- 1Massachusetts General Hospital Institute of Health Professions, Boston, MA, USA
- 2Massachusetts Institute of Technology, Cambridge, MA, USA
- 3Marquette University, Milwaukee, WI, USA
- 4The Ohio State University, Columbus, OH, USA
Communication disorders have complex genetic origins, with constellations of relevant gene markers that vary across individuals. Some genetic variants are present in healthy individuals as well as those affected by developmental disorders. Growing evidence suggests that some variants may increase susceptibility to these disorders in the presence of other pathogenic gene mutations. In the current study, we describe eight children with specific language impairment and four of these children had a copy number variant in one of these potential susceptibility regions on chromosome 15. Three of these four children also had variants in other genes previously associated with language impairment. Our data support the theory that 15q11.2 is a susceptibility region for developmental disorders, specifically language impairment.
Introduction
Specific language impairment (SLI) is a developmental language disorder characterized by impaired oral language skills (Leonard et al., 1999; Catts et al., 2005). The disorder is typically diagnosed in the preschool years, when children normally begin speaking in more complex and complete sentences. These children have normal non-verbal IQ in spite of their problems with semantics, syntax, and discourse (Paul, 2007). Hallmark grammatical errors include the omission of articles (such as “the”), pronoun mistakes (e.g., “him” in place of “he”), grammatical inflection (e.g., “go” instead of “goes”), and tense errors (e.g., switching present for past tense).
Children with language impairments rarely have a single gene mutation and it is agreed that even individuals with the same disorder are unlikely to have the exact same set of genetic markers (Bishop, 2002). The lack of a consistent causal gene has led some to speculate that complex developmental disorders such as dyslexia, attention deficits, and SLI are instead due to any one of several combinations of genetic markers. Recently, evidence has suggested that some genetic variants may create a susceptibility to developmental disorders, and these susceptibility variants may be common in many individuals, even if the remainder of their genetic variants differ (Donlon, 1988; Wang et al., 2009; Burnside et al., 2011; Centanni et al., 2015; Hashemi et al., 2015).
The effect of copy number variants (CNVs) on chromosome 15 (q11.2) has been a subject of debate in the field, both anecdotally and in scholarly articles. Microdeletions and microduplications in this region have been associated with a variety of disorders, including autism, schizophrenia, Prader–Willi, and Angelman’s syndromes (Kirov et al., 2009; Hogart et al., 2010; Mefford et al., 2010; Dimitropoulos et al., 2013; Hashemi et al., 2015). However, duplications at this location are commonly seen in typically developing individuals (Mefford et al., 2010), which raises questions about whether variants at this location play a role in the disorders mentioned above. The consistent association between this region and a variety of developmental disorders suggests that variants in this region do contribute to the disordered state, even if they are not causal on their own. Though copy number variations in this region have been suggested as a susceptibility variant in many disorders, it is currently unknown if these variants are susceptibility factors for disorders such as SLI.
In the current report, we discuss the behavioral and genetic profiles of eight children with SLI who took part in a larger study on the biological basis of language impairment. Due to the current controversy regarding the definition of SLI and its diagnostic criterion (Reilly et al., 2014), we used strict assessment score cutoffs that are in line with other studies on the genetics of SLI (Rice et al., 2009). Four of these children all had gains in this region of chromosome 15 as well as additional CNVs in multiple other regions previously linked to language impairments.
Materials and Methods
Participants
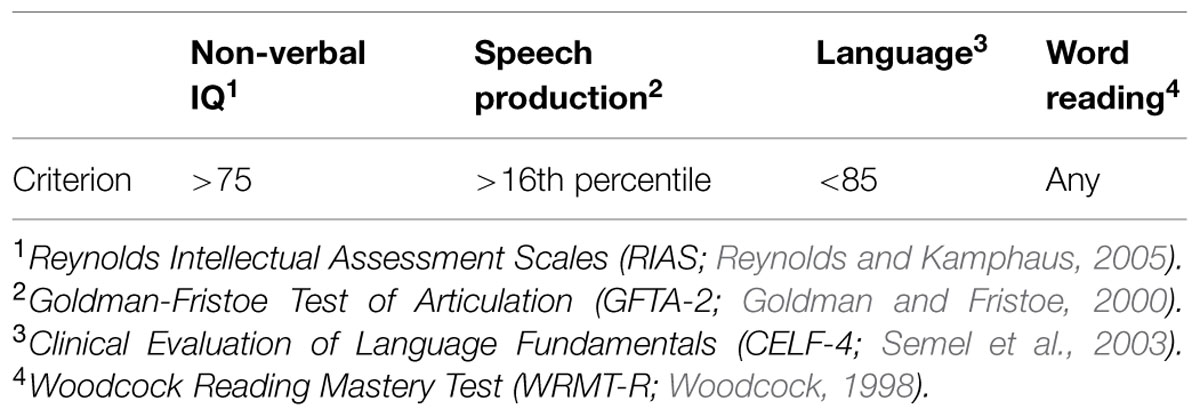
In the current study, we discuss four children who were part of a cohort of eight children with SLI, ranging in age from 4;5 to 17;2 (years;months), that participated in a larger study on the biological pathways of speech and language disorders. All procedures were approved by the Institutional Review Board of the University of Nebraska Medical Center and all participants were consented prior to participation. Participants completed a series of commonly administered, age-appropriate speech, language, reading, and cognitive assessments including the Goldman Fristoe Test of Articulation-Second Edition (GFTA-2; Goldman and Fristoe, 2000), the Clinical Evaluation of Language Fundamentals-Fourth Edition (CELF-4; Semel et al., 2003), Reynolds Intellectual Assessment Scales (RIAS; Reynolds and Kamphaus, 2005), and the Woodcock Reading Mastery Test-Revised (WRMT-R; Woodcock, 1998). All participants were required to have normal cognition based on a standard score higher of 75 or higher on the RIAS.
Children were assigned to the SLI group based on GFTA-2 percentile scores of 16 or higher and a CELF-4 standard score below 85. Inclusionary criteria are presented in Table 1.
DNA Collection and Isolation
Buccal cell samples were collected from all eight participants with SLI using the Isohelix DNA swab packs (Cell Projects, Ltd., Kent, UK), and DNA was extracted per manufacturer’s recommendations using the QIACube (Qiagen, Valencia, CA, USA). DNA quantity and quality were determined using the NanoDrop ND-1000® spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and agarose gel electrophoresis, respectively.
CNV Detection
High-resolution genome-wide analysis was performed on genomic DNA using the CytoScanHDTM array (Affymetrix, Santa Clara, CA, USA) according to manufacturer’s instruction. This array contains more than 2.6 million markers for high-resolution whole-genome copy number analysis and 750,000 genotype-able single nucleotide polymorphisms (SNPs) for reliable detection of copy neutral loss of heterozygosity (CN-LOH). Data were visualized and analyzed with the Chromosome Analysis Suite (ChAS) software (Affymetrix) using the following filter parameters: (1) ≥25 markers and ≥5 kilobases (kb) for CNVs and (2) ≥5 megabases (Mb) for CN-LOH. All basepairs are mapped to Build 37/hg19. Parental DNA samples were not available for these children, so it was not possible to determine whether these were de novo variants.
Statistical Analysis
We used Pearson’s correlation to evaluate the relationship between gain size in 15q11.2 and phenotype characteristics (p < 0.05).
Results
Behavioral Profile
All eight children were administered a number of speech, language, and cognitive assessments to ensure a diagnosis of SLI in the absence of any comorbid conditions (Table 1). All children were classified as having SLI since they scored below 85 on the language measure in the presence of normal non-verbal IQ and no articulation impairments (Table 2). Because children five through eight did not evidence any variants at 15q11.2, their data were excluded from further consideration in this paper. Child 1 was 10;3 (years;months) and female, child 2 was 9;3 and female, child 3 was 10;1 and female, and child 4 was 11;3 and male. None of the children scored within the impaired range on the word reading measure (<85), but they did display a wide range of typical word reading abilities, from the 30th percentile (child 1, 30; child 2, 32; child 3, 45) up to the 73rd percentile (child 4).
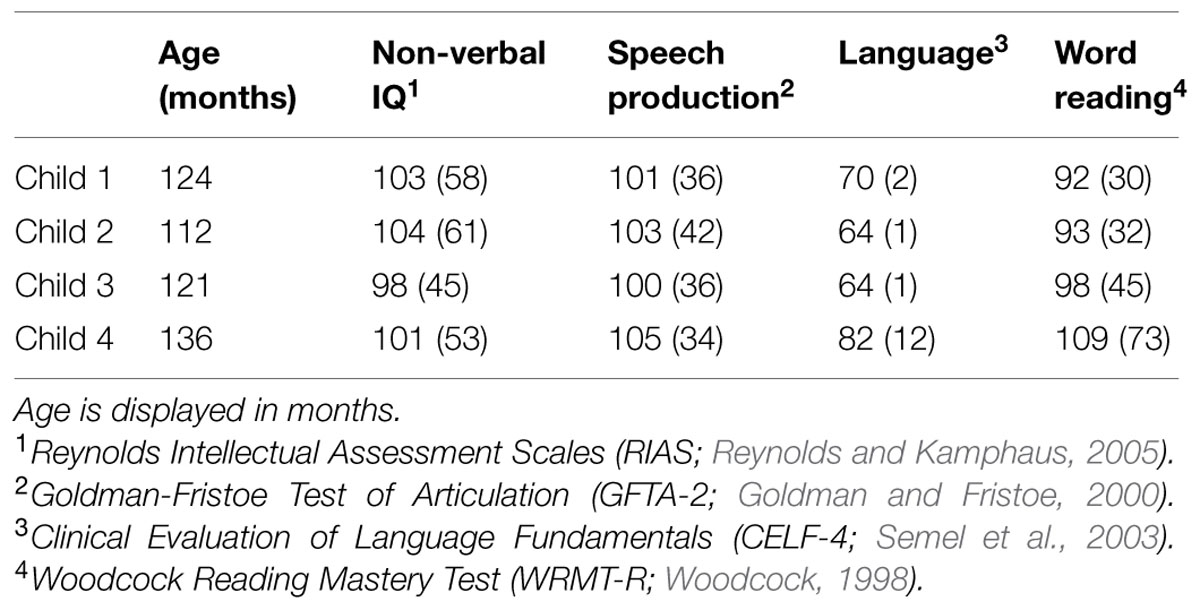
TABLE 2. Assessment standard scores (percentiles in parenthesis where applicable) for four children with copy number variants (CNVs) at 15q11.2.
Genetic Profile
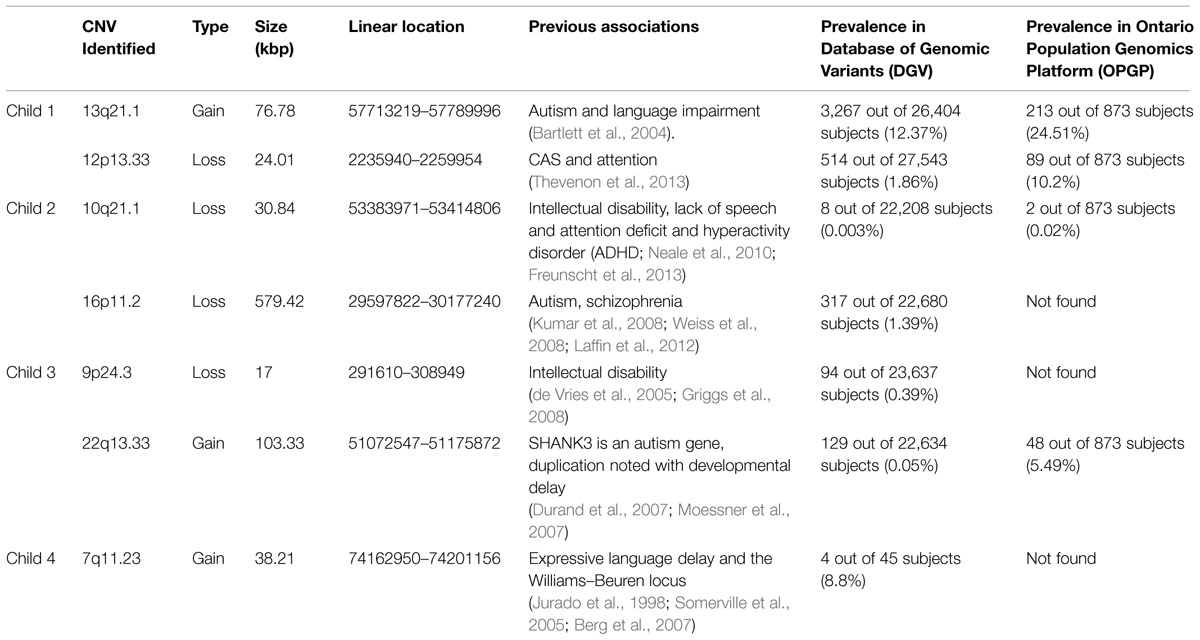
Of the eight children with SLI that were genotyped, four of these children had large gains in an overlapping region at 15q11.2. Child 1’s gain was 54.34 kilo-bases in length (25283093–25337431), child 2 had a gain of 41.70 kb (25295728–25337431), child 3 had a gain of 32.94 kb (25291742–25324677), and child 4 had a gain of 14.81 kb (25306864–25321675). These gains are large and encompass a variety of genes. Because of the size of these gains, exons and introns for a variety of genes were affected. Most hits among these four children included the genes SNORD109A, SNORD109B, and SNORD116-(1-23). These genes are commonly associated with Prader–Willi syndrome, with evidence suggesting that several genes, including SNORD116, are a key pathogenic component (Rabinovitz et al., 2012; Anderlid et al., 2014).
We also annotated copy number at other known language, and more broadly, neurodevelopmental loci. Overall, the range of assessment scores and the variety of gain sizes in the sample suggest that other genetic factors may be contributing to the observed phenotypes (Table 3). Child 1 had two additional significant gains or losses (hereafter collectively called ‘hits’). The first was a gain at 13q21.1, which has been seen in individuals with autism and language impairment (Bartlett et al., 2004). The second was a loss at 12p13.33, which has been previously associated with childhood apraxia of speech (CAS) and attention difficulties (Thevenon et al., 2013). Child 2 had two hits of clinical significance in addition to 15q11.2. The first was a loss at 10q21.1, which has been associated with intellectual disability, lack of expressive speech, and attention deficit and hyperactivity disorder (ADHD; Neale et al., 2010; Freunscht et al., 2013). The second was a loss at 16p11.2, which has been previously associated with autism (Kumar et al., 2008; Weiss et al., 2008; Laffin et al., 2012). This gene is often characterized as pathogenic and likely contributed to the phenotype of this child.
Child 3 had two CNVs in addition to the deletion at 15q11.2, both of which may be pathogenic. The first was a loss at 9p24.3 involving the gene DOCK8 and has been previously linked with intellectual disability (de Vries et al., 2005; Griggs et al., 2008). In fact, this child did have the lowest non-verbal IQ (standard score of 98) of the four children described here (Table 2). The second was a gain at 22q13.33. A duplication of this region is linked with developmental delay and the region also contains the gene SHANK3, which has been associated with autism (Durand et al., 2007; Moessner et al., 2007). Finally, child 4 had one CNV in addition to the deletion at 15q11.2: a gain at 7q11.23, which has been associated with language delay and the Williams–Beuren locus (Jurado et al., 1998; Somerville et al., 2005; Berg et al., 2007). The result that these children all exhibited other genetic variants previously associated with speech and language impairments support the idea that a variant at 15q11.2 is a susceptibility locus and not necessarily one that is deleterious by itself.
The genetic profiles have an interesting phenotypic context for evaluating the function of 15q11.2 relative to reading and language. We consider the clustering of percentiles for children 1–3 and the relative outlier of child 4. Child 4 had the smallest gain (18.13 kb less than the next largest gain, in child 3) and also had the highest scores on the language and word reading measures. Although this child is the oldest in our sample, it is unlikely that age was a factor considering that the reading scores were normed for age. Although there are just four data points, there is a significant linear association between the size of the gain observed and the scores on the word reading measure (r = -0.96, p = 0.04; Figure 1). In spite of the age correction, Child 4’s data point could be a possible outlier. Future studies with a larger group of children are required to validate this potential association between gain size and word reading scores.
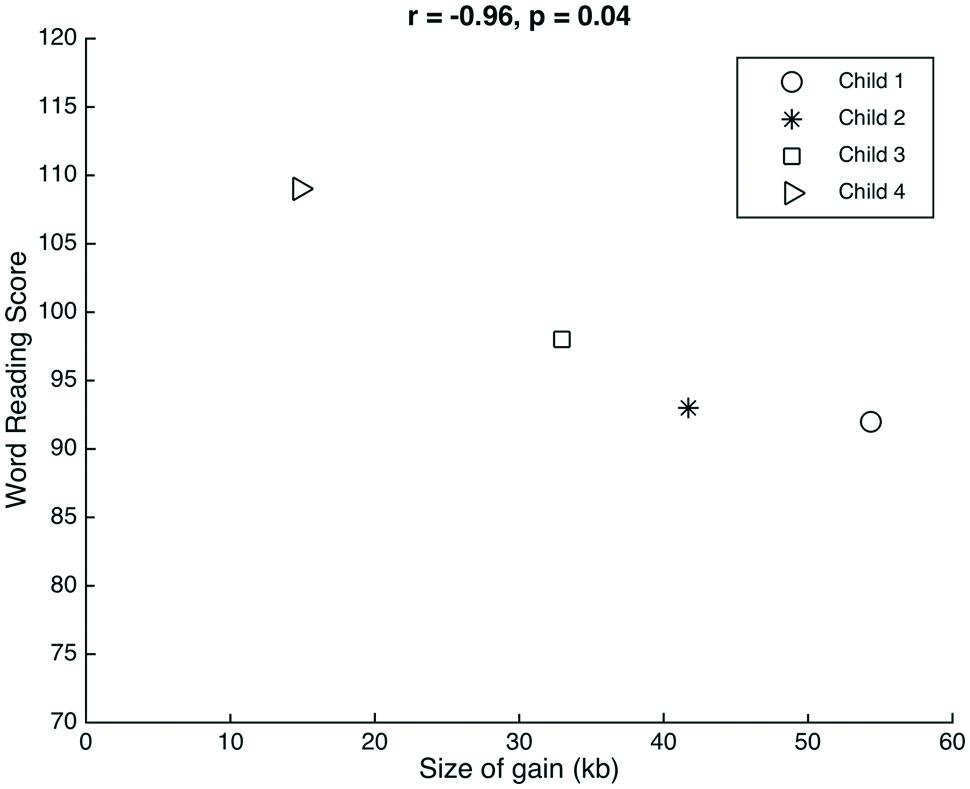
FIGURE 1. Relation between gain size and word reading. There was a significant correlation between the size of the gain observed at 15q11.2 in each of the children and their respective scores on the word reading measure.
Prevalence of Observed Hits in the General Population
Search of the Database of Genomic Variants (DGV; http://dgv.tcag.ca/) yielded one unselected sample with variation at 15q11.2. A single deletion was found (1 out of N = 873 subjects) in the Ontario Population Genomics Platform (OPGP) controls (Costain et al., 2013). This study used the same technology as our study (Affymetrix-CytoScanHD). To evaluate the population prevalence of the secondary hits observed in our four children, we also searched the DGV and the OPGP for each of the variants reported here. Population frequencies in these two populations are shown in Table 3.
Discussion
In the current study, we report the behavioral phenotypes of four children with SLI who also had large gains in the q11.2 region of chromosome 15. These children all had poor oral language abilities compared to typical peers. In spite of normal non-verbal intelligence and normal speech articulation, these children had a wide range of abilities in word reading. Three of the four children also had additional genetic variants located in areas previously associated with speech and language impairments. These results support the theory that variants at 15q11.2 may create an increased predisposition to displaying a language disorder.
Strengths and Caveats of the Current Study
A strength of the current study is the strict criterion used to identify children with SLI. Because this disorder often co-occurs with dyslexia (Leonard et al., 2002; McCarthy et al., 2012), it has been difficult to determine which genes are related to SLI specifically and which are related to dyslexia. Though our sample size was small (eight children with SLI), the result that four of the eight had a duplication at 15q11.2 supports previous work linking developmental disorders with microdeletions or microduplications in this region (de Kovel et al., 2010; Hashemi et al., 2015). Since all the children in our study were confirmed as having SLI, we were unable to provide support for previous reports that this CNV can occur in typically developing individuals. Future studies should investigate this marker in a larger population of typically developing children as well as those with SLI in the absence of comorbid conditions.
The Multiple Hits Model of Developmental Disorders
To date, no single genetic marker reliably predicts the occurrence of SLI. It is likely that SLI, and perhaps other communication disorders, are caused by a constellation of genes (Rice et al., 2009). An existing hypothesis states that region 15q11.2 is a susceptibility variant. If so, a hit in this region could increase the likelihood that an individual will exhibit a developmental disorder phenotype when additional risk variants are also present. Microdeletions in this region are commonly associated with developmental disorders like Prader–Willi Syndrome (Dimitropoulos et al., 2013) and Angelman Syndrome (Donlon, 1988), as well as epilepsy (Mefford et al., 2010) and autism (de Kovel et al., 2010). For example, a microdeletion in 15q11.2 was observed in 1% of individuals with idiopathic generalized epilepsies (12 of 1234; de Kovel et al., 2010). These deletions are often seen in unaffected family members in addition to affected offspring.
Though the variants seen in the current study were microduplications rather than microdeletions, recent evidence suggests that this type of variant may also indicate susceptibility to developmental disorders, including autism (Hogart et al., 2010; Kitsiou-Tzeli et al., 2010; van der Zwaag et al., 2010) and speech delay (Burnside et al., 2011). The consistent observation that microduplications in 15q11.2 are associated with SLI in our sample, together with previous evidence that microdeletions in this area are related to other developmental disorders, suggests that this region is sensitive to mutations of various forms. It is interesting to note that the four children with gains at 15q11.2 did not evidence any variants in regions previously associated with SLI, including 16q (SLI1), 19q (SLI2), and 13q (SLI3) (Bartlett et al., 2004; Consortium, 2004; Monaco, 2007; Newbury et al., 2011). Specifically, a locus at 16q known as SLI1, has not only been linked with SLI in a large sample, but is also associated with basic reading, spelling, and reading comprehension measures (Consortium, 2004). The observation that none of our participants exhibited variants in these notable regions is likely due to a combination of study design and sample size. Developmental language and communication disorders are notorious for having a complicated genetic picture, without a single causal gene (Bishop, 2002). It is possible that the variant at 15q represents another path to SLI in the absence of variants at the previously associated areas.
Our result provides additional support to 15q11.2 as a susceptibility locus, though larger studies of persons with language and related cognitive phenotypes are needed to establish the prevalence of this variant in the general population compared with a variety of developmental disorders.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the University of Nebraska Health Research Consortium (Co-PIs Hogan and Green) and the Barkley Memorial Trust. The authors wish to thank Jennifer Sanmann and Warren Sanger, for their expertise in genetic data analysis and their invaluable comments on previous versions of this manuscript. Thank you also to the following individuals: Kimber Green, Sara Benham, Dyann Rupp, Tacy Corson, Phoebe Chung, Natalie Vanderveen, and Kristin Schneller for their assistance with data collection and Diane Pickering and Danielle Bishay for specimen processing and genetic data analysis.
References
Anderlid, B., Lundin, J., Malmgren, H., Lehtihetm, M., and Nordgren, A. (2014). Small mosaic deletion encompassing the snoRNAs and SNURF–SNRPN results in an atypical Prader–Willi syndrome phenotype. Am. J. Med. Genet. 164A, 425–431. doi: 10.1002/ajmg.a.36307
Bartlett, C., Flax, J., Logue, M., Smith, B., Vieland, V. J., Tallal, P., et al. (2004). Examination of potential overlap in autism and language loci on chromosomes 2, 7, and 13 in two independent samples ascertained for specific language impairment. Hum. Hered. 57, 10–20. doi: 10.1159/000077385
Berg, J., Brunetti-Pierri, N., Peters, S. Kang, S. H., Fong, C. T., Salamone, J., et al. (2007). Speech delay and autism spectrum behaviors are frequently associated with duplication of the 7q11. 23 Williams-Beuren syndrome region. Genet. Med. 9, 427–441. doi: 10.1097/GIM.0b013e3180986192
Bishop, D. (2002). The role of genes in the etiology of specific language impairment. J. Commun. Disord. 35, 311–328. doi: 10.1016/S0021-9924(02)00087-4
Burnside, R., Pasion, R., Mikhail, F., Carroll, A. J., Robin, N. H., Youngs, E. L., et al. (2011). Microduplication of proximal 15q11. 2 between BP1 and BP2: a susceptibility region for neurological dysfunction including developmental and language delay. Hum. Genet. 130, 517–528. doi: 10.1007/s00439-011-0970-4
Catts, H. W., Adlof, S. M., Hogan, T. P., and Weismer, S. E. (2005). Are Specific Language Impairment and Dyslexia Distinct Disorders? J. Speech Lang. Hear. Res. 48, 1378–1396. doi: 10.1044/1092-4388(2005/096)
Centanni, T., Sanmann, J., Green, J. R., Iuzzini-Seigel, J., Bartlett, C., Sanger, W. G., et al. (2015). The role of candidate–gene CNTNAP2 in childhood apraxia of speech and specific language impairment. Am. J. Med. Genet. B Neuropsychiatr. Genet. doi: 10.1002/ajmg.b.32325 [Epub ahead of print].
Consortium, S. (2004). Highly significant linkage to the SLI1 locus in an expanded sample of individuals affected by specific language impairment. Am. J. Hum. Genet. 74, 1225–1238. doi: 10.1086/421529
Costain, G., Lionel, A. C., Merico, D., Forsythe, P., Russell, K., Lowther, C., et al. (2013). Pathogenic rare copy number variants in community-based schizophrenia suggest a potential role for clinical microarrays. Hum. Mol. Genet. 22, 4485–4501. doi: 10.1093/hmg/ddt297
de Kovel, C., Trucks, H., Helbig, I., Mefford, H., Baker, C., Leu, C., et al. (2010). Recurrent microdeletions at 15q11. 2 and 16p13. 11 predispose to idiopathic generalized epilepsies. Brain 133(Pt 1), 23–32. doi: 10.1093/brain/awp262
de Vries, B. B. A., Pfundt, R., Leisink, M., Koolen, D. A., Vissers, L. E. L. M., Janssen, I. M., et al. (2005). Diagnostic genome profiling in mental retardation. Am. J. Hum. Genet. 77, 606–616. doi: 10.1086/491719
Dimitropoulos, A., Ferranti, A., and Lemler, M. (2013). Expressive and receptive language in Prader-Willi syndrome: report on genetic subtype differences. J. Commun. Disord. 46, 193–201. doi: 10.1016/j.jcomdis.2012.12.001
Donlon, T. (1988). Similar molecular deletions on chromosome 15q11. 2 are encountered in both the Prader-Willi and Angelman syndromes. Hum. Genet. 80, 322–328. doi: 10.1007/BF00273644
Durand, C., Betancur, C., Boeckers, T., Bockmann, J., Chaste, P., Fauchereau, F., et al. (2007). Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 39, 25–27. doi: 10.1038/ng1933
Freunscht, I., Popp, B., Blank, R., Endele, S., Moog, U., Petri, H., et al. (2013). Behavioral phenotype in five individuals with de novo mutations within the GRIN2B gene. Behav. Brain Funct. 9, 20. doi: 10.1186/1744-9081-9-20
Goldman, R., and Fristoe, M. (2000). Goldman-Fristoe Test of Articulation-2 (GFTA-2). Circle Pines, MN: American Guidance Service.
Griggs, B. L., Ladd, S., Saul, R. A., DuPont, B. R., and Srivastava, A. K. (2008). Dedicator of cytokinesis 8 is disrupted in two patients with mental retardation and developmental disabilities. Genomics 91, 195–202. doi: 10.1016/j.ygeno.2007.10.011
Hashemi, B., Bassett, A., Chitayat, D., Chong, K., Feldman, M., Flanagan, J., et al. (2015). Deletion of 15q11.2(BP1-BP2) region: further evidence for lack of phenotypic specificity in a pediatric population. Am. J. Med. Genet. A doi: 10.1002/ajmg.a.37134 [Epub ahead of print].
Hogart, A., Wu, D., LaSalle, J. M., and Schanen, N. C. (2010). The comorbidity of autism with the genomic disorders of chromosome 15q11.2–q13. Neurobiol. Dis. 38, 181–191. doi: 10.1016/j.nbd.2008.08.011
Jurado, L., Wang, Y. K., Peoples, R., Coloma, A., Cruces, J., and Francke, U. (1998). A duplicated gene in the breakpoint regions of the 7q11. 23 Williams-Beuren syndrome deletion encodes the initiator binding protein TFII-I and BAP-135, a. Hum. Mol. Genet. 7, 325–334. doi: 10.1093/hmg/7.3.325
Kirov, G., Grozeva, D., Norton, N., Ivanov, D., Mantripragada, K. K., Holmans, P., et al. (2009). Support for the involvement of large copy number variants in the pathogenesis of schizophrenia. Hum. Mol. Genet. 18, 1497–1503. doi: 10.1093/hmg/ddp043
Kitsiou-Tzeli, S., Tzetis, M., Sofocleous, C., Vrettou, C., Xaidara, A., Giannikou, K., et al. (2010). De novo interstitial duplication of the 15q11.2-q14 PWS/AS region of maternal origin: clinical description, array CGH analysis, and review of the literature. Am. J. Med. Genet. A 152A, 1925–1932. doi: 10.1002/ajmg.a.33447
Kumar, R., KaraMohamed, S., Sudi, J., Conrad, D. F., Brune, C., Badner, J. A., et al. (2008). Recurrent 16p11. 2 microdeletions in autism. Hum. Mol. Genet. 17, 628–638. doi: 10.1093/hmg/ddm376
Laffin, J. J. S., Raca, G., Jackson, C. A., Strand, E. A., Jakielski, K. J., and Shriberg, L. D. (2012). Novel candidate genes and regions for childhood apraxia of speech identified by array comparative genomic hybridization. Genet. Med. 14, 928–936. doi: 10.1038/gim.2012.72
Leonard, C., Lombardino, L., Walsh, K., Eckert, M. A., Mockler, J. L., Rowe, L. A., et al. (2002). Anatomical risk factors that distinguish dyslexia from SLI predict reading skill in normal children. J. Commun. Disord. 35, 501–531. doi: 10.1016/S0021-9924(02)00120-X
Leonard, L., Miller, C., and Gerber, E. (1999). Grammatical morphology and the lexicon in children with specific language impairment. J. Speech Lang. Hear. Res. 42, 678–689. doi: 10.1044/jslhr.4203.678
McCarthy, J. H., Hogan, T. P., and Catts, H. W. (2012). Is weak oral language associated with poor spelling in school-age children with specific language impairment, dyslexia or both? Clin. Linguist. Phonet. 26, 791–805. doi: 10.3109/02699206.2012.702185
Mefford, H. C., Muhle, H., Ostertag, P., von Spiczak, S., Buysse, K., Baker, C., et al. (2010). Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 6:e1000962. doi: 10.1371/journal.pgen.1000962
Moessner, R., Marshall, C., Sutcliffe, J., Skaug, J., Pinto, D., Vincent, J., et al. (2007). Contribution of SHANK3 mutations to autism spectrum disorder. Am. J. Med. Genet. 81, 1289–1297. doi: 10.1086/522590
Monaco, A. (2007). Multivariate linkage analysis of specific language impairment (SLI). Ann. Hum. Genet. 71(Pt 5), 660–673. doi: 10.1111/j.1469-1809.2007.00361.x
Neale, B. M., Medland, S., Ripke, S., Anney, R. J. L., Asherson, P., Buitelaar, J., et al. (2010). Case-control genome-wide association study of attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 49, 906–920. doi: 10.1016/j.jaac.2010.06.007
Newbury, D. F., Paracchini, S., Scerri, T. S., Winchester, L., Addis, L., Richardson, A. J., et al. (2011). Investigation of dyslexia and SLI risk variants in reading-and language-impaired subjects. Behav. Genet. 41, 90–104. doi: 10.1007/s10519-010-9424-3
Paul, R. (2007). Language Disorders from Infancy Through Adolescence: Assessment & Intervention. Available at: http://books.google.com/books?hl=en&lr=&id=QxLfgByBvToC&oi=fnd&pg=PA3&dq=Language+disorders+from+infancy+to+adolescence:+Assessment+and+intervention&ots=znsQqiYxNZ&sig=F1v_tzryt-quX3GmmCBK9v_1Wh0
Rabinovitz, S., Kaufman, Y., Ludwig, G., Razin, A., and Shemer, R. (2012). Mechanisms of activation of the paternally expressed genes by the Prader-Willi imprinting center in the Prader-Willi/Angelman syndromes domains. Proc. Natl. Acad. Sci. U.S.A. 109, 7403–7408. doi: 10.1073/pnas.1116661109
Reilly, S., Tomblin, B., Law, J., McKean, C., Mensah, F. K., Morgan, A., et al. (2014). Specific language impairment: a convenient label for whom? Int. J. Lang. Commun. Disord. 49, 416–451. doi: 10.1111/1460-6984.12102
Reynolds, C. R., and Kamphaus, R. W. (2005). “Introduction to the Reynolds intellectual assessment scales and the Reynolds intellectual screening test,” in Contemporary Intellectual Assessment: Theories, Tests, and Issues, 2nd Edn, eds D. P. Flanagan and P. L. Harrison (New York, NY: Guilford Press), 461–483.
Rice, M. L., Smith, S. D., and Gayán, J. (2009). Convergent genetic linkage and associations to language, speech and reading measures in families of probands with specific language impairment. J. Neurodev. Disord. 1, 264–282. doi: 10.1007/s11689-009-9031-x
Semel, E., Wiig, E., and Secord, W. (2003). Clinical Evaluation of Language Fundamentals-IV. Marickville: Harcourt Assessment.
Somerville, M. J., Mervis, C. B., Young, E. J., Seo, E.-J., del Campo, M., Bamforth, S., et al. (2005). Severe expressive-language delay related to duplication of the Williams-Beuren locus. N. Engl. J. Med. 353, 1694–1701. doi: 10.1056/NEJMoa051962
Thevenon, J., Callier, P., Andrieux, J., Delobel, B., David, A., Sukno, S., et al. (2013). 12p13.33 microdeletion including ELKS/ERC1, a new locus associated with childhood apraxia of speech. Eur. J. Hum. Genet. 21, 82–88. doi: 10.1038/ejhg.2012.116
van der Zwaag, B., Staal, W. G., Hochstenbach, R., Poot, M., Spierenburg, H. A., de Jonge, M. V., et al. (2010). A co-segregating microduplication of chromosome 15q11.2 pinpoints two risk genes for autism spectrum disorder. Am. J. Med. Genet. B Neuropsychiat. Genet. 153B, 960–966. doi: 10.1002/ajmg.b.31055
Wang, K., Zhang, H., Ma, D., Bucan, M., Glessner, J. T., Abrahams, B. S., et al. (2009). Common genetic variants on 5p14. 1 associate with autism spectrum disorders. Nature. 459, 528–533. doi: 10.1038/nature07999
Weiss, L., Shen, Y., Korn, J., Arking, D. E., Miller, D. T., Fossdal, R., et al. (2008). Association between microdeletion and microduplication at 16p11. 2 and autism. Engl. J. Med. Available at: http://www.nejm.org/doi/full/10.1056/NEJMoa075974
Keywords: specific language impairment, multiple hit model, gene association, language disorders, 15q11.2
Citation: Centanni TM, Green JR, Iuzzini-Seigel J, Bartlett CW and Hogan TP (2015) Evidence for the multiple hits genetic theory for inherited language impairment: a case study. Front. Genet. 6:272. doi: 10.3389/fgene.2015.00272
Received: 28 May 2015; Accepted: 07 August 2015;
Published: 24 August 2015.
Edited by:
Jenae Neiderhiser, The Pennsylvania State University, USAReviewed by:
Juan R. Ordonana, University of Murcia, SpainDimitrios Avramopoulos, Johns Hopkins University, USA
Copyright © 2015 Centanni, Green, Iuzzini-Seigel, Bartlett and Hogan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiffany P. Hogan, Massachusetts General Hospital Institute of Health Professions, 36 1st Avenue, Boston, MA 02129, USA, thogan@mghihp.edu
 Tracy M. Centanni
Tracy M. Centanni Jordan R. Green1
Jordan R. Green1 Jenya Iuzzini-Seigel
Jenya Iuzzini-Seigel Christopher W. Bartlett
Christopher W. Bartlett Tiffany P. Hogan
Tiffany P. Hogan