (Cold control), (3) anesthesia and dorsal injuries (Injured), (4) anesthesia and spine removal (Spineless). (n = 20 larvae for each treatment).
The whole larval development was relatively unaffected by the different treatments even if a significant effect on larval weight was detected on the day following the surgery [Table 1, Treatment: Likelihood Ratio (LR) = 36.2, d.f. = 3, P < 0.001]. The first day after surgery, the larvae of the spineless and injured groups had a lower weight relative to the control (Table 2, Treat.Spineless: Credible Interval (CI) [−0.8512, −0.4092], P < 0.001; Treat.Injuried CI [−0.6898, −0.2724], P < 0.001) in contrast to the cold control group that has a mean weight similar to the control group (Treat.Cold control: CI [−0.2743, 0.132], P = 0.516). However, after this initial weight difference, the growth rates were not statistically different between the four treatments, i.e., the weight gains across time were similar to those of the control (Time*Treatment: LR = 2.29, d.f. = 3, P = 0.51). This was also the case before the application of the treatment (Time*Treatment*Surgery: LR = 0.01, d.f. = 3, P = 0.99; Figure 5). The mean development time to prepupal stage was similar in the injured, spineless, and control groups: 9.7, 10.2, and 10.1 days, respectively (Tukey's range test with P > 0.4), whereas the cold control group developed significantly faster (9.3 days) than the control and the spineless groups (Tukey corrected P = 0.02 in both cases).
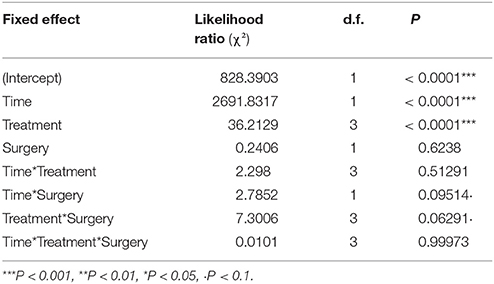
Table 1. Analysis of deviance table (Likelihood Ratio tests—Type III tests) for the linear mixed model describing the change of the larval weight of H. axyridis across time in the four treatments before and after surgery.
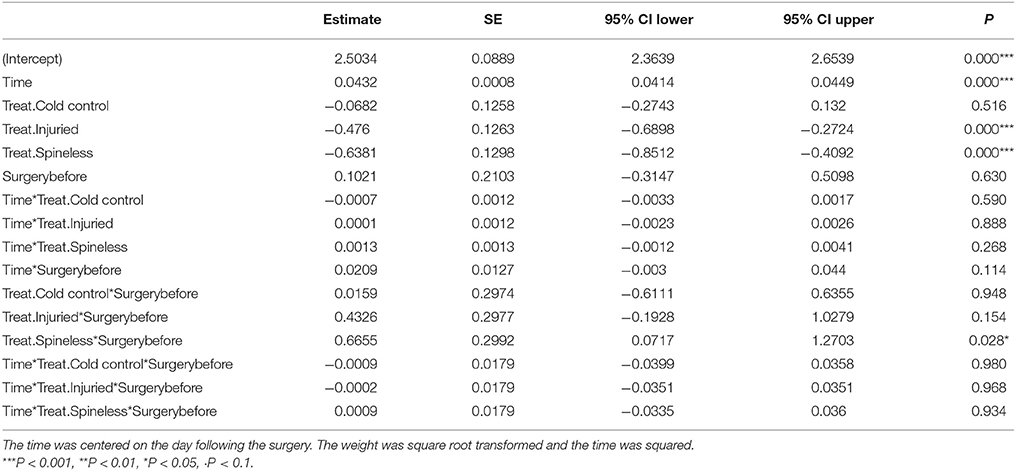
Table 2. Model parameter estimates, standard error (SE) and credible intervals (CI) of the linear mixed model describing the change of the larval weight of H. axyridis across time in the four treatments (Treat.) before and after surgery.
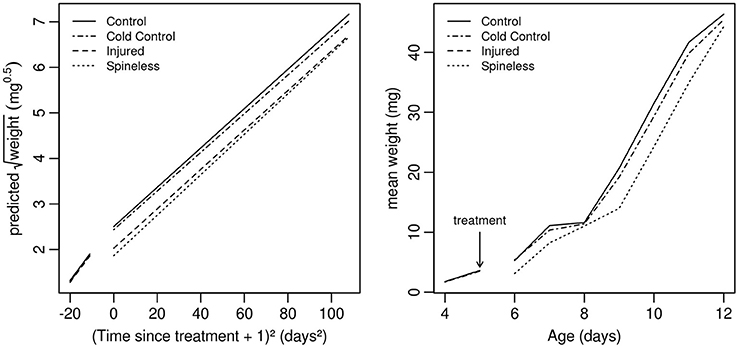
Figure 5. Development curves for H. axyridis larvae that, at the second instar, had: (1) no treatment (Control), (2) only anesthesia with ice (Cold control), (3) anesthesia and dorsal injuries (Injured), (4) anesthesia and spine removal (Spineless). (Right) Weight predicted by the model with transformed data. (Left) Observed mean weight on the original scale (n = 20 larvae for each treatment).
Predation Experiments
Before the tests, no significant difference in larval weights was observed between treatments for both the IG-prey [third instar H. axyridis larvae: F(2, 85) = 0.28; P = 0.75] and the IG-predators [fourth instar C. septempunctata larvae: F(2, 85) = 1.2; P = 0.3]. The mean weight of the IG-prey was about 1.8 times lower than that of the IG-predators for each treatment: 11.5 vs. 21.2 mg, 11.8 vs. 21.4 mg, 11.8 vs. 21.5 mg for the control, injured and spineless group, respectively.
During the 30 min observations, the attack rate by the IG-predators was similar toward the spined, injured and spineless IG-prey: 0.66 (predicted value), 0.62, 0.62 (Figure 6A: LR = 0.88, d.f. = 2, P = 0.64). Following these attacks, the spineless larvae tended to escape less often than the spined and injured larvae: 0.106 vs. 0.222 and 0.198, respectively, but this trend was only marginally significant (Figure 6B: LR = 4.8, d.f. = 2, P = 0.089). On the contrary, significant differences between the three pairs studied were observed for the bite rate (Figure 6C: LR = 18.7, d.f. = 2, P < 0.0001) and the dorsal bite rate (Figure 6D: LR = 20.4, d.f. = 2, P < 0.0001). Prey catches by IG-predators led to bites, on the average in 22.0, 31.5, and 53.7% of the catching events involving the spined, injured and spineless IG-prey, respectively. The spineless IG-prey were thus bitten significantly more frequently (ca. 1.7 times) than the control (post-hoc paired comparison, Z = 4.219, corrected P = < 0.001) and injured larvae (Z = 2.635, P = 0.023) but no significant difference was observed between the spined and injured groups (Z = 1.422, P = 0.329). These bites were significantly concentrated (ca. 2.3 times more frequently) on the dorsal face of the spineless larvae as compared to the spined (Z = 3.574, P = 0.001) and injured larvae (Z = 3.576, P = < 0.001). No significant difference in dorsal bites was observed between the spined and injured larvae (Z = −0.088, P = 0.996). Supplementary Videos 2, 3 respectively show a C. septempunctata larva attacking a spined and a spineless H. axyridis larva. Dorsal bites on the thorax were observed only at the junction between the nota, devoid of spines and with a softer cuticle (Figure 1). In addition, despite the bites, H. axyridis larvae tried to turn round to the predator and were able to pull it, underlining the strength of H. axyridis larvae.
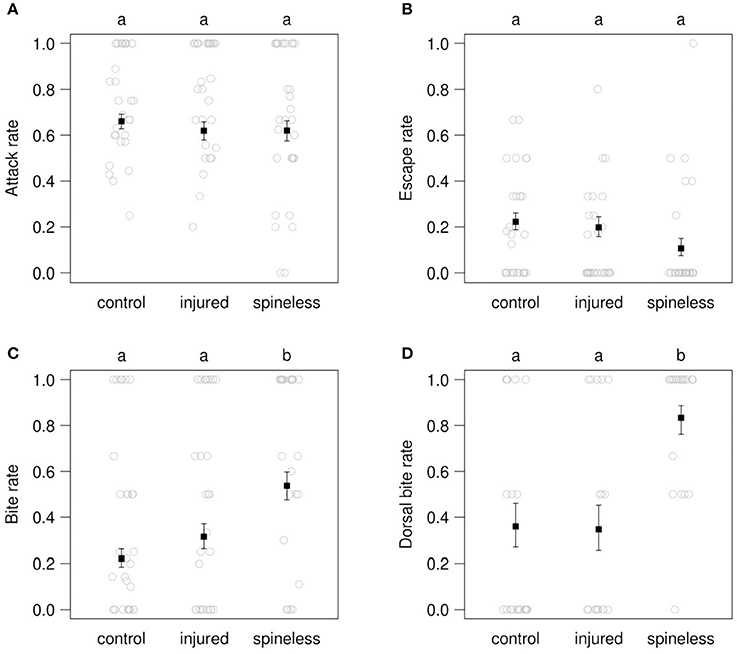
Figure 6. Rates of attack (A), escape (B), bite (C), and dorsal bite (D) in the contests between IG-predators (fourth instar C. septempunctata larvae) and IG-prey (third instar H. axyridis larvae, either spined, injured, or spineless). Open circles: observed values. Solid squares: value predicted by a binomial GLM (±SE). Different letters above each plot indicate significant differences among treatments (α = 0.05) after correction for multiple testing.
After 30 min contest in Petri dishes, no significant difference in mortality between the groups was observed (LR = 0.45, d.f. = 2, P = 0.79). Only 1 out of 30 IG prey died from the interactions with an IG-predator in the control group, 1 out of 28 in the injured group, and 2 out of 30 in the spineless group. The survival time of spineless larvae was lower, estimated value: 128.9 min, than that of injured and spined larvae: respectively 179.3 and 205.9 min (Figure 7), although these differences were only marginally significant [F(2, 85) = 2.6, P = 0.08]. Most often, numerous bites were required to kill a H. axyridis larva.
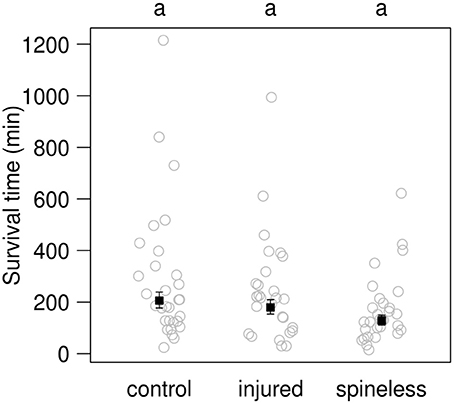
Figure 7. Survival time for third instar control, injured or spineless H. axyridis larvae in the presence of a fourth instar C. septempunctata larva. Open circles: observed values. Solid squares: value predicted by a simple linear model (±SE). Different letters above each plot indicate significant differences among treatments (α = 0.05) after correction for multiple testing.
Discussion
The dorsal cuticular protuberances in H. axyridis larva are not a vital structure. Removing the spines of H. axyridis larvae did not hamper larval development. Development time, molting, and pupation of spineless individuals were comparable to those of the controls. Likewise, anesthesia (cold control) and injuries with an entomological pin did not affect larval development. Spines removal affected larval survival by creating lethal injuries. However, larvae that survived later developed quite normally. Surgery seemed to generate a temporary stress. Larval growth was stopped for ~1 day leading to a lower weight compared to the controls. No differences in growth between the treatments, however, were detected. We thus consider that the spineless larvae used in the predation experiment were not weakened compared to the spined larvae and can be used to test the role of spines as a physical defense.
Our hypothesis that spined larvae would suffer less from IG-predators bitings than spineless larvae was supported under our experimental conditions where strong intraguild pressure was created due to wide size differences between the interacting species. However, the influence of this pressure on survival time was similar for spined and spineless larvae, which suggests that this defense is not sufficient per se, at least in our artificial experimental conditions.
Predation is a strong selection pressure toward morphological antipredator adaptations and behavioral decisions (Lima and Dill, 1990). Spines are known to serve as physical defenses in plants and animals and are often highly conspicuous (Inbar and Lev-Yadun, 2005) as, for example in acacias, thistles and cacti (Cooper and Ginnett, 1998; Young and Okello, 1998). Different animal phyla have developed spines, in terrestrial habitats (e.g., arthropods, mammalians) as well as in aquatic habitats (e.g., arthropods, fish, gastropods) as a direct protection against predators or by increasing handling time or as camouflage (Edmunds, 1974). For example the length and shape of spines in Odonata larvae are influenced by the presence of fish predators (Johansson and Samuelsson, 1994). However, spines are not always a response to predation and can have several other functions such as anchoring or decreasing the mass/area ratio (Willman, 2007). In coccinellids, different form and size of spines cover the larvae and are characteristic for each coccinellid tribe (Hodek, 1973). These spines are not linked to a particular kind of food resource and are present in aphidophagous (e.g., A. ocellata, C. quatuordecimguttata), coccidophagous (e.g., Chilocorus spp.), phytophagous [e.g., Subcoccinella vigintiquattuorpunctata (L.)], and polyphagous species (e.g., Exochomus quadripustulatus L.). Coccinellids are attacked by parasitoids and by vertebrate and invertebrate predators (Ceryngier et al., 2012). However, in contrast to predators, parasitoids rarely attack coccinellid larvae. Predators of coccinellids can be mammals, birds, reptiles, or arthropods (Hodek and Honek, 1996) but the frequency of encounters and hence the risk is probably higher with arthropod members of the same guilds than with larger and generalist predators. The highest risk lies with ants that protect aphids and may attack and kill aphidophagous and coccidophagous coccinellid larvae (Majerus et al., 2007). However, larvae of myrmecophilous species like Coccinella magnifica Redtenbacher, Platynaspis luteorubra (Goeze) do not develop abdominal spines and some species are protected by wax and white smear (Richards, 1980; Völkl and Vohland, 1996; Schwartzberg et al., 2010). In the pupae, the dense hair cover acts as a protection against ants. The main threats to coccinellid larvae may come from species that exploit the same resources, such as heterospecific coccinellids (Agarwala and Dixon, 1992; Obrycki et al., 1998), syrphids (Hindayana et al., 2001), chrysopids (Sengonca and Frings, 1985; Lucas et al., 1998; Phoofolo and Obrycki, 1998), and pentatomids (Mallampalli et al., 2002; De Clercq et al., 2003). However, little predation has been directly observed toward H. axyridis larvae except by coccinellid larvae (Yasuda et al., 2001; Ware and Majerus, 2008) or by the pentatomid bug Podisus maculiventris (Say) (De Clercq et al., 2003). Thus, to survive on ephemeral food resources with numerous competitors and potential IG-predators that are often older (Takahashi, 1989; Hironori and Katsuhiro, 1997; Jansen and Hautier, 2008), H. axyridis must be well defended. Our observations demonstrate that, in addition to chemical and behavioral defenses, the dorsal spines of H. axyridis significantly reduce biting by an IG-predator, even though they do not reduce capture success as observed in an invasive amphipod (Bollache et al., 2006). We thus suggest that spines play a protective role against intraguild predation during larval development. By reducing the risks of being bitten, spines not only reduce risks of injury but they also increase opportunities for behavioral reactions such as biting back or escaping. However, little effect on survival time was observed in our experiments; this was probably due to experimental settings that did not allow for escape.
The protective role of spines could be more crucial during the first and second instars which are more vulnerable because of their small size. Another particular moment when spine protection might be crucial is molting. Sessile individuals are more vulnerable because of they cannot escape or display aggressive behaviors (Lucas et al., 2000) or use chemical defenses by reflex bleeding (L. Hautier pers obs.). However, newly ecdysed larvae might not be well protected by their spines, as it takes times for their integument to harden (Ware and Majerus, 2008).
Novel weapons can explain the success of invasive species as suggested by Callaway and Ridenour (2004). Novel or superior defenses are also a huge asset. H. axyridis is known as a fast invader (Brown et al., 2011; Hemptinne et al., 2012), which could be a consequence of several traits such as a wide trophic niche and a high phenotypic plasticity (Grill et al., 1997), strong dispersal capacities (Osawa, 1993; Brown et al., 2011), high fertility (Hodek and Honek, 1996), release from natural enemies (Roy et al., 2011), chemical and behavioral defenses (Sloggett et al., 2011). Here we suggest that morphological defenses might also contribute to the invasiveness of H. axyridis in niches already exploited by other aphidophages. This trait has already been reported by Bollache et al. (2006), who observed lower predation on a spined invasive amphipod in comparison to non spined native species.
Author Contributions
LH, J-CG conceived and designed the experiments. LH performed all experiments and observation. GSM and LH analyzed data. LH and J-CG wrote paper. All authors provided editorial advice.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Brigitte Jonard, Marjorie Jonard, and Virginie Maeck for their help in the ladybird rearings. We would like to thank Jacques Pasteels and Mike Majerus for the fruitful discussions about the spines in ladybirds.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2017.00135/full#supplementary-material
Supplementary Video 1. The removal of abdominal top spines of third instar H. axyridis.
Supplementary Video 2. A fourth instar C. septempunctata attacking a spined third instar H. axyridis.
Supplementary Video 3. A fourth instar C. septempunctata attacking a spineless third instar H. axyridis.
References
Agarwala, B. K., and Dixon, A. F. G. (1992). Laboratory study of cannibalism and interspecific predation in ladybirds. Ecol. Entomol. 17, 303–309. doi: 10.1111/j.1365-2311.1992.tb01062.x
Agarwala, B. K., and Yasuda, H. (2001). Larval interactions in aphidophagous predators: effectiveness of wax cover as defence shield of Scymnus larvae against predation from syrphids. Entomol. Exp. Appl. 100, 101–107. doi: 10.1046/j.1570-7458.2001.00852.x
Agarwala, B. K., Yasuda, H., and Kajita, Y. (2003). Effect of conspecific and heterospecific feces on foraging and oviposition of two predatory ladybirds: role of fecal cues in predator avoidance. J. Chem. Ecol. 29, 357–376. doi: 10.1023/A:1022681928142
Bates, D., Maechler, M., and Bolker, B. (2011). lme4: Linear Mixed-Effects Models using S4 Classes. R package version 0.999375-42. Available online at: http://CRAN.R-project.org/package=lme4
Berkvens, N., Bonte, J., Berkvens, D., Deforce, K., Tirry, L., and De Clercq, P. (2008). Pollen as an alternative food for Harmonia axyridis. BioControl 53, 201–210. doi: 10.1007/s10526-007-9128-7
Bollache, L., Kaldonski, N., Troussard, J.-P., Lagrue, C., and Rigaud, T. (2006). Spines and behaviour as defences against fish predators in an invasive freshwater amphipod. Anim. Behav. 72, 627–633. doi: 10.1016/j.anbehav.2005.11.020
Braekman, J. C., Daloze, D., and Pasteels, J. M. (1998). “Alkaloids in animals,” in Alkaloids Biochemistry, Ecology, and Medicinal Applications, eds M. F. Roberts and M. Wink (Boston, MA: Springer), 349–378.
Bretz, F., Hothorn, T., Westfall, P., and Westfall, P. H. (2010). Multiple Comparisons Using R. Boca Raton, FL: Chapman and Hall/CRC.
Brown, P. M. J., Thomas, C. E., Lombaert, E., Jeffries, D. L., Estoup, A., and Lawson Handley, L.-J. (2011). The global spread of Harmonia axyridis (Coleoptera: Coccinellidae): distribution, dispersal and routes of invasion. BioControl 56, 623–641. doi: 10.1007/s10526-011-9379-1
Callaway, R. M., and Ridenour, W. M. (2004). Novel weapons: invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2, 436–443. doi: 10.1890/1540-9295(2004)002[0436:NWISAT]2.0.CO;2
Ceryngier, P., Roy, H. E., and Poland, R. L. (2012). “Natural enemies of ladybird beetles,” in Ecology and Behaviour of the Ladybird Beetles (Coccinellidae), eds I. Hodek, H. F. van Emden, and A. Honek (Chichester: Blackwell), 375–443.
Cooper, S. M., and Ginnett, T. F. (1998). Spines protect plants against browsing by small climbing mammals. Oecologia 113, 219–221. doi: 10.1007/s004420050371
Daloze, D., Braekman, J.-C., and Pasteels, J. M. (1994). Ladybird defence alkaloids: structural, chemotaxonomic and biosynthetic aspects (Col.: Coccinellidae). Chemoecology 5, 173–183. doi: 10.1007/BF01240602
De Clercq, P., Peeters, I., Vergauwe, G., and Thas, O. (2003). Interaction between Podisus maculiventris and Harmonia axyridis two predators used in augmentative biological control in greenhouse crops. BioControl 48, 39–55. doi: 10.1023/A:1021219714684
Dixon, A. F. G. (2000). Insect Predator-Prey Dynamics: Ladybird Beetles and Biological Control. Cambridge, UK: Cambridge University Press.
Gagnon, A.-È., Heimpel, G. E., and Brodeur, J. (2011). The ubiquity of intraguild predation among predatory arthropods. PLoS ONE 6:e28061. doi: 10.1371/journal.pone.0028061
Glisan King, A., and Meinwald, J. (1996). Review of the defensive chemistry of Coccinellids. Chem. Rev. 96, 1105–1122. doi: 10.1021/cr950242v
Grill, C. P., Moore, A. J., and Brodie, E. D. III (1997). The genetics of phenotypic plasticity in a colonizing population of the ladybird beetle, Harmonia axyridis. Heredity 78, 261–269. doi: 10.1038/sj.hdy.6881030
Hautier, L. (2003). Impacts sur L'entomofaune Indigene D'une Coccinelle Exotique Utilisée en Lutte Biologique. TFE Univ. Libre Brux: IGEAT Bruss.
Hautier, L., San Martin, G., Callier, P., Biseau, J.-C., and Grégoire, J.-C. (2011). Alkaloids provide evidence of intraguild predation on native coccinellids by Harmonia axyridis in the field. Biol. Invasions 13, 1805–1814. doi: 10.1007/s10530-010-9935-0
Hemptinne, J. L., Lognay, G., Gauthier, C., and Dixon, A. F. G. (2000). Role of surface chemical signals in egg cannibalism and intraguild predation in ladybirds (Coleoptera: Coccinellidae). Chemoecology 10, 123–128. doi: 10.1007/PL00001813
Hemptinne, J. L., Magro, A., Evans, E., and Dixon, A. F. G. (2012). Body size and the rate of spread of invasive ladybird beetles in North America. Biol. Invasions 14, 1–11. doi: 10.1007/s10530-011-0101-0
Hindayana, D., Meyhöfer, R., Scholz, D., and Poehling, H. M. (2001). Intraguild predation among the hoverfly Episyrphus balteatus de Geer (Diptera: Syrphidae) and other aphidophagous predators. Biol. Control 20, 236–246. doi: 10.1006/bcon.2000.0895
Hironori, Y., and Katsuhiro, S. (1997). Cannibalism and interspecific predation in two predatory ladybirds in relation to prey abundance in the field. BioControl 42, 153–163. doi: 10.1007/BF02769893
Hodek, I. (1973). Biology of Coccinellidae. Prague: Academia, Publishing House of the Czechoslovak Academy of Sciences.
Inbar, M., and Lev-Yadun, S. (2005). Conspicuous and aposematic spines in the animal kingdom. Naturwissenschaften 92, 170–172. doi: 10.1007/s00114-005-0608-2
Jansen, J., and Hautier, L. (2008). Ladybird population dynamics in potato: comparison of native species with an invasive species, Harmonia axyridis. BioControl 53, 223–233. doi: 10.1007/s10526-007-9134-9
Johansson, F., and Samuelsson, L. (1994). Fish-induced variation in abdominal spine length of Leucorrhinia dubia (Odonata) larvae? Oecologia 100, 74–79.
Kajita, Y., Obrycki, J. J., Sloggett, J. J., and Haynes, K. F. (2010). Intraspecific alkaloid variation in ladybird eggs and its effects on con- and hetero-specific intraguild predators. Oecologia 163, 313–322. doi: 10.1007/s00442-009-1551-2
Koch, R. L., Venette, R. C., and Hutchison, W. D. (2006). Invasions by Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) in the western hemisphere: implications for South America. Neotrop. Entomol. 35, 421–434. doi: 10.1590/S1519-566X2006000400001
Laurent, P., Braekman, J. C., and Daloze, D. (2005). Insect chemical defense. Top. Curr. Chem. 240, 167–230. doi: 10.1007/b98317
Lima, S. L., and Dill, L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. doi: 10.1139/z90-092
Lucas, E., Coderre, D., and Brodeur, J. (1998). Intraguild predation among aphid predators: characterization and influence of extraguild prey density. Ecology 79, 1084–1092. doi: 10.1890/0012-9658(1998)079[1084:IPAAPC]2.0.CO;2
Lucas, É., Coderre, D., and Brodeur, J. (2000). Selection of molting and pupation sites by Coleomegilla maculata (Coleoptera: Coccinellidae): avoidance of intraguild predation. Environ. Entomol. 29, 454–459. doi: 10.1603/0046-225X-29.3.454
Majerus, M. E. N. (1994). Ladybirds. The New Naturalist Series. London, UK: Harper Collins Publishers.
Majerus, M., Sloggett, J., Godeau, J.-F., and Hemptinne, J.-L. (2007). Interactions between ants and aphidophagous and coccidophagous ladybirds. Popul. Ecol. 49, 15–27. doi: 10.1007/s10144-006-0021-5
Mallampalli, N., Castellanos, I., and Barbosa, P. (2002). Evidence for intraguild predation by Podisus maculiventris on a ladybeetle, Coleomegilla maculata: implications for biological control of Colorado potato beetle, Leptinotarsa decemlineata. BioControl 47, 387–398. doi: 10.1023/A:1015667004364
Michaud, J. P., and Grant, A. K. (2003). Intraguild predation among ladybeetles and a green lacewing: do the larval spines of Curinus coeruleus (Coleoptera: Coccinellidae) serve a defensive function? Bull. Entomol. Res. 93, 499–505. doi: 10.1079/BER2003269
Moser, S. E., and Obrycki, J. J. (2009). Competition and intraguild predation among three species of coccinellids (Coleoptera: Coccinellidae). Ann. Entomol. Soc. Am. 102, 419–425. doi: 10.1603/008.102.0310
Obrycki, J. J., Giles, K. L., and Ormord, A. M. (1998). Interactions between an introduced and indigenous coccinellid species at different prey densities. Oecologia 117, 279–285. doi: 10.1007/s004420050659
Osawa, N. (1993). Population field studies of the aphidophagous ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae): life tables and key factor analysis. Popul. Ecol. 35, 335–348. doi: 10.1007/BF02513605
Pasteels, J. M., Deroe, C., Tursch, B., Braekman, J. C., Daloze, D., and Hootele, C. (1973). Distribution et activités des alcaloïdes défensifs des Coccinellidae. J. Insect Physiol. 19, 1771–1784. doi: 10.1016/0022-1910(73)90046-2
Phoofolo, M. W., and Obrycki, J. J. (1998). Potential for intraguild predation and competition among predatory Coccinellidae and Chrysopidae. Entomol. Exp. Appl. 89, 47–55. doi: 10.1046/j.1570-7458.1998.00380.x
R Development Core Team (2012). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org/
Richards, A. M. (1980). Defensive adaptations and behaviour in Scymnodes lividigaster (Coleoptera: Coccinellidae). J. Zool. 192, 157–168. doi: 10.1111/j.1469-7998.1980.tb04227.x
Roy, H. E., Brown, P. M. J., Adriaens, T., Berkvens, N., Borges, I., Clusella-Trullas, S., et al. (2016). The harlequin ladybird, Harmonia axyridis: global perspectives on invasion history and ecology. Biol. Invasions 18, 997–1044. doi: 10.1007/s10530-016-1077-6
Roy, H. E., Rhule, E., Harding, S., Lawson Handley, L.-J., Poland, R. L., Riddick, E. W., et al. (2011). Living with the enemy: parasites and pathogens of the ladybird Harmonia axyridis. BioControl 56, 663–679. doi: 10.1007/s10526-011-9387-1
Sato, S., Anthony, F. G. D., and Hironori, Y. (2003). Effect of emigration on cannibalism and intraguild predation in aphidophagous ladybirds. Ecol. Entomol. 28, 628–633. doi: 10.1046/j.1365-2311.2003.00542.x
Sato, S., Hironori, Y., and Edward, W. E. (2005). Dropping behaviour of larvae of aphidophagous ladybirds and its effects on incidence of intraguild predation: interactions between the intraguild prey, Adalia bipunctata (L.) and Coccinella septempunctata (L.), and the intraguild predator, Harmonia axyridis pallas. Ecol. Entomol. 30, 220–224. doi: 10.1111/j.0307-6946.2005.00688.x
Schellhorn, N. A., and Andow, D. A. (1999). Cannibalism and interspecific predation: role of oviposition behavior. Ecol. Appl. 9, 418–428. doi: 10.2307/2641129
Schwartzberg, E. G., Haynes, K. F., Johnson, D. W., and Brown, G. C. (2010). Wax structures of Scymnus louisianae attenuate aggression from aphid-tending ants. Environ. Entomol. 39, 1309–1314. doi: 10.1603/EN09372
Sengonca, Ç., and Frings, B. (1985). Interference and competitive behaviour of the aphid predators, Chrysoperla carnea and Coccinella septempunctata in the laboratory. BioControl 30, 245–251.
Sloggett, J. J., Haynes, K. F., and Obrycki, J. J. (2009). Hidden costs to an invasive intraguild predator from chemically defended native prey. Oikos 118, 1396–1404. doi: 10.1111/j.1600-0706.2009.17407.x
Sloggett, J. J., Magro, A., Verheggen, F. J., Hemptinne, J.-L., Hutchison, W. D., and Riddick, E. W. (2011). The chemical ecology of Harmonia axyridis. BioControl 56, 643–661. doi: 10.1007/s10526-011-9376-4
Snyder, W. E., and Hurd, L. E. (1995). Egg-hatch phenology and intraguild predation between two mantid species. Oecologia 104, 496–500. doi: 10.1007/BF00341347
Takahashi, K. (1989). Intra- and inter-specific predations of lady beetles in spring alfalfa fields. Jpn. J. Entomol. 57, 199–203.
Völkl, W., and Vohland, K. (1996). Wax covers in larvae of two Scymnus species: do they enhance coccinellid larval survival? Oecologia 107, 498–503.
Ware, R., and Majerus, M. (2008). Intraguild predation of immature stages of British and Japanese Coccinellids by the invasive ladybird Harmonia axyridis. BioControl 53, 169–188. doi: 10.1007/s10526-007-9135-8
Willman, S. (2007). Testing the role of spines as predatory defense. J. Shellfish Res. 26, 261–266. doi: 10.2983/0730-8000(2007)26[261:TTROSA]2.0.CO;2
Wissinger, S. A. (1992). Niche overlap and the potential for competition and intraguild predation between size-structured populations. Ecology 73, 1431–1444. doi: 10.2307/1940688
Woodward, G., and Hildrew, A. G. (2002). Body-size determinants of niche overlap and intraguild predation within a complex food web. J. Anim. Ecol. 71, 1063–1074. doi: 10.1046/j.1365-2656.2002.00669.x
Yasuda, H., Kikuchi, T., Kindlmann, P., and Sato, S. (2001). Relationships between attack and escape rates, cannibalism, and intraguild predation in larvae of two predatory ladybirds. J. Insect Behav. 14, 373–384. doi: 10.1023/A:1011175430247
Yasuda, H., Takagi, T., and Kogi, K. (2000). Effects of conspecific and heterospecific larval tracks on the oviposition behaviour of the predatory ladybird, Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 103, 757–763. doi: 10.14411/eje.2000.085
Keywords: coccinellids, Coccinella septempunctata, defense, Harmonia axyridis, intraguild predation, invasive alien species
Citation: Hautier L, San Martin G, Jansen J-P, Branquart E and Grégoire J-C (2017) Spiny Prey, Fortunate Prey. Dorsal Spines Are an Asset in Intraguild Interactions among Lady Beetles. Front. Ecol. Evol. 5:135. doi: 10.3389/fevo.2017.00135
Received: 30 August 2017; Accepted: 23 October 2017;
Published: 07 November 2017.
Edited by:
Eric W. Riddick, Agricultural Research Service (USDA), United StatesReviewed by:
Matthew R. E. Symonds, Deakin University, AustraliaShawn M. Wilder, Oklahoma State University, United States
Copyright © 2017 Hautier, San Martin, Jansen, Branquart and Grégoire. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Louis Hautier, l.hautier@cra.wallonie.be
 Louis Hautier
Louis Hautier Gilles San Martin1
Gilles San Martin1  Jean-Claude Grégoire
Jean-Claude Grégoire