- 1Department of Biochemistry, University of Otago, Dunedin, New Zealand
- 2Department of Medicine, University of Otago, Christchurch, New Zealand
- 3Department of Medicine, University of Auckland, Auckland, New Zealand
The SLC2A9 gene, that encodes a renal uric acid reuptake transporter, has genetic variants that explain ∼3% of variance in urate levels. There are previous reports of non-additive interaction between SLC2A9 genotype and environmental factors which influence urate control. Therefore, our aim was to further investigate the general phenomenon that such non-additive interactions contribute to genotype-specific association with variance at SLC2A9. Data from 14135 European individuals were used in this analysis. The measure of variance was derived from a ranked inverse normal transformation of residuals obtained by regressing known urate-influencing factors (sex, age, and body mass index) against urate. Variant rs6449173 showed the most significant effect on serum urate variance at SLC2A9 (P = 7.9 × 10-14), which was maintained after accounting for the effect on average serum urate levels (P = 0.022). Noting the stronger effect in a sub-cohort that consisted of pre-menopausal women and younger men, the participants were stratified into males and pre-menopausal and post-menopausal women. This revealed a strong effect on variance in pre-menopausal women (P = 3.7 × 10-5) with a weak effect in post-menopausal women (P = 0.032) and no effect in men (P = 0.22). The T-allele of rs6449173, which associates with increased urate levels, was associated with the greater variance in urate. There was a non-additive interaction between rs6449173 genotype and female gender in control of serum urate levels that was driven by a greater increase in urate levels associated with the T-allele in women. Female hormones, and/or other factors they influence or are associated with (such as iron levels, temperature, testosterone) interact with SLC2A9 genotype in women to determine urate levels. The association of SLC2A9 with greater variance in pre-menopausal women may reflect the cyclical changes resulting from menstruation.
Introduction
Heterogeneity in genetic variance exists when the effect a genotype has on phenotype is influenced by external factors. Such factors include differing environmental exposures, internal factors (such as epistatic interactions with other genetic variants), or other biological phenomena. An example of the latter are the stochastic processes underlying photoreceptor choice of cone cells in developing tri-chromatic vision or increased variation with aging within individuals of a given genotype (Jacobs, 2009; Paré et al., 2010; Geiler-Samerotte et al., 2013). In humans, genotypic control of phenotypic variability has been demonstrated for FTO/IRX3 in body mass index (BMI; Yang et al., 2012), LEPR in C-reactive protein levels and ICAM1 and PNPLA3 in soluble ICAM1 levels (Paré et al., 2010). A total of 23 genome-wide significant variance expression quantitative trait loci single nucleotide polymorphisms (SNPs) have been reported in lymphoblastoid cell lines, of which ∼70% could be attributed to non-additive gene by environment (GxE) interactions (Brown et al., 2014).
Urate is a medically important metabolite. Elevated serum urate (hyperuricemia) is a central cause of gout, the most common form of inflammatory arthritis characterized by severe pain, disability, and joint damage. A genome-wide association study (GWAS) has demonstrated that levels of serum urate are influenced by genetic variants in 28 loci, with the strongest effects observed in renal and gut transporters of uric acid (Köttgen et al., 2013). In particular, variants in SLC2A9 have a very large effect on urate levels (e.g., rs12498742) and gout [e.g., rs11942223; in strong linkage disequilibrium (LD) with rs12498742], explaining 2–3% of the variance in serum urate in European individuals and a substantially stronger effect in women than in men (Hollis-Moffatt et al., 2009; Köttgen et al., 2013). Sex is the strongest reported interacting variable with SLC2A9 genotype to control urate levels (P = 8.2 × 10-6 for sex, P = 0.02–0.03 for age and alcohol intake, P > 0.38 for BMI, diabetes and hypertension status; Voruganti et al., 2014).
In addition, non-additive interactions between SLC2A9 genetic variants, food items, and diuretic medication have been reported. The influence of diet and diuretic medication on serum urate is well-established. Use of diuretics and consumption of seafood, red meat, alcohol and sugar-sweetened beverage (SSB) and tomatoes all associate with increased urate and the risk of gout (Choi and Curhan, 2004, 2008; Choi et al., 2004a,b, 2005, 2008, 2012; Rasheed et al., 2013; Batt et al., 2014; Flynn et al., 2015). Non-additive interaction between rs6449173 (in strong LD with rs12498742 and rs11942223) genotype at SLC2A9 and SSB consumption in control of serum urate and risk of gout has been reported (Batt et al., 2014). A similar interaction between rs6449213 genotype (in strong LD with rs6449173) and alcohol has been reported in American Indian individuals (Voruganti et al., 2014). There is evidence that SLC2A9 (rs13129697) and SLC22A11 (rs2078267) genotype interact with diuretics to determine the risk of gout (McAdams-DeMarco et al., 2013), although this was not replicated in a larger study (Bao et al., 2015). Whilst these findings require further validation, the data suggest that non-additive gene–environment interactions are involved in control of urate levels at SLC2A9. Such interactions are important to understand in order to increase insight into the molecular pathogenesis of hyperuricemia.
To further investigate non-additive interactions between SLC2A9 genotype and environmental exposures in control of urate levels and risk of gout a series of classical interaction tests focused on putative instrinsic and extrinsic interactors could be conducted as has been performed previously (McAdams-DeMarco et al., 2013; Batt et al., 2014; Voruganti et al., 2014). Alternatively, because an interacting genotype would be expected to result in larger variance (Paré et al., 2010; Struchalin et al., 2010), a single dimensional analysis for genotypes influencing phenotypic variance could be used. Therefore, the aim of this study was to test for association with variance in serum urate at SLC2A9 and potentially identify other environmental interactions with SLC2A9 in serum urate.
Materials and Methods
Participants
Participants of European ancestry were included from five separate sample sets (Table 1). Two were from the Atherosclerosis Risk in Communities study (ARIC; n = 5362) and the Framingham Heart Study (FHS Generation 3; n = 3282) from which people taking antihypertensive or urate-lowering medication, or who self-reported physician-diagnosed kidney disease or gout were excluded. Two were from the Coronary Artery Risk Development in Young Adults study (CARDIA; n = 1496) and the Cardiovascular Health Study (CHS; n = 2799), from which individuals taking urate-lowering medication and who self-reported physician-diagnosed kidney disease or gout were excluded. ARIC individuals self-reporting as taking diuretics (n = 1196) were also included as the fifth sample set. No individuals were excluded based on estimated glomerular filtration rate (eGFR) – there were 47 (0.33%) individuals with eGFR < 30, 46 of whom were from CHS and one from ARIC. The research procedures were in accordance with the ethical standards of the institutional review boards relevant to the various data sets. Written informed consent was given by all participants. The ARIC, FHS, CHS, and CARDIA analyses (project #834) were approved by the relevant Database of Genotype and Phenotype1 Data Access Committees. The overall project was approved by the New Zealand Health and Disability Ethics Committee (ref: 05/10/130).
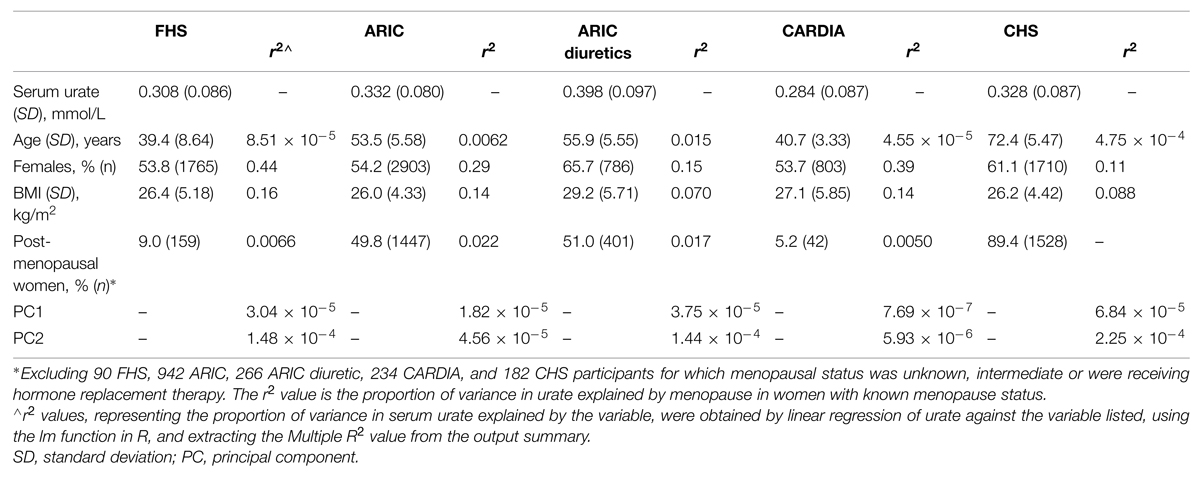
TABLE 1. Demographic and clinical details of the three data sets, and associations of clinical features with serum urate concentrations.
Phenotypes
Phenotypes from baseline exams were used for all studies with the exception of CARDIA, where phenotypes from exam six were used. For the total 7967 European female participants menopause status was determined by self-report. Those who were pregnant, breastfeeding, taking hormone replacement therapy, or did not report menopause status were excluded from the menopause analysis. Subjects who reported as post-menopausal, but had menstruated in the last 12 months were also excluded from the menopause analysis. Serum urate levels were measured using a standard uricase assay (precision value of 8.6%) in the ARIC and CARDIA datasets (Henry et al., 1957; ARIC Investigators, 1989; Dyer et al., 1999). CHS used a Kodak Ektachem 700 analyzer with reagents (Eastman Kodak, Rochester, NY, USA), which had a coefficient of variation of 2.4% (Cushman et al., 1995). A phosphotungstic acid reagent autoanalyzer was used to measure serum urate levels in the FHS data set participants (Crowley, 1964). This method has a precision value of 2.8% (Henry et al., 1957; Crowley, 1964).
Genotypes
Publicly available genome-wide genotype data (Affymetrix 6.0) from the ARIC and CARDIA data sets, combined Affymetrix 50K and 500K platform data from the FHS data set and CHS genotypes imputed from Illumina Human CNV370v1 was used to impute the full SLC2A9 region (±200 kb) using Impute2 version 2.3.0 with the 1000 Genomes Phase 1 integrated variant set phased with SHAPEIT2 as the reference haplotype panel (Delaneau et al., 2014).
Statistical Analysis
Analysis was done using the R statistical software package (version 3.22). R code is presented in Supplementary Table S1.
The variable used as a measure of variance was derived from residuals obtained from sex- and cohort-specific analysis regressing age and BMI (BMI causally affects urate levels Lyngdoh et al., 2012; Palmer et al., 2013). The top two principal component eigenvectors (calculated using default parameters with SMARTPCA Patterson et al., 2006) were also included to account for cryptic relatedness within sample sets. A ranked inverse normal transformation of the absolute residual values yielded the z-score, with the z2-score being the variance variable. The inverse normal transformation, while likely to be overly conservative, minimizes a possible mean-variance relationship of phenotype (Yang et al., 2012). To account for the influence of the mean effect of rs6449173 genotype on the variance effect, using the approach of Yang et al. (2012), the genotype-specific mean urate was subtracted from the urate level of each individual participant and the genotype effect on variance was retested on squared residuals as described above. Data sets were combined by inverse-variance weighted meta-analysis in R (meta version 4.2-03) using a fixed effects model, except where there was evidence for heterogeneity (PHet < 0.05) whereupon a random effects model was used.
Interaction analysis between menopausal status and rs6449173 was conducted using the R lm function with a linear model regressing urate against age, BMI, rs6449173 allele, menopausal status and the interaction term between menopause and rs6449173 allele. Post-menopausal and pre-menopausal women were compared to men (as the referent group) in separate models and the effect of the interaction term reported.
Results
Analysis of all SLC2A9 variants within ±200 kb of the gene for association with variance in serum urate levels resulted in a single association peak (Figure 1). We chose to analyze SNP rs6449173 which is one of a large number (n = 136) of SNPs (Supplementary Figure S1) in a haplotype block including the variant (rs12498742, r2 = 0.96 with rs6449173) previously reported as most strongly associated with average serum urate by GWAS (Köttgen et al., 2013). Rs6449173 demonstrated the strongest effect on serum urate variance at this locus (Figure 1; Table 2; βTallele = -0.152, P = 7.9 × 10-14). The initial region-wide analysis (Figure 1) was unadjusted for the possible confounding effect of genotype-specific mean urate levels. After adjustment of the mean effect the genotype-specific effect on the variance was reduced in magnitude, and the direction of effect was reversed, with the major T allele associated with greater variance in urate (βTallele = 0.047, P = 0.022).
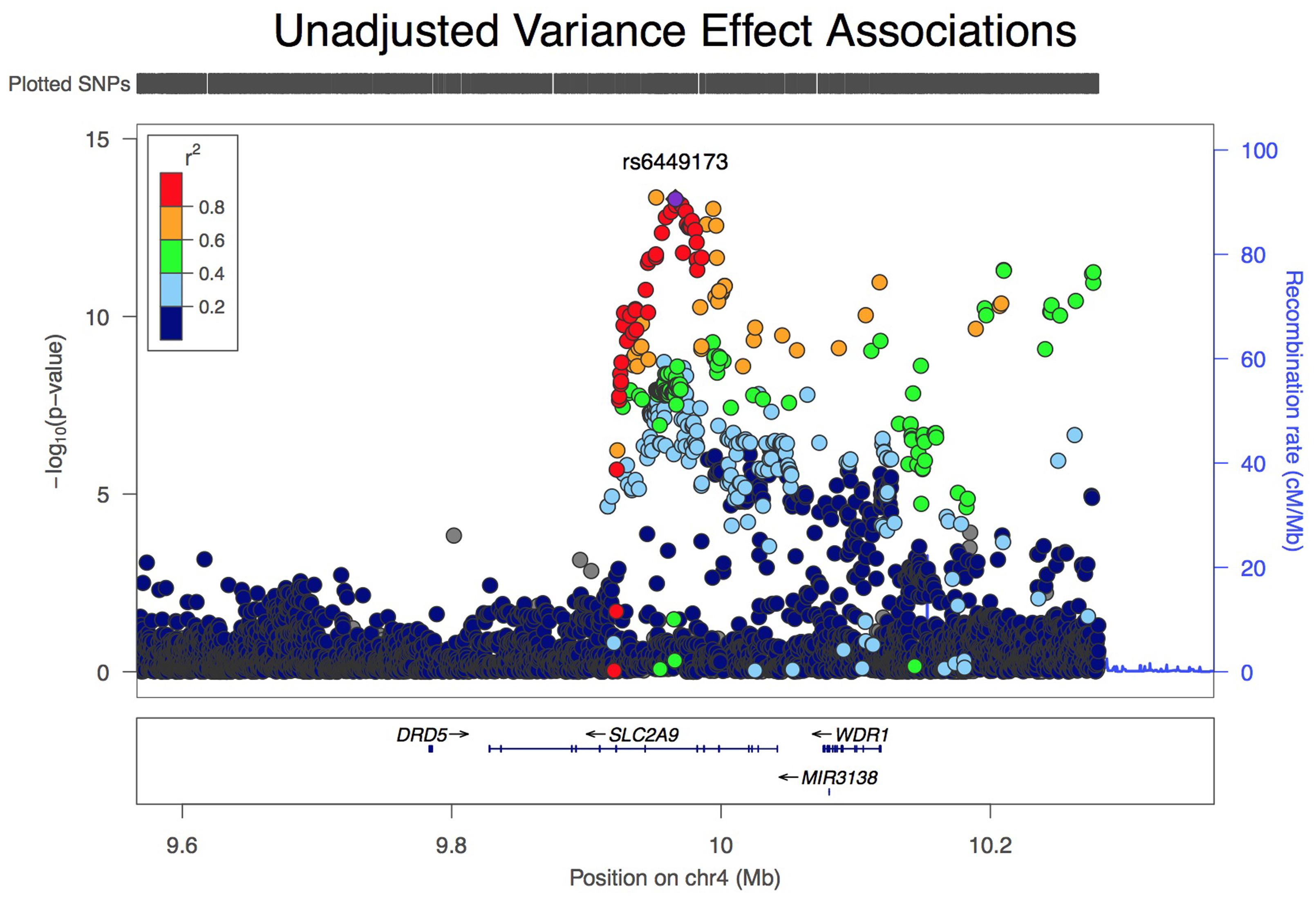
FIGURE 1. LocusZoom view of association of 5544 variants at the SLC2A9 locus with variance, unadjusted for effect on mean urate level.
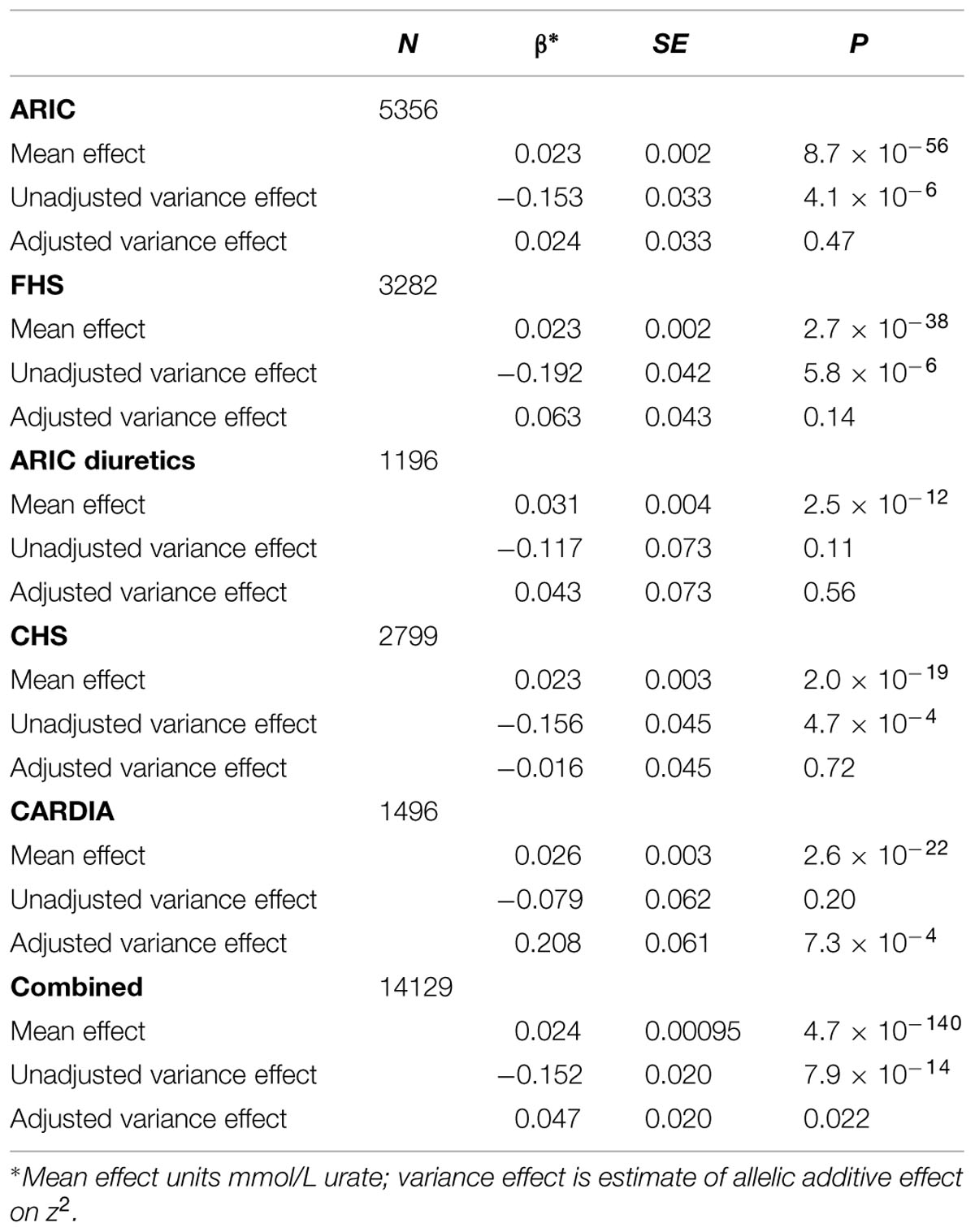
TABLE 2. The influence of accounting for the rs6449173 average effect on the estimated serum urate variance effect.
The adjusted variance effect was statistically significant only in the CARDIA data set (Table 2; β = 0.208, P = 7.3 × 10-4; all other European sample sets β ≤ 0.043, P ≥ 0.14). Noting that this sample set was comprised entirely of younger individuals (Table 1; men and predominantly pre-menopausal woman); noting that the effect of SLC2A9 on average urate levels is stronger in women (Köttgen et al., 2013); and noting the association of menopause with serum urate levels (Hak and Choi, 2008) we therefore reanalyzed the SLC2A9 genotype effect on variance in men and pre-menopausal and post-menopausal women separately. This revealed that the variance effect was stronger in pre-menopausal women in the combined sample set (Table 3; β = 0.191, P = 3.7 × 10-5) than post-menopausal women (β = 0.087, P = 0.032) or men (β = -0.038, P = 0.22). The variance effect was visualized using box plots (Figure 2). This showed that increased median z2-scores and increased standard deviation were observed with the TT-genotype and decreased median z2-scores and standard deviation were associated with the GG genotype in pre-menopausal women. This effect was less obvious in post-menopausal women and was not observed in men.
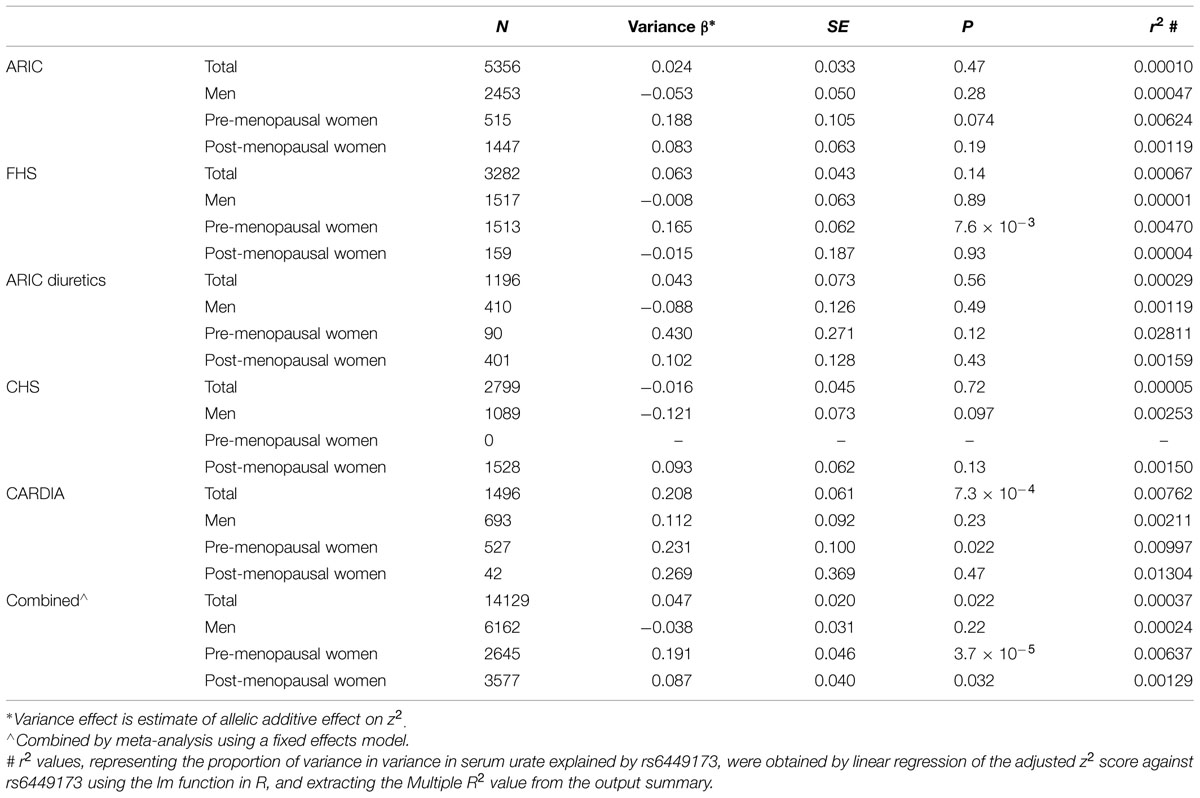
TABLE 3. Association of rs6449173 genotype with serum urate variance in sample sets stratified into men, pre-menopausal women and post-menopausal women, with adjustment for rs6449173 mean effect.
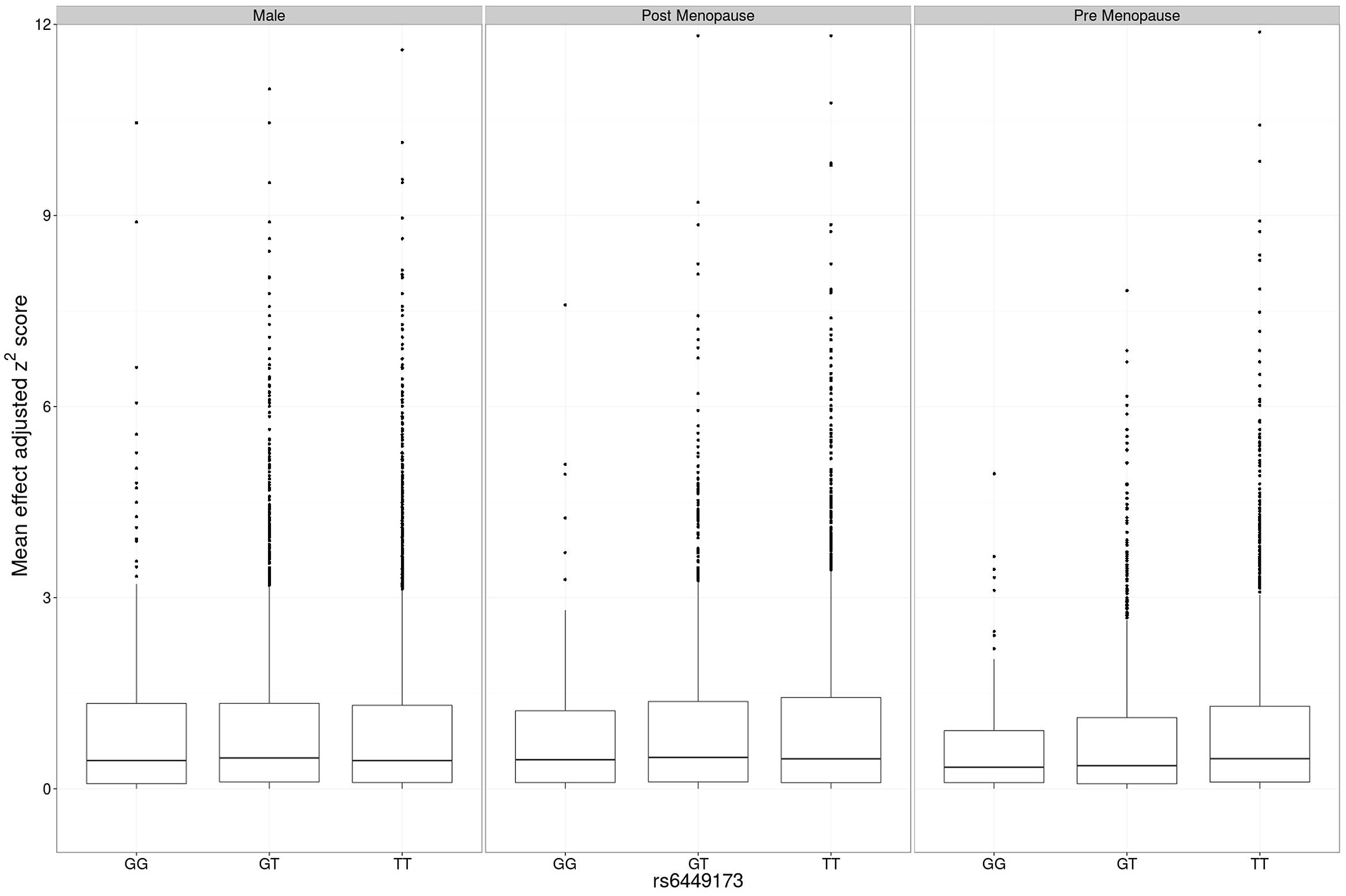
FIGURE 2. Main effect adjusted z2-scores at rs6449173. The genotype-specific median (standard deviation) z2 for men was GG 0.439 (1.458), GT 0.487 (1.443), TT 0.441 (1.380), for post-menopausal women was GG 0.411 (1.103), GT 0.465 (1.304), TT 0.451 (1.470), and for pre-menopausal women was GG 0.408 (0.891), GT 0.373 (1.215), TT 0.507 (1.514).
The hypothesis that there was non-additive interaction between genotype and female hormone status in determining serum urate (adjusting for age and BMI) was evaluated. Men and post-menopausal women have similar estrogen levels and estrogen has a similar paracrine role, not acting solely as an endocrine factor produced by the ovaries in each group (Khosla et al., 1998; Simpson and Davis, 2001). We therefore expected pre-menopausal women to have an interaction effect of greater magnitude than post-menopausal women (when both groups are compared to men), reflective of the variance results. However, allelic interaction terms were of approximately equal effect for both pre-menopausal and post-menopausal women as compared to men (βInteraction = 0.013 mmol/L, P = 2.4 × 10-6 and βInteraction = 0.012 mmol/L, P = 3.5 × 10-6, respectively). This effect was driven by a greater increase in serum urate levels when the T allele is present in pre-menopausal women (βTT – βGG = 0.075) and post-menopausal women (βTT – βGG = 0.065) compared to men (βTT – βGG = 0.038; Table 4).
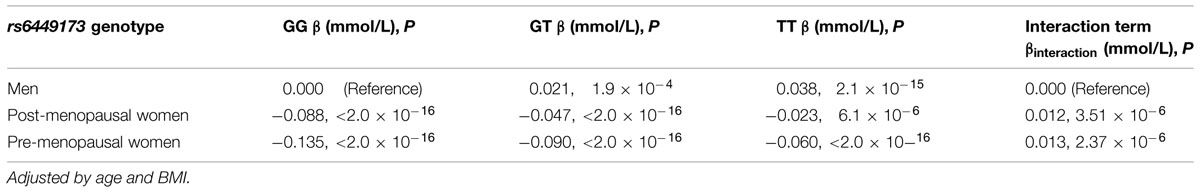
TABLE 4. Association by linear regression and interaction of rs6449173 genotype with average serum urate levels in pre-menopausal women and post-menopausal women and men stratified by genotype.
We included an interaction term in the variance model (adjusted for mean effect) resulting in βInteraction = 0.228, P = 4.49 × 10-5 for pre-menopausal women and βInteraction = 0.125, P = 0.015 for post-menopausal women, both with men as referent group. In this model, for pre-menopausal women the proportion of variance (r2) in phenotypic variance explained by adding the interaction term increased from 0.00018 to 0.0021 and in post-menopausal women increased from 0.000011 to 0.00062. The increase in r2 indicates that non-additive interaction with menopausal status also contributes to the observed association between rs6449173 and variance in urate levels. This interaction is stronger in pre-menopausal than post-menopausal women. This phenomenon is separate to the non-additive interaction between rs6449173 and sex per se in determining mean urate levels (Table 4).
Discussion
We present evidence that the SLC2A9 genotype associated with average serum urate levels also differentially associates with variance in urate levels in pre-menopausal women. This may reflect the cyclical changes resulting from menstruation. There was also non-additive interaction between sex and SLC2A9 in determining urate levels, replicating the findings of Voruganti et al. (2014). We interpret these findings to indicate that the intrinsic biological phenomenon of female hormones (which change upon menopause) and/or other factors that they directly affect (such as temperature, iron levels, testosterone) interact with SLC2A9 genotype in a non-additive fashion in women to determine urate levels. The effect of the rs6449173 T-allele in raising urate is greater in women.
Our data can be compared to the findings of Yang et al. (2012) who associated FTO/IRX3 with genotype-specific variance in BMI. This locus, like SLC2A9, has the strongest mean effect size on phenotype in the genome. At the FTO SNP rs7202116 the allelic effect on average phenotype did not contribute to the observed effect on variance, in contrast with SLC2A9 rs6449173 where the allelic effect on mean phenotype contributed considerably to the genotype-specific association with variance in phenotype. Stratifying the sample set in our study clarified the analysis and clearly showed a genotype-specific effect on variance in urate in pre-menopausal women after accounting for the average effect. While a number of changes occur throughout the menstrual cycle (e.g., iron levels, temperature, estrogen and progesterone levels) the factors with the most evidence supporting a role in urate control are iron levels (Ghio et al., 2005; Mainous et al., 2011) and hormones. Female hormones (estrogens) increase the fractional excretion of uric acid and reduce serum urate levels (Yahyaoui et al., 2008). Our data are consistent with a model whereby female hormones contribute directly via SLC2A9 in a genotype-specific fashion both to the mean urate levels and variance in urate levels. In animals estrogen reduces renal urate reabsorption by reducing Slc2a9 protein levels (Takiue et al., 2011), so it is conceivable that in human females estrogen could contribute to the SLC2A9-mediated mean effect by a rs6449173 genotype-specific effect on expression of SLC2A9.
In pre-menopausal women urate levels vary across the menstrual cycle with endogenous estradiol associated with reduced, and follicle stimulating hormone associated with increased, urate (Mumford et al., 2013). Thus, also in a genotype specific manner, female hormones would be expected to contribute to variance potentially owing to the cyclical changes in levels of female hormones in pre-menopausal women or other factors influenced or associated with menstrual cycling in pre-menopausal women (e.g., oral contraceptive use Stöckl et al., 2012). Whilst estrogen levels in post-menopausal women are more similar to levels in men than pre-menopausal women (Khosla et al., 1998), and serum urate levels rise to levels approximately equivalent to those of men after menopause, the data in Table 4 suggest that post-menopausal women and men still control serum urate levels differently. However we were unable to test for a direct interaction between female hormone levels and SLC2A9 genotype. Owing to the use of cross-sectional data we were also unable to test for any genotype-specific effect on intra-individual variability in pre-menopausal women. Such a study would allow some evaluation of the hypothesis that SLC2A9 genotype interacts non-additively with female hormones or another variable factor associated with menstruation.
The association with variance was largely restricted to pre-menopausal women. There is epidemiological evidence from cross-sectional observational data that menopause associates (independent of measured confounders) with increased urate, that post-menopausal hormone replacement therapy associates with reduced urate (Simon et al., 2006; Hak and Choi, 2008; Stöckl et al., 2012) and that estrogen levels are inversely associated with urate levels during the menstrual cycle (Mumford et al., 2013). This is consistent with clinical studies demonstrating a urate-lowering effect of hormone replacement therapy (Nicholls et al., 1973; Gotfredsen et al., 1983; Sumino et al., 1999), however, there is little definitive evidence that this effect occurs through an influence on renal uric acid handling (Nicholls et al., 1973; Gotfredsen et al., 1983; Antón et al., 1986; Ghio et al., 2005). The increased urate-associated TT genotype of rs6449173 drives the association with variance in urate in European pre-menopausal women (Figure 2), suggesting that understanding the molecular consequence of the genetic effect that this allele tags is key to understanding the mechanism for the observed genotype-specific effects of SLC2A9 on average urate and variability in urate. To this end, determining if rs6449173 is in fact associated with the separate SLC2A9 isoforms (full length and missing 28 cytoplasmic residues), as published data suggest (Döring et al., 2008; Vitart et al., 2008), will be important.
There are multiple independent effects at SLC2A9 with the urate association signal at SLC2A9 encompassing 100s of extremely strongly associated genetic variants over a very large region (500 kb; Köttgen et al., 2013). In a GWAS of serum urate levels in East Asians (Okada et al., 2012), the strongest genome-wide association with urate was at SLC2A9, but with a different SNP variant (rs3775948). The most strongly associated European variant [rs12498742, in strong LD (r2 = 0.86) with rs6449173; Köttgen et al., 2013] was not associated in the East Asian GWAS probably because of the rarity of the minor allele (prevalence of ∼1%). Interestingly the rs3775948 mean effect in East Asians also has, by conditional analysis, an effect in Europeans independent of the European mean effect (Stahl et al., 2014). Furthermore, a GWAS testing for association of common copy number variation with serum urate in Europeans (Scharpf et al., 2014) found association with two copy number variations 200 and 350 kb upstream of SLC2A9 that were each genetically independent of the rs12498742 effect at SLC2A9. Thus there is evidence for at least three independent variants in SLC2A9 that influence urate levels in Europeans, and a separate variant in East Asians. The study of Wei et al. (2014) is consistent with the above studies in providing evidence for multiple independent genetic effects at the SLC2A9 locus – five independent genetic effects were reported. Additional complexity in genetic control of urate levels at SLC2A9 was revealed with epistasis between genetic variants at the SLC2A9 locus influencing urate levels. [Note that rs6449173 and SNPs in strong LD were not amongst SNP pairs in Wei et al. (2014) exhibiting epistasis.] Combined with the evidence here for a genotype-dependent effect at SLC2A9 on variance, previous reports of non-additive GxE interaction at SLC2A9 (McAdams-DeMarco et al., 2013; Batt et al., 2014; Voruganti et al., 2014) and evidence for a population-specific influence of genotype to fructose response (Dalbeth et al., 2013), it is clear that this is an extremely complex urate and gout locus that will be very challenging to understand using genetic epidemiology.
The contribution of non-additive GxE interactions to the phenomenon of ‘missing’ heritability (predicted genetic variance not explained by genome-wide studies assessing the contribution of common genetic variants) is unclear, although it has been suggested that a failure to include the possibility of interactions in an inheritance model can lead to over-estimation of the genetic heritability of a phenotype (Manolio et al., 2009; Zuk et al., 2012). Urate levels are an ideal phenotype to address this question given that there are established dietary and drug environmental exposures (see Introduction) that have relatively immediate temporal effects on urate levels via hepatic production and perhaps also by interfering with excretion (Dalbeth and Merriman, 2013; Batt et al., 2014). This means that data on environmental exposures that are likely causal of changes in urate levels are able to be collected at the same time as phenotype in cross-sectional study designs. To facilitate identification of non-additive GxE interactions, systematically identifying genetic variants with a genotype-specific effect on variance in phenotype, in genome-wide approaches using very large sample sets and accounting for the average effect, can prioritize variants that can be tested for non-additive GxE with specific environmental exposures in linear and logistic models that incorporate interaction terms. Furthermore, identification of variance-associated genetic variants could allow identification of new urate loci which may have average main effects obscured in genome-wide studies that do not incorporate environmental exposures.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Health Research Council of New Zealand, Arthritis New Zealand, New Zealand Lottery Health, and the University of Otago. The authors would like to thank Jill Drake, Roddi Laurence, Meaghan House, Christopher Franklin, and Gabrielle Sexton for assistance in recruitment of the NZ data set. The ARIC study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, N01-HC-55022, R01HL087641, R01HL59367, and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. Infrastructure was partly supported by grant number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. The FHS and the Framingham SHARe project are conducted and supported by the NHLBI in collaboration with Boston University. The Framingham SHARe data used for the analyses described in this manuscript were obtained through dbGaP. The CHS research reported in this article was supported by contract numbers N01-HC-85079, N01-HC-85080, N01-HC-85081, N01-HC-85082, N01-HC-85083, N01-HC-85084, N01-HC-85085, N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, N01-HC-85239, and HHSN268201200036C; grant number U01 HL080295 from the NHLBI and R01 AG-023629 from the National Institute on Aging, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. The CARDIA is conducted and supported by the NHLBI in collaboration with the University of Alabama at Birmingham (N01-HC95095 and N01-HC48047), University of Minnesota (N01-HC48048), Northwestern University (N01-HC48049), and Kaiser Foundation Research Institute (N01-HC48050). The authors thank the staff and participants of the FHS, CHS, ARIC, and CARDIA studies for their important contributions. This manuscript was not prepared in collaboration with, nor approved by, investigators of the FHS, the CHS, the ARIC or CARDIA studies and does not necessarily reflect the opinions or views of the FHS, the CHS, the ARIC or CARDIA studies, Boston University, or the NHLBI.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fgene.2015.00313
FIGURE S1 | Haploview plot depicting intermarker linkage disequilibrium of 136 SLC2A9 variants associated with mean serum urate level by Köttgen et al. (2013) that include rs6449173 (right arrow) and the variant with the strongest association, rs12498742 (left arrow). Linkage disequilibrium range in the haplotype block is from 0 to 1, mean is 0.55, SD is 0.36.
Footnotes
References
Antón, F. M., Garcia Puig, J., Ramos, T., González, P., and Ordás, J. (1986). Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal h andling of urate. Metabolism 35, 343–348. doi: 10.1016/0026-0495(86)90152-6
Bao, Y., Curhan, G., Merriman, T., Plenge, R., Kraft, P., and Choi, H. K. (2015). Lack of gene-diuretic interactions on the risk of incident gout: the Nurses’ health study and health professionals follow-up study. Ann. Rheum. Dis. 74, 1394–1389. doi: 10.1136/annrheumdis-2014-206534
Batt, C., Phipps-Green, A. J., Black, M. A., Cadzow, M., Merriman, M. E., Topless, R., et al. (2014). Sugar-sweetened beverage consumption: a risk factor for prevalent gout with SLC2A9 genotype-specific effects on serum urate and risk of gout. Ann. Rheum. Dis. 73, 2101–2106. doi: 10.1136/annrheumdis-2013-203600
Brown, A. A., Buil, A., Viñuela, A., Lappalainen, T., Zheng, H.-F., Richards, J. B., et al. (2014). Genetic interactions affecting human gene expression identified by variance association mapping. eLife 3:e01381. doi: 10.7554/eLife.01381
Choi, H. K., Atkinson, K., Karlson, E. W., Willett, W., and Curhan, G. (2004a). Alcohol intake and risk of incident gout in men: a prospective study. Lancet 363, 1277–1281. doi: 10.1016/S0140-6736(04)16000-5
Choi, H. K., Atkinson, K., Karlson, E. W., Willett, W., and Curhan, G. (2004b). Purine-rich foods, dairy and protein intake, and the risk of gout in men. N. Engl. J. Med. 350, 1093–1103. doi: 10.1056/NEJMoa035700
Choi, H. K., and Curhan, G. (2004). Beer, liquor, and wine consumption and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 51, 1023–1029. doi: 10.1002/art.20821
Choi, H. K., and Curhan, G. (2008). Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ 336, 309–312. doi: 10.1136/bmj.39449.819271.BE
Choi, H. K., Liu, S., and Curhan, G. (2005). Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 52, 283–289. doi: 10.1002/art.20761
Choi, J. W., Ford, E. S., Gao, X., and Choi, H. K. (2008). Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 59, 109–116. doi: 10.1002/art.23245
Choi, J. W., Ford, E. S., Gao, X., and Choi, H. K. (2012). Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 344:d8190. doi: 10.1136/bmj.d8190
Crowley, L. V. (1964). Determination of uric acid an automated analysis based on a carbonate method. Clin. Chem. 10, 838–844.
Cushman, M., Cornell, E. S., Howard, P. R., Bovill, E. G., and Tracy, R. P. (1995). Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin. Chem. 41, 264–270.
Dalbeth, N., House, M. E., Gamble, G. D., Horne, A., Pool, B., Purvis, L., et al. (2013). Population-specific influence of SLC2A9 genotype on the acute hyperuricaemic response to a fructose load. Ann. Rheum. Dis. 72, 1868–1873. doi: 10.1136/annrheumdis-2012-202732
Dalbeth, N., and Merriman, T. (2013). “Hyperuricemia and gout,” in The online metabolic and molecular bases of inherited disease (OMMBID), eds D. Valle, A. Beaudet, B. Vogelstein, K. Kinzler, S. Antonarakis, and A. Ballabio (New York, NY: McGraw-Hill Medical), 11, 106.
Delaneau, O., Marchini, J., and 1000 Genomes Project Consortium. (2014). Integrating sequence and array data to create an improved 1000 Genomes Project haplotype reference panel. Nat. Commnu. 5:3934. doi: 10.1038/ncomms4934
Döring, A., Gieger, C., Mehta, D., Gohlke, H., Prokisch, H., and Coassin, S,. et al. (2008). SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat. Genet. 40, 430–436. doi: 10.1038/ng.107
Dyer, A., Liu, K., Walsh, M., Kiefe, C., Jacobs, D. R. Jr., and Bild, D. E. (1999). Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J. Hum. Hypertenns. 13, 13–21.
Flynn, T. J., Cadzow, M., Dalbeth, N., Jones, P. B., Stamp, L. K., Harré Hindmarsh, J., et al. (2015). Positive association of tomato consumption with serum urate: support for tomato consumption as an anecdotal trigger of gout flares. BMC Musculoskelet. Disord. 16:196. doi: 10.1186/s12891-015-0661-8
Geiler-Samerotte, K., Bauer, C., Li, S., Ziv, N., Gresham, D., and Siegal, M. (2013). The details in the distributions: why and how to study phenotypic variability. Curr. Opin. Biotechnol. 24, 752–759. doi: 10.1016/j.copbio.2013.03.010
Ghio, A. J., Ford, E. S., Kennedy, T. P., and Hoidal, J. R. (2005). The association between serum ferritin and uric acid in humans. Free Radic. Res. 39, 337–342. doi: 10.1080/10715760400026088
Gotfredsen, A., Christiansen, C., and Transbøl, I. (1983). Effect of natural oestrogen/gestagen therapy on uric acid metabolism in post-menopausal women. Maturitas 5, 9–15. doi: 10.1016/0378-5122(83)90016-6
Hak, A. E., and Choi, H. K. (2008). Menopause, postmenopausal hormone use and serum uric acid levels in US women–the Third National Health and Nutrition Examination Survey. Arthritis Res. Ther. 10:R116. doi: 10.1186/ar2519
Henry, R., Sobel, C., and Kim, J. (1957). A modified carbonate-phosphotungstate method for the determination of uric acid and comparison with the spectrophotometric uricase method. Am. J. Clin. Pathol. 28, 152–160.
Hollis-Moffatt, J. E., Xu, X., Dalbeth, N., Merriman, M. E., Topless, R., Waddell, C., et al. (2009). Role of the urate transporter SLC2A9 gene in susceptibility to gout in New Zealand Maori, Pacific Island, and Caucasian case-control sample sets. Arthritis Rheum. 60, 3485–3492. doi: 10.1002/art.24938
Investigators, A. R. I. C. (1989). The atherosclerosis risk in communities (aric) study: design and objectives. Am. J. Epidemiol. 129, 687–702.
Jacobs, G. H. (2009). Evolution of colour vision in mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2957–2967. doi: 10.1098/rstb.2009.0039
Khosla, S., Melton, L. J. III, Atkinson, E. J., O’Fallon, W. M., Klee, G. G., and Riggs, B. L. (1998). Relationship of serum sex steroids and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J. Clin. Endocrinol. Metab. 83, 2266–2274. doi: 10.1210/jc.83.7.2266
Köttgen, A., Albrecht, E., Teumer, A., Vitart, V., Krumsiek, J., Hundertmark, C., et al. (2013). Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 45, 145–154. doi: 10.1038/ng.2500
Lyngdoh, T., Vuistiner, P., Marques-Vidal, P., Rousson, V., Waeber, G., Vollenweider, P., et al. (2012). Serum uric acid and adiposity: deciphering causality using a bidirectional Mendelian randomization approach. PLoS ONE 7:e39321. doi: 10.1371/journal.pone.0039321
Mainous, A. G., Knoll, M. E., Everett, C. J., Matheson, E. M., Hulihan, M. M., and Grant, A. M. (2011). Uric acid as a potential cue to screen for iron overload. J. Am. Board Fam. Med. 24, 415–421. doi: 10.3122/jabfm.2011.04.110015
Manolio, T. A., Collins, F. S., Cox, N. J., Goldstein, D. B., Hindorff, L. A., Hunter, D. J., et al. (2009). Finding the missing heritability of complex diseases. Nature 461, 747–753. doi: 10.1038/nature08494
McAdams-DeMarco, M. A., Maynard, J. W., Baer, A. N., Kao, L. W., Kottgen, A., and Coresh, J. (2013). A urate gene-by-diuretic interaction and gout risk in participants with hypertension: results from the ARIC study. Ann. Rheum. Dis. 72, 701–706. doi: 10.1136/annrheumdis-2011-201186
Mumford, S. L., Dasharathy, S. S., Pollack, A. Z., Perkins, N. J., Mattison, D. R., Cole, S. R., et al. (2013). Serum uric acid in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle study. Hum. Reprod. 28, 1853–1862. doi: 10.1093/humrep/det085
Nicholls, A., Snaith, M., and Scott, J. (1973). Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br. Med. J. 1, 449–451. doi: 10.1136/bmj.1.5851.449
Okada, Y., Sim, X., Go, M. J., Wu, J.-Y., Gu, D., Takeuchi, F., et al. (2012). Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat. Genet. 44, 904–909. doi: 10.1038/ng.2352
Palmer, T. M., Nordestgaard, B. G., Benn, M., Tybjaerg-Hansen, A., Davey Smith, G., Lawlor, D. A., et al. (2013). Association of plasma uric acid with ischaemic heart disease and blood pressure: mendelian randomisation analysis of two large cohorts. BMJ 347, f4262. doi: 10.1136/bmj.f4262
Paré, G., Cook, N. R., Ridker, P. M., and Chasman, D. I. (2010). On the use of variance per genotype as a tool to identify quantitative trait interaction effects: a report from the Women’s Genome Health Study. PLoS Genet. 6:e1000981. doi: 10.1371/journal.pgen.1000981
Patterson, N., Price, A. L., and Reich, D. (2006). Population structure and eigenanalysis. PLoS Genet. 2:e190. doi: 10.1371/journal.pgen.0020190
Rasheed, H., Phipps-Green, A., Topless, R., Hollis-Moffatt, J. E., Harre Hindmarsh, J., Franklin, C., et al. (2013). Association of the lipoprotein receptor-related protein 2 gene with gout and non-additive interaction with alcohol. Arthritis Res. Ther. 15:R177. doi: 10.1186/ar4366
Scharpf, R. B., Mireles, L., Yang, Q., Köttgen, A., Ruczinski, I., Susztak, K., et al. (2014). Copy number polymorphisms near SLC2A9 are associated with serum uric acid concentrations. BMC Genet. 15:81. doi: 10.1186/1471-2156-15-81
Simon, J. A., Lin, F., Vittinghoff, E., and Bittner, V. (2006). The relation of postmenopausal hormone therapy to serum uric acid and the risk of coronary heart disease events: the Heart and Estrogen–Progestin Replacement Study (HERS). Ann. Epidemiol. 16, 138–145. doi: 10.1016/j.annepidem.2005.04.003
Simpson, E. R., and Davis, S. R. (2001). Minireview: aromatase and the regulation of estrogen biosynthesis—some new perspectives. Endocrinology 142, 4589–4594. doi: 10.1210/en.142.11.4589
Stahl, E., Choi, H., Cadzow, M., Flynn, T., Topless, R., and Merriman, T. R. (2014). Conditional analysis of 30 serum urate loci identifies 25 additional independent effects. Arthritis Rheum. 65S:2961.
Stöckl, D., Döring, A., Thorand, B., Heier, M., Belcredi, P., and Meisinger, C. (2012). Reproductive factors and serum uric acid lebels in females from the general population: the KORA F4 study. PLoS ONE 7:e32668. doi: 10.1371/journal.pone.0032668
Struchalin, M. V., Dehghan, A., Witteman, J. C., van Duijn, C., and Aulchenko, Y. S. (2010). Variance heterogeneity analysis for detection of potentially interacting genetic loci: method and its limitations. BMC Genet. 11:92. doi: 10.1186/1471-2156-11-92
Sumino, H., Ichikawa, S., Kanda, T., Nakamura, T., and Sakamaki, T. (1999). Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet 354, 650. doi: 10.1016/S0140-6736(05)77129-4
Takiue, Y., Hosoyamada, M., Kimura, M., and Saito, H. (2011). The effect of female hormones upon urate transport systems in the mouse kidney. Nucleosides Nucleotides Nucleic Acids 30, 113–119. doi: 10.1080/15257770.2010.551645
Vitart, V., Rudan, I., Hayward, C., Gray, N. K., Floyd, J., Palmer, C. N., et al. (2008). SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 40, 437–442. doi: 10.1038/ng.106
Voruganti, V. S., Franceschini, N., Haack, K., Laston, S., MacCleur, J. W., Umans, J. G., et al. (2014). Replication of the effect of SLC2A9 genetic variation on serum uric acid levels in American Indians. Eur. J. Hum. Genet. 22, 938–943. doi: 10.1038/ejhg.2013.264
Wei, W.-H., Guo, Y., Kindt, A. S., Merriman, T. R., Semple, C. A., Wang, K., et al. (2014). Abundant local interactions in the 4p16. 1 region suggest functional mechanisms underlying SLC2A9 associations with human serum uric acid. Hum. Mol. Genet. 23, 5061–5068. doi: 10.1093/hmg/ddu227
Yahyaoui, R., Esteva, I., Haro-Mora, J. J., Almaraz, M. C., Morcillo, S., Rojo-Martinez, G., et al. (2008). Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J. Clin. Endocrinol. Metab. 93, 2230–2233. doi: 10.1210/jc.2007-2467
Yang, J., Loos, R. J., Powell, J. E., Medland, S. E., Speliotes, E. K., Chasman, D. I., et al. (2012). FTO genotype is associated with phenotypic variability of body mass index. Nature 490, 267–272. doi: 10.1038/nature11401
Keywords: genotype, exposure, interaction, urate, SLC2A9, variance, gout, uric acid
Citation: Topless RK, Flynn TJ, Cadzow M, Stamp LK, Dalbeth N, Black MA and Merriman TR (2015) Association of SLC2A9 genotype with phenotypic variability of serum urate in pre-menopausal women. Front. Genet. 6:313. doi: 10.3389/fgene.2015.00313
Received: 21 July 2015; Accepted: 02 October 2015;
Published: 14 October 2015.
Edited by:
Jill Barnholtz-Sloan, Case Western Reserve University, USAReviewed by:
Cheryl L. Thompson, Case Western Reserve University, USARobin Taylor Wilson, The Pennsylvania State University, USA
Matthew B. B. Schabath, Moffitt Cancer Center, USA
Copyright © 2015 Topless, Flynn, Cadzow, Stamp, Dalbeth, Black and Merriman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tony R. Merriman, tony.merriman@otago.ac.nz
 Ruth K. Topless
Ruth K. Topless Tanya J. Flynn
Tanya J. Flynn Murray Cadzow
Murray Cadzow Lisa K. Stamp
Lisa K. Stamp Nicola Dalbeth
Nicola Dalbeth Michael A. Black
Michael A. Black Tony R. Merriman
Tony R. Merriman