Autumn Warming Delays the Downregulation of Photosynthesis and Does Not Increase the Risk of Freezing Damage in Interior and Coastal Douglas-fir
- 1Department of Biology, University of Toronto Mississauga, Mississauaga, ON, Canada
- 2Graduate Department of Cell and Systems Biology, University of Toronto, Toronto, ON, Canada
- 3Graduate Department of Ecology and Evolutionary Biology, University of Toronto, Toronto, ON, Canada
During autumn, evergreen conifers utilize the decrease in daylength and temperature as environmental signals to trigger cold acclimation, a process that involves the downregulation of photosynthesis, upregulation of photoprotection, and development of cold hardiness. Global warming will delay the occurrence of autumn low temperatures while daylength remains unaffected. The impact of autumn warming on cold acclimation and the length of the carbon uptake period of species with ranges that encompass diverse climates, such as Douglas-fir (Pseudotsuga menziesii), remains unclear. Our study investigated intraspecific variation in the effects of autumn warming on photosynthetic activity, photosynthetic pigments, and freezing tolerance in two interior (var. glauca) and two coastal (var. menziesii) Douglas-fir provenances. Following growth under simulated summer conditions with long days (16 h photoperiod) and summer temperatures (22/13°C day/night), Douglas-fir seedlings were acclimated to simulated autumn conditions with short days (8 h photoperiod) and either low temperatures (cool autumn, CA; 4/−4°C day/night) or elevated temperatures (warm autumn, WA; 19/11°C day/night). Exposure to low temperatures in the CA treatment induced the downregulation of photosynthetic carbon assimilation and photosystem II efficiency, increased the size and de-epoxidation of the xanthophyll cycle pigment pool, and caused the development of sustained nonphotochemical quenching (NPQ). Seedlings in the WA treatment exhibited no downregulation of photosynthesis, no change in xanthophyll cycle pigment de-epoxidation, and no development of sustained NPQ. Albeit these changes, freezing tolerance was not impaired under WA conditions compared with CA conditions. Interior Douglas-fir seedlings developed greater freezing tolerance than coastal seedlings. Our findings suggest that autumn warming, i.e., short photoperiod alone, does not induce the downregulation of photosynthesis in Douglas-fir. Although autumn warming delays the downregulation of photosynthesis, the prolonged period of photosynthetic activity does not bear a trade-off of impaired freezing tolerance.
Introduction
Climate change is projected to increase global average surface temperatures by 2.5–5°C by the end of the century (IPCC, 2014). Even larger increases are projected for the middle to high latitudes of the Northern Hemisphere, which are dominated by temperate and boreal forests. In these forests, evergreen conifers undergo cold acclimation during autumn to protect their overwintering tissues (Chang et al., 2020). This cold acclimation process involves the cessation of growth, downregulation of photosynthesis, upregulation of photoprotection, and development of cold hardiness (Öquist and Hüner, 2003; Chang et al., 2020). Evergreen conifers rely upon decreases in temperature and photoperiod during autumn as signals to trigger these physiological changes (Welling et al., 2002; Rossi et al., 2008; Singh et al., 2017). Autumn warming will delay the low temperature signal while the short photoperiod signal will remain unaffected; this desynchronization has the potential to disrupt the cold acclimation process (Hänninen, 2016; Chang et al., 2020). Thus, the impact of projected autumn warming on evergreen conifer species will be strongly influenced by the importance of photoperiod versus temperature for the induction of the physiological changes that constitute cold acclimation (Way and Montgomery, 2015).
Evergreen conifer cold acclimation begins during late summer and early autumn with the initiation of growth cessation (Repo et al., 2000; Hamilton et al., 2016) and bud formation (Chen et al., 2012; Maurya and Bhalerao, 2017). Most evergreen conifers utilize short photoperiod as the dominant signal for initiation of growth cessation (Rossi et al., 2006). In these species, growth cessation likely involves a preemptive redirection of photoassimilates from sink tissues to storage tissues in early autumn (Oleksyn et al., 2000; Palacio et al., 2014). During late autumn, low temperatures begin to limit cell division, cell differentiation, and carbon allocation (Rossi et al., 2008). These changes decrease metabolic sink capacity and further cease growth. Photosynthesis continues to fuel the cold acclimation process (Wong et al., 2019; Fréchette et al., 2020); however, the combined effects of growth cessation and low temperatures eventually induce a downregulation of photosynthetic activity (Öquist and Hüner, 2003; Chang et al., 2020). This downregulation is due to limitation of the rates of photosynthetic electron transport and the Calvin cycle (Kingston-Smith et al., 1997; Öquist and Hüner, 2003; Crosatti et al., 2013). Comparatively, the rates of the primary photophysical and photochemical reactions of photosystem II (PSII) are largely temperature independent (Hüner et al., 1998; Ensminger et al., 2006). This disparity in temperature sensitivities creates potential for the accumulation of excess light energy during late autumn and winter (Öquist and Hüner, 2003; Hüner et al., 2013). Excess light energy can lead to the generation of reactive oxygen species (ROS) that cause photooxidative damage to PSII (Barber and Andersson, 1992; Apel and Hirt, 2004). This is especially threatening since low temperatures also inhibit the repair of damaged PSII subunits, such as the reaction center protein D1 (Ottander et al., 1995; Ensminger et al., 2006).
To protect against the harmful effects of low temperatures, evergreen conifers reorganize their photosynthetic apparatus into large aggregates that exhibit downregulated photosynthesis and upregulated photoprotection (Ottander et al., 1995; Savitch et al., 2002; Chang et al., 2020). In Scots pine (Pinus sylvestris), the downregulation of photosynthetic activity involves the moderate degradation of chlorophyll pigments and reaction center proteins, which causes a decrease in the size of PSII light-harvesting complexes (LHCs; Ottander et al., 1995; Porcar-Castell et al., 2008). The upregulation of photoprotective capacity usually involves the accumulation of carotenoid pigments that perform ROS scavenging, such as β-carotene, neoxanthin, and lutein (Krieger-Liszkay et al., 2008; Jahns and Holzwarth, 2012; Chang et al., 2015). It also involves the accumulation of carotenoid pigments that contribute to nonphotochemical quenching (NPQ), particularly xanthophyll pigments (Demmig-Adams and Adams, 1992; Ensminger et al., 2006; Verhoeven, 2014). In response to excess light energy, light-harvesting violaxanthin is de-epoxidated to form antheraxanthin, which is further de-epoxidated to form energy quenching zeaxanthin (Müller et al., 2001; Horton et al., 2008). During the growing season, these reactions occur in a fast and reversible process called the xanthophyll cycle, which provides a dynamic mechanism of NPQ in response to short-term stress (Demmig-Adams et al., 2012; Janka et al., 2015). During winter, the pigment quantity (VAZ) and de-epoxidation state (DEPS) of the xanthophyll cycle are increased, providing a sustained mechanism of NPQ in response to long-term stress (Sveshnikov et al., 2006; Demmig-Adams et al., 2012).
Cold acclimation also involves the development of cold hardiness, which is initiated during early autumn in response to decreasing photoperiod and evolves during late autumn in response to the combination of short photoperiod and low temperature (Chang et al., 2015; Strimbeck et al., 2015). Freezing temperatures can induce the formation of extracellular ice crystals that impose mechanical and osmotic stresses, causing tissue damage and cellular dehydration (Sutinen et al., 2001; Crosatti et al., 2013). Freezing and thawing in leaves can also disrupt the integrity of plasma and thylakoid membranes, resulting in leakage of solutes and collapse of cells and chloroplasts (Steponkus, 1984). To protect against this, evergreen conifers, such as Siberian spruce (Picea obovata), undergo large changes in lipid and carbohydrate metabolism during the development of cold hardiness (Angelcheva et al., 2014). These metabolic changes help maintain membrane fluidity (Moellering et al., 2010; Crosatti et al., 2013) and provide cellular osmo- and cryoprotection (Angelcheva et al., 2014; Chang et al., 2015). In addition, specific dehydrin proteins that have osmo- and cryoprotective functions are expressed (Close, 1997; Chang et al., 2015, 2016). Together, these cold hardening processes provide evergreen conifers, such as Douglas-fir, with tolerance to prolonged exposure to winter temperatures below −40°C (Strimbeck et al., 2015).
Several studies have investigated the effects of autumn warming on cold acclimation in evergreen conifers in order to assess the impact of climate change on temperate and boreal forests. Growth chamber experiments employing elevated temperatures ranging from +5 to +15°C have demonstrated delayed downregulation of photosynthesis in seedlings of white pine (Pinus strobus; Chang et al., 2016; Fréchette et al., 2016) and white spruce (Picea glauca; Hamilton et al., 2016; Stinziano and Way, 2017). These findings are consistent with those of open-top chamber field experiments on mature Scots pine (Wang, 1996). Delayed development of freezing tolerance due to autumn warming has also been demonstrated in both growth chamber and open-top chamber experiments (Repo et al., 1996; Guak et al., 1998; Chang et al., 2016). However, a +15/+13°C (day/night) autumn warming treatment caused the downregulation of photosynthetic carbon assimilation but not PSII efficiency in jack pine seedlings (Pinus banksiana) (Busch et al., 2007). These seedlings responded to the resulting excess light energy via enhanced dynamic NPQ and increased quantities of ROS-scavenging β-carotene. In addition, the development of freezing tolerance in white spruce seedlings was unaffected under a +14/+12°C autumn warming treatment (Hamilton et al., 2016). These contrasting results highlight the variation among evergreen conifer species in the photoperiod sensitivity of the different cold acclimation processes.
Responses to shorter photoperiod and lower temperature during late summer and autumn can also vary within species and among populations originating from different geographical areas (i.e., provenances) (Savolainen et al., 2007). Fréchette et al. (2020) observed in white pine that the timing of the downregulation of photosynthesis in response to shorter photoperiod was delayed in southern provenances compared with northern provenances. In the same field experiment, Fréchette et al. (2020) also simulated autumn warming (+1.5°C above ambient during the day and +3°C above ambient during the night) and observed that warming caused a larger delay in the downregulation of photosynthesis in southern provenances than in northern provenances. Beuker et al. (1998) observed that northern provenances of Scots pine and Norway spruce (Picea abies) initiated the development of freezing tolerance in response to shorter photoperiods. These examples of intraspecific variation emerge from selective pressure for local adaptation to climate (Aitken et al., 2008). Local adaptation often results in patterns of trait variability that follow latitudinal and elevational gradients across the ranges of evergreen conifer species (Howe et al., 2003; Savolainen et al., 2007; Rehfeldt et al., 2014). However, local adaptation in many populations is lagging behind the shifts in climate caused by rapid warming (Carter, 1996; Corlett and Westcott, 2013; Gray and Hamann, 2013). As sessile species with long generation times, evergreen conifers are limited in their capacity to migrate and adapt in response to these shifts (Savolainen et al., 2007; Aitken et al., 2008). Thus, their capacity for phenotypic plasticity will be a major factor determining their ability to avoid maladaptation to autumn warming.
Douglas-fir (Pseudotsuga menziesii) has been used in many studies to demonstrate local adaptation (Eckert et al., 2009; Eilmann et al., 2013) and phenotypic plasticity (Isaac-Renton et al., 2014; Hess et al., 2016). It is a dominant and economically valuable evergreen conifer in western North America with a range that encompasses diverse climates (Howe et al., 2006; Mullin et al., 2011). In British Columbia specifically, mean annual temperature can vary as much as 6°C among Douglas-fir provenances (Wang et al., 2012). High levels of intraspecific variation in growth cessation and freezing tolerance development have been observed across its latitudinal and elevational gradients (Bansal et al., 2015; Ford et al., 2017). Intraspecific variation in the response of photosynthetic and photoprotective mechanisms to photoperiod and temperature is thus expected as well. The coastal Douglas-fir variety (var. menziesii) generally originates from low- to mid-elevation environments with milder climates, whereas the interior variety (var. glauca) generally originates from mid- to high-elevation environments with colder climates and larger daily temperature ranges (Howe et al., 2006). Adaptation of these varieties in part reflects trade-offs between traits that improve vigor in milder climates and traits that improve tolerance to early frost (St Clair et al., 2005). However, selection pressures are changing rapidly; an increase in average autumn temperature of up to 6°C is projected in British Columbia by the end of the century (Arora et al., 2011; Wang et al., 2012). The impact that this will have on the physiological mechanisms that regulate the autumn cold acclimation process in Douglas-fir remains unclear.
In this study, we assessed the effects of simulated autumn warming on cold acclimation in two interior and two coastal provenances of Douglas-fir seedlings. We aimed to characterize the intraspecific variation in (1) the autumn cold acclimation process as well as (2) the plasticity of this process in response to warming. We hypothesized that (1) the autumn cold acclimation process requires both low temperature and short photoperiod signals in Douglas-fir. Autumn warming therefore delays the downregulation of photosynthesis and the corresponding upregulation of photoprotection, which causes an extension of the carbon uptake period at the cost of impairment of the development of freezing tolerance. We further hypothesized that (2) interior Douglas-fir is maladapted to warm autumn (WA) temperatures at lower latitudes and elevations, whereas coastal Douglas-fir is maladapted to frost risk at higher latitudes and elevations.
Materials and Methods
Plant Materials and Experimental Conditions
Seeds of interior and coastal Douglas-fir provenances (P. menziesii var. glauca and var. menziesii, respectively) in British Columbia were obtained from the Tree Seed Centre (Surrey, British Columbia, Canada). Interior Douglas-fir seeds originated from Little Elk Creek (LIT; 50°32′24.0′′N, 115°37′12.0′′W, 1,525 m) and Meldrum Creek (MEL; 52°02′24.0′′N, 122°19′48.0′′W, 900 m), and coastal Douglas-fir seeds originated from Pemberton (PEM; 50°19′12.0′′N, 122°43′48.0′′W, 550 m) and Tsowwin River (TSO; 49°46′48.0′′N, 126°37′48.0′′W, 225 m) (Figure 1). These four provenances were selected because they represent a wide scope of the geographic and climatic conditions present across Douglas-fir’s range in British Columbia (Table 1).
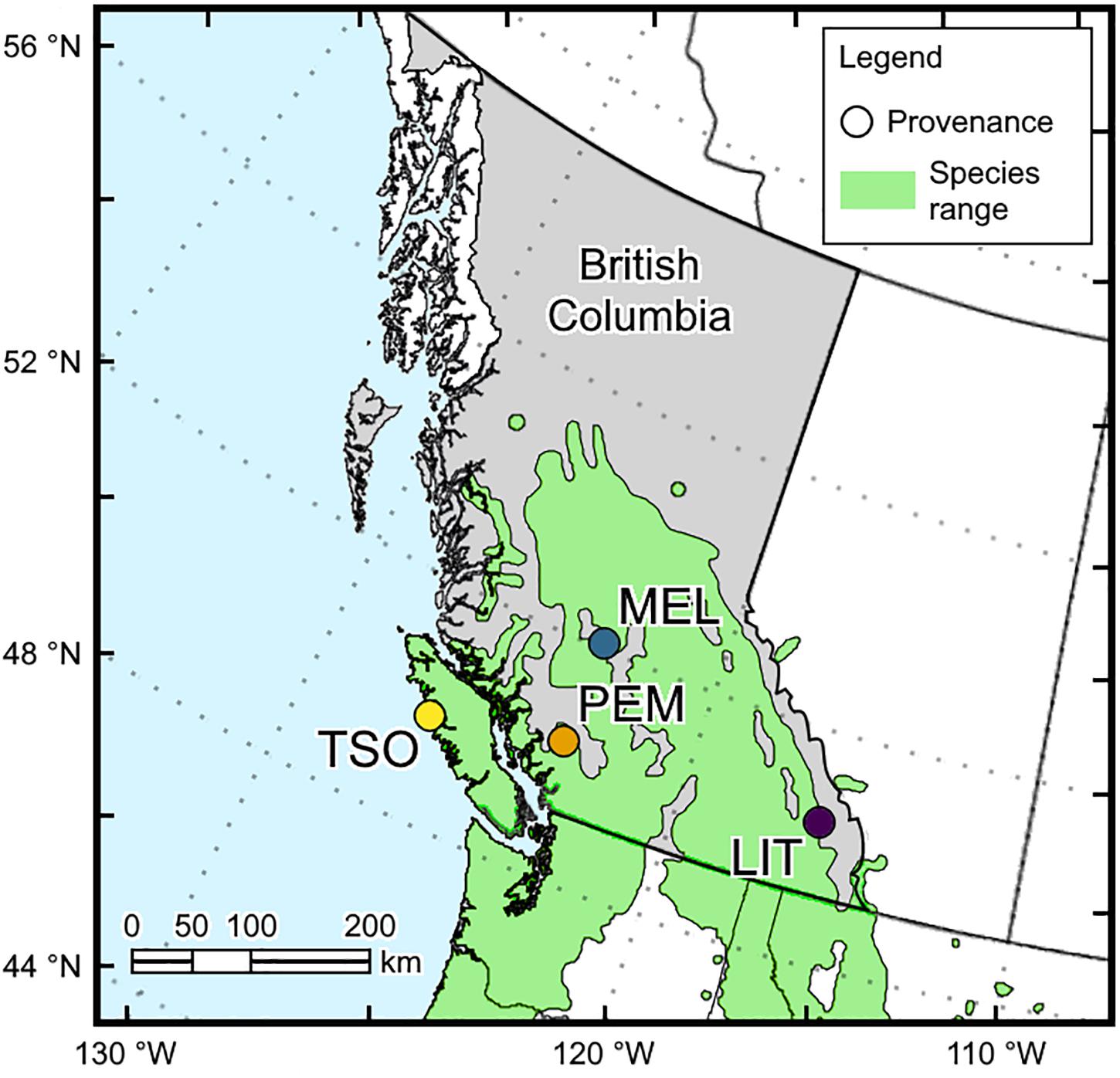
Figure 1. Locations of the interior (LIT, MEL) and coastal (PEM, TSO) Douglas-fir provenances used in this experiment.
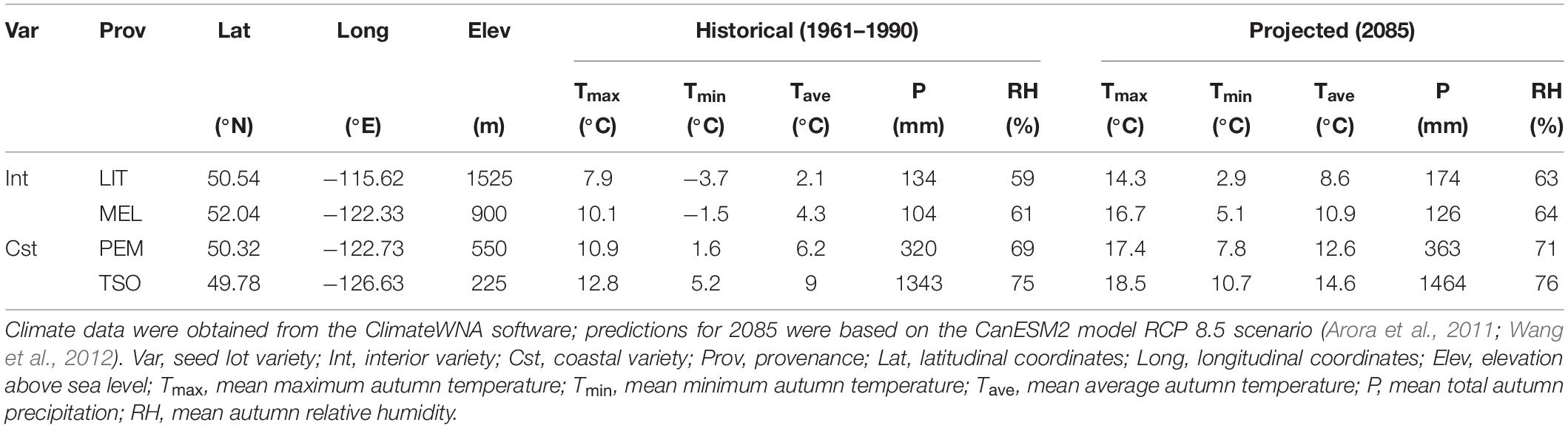
Table 1. Historical and projected autumn climate of the Douglas-fir provenances used in this experiment.
Seeds were soaked for 24 h in distilled water at room temperature, surface-sterilized for 5 h in 30 ml of 3% hydrogen peroxide, and stratified for 3 weeks in the dark at 4°C. pH 4.5 potting soil was prepared with 21.6% (v/v) silica sand (Cat. No. 1240s; Bell & Mackenzie, Hamilton, ON, Canada), 13.5% (v/v) sphagnum peatmoss (Premier Tech, Rivière-du-Loup, PQ, Canada), 10.8% (v/v) Turface (PROFILE, Buffalo Grove, IL, United States), 7.6% (v/v) coarse perlite (Therm-O-Rock, New Eagle, PA, United States), 3.2% (v/v) medium vermiculite (Therm-O-Rock), 0.1% (v/v) dolomitic limestone (National Lime & Stone, Findlay, OH, United States), and 43.2% (v/v) distilled water. Seeds were planted in 168 ml cones lightly packed with potting soil and covered with 5 mm of silica sand. Seeds were germinated for 4 weeks in greenhouse under 17 h photoperiod and 25/17°C day/night, 400–1,200 μmol photos m–2 s–1 of photosynthetically active radiation, and 55% relative humidity (RH). At 4 weeks after planting, seedlings were transplanted into 25 l square pots containing one seedling from each provenance. Seedlings were grown for 6 months under 18 h photoperiod and a temperature regime based on Wang et al. (2016). Following this growth phase, seedlings were transferred to growth chambers (BioChambers, Winnipeg, MB, Canada) and chilled for 2 months under 8 h photoperiod, 8/4°C day/night, and 50–300 μmol photons m–2 s–1. This was followed by a second 6-month-long growth phase in greenhouse. During growth phases, seedlings were watered once per week and fertilized twice per week according to Wenny and Dumroese (1992).
At the start of the experiment, 2-year-old seedlings were transferred to four growth chambers and acclimated to simulated summer control (SC) conditions. This SC treatment consisted of long days and average summer temperatures (16 h photoperiod; 22/13°C day/night). Following 6 weeks of acclimation, sampling and measurements were performed for the SC treatment. Seedlings were then shifted to simulated WA or cool autumn (CA) conditions (two growth chambers per treatment). The WA treatment consisted of short days and elevated temperature (8 h photoperiod; 19/11°C day/night), designed to simulate the projected autumn temperature range for Tsowwin River in 2085 (Wang et al., 2012) according to the representative concentration pathway (RCP) 8.5 climate change scenario of the CanESM2 climate model (Arora et al., 2011). The CA treatment consisted of short days and low temperatures (8 h photoperiod; 4/-4°C day/night), designed to reproduce the average autumn temperature range for Little Elk Creek from 1961 to 1990. Following 6 weeks of acclimation, sampling and measurements were performed for the WA and CA treatments. Ten seedlings per treatment and provenance were randomly selected for sampling and measurements. All analyses were performed on 2-year-old needles. Seedlings were rotated within each growth chamber once per week. Photosynthetically active radiation (PAR) was maintained at 1,200 μmol photons m–2 s–1 midday and 400 μmol photons m–2 s–1 during the first and last hours of each day. Light was provided using metal halide and high-pressure sodium bulbs. RH was set to 55%.
Photosynthetic Gas Exchange and Chlorophyll Fluorescence
Photosynthetic gas exchange was measured using a GFS-3000 portable gas exchange system with a standard cuvette and a 3056-FL PAM-Fluorometer (Walz, Effeltrich, Germany). Measurements were started at least 1 h after growth lights were turned on. The measuring cuvette settings were: 400 ml min–1 flow rate; 400 ppm CO2; 22 (SC treatment), 19 (WA treatment), or 4°C (CA treatment); and 55% RH. Dark respiration (Rd) was measured after 40 min of dark adaptation. Net photosynthetic carbon assimilation (Anet) and stomatal conductance (gs) were measured during steady-state assimilation at 1,500 μmol m–2 s–1 PAR after at least 3 min of exposure. Gas exchange measurements were normalized to leaf surface area measurements estimated using WinSEEDLE Pro v.2011b (Regent Instruments, Québec City, QC, Canada).
Chlorophyll fluorescence was measured simultaneously with photosynthetic gas exchange. Dark adapted minimum PSII fluorescence (Fo) and maximum PSII fluorescence (Fm) were measured after 40 min of dark adaptation. This was followed by measurement of light-adapted minimum PSII fluorescence (Fo’), maximum PSII fluorescence (Fm’), and transient PSII fluorescence (Ft) at 1,500 μmol m–2 s–1 PAR after at least 3 min of exposure. Maximum quantum yield of PSII (Fv/Fm) was calculated as according to Genty et al. (1989).
Light energy partitioning was calculated using the parameters ΦPSII, ΦNPQ, and Φf,D (Hendrickson et al., 2004).
Photosynthetic Pigments
Photosynthetic pigments were extracted according to Junker and Ensminger (2016). Samples were flash-frozen in liquid nitrogen and stored at −80°C. Frozen samples were transferred to a pre-cooled mortar and pestle filled with liquid nitrogen and homogenized to a fine powder. Approximately 50–60 mg of homogenized frozen needle tissue was then transferred to a 2 ml amber vial, and pigments were then extracted for 2 h at 4°C in 98% methanol buffered with 2% 0.5 M ammonium acetate in the dark. The extract was centrifuged for 5 min at 4°C at 14,000 rpm, the supernatant was collected, and the pellet was washed with 100% methanol. This step was repeated twice. The supernatants were combined and filtered using 0.2 μm pore PTFE syringe filters (Thermo Scientific, Rockwood, TN, United States). Photosynthetic pigments were separated using a reverse-phase C30 column (5 μm, 250 × 4.6 mm; YMC Co., Ltd., Kyoto, Japan) and analyzed with a 1260 Infinity high performance liquid chromatography (HPLC) system equipped with a UV-diode array detector (Agilent Technologies, Santa Clara, CA, United States). Pigments were eluted using a mobile phase with a gradient of methanol, water buffered with 0.2% ammonium acetate, and methyl tert-butyl ether. Elution was performed at a flow rate of 1 ml min–1 and a column temperature of 25°C. Calibration was performed using standards for chlorophyll a and chlorophyll b (St. Louis, MO, United States) and antheraxanthin, α-carotene, β-carotene, lutein, neoxanthin, violaxanthin, and zeaxanthin from DHI Lab (Hørsholm, Denmark). Peak detection and pigment quantification were performed using ChemStation (Agilent Technologies).
Total chlorophyll content (Chl) was calculated as the sum of chlorophyll a and b contents per gram of fresh weight. Total carotenoid content (Car) was calculated as the sum of violaxanthin (Vio), antheraxanthin (Ant), zeaxanthin (Zea), neoxanthin (Neo), lutein (Lut), α-carotene (α-Car), and β-carotene (β-Car), normalized to Chl. Total xanthophyll cycle pigment content (VAZ) was calculated as the sum of Vio, Ant, and Zea. DEPS of xanthophyll cycle pigments was calculated according to Thayer and Bjorkman (1990).
Freezing Tolerance
Cold hardiness was assessed by measuring chlorophyll fluorescence to determine leaf freezing tolerance, following a modified protocol after Chang et al. (2016). Samples were taken after 9 weeks of exposure to WA or CA treatments. Needles were excised and placed proximal-end-down within 1.5 ml microcentrifuge tubes containing 0.10 ml of distilled water. Needles were exposed to a range of freezing temperatures at 5°C intervals from 0 to −40°C using a Thermotron SM-16-8200 environmental test chamber (Thermotron Industries, Holland, MI, USA). The initial decrease from 0 to −1°C occurred over 1 h, followed by a maximum cooling rate of −5°C per h to reach target temperature. Each target temperature was held for 10–12 h. After each freezing interval, needles were transferred to thaw in a stepwise manner: −20°C refrigeration for 24 h (if target temperature was >30°C), 4°C refrigeration for 24 h, and room temperature for 24 h. Following recovery, needles were exposed to 800 μmol photons m–2 s–1 for 1 h. Needles were then dark-adapted for 40 min, and Fv/Fm was assessed. The temperature corresponding to a 50% reduction in post-recovery Fv/Fm (LT50) was used as a proxy for freezing tolerance of the photosynthetic apparatus. LT50 was calculated using the midpoints of sigmoidal vulnerability curves constructed using a modified generalized logistic function.
Statistical Analysis
All statistical tests were performed in R v3.5.2 (R Development Core Team, 2010). For all photosynthetic gas exchange, chlorophyll fluorescence, and photosynthetic pigment parameters, the effects of treatment, provenance, and the interaction thereof were assessed via two-way mixed-design ANOVA models using the lmerTEST package (Kuznetsova et al., 2017), where treatment and provenance represented categorical fixed factors, and chamber and pot represented random factors. For each parameter, the best-fit model was chosen according to lowest Akaike information criterion (AIC; Akaike, 1998). Following the determination of estimated marginal means between provenances for all parameters where provenance was significant, using the emmeans package (Lenth, 2016), significance of pairwise differences between provenances was assessed via Tukey’s HSD test. Freezing tolerance was compared between treatments and provenances via Satterthwaite’s approximate F test using the drc package (Ritz et al., 2015). Normality of distributions and equality of variances (homoscedasticity) for model residuals were assessed via Shapiro–Wilk test and Levene’s test, respectively.
Results
Photosynthetic Gas Exchange
Under simulated SC conditions, net photosynthetic carbon assimilation (Anet) was similar in seedlings of the provenances MEL, PEM, and TSO (Figure 2A). The lowest Anet was observed in the interior provenance LIT, which exhibited levels that were significantly lower than those in the interior provenance MEL (P < 0.05). After 6 weeks of acclimation to WA conditions, there were no significant changes in Anet. In contrast, after 6 weeks of acclimation to CA conditions, Anet significantly decreased by 85–95% (P < 0.001). This trend of treatment effects was also observed for stomatal conductance (gs) (Figure 2B). Under SC, dark respiration (Rd) was significantly higher (approximately 50–60%) in seedlings of the interior provenances LIT and MEL than in those of the coastal provenances PEM and TSO (P < 0.001; Figure 2C). After acclimation to WA, only MEL seedlings exhibited a strong decrease in Rd. After acclimation to CA, LIT, MEL, and TSO seedlings all exhibited strong decreases in Rd. For LIT seedlings in particular, Rd significantly decreased by almost 50% under CA (P < 0.001).
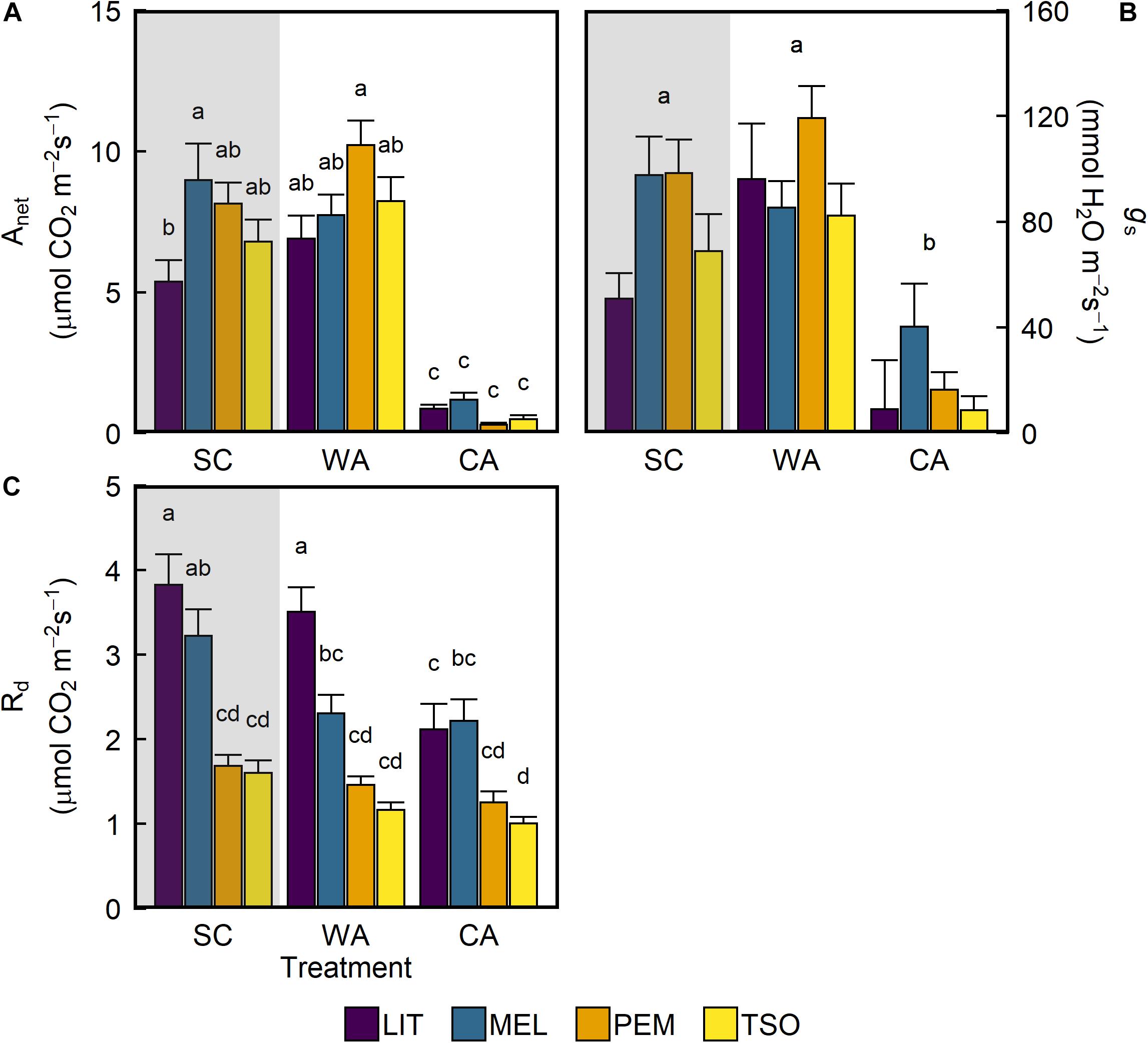
Figure 2. Response of photosynthetic gas exchange of interior (LIT, MEL) and coastal (PEM, TSO) Douglas-fir seedlings to summer control (SC), warm autumn (WA), and cool autumn (CA) conditions. (A) Net photosynthetic carbon assimilation (Anet); (B) stomatal conductance (gs); (C) dark respiration (Rd). Gray background indicates long photoperiod (summer); white background indicates short photoperiod (autumn). Measurements for Anet and gs were taken at 1,500 μmol m– 2 s– 1 light intensity under growth conditions. Bars represent the mean of n = 7–10 ± SE. Letters where present indicate statistically different groups (P < 0.05) as determined by Tukey’s HSD test.
Chlorophyll Fluorescence
Analysis of the maximum quantum yield of PSII (Fv/Fm) revealed values of approximately 0.81 in seedlings of all provenances under SC conditions (Figure 3). Acclimation to WA did not significantly affect Fv/Fm. However, Fv/Fm was approximately 75–85% lower under CA conditions than under SC and WA conditions (P < 0.001). There were no significant differences in Fv/Fm between provenances under any treatment (Table 2). Analysis of the proportion of absorbed light energy used for photochemistry (ΦPSII), dynamic NPQ (ΦNPQ), and the sum of fluorescence and sustained NPQ (Φf,D) revealed that, under SC conditions, decreases in ΦPSII under increasing light intensity were compensated for via increases in ΦNPQ (Figure 4 and Supplementary Figure 2). Acclimation to WA did not significantly alter this pattern of light energy partitioning (Table 2). However, acclimation to CA caused an almost fivefold increase in quenching of excess light energy via Φf,D (P < 0.001). This shift in light energy partitioning primarily replaced ΦNPQ. LIT seedlings exhibited significantly higher ΦPSII and lower ΦNPQ than seedlings of other provenances (P < 0.001; Figure 4).
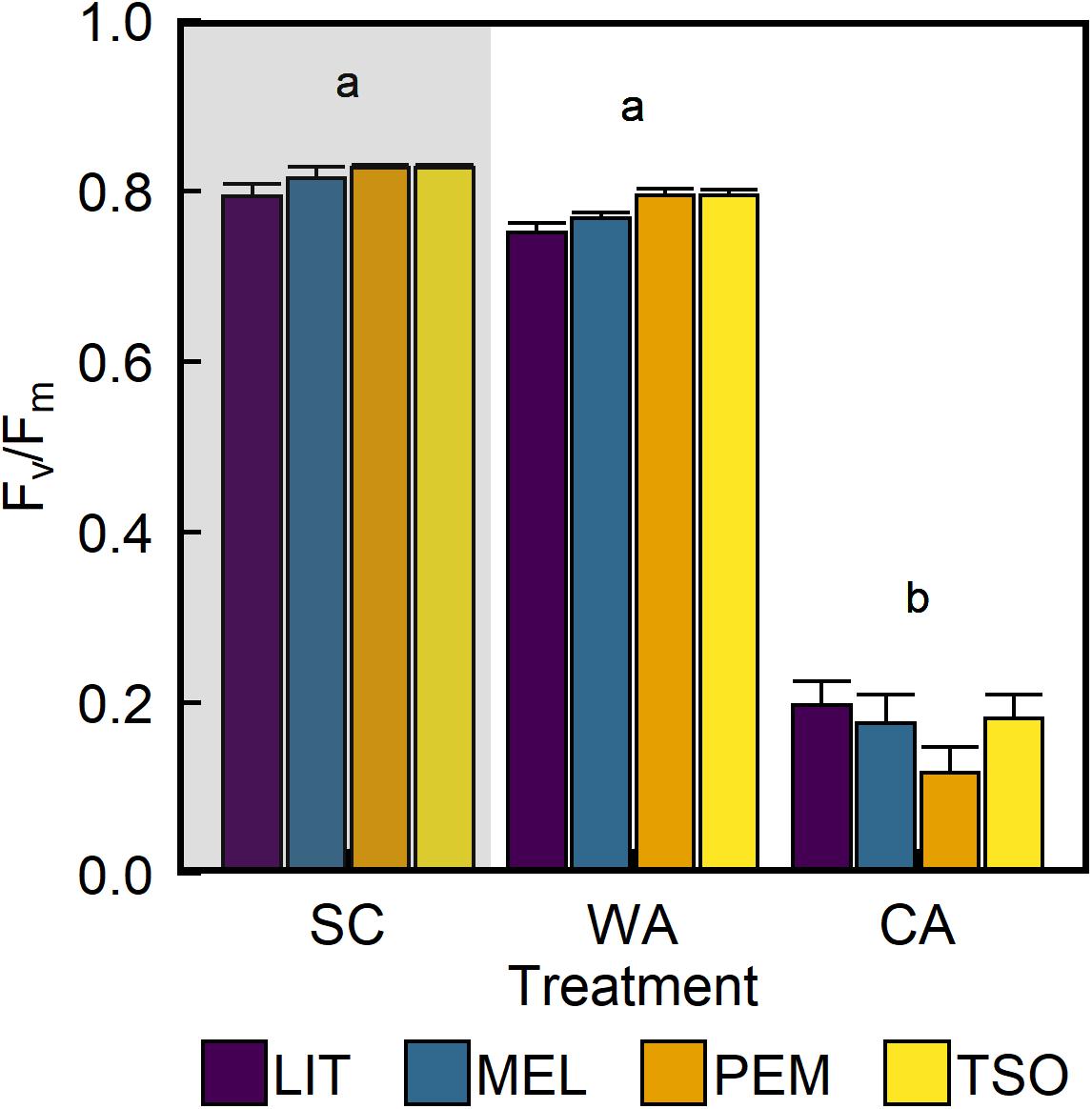
Figure 3. Response of maximum quantum efficiency of photosystem II (Fv/Fm) of interior (LIT, MEL) and coastal (PEM, TSO) Douglas-fir seedlings to summer control (SC), warm autumn (WA), and cool autumn (CA) conditions. Gray background indicates long photoperiod (summer); white background indicates short photoperiod (autumn). Bars represent the mean of n = 10 ± SE. Letters where present indicate statistically different groups (P < 0.05) as determined by Tukey’s HSD test.
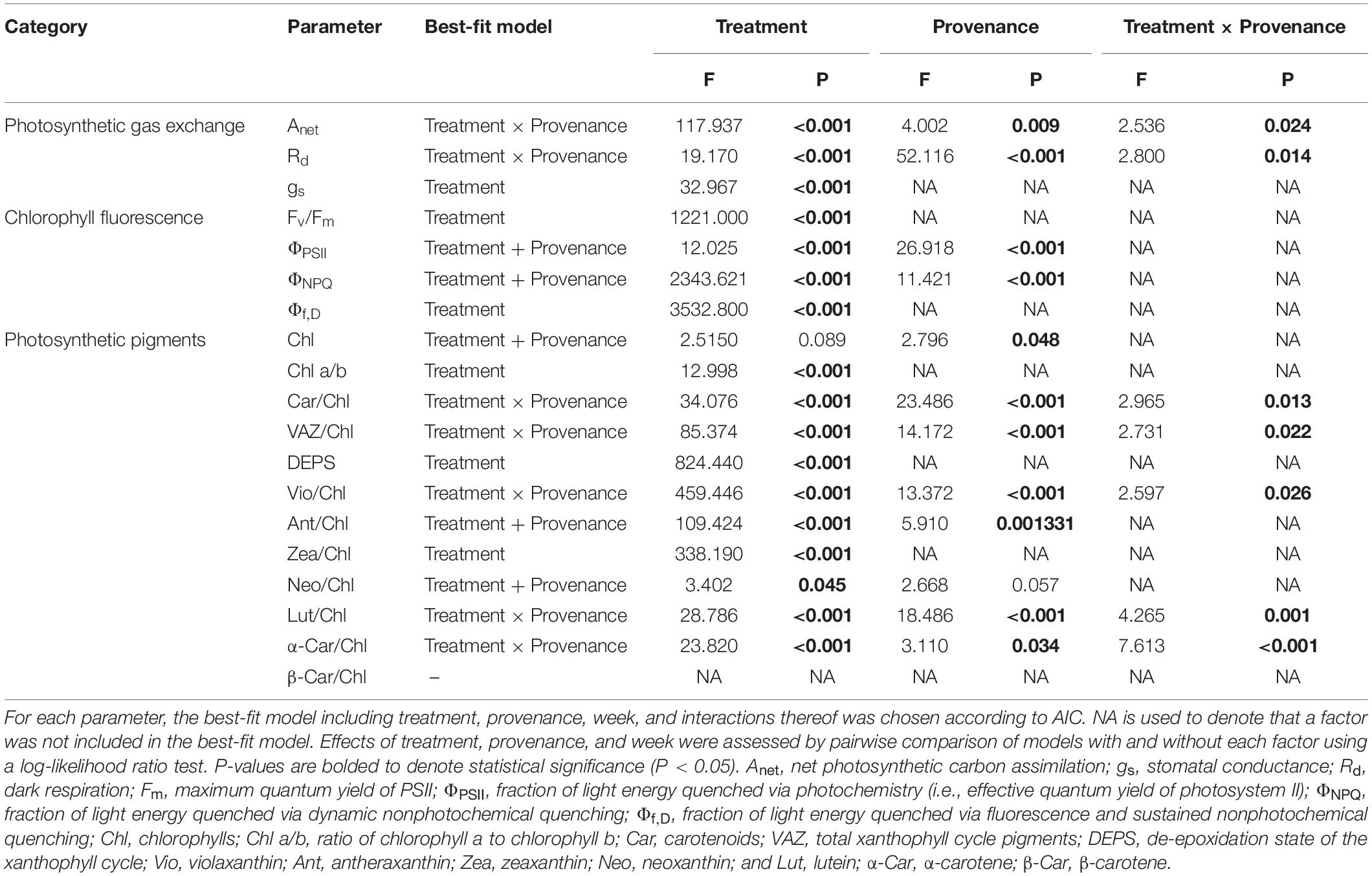
Table 2. Effect of treatment and provenance on photosynthetic gas exchange, chlorophyll fluorescence, photosynthetic pigments, and freezing tolerance.
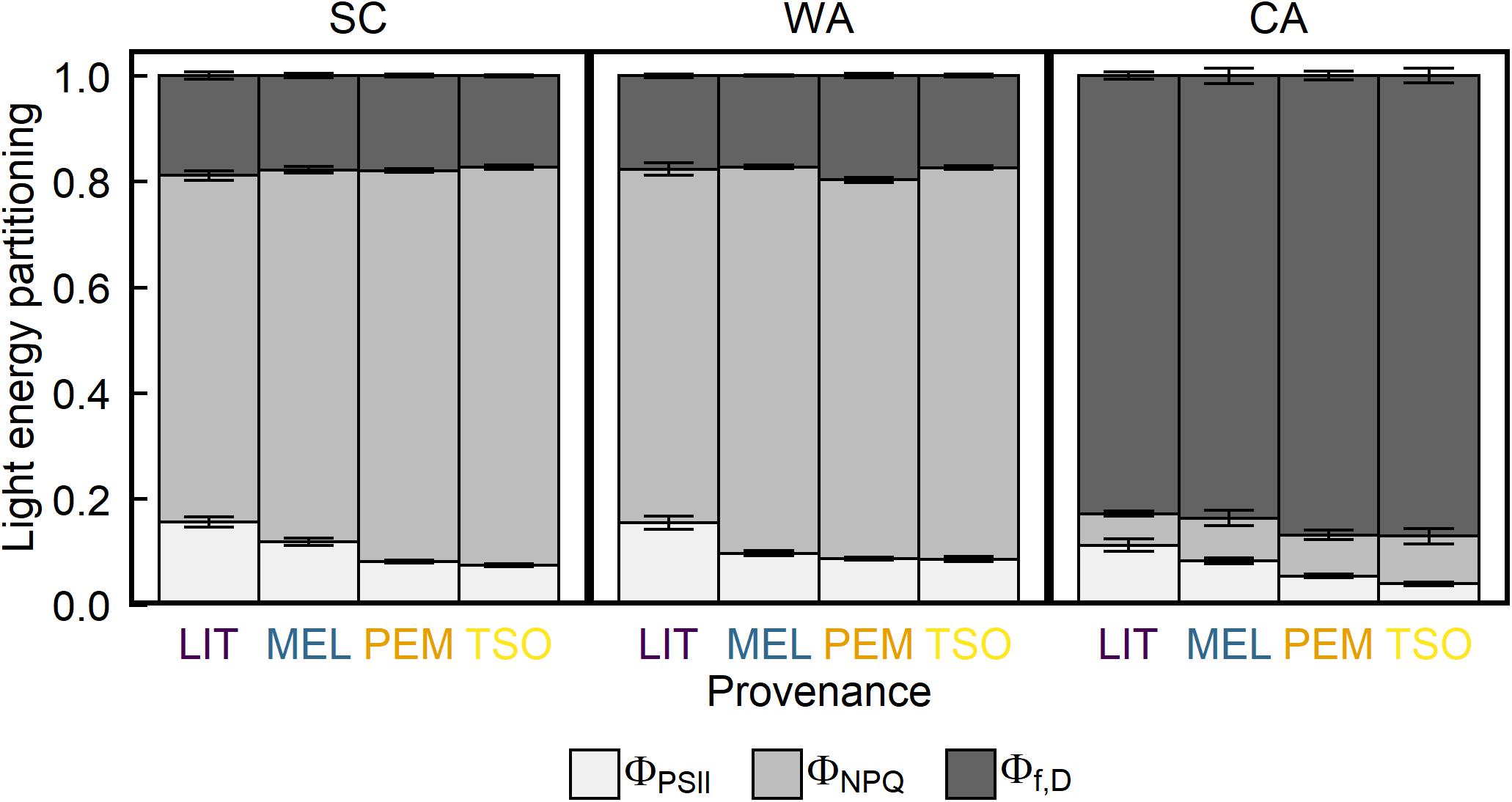
Figure 4. Response of light energy partitioning of interior (LIT, MEL) and coastal (PEM, TSO) Douglas-fir seedlings to summer control (SC), warm autumn (WA), and cool autumn (CA) conditions. Off-white represents ΦPSII, the fraction of absorbed light used for photochemistry; light gray represents ΦNPQ, dynamic NPQ; and dark gray represents Φf,D, the sum of fluorescence and sustained NPQ. Measurements were taken at 1,500 μmol m– 2 s– 1 light intensity under growth conditions. Bars represent the mean of n = 10 ± SE.
Photosynthetic Pigments
After acclimation to WA and CA, there were no significant changes in total chlorophylls (Chl) (Table 2). However, there were small but significant decreases in the ratio of chlorophyll a to chlorophyll b (Chl a/b) for all provenances under WA and CA (P < 0.01; Figure 5B). In contrast, acclimation to WA and CA caused increases in total carotenoids (Car), lutein (Lut), and total xanthophyll cycle pigments (VAZ) (Figures 5C–E). After acclimation to WA, Car, Lut, and VAZ increased in seedlings of the interior provenances only. After acclimation to CA, however, they increased in seedlings of both the interior and coastal provenances. Under CA compared with SC and WA, VAZ was significantly higher (approximately 40–50%) in seedlings of all provenances (P < 0.01; Figure 5E). The DEPS of the xanthophyll cycle increased only in response to CA conditions (Figure 5F). Under CA compared with SC and WA, DEPS was 3.5–5 times higher in seedlings of all provenances (P < 0.01).
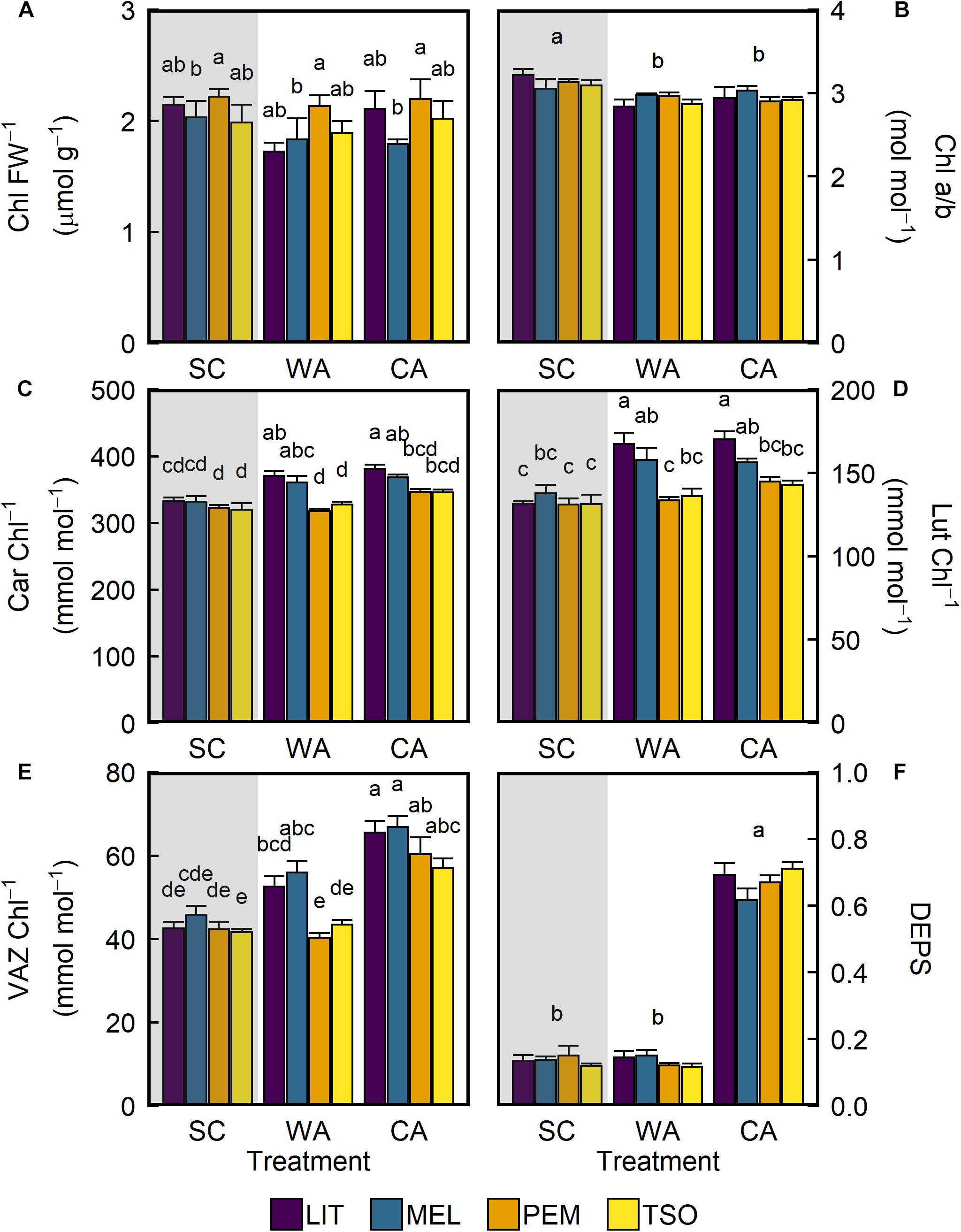
Figure 5. Response of photosynthetic leaf pigments of interior (LIT, MEL) and coastal (PEM, TSO) Douglas-fir seedlings to summer control (SC), warm autumn (WA), and cool autumn (CA) conditions. (A) Total chlorophylls per fresh weight (Chl); (B) ratio of chlorophyll a to chlorophyll b (Chl a/b); (C) total carotenoids per Chl (Car); (D) amount of lutein per Chl (Lut); (E) total xanthophyll cycle pigments per Chl (VAZ); (F) de-epoxidation state of the xanthophyll cycle (DEPS). Gray background indicates long photoperiod (summer); white background indicates short photoperiod (autumn). Bars represent the mean of n = 5 ± SE. Letters where present indicate statistically different groups (P < 0.05) as determined by Tukey’s HSD test.
Freezing Tolerance
The freezing temperature corresponding to 50% reduction in Fv/Fm (LT50) did not significantly differ between seedlings acclimated to WA and seedlings acclimated to CA (Figure 6). However, LT50 of the interior provenances LIT and MEL was approximately 8–9°C higher than that of the coastal provenances PEM and TSO (P < 0.01).
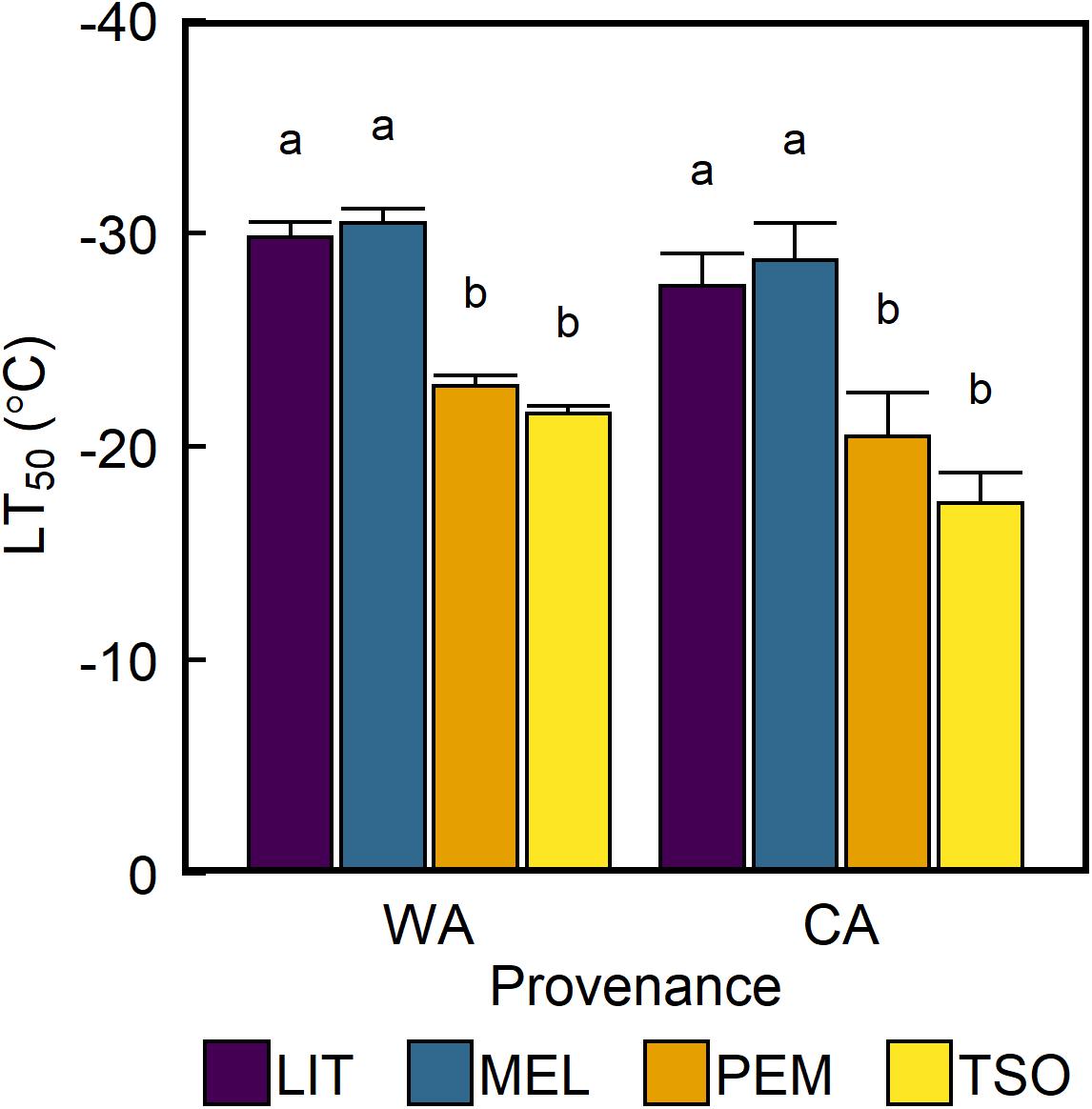
Figure 6. Response of leaf freezing tolerance of interior (LIT, MEL) and coastal (PEM, TSO) Douglas-fir seedlings to warm autumn (WA) and cool autumn (CA) conditions. Bars represent the mean LT50 of n = 5 ± SE, estimated using sigmoidal vulnerability curves fit to the data (Supplementary Figure 3). Different letters where present indicate statistically different groups (P < 0.05) as determined by log-likelihood ratio test.
Discussion
Downregulation of Photosynthesis and Upregulation of Photoprotection in Response to CA Conditions
The transition from simulated SC conditions to simulated CA conditions caused substantial decreases in photosynthetic carbon assimilation (Figure 2A) and PSII efficiency (Figure 3). These changes are indicative of the downregulation of photosynthesis that is expected in evergreen conifers undergoing cold acclimation during autumn (Wong et al., 2019; Chang et al., 2020). The near-zero levels of Anet and Fv/Fm in our seedlings after 6 weeks of acclimation to CA were comparable with those observed in mature interior Douglas-fir growing under field conditions during mid-winter (Adams et al., 2002). Unlike in other evergreen conifer species, such as white pine (Chang et al., 2016; Fréchette et al., 2016) and Scots pine (Ottander et al., 1995; Ensminger et al., 2004), we did not observe that the degradation of chlorophyll pigments contributed to the downregulation of photosynthesis in our Douglas-fir seedlings following acclimation to low temperatures (Figure 5A). However, it is likely that the degradation of the PSII protein D1 contributed to the observed downregulation of photosynthesis, as has been demonstrated in field-grown white pine (Verhoeven et al., 2009) and Douglas-fir (Ebbert et al., 2005). The additional reduction of Rd in all provenances except PEM under CA conditions suggests a general downregulation in metabolic processes in these seedlings. While counterintuitive, the higher levels of Rd observed in the interior provenances LIT and MEL compared with the coastal provenances PEM and TSO are consistent with studies that report a general trend of higher Rd in trees originating from colder, high-elevation environments (Mitchell et al., 1999). This trend has been found to persist even in common growing environments similar to our own (Reich et al., 1996; Oleksyn et al., 1998).
The low Fv/Fm observed in our CA seedlings indicates photoinhibition of PSII caused by excess light energy, which was likely caused by the combination of light and low temperature (Ensminger et al., 2006). The transition from simulated summer conditions to simulated CA conditions caused a substantial shift in the quenching of excess light energy from dynamic NPQ to sustained NPQ (Figure 4). Levels of Φf,D in our seedlings after 6 weeks of acclimation to CA were comparable with those observed in field-grown white spruce seedlings during mid-winter (D’Odorico et al., 2020). This change in energy partitioning corresponded with increased quantities of the photoprotective xanthophyll carotenoids lutein, violaxanthin, antheraxanthin, and zeaxanthin (Figures 5D,E). Increased lutein content indicates an increased capacity for quenching of chlorophyll triplet states that generate photodamaging ROS (Dall’Osto et al., 2006; Jahns and Holzwarth, 2012). Increased violaxanthin, antheraxanthin, and zeaxanthin contents imply a general upregulation of xanthophyll cycle-dependent NPQ mechanisms. However, the increased xanthophyll cycle DEPS (Figure 5F) reflects a longer retention of energy quenching zeaxanthin, which indicates an upregulation of a sustained mechanism of zeaxanthin-dependent thermal energy dissipation (Demmig-Adams and Adams, 2006; Verhoeven, 2014). Similar to the findings of Fréchette et al. (2016), our Douglas-fir seedlings also retained some dynamic NPQ functionality under CA conditions. ΦNPQ increased in response to increasing light intensity (Supplementary Figure 1), highlighting its continued importance as a short-term response to light stress even under low temperatures.
Warm Temperature Delays the Downregulation of Photosynthesis and Development of Sustained NPQ in Seedlings Growing Under Short Autumn Photoperiod
In contrast to CA, the transition from simulated summer conditions to conditions where we simulated the anticipated future warmer autumn did not affect photosynthetic carbon assimilation (Figure 2A) or PSII efficiency (Figure 3). Even after 6 weeks of exposure to short photoperiod, Anet and Fv/Fm remained largely unchanged under WA temperature. This suggests that short photoperiod alone does not trigger the downregulation of photosynthesis in Douglas-fir seedlings. This is in contrast to previous findings in white pine (Fréchette et al., 2016, 2020), jack pine (P. banksiana; Busch et al., 2007), and Scots pine (Vogg et al., 1998). The downregulation of photosynthesis has been linked to reduced metabolic sink capacity due to short photoperiod-induced growth cessation (Savitch et al., 2002; Busch et al., 2007; Hamilton et al., 2016). We did not measure growth cessation or bud formation, so it is unclear to what degree growth acted as a metabolic sink in our WA seedlings. Ford et al. (2017) used a modeling approach to determine that growth cessation is primarily induced by short photoperiod in provenances originating from low elevations that are adapted to warmer autumn temperatures compared with provenances originating from higher elevations that are adapted to colder autumn temperatures. The lack of downregulation of photosynthesis under WA conditions in our warmer low-elevation coastal provenances suggests that growth cessation may not be a major factor influencing the downregulation of photosynthesis in Douglas-fir. Photoassimilates produced during continued photosynthesis under WA were likely used to drive the metabolically expensive alterations that contribute to the accumulation of photoprotective carotenoid pigments and the development of cold hardiness (discussed below). Distribution of carbon through roots to soil organisms has been demonstrated to increase substantially at the end of the growing season in Scots pine forests (Högberg et al., 2010), presenting another potential sink for photoassimilates produced during autumn under warm temperatures.
In Interior Seedlings, Warm Temperature Does Not Impair the Accumulation of Photoprotective Carotenoid Pigments That Is Observed During Cold Acclimation and Growth Under Short Autumn Photoperiod
Following the transition from simulated summer conditions to simulated WA conditions, absorbed light energy that was in excess of the capacity for photochemistry and required safe dissipation was primarily quenched via dynamic NPQ (Figure 4). This indicates that the development of sustained NPQ during autumn cold acclimation requires low temperature in Douglas-fir, which is consistent with recent findings in white pine (Chang et al., 2016; Fréchette et al., 2016). Accordingly, the DEPS of the photoprotective xanthophyll cycle also remained unchanged under WA conditions (Figure 5F). Interestingly, the interior provenances LIT and MEL increased their photoprotective capacity during WA through increases in carotenoid pigments (Figure 5C). The increased levels of lutein under WA were identical to those under CA (Figure 5D), suggesting that lutein accumulates only in response to short photoperiod in these provenances. The increase in xanthophyll cycle pigments in LIT and MEL under WA is surprising (Figure 5E) considering that there were no corresponding increases in dynamic or sustained NPQ (Figure 4). The short photoperiod sensitivity of these metabolic changes may reflect an adaptation to the sub-zero temperatures that colder high elevation provenances, such as LIT and MEL, experience during autumn (Table 1). Since temperature is a less reliable signal during the summer–autumn transition (Way and Montgomery, 2015), these seedlings may utilize shortening photoperiod to initiate changes in photoprotective pigments prior to low temperature exposure that causes excess light stress. Xanthophyll cycle activity can be rapidly induced by exposure to low temperature under controlled conditions in white pine (Chang et al., 2016; Fréchette et al., 2016). If this is also true in Douglas-fir under field conditions, the short photoperiod sensitivity of carotenoid accumulation in interior Douglas-fir may actually provide better photoprotection during sporadic cold exposure in future warmer autumns.
Warm Temperature Does Not Impair the Development of Freezing Tolerance in Seedlings Growing Under Short Autumn Photoperiod
Levels of freezing tolerance in our seedlings after 6 weeks of acclimation to CA (Figure 6) were consistent with those exhibited by Douglas-fir seedlings under late autumn field conditions in a coastal site (Guak et al., 1998) as well as by mature Douglas-fir under mid-autumn field conditions in a mountain site (Bansal et al., 2016). In field-grown white pine seedlings, the development of freezing tolerance begins in response to shortening photoperiod and rapidly increases following exposure to sub-zero temperatures (Chang et al., 2016). Field experiments employing elevated temperatures of approximately +1.5/+3°C day/night have shown no change in freezing tolerance development compared with ambient autumn conditions (Riikonen et al., 2012; Chang et al., 2015). However, chamber experiments using larger temperature increases in the same range as the one used in our experiment have demonstrated delayed development of freezing tolerance under autumn warming (Repo et al., 1996; Guak et al., 1998; Chang et al., 2016). Guak et al. (1998) observed delayed development of freezing tolerance in Douglas-fir seedlings in response to only +4°C of warming applied continuously year round in open-top chambers. In contrast to this experiment and our expectations, we observed no difference in freezing tolerance between seedlings acclimated to CA and WA conditions. We also observed higher freezing tolerance in seedlings of the interior provenances versus coastal provenances, which is consistent with the observations of Bansal et al. (2016) that indicated provenances with colder autumn climates develop greater freezing tolerance. Nevertheless, WA conditions induced in all seedlings the development of freezing tolerance sufficient for the winter temperature minimums projected across the range of Douglas-fir in British Columbia (Arora et al., 2011; Wang et al., 2012), even under the highest emissions scenario. These results suggest that Douglas-fir possesses a degree of short photoperiod sensitivity that will help conserve their freezing tolerance development under future warmer climates.
Conclusion
Our findings indicate that low temperature is the dominant signal for the downregulation of photosynthesis and upregulation of photoprotection in Douglas-fir seedlings during autumn. As we hypothesized, warming disrupts these key components of the autumn cold acclimation process, resulting in prolonged photosynthetic activity. Interior Douglas-fir does not appear to be maladapted to WA temperatures compared with coastal Douglas-fir. They exhibited unique accumulation of photoprotective carotenoid pigments in response to short photoperiod, which may be a benefit during low temperature events in future warmer autumns. Contrary to our hypothesis, the development of freezing tolerance does not appear to require both low temperature and short photoperiod signals in Douglas-fir. The short photoperiod sensitivity of the development of freezing tolerance appears to provide a degree of frost resistance that is sufficient for winter temperatures projected across Douglas-fir’s range. Thus, even coastal Douglas-fir appears to be adapted to frost risk at higher latitudes and elevations. Based on our findings, Douglas-fir from interior and coastal origin may benefit from an extended carbon uptake period resulting from autumn warming due to changing climate, without the costs associated with the disruption of cold acclimation. This distinguishes Douglas-fir from species of pine assessed in similar studies, which have demonstrated both limitation of photosynthesis under short autumn photoperiod and limitation of cold hardiness under warm autumn temperature (Repo et al., 1996; Vogg et al., 1998; Chang et al., 2016; Fréchette et al., 2016, 2020). While our study focused on the effects of warming, rising CO2 and episodes of drought are concurrent with rising temperature under natural climate conditions. Elevated CO2 was demonstrated to additionally delay the downregulation of photosynthesis in white pine (Chang et al., 2016) and Scots pine (Wang, 1996). If this pattern holds for Douglas-fir, rising CO2 may further prolong the period of photosynthetic activity under future climate. Albeit the potential carbon gains caused by elevated CO2 and warming during autumn, it is crucial to emphasize that these carbon gains might be offset by loss in carbon uptake resulting from water stress and drought due to climate warming (Spittlehouse, 2003).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
DN performed all experiments and collected the experimental data. DN and VV analyzed the data with input from IE. DN and IE wrote the manuscript. All authors designed the experiments, and reviewed, and edited the final manuscript.
Funding
IE acknowledges support by the NSERC (RGPIN-2020-06928), the Canadian Foundation for Innovation (CFI) (grant no. 27330), and the Ontario Ministry of Research and Innovation (grant no. ER10-07-015). Funding for the CoAdapTree project is provided by Genome Canada (241REF), Genome British Columbia, and 16 other sponsors (https://coadaptree.forestry.ubc.ca/sponsors/).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the CoAdapTree project seed contributors (https://coadaptree.forestry.ubc.ca/seed-contributors/). We are grateful to Chris Wong for his expertise and assistance with photosynthetic pigment analysis, as well as Sepideh Torabi, Tomoyuki Sen, and Annika Elimelech for their assistance with growing the plants used for this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2021.688534/full#supplementary-material
References
Adams, W. W. III, Demmig-Adams, B., Rosenstiel, T. N., Brightwell, A. K., and Ebbert, V. (2002). Photosynthesis and photoprotection in overwintering plants. Plant Biol. 4, 545–557. doi: 10.1055/s-2002-35434
Aitken, S. N., Yeaman, S., Holliday, J. A., Wang, T., and Curtis-McLane, S. (2008). Adaptation, migration or extirpation: climate change outcomes for tree populations. Evol. Appl. 1, 95–111. doi: 10.1111/j.1752-4571.2007.00013.x
Akaike, H. (1998). “Information theory and an extension of the maximum likelihood principle,” in Selected Papers of Hirotugu Akaike Springer Series in Statistics (Perspectives in Statistics), eds E. Parzen, K. Tanabe, and G. Kitagawa (New York, NY: Springer), 199–213. doi: 10.1007/978-1-4612-1694-0_15
Angelcheva, L., Mishra, Y., Antti, H., Kjellsen, T. D., Funk, C., Strimbeck, R. G., et al. (2014). Metabolomic analysis of extreme freezing tolerance in Siberian spruce (Picea obovata). New Phytol. 204, 545–555. doi: 10.1111/nph.12950
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Arora, V. K., Scinocca, J. F., Boer, G. J., Christian, J. R., Denman, K. L., Flato, G. M., et al. (2011). Carbon emission limits required to satisfy future representative concentration pathways of greenhouse gases. Geophys. Res. Lett. 38:L05805. doi: 10.1029/2010GL046270
Bansal, S., Harrington, C. A., Gould, P. J., and St.Clair, J. B. (2015). Climate-related genetic variation in drought-resistance of Douglas-fir (Pseudotsuga menziesii). Glob. Change Biol. 21, 947–958. doi: 10.1111/gcb.12719
Bansal, S., Harrington, C. A., and St.Clair, J. B. (2016). Tolerance to multiple climate stressors: a case study of Douglas-fir drought and cold hardiness. Ecol. Evol. 6, 2074–2083. doi: 10.1002/ece3.2007
Barber, J., and Andersson, B. (1992). Too much of a good thing: light can be bad for photosynthesis. Trends Biochem. Sci. 17, 61–66. doi: 10.1016/0968-0004(92)90503-2
Beuker, E., Valtonen, E., and Repo, T. (1998). Seasonal variation in the frost hardiness of Scots pine and Norway spruce in old provenance experiments in Finland. For. Ecol. Manage. 107, 87–98. doi: 10.1016/S0378-1127(97)00344-7
Busch, F., Hüner, N. P. A., and Ensminger, I. (2007). Increased air temperature during simulated autumn conditions does not increase photosynthetic carbon gain but affects the dissipation of excess energy in seedlings of the evergreen conifer jack pine. Plant Physiol. 143, 1242–1251. doi: 10.1104/pp.106.092312
Carter, K. K. (1996). Provenance tests as indicators of growth response to climate change in 10 north temperate tree species. Can. J. For. Res. 26, 1089–1095. doi: 10.1139/x26-120
Chang, C. Y., Bräutigam, K., Hüner, N. P. A., and Ensminger, I. (2020). Champions of winter survival: cold acclimation and molecular regulation of cold hardiness in evergreen conifers. New Phytol. 229, 675–691. doi: 10.1111/nph.16904
Chang, C. Y., Unda, F., Zubilewich, A., Mansfield, S. D., and Ensminger, I. (2015). Sensitivity of cold acclimation to elevated autumn temperature in field-grown Pinus strobus seedlings. Front. Plant Sci. 6:165. doi: 10.3389/fpls.2015.00165
Chang, C. Y.-Y., Fréchette, E., Unda, F., Mansfield, S. D., and Ensminger, I. (2016). Elevated temperature and CO2 stimulate late season photosynthesis but impair cold hardening in pine. Plant Physiol. 172, 802–818. doi: 10.1104/pp.16.00753
Chen, J., Källman, T., Ma, X., Gyllenstrand, N., Zaina, G., Morgante, M., et al. (2012). Disentangling the roles of history and local selection in shaping clinal variation of allele frequencies and gene expression in Norway spruce (Picea abies). Genetics 191, 865–881. doi: 10.1534/genetics.112.140749
Close, T. J. (1997). Dehydrins: a commonalty in the response of plants to dehydration and low temperature. Physiol. Plant. 100, 291–296. doi: 10.1111/j.1399-3054.1997.tb04785.x
Corlett, R. T., and Westcott, D. A. (2013). Will plant movements keep up with climate change? Trends Ecol. Evol. 28, 482–488. doi: 10.1016/j.tree.2013.04.003
Crosatti, C., Rizza, F., Badeck, F. W., Mazzucotelli, E., and Cattivelli, L. (2013). Harden the chloroplast to protect the plant. Physiol. Plant. 147, 55–63. doi: 10.1111/j.1399-3054.2012.01689.x
Dall’Osto, L., Lico, C., Alric, J., Giuliano, G., Havaux, M., and Bassi, R. (2006). Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 6:32. doi: 10.1186/1471-2229-6-32
Demmig-Adams, B., and Adams, W. W. (1992). Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 599–626. doi: 10.1146/annurev.pp.43.060192.003123
Demmig-Adams, B., and Adams, W. W. (2006). Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol. 172, 11–21. doi: 10.1111/j.1469-8137.2006.01835.x
Demmig-Adams, B., Cohu, C. M., Muller, O., and Adams, W. W. (2012). Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth. Res. 113, 75–88. doi: 10.1007/s11120-012-9761-6
D’Odorico, P., Besik, A., Wong, C. Y. S., Isabel, N., and Ensminger, I. (2020). High-throughput drone-based remote sensing reliably tracks phenology in thousands of conifer seedlings. New Phytol. 226, 1667–1681. doi: 10.1111/nph.16488
Ebbert, V., Adams Iii, W. W., Mattoo, A. K., Sokolenko, A., and Demmig-Adams, B. (2005). Up-regulation of a photosystem II core protein phosphatase inhibitor and sustained D1 phosphorylation in zeaxanthin-retaining, photoinhibited needles of overwintering Douglas fir. Plant Cell Environ. 28, 232–240. doi: 10.1111/j.1365-3040.2004.01267.x
Eckert, A. J., Bower, A. D., Wegrzyn, J. L., Pande, B., Jermstad, K. D., Krutovsky, K. V., et al. (2009). Association genetics of coastal Douglas fir (Pseudotsuga menziesii var. menziesii, Pinaceae). I. Cold-hardiness related traits. Genetics 182, 1289–1302. doi: 10.1534/genetics.109.102350
Eilmann, B., de Vries, S. M. G., den Ouden, J., Mohren, G. M. J., Sauren, P., and Sass-Klaassen, U. (2013). Origin matters! Difference in drought tolerance and productivity of coastal Douglas-fir (Pseudotsuga menziesii (Mirb.)) provenances. For. Ecol. Manage. 302, 133–143. doi: 10.1016/j.foreco.2013.03.031
Ensminger, I., Busch, F., and Huner, N. P. A. (2006). Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiol. Plant. 126, 28–44. doi: 10.1111/j.1399-3054.2006.00627.x
Ensminger, I., Sveshnikov, D., Campbell, D. A., Funk, C., Jansson, S., Lloyd, J., et al. (2004). Intermittent low temperatures constrain spring recovery of photosynthesis in boreal Scots pine forests. Glob. Change Biol. 10, 995–1008. doi: 10.1111/j.1365-2486.2004.00781.x
Ford, K. R., Harrington, C. A., and St.Clair, J. B. (2017). Photoperiod cues and patterns of genetic variation limit phenological responses to climate change in warm parts of species’ range: Modeling diameter-growth cessation in coast Douglas-fir. Glob. Change Biol. 23, 3348–3362. doi: 10.1111/gcb.13690
Fréchette, E., Chang, C. Y., and Ensminger, I. (2020). Variation in the phenology of photosynthesis among eastern white pine provenances in response to warming. Glob. Change Biol. 26, 5217–5234. doi: 10.1111/gcb.15150
Fréchette, E., Chang, C. Y.-Y., and Ensminger, I. (2016). Photoperiod and temperature constraints on the relationship between the photochemical reflectance index and the light use efficiency of photosynthesis in Pinus strobus. Tree Physiol. 36, 311–324. doi: 10.1093/treephys/tpv143
Genty, B., Briantais, J.-M., and Baker, N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA) Gen. Subjects 990, 87–92. doi: 10.1016/S0304-4165(89)80016-9
Gray, L. K., and Hamann, A. (2013). Tracking suitable habitat for tree populations under climate change in western North America. Clim. Change 117, 289–303. doi: 10.1007/s10584-012-0548-8
Guak, S., Olsyzk, D. M., Fuchigami, L. H., and Tingey, D. T. (1998). Effects of elevated CO2 and temperature on cold hardiness and spring bud burst and growth in Douglas-fir (Pseudotsuga menziesii). Tree Physiol. 18, 671–679. doi: 10.1093/treephys/18.10.671
Hamilton, J. A., El Kayal, W., Hart, A. T., Runcie, D. E., Arango-Velez, A., and Cooke, J. E. K. (2016). The joint influence of photoperiod and temperature during growth cessation and development of dormancy in white spruce (Picea glauca). Tree Physiol. 36, 1432–1448. doi: 10.1093/treephys/tpw061
Hänninen, H. (2016). Boreal and Temperate Trees in a Changing Climate. Dordrecht: Springer, doi: 10.1007/978-94-017-7549-6
Hendrickson, L., Furbank, R. T., and Chow, W. S. (2004). A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth. Res. 82, 73–81. doi: 10.1023/B:PRES.0000040446.87305.f4
Hess, M., Wildhagen, H., Junker, L. V., and Ensminger, I. (2016). Transcriptome responses to temperature, water availability and photoperiod are conserved among mature trees of two divergent Douglas-fir provenances from a coastal and an interior habitat. BMC Genomics 17:780. doi: 10.1186/s12864-016-3022-6
Högberg, M. N., Briones, M. J. I., Keel, S. G., Metcalfe, D. B., Campbell, C., Midwood, A. J., et al. (2010). Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytol. 187, 485–493. doi: 10.1111/j.1469-8137.2010.03274.x
Horton, P., Johnson, M. P., Perez-Bueno, M. L., Kiss, A. Z., and Ruban, A. V. (2008). Photosynthetic acclimation: Does the dynamic structure and macro-organisation of photosystem II in higher plant grana membranes regulate light harvesting states? FEBS J. 275, 1069–1079. doi: 10.1111/j.1742-4658.2008.06263.x
Howe, G., Jayawickrama, K., and Cherry, M. (2006). “Breeding douglas-fir,” in Plant Breeding Reviews, ed. J. Janick (Hoboken, NJ: John Wiley & Sons), 245–353. doi: 10.1002/9780470650349.ch6
Howe, G. T., Aitken, S. N., Neale, D. B., Jermstad, K. D., Wheeler, N. C., and Chen, T. H. (2003). From genotype to phenotype: unraveling the complexities of cold adaptation in forest trees. Can. J. Bot. 81, 1247–1266. doi: 10.1139/b03-141
Hüner, N. P. A., Bode, R., Dahal, K., Busch, F. A., Possmayer, M., Szyszka, B., et al. (2013). Shedding some light on cold acclimation, cold adaptation, and phenotypic plasticity. Botany 91, 127–136. doi: 10.1139/cjb-2012-0174
Hüner, N. P. A., Öquist, G., and Sarhan, F. (1998). Energy balance and acclimation to light and cold. Trends Plant Sci. 3, 224–230. doi: 10.1016/S1360-1385(98)01248-5
IPCC (2014). in Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds Core Writing Team R. K. Pachauri and L. E. Mayer (Geneva: IPCC). doi: 10.1016/s1360-1385(98)01248-5
Isaac-Renton, M. G., Roberts, D. R., Hamann, A., and Spiecker, H. (2014). Douglas-fir plantations in Europe: a retrospective test of assisted migration to address climate change. Glob. Change Biol. 20, 2607–2617. doi: 10.1111/gcb.12604
Jahns, P., and Holzwarth, A. R. (2012). The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta (BBA) Bioenerg. 1817, 182–193. doi: 10.1016/j.bbabio.2011.04.012
Janka, E., Körner, O., Rosenqvist, E., and Ottosen, C.-O. (2015). Using the quantum yields of photosystem II and the rate of net photosynthesis to monitor high irradiance and temperature stress in chrysanthemum (Dendranthema grandiflora). Plant Physiol. Biochem. 90, 14–22. doi: 10.1016/j.plaphy.2015.02.019
Junker, L. V., and Ensminger, I. (2016). Fast detection of leaf pigments and isoprenoids for ecophysiological studies, plant phenotyping and validating remote-sensing of vegetation. Physiol. Plant. 158, 369–381. doi: 10.1111/ppl.12512
Kingston-Smith, A. H., Harbinson, J., Williams, J., and Foyer, C. H. (1997). Effect of chilling on carbon assimilation, enzyme activation, and photosynthetic electron transport in the absence of photoinhibition in maize leaves. Plant Physiol. 114, 1039–1046. doi: 10.1104/pp.114.3.1039
Krieger-Liszkay, A., Fufezan, C., and Trebst, A. (2008). Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 98, 551–564. doi: 10.1007/s11120-008-9349-3
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. B. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82:26. doi: 10.18637/jss.v082.i13
Lenth, R. V. (2016). Least-squares means: the R package lsmeans. J. Stat. Softw. 69, 1–33. doi: 10.18637/jss.v069.i01
Maurya, J. P., and Bhalerao, R. P. (2017). Photoperiod- and temperature-mediated control of growth cessation and dormancy in trees: a molecular perspective. Ann. Bot. 120, 351–360. doi: 10.1093/aob/mcx061
Mitchell, K. A., Bolstad, P. V., and Vose, J. M. (1999). Interspecific and environmentally induced variation in foliar dark respiration among eighteen southeastern deciduous tree species. Tree Physiol. 19, 861–870. doi: 10.1093/treephys/19.13.861
Moellering, E. R., Muthan, B., and Benning, C. (2010). Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330, 226–228. doi: 10.1126/science.1191803
Müller, P., Li, X.-P., and Niyogi, K. K. (2001). Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125, 1558–1566. doi: 10.1104/pp.125.4.1558
Mullin, T., Andersson, B., Bastien, J., Beaulieu, J., Burdon, R., Dvorak, W., et al. (2011). “Economic importance, breeding objectives and achievements,” in Genetics, Genomics and Breeding of Conifers, ed. C. Kole (Enfield, NH: Science Publishers), doi: 10.1201/b11075-3
Oleksyn, J., Modrzynski, J., Tjoelker, M. G., Z.ytkowiak, R., Reich, P. B., and Karolewski, P. (1998). Growth and physiology of Picea abies populations from elevational transects: common garden evidence for altitudinal ecotypes and cold adaptation. Funct. Ecol. 12, 573–590. doi: 10.1046/j.1365-2435.1998.00236.x
Oleksyn, J., Zytkowiak, R., Karolewski, P., Reich, P. B., and Tjoelker, M. G. (2000). Genetic and environmental control of seasonal carbohydrate dynamics in trees of diverse Pinus sylvestris populations. Tree Physiol. 20, 837–847. doi: 10.1093/treephys/20.12.837
Öquist, G., and Hüner, N. P. A. (2003). Photosynthesis of overwintering evergreen plants. Annu. Rev. Plant Biol. 54, 329–355. doi: 10.1146/annurev.arplant.54.072402.115741
Ottander, C., Campbell, D., and Oquist, G. (1995). Seasonal changes in photosystem II organisation and pigment composition in Pinus syivestris. Planta 197, 176–183.
Palacio, S., Hoch, G., Sala, A., Körner, C., and Millard, P. (2014). Does carbon storage limit tree growth? New Phytol. 201, 1096–1100. doi: 10.1111/nph.12602
Porcar-Castell, A., Juurola, E., Ensminger, I., Berninger, F., Hari, P., and Nikinmaa, E. (2008). Seasonal acclimation of photosystem II in Pinus sylvestris. II. Using the rate constants of sustained thermal energy dissipation and photochemistry to study the effect of the light environment. Tree Physiol. 28, 1483–1491. doi: 10.1093/treephys/28.10.1483
R Development Core Team (2010). R: A Language and Environment for Statistical Computing. Vienna: R Core Team.
Rehfeldt, G. E., Leites, L. P., St Clair, J. B., Jaquish, B. C., Sáenz-Romero, C., López-Upton, J., et al. (2014). Comparative genetic responses to climate in the varieties of Pinus ponderosa and Pseudotsuga menziesii: Clines in growth potential. For. Ecol. Manage. 324, 138–146. doi: 10.1016/j.foreco.2014.02.041
Reich, P. B., Oleksyn, J., and Tjoelker, M. G. (1996). Needle respiration and nitrogen concentration in Scots pine populations from a broad latitudinal range: a common garden test with field-grown trees. Funct. Ecol. 10, 768–776. doi: 10.2307/2390512
Repo, T., Hanninen, H., and Kellomaki, S. (1996). The effects of long-term elevation of air temperature and CO2 on the frost hardiness of Scots pine. Plant Cell Environ. 19, 209–216. doi: 10.1111/j.1365-3040.1996.tb00242.x
Repo, T., Zhang, G., Ryyppö, A., Rikala, R., and Vuorinen, M. (2000). The relation between growth cessation and frost hardening in Scots pines of different origins. Trees 14, 456–464. doi: 10.1007/s004680000059
Riikonen, J., Kontunen-Soppela, S., Ossipov, V., Tervahauta, A., Tuomainen, M., Oksanen, E., et al. (2012). Needle metabolome, freezing tolerance and gas exchange in Norway spruce seedlings exposed to elevated temperature and ozone concentration. Tree Physiol. 32, 1102–1112. doi: 10.1093/treephys/tps072
Ritz, C., Baty, F., Streibig, J. C., and Gerhard, D. (2015). Dose-response analysis using R. PLoS One 10:e0146021. doi: 10.1371/journal.pone.0146021
Rossi, S., Deslauriers, A., Anfodillo, T., Morin, H., Saracino, A., Motta, R., et al. (2006). Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 170, 301–310. doi: 10.1111/j.1469-8137.2006.01660.x
Rossi, S., Deslauriers, A., Griçar, J., Seo, J.-W., Rathgeber, C. B., Anfodillo, T., et al. (2008). Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 17, 696–707. doi: 10.1111/j.1466-8238.2008.00417.x
Savitch, L. V., Leonardos, E. D., Krol, M., Jansson, S., Grodzinski, B., Huner, N. P. A., et al. (2002). Two different strategies for light utilization in photosynthesis in relation to growth and cold acclimation. Plant Cell Environ. 25, 761–771. doi: 10.1046/j.1365-3040.2002.00861.x
Savolainen, O., Pyhäjärvi, T., and Knürr, T. (2007). Gene flow and local adaptation in trees. Annu. Rev. Ecol. Evol. Syst. 38, 595–619. doi: 10.1146/annurev.ecolsys.38.091206.095646
Singh, R. K., Svystun, T., AlDahmash, B., Jönsson, A. M., and Bhalerao, R. P. (2017). Photoperiod- and temperature-mediated control of phenology in trees–a molecular perspective. New Phytol. 213, 511–524. doi: 10.1111/nph.14346
Spittlehouse, D. L. (2003). Water availability, climate change and the growth of Douglas-Fir in the Georgia Basin. Can. Water Resour. J. Rev. Can. Ressour. Hydriques 28, 673–688. doi: 10.4296/cwrj2804673
St Clair, J. B., Mandel, N. L., and Vance-Borland, K. W. (2005). Genecology of douglas fir in Western Oregon and Washington. Ann. Bot. 96, 1199–1214. doi: 10.1093/aob/mci278
Steponkus, P. L. (1984). Role of the plasma membrane in freezing injury and cold acclimation. Annu. Rev. Plant Physiol. 35, 543–584. doi: 10.1146/annurev.pp.35.060184.002551
Stinziano, J. R., and Way, D. A. (2017). Autumn photosynthetic decline and growth cessation in seedlings of white spruce are decoupled under warming and photoperiod manipulations. Plant Cell Environ. 40, 1296–1316. doi: 10.1111/pce.12917
Strimbeck, G. R., Schaberg, P. G., Fossdal, C. G., Schröder, W. P., and Kjellsen, T. D. (2015). Extreme low temperature tolerance in woody plants. Front. Plant Sci. 6:884. doi: 10.3389/fpls.2015.00884
Sutinen, M.-L., Arora, R., Wisniewski, M., Ashworth, E., Strimbeck, R., and Palta, J. (2001). “Mechanisms of frost survival and freeze-damage in nature,” in Conifer Cold Hardiness Tree Physiology, eds F. J. Bigras and S. J. Colombo (Dordrecht: Springer), 89–120. doi: 10.1007/978-94-015-9650-3_4
Sveshnikov, D., Ensminger, I., Ivanov, A. G., Campbell, D., Lloyd, J., Funk, C., et al. (2006). Excitation energy partitioning and quenching during cold acclimation in Scots pine. Tree Physiol. 26, 325–336. doi: 10.1093/treephys/26.3.325
Thayer, S. S., and Bjorkman, O. (1990). Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth. Res. 23, 331–343. doi: 10.1007/BF00034864
Verhoeven, A. (2014). Sustained energy dissipation in winter evergreens. New Phytol. 201, 57–65. doi: 10.1111/nph.12466
Verhoeven, A., Osmolak, A., Morales, P., and Crow, J. (2009). Seasonal changes in abundance and phosphorylation status of photosynthetic proteins in eastern white pine and balsam fir. Tree Physiol. 29, 361–374. doi: 10.1093/treephys/tpn031
Vogg, G., Heim, R., Hansen, J., Schäfer, C., and Beck, E. (1998). Frost hardening and photosynthetic performance of Scots pine (Pinus sylvestris L.) needles. I. Seasonal changes in the photosynthetic apparatus and its function. Planta 204, 193–200. doi: 10.1007/s004250050246
Wang, K.-Y. (1996). Canopy CO2 exchange of Scots pine and its seasonal variation after four-year exposure to elevated CO2 and temperature. Agric. For. Meteorol. 82, 1–27. doi: 10.1016/0168-1923(96)02342-8
Wang, T., Hamann, A., Spittlehouse, D., and Carroll, C. (2016). Locally downscaled and spatially customizable climate data for historical and future periods for North America. PLoS One 11:e0156720. doi: 10.1371/journal.pone.0156720
Wang, T., Hamann, A., Spittlehouse, D. L., and Murdock, T. Q. (2012). ClimateWNA–high-resolution spatial climate data for western North America. J. Appl. Meteorol. Climatol. 51, 16–29. doi: 10.1175/JAMC-D-11-043.1
Way, D. A., and Montgomery, R. A. (2015). Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant Cell Environ. 38, 1725–1736. doi: 10.1111/pce.12431
Welling, A., Moritz, T., Palva, E. T., and Junttila, O. (2002). Independent activation of cold acclimation by low temperature and short photoperiod in hybrid aspen. Plant Physiol. 129, 1633–1641. doi: 10.1104/pp.003814
Wenny, D. L., and Dumroese, R. K. (1992). A Growing Regime for Container-Grown Douglas-fir Seedlings, Report Number: Bulletin 49. Moscow, ID: University of Idaho.
Keywords: Pseudotsuga menziesii, autumn cold acclimation, intraspecific variation, photosynthesis, photoprotection, freezing tolerance, climate change, autumn warming
Citation: Noordermeer D, Velasco VME and Ensminger I (2021) Autumn Warming Delays the Downregulation of Photosynthesis and Does Not Increase the Risk of Freezing Damage in Interior and Coastal Douglas-fir. Front. For. Glob. Change 4:688534. doi: 10.3389/ffgc.2021.688534
Received: 31 March 2021; Accepted: 23 April 2021;
Published: 03 June 2021.
Edited by:
James Blande, University of Eastern Finland, FinlandReviewed by:
Rongzhou Man, Ontario Ministry of Natural Resources and Forestry, CanadaJosé Javier Peguero-Pina, Aragon Agrifood Research and Technology Center (CITA), Spain
Copyright © 2021 Noordermeer, Velasco and Ensminger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ingo Ensminger, ingo.ensminger@utoronto.ca
 Devin Noordermeer
Devin Noordermeer Vera Marjorie Elauria Velasco
Vera Marjorie Elauria Velasco Ingo Ensminger
Ingo Ensminger