A Review of the Factors That Determine Whether Stormwater Ponds Are Ecological Traps And/or High-Quality Breeding Sites for Amphibians
- Pantheon-Sorbonne University, Paris, France
Stormwater ponds were originally constructed to control the quantity and quality of runoff on urban roads and highways before it was released to the environment. Often, stormwater ponds were designed in a technical feat of civil engineering, with no particular ecological or landscape objective in mind. Nevertheless, they are colonized spontaneously by diverse species, including amphibians. Through an initial review of the scientific literature, the objective of this study was to understand which factors determine whether a pond can be considered as an ecological trap or a valuable breeding site for amphibians. The first step was to question the role of the pond environment as a major factor in its colonization by amphibians, demonstrating that not all ponds are colonized by the same variety of species. The internal factors in the ponds that define them as ecological traps or sustainable breeding sites for amphibians was also considered. After confirming the functional and structural similarity between highway and urban stormwater ponds, 25 publications were compared, with study sites mostly located in Europe and North America, which concern the colonization of stormwater ponds by amphibians in urban or highway areas. Several factors were identified that may affect the ecological viability of these basins: (1) the factors related to the shape of the ponds (inclination of the banks, materials used, etc.,); (2) the biotic factors (aquatic vegetation, presence of predators, etc.,); (3) the abiotic factors (luminosity, water level in the ponds, etc.,); and (4) water pollutants. The low number of publications on this subject, as well as the low variety in the location of study sites, only allow cautious conclusions to be drawn. In particular, stormwater ponds located in highly anthropogenic landscapes can be both ecological traps and suitable habitats for amphibian breeding. This depends on the species that colonize each pond, many internal factors, and the environmental context in which it is embedded. Additional research is therefore needed in other parts of the world—particularly in amphibian biodiversity hotspots—as well as other impact factors such as the effects of different maintenance practices.
Introduction
There is no doubt that there is an increase of the extension of the urbanized land and transport infrastructure. The World Urbanization Prospects report notes that “among 233 countries or areas, just 24 per cent had levels of urbanization greater than 50 per cent in 1950 and only 8 per cent were more than 75 per cent urban. By 2014, 63 per cent of countries were more than half urban and one-third was more than 75 percent urban” (United Nations, 2014). This trend is accompanied by a rise in the number of stormwater ponds in the urban landscape. This has resulted in the creation of a vast network of wetland micro-zones and ecological spaces, which have been quickly colonized by a wide variety of organisms (Scher, 2005), including various amphibian species (Le Viol et al., 2009, 2012; Simon et al., 2009; McCarthy and Lathrop, 2011).
The main role of stormwater ponds is to reduce the environmental impact of water pollution by controlling the quantity and quality of water that is discharged into the receiving environment (Skriabine et al., 2004; Andrews et al., 2015). They are defined in literature as moderately-open surfaces and deep-water systems, initially built to control water runoff and pollution (Scher, 2005; Fayoux and Pelletier, 2009; Le Viol et al., 2009, 2012; Tixier et al., 2011). As Geai et al. (1997) clearly explain, the traditional design of highway ponds is essentially based on technical recommendations from civil engineering, which favors the construction of ponds with regular geometric shapes and varying slope inclination, made of artificial materials (often concrete) that often do not take into account the pond's aesthetic or ecological quality. Two types of stormwater ponds may be distinguished: those located in urban areas and those located on the edge of highways. When the biotic and abiotic characteristics of highway and urban stormwater ponds are compared (see Table in Supplementary Materials), similarities can be found. Although both tend to have a similar average size, the size of stormwater ponds can vary from 173 to 7,000 m2 for highways ponds (Scher, 2005; Simon et al., 2009; Pohl et al., 2015) and from 49 to 14,784 m2 for urban ponds (Bishop et al., 2000; Simon et al., 2009; Brand and Snodgrass, 2010; Scheffers and Paszkowski, 2013). Aquatic vegetation generally covers part of the pond water surface, with an average of 55% for highway ponds (Le Viol et al., 2009) and 38% for urban ponds (Bishop et al., 2000; Gledhill et al., 2008; Scheffers and Paszkowski, 2013; Holzer, 2014). Fish are found in 28% of the highway ponds (Le Viol et al., 2009) and 25% of urban ponds (Holzer, 2014). Water features are often similar, with temperatures in temperate regions hovering around 16°C in March (Scher, 2005; Gallagher et al., 2014), a slightly basic pH and a conductivity of around 0.80 mS/cm (e.g., Scher, 2005; Gledhill et al., 2008; Le Viol et al., 2012; Pohl et al., 2015). Whether on highways or in urban areas, a similar proportion of each category of ponds can be found, characterized by the variation of the water level. On highways, Le Viol et al. (2012) found 58% of permanent ponds and 42% of temporary ponds while Scher (2005) found 16% of permanent ponds, 66% of semi-permanent ponds and 18% of temporary ponds. In urban areas, Gallagher et al. (2014) found 36.5% of temporary ponds, 20.6 % of seasonal ponds and 42.8 % of quasi-permanent ponds while Holzer (2014) found 43.5 % of temporary ponds and 56.4% of permanent ponds. Dissolved oxygen levels are quite different. In highway ponds, Pohl et al. (2015) and Scher (2005) found 108% on average. In urban ponds, Gledhill et al. (2008) found 50% but this difference can be due to the fact that only one publication mentions this rate for urban ponds. Finally, in urban ponds, (Scheffers and Paszkowski, 2013; Gallagher et al., 2014; Holzer, 2014) found on average 54 mg/kg of chromium, 29.6 mg/kg of nitrate, 51.3 mg/kg of copper, 212.5 mg/kg of zinc, 0.3 mg/kg of cadmium, 30.1 mg/kg of lead and 662 mg/kg of carbon-hydrogen. There is no data on highway ponds. Although they were not built to host biodiversity, stormwater ponds are colonized by many species, both flora and fauna, common or rare (e.g., Bishop et al., 2000; Ackley and Meylan, 2010; Le Viol et al., 2012; Moore and Hunt, 2012). In some conditions, stormwater ponds biodiversity has been considered equivalent to that of semi-natural wetlands (Hassall and Anderson, 2015).
The 12th session of the Ramsar Convention on Wetlands, held in Uruguay in June 2015, estimated that the global extent of wetlands had declined between 64% and 71% in the twentieth century and that wetland losses and degradation continue worldwide (Gardner et al., 2015). According to Bateman (2014), wetland decline is accompanied by a significant decrease in the global population of amphibians, especially over the past few decades (Blaustein et al., 1994; Houlahan and Findlay, 2003; Dodd, 2010). Because of their biphasic lifestyles, amphibians are subject to both aquatic and terrestrial threats, including habitat loss and degradation (e.g., Berger et al., 1998; Dodd and Cade, 1998; Thomas et al., 2004; Todd et al., 2009; Becker et al., 2010; Bancroft et al., 2011). According to Hayes et al. (2010), death and reproductive failure are the two immediate causes of amphibian decline. Atmospheric change, environmental pollutants, habitat modification, and invasive species are considered as the 4 indirect factors contributing to amphibian decline caused by reproductive failure (Hayes et al., 2010). It is therefore not uncommon to notice the presence of several species of amphibians in urban or highway stormwater ponds. One of the first reactions of the pond managers to this spontaneous colonization was trying to prevent them from entering the ponds, particularly with nets or screens. Few studies have been done to confirm or refute their effectiveness. Another solution to be considered is the possibility to design and maintain these ponds to be viable sites for amphibian reproduction but it is first necessary to determine the features that have an influence on whether the ponds are ecological traps or valuable breeding sites for amphibians.
The concept of ecological trap was first described by Dwernychuk and Boag (1972), but has only been studied in recent years (Battin, 2004). According to Brand and Snodgrass (2010), considering the principle that organisms select high-quality habitats from environmental signals, an ecological trap occurs when environmental clues provide an inaccurate representation of a habitat's suitability for reproduction and survival (Schlaepfer et al., 2002; Battin, 2004; Robertson and Hutto, 2006). A stormwater pond could be defined as an ecological trap if it leads to direct mortality of individuals or if, as a breeding site, reproductive success is not high enough to support a stable or growing population without immigration (Battin, 2004). Due to the proximity of roads that may cause an increase of water pollutants in the pond and also other impacts such as a barrier effect and an increased risk of mortality, highway stormwater ponds do not appear to be suitable breeding sites for amphibians. The presence of water during the breeding period (from spring to early summer) and of vegetation in the ponds may attract amphibians, thus turning these ponds into ecological traps (Schlaepfer et al., 2002; Battin, 2004; Robertson and Hutto, 2006; Brand and Snodgrass, 2010). The purpose of this literature review is to ascertain to which extent stormwater ponds are colonized by amphibians and to identify the factors that would make a stormwater pond an ecological trap vs. a viable breeding site.
Methods and Materials
The selection of papers to be evaluated in the study was undertaken using several databases specialized in ecology and geography (Science Direct, Springer, Jstor). Words used in the search were “stormwater pond” (1980 results) associated with “amphibians” (96 results), “highways” (390 results) and “road” (890 results). The publications studied cover all types of environmental contexts (agricultural, urban, and forest environments), though most of them are located in developed countries, in particular on the European and North American continents. Though the subject of this study concerns highway stormwater ponds and amphibians, the limited number of publications on this subject (5) resulted in a complementary search through other publications referring to “urban stormwater ponds”. As highways and urban ponds show high similarities (see Table in Supplementary Materials), 25 publications were selected on both urban and highway ponds: 50% of these publications were from journals of biology, 36% from conservation ecology journals and 14% from environmental science journals, the categories of journals having been determined according to the keywords used. Then, using this bibliographic database from Science Direct, Springer, Jstor as well as a complementary panel of publications on the ecology of amphibians (46), several tables were built to compare the species identified in ponds and the factors influencing—both positively and negatively—the species richness present in the stormwater ponds. There are many factors that can influence the ecological viability of stormwater ponds as a breeding site for amphibians. The effect of 4 categories of factors has been analyzed according to the definitions proposed by Jumeau (2017): (1) the factors related to the design of the ponds (inclination of the banks, materials used, etc.,); (2) the biotic factors (aquatic vegetation, presence of predators, etc.,); (3) the so-called “immediate” factors (luminosity, winds, water level in the ponds, etc.,) and (4) water pollutants. This results in a total of 77 factors. However, some of these factors have not been studied in other research. Therefore, only 37 factors were included in the comparison as they were considered in at least 2 publications.
Results
The Number of Colonizing Species Depends on the Pond's Environment
44 amphibian species were identified colonizing stormwater ponds in the 25 publications included in the analysis (Table 1). Three publications on highway ponds referred to 13 amphibian species. 22 publications on urban ponds referred to 37 amphibian species and 17 of these species are cited in at least 2 publications. 6 species were found to be the most common to both urban and highway ponds: the green frog (Rana clamitans) and the pickerel frog (Rana palustris), which were identified in 5 studies; the American toad (Bufo americanus) and the green frog (R. clamitans) identified in 6 studies; the spring peeper (Pseudacris crucifer) and the wood frog (Rana sylvatica), which were identified in 7 studies.
Despite these findings, several articles have reported that species richness depends on the pond's environment and can therefore vary from one site to another. The importance of the landscape matrix for amphibians is regularly examined in the literature, within a radius of up to 500 m around the ponds (e.g., Semlitsch and Bodie, 2003; Simon et al., 2009). The species richness of amphibians is often correlated with a small impervious surface, the proximity to woodlands (Dodd and Cade, 1998; Le Viol et al., 2009, 2012; McCarthy and Lathrop, 2011) and the presence of a dispersal corridor in the case where the breeding site is not directly connected with woodlands (Semlitsch and Bodie, 2003; Ouellet and Leheurteux, 2007; Hamer and McDonnell, 2008; McCarthy and Lathrop, 2011). A terrestrial suitable habitat also provides food and the necessary overwintering sites for amphibians to survive (deMaynadier and Hunter, 1995).
Many studies point out that the density of forest cover has a moderate influence on the presence of amphibians in ponds (Bishop et al., 2000), but this influence varies according to species (e.g., Simon et al., 2009; Birx-Raybuck et al., 2010; Le Viol et al., 2012; Holzer, 2014). As shown by Gallagher et al. (2014), sensitive species such as the wood frog (R. sylvatica) occupy only ponds surrounded by a high proportion of forest cover, contrary to more tolerant species such as toads (Scher and Thièry, 2005; Simon et al., 2009). Thus, the literature review shows a correlation between the decrease in forest cover in the surrounding landscape and the decrease in the amphibian species richness identified in the ponds (Le Viol et al., 2009, 2012; Simon et al., 2009). Conversely they demonstrate that species richness and the occurrence of individual species were negatively related to impervious built surface cover (Scher and Thièry, 2005; Simon et al., 2009). The surrounding agricultural matrix is also identified as having a negative influence on species richness, particularly where intensive farming is practiced, probably due to the release of fertilizers and pesticides (Beja and Alcazar, 2003; Le Viol et al., 2012) and/or because it creates a break in the connectivity of the pond and the natural habitat that amphibians depend on (Trenham et al., 2003; Parris, 2006).
Stormwater ponds therefore host a wide variety of amphibian species, but species richness may vary depending on the presence of more or less appropriate amphibian habitats in their surroundings.
Factors That Positively or Negatively Influence the Ecological Viability of Stormwater Ponds as Breeding Sites
The factors that can affect the viability of stormwater ponds as good breeding sites for amphibians cannot be overlooked. Indeed, although they host many amphibians, stormwater ponds are not necessarily favorable habitats for the species that breed there.
Water pollution is one of the most studied factors in the relationship between stormwater ponds and amphibians (Table 3). Pollutants such as road salt may have sub-lethal effects on amphibians, which could lead to death in the long term (e.g., Bishop et al., 2000; Sanzo and Hecnar, 2006; Karraker et al., 2008; Snodgrass et al., 2008; Collins and Russell, 2009). However, some authors point out that road salt remains a factor that can slow down the development of the larvae but that it is not decisive in assessing the viability of the ponds as amphibian breeding sites (Scher and Thièry, 2005; Brand et al., 2010; Hassall and Anderson, 2015). Moderate levels of nitrogen in ponds appear to have little or no direct risk on the development of amphibian embryos and larvae identified on site (Mayer et al., 1996; Bishop et al., 2000; Massal et al., 2007). Snodgrass et al. (2008) point out that the impact of pollution on the populations of amphibians present in the ponds studied depends on the tolerance of each species to each of the pollutants. For example, nitrate () emissions from cars may be an important nutrient for aquatic vegetation (Camargo et al., 2005 in Holzer, 2014). Conversely, high levels of nitrate may have detrimental effects on amphibian larvae due to its toxicity or due to anoxia resulting from eutrophication (Marco et al., 1999; Hatch and Blaustein, 2003; Holzer, 2014). The impact of the nitrate levels also varies positively (Scheffers and Paszkowski, 2013) or negatively (Houlahan and Findlay, 2003) according to the species considered. In fact, stormwater ponds containing moderate nitrate levels may be suitable for the breeding and development of amphibian larvae because it contributes to the development of algae, micro-organisms, and decaying material which amphibian larvae feed on (Duguet et al., 2003; Pohl et al., 2015). Neighboring agricultural areas can also contribute to the development of algae in ponds through the runoff of nitrate-rich fertilizer (Beja and Alcazar, 2003). The odors produced by algae proliferation attract frogs when they're on a reproductive migration (Savage, 1961; Grubb, 1973, 1975; McCarthy and Lathrop, 2011). Algae also nourish amphibian larvae (Bateman, 2014; Holzer, 2014). However, nitrates may also be harmful when present in high concentration, as excessive algae growth can lead to eutrophication (Bishop et al., 2000). In conclusion, agricultural land-use near ponds can have a variable impact, depending on agricultural practices and the sensibility of amphibian species to euthophication (Le Viol et al., 2012).
Some studies point to hydroperiod (Tables 2, 3) as a factor affecting species present on studied sites (Hamer and McDonnell, 2008; Chester and Robson, 2013). Hydroperiod is defined as the time of inundation during which the soil becomes saturated in water, resulting in anoxia (Bonis, 2014). These alternations of flood and wet stages can lead to the coexistence of species with a wide range of tolerance and ecological requirements (Bonis, 2014). The literature has shown that a too short hydroperiod can be harmful to species that have a long developing period, which are unable to reach metamorphosis before the pond dries. Those species die from dehydration (Ostergaard et al., 2008; Brand and Snodgrass, 2010; McCarthy and Lathrop, 2011). Conversely, a too long hydroperiod (i.e., a prolonged duration of submersion) is often associated with the presence of fish, which represent a significant risk of predation for the communities of amphibians present in ponds (e.g., Hamer and Parris, 2011).
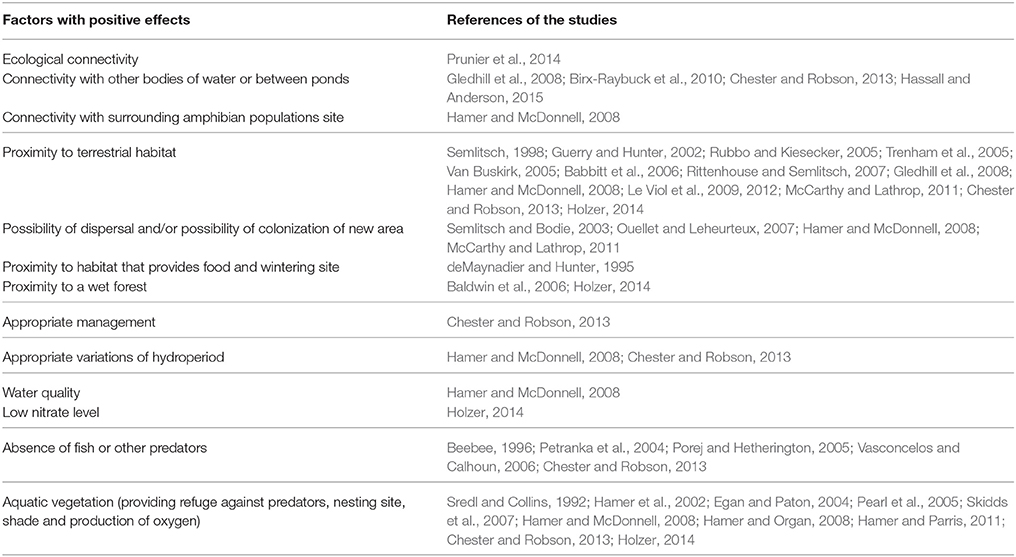
Table 2. Factors identified in the literature review that have a positive effect on the use of stormwater ponds as breeding sites for amphibians.
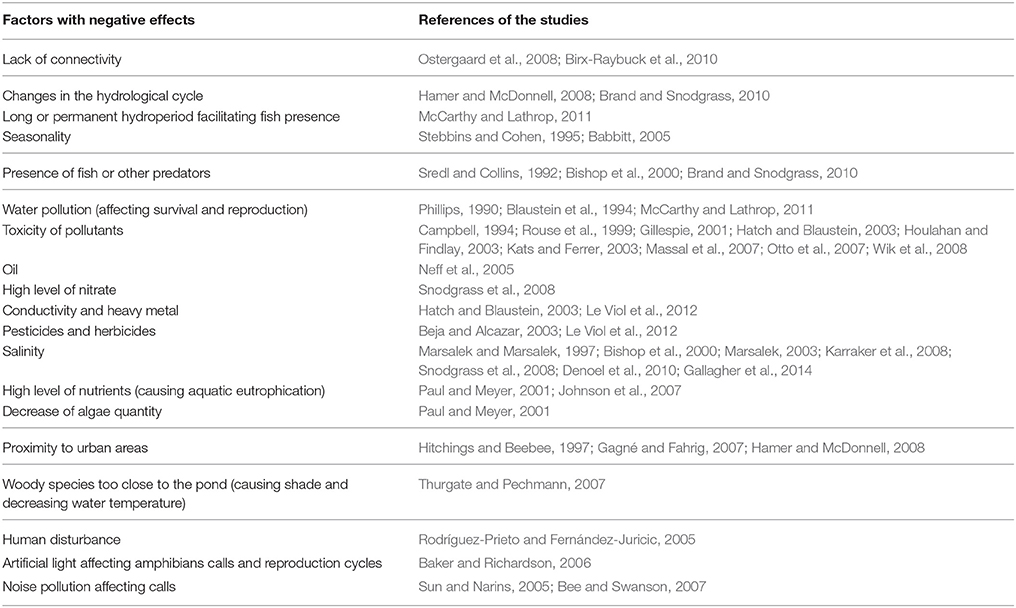
Table 3. Factors identified in the literature review that have a negative effect on the use of stormwater ponds as breeding sites for amphibians.
A comparison of both Tables 2, 3 shows a divergence of opinions on the predation issue. The absence of fish is considered a positive factor in five studies (Beebee, 1996; Petranka et al., 2004; Porej and Hetherington, 2005; Vasconcelos and Calhoun, 2006; Chester and Robson, 2013) and a negative factor in three other studies (Sredl and Collins, 1992; Bishop et al., 2000; Brand and Snodgrass, 2010). The presence of fish has direct and indirect negative impacts on frog larvae (e.g., Porej and Hetherington, 2005; Hamer and Parris, 2011) but some species show resistance to fish predation (Kats et al., 1988; Gunzburger and Travis, 2005; McCarthy and Lathrop, 2011). Brand and Snodgrass (2010) recommend a seasonal hydroperiod, natural or artificial, with a late drainage (i.e., at the end of summer), to increase the suitability of stormwater ponds as amphibian breeding sites. In addition, this practice can be adopted in the maintenance of highway and urban ponds in temperate areas where spring and autumn periods show high levels of rainfall while in summer they have less precipitation.
The pond banks inclination is cited in the literature as a characteristic that can make ponds traps for amphibians (Table 3). For example, Parris (2006) registers, on the basis of a predictive model, a decrease of more than 40% in the species richness measured in stormwater ponds due to the presence of a vertical wall. Other factors are mentioned as having an effect on the amphibian presence in stormwater ponds, but do not appear to be conclusive. This is the case for the age of ponds (e.g., Birx-Raybuck et al., 2010; Pohl et al., 2015). Although Birx-Raybuck et al. (2010) identified the presence of amphibians in recent ponds, some species may be slower to colonize new wetlands and are therefore likely to occupy older ponds. The presence of aquatic vegetation within the pond studied is also mentioned as a factor favorable for amphibian development (Table 2), without being defined as a determining factor (Brand and Snodgrass, 2010; Hamer and Parris, 2011; Scheffers and Paszkowski, 2013). Similarly, the connectivity of the ponds studied to the surrounding wetlands (Tables 2, 3) is also correlated to the species richness and abundance (Gledhill et al., 2008; Birx-Raybuck et al., 2010; McCarthy and Lathrop, 2011; Hassall and Anderson, 2015).
Certain Negative Factors May Be Modified by Stormwater Pond Managers to Make Them Ecologically Viable for Amphibian Breeding
As previously mentioned in the results, some factors influencing the suitability of stormwater ponds as amphibian breeding sites can be modified during pond maintenance operations (e.g., the presence of predators, hydroperiod or pollution accumulation) (e.g., Snodgrass et al., 2008; Birx-Raybuck et al., 2010; Brand and Snodgrass, 2010; Hamer and Parris, 2011). The connectivity between the ponds and suitable natural habitats in the surroundings can be enhanced by vegetation management (Hamer and Parris, 2011; McCarthy and Lathrop, 2011). Similarly, the connectivity of the studied ponds to other wetland habitats (including other stormwater ponds) can be enhanced by vegetation management (Gledhill et al., 2008; Birx-Raybuck et al., 2010; Chester and Robson, 2013; Hassall and Anderson, 2015) in order to support the creation of a dispersal corridor and form a network (Hamer and McDonnell, 2008; Le Viol et al., 2009; Hamer and Parris, 2011). Bateman (2014) suggests that ponds be managed in groups rather than individually to ensure that the habitat requirements of the different species are respected, while improving the species richness on a regional scale.
Finally, other authors Geai et al. (1997); Chang et al. (2011), and Scheffers and Paszkowski (2013) recommend the construction of gently sloping banks, which facilitate the growth of aquatic and semi-aquatic vegetation, as is already the case in many ponds. Chang et al. (2011) recommend that ponds with vegetated banks have slopes ≦ 45°, those designed with concrete subtract ≦ 60° and those designed with clay ≦ 30° in order to allow amphibians to climb more easily. In addition to facilitating the entry and exit of amphibians, a structure covered by vegetation provides shelter against predators (Geai et al., 1997; Scheffers and Paszkowski, 2013) and facilitates access for maintenance (Geai et al., 1997).
Discussion
This literature review demonstrates that the viability of stormwater ponds as breeding sites depends largely on characteristics of ponds and their surroundings but also on the ecology of the colonizing species. While water pollution seems to be one of the main characteristics for defining stormwater ponds as ecological traps for amphibians (Bishop et al., 2000; Collins and Russell, 2009; Gallagher et al., 2014), many studies show that its effect varies depending on species and pollution levels, (McCarthy and Lathrop, 2011; Bateman, 2014) even though some pollutants do not directly threaten the development of amphibian embryos and larvae (Massal et al., 2007). We could therefore conclude that stormwater ponds may constitute suitable additional or alternative breeding sites for pollutant-tolerant species (Snodgrass et al., 2008; Holzer, 2014; Pohl et al., 2015). A low level of water pollution, such as a low presence of nitrate, may also be positive for amphibians because it contributes to the development of microorganisms that larvae feed on. However, there is a need to establish which levels of pollution can be tolerated, and by which species.
Thus, for several factors such as pollution levels or hydroperiod, it is difficult to make precise recommendations because of the heterogeneity of evaluation criteria presented in the publications, which limits the comparisons. A good example of this heterogeneity is the characterization of ponds in terms of hydroperiod variations. Stormwater ponds can be divided into two categories: temporary or permanent (Le Viol et al., 2012; Holzer, 2014); or seasonal or semi-permanent (Brand and Snodgrass, 2010). However, the classification may be more subtle and may include three categories based on annual observations of ponds. Scher and Thièry (2005) suggest a classification of highway ponds according to whether they are always full (permanent), have submerged depths (semi-permanent), or have a total drying phase exceeding 1 month (temporary). Similarly, Gallagher et al. (2014) define three categories of urban ponds according to the duration of flooding, which can be considered temporary (<50% of the time), seasonal (50–90% of the time) or quasi-permanent (>90% of the time) ponds. Thus, the categories of hydroperiods show a high variation in stormwater ponds (Scher and Thièry, 2005; Brand and Snodgrass, 2010; Le Viol et al., 2012; Gallagher et al., 2014; Holzer, 2014) and is probably the determining factor in the suitability of ponds as habitats for amphibians. Nevertheless, the lack of homogeneity within the hydroperiod classification does not allow the comparison of the results published.
Concerning the surrounding land, the negative influence of the adjacent intensive agricultural areas on the presence of amphibians in stormwater ponds can be explained by a strong tendency of these species to avoid these areas (Joly et al., 2001; Rothermel and Semlitsch, 2002). These agricultural land appear to be obstacles to species dispersal. In addition, they can cause a high concentration of pesticides in the water, which can be lethal for some species (Sparling et al., 2001). On the other hand, the positive influence of the presence of other wetlands near the ponds can be explained by the fact that the size of the regional population is often small. Consequently, the persistence of these populations depends on functional metapopulations composed of a network of different ponds (Semlitsch and Bodie, 2003). Finally, the positive influence of a forest environment can be explained by the fact that many species require habitats covered by natural vegetation where they can find refuge and food, such as forests. The close proximity of the pond to a forest environment leads to a lower cost of dispersal for amphibians during seasonal migration phases (Bonte et al., 2012). Otherwise, most of the data presented in the publications analyzed here concerning the pond environment are studied within a radius of 500 m, considered as the distance of influence of an environment in relation to a pond (Simon et al., 2009). This distance is justified by the fact that dispersal movements may range from several hundred meters to one kilometer (Joly and Grolet, 1996; Denoel, 2005; Kovar et al., 2009). Nevertheless, the area studied should be adapted regarding the dispersal ability of each species.
It is important to underline the low number of publications and their restricted location as a large majority of the articles relate to studies conducted on sites located in Europe and North America. Stormwater ponds are used around the world in a variety of forms and environmental contexts that are currently difficult to evaluate in a review article as the literature does not provide information about functionality, maintenance practices and environment features surrounding the pond. Additional studies are needed, including multi-factorial studies to investigate the influence of the combination of factors listed in this article on amphibian development.
It is also necessary to conduct more targeted studies on the different types of stormwater ponds whose operational objectives and management methods may vary. It might be relevant to include a wider range of factors in the analysis and to make comparative studies between different types of ponds. The difficulty in drawing accurate conclusions also comes from the lack of homogeneity in the few publications concerning this subject as shown with the example of the term “hydroperiod.”
Therefore, it is difficult to state on the basis of this review, that stormwater ponds are or are not ecological traps, insofar as this depends on many criteria that vary according to the type of pond, its design, the climate and the land uses in the areas adjacent to the pond's location. The ecology of the species that colonize it is also an important factor. However, it is interesting to note that several authors point out that stormwater ponds could be beneficial breeding sites for amphibian species (Bishop et al., 2000), especially in man-made landscapes where aquatic habitats are increasingly rare (Le Viol et al., 2009, 2012; Gallagher et al., 2014). If so, they could make a substantial contribution toward enhancing local and even regional biodiversity (Gledhill et al., 2008).
It is important to remark that many factors that can affect the sustainability of stormwater ponds to provide quality habitat for amphibians have not been studied. This review of the scientific literature raises the question of whether or not stormwater pond maintenance practices can play a role in the adaptation of the ponds as breeding sites for amphibians (Hamer and Parris, 2011; Gallagher et al., 2014; Hassall and Anderson, 2015).
In Tables 2, 3, pond maintenance is only noted as a potential positive factor and is absent from the negative factors (Chester and Robson, 2013). The different maintenance practices include dredging the pond (i.e., removing the sludge that accumulates at the bottom of the ponds) and clearing vegetation (IFSTTAR-LCPC., 2006; Le Viol et al., 2009). Dredging can have a deadly impact on amphibian populations present in the pond if the activity is carried out during the breeding season. Nevertheless, it can also have other potential benefits because it prevents the pond from being filled by mud and consequently from drying out (Duguet et al., 2003; Ruban et al., 2003). In addition, it prevents the pond from the proliferation of invasive species. The management of stormwater ponds also involves vegetation control in and around the ponds. Although aquatic vegetation plays a positive role in spawning and providing refuges against predators (Duguet et al., 2003), an excessive development can result in eutrophication as well as difficulties in water circulation (Hamer and Parris, 2011). There is a need to find a balance between ecological and technical management in order to support the development of amphibian populations without hindering the functioning of the ponds. Further studies are needed to reconsider pond management and to identify the best practices to reduce the negative factors and to enhance positive ones for amphibian development in stormwater ponds.
This literature review suggests a possible compatibility between a purely technical management and ecological management of ponds that can benefit amphibians while maintaining the functionality of the pond. In addition, this review highlights the importance of a proper vegetation management to link these ponds to a terrestrial habitat, which is necessary for the lifecycle of amphibians. Finally, it seems important to consider the temporality of the process in the analysis of the suitability of stormwater ponds as habitats for amphibians. Whether it be short-term because of a variable hydroperiod, or long-term because of the need to be cleaned regularly to avoid filling, the question remains: Can stormwater ponds be considered temporary wetlands, such as the natural temporary Mediterranean pools mentioned by Babbitt and Tanner (2000); Beja and Alcazar (2003); Jakob et al. (2003); Bagella et al. (2010); Ruhí et al. (2012) or the continental pools mentioned by Lukács et al. (2013)? If so, should these ponds be included in local or regional plans to enhance biodiversity?
However, it is possible to conclude that stormwater ponds located in highly anthropogenic landscapes, as is the case in Europe and North America, can be both ecological traps and suitable habitats for amphibian breeding, depending on a number of factors, including the species that colonize them, pond design, and the environmental context in which they are embedded. Additional studies are therefore needed in other parts of the world, particularly where amphibian biodiversity hotspots are located, but also on possible management and maintenance practices and how to link stormwater ponds to quality terrestrial habitats through the creation of ecological corridors.
Author Contributions
LC, CC and PP: Contributed conception and design of the study; LC: Organized the database; LC: Performed the statistical analysis; LC: Wrote the first draft of the manuscript; LC: Wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This contribution would have been impossible without the support of Karine Tourret and Eiffage group. The authors would also like to thank Constance Schéré, Paul Boos, Eric Bezault and Neil Minkley for their language review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00040/full#supplementary-material
References
Ackley, J. W., and Meylan, P. A. (2010). Watersnake Eden: use of stormwater retention ponds by mangrove salt marsh snakes (Nerodia clarkii compressicauda) in urban Florida. Her Petol. Conserv. Biol. 5, 17–22.
Andrews, K. M., Nanjappa, P., and Riley, S. P. (Eds.). (2015). Roads and Ecological Infrastructure: Concepts and Applications for Small Animals. Baltimore, MD: JHU Press.
Babbitt, K. J. (2005). The relative importance of wetland size and hydroperiod for amphibians in southern New Hampshire, USA. Wetlands Ecol. Manag. 13, 269–179. doi: 10.1007/s11273-004-7521-x
Babbitt, K. J., Baber, M. J., and Brandt, L. A. (2006). The effect of woodland proximity and wetland characteristics on larval anuran assemblages in an agricultural landscape. Can. J. Zool. 84, 510–519. doi: 10.1139/z06-020
Babbitt, K. J., and Tanner, G. W. (2000). Use of temporary wetlands by anurans in a hydrologically modified landscape. Wetlands 20, 313–322. doi: 10.1672/0277-5212(2000)020[0313:UOTWBA]2.0.CO;2
Bagella, S., Caria, M. C., and Zuccarello, V. (2010). Patterns of emblematic habitat types in Mediterranean temporary wetlands. C. R. Biol. 333, 694–700. doi: 10.1016/j.crvi.2010.06.006
Baker, B. J., and Richardson, J. M. L. (2006). The effect of artificial light on male breeding-season behaviour in green frogs, Rana clamitans melanota. Can. J. Zool. 84, 1528–1532. doi: 10.1139/z06-142
Baldwin, R. F., Calhoun, A. J. K., and deMaynadier, P. G. (2006). Conservation planning for amphibian species with complex habitat requirements: a case study using movements and habitat selection of the Wood Frog Rana sylvatica. J. Herpetol. 40, 442–453. doi: 10.1670/0022-1511(2006)40[442:CPFASW]2.0.CO;2
Bancroft, B., Han, B., Searle, C., Biga, L., Olson, D., Kats, D., et al. (2011). Species-level correlates of susceptibility to the pathogenic amphibian fungus Batrachochytrium dendrobates in the United States. Biodiver. Conserv. 20, 1911–1920. doi: 10.1007/s10531-011-0066-4
Bateman, J. A. (2014). Effects of Stormwater Ponds on Calling Amphibian Communities in Monroe County, NY. thesis, Environmental Science and Biology, 65. Available online at: https://digitalcommons.brockport.edu/env_theses/91
Battin, J. (2004). When good animals love bad habitats: ecological traps and the conservation of animal populations. Conserv. Biol. 8, 1482–1491. doi: 10.1111/j.1523-1739.2004.00417
Becker, C. G., Fonseca, C. R., Haddad, C. F. B., and Prado, P. I. (2010). Habitat split as a cause of local population declines of amphibians with aquatic larvae: contributed paper. Conserv. Biol. 24, 287–294. doi: 10.1111/j.1523-1739.2009.01324.x
Bee, M. A., and Swanson, E. M. (2007). Auditory masking of anuran advertisement calls by road traffic noise. Anim. Behav. 74, 1765–1776. doi: 10.1016/j.anbehav.2007.03.019
Beja, P., and Alcazar, R. (2003). Conservation of Mediterranean temporary ponds under agricultural intensification: an evaluation using amphibians. Biol. Conserv. 114, 317–326. doi: 10.1016/S0006-3207(03)00051-X
Berger, L., Speare, R., Daszak, P., Green, D. E., Cunningham, A. A., Goggin, C. L., et al. (1998). Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. U.S.A. 95, 9031–9036. doi: 10.1073/pnas.95.15.9031
Birx-Raybuck, D. A., Price, S. J., and Dorcas, M. E. (2010). Pond age and riparian zone proximity influence anuran occupancy of urban retention ponds. Urban Ecosyst. 13, 181–190. doi: 10.1007/s11252-009-0116-9
Bishop, C. A., Struger, J., Barton, D. R., Shirose, L. J., Dunn, L., Lang, A. L., et al. (2000). Contamination and wildlife communities in stormwater detention ponds in Guelph and the Greater Toronto Area, Ontario, 1997 and 1998. Part I - Wildlife communities. Water Q. Res. J. Can. 35, 399–435.
Blaustein, A. R., Wake, D. B., and Sousa, W. P. (1994). Amphibian declines: judging stability, persistence, and susceptibility of populations to local and global extinctions. Conserv. Biol. 8, 60–71. doi: 10.1046/j.1523-1739.1994.08010060.x
Bonis, A. (2014). “Hydropériode des zones humides : un enjeu décisif pour la structure des communautés végétales et leur diversité,” in Ecologie des Zones Humides : Concepts, Méthodes et Démarches, ed J. B. Bouzillé (Lavoisier), 102–152.
Bonte, D., Van Dyck, H., Bullock, J. M., Coulon, A., Delgado, M., Gibbs, M., et al. (2012). Costs of dispersal. Biol. Rev. 87, 290–312. doi: 10.1111/j.1469-185X.2011.00201.x
Brand, A. B., and Snodgrass, J. W. (2010). Value of artificial habitats for amphibian reproduction in altered landscapes: contributed paper. Conserv. Biol. 24, 295–301. doi: 10.1111/j.1523-1739.2009.01301.x
Brand, A. B., Snodgrass, J. W., Gallagher, M. T., Casey, R. E., and Van Meter, R. (2010). Lethal and sublethal effects of embryonic and larval exposure of Hyla versicolor to stormwater pond sediments. Arch. Environ. Contam. Toxicol. 58, 325–331. doi: 10.1007/s00244-009-9373-0
Camargo, J. A., Alonso, A., and Salamanca, A. (2005). Nitrate toxicity to aquatic animals: a review with new data for freshwater invertebrates. Chemosphere 58, 1255–1267. doi: 10.1016/j.chemosphere.2004.10.044
Campbell, K. R. (1994). Concentrations of heavy metals associated with urban runoff in fish living in stormwater treatment ponds. Arch. Environ. Contam. Toxicol. 27, 352–356. doi: 10.1007/BF00213171
Chang, Y. H., Wang, H. W., and Hou, W. S. (2011). Effects of construction materials and design of lake and stream banks on climbing ability of frogs and salamanders. Ecol. Eng. 37, 1726–1733. doi: 10.1016/j.ecoleng.2011.07.005
Chester, E. T., and Robson, B. J. (2013). Anthropogenic refuges for freshwater biodiversity: their ecological characteristics and management. Biol. Conserv. 166, 64–75. doi: 10.1016/j.biocon.2013.06.016
Collins, S. J., and Russell, R. W. (2009). Toxicity of road salt to Nova Scotia amphibians. Environ. Pollut. 157, 320–324. doi: 10.1016/j.envpol.2008.06.032
deMaynadier, P. G., and Hunter, M. L. (1995). The relationship between forest management and amphibian ecology: a review of the North American literature. Environ. Rev. 3, 230–261 doi: 10.1139/a95-012
Denoel, M. (2005). Persistence and dispersion of an introduced population of alpine newt (Triturus alpestris) in the limestone plateau of Larzac (Southern France). Revue d'Ecologie - La Terre et la Vie. 60, 139–148.
Denoel, M., Bichot, M., Ficetola, G., Delcourt, J., Ylieff, M., Kestemont, P., et al. (2010). Cumulative effects of road de-icing salt on amphibian behavior. Aquat. Toxicol. 99, 275–280. doi: 10.1016/j.aquatox.2010.05.007
Dodd, C. (2010). Amphibian Ecology and Conservation: A Handbook of Techniques. New York, NY: Oxford University Press.
Dodd, C. K., and Cade, B. S. (1998). Movement patterns and the conservation of amphibians breeding in small temporary wetlands. Conserv. Biol. 12, 331–339. doi: 10.1111/j.1523-1739.1998.97183
Duguet, R., Melki, F., and Acemav Association (2003). Les Amphibiens de France, Belgique et Luxembourg. Méze: Biotope, 480.
Dwernychuk, L. W., and Boag, D. A. (1972). Ducks nesting in association with gulls: an ecological trap? Can. J. Zool. 50, 559–563. doi: 10.1139/z72-076
Egan, R. S., and Paton, P. W. C. (2004). Within-pond parameters affecting oviposition by wood frogs and spotted salamanders. Wetlands 24, 1–13. doi: 10.1672/0277-5212(2004)024[0001:WPAOBW]2.0.CO;2
Fayoux, D., and Pelletier, D. (2009). Étanchéité des bassins de lagunage et routiers – Règles pratiques issues de l'expérience. Ingénierie 21–31.
Gagné, S. A., and Fahrig, L. (2007). Effect of landscape context on anuran communities in breeding ponds in the National Capital Region, Canada. Landsc. Ecol. 22, 205–215. doi: 10.1007/s10980-006-9012-3
Gallagher, M. T., Snodgrass, J. W., Brand, A. B., Casey, R. E., Lev, S. M., and Van Meter, R. J. (2014). The role of pollutant accumulation in determining the use of stormwater ponds by amphibians. Wetlands Ecol. Manag. 22, 551–564. doi: 10.1007/s11273-014-9351-9
Gardner, R. C., Barchiesi, F., Beltrame, C., Finlayson, C., Galewski, T., Harrison, I., et al. (2015). State of the World's Wetlands and their Services to People: A Compilation of Recent Analyses. Ramsar Briefing Note no. 7. Gland, Switzerland: Ramsar Convention Secretariat.
Geai, S., Guy, D., Gaber, J., Gay, L., Hurtevent, J., and Sivestre, P. (1997). L'eau et la route : dispositif de traitement des eaux pluviales. Setra 7, 166.
Gillespie, G. R. (2001). The role of introduced trout in the decline of the spotted tree frog (Litoria Spenceri) in south-eastern Australia. Biol. Conserv. 100, 187–198. doi: 10.1016/S0006-3207(01)00021-0
Gledhill, D. G., James, P., and Davies, D. H. (2008). Pond density as a determinant of aquatic species richness in an urban landscape. Landsc. Ecol. 23, 1219–1230. doi: 10.1007/s10980-008-9292-x
Grubb, J. (1973). Olfactory orientation in Bufo woodhousei fowleri, Pseudacris clarki and Pseudacris streckeri. Anim. Behav. 21, 726–732. doi: 10.1016/S0003-3472(73)80098-3
Grubb, J. (1975). Olfactory orientation in southern leopard frogs, Rana utricularia. Herpetologica 31, 219–221.
Guerry, A. D., and Hunter, M. L. Jr. (2002). Amphibian distributions in a landscape of forests and agriculture: an examination of landscape composition and configuration. Conserv. Biol. 16, 745–754. doi: 10.1046/j.1523-1739.2002.00557.x
Gunzburger, M. S., and Travis, J. (2005). Critical literature review of the evidence for unpalatability of amphibian eggs and larvae. J. Herpetol. 39, 547–571. doi: 10.1670/1-05A.1
Hamer, A. J., and McDonnell, M. J. (2008). Amphibian ecology and conservation in the urbanising world: a review. Biol. Conserv. 141, 2432–2449. doi: 10.1016/j.biocon.2008.07.020
Hamer, A. J., and Organ, A. K. (2008). Aspects of the ecology and conservation of the Growling Grass Frog Litoria raniformis in an urban-fringe environment, southern Victoria. Austral. Zool. 34, 393–407. doi: 10.7882/AZ.2008.017
Hamer, A. J., and Parris, K. M. (2011). Local and landscape determinants of amphibian communities in urban ponds. Ecol. Appl. 21, 378–390. doi: 10.1890/10-0390.1
Hamer, A. J., Lane, S. J., and Mahony, M. J. (2002). The role of introduced mosquitofish (Gambusia holbrooki) in excluding the native green and golden bell frog (Litoria aurea) from original habitats in south-eastern Australia. Oecologia 132, 445–452. doi: 10.1007/s00442-002-0968-7
Hassall, C., and Anderson, S. (2015). Stormwater ponds can contain comparable biodiversity to unmanaged wetlands in urban areas. Hydrobiologia 745, 137–149. doi: 10.1007/s10750-014-2100-5
Hatch, A. C., and Blaustein, A. R. (2003). Combined effects of UV-B radiation and nitrate fertilizer on larval amphibians. Ecol. Appl. 13, 1083–1093. doi: 10.1890/1051-0761(2003)13[1083:CEOURA]2.0.CO;2
Hayes, T. B., Falso, P., Gallipeau, S., and Stice, M. (2010). The cause of global amphibian declines: a developmental endocrinologist's perspective. J. Exp. Biol. 213, 921–933. doi: 10.1242/jeb.040865
Hitchings, S. P., and Beebee, T. J. C. (1997). Genetic substructuring as a result of barriers to gene flow in urban Rana tempo- raria (common frog) populations: implications for biodiversity conservation. Heredity 79, 117–127. doi: 10.1038/hdy.1997.134
Holzer, K. A. (2014). Amphibian use of constructed and remnant wetlands in an urban landscape. Urban Ecosyst. 17, 955–968. doi: 10.1007/s11252-014-0373-0
Houlahan, J. E., and Findlay, C. S. (2003). The effects of adjacent land use on wet-land amphibian species richness and community composition. Can. J. Fish. Aquat. Sci. 60, 1078–1094. doi: 10.1139/f03-095
IFSTTAR-LCPC. (2006). Recommandations Pratiques pour la Gestion Des Produits de L'assainissement Pluvial (Practical recommendations for the management of stormwater sediments). Guide technique LCPC. Laboratoire Central des Ponts et Chaussées DISTC, Paris.
Jakob, C., Poizat, G., Veith, M., Seitz, A., and Crivelli, A. J. (2003). Breeding phenology and larval distribution of amphibians in a Mediterranean pond network with unpredictable hydrology. Hydrobiologia 499, 51–61. doi: 10.1023/A:1026343618150
Johnson, J. R., Knouft, J. H., and Semlitsch, R. D. (2007). Sex and seasonal differences in the spatial terrestrial distribution of gray treefrog (Hyla versicolor) populations. Biol. Conserv. 140, 250–258. doi: 10.1016/j.biocon.2007.08.010
Joly, P., and Grolet, O. (1996). Colonization dynamics of new ponds, and the age structure of colonizing alpine newts (Triturus alpestris). Acta Oecol. 17, 599–608.
Joly, P., Miaud, C., Lehmann, A., and Grolet, O. (2001). Habitat matrix effects on pond occupancy in newts. Conserv. Biol. 1, 239–248. doi: 10.1111/j.1523-1739.2001.99200.x
Jumeau, J. (2017). Les Possibilités de Dispersion et Éléments D'habitat-Refuge Dans un Paysage D'agriculture Intensive Fragmenté Par un Réseau Routier Dense : Le cas de la Petite Faune Dans la Plaine du Bas-Rhin. Dissertation thesis, Strasbourg: Strasbourg University.
Karraker, N. E., Gibbs, J. P., and Vonesh, J. R. (2008). Impacts of road deicing salt on the demography of vernal pool-breeding amphibians. Ecol. Appl. 18, 724–734. doi: 10.1890/07-1644.1
Kats, L. B., and Ferrer, R. P. (2003). Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Diver. Distribut. 9, 99–110. doi: 10.1046/j.1472-4642.2003.00013.x
Kats, L. B., Petranka, J. W., and Sih, A. (1988). Antipredator defenses and the persistence of amphibian larvae with fishes. Ecology 69, 1865–1870. doi: 10.2307/1941163
Kovar, R., Brabec, M., Vita, R., and Bocek, R. (2009). Spring migration distances of some Central European amphibian species. Amphib. Reptil. 30, 367–378. doi: 10.1163/156853809788795236
Le Viol, I., Chiron, F., Julliard, R., and Kerbiriou, C. (2012). More amphibians than expected in highway stormwater ponds. Ecol. Eng. 47, 146–154. doi: 10.1016/j.ecoleng.2012.06.031
Le Viol, I., Mocq, J., Julliard, R., and Kerbiriou, C. (2009). The contribution of motorway stormwater retention ponds to the biodiversity of aquatic macroinvertebrates. Biol. Conserv. 142, 3163–3171. doi: 10.1016/j.biocon.2009.08.018
Lukács, B. A., Sramkó, G., and Molnár, V. A. (2013). Plant diversity and conservation value of continental temporary pools. Biol. Conserv. 158, 393–400. doi: 10.1016/j.biocon.2012.08.024
Marco, A., Quilchano, C., and Blaustein, A. R. (1999). Sensitivity to nitrate and nitrite in pond-breeding amphibians from the Pacific Northwest. Environ. Toxicol. Chem. 18, 2836–2839. doi: 10.1002/etc.5620181225
Marsalek, J. (2003). Road salts in urban stormwater: an emerging issue in stormwater management in cold climates. Water Sci. Technol. 48, 61–70.
Marsalek, J., and Marsalek, P. M. (1997). Characteristics of sediments from a stormwater management pond. Water Sci. Technol. 36, 117–122.
Massal, L. R., Snodgrass, J. W., and Casey, R. E. (2007). Potential for toxic effects on amphibian embryos and larvae. Appl. Herpetol. 1, 1–11. doi: 10.1163/157075407779766714
Mayer, T., Marsalek, J., and Delos Reyes, E. (1996). Nutrients and metal contaminants status of urban stormwater ponds. Lake Reservoir Manag. 12, 348–363. doi: 10.1080/07438149609354276
McCarthy, K., and Lathrop, R. G. (2011). Stormwater basins of the New Jersey coastal plain: subsidies or sinks for frogs and toads? Urban Ecosyst. 14, 395–413. doi: 10.1007/s11252-011-0161-z
Moore, T. L., and Hunt, W. F. (2012). Ecosystem service provision by stormwater wetlands and ponds - a means for evaluation? Water Res. 46, 6811–6823. doi: 10.1016/j.watres.2011.11.026
Neff, J. M., Stout, S. A., and Gunster, D. G. (2005). Ecological risk assessment of polycyclic aromatic hydrocarbons in sediments: identifying sources and ecological hazard. Integr. Environ. Assess. Manag. 1, 22–33. doi: 10.1897/IEAM_2004a-016.1
Ostergaard, E., Richter, K., and West, S. (2008). “Amphibian use of stormwater ponds in the Puget lowlands of Washington, USA,” in Urban Herpetology. eds J. C. Mitchell, R. E. Jung, and B. Bartholomew (Salt lake City, UT: Society for the Study of Amphibians and Reptiles), 259–270.
Otto, C. R., Forester, D. C., and Snodgrass, J. W. (2007). Influences of wetland and landscape characteristics on the distribution of carpenter frogs. Wetlands 27, 261–269. doi: 10.1672/0277-5212(2007)27[261:IOWALC]2.0.CO;2
Ouellet, M., and Leheurteux, C. (2007). Principes de conservation et d'aménagement des habitats des amphibiens : revue de littérature et recommandations suggérées pour la rainette faux-grillon de l'Ouest (Pseudacris triseriata). Amphibia-Nature et Ministère Des Ressources Naturelles et de La Faune (Québec). 52.
Parris, K. M. (2006). Urban amphibian assemblages as metacommunities. J. Anim. Ecol. 75, 757–764. doi: 10.1111/j.1365-2656.2006.01096.x
Paul, M. J., and Meyer, J. L. (2001). Streams in the urban landscape. Annu. Rev. Ecol. Syst. 32, 333–365. doi: 10.1146/annurev.ecolsys.32.081501.114040
Pearl, C. A., Adams, M. J., Leuthold, N., and Bury, R. B. (2005). Amphibian occurrence and aquatic invaders in a changing landscape: Implications for wetland mitigation in the Willamette valley, Oregon, U. S. A. Wetlands 25, 76–88. doi: 10.1672/0277-5212(2005)025[0076:AOAAII]2.0.CO;2
Petranka, J. W., Smith, C. K., Scott, A. F., and Floyd, A. A. (2004). Identifying the minimal demographic unit for monitoring pond-breeding amphibians. Ecol. Appl. 14, 1065–1078. doi: 10.1890/02-5394
Phillips, K. (1990). Where have all the frogs and toads gone? Bioscience 40, 422–424. doi: 10.2307/1311385
Pohl, J., Örn, S., Norrgren, L., and Carlsson, G. (2015). Toxicological evaluation of water from stormwater ponds using Xenopus tropicalis embryos. Wetlands Ecol. Manag. 23, 1091–1098. doi: 10.1007/s11273-015-9444-0
Porej, D., and Hetherington, T. E. (2005). Designing wetlands for amphibians: the importance of predatory fish and shallow littoral zones in structuring of amphibian communities. Wetlands Ecol. Manag. 13, 445–455. doi: 10.1007/s11273-004-0522-y
Prunier, J. G., Kaufmann, B., Léna, J. P., Fenet, S., Pompanon, F., and Joly, P. (2014). A 40-year-old divided highway does not prevent gene flow in the alpine newt Ichthyosaura alpestris. Conserv. Genet. 15, 453–468. doi: 10.1007/s10592-013-0553-0
Rittenhouse, T. A. G., and Semlitsch, R. D. (2007). Distribution of amphibians in terrestrial habitat surrounding wetlands. Wetlands 27, 153–161. doi: 10.1672/0277-5212(2007)27[153:DOAITH]2.0.CO;2
Robertson, B. A., and Hutto, R. L. (2006). A framework for understanding ecological traps and an evaluation of existing evidence. Ecology 87, 1075–1085. doi: 10.1890/0012-9658(2006)87[1075:AFFUET]2.0.CO;2
Rodríguez-Prieto, I., and Fernández-Juricic, E. (2005). Effects of direct human disturbance on the endemic Iberian frog (Rana iberica) at individual and population levels. Biol. Conserv. 123, 1–9. doi: 10.1016/j.biocon.2004.10.003
Rothermel, B. B., and Semlitsch, R. D. (2002). An experimental investigation of landscape resistance of forest versus old-field habitats to emigrating juvenile amphibians. Conserv. Biol. 16, 1324–1332. doi: 10.1046/j.1523-1739.2002.01085.x
Rouse, J. D., Bishop, C. A., and Struger, J. (1999). Nitrogen pollution: an assessment of its threat to amphibian survival. Environ. Health Perspect. 107, 799–803. doi: 10.1289/ehp.99107799
Ruban, V., Clozel, B., Conil, C., and Durand, D. (2003). Origine, Caractérisation et Gestion des Boues de L'assainissement Pluvial Routier et Urbain. Points sur les connaissances actuelles et perspectives. Bull. Labo. Ponts et Chaussées. 246–247.
Rubbo, M. J., and Kiesecker, J. M. (2005). Amphibian Breeding distribution in an urbanized landscape. Conserv. Biol. 19, 504–511. doi: 10.1111/j.1523-1739.2005.000101.
Ruhí, A., Sebastian, O. S., Feo, C., Franch, M., Gascón, S., Richter-Boix, À., et al. (2012). Man-made Mediterranean temporary ponds as a tool for amphibian conservation. Annal. Limnol. Int. J. Limnol. 48, 81–93. doi: 10.1051/limn/2011059
Sanzo, D., and Hecnar, S. J. (2006). Effects of road de-icing salt (NaCl) on larval wood frogs (Rana sylvatica). Environ. Pollut. 14, 247–256. doi: 10.1016/j.envpol.2005.07.013
Savage, R. M. (1961). The Ecology and Life History of the Common Frog (Rana Temporaria Temporaria). London: Pitman.
Scheffers, B. R., and Paszkowski, C. A. (2013). Amphibian use of urban stormwater wetlands: the role of natural habitat features. Landsc. Urban Plan. 113, 139–149. doi: 10.1016/j.landurbplan.2013.01.001
Scher, O. (2005). Les Bassins d'eau Pluviale Autoroutiers en Région Méditerranéenne: Fonctionnement et Biodiversité - Evaluation de l'impact de la Pollution Routière sur les Communautés Animales Aquatiques [Doctoral dissertation] Université de Provence - Aix-Marseille.
Scher, O., and Thièry, A. (2005). Odonata, Amphibia and environmental characteristics in motorway stormwater retention ponds (Southern France). Hydrobiologia 551, 237–251. doi: 10.1007/s10750-005-4464-z
Schlaepfer, M. A., Runge, M. C., and Sherman, P. W. (2002). Ecological and evolutionary traps. Trends Ecol. Evol. 17, 474–480. doi: 10.1016/S0169-5347(02)02580-6
Semlitsch, R. D. (1998). Biological delineation of terrestrial buffer zones for pond-breeding salamanders. Conserv. Biol. 12, 1113–1119. doi: 10.1046/j.1523-1739.1998.97274.x
Semlitsch, R. D., and Bodie, J. R. (2003). Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conserv. Biol. 17, 1219–1228. doi: 10.1046/j.1523-1739.2003.02177.x
Simon, J. A., Snodgrass, J. W., Casey, R. E., and Sparling, D. W. (2009). Spatial correlates of amphibian use of constructed wetlands in an urban landscape. Landsc. Ecol. 24, 361–373. doi: 10.1007/s10980-008-9311-y
Skidds, D. E., Golet, F. C., Paton, P. W. C., and Mitchell, J. C. (2007). Habitat correlates of reproductive effort in wood frogs and spotted salamanders in an urbanizing watershed. J. Herpetol. 41, 439–450. doi: 10.1670/0022-1511(2007)41[439:HCOREI]2.0.CO;2
Skriabine, P., Billon, V., Geai, S., Calovi, L., and Ruperd, Y. (2004). Nomenclature de la loi sur l'eau - application aux infrastructures routières-guide technique. Setra 111.
Snodgrass, J. W., Casey, R. E., Joseph, D., and Simon, J. A. (2008). Microcosm investigations of stormwater pond sediment toxicity to embryonic and larval amphibians: variation in sensitivity among species. Environ. Pollut. 154, 291–297. doi: 10.1016/j.envpol.2007.10.003
Sparling, D. W., Fellers, G. M., and McConnell, L. L. (2001). Pesticides and amphibian population declines in California, USA. Environ. Toxicol. Chem. 20, 1591–1595. doi: 10.1002/etc.5620200725
Sredl, M. J., and Collins, J. P. (1992). The interaction of predation, competition, and habitat complexity in structuring an amphibian community. Copeia 3, 607–614. doi: 10.2307/1446138
Stebbins, R. C., and Cohen, N. W. (1995). A Natural History of Amphibians. Princeton, NJ: Princeton University Press.
Sun, J. W. C., and Narins, P. M. (2005). Anthropogenic sounds differentially affect amphibian call rate. Biol. Conserv. 121, 419–427. doi: 10.1016/j.biocon.2004.05.017
Thomas, C. D., Cameron, A., Green, R. E., Bakkenes, M., Beaumont, L. J., Collingham, Y. C., et al. (2004). Extinction risk from climate change. Nature 427, 145–148. doi: 10.1038/nature02121
Thurgate, N. Y., and Pechmann, J. H. K. (2007). Canopy closure, competition, and the endangered dusky gopher frog. J. Wildlife Manag. 71, 1845–1852 doi: 10.2193/2005-586
Tixier, G., Lafont, M., Grapentine, L., Rochfort, Q., and Marsalek, J. (2011). Ecological risk assessment of urban stormwater ponds: literature review and proposal of a new conceptual approach providing ecological quality goals and the associated bioassessment tools. Ecol. Indic. 11, 1497–1506. doi: 10.1016/j.ecolind.2011.03.027
Todd, B. D., Luhring, T. M., Rothermel, B. B., and Gibbons, J. W. (2009). Effects of forest removal on amphibian migrations: implications for habitat and land- scape connectivity. J. Appl. Ecol. 46, 554–561. doi: 10.1111/j.1365-2664.2009.01645.x
Trenham, P. C., and Shaffer, H. B. (2005). Amphibian upland habitat use and its consequences for population viability. Ecol. Appl. 15, 1158–1168. doi: 10.1890/04-1150
Trenham, P. C., Koenig, W. D., Mossman, M. J., Stark, S. L., and Jagger, L. A. (2003). Regional dynamics of wetland-breeding frogs and toads: turnover and synchrony. Ecol. Appl. 13, 1522–1532. doi: 10.1890/02-5206
United Nations (2014). World Urbanization Prospects: The 2014 Revision, Highlights. Department of Economic and Social Affairs. Population Division, United Nations.
Van Buskirk, J. (2005). Local and landscape influence on amphibian occurrence and abundance. Ecology 86, 1936–1947. doi: 10.1890/04-1237
Vasconcelos, D., and Calhoun, A. J. K. (2006). Monitoring created seasonal pools for functional success: a six-year case study of amphibian responses, Sears Island, Maine, U. S. A. Wetlands 26, 992–1003. doi: 10.1672/0277-5212(2006)26[992:MCSPFF]2.0.CO;2
Keywords: stormwater ponds, amphibians ecology, transport infrastructures, urban ecology, ecological management
Citation: Clevenot L, Carré C and Pech P (2018) A Review of the Factors That Determine Whether Stormwater Ponds Are Ecological Traps And/or High-Quality Breeding Sites for Amphibians. Front. Ecol. Evol. 6:40. doi: 10.3389/fevo.2018.00040
Received: 03 July 2017; Accepted: 26 March 2018;
Published: 12 April 2018.
Edited by:
Carme Rosell, Minuartia, SpainReviewed by:
Iryna Dronova, University of California, Berkeley, United StatesTom Langen, Clarkson University, United States
Copyright © 2018 Clevenot, Carré and Pech. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Clevenot, lauraclevenot@gmail.com
 Laura Clevenot
Laura Clevenot Catherine Carré
Catherine Carré  Pierre Pech
Pierre Pech