Harmonia axyridis Does Not Have Obvious Fitness Gain and Preference to the Red Morph of Acyrthosiphon pisum: A Case Study on a Laboratory Strain
- 1State Key Laboratory of Crop Stress Biology for Arid Areas and Key Laboratory of Northwest Loess Plateau Crop Pest Management of Ministry of Agriculture, Northwest A&F University, Yangling, China
- 2Biocontrol Engineering Laboratory of Crop Diseases and Pests of Gansu Province, College of Plant Protection, Gansu Agricultural University, Lanzhou, China
Prey selection was assumed to be a vital strategy to maximize the fitness of predators. Visual cues are important for predators to locate a prey, and several species of lady beetles have been reported to prefer the red morph pea aphid Acyrthosiphon pisum rather than the green morph in empty containers with different colors. However, the preference of a predator on the red and green color morphs of A. pisum on green plant leaves and the relationship between the fitness of the predator that feeds on the two color morphs have not been revealed. In this study, two colonies of the multicolored Asian lady beetle, Harmonia axyridis, a generalist predator, fed on a red morph and a green morph of A. pisum (indicated as RA-Har and GA-Har, respectively) were used to determine their prey preferences on the two morphs of A. pisum on leaf discs. The fourth instars and newly emerged adults of H. axyridis that fed on the two aphid morphs did not show any significant prey preference, except that GA-Har males consumed significantly more green morph than red morph. The fitness of RA-Har and GA-Har, including larval development and body weights of the fourth instars and the newly emerged female adults were not significantly different, and so were the predation capability and fecundity. However, RA-Har had significantly greater larval hatch rate than GA-Har, and tended to produce more eggs on cage walls or plant pots than on broad bean leaves in the cage assays. Our results provide evidences that H. axyridis did not prefer either morph of A. pisum, and also had no significant differences of the fitness. We hope that results from this study will stimulate more studies to reveal the mechanism of colored prey preference of predators.
Introduction
The optimal foraging theory assumes that prey selection is to maximize the fitness of predators (Stephens and Krebs, 1986). Base on this theory, the predators will make a decision with the optimal balance of caloric and nutritive value and associated foraging costs when several preys are simultaneously presented (Stephens and Krebs, 1986). In addition, visual cues of prey are important signals for the predators (Bahlai et al., 2008). Coccinellid adults generally have three types of visual receptors (UV, blue, and green) that allow them to respond to wavelengths from 310 to 600 nm (Agee et al., 1990; Lin, 1993). For example, the seven-spotted lady beetle, Coccinella septempunctata had high phototactic response rates to ultraviolet (UV) (340 nm) (Zhou et al., 2013). Practically, visual cues have been proved to play important roles in host-finding of many coccinellids (Nakamuta, 1984; Hattingh and Samways, 1995; Adedipe and Park, 2010). Several coccinellid species have been shown to prefer aphids that display colors contrast with different environmental backgrounds (Harmon et al., 1998; Mondor and Warren, 2000).
Most research on color morph preference was conducted on the pea aphid, Acyrthosiphon pisum, which displays noteworthy color-polymorphism of red and green. The color morphs of A. pisum have been proved to be genetically determined, with red being dominant over green (Markkula, 1963; Caillaud and Losey, 2010). Under field conditions, the two morphs of A. pisum generally coexisted at a relative stable ratio within a population (Lowe and Taylor, 1964; Balog and Schmitz, 2013). The red morph preference of C. septempunctata has been proved to be an important factor to maintain the color-polymorphism of A. pisum (Losey et al., 1997; Balog and Schmitz, 2013). However, the color morph preference of predators showed to be variable on different colored backgrounds. For example, in green containers, predation by C. septempunctata adults was higher on red morph than on green morph; whereas, in red containers, higher predation was found on green morph, and no difference was detected in white containers (Losey et al., 1997; Harmon et al., 1998). Moreover, preferences of the two morphs of A. pisum vastly varied among different coccinellid species. For example, under similar condition as that conducted for C. septempunctata, both the adults of Hippodamia convergens and Coleomegilla maculata had similar predation rates on the two morphs of A. pisum (Harmon et al., 1998). However, almost all studies regarded to color morph preference were conducted in empty containers. Actually, to locate a prey on plants, the conditions were much more complicated (e.g., olfactory and gustatory cues could also provide important signals for the coccinellids) (Obata, 1986; Bahlai et al., 2008; Gencer et al., 2009). Thus, the cases of color morph preferences by coccinellids under conditions similar to those on plants needed to be explored.
The multicolored Asian lady beetle, Harmonia axyridis, is a generalist predator and mainly an aphidophagous predator, but also feeds on many other soft bodied insects (Koch, 2003; Provost et al., 2006). This lady beetle has been widely introduced into many parts of the world as an important biological control agent (Koch, 2003). For A. pisum, H. axyridis has been shown to prefer the red morph to the green morph regardless of background coloration in an empty container (Harmon et al., 1998). But whether H. axyridis exhibits any preference of the two color morphs of A. pisum on green plant leaves has not been explored. In this study, we conducted the prey preference experiments on green leaf discs. In addition, we determined the fitness of H. axyridis preying on the two morphs of A. pisum because the information may help to understand the color morph preference mechanism of the predator.
Materials and Methods
Insects
Harmonia axyridis were obtained from a continuous rearing colony in our laboratory (24 ± 1°C, 65% RH and 14:10 h L:D). They were reared with the green peach aphid, Myzus persicae, infesting on pepper seedlings (Capsicum annum fasciculatum, var. “Changfeng”). One pair of newly emerged adults were collected from the laboratory colony and maintained in a plastic Petri dish (9 cm in diameter and 1.5 cm in depth) with sufficient M. persicae, and their offspring were used in all subsequent experiments.
The green and red morphs of A. pisum were collected from Lanzhou, Gansu, China, and were separately reared on broad bean seedlings (Vicia faba L., var. “Jinnong”) in different cages (60 × 60 × 45 cm) in an insectary (24 ± 1°C, 65% RH and 14:10 h L:D). Eggs laid by the stock pair of H. axyridis were collected and incubated in plastic Petri dishes (9 cm in diameter and 1.5 cm in depth). Thirty to forty newly hatched H. axyridis larvae in each day were equally separated and raised with one of the two morphs of A. pisum in separate cages (these two colonies of H. axyridis were named as RA-Har and GA-Har, respectively). Larval development of H. axyridis was monitored daily. The pupae (almost 3 days old) were picked out with a fine brush and maintained in plastic Petri dishes (9 cm in diameter and 1.5 cm in depth) until adult emergence. Fifteen pupae were placed in one Petri dish and the detached pupae were re-attached on the bottom surface of Petri dish with double-faced adhesive tapes to maintain the natural dorsal-ventral position of the pupae. To avoid sticking the newly emerged adults, the upper-surface of tapes was gently wiped with a piece of filter paper to decrease the stickiness. The fourth instars and newly emerged adults of RA-Har and GA-Har were used in subsequent experiments. All experiments were conducted in bioclimatic chambers (24 ± 1°C, 65% RH and 14:10 h L:D).
Color Morph Preference Experiment
Color morph preference of the fourth instars, newly emerged adults (48 h post-emergence) of RA-Har and GA-Har were determined on leaf discs (9 cm in diameter and 1.5 cm in depth) with 20 replicates conducted at five different times, and each replicate had four individuals. The leaf discs were maintained fresh using a thin layer of agar (10 ml, 1% concentration) at the bottom. A hole (1 cm in diameter) on the center of the Petri dish lid was covered with gauze (100 mesh) for ventilation. The leaf disc was cut to fit the bottom of the Petri dish. Twenty adult A. pisum were selected from the stock colonies, and were weighted individually using an electronic microbalance (Sartorius MSA 3.6P-000-DM, Gottingen, Germany). The average weight of one red and green morph was about 3.399 ± 0.089 mg and 4.687 ± 0.079 mg, respectively. The aphids were then introduced onto each leaf disc (10 for each morph) and kept in 4°C for 1 h to minimize nymph production and slow down their activity. After a 20-min recovery of the aphids, a fourth instar larva or an adult was introduced onto each leaf disc and was allowed to feed on the aphids for 6 h. Number of aphids of each morph consumed by the predator was recorded.
Fitness Evaluation
Development
Eggs laid by the stock pair of H. axyridis were collected and incubated in plastic Petri dishes (3 cm in diameter and 1.5 cm in depth). Newly hatched larvae were individually reared in the plastic Petri dishes with sufficient red or green morph of A. pisum (approximately 60 mg for first and second instar larvae, and 200 mg for third and fourth instar larvae) until pupation. Larval development was recorded daily. The fourth instars and newly emerged adults (<24 h) were individually weighted using an electronic balance (Mettler Toledo AL204, Shah Alam Selangor, Malaysia). For each colony, 20 replicates (four individuals by five different times) were measured.
Predation Capacity
The predation capacity of fourth instars and newly emerged adults (48 h post-emergence) of RA-Har and GA-Har was measured on leaf discs as described previously. Ten adult aphids of each morph were selected from the stock colonies and introduced onto a leaf disc. The aphids were kept in 4°C for 1 h to minimize nymph production and slow down the activity. After a 20-min recovery of the aphids, one lady beetle was introduced onto each leaf disc for 6 h, and number of aphids consumed was recorded. A total of 20 lady beetles were used for each colony.
Reproduction
Reproduction of RA-Har and GA-Har was compared using cage experiments in an air-conditioned insectary (24 ± 1°C, 65% RH and 14:10 h L:D). Newly emerged adults (48 h post-emergence) were sexed, and four pairs were introduced into each cage (30 cm in diameter and 45 cm in height). Four broad bean plants (about 30 cm in height) that were infested with A. pisum were introduced into each cage (red morph was supplied for RA-Har, and green morph for GA-Har). The infested plants were replaced every 4 days, and egg production of H. axyridis was carefully checked daily (female stared to lay eggs 6–7 days after emergence). The eggs were gently removed from the attached substance with a water-dampened fine brush (had been confirmed to be no negative effect to the eggs), and then were transferred to plastic Petri dishes (9 cm in diameter and 1.5 cm in depth) with an immersed cotton ball for maintaining moisture. The eggs deposited on broad bean leaves vs. cage walls or plant pots were separately counted and incubated in different Petri dishes. Newly hatched larvae were removed with a fine brush to avoid egg cannibalism. Oviposition of H. axyridis was monitored for 10 days, and number of eggs laid was examined daily. The development of eggs was monitored, and the larval hatch rates were calculated following the equation: number of larvae/total number of eggs × 100%. Five replicates (in total of 20 pairs) were conducted for RA-Har and GA-Har, respectively.
Data Analysis
Mean numbers of red and green morphs consumed by H. axyridis in the color morph preference experiment were compared with a paired two-tailed, Student t-test (p < 0.05). The fitness parameters between GA-Har and RA-Har were compared using the independent sample t-test (p < 0.05). The data were analyzed after testing for homogeneity of variances using the Levene test. SPSS (version 20; SPSS Inc., Chicago, IL, USA) was used for all statistical analysis.
Results
Color Morph Preference
When the predators were exposed to both red and green morphs of A. pisum, the fourth instars and newly emerged adults (female and male) of RA-Har did not exhibit any preference as shown by the similar numbers of the two morphs of A. pisum consumed (df = 19, fourth instars: t = 1.44, p = 0.17; female: t = −0.99, p = 0.34; male: t = 0.78, p = 0.45; Figure 1A). For GA-Har, the numbers of red and green morphs consumed by the fourth instars and the newly emerged female adults were also not significantly different (df = 19, fourth instars: t = 0.73, p = 0.47; female: t = 0.12, p = 0.91), while the males showed preference for green over red morph of A. pisum (df = 19, t = −2.65, p = 0.02; Figure 1B).
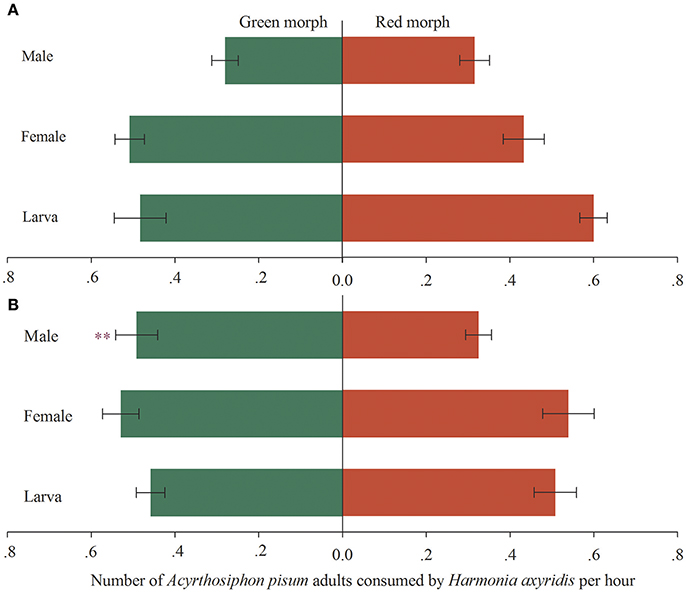
Figure 1. Preference of H. axyridis on two morphs of A. pisum. Green areas represent the predation on the green morph, while red areas represent the predation on the red morph. Asterisk indicates a significant difference between the average number of the red and the green morphs consumed by H. axyridis (p < 0.05). (A) Red morph aphid raised H. axyridis (RA-Har), (B) green morph aphid raised H. axyridis (GA-Har).
Fitness Evaluation
Development
The development times of H. axyridis feeding on red or green morph of A. pisum were not significantly different at each larval stage, and consequently, the whole pre-imaginal development times of RA-Har and GA-Har were almost the same (Table 1). The weights of the fourth instars and the newly emerged female adults of RA-Har also did not significantly differ from those of GA-Har (df = 38, fourth instars: t = 0.30, p = 0.77; female: t = 0.59, p = 0.56). However, the males of RA-Har were significantly heavier (28.3 mg) than those of GA-Har (26.5 mg) (df = 38, t = 3.04, p < 0.01; Table 1).
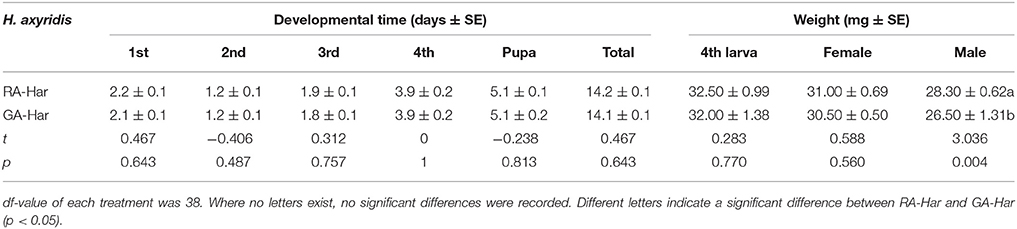
Table 1. Pre-imaginal development time and the weights of fourth instars and newly emerged adults of H. axyridis.
Predation Capacity
H. axyridis did not show significant differences in consumption between the two color morphs of A. pisum (df = 38, fourth instars: t = −1.81, p = 0.08; female: t = 0.93, p = 0.36; male: t = 0.31, p = 0.76). Specifically, nearly one aphid was consumed by a fourth instar larva in 1 h, and to be almost 0.9 and 0.7, respectively, for a newly emerged female and male adult (Figure 2).
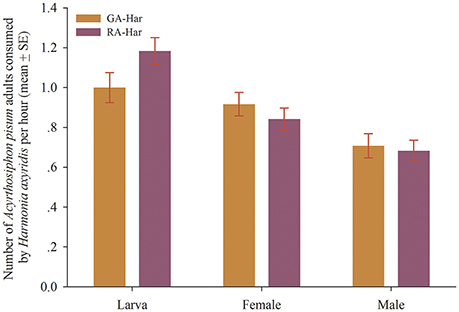
Figure 2. Predation capacity of H. axyridis on the red and the green morphs of A. pisum. The two lady beetle colonies were named as RA-Har and GA-Har, respectively.
Reproduction
Each RA-Har female oviposited an average of 46 eggs in each day during a period of 10 days, and each GA-Har female oviposited an average of 37 eggs daily during the same period, and there were no significant differences between RA-Har and GA-Har (df = 8, t = 2.10, p = 0.07; Figure 3A). However, RA-Har females produced significantly fewer eggs on broad bean leaves than on the cage walls and plant pots (36.8% vs. 63.3%) (df = 4, t = −2.98, p = 0.04), whereas GA-Har oviposited a similar proportion of eggs on broad bean leaves (50.6%) and the cage walls and plant pots (49.4%) (df = 4, t = 0.09, p = 0.93). Of the eggs produced by RA-Har, 59.3% were hatched, which was significantly more than those by GA-Har (48.2%) (df = 8, t = 2.33, p = 0.048; Figure 3B).
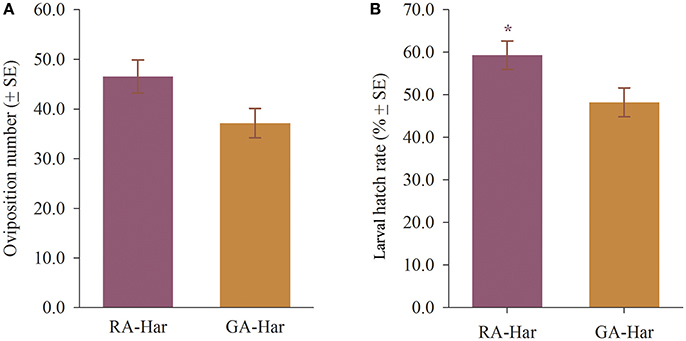
Figure 3. Reproduction of H. axyridis on red and green morphs of A. pisum. The two lady beetle colonies were named as RA-Har and GA-Har, respectively. (A) Average number of eggs laid by a female, (B) larval hatch rate. Asterisk indicates a significant difference between RA-Har and GA-Har (p < 0.05).
Discussion
The fourth instars and newly emerged adults of H. axyridis, regardless of which morph of A. pisum they fed on, did not exhibit significant color morph preference on either red or green morph. This was different from the results that were found in empty container in previous studies. For example, Harmon et al. (1998) reported that the adults of H. axyridis preferred to choose red morph of A. pisum in green container. This difference could be caused by the different substrates of an empty container and a leaf disc. As we know, herbivore-induced leaf volatiles and honeydew excreted by aphids when feeding plants usually provide important signals to foraging predators for location of their prey (Havelka and Syrovatka, 1991; Dicke et al., 1993; Zhu and Park, 2005). However, it seems that these chemical signals were not be used by H. axyridis inside a Petri dish due to the short distance, high prey density and weak airflow (Liu and Sengonca, 1994; Pinto et al., 2007). Several other reasons might be considered. Firstly, coccinellid adults generally have several specific types of visual receptors (e.g., UV, blue, and green), and they might only be sensitive to a limited range of light spectrum or wavelengths (Agee et al., 1990; Lin, 1993). The reflect wavelengths of the green broad bean leaves should be different from those of the green container, and the color contrast of the red morph A. pisum on the green leaf disc might be undetectable by H. axyridis. Secondly, the red morph of A. pisum, especially the adults, exhibited more intense defensive reactions as compared to the green morph (Lowe and Taylor, 1964; Braendle and Weisser, 2001), which are expected to partially compensate its visual attractiveness (Farhoudi et al., 2014). In contrast to the adults, coccinellid larvae do not use their vision to locate prey (Banks, 1954; Storch, 1976; Stubbs, 1980; Hattingh and Samways, 1995). Thus, the fourth instars of H. axyridis could not distinguish the difference between the red and green morphs of the aphid. Like other aphid species, the two morphs of A. pisum could cause plant foliage becoming yellowish after feeding (Seagraves, 2009; Ramezani et al., 2013), and H. axyridis exhibited obvious preference to yellowish plants on which aphids likely occur (Seagraves, 2009; Adedipe and Park, 2010). All these characteristics make H. axyridis an effective biological control agent of A. pisum.
We found that H. axyridis feeding on the red and green morphs of A. pisum did not have significant differences in developmental time and egg production. Ahsaei et al. (2013) found that the two morphs of A. pisum had similar content of total energy reserves. Thus, the two morphs of A. pisum seemed to be equal in satisfying the nutritional requirements of development and egg production of H. axyridis. However, the larval hatch rate of RA-Har was significantly greater than that of GA-Har, and this difference could be caused by the fact that RA-Har males were heavier or larger than GA-Har males. In insects, adult body size is an important indicator of quality, and large males generally have advantages in mating success over small males (Himuro et al., 2006). It has been reported that the red morph of A. pisum had higher percentage of water-soluble carbohydrates and lipids than the green morph which stored a higher percentage of protein than the red morph (Ahsaei et al., 2013). The different compositions of energy reserves of the two morphs of A. pisum might result in the differences of body weights of male H. axyridis. In fact, the enzyme equipment and nutritional budgets of aphids (Klingauf, 1988; Srivastava, 1988; Dixon, 1998) as well as the nutritive requirements of predators (Eubanks and Denno, 2000) could affect the fitness of predators on an aphid species. For example, H. axyridis larvae could not complete their development when fed exclusively on Megoura viciae, but successfully developed into adult on Aphis gossypii. However, when the first instar H. axyridis first fed on A. gossypii and then on M. viciae in subsequent larval stages, the larvae completed their development, and their adults consumed a greater weight of M. viciae than A. gossypii (Tsaganou et al., 2004).
We also found that RA-Har females tended to lay their eggs on plant pots and cage walls rather than on broad bean leaves. As we know, coccinellid females prefer to lay eggs on sites where distributed sufficient aphids to ensure the survival of their offspring (Dixon, 1959; Wratten, 1973; Seagraves, 2009). However, when coexist with predators, the red morph of A. pisum drop more frequently than the green morph (Braendle and Weisser, 2001), which might prompt RA-Har females to lay eggs dispersedly. This phenomenon can be regarded as an evolved strategy to improve offspring survival. In addition, H. axyridis females could balance the direct distance from a cluster to the nearest aphid colony to decrease the intensity of non-sibling cannibalism (Osawa, 2003). Oviposition-site choice is a major maternal effect which might be affected by many factors (Refsnider and Janzen, 2010). The mechanism of different oviposition-site choice of H. axyridis feeding on the two morphs of A. pisum remains unclear and needs more studies.
Our study provide sufficient evidences that H. axyridis exhibited no-preference on the red morph of A. pisum on green leaf disc, which might be due to that feeding on the red morph aphids did not result in greater fitness for H. axyridis than those fed on the green morph aphids. These results might encourage more studies to reveal the biological significance of the predators when they exhibit obvious preference for a specific prey.
Author Contributions
Y-XS and T-XL designed research; Y-XS performed research; Y-XS and T-XL wrote the paper.
Funding
Funding of this research was partially supported by the following grants: the National Natural Science Foundation of China (No. 31471819; No. 31660522), National Basic Research Program of China (973 Project No. 2013CB127600), and China Agriculture Research System (No. CARS-25-B-06).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful for the assistance of all staff and students in the Key Laboratory of Applied Entomology, Northwest A&F University at Yangling, Shaanxi, China.
References
Adedipe, F., and Park, Y. L. (2010). Visual and olfactory preference of Harmonia axyridis (Coleoptera: Coccinellidae) adults to various companion plants. J. Asia-Pac. Entomol. 13, 319–323. doi: 10.1016/j.aspen.2010.07.004
Agee, H. R., Mitchell, E. R., and Flanders, R. V. (1990). Spectral sensitivity of the compound eye of Coccinella septempunctata (Coleoptera: Coccinellidae). Ann. Entomol. Soc. Am. 83, 817–819. doi: 10.1093/aesa/83.4.817
Ahsaei, S. M., Tabadkani, S. M., Hosseininaveh, V., Allahyari, H., and Bigham, M. (2013). Differential accumulation of energy by the colour morphs of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphididae) mirrors their ecological adaptations. Eur. J. Entomol. 110, 241–245. doi: 10.14411/eje.2013.035
Bahlai, C. A., Welsman, J. A., Macleod, E. C., Schaafsma, A. W., Hallett, R. H., and Sears, M. K. (2008). Role of visual and olfactory cues from agricultural hedgerows in the orientation behavior of multicolored Asian lady beetle (Coleoptera: Coccinellidae). Environ. Entomol. 37, 973–979. doi: 10.1093/ee/37.4.973
Balog, A., and Schmitz, O. J. (2013). Predation drives stable coexistence ratios between red and green pea aphid morphs. J. Evolution. Biol. 26, 545–552. doi: 10.1111/jeb.12070
Banks, C. J. (1954). The searching behaviour of coccinellid larvae. Br. J. Anim. Behav. 2, 37–38. doi: 10.1016/S0950-5601(54)80082-7
Braendle, C., and Weisser, W. W. (2001). Variation in escape behavior of red and green clones of the pea aphid. J. Insect Behav. 14, 497–509. doi: 10.1023/A:1011124122873
Caillaud, M. C., and Losey, J. E. (2010). Genetics of color polymorphism in the pea aphid, Acyrthosiphon pisum. J. Insect Sci. 10:95. doi: 10.1673/031.010.9501
Dicke, M., van Baarlen, P., Wessels, R., and Dijkman, H. (1993). Herbivory induces systemic production of plant volatiles that attract predators of the herbivore: extraction of endogenous elicitor. J. Chem. Ecol. 19, 581–599. doi: 10.1007/BF00994327
Dixon, A. F. G. (1959). An experimental study of the searching behaviour of the predatory coccinellid beetle Adalia decempunctata (L.). J. Anim. Ecol. 28, 259–281. doi: 10.2307/2082
Eubanks, M. D., and Denno, R. F. (2000). Health food versus fast food: the effects of prey quality and mobility on prey selection by a generalist predator and indirect interactions among prey species. Ecol. Entomol. 25, 140–146. doi: 10.1046/j.1365-2311.2000.00243.x
Farhoudi, F., Allahyari, H., Tabadkani, S. M., and Gholizadeh, M. (2014). Prey preference of Aphidoletes aphidimyza on Acyrthosiphon pisum: effect of prey color and size. J. Insect Behav. 27, 776–785. doi: 10.1007/s10905-014-9470-4
Gencer, N. S., Kumral, N. A., Sivritepe, H. O., Seidi, M., Susurluk, H., and Senturk, B. (2009). Olfactory response of the ladybird beetle Stethorus gilvifrons to two preys and herbivore-induced plant volatiles. Phytoparasitica 37, 217–224. doi: 10.1007/s12600-009-0032-9
Harmon, J. P., Losey, J. E., and Ives, A. R. (1998). The role of vision and color in the close proximity foraging behavior of four coccinellid species. Oecologia 115, 287–292. doi: 10.1007/s004420050518
Hattingh, V., and Samways, M. J. (1995). Visual and olfactory location of biotypes, prey patches, and individual prey by the ladybeetle Chilocorus nigritus. Entomol. Exp. Appl. 75, 87–98. doi: 10.1111/j.1570-7458.1995.tb01914.x
Havelka, J., and Syrovatka, O. (1991). Stimulatory effect of the honeydew of several aphid species on the females of gall midge Aphidoletes aphidimyza (Rondani) (Dipt., Cecidomyiidae): electroantennograph studies. J. Appl. Entomol. 112, 341–344. doi: 10.1111/j.1439-0418.1991.tb01065.x
Himuro, C., Hosokawa, T., and Suzuki, N. (2006). Alternative mating strategy of small male Megacopta punctatissima (Hemiptera: Plataspidae) in the presence of large intraspecific males. Ann. Entomol. Soc. Am. 99, 974–977. doi: 10.1603/0013-8746(2006)99[974:AMSOSM]2.0.CO;2
Klingauf, F. A. (1988). “Feeding, adaptation and excretion,” in Aphids: Their Biology, Natural Enemies and Control, eds A. K. Minks and P. Harrewijn (Amsterdam: Elsevier), 225–253.
Koch, R. L. (2003). The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. J. Insect Sci. 3:32. doi: 10.1093/jis/3.1.32
Lin, J. T. (1993). Identification of photoreceptor locations in the compound eye of Coccinella septempunctata Linnaeus (Coleoptera, Coccinellidae). J. Insect Physiol. 39, 555–562. doi: 10.1016/0022-1910(93)90037-R
Liu, B., and Sengonca, Ç. (1994). Development of 8-armed airflow olfactometers for measuring olfactory responses of insect predators. Anz. Schädlingsk. Pfl. Umweltsch. 67, 30–34. doi: 10.1007/BF01907347
Losey, J. E., Ives, A. R., Harmon, J., Ballantyne, F., and Brown, C. (1997). A polymorphism maintained by opposite patterns of parasitism and predation. Nature 388, 269–272. doi: 10.1038/40849
Lowe, H. J. B., and Taylor, L. R. (1964). Population parameters, wing production and behaviour in red and green Acyrthosiphon pisum (Harris) (Homoptera: Aphididae). Entomol. Exp. Appl. 7, 287–295. doi: 10.1111/j.1570-7458.1964.tb00730.x
Markkula, M. (1963). Studies on the pea aphid, Acyrthosiphon pisum, with special reference to the differences in the biology of the green and red forms. Ann. Agric. Fenn. 2, 1–30.
Mondor, E. B., and Warren, J. L. (2000). Unconditioned and conditioned responses to colour in the predatory coccinellid, Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 97, 463–468. doi: 10.14411/eje.2000.071
Nakamuta, K. (1984). Visual orientation of a ladybeetle, Coccinella septempunctata L., (Coleoptera: Coccinellidae), toward its prey. Appl. Entomol. Zool. 19, 82–86. doi: 10.1303/aez.19.82
Obata, S. (1986). Mechanisms of prey finding in the aphidophagous ladybird beetle, Harmonia axyridis (Coleoptera: Coccinellidae). Entomophaga 31, 303–311. doi: 10.1007/BF02373340
Osawa, N. (2003). The influence of female oviposition strategy on sibling cannibalism in the ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 100, 43–48. doi: 10.14411/eje.2003.009
Pinto, D. M., Blande, J. D., Nykänen, R., Dong, W. X., Nerg, A. M., and Holopainen, J. K. (2007). Ozone degrades common herbivore-induced plant volatiles: does this affect herbivore prey location by predators and parasitoids?. J. Chem. Ecol. 33, 683–694. doi: 10.1007/s10886-007-9255-8
Provost, C., Lucas, E., Coderre, D., and Chouinard, G. (2006). Prey selection by the lady beetle Harmonia axyridis: the influence of prey mobility and prey species. J. Insect Behav. 19, 265–277. doi: 10.1007/s10905-006-9023-6
Ramezani, Z., Talaei-Hassanloui, R., and Allahyari, H. (2013). Simultaneous use of entomopathogenic fungus Lecanicillium and predatory ladybird Hippodamia variegata in control of pea aphid, Acyrthosiphon pisum. Int. J. Agri. Innov. Res. 2, 246–250.
Refsnider, J. M., and Janzen, F. J. (2010). Putting eggs in one basket: ecological and evolutionary hypotheses for variation in oviposition-site choice. Annu. Rev. Ecol. Evol. Syst. 41, 39–57. doi: 10.1146/annurev-ecolsys-102209-144712
Seagraves, M. P. (2009). Lady beetle oviposition behavior in response to the trophic environment. Biol. Control 51, 313–322. doi: 10.1016/j.biocontrol.2009.05.015
Srivastava, P. N. (1988). “Nutritional physiology,” in Aphids: Their Biology, Natural Enemies and Control, eds A. K. Minks, and P. Harrewijn (Amsterdam: Elsevier), 99–121.
Storch, R. H. (1976). Prey detection by fourth stage Coccinella transversoguttata larvae (Coleoptera: Coccinellidae). Anim. Behav. 24, 690–693. doi: 10.1016/S0003-3472(76)80082-6
Stubbs, M. (1980). Another look at prey detection by coccinellids. Ecol. Entomol. 5, 179–182. doi: 10.1111/j.1365-2311.1980.tb01139.x
Tsaganou, F. C., Hodgson, C. J., Athanassiou, C. G., Kavallieratos, N. G., and Tomanovic, Ž. (2004). Effect of Aphis gossypii Glover, Brevicoryne brassicae (L.), and Megoura viciae Buckton (Hemiptera: Aphidoidea) on the development of the predator Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Biol. Control 31, 138–144. doi: 10.1016/j.biocontrol.2004.04.017
Wratten, S. D. (1973). The effectiveness of the coccinellid beetle, Adalia bipunctata (L.), as a predator of the lime aphid, Eucallipterus tiliae L. J. Anim. Ecol. 42, 785–802. doi: 10.2307/3139
Zhou, J. X., Kuang, R. P., Chen, Z., Wang, S. M., and Liu, X. (2013). Phototactic behavior of Coccinella septempunctata L. (Coleoptera: Coccinellidae). Coleopts. Bull. 67, 33–39. doi: 10.1649/072.067.0108
Keywords: fitness, prey preference, signal, Harmonia axyridis, Acyrthosiphon pisum, red morph
Citation: Sun Y-X and Liu T-X (2018) Harmonia axyridis Does Not Have Obvious Fitness Gain and Preference to the Red Morph of Acyrthosiphon pisum: A Case Study on a Laboratory Strain. Front. Ecol. Evol. 6:1. doi: 10.3389/fevo.2018.00001
Received: 04 August 2017; Accepted: 09 January 2018;
Published: 13 February 2018.
Edited by:
Eric W. Riddick, Agricultural Research Service (USDA), United StatesReviewed by:
Ted Cottrell, Agricultural Research Service (USDA), United StatesChristos Athanassiou, University of Thessaly, Greece
Copyright © 2018 Sun and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tong-Xian Liu, txliu@nwsuaf.edu.cn
 Yuan-Xing Sun
Yuan-Xing Sun Tong-Xian Liu
Tong-Xian Liu