Mycorrhizae Alter Toxin Sequestration and Performance of Two Specialist Herbivores
- Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, MI, United States
Multitrophic species interactions are shaped by both top-down and bottom-up factors. Belowground symbionts of plants, such as arbuscular mycorrhizal fungi (AMF), can alter the strength of these forces by altering plant phenotype. For example, AMF-mediated changes in foliar toxin and nutrient concentrations may influence herbivore growth and fecundity. In addition, many specialist herbivores sequester toxins from their host plants to resist natural enemies, and the extent of sequestration varies with host plant secondary chemistry. Therefore, by altering plant phenotype, AMF may affect both herbivore performance and their resistance to natural enemies. We examined how inoculation of plants with AMF influences toxin sequestration and performance of two specialist herbivores feeding upon four milkweed species (Asclepias incarnata, A. curassavica, A. latifolia, A. syriaca). We raised aphids (Aphis nerii) and caterpillars (Danaus plexippus) on plants for 6 days in a fully factorial manipulation of milkweed species and level of AMF inoculation (zero, medium, and high). We then assessed aphid and caterpillar sequestration of toxins (cardenolides) and performance, and measured defensive and nutritive traits of control plants. Aphids and caterpillars sequestered higher concentrations of cardenolides from plants inoculated with AMF across all milkweed species. Aphid per capita growth rates and aphid body mass varied non-linearly with increasing AMF inoculum availability; across all milkweed species, aphids had the lowest performance under medium levels of AMF availability and highest performance under high AMF availability. In contrast, caterpillar survival varied strongly with AMF availability in a plant species-specific manner, and caterpillar growth was unaffected by AMF. Inoculation with AMF increased foliar cardenolide concentrations consistently among milkweed species, but altered aboveground biomasses and foliar phosphorous concentrations in a plant species-specific fashion. Increased herbivore sequestration of cardenolides followed AMF-mediated increases in foliar cardenolide concentrations. Aphid performance declined with increasing foliar cardenolide concentrations, while caterpillar survival increased with aboveground biomass. Our findings suggest that by altering plant phenotype, the availability of AMF in soil has the potential to influence both top-down (via sequestration) and bottom up (via plant defense and nutrition) forces that operate on herbivores.
Introduction
Multitrophic species interactions are governed by a mixture of top-down forces, such as predators and parasites, and bottom-up forces, such as resource availability (Hunter and Price, 1992; Schmitz et al., 2000). In terrestrial ecosystems, both top-down and bottom-up forces travel with ease across the traditional soil “boundary,” with plants connecting the interactions that occur between above and belowground organisms (van der Putten et al., 2001; van Dam and Heil, 2011; Hunter, 2016). As a result, soil organisms that are associated with plant roots have the potential to affect herbivore populations above ground both by affecting plant quality for herbivores from the bottom-up (Hartley and Gange, 2009; Koricheva et al., 2009; Jung et al., 2012) and the resistance of herbivores to their natural enemies from the top down (Gange et al., 2003; Rasmann et al., 2017; Tao et al., 2017).
Arbuscular mycorrhizal fungi (AMF) engage in one of the most ubiquitous root-microbe symbioses in terrestrial ecosystems (Smith and Read, 2008), associating with over 80 percent of plant species globally (Wang and Qiu, 2006; Smith and Read, 2008; Soudzilovskaia et al., 2015). AMF provide nutrients to plants, such as phosphorous, in exchange for plant sugars (Smith and Read, 2008). In establishing and maintaining the symbiosis, AMF also interact with plant defensive signaling pathways, including the jasmonic acid and salicylic acid pathways (Jung et al., 2012; Cameron et al., 2013; Bucher et al., 2014; Gutjahr, 2014). As a result, AMF alter plant nutritive quality and a diversity of plant primary and secondary metabolites (Bennett et al., 2009; Roger et al., 2013; Vannette et al., 2013; Schweiger et al., 2014; Schweiger and Müller, 2015), affecting plant quality for insect herbivores substantially (Hartley and Gange, 2009; Koricheva et al., 2009).
The response of insect herbivores to AMF colonization of their host plants varies widely, from positive to neutral or negative (Koricheva et al., 2009). Much of this variation is explained by the degree of specialization and feeding mode of the herbivore (Hartley and Gange, 2009; Koricheva et al., 2009). For instance, both generalist and specialist phloem-feeding insects, such as aphids, generally benefit from AMF colonization of their host plants. Specialist chewing herbivores, such as caterpillars, also benefit, but generalist chewing herbivores are negatively affected by AMF colonization of their host plants (Hartley and Gange, 2009; Koricheva et al., 2009). Phloem-feeding insects may avoid AMF-mediated increases in plant defenses because phloem lacks or contains far lower concentrations of plant secondary metabolites than leaves (Züst and Agrawal, 2016a). In addition, phloem-feeding insects may benefit from AMF-mediated increases in the size of plant vascular bundles (Krishna et al., 1981; Simon et al., 2017). Generalist chewers may be more susceptible to AMF-mediated increases in plant defenses (Schoonhoven et al., 2005), while specialist chewers may benefit from increased nutritive quality of host plants colonized by AMF (Koricheva et al., 2009).
Even within these trends, there is large variation in herbivore responses to AMF, and we lack an understanding of what is driving this variation. For instance, aphids generally benefit from AMF colonization of their host plants; aphids are more attracted to plants colonized by AMF and have greater body masses, growth rates, and fecundity on host plants colonized by AMF (Gange and West, 1994; Gange et al., 1999, 2002; Koricheva et al., 2009; Babikova et al., 2014a,b; Simon et al., 2017). However, aphids have also been found to not respond to AMF colonization of their host plants (Pacovsky et al., 1985; Wurst et al., 2004; Colella et al., 2014; Grabmaier et al., 2014; Williams et al., 2014; Bennett et al., 2016) or to have reduced population growth on plants colonized by AMF (Gehring and Whitham, 2002; Hempel et al., 2009; Abdelkarim et al., 2011). Similarly, while some specialist chewers benefit from AMF colonization of their host plants (Borowicz, 1997; Goverde et al., 2000; Vannette and Hunter, 2013), others are unaffected (Laird and Addicott, 2008; Cosme et al., 2011). Some of this variation may be explained by the stage of the association between the plant and AMF; aphids, for example, tend to benefit only after at least a month of AMF establishment (Tomczak and Müller, 2017). This variation in herbivore responses to AMF may also be a consequence of plant species-specific responses of plant traits to the presence of AMF (e.g., Grman, 2012; Barber et al., 2013; Anacker et al., 2014; Tao et al., 2016a) and the density or identity of AMF inoculum available to the plant (Garrido et al., 2010; Vannette and Hunter, 2011, 2013; Barber et al., 2013).
In addition to being shaped by host plant quality, herbivore populations are also affected by their natural enemies. Root-associated microbes, such as AMF, affect herbivore-natural enemy interactions indirectly by altering plant phenotype (Rasmann et al., 2017; Tao et al., 2017). For instance, AMF increase the attractiveness of plants to natural enemies by changing the volatile emissions of their host plants (Guerrieri et al., 2004; Fontana et al., 2009; Hoffmann et al., 2011; Schausberger et al., 2012; Babikova et al., 2013). AMF also influence the searching efficiency of natural enemies, likely by changing plant size (Gange et al., 2003), and can improve natural enemy performance (Hempel et al., 2009; Bennett et al., 2016). AMF mediation of herbivore-natural enemy interactions can ultimately benefit host plants. For instance, AMF colonization increases herbivorous mite densities on Phaseolus vulgaris plants, yet improves plant productivity by enhancing the population growth of predatory mites and plant tolerance sufficiently to compensate for the increase in herbivores (Hoffmann et al., 2011).
Many specialist herbivores are able to resist their natural enemies by sequestering secondary metabolites from their host plants (Nishida, 2002; Opitz and Müller, 2009; Ode, 2013; Erb and Robert, 2016; Petschenka and Agrawal, 2016). The concentration and composition of secondary metabolites that herbivores sequester are tied closely with host plant secondary chemical profiles (Malcolm, 1990, 1994; Agrawal et al., 2015; Petschenka and Agrawal, 2015), and are affected by environmental factors, such as soil nutrient availability (Jamieson and Bowers, 2012; Tao and Hunter, 2015). Herbivores that sequester higher concentrations of secondary metabolites from their host plants are more toxic and deterrent to their natural enemies (Brower et al., 1968; Reichstein et al., 1968; Brower and Moffitt, 1974; Malcolm, 1992; Dyer and Bowers, 1996; Camara, 1997). Therefore, by increasing plant chemical defenses, AMF may increase toxin sequestration by herbivores, thereby improving herbivore resistance to their natural enemies. Despite widespread recognition of sequestration as an integral component of host plant specialization and an important factor shaping ecological networks (Duffey, 1980; Lampert et al., 2014; Petschenka and Agrawal, 2016; Züst and Agrawal, 2016b), no study to date has considered how microbial root mutualists of plants, including AMF, affect herbivore sequestration of plant toxins.
Here, we evaluate how AMF affect toxin sequestration and performance of specialist herbivores of milkweed (Asclepias) species. Milkweed species provide an ideal system in which to address these questions because milkweed species produce a suite of resistance traits and are fed upon by specialized herbivores that can tolerate and sequester milkweed defenses. Milkweed tissues, including leaves and phloem, contain cardenolides, bitter tasting steroids that disrupt the functioning of sodium-potassium channels in animal cells by inhibiting an essential cation transporter, Na+/K+-ATPase (Agrawal et al., 2012; Pringle et al., 2014; Züst and Agrawal, 2016b). In response to leaf damage, milkweeds exude latex, a sticky isoprene polymer that gums up the mouths of chewing herbivores (Zalucki et al., 2001a; Agrawal and Konno, 2009). In addition, milkweed species vary in leaf toughness (Agrawal and Fishbein, 2006), which is tightly correlated with specific leaf mass (SLM) (Frost and Hunter, 2008).
We used two specialist herbivores of milkweed that vary in their feeding mode: oleander aphids (Aphis nerii; phloem-feeding) and monarch caterpillars (Danaus plexippus; leaf-chewing). Oleander aphids tolerate cardenolides through regulation of a narrow set of genes involved in canonical detoxification processes (Birnbaum et al., 2017). Monarch caterpillars, in contrast, have NA+/K+- ATPases that are insensitive to cardenolides (Dobler et al., 2012; Petschenka and Agrawal, 2015). Despite being able to tolerate cardenolides, both oleander aphids and monarch caterpillars exhibit reduced performance on host plants with high concentrations of cardenolides (Zalucki et al., 2001a; Agrawal, 2004, 2005; Rasmann et al., 2009; de Roode et al., 2011; Colvin et al., 2013; Tao et al., 2016b; Birnbaum et al., 2017). Furthermore, both oleander aphids and monarch caterpillars sequester cardenolides (Rothschild et al., 1970; Malcolm and Brower, 1989; Malcolm, 1990; Züst and Agrawal, 2016b), providing an effective defense against aphid predators (Pasteels, 1978; Malcolm, 1989, 1992; Pappas et al., 2007; Mooney et al., 2008) and monarch predators and parasites (Brower et al., 1968; Reichstein et al., 1968; Brower and Moffitt, 1974; Sternberg et al., 2012). Oleander aphids appear to sequester cardenolides passively through diffusion of non-polar (lipophilic) cardenolides (Malcolm, 1990; Züst and Agrawal, 2016b). In contrast, monarch caterpillars sequester polar cardenolides selectively (Malcolm and Brower, 1989; Petschenka and Agrawal, 2015; Tao and Hunter, 2015; Erb and Robert, 2016), likely through active translocation by transport proteins through gut membranes (Frick and Wink, 1995). Nonetheless, cardenolide sequestration by both oleander aphids and monarch caterpillars is closely correlated with their host plant cardenolides (Malcolm, 1990, 1994; Agrawal et al., 2015; Petschenka and Agrawal, 2015). Thus, AMF-mediated changes in plant cardenolide expression may influence aphid and caterpillar sequestration.
We performed a full-factorial experiment, manipulating oleander aphids and monarch caterpillars on four closely related milkweed species provided with different amounts of AMF inoculum. We expected herbivores to sequester higher concentrations of cardenolides on AMF-colonized plants due to AMF-mediated increases in the cardenolide concentrations of their host plants. Furthermore, we expected that AMF colonization would improve the performance of aphids and caterpillars by increasing plant nutritive quality and biomass, outweighing the negative effects of increased cardenolide concentrations on the herbivores. Because the outcomes of many AMF-plant associations are specific to the AMF and plant species (e.g., Grman, 2012; Barber et al., 2013; Anacker et al., 2014; Tao et al., 2016a), we expected the magnitude of the effects of AMF on herbivore sequestration and performance to vary among plant species and with the level of AMF inoculum available to the plant.
Materials and Methods
Plants and Insects
We used four North American milkweed species (Asclepias curassavica, A. latifolia, A. syriaca, and A. incarnata) that show constitutive and AMF-mediated variation in milkweed defenses and nutritive quality (Vannette et al., 2013; Tao et al., 2016a). Asclepias incarnata and A. syriaca seeds were collected from naturally occurring populations in Livingston County, MI, and A. latifolia and A. curassavica seeds were purchased from commercial sources (Alplains and Butterfly Encounters Inc., respectively). We obtained fungal inoculum from Mycorrhizal Applications (Grants Pass, OR, USA), which was comprised of equal proportions of four AMF species including Rhizophagus intraradices, Funneliformis mosseae, Glomus aggregatum, and Claroideoglomus etunicatum (33 spores of each AMF species per gram of inoculum, www.plant-success.com). However, cloning and sequencing of the inoculum with AMF-specific primers (Krüger et al., 2009) revealed the mix to consist only of F. mosseae (details in Supplementary Material). Milkweeds grow in habitats that host a diversity of AMF taxa (Öpik et al., 2006), and can form associations with these cosmopolitan AMF species in natural and experimental populations (Landis et al., 2004; Vannette and Hunter, 2011; Vannette et al., 2013; Tao et al., 2015, 2016a). However, as with most systems, the frequency of these relationships is not known.
To assess how the availability of AMF inoculum influences the performance of herbivores, we used two specialist herbivores: oleander aphids (A. nerii; phloem-feeding) and monarch caterpillars (D. plexippus; leaf-chewing). All oleander aphids used in the experiment were clones derived from a single aphid collected in March 2014 from the Emory University greenhouses (Atlanta, GA) and reared indoors on A. tuberosa, which does not produce cardenolides, for 1 month prior the experiment. Monarch larvae were the second generation of outcrossed progeny of butterflies obtained from Shady Oak Farms (www.butterfliesetc.com), Mr. Butterfly (www.mrbutterflies.com), and Butterfly Release Company (www.butterflyreleasecompany.com). Monarch larvae were raised on a combination of A. syriaca, A. incarnata, and A. curassavica in a growth room with photoperiod of 16:8 L:D and adults were reared on a 10% honey solution.
Experimental Protocols
After 6 weeks of cold, moist stratification at 4°C, we surface-sterilized seeds in 5% bleach and germinated them at room temperature (A. curassavica did not require stratification) in March 2014. We planted individual seedlings in conical deepots (D40H, Steuwe and Sons Inc., Corvalis, OR, USA) filled with 600 ml of a 3:1 mix of autoclaved soil (Metro-Mix 380; MetroMix Sun Gro Horticulture Canada CM Ltd., Vancouver, BC, Canada) and sand containing AMF inoculum. We manipulated the amount of live and autoclaved (dead) AMF inoculum available to experimental plants to generate zero, medium, and high levels of root colonization, which is possible because the amount of AMF inoculum available to milkweed plants affects the levels of AMF colonization of roots (Vannette and Hunter, 2011; Tao et al., 2015, 2016a). Specifically, we homogenized 4.20 g autoclaved AMF inoculum (zero treatment), 1.20 g live and 3.00 g autoclaved inoculum (medium treatment), or 4.20 g live inoculum (high treatment) in 200 ml of autoclaved soil, which was placed between 400 ml of autoclaved soil and sand to prevent the transfer of mycorrhizal spores or hyphae among treatments. To return the natural bacterial community of the potting soil to the autoclaved soil of each pot, we added 20 ml of bacterial solution made by suspending 100 ml potting soil in 1 L deionized water and filtering the suspension through an ultra-fine soil sieve (38 μm) to remove AMF hyphae and spores. Plants were grown at the Matthaei Botanical Gardens greenhouses (Ann Arbor, MI) with a photoperiod of 16:8 L:D for 3 months. Plants were watered ad libitum and fertilized biweekly with 90 ml of a low concentration (94 ppm) of 15-0-15 (N-P-K) dark weather fertilizer (JR Peters Inc., Allentown, PA). All experimental plants were exposed to colonization and damage by greenhouse thrips and sprayed monthly with a mixture of Enstar, Lucid, and MPede to minimize damage. No pesticides were sprayed for 3 weeks prior to the addition of herbivores; thrips were killed weekly by hand during this period.
In a fully factorial design, we placed oleander aphids, monarch caterpillars, or no herbivores on plants of each plant species x AMF treatment and allowed herbivores to feed for 6 days in June 2014. The 6 days of feeding represent approximately one generation for oleander aphids (Zehnder and Hunter, 2009) and 50% of the average larval period of monarchs under our rearing conditions (Vannette and Hunter, 2013). Effects of plant quality on monarch growth are most important during early instars (Zalucki et al., 2001b). All plants were covered with white, nylon mesh bags (5-gallon paint strainer bags) to prevent insect movement among experimental plants. Five reproductive, apterous oleander aphids were placed at the apex of 15 replicates of each plant species x AMF treatment and allowed to reproduce naturally for 6 days (n = 180). Dead or missing reproductive aphids were replaced on the second day. One newly hatched monarch caterpillar was placed on each of 20 replicates of each plant species x AMF treatment and allowed to feed for 6 days (n = 240). Missing or dead caterpillars were replaced on the second day. Twenty plants of each plant species × AMF treatment experienced no herbivory but were covered with white, nylon mesh bags to control for effects of mesh on plant traits (n = 240). We used these control plants to evaluate the effects of AMF on plant traits that may influence herbivore performance, and to determine the levels of AMF colonization of plant roots (n = 240). We could not use the plants upon which the herbivores fed, because aphid and caterpillar feeding alters milkweed defenses, nutritive quality, and levels of AMF colonization (A. R. Meier, unpublished data). Therefore, the traits measured in herbivore-damaged plants would not be representative of the initial plant quality experienced by aphids and caterpillars. We conducted this experiment in four temporal blocks separated by 1 day, with each treatment equally represented in each temporal block.
Analysis of Herbivore Traits
After the 6 days, aphids were counted and collected, allowed to void their guts for 24 h, frozen, lyophilized, and weighed. Caterpillars were also collected, allowed to void their guts for 24 h, frozen, dried at 50°C, and weighed. Simultaneously, control plants were harvested destructively to measure plant resistance and nutritive traits, biomass, and AMF colonization of roots. Aphid per capita growth rate per plant (r) was calculated by taking the natural log of the final aphid population size divided by the initial aphid population size (5 aphids) (Speight et al., 2008). Aphid individual mass was calculated by weighing each aphid population (i.e., all aphids present on one experimental plant) and dividing by the number of aphids in the population. Mean caterpillar growth rate per day was calculated by dividing the final, dry caterpillar mass by the 6 days for which it fed (Waldbauer, 1968). Leaves damaged by caterpillars were removed, scanned, and the area consumed by caterpillars (consumed leaf area, CLA) was determined with Image J (Schneider et al., 2012; Roger et al., 2013). To calculate the efficiency of conversion of ingested biomass (ECI) for caterpillars, we first determined the mass of leaves consumed by caterpillars. To do so, we calculated a mass/area ratio per plant by weighing and photographing two to three dried leaves from leaf pairs neighboring those consumed by caterpillars, and measuring the leaf area using Image J. Using this mass/area ratio, we calculated the mass of leaves consumed by caterpillars from the consumed leaf area that we measured. We calculated ECI per caterpillar as the final dry mass of the caterpillar divided by the dry mass of food it consumed (Waldbauer, 1968). Nine caterpillars that consumed flower buds in addition to leaves on A. curassavica plants were excluded from analyses of CLA and ECI. No other plant species produced flowers during the experiment.
After being dried and weighed, aphid populations and individual caterpillars were placed in 1 mL of methanol and stored at −10°C until cardenolide analysis. We assessed the cardenolides that herbivores sequestered following well-established methods (Zehnder and Hunter, 2007; Tao and Hunter, 2015). Aphids and caterpillars were ground for 3 min in methanol, sonicated for 1 h, and then centrifuged for 6 min. The supernatant was evaporated under vacuum at 45°C until dry and resuspended in 150 μl methanol containing 0.15 mg ml−1 digitoxin as an internal standard. Samples were then separated by ultra-performance liquid chromatography (UPLC; Waters Inc., Milford, MA, USA) using a Luna 2.5 μm C18(2) column (50 × 2 mm, Phenomenex Inc., Torrance, CA, USA). Each 2 μl injection was eluted at a constant flow of 0.7 ml per min with a gradient of acetonitrile and water for the 9 min run, maintaining first at 20% acetonitrile for 3 min, increasing to 45% acetonitrile for 5 min, and then maintaining at 20% acetonitrile for 1 min. Peaks were detected by a diode array detector at 218 nm, and absorbance spectra recorded from 200 to 400 nm. Symmetric peaks with maximum absorbance between 217 and 222 nm were quantified as cardenolides. Cardenolide concentrations were calculated using the digitoxin internal standard and total cardenolide concentrations were calculated as the sum of individual peaks. The masses of some aphid populations were too small to obtain enough dried material to detect cardenolides, and those samples were not included in our analyses of cardenolide sequestration (Table S1). In total, we analyzed 107 aphid populations (= replicate plants) with masses from 1.0 to 13.3 mg.
Analysis of Plant Traits
To measure foliar traits, we punched three fresh leaf disks from each leaf of the sixth leaf pair (six hole punches, 424 mm2 total) of each plant, placed the disks in 1 mL of methanol, and stored them at −10°C until cardenolide analysis. Foliar cardenolide concentrations were later assessed following the same procedure as for aphids and caterpillars (above). Latex that exuded from the hole punches was collected on pre-weighed cellulose disks, dried at 50°C, and weighed. Six additional leaf disks were taken from the same leaves, stored in glassine envelopes, and dried at 50°C. These leaf disks were weighed to estimate SLM and dry mass of foliar material used in cardenolide analyses. SLM was estimated by dividing the mass of dried leaf disks by the total disk area as a proxy for leaf toughness (Frost and Hunter, 2008). Additional leaves from neighboring leaf pairs were removed and dried at 50°C for subsequent carbon, nitrogen, and phosphorus analyses. Remaining plant material was dried at 50°C in paper bags and weighed to measure aboveground biomass after correcting for foliar tissue removed for chemistry sampling.
Carbon (C) and nitrogen (N) concentrations of foliar tissues were measured with a TruMac elemental analyzer (Leco Corporation, St. Joseph, MI, 49085, USA). Phosphorous (P) concentrations of foliar samples were determined by dry combusting ground samples in a muffle furnace at 550°C overnight, followed by persulfate digestion at 121°C for 60 min in an autoclave, and analysis by the molybdenum blue method on a PowerWave XS plate reader reading at 880 nm (Bio-Tek, Highland Park, Winooski, Vermont, 05404, USA). We calculated P concentrations of samples from a potassium phosphate standard curve and assessed quality control with NIST apple leaf standard analyzed with all samples. Only a subset of all experimental treatments were analyzed for nutritive traits, due to time and financial constraints (10 replicates of each plant species × AMF treatment, n = 120).
After washing the roots in deionized water, we stored 150 mg of 1 cm pieces of fresh fine root tissue in 60% ethanol at 4°C until we could quantify AMF colonization. We also took 400 mg of fresh fine root, dried it at 50°C, and reweighed it to calculate wet weight/dry weight ratios from which to estimate the dry mass of the subsample taken to quantify AMF colonization. We dried all remaining root tissue at 50°C and weighed its contribution to total root biomass. We analyzed a subset of roots of all experimental treatments (10 replicates of each AMF x plant species treatment, n = 120) due to time constraints in harvesting.
To quantify AMF colonization, roots were cleared with 10% KOH for 10 min, acidified using 2% HCl, and stained in 0.05% trypan blue in 1:1:1 water:glycerol:lactic acid (Vannette and Hunter, 2011). We mounted stained roots on slides and scored AMF colonization using the magnified gridline intersect method (McGonigle et al., 1990) with a Nikon compound microscope (Melville, NY, USA). A root intersection was considered colonized if hyphae, arbuscules, or vesicles were present. At least 100 root intersections were analyzed per plant.
Data Analyses
Some aphid populations did not sequester detectable concentrations of cardenolides on plants that contained cardenolides (Table S1), so we first determined whether the probability that aphids would sequester cardenolides was a function of plant species, AMF inoculum availability, or their interaction using a generalized linear mixed model with a binomial distribution and logit link function. Unlike aphids, all caterpillars sequestered cardenolides, except for those feeding on A. incarnata, so we did not evaluate the probability of caterpillar sequestration. For the aphid populations that did sequester cardenolides and all individual caterpillars, we used general linear mixed models to evaluate the effects of AMF inoculum availability and milkweed species on herbivore sequestration. In all models, temporal block was a random effect while milkweed species, AMF inoculum availability, and their interaction were fixed effects. For monarchs, we also included the family from which the caterpillar originated as a random effect. Using these models, we evaluated the effects of AMF inoculum availability on three measures of cardenolides sequestered by herbivores; total cardenolide concentration (sum of all cardenolide peaks), cardenolide diversity (using Shannon's index), and cardenolide polarity (relative representation of lipophilic cardenolides), calculated by summing the relative peak areas multiplied by each peaks' retention time (Rasmann and Agrawal, 2011; Sternberg et al., 2012). A greater diversity of cardenolides and more lipophilic cardenolides are considered more toxic than lower diversity or more polar mixes (Fordyce and Malcolm, 2000; Zehnder and Hunter, 2007; Sternberg et al., 2012). Because herbivores feeding upon A. incarnata rarely sequestered cardenolides (Table S1), they were excluded from all sequestration analyses.
For these and the following analyses, data were natural log- and log-transformed when necessary. In addition, we used Tukey's adjustment for multiple comparisons to identify significant differences among treatment means. We considered differences to be significant at P < 0.05, except when evaluating differences among AMF treatments within plant species. For these analyses, we considered differences to be significant at P < 0.1 due to the reduced sample size of these analyses. All statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, NC, USA). Because several caterpillars died before the end of the experiment and several samples were lost during processing and chemical analyses, final sample sizes were smaller than initial (details in Table S2).
We also tested for differences in the composition (i.e., identity and relative abundance) of cardenolides sequestered by herbivores and present in leaves, among plant species, AMF treatments, and their interaction using permutational multivariate ANOVA (PERMANOVA; McCune et al., 2002). We used the adonis function in the vegan package (Oksanen et al., 2016) in R v 3.3.1 and calculated dissimilarities among samples using the Bray-Curtis metric for PERMANOVA. To evaluate how AMF influenced the composition of cardenolides in herbivore and foliar tissue, we used non-metric multidimensional scaling (NMDS) through the vegan package.
We also used general linear mixed models to compare the effects of AMF inoculum availability and milkweed species on aphid and caterpillar performance. As before, temporal block was a random effect while AMF inoculum availability, milkweed species, and their interaction were fixed effects. For monarchs, we included the family from which the caterpillar originated as a random effect. Each herbivore performance measure (aphid per capita growth rate, aphid mass per individual, caterpillar growth rate, ECI, CLA) was a dependent variable. Not all caterpillars survived through the 6th day of feeding, so we assessed the probability of caterpillar survival among treatments using a generalized linear mixed model with a binomial distribution and logit link function.
We used general linear mixed models to evaluate the effects of AMF inoculum availability and milkweed species on plant traits. In all models, temporal block was a random effect while milkweed species, AMF inoculum availability, and their interaction were fixed effects. Each plant trait (i.e., foliar defensive traits, foliar nutritive traits, aboveground biomass, and levels of AMF colonization of roots) was a dependent variable. A. incarnata produced no foliar cardenolides in this study, and were therefore excluded from analyses of foliar cardenolides.
To gain some insight into the phenotypic traits of plants through which AMF influenced herbivores, we assessed the effects of measured plant traits on herbivore performance and sequestration using multiple regression. However, because herbivore and plant traits were measured from different groups of plants (above), we could only assess relationships among average values for each plant species x AMF treatment, yielding only 8–12 data points for these analyses. Therefore, we present these analyses in the Supplementary Materials.
Results
We summarize the effects of milkweed species, AMF inoculum availability, and their interaction on plant traits and herbivore traits (toxin sequestration and performance) in Tables 1, 2, respectively. We describe key results in more detail below.
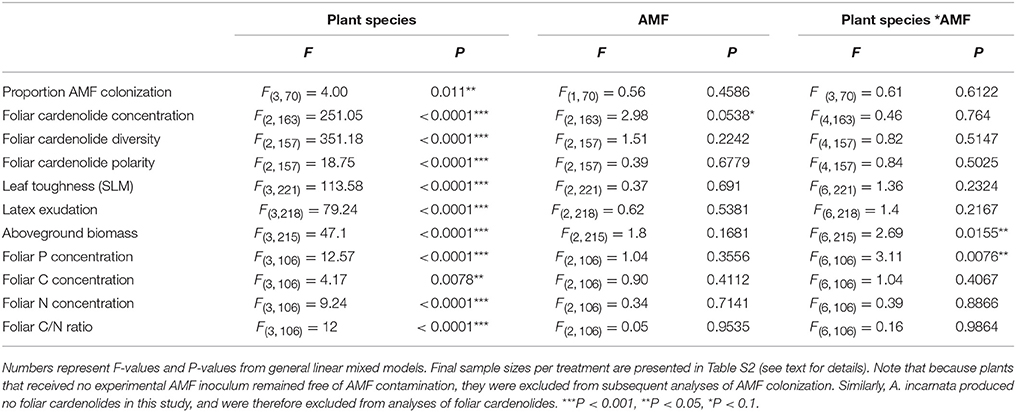
Table 1. Effects of plant species, arbuscular mycorrhizal fungi (AMF) inoculum availability, and their interaction on plant traits, including the proportion of roots colonized by AMF, natural log-transformed foliar cardenolide concentrations, foliar cardenolide diversity, foliar cardenolide polarity, leaf toughness (specific leaf mass, SLM; mg/cm2), natural log-transformed latex exudation (mg), aboveground biomass (mg), foliar P concentration (%), foliar C concentration (%), foliar N concentration (%), foliar C/N ratio.
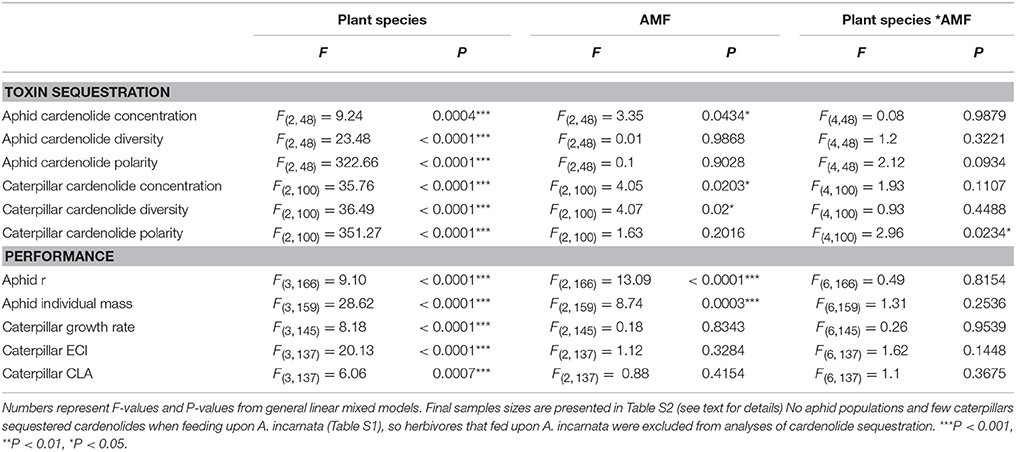
Table 2. Effects of plant species, arbuscular mycorrhizal fungi (AMF) inoculum availability, and their interaction on measures of herbivore toxin sequestration and performance, including natural log-transformed cardenolide concentration sequestered by aphids (mg/g dry mass), diversity of cardenolides sequestered by aphids, natural log-transformed polarity of cardenolides sequestered by aphids, natural log-transformed cardenolide concentration sequestered by caterpillars (mg/g dry mass), diversity of cardenolides sequestered by caterpillars, natural log-transformed polarity of cardenolides sequestered by caterpillars, aphid per capita growth rate (r), individual aphid dry mass (μg), caterpillar growth rate (mg/day), log-transformed caterpillar efficiency of conversion (ECI) of ingested biomass, and log-transformed leaf area consumed (CLA) by caterpillars (cm2).
AMF Colonization
The proportion of roots colonized by AMF arbuscules was tightly correlated with root colonization by all fungal structures (R2 = 0.95, P < 0.0001), so we report only the latter here. Inoculation with AMF led to successful root colonization, while control plants remained AMF-free [F(2, 106) = 43.91, P < 0.0001]. Analysis of plants inoculated with live AMF (medium and high AMF treatments only) illustrated that AMF colonization was not a simple function of inoculum availability. Rather, levels of colonization varied substantially among plant species [F(3, 70) = 4.00, P = 0.011; Figure S1], but were similar in medium and high AMF treatments [F(1, 70) = 0.56, P = 0.4586; Figure S1]. However, because herbivore performance varied substantially between medium and high AMF treatments (below), we conclude that the availability of inoculum had effects on plant phenotype beyond those observed by estimates of colonization alone. We have therefore continued to treat medium and high AMF treatments separately in all following analyses.
Herbivore Sequestration of Cardenolides
As expected (Malcolm, 1990, 1994; Agrawal et al., 2015; Petschenka and Agrawal, 2015), the concentration, diversity, polarity, and composition of cardenolides sequestered by aphids and caterpillars varied strongly among plant species, following plant species-specific differences in cardenolide expression (Table 2, PERMANOVA aphid: Plant species [F(2, 50) = 22.2694, P < 0.001]; caterpillar: Plant species [F(2, 110) = 98.086, P < 0.001]. For instance, aphids and caterpillars sequestered the highest cardenolide concentration and diversity, and most lipophilic (non-polar) cardenolides, when feeding upon the high cardenolide-containing A. curassavica and the least when feeding upon the low cardenolide-containing A. syriaca.
Importantly, the amount of AMF inoculum available to the milkweed hosts of aphids and caterpillars influenced the concentration of cardenolides that aphid populations and caterpillars sequestered (aphid: AMF [F(2, 48) = 3.35, P = 0.0434]; Figure 1A; caterpillar: AMF [F(2, 100) = 4.05, P = 0.0203]; Figure 1B). Across milkweed species, aphids sequestered, on average, 87% and 36% higher cardenolide concentrations when feeding upon plants under medium and high AMF availability, respectively, than when feeding upon plants without AMF (Figure 1A). Similarly, caterpillars sequestered 38 and 25% higher cardenolide concentrations when they fed upon plants under medium and high AMF inoculum availability, respectively, than caterpillars that fed upon plants without AMF (Figure 1B). The probability that aphid populations would sequester cardenolides did not vary among plant species or with AMF inoculum availability {Plant species [F(2, 93) = 2.56, P = 0.0824]; AMF [F(2, 93) = 0.65, P = 0.5264]}.
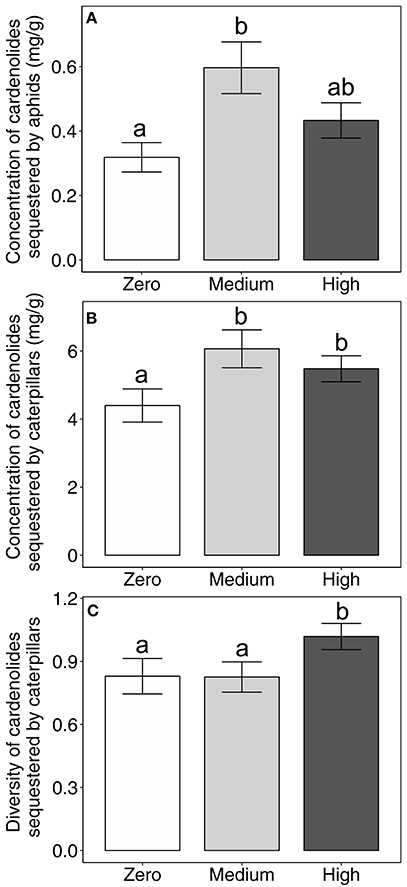
Figure 1. Effects of AMF inoculum availability on the concentration of cardenolides sequestered by (A) aphid populations and (B) individual caterpillars, and on (C) the diversity of cardenolides sequestered by individual caterpillars reared on three milkweed species. Sample sizes range from 15 to 24 aphid populations (= replicate plants) for aphid cardenolide concentrations, 39–43 individual caterpillars (= replicate plants) for the concentration and diversity of cardenolides sequestered by caterpillars per AMF treatment. Bars display the mean ± 1 SE. Different letters indicate significantly (P < 0.05) different means (Tukey post-hoc test of the ANOVA).
The availability of AMF inoculum also shifted the community of cardenolides that aphids and caterpillars sequestered {PERMANOVA aphid: AMF [F(2, 50) = 2.2045, P = 0.047]; caterpillar: [Plant species ∗ AMF F(4, 110) = 2.022, P = 0.035]}. In addition, caterpillars feeding on plants under high AMF availability sequestered more diverse communities of cardenolides, by an average of 23%, than did caterpillars feeding upon plants under zero or medium AMF availability {AMF [F(2, 100) = 4.07, P = 0.02; Figure 1C]}. There were also minor, plant species-specific effects of AMF on the polarity of cardenolides that caterpillars sequestered {Plant species*AMF [F(4, 100) = 2.96, P = 0.0234]}. Caterpillars sequestered 22% more lipophilic (non-polar) cardenolides when feeding upon A. syriaca plants under high AMF availability than on A. syriaca plants under zero or medium AMF availability. However, the polarity of cardenolides that caterpillars sequestered was unaffected by the amount of AMF available to A. curassavica and A. latifolia. AMF availability also did not influence the diversity or polarity of cardenolides that aphids sequestered (Table 2).
Herbivore Performance
Aphid performance varied non-linearly with increasing AMF availability; it was lowest on plants under medium AMF availability, but highest on plants under high AMF availability (Table 2, Figure 2). Specifically, aphid per capita growth rates were 19% greater under high AMF availability than under medium AMF availability, with intermediate per capita growth rates on plants without AMF [F(2, 166) = 13.09, P < 0.0001; Figure 2A]. Similarly, individual aphids were 24% heavier on plants under high AMF availability than were aphids on plants under medium AMF availability [F(2, 159) = 8.74, P = 0.0003; Figure 2B]. As expected from previous work (Agrawal, 2004), aphid per capita growth rates and masses varied among milkweed species {r: [F(3, 166) = 9.10, P < 0.0001; mass: F(3, 159) = 28.62, P < 0.0001]}.
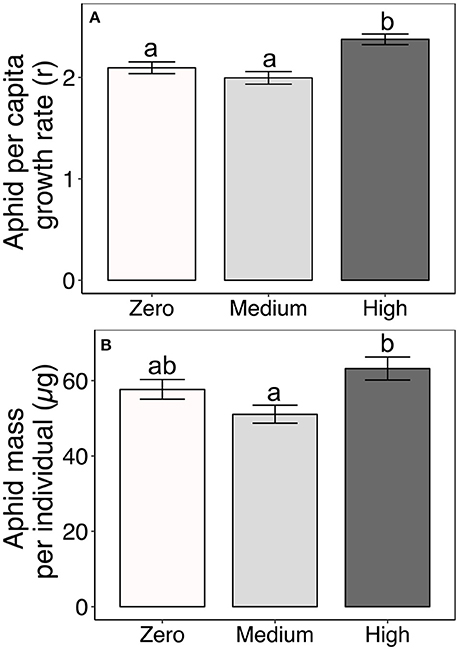
Figure 2. Effects of AMF inoculum availability on (A) per capita growth rates of aphids (r over 6 days) and (B) average dry mass of individual aphids reared on four milkweed species. Sample sizes are 60 populations of aphids (= replicate plants) for aphid per capita growth rates and range from 55 to 59 populations for average individual aphid mass per AMF treatment. Bars display the mean ± 1SE. Different letters indicate significantly (P < 0.05) different means (Tukey post-hoc test of the ANOVA).
The availability of AMF inoculum had striking effects on caterpillar survival, but those effects varied among milkweed species (Plant species*AMF χ2 = 14.1, df = 6, P = 0.0286; Figure 3). For example, caterpillars feeding on A. incarnata and A. syriaca were 13 and 44% more likely to survive on plants without AMF than on plants with AMF, respectively. In contrast, caterpillars feeding on A. latifolia were 38% more likely to survive on plants grown under medium AMF inoculum availability than on plants without AMF. Caterpillars feeding on A. curasssavica were affected minimally by AMF inoculum availability (Figure 3). Caterpillar growth rates, efficiency of conversion of ingested biomass (ECI), and consumption of leaf area (CLA) varied widely among milkweed species, but were unaffected by the availability of AMF inoculum (Table 2).
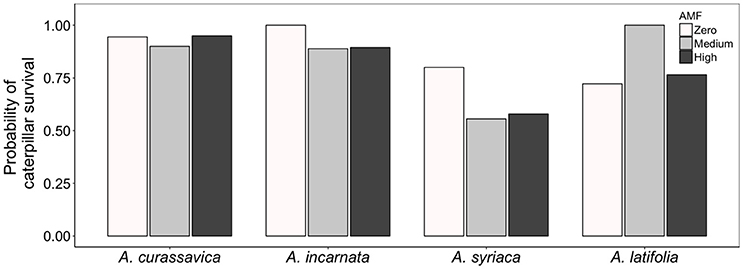
Figure 3. Effect of AMF inoculum availability on the probability of caterpillar survival on four milkweed species. Sample sizes range from 17 to 20 caterpillars (= replicate plants) per plant species × AMF treatment.
Effects of AMF on Plant Traits
Consistent with the effects of AMF on cardenolide sequestration by herbivores (above), foliar cardenolide concentrations in milkweed plants under medium and high AMF availability were an average of 17 and 19% greater, respectively, than were concentrations in AMF-free plants {AMF [F(2, 163) = 2.98, P = 0.0538; Figure 4]}. As expected (Rasmann and Agrawal, 2011; Sternberg et al., 2012; Vannette et al., 2013), milkweed species varied in the diversity, polarity, and composition of cardenolides in their leaves, as well as in leaf toughness (SLM) and latex exudation {Table 1, PERMANOVA for composition [F(2, 160) = 131.51, P < 0.001]}. However, we observed no influence of AMF inoculum availability on any of these chemical or physical resistance traits {Table 1, PERMANOVA for cardenolide composition: AMF [F(2, 160) = 1.62, P = 0.128]}.
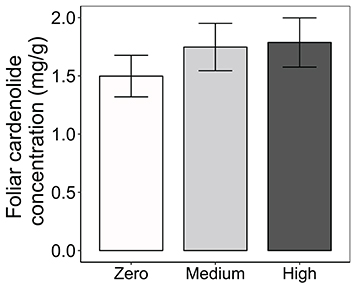
Figure 4. Effects of AMF inoculation on foliar cardenolide concentrations of three milkweed species. Samples sizes range from 58 to 59 plants per AMF treatment. Bars display the mean ± 1SE. Foliar cardenolide concentrations vary among AMF treatments (P = 0.0538), but treatment means are not significantly different at P < 0.05 (Tukey post-hoc test of the ANOVA).
In contrast to their consistent effects on foliar cardenolide concentrations, AMF altered plant growth and nutritive traits in a plant species-specific fashion (Table 1, Figures 5A,B). AMF inoculation decreased the aboveground biomass of most milkweed species by 8–29%. The exception was A. curassavica, in which AMF inoculation increased aboveground biomass by an average of 28% {Plant species*AMF [F(6, 215) = 2.69, P = 0.0155, Figure 5A]}. AMF inoculation increased foliar P concentrations in A. curassavica and A. latifolia by an average of 25 and 16%, respectively, but decreased P concentrations in A. incarnata and A. syriaca by an average of 8 and 13%, respectively {Plant species*AMF [F(6, 106) = 3.11, P = 0.0076; Figure 5B]}. In contrast, AMF inoculum availability did not affect foliar C or N concentrations, or foliar C/N ratios, although these traits did vary among plant species (Table 1).
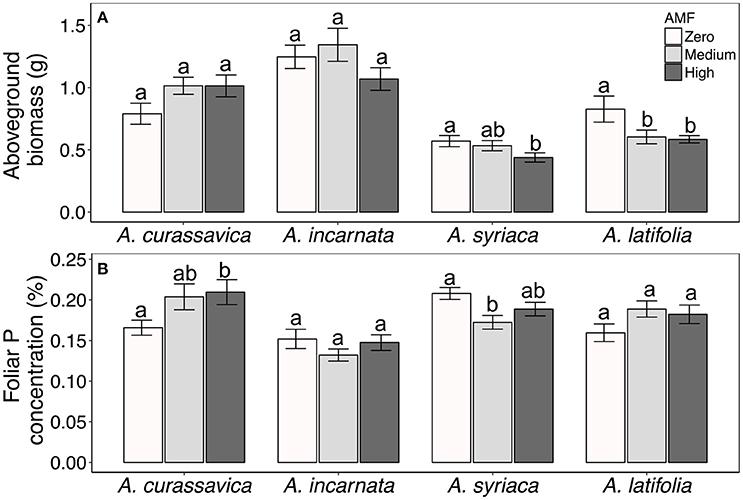
Figure 5. (A) Aboveground biomass and (B) foliar phosphorus (P) concentrations of four milkweed species. Sample sizes range from 17 to 20 plants per treatment for aboveground biomass and 9–10 plants per treatment for P concentrations. Bars display the mean ± 1SE. Different letters indicate significantly (P < 0.1) different AMF treatment means within each plant species (Tukey post-hoc test of the ANOVA within plant species).
Discussion
Our study is among the first to document the impacts of AMF on toxin sequestration by specialist herbivores, while measuring simultaneously effects on herbivore performance. We demonstrate that (1) aphids and caterpillars sequester higher concentrations of cardenolides from plants inoculated with AMF, following AMF-mediated increases in foliar cardenolide concentrations. (2) AMF availability influences the performance of both aphids and caterpillars on milkweed, though in different ways. On all milkweed species, aphid performance varies non-linearly with increasing AMF inoculum availability, with lowest performance under medium levels of inoculum availability and highest performance under high inoculum availability. In contrast, while caterpillar survival varies markedly with AMF inoculum availability, it does so in a plant species-specific manner, and caterpillar growth is unaffected by AMF. Our findings suggest that by altering plant phenotype, the availability of AMF in soil has the potential to influence both the top-down (via sequestration) and the bottom up (via plant defense and nutrition) forces that operate on milkweed herbivores.
Inoculation of plants with medium or high amounts of AMF inoculum resulted in equal levels of root colonization (Figure S1). Nonetheless, we observed that the availability of AMF inoculum (medium versus high) influenced herbivore performance and plant phenotype (Tables 1, 2). Because the commercial AMF mix that we used was purported to consist of four AMF species, the different effects of AMF availability on herbivore performance may be a function of differential colonization by AMF species under medium and high AMF availability. AMF species vary in their relative trading of nutrients (Lendenmann et al., 2011; Thonar et al., 2014; Argüello et al., 2016) and effects on plant phenotype (Gehring and Bennett, 2009; Bennett et al., 2013) which can alter herbivore performance (Roger et al., 2013; Vannette and Hunter, 2013). However, cloning and sequencing of the AMF mix, and roots from milkweed plants grown under the same experimental conditions, with AMF-specific primers (Krüger et al., 2009) demonstrated that the AMF mix consisted only of F. mosseae (details in Supplementary Material).
Instead, the differential effects of medium and high AMF inoculum availability on herbivore performance and plant phenotype are more likely due to differential regulation of AMF colonization by plants under medium and high AMF availability. Although AMF colonization levels increase with increasing inoculum availability (Garrido et al., 2010; Vannette and Hunter, 2011), plants maintain a maximum level of AMF colonization of roots (Vierheilig et al., 2000a,b; Meixner et al., 2005) and suppress further colonization after reaching a critical level (Vierheilig, 2004). Plant regulation of AMF development in roots is controlled by the same plant hormones (Staehelin et al., 2011; Bucher et al., 2014; Gutjahr, 2014; and references therein) that are integral to the development of plant vascular tissues (Lucas et al., 2013) and the resistance responses of plants to insect herbivores (Pieterse et al., 2012, 2014). In our medium AMF treatment, there may have been sufficient inoculum to attain maximum levels of AMF colonization of plant roots. Therefore, under high AMF availability, plants may have suppressed AMF development in roots more strongly by altering phytohormone levels, resulting in the observed differences in herbivore performance and plant phenotype between medium and high AMF treatments.
Sequestration by Specialist Herbivores Is Altered by AMF Availability
Both aphids and caterpillars sequestered higher concentrations of cardenolides when feeding upon plants under medium and high AMF inoculum availability (Figures 1A,B), following AMF-mediated increases in foliar cardenolide concentrations (Figure 4 and Figures S2A,B; Table S3). This is consistent with previous reports of tight links between aphid and caterpillar sequestration and host plant cardenolide concentrations (Malcolm, 1990, 1994; Agrawal et al., 2015; Petschenka and Agrawal, 2015). However, while AMF inoculum availability did not influence the composition of cardenolides in foliage, AMF did affect the composition of cardenolides sequestered by aphids and caterpillars. Sequestration of cardenolides by A. nerii occurs through passive diffusion (Malcolm, 1990; Züst and Agrawal, 2016b). Therefore, AMF-mediated changes in the composition of cardenolides sequestered by aphids may result from AMF changing the relative concentrations of cardenolides present in phloem, but not leaves. While milkweed phloem contains the same variety of cardenolides as leaves, the concentrations of specific cardenolides may vary between phloem and leaves (Züst and Agrawal, 2016b).
In contrast, monarch caterpillars may control the uptake of particular cardenolides and their amounts (Malcolm, 1994; Tao and Hunter, 2015) by sequestering cardenolides actively and selectively (Malcolm and Brower, 1989; Frick and Wink, 1995; Petschenka and Agrawal, 2015; Erb and Robert, 2016). AMF may have affected the composition of cardenolides sequestered by caterpillars, without affecting the composition of foliar cardenolides, by altering aspects of plant quality that may affect active sequestration, such as nutrient availability. We did not find correlations between foliar nutrient content and sequestration, potentially due to low sample sizes, but variation in soil N and P availability has been found to alter the efficiency of monarch caterpillar sequestration and the composition of cardenolides that monarch caterpillars sequester (Tao and Hunter, 2015). Alternatively, interactions between AMF and caterpillar feeding may have altered the composition of foliar cardenolides (Bennett et al., 2009; Agrawal et al., 2014; Wang et al., 2015), resulting in the observed, AMF-mediated differences in caterpillar sequestration. However, milkweed responses to monarch caterpillar feeding can take up to 5 days to occur (Agrawal et al., 2014) and monarch caterpillars fed on our experimental plants for only 6 days. Therefore, we think it unlikely that AMF-mediated changes in caterpillar sequestration were driven by interactions between AMF and caterpillar induction of foliar cardenolides.
AMF Abundance Alters Specialist Herbivore Performance and Survival
The availability of AMF inoculum had consistent, non-linear effects on aphid performance, regardless of milkweed species (Figure 2). Aphids had the lowest per capita growth rates and individual masses on plants under medium AMF availability, yet had the highest per capita growth rates and masses on plants under high AMF availability (Figure 2). Thus, we found within a single study the range of aphid responses to AMF from the literature, from positive to negative (Pacovsky et al., 1985; Gange and West, 1994; Gange et al., 1999, 2002; Gehring and Whitham, 2002; Wurst et al., 2004; Hempel et al., 2009; Koricheva et al., 2009; Abdelkarim et al., 2011; Babikova et al., 2014a; Colella et al., 2014; Grabmaier et al., 2014; Williams et al., 2014; Bennett et al., 2016; Simon et al., 2017; Tomczak and Müller, 2017). Our findings suggest that some of the previously found variation in aphid responses may result from differences in AMF inoculum availability among studies.
AMF may have affected aphid performance by altering foliar cardenolide concentrations; we found that aphid masses declined with increasing foliar cardenolide concentrations (Table S3, Figure S2d). Indeed, aphids had lower masses and per capita growth rates on plants under medium AMF availability (Figure 2), which had greater foliar cardenolide concentrations than plants without AMF (Figure 4). Although A. nerii tolerate cardenolides, they are negatively affected by high cardenolide concentrations (Agrawal, 2004; de Roode et al., 2011; Birnbaum et al., 2017). Nonetheless, we interpret the regressions with caution due to low sample sizes and plant species-specific differences in traits. AMF-mediated increases in aphid performance under high AMF availability may also be a consequence of increased vascular bundle size; AMF colonization increases the size of vascular bundles in plants (Krishna et al., 1981), increasing aphid phloem feeding and reproductive success (Simon et al., 2017). Although aphids are often responsive to changes in amino acid content of phloem (Züst and Agrawal, 2016a), we think it unlikely that AMF influenced A. nerii performance by changing phloem soluble sugar or amino acid content because previous studies found no correlations among AMF-mediated changes in aphid performance and foliar or phloem nutrient content (Gange and West, 1994; Hempel et al., 2009; Grabmaier et al., 2014).
Although AMF colonization of plants has been found to increase the survival of specialist caterpillars (Goverde et al., 2000), we found that AMF inoculum availability improved, did not affect, or reduced the survival of a specialist caterpillar, depending on the plant species and density of AMF inoculum available to the plant (Figure 3). This breadth of responses of monarch caterpillars to AMF among plant species may result from plant species-specific effects of AMF on plant biomass (Figure 5A); caterpillar survival increased with increasing aboveground biomass (Tables S3, Figure S2e). Although caterpillars were never food limited in our study, AMF-mediated declines in plant biomass may have reduced caterpillar survival by decreasing the availability of young leaves because monarch caterpillars prefer younger leaves (Bingham and Agrawal, 2010). AMF-mediated increases in foliar cardenolide concentrations did not correlate with declines in caterpillar survival in this study, although high cardenolide concentrations often reduce monarch caterpillar performance and survival (Zalucki et al., 2001a; Agrawal, 2005; Rasmann et al., 2009; Tao et al., 2016b).
Interestingly, despite finding strong effects of AMF on monarch survival, we found no influence of AMF on monarch caterpillar growth rates (Table 2). Our findings confirm those for other specialist chewers, such as specialist beetle larvae and adult weevils (Laird and Addicott, 2008; Cosme et al., 2011), whose growth rates are also unaffected by AMF. However, our findings contrast with previous work that found monarch caterpillar growth rates to increase on milkweed plants under higher AMF inoculum availability (Vannette and Hunter, 2013). These conflicting findings may result from experimental milkweed plants being inoculated with different AMF species; individual AMF taxa and mixes alter plant phenotype differently (Bennett et al., 2009; Vannette and Hunter, 2011), affecting caterpillar performance (Goverde et al., 2000; Roger et al., 2013). Indeed, AMF-mediated increases in monarch caterpillar growth rates were attributed to AMF-mediated declines in milkweed leaf toughness (SLM) and latex exudation (Vannette and Hunter, 2013) and we found no influence of AMF on these traits (Table 1). In addition, it is possible that our plants were already induced by thrip activity, whereas plants in previous studies were not. However, because plants of all treatments were attacked equally, we do not believe that the minor thrip damage altered the quality of our results.
Effects of AMF on Herbivore Performance and Toxin Sequestration May Have Community-Wide Consequences
Because the availability of AMF inoculum altered both toxin sequestration and performance of specialist herbivores, AMF may affect herbivore populations by altering both top-down and bottom-up factors. For instance, aphids that fed upon milkweeds under medium AMF availability sequestered nearly twice the concentration of cardenolides that they did when feeding upon AMF-free plants, potentially improving aphid resistance to natural enemies. Aphid predators exhibit high rates of mortality when fed oleander aphids from high cardenolide milkweeds, but experience low rates of mortality when fed aphids from low cardenolide milkweeds (Malcolm, 1992). Accordingly, in the field, oleander aphid populations are smaller and more influenced by predators when feeding on low cardenolide milkweed species than when feeding on high cardenolide milkweed species (Malcolm, 1992; Mohl et al., 2016). Similarly, monarch caterpillars that sequester higher concentrations of cardenolides are more toxic to their predators (Brower et al., 1968; Reichstein et al., 1968; Brower and Moffitt, 1974) and may be more resistant to their parasites (Lefèvre et al., 2010; Sternberg et al., 2012). Therefore, monarch caterpillars may be better protected against their natural enemies when their host plants are inoculated with AMF.
The strong effects of AMF on aphid per capita growth rates and caterpillar survival suggest that the availability of AMF in soil may also influence the population dynamics of herbivores by changing host plant quality. Furthermore, by altering aphid densities and individual masses, AMF may influence aphid-parasitoid interactions. Parasitism rates of A. nerii are density dependent (Helms et al., 2004), and parasitoids that develop in larger herbivore hosts have larger clutch sizes, bigger individual offspring, greater proportions of female offspring, and increased longevity (Hunter, 2003; Bukovinszky et al., 2008; van Veen and Godfray, 2012). AMF colonization of plants has been found to increase parasitoid attack rates, shorten parasitoid developmental times, and increase successful emergence of aphid parasitoids (Hempel et al., 2009; Bennett et al., 2016), even in the absence of plant-derived cues such as volatiles (Bennett et al., 2016). Our study suggests that AMF-mediated increases in aphid size may be a simple mechanism by which AMF improve parasitoid success. In support of this, communities of other belowground organisms, such as soil-dwelling nematodes, have been found to improve parasitoid performance, potentially by increasing aphid size (Bezemer et al., 2005).
Conclusion
In summary, we found that AMF inoculum availability influences strongly toxin sequestration and performance of two specialist herbivores, suggesting that AMF availability may substantially alter interactions among plants, herbivores, and their natural enemies. Furthermore, the availability of AMF inoculum, measured as infectivity and spore abundances, varies on small scales, such as centimeters (Wolfe et al., 2007) and meters (Carvalho et al., 2003). Therefore, plants within a single population may experience substantial variation in AMF availability in soils. This variation in AMF abundance may result in spatial variation in plant quality for herbivores, and herbivore quality for their natural enemies, ultimately affecting large scale population dynamics (Riolo et al., 2015). Future studies should consider how natural AMF abundances influence plant phenotype and the resulting herbivore and natural enemy population dynamics in the field.
Data Accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.8985578.
Author Contributions
AM and MH: Conceived the ideas and designed methodology; AM: Collected the data; AM and MH: Analyzed the data; AM: Led the writing of the manuscript. Both authors contributed to the drafts and gave final approval for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the Matthaei Botanical Gardens for greenhouse space and help with plant care. We gratefully acknowledge Lucas Michelotti, Jordan McMahon, Hillary Streit, Skye Huerta, Sam Clinton, and Riley Peterson for providing assistance with the experiment and chemical analyses. We thank Leslie Decker, Katherine Crocker, Kristel Sanchez, Anne Elise Stratton, and Tim James for constructive comments on an earlier draft. We also thank two reviewers for their constructive comments on an earlier version of the paper. The work was supported by a Block Grant, Matthaei Botanical Gardens Research Award, and Rackham Graduate Student Research Grant from the University of Michigan to AM, NSF DEB 1256115 to MH and a NSF GRFP to AM.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00033/full#supplementary-material
References
Abdelkarim, M., Ownley, B. H., Klingeman, W. E., and Gwinn, K. D. (2011). Effect of arbuscular mycorrhizae on aphid infestation of wheat. Phytopathology 101:S2.
Agrawal, A. A. (2004). Plant defense and density dependence in the population growth of herbivores. Am. Nat. 164, 113–120. doi: 10.1086/420980
Agrawal, A. A. (2005). Natural selection on common milkweed (Asclepias syriaca) by a community of specialized insect herbivores. Evol. Ecol. Res. 7, 651–667.
Agrawal, A. A., Ali, J. G., Rasmann, S., and Fishbein, M. (2015). “Macroevolutionary trends in the defense of milkweeds against monarchs,” in Monarchs in a Changing World: Biology and Conservation of an Iconic Insect, eds K. Oberhauser, S. Altizer, and K. Nail (Ithaca, NY: Cornell University Press), 47–59.
Agrawal, A. A., and Fishbein, M. (2006). Plant defense syndromes. Ecology 87, S132–S149. doi: 10.1890/0012-9658(2006)87[132:PDS]2.0.CO;2
Agrawal, A. A., Hastings, A. P., Patrick, E. T., and Knight, A. C. (2014). Specificity of herbivore-induced hormonal signaling and defensive traits in five closely related milkweeds (Asclepias spp.). J. Chem. Ecol. 40, 717–729. doi: 10.1007/s10886-014-0449-6
Agrawal, A. A., and Konno, K. (2009). Latex: a model for understanding mechanisms, ecology, and evolution of plant defense against herbivory. Annu. Rev. Ecol. Evol. Syst. 40, 311–331. doi: 10.1146/annurev.ecolsys.110308.120307
Agrawal, A. A., Petschenka, G., Bingham, R. A., Weber, M. G., and Rasmann, S. (2012). Toxic cardenolides: chemical ecology and coevolution of specialized plant-herbivore interactions. New Phytol. 194, 28–45. doi: 10.1111/j.1469-8137.2011.04049.x
Anacker, B. L., Klironomos, J. N., Maherali, H., Reinhart, K. O., and Strauss, S. Y. (2014). Phylogenetic conservatism in plant-soil feedback and its implications for plant abundance. Ecol. Lett. 17, 1613–1621. doi: 10.1111/ele.12378
Argüello, A., O'Brien, M. J., van der Heijden, M. G., Wiemken, A., Schmid, B., and Niklaus, P. A. (2016). Options of partners improve carbon for phosphorus trade in the arbuscular mycorrhizal mutualism. Ecol. Lett. 19, 648–656. doi: 10.1111/ele.12601
Babikova, Z., Gilbert, L., Bruce, T., Dewhirst, S. Y., Pickett, J. A., and Johnson, D. (2014a). Arbuscular mycorrhizal fungi and aphids interact by changing host plant quality and volatile emission. Funct. Ecol. 28, 375–385. doi: 10.1111/1365-2435.12181
Babikova, Z., Gilbert, L., Bruce, T. J., Birkett, M., Caulfield, J. C., Woodcock, C., et al. (2013). Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecol. Lett. 16, 835–843. doi: 10.1111/ele.12115
Babikova, Z., Gilbert, L., Randall, K. C., Bruce, T. J. A., Pickett, J. A., and Johnson, D. (2014b). Increasing phosphorus supply is not the mechanism by which arbuscular mycorrhiza increase attractiveness of bean (Vicia faba) to aphids. J. Exp. Bot. 65, 5231–5241. doi: 10.1093/jxb/eru283
Barber, N. A., Kiers, E. T., Hazzard, R. V., and Adler, L. S. (2013). Context-dependency of arbuscular mycorrhizal fungi on plant-insect interactions in an agroecosystem. Front. Plant Sci. 4:338. doi: 10.3389/fpls.2013.00338
Bennett, A. E., Bever, J. D., and Bowers, M. D. (2009). Arbuscular mycorrhizal fungal species suppress inducible plant responses and alter defensive strategies following herbivory. Oecologia 160, 771–779. doi: 10.1007/s00442-009-1338-5
Bennett, A. E., Macrae, A. M., Moore, B. D., Caul, S., and Johnson, S. N. (2013). Early root herbivory impairs arbuscular mycorrhizal fungal colonization and shifts defence allocation in establishing Plantago lanceolata. PLoS ONE 8:e66053. doi: 10.1371/journal.pone.0066053
Bennett, A. E., Millar, N. S., Gedrovics, E., and Karley, A. J. (2016). Plant and insect microbial symbionts alter the outcome of plant–herbivore–parasitoid interactions: implications for invaded, agricultural and natural systems. J. Ecol. 104, 1734–1744. doi: 10.1111/1365-2745.12620
Bezemer, T. M., De Deyn, G. B., Bossinga, T. M., Van Dam, N. M., Harvey, J. A., and Van Der Putten, W. H. (2005). Soil community composition drives aboveground plant-herbivore-parasitoid interactions. Ecol. Lett. 8, 652–661. doi: 10.1111/j.1461-0248.2005.00762.x
Bingham, R. A., and Agrawal, A. A. (2010). Specificity and trade-offs in the induced plant defence of common milkweed Asclepias syriaca to two lepidopteran herbivores. J. Ecol. 98, 1014–1022. doi: 10.1111/j.1365-2745.2010.01681.x
Birnbaum, S. S. L., Rinker, D. C., Gerardo, N. M., and Abbot, P. (2017). Transcriptional profile and differential fitness in a specialist milkweed insect across host plants varying in toxicity. Mol. Ecol. 12, 3218–3221. doi: 10.1111/mec.14401
Borowicz, V. A. (1997). A fungal root symbiont modifies plant resistance to an insect herbivore. Oecologia 112, 534–542. doi: 10.1007/s004420050342
Brower, L. P., and Moffitt, C. M. (1974). Palatability dynamics of cardenolides in the monarch butterfly. Nature 249, 280–283. doi: 10.1038/249280b0
Brower, L. P., Ryerson, W. N., Coppinger, L. L., and Glazier, S. C. (1968). Ecological chemistry and the palatability spectrum. Science 161, 1349–1350. doi: 10.1126/science.161.3848.1349
Bucher, M., Hause, B., Krajinski, F., and Küster, H. (2014). Through the doors of perception to function in arbuscular mycorrhizal symbioses. New Phytol. 204, 833–840. doi: 10.1111/nph.12862
Bukovinszky, T., van Veen, F. J., Jongema, Y., and Dicke, M. (2008). Direct and indirect effects of resource quality on food web structure. Science 319, 804–807. doi: 10.1126/science.1148310
Camara, M. D. (1997). Predator responses to sequestered plant toxins in buckeye caterpillars: are tritrophic interactions locally variable? J. Chem. Ecol. 23, 2093–2106. doi: 10.1023/B:JOEC.0000006431.34359.c2
Cameron, D. D., Neal, A. L., van Wees, S. C., and Ton, J. (2013). Mycorrhiza-induced resistance: more than the sum of its parts? Trends Plant Sci. 18, 539–545. doi: 10.1016/j.tplants.2013.06.004
Carvalho, L. M., Correia, P. M., Ryel, R. J., and Martins-Loução, M. A. (2003). Spatial variability of arbuscular mycorrhizal fungal spores in two natural plant communities. Plant Soil 251, 227–236. doi: 10.1023/A:1023016317269
Colella, T., Candido, V., Campanelli, G., Camele, I., and Battaglia, D. (2014). Effect of irrigation regimes and artificial mycorrhization on insect pest infestations and yield in tomato crop. Phytoparasitica 42, 235–246. doi: 10.1007/s12600-013-0356-3
Colvin, S. H., Snyder, J. C., Thacker, R., and Yeargan, K. V. (2013). Thinking outside the Asclepias box: oleander aphids and honeyvine milkweed. Ann. Entomol. Soc. Am. 106, 214–221. doi: 10.1603/AN11189
Cosme, M., Stout, M. J., and Wurst, S. (2011). Effect of arbuscular mycorrhizal fungi (Glomus intraradices) on the oviposition of rice water weevil (Lissorhoptrus oryzophilus). Mycorrhiza 21, 651–658. doi: 10.1007/s00572-011-0399-6
de Roode, J. C., Rarick, R. M., Mongue, A. J., Gerardo, N. M., and Hunter, M. D. (2011). Aphids indirectly increase virulence and transmission potential of a monarch butterfly parasite by reducing defensive chemistry of a shared food plant. Ecol. Lett. 14, 453–461. doi: 10.1111/j.1461-0248.2011.01604.x
Dobler, S., Dalla, S., Wagschal, V., and Agrawal, A. A. (2012). Community-wide convergent evolution in insect adaptation to toxic cardenolides by substitutions in the Na,K-ATPase. Proc. Natl. Acad. Sci. U.S.A. 109, 13040–13045. doi: 10.1073/pnas.1202111109
Duffey, S. S. (1980). Sequestration of plant natural products by insects. Annu. Rev. Entomol. 25, 447–477. doi: 10.1146/annurev.en.25.010180.002311
Dyer, L. A., and Bowers, M. (1996). The importance of sequestered iridoid glycosides as a defense against an ant predator. J. Chem. Ecol. 22, 1527–1539. doi: 10.1007/BF02027729
Erb, M., and Robert, C. A. (2016). Sequestration of plant secondary metabolites by insect herbivores: molecular mechanisms and ecological consequences. Curr. Opin. Insect Sci. 14, 8–11. doi: 10.1016/j.cois.2015.11.005
Fontana, A., Reichelt, M., Hempel, S., Gershenzon, J., and Unsicker, S. B. (2009). The effects of arbuscular mycorrhizal fungi on direct and indirect defense metabolites of Plantago lanceolata L. J. Chem. Ecol. 35, 833–843. doi: 10.1007/s10886-009-9654-0
Fordyce, J. A., and Malcolm, S. B. (2000). Specialist weevil, Rhyssomatus lineaticollis, does not spatially avoid cardenolide defenses of common milkweed by ovipositing into pith tissue. J. Chem. Ecol. 26, 2857–2874. doi: 10.1023/A:1026450112601
Frick, C., and Wink, M. (1995). Uptake and sequestration of ouabain and other cardiac glycosides in Danaus plexippus (Lepidoptera: Danaidae): evidence for a carrier-mediated process. J. Chem. Ecol. 21, 557–575. doi: 10.1007/BF02033701
Frost, C. J., and Hunter, M. D. (2008). Insect herbivores and their frass affect Quercus rubra leaf quality and initial stages of subsequent litter decomposition. Oikos 117, 13–22. doi: 10.1111/j.2007.0030-1299.16165.x
Gange, A. C., Bower, E., and Brown, V. K. (1999). Positive effects of an arbuscular mycorrhizal fungus on aphid life history traits. Oecologia 120, 123–131. doi: 10.1007/s004420050840
Gange, A. C., Brown, V. K., and Aplin, D. M. (2003). Multitrophic links between arbuscular mycorrhizal fungi and insect parasitoids. Ecol. Lett. 6, 1051–1055. doi: 10.1046/j.1461-0248.2003.00540.x
Gange, A. C., Stagg, P. G., and Ward, L. K. (2002). Arbuscular mycorrhizal fungi affect phytophagous insect specialism. Ecol. Lett. 5, 11–15. doi: 10.1046/j.1461-0248.2002.00299.x
Gange, A. C., and West, H. M. (1994). Interactions between arbuscular mycorrhizal fungi and foliar-feeding insects in Plantago lanceolata L. New Phytol. 128, 79–87. doi: 10.1111/j.1469-8137.1994.tb03989.x
Garrido, E., Bennett, A. E., Fornoni, J., and Strauss, S. Y. (2010). Variation in arbuscular mycorrhizal fungi colonization modifies the expression of tolerance to above-ground defoliation. J. Ecol. 98, 43–49. doi: 10.1111/j.1365-2745.2009.01586.x
Gehring, C. A., and Whitham, T. G. (2002). “Mycorrhizae-herbivore interactions: population and community consequences,” in Mycorrhizal Ecology, eds M. G. A. van der Heijden and I. R. Sanders (Berlin; Heidelberg: Springer), 295–320.
Gehring, C., and Bennett, A. (2009). Mycorrhizal fungal–plant–insect interactions: the importance of a community approach. Environ. Entomol. 38, 93–102. doi: 10.1603/022.038.0111
Goverde, M., van der Heijden, M., Wiemken, A., Sanders, I., and Erhardt, A. (2000). Arbuscular mycorrhizal fungi influence life history traits of a lepidopteran herbivore. Oecologia 125, 362–369. doi: 10.1007/s004420000465
Grabmaier, A., Heigl, F., Eisenhauer, N., van der Heijden, M. G. A., and Zaller, J. G. (2014). Stable isotope labelling of earthworms can help deciphering belowground-aboveground interactions involving earthworms, mycorrhizal fungi, plants and aphids. Pedobiologia 57, 197–203. doi: 10.1016/j.pedobi.2014.10.002
Grman, E. (2012). Plant species differ in their ability to reduce allocation to non-beneficial arbuscular mycorrhizal fungi. Ecology 93, 711–718. doi: 10.1890/11-1358.1
Guerrieri, E., Lingua, G., Digilio, M. C., Massa, N., and Berta, G. (2004). Do interactions between plant roots and the rhizosphere affect parasitoid behaviour? Ecol. Entomol. 29, 753–756. doi: 10.1111/j.0307-6946.2004.00644.x
Gutjahr, C. (2014). Phytohormone signaling in arbuscular mycorhiza development. Curr. Opin. Plant Biol. 20, 26–34. doi: 10.1016/j.pbi.2014.04.003
Hartley, S. E., and Gange, A. C. (2009). Impacts of plant symbiotic fungi on insect herbivores: mutualism in a multitrophic context. Annu. Rev. Entomol. 54, 323–342. doi: 10.1146/annurev.ento.54.110807.090614
Helms, S. E., Connelly, S. J., and Hunter, M. D. (2004). Effects of variation among plant species on the interaction between a herbivore and its parasitoid. Ecol. Entomol. 29, 44–51. doi: 10.1111/j.0307-6946.2004.00566.x
Hempel, S., Stein, C., Unsicker, S. B., Renker, C., Auge, H., Weisser, W. W., et al. (2009). Specific bottom-up effects of arbuscular mycorrhizal fungi across a plant-herbivore-parasitoid system. Oecologia 160, 267–277. doi: 10.1007/s00442-009-1294-0
Hoffmann, D., Vierheilig, H., and Schausberger, P. (2011). Arbuscular mycorrhiza enhances preference of ovipositing predatory mites for direct prey-related cues. Physiol. Entomol. 36, 90–95. doi: 10.1111/j.1365-3032.2010.00751.x
Hunter, M. D. (2003). Effects of plant quality on the population ecology of parasitoids. Agric. For. Entomol. 5, 1–8. doi: 10.1046/j.1461-9563.2003.00168.x
Hunter, M. D. (2016). The Phytochemical Landscape: Linking Trophic Interactions and Nutrient Dynamics. Princeton, NY: Princeton University Press.
Hunter, M. D., and Price, P. W. (1992). Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73, 724–732.
Jamieson, M. A., and Bowers, M. D. (2012). Plant-mediated effects of soil nitrogen enrichment on a chemically defended specialist herbivore, Calophasia lunula. Ecol. Entomol. 37, 300–308. doi: 10.1111/j.1365-2311.2012.01366.x
Jung, S. C., Martinez-Medina, A., Lopez-Raez, J. A., and Pozo, M. J. (2012). Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 38, 651–664. doi: 10.1007/s10886-012-0134-6
Koricheva, J., Gange, A. C., and Jones, T. (2009). Effects of mycorrhizal fungi on insect herbivores: a meta-analysis. Ecology 90, 2088–2097. doi: 10.1890/08-1555.1
Krishna, K. R., Suresh, H. M., Syamsunder, J., and Bagyaraj, D. J. (1981). Changes in the leaves of finger millet due to VA mycorrhizal infection. New Phytol. 87, 717–722. doi: 10.1111/j.1469-8137.1981.tb01706.x
Krüger, M., Stockinger, H., Krüger, C., and Schüßler, A. (2009). DNA-based species level detection of Glomeromycota: one PCR primer set for all arbuscular mycorrhizal fungi. New Phytol. 183, 212–223. doi: 10.1111/j.1469-8137.2009.02835.x
Laird, R. A., and Addicott, J. F. (2008). Neutral indirect effects of mycorrhizal fungi on a specialist insect herbivore. Environ. Entomol. 37, 1017–1024. doi: 10.1093/ee/37.4.1017
Lampert, E. C., Dyer, L. A., and Bowers, M. D. (2014). Dietary specialization and the effects of plant species on potential multitrophic interactions of three species of nymphaline caterpillars. Entomol. Exp. Appl. 153, 207–216. doi: 10.1111/eea.12242
Landis, F. C., Gargas, A., and Givnish, T. J. (2004). Relationships among arbuscular mycorrhizal fungi, vascular plants and environmental conditions in oak savannas. New Phytol. 164, 493–504. doi: 10.1111/j.1469-8137.2004.01202.x
Lefèvre, T., Oliver, L., Hunter, M. D., and De Roode, J. C. (2010). Evidence for trans-generational medication in nature. Ecol. Lett. 13, 1485–1493. doi: 10.1111/j.1461-0248.2010.01537.x
Lendenmann, M., Thonar, C., Barnard, R. L., Salmon, Y., Werner, R. A., Frossard, E., et al. (2011). Symbiont identity matters: carbon and phosphorus fluxes between Medicago truncatula and different arbuscular mycorrhizal fungi. Mycorrhiza 21, 689–702. doi: 10.1007/s00572-011-0371-5
Lucas, W. J., Groover, A., Lichtenberger, R., Furuta, K., Yadav, S. R., Helariutta, Y., et al. (2013). The plant vascular system: evolution, development and functions. J. Integr. Plant Biol. 55, 294–388. doi: 10.1111/jipb.12041
Malcolm, S. B. (1989). Disruption of web structure and predatory behavior of a spider by plant-derived chemical defenses of an aposematic aphid. J. Chem. Ecol. 15, 1699–1716. doi: 10.1007/BF01012259
Malcolm, S. B. (1990). Chemical defence in chewing and sucking insect herbivores: plant-derived cardenolides in the monarch butterfly and oleander aphid. Chemoecology 1, 12–21. doi: 10.1007/BF01240581
Malcolm, S. B. (1992). “Prey defence and predator foraging,” in Natural Enemies, ed M. J. Crawley (Oxford: Blackwell Scientific Publications), 458–475.
Malcolm, S. B. (1994). Milkweeds, monarch butterflies and the ecological significance of cardenolides. Chemoecology 5–6, 101–117. doi: 10.1007/BF01240595
Malcolm, S. B., and Brower, L. P. (1989). Evolutionary and ecological implications of cardenolide sequestration in the monarch butterfly. Experientia 45, 284–295. doi: 10.1007/BF01951814
McCune, B., Grace, J. B., and Urban, D. L. (2002). Analysis of Ecological Communities. Oregon, OR: MjM Software Design.
McGonigle, T. P., Miller, M. H., Evans, D. G., Fairchild, G. L., and Swan, J. A. (1990). A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 115, 495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x
Meixner, C., Ludwig-Müller, J., Miersch, O., Gresshoff, P., Staehelin, C., and Vierheilig, H. (2005). Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta 222, 709–715. doi: 10.1007/s00425-005-0003-4
Öpik, M., Moora, M., Liira, J., and Zobel, M. (2006). Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J. Ecol. 94, 778–790. doi: 10.1111/j.1365-2745.2006.01136.x
Mohl, E. K., Santa-Martinez, E., and Heimpel, G. E. (2016). Interspecific differences in milkweeds alter predator density and the strength of trophic cascades. Arthropod. Plant. Interact. 10, 249–261. doi: 10.1007/s11829-016-9430-3
Mooney, K. A., Jones, P., and Agrawal, A. A. (2008). Coexisting congeners: demography, competition, and interactions with cardenolides for two milkweed-feeding aphids. Oikos 117, 450–458. doi: 10.1111/j.2007.0030-1299.16284.x
Nishida, R. (2002). Sequestration of defensive substances from plants by LepidopterA. Annu. Rev. Entomol. 47, 57–92. doi: 10.1146/annurev.ento.47.091201.145121
Ode, P. J. (2013). “Plant defences and parasitoid chemical ecology,” in Chemical Ecology of Insect Parasitoids, eds E. Wajnberg and S. Colazza (Oxford: John Wiley & Sons, Ltd), 9–36.
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2016). vegan: Community Ecology Package. R package version 2.4-0. Available online at: https://cran.r-project.org/web/packages/vegan/index.html
Opitz, S. E. W., and Müller, C. (2009). Plant chemistry and insect sequestration. Chemoecology 19, 117–154. doi: 10.1007/s00049-009-0018-6
Pacovsky, R. S., Rabin, L. B., Montllor, C. B., and Waiss, A. C. J. (1985). “Host-plant resistance to insect pests altered by Glomus fasciculatum colonization,” in Proceeding of 6th North American Conference Mycorrhiza, ed R. Molina (Corvallis: Oregon State University), 288.
Pappas, M. L., Broufas, G. D., and Koveos, D. S. (2007). Effects of various prey species on development, survival and reproduction of the predatory lacewing Dichochrysa prasina (Neuroptera: Chrysopidae). Biol. Control 43, 163–170. doi: 10.1016/j.biocontrol.2007.07.006
Pasteels, J. M. (1978). Apterous and brachypterous coccinellids at the end of the food chain, Cionura ereca (Asclepiadaceae) - Aphis nerii. Entomol. Exp. Appl. 24, 579–584.
Petschenka, G., and Agrawal, A. A. (2015). Milkweed butterfly resistance to plant toxins is linked to sequestration, not coping with a toxic diet. Proc. R. Soc. B Biol. Sci. 282:2015.1865. doi: 10.1098/rspb.2015.1865
Petschenka, G., and Agrawal, A. A. (2016). How herbivores coopt plant defenses: natural selection, specialization, and sequestration. Curr. Opin. Insect Sci. 14, 17–24. doi: 10.1016/j.cois.2015.12.004
Pieterse, C. M., Van der Does, D., Zamioudis, C., Leon-Reyes, A., and Van Wees, S. C. M. (2012). Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28, 489–521. doi: 10.1146/annurev-cellbio-092910-154055
Pieterse, C. M., Zamioudis, C., Berendsen, R. L., Weller, D. M., Van Wees, S. C., and Bakker, P. A. H. (2014). Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347–375. doi: 10.1146/annurev-phyto-082712-102340
Pringle, E. G., Novo, A., Ableson, I., Barbehenn, R. V., and Vannette, R. L. (2014). Plant-derived differences in the composition of aphid honeydew and their effects on colonies of aphid-tending ants. Ecol. Evol. 4, 4065–4079. doi: 10.1002/ece3.1277
Rasmann, S., and Agrawal, A. A. (2011). Latitudinal patterns in plant defense: evolution of cardenolides, their toxicity and induction following herbivory. Ecol. Lett. 14, 476–483. doi: 10.1111/j.1461-0248.2011.01609.x
Rasmann, S., Bennett, A., Biere, A., Karley, A., and Guerrieri, E. (2017). Root symbionts: powerful drivers of plant above- and belowground indirect defenses. Insect Sci. 24, 947–960. doi: 10.1111/1744-7917.12464
Rasmann, S., Johnson, M. D., and Agrawal, A. A. (2009). Induced responses to herbivory and jasmonate in three milkweed species. J. Chem. Ecol. 35, 1326–1334. doi: 10.1007/s10886-009-9719-0
Reichstein, T., von Euw, J., Parsons, J. A., and Rothschild, M. (1968). Heart poisons in the monarch butterfly. some aposematic butterflies obtain protection from cardenolides present in their food plants. Science 161, 861–866. doi: 10.1126/science.161.3844.861
Riolo, M. A., Rohani, P., and Hunter, M. D. (2015). Local variation in plant quality influences large-scale population dynamics. Oikos 124, 1160–1170. doi: 10.1111/oik.01759
Roger, A., Gétaz, M., Rasmann, S., and Sanders, I. R. (2013). Identity and combinations of arbuscular mycorrhizal fungal isolates influence plant resistance and insect preference. Ecol. Entomol. 38, 330–338. doi: 10.1111/een.12022
Rothschild, M., von Euw, J., and Reichstein, T. (1970). Cardiac glycosides in the oleander aphid, Aphis nerii. J. Insect Physiol. 16, 1141–1145. doi: 10.1016/0022-1910(70)90203-9
Schausberger, P., Peneder, S., Jürschik, S., and Hoffmann, D. (2012). Mycorrhiza changes plant volatiles to attract spider mite enemies. Funct. Ecol. 26, 441–449. doi: 10.1111/j.1365-2435.2011.01947.x
Schmitz, O. J., Hambäck, P. A., and Beckerman, A. P. (2000). Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am. Nat. 155, 141–153. doi: 10.1086/303311
Schneider, C. A., Rasband, W. S., and Eliceiri, K. W. (2012). NIH Image to Image J: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Schoonhoven, L., van Loon, J., and Dicke, M. (2005). Insect-Plant Biology. Oxford: Oxford University Press.
Schweiger, R., Baier, M. C., Persicke, M., and Müller, C. (2014). High specificity in plant leaf metabolic responses to arbuscular mycorrhizA. Nat. Commun. 5:3886. doi: 10.1038/ncomms4886
Schweiger, R., and Müller, C. (2015). Leaf metabolome in arbuscular mycorrhizal symbiosis. Curr. Opin. Plant Biol. 26, 120–126. doi: 10.1016/j.pbi.2015.06.009
Simon, A. L., Wellham, P. A., Aradottir, G. I., and Gange, A. C. (2017). Unravelling mycorrhiza-induced wheat susceptibility to the English grain aphid Sitobion avenae. Sci. Rep. 7:46497. doi: 10.1038/srep46497
Soudzilovskaia, N. A., Douma, J. C., Akhmetzhanova, A. A., van Bodegom, P. M., Cornwell, W. K., Moens, E. J., et al. (2015). Global patterns of plant root colonization intensity by mycorrhizal fungi explained by climate and soil chemistry. Glob. Ecol. Biogeogr. 24, 371–382. doi: 10.1111/geb.12272
Speight, M. R., Hunter, M. D., and Watt, A. D. (2008). Ecology of Insects: Concepts and Applications. 2nd Edn. Oxford: Blackwell Science Ltd.
Staehelin, C., Xie, Z. P., Illana, A., and Vierheilig, H. (2011). Long-distance transport of signals during symbiosis. Plant Signal. Behav. 6, 372–377. doi: 10.4161/psb.6.3.13881
Sternberg, E. D., Lefèvre, T., Li, J., de Castillejo, C. L. F., Li, H., Hunter, M. D., et al. (2012). Food plant derived disease tolerance and resistance in a natural butterfly-plant-parasite interaction. Evolution 66, 3367–3376. doi: 10.1111/j.1558-5646.2012.01693.x
Tao, L., Ahmad, A., de Roode, J. C., and Hunter, M. D. (2016a). Arbuscular mycorrhizal fungi affect plant tolerance and chemical defences to herbivory through different mechanisms. J. Ecol. 104, 561–571. doi: 10.1111/1365-2745.12535
Tao, L., Gowler, C. D., Ahmad, A., Hunter, M. D., and de Roode, J. C. (2015). Disease ecology across soil boundaries: effects of below-ground fungi on above-ground host-parasite interactions. Proc. R. Soc. B Biol. Sci. 282:20151993. doi: 10.1098/rspb.2015.1993
Tao, L., Hoang, K. M., Hunter, M. D., and de Roode, J. C. (2016b). Fitness costs of animal medication: antiparasitic plant chemicals reduce fitness of monarch butterfly hosts. J. Anim. Ecol. 85, 1246–1254. doi: 10.1111/1365-2656.12558
Tao, L., and Hunter, M. D. (2015). Effects of soil nutrients on the sequestration of plant defence chemicals by the specialist insect herbivore, Danaus plexippus. Ecol. Entomol. 40, 123–132. doi: 10.1111/een.12168
Tao, L., Hunter, M. D., and de Roode, J. C. (2017). Microbial root mutualists affect the predators and pathogens of herbivores above ground: mechanisms, magnitudes, and missing links. Front. Ecol. Evol. 5:160. doi: 10.3389/fevo.2017.00160
Thonar, C., Frossard, E., Smilauer, P., and Jansa, J. (2014). Competition and facilitation in synthetic communities of arbuscular mycorrhizal fungi. Mol. Ecol. 23, 733–746. doi: 10.1111/mec.12625
Tomczak, V. V., and Müller, C. (2017). Influence of arbuscular mycorrhizal stage and plant age on the performance of a generalist aphid. J. Insect Physiol. 98, 258–266. doi: 10.1016/j.jinsphys.2017.01.016
van Dam, N. M., and Heil, M. (2011). Multitrophic interactions below and above ground: en route to the next level. J. Ecol. 99, 77–88. doi: 10.1111/j.1365-2745.2010.01761.x
van der Putten, W. H., Vet, L. E. M., Harvey, J. A., and Wäckers, F. L. (2001). Linking above- and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol. Evol. 16, 547–554. doi: 10.1016/S0169-5347(01)02265-0
Vannette, R. L., and Hunter, M. D. (2011). Plant defence theory re-examined: nonlinear expectations based on the costs and benefits of resource mutualisms. J. Ecol. 99, 66–76. doi: 10.1111/j.1365-2745.2010.01755.x
Vannette, R. L., and Hunter, M. D. (2013). Mycorrhizal abundance affects the expression of plant resistance traits and herbivore performance. J. Ecol. 101, 1019–1029. doi: 10.1111/1365-2745.12111
Vannette, R. L., Hunter, M. D., and Rasmann, S. (2013). Arbuscular mycorrhizal fungi alter above- and below-ground chemical defense expression differentially among Asclepias species. Front. Plant Sci. 4:361. doi: 10.3389/fpls.2013.00361
van Veen, F. J. F., and Godfray, H. C. J. (2012). “Consequences of trait changes in host-parasitoid interactions in insect communities,” in Interaction Richness and Complexity: Ecological and Evolutionary Aspects of Trait-Mediated Indirect Interactions, eds T. Ohgushi., O. Schmitz, and R. Holt (Cambridge: Cambridge University Press), 28–46.
Vierheilig, H. (2004). Further root colonization by arbuscular mycorrhizal fungi in already mycorrhizal plants is suppressed after a critical level of root colonization. J. Plant Physiol. 161, 339–341. doi: 10.1078/0176-1617-01097
Vierheilig, H., Garcia-Garrido, J. M., Wyss, U., and Piché, Y. (2000a). Systemic suppression of mycorrhizal colonization of barley roots already colonized by AM fungi. Soil Biol. Biochem. 32, 589–595. doi: 10.1016/s0038-0717(99)00155-8
Vierheilig, H., Maier, W., Wyss, U., Samson, J., Strack, D., and Piché, Y. (2000b). Cyclohexenone derivative- and phosphate-levels in split-root systems and their role in the systemic suppression of mycorrhization in precolonized barley plants. J. Plant Physiol. 157, 593–599. doi: 10.1016/s0176-1617(00)80001-2
Waldbauer, G. P. (1968). The consumption and utilization of food by insects. Adv. Insect Phys. 5, 229–288. doi: 10.1016/S0065-2806(08)60230-1
Wang, B., and Qiu, Y. L. (2006). Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16, 299–363. doi: 10.1007/s00572-005-0033-6
Wang, M., Bezemer, T. M., van der Putten, W. H., and Biere, A. (2015). Effects of the timing of herbivory on plant defense induction and insect performance in ribwort plantain (Plantago lanceolata L.) depend on plant mycorrhizal status. J. Chem. Ecol. 41, 1006–1017. doi: 10.1007/s10886-015-0644-0
Williams, A., Birkhofer, K., and Hedlund, K. (2014). Above- and below-ground interactions with agricultural management: effects of soil microbial communities on barley and aphids. Pedobiologia. 57, 67–74. doi: 10.1016/j.pedobi.2014.01.004
Wolfe, B. E., Mummey, D. L., Rillig, M. C., and Klironomos, J. N. (2007). Small-scale spatial heterogeneity of arbuscular mycorrhizal fungal abundance and community composition in a wetland plant community. Mycorrhiza 17, 175–183. doi: 10.1007/s00572-006-0089-y
Wurst, S., Dugassa-Gobena, D., Langel, R., Bonkowski, M., and Scheu, S. (2004). Combined effects of earthworms and vesicular-arbuscular mycorrhizas on plant and aphid performance. New Phytol. 163, 169–176. doi: 10.1111/j.1469-8137.2004.01106.x
Zalucki, M. P., Brower, L. P., and Alonso, M., A. (2001a). Detrimental effects of latex and cardiac glycosides on survival and growth of first-instar monarch butterfly larvae Danaus plexippus feeding on the sandhill milkweed Asclepias humistrata. Ecol. Entomol. 26, 212–224. doi: 10.1046/j.1365-2311.2001.00313.x
Zalucki, M. P., Malcolm, S. B., Paine, T. D., Hanlon, C. C., Brower, L. P., and Clarke, A. R. (2001b). It's the first bites that count: survival of first-instar monarchs on milkweeds. Austral Ecol. 26, 547–555. doi: 10.1046/j.1442-9993.2001.01132.x
Zehnder, C. B., and Hunter, M. D. (2007). Interspecific variation within the genus Asclepias in response to herbivory by a phloem-feeding insect herbivore. J. Chem. Ecol. 33, 2044–2053. doi: 10.1007/s10886-007-9364-4
Zehnder, C. B., and Hunter, M. D. (2009). More is not necessarily better: the impact of limiting and excessive nutrients on herbivore population growth rates. Ecol. Entomol. 34, 535–543. doi: 10.1111/j.1365-2311.2009.01101.x
Züst, T., and Agrawal, A. A. (2016a). Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2, 1–9. doi: 10.1038/nplants.2015.206
Keywords: above-belowground interactions, sequestration, plant-herbivore interactions, plant-microbe interactions, arbuscular mycorrhizal fungi (AMF), Asclepias, Danaus plexippus, Aphis nerii
Citation: Meier AR and Hunter MD (2018) Mycorrhizae Alter Toxin Sequestration and Performance of Two Specialist Herbivores. Front. Ecol. Evol. 6:33. doi: 10.3389/fevo.2018.00033
Received: 08 January 2018; Accepted: 19 March 2018;
Published: 23 April 2018.
Edited by:
Ainhoa Martinez Medina, German Center for Integrative Biodiversity Research, GermanyReviewed by:
Maria Pappas, Democritus University of Thrace, GreeceArjen Biere, Netherlands Institute of Ecology (NIOO-KNAW), Netherlands
Copyright © 2018 Meier and Hunter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda R. Meier, armeier@umich.edu
 Amanda R. Meier
Amanda R. Meier Mark D. Hunter
Mark D. Hunter