Exposure to Androstenes Influences Processing of Emotional Words
- 1Laboratoire d'Ethologie Expérimentale et Comparée, Université Paris 13 - Sorbonne Paris Cité, Villetaneuse, France
- 2Unité Transversale de Recherche Psychogenèse et Psychopathologie, EA4403, Université Paris 13 - Sorbonne Paris Cité, Villetaneuse, France
- 3Centre des Sciences du Goût, Centre National de la Recherche Scientifique UMR 6265, Université de Bourgogne, Dijon, France
- 4Division of Psychology, University of Stirling, Stirling, Scotland
There is evidence that human-produced androstenes affect attitudinal, emotional, and physiological states in a context-dependent manner, suggesting that they could be involved in modulating social interactions. For instance, androstadienone appears to increase attention specifically to emotional information. Most of the previous work focused on one or two androstenes. Here, we tested whether androstenes affect linguistic processing, using three different androstene compounds. Participants (90 women and 77 men) performed a lexical decision task after being exposed to an androstene or to a control treatment (all compounds were applied on the philtrum). We tested effects on three categories of target words, varying in emotional valence: positive, competitive, and neutral words (e.g., hope, war, and century, respectively). Results show that response times were modulated by androstene treatment and by emotional valence of words. Androstenone, but not androstadienone and androstenol, significantly slowed down the reaction time to words with competitive valence. Moreover, men exposed to androstenol showed a significantly reduced error rate, although men tended to make more errors than women in general. This suggests that these androstenes modulate the processing of emotional words, namely some particular lexical emotional content may become more salient under the effect of androstenes.
Introduction
Despite the conventional belief that olfaction is not as efficient and functional in humans as in other mammals, humans have a very good sense of smell (Stoddart, 1990; Schaal and Porter, 1991; Wyatt, 2014; Wyatt and, 2015; McGann, 2017). Humans are reported to be able to discriminate one trillion olfactory stimuli (Bushdid et al., 2014) and at best an innumerable amount of odors (Gerkin and Castro, 2015). Humans use this sensory channel extensively and chemical communication plays an important role in our everyday life, consciously or unconsciously. Odorants may induce basic emotions: a sniff of lavender odor will activate the autonomic nervous system and elicit “happiness,” while the odor of butyric acid will induce negative emotions such as “anger” and “disgust” (Vernet-Maury et al., 1999). Odorants have an effect on taste perception by enhancing or suppressing both sweet and sour tastes (Stevenson et al., 1999). Perhaps one of the most surprising ways by which odorants influence human behavior is through the modulation of other sensory inputs. For instance, we automatically adjust the spread of our fingers to match the size of an object when grasping. However, if we smell the odor of an orange when grasping a strawberry the amplitude of our hand opening will be larger than when the odor evokes an object of a similar size as the target; conversely, if we smell the odor of a small object (e.g., an almond) when grasping a large item (e.g., a peach) the amplitude of hand opening is reduced (Castiello et al., 2006). Odorants presented at a sub-threshold level, and thus not consciously perceived, can affect visual processing by facilitating the identification of an object in a complex scene that is congruent with the odor (e.g., looking at the picture of an orange among 12 other items after being exposed to orange odor; Seigneuric et al., 2010).
Humans, as well as other animals, are constantly exposed to olfactory stimuli in the environment, some of which may modulate attitudes, behavior, and physiology. In particular, we are regularly exposed to compounds produced by our own body and those of others. Among these are 16-unsaturated C19 steroids (16-androstenes), which have been found in human axillary sweat, saliva, semen, and milk (reviewed in Havlicek et al., 2010). During the last couple of decades, studies have focused on three of these androstenes supposedly produced prevalently, or in greater amounts, by men: 5α-androst-16-en-3-one (androstenone), 5α-androst-16-en-3α-ol (androstenol), and 4,16-androstadien-3-one (androstadienone) (reviewed in Havlicek et al., 2010; Wyatt and, 2015). This does not imply that these androstenes are the only possible candidates for human semiochemicals (cf. Wyatt and, 2015); yet, generally in laboratory experiments, these compounds appear to play a significant role in modulating human behavior, psychology, and physiology, usually in a gender-specific manner (e.g., Gustavson et al., 1987; Bensafi et al., 2003). For instance, androstenes increase: (i) women's ratings of attractiveness of both men and women (Cowley et al., 1977; Kirk-Smith et al., 1978; Saxton et al., 2008; but see Ferdenzi et al., 2016; Hare et al., 2017 where effects are not gender-specific); (ii) positive mood (Jacob and McClintock, 2000; Jacob et al., 2002); and (iii) social interactions with the opposite sex (Cowley and Brooksbank, 1991). Results are not always consistent possibly because different compounds play different roles, for example they might be involved in either intersexual (i.e., in regulating attractiveness of men to women) or intrasexual (i.e., in regulating male-male competition) processes (Havlicek et al., 2010). In addition to effects on behavior, exposure to androstenes may modulate physiological responses such as variation in skin conductance, respiration rate and heart rate, sometimes with opposite effects in men and women (Grosser et al., 2000; Bensafi et al., 2003). Being exposed to one of the commonly investigated androstenes, androstadienone, raises the level of cortisol in women, a hormone linked to arousal and mood (Wyart et al., 2007). If the chemistry of sexual attraction involves states of mind, such as being relaxed, being in a good mood or being open to men's or women's advances, then androstenes may help in “setting the stage” for physical attraction. Yet, androstenol and androstenone did not seem to increase female sexual arousal in response to erotic prose (McCollough et al., 1981; Benton and Wastell, 1986).
In the various experimental studies available in the literature, only one or two of these androstenes have been tested with the same protocol (reviewed in Havlicek et al., 2010). We thus believe that it is important to test the effect of these three different androstenes using the same experimental paradigm, in order to assess their relative importance as potential chemosignals. In the present study we investigated whether and how exposure to androstenes may have an impact on linguistic processing. Our rationale is based on previous studies showing that the semantic value or the emotional valence of olfactory stimulations can interact with lexical access. For instance, by using a lexical decision task in the presence of an odor, Holland et al. (2005) found that odors facilitated the recognition of odor-related words in a lexical decision task (the possible effect of gender was not tested). In a study manipulating the affective valence of stimuli, Hermans et al. (1998) found that the response time to evaluate words as “positive” or “negative” was shortened if preceded by an affectively congruent odor compared to an affectively incongruent odor. This result was only observed in female participants, and interpreted by the authors as being related to general gender differences in odor perception.
Recently, androstadienone has been suggested to specifically increase attention to visual stimuli with emotional significance (Hummer and McClintock, 2009). To our knowledge, this is one of the few studies using, among other psychological tests, a language paradigm, namely the “Stroop task” (Stroop, 1935; for a review, see MacLeod, 1991) in which participants are required to identify the ink color of written words. Naming response (reaction) times are recorded. The Stroop effect occurs when a psychological process (word reading) interferes with the goal of the task (naming the ink color of words). In Hummer and McClintock's (2009) experiment, the emotional Stroop effect was defined by calculating the difference between naming times of the ink color of emotional words (positive or negative valence such as holiday and pathetic) and of control pseudo-words. The authors also compared matching and mismatching words (e.g., green written in green and green written in blue) to control pseudo-words. Androstadienone slowed ink color identification only for emotional words, both positive and negative, and the effect was similar in men and women. The authors suggested a selective effect of androstadienone on emotional attention, i.e., the lexical emotional content was more difficult to ignore when exposed to androstadienone. This opens novel research questions aimed at exploring the possible role of odor-active compounds, and specifically of androstenes, in the modulation of linguistic processes.
The aim of the present study was to investigate possible interactions between androstenes and language by testing cross-modal modulation between the presentation of androstenes and the processing of words. In particular, we investigated whether androstenone, androstenol, and androstadienone may affect the processing of emotional words with a paradigm classically used in psycholinguistic studies (e.g., Meyer and Schvaneveldt, 1971; Neely, 1991; McNamara, 2005; Bueno and Frenck-Mestre, 2008). In the lexical decision task, participants must determine whether or not a letter string is a real word (for instance, in English: boat vs. trui). Response times and errors are recorded. In our experiment, three types of words were studied representing three emotional conditions: positive emotion words (e.g., love), competitive emotion words (e.g., fight), and control, neutral words (e.g., portion). We used positive and competitive words because the effects of androstenes might be linked to positive (intersexual) or competitive (intrasexual) cue processing. There were three experimental groups of participants (exposed to either androstenone, androstenol, or androstadienone) and one control group (mineral oil), each of which composed of about half women and half men (Table 1). All participants performed the lexical decision task.
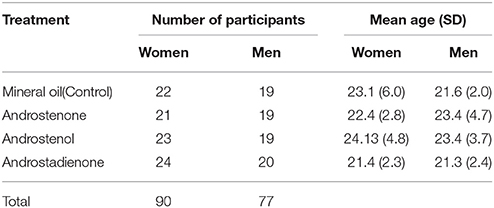
Table 1. Sample size and age of participants for the three androstene treatments and one control group.
In the present study, participants were tested in the context of the priming paradigm, generally used in a large variety of circumstances (e.g., McRae and Boisvert, 1998; Plaut and Booth, 2000; review in McNamara, 2005; Bueno and Frenck-Mestre, 2008). In the experimental condition, participants are exposed to a first event (the prime) that is supposed to facilitate the recognition/categorization/naming (and more generally the psychological or motor behavior) of a second one, the target, whenever they are linked. In the control condition, this prime is replaced by another prime not related to the target. If a mental relationship exists between the prime and the target, then the target is processed more rapidly in the experimental condition. For instance, presenting a prime word such as doctor facilitates (i.e., accelerates) the recognition of the target word nurse (pinpointing the semantic proximity of these concepts in memory), compared to the situation where the prime (e.g., window) is not related to the same target word nurse. The present study falls into the framework of the semantic priming effect since it compares the exposure to one event (the androstrene treatment) and its effect on a target word.
We expected that exposure to androstenes would influence processing of emotional words, and that these processing effects might differ according to emotional valence of words (positive, competitive, or neutral). In a lexical decision task, we recorded response times (the latency to decide whether a sequence of letters is a real word or not) and error rates (correctly distinguishing between words and pseudo-words). In lexical decisions, responses to positive stimuli are generally faster than to negative stimuli (review in Kuperman et al., 2014).
We hypothesized that androstenes could act at two levels. First, at the level of attentional processes. Knowing that androstadienone enhances attention to emotional stimuli (e.g., Hummer and McClintock, 2009), we expected that androstenes would increase attention to emotional words, compared to control words. Moreover, given that androstadienone enhances the feeling of being focused (Lundström et al., 2003), we expected that error rates in androstene-exposed groups would be lower than in the control group.
The second level addresses the possible function of androstenes in sexual selection. If androstenes are involved in inter-sexual relationships, exposure to androstenes would facilitate the processing of positive words. We would therefore expect faster response time and lower error rates for positive words compared to the other categories of words in androstene-exposed participants. Moreover, if androstenes are involved in intra-sexual processes, we would expect that the processing of competitive words may be facilitated (faster responses, lower error rate) compared to the other categories of words. Different androstenes may differ in their effects; this is why it is important to test different androstenes using the same protocol.
The effects of androstenes have been shown to be gender-specific in some studies (e.g., Saxton et al., 2008) but not in others (e.g., Ferdenzi et al., 2016). Therefore, we could not formulate a clear prediction in this respect; however, we tested whether the effects of androstenes on response time and error rates were modulated by sex.
Methods
Participants
A total of 171 adults participated in the experiment. Their age ranged from 18 to 40 (mean ± SD = 22.75 ± 4.39 years, details in Table 1). Participants were assigned to one of four groups (3 androstene treatments and 1 control, sample size in Table 1). Among them, four were discarded from subsequent analyses: one because she was aged more than 40 years; two because they made more than 10% errors on the lexical decision task; and one because 10% of his response times were outliers according to the classical criterion used in studies dealing with this kind of task (mean ± 2 SD; see details below). Thus, analyses were performed on a sample of 167 participants (77 men and 90 women) who were mostly students enrolled at the University of Paris 13, Sorbonne Paris Cité.
Overall, the percentage of women participating in this study and taking hormonal contraceptives was 54.1%. The percentage of women taking hormonal contraceptives did not differ significantly among treatments (chi-square test: = 1.575, P = 0.665): control group: 61.9%, androstenone: 45.0%, androstenol: 61.9%, and androstadienone: 56.5%. Furthermore, the phase of cycle in women who did not take hormonal contraceptives did not differ among treatments, as we did not find significant differences among the four treatment groups in the time delay between the women's last period and their date of participation in the study [one-way Anova: F(3, 33) = 0.102, P = 0.959].
Participants were remunerated with a 15-euro voucher at the end of the experiment. Written informed consent was obtained from all participants before testing. Data management and experimental protocol have been approved by both digital-data collection and French ethics committees [Commission Nationale de l'Informatique et des Libertés (CNIL) and Comité de Protection des Personnes Ile de France X - Hôpital Robert Ballanger, Aulnay-sous-Bois, respectively].
Chemicals
We prepared three test solutions of 16-unsaturated C19 steroids (16-androstenes) and one control. Test chemicals were 5α-androst-16-en-3-one (androstenone, SIGMA-Aldrich, France), 5α-androst-16-en-3α-ol (androstenol, Steraloids, U.S.A.), and androsta-4,16-dien-3-one (androstadienone, Steraloids, U.S.A.) dissolved in mineral oil (SIGMA-Aldrich, France) to obtain a 250 μM solution. This concentration was used in previous studies of androstadienone (e.g., Jacob and McClintock, 2000; Saxton et al., 2008), and when comparing effects of androstadienone and androstenol (Jacob et al., 2002). Other studies have used widely differing concentrations, including some with even higher concentrations of androstadienone (Bensafi et al., 2004) and several using lower concentrations for the stronger-smelling androstenone and androstenol (reviewed by Havlicek et al., 2010). Because it would be challenging to obtain perceptually equivalent concentrations for each individual, given that there are substantial individual differences in sensitivity to different androstene compounds, we used the same concentration across all three compounds. The control stimulus was mineral oil alone.
After the completion of the lexical decision task, we asked the participants to indicate whether and how they had perceived the smell of the substance (androstenes or mineral oil) on their upper lip, using a score ranging from −2 to +2 (−2: very unpleasant; −1: unpleasant; 0: neutral; +1: pleasant; +2: very pleasant). A statistical comparison of these scores revealed no significant differences in the perception of the substances among the 4 treatment groups (Kruskal Wallis H test: = 2.397, P = 0.494).
Linguistic Material
Three categories of real words were used: 15 words with emotional positive value (e.g., kindness, mean frequency of positive words = 50.00 per million, SD = 66.03; mean length, i.e., number of letters = 6.53, SD = 1.68), 15 words with emotional competitive value (e.g., fight, mean frequency = 40.96, SD = 93.14; mean length = 5.06, SD = 2.07), and 15 neutral words (e.g., portion, mean frequency = 35.96, SD = 61.10; mean length = 5.93, SD = 1.87). The three categories of words did not differ significantly in frequency, i.e., in the occurrence per million words used [one-way Anova: F(2, 37) = 0.173, P = 0.842] or in length [i.e., in the number of letters: F(2, 37) = 1.090, P = 0.347]. The list of words is available as Supplementary Material. Furthermore, the correlation between number of letters and number of phonemes in French words is significantly high (r = 0.77; New et al., 2001). To avoid the possibility that participants might become aware that they were tested on the valence of words, 45 “filler” words were added. Filler words were neutral and were controlled for frequency and length. In order to have the same amount of “yes” and “no” answers (so to avoid possible answering strategies), 90 pseudo-words were created based on legal French letter strings (New et al., 2001). Half of the items were real (French) words and half were pseudo-words (e.g., patrule). The resulting list of words was composed of 180 items in total.
Two preliminary tests assessed the valence of the words used in the lexical decision task, one for selecting the positive and neutral words (pre-test 1), and one for selecting the competitive words (pre-test 2). Forty-eight participants were recruited for pre-test 1, and 42 additional participants for pre-test 2. To select the neutral and positive words, 220 words were rated. To avoid fatigue, each participant was asked to judge the emotional valence of 110 words on a scale ranging from −3 (very negative) to +3 (very positive), with 0 being considered neutral. Two series of 110 words were created. To avoid a rank effect, three orders of these words were created for each series, yielding six lists. Participants were allocated to one of these lists. For pre-test 2, aiming at selecting the competitive words, participants judged how much a word would refer to a competitive situation using a scale ranging from 0 (no competition) to 10 (very competitive situation). Here, a total of 30 words were pre-tested and three different orders of these words were created.
For the positive emotional words, we selected the 15 words that had the highest score according to the pre-test results. Similarly, for neutral words we selected 15 words with a value of 0. Selected competitive words were the 15 highest rated words on the 10-point scale. This selection procedure allowed selecting words for which the judgment reached 75% of agreement among the raters.
Procedure
Each participant entered a quiet experimental room individually, a male experimenter gave the participant the informed consent document to read and complete. Participants were pseudo-randomly allocated to one of the four experimental groups by trying to balance the number of men and women in each group. Afterwards, the experimenter applied 100 μL of solution (either one of the three androstene solutions or the control) to the philtrum of the participant using a cotton swab. Participants did not begin the experimental session until 10 min after the administration of the solution. During this interval, the participant completed a questionnaire about general background information (e.g., gender, age) and was given instructions about the forthcoming computer task.
Each participant then completed the lexical decision task on a computer, while seated on an office chair in the experimental room. Participants were asked to decide, as quickly and accurately as possible, whether the letter string appearing on the computer screen was an existing word or not, by pressing a key (designed as “yes” or “no”) on the computer keyboard. A sequence was composed of three events: a blank screen for 2 s, then 12 hashmarks for 500 ms in the middle of the screen, and finally the target item, which remained on the screen until the decision was made. The DMDX software (Forster and Forster, 2003) was used to control word display, recording of response times (i.e., the latency to make a decision whether a sequence of letters constitutes a word) with millisecond accuracy, and the occurrence of errors (false negatives, i.e., a real word was classified as a pseudo-word by the participant answering “no”). False positives, i.e., cases where pseudo-words were incorrectly considered as a word were rare and not taken into account for further analyses. Prior to the task, participants performed a short training session on a few items. In total, the experimental session lasted about 30 min, during which the experimenter remained in the room seated quietly in a corner far from the computer.
Statistics
The word ring (belonging to the category of words with competitive valence) was excluded from statistical analyses because of the high error rate participants made with respect to this word (33% of errors). During manuscript revision, we also excluded the words champion and victory from analyses. These words belonged to the competitive valence group, but may entail elements of positive valence. These exclusion criteria reduced the number of words with competitive valence from 15 to 12. An analysis based on the complete list of competitive words (n = 15) gave substantially the same results.
Statistical analyses were performed with R, version 3.4.1 (R Core Team, 2017). Data were analyzed by multifactorial (generalized) linear mixed-effects models, using the R package lme4 (Bates et al., 2015). Models included two random (intercept) factors: Individual (subject) identity was included to account for repeated measurements of subjects across words with different valence within each treatment. Furthermore, the identity of the words (in total: 42 different words) was included as a random factor in order to account for potentially different responses (in response time or in error probability) to these words.
We analyzed the effects of three independent (predictor) variables: treatment (factor with 4 levels), sex (factor with 2 levels), and valence (factor with 3 levels) on the dependent variables: (a) response time (continuous variable) and (b) the occurrence of errors (false negatives) in determining the existence of a given word (dichotomous variable). The analysis of response time was carried out by multifactorial linear mixed-effects models (LMM). To this end, the variable “response time,” which showed a right skewed distribution, was log[x]-transformed to adjust its distribution to normal. The analysis of occurrence of errors (hereafter referred to as “error rate”) was carried out by multifactorial generalized linear mixed-effects models (with a logit link) for binomially distributed data (GLMM). P-values for LMM were calculated by F-tests based on the Satterthwaite's approximation (Bolker et al., 2009). P-values for GLMM were calculated by Wald chi-square tests. For LMM, we checked for homogeneity of variances by plotting residual vs. fitted values, and we verified that model residuals were well-adjusted to a normal distribution by normal probability plots (Faraway, 2006).
Full models included 3-way and all 2-way interactions between the three independent (predictor) variables: treatment, sex, and valence (Table 2). We present minimal adequate models with respect to interaction terms (Crawley, 2007); that is, non-significant interaction terms were step-wise removed from the models before these were recalculated; however, the P-values of the last step prior to removal from the models are given in the tables (Tables 2–4) for completeness. In addition, we applied likelihood ratio tests (Faraway, 2006) by which we compared the full models with less parameterized models excluding one of the interaction terms (see Tables S1, S2 in the supplementary material). The aim of this analysis was to identify interaction terms, which did not provide significant information to the model and thus could be excluded. These additional analyses provided the same results as our model selection strategy of a stepwise removal of interaction terms via F-tests based on the Satterthwaite's approximation, as presented in Tables 2–4.
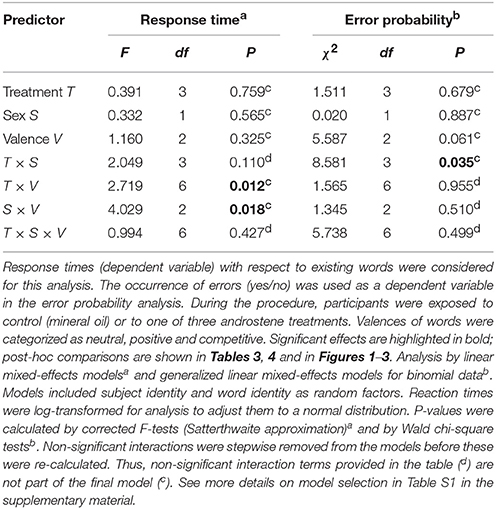
Table 2. Effects of different predictor variables including their interactions on the response time (latency) of participants to decide whether the letter string was an existing word or not, and the occurrence of false negatives in this decision.
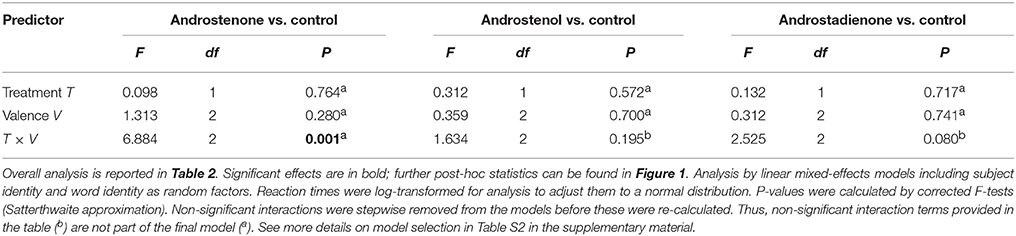
Table 3. Post-hoc analyses to the significant interaction between treatment and valence with respect to participants' response times.
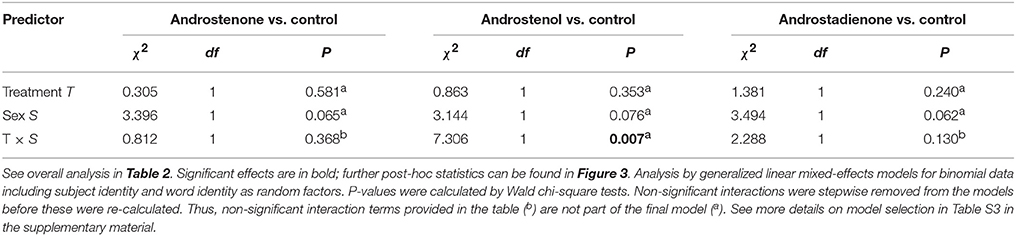
Table 4. Post-hoc analyses to the significant interaction between treatment and sex with respect to participants' error probability.
For significant interaction terms including “treatment,” we carried out multifactorial post-hoc analyses based on comparisons between the different androstene treatments and the control treatment (Tables 3, 4). Pairwise post-hoc comparisons after significant LMM (Figures 1, 2) and GLMM (Figure 3) were carried out using the same principal model, respectively—although based on a subset of the data. Alpha levels of these multiple comparisons (Figures 1–3) were Bonferroni corrected by a sequential correction following the methods as described in Holm (1979).
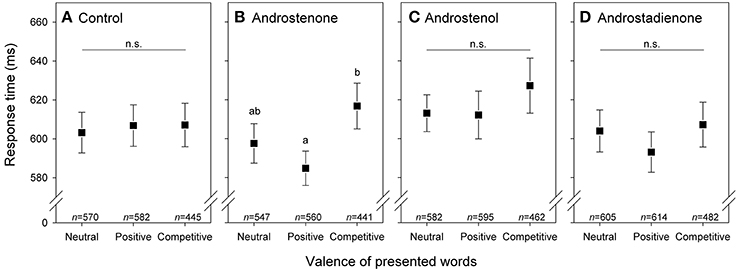
Figure 1. Response times (means with 95% confidence intervals) of subjects (n = 167) to words with neutral, positive, and competitive valence while exposed to (A) control (mineral oil) or to (B–D) one of three androstene treatments. Significant differences based on pairwise comparisons (after sequential Bonferroni correction: Holm, 1979) between words with different valence are indicated by different letters. Sample sizes of presented words are given; see also Tables 2, 3.
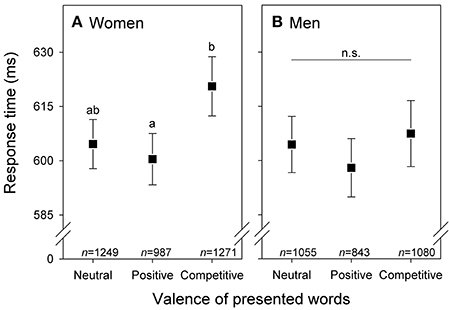
Figure 2. Response times (means with 95% confidence intervals) of (A) women (n = 90) and (B) men (n = 77) to words with neutral, positive, and competitive valence. Significant differences based on pairwise comparisons (after sequential Bonferroni correction: Holm, 1979) between words with different valence are indicated by different letters. Sample sizes of presented words are given; details on statistics in Table 2.
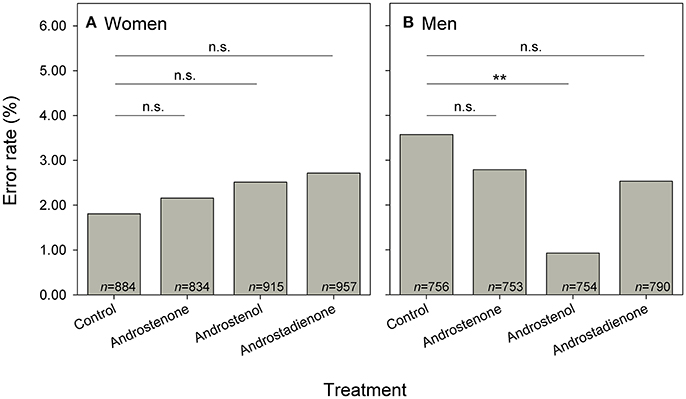
Figure 3. Error rates of (A) women (n = 90) and (B) men (n = 77) in distinguishing words when exposed to control (mineral oil) or one of three androstene treatments. Significant pairwise comparisons (post-hoc to significant general differences, see Tables 2, 4; sequential Bonferroni correction: Holm, 1979) between different treatment groups are indicated by asterisks (**P < 0.010). Sample sizes of presented words are given.
As usual with analysis of response time data, outliers might result from distraction, boredom, tiredness or similar factors, and were consistently removed from the dataset (e.g., Holland et al., 2005; Bueno and Frenck-Mestre, 2008). Outliers were identified as those response times over 2 standard deviations from the mean, with limits separately calculated for each treatment, gender, word valence and participant (Ratcliff, 1993; Baayen and Milin, 2010). Overall, 5.1% of the data were excluded as outliers from the analysis of response times. However, an analysis based on the full data set including these outliers gives very similar results.
Results
Response Times
Multifactorial analysis revealed no significant 3-way interactive effects between androstene treatment, valence of words, and sex on the response time of subjects (Table 2). However, the significant 2-way interaction between treatment and valence (Table 2) indicates modulating effects of androstene treatment on the reaction time to words with different valence, independently of the participants' gender. Post-hoc comparisons, given in Table 3, showed that such a significant modulation occurred only under androstenone treatment: subjects showed significantly longer response times when dealing with words with competitive valence compared to words with positive valence, whereas response times to both groups of words did not differ significantly from words with neutral valence (pair-wise comparisons in Figure 1B). Additional, pair-wise comparisons between control treatment and androstenone treatment revealed that response times to words with positive valence were significantly shorter in the androstenone group than in the control group [LMM: F(1, 1,127) = 9.430, P = 0.002]; whereas response times to words with neutral or competitive valence did not differ significantly between mineral oil (control) and androstenone treatment (all P > 0.10; see Figure S1 in the supplementary material).
Furthermore, in the overall analysis, the significant 2-way interaction between sex and valence of words indicates that women and men generally showed significantly different patterns in their response times, independently of the treatment (Table 2). Post-hoc comparisons revealed that women had significantly longer response times when dealing with words with competitive valence than with words with positive valence, whilst response times associated to words with neutral valence did not differ significantly from the two latter groups (statistics in Figure 2A). In contrast, men did not differ significantly in their response times with respect to words with different valence (Figure 2B).
Error Rates
We did not find a significant 3-way interaction with respect to the occurrence of errors in correctly determining whether a string of letters constituted an existing word (Table 2). However, there was a significant 2-way interaction between sex and treatment, indicating that sex-specific error rates were modulated by exposure to particular androstenes, independently of the valence of the words (Table 2). Post-hoc comparisons (in Table 4, Figure 3B) revealed that men exposed to androstenol made significantly fewer errors compared to men under control treatment, whereas there were no significant differences in error rates between the control and any other treatment (Figure 3B). In contrast, there were no such statistically significant differences between the control group and the different androstene treatments in women (Figure 3A). Overall, none of the applied androstene treatments significantly affected women's error rates.
Furthermore, gender-specific post-hoc comparisons revealed that men showed significantly lower error rates (by 1.58% lower) than women after androstenol treatment (GLMM: = 4.184, P = 0.040) but tended to show higher error rates in comparison to women (by 1.76% higher) in the control group ( = 3.030, P = 0.082; see Figure S2 in the supplementary material). There were no statistically significant differences or trends indicating differences in error rates between women and men after the treatment with androstenone or androstadienone (both P > 0.10).
There was also a statistical tendency of a main effect of valence (Table 2). Post-hoc comparisons revealed that the overall error rate for words with competitive valence (3.48%) was higher than for words with positive valence (1.26%) (GLMM: = 5.463, P = 0.019). Words with neutral valence were associated to an intermediate error rate (2.6%), which did not differ significantly from the two other groups (neutral vs. positive: = 2.811, P = 0.094; neutral vs. competitive: = 0.566, P = 0.452).
Discussion
In this study we used a lexical decision task to investigate the possible effect of exposure to 16-androstenes on language processing. Slower responses in the task indicate increased attentional allocation to the presented words. We tested the effects of three androstenes most commonly used in previous research: androstenone, androstenol and androstadienone. Previous studies typically used only one of these compounds in a given experimental paradigm (e.g., Hummer and McClintock, 2009), which precludes the possibility to determine whether reported effects are elicited by any androstene or whether they are compound-specific (Havlicek et al., 2010). We compared effects of these androstene compounds on three categories of target words. We had three emotional conditions: positive emotion words, competitive emotion words, and control words. Our results show that priming of distinct androstene compounds had different effects on linguistic processes. We report two main findings. First, we analyzed the response time of participants in a lexical decision task, i.e., the latency to make a decision whether a sequence of letters is a real word or not. We show a modulating effect of one androstene on response time to words with different valence: androstenone differentially altered the response time of words with competitive and positive valence, independently of the participants' gender (Figure 1). Both men and women exposed to androstenone were slower when presented with competitive words than with positive words. This effect was not observed for the other two compounds, suggesting a compound-specific effect on semantic processing. When comparing the androstenone-exposed group and the control group (exposed to mineral oil), we observed that, for both sexes, the response time to positive words was faster in the androstenone group (Figure S1). Second, we analyzed the occurrence of errors (false negatives, i.e., a real word was not identified as such). Here, we observed gender-specific errors modulated by a different androstene: men exposed to androstenol made significantly fewer errors compared with those in the no-odor control group (Figure 3). Such an effect was not observed in women.
These results support the hypothesis that androstenes modulate attentional processes. Several studies suggest that attention is disengaged more slowly from negative stimuli than from positive ones and the consequence of this delayed disengagement is slower responding in lexical decisions (review in Kuperman et al., 2014). Our results that exposure to androstenone induced a slower response time to competitive words compared with positive (but not neutral) words is in agreement with these findings. Slower response times may indicate greater allocation of attentional resources toward emotional information contained in this category of words, which hold attention longer. This might be important in the context of partner choice and/or intrasexual signaling. However, our predictions stemming from the sexual selection hypothesis were not verified.
Regardless of treatment, women were overall slower to react to competitive than positive (but not neutral) words (Figure 2); women may particularly attend to competitive words because they reveal something about male competitive attitude (where high competitiveness is desirable), or alternatively because the use of competitive words in a conversation might reveal high dominance or aggressiveness in men, characteristics that may be undesirable for long-term relationships (Puts et al., 2012; Valentine et al., 2014). It appears that androstenone accentuates the impact of emotional information conveyed by words because positive words, which in general elicit lower response time (Kuperman et al., 2014), were identified even faster under androstenone exposure compared to exposure to control (Figure S1).
A different androstene had an effect on error rates, and this effect was sex-specific (Figure S2). Men exposed to androstenol made fewer errors than controls (Figure 3), thus appearing more focused on the task under this prime. This could be the effect of general arousal and/or feeling in a competition context when perceiving certain 16-androstenes. Indeed, previous studies showed that men avoid sitting on chairs that were treated with androstenone in a waiting room (Kirk-Smith and Booth, 1980; Pause, 2004) and exposure to androstenol made men consumers evaluate male magazines as more masculine (Ebster and Kirk-Smith, 2005). Interestingly, the effect of androstenol was sex-specific in a study showing that men avoided androstenol-sprayed stalls in restrooms while women showed no preference. This was interpreted as supporting the idea of androstenol as “human spacing signal,” which would be relevant particularly in male-male competition (Gustavson et al., 1987). Although the effects of androstenes on, for instance, ratings of attractiveness appeared to be gender-specific in some studies (e.g., Cowley et al., 1977; Kirk-Smith et al., 1978; Saxton et al., 2008) but not in others (Ferdenzi et al., 2016; Hare et al., 2017), our results are partially consistent with studies in which sex differences have been described in semantic processing and in priming processes. Using event-related potentials, for example, Wirth et al. (2007) showed that women and men differ in the depth of semantic elaboration and integration; in general, women appear to conduct a deeper semantic analysis than men. However, gender-specific effects might be amplified when women participants face a male experimenter, or vice versa. The effects of androstenes have indeed been shown to be context-dependent, the reactions of women being more prevalent in presence of a male experimenter (Jacob et al., 2001; Lundström and Olsson, 2005). Future research in this area should consider systematic counterbalancing of the experimenter's gender.
In our dataset, androstadienone exposure did not induce statistically significant effects, despite it being a widely used and “popular” compound (see Wyatt and, 2015), which is often associated with changes in behavior. Several studies suggest that androstadienone enhances women's feeling of being focused and modulates positively psychological arousal and mood (Lundström et al., 2003; Lundström and Olsson, 2005). In particular, Hummer and McClintock (2009) obtained positive results using the Stroop task (see introduction). The difference between the current and these previous studies might be due to the fact that the information processing strategy needed for lexical decisions is different from that underlying identification (reading aloud) of words (Schmidt et al., 2013). Furthermore, although we tested the responses to differently valenced words in the same participants, tests of the effects of treatment (exposure to one of three different androstene compounds or mineral oil control) were carried out between-subjects. Further tests of compound-specific effects might benefit from exposing the same participants to the different odor compounds. Although Holland et al. (2005) have shown an effect of odor priming on linguistic and other overt and covert behavioral processes, the present study investigated such effects using three distinct human-produced odorant androstenes. The priming effect induced separately by two of these androstenes had an influence on the response times (or error rate) to the subsequent linguistic material, thus confirming a direct link between chemo-perception and activation of high-level integration processes such as memory and language comprehension. Further steps should involve investigating the effects of androstenes in experiments using larger linguistic units such as sentences. If androstenes can truly affect behavior, their effects should be revealed in the processing of sentences describing obviously conflicting or positive situations, for instance. We thus expect that androstenes would also modulate response times in complex linguistic tasks. Another point open to future enquiry relates to the fact that these androstenes are emitted as mixtures in human body secretions/excretions. Thus, another step would be to assess the priming effects of mixtures of androstenes, possibly in the context of other odor-active compounds of body odor headspace.
In conclusion, whole body odors, including axillary odor, have been shown to influence a range of human judgments, attitudes and behaviors, just as they do in other animals (e.g., Gosling and Roberts, 2001; Wyatt, 2014). There is currently uncertainty concerning the nature of the odorants conveying such effects, and individual androstenes have been intensively assessed for their particular and specific influence on behavior (Havlicek et al., 2010; Wyatt and, 2015). We concede that androstenes are just one set of compounds within a much larger and complex mixture (e.g.,Curran et al., 2005; Gallagher et al., 2008; Dormont et al., 2013), and therefore we do not wish to make claims about whether the effects reported in the literature are exclusively produced by androstenes. Other types of odorants present in the body odor mixture need indeed to be tested. However, androstene compounds certainly contribute to axillary odor profiles and could therefore provide functional cues (or even signals) that are used in inter-individual assessment. Our results are consistent with other studies in showing that they elicit measurable behavioral effects, and suggest some androstenes at least have the potential to make a contribution to the effects seen using whole body odor. Furthermore, because we set out to compare effects of three different androstene compounds, and in view of the results in which we find compound-specific effects, it is possible that individual androstene compounds (or their relative concentrations) may have functional effects in modulating social interactions. Androstenes, which are produced in higher quantities in males, might be involved in intersexual and intrasexual communication (Pause, 2012; Lübke and Pause, 2015), and particularly in modulating responses to words in social contexts that are associated with specific emotions.
Author Contributions
Pd, SB, HM, and AS conceived the study; Pd, SB, HM, AS, BS, and SR designed the study; Pd, SB, HM, and AS carried out the experimental work with the help of students and prepared the dataset; HR analyzed the data; Pd and HR wrote the manuscript with the contribution of SB and SR. All authors read, commented, and approved the manuscript.
Funding
The study was funded by a grant from University of Paris 13, Sorbonne Paris Cité to Pd (Interdisciplinary BQR-2012: “Are body odours modulating linguistic processes?”). PdE is also supported by the French National Research Agency ANR (project PheroMod, ANR-14-CE18-0003).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Many thanks to Jean-Baptiste Goin, Emmanuel Terrine, and Aleksandar Ivkovic for help with data collection; part of the data are included in the final report to obtain the Master degree in Ethology by J-B Goin. Pd thanks Fabien Florimond for assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2017.00169/full#supplementary-material
References
Baayen, R. H., and Milin, P. (2010). Analyzing reaction times. Int. J. Psychol. Res. 3, 12–28. doi: 10.21500/20112084.807
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48. doi: 10.18637/jss.v067.i01
Bensafi, M., Brown, W. M., Tsutsui, T., Mainland, J. D., Johnson, B. N., Bremner, E., et al. (2003). Sex steroid derived compounds induce sex-specific effects on autonomic nervous system function in humans. Behav. Neurosci. 117, 1125–1134. doi: 10.1037/0735-7044.117.6.1125
Bensafi, M., Tsutsui, T., Khan, R., Levenson, R. W., and Sobel, N. (2004). Sniffing a human sex-steroid derived compound affects mood and autonomic arousal in a dose-dependent manner. Psychoneuroendocrinology 29, 1290–1299. doi: 10.1016/j.psyneuen.2004.03.007
Benton, D., and Wastell, V. (1986). Effects of androstenol on human sexual arousal. Bio. Psychol. 22, 141–147. doi: 10.1016/0301-0511(86)90041-4
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H. H., et al. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. doi: 10.1016/j.tree.2008.10.008
Bueno, S., and Frenck-Mestre, C. (2008). The activation of semantic memory: effects of prime exposure, prime-target relationship, and task demands. Mem. Cogn. 36, 882–898. doi: 10.3758/MC.36.4.882
Bushdid, C., Magnasco, M. O., Vosshall, L. B., and Keller, A. (2014). Humans discriminate more than 1 trillion olfactory stimuli. Science 343, 1370–1372. doi: 10.1126/science.1249168
Castiello, U., Zucco, G. M., Parma, V., Ansuini, C., and Tirindelli, R. (2006). Cross-modal interactions between olfaction and vision when grasping. Chem. Senses 31, 665–671. doi: 10.1093/chemse/bjl007
Cowley, J. J., Johnson, A. L., and Brooksbank, B. W. (1977). The effect of two odorous compounds on performance in an assessment-of-people test. Psychoneuroendocrinology 2, 159–172. doi: 10.1016/0306-4530(77)90021-X
Cowley, J. J., and Brooksbank, B. W. (1991). Human exposure to putative pheromones and changes in aspects of social behaviour. J. Steroid. Biochem. Mol. Biol. 39, 647–659. doi: 10.1016/0960-0760(91)90264-6
Curran, A. M., Rabin, S. I., Prada, P. A., and Furton, K. G. (2005). Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J. Chem. Ecol. 31, 1613–1625. doi: 10.1007/s10886-005-5801-4
Dormont, L., Bessière, J. M., and Cohuet, A. (2013). Human skin volatiles: a review. J. Chem. Ecol. 39, 569–578. doi: 10.1007/s10886-013-0286-z
Ebster, C., and Kirk-Smith, M. (2005). The effect of the human pheromone androstenol on product evaluation. Psychol. Market. 22, 739–749. doi: 10.1002/mar.20082
Faraway, J. J. (2006). Extending the Linear Model with R. Generalized Linear, Mixed Effects and Nonparametric Regression Models. Boca Raton, FL: Chapman & Hall.
Ferdenzi, C., Delplanque, S., Atanassova, R., and Sander, D. (2016). Androstadienone's influence on the perception of facial and vocal attractiveness is not sex specific. Psychoneuroendocrinology 66, 166–175. doi: 10.1016/j.psyneuen.2016.01.016
Forster, K., and Forster, J. (2003). A Window display program with millisecond accuracy. Behav. Res. Methods 35, 116–124. doi: 10.3758/BF03195503
Gallagher, M., Wysocki, C. J., Leyden, J. J., Spielman, A. I., Sun, X., and Preti, G. (2008). Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 159, 780–791. doi: 10.1111/j.1365-2133.2008.08748.x
Gerkin, R. C., and Castro, J. B. (2015). The number of olfactory stimuli that humans can discriminate is still unknown. Elife 4:e08127. doi: 10.7554/eLife.08127
Gosling, L. M., and Roberts, S. C. (2001). Scent-marking by male mammals: cheat-proof signals to competitors and mates. Adv. Stud. Behav. 30, 169–217. doi: 10.1016/S0065-3454(01)80007-3
Grosser, B. I., Monti-Bloch, L., Jennings-White, C., and Berliner, D. L. (2000). Behavioral and electrophysiological effects of androstadienone, a human pheromone. Psychoneuroendocrinology 25, 289–299. doi: 10.1016/S0306-4530(99)00056-6
Gustavson, A. R., Dawson, M. E., and Bonett, D. G. (1987). Androstenol, a putative human pheromone, affects human (Homo sapiens) male choice performance. J. Comp. Psychol. 101, 210–212. doi: 10.1037/0735-7036.101.2.210
Hare, R. M., Schlatter, S., Rhodes, G., and Simmons, L. W. (2017). Putative sex-specific human pheromones do not affect gender perception, attractiveness ratings or unfaithfulness judgements of opposite sex faces. R. Soc. Open Sci. 4:160831. doi: 10.1098/rsos.160831
Havlicek, J., Murray, A. K., Saxton, T. K., and Roberts, S. C. (2010). Current issues in the study of androstenes in human chemosignaling. Vitamins Horm. 83, 47–82. doi: 10.1016/S0083-6729(10)83003-1
Hermans, D., Baeyens, F., and Eelen, P. (1998). Odours as affective-processing context for word evaluation: a case of cross-modal affective priming. Cogn. Emot. 12, 601–613. doi: 10.1080/026999398379583
Holland, R. W., Hendriks, M., and Aarts, H. (2005). Smells like clean spirit: nonconscious effects of scent on cognition and behavior. Psychol. Sci. 16, 689–693. doi: 10.1111/j.1467-9280.2005.01597.x
Holm, S. (1979). A simple sequential rejective multiple test procedure. Scand. J. Stat. 6, 65–70. doi: 10.2307/4615733
Hummer, T. A., and McClintock, M. K. (2009). Putative human pheromone androstadienone attunes the mind specifically to emotional information. Horm. Behav. 55, 548–559. doi: 10.1016/j.yhbeh.2009.01.002
Jacob, S., Garcia, S., Hayreh, D., and McClintock, M. K. (2002). Psychological effects of musky compounds: comparison of androstadienone with androstenol and muscone. Horm. Behav. 42, 274–283. doi: 10.1006/hbeh.2002.1826
Jacob, S., Hayreh, D. J., and McClintock, M. K. (2001). Context-dependent effects of steroid chemosignals on human physiology and mood. Physiol. Behav. 74, 15–27. doi: 10.1016/S0031-9384(01)00537-6
Jacob, S., and McClintock, M. K. (2000). Psychological state and mood effects of steroidal chemosignals in women and men. Horm. Behav. 37, 57–78. doi: 10.1006/hbeh.1999.1559
Kirk-Smith, M. D., and Booth, D. A. (1980). “Effect of androstenone on choice of location in others' presence,” in International Symposium on Olfaction and Taste VII (Noordwijkerhout: IRL Press Limited Netherlands), 397–400.
Kirk-Smith, M. D., Booth, D. A., Carroll, D., and Davies, P. (1978). Human social attitudes affected by androstenol. Res. Commun. Psychol. Psychiatry Behav. 3, 379–384.
Kuperman, V., Estes, Z., Brysbaert, M., and Warriner, A. (2014). Emotion and language: valence and arousal affect word recognition. J. Exp. Psychol. Gen. 143, 1065–1081. doi: 10.1037/a0035669
Lübke, K. T., and Pause, B. M. (2015). Always follow your nose: the functional significance of social chemosignals in human reproduction and survival. Horm. Behav. 68, 134–144. doi: 10.1016/j.yhbeh.2014.10.001
Lundström, J. N., Goncalves, M., Esteves, F., and Olsson, M. J. (2003). Psychological effects of subthreshold exposure to the putative human pheromone 4,16-androstadien-3-one. Horm. Behav. 44, 395–401. doi: 10.1016/j.yhbeh.2003.06.004
Lundström, J. N., and Olsson, M. J. (2005). Subthreshold amounts of social odorant affect mood, but not behavior, in heterosexual women when tested by a male, but not a female, experimenter. Biol. Psychol. 70, 197–204. doi: 10.1016/j.biopsycho.2005.01.008
McCollough, P. A., Owen, J. W., and Pollak, E. I. (1981). Does androstenol affect emotion? Ethol. Sociobiol. 2, 85–88. doi: 10.1016/0162-3095(81)90036-4
MacLeod, C. M. (1991). Half a century of research on the Stroop effect: an integrative review. Psychol. Bull. 109, 163–203. doi: 10.1037/0033-2909.109.2.163
McGann, J. P. (2017). Poor human olfaction is a 19th Century myth. Science 356:eaam7263. doi: 10.1126/science.aam7263
McNamara, T. P. (2005). Semantic Priming. Perspectives from Memory and Word Recognition. New York, NY: Psychology Press.
McRae, K., and Boisvert, S. (1998). Automatic semantic similarity priming. J. Exp. Psychol. 24, 558–572. doi: 10.1037/0278-7393.24.3.558
Meyer, D. E., and Schvaneveldt, R. W. (1971). Facilitation in recognizing pairs of words: evidence of dependence between retrieval operations. J. Exp. Psychol. 90, 227–234. doi: 10.1037/h0031564
Neely, J. H. (1991). “Semantic priming effects in visual word recognition: a selective review of current findings and theory,” in Basic Processes in Reading: Visual Word Recognition, eds D. Besner and G. W. Humphreys (Hillsadale, NJ: Erlbaum), 264–336.
New, B., Pallier, C., Ferrand, L., and Matos, R. (2001). Une base de données lexicales du français contemporain sur internet: LEXIQUE. Ann. Psychol. 101, 447–462. doi: 10.3406/psy.2001.1341
Pause, B. M. (2004). Are androgen steroids acting as pheromones in humans? Physiol. Behav. 83, 21–29. doi: 10.1016/S0031-9384(04)00345-2
Pause, B. M. (2012). Processing of body odor signals by the human brain. Chemosens. Percept. 5, 55–63. doi: 10.1007/s12078-011-9108-2
Plaut, D. C., and Booth, J. R. (2000). Individual and developmental differences in semantic priming: empirical and computational support for a single-mechanism account of lexical processing. Psychol. Rev. 107, 786–823. doi: 10.1037/0033-295X.107.4.786
Puts, D. A., Jones, B. C., and DeBruine, L. M. (2012). Sexual selection on human faces and voices. J. Sex Res. 49, 227–243. doi: 10.1080/00224499.2012.658924
R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: www.R-project.org
Ratcliff, R. (1993). Methods for dealing with reaction time outliers. Psychol. Bull. 114, 510–532. doi: 10.1037/0033-2909.114.3.510
Saxton, T. K., Lyndon, A., Little, A. C., and Roberts, S. C. (2008). Evidence that androstadienone, a putative human chemosignal, modulates women's attributions of men's attractiveness. Horm. Behav. 54, 597–601. doi: 10.1016/j.yhbeh.2008.06.001
Schaal, B., and Porter, R. H. (1991). Microsmatic humans revisited: the generation and perception of chemical signals. Adv. Stud. Behav. 20, 135–200. doi: 10.1016/S0065-3454(08)60321-6
Schmidt, J. R., Cheesman, J., and Besner, D. (2013). You can't Stroop a lexical decision: is semantic processing fundamentally facilitative? Can. J. Exp. Psychol. 67, 130–139. doi: 10.1037/a0030355
Seigneuric, A., Durand, K., Jiang, T., Baudouin, J.-Y., and Schaal, B. (2010). The nose tells it to the eyes: crossmodal associations between olfaction and vision. Perception 39, 1541–1554. doi: 10.1068/p6740
Stevenson, R. J., Prescott, J., and Boakes, R. A. (1999). Confusing tastes and smells: how odours can influence the perception of sweet and sour tastes. Chem. Senses 24, 627–635. doi: 10.1093/chemse/24.6.627
Stoddart, D. M. (1990). The Scented Ape: The Biology and Culture of Human Odour. Cambridge: Cambridge University Press.
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. J. Exp. Psychol. 18, 643–662. doi: 10.1037/h0054651
Valentine, K. A., Li, N. P., Penke, L., and Perrett, D. I. (2014). Judging a man by the width of his face: the role of facial ratios and dominance in mate choice at speed-dating events. Psychol. Sci. 25, 806–811. doi: 10.1177/0956797613511823
Vernet-Maury, E., Alaoui-Ismaili, O., Dittmar, A., Delhomme, G., and Chanel, J. (1999). Basic emotions induced by odorants: a new approach based on autonomic pattern results. J. Auton. Nerv. Syst. 75, 176–183. doi: 10.1016/S0165-1838(98)00168-4
Wirth, M., Horn, H., Koenig, T., Stein, M., Federspiel, A., Meier, B., et al. (2007). Sex differences in semantic processing: event-related brain potentials distinguish between lower and higher order semantic analysis during word reading. Cereb. Cortex 17, 1987–1997. doi: 10.1093/cercor/bhl121
Wyart, C., Webster, W. W., Chen, J. H., Wilson, S. R., McClary, A., Khan, R. M., et al. (2007). Smelling a single component of male sweat alters levels of cortisol in women. J. Neurosci. 27, 1261–1265. doi: 10.1523/JNEUROSCI.4430-06.2007
Wyatt, T. D. (2014). Pheromones and Animal Behavior, 2nd Edn. Cambridge: Cambridge University Press.
Keywords: 16-androstenes, lexical decision task, olfaction, emotions, humans
Citation: d'Ettorre P, Bueno S, Rödel HG, Megherbi H, Seigneuric A, Schaal B and Roberts SC (2018) Exposure to Androstenes Influences Processing of Emotional Words. Front. Ecol. Evol. 5:169. doi: 10.3389/fevo.2017.00169
Received: 16 December 2016; Accepted: 13 December 2017;
Published: 10 January 2018.
Edited by:
Luisa Amo, Consejo Superior de Investigaciones Científicas (CSIC), SpainReviewed by:
Camille Ferdenzi, Centre National de la Recherche Scientifique (CNRS), FranceEmiel Krahmer, Tilburg University, Netherlands
Copyright © 2018 d'Ettorre, Bueno, Rödel, Megherbi, Seigneuric, Schaal and Roberts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrizia d'Ettorre, dettorre@leec.univ-paris13.fr
 Patrizia d'Ettorre
Patrizia d'Ettorre Steve Bueno
Steve Bueno Heiko G. Rödel
Heiko G. Rödel Hakima Megherbi2
Hakima Megherbi2  Benoist Schaal
Benoist Schaal S. Craig Roberts
S. Craig Roberts